Asthma in Children and Adults—What Are the Differences and What Can They Tell us About Asthma?
- 1Division of Pediatric Pulmonology, Department of Pediatrics, University of Massachusetts Medical School, Worcester, MA, United States
- 2Department of Population and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, MA, United States
- 3Department of Respiratory Medicine, Alfred Hospital, Melbourne, VIC, Australia
- 4Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
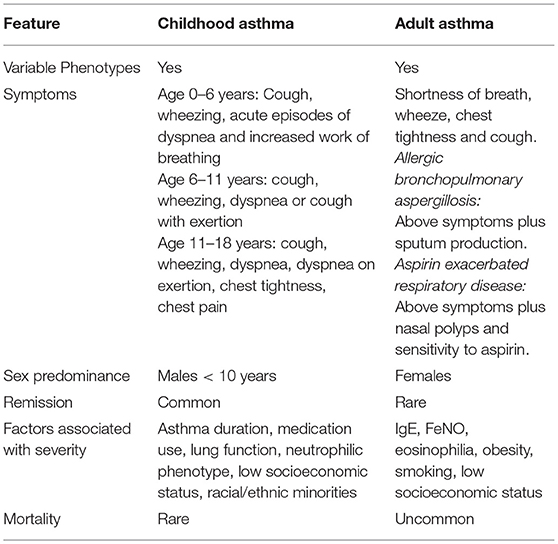
Asthma varies considerably across the life course. Childhood asthma is known for its overall high prevalence with a male predominance prior to puberty, common remission, and rare mortality. Adult asthma is known for its female predominance, uncommon remission, and unusual mortality. Both childhood and adult asthma have variable presentations, which are described herein. Childhood asthma severity is associated with duration of asthma symptoms, medication use, lung function, low socioeconomic status, racial/ethnic minorities, and a neutrophilic phenotype. Adult asthma severity is associated with increased IgE, elevated FeNO, eosinophilia, obesity, smoking, and low socioeconomic status. Adult onset disease is associated with more respiratory symptoms and asthma medication use despite higher prebronchodilator FEV1/FVC. There is less quiescent disease in adult onset asthma and it appears to be less stable than childhood-onset disease with more relapses and less remissions.
Childhood Onset Asthma
Introduction to Childhood Asthma
Childhood asthma is not a singular disease, but rather a uniquely diverse disorder with variable presentation throughout childhood. Asthma affects 8.3% of children in the United States and is the most common chronic disease of childhood (1, 2). Childhood asthma is responsible for 50 billion dollars in annual healthcare expenditures and is a major cause of emergency room visits, hospital admissions, school absences, and loss of parental workdays (1–3).
Asthma is characterized by inflammation leading to bronchoconstriction, edema, and increased mucous production in the airways. Interestingly, the disorder is more prevalent in boys in the first decade of life. However, after puberty and in the second decade of life, it appears that asthma is more prevalent in young women (4). Asthma disproportionately affects minority and low-income children with African American and Hispanic children having the highest prevalence rates, morbidity and mortality due to asthma (5, 6).
Asthma is considered a chronic disease of childhood however there are periods of time during which disease can go into remission or resolve altogether. Important risk factors for the development of childhood asthma have been identified. The phenotypes of childhood asthma and varied presentations are best defined through the periods of the pediatric life course and are described herein.
Childhood Asthma Risk Factors
The perinatal period has been implicated in the development of childhood asthma. Several cohort studies have unveiled risk factors for the development of asthma in offspring, with factors that span from genetic and environmental risk factors to features such as child's sex and presence of atopy.
Genetic Risk Factors
The genetics of asthma are an emerging and complicated topic. Multiple genes are thought to contribute to asthma and rapidly changing technology continues to build our current understanding of the genetic risk factors for asthma development. This is a complex topic that we will only briefly describe herein. Genome-wide Association Studies (GWAS) have dramatically improved our understanding of asthma susceptibility genes. Briefly, the following genes have been determined to have significant association with asthma susceptibility: the 17q21 locus with the ORMDL3 and GSDML genes, the IL33 gene on chromosome 9p24, the HLA- DR/DQ gene on chromosome 6p21, the IL1RL1/IL18R1gene on chromosome 2q12, the WDR36/ TSLP gene on chromosome 5q22 and the IL13gene on chromosome 5q31 (7). Interestingly, GWAS have shown evidence that loci may be specific to racial/ethnic populations, such PHYNN1 observed in African-Americans with asthma (8). However, as with other common diseases, for individuals, only a small degree of heritability of asthma can be explained by the genes observed in GWAS. Therefore, emerging and multi-genetic approaches are needed to further study the genetic susceptibility to asthma (7).
Environmental Risk Factors
Environmental perinatal risk factors are also important to consider for childhood asthma. Maternal tobacco smoking during pregnancy has been shown to increase the risk of childhood asthma (9). Maternal diet in pregnancy has also been implicated as an asthma risk factor with reports of maternal diets higher in vitamin E, zinc, and polyunsaturated fatty acids as protective against the development of childhood asthma (10–12). In contrast, high sugar intake in the maternal diet during pregnancy has been associated with increased risk of asthma in offspring (13). Other maternal dietary factors have been studied but with less conclusive results including the intake of vitamin D, vitamin C, and a Mediterranean diet. Other perinatal risk factors for childhood asthma that have been reported are neonatal jaundice, maternal preeclampsia, and cesarean section delivery, all which have been associated with higher risk of childhood asthma development (14–16).
Ultimately gene-environment interactions (the genetic-environmental axis) are critical for the development of asthma in a child (8).
Natal Risk Factors
Chronic lung disease of prematurity is known to increase the risk of asthma development in children (17). Specifically, extreme preterm birth (23–27 weeks gestation) is associated with an increased risk of asthma into young adulthood (18). Additionally, cesarean section delivery as mode of delivery (16, 19) and low birth weight have been associated with asthma diagnosis in mid-childhood with symptoms persisting into adult life (20).
Sex
Boys are more likely to develop childhood asthma, as compared with girls, at least until the point of puberty. This has been explained by smaller airway size in boys compared with girls under age 10 years, which predisposes to worsened airway reactivity, as compared with girls of the same age, height and weight (21).
Family History
Both maternal and paternal histories of asthma are associated with increased risk of asthma in offspring. Interestingly, maternal asthma history is more strongly associated with asthma development in the child (22).
Medical History
Presence of atopy (having IgE antibodies to specific allergens) is strongly associated with childhood asthma (23). Specifically the “atopic march” is a pattern that is described clinically in individuals with atopic disease. This “atopic march” begins as atopic dermatitis (or eczema) in infancy, develops on to allergic rhinitis (or hayfever) and then asthma later in childhood (24). Specific indoor allergen sensitization in early life have been of interest with regard to asthma development. Sensitization to house dust mite, alternaria mold, and cockroach allergens have been associated with increased risk of asthma (25, 26), whereas early life exposure to cat and dog allergens have been associated with both increased and decreased risk of asthma in different studies (27, 28).
Medication Exposure
Exposures to antibiotics (29) and antipyretics (30) in infancy have been described to be associated with increased risk of developing childhood asthma however the data has been conflicting and the study results have been concerning for uncontrolled confounding bias. Therefore, further studies are warranted before conclusions can be made about these associations.
Presentation of Asthma: Early Childhood (0–6 Years)
Studies of asthma's natural history have shown that almost 80% of cases begin during the first 6 years of life (31). The symptoms of pediatric asthma in this age group are varied and not specific to asthma making the diagnosis challenging. The primary symptoms of asthma in infancy and early childhood include cough, both dry and productive (albeit young children rarely expectorate), wheeze, shortness of breath, and work of breathing. Asthma symptoms are a result of airway inflammation, bronchospasm, airway edema, and airway mucous gland hypertrophy. Interestingly, these symptoms can also present with a multitude of other pediatric diseases including respiratory tract infections and congenital airway anomalies posing a diagnostic challenge. It is well-established that asthma in this age group is frequently under-diagnosed and undertreated (32, 33).
Often, clinicians including pediatric asthma specialists (pulmonologists and allergists) define asthma in this age group as symptoms of airway inflammation that reverse with bronchodilator therapy. However, given the diagnostic challenge in this age group, the Asthma Predictive Index (API) was developed to guide the diagnosis of childhood asthma in children under age 3 years (34). The API has limited sensitivity but reasonable specificity. The API major criteria were defined as physician-diagnosed eczema or parental asthma. The minor criteria were defined as physician-diagnosed allergic rhinitis, wheezing apart from colds, and serum eosinophilia >4%. Positive loose index were defined as parental report of wheezing on surveys at age 2 or 3 years and either 1 major or 2 minor criteria. Positive stringent index was defined as frequent wheezing on surveys at age 2 or 3 years and either 1 major or 2 minor criteria. Children with a positive loose index were found to be four times more likely to have active asthma on surveys at age 6, 8, 11, and 13 years (sensitivity 42%, specificity 85%). Children with a positive stringent index were seven times more likely to have asthma on a school-age survey (sensitivity 16%, specificity 97%). The API is most useful for its negative predictive value and thus, when negative, is an essential tool for determining who is not likely to go on to having later asthma. While not a perfect tool, a slightly modified API, with criteria of a higher score of frequent wheezing and replacing “physician-diagnosed allergic rhinitis” with skin prick testing, is endorsed by the US National Asthma Education and Prevention Program Expert Panel Report 3 for use in diagnosing asthma in this young age group (35).
