- 1Faculty of Human Science and Public Health, School of Health and Social Sciences, Bournemouth University, Bournemouth, United Kingdom
- 2School of Psychology, Ulster University, Coleraine, United Kingdom
- 3Norwich Medical School, University of East Anglia, Norwich, United Kingdom
- 4Acquired Brain Injury Rehabilitation Alliance, School of Health Sciences, University of East Anglia, Norwich, United Kingdom
Background: Better upper limb recovery after stroke could be achieved through tailoring rehabilitation interventions directly at movement deficits.
Aim: To identify potential; targets for therapy by synthesizing findings of differences in kinematics and muscle activity between stroke survivors and healthy adults performing reach-to-target tasks.
Methods: A systematic review with identification of studies, data extraction, and potential risk of bias was completed independently by two reviewers. Online databases were searched from their inception to November 2017 to find studies of reach-to-target in people-with-stroke and healthy adults. Potential risk-of-bias was assessed using the Down's and Black Tool. Synthesis was undertaken via: (a) meta-analysis of kinematic characteristics utilizing the standardized mean difference (SMD) [95% confidence intervals]; and (b), narrative synthesis of muscle activation.
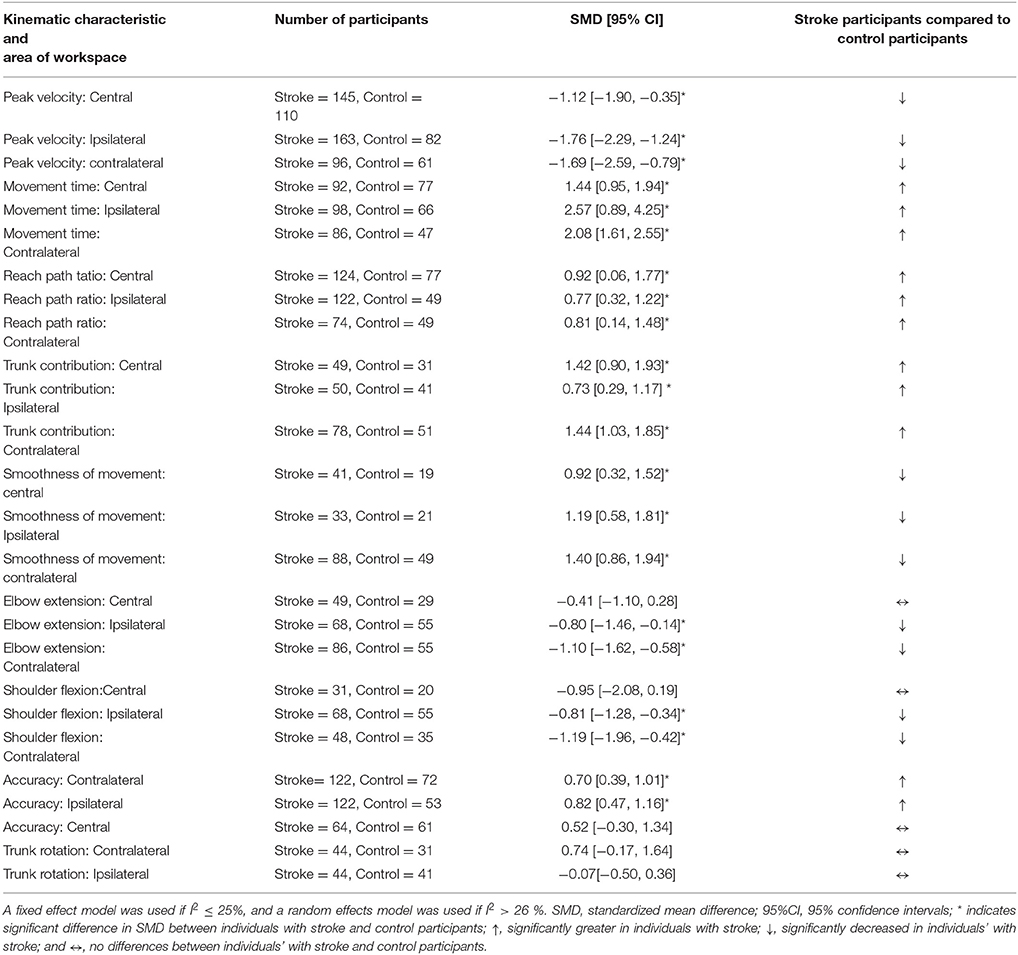
Results: Forty-six studies met the review criteria but 14 had insufficient data for extraction. Consequently, 32 studies were included in the meta-analysis. Potential risk-of-bias was low for one study, unclear for 30, and high for one. Reach-to-target was investigated with 618 people-with-stroke and 429 healthy adults. The meta-analysis found, in all areas of workspace, that people-with-stroke had: greater movement times (seconds) e.g., SMD 2.57 [0.89, 4.25]; lower peak velocity (millimeters/second) e.g., SMD −1.76 [−2.29, −1.24]; greater trunk displacement (millimeters) e.g. SMD 1.42 [0.90, 1.93]; a more curved reach-path-ratio e.g., SMD 0.77 [0.32, 1.22] and reduced movement smoothness e.g., SMD 0.92 [0.32, 1.52]. In the ipsilateral and contralateral workspace, people-with-stroke exhibited: larger errors in target accuracy e.g., SMD 0.70 [0.39, 1.01]. In contralateral workspace, stroke survivors had: reduced elbow extension and shoulder flexion (degrees) e.g., elbow extension SMD −1.10 [−1.62, −0.58] and reduced shoulder flexion SMD −1.91 [−1.96, −0.42]. Narrative synthesis of muscle activation found that people-with-stroke, compared with healthy adults, exhibited: delayed muscle activation; reduced coherence between muscle pairs; and use of a greater percentage of muscle power.
Conclusions: This first-ever meta-analysis of the kinematic differences between people with stroke and healthy adults performing reach-to-target found statistically significant differences for 21 of the 26 comparisons. The differences identified and values provided are potential foci for tailored rehabilitation interventions to improve upper limb recovery after stroke.
Introduction
Reaching is essential for everyday activities such as drinking, using a touch screen or operating buttons on an elevator. Rehabilitation therefore gives emphasis to regaining reaching ability through evidenced-based task-specific training. Many people after stroke have upper limb disability, for example: approximately 48% of a consecutive admissions sample at three days after stroke(1); and 65% of individuals with severe stroke not regaining the ability to reach and grasp everyday objects despite participation in rehabilitation (2).
There are many different therapy approaches available to clinicians to progress upper limb motor function. An alternative to best conventional therapy is offered by impairment-orientated therapy (3). This impairment-orientated training involves targeting interventions at the movement control deficits underlying difficulty and inability to perform everyday functional tasks. Therefore, a precursor to continuing investigation of impairment-orientated training is to identify the exact movement control deficits experienced by stroke survivors.
Movement control deficits can be identified by kinematic assessment providing sensitive, objective and reliable measurement (4–9). Therefore, kinematic assessment can be used to identify movement control deficits as targets for impairment-orientated training after stroke. Indeed, reaching kinematics has been studied widely in both healthy populations (10–12) and in people after stroke (13–16). Even more information can be gained by combining kinematics with measurement of muscle activity (17). For example, electromyography (EMG) provides neurological measures such as spatial-temporal patterns of muscle activity for enhanced understanding of the movement control (kinematics and muscle activity) underlying the performance of everyday tasks (18).
Knowledge of the kinematics of all forms of reaching (4, 19) and more specifically, coordination of reach and grasp components (20), has been drawn together in narrative reviews. These reviews are valuable as they provide an expert overview of the kinematics of reaching activity. However, narrative reviews have potential for bias in at least two aspects: identification of the primary studies included (selection bias); and the possibility that synthesis is influenced by author opinion (expert opinion bias). A robust systematic review is required to minimize the risk of potential bias. In addition, review of the neural components of reaching is required alongside the kinematics.
To understand reaching impairment we need to consider the different forms of reaching required for everyday activity e.g., reach-to-target (operate elevator buttons), reach-to-release (put can on shelf); reach-to manipulate (cut paper with scissors); and reach-to-pull (open cupboard). In addition, reaching activity takes place in many workspace areas including: above the head, behind the trunk and to the contralateral side of the reaching upper limb. Diverse forms of reaching for performance of everyday tasks require the ability to utilize different spatial-temporal patterns of muscle activity and limb segment orientations (4, 19). Indeed, kinematic characteristics vary depending on the reaching task and goal (21, 22). A prerequisite for development of impairment-orientated rehabilitation, therefore, requires knowledge of the movement control deficits underlying difficulty performing everyday reaching tasks to enable therapy to be targeted at what needs to change.
The aims of this systematic review were to: (1) systematically synthesise the differences between individuals with stroke and healthy adults for the kinematics and muscle activations of reach-to-target; and (2) determine the potential influence of object location on the differences in kinematics and muscle activity. Reach-to-target was chosen because it is the precursor component of most everyday upper limb tasks and is essential for many daily activities such as a using touch screen (tablet, computer), turning on/off light switch, and using a doorbell, or elevator.
Methods
The systematic review methodology was based on guidelines by the Cochrane Collaboration (23). Two reviewers worked independently at each stage: title and abstract screening, full text screening, assessment of potential risk of bias, and data extraction. Each reviewer recorded their assessment on a pre-agreed proforma. If there were disagreements the two reviewers referred to the original document in question. If agreement could not be reached then a third researcher was consulted.
Searching for Studies
The search strategy was developed in collaboration with a research librarian. The search was limited to studies published in the English language. The search terms used included: reaching, upper limb, kinematics, biomechanics, movement analysis, electromyography, and stroke. The terms were a combination of MeSH and non-MeSH terms used as text words. Three online databases were searched: MEDLINE, AMED, and EMBASE; the databases were searched from their inception to November 2017. Due to the differences between databases the search strategy was modified for each individual database; an example of the search strategy used for MEDLINE is in Table 1. In addition, the reference lists of relevant papers were hand searched for potential articles that were not retrieved in the electronic search.
Study Eligibility Criteria
Types of Studies
All study designs were included except for single case studies, and reviews. Included studies of people after stroke also needed to investigate healthy adults (control) completing identical reach-to-target tasks.
Types of Participants
The participants in eligible studies had to be at least 18 years of age. For people after stroke there were no limitations placed on lesion location, time since ictus, or number of strokes. Healthy adult participants needed to have no diagnosis of a neurological or musculoskeletal disorder that could potentially influence movement control or reaching.
Types of Reaching Tasks
Studies were eligible if reaching to a target was assessed with the paretic upper limb of the people after stroke and either upper limb of the healthy adult participants. Specific exclusion criteria were: reach-to-grasp of an object, tapping, tracing, drawing tasks, or reaching with the non-paretic limb (stroke survivors).
Types of Measures
Eligible studies employed kinematic assessment (motion analysis); muscle activity (electromyography, EMG); and/or corticospinal pathway excitability (transcranial magnetic stimulation, TMS) during the reach-to-target task.
Identification of Studies
Studies were assessed as not relevant, probably relevant, or relevant. Title and abstract were screened together. For those studies deemed as either relevant or probably relevant their full texts were then screened (23, 24). Those studies which met the eligibility criteria were included in this review.
Potential Risk of Bias
The majority of included studies used observational designs, therefore, the Downs and Black tool was used to assess potential risk of bias (25). The tool was modified by using just the criteria pertinent to potential risk of bias of observational study designs (23, 26). For example: the removal of questions relating to randomization, group allocation, and group concealment (26–28).
Data Extraction
The data extracted were: number of participants, participants' age, time since stroke, reach-to-target task description, use of trunk restraint, upper limb motor ability, kinematic characteristics (e.g., velocity), EMG data (e.g., muscle activity). Some included studies evaluated the effect of an intervention. For these, only the baseline data (pre-intervention) were extracted. For studies in which the published data were unclear or missing then the authors were emailed to request clarification/more details.
Synthesis
A meta-analysis was undertaken for measures where two or more included studies reported measurement values of the same movement characteristic. A narrative synthesis was performed if there was insufficient similarity across included studies.
If a study included data for multiple reach-to-target tasks one task was selected to be included in the meta-analysis. The task selected was the one most similar to the rest of the studies in the meta-analysis. For example, reaching at a self-paced speed versus fast speeds, tasks in which reaching distances were most similar, and most similar grip (23).
The meta-analysis used the Cochrane Statistical package, RevMan 5.2, to compare the group means and standard deviations of the kinematic characteristics of people after stroke and healthy adult participants. The heterogeneity of data was assessed using the I2 statistic and interpreted as low for a value ≤ 25%, high for a value of ≥ 75% and moderate for all values in between (23, 29, 30). If heterogeneity was low a fixed effect model was used; if heterogeneity was moderate or high a random effects model was used (23, 30). The standardized mean difference (SMD) was calculated (23).
Results
Identification of Studies
The flowchart describing the results of the search is provided the PRISMA diagram Figure 1. In summary, 2,222 records were identified after duplicates were removed. Following title, abstract, and full text screening 46 studies met the inclusion criteria, however, 14 were subsequently excluded because the relevant data could not be extracted (9, 31–43). Therefore, there are 33 studies included in the synthesis (5, 7, 13, 14, 16, 44–71). There were two pairs of studies that reported two reaching tasks in the same cohort (16, 63, 67, 68) so participants were only counted once in any particular meta-analysis.
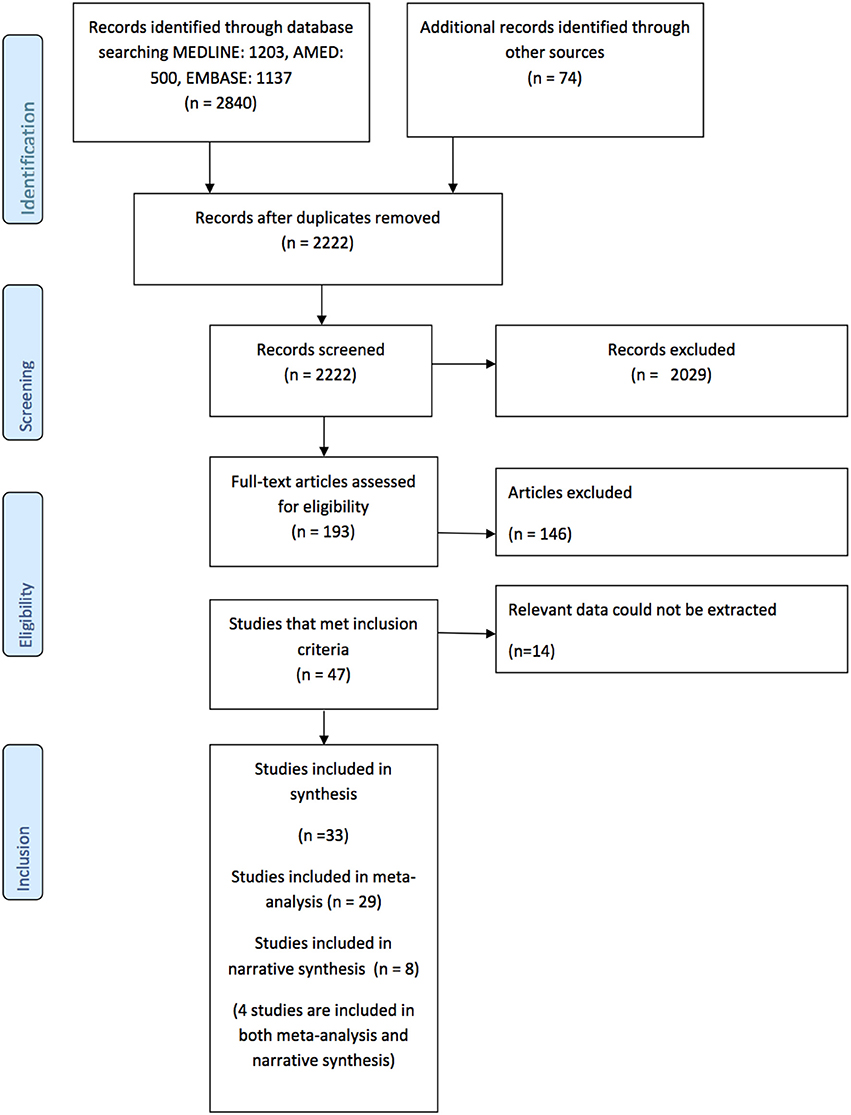
Figure 1. Prisma Diagram detailing the search and processes of identification of studies included in the systematic review.
Included Studies
Observational designs were used by 27 of the 32 studies and five studies used experimental designs (5, 45, 47, 48, 61, 69). The included studies investigated reach-to-target with 618 people after stroke and 429 healthy adult participants. The mean number (standard deviation, SD) of individuals per included study was 17.2 ± 9.9 people after stroke and 11.9 ± 9.3 control participants.
Participants
The mean age (SD) of: people after stroke was 58.4 ± 9.3 years whilst healthy adult participants were a mean (SD) of 54.0 ± 10.0 years. The mean time after stroke, calculated from the data reported, was 25.6 ± 23.1 months. Full details of participants are provided in Tables 2–5 according to the placement of the target in the workspace.
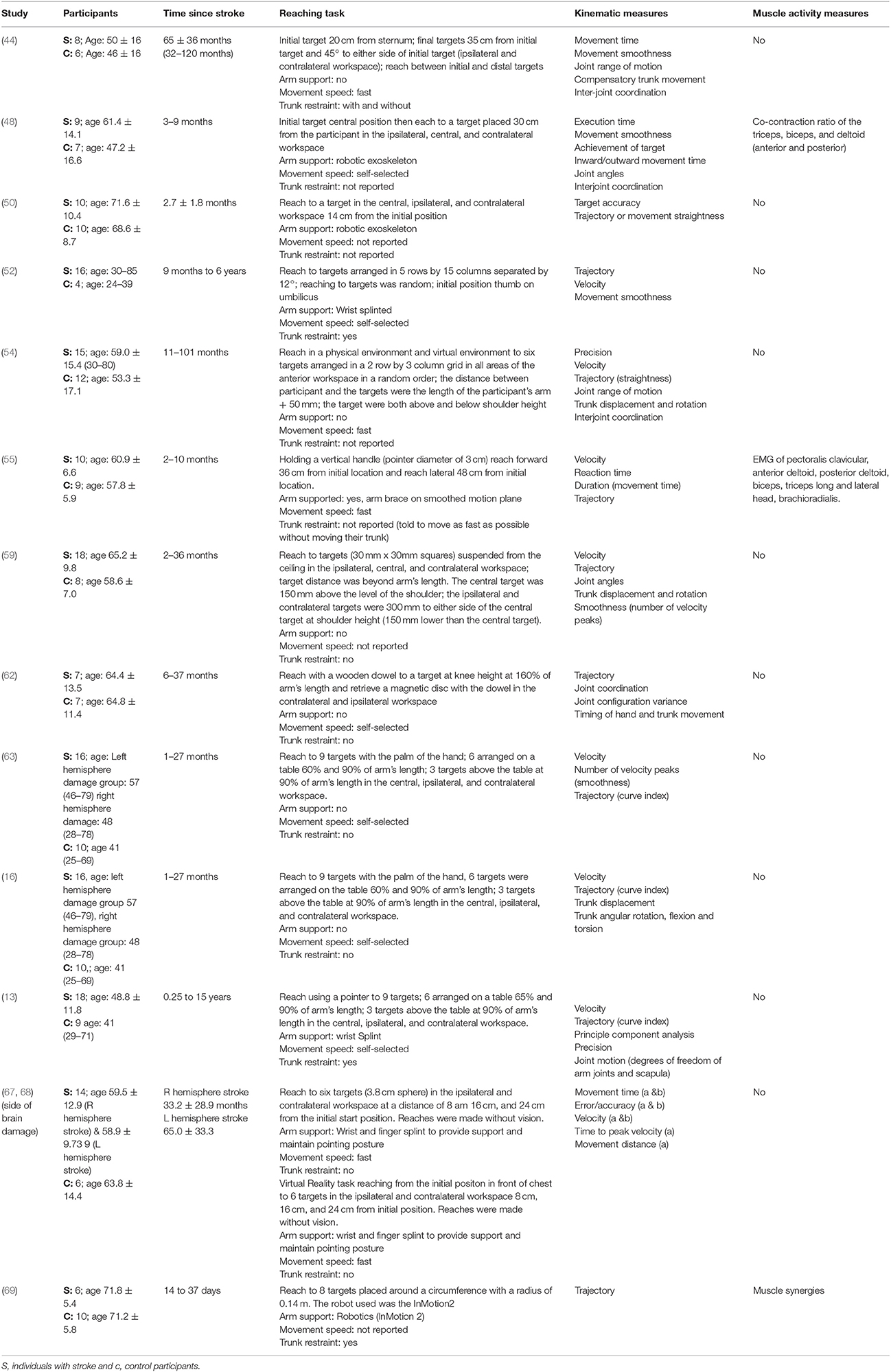
Table 2. Characteristics of included studies investigating reach-to-target in multiple anterior areas of the workspace.
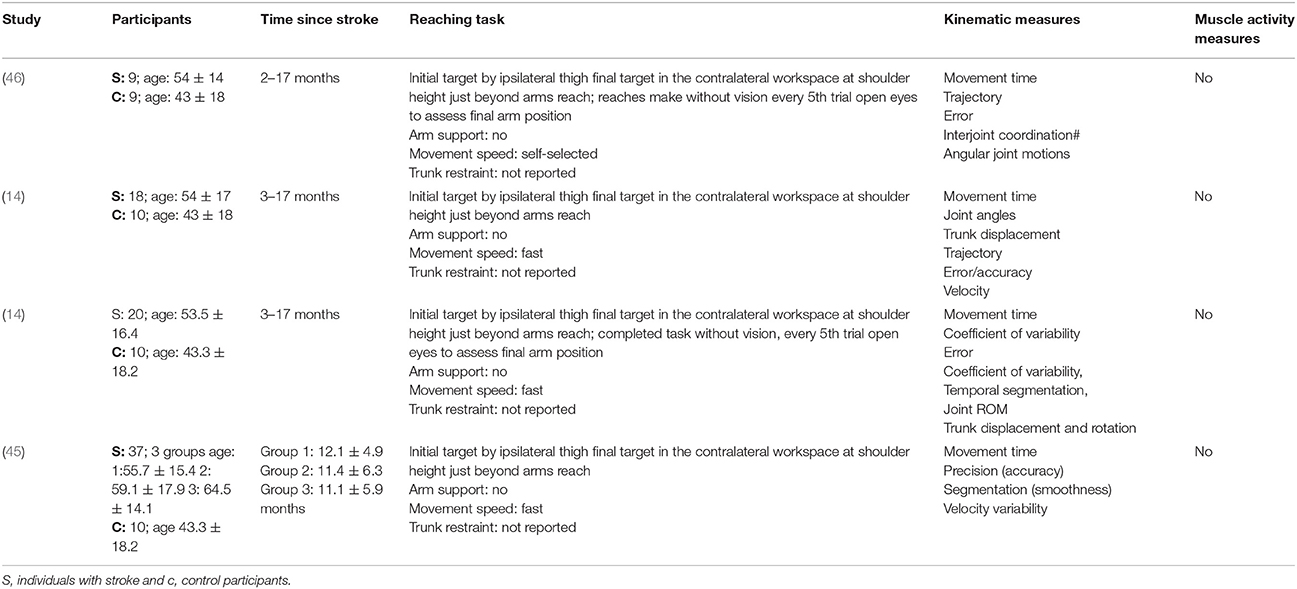
Table 3. Characteristics of included studies investigating reach-to-target task in the contralateral workspace.
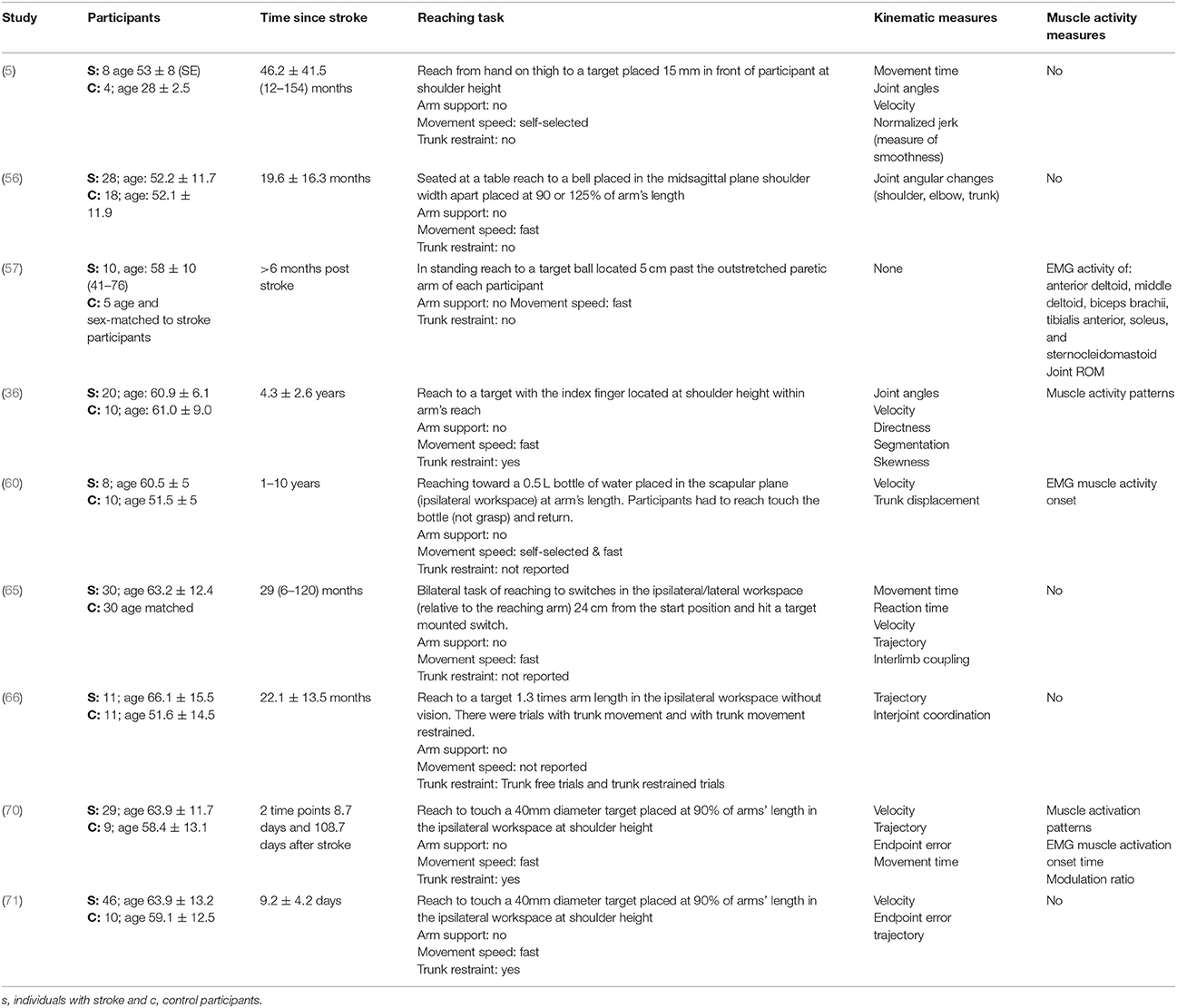
Table 4. Characteristics of included studies investigating reach-to-target in the ipsilateral workspace.
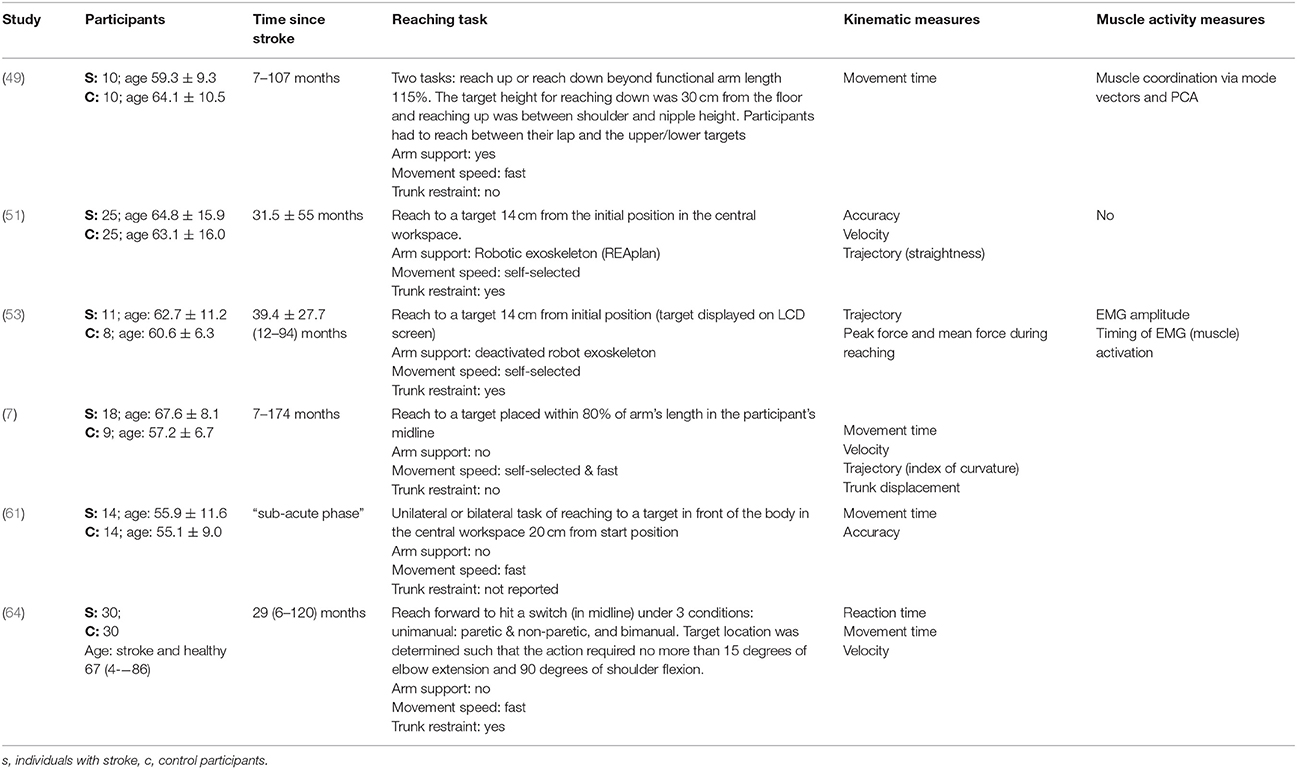
Table 5. Characteristics of included studies investigating reach-to-target in the central workspace.
Reach-to-Target Task
The reach-to-target task varied across studies. Heterogeneity was present in: the target distance; target size; target location; reaching speed; trunk restraint; and use of vision for reaching. A description of the reaching tasks, grouped by the location of the target in the workspace, is provided in Tables 2–5. Location of the target in the workspace was considered the pertinent grouping variable because of the expectation of related differences in joint angles, joint trajectories and spatial-temporal patterns of muscle activity.
Outcome Measures
The methods of data collection, kinematic, and EMG outcomes assessed across all studies were diverse. The kinematic characteristics most frequently assessed were: movement time; peak velocity; reach-path-ratio/trajectory; movement smoothness; target accuracy; joint range of motion; and trunk contribution to movement. The EMG-derived assessments most frequently made were: muscle coupling; muscle onset time; and the percentage of muscle used.
Risk of Potential Bias
The detailed assessment of risk of potential bias is provided in Table 6. In summary, one study (45) had a low risk of bias across all 13 items of the modified Downs and Black tool (Table 6). There was only one study that was judged to have a high risk of bias for one item (52). This was for participant description. Most of the risk of potential bias was due to unclear reporting of (a) adverse events during the studies and (b) the use of assessors blinded to the intervention/task being investigated.
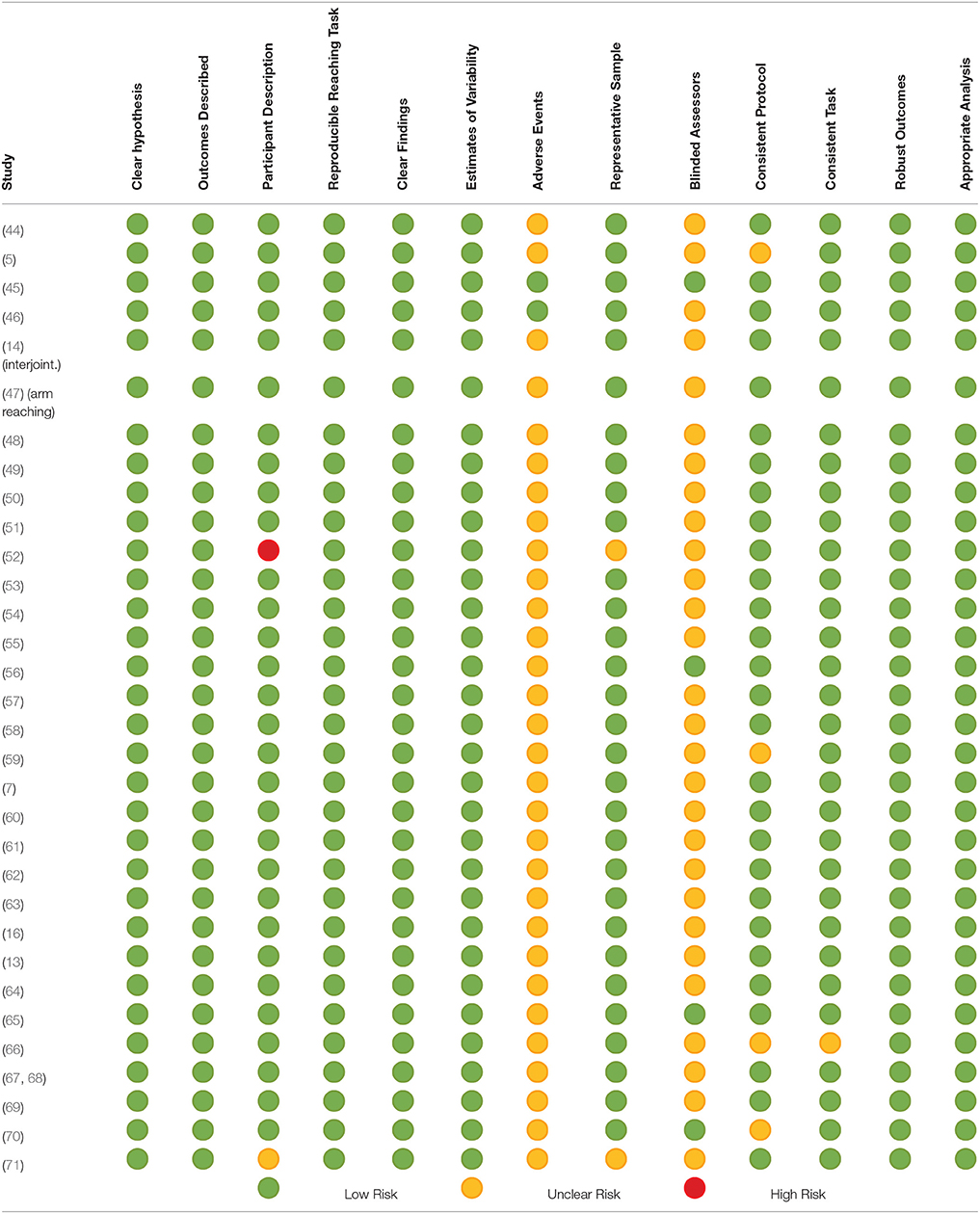
Table 6. Potential risk of bias of included studies assessed using the modified Down's and Black Tool.
There were seven studies in which the experimental protocol differed for people after stroke and healthy adult participants (9, 61, 63, 69, 72, 73). This was primarily because people after stroke were receiving some rehabilitation and thus had pre/post assessments whereas the healthy adult participants had one assessment only. The reach-to-target task protocols did not differ, thus as the review is utilizing baseline data only this difference in protocol does not impact on the findings and does not contribute to potential bias.
Synthesis
The synthesis is grouped by workspace location of the target for reach-to-target: central, ipsilateral, contralateral and multiple. Data from 27 of the 32 studies were included in the meta-analysis. The narrative synthesis included data from 8 of the 32 studies.
Meta-Analysis of Kinematic Data
Meta-analysis was possible for the kinematic characteristics of: peak velocity; movement time; reach-path-ratio; smoothness of movement; elbow range of motion (extension); shoulder range of motion (flexion); accuracy; trunk contribution during reaching; and trunk rotation during reaching. Two or more included studies investigated these characteristics. Twenty-six meta-analyses were undertaken. The heterogeneity of the meta-analyses, as measured by the I2 statistic, was low (I2 = ≤ 25%) for 10, moderate (I2 = 26–74%) for 13, and high (I2 ≥ 75%) for three (Figures 2–8).
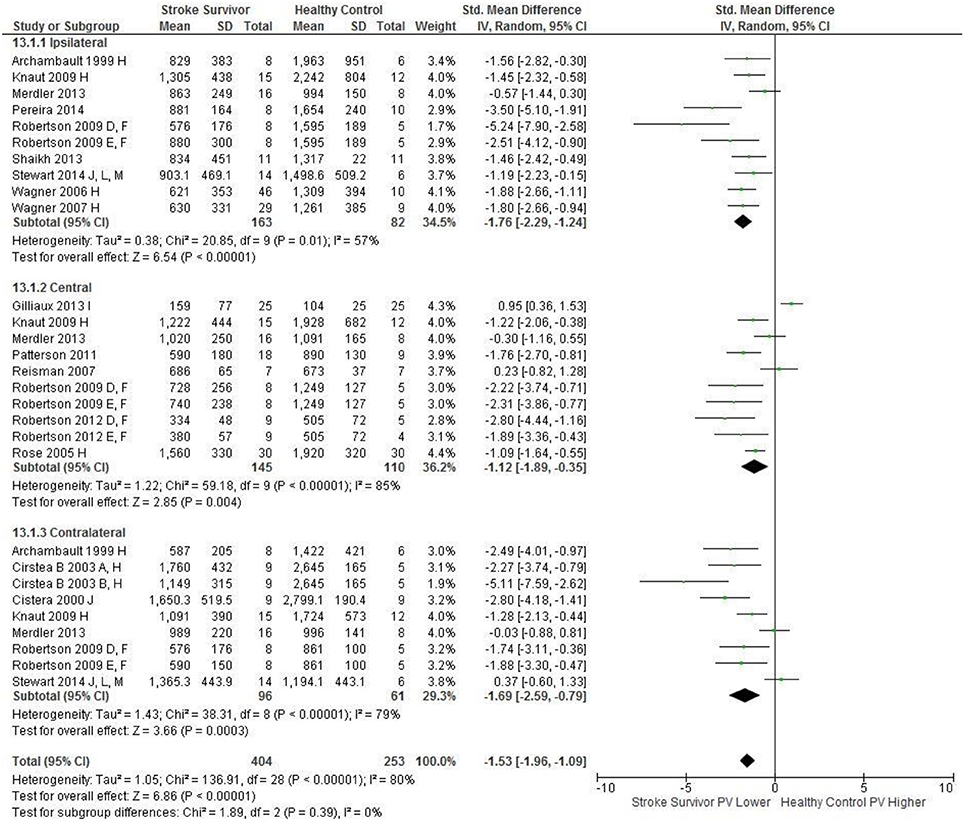
Figure 2. The standardized mean difference (SMD) of peak velocity (mm/s) during reach-to-target in the: ipsilateral, central, and contralateral workspace. D, right hemisphere stroke; E, left hemisphere stroke; F, target placed 90% of arm's length; H, fast speed; I, robotics; J, reaches without vision; L, 24 cm target distance; M, virtual environment.
An overview of the meta-analyses is provided in Table 7 and details in Figures 2–8. In summary, 21 of the 26 meta-analyses found significant differences in kinematics between stroke survivors and control participants.
The SMD (95% CIs) for the significant differences in kinematic characteristics between people after stroke and healthy adult participants ranged from: −1.76 (−2.29, −1.24) for peak velocity in the ipsilateral workspace to 2.57 (0.89, 4.25) for movement time in the ipsilateral workspace. Individuals with stroke demonstrated lower peak velocities and longer movement times in all areas of the workspace (Figures 2, 3). A more curved reach-path-ratio associated with less efficient reaching was demonstrated by individuals with stroke (Figure 4) as well as less smooth more segmented movement due to a greater number of velocity peaks in all areas of the workspace (Figure 6). Individuals with stroke demonstrated greater trunk displacement during reaching (Figure 5), less upper limb range of motion in all areas of the workspace (Figure 7) and reduced reaching accuracy (Figure 8).
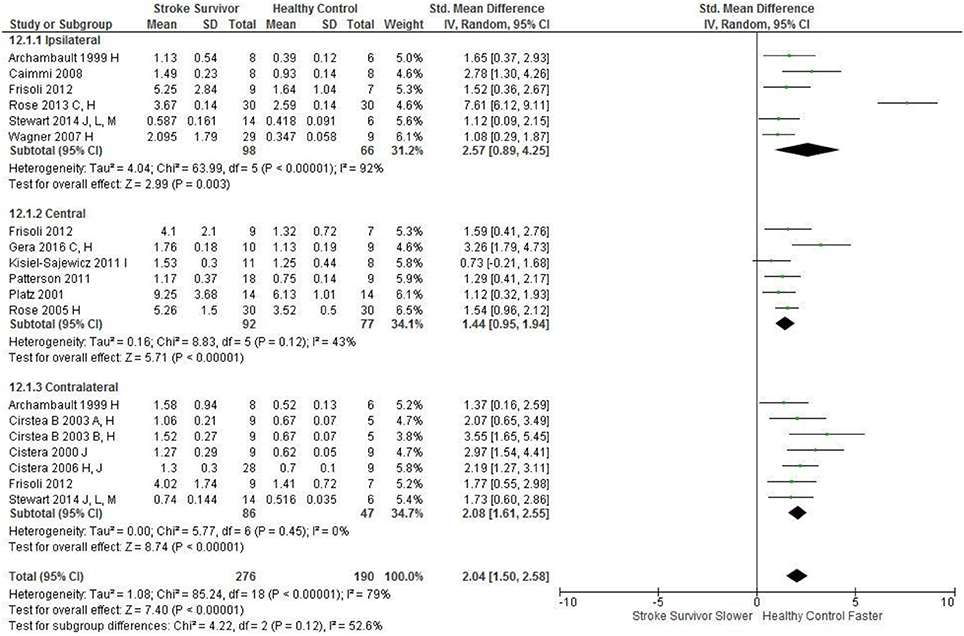
Figure 3. The standardized mean difference (SDM) of movement time (s) during reach-to-target in the: ipsilateral, central, and contralateral workspace. A, mild motor impairment; B, moderate motor impairment; C, bilateral task; F, target placed 90% of arm's length; C, bimanual task; H, fast speed; I, robotics; J, reaches without vision; L, 24 cm target distance; M, virtual environment.
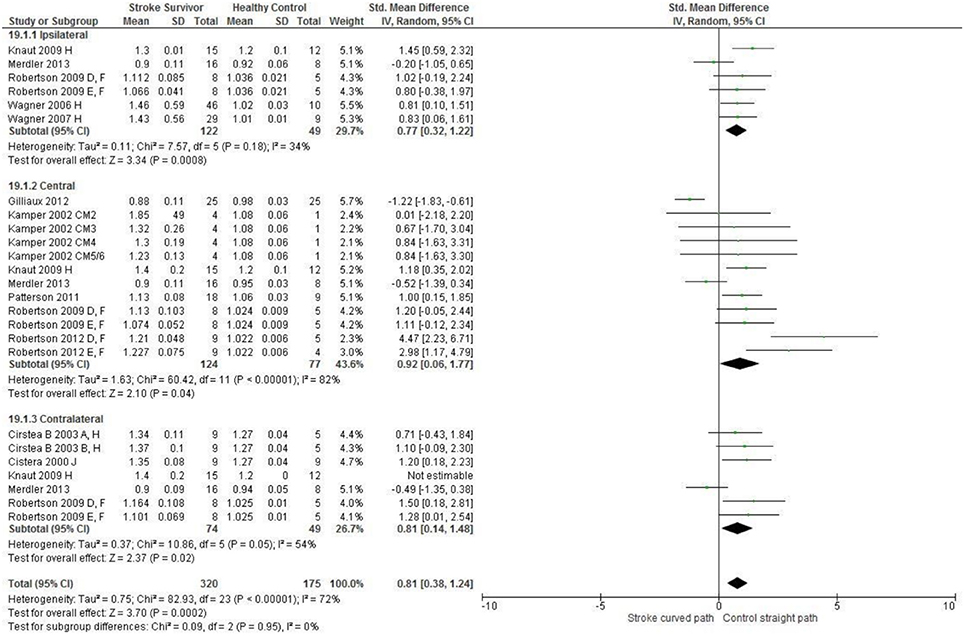
Figure 4. The standardized mean difference (SDM) of reach-path ratio in the: ipsilateral, central, and contralateral workspace. A, mild motor impairment; B, moderate motor impairment; D, right hemisphere stroke; E, left hemisphere stroke; F, target placed 90% of arm's length; H, fast speed; J, reaches without vision; CM, Chedoke-McMaster Stroke Assessment Scale; and corresponding stage (2–6).
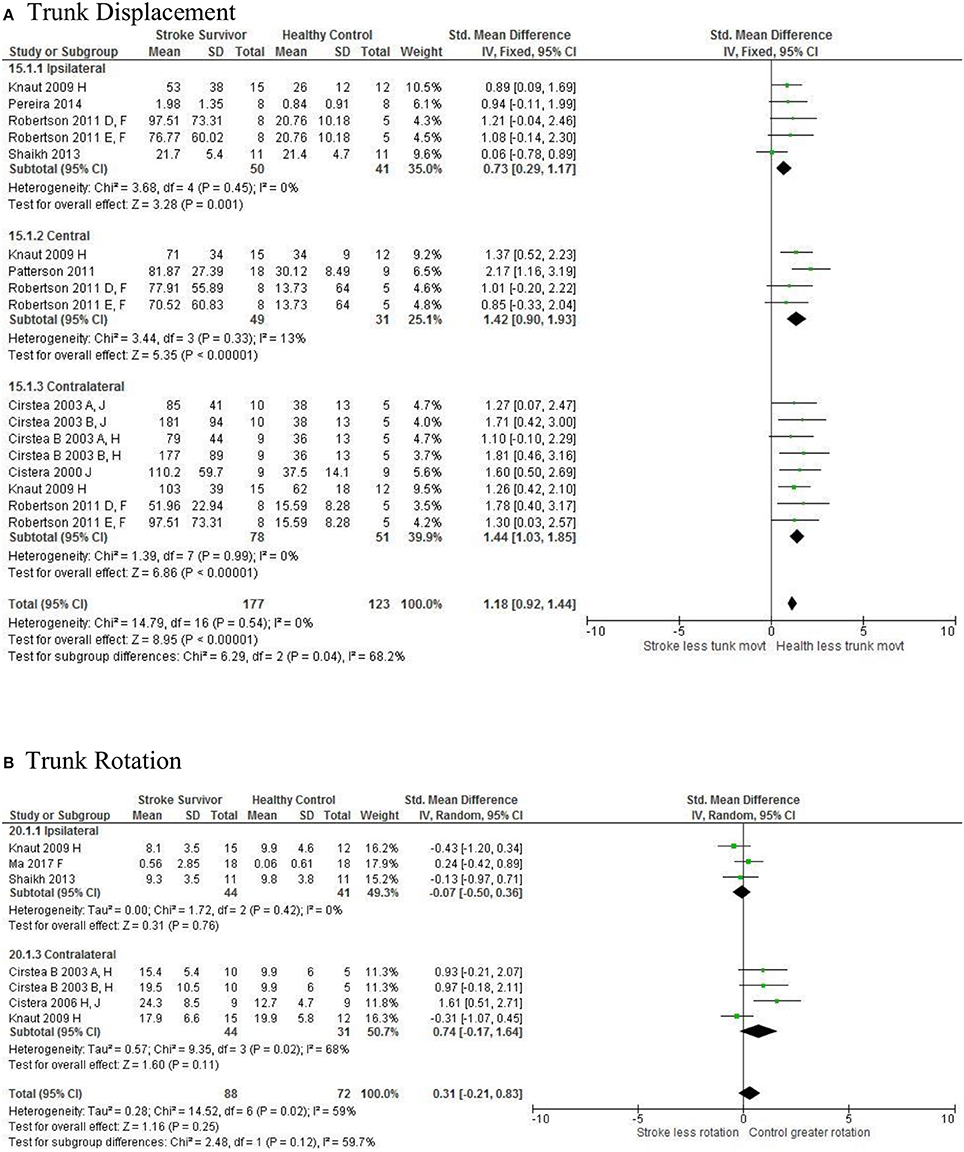
Figure 5. The standardized mean difference (SDM) of trunk displacement (mm) during reach-to-target in the ipsilateral, central, and contralateral workspace. A, mild motor impairment; B, moderate motor impairment; C, bilateral task; D, right hemisphere stroke; E, left hemisphere stroke; F, target placed 90% of arm's length; H, fast speed; LK robotics; J, reaches without vision.
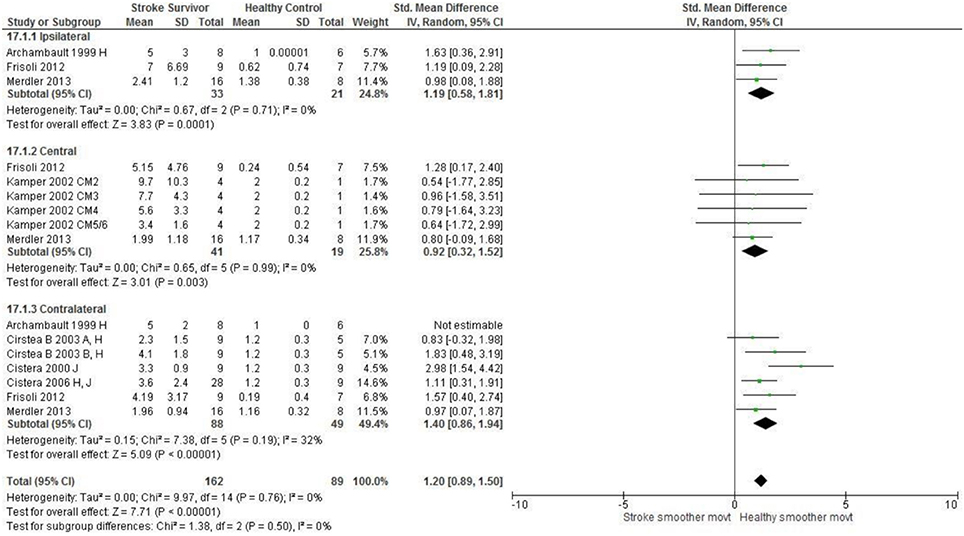
Figure 6. The standardized mean difference (SDM) of movement smoothness during reach-to-target in the ipsilateral, central, and contralateral workspace. A, mild motor impairment; B, moderate motor impairment; H, fast speed; I, robotics; J, reaches without vision; M, virtual environment; CM, Chedoke-McMaster Stroke Assessment Scale; and corresponding stage (2–6).
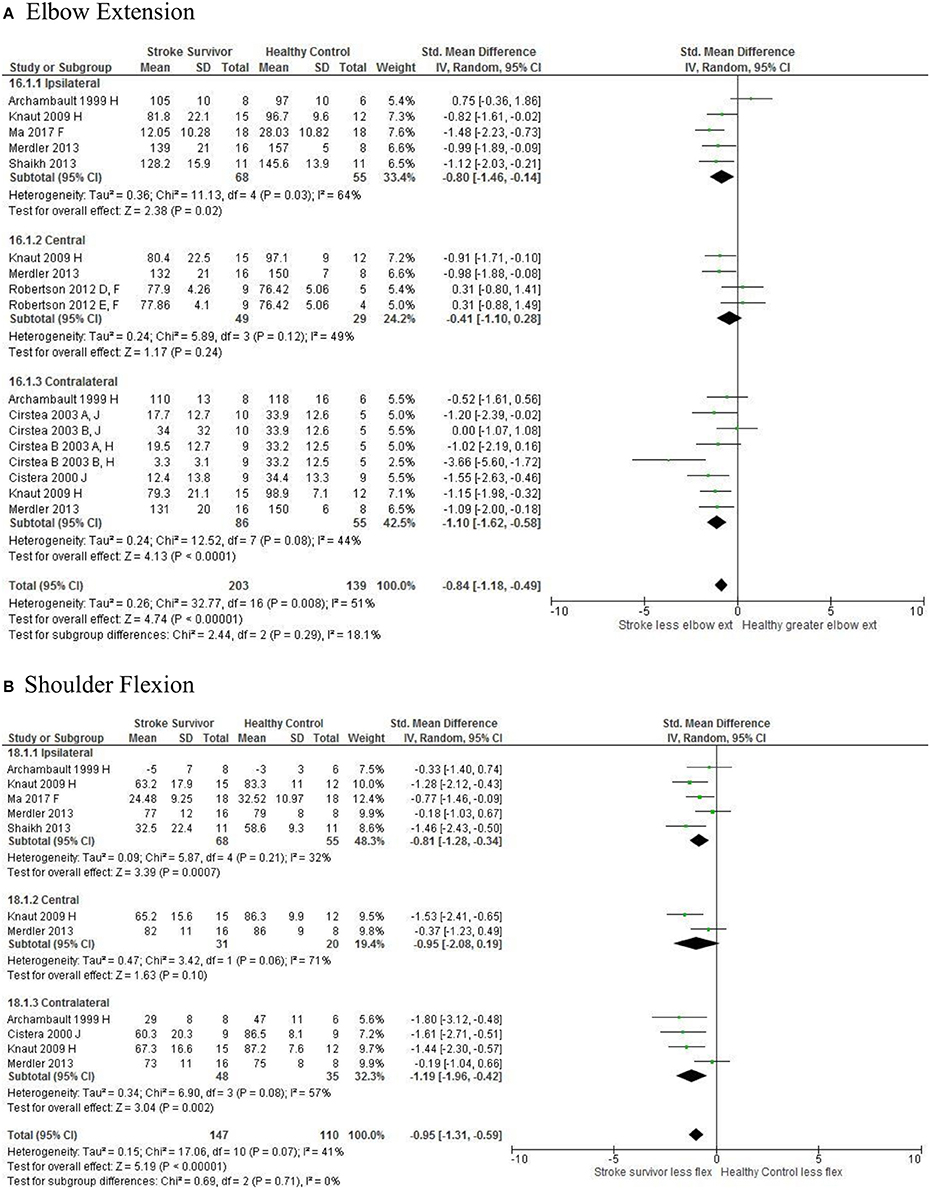
Figure 7. The standardized mean difference (SDM) of joint kinematics in the ipsilateral, central, and contralateral workspace. D, right hemisphere stroke; E, left hemisphere stroke; F, target at 90% of arm's length; H, fast speed; J, reaches without vision.
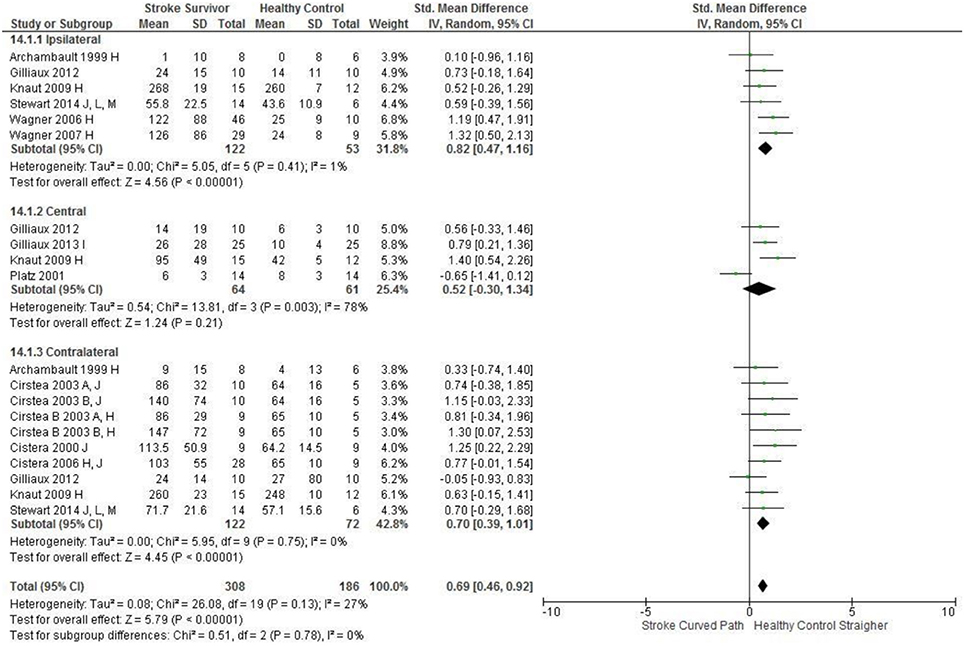
Figure 8. The standardized mean difference (SDM) of accuracy (mm) in the ipsilateral, central, and contralateral workspace. A, mild motor impairment; B, moderate motor impairment; C, bilateral task; H, fast speed; I, robotics; J, reaches without vision; M, virtual environment.
The non-significant differences between people after stroke and healthy adults for kinematics during reaching were: elbow extension in the central workspace SMD = −0.41 [−1.10, 0.28]; target accuracy in the central workspace SMD = 0.52 [−0.30, 1.34]; trunk rotation in the contralateral workspace SMD = 0.74 [−0.17, 1.54], trunk rotation in the ipsilateral workspace SMD = −0.07 [−0.50, 0.36]; and shoulder flexion in the central workspace SMD = −0.95 [−2.08, 0.19].
Narrative Synthesis of Muscle Activity Data
The muscles most frequently investigated were the: triceps, biceps, deltoid (anterior, posterior, and middle), trapezius, pectoralis, and latissimus dorsi. Six studies investigated interaction between muscle pairs (48, 53, 69, 70, 72, 74). Also investigated were muscle activation patterns (58, 60), muscle timing (57, 60), and the percentage of muscle activity used in relation to the maximal voluntary contraction (MVC) (55, 58, 69, 70).
There were comparable findings across studies. For example, compared to healthy adult participants the people after stroke used a greater percentage of MVC (58, 70), higher background muscle activity (55, 69), a reduced level of coherence between antagonistic muscle pairs (48, 53, 74), and prolonged co-contraction between muscles after achieving the task (55).
There were also differences between studies. For example, delayed onset of muscle activation in stroke survivors compared to healthy adult participants (57, 60, 74), contrasts with findings of no significant difference between the two groups (53).
The synthesis also suggests that just examining one aspect of muscle activity might not be sufficient for identification of potential therapy targets after stroke. For example, people after stroke and healthy adult participants were found to utilize a similar number of muscle synergies during reaching (69, 72). But, a notable difference was that healthy adult participants during arm abduction and flexion recruited the anterior deltoid and pectoralis major whereas people after stroke recruited additional muscle of the brachioradialis and brachial (69).
Discussion
The meta-analysis reported here found that people after stroke, compared with healthy adult participants, demonstrate: longer movement time, decreased peak velocity, greater trunk contribution, less smooth movement, and a more curved reach path when performing reach-to-target in all areas of the workspace. Furthermore, people after stroke exhibit less accurate reaches and decreased elbow extension reaching to objects in the ipsilateral and contralateral workspace; and less shoulder flexion when reaching in the contralateral and ipsilateral workspace. Object location in the workspace influenced joint range of motion and target accuracy such that there were no differences between individuals with and without stroke in the central workspace. These kinematic elements of movement skill are potential targets for rehabilitation therapy.
The narrative analysis reported here suggests that compared with healthy adult participants, people after stroke performing reach-to-target: use a greater percentage of MVC, have higher background muscle activity, and decreased coherence between muscle pairs. Meta-analysis was precluded by heterogeneity between included studies therefore caution needs to be used in considering these elements of movement skill as potential targets for rehabilitation therapy.
The meta-analysis finding reported here are applicable to individuals with stroke that exhibit similar levels of motor function to those individuals within the studies e.g., have the motor control to reach and point (mild to moderate upper limb deficits).
Comparison With Earlier Published Findings
Interpretation of the present findings needs to be made considering the risk of potential bias of included studies. Most items were assessed as low risk; however, there was one area of one study assessed as high risk. Overall there was unclear reporting of both adverse events and blinded assessment for most included studies. The influence of these indications of risk of potential bias is debatable. It is reasonable to propose that reporting adverse events is irrelevant to this review because most included studies did not investigate an intervention and for those that did, only the baseline measures were included. It is also possible that unclear reporting of blinded assessment is not directly relevant to the results of this systematic review as measures derived from kinematic assessment and EMG are objective. However, the risk of potential bias from unclear reporting of blinded assessment remains if the same researcher conducted the assessments and those conducting processing and statistical analysis of the movement data. So, caution remains in respect of unclear reporting of blinded assessment. Otherwise, there is mostly low risk of potential bias and therefore the meta-analysis results are considered to be strong.
The identified kinematic differences during reach-to-target are mostly in accordance with previous narrative reviews (4, 19, 20). However, the study reported here is the first-ever meta-analysis of reach-to-target, using a systematic literature search unlike two of the earlier reviews (4, 20) and employed a systematic approach for reviewers to identify relevant studies and extract data unlike any of the earlier reviews (4, 19, 20). The results therefore are less likely to be confounded by reviewer bias than the earlier reviews. The results reported here provide the kinematic differences, and their variances, during reach-to-target performed by people after stroke and healthy adult participants. Objective reference values that could be used for target setting for upper limb rehabilitation after stroke can also be derived from this review. Consequently, the review reported here has provided additional knowledge to that provided in the earlier narrative reviews. Especially as the earlier reviews examined a variety of tasks involving reaching (19); reach-to-grasp rather than reach-to-target; and did not specify the aspects of reaching that were reviewed. This difference between reviews is important as it has been known for some time that kinematic characteristics differ between different reaching tasks (21, 22, 75).
Unlike the earlier narrative reviews (4, 19, 20) the review has examined EMG-derived measures of reach-to-target. It is possible that reduced coherence between muscles contributes to the kinematic differences between individuals with and without stroke such as reduced peak velocity and decreased movement smoothness. Suchc an association has been found between a reduced number of muscle synergies and reduced gait speed after stroke which was subsequently correlated with walking dysfunction (76).
This review found conflicting findings for timing of muscle activation. One study identified no difference in muscle onset time in comparison to control participants (53). Whereas, another found that individuals with stroke have delayed muscle onset/activation (57, 60, 74). Clearly this is an area for future research.
Interestingly 12 reach-to-target studies included reaching into the contralateral workspace. Yet healthy adults, when given the option to use their preferred arm to reach to target in any area of the workspace, utilize ipsilateral reaches rather than contralateral reaches during spontaneous activity (left arm for left targets, and right arm for (77) right targets) (34, 78, 79). Potential explanations for preferred ipsilateral reaches are that contralateral reaches are less biomechanically efficient thus require greater energy (79). Workspace location had minimal influence on the differences in kinematics between individuals with stroke and control with there being consistent significant differences in all areas of the workspace. However, in the central workspace there were no differences in shoulder/elbow range of motion or accuracy between individuals with and without stroke. This could be due to the joint combinations needed to reach to the central workspace (e.g. elbow extension with shoulder adduction) are part of the flexor synergy in individuals with stroke, an often used movement pattern (77).
Strengths and Limitations
The studies included in the systematic review were heterogeneous, for example: the reaching task; movement speed; object location; use of trunk restraint; upper limb motor ability of individuals with stroke; and varied time since stroke. The I2 statistic demonstrated that of the 26 meta-analyses three meta-analyses had high heterogeneity (I2 ≥ 75%) reach-path-ratio (central workspace), peak velocity (central workspace), and movement time in the ipsilateral workspace. The remaining twenty three meta analyses exhibited low (10/26) and moderate heterogeneity (13/26) (23, 30). Evaluation of the forest plots demonstrates that many of the confidence intervals are overlapping and the mean differences fall on the same side of the line of no effect (23, 30) suggesting the studies are comparable. However the possibility remains that combing heterogeneous studies with in a meta-analysis could be a limitation as the findings may be biased (23).
There are two additional potential limitations to this review. First, limitation of the search to articles published in the English language. However, a strength is that the search strategy was robust and carried out in multiple data-bases. The second limitation is that participants with stroke had to have sufficient upper limb motor function to complete the reaching task, so, the findings may not be applicable to those with severe paresis.
Conclusion
This first-ever meta-analysis of the kinematics of reach-to-target by people with stroke and healthy adults performing reach-to-target found 21 elements that could provide targets for impairment-orientated therapy for better upper limb recovery. Of the kinematic characteristics, object location influenced joint range of motion and target accuracy.The findings also quantify the differences which should inform measurement of the efficacy of rehabilitation. Subsequent studies need to investigate whether tailoring therapy at the identified differences reported here, does enhance upper limb recovery after stroke.
Author Contributions
KC led the conception, design, analysis and interpretation of this systematic review, prepared the initial drafts of the report, provided approval for publication of the content and agreed to be accountable for all aspects of the work. NK made substantial contributions to the conception, design, analysis and interpretation of this systematic review, contributed to drafts of the report, provided approval for publication of the content and agreed to be accountable for all aspects of the work. AC made substantial contributions to the conception, design, analysis and interpretation of this systematic review, contributed to drafts of the report, provided approval for publication of the content and agreed to be accountable for all aspects of the work. VP made substantial contributions to the conception, design, analysis and interpretation of this systematic review, contributed to drafts of the report, prepared the final version of this report, provided approval for publication of the content and agreed to be accountable for all aspects of the work.
Funding
No external funding was provided for the systematic review reported here which was undertaken as part of the Ph.D. studies of KC (first author).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Persson HC, Parziali M, Danielsson A, Sunnerhagen KS. Outcome and upper extremity function within 72 hours after first occasion of stroke in an unselected population at a stroke unit. A part of the SALGOT study. BMC Neurol. (2012) 12:162. doi: 10.1186/1471-2377-12-162
2. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb impact of severity of paresis and time since onset in acute stroke. Stroke (2003) 34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD
3. Platz T, van Kaick S, Mehrholz J, Leidner O, Eickhof C, Pohl M. Best conventional therapy versus modular impairment-oriented training for arm paresis after stroke: a single-blind, multicenter randomized controlled trial. Neurorehabil Neural Repair (2009) 23:706–17. doi: 10.1177/1545968309335974
4. McCrea PH, Eng JJ, Hodgson AJ. Biomechanics of reaching: clinical implications for individuals with acquired brain injury. Disabil Rehabil. (2002) 24:534–41. doi: 10.1080/09638280110115393
5. Caimmi M, Carda S, Giovanzana C, Maini E, Sabatini A, Smania N, et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neuror ehabil Neural Repair. (2008) 22:31–9. doi: 10.1177/1545968307302923
6. Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Topics Stroke Rehabil. (2009) 16:237–53. doi: 10.1310/tsr1604-237
7. Patterson T, Bishop M, McGuirk T, Sethi A, Richards L. Reliability of upper extremity kinematics while performing different tasks in individuals with stroke. J Motor Behav. (2011) 43:121–30. doi: 10.1080/00222895.2010.548422
8. Nowak DA. The impact of stroke on the performance of grasping: usefulness of kinetic and kinematic motion analysis. Neurosci Biobehav Rev. (2008) 32:1439–50. doi: 10.1016/j.neubiorev.2008.05.021
9. Platz T, Prass K, Denzler P, Bock S, Mauritz K-H. Testing a motor performance series and a kinematic motion analysis as measures of performance in high-functioning stroke patients: reliability, validity, and responsiveness to therapeutic intervention. Arch Phys Med Rehabil. (1999) 80:270–7. doi: 10.1016/S0003-9993(99)90137-5
10. Vandenberghe A, Levin O, De Schutter J, Swinnen S, Jonkers I. Three-dimensional reaching tasks: effect of reaching height and width on upper limb kinematics and muscle activity. Gait Posture. (2010) 32:500–7. doi: 10.1016/j.gaitpost.2010.07.009
11. Micera S, Carpaneto J, Posteraro F, Cenciotti L, Popovic M, Dario P. Characterization of upper arm synergies during reaching tasks in able-bodied and hemiparetic subjects. Clin Biomech. (2005) 20:939–46. doi: 10.1016/j.clinbiomech.2005.06.004
12. Rundquist PJ, Obrecht C, Woodruff L. Three-dimensional shoulder kinematics to complete activities of daily living. Am J Phys Med Rehabil. (2009) 88:623–9. doi: 10.1097/PHM.0b013e3181ae0733
13. Robertson JV, Roche N, Roby-Brami A. Influence of the side of brain damage on postural upper-limb control including the scapula in stroke patients. Exp Brain Res. (2012) 218:141–55. doi: 10.1007/s00221-012-3014-y
14. Cirstea MC, Mitnitski AB, Feldman AG, Levin MF. Interjoint coordination dynamics during reaching in stroke. Exp Brain Res. (2003) 151:289–300. doi: 10.1007/s00221-003-1438-0
15. Michaelsen SM, Jacobs S, Roby-Brami A, Levin MF. Compensation for distal impairments of grasping in adults with hemiparesis. Exp Brain Res. (2004) 157:162–73. doi: 10.1007/s00221-004-1829-x
16. Robertson JV, Roby-Brami A. The trunk as a part of the kinematic chain for reaching movements in healthy subjects and hemiparetic patients. Brain Res. (2011) 1382:137–46. doi: 10.1016/j.brainres.2011.01.043
17. Flanders M, Pellegrini JJ, Geisler SD. Basic features of phasic activation for reaching in vertical planes. Exp Brain Res. (1996) 110:67–79. doi: 10.1007/BF00241376
18. Criswell E. Cram's Introduction to Surface Electromyography. Boston, MA: Jones & Bartlett Publishers (2010).
19. Alt Murphy M, Häger CK. Kinematic analysis of the upper extremity after stroke-how far have we reached and what have we grasped? Phys Ther Rev. (2015) 20:137–55. doi: 10.1179/1743288X15Y.0000000002
20. van Vliet P, Pelton TA, Hollands KL, Carey L, Wing AM. Neuroscience findings on coordination of reaching to grasp an object implications for research. Neurorehabil Neural Repair. (2013) 27:622–35. doi: 10.1177/1545968313483578
21. Wu C, Trombly C, Lin K, Tickle-Degnen L. A kinematic study of contextual effects on reaching performance in persons with and without stroke: influences of object availability. Arch Phys Med Rehabi. (2000) 81:95–101. doi: 10.1016/S0003-9993(00)90228-4
22. Schaefer SY, Hengge C. Testing the concurrent validity of a naturalistic upper extremity reaching task. Exp Brain Res. (2016) 234:229–40. doi: 10.1007/s00221-015-4454-y
23. Higgins JP, Green S, Collaboration C. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library (2008).
24. Mateen FJ, Oh J, Tergas AI, Bhayani NH, Kamdar BB. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. (2013) 6:89. doi: 10.2147/CLEP.S43118
25. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health (1998) 52:377–84. doi: 10.1136/jech.52.6.377
26. Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol. (2006) 59:765–9. doi: 10.1016/j.jclinepi.2005.12.010
27. Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obesity Rev. (2007) 8:307–26. doi: 10.1111/j.1467-789X.2007.00347.x
28. Monteiro POA, Victora C. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obesity Rev. (2005) 6:143–54. doi: 10.1111/j.1467-789X.2005.00183.x
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. (2003) 327:557. doi: 10.1136/bmj.327.7414.557
30. Ried K. Interpreting and understanding meta-analysis graphs: a practical guide. Aust Family Phys. (2006) 35:635–8.
31. Coderre AM, Amr Abou Z, Dukelow SP, Demmer MJ, Moore KD, Demers MJ, et al. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair. (2010) 24:528–41. doi: 10.1177/1545968309356091
32. Finley M, Combs S, Carnahan K, Peacock S, Van BA. Comparison of 'less affected limb' reaching kinematics in individuals wth chronic stroke and healthy age-matched controls. Phys Occup Ther Geriatr. (2012) 30:245–59. doi: 10.3109/02703181.2012.716506
33. Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain (1996) 119:281–93.
34. Mani S, Przybyla A, Good DC, Haaland KY, Sainburg RL. Contralesional arm preference depends on hemisphere of damage and target location in unilateral stroke patients. Neurorehabil Neural Repair. (2014) 28:584–93. doi: 10.1177/1545968314520720
35. Mazzoleni S, Sale P, Tiboni M, Franceschini M, Carrozza M, Posteraro F. Upper limb robot-assisted therapy in chronic and subacute stroke patients, A kinematic analysis. Am J Phys Med Rehabil. (2013) 92:e26-e37. doi: 10.1097/PHM.0b013e3182a1e852
36. McCrea PH, Eng JJ. Consequences of increased neuromotor noise for reaching movements in persons with stroke. Exp Brain Res. (2005) 162:70–7. doi: 10.1007/s00221-004-2106-8
37. Panarese A, Pirondini E, Tropea P, Cesqui B, Posteraro F, Micera S. Model-based variables for the kinematic assessment of upper-extremity impairments in post-stroke patients. J Neuroeng Rehabil. (2016) 13:81. doi: 10.1186/s12984-016-0187-9
38. Reinkensmeyer DJ, Cole AM, Kahn LE, Kamper DG. Directional control of reaching is preserved following mild/moderate stroke and stochastically constrained following severe stroke. Exp Brain Res. (2002) 143:525–30. doi: 10.1007/s00221-002-1055-3
39. Reisman D, Scholz J. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain (2003) 126(Pt 11):2510–27. doi: 10.1093/brain/awg246
40. Reisman DS, Scholz JP. Workspace location influences joint coordination during reaching in post-stroke hemiparesis. Exp Brain Res. (2006) 170:265–76. doi: 10.1007/s00221-005-0209-5
41. Rodrigues MR, Slimovitch M, Chilingaryan G, Levin M.F. Does the finger-to-nose test measure upper limb coordination in chronic stroke? J Neuroeng Rehabil. (2017) 14:6. doi: 10.1186/s12984-016-0213-y
42. Sethi A, Stergiou N, Patterson TS, Patten C, Richards LG. Speed and rhythm affect temporal structure of variability in reaching poststroke: a pilot study. J Motor Behav. (2016) 49:35–45. doi: 10.1080/00222895.2016.1219304
43. Zackowski K, Dromerick A, Sahrmann S, Thach W, Bastian A. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain (2004) 127:1035–46. doi: 10.1093/brain/awh116
44. Archambault P, Pigeon P, Feldman A, Levin M. Recruitment and sequencing of different degrees of freedom during pointing movements involving the trunk in healthy and hemiparetic subjects. Exp Brain Res. (1999) 126:55–67. doi: 10.1007/s002210050716
45. Cirstea C, Ptito A, Levin M. Feedback and cognition in arm motor skill reacquisition after stroke. Stroke (2006) 37:1237–42. doi: 10.1161/01.STR.0000217417.89347.63
46. Cirstea M, Levin MF. Compensatory strategies for reaching in stroke. Brain (2000) 123:940–53. doi: 10.1093/brain/123.5.940
47. Cirstea MC, Ptito A, Levin MF. Arm reaching improvements with short-term practice depend on the severity of the motor deficit in stroke. Exp Brain Res. (2003) 152:476–88. doi: 10.1007/s00221-003-1568-4
48. Frisoli A, Procopio C, Chisari C, Creatini I, Bonfiglio L, Bergamasco M, et al. Positive effects of robotic exoskeleton training of upper limb reaching movements after stroke. J Neuroeng Rehabil. (2012) 9:36. doi: 10.1186/1743-0003-9-36
49. Gera G, McGlade KE, Reisman DS, Scholz JP. Trunk muscle coordination during upward and downward reaching in stroke survivors. Motor Control. (2016) 20:50–69. doi: 10.1123/mc.2014-0038
50. Gilliaux M, Lejeune T, Detrembleur C, Sapin J, Dehez B, Stoquart G. A robotic device as a sensitive quantitative tool to assess upper limb impairments in stroke patients: a preliminary prospective cohort study. J Rehabil Med. (2012) 44:210–7. doi: 10.2340/16501977-0926
51. Gilliaux M, Lejeune TM, Detrembleur C, Sapin P, Dehez B, Selves C, et al. Using the robotic device REAplan as a valid, reliable, and sensitive tool to quantify upper limb impairments in stroke patients. J Rehabil Med. (2014) 46:117–25. doi: 10.2340/16501977-1245
52. Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. (2002) 83:702–7. doi: 10.1053/apmr.2002.32446
53. Kisiel-Sajewicz K, Fang Y, Hrovat K, Yue G, Siemionow V, Sun C-K, et al. Weakening of synergist muscle coupling during reaching movement in stroke patients. Neurorehabil Neural Repair. (2011) 25:359–68. doi: 10.1177/1545968310388665
54. Knaut L, Subramanian S, McFadyen B, Bourbonnais D, Levin M. Kinematics of pointing movements made in a virtual versus a physical 3-dimensional environment in healthy and stroke subjects. Arch Phys Med Rehabil. (2009) 90:793–802. doi: 10.1016/j.apmr.2008.10.030
55. Li S, Zhuang C, Niu CM, Bao Y, Xie Q, Lan N. Evaluation of functional correlation of task-specific muscle synergies with motor performance in patients poststroke. Front Neurol. (2017) 8:337. doi: 10.3389/fneur.2017.00337
56. Ma H-I, Lin K-C, Hsieh F-H, Chen C-L, Tang SF, Wu C-Y. Kinematic manifestation of arm-trunk performance during symmetric bilateral reaching after stroke: within vs. beyond arm's length. Am J Phys Med Rehabil. (2017) 96:146–51. doi: 10.1097/PHM.0000000000000554
57. McCombe Waller S, Yang C-L, Magder L, Yungher D, Creath R, Gray V, et al. Corrigendum to “Impaired motor preparation and execution during standing reach in people with chronic stroke” [Neurosci. Lett. 630 (2016) 38-44]. Neurosci Lett. (2016) 633:290. doi: 10.1016/j.neulet.2016.09.035
58. McCrea PH, Eng JJ, Hodgson AJ. Saturated muscle activation contributes to compensatory reaching strategies after stroke. J Neurophysiol. (2005) 94:2999–3008. doi: 10.1152/jn.00732.2004
59. Merdler T, Liebermann DG, Levin MF, Berman S. Arm-plane representation of shoulder compensation during pointing movements in patients with stroke. J Electromyogr Kinesiol. (2013) 23:938–47. doi: 10.1016/j.jelekin.2013.03.006
60. Pereira S, Silva CC, Ferreira S, Silva C, Oliveira N, Santos R, et al. Anticipatory postural adjustments during sitting reach movement in post-stroke subjects. J Electromyogr Kinesiol. (2014) 24:165–71. doi: 10.1016/j.jelekin.2013.10.001
61. Platz T, Bock S, Prass K. Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia (2001) 39:687–98. doi: 10.1016/S0028-3932(01)00005-7
62. Reisman D, Scholz J. Deficits in surface force production during seated reaching in people after stroke. Phys Ther. (2007) 87:326–36. doi: 10.2522/ptj.20050303
63. Robertson JV, Hoellinger T, Lindberg P, Bensmail D, Hanneton S, Roby-Brami A. Effect of auditory feedback differs according to side of hemiparesis: a comparative pilot study. J Neuroeng Rehabil. (2009) 6:45. doi: 10.1186/1743-0003-6-45
64. Rose D, Winstein C. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil. (2005) 19:452–62. doi: 10.1191/0269215505cr806oa
65. Rose DK, Winstein CJ. Temporal coupling is more robust than spatial coupling: an investigation of interlimb coordination after stroke. J Motor Behav. (2013) 45:313–24. doi: 10.1080/00222895.2013.798250
66. Shaikh T, Goussev V, Feldman AG, Levin MF. Arm–trunk coordination for beyond-the-reach movements in adults with stroke. Neurorehabil Neural Repair. (2013) 28:355–66. doi: 10.1177/1545968313510973
67. Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke: kinematic differences based on side of brain damage. Exp Brain Res. (2014) 232:2407–19. doi: 10.1007/s00221-014-3938-5
68. Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke II: planning and adjustments to control movement distance. Exp Brain Res. (2014) 232:3431–43. doi: 10.1007/s00221-014-4025-7
69. Tropea P, Monaco V, Coscia M, Posteraro F, Micera S. Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: a pilot study. J Neuroeng Rehabil. (2013) 10:1. doi: 10.1186/1743-0003-10-103
70. Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clin Neurophysiol. (2007) 118:164–76. doi: 10.1016/j.clinph.2006.09.022
71. Wagner J, Lang C, Sahrmann S, Hu Q, Bastian A, Edwards D, et al. Relationships between sensorimotor impairments and reaching deficits in acute hemiparesis. Neurorehabil Neural Repair. (2006) 20:406–16. doi: 10.1177/1545968306286957
72. Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci USA. (2009) 106:19563–8. doi: 10.1073/pnas.0910114106
73. Wagner JM, Lang CE, Sahrmann SA, Edwards DF, Dromerick AW. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys Ther. (2007) 87:751–65. doi: 10.2522/ptj.20060135
74. Barker RN, Brauer S, Carson R. Training-induced changes in the pattern of triceps to biceps activation during reaching tasks after chronic and severe stroke. Exp Brain Res. (2009) 196:483–96. doi: 10.1007/s00221-009-1872-8
75. Lin KC, Wu CY, Trombly CA. Effects of task goal on movement kinematics and line bisection performance in adults without disabilities. Am J Occup Ther (1998) 52:179–87. doi: 10.5014/ajot.52.3.179
76. Ambrosini E, Marchis C, Pedrocchi A, Ferrigno G, Monticone M, Schmid M, et al. Neuro-mechanics of recumbent leg cycling in post-acute stroke patients. Ann Biomed Eng. (2016) 44:3238–51. doi: 10.1007/s10439-016-1660-0
77. Van Kordelaar J, Van Wegen EEH, Kwakkel G. Unraveling the interaction between pathological upper limb synergies and compensatory trunk movements during reach-to-grasp after stroke: a cross-sectional study. Exp Brain Res. (2012) 221:251–62. doi: 10.1007/s00221-012-3169-6
78. Stins JF, Kadar EE, Costall A. A kinematic analysis of hand selection in a reaching task. Laterality (2001) 6:347–67. doi: 10.1080/713754421
Keywords: stroke rehabilitation, reaching, upper limb, kinematics, movement performance
Citation: Collins KC, Kennedy NC, Clark A and Pomeroy VM (2018) Kinematic Components of the Reach-to-Target Movement After Stroke for Focused Rehabilitation Interventions: Systematic Review and Meta-Analysis. Front. Neurol. 9:472. doi: 10.3389/fneur.2018.00472
Received: 08 March 2018; Accepted: 31 May 2018;
Published: 25 June 2018.
Edited by:
Pavel Lindberg, INSERM U894 Centre de Psychiatrie et Neurosciences, FranceReviewed by:
Margit Alt Murphy, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, SwedenDenis Mottet, University of Montpellier 1, France
Copyright © 2018 Collins, Kennedy, Clark and Pomeroy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie M. Pomeroy, v.pomeroy@uea.ac.uk
 Kathryn C. Collins
Kathryn C. Collins Niamh C. Kennedy
Niamh C. Kennedy Allan Clark
Allan Clark Valerie M. Pomeroy
Valerie M. Pomeroy