- 1Department of Medical Oncology, Tongji Medical College, Hubei Cancer Hospital, Huazhong University of Science and Technology, Wuhan, China
- 2Colorectal Cancer Clinical Research Center of Hubei Province, Wuhan, China
- 3Colorectal Cancer Clinical Research Center of Wuhan, Wuhan, China
- 4Department of Gastrointestinal Surgery, Tongji Medical College, Hubei Cancer Hospital, Huazhong University of Science and Technology, Wuhan, China
- 5Division of Nephrology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
Background: Inflammatory factors and nutritional status are critical to the prognosis of colorectal cancer patients. This study aimed to investigate the prognostic value of the combination of preoperative lymphocytes, albumin, and neutrophils (LANR) in patients with resectable colorectal cancer.
Methods: A total of 753 patients with pathologically diagnosed primary colorectal cancer were included in the study. The value of LANR was defined as follows: LANR, lymphocyte × albumin/neutrophil. The ROC curve, subgroup analysis and Cox proportional hazard regression analysis were used to assess the prognostic value of LANR in overall survival and progression-free survival.
Results: The median age of the patients was 60 years (range 52–67 years). In overall survival, the area under the curve of LANR was 0.6276, and the HR (95% CI) was 0.551 (0.393–0.772). And in progression-free survival, the area under the curve of LANR was 0.5963, and the HR (95% CI) was 0.697 (0.550–0.884). The results indicate that preoperative LANR may be a reliable predictor of overall and progression-free survival in resectable colorectal cancer patients.
Conclusions: LANR is an important prognostic indicator for patients with resectable colorectal cancer, and it can also provide a reference for clinicians and patients to choose a treatment plan.
Introduction
Colorectal cancer (CRC) is an important public health problem. In 2018, there were more than 1.8 million new cases of colorectal cancer and 881,000 deaths worldwide, accounting for about one-tenth of global cancer incidence and deaths. And the incidence of colorectal cancer ranks third and the mortality rate ranks second (1). From a clinical perspective, surgery has been established as the main treatment for colorectal cancer (2). Although advances in medical treatment have gradually improved the survival of patients (3). In fact, even after surgery, the prognosis of colorectal cancer is far from satisfactory (4, 5). Therefore, finding more effective biomarkers to predict the prognosis of colorectal cancer becomes particularly important.
With the continuous development of tumor prognosis research, more and more evidence indicates the role of inflammatory factors and nutritional status in cancer prognosis (6–8). Systemic inflammatory factors, such as lymphocytes (9), monocytes (10) and neutrophils (11), and blood biochemical indicators related to nutritional status, such as C-reactive protein levels (CRP) (12) and albumin levels (ALB) (13), are valuable prognostic indicators for cancers including colorectal cancer. In addition, studies have shown that the integration of these biochemical indicators such as the modified Glasgow Prognosis Score (mGPS) (14), C-reactive protein to albumin ratio (CAR) (15, 16), and neutrophil to lymphocyte ratio (NLR) (17) can effectively improve the accuracy of cancer prognosis prediction.
However, the prognostic significance of the combination of lymphocyte, albumin, and neutrophil (LANR) in colorectal cancer has not been well-investigated to date. Therefore, in this study, we retrospectively analyzed the preoperative blood biochemical indicators of 753 colorectal cancer patients, and systematically evaluated the survival prognostic value of LANR.
Materials and Methods
Patient Cohort
We retrospectively collected 829 patients with pathologically diagnosed primary colorectal cancer at Hubei Cancer Hospital from January 2013 to December 2016. The exclusion criteria were as follows: (1) having history of malignant tumors; (2) having incomplete clinical data; (3) having concurrent malignant tumors other than colorectal cancer; or (4) having other diseases with serious impacts on prognosis, such as ischemic heart disease and stroke. Based on the exclusion criteria, we eventually included 753 patients with colorectal cancer in our study (Supplementary Figure 1). Tumor stage was determined based on the Eighth Edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (18). The study was supported by the Ethics Committee and Institutional Review Board of Hubei Cancer Hospital. And all patients provided informed consent.
