- Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, China
Oral-gut pathogens are closely associated with pancreatic cancer, such as Campylobacter jejuni, Clostridium difficile, Enterococcus faecalis, Escherichia coli, Fusobacterium nucleatum, Helicobacter pylori, Porphyromonas gingivalis, and Vibrio cholera, but the related mechanisms remain not well understood. Phosphatase and tensin homolog (PTEN, a widely known tumor suppressor) play a key role in the anti-cancer immune system. Pancreatic cancer cells with PTEN loss are often in the immunosuppressive tumor microenvironment regulated by myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2 macrophages, which are regarded as the mechanism in the immune escape of cancers. The miR-21, as an oncogene in human cancers, plays an important role in pancreatic cancer progression, downregulates the levels of PTEN, and may promote cancer to evade host immune surveillance. Some oral-gut pathogens have been found to promote miR-21 expression and reduce PTEN expression. On the other hand, most gut pathogens infection is thought to produce reactive oxygen species (ROS) or activate inflammatory cytokines, which may also induce ROS-mediated miR-21 expression. These pathogens' infection is involved with the cell density of MDSCs, Tregs, and M2 macrophages. Therefore, it is quite reasonable to propose that oral-gut pathogens possibly promote pancreatic cancer escaping from host immune surveillance by activating the miR-21/PTEN axis and immune-suppressive cells. The present exploration suggests that an increased understanding of the pattern of the effects of gut pathogens on the miR-21/PTEN axis will lead to better insights into the specific mechanisms associated with the immune escape of pancreatic cancer caused by oral-gut microbiota.
Introduction
Pancreatic cancer is highly metastatic and lethal (Pourshams et al., 2019). It is urgent to understand the cause of pancreatic cancer progression and possible mechanisms. Most oral-gut microbiota forms a symbiotic relationship with humans after long-term mutual adaptation. More and more researchers have begun to pay attention to the link between human microbiota and pancreatic cancer (Zhang et al., 2020a), but the related mechanisms remain not well understood.
Lipopolysaccharide (LPS) is the main ingredient of bacterial cell walls and may increase pancreatic cancer risk by secreting pro-inflammatory cytokines (Yaw et al., 2020). Oral-gut pathogen infection has long-term effects to produce ROS or stimulate inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) (Supplementary Table 1). IL-1β induces ROS and nitric oxide (NO) production via phosphatidylinositol-3-kinase (PI3K)/Akt signaling (Rao et al., 2017). IL-6 promotes Epithelial-Mesenchymal transition (EMT) and metastasis through ROS production in cancer cells (Dong et al., 2018). TNF-α induces ROS production in human leukemia U937 cells (Moon et al., 2010). ROS often causes DNA damage, which is closely associated with radiotherapy resistance to pancreatic cancer therapy (Zhang et al., 2022). Furthermore, ROS has been confirmed to activate miR-21 (multiple-faceted biomarkers in various types of cancers) expression and functions (Zhang et al., 2020b). Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a direct target of miR-21 and the downregulation of miR-21 will upregulate PTEN levels and inhibit pancreatic cancer metastasis (Zhang et al., 2019).
Cancer cells with PTEN downregulation are often in the immunosuppressive tumor microenvironment regulated by myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2 macrophages (Vidotto et al., 2020). Therefore, the miR-21/PTEN axis plays an important role in the immune escape of pancreatic cancer. The importance of the relationship between gut pathogens and pancreatic cancer risk was explored by investigating the miR-21/PTEN axis.
