- Key Laboratory of Crop Genetics and Physiology of Jiangsu Province, College of Horticulture and Plant Protection, Yangzhou University, Yangzhou, China
Flower color is one of the most important features of ornamental plants. Its development and regulation are influenced by many internal and external factors. Therefore, understanding the mechanism of color development and its regulation provides an important theoretical basis and premise for the cultivation and improvement of new color varieties of ornamental plants. This paper outlines the functions of petal tissue structure, as well as the distribution and type of pigments, especially anthocyanins, in color development. The progress of research on flower color regulation with a focus on physical factors, chemical factors, and genetic engineering is introduced. The shortcomings of flower color research and the potential directions for future development are explored to provide a broad background for flower color improvements in ornamental plants.
Introduction
Flower color can attract pollinators and protect floral organs. Furthermore, people enjoy these colors in daily life. For ornamental plants, flower color is an important quality determinant that not only affects the ornamental merit of a plant but also directly influences its commercial value. Although there is a wide range of natural flower colors, colors are limited in some important ornamental plants. For example, Chinese rose and chrysanthemum lack blue, and herbaceous peony and cyclamen lack yellow. Therefore, making flower color improvements has always been an important goal for breeders.
Over the years, much research has been conducted on the development and regulation of ornamental plant color. Researchers have found that the development of flower color is related to petal tissue structure, pigment distribution and its types; it can be regulated through environmental factors and genetic engineering. In this review, we described recent advances toward a better understanding of the development and regulation of flower color in ornamental plants.
Mechanism of Flower Color Development
When a petal is exposed to light, the light penetrates the pigment layer and is partially absorbed. Some of the remaining light is reflected by the sponge tissue and passes back through the pigment layer. Therefore, it is sensed by our eyes as color. The color of flowers is related to the internal or surface tissue structure of a petal and the type and amount of pigments in the petal cells, but pigment plays a major role.
Petal Tissue Structure and Pigment Distribution
Petal tissue structure is similar to leaf blade structure, which can be divided into four parts: upper epidermis, palisade tissue, sponge tissue, and lower epidermis. Under normal circumstances, petal pigments are mainly distributed in the upper epidermal cells, but they can also be found in the palisade tissue and the lower epidermis of dark colored petals. For example, pigment exists in the palisade tissue of pale blue grape hyacinth (Qi et al., 2013) as well as in the petal lower epidermis of tulip (Shoji et al., 2007), Ipomoea tricolor (Figure 1; Yoshida et al., 2009) and meconopsis (Yoshida et al., 2006). Typically, no pigment is distributed in the sponge tissue. However, its thickness and density is related to the brightness of flower color. The thicker and denser the sponge tissue, the brighter the color (An, 1989).
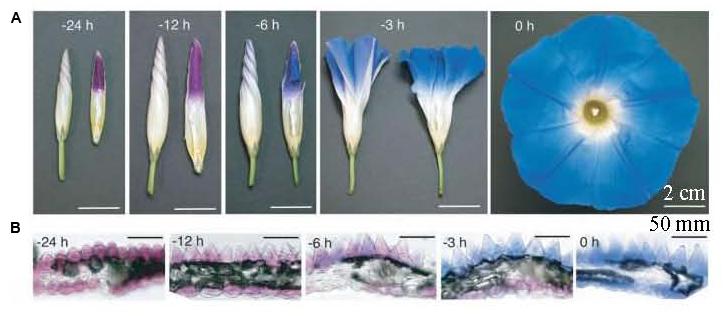
FIGURE 1. Petal color change and transverse sections in the open process of Ipomoea tricolor (Yoshida et al., 2009). (A) Whole flower growth. The right photos are half-cut buds; (B) Transverse sections of petals.
Different pigments in the same tissue can exhibit different subcellular localization. In general, carotenoids are deposited in the plastids of cytoplasm, and flavonoids are deposited in vacuoles. It has also been found that flavonoids can exist in different forms in cells, Markham et al. (2000) reported the presence of flavonoids in lisianthus petal epidermal cell walls.
In addition, various shapes of petal epidermal cells can also have an important impact on flower color. Conical cells can increase the proportion of the incident light on epithelial cells, which enhances the light absorption by pigments, thereby leading to darkened flower color and enhanced color saturation. Flat cells can reflect more incident light, leading to lighter flower color. The epidermal cells with protruding papillae can generate a velvet sheen on the petals. Noda et al. (1994) found that when magenta snapdragon was mutated to pink, conical epidermal cells became flat (Figure 2), this transformation was regulated by a MYB family transcription factor, MIXTA. And Vignolini et al. (2015) found that the diffraction from the regularly folded cuticle overlying the petal epidermal cells in Hibiscus trionum generated the iridescent effect. In addition, Yoshida et al. (1995) believed that the length and arrangement of iris petal epidermal cells had certain influences on the flower color.
