- 1Department of Neurosciences, Laboratory for Molecular Neurobiomarker Research, KU Leuven, Leuven, Belgium
- 2Laboratory Medicine, University Hospitals Leuven, Leuven, Belgium
- 3Laboratory of Neurobiology, Department of Neurosciences, KU Leuven and Center for Brain & Disease Research VIB Leuven, Leuven, Belgium
- 4Department of Neurology, Neuromuscular Reference Centre, University Hospitals Leuven, Leuven, Belgium
There is a need for biomarkers for amyotrophic lateral sclerosis (ALS), to support the diagnosis of the disease, to predict disease progression and to track disease activity and treatment responses. Over the last decade multiple studies have investigated the potential of neurofilament levels, both in cerebrospinal fluid and blood, as biomarker for ALS. The most widely studied neurofilament subunits are neurofilament light chain (NfL) and phosphorylated neurofilament heavy chain (pNfH). Neurofilament levels are reflecting neuronal injury and therefore potentially of value in ALS and other neurological disorders. In this mini-review, we summarize and discuss the available evidence about neurofilaments as diagnostic and prognostic biomarker for human ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder primarily affecting the motor system network, giving rise to progressive muscle weakness in the limbs, the bulbar region, but also of the respiratory muscles. Survival is typically between 2 and 5 years after disease onset, but in about 15% of patients a slower disease progression is present (1). The most important extramotor manifestations of the disease include behavioral changes, executive dysfunction and language problems, reminiscent of frontotemporal dementia.
As of today, the diagnosis of ALS remains based on clinical judgement and requires a combination of signs of upper and lower motor neuron involvement in a patients with progressive muscle weakness, without alternative explanation for the presenting symptoms and signs (2). Despite efforts to make the diagnostic criteria more sensitive (3, 4), the diagnostic delay remains about 10–12 months after symptom onset (5). The current clinical criteria also do not discriminate between different subtypes of ALS, although they may have very different disease trajectories. Combinations of clinical parameters allow to predict disease progression and survival in ALS patients, but they do not reflect the underlying biological processes (6).
Biomarkers, which reflect hallmarks of the disease, may not only aid in the diagnostic algorithm of ALS, but could also be of value in defining homogeneous subgroups of patients. Potentially, they could also be helpful to track disease progression and treatment responses (7). Neurofilaments (NF) have been studied extensively in different neurological conditions, and are considered to be useful as marker of acute and chronic neuronal injury (8). Neurofilaments are intermediate filaments of 10 nm in neurons, composed of heteropolymers of different subunits, neurofilament light chain (NfL), neurofilament medium chain (NfM), and neurofilament heavy chain (NfH) (9). Phosphorylation and O-glycosylation are believed to be important for NF assembly (9) and especially NfM and NfH undergo these posttranslational modifications. NF are highly expressed in neurons, provide structural support for neurons and determine axon caliber and conduction velocity (10). Mutations in the genes encoding NfH and NfL can cause the inherited neuropathy Charcot-Marie-Tooth disease (11), inframe deletions or insertions in the side arm domain or C-terminal tail domain of NfH have also been linked to ALS (12). Neurofilamentous abnormalities and elevated NF levels are not restricted to ALS. However, NF have been implicated in the pathogenesis of ALS for more than 2 decades (13). In post mortem spinal cord of ALS patients, accumulations of NF are seen in the perikaryon and axons of motor neurons (14) and motor neurons display reduced NfL mRNA levels (15). Overexpression of NfH causes a motor axonopathy with NF inclusions in mice, which can be rescued by NfL overexpression (16), suggesting that an imbalance between the relative expression levels of the different NF subunits may be important. In line with this hypothesis, reducing the NfL levels and overexpression of NfH levels in the SOD1 mouse model of ALS, increased the lifespan of these animals (17, 18). In this model of ALS, the degeneration of motor neurons is accompanied by a progressive rise in blood NF levels, and these levels have been shown to be able to capture treatment responses (19, 20).
In this review, we will give an overview of the current knowledge about the diagnostic and prognostic value of NF levels in cerebrospinal fluid and blood for human ALS.