The Gut Pathogens Associated with Pancreatic Cancer Risk
Campylobacter jejuni is a bacterial enteric pathogen often causing diarrhea and enterocolitis, and its Cas9 protein contributes to the carcinogenesis of pancreatic cancer (Chang et al., 2020). Clostridium difficile is a bacterium that causes mild to severe diarrhea and its infection is mostly found in patients with pancreatic ductal adenocarcinoma (PDAC) (Sumiyoshi et al., 2022). Enterococcus faecalis is a commensal Gram-positive pathogen and its infection may induce chronic pancreatitis and cancer (Maekawa et al., 2018). The presence of Escherichia coli in the bloodstream can promote the progression of pancreatic cancer (Bai et al., 2022). Fusobacterium nucleatum is a periodontal bacterium linked with a wide spectrum of human diseases and its infection occurrence in pancreatic cancer can cause shorter survival of patients (Mitsuhashi et al., 2015). Helicobacter pylori is a widely reported bacillus that can damage the tissue in the human stomach and duodenum, and its infection is linked to the development of pancreatic cancer (Kunovsky et al., 2021). Porphyromonas gingivalis is a widely reported dental pathogen, and pancreatic cancer development is promoted by its infection (Chen et al., 2020). Vibrio cholerae is a halophilic, facultative, and anaerobic bacillus, and is linked to pancreatic cancer metastasis (Cavuoti et al., 2002). The oral-gut pathogens related to pancreatic cancer risk were chosen for exploring the possible molecular mechanisms for the effects of oral-gut microbiota on pancreatic cancer progression (Supplementary Table 1).
Gut Pathogens Possibly Promote Pancreatic Cancer to Escape from Immune Surveillance by Activating the MIR-21/PTEN Axis
The levels of miR-21 were found to be upregulated and the levels of PTEN were downregulated in the pancreatic cancer cell lines, such as CAPAN-1, BxPC-3, JF305, PANC-1, and SW1990, when compared with those in normal human pancreatic HPDE6-C7 cells. The upregulation of miR-21 and the downregulation of PTEN will promote these pancreatic cancer cell metastasis (Zhang et al., 2019). The miR-21 stimulates chemoresistance toward 5-fluorouracil in pancreatic cancer cells PATU8988 and PANC-1 by downregulating the levels of PTEN. The inhibition of miR-21 will rescue PTEN levels and increase the chemotherapy sensitivity of these pancreatic cancer cells (Wei et al., 2016). Similar results were also reported for other chemotherapy resistance in human pancreatic cancer cell lines MIAPaCa-E, MIAPaCa-M, and BxPC-3, which were resistant to gemcitabine. The downregulation of miR-21 caused the increase in tumor suppressor PTEN levels, which improved the sensitivity of these pancreatic cancer cells to gemcitabine (Ali et al., 2010). Among all the pathogens, Bacteroidetes, E. coli, F. nucleatum, Helicobacter pylori, P. gingivalis, and P. aeruginosa infection will result in an increase in miR-21 level and a decrease in PTEN levels (Supplementary Table 1). According to the above results, the upregulation of miR-21 and downregulation of PTEN will certainly promote pancreatic cancer cell metastasis and chemotherapy resistance. Campylobacter jejuni, Citrobacter rodentium, Clostridium difficile, Enterococcus faecalis, Streptococcus B, and Staphylococcus aureus infection can cause an increase in miR-21 levels, which will also result in the reduction in the level of PTEN (Supplementary Table 1). In this case, the pathogens' infection will cause pancreatic cancer cell metastasis, as well as chemoresistance. Salmonella typhimurium and Treponema infection will lead to a reduction in PTEN levels (Supplementary Table 1), which will promote pancreatic cancer cell proliferation since PTEN is a widely reported tumor suppressor.