Data Collection
We collected the clinicopathological information and preoperative blood biochemical indicators of patients with colorectal cancer through electronic and paper medical records from the hospital. Such as gender, age, tumor size, vascular tumor thrombus, nerve invasion, circumferential margin, radiotherapy, chemotherapy, tumor location, TNM stage, differentiation, ALB, lymphocyte, and neutrophil. Based on previous studies (19–22), we found that ALB, lymphocyte, and neutrophil were key biochemical indicators related to tumor prognosis. And we also used these three indicators to construct a new prognostic marker-LANR, which was defined as Lymphocyte × Albumin/Neutrophil. The study follow-up until August 2019. The overall survival (OS) was set as the first outcome and progression-free survival (PFS) as the secondary outcome. The PFS was defined as the time from the date of tumor resection to the date of cancer recurrence, metastasis, death, or the end of follow-up whichever came first, and the OS was calculated as the time from the date of tumor resection to the date of death or the end of follow-up.
Statistical Analysis
The continuous variables were expressed as mean ± standard deviation ( ± SD) or median (interquartile range), and the categorical variables were presented by the number of cases (percentage). Student's t-test or Wilcoxon test was used to compare differences between groups of continuous variables. Chi-square or Fisher's exact tests were used to evaluate categorical variables. By using the inflection point as the cut-offs, the receiver operating characteristic (ROC) curve was used to convert continuous variables (albumin, neutrophils, lymphocytes, and LANR) into dichotomous variables. Kaplan–Meier survival curves and log-rank test were used to compare the survival difference between groups classified by dichotomized biochemical indicators. Cox proportional hazard regression model was used for univariate and multivariate regression analysis. Statistically significant variables (P < 0.05) in univariate analysis were included in multivariate analysis. Subgroup analysis were performed to show the prognostic association between patients with different characteristics and the new index, and the results were shown in the forest plots (23). Statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina, USA) and R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All analyses were two-sided, and P < 0.05 were considered statistically significant.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Patient Characteristics