Often in this age group, particularly over 0–3 years, symptoms are virally triggered rather than allergically triggered. Infants will often have very few symptoms until they experience an upper respiratory infection, which can trigger a significant and severe inflammatory cascade.
In children, the initial few years following asthma diagnosis are critical. For both physician visits and hospitalizations, the number of children having had a second asthma encounter peaked at 3 years after diagnosis and then stabilized (36). Overall, 75% of children had a second asthma episode within 3 years of diagnosis, suggesting that it takes ~3 years to control and stabilize asthma episodes (36). The frequency of asthma episodes soon after diagnosis points to the need for attentive follow-up and aggressive management and education strategies in the early years (36). The mainstays of therapy in this age group are based on recurrence of wheezing symptoms or in severity. For those children with recurrent wheezing or significant morbidity with multiple emergent visits, oral steroid courses or hospital admissions, inhaled corticosteroids are the main therapy. There are a limited number of pathophysiological asthma studies in children under 5 years of age, which presents a challenge in the evidence base for the management of childhood asthma.
Presentation of Asthma: Late Childhood (7–11 Years)
By this age, children can more reliably perform spirometry, and reversible airway obstruction on spirometry can be a helpful diagnostic tool. However, it is important to note that in children with asthma, spirometry values can be normal despite significant disease and morbidity (37). Therefore, in children, spirometry is often used as a monitoring tool for asthma symptoms after the diagnosis has been established through other assessments (38).
Symptoms in this age group transition more from discrete episodes of wheezing in response to viral infections to allergic triggered exacerbations. In this age group, exercise-induced symptoms manifest more clearly which may be due to a true change in the clinical presentation of asthma in this age group or also due to sports and exercise becoming a more discreet activity for children of this age wherein caretakers are able to appreciate the symptoms of dyspnea or cough with exertion. In children who avoid or develop a loss of interest in exercise or physical activities, it is important to consider that asthma may be underlying.
Some children in this age group will have few day-to-day symptoms, but have severe asthma attacks in response to specific triggers such as cold weather, cigarette smoke, or seasonal allergies. Virally triggered asthma exacerbations occur in this age group but less often than in the 0–6 year age range and may contribute to the lower rates of healthcare utilization in this age group as compared with younger years of 0–4 years (2).
Presentation of Asthma: Adolescence (12–18 Years)
Puberty has an interesting impact on childhood asthma, specifically relating to sex. Prior to puberty, asthma risk is higher among male children. At the time of puberty, the risk of asthma is approximately equal between males and females, and after puberty, girls have a higher risk of asthma (39). Some of these differences could be explained by the differences in airway development between the sexes. The fetal lung is less developed in boys from 16 to 26 weeks, measured by mouth movements that reflect fetal breathing, a critical determinant of lung development. In the last 4 weeks of gestation, airway resistance is higher in males (21). Boys up to 10 years old appear to have smaller airways in relation to lung size as compared with girls of the same age, height, and weight (21). After puberty, smaller airway caliber is then observed in the female sex (39). The known sex differences in asthma may also be due to other factors such as hormonal effects, genetic susceptibility, immunologic response, and differences in consultation practices and health-seeking behaviors by sex (4, 40).
Asthma symptoms in this age group are most predominantly shortness of breath with exertion, wheezing in response to triggers, chest pain, chest tightness, and cough. In this age group, asthma symptoms can significantly impact sleep, school, sports, and social engagements. Children are more aware of symptoms in this age range and often feel more embarrassment or stigma around using an inhaler and in particular a spacer, often leading to under treatment of asthma symptoms (40).
Remission is common in adolescence, with remission rates reported at 16–60% (41). Factors that have been implicated in an increased probability of asthma remission include mild disease and minor airway inflammation before adolescence, male sex, and the absence of allergic sensitization (42, 43).
Wheezing and Asthma Phenotypes in Childhood
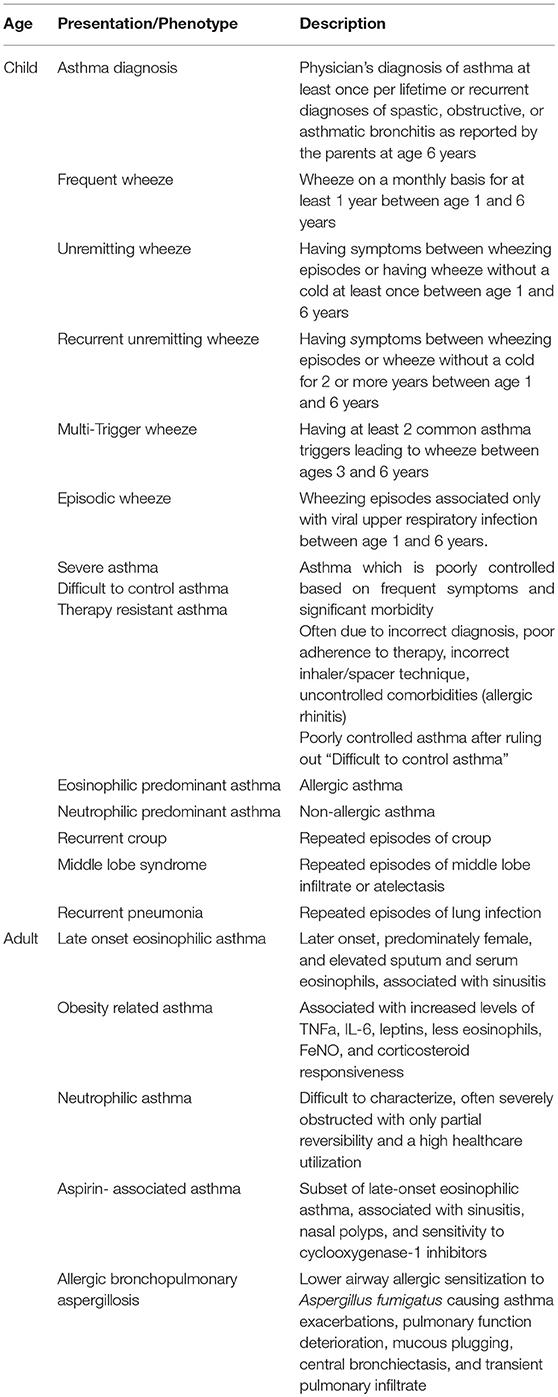
Childhood wheezing phenotypes have been explored given that nearly 50% of children experience wheezing before age 1, yet only 20% of those children progress to have continued wheezing later in childhood (44). While there are several longitudinal birth cohorts that have described wheezing phenotypes, we will describe the classifications according to the earliest of these studies: the Tucson Children's Respiratory Study and the most recent systematic comparison of the clinical and epidemiologic classifications (45).
Wheezing Phenotypes (44, 45):
Never/Infrequent Wheeze
This Describes Children who do not Experience any Wheezing.
The Transient Wheeze
This describes children who have their first wheeze before the age of 3 years with resolved wheezing by age 6 years. Transient wheeze is not strongly related to atopy and genetic risk; there are only mild impairments in lung function in this phenotype, and medication use is very uncommon (45).
The Persistent Wheeze
This describes children who experience first wheezing before age 3 year, however go on to have continued wheezing at age 6 years. Persistent wheeze was strongly related to the asthma risk locus on chromosome 17, however this phenotype appears to be unrelated to environmental determinants. Interestingly, bronchodilator administration dramatically improved any compromises in lung function for children with this phenotype (45).
Intermediate Onset Wheeze
This describes children who experience rare (or no) wheezing before 18 months of age, but persistent wheeze thereafter. Intermediate-onset wheeze has associations with atopy, but only to pollen sensitization (45).
The Late Onset Wheeze
These children develop wheezing between age 3 and 6 years. Late-onset wheeze is strongly associated with fractional exhaled nitric oxide levels and sensitization to inhaled allergens at 6 years and at 4 years. There appears to be severe and irreversible reduction in lung function in this phenotype and asthma medication use is common (45).
Childhood Asthma Clinical Phenotypes
Asthma Diagnosis (45)
Physician's diagnosis of asthma at least once per lifetime or recurrent diagnoses of spastic, obstructive, or asthmatic bronchitis as reported by the parents at age 6 years.
Frequent Wheeze (45)
Wheeze on a monthly basis for at least 1 year between age 1 and 6 years.
Unremitting Wheeze (45)
Having symptoms between wheezing episodes or having wheeze without a cold at least once between age 1 and 6 years.
Recurrent Unremitting Wheeze (45)
Having symptoms between wheezing episodes or wheeze without a cold for 2 or more years between age 1 and 6 years.
Multi-Trigger Wheeze (45)
Having at least 2 common asthma triggers leading to wheeze between ages 3–6 years.
Episodic Wheeze (45)
Wheezing episodes associated only with viral upper respiratory infection between age 1 and 6 years.
Severe, Difficult to Control Asthma, Steroid-Resistant Asthma
Children that do not seem to respond to standard treatment are referred to as severe or difficult to control asthma, and these children experience substantial morbidity from asthma symptoms. To classify a child into this phenotype, the first step is to exclude an incorrect diagnosis, poor adherence to treatment, or incorrect technique with an inhaler and spacer (46, 47). Supervised asthma therapy programs can be extremely useful in managing asthma symptoms and reducing healthcare utilization for children with poor medication adherence and inhaler and spacer technique (48, 49). It is important to differentiate between severe therapy-resistant asthma and difficult-to-treat asthma due to comorbidities Difficult to treat asthma is a much more common reason for persistent symptoms and exacerbations and can be managed if comorbidities, such as allergic rhinitis and chronic exposure to asthma triggers, are directly targeted. Home visiting programs and assessment of the school environment are important features of the evaluation for children with concern for chronic exposure to asthma triggers (50–52). Children with persistent symptoms and exacerbations despite correct inhaler technique and good medical adherence to standard asthma therapy (steroid-resistant or therapy resistant asthma) should be referred to an asthma specialist to consider more potent biologic therapies such as anti-IgE, anti-IL-5, or anti-IL-13 therapies and further evaluation (47).
Eosinophilic Predominant
Eosinophilic inflammation is considered to be the main feature of allergic asthma in children (53). Sputum eosinophils and serum periostin are biomarkers that have been proposed for defining which children with asthma will respond to anti-IgE, anti-IL-5, or anti-IL-13 asthma treatment (54). However bronchoscopy is not routinely done in children with asthma, therefore serum eosinophils are often the least invasive and most common biomarker utilized to indicate the presence of eosinophilic predominant asthma and help predict responsiveness to inhaled corticosteroid therapy (55).
Neutrophilic Predominant
While initially most childhood asthma was thought to be eosinophilic in nature, a neutrophilic predominance has emerged as an important phenotype. In this phenotype, children generally have low IgE levels, low serum and sputum eosinophil counts with very little allergic symptoms. Neutrophil-predominant asthma is the most severe asthma phenotype with poor corticosteroid response (54) and may explain some of the children who have not respond well to standard asthma therapy.
Other Childhood Asthma Clinical Presentations:
In clinical practice, there are different clinical presentations of symptoms that point to an underlying diagnosis of childhood asthma, and clinical improvement can occur in response to starting a child on preventive asthma therapy, such as a daily-inhaled corticosteroid and use of bronchodilator therapy for acute episodes.
Recurrent Croup
Croup, inflammation of the upper airway, which presents as barky cough and stridor, is a common isolated entity in infancy and early childhood. However, the presence of recurrent croup may indicate the presentation of underlying asthma in childhood. Recurrent croup has been shown to be a risk factor for childhood asthma and airway hyperreactivity (56) as well as strongly associated with bronchial asthma in children (57).
Middle Lobe Syndrome
Asthma in children is associated with significant atelectasis and specifically with middle lobe and lingula collapse, (58) and often infants and children who ultimately develop asthma, present as recurrent atelectasis, or mucous plugging in the right middle lobe and lingula. In contrast to adults, children are thought to have higher resistance to collateral airflow, possibly due to increasing number and size of collateral alveolar ventilation, through pores of Kohn and bronchoalveolar Lambert's channels, that develop from birth to adulthood (59). This theory is supported by the finding that pores of Kohn are absent in newborns, and develop around 4 years of age, with the greatest numbers of pores of Kohn found in the apical portions of upper and lower lobes, as well as in peribronchial, perivascular, and subpleural areas, (60, 61), leaving the right middle lobe and lingual vulnerable to atelectasis and mucous plugging, particularly in children with asthma.
Recurrent Pneumonia
In a child with recurrent multi-lobar pneumonia, with a normal immune function evaluation, asthma should be considered as an underlying cause of the recurrent chest infections. Many children referred to specialty care with recurrent chest infections will be found to have undiagnosed or undertreated asthma. Often the history reveals that most have recurrent episodes of cough, wheeze and breathlessness, with trigger factors of upper respiratory tract infections, exercise, cold air, emotional upset, or exposure to pets and other aero-allergens suggestive of asthma (62). In several studies, asthma was the main underlying cause for recurrent pneumonia in children (63).
Association Between Childhood Asthma and COPD
Children with asthma have an increased risk of developing chronic obstructive pulmonary disease (COPD) in adulthood. Specifically, it has been shown that children who smoke tobacco and also have asthma are at increased risk for developing low lung function and COPD as adults, when compared to smokers who did not have asthma in childhood (64).
Remission and Mortality in Childhood Asthma
Asthma remission occurs most commonly between the ages of 14–21 years (65). However, large longitudinal studies have also shown that, among children who wheezed before age 3 years, more than 50% had stopped wheezing either by 6 years of age (44) or by 12 years of age, (36, 66) depending on the study. Remission rates of childhood asthma have been reported between 16 and 60% by early adulthood, according to prior longitudinal studies (41, 65, 67). The wide variation in reported remission rates is likely due to diverse study designs, varying follow-up periods, and different study populations. In longitudinal studies, children with the following characteristics had higher remission rates: episodic asthma (rather than persistent asthma), milder initial asthma severity, less allergic sensitization, less allergic rhinitis, less atopic dermatitis, and male sex (36, 65).
While the morbidity of childhood asthma is significant, fortunately, mortality from childhood asthma is rare with an estimated 28 deaths per million children with asthma (2). As with childhood asthma morbidity, there are grave racial disparities in childhood asthma mortality, and black, and Hispanic children suffer disproportionately from the highest mortality rates (2).
Adult-Onset Asthma
Introduction to Adult-Onset Asthma
Asthma is increasingly recognized as an umbrella term for a heterogenous group of conditions that has been likened to the term “arthritis” in Rheumatology—not a specific diagnosis but a term that describes a diverse group of conditions clinically and biologically (68). Some have suggested discarding the term asthma altogether (69).
Asthma is a common condition amongst adults, estimated to affect 235 million people worldwide, and is estimated to cause more than 350,000 deaths per year (70). It carries a huge economic, morbidity and mortality burden in both developing and developed nations (70). The mortality in developed countries from asthma has remained static for more than a decade and it is clear that a better understanding and management of this diverse group of conditions is required (71).
Asthma is considered a childhood disease by many but this is erroneous as longitudinal studies have shown that approximately half of middle aged patients with asthma have had onset in adulthood rather than childhood (72–74). This proportion of adult onset asthma increases with age (73). The annual incidence of asthma amongst adults is estimated to be 0.5%, similar to the incidence in childhood and it remains unclear as to whether adult-onset asthma is a different disease to that occurring in childhood (75).
The area of asthma phenotyping amongst adults has been developing at a rapid rate. Initially clinical phenotypes were identified that help to categorize asthma in adults by clinical traits including that of early and late onset, obese vs. non-obese, and atopic vs. non-atopic (76, 77). More recently biologic phenotyping has been increasingly recognized and performed clinically, particularly with the availability of targeted biologic agents, and this phenotyping is currently based on presence of allergic or eosinophilic inflammation although this is a rapidly evolving field (68). This has led on to more complex molecular phenotyping which may well-inform future precision medicine in asthma (78).
The natural history of asthma occurring in adulthood is complex because it is such a heterogenous disease. Despite the complexities adult-onset asthma appears to run a different course to that of childhood-onset disease where the majority of the disease is mild and remission is common (79) (Table 1). In adults with asthma remission is uncommon and disease is often more severe and progressive (80).
Phenotypes
Although there has been significant development and research into asthma phenotypes in the past two decades the concept has developed slowly and to some remains controversial (68). In this context phenotype refers to subtypes of the disease that have recognizable properties produced by interactions of the genotype and the environment (81). From this concept has emerged the concept of asthma endotypes where a biologic pathway is identified that leads to the clinical phenotype (82). Phenotypes and endotypes are an attractive concept but unfortunately patients rarely fit into these classifications perfectly with many factors that muddy the waters including presence of comorbidities and confounders. For this reason a more pragmatic concept of treatable traits has gained momentum (83). Rather than attempting to categorize people with asthma, this approach seeks to describe traits associated with an individual's asthma including clinical aspects, biological characteristics, and comorbidities (83).
Phenotypes of asthma can be broadly categorized into clinical, biological, and molecular although there is considerable overlap between these and, ultimately, it is the combination of the three that is likely to form the asthma phenotypes of the future.
Clinical
The attempt to classify asthma is not a new concept with clinical phenotypes having been described since the 1940s (84, 85). One of the oldest approaches to clinical phenotyping was between allergic (extrinsic) and non-allergic (intrinsic) asthma that described two clinically distinct entities—that of early onset asthma associated with sensitization to allergens and other allergic diseases, and that of the poorly understood late onset asthma that was non-allergic (84). Because these phenotypes did not confer specific management there was little clinical utility in the distinction.
More recently different clinical phenotypes have been identified by unbiased cluster analysis of cohorts of asthma patients, with the most detailed analysis performed on the Severe Asthma Research Program cohort in the United States (76, 77). This analysis resulted in the development of five clinical groups, two with early onset, and three other groups. The two groups with early onset are those with normal lung function requiring minimal controller medications, and those with preserved lung function but requiring more medications and healthcare utilization (77). The first of the other three groups comprises obese women with late onset non-atopic asthma, moderately impaired lung function and frequent oral corticosteroid use for asthma exacerbations. The remaining two groups comprise those with severe airflow obstruction and bronchodilator responsiveness, and a less well-defined group with variable ability to attain normal lung function, age of onset, atopic status, and use of oral steroids.
These phenotypes have been further refined with the addition of biologic markers to five groups; early-onset allergic, late-onset eosinophilic, exercise-induced, obesity-related and neutrophilic (68). These will be discussed in more detail below.
The extension of the development of more detailed clinical phenotypes is whether these phenotypes represent distinct clinical entities with different treatment strategies. There is considerable work being done currently in this space.
There are, however, a number of clinical phenotypes amongst adults with asthma that are distinct, including those with occupational asthma, aspirin-associated asthma, and asthma associated with other conditions such as allergic bronchopulmonary aspergillosis and chronic obstructive airways disease (COPD) (86–88). Some of these subtypes have more developed clinical characteristics associated with them, biological mechanisms, management, and natural history.
Biologic
As phenotyping of asthma has progressed there has been progressive attempts to describe the endotype, or biologic pathway, behind different clinical phenotypes.
The most developed of these is the distinction between the presence or absence of Type 2 inflammation (T2) in asthma (89). It has long been recognized that for many, but not all, patients with asthma there is upregulation of Type 2 inflammation. This is characterized by an stimulus at the level of the airway epithelium that results in production of IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) that stimulate release of IL-4, IL-5, and IL-13 and, in turn eosinophils and antibodies that lead to the pathogenic airway changes characteristic of asthma (90). This upregulation of the Type 2 inflammatory response in asthma has important clinical implications because this inflammation tends to be responsive to corticosteroids.
Unlike in children, amongst adults there is a significant proportion of non-T2 high disease. This group of patients is less well-understood than their T2-high disease counterparts and accounts for a significant proportion of mild-moderate adult-onset asthma (91). Although these patients are poorly characterized there are significant implications for treatment as they tend to be less steroid responsive than those with T2-high disease and it is unclear what the best treatment strategy for these patients is (92).
Most asthma, even amongst adults, still appears to have T2-high etiology (76, 77). Even clinical subtypes such as exercise-induced asthma appears to be T2-mediated (93). With regard to the asthma phenotypes discussed above, different biologic mechanisms have been described. Early-onset allergic asthma is the predominate form in children, varies in severity, is associated with allergic symptoms and other allergic diseases and characterized by elevated specific IgE, T2 cytokines and is responsive to inhaled corticosteroids (68). A similar form does also occur in adults. Exercise-induced asthma can be diagnosed in children or adults and is characterized by relatively mild, intermittent symptoms. Biologically there is mast cell activation, T2 high and it tends to respond to leukotriene antagonists, beta agonists and IL-9 agonists (94).
Molecular
More recently molecular phenotyping has also been attempted. In one study on mild to moderate asthma patients upregulated genes were identified in epithelial brushings to molecularly classify those with T2-high and T2-low asthma (90). Those with T2-low asthma were found to have similar gene-expression patterns to the control group (90). Those with T2-high asthma on genetic profile were found to have increased IL-13, IL-5, eosinophils, and mast cells as well as more atopy (95).
Molecular phenotyping has potential implications for treatment with those with T2-high asthma based on genetic profile having a response to inhaled corticosteroids and those with T2-low disease having no response (90, 95).
Adult Phenotypes of Asthma
Late Onset Eosinophilic
Characterized by both clinical and biologic features of later onset, predominately female, and elevated sputum and serum eosinophils. Late-onset eosinophilic asthma is defined clinically by adult-onset, severe disease and is associated with sinusitis and less allergic sensitization compared to early onset disease. Biologically patients have increased IL-5 and IL-13 in the airways and elevated eosinophils in the sputum and serum (96–98). No cut off for sputum and serum eosinophils have been universally agreed upon however it is generally accepted that a sputum eosinophil count of >2% or a serum eosinophil count of >300 cells/uL (or in some cases >150 cells/uL) indicates eosinophilic asthma (99–101). Despite a high prevalence of positive skin prick tests this form of asthma appears to be less allergic but is often associated with sinusitis, nasal polyps, and aspirin exacerbated respiratory disease (96). A family history of asthma is seen less frequently than those with early onset asthma (96). This type of asthma can be relatively steroid resistant but biologic therapies targeting T2 pathways have been shown to be highly effective in this group of patients (102–105).
Obesity-Related Asthma
Obesity-related asthma is not well-understood. It is unclear whether it is a comorbidity common in asthma that confers greater likelihood of breathing pattern disorder, gastroesophageal reflux and deconditioning or whether it is the driver for a proinflammatory state that results in asthma (106–109). Higher body mass index (BMI) is associated with increased levels of TNFa, IL-6, and leptins and less eosinophils, FeNO and corticosteroid responsiveness (108, 109). Clinically there appears to be a group of older non-allergic, obese females who have significant symptoms but minimal healthcare utilization (76, 77). The interaction between BMI and eosinophils is more complex, with those who have early onset T2-high asthma having a correlation between duration of asthma and BMI, lower activity levels, and corticosteroid exposure that does not appear to exist in those with T2-low disease (110). Bariatric surgery has been shown to improve outcomes in asthma patients with late onset, non-allergic asthma but not in those with allergic disease (111).
Neutrophilic Asthma
Neutrophilic asthma is poorly defined and there is no consensus about the characterization of this entity. Adding to the confusion is the fact that corticosteroid treatment commonly suppresses eosinophils and causes neutrophilia making the assessment of corticosteroid-dependant patients difficult (112–114). The clinical phenotype remains controversial and inconsistent but has been suggested to be that of adult-onset, severely obstructed with only partial reversibility and a high healthcare utilization (77, 78). Smoking may also play a role. Furthermore, neutrophilic asthma can occur in those with elevated eosinophils conferring a particularly severe clinical phenotype (115). These patients tend to be less corticosteroid responsive and other treatment strategies have been tried including use of macrolide antibiotics (116). Although IL-17 has been suggested as a potential therapeutic target in neutrophilic asthma a biologic targeted at this interleukin did not result in improvement in asthma control and so far this area remains disappointing (117, 118). The initial disappointment with targeted therapies such as the anti-IL-17 and anti-TNFa agents has been tempered by positive preliminary results with a newer biologic agent targeting TSLP that has showed promise in Phase 2 studies in a non-eosinophilic group (119, 120).
Aspirin-Associated Asthma
Aspirin-associated asthma, a subset of aspirin exacerbated respiratory disease (AERD) has been described for many years (85). It tends to occur in adulthood at an average age of 34 years and is more common amongst females (86). This is a subset of late-onset eosinophilic asthma and is associated with sinusitis, nasal polyps and sensitivity to cyclooxygenase-1 inhibitors including aspirin (86). Biologically it is characterized by upregulation of the cysteinyl leukotriene pathway and elevated eosinophils (96). Molecularly genes related to the leukotriene pathway have been implicated and periostin, a biomarker of IL-13 activity, has been found in nasal polyps present in patients with AERD (97, 121). These patients are often relatively corticosteroid resistant, requiring high doses for control, but can be responsive to leukotriene antagonists (122, 123). More recently biologic therapies that target T2 pathways including IL-4, IL-13, and IL-5 have been shown to be effective in patients with asthma and nasal polyps (124–126).
Allergic Bronchopulmonary Aspergillosis
Allergic bronchopulmonary aspergillosis (ABPA) was first described in the 1950s and is caused by allergic sensitization to fungal colonization of the lower airways with Aspergillus fumigatus (127, 128). It occurs in 1–2% of asthma patients, although has been detected in up to 13% of the population in asthma clinics, predominately adults, and causes asthma exacerbations, deterioration of pulmonary function, mucous plugging, central bronchiectasis, and transient pulmonary infiltrates with characteristic biologic features including elevated total and Aspergillus-specific IgE as well as peripheral eosinophilia (88, 128, 129). The diagnosis is based on presence of asthma, proximal bronchiectasis, sensitization to Aspergillus, and an elevated total IgE (129). It is important to diagnose ABPA because of the progressive nature of the bronchiectasis in the absence of treatment (129). The mainstay of treatment for ABPA is systemic corticosteroids and, in some cases, antifungal agents (88).
Link Between Early Transient Wheeze and COPD
There is a link between childhood asthma and COPD (130). Both childhood asthma and childhood wheezy bronchitis have been associated with an increased risk of COPD in adults (131). Severe childhood asthma has been shown to confer a 32 times higher risk of COPD in adults despite the fact that just under half of those diagnosed with COPD in this cohort had never smoked (132). Early transient wheeze has been thought of as a benign condition but did significantly increase risk of the presence of COPD in adulthood with long term follow up (131).
Asthma-smoking associations have been described in both early and late onset asthma (133). Smoking remains a key risk factor for airflow obstruction in normal and asthmatic individuals, however the risk appears to be greatest in those with late onset disease (74).
Natural History
The natural history of asthma in adults is different to that of asthma in children with less remission of adult-onset asthma than that occurring in childhood.
Many adults with asthma have childhood-onset disease that has persisted and there have been many risk factors that predict the persistence of asthma into adulthood including the severity of childhood disease, the presence of bronchial hyperresponsiveness, atopy, exposure to allergens and a parental history of asthma (134–136). In longitudinal studies the amount of wheeze in early adolescence has been shown to predict the severity in later life. In an Australian-based cohort of second graders with wheezing of various severities followed up to age 50 there was remission of asthma in 64% of those with mild wheezy bronchitis or wheezy bronchitis, 47% of those with persistent asthma and only 15% of those with severe asthma (137). In this group risk factors for persistence of asthma at age 50 were severe childhood asthma, childhood allergic rhinitis, and female sex (137). In another longitudinal cohort 73% with few symptoms at 14 years had little or no asthma 14 years later whereas 68% of those with frequent wheeze at 14 years still had asthma 14 years later (138). Most who had frequent wheeze at 21 still had wheeze at 28 years and of those with infrequent wheeze at 21 years 44% had worsened at 28 years (138). These findings have been replicated in more recent studies—three quarters of children with childhood asthma will outgrow the disease by middle age although overall our understanding of the natural history of childhood asthma remains poorly understood (139).
Adult onset asthma has many different forms and the risk factors appear to be different to that of childhood-onset disease. Compared to childhood asthma, major associations with adult-onset disease are female sex, current smoking, and low socio-economic status but not atopy or a family history of asthma (74). Other risk factors include; clinical—historical symptoms of wheeze, rhinitis, chronic cough; physiological—lower lung function, bronchial hyperresponsiveness, lower height; comorbidities—higher BMI, nocturnal gastroesophageal reflux disease, habitual snoring, IgE reactivity to Timothy grass; and lifestyle—low physical activity amongst men (75, 107, 140–144). The evidence for smoking as a risk factor is mixed with two large cohort studies from Australia and Sweden showing that this is a risk factor for adult-onset asthma and other studies showing that it doesn't appear to be (74, 75, 141, 144).
In a large cohort with severe asthma with onset between 14 and 55 who were followed for 10 years 83% had less severe asthma at 10 years (145). Risk factors for the persistent presence of severe asthma was low socioeconomic status, high comorbidity burden and high adherence to medications in the first year after diagnosis (145). In this cohort sex and other risk factors important in childhood asthma were not associated with continued presence of severe asthma (145).
The sex differences in asthma amongst different age groups are interesting. Amongst children males are more commonly affected by asthma than females and male sex is a risk factor for developing asthma (39). Around the time of puberty this risk becomes equal and after puberty the risk in females is greater than that amongst males, an observation partially but not fully explained by women's smaller airway caliber in adults (39). There is emerging evidence of the association between female hormonal changes and asthma that may partially explain the female predominance of asthma after puberty (146, 147).
Some factors that don't appear to be risk factors for adult onset asthma include level of education, atopy (either baseline or newly positive skin prick tests), occupational exposures or maternal asthma (141, 143, 144).
Despite the differences in risk factors for adult-onset asthma compared to childhood onset disease the prevalence of asthma amongst adults was shown to be increasing in the second half of the twentieth century similar to that of childhood asthma (73, 148).
Young Adults
In those who develop asthma as young adults the natural history appears to be more similar to that of childhood asthma with atopy an important a risk factor and more remissions (149). The 23 year follow up of one study in the US examined asthma incidence in participants 23 years after college, finding that the cumulative prevalence of asthma increases with age and that three-quarters of those who had asthma at the first visit were in remission or had improved symptoms at 40 years (149). Only a small number who had asthma at the baseline visit were worse (149).
Middle Age
Many patients are diagnosed with asthma in middle age and this disproportionately affects women. In fact for women most asthma occurring in middle age is adult-onset asthma and by 40 years more than half of asthma in women is adult onset (73). The proportion of adult onset asthma was even higher in women who were obese, non-atopic, ever smokers, white, and lower socio-economic status (73, 74). In contrast for men by 50 years only one third of asthma is adult-onset asthma (73). Therefore it is clear that adult-onset asthma is more common in women (73). Overall those with adult onset asthma are more likely to experience symptoms including wheeze, new rhinitis, snoring, and weight gain (143). Those with adult onset disease are also more likely to have a decline in lung function than those with childhood onset disease (143).
Older Adults
For those who first experience asthma in their senior year atopy does not appear to be a risk factor (149). Older adults who develop asthma have a similar incidence as younger people of ~100 per 100,000 (150). However, disease severity is worse amongst older adults developing asthma compared to younger people and is also more progressive with poorer lung function and more fixed airflow obstruction (151). This poorer lung function and more fixed airflow obstruction is thought to be largely the result of the more common presence of comorbid lung diseases such as bronchiectasis, pulmonary fibrosis, and COPD (151). Older adults with asthma are less likely to experience remission than their younger counterparts (151).
Remissions
In adults with asthma remissions have been found to be uncommon after the second decade of life and particularly uncommon in those between 30 and 60 years old (79). In adult-onset, non-allergic asthma remission is especially unusual occurring in only 20% of patients (152). Risk factors for those not achieving remission were severe symptoms, impaired lung function, and a comorbid diagnosis of chronic bronchitis or emphysema (79). Amongst adults with asthma who do remit relapses have been found to be common and increase until the age of 70 years particularly if there was persistent symptoms despite remission (79, 153).
Lung Function
Lung function has been found to decline in some but not all adult-onset asthma patients (154–156). In those with persistent asthma there is generally a decline in lung function that eventually leads to a restrictive pattern of spirometry (157). Adult lung function is influenced by initial FEV1 and sex, with a larger decline observed amongst females, but not initial asthma severity or allergic sensitization (136). Lower lung function has also been found to be related to presence of bronchial hyperresponsiveness (136).
In aspirin-exacerbated asthma a steady progress in severity has been found (86). Rhinitis generally appears around the age of 30 years followed by asthma, aspirin sensitivity, then nasal polyps (158). Women generally have more severe disease with earlier onset, one third of patients are atopic and they tend to have an earlier onset, and lower FEV1 (159).
The natural history of ABPA is variable with five stages identified including acute, active; remission; recurrent; chronic; and severe, end-stage disease (160). Early diagnosis and treatment is associated with a reduced likelihood of progressive disease (146, 160). Serum IgE level is often used to track progress of disease and help to identify remission and relapse of ABPA (160). Overall those with ABPA have been observed to have a progressive decline in lung function although there are those who undergo complete remission (88).
Mortality
Thankfully mortality in asthma remain uncommon (71). However mortality rates are not declining and in fact have increased in some developed countries (71, 161). Asthma deaths are more common amongst adolescents and young adults, those of low socioeconomic status, black race, those with substance abuse, and are uncommon in young children and older adults (162). Other risk factors for death include previous near-fatal asthma, hospitalization or emergency department visit for asthma in the past year, current or recent oral corticosteroid use, non-adherence with inhaled corticosteroids, a history of psychiatric disease, lack of a written asthma action plan and the presence of comorbid food allergy (161). Overall the average life expectancy for those with asthma is not reduced compared to the general population (163). Elderly patients die more frequently from respiratory diseases and are more at risk of complications of medications (150).
Contrasts
It is difficult to accurately compare and contrast childhood and adult-onset asthma due to existing gaps in the literature and we acknowledge this limitation. Additionally because some findings are reported more in adults, this does not necessarily mean they are more prevalent, but rather a possible manifestation of publication bias. Nevertheless, we have provided a reflection of the similarities and differences based on the currently available literature (Tables 1, 2). Adult onset disease differed from pediatric onset disease in regard to increased prevalence in women, non-atopic individuals, and obese patients (73). Adult onset disease is associated with more respiratory symptoms and asthma medication use despite higher prebronchodilator FEV1/FVC (73). There is less quiescent disease in adult onset asthma and it appears to be less stable than childhood-onset disease with more relapses and less remissions (73).
Severe asthma in children is distinct from severe asthma in adults and approaches to severe asthma in adults should not be extrapolated to children. In children the factors associated with severity have been found to be asthma duration, medication use and lung function rather than Type 2 inflammatory markers such as increased IgE and elevated FENO that are markers of severity in adult-onset disease (93).
Author Contributions
MT and ED made substantial contributions to: The conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Provide approval for publication of the content; Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The manuscript described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001-2009. MMWR Morb Mortal Wkly Rep. (2011) 60:547–52. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6017a4.htm
2. Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. (2009) 123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C
3. Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. (2016) 137:e20152354. doi: 10.1542/peds.2015-2354
4. Wright AL, Stern DA, Kauffmann F, Martinez FD. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: the Tucson Children's Respiratory Study. Pediatr Pulmonol. (2006) 41:318–25. doi: 10.1002/ppul.20373
5. Mitchell SJ, Bilderback AL, Okelo SO. Racial disparities in asthma morbidity among pediatric patients seeking asthma specialist care. Acad Pediatr. (2016) 16:64–7. doi: 10.1016/j.acap.2015.06.010
6. Flores G, Snowden-Bridon C, Torres S, Perez R, Walter T, Brotanek J, et al. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. (2009) 46:392–8. doi: 10.1080/02770900802712971
7. Meyers DA, Bleecker ER, Holloway JW, Holgate ST. Asthma genetics and personalised medicine. Lancet Respir Med. (2014) 2:405–15. doi: 10.1016/S2213-2600(14)70012-8
8. Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. (2011) 43:887–92. doi: 10.1038/ng.888
9. Neuman A, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. (2012) 186:1037–43. doi: 10.1164/rccm.201203-0501OC
10. Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. (2006) 174:499–507. doi: 10.1164/rccm.200512-1946OC
11. Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA Jr, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. (2006) 84:903–11. doi: 10.1093/ajcn/84.4.903
12. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. (2016) 375:2530–9. doi: 10.1056/NEJMoa1503734
13. Bedard A, Northstone K, Henderson AJ, Shaheen SO. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur Respir J. (2017) 50:1700073. doi: 10.1183/13993003.00073-2017
14. Ku MS, Sun HL, Sheu JN, Lee HS, Yang SF, Lue KH. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol. (2012) 23:623–8. doi: 10.1111/j.1399-3038.2012.01345.x
15. Stokholm J, Sevelsted A, Anderson UD, Bisgaard H. Preeclampsia associates with asthma, allergy, and eczema in childhood. Am J Respir Crit Care Med. (2017) 195:614–21. doi: 10.1164/rccm.201604-0806OC
16. Tollanes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr. (2008) 153:112–6. doi: 10.1016/j.jpeds.2008.01.029
17. Kallen B, Finnstrom O, Nygren KG, Otterblad Olausson P. Association between preterm birth and intrauterine growth retardation and child asthma. Eur Respir J. (2013) 41:671–6. doi: 10.1183/09031936.00041912
18. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics. (2011) 127:e913–20. doi: 10.1542/peds.2010-2603
19. Sevelsted A, Stokholm J, Bisgaard H. Risk of asthma from cesarean delivery depends on membrane rupture. J Pediatr. (2016) 171:38–42.e1–4. doi: 10.1016/j.jpeds.2015.12.066
20. Johnson CC, Peterson EL, Joseph CL, Ownby DR, Breslau N. Birth weight and asthma incidence by asthma phenotype pattern in a racially diverse cohort followed through adolescence. J Asthma. (2015) 52:1006–12. doi: 10.3109/02770903.2015.1054405
21. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. (1999) 54:1119–38. doi: 10.1136/thx.54.12.1119
22. Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE. (2010) 5:e10134. doi: 10.1371/journal.pone.0010134
23. Arbes SJ Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. (2007) 120:1139–45. doi: 10.1016/j.jaci.2007.07.056
24. Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. (2014) 5:202. doi: 10.4172/2155-9899.1000202
25. Porsbjerg C, von Linstow ML, Ulrik CS, Nepper-Christensen S, Backer V. Risk factors for onset of asthma: a 12-year prospective follow-up study. Chest. (2006) 129:309–16. doi: 10.1378/chest.129.2.309
26. Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy. (2016) 71:463–74. doi: 10.1111/all.12827
27. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. (2014) 134:593–601.e12. doi: 10.1016/j.jaci.2014.04.018
28. Carlsten C, Brauer M, Dimich-Ward H, Dybuncio A, Becker AB, Chan-Yeung M. Combined exposure to dog and indoor pollution: incident asthma in a high-risk birth cohort. Eur Respir J. (2011) 37:324–30. doi: 10.1183/09031936.00187609
29. Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, et al. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. (2006) 129:610–8. doi: 10.1378/chest.129.3.610
30. Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA Jr, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. (2015) 135:441–8. doi: 10.1016/j.jaci.2014.07.065
31. Yunginger JW, Reed CE, O'Connell EJ, Melton LJ III, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. (1992) 146:888–94. doi: 10.1164/ajrccm/146.4.888
32. Galant SP, Morphew T, Amaro S, Liao O. Current asthma guidelines may not identify young children who have experienced significant morbidity. Pediatrics. (2006) 117:1038–45. doi: 10.1542/peds.2005-1076
33. Kuehni CE, Frey U. Age-related differences in perceived asthma control in childhood: guidelines and reality. Eur Respir J. (2002) 20:880–9. doi: 10.1183/09031936.02.00258502
34. Castro-Rodriguez JA. The asthma predictive index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. (2010) 126:212–6. doi: 10.1016/j.jaci.2010.06.032
35. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. (2007) 120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.029
36. To T, Gershon A, Wang C, Dell S, Cicutto L. Persistence and remission in childhood asthma: a population-based asthma birth cohort study. Arch Pediatr Adolesc Med. (2007) 161:1197–204. doi: 10.1001/archpedi.161.12.1197
37. Murray C, Foden P, Lowe L, Durrington H, Custovic A, Simpson A. Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolesc Health. (2017) 1:114–23. doi: 10.1016/S2352-4642(17)30008-1
38. Nair SJ, Daigle KL, DeCuir P, Lapin CD, Schramm CM. The influence of pulmonary function testing on the management of asthma in children. J Pediatr. (2005) 147:797–801. doi: 10.1016/j.jpeds.2005.07.023
39. de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. (2000) 162:68–74. doi: 10.1164/ajrccm.162.1.9907008
40. de Benedictis D, Bush A. Asthma in adolescence: Is there any news? Pediatr Pulmonol. (2017) 52:129–38. doi: 10.1002/ppul.23498
41. Burgess JA, Matheson MC, Gurrin LC, Byrnes GB, Adams KS, Wharton CL, et al. Factors influencing asthma remission: a longitudinal study from childhood to middle age. Thorax. (2011) 66:508–13. doi: 10.1136/thx.2010.146845
42. Bjerg-Backlund A, Perzanowski MS, Platts-Mills T, Sandstrom T, Lundback B, Ronmark E. Asthma during the primary school ages–prevalence, remission and the impact of allergic sensitization. Allergy. (2006) 61:549–55. doi: 10.1111/j.1398-9995.2006.01027.x
43. Vonk JM, Boezen HM. Predicting adult asthma in childhood. Curr Opin Pulm Med. (2006) 12:42–7. doi: 10.1097/01.mcp.0000188371.30508.54
44. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. (1995) 332:133–8. doi: 10.1056/NEJM199501193320301
45. Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvarinen A, Kaulek V, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. (2014) 189:129–38. doi: 10.1164/rccm.201307-1198OC
46. Bush A, Saglani S. Management of severe asthma in children. Lancet. (2010) 376:814–25. doi: 10.1016/S0140-6736(10)61054-9
47. Haktanir Abul M, Phipatanakul W. Severe asthma in children: evaluation and management. Allergol Int. (2019) 68:150–7. doi: 10.1016/j.alit.2018.11.007
48. Trivedi M, Patel J, Lessard D, Kremer T, Byatt N, Phipatanakul W, et al. School nurse asthma program reduces healthcare utilization in children with persistent asthma. J Asthma. 2017:1–7. doi: 10.1080/02770903.2017.1396473
49. Gerald LB, McClure LA, Mangan JM, Harrington KF, Gibson L, Erwin S, et al. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school-based supervised asthma therapy. Pediatrics. (2009) 123:466–74. doi: 10.1542/peds.2008-0499
50. Bhaumik U, Sommer SJ, Giller-Leinwohl J, Norris K, Tsopelas L, Nethersole S, et al. Boston children's hospital community asthma initiative: Five-year cost analyses of a home visiting program. J Asthma. (2017) 54:134–42. doi: 10.1080/02770903.2016.1201837
51. Hauptman M, Phipatanakul W. The school environment and asthma in childhood. Asthma Res Pract. (2015) 1:12. doi: 10.1186/s40733-015-0010-6
52. Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A practical approach to severe asthma in children. Ann Am Thorac Soc. (2018) 15:399–408. doi: 10.1513/AnnalsATS.201708-637FR
53. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. (2002) 57:643–8. doi: 10.1136/thorax.57.7.643
54. Su MW, Lin WC, Tsai CH, Chiang BL, Yang YH, Lin YT, et al. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy. (2018) 73:2024–32. doi: 10.1111/all.13439
55. Venge P. Role of eosinophils in childhood asthma inflammation. Pediatr Pulmonol Suppl. (1995) 11:34–5. doi: 10.1002/ppul.1950191119
56. Lin SC, Lin HW, Chiang BL. Association of croup with asthma in children: a cohort study. Medicine (Baltimore). (2017) 96:e7667. doi: 10.1097/MD.0000000000007667
57. Van Bever HP, Wieringa MH, Weyler JJ, Nelen VJ, Fortuin M, Vermeire PA. Croup and recurrent croup: their association with asthma and allergy. An epidemiological study on 5–8-year-old children. Eur J Pediatr. (1999) 158:253–7. doi: 10.1007/s004310051062
58. Sekerel BE, Nakipoglu F. Middle lobe syndrome in children with asthma: review of 56 cases. J Asthma. (2004) 41:411–7. doi: 10.1081/JAS-120033983
59. Terry PB, Traystman RJ. The clinical significance of collateral ventilation. Ann Am Thorac Soc. (2016) 13:2251–7. doi: 10.1513/AnnalsATS.201606-448FR
60. Boyden EA. Notes on the development of the lung in infancy and early childhood. Am J Anat. (1967) 121:749–61. doi: 10.1002/aja.1001210317
61. Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol. (1996) 21:383–97. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M
62. Couriel J. Assessment of the child with recurrent chest infections. Br Med Bull. (2002) 61:115–32. doi: 10.1093/bmb/61.1.115
63. Lodha R, Puranik M, Natchu UC, Kabra SK. Recurrent pneumonia in children: clinical profile and underlying causes. Acta Paediatr. (2002) 91:1170–3. doi: 10.1080/080352502320777388
64. Hayden LP, Cho MH, Raby BA, Beaty TH, Silverman EK, Hersh CP, et al. Childhood asthma is associated with COPD and known asthma variants in COPDGene: a genome-wide association study. Respir Res. (2018) 19:209. doi: 10.1186/s12931-018-0890-0
65. Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. (2014) 133:1572–8.e3. doi: 10.1016/j.jaci.2013.12.1033
66. Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. (1996) 312:1195–9. doi: 10.1136/bmj.312.7040.1195
67. Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. (2003) 349:1414–22. doi: 10.1056/NEJMoa022363
68. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. (2012) 18:716–25. doi: 10.1038/nm.2678
69. The Lancet. A plea to abandon asthma as a disease concept. Lancet. (2006) 368:705. doi: 10.1016/S0140-6736(06)69257-X
70. World Health Organisation. Asthma. Available online at: www.who.int/en/news-room/fact-sheets/detail/asthma (2017).
71. Akinbami LJ, Bailey CM, Johnson CA, King ME, Liu X, Moorman JE, et al. Trends in Asthma Prevalence, Health Care Use, And Mortality in the United States, 2001–2010. NCHS Data Brief. (2012) 1–8. Available online at: https://stacks.cdc.gov/view/cdc/12331#tabs-2
72. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. (2004) 59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x
73. Sood A, Qualls C, Schuyler M, Arynchyn A, Alvarado JH, Smith LJ, et al. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. The longitudinal CARDIA study. Anna Am Thor Soc. (2013) 10:188–97. doi: 10.1513/AnnalsATS.201212-115OC
74. Tan DJ, Walters EH, Perret JL, Burgess JA, Johns DP, Lowe AJ, et al. Clinical and functional differences between early-onset and late-onset adult asthma: a population-based Tasmanian Longitudinal Health Study. Thorax. (2016) 71:981–7. doi: 10.1136/thoraxjnl-2015-208183
75. Rönmark E, Lundbäck B, Jonsson E, Jonsson AC, Lindström M, Sandström T. Incidence of asthma in adults – report from the obstructive lung disease in northern sweden study. Allergy. (1997) 52:1071–8. doi: 10.1111/j.1398-9995.1997.tb00178.x
76. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respirat Crit Care Med. (2008) 178:218–24. doi: 10.1164/rccm.200711-1754OC
77. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respirat Crit Care Med. (2010) 181:315–23. doi: 10.1164/rccm.200906-0896OC
78. Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. (2011) 127:153–60.e9. doi: 10.1016/j.jaci.2010.10.024
79. Bronnimann S, Burrows B. A prospective study of the natural history of asthma: remission and relapse rates. Chest. (1986) 90:480–4. doi: 10.1378/chest.90.4.480
80. Maestrelli P. Natural history of adult-onset asthma. Am J Respirat Crit Care Med. (2004) 169:331–2. doi: 10.1164/rccm.2312012
81. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. (2006) 368:804–13. doi: 10.1016/S0140-6736(06)69290-8
82. Lotvall J, Akdis CB, Bacharier L, Bjermer LB, Casale T, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allerg Clin Immunol. (2011) 127:355–60. doi: 10.1016/j.jaci.2010.11.037
83. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respirat J. (2016) 47:410–9. doi: 10.1183/13993003.01359-2015
84. Rackemann FM. A working classification of asthma. Am J Med. (1947) 3:601–6. doi: 10.1016/0002-9343(47)90204-0
85. Samter M, Beers RF Jr. Concerning the nature of intolerance to aspirin. J Allergy. (1967) 40:281–93. doi: 10.1016/0021-8707(67)90076-7
86. Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Annals Allergy Asthma Immunol. (2002) 89:474–8. doi: 10.1016/S1081-1206(10)62084-4
87. Maghni K, Lemière C, Ghezzo H, Yuquan W, Malo JL. Airway inflammation after cessation of exposure to agents causing occupational asthma. Am J Resp Crit Care Med. (2004) 169:367–72. doi: 10.1164/rccm.200309-1238OC
88. Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. (2002) 110:685–92. doi: 10.1067/mai.2002.130179
89. Fahy JV. Type 2 inflammation in asthma — present in most, absent in many. Nat Rev Immunol. (2014) 15:57–65. doi: 10.1038/nri3786
90. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper Type 2–driven Inflammation defines major subphenotypes of asthma. Am J Respirat Crit Care Med. (2009) 180:388–95. doi: 10.1164/rccm.200903-0392OC
91. Martin PE, Matheson MC, Fau - Gurrin L, Gurrin L, Fau - Burgess JA, Burgess Ja Fau - Osborne N, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. (2011) 127:1473–9.e1. doi: 10.1016/j.jaci.2011.02.041
92. Samitas K, Zervas E, Gaga M. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulmonary Med. (2017) 23:48–55. doi: 10.1097/MCP.0000000000000342
93. Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the national institutes of health/national heart, lung, and blood institute severe asthma research program. J Allergy Clin Immunol. (2011) 127:382–9.e13. doi: 10.1016/j.jaci.2010.11.015
94. Hallstrand TS, Moody MW, Aitken ML, Henderson WR. Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. (2005) 116:586–93. doi: 10.1016/j.jaci.2005.04.035
95. Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. (2010) 125:1046–53.e8. doi: 10.1016/j.jaci.2010.03.003
96. Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. (2004) 113:101–8. doi: 10.1016/j.jaci.2003.10.041
97. Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. (2008) 121:685–91. doi: 10.1016/j.jaci.2008.01.005
98. Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. (2002) 32:1558–65. doi: 10.1046/j.1365-2222.2002.01477.x
99. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. (2002) 360:1715–21. doi: 10.1016/S0140-6736(02)11679-5
100. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. (2013) 132:72–80.e12. doi: 10.1016/j.jaci.2013.03.044
101. Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respirat Med. (2015) 3:849–58. doi: 10.1016/S2213-2600(15)00367-7
102. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. (2011) 364:588. doi: 10.1056/NEJMx110005
103. Nair P, Pizzichini MMM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. (2009) 360:985–93. doi: 10.1056/NEJMoa0805435
104. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. (2016) 388:2115–27. doi: 10.1016/S0140-6736(16)31324-1
105. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2016) 388:2128–41. doi: 10.1016/S0140-6736(16)31322-8
106. Sin DD, Jones RL, Man SFP. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. (2002) 162:1477–81. doi: 10.1001/archinte.162.13.1477
107. Gunnbjörnsdóttir MI, Omenaas E, Gíslason T, Norrman E, Olin AC, Jõgi R, et al. Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Resp J. (2004) 24:116–121. doi: 10.1183/09031936.04.00042603
108. Pakhale S, Doucette S, Vandemheen K, Boulet LP, McIvor RA, FitzGerald JM, et al. A comparison of obese and nonobese people with asthma: exploring an asthma-obesity interaction. Chest. (2010) 137:1316–23. doi: 10.1378/chest.09-2491
109. Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Resp J. (2006) 27:495–503. doi: 10.1183/09031936.06.00077205
110. Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. (2011) 127:1486–93.e2. doi: 10.1016/j.jaci.2011.03.036
111. Reddy CR, Baptist A, Fan Z, Carlin MA, Birkmeyer NJO. The effects of bariatric surgery on asthma severity. Obes Surg. (2011). 200–6. doi: 10.1007/s11695-010-0155-6
112. Jatakanon A, Uasuf C, Maziak W, Lim SAM, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respirat Crit Care Med. (1999) 160:1532–9. doi: 10.1164/ajrccm.160.5.9806170
113. Kato T, Takeda Y, Nakada T, Sendo F. Inhibition by dexamethasone of human neutrophil apoptosis in vitro. Nat Immun. (1995) 14:198–208.
114. Schleimer R, S Freeland H, Peters S, E Brown K, P Derse C. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. (1989) 25:598–605.
115. Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. (2010) 125:1028–36.e13. doi: 10.1016/j.jaci.2010.02.008
116. Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. (2017) 390:659–68. doi: 10.1016/S0140-6736(17)31281-3
117. Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti–IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respirat Crit Care Med. (2013) 188:1294–302. doi: 10.1164/rccm.201212-2318OC
118. Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. where do we stand? Am J Respirat Crit Care Med. (2014) 190:1094–101. doi: 10.1164/rccm.201405-0859PP
119. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. (2017) 377:936–46. doi: 10.1056/NEJMoa1704064
120. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén S-E, et al. A Randomized, double-blind, placebo-controlled study of tumor necrosis factor-α blockade in severe persistent asthma. Am J Respirat Crit Care Med. (2009) 179:549–58. doi: 10.1164/rccm.200809-1512OC
121. Sanak M, Simon H-U, Szczeklik A. Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet. (1997) 350:1599–600. doi: 10.1016/S0140-6736(05)64015-9
122. Wenzel S, Schwartz LB, Langmack E, Halliday JL, Trudeau J, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respair Crit Care Med. (1999) 160:1001–8. doi: 10.1164/ajrccm.160.3.9812110
123. DahlÉN SE, Malmströ MK, Nizankowska EWA, DahlÉN B, Kuna P, Kowalski M, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist. Am J Respirat Crit Care Med. (2002) 165:9–14. doi: 10.1164/ajrccm.165.1.2010080
124. Pavord ID, Ford L, Sher L, Rabe KF, Park H-S, Cosio BG, et al. Dupilumab efficacy in asthma patients with comorbid chronic rhinosinusitis or nasal polyposis (CRS/NP) in Liberty Asthma Quest. Eur Respirat J. (2018) 52(Suppl 62):OA1651. doi: 10.1183/13993003.congress-2018.OA1651
125. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trialsubcutaneous treatment for chronic sinusitis with nasal polyposissubcutaneous treatment for chronic sinusitis with nasal polyposis. JAMA. (2016) 315:469–79. doi: 10.1001/jama.2015.19330
126. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. (2016) 388:31–44. doi: 10.1016/S0140-6736(16)30307-5
127. Hinson KFW, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax. (1952) 7:317–33. doi: 10.1136/thx.7.4.317
128. Knutsen A. Allergic bronchopulmonary aspergillosis in asthma AU - Knutsen, Alan P. Exp Rev Clin Immunol. (2017) 13:11–4. doi: 10.1080/1744666X.2017.1232620
129. Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. (2009) 135:805–26. doi: 10.1378/chest.08-2586
130. Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a Risk Factor for COPD in a longitudinal study. Chest. (2004) 126:59–65. doi: 10.1378/chest.126.1.59
131. Tagiyeva N, Fielding S, Devereux G, Turner S, Douglas G. Childhood wheeze – A risk factor for COPD? A 50-year cohort study. Eur Respirat J. (2015) 46(Suppl 59):OA2000. doi: 10.1183/13993003.congress-2015.OA2000
132. Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. (2014) 69:805. doi: 10.1136/thoraxjnl-2013-204815
133. Perret JL, Dharmage SC, Matheson MC, Matheson MC, Johns DP, Gurrin LC, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. Am J Respair Crit Care Med. (2013) 187:42–8. doi: 10.1164/rccm.201205-0788OC
134. Russell G. Asthma in the transition from childhood to adulthood. Thorax. (2002) 57:96. doi: 10.1136/thorax.57.2.96
135. Xuan W, Marks GB, Toelle BG, Belousova E, Peat JK, Berry G, et al. Risk factors for onset and remission of atopy, wheeze, and airway hyperresponsiveness. Thorax. (2002) 57:104. doi: 10.1136/thorax.57.2.104
136. Gustafsson PM, Kjellman B. Asthma from childhood to adulthood: course and outcome of lung function. Respir Med. (2000) 94:466–74. doi: 10.1053/rmed.1999.0763
137. Banks JR, Andrews T. Outcomes of childhood asthma to the age of 50 years. Pediatrics. (2015) 136(Supplement 3):S266. doi: 10.1542/peds.2015-2776JJJJ
138. Kelly WJW, Hudson I, Phelan PD, Pain MCF, Olinsky A. Childhood asthma in adult life: a further study At 28 years of age. Br Med J. (1987) 294:1059–62. doi: 10.1136/bmj.294.6579.1059
139. Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. (2010) 126:187–97. doi: 10.1016/j.jaci.2010.07.011
140. Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. Eur Respir J. (2004) 24:740–4. doi: 10.1183/09031936.04.00088003
141. Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. (2003) 97:273–80. doi: 10.1053/rmed.2003.1419
142. Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol. (2004) 160:969–76. doi: 10.1093/aje/kwh303
143. Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. (2009) 14:814–21. doi: 10.1111/j.1440-1843.2009.01562.x
144. BasagaÑA X, Sunyer J, Zock JP, Kogevinas M, Urrutia I, Maldonado JA, et al. Incidence of asthma and its determinants among adults in spain. Am J Respir Crit Care Med. (2001) 164:1133–7. doi: 10.1164/ajrccm.164.7.2012143
145. Chen W, Marra CA, Lynd LD, FitzGerald JM, Zafari Z, Sadatsafavi M. The natural history of severe asthma and influences of early risk factors: a population-based cohort study. Thorax. (2016) 71:267. doi: 10.1136/thoraxjnl-2015-207530
146. Almqvist C, Worm M, Leynaert B, for the working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. (2008) 63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x
147. Jenkins MA, Dharmage SC, Flander LB, Douglass JA, Ugoni AM, Carlin JB, et al. Parity and decreased use of oral contraceptives as predictors of asthma in young women. Clin Exp Allergy. (2006) 36:609–13. doi: 10.1111/j.1365-2222.2006.02475.x
148. Dubois P, Degrave E, Vandenplas O. Asthma and airway hyperresponsiveness among Belgian conscripts, 1978-91. Thorax. (1998) 53:101. doi: 10.1136/thx.53.2.101
149. Settipane GA, Greisner WA III, Settipane RJ. Natural history of asthma: a 23-year followup of college students. Ann Allergy Asthma Immunol. (2000) 84:499–503. doi: 10.1016/S1081-1206(10)62512-4
150. Bauer B, Reed CE, Yunginger JW, Wollan PC, Silverstein MD. Incidence and outcomes of asthma in the elderly: a population-based study in rochester, Minnesota. Chest. (1997) 111:303–10. doi: 10.1378/chest.111.2.303
151. Reed CE. The natural history of asthma in adults: the problem of irreversibility. J Allergy Clin Immunol. (1999) 103:539–47. doi: 10.1016/S0091-6749(99)70221-6
152. Rackemann FM, Edwards MC. Is intrinsic asthma a reversible disease?: a follow-up study. J Allergy Clin Immunol. (1958) 29:528–34. doi: 10.1016/0021-8707(58)90025-X
153. Rönmark E, Jönsson E, Lundbäck B. Remission of asthma in the middle aged and elderly: report from the obstructive lung disease in northern sweden study. Thorax. (1999) 54:611–3. doi: 10.1136/thx.54.7.611
154. Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J. (1999) 13:904–18. doi: 10.1034/j.1399-3003.1999.13d35.x
155. McFadden ER Jr. Natural history of chronic asthma and its long-term effects on pulmonary function. J Allergy Clin Immunol. (2000) 105:S535–S9. doi: 10.1016/S0091-6749(00)90057-5
156. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-Year follow-up study of ventilatory function in adults with asthma. N Engl J Med. (1998) 339:1194–200. doi: 10.1056/NEJM199810223391703
157. Savage-Brown A, Mannino DM, Redd SC. Lung disease and asthma severity in adults with asthma: data from the third national health and nutrition examination. J Asthma. (2005) 42:519–23. doi: 10.1081/JAS-200067605
158. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. (2000) 16:432. doi: 10.1034/j.1399-3003.2000.016003432.x
159. Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. (2005) 116:970–5. doi: 10.1016/j.jaci.2005.08.035
160. Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thora Soc. (2010) 7:237–44. doi: 10.1513/pats.200908-086AL
161. D'Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A, et al. Asthma-related deaths. Multidiscipl Respir Med. (2016) 11:37. doi: 10.1186/s40248-016-0073-0
162. Thomas SD, Whitman S. Asthma hospitalizations and mortality in chicago: an epidemiologic overview. Chest. (1999) 116:135S−41S. doi: 10.1378/chest.116.suppl_2.135S
Keywords: asthma, pediatric, adult, childhood, airway
Citation: Trivedi M and Denton E (2019) Asthma in Children and Adults—What Are the Differences and What Can They Tell us About Asthma? Front. Pediatr. 7:256. doi: 10.3389/fped.2019.00256
Received: 12 April 2019; Accepted: 06 June 2019;
Published: 25 June 2019.
Edited by:
Steve Turner, University of Aberdeen, United KingdomReviewed by:
Basil Elnazir, Tallaght Hospital, IrelandAroonwan Preutthipan, Mahidol University, Thailand
Copyright © 2019 Trivedi and Denton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Trivedi, michelle.trivedi@umassmemorial.org
 Michelle Trivedi
Michelle Trivedi Eve Denton3,4
Eve Denton3,4