Just as was previously mentioned, PTEN loss will increase immune suppressive cells, such as MDSCs, Tregs, and M2 macrophages. Pancreatic cancer cells are mediated by multiple immune cells under a powerful immunosuppressive environment, in which the cancer cells still can easily proliferate. MDSCs are significantly increased in both the circulation and the microenvironment of pancreatic cancer cells and are closely associated with the clinical cancer stages of PDAC (Markowitz et al., 2015). The prevalence of Tregs is also prevalent in the peripheral blood, as well as in the tumor microenvironment of pancreatic cancer, which is linked with pancreatic cancer progression and premalignant lesions (Lytle et al., 2019). M2 macrophages create an anti-inflammatory microenvironment of pancreatic cancer cells and also facilitate its progression (Attri et al., 2018). The present summary shows that the changes in the cell density of MDSCs, Tregs, and M2 macrophages have been found in the most pathogen infection, except for Campylobacter jejuni (Supplementary Table 1), suggesting that the most oral-gut pathogens may help pancreatic cancer escape from host immune by affecting immune suppressive cells via miR-21 and PTEN axis.
From these results, a miR-21/PTEN axis may be involved with the immune escape of pancreatic cancer caused by an oral-gut pathogen. The upregulation of miR-21 inhibits PTEN expression, and its loss will increase in immune-suppressive cells, MDSCs, Tregs, and M2 macrophages. In this case, pancreatic cancer can evade host immune inhibition (Figure 1). For normal flora, if inflammatory cytokines and ROS maintain at a balanced level, the miR-21/PTEN axis will be inactivated. PTEN reaches a suitable level to control the immune-suppressive cells. CD8+ T cells, acting as mediators of adaptive immunity, selectively detect and eradicate cancer cells (Figure 1) (Philip and Schietinger, 2021). Furthermore, most pathogens' infections can cause an increase in inflammatory cytokine production. In the cytokine-rich microenvironment, pancreatic cancer develops a tolerance state to escape from host immune surveillance as well (Wang and Yao, 2019).
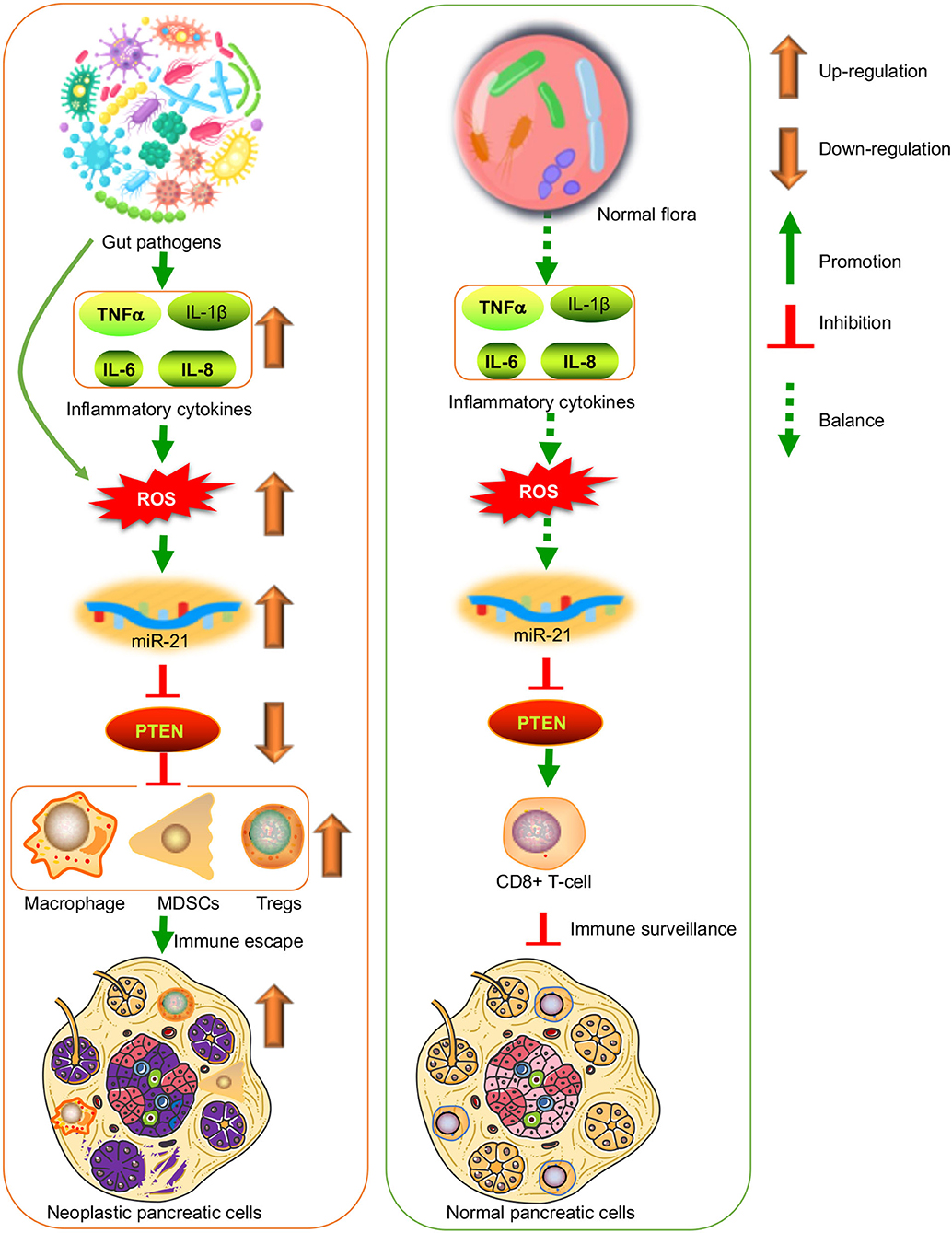