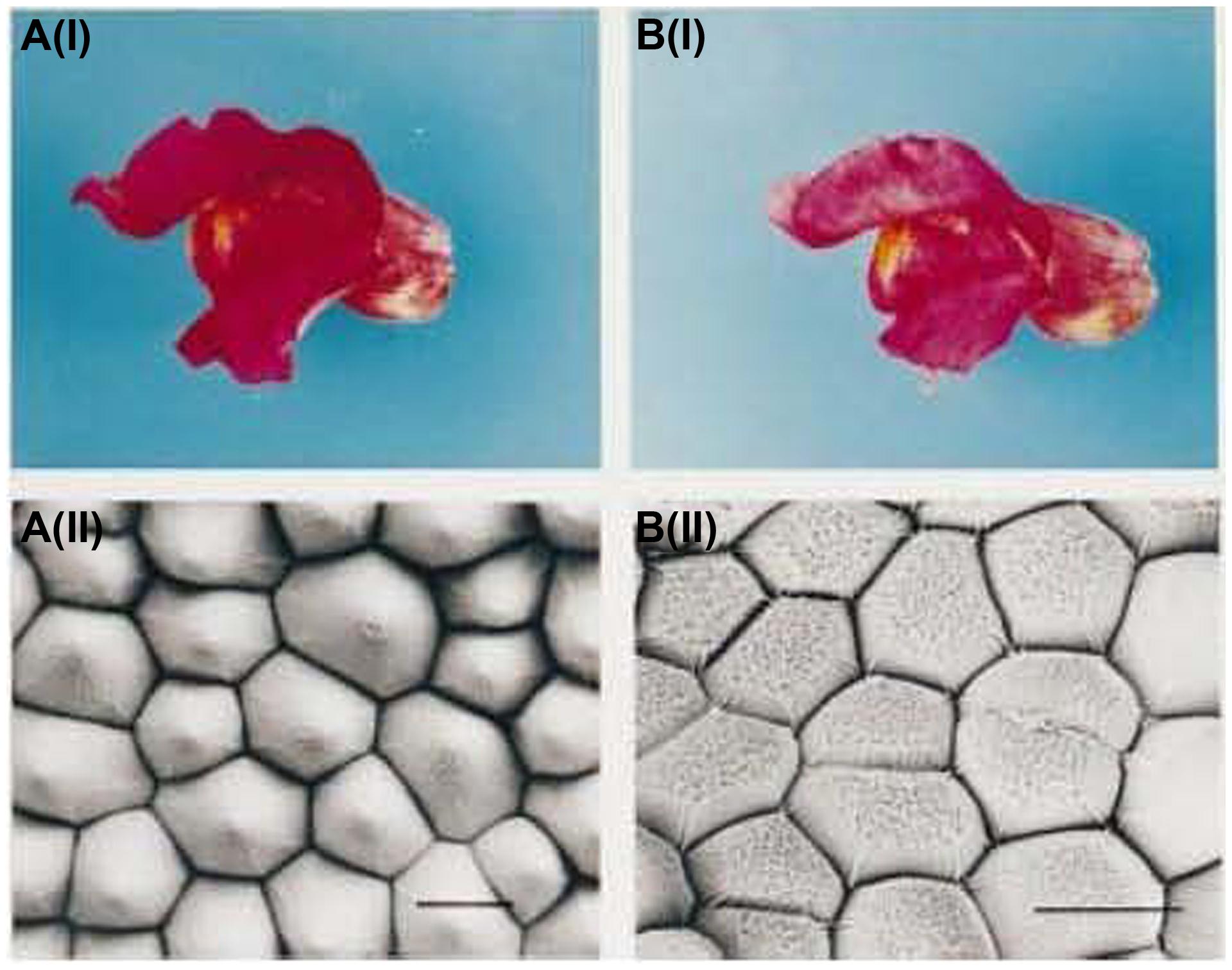
FIGURE 2. Petal color and scanning electron micrograph of snapdragon and its mutant (Noda et al., 1994). A(i) Wild-type flower with magenta petals; A(ii) Scanning electron micrograph of wild-type petal; B(i) Mutant flower with pink petals; B(ii); Scanning electron micrograph of a mutant petal.
Pigment Types
People have extracted pigments from colorful flowers to study their components since the mid-19th century. After more than 150 years of research, a wide variety of pigments have been found which could be generally divided into three groups, carotenoids, flavonoids, and alkaloids according to their chemical structures, cellular localizations and biochemical synthesis pathways.