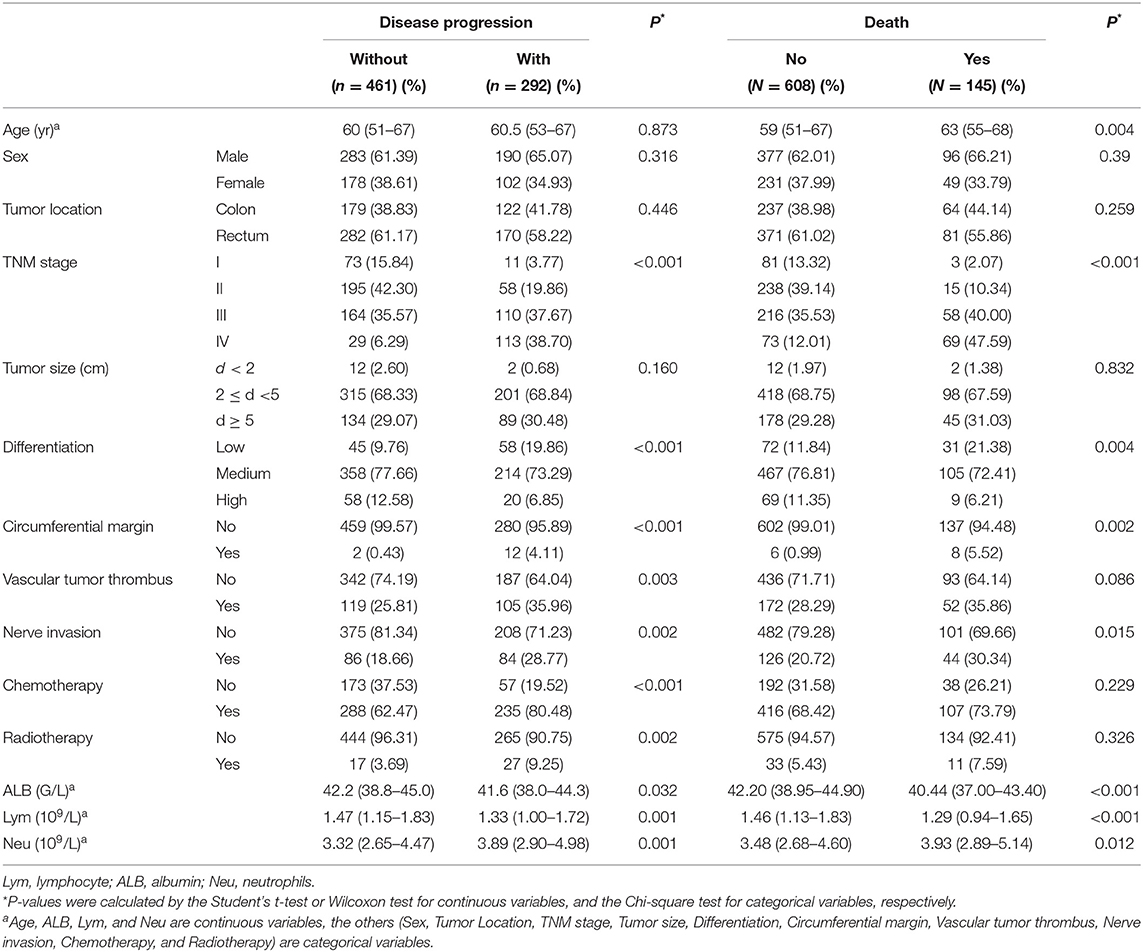
A total of 753 patients with colorectal cancer were included in this study. The demographic and clinicopathological characteristics are shown in Table 1. There were 280 females (37.18%) and 473 males (62.82%). The median age of the patients was 60 years (range 52–67 years). There were 84 patients (11.16%) in stage I, 253 (33.60%) in stage II, 274 (36.39%) in stage III, and 142 (18.86%) in stage IV. Of these patients, 452 (60.03%) had rectal tumors, and 301 (39.97%) had colon tumors. A total of 292 (38.78%) patients progressed and 145 patients (19.26%) died. The study's median progression-free survival was 63.47 months, and the median follow-up time was 37.03 months.
Prognostic Value of LANR in Overall Survival
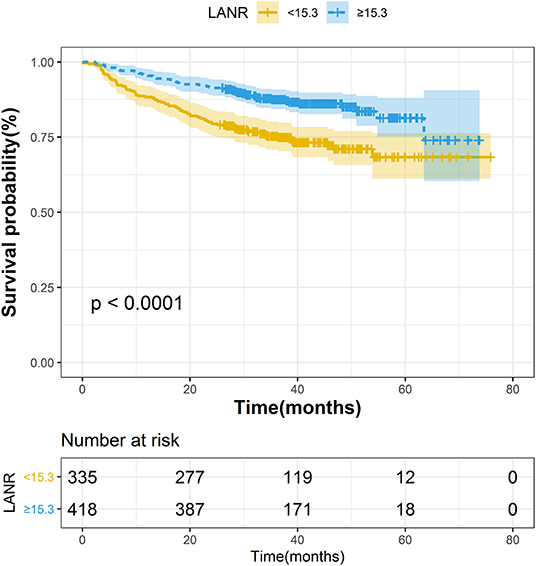
The areas under the ROC curves and inflection points of albumin, neutrophils, lymphocyte counts, and LANR for OS are listed in Table 2. According to the ROC curve, we found that the area under the curve of LANR was the best at 0.6276 (Supplementary Figure 2). We divided LANR into high-level (n = 418, 55.51%) and low-level (n = 335, 44.49%) groups based on cut-off values (Supplementary Table 1), and the Kaplan-Meier survival curve showed that patients with high-level LANR had longer overall survival (Figure 1). Univariate analysis revealed that TNM stage, differentiation, circumferential margin, nerve invasion, albumin, neutrophils, lymphocytes, and LANR all showed significant association with OS (all P < 0.05; Table 2). Multivariate analysis showed that high levels of albumin, neutrophils, lymphocytes and LANR had 0.681 (95% CI: 0.476–0.974), 1.512 (95% CI: 1.085–2.107), 0.634 (95% CI: 0.445–0.903), and 0.551 (95% CI: 0.393–0.772)-fold risk of death (Table 2). LANR presented significant associations with OS among patients in different genders, age (<65 yr), tumor locations (colon), differentiation (medium), and TNM stages (III/IV) (Supplementary Figure 3). Combining the area under the curve and the results of multivariate cox regression, we found that LANR is a valuable new prognostic indicator in overall survival.

Table 2. Univariate and multivariate Cox regression analysis of overall survival in patients with colorectal cancer.