Figure 1. Oral-gut pathogens induce the immune escape of pancreatic cancer by affecting miR-21/PTEN axis via inflammatory cytokines-induced reactive oxygen species (ROS). It is suggestive that pathogen infection has long-term effects to stimulate inflammatory cytokines. Most of these cytokines can induced ROS products, which induce miR-21 expression and functions. Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a direct target of miR-21 and the upregulation of miR-21 will cause PTEN loss. Pancreatic cancer cells with PTEN downregulation are often in the immunosuppressive tumor microenvironment regulated by myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2 macrophages. On the other hand, the healthy gut microbiota will maintain normal cancer immune surveillance possibly via CD8+ T cells.
Discussion
This present exploration indicates that oral-gut pathogens are closely associated with pancreatic cancer metastasis, possibly by arousing the immune escape of cancer cells via the miR-21/PTEN axis and immune-suppressive cells. The pattern of the effects of gut pathogens on the miR-21/PTEN axis will lead to better insights into the specific mechanisms associated with the immune escape of pancreatic cancer caused by oral-gut microbiota. Notably, not all pathogens will promote pancreatic cancer progression or metastasis. In contrast, some pathogens are beneficial to control pancreatic cancer development. Listeria monocytogenes expressing mesothelin are potential vaccines for preventing pancreatic cancer metastasis (Le et al., 2015). A genetically-modified Salmonella typhimurium may provide an effective approach to the prevention of orthotopic human pancreatic cancer (Nagakura et al., 2009). Shigella flexneri shows anti-tumor impacts on pancreatic cancer cells (Khodavirdipour et al., 2021). Therefore, some gut pathogens may provide effective tools to develop clinical immunotherapies for pancreatic cancer.
There are some limitations to this mini-review. Only a few types of oral-gut pathogens associated with the metastasis of pancreatic cancer were analyzed in this mini-review. We only analyzed that miR-21/PTEN axis regulates the immune escape of pancreatic cancer. PTEN can be regulated by other miRNA and another miRNA can mediate PTEN levels, which were not explored in this mini-review. Still, there is no complete job to approve that oral or gut pathogen causes the immune escape of pancreatic cancer via the miR-21/PTEN axis, although the clues between the pathogen infection and miR-21/PTEN axis activation can be traced from different kinds of literature. Much work needs to be done to better understand the specific mechanism for the effects of oral-gut microbiota on pancreatic cancer progression.
Prospects and Challenges
A high prevalence of chemo-resistant pancreatic cancer may be caused by oral-gut pathogens. It is critical to diagnose and treat most people with oral-gut pathogens at the primary healthcare level. Most pathogens can also develop drug resistance and their therapy and control will become great challenges. Based on these common pathogen antigens, it may be an effective approach to explore multi-pathogen vaccines. According to the present summary, it is vital to maintain inflammatory and oxidative stress balance to prevent the development of pancreatic cancer. Furthermore, it will be a potential method to explore miR-21 and PTEN regulation via modern gene-editing techniques in a safe manner.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.928846/full#supplementary-material
References
Ali, S., Ahmad, A., and Banerjee, S. (2010). Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 70, 3606–3617. doi: 10.1158/0008-5472.CAN-09-4598
Attri, K. S., Mehla, K., and Singh, P. K. (2018). Evaluation of macrophage polarization in pancreatic cancer microenvironment under hypoxia. Hypoxia. 2018, 265–276. doi: 10.1007/978-1-4939-7665-2_23
Bai, C., Zhang, X., Yang, D., Li, D., Feng, H., and Li, Y. (2022). Clinical Analysis of Bloodstream Infection of Escherichia coli in Patients with Pancreatic Cancer from 2011 to 2019. Can. J. Infect. Dis. Med. Microbiol. 2022:1338188. doi: 10.1155/2022/1338188
Cavuoti, D., Fogli, M., Quinton, R., Gander, R. M., and Southern, P. M. (2002). Splenic abscess with Vibrio cholerae masking pancreatic cancer. Diag. Microbiol. Infect. Dis. 43, 311–313. doi: 10.1016/S0732-8893(02)00412-1
Chang, Y. J., Bae, J., and Zhao, Y. (2020). In vivo multiplex gene targeting with Streptococcus pyogens and Campylobacter jejuni Cas9 for pancreatic cancer modeling in wild-type animal. J. Vet. Sci. 21 doi: 10.4142/jvs.2020.21.e26
Chen, S-. M., Hsu, L-. J., and Lee, H-. L. (2020). Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-rasG12D Transgenic Mice. Cancers. 12, 3522. doi: 10.3390/cancers12123522
Dong, Y., Wu, Z., and He, M. (2018). ADAM9 mediates the interleukin-6-induced Epithelial–Mesenchymal transition and metastasis through ROS production in hepatoma cells. Cancer Lett. 421, 1–14. doi: 10.1016/j.canlet.2018.02.010
Khodavirdipour, A., Jamshidi, F., Nejad, H. R., Zandi, M., and Zarean, R. (2021). To study the anti-cancer effects of Shigella flexneri in AspC-1 pancreatic cancer cell line in approach to Bax and bcl-2 genes. J. Gastrointest. Cancer. 52, 593–599. doi: 10.1007/s12029-020-00433-9
Kunovsky, L., Dite, P., and Jabandziev, P. (2021). Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 13, 835. doi: 10.4251/wjgo.v13.i8.835
Le, D. T., Wang-Gillam, A., and Picozzi, V. (2015). Safety and survival with GVAX pancreas prime and Listeria monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 33, 1325. doi: 10.1200/JCO.2014.57.4244
Lytle, N. K., Ferguson, L. P., and Rajbhandari, N. (2019). A multiscale map of the stem cell state in pancreatic adenocarcinoma. Cell. 177, 572–586. e22. doi: 10.1016/j.cell.2019.03.010
Maekawa, T., Fukaya, R., and Takamatsu, S. (2018). Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Comm. 506, 962–969. doi: 10.1016/j.bbrc.2018.10.169
Markowitz, J., Brooks, T. R., and Duggan, M. C. (2015). Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol. Immunother. 64, 149–159. doi: 10.1007/s00262-014-1618-8
Mitsuhashi, K., Nosho, K., and Sukawa, Y. (2015). Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 6, 7209. doi: 10.18632/oncotarget.3109
Moon, D-. O., Kim, M-. O., Lee, J-. D., Choi, Y. H., and Kim, G-. Y. (2010). Rosmarinic acid sensitizes cell death through suppression of TNF-α-induced NF-κB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 288, 183–191. doi: 10.1016/j.canlet.2009.06.033
Nagakura, C., Hayashi, K., and Zhao, M. (2009). Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 29, 1873–1878.
Philip, M., and Schietinger, A. (2021). CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 12:1–15. doi: 10.1038/s41577-021-00574-3
Pourshams, A., Sepanlou, S. G., and Ikuta, K. S. (2019). The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 4, 934–947. doi: 10.1016/S2468-1253(19)30347-4
Rao, Z., Wang, S., and Wang, J. (2017). Peroxiredoxin 4 inhibits IL-1β-induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed. Pharmacother. 90, 414–420. doi: 10.1016/j.biopha.2017.03.075
Sumiyoshi, T., Uemura, K., and Aoki, G. (2022). Increased clostridium difficile infection in the era of preoperative chemotherapy for pancreatic cancer. Pancreatology. 22, 258–263. doi: 10.1016/j.pan.2021.12.009
Vidotto, T., Melo, C. M., Castelli, E., Koti, M., Dos Reis, R. B., and Squire, J. A. (2020). Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer. 122, 1732–1743. doi: 10.1038/s41416-020-0834-6
Wang, L. i. Z., and Yao, M. (2019). X, et al. Development of a novel EGFR-targeting antibody-drug conjugate for pancreatic cancer therapy. Target. Oncol. 14, 93–105. doi: 10.1007/s11523-018-0616-8
Wei, X., Wang, W., and Wang, L. (2016). Micro RNA-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating PTEN and PDCD 4. Cancer Med. 5, 693–702. doi: 10.1002/cam4.626
Yaw, A. C. K., Chan, E. W. L., Yap, J. K. Y., and Mai, C. W. (2020). The effects of NLRP3 inflammasome inhibition by MCC950 on LPS-induced pancreatic adenocarcinoma inflammation. J. Cancer Res. Clin. Oncol. 146, 2219–2229. doi: 10.1007/s00432-020-03274-y
Zhang, H., Feng, X., and Zhang, M. (2019). Long non-coding RNA CASC2 upregulates PTEN to suppress pancreatic carcinoma cell metastasis by downregulating miR-21. Cancer Cell Int. 19, 1–8. doi: 10.1186/s12935-019-0728-y
Zhang, X., Liu, Q., Liao, Q., and Zhao, Y. (2020a). Pancreatic cancer, gut microbiota, and therapeutic efficacy. J. Cancer. 11, 2749. doi: 10.7150/jca.37445
Zhang, Y., Houchen, C.W., and Li, M. (2022). Attenuating DNA damage response and immunosuppression radiosensitizes pancreatic cancer. EBioMedicine. 76:103822. doi: 10.1016/j.ebiom.2022.103822
Keywords: oral-gut pathogens, pancreatic cancer, metastasis, miR-21/PTEN axis, immune escape
Citation: Li R, Hu Y and Hou S (2022) An Exploration of Oral-Gut Pathogens Mediating Immune Escape of Pancreatic Cancer via miR-21/PTEN Axis. Front. Microbiol. 13:928846. doi: 10.3389/fmicb.2022.928846
Received: 26 April 2022; Accepted: 10 May 2022;
Published: 22 June 2022.
Edited by:
George Grant, University of Aberdeen, United KingdomReviewed by:
Italia Falcone, Regina Elena National Cancer Institute (IRCCS), ItalyCopyright © 2022 Li, Hu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhong Hou, housh@sj-hospital.org
 Rui Li
Rui Li Yaoyuan Hu
Yaoyuan Hu Shuhong Hou
Shuhong Hou