Carotenoids are the most widely distributed pigments in nature. In addition to flowers, they can also be found in fruits, leaves and roots in higher plants. Carotenoids can be divided into the two major categories of carotene and lutein. Both groups are cyclization-produced organic molecules of a C40 polyene backbone with an ionone ring at the end. This structure makes carotenoids able to absorb visible light of short wavelengths. The wavelength of light being absorbed is determined by the number and properties of double bonds. Therefore, carotenoids can be brilliant red, orange and yellow (Britton et al., 2004). Although carotenoids exist in the petals of different ornamental species, their specific compositions are not the same in all species. Han et al. (2014) found that Osmanthus fragrans yellow petals contained small amounts of β-carotene, golden yellow petals had high levels of lutein, as well as low levels of α-carotene and β-carotene, and orange–red petals accumulated considerable concentrations of α-carotene and β-carotene. Previous studies have shown that the petals of marigold ‘Lady’ and chrysanthemum ‘Yellow Paragon’ contain only lutein (Moehs et al., 2001; Ohmiya et al., 2006). Large amounts of violaxanthin and zeaxanthin, as well as small amounts of neoxanthin, lutein, zeaxanthin and β-carotene, are the carotenoid components that make lotus root yellow (Suzuki et al., 2007). The major carotenoid components in yellow oncidium petals are trans-violaxanthin and 9-cis-violaxanthin (Hieber et al., 2006), whereas zeaxanthin, β-carotene and ζ-carotene are mainly found in saffron petals (Castillo et al., 2005). Other zeaxanthins, 9-Z-violaxanthin and cis-lutein are the main components of the yellow lily ‘Connecticut King’ petals (Zhu et al., 2010).
Flavonoids are a large class of secondary metabolites, which are widely distributed in plants. Chemically, flavonoids are a collection of substances based on the structure of the 2-phenylchromone nucleus. Flavonoids are the most important pigment group and produce the widest spectrum of colors, ranging from pale yellow to blue-purple. They are one of the most important pigments in a variety of ornamental plant petals, such as chrysanthemum (Chen, 2012), dahlia (Thill et al., 2012), groundcover rose (Schmitzer et al., 2010), violet (Fumi et al., 2012), and herbaceous peony (Zhao et al., 2012a,b, 2013, 2014). The composition of flavonoids may vary greatly among different color petals of the same species. Chen et al. (2012) analyzed the pigment composition of chrysanthemum in two different color flowers and found that the white flower contained only flavones and flavonols, whereas the pink flowers mainly contained anthocyanins, flavones and flavonols. He et al. (2011) analyzed the pigments of purple, red, orange, yellow, and white Lycoris longituba and found that only one of the four identified anthocyanins was present in all purple, red, and orange samples; no anthocyanins were detected within white and yellow samples. This result occurs mainly because among flavonoids, anthocyanin belongs to the red series and controls pink to blue-violet flower colors. Other flavonoids belong to the pure yellow series, among which chalcone and aurone are deep yellow, and flavones, flavonols and flavanones are light yellow or nearly colorless.
Alkaloids are a class of cyclic organic substances that contain negative oxidized nitrogen atoms, including betalain, papaverine and berberine. Among them, betalain is a water-soluble nitrogen compound present in red beets (also known as purple beetroots) and some flowers, fruits, roots and leaves. Betacyanin and betaxanthin are present in these plants, with betacyanin being the main component, accounting for ∼75–95% of the total betalain (Strack et al., 2003). To date, betalain has been found only in Caryophyllales plants (except Caryophyllaceae and Molluginaceae whose colors are produced by anthocyanin). The two types of pigments, betalains and anthocyanins, have never been found in the same plant (Gandía-Herrero and García-Carmona, 2013). Betalains are very important for flower color development. The difference between a flower being red or yellow depends on the presence of betacyanin or betaxanthin in the petals. Orange to red or variegated colors may be produced if both pigments co-exist in a flower (Gandía-Herrero et al., 2005; Felker et al., 2008). Kugler et al. (2007) researched amaranth and bougainvillea in three different colors, and found that the orange petals contained mostly betaxanthin and a minor amount of betacyanin; the red petals contained essentially equal amounts of the two pigments. A large amount of betacyanin was accompanied by a trace amount of betaxanthin in the purple petals.
Anthocyanins and Color Development
Among of the aforementioned pigments, water soluble flavonoids containing anthocyanins and anthoxanthins can produce the full spectrum of colors from pale yellow to blue–purple. Anthoxanthins mainly produce the colors from white to dark yellow in flowers. And anthocyanins are the main flavonoid group, they play an irreplaceable role in the color development of plants, exhibiting a wide range of colors, from pink to blue–purple. Therefore, this section will review the role of anthocyanins in flower color development.
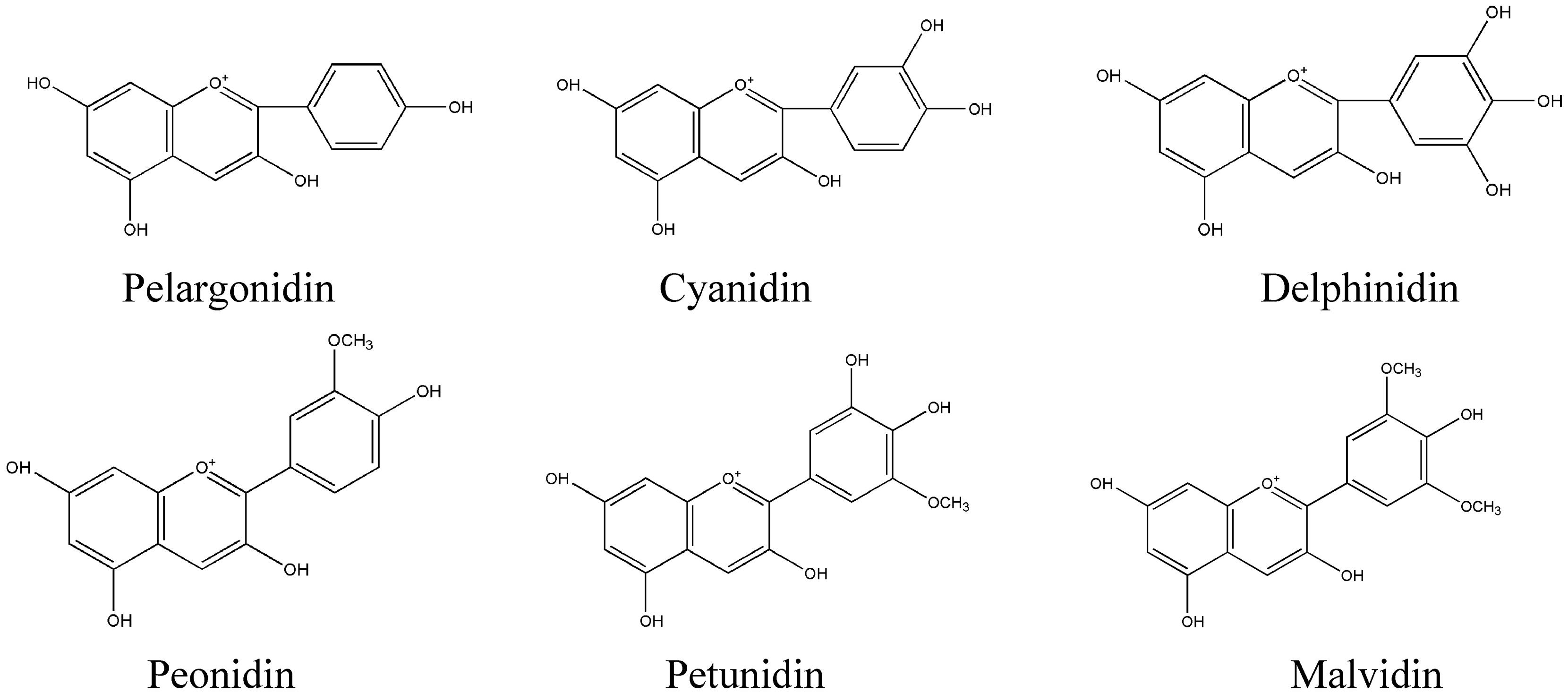
As flavonoids, anthocyanidins have a highly characteristic C6-C3-C6 carbon skeleton and the same biosynthetic origins. Due to the instability of anthocyanidins, they exist mainly as anthocyanins (i.e., sugar-containing counterparts) in plants. Approximately 100 anthocyanins have been reported (Veitch and Grayer, 2008), primarily derived from six common types of anthocyanidins, namely, pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin (Figure 3). In terms of biosynthesis, peonidin is derived from cyanidin, and petunidin and malvidin are derived from delphinidin; thus, pelargonidin, cyanidin, and delphinidin are the three main anthocyanidins (Tanaka et al., 2009). The anthocyanin sugar groups mainly include glucose, rhamnose, xylose, galactose and arabinose, and the monosaccharaides compose uniform or non-uniform disaccharides and trisaccharides; 3-monoglucoside, 5-diglucoside, 3,5-diglycoside and 3,7-diglycoside are the most common (Liu, 1998). The colors of the different anthocyanins are related to the environment and the substituents linked to the parental C6-C3-C6 carbon backbone.
Anthocyanins are a class of pigments that are soluble in water, methanol, ethanol, and acetone; they are insoluble in ether and chloroform. They can be precipitated by lead acetate and absorbed by activated carbon. Anthocyanin extract is distinguished from other flavonoids by strong visible light absorption. It exhibits a significant characteristic absorption peak at 500–550 nm in the visible region (Zhao et al., 2012b). Anthocyanins are very unstable. Light, temperature, pH, oxidants and reducing agents can significantly affect their stability (Bordignon-Luiz et al., 2007; Zhao et al., 2011). For example, the color of anthocyanins is red in acidic pH, colorless in neutral or nearly neutral pH and blue in alkaline pH. This effect is due to the existence of four anthocyanin tautomers in different pH values: alkali blue quinone A, red–yellow molten cation AH+, colorless false base B and colorless chalcone C. The three balance conversions between them are readily affected by pH (Pina, 2014).
Anthocyanins are glycosides, which are naturally formed by anthocyanidins and various sugars. They are stably localized in plant organs, such as petals, and are red, purple, blue, and black (Li et al., 2003). Previous studies have shown that the color differences are related to the anthocyanin content. Kazuma et al. (2003) measured the amount of anthocyanins in a series of butterfly pea petals from white to blue and found that the anthocyanin content was significantly higher in the blue petals than in the other petals; there were no anthocyanins in the white petals. The differences in anthocyanins are one of the important reasons for the development of a variety of colors. In cineraria, the blue and red flower colors are mainly determined by delphinidin aglycone and cyanidin aglycone, respectively. The pink flowers contain cyanidin aglycone and pelargonidin aglycone as the core anthocyanins, and purple flowers contain mainly delphinidin aglycone and cyanidin aglycone as the core anthocyanins (Sun et al., 2009). The red pigments, pelargonidin and cyanidin, appear differently in lagenaria. Cyanidin appears in red, while pelargonidin leans toward scarlet (Zhang et al., 2011). Moreover, the glycoside types of the same anthocyanidins are also closely linked to flower color development. In tropical water lily, the cultivars which are detected delphinidin 3-galactoside (Dp3Ga) present amaranth, and detected delphinidin 3′-galactoside (Dp3′Ga) present blue (Zhu et al., 2012). In addition, the co-coloring effect, the pH in the vacuole and chelation are all important in affecting the color of anthocyanins, which have been described in detail by Tanaka et al. (2009).
Anthocyanin Biosynthetic Pathway and Key Genes
Anthocyanin biosynthesis has been a research hotspot in the field of plant secondary metabolism, and its biosynthetic pathway and key genes in plants have been clarified (Cheynier et al., 2013). Anthocyanin biosynthesis, beginning with the direct precursor of phenylalanine, can be divided into three stages (Figure 4). The first stage is the conversion of phenylalanine to coumarate-CoA by phenylalanine ammonia lyase (PAL), cinnamate-4-hydroxylase (C4H) and 4-coumarate: CoA ligase (4CL), which is a common step in many secondary metabolic pathways. The second stage is the formation of dihydroflavonol by one molecule of coumarate-CoA and three molecules of malonyl-CoA catalyzed by chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H) and flavonoid 3′,5′-hydroxylase (F3′5′H), which is a key reaction in the metabolism of flavonoids. The third stage is the formation of various anthocyanidins by dihydroflavonols catalyzed by dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (ANS). The synthesized anthocyanidins are then modified through a series of glycosylation and methylation steps to form stable anthocyanins catalyzed by UDP-glucose: flavonoid glucosyltransferase (UFGT) and methyl transferase (MT).
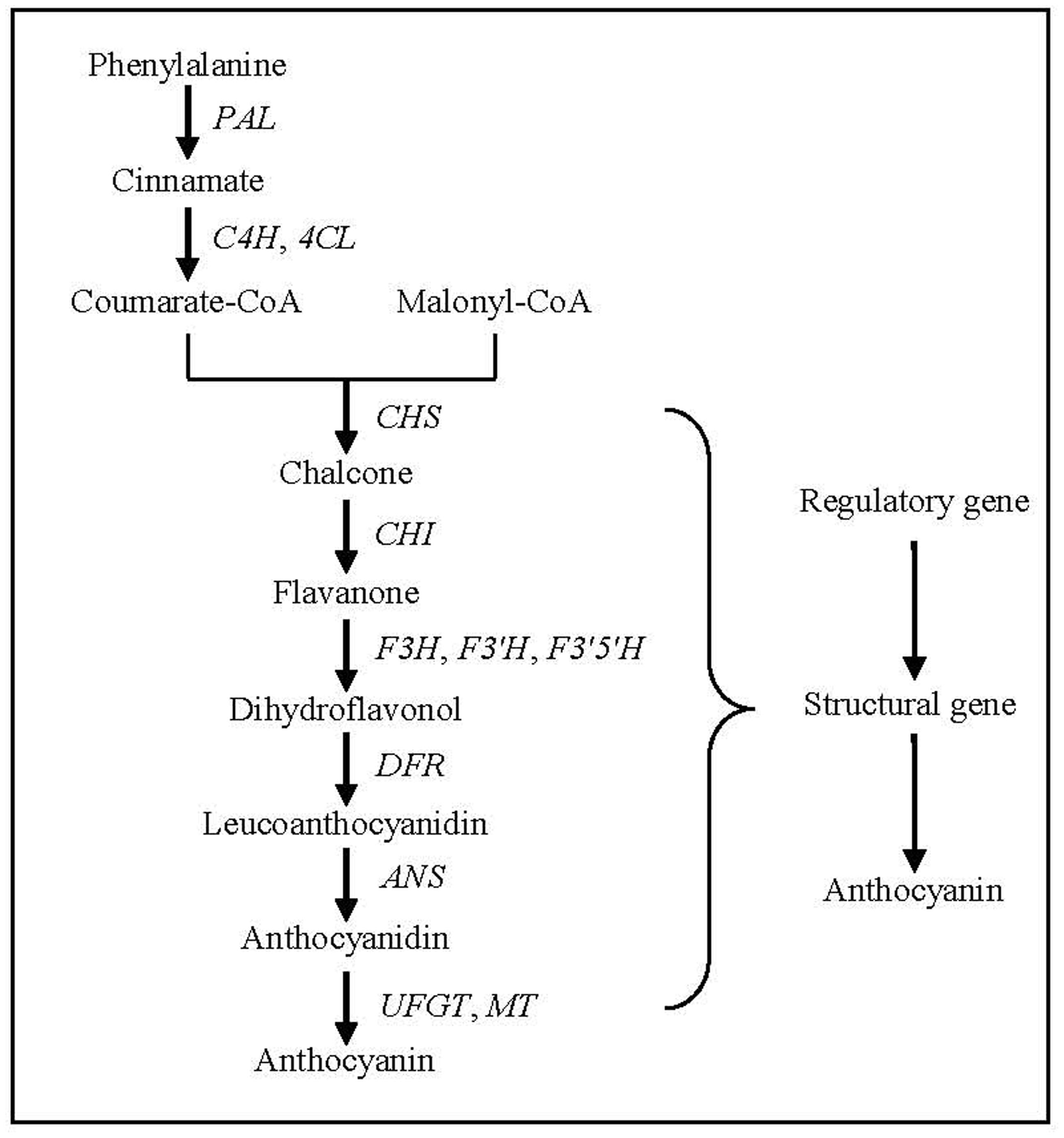
FIGURE 4. Anthocyanin biosynthesis pathway in plants. PAL, phenylalanine ammonia lyase gene; C4H, cinnamate-4-hydroxylase gene; 4CL, 4-coumarate: CoA ligase gene; CHS, chalcone synthase gene; CHI, chalcone isomerase gene; F3H, flavanone 3-hydroxylase gene; F3′H, flavonoid 3′-hydroxylase gene; F3′,5′H. flavonoid 3′,5′-hydroxylase gene; DFR, dihydroflavonol 4-reductase gene; ANS, anthocyanidin synthase gene; UFGT, UDP-glucose: flavonoid glucosyltransferase gene; MT, methyl transferase gene.
CHS encodes the first key enzyme gene in anthocyanin biosynthesis in plants, which combines one molecule of coumarate-CoA and three molecules of malonyl CoA to form chalcone. Molecular evolution analysis of CHS has shown that it is ubiquitous in plants including early land plants and algae of the charophyceae (Schroder, 1997). In ornamental plants, CHS has largely been isolated, including petunia (Morgret et al., 2005), phalaenopsis (Han et al., 2006) and herbaceous peony (Zhao et al., 2012b), since it was first reported in parsley by Reimold et al. (1983). And their protein sequences are highly conserved among different plants with ∼80–90% homologies (Beerhues and Wiermann, 1988). CHS plays an important role in the synthesis and accumulation of anthocyanins, which induce the results of altering flower color. Transgenic petunia expressing CHS1 of Freesia hybrid shows flower color alteration from white to pink (Sun et al., 2015), and transgenic tobacco expressing CHS of Malus crabapple displays a higher anthocyanin accumulation and a deeper red petal color compared with control untransformed lines (Tai et al., 2014). In addition, CHS expression is often regulated by tissue specificity and different developmental stages, and it has varied sensitivity to environmental stimuli. For example, CHS of safflower is responsive to wounding, salicylic acid treatment and salinity stress (Dehghan et al., 2014), temperature and UV can induce the expression of CHS in Dryopteris fragrans (Sun et al., 2014).
CHI encodes the second key enzyme gene in plant anthocyanin biosynthesis, which catalyzes the isomerization of chalcone. Chalcone is modified by CHI to form flavanone. This product is required in the metabolic branch pathways of flavone, flavonol, proanthocyanidin, and anthocyanin synthesis. Currently, CHI has been occurred from Bryophytes through to Angiosperms (Ngaki et al., 2011), and it in plants can be divided into two types according to their catalytic substrates, one type uses 6′-hydroxy chalcone as a substrate, as well as the other can catalyze the isomerization of 6′-hydroxy chalcone and 6′-deoxy chalcone (Chmiel et al., 1983). Regardless of type, whether CHI is expressed and its expression level affect flavonoid metabolism in plants, thus affecting flower color development. For example, a decrease in CHI expression in carnations, asters, cyclamen and tobacco can result in a greater accumulation of chalcone in petals, turning them yellow (Nishihara et al., 2005).
F3H encodes the enzyme gene that catalyzes the hydroxylation of flavanones at C3 to form dihydroflavonol. It is considered a key enzyme at the branch point of the flavonoid biosynthetic pathway. The enzyme can independently regulate metabolism, but often collaborates with the upstream CHS and CHI products to catalyze the formation of downstream products (Owens et al., 2008). It was shown that the expression patterns and levels of the F3H were similar in white, red and blue cineraria petals (Hu et al., 2009). Therefore, the gene was not used in color breeding until 2001 when Zuker et al. (2002) reported that the inhibition of F3H expression in an F3′H and F3′5′H null carnation mutant made orange flowers colorless. To date, the gene has been isolated from ornamental plants, including cineraria (Hu et al., 2009), saussurea (Jin et al., 2005), and herbaceous peony (Zhao et al., 2012b).
DFR is another gene encoding a key enzyme in the plant anthocyanin biosynthetic pathway that plays an important role in flower color development. DFR belongs to the reduced coenzyme II (nicotinamide adenine dinucleotide phosphate, NADPH)-dependent short-chain reductase family and is encoded by single or multiple gene(s). This enzyme can reduce three types of dihydroflavonols, dihydromyricetin flavonoids, dihydroquercetins, and dihydrokaempferols, to their corresponding colorless anthocyanidins with NADPH. These molecules are further modified to various anthocyanins by downstream gene products (Petit et al., 2007). Because the differences in DFR expression and its substrate specificity create color variation in flowers, studies of the mechanisms of its regulation of flower color development have become an important research direction. Currently, DFR has been reported to exist in ornamental plants, including Asia lily (Nakatsuka et al., 2003), gentian (Nakatsuka et al., 2005), herbaceous peony (Zhao et al., 2012b), and saussurea (Li et al., 2012). In the study of gene function, Zhao et al. (2012b) studied the expression of DFR in different herbaceous peony organs and found that it had the highest expression in the petals that accumulated large amounts of anthocyanins. Similar results have been reported in Asian lily (Nakatsuka et al., 2003) and gentian (Nakatsuka et al., 2005), suggesting that DFR may regulate flower color development at the transcriptional level.
ANS encodes one of the key enzyme genes in the late stage of anthocyanin biosynthesis. This gene catalyzes the conversion of leucoanthocyanidin to colored anthocyanidin using Fe2+ and 2-oxoglutarate (Heller et al., 1985). Studies have shown that ANS is encoded by a small gene family in many plants, and these genes have been cloned from ornamental plants, including Forsythia supensa (Rosati et al., 1999), gerbera (Wellmann et al., 2006) and herbaceous peony (Zhao et al., 2012b). Rosati et al. (1999) studied the ANS gene expression pattern in Forsythia supensa and found that null expression of the ANS gene resulted in little accumulation of anthocyanins in petals; similarly, the absence of the ANS gene sequence was the underlying reason for the color change of lisianthus flowers (Shimizu et al., 2011), suggesting its importance in the regulation of plant colors.
In addition to the structural genes in the anthocyanin biosynthetic pathway, transcription factors also play important roles in flower color development through regulating the temporal and spatial expression of structural genes (Xie et al., 2006). Transcription regulatory genes, also known as transcription factors, are DNA-binding proteins located in the nucleus. They can bind to cis-acting elements in promoter regions and regulate the expression of target genes. Currently, there are three main types of transcription factors that affect flower color, MYB, bHLH and WD40 (Ramsay and Glover, 2005). These transcription factors activate or suppress the transcription and expression of target genes through binding to specific DNA sequences and affect protein–protein interactions. Therefore, they regulate anthocyanin synthesis (Zhang et al., 2003). Among the three types of transcription factors that regulate the synthesis of plant anthocyanins, MYB transcription factors have been the most intensively studied. MYB genes have been widely found to regulate the synergistic expression of the structural genes in the plant anthocyanin synthetic pathway at the transcriptional level (Allan et al., 2008; Feng et al., 2010; Pattanalk et al., 2010). Among the three subtypes of MYB transcription factors, R2R3-MYB has commonly been considered closely related to anthocyanin metabolism and regulation (Petroni and Tonelli, 2011; Davies et al., 2012). At present, in-depth studies on this subtype of transcription factor have been reported in vegetables and fruit trees (Kobayashi et al., 2004; Takos et al., 2006; Ballester et al., 2010; Niu et al., 2010). Studies on ornamental plants, in addition to some model plants that were studied in some early reports, such as petunia and snapdragon (Sablowski et al., 1994; Quattrocchio et al., 1999), have been recently reported. For example, in gerbera, GhMYB10 is closely related to anthocyanin synthesis in leaves, scapes and flowers, and it specifically promotes anthocyanin synthesis in undifferentiated callus tissues and asexual reproductive organs (Roosa et al., 2008). Other examples include the bleaching of gentian flowers due to mutations in GtMYB3 (Nakatsuka et al., 2008) and the positive regulation of anthocyanin biosynthesis and its effects on organ and tissue-specific anthocyanin accumulation in lily via LhMYB6 and LhMYB12 (Yamagishi et al., 2010). Further studies discovered that sequence variations and methylation levels in MYB transcription factor genes also affected anthocyanin accumulation, but this observation was only reported in studies with fruit trees (Espley et al., 2009; Xu et al., 2012) and maize (Das and Messing, 1994; Cocciolone et al., 2001; Robbins et al., 2009).
Regulation of Flower Color
As one of the major flavonoid pigments, anthocyanins are discussed as above. The flower color development predominantly mediated by anthocyanins can also be regulated by physical and chemical factors and genetic engineering. Therefore, the regulatory factors in flower color development are discussed below.
Physical Factors
Temperature is a major physical factor which affects flower color. Extreme temperatures will have an impact on flower color development in plants, primarily due to the effect of temperature on anthocyanin accumulation (Lai et al., 2011). In general, high temperatures lead to lighter flower colors due to reduced anthocyanin content in plants such as oriental lily (Lai et al., 2011), rose (Dela et al., 2003), chrysanthemum (Nozaki et al., 2006), and tuberose (Huang et al., 2000). Conversely, low temperatures result in darker flowers because of increased anthocyanin content in plants, such as plantain (Stiles et al., 2007). These phenomena are the result of the suppressed expression of genes involved in anthocyanin biosynthesis, such CHS, F3H and DFR, and thus, the anthocyanin biosynthesis rate is reduced at high temperatures affecting the concentrations of anthocyanins (Lai et al., 2011). In addition, Chen et al. (2000) believed that temperature altered flower color by affecting the cellular structures of petal epidermal cells. At 30°C, the epidermal cells in petals are arranged in arrays of flat cells, whereas the thickness of the upper epidermis of petals increases at 10–20°C, which changes the distribution of anthocyanins in these cells, leading to darker petals.
Light is another major factor that affects flower color, particularly light intensity, light quality and photoperiod. Based on their requirements for light intensity, plants are classified into heliophytes and sciophytes, and they can only grow well under appropriate light intensities. For example, as a heliophyte, flowers of tuberose are purplish red under strong light intensities, but their color fades under weak light intensities (Huang et al., 2000). This effect also occurs in boronia (KangMo et al., 2007). Shade is a commonly used gardening method for the modification of light exposure. Huang et al. (2000) found that the tuberose flowers cultivated at 25°C were almost white by 45% shading, but pale reddish-purple under 25 or 0% shade treatment, which was related to the enzyme activity participating the biosynthesis of anthocyanins. Meanwhile, in herbaceous peony, 60% shade caused significantly reduced anthocyanin content and lighter flower color (Figure 5), mediated by the synergistic action of structural genes involved in anthocyanin biosynthesis and especially the downregulated expression of PlPAL, PlCHS, PlF3H, and PlF3′H (Zhao et al., 2012a).

FIGURE 5. Herbaceous peony flowers in the bloom stage under sun exposure and shade treatments (Zhao et al., 2012a).
Additionally, light quality has an impact on flower color. High red light could result in darker flower color of Hibiscus syriacus by decreasing Hunter L value but increasing Hunter a value (Young et al., 1997). Moreover, ultraviolet light can also enhance anthocyanin accumulation, UV-B radiation induced an increase in F3H enzyme activity of Reaumuria soongorica and the accumulation of the products in the flavonoid biosynthetic pathway (total flavonoid and anthocyanin; Liu et al., 2013). And the rich and bright colors of alpine and tropical flowers are all related to the strong ultraviolet light in these regions. In addition, photoperiod also has an impact on flower color. When insolation duration reached more than 12 h, the leaf colors of colored-leaf trees, such as purple-leaf plum, became more vivid and bright (Li and Liu, 1998). The bract color of poinsettia deteriorated when the short-day treatment was ended before bolting (Sun et al., 2006). A prolonged photoperiod led to gradually increased anthocyanin contents in the petals of lisianthus (Uddin et al., 2001).
Water controls the chromaticity of plant organs through its effect on the accumulation of anthocyanins in vacuoles (Zhi et al., 2012). Appropriate water content allows plants to maintain their inherent flower colors for a longer period of time, while water deficiency causes flowers to turn darker (Lai et al., 2011). For example, drought stress induced an increase in F3H enzyme activity of Reaumuria soongorica and the accumulation of the products in the flavonoid biosynthetic pathway (total flavonoid and anthocyanin; Liu et al., 2013). However, prolonged stress can also cause reductions in anthocyanin content (Li et al., 2009). All these alterations in anthocyanin content led to changes in the colors of plant organs.
In addition to the three physical factors discussed above, pollinators (Adriana et al., 2011), ion beam irradiation (Figure 6; Hase et al., 2010; Masayoshi et al., 2012) and gamma rays (Dwivedi et al., 2000; Bala and Singh, 2013) also affect the flower color of ornamental plants.