Prognostic Value of LANR in Progression-Free Survival
The areas under the ROC curves and inflection points of albumin, neutrophils, lymphocyte counts, and LANR for PFS are listed in Table 3. According to the ROC curve, we found that the area under the curve of LANR was the best at 0.5963 (Supplementary Figure 4). We divided LANR into high-level (n = 367, 48.74%) and low-level (n = 386, 51.26%) groups based on cut-off values (Supplementary Table 2), and the Kaplan-Meier survival curve showed that patients with high-level LANR had longer progression-free survival (Figure 2). Univariate analysis revealed that TNM stage, differentiation, circumferential margin, vascular tumor thrombus, nerve invasion, chemotherapy, radiotherapy, albumin, neutrophils, lymphocytes, and LANR all showed significant association with PFS (all P < 0.05; Table 3). Multivariate analysis showed that high levels of albumin, neutrophils, lymphocytes, and LANR had 0.800 (95% CI: 0.624–1.026), 1.450 (95% CI: 1.141–1.843), 0.727 (95% CI: 0.570–0.926), and 0.697 (95% CI: 0.550–0.884)-fold risk of disease progression (Table 3). LANR presented significant associations with PFS among patients in age (≥65 yr), gender (female), tumor locations (rectum), differentiation (medium), and TNM stages (III/IV) (Supplementary Figure 5). Combining the area under the curve and the results of multivariate cox regression, we found that LANR is also a valuable new prognostic indicator in 5-year progression-free survival.
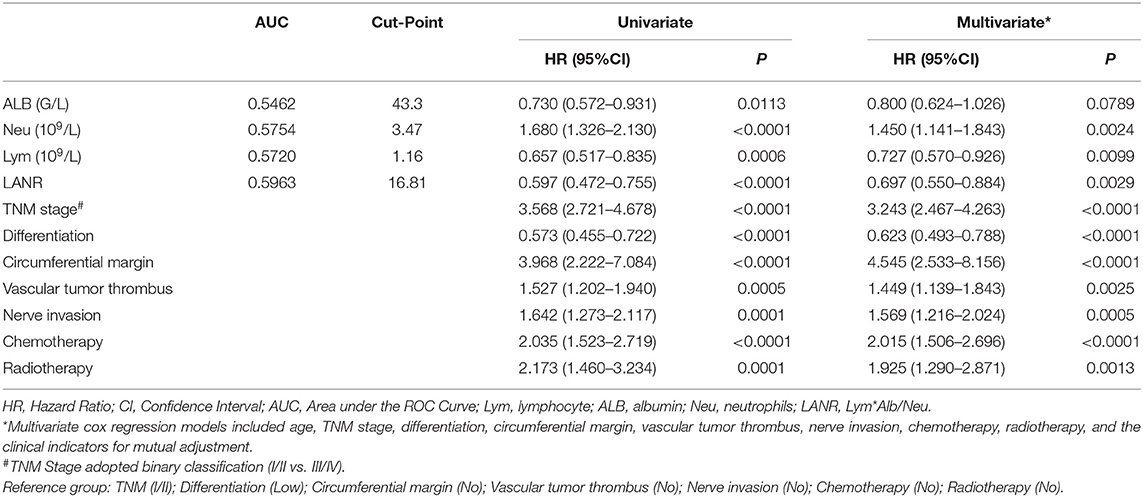
Table 3. Univariate and multivariate cox regression analysis of progression-free survival in patients with colorectal cancer.
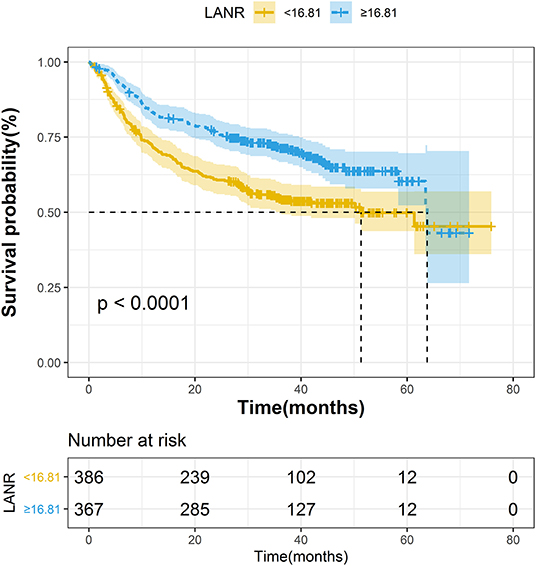
Figure 2. The Kaplan–Meier curves for progression-free survival of colorectal cancer patients based on LANR.