Available Methods to Measure Neurofilaments Levels
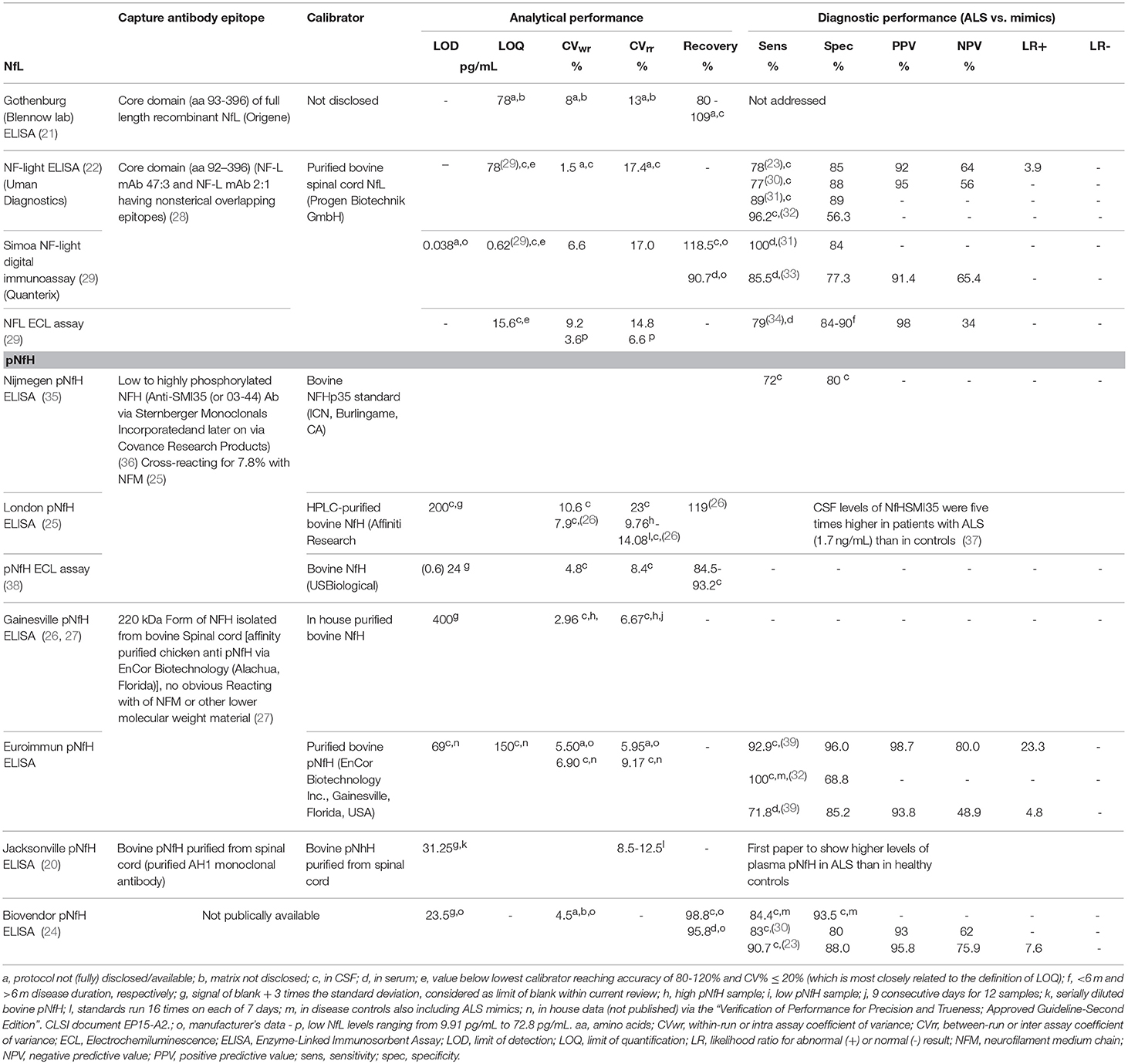
Numerous studies employed in house developed assays or commercial “for research use only” ELISAs for NF measurements (20–27). Although the precision and recovery profile of such kits was acceptable (Table 1), the analytical sensitivity in terms of limit of detection and limit of quantification was insufficient to precisely detect NF levels in CSF of controls or in blood of most patients with ALS (30). Using the same antibodies against NfL, novel technologies including electrochemiluminescence (ECL) and Single Molecule Array (SIMOA) enabled to precisely and sensitively quantify NfL in CSF and blood (22, 29, 40). Furthermore, an improved ELISA assay allowed to accurately quantify pNfH in blood and CSF of patients with ALS (39).
Diagnostic Value of Neurofilaments
It is already known for more than 2 decades that NF levels are roughly 5–10 times higher in ALS patients compared to healthy controls (41). Numerous studies since then, have shown that NF levels are increased in patients with ALS, not only in CSF, but also in serum or plasma (42). As NFs are produced by neurons, the serum/plasma levels are 10 fold lower compared to CSF levels.
Several studies showed that NfL and pNfH are elevated in CSF and serum/plasma in patients with ALS (20, 23, 30–32, 35, 37–39, 43–55). There is a good correlation between NF levels in CSF and in blood, and this is the case for NfL and pNfH (34, 39, 40). Nevertheless, the diagnostic performance was found to be better in CSF compared to blood (39, 54). Most studies compared ALS patients to healthy controls, only few studies tested the diagnostic performance in comparison to ALS mimicking disorders (23, 30, 31, 39). The sensitivity and specificity for ALS was better for pNfH than for NfL in studies comparing both neurofilament subunits (23, 32, 39). Even though there is considerable elevation in NF in some of the ALS mimicking disorders, the diagnostic accuracy to detect ALS is still good. The diagnostic performance of NfL and pNfH assays is shown in Table 1. One study suggested that the discrimination from disease controls improved by using the CSF pNfH/complement C3 ratio (24). For implementation in the routine clinical practice, assay standardization, and characterization, and independent validation of the cut-offs are required. Indeed, the development of reference methods for NF measurements, e.g., by means of mass spectrometry (56, 57), and of certified reference materials for traceability of the calibrators and to demonstrate commutability among the different assays should be encouraged (58). Independent evaluation of the performance characteristics of the NF assays enables the public availability of data on the analytical quality of the different commercially available assays. Furthermore, automation of immunoassay facilitates single measurements with similar precision profiles as duplicate measurements in manually performed ELISAs, the former significantly reducing the implementation costs for patients (59). As the range of NF levels in ALS mimicking disorders is rather wide, the robustness of reported cut-offs might be challenged by the rather low number of ALS mimicking disorders included in most studies (23, 39). Multicenter studies are warranted to establish universally applicable cut-offs for NF.
Importantly, the increase in NF is already measurable early in the disease course (23, 31, 40). A recent study showed that NfL levels increase already several months prior to symptom onset in SOD1 mutation carriers (60). NFs are elevated in sporadic and familial ALS patients, although slightly lower in confirmed SOD1 cases (43) and higher in C9orf72 positive patients (51).
The neuroanatomical correlate of elevated NF levels in ALS is not entirely clear. Both NfL and pNFH correlate with the extent of clinical upper and lower motor neuron involvement (23), although pNfH levels correlate better with lower motor neuron involvement and NfL levels better with upper motor neuron involvement (23, 34). An imaging study revealed that NfL levels in CSF correlate with the extent of corticospinal tract involvement on DTI (48).
Prognostic Value of Neurofilaments
The levels of both NfL and pNfH have been shown to correlate with parameters of disease severity, such as the decline on the ALS functional rating scale-revised or ALSFRS-R (23, 30, 34). They also predict survival of ALS patients, with higher NF levels being unfavorable. In Cox regression analyses both NfL and pNfH have been shown to be independent predictors of survival, when taking other prognostic factors into account (30, 34, 45, 61). Patients with very long survival typically have low levels of NFs (23, 53). The predictive value of NFs is present when using both CSF and blood samples. As higher NF levels are associated with a faster disease progression in typical ALS patients, NF levels could theoretically be used to stratify patients in clinical trials. However, data on this topic are currently lacking.
The difference in disease progression between different clinical subtypes of ALS is not always reflected in NF levels. Patients with C9orf72 ALS have been reported to have higher pNfH CSF levels (51), but further studies on NF levels are needed in different motor neuron disease subtypes. In patients with primary lateral sclerosis (PLS), the levels can also be increased, but mostly to a lesser extent (30, 31, 34). ALS patients with cognitive/behavioral impairment or comorbid FTD have a worse outcome (62, 63), but if this is reflected in NF levels requires further study (64). The unfavorable outcome of patients with bulbar onset or respiratory onset ALS may not be reflected in NF levels.
Value of Neurofilaments to Track Treatment Response?
NFs may not only have value to help with the diagnosis and prediction of disease severity in ALS, they may also become of value to track the response to treatments. As marker of neuronal injury it is anticipated that neuroprotective treatments would result in lower NF levels. For ALS, there are no studies in patients that report a treatment response on NF levels at present. Whether the effect of riluzole on survival can be captured by measuring NF levels remains unknown. On the other hand, a recent study using rodent mutant SOD1 models, showed a clear survival benefit of treatment with antisense oligonucleotides, which was accompanied by a reduction in serum pNfH levels (65). In addition, in other neurological disorders, such as multiple sclerosis, NFs levels reflect the effect of disease-modifying therapies (66).
In patients with ALS, it is know that NFs levels are relatively stable during the course of the disease in many patients (51, 67). However, there is some evidence that the levels may increase during the first phase of the disease (53). This is backed up by data from a recent study in SOD1 mutation carriers, which showed that the levels slowly increase up to 12 months prior to symptom onset and can continue to rise the months following symptom onset (60). The NF levels also correlate with the number of body regions affected by ALS and the ALS progression rate (23, 34), suggesting that they reflect the extent and rate of motor neuron degeneration. Several cross-sectional studies have reported a negative correlation of NF levels with survival (30, 34, 53). This may suggest that the levels drop slightly in later disease stages, although there certainly is a bias introduced by the enrichment for patients with a longer survival at later time points. Longitudinal sampling shows a tendency to lower levels upon follow up, especially in fast-progressing patients (67).
Conclusion
Evidence is emerging that NF levels can become valuable biomarker for ALS, both for diagnosing ALS, for predicting outcome, and potentially for the monitoring of treatment effects. The CSF pNfH level seems to be the most accurate diagnostic marker, but both pNfH and NfL serum or plasma measurements perform good to predict survival and disease progression. Further research is needed to establish the value of NF levels for stratification and for disease monitoring in clinical trials.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PV is supported by grants from KU Leuven (C14/17/107), Opening the Future Fund (KU Leuven), the Interuniversity Attraction Poles (IUAP) program P7/16 of the Belgian Federal Science Policy Office, the Alzheimer Research Foundation (SAO-FRA), the Flemish Government initiated Flanders Impulse Program on Networks for Dementia Research (VIND), the Fund for Scientific Research Vlaanderen (FWO-Vlaanderen), under the frame of E-RARE-2 (PYRAMID) and JPND (STRENGTH and RiMod-FTD), the ALS Liga België and the KU Leuven funds Een Hart voor ALS and Laeversfonds voor ALS Onderzoek. PV holds a senior clinical investigatorship from FWO-Vlaanderen.
References
1. Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers (2017) 3:17085. doi: 10.1038/nrdp.2017.85
2. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:293–9. doi: 10.1080/146608200300079536
3. de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. (2008) 119:497–503. doi: 10.1016/j.clinph.2007.09.143
4. Schrooten M, Smetcoren C, Robberecht W, Van Damme P. Benefit of the Awaji diagnostic algorithm for amyotrophic lateral sclerosis: a prospective study. Ann Neurol. (2011) 70:79–83. doi: 10.1002/ana.22380
5. Paganoni S, Macklin EA, Lee A, Murphy A, Chang J, Zipf A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotr Lateral Scler Frontotem Degener. (2014) 15:453–6. doi: 10.3109/21678421.2014.903974
6. Westeneng HJ, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, Calvo A, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. (2018) 17:423–33. doi: 10.1016/S1474-4422(18)30089-9
7. Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. (2009) 8:94–109. doi: 10.1016/S1474-4422(08)70293-X
8. Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron (2016) 91:56–66. doi: 10.1016/j.neuron.2016.05.018
9. Lee MK, Cleveland DW. Neuronal intermediate filaments. Ann Rev Neurosci. (1996) 19:187–217. doi: 10.1146/annurev.ne.19.030196.001155
10. Yuan A, Rao MV, Veeranna Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harbor Perspect Biol. (2017) 9:4. doi: 10.1101/cshperspect.a018309
11. Hoyle JC, Isfort MC, Roggenbuck J, Arnold WD. The genetics of Charcot-Marie-Tooth disease: current trends and future implications for diagnosis and management. Appl Clin Genet. (2015) 8:235–43. doi: 10.2147/TACG.S69969
12. Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. (1994) 3:1757–61. doi: 10.1093/hmg/3.10.1757
13. Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell (2001) 104:581–91. doi: 10.1016/S0092-8674(01)00244-6
14. Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. (1984) 43:471–80. doi: 10.1097/00005072-198409000-00002
15. Wong NK, He BP, Strong MJ. Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic amyotrophic lateral sclerosis (ALS). J Neuropathol Exp Neurol. (2000) 59:972–82. doi: 10.1093/jnen/59.11.972
16. Meier J, Couillard-Despres S, Jacomy H, Gravel C, Julien JP. Extra neurofilament NF-L subunits rescue motor neuron disease caused by overexpression of the human NF-H gene in mice. J Neuropathol Exp Neurol. (1999) 58:1099–110. doi: 10.1097/00005072-199910000-00009
17. Williamson TL, Bruijn LI, Zhu Q, Anderson KL, Anderson SD, Julien JP, et al. Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci USA. (1998) 95:9631–6. doi: 10.1073/pnas.95.16.9631
18. Couillard-Despres S, Zhu Q, Wong PC, Price DL, Cleveland DW, Julien JP. Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc Natl Acad Sci USA. (1998) 95:9626–30. doi: 10.1073/pnas.95.16.9626
19. Lu CH, Petzold A, Kalmar B, Dick J, Malaspina A, Greensmith L. Plasma neurofilament heavy chain levels correlate to markers of late stage disease progression and treatment response in SOD1(G93A) mice that model ALS. PLoS ONE (2012) 7:e40998. doi: 10.1371/journal.pone.0040998
20. Boylan K, Yang C, Crook J, Overstreet K, Heckman M, Wang Y, et al. Immunoreactivity of the phosphorylated axonal neurofilament H subunit (pNF-H) in blood of ALS model rodents and ALS patients: evaluation of blood pNF-H as a potential ALS biomarker. J Neurochem. (2009) 111:1182–91. doi: 10.1111/j.1471-4159.2009.06386.x
21. Gaetani L, Hoglund K, Parnetti L, Pujol-Calderon F, Becker B, Eusebi P, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. (2018) 10:8. doi: 10.1186/s13195-018-0339-1
22. Koel-Simmelink MJ, Vennegoor A, Killestein J, Blankenstein MA, Norgren N, Korth C, et al. The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated SinglePlex Luminex assay and ELISA. J Immunol Methods (2014) 402:43–9. doi: 10.1016/j.jim.2013.11.008
23. Poesen K, De Schaepdryver M, Stubendorff B, Gille B, Muckova P, Wendler S, et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology (2017) 88:2302–9. doi: 10.1212/WNL.0000000000004029
24. Ganesalingam J, An J, Shaw CE, Shaw G, Lacomis D, Bowser R. Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem (2011) 117:528–37. doi: 10.1111/j.1471-4159.2011.07224.x
25. Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J Immunol Methods (2003) 278:179–90. doi: 10.1016/S0022-1759(03)00189-3
26. Petzold A, Shaw G. Comparison of two ELISA methods for measuring levels of the phosphorylated neurofilament heavy chain. J Immunol Methods (2007) 319:34–40. doi: 10.1016/j.jim.2006.09.021
27. Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. (2005) 336:1268–77. doi: 10.1016/j.bbrc.2005.08.252
28. Norgren N, Karlsson JE, Rosengren L, Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybridoma Hybridom. (2002) 21:53–9. doi: 10.1089/15368590252917647
29. Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. (2016) 54:1655–61. doi: 10.1515/cclm-2015-1195
30. Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry (2016) 87:12–20. doi: 10.1136/jnnp-2015-311387
31. Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology (2018) 90:e22–e30. doi: 10.1212/WNL.0000000000004761
32. Li DW, Ren H, Jeromin A, Liu M, Shen D, Tai H, et al. Diagnostic performance of neurofilaments in chinese patients with amyotrophic lateral sclerosis: a prospective study. Front Neurol. (2018) 9:726. doi: 10.3389/fneur.2018.00726
33. Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S, et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry (2018). doi: 10.1136/jnnp-2018-318704 [Epub ahead of print].
34. Gille B, De Schaepdryver M, Goossens J, Dedeene L, De Vocht J, Oldoni E, et al. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. (2018). doi: 10.1111/nan.12511 [Epub ahead of print].
35. Reijn TS, Abdo WF, Schelhaas HJ, Verbeek MM. CSF neurofilament protein analysis in the differential diagnosis of ALS. J Neurol. (2009) 256:615–9. doi: 10.1007/s00415-009-0131-z
36. Goldstein ME, Sternberger LA, Sternberger NH. Varying degrees of phosphorylation determine microheterogeneity of the heavy neurofilament polypeptide (Nf-H). J Neuroimmunol. (1987) 14:135–48. doi: 10.1016/0165-5728(87)90048-8
37. Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology (2006) 66:852–6. doi: 10.1212/01.wnl.0000203120.85850.54
38. Kuhle J, Regeniter A, Leppert D, Mehling M, Kappos L, Lindberg RL, et al. A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol. (2010) 220:114–9. doi: 10.1016/j.jneuroim.2010.01.004
39. De Schaepdryver M, Jeromin A, Gille B, Claeys KG, Herbst V, Brix B, et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry (2017). 89:367–73. doi: 10.1136/jnnp-2017-316605
40. Weydt P, Oeckl P, Huss A, Muller K, Volk AE, Kuhle J, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. (2016) 79:152–8. doi: 10.1002/ana.24552
41. Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. (1996) 67:2013–8. doi: 10.1046/j.1471-4159.1996.67052013.x
42. Xu Z, Henderson RD, David M, McCombe PA. Neurofilaments as biomarkers for amyotrophic lateral sclerosis: a systematic review and meta-analysis. PLoS ONE (2016) 11:e0164625. doi: 10.1371/journal.pone.0164625
43. Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM. Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur J Neurol. (2007) 14:1329–33. doi: 10.1111/j.1468-1331.2007.01972.x
44. Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE (2013) 8:e75091. doi: 10.1371/journal.pone.0075091
45. Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology (2015) 84:2247–57. doi: 10.1212/WNL.0000000000001642
46. Tortelli R, Ruggieri M, Cortese R, D'Errico E, Capozzo R, Leo A, et al. Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur J Neurol. (2012) 19:1561–7. doi: 10.1111/j.1468-1331.2012.03777.x
47. Tortelli R, Copetti M, Ruggieri M, Cortese R, Capozzo R, Leo A, et al. Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol. (2015) 22:215–8. doi: 10.1111/ene.12421
48. Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Trans Neurol. (2015) 2:748–55. doi: 10.1002/acn3.212
49. Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R, et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotr Later Scler Frontotemp Degen. (2016) 17:404–13. doi: 10.3109/21678421.2016.1167913
50. Boylan KB, Glass JD, Crook JE, Yang C, Thomas CS, Desaro P, et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry (2013) 84:467–72. doi: 10.1136/jnnp-2012-303768
51. Gendron TF, Group CONS, Daughrity LM, Heckman MG, Diehl NN, Wuu J, et al. Phosphorylated neurofilament heavy chain: A biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol. (2017) 82:139–46. doi: 10.1002/ana.24980
52. Lehnert S, Costa J, de Carvalho M, Kirby J, Kuzma-Kozakiewicz M, Morelli C, et al. Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotr Later Scler Frontotemp Degen. (2014) 15:344–50. doi: 10.3109/21678421.2014.884592
53. McCombe PA, Pfluger C, Singh P, Lim CY, Airey C, Henderson RD. Serial measurements of phosphorylated neurofilament-heavy in the serum of subjects with amyotrophic lateral sclerosis. J Neurol Sci. (2015) 353:122–9. doi: 10.1016/j.jns.2015.04.032
54. Li S, Ren Y, Zhu W, Yang F, Zhang X, Huang X. Phosphorylated neurofilament heavy chain levels in paired plasma and CSF of amyotrophic lateral sclerosis. J Neurol Sci. (2016) 367:269–74. doi: 10.1016/j.jns.2016.05.062
55. Chen X, Chen Y, Wei Q, Ou R, Cao B, Zhao B, et al. Assessment of a multiple biomarker panel for diagnosis of amyotrophic lateral sclerosis. BMC Neurol. (2016) 16:173. doi: 10.1186/s12883-016-0689-x
56. Oeckl P, Steinacker P, Otto M. Comparison of internal standard approaches for SRM analysis of alpha-synuclein in cerebrospinal fluid. J Proteome Res. (2018) 17:516–23. doi: 10.1021/acs.jproteome.7b00660
57. Leinenbach A, Pannee J, Dulffer T, Huber A, Bittner T, Andreasson U, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem. (2014) 60:987–94. doi: 10.1373/clinchem.2013.220392
58. Andreasson U, Kuhlmann J, Pannee J, Umek RM, Stoops E, Vanderstichele H, et al. Commutability of the certified reference materials for the standardization of beta-amyloid 1–42 assay in human cerebrospinal fluid: lessons for tau and beta-amyloid 1–40 measurements. Clin Chem Lab Med. (2018). 56:2058–66. doi: 10.1515/cclm-2018-0147
59. Gille B, Dedeene L, Stoops E, Demeyer L, Francois C, Lefever S, et al. Automation on an Open-Access Platform of Alzheimer's Disease Biomarker Immunoassays. SLAS Technol. (2018) 23:188–97. doi: 10.1177/2472630317750378
60. Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. (2018) 84:130–9. doi: 10.1002/ana.25276
61. Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, et al. Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol. (2017) 74:525–32. doi: 10.1001/jamaneurol.2016.5398
62. Chio A, Ilardi A, Cammarosano S, Moglia C, Montuschi A, Calvo A. Neurobehavioral dysfunction in ALS has a negative effect on outcome and use of PEG and NIV. Neurology (2012) 78:1085–9. doi: 10.1212/WNL.0b013e31824e8f53
63. Elamin M, Bede P, Byrne S, Jordan N, Gallagher L, Wynne B, et al. Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology (2013) 80:1590–7. doi: 10.1212/WNL.0b013e31828f18ac
64. Illan-Gala I, Alcolea D, Montal V, Dols-Icardo O, Munoz L, de Luna N, et al. CSF sAPPbeta, YKL-40, and NfL along the ALS-FTD spectrum. Neurology (2018) 91:e1619–e28. doi: 10.1212/WNL.0000000000006383
65. McCampbell A, Cole T, Wegener AJ, Tomassy GS, Setnicka A, Farley BJ, et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J Clin Invest. (2018) 128:3558–67. doi: 10.1172/JCI99081
66. Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. (2017) 81:857–70. doi: 10.1002/ana.24954
Keywords: amyotrophic lateral sclerosis, frontotemporal dementia, neurofilament, cerebrospinal fluid, serum, plasma, NfL, pNfH
Citation: Poesen K and Van Damme P (2019) Diagnostic and Prognostic Performance of Neurofilaments in ALS. Front. Neurol. 9:1167. doi: 10.3389/fneur.2018.01167
Received: 31 October 2018; Accepted: 17 December 2018;
Published: 18 January 2019.
Edited by:
Peter Bede, Trinity College Dublin, IrelandReviewed by:
Siw Johannesen, University of Regensburg, GermanyPatrizia Longone, Fondazione Santa Lucia (IRCCS), Italy
Copyright © 2019 Poesen and Van Damme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koen Poesen, koen.poesen@uzleuven.be
Philip Van Damme, philip.vandamme@uzleuven.be
 Koen Poesen1,2*
Koen Poesen1,2* Philip Van Damme
Philip Van Damme