Discussion
In this study, we developed a novel indicator—LANR, which was based on lymphocytes, neutrophils, and albumin, and the results showed that the indicator was significantly correlated with the prognosis of colorectal cancer patients. And to our knowledge, this is the first study to investigate the prognostic value of LANR in colorectal cancer patients.
The development of cancer and its response to treatment are strongly influenced by innate and adaptive immunity, which promote or reduces tumorigenesis and may have opposite effects on the outcome of treatment. At the same time, chronic inflammation promote tumor development, progression, metastatic spread and treatment resistance (24). In addition, studies have shown that systemic inflammation is a marker of poor prognosis present in around 20–40% of colorectal cancer patients (25). Neutrophils are the main circulating granulocytes in humans. They reflect the state of host inflammation and are a hallmark of cancer (26). Neutrophils are involved in different stages of the carcinogenic process, including tumorigenesis, growth, proliferation, or metastatic spread (27, 28). It promotes tumorigenesis by releasing reactive oxygen species (ROS), reactive nitrogen (RNS), or proteases (29), promotes tumor proliferation by weakening the immune system, and also promotes metastatic spread by inhibiting natural killing function and promoting tumor cell extravasation (30). Studies have shown that neutrophils are associated with poor prognosis (31). And the higher the neutrophil level, the higher the risk of progression and death (20, 32). As one of the main cells of human immunity, lymphocytes can produce an immune response to tumor cells, and the decrease of the lymphocytes leads to a decrease in the body's ability to inhibit tumors (33). At the same time, lymphocytes participate in cytotoxic cell death and inhibition of tumor cell proliferation and migration (34). As research continues to develop, more and more evidence suggests that lymphocytes play an important role in predicting the prognosis in colorectal cancer (35–37). And high levels of lymphocytes were significantly associated with good tumor behavior and better survival (38). In addition to inflammatory factors, nutritional indicators can also predict complications, recurrence and prognosis in patients with colorectal cancer. Using prognostic nutrition indicators to investigate the nutrition and immune status of patients may be a useful clinical approach (39). Serum albumin is a common indicator of nutritional status (40). Studies have shown that serum albumin levels were significantly related to overall survival (41). And low levels of serum albumin are associated with a poor overall prognosis in patients with colorectal cancer (42, 43). In this study, we combined these valuable indicators to construct a new prognostic indicator that has good prognostic performance for both overall survival (HR: 0.551; 95% CI: 0.393–0.772) and progression-free survival (HR: 0.697; 95% CI: 0.550–0.884). But we also have some limitations. First, the sample size included in the study was relatively small. Second, as a single-center study, the conclusion may be biased. Therefore, a lot of research is needed to further confirm our findings. In this study, we found a new index that is easily available and has good prognostic performance, which will provide a reference for clinicians and patients to choose a treatment method.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was supported by the Ethics Committee and Institutional Review Board of Hubei Cancer Hospital. And all patients provided informed consent.
Author Contributions
SW and YL contributed to the conception and design of the study, reviewed and edited the manuscript, guarantors of this work, who have full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. XL, SY, PL, YM, HX, ZY, JH, SW, and YL contributed to the acquisition of data, reviewed, and commented on various versions of the manuscript. XL and SY analyzed the data and wrote the first draft of the manuscript. All authors agree to be responsible for all aspects of the work and give final approval for the submission.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2017YFC0908204), the Health commission of Hubei Province scientific research project (Grant Nos. WJ2021Z001 and WJ2019H121), the Natural Science Foundation of Hubei Province (Grant No. 2019ACA135), the applied Basic Research Program of Wuhan Science and Technology Bureau (Grant No. 2020020601012250), the Foundation of Chinese Society of Clinical Oncology (CSCO: Y-QL2019-0351 and Y-HS2019-39).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all participants who volunteered to provide data and samples in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.610264/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Rentsch M, Schiergens T, Khandoga A, Werner J. Surgery for colorectal cancer - trends, developments, and future perspectives. Visc Med. (2016) 32:184–91. doi: 10.1159/000446490
3. Zheng C, Jiang F, Lin H, Li S. Clinical characteristics and prognosis of different primary tumor location in colorectal cancer: a population-based cohort study. Clin Transl Oncol. (2019) 21:1524–31. doi: 10.1007/s12094-019-02083-1
4. Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M, et al. Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer-central evaluation of FIRE-3. Eur J Cancer. (2018) 88:77–86. doi: 10.1016/j.ejca.2017.10.028
5. Peng F, Hu D, Lin X, Liang B, Chen Y, Zhang H, et al. Impact of long-term antihypertensive and antidiabetic medications on the prognosis of post-surgical colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Aging. (2018) 10:1166–81. doi: 10.18632/aging.101459
6. Miyamoto Y, Hiyoshi Y, Daitoku N, Okadome K, Sakamoto Y, Yamashita K, et al. Naples prognostic score is a useful prognostic marker in patients with metastatic colorectal cancer. Dis Colon Rectum. (2019) 62:1485–93. doi: 10.1097/dcr.0000000000001484
7. Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, et al. Combination of serum albumin and cholinesterase levels as prognostic indicator in patients ith colorectal cancer. Anticancer Res. (2019) 39:1085–90. doi: 10.21873/anticanres.13217
8. Hua X, Long ZQ, Huang X, Deng JP, Wen W, He ZY, et al. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. (2020) 44:100560. doi: 10.1016/j.currproblcancer.2020.100560
9. Yamamoto M, Saito H, Uejima C, Tanio A, Takaya S, Ashida K, et al. Combined pre- and postoperative lymphocyte count accurately predicts outcomes of patients with colorectal cancer. Dig Surg. (2019) 36:487–94. doi: 10.1159/000492340
10. Hu S, Zou Z, Li H, Zou G, Li Z, Xu J, et al. The preoperative peripheral blood monocyte count is associated with liver metastasis and overall survival in colorectal cancer patients. PLoS ONE. (2016) 11:e0157486. doi: 10.1371/journal.pone.0157486
11. Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, et al. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. (2018) 267:311–8. doi: 10.1097/sla.0000000000002058
12. Kostner AH, Kersten C, Lowenmark T, Ydsten KA, Peltonen R, Isoniemi H, et al. The prognostic role of systemic inflammation in patients undergoing resection of colorectal liver metastases: C-reactive protein (CRP) is a strong negative prognostic biomarker. J Surg Oncol. (2016) 114:895–9. doi: 10.1002/jso.24415
13. Egenvall M, Morner M, Martling A, Gunnarsson U. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis. (2018) 20:26–34. doi: 10.1111/codi.13807
14. Tsuchihashi K, Ito M, Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, et al. Role of predictive value of the modified glasgow prognostic score for later-line chemotherapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. (2018) 17:e687–97. doi: 10.1016/j.clcc.2018.07.004
15. Fan Y, Xiang S, Dai Z, Zou C, Wang X, Gao Z. Prognostic significance of C-reactive protein to albumin ratio in colorectal cancer patients: a meta-analysis. Int J Colorectal Dis. (2019) 34:1105–11. doi: 10.1007/s00384-019-03299-x
16. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. (2016) 23:900–7. doi: 10.1245/s10434-015-4948-7
17. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition ajcc cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–99. doi: 10.3322/caac.21388
19. Wei Y, Xu H, Dai J, Peng J, Wang W, Xia L. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Res Int. (2018) 2018:1804086. doi: 10.1155/2018/1804086
20. Sjoquist KM, Renfro LA, Simes RJ, Tebbutt NC, Clarke S, Seymour MT, et al. Personalizing survival predictions in advanced colorectal cancer: the ARCAD nomogram project. J Natl Cancer Inst. (2018) 110:638–48. doi: 10.1093/jnci/djx253
21. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261
22. Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, et al. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer. (2017) 17:744. doi: 10.1186/s12885-017-3752-0
23. Sedgwick P. How to read a forest plot in a meta-analysis. BMJ. (2015) 351:h4028. doi: 10.1136/bmj.h4028
24. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. (2015) 125:3347–55. doi: 10.1172/jci80007
25. Tuomisto AE, Makinen MJ, Vayrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. (2019) 25:4383–404. doi: 10.3748/wjg.v25.i31.4383
26. Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
27. Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. (2015) 34:735–51. doi: 10.1007/s10555-015-9594-9
28. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
29. Antonio N, Bonnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. (2015) 34:2219–36. doi: 10.15252/embj.201490147
30. Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK Cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. (2016) 6:630–49. doi: 10.1158/2159-8290.cd-15-1157
31. Xiong Y, Wang K. Profiles of immune infiltration in colorectal cancer and their clinical significant: a gene expression-based study. Cancer Med. (2018) 7:4496–508. doi: 10.1002/cam4.1745
32. Dimitriou N, Felekouras E, Karavokyros I, Alexandrou A, Pikoulis E, Griniatsos J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer. (2018) 18:1202. doi: 10.1186/s12885-018-5042-x
33. Jin LJ, Chen WB, Zhang XY, Bai J, Zhao HC, Wang ZY. Analysis of factors potentially predicting prognosis of colorectal cancer. World J Gastrointest Oncol. (2019) 11:1206–17. doi: 10.4251/wjgo.v11.i12.1206
34. Wu Q, Hu T, Zheng E, Deng X, Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine. (2017) 96:e7051. doi: 10.1097/md.0000000000007051
35. Kong JC, Guerra GR, Pham T, Mitchell C, Lynch AC, Warrier SK, et al. Prognostic impact of tumor-infiltrating lymphocytes in primary and metastatic colorectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. (2019) 62:498–508. doi: 10.1097/dcr.0000000000001332
36. Berntsson J, Svensson MC, Leandersson K, Nodin B, Micke P, Larsson AH, et al. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. Int J Cancer. (2017) 141:1654–66. doi: 10.1002/ijc.30869
37. Palin RP, Devine AT, Hicks G, Burke D. Association of pretreatment neutrophil-lymphocyte ratio and outcome in emergency colorectal cancer care. Ann R Coll Surg Engl. (2018) 100:308–15. doi: 10.1308/rcsann.2017.0232
38. Ko YS, Pyo JS. Clinicopathological significance and prognostic role of tumor-infiltrating lymphocytes in colorectal cancer. Int J Biol Markers. (2019) 34:132–8. doi: 10.1177/1724600818817320
39. Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. (2015) 58:1048–57. doi: 10.1097/dcr.0000000000000458
40. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in Patients with colorectal cancer. Anticancer Res. (2016) 36:995–1001
41. Ihara K, Yamaguchi S, Shida Y, Ogata H, Domeki Y, Okamoto K, et al. Poor nutritional status before and during chemotherapy leads to worse prognosis in unresectable advanced or recurrent colorectal cancer. Int Surg. (2015). doi: 10.9738/intsurg-d-15-00079.1. [Epub ahead of print].
42. Zhou QP, Li XJ. C-Reactive protein to albumin ratio in colorectal cancer: a meta-analysis of prognostic value. Dose Response. (2019) 17:1559325819889814. doi: 10.1177/1559325819889814
Keywords: lymphocytes, albumin, neutrophils, colorectal cancer, prognosis
Citation: Liang X, Yao S, Lu P, Ma Y, Xu H, Yin Z, Hu J, Liu Y and Wei S (2021) The Prognostic Value of New Index (LANR) Composed of Pre-operative Lymphocytes, Albumin, and Neutrophils in Patients With Resectable Colorectal Cancer. Front. Oncol. 11:610264. doi: 10.3389/fonc.2021.610264
Received: 25 September 2020; Accepted: 08 February 2021;
Published: 25 May 2021.
Edited by:
Jaw-Yuan Wang, Kaohsiung Medical University Hospital, TaiwanReviewed by:
Feng Fan Chiang, Taichung Veterans General Hospital, TaiwanWan-Hsiang Hu, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Copyright © 2021 Liang, Yao, Lu, Ma, Xu, Yin, Hu, Liu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaozhong Wei, weishaozhong@163.com; Yanyan Liu, liuyy1919@163.com
†These authors have contributed equally to this work
 Xinjun Liang
Xinjun Liang Shuang Yao1,2,3†
Shuang Yao1,2,3† Ping Lu
Ping Lu Yifei Ma
Yifei Ma Yanyan Liu
Yanyan Liu Shaozhong Wei
Shaozhong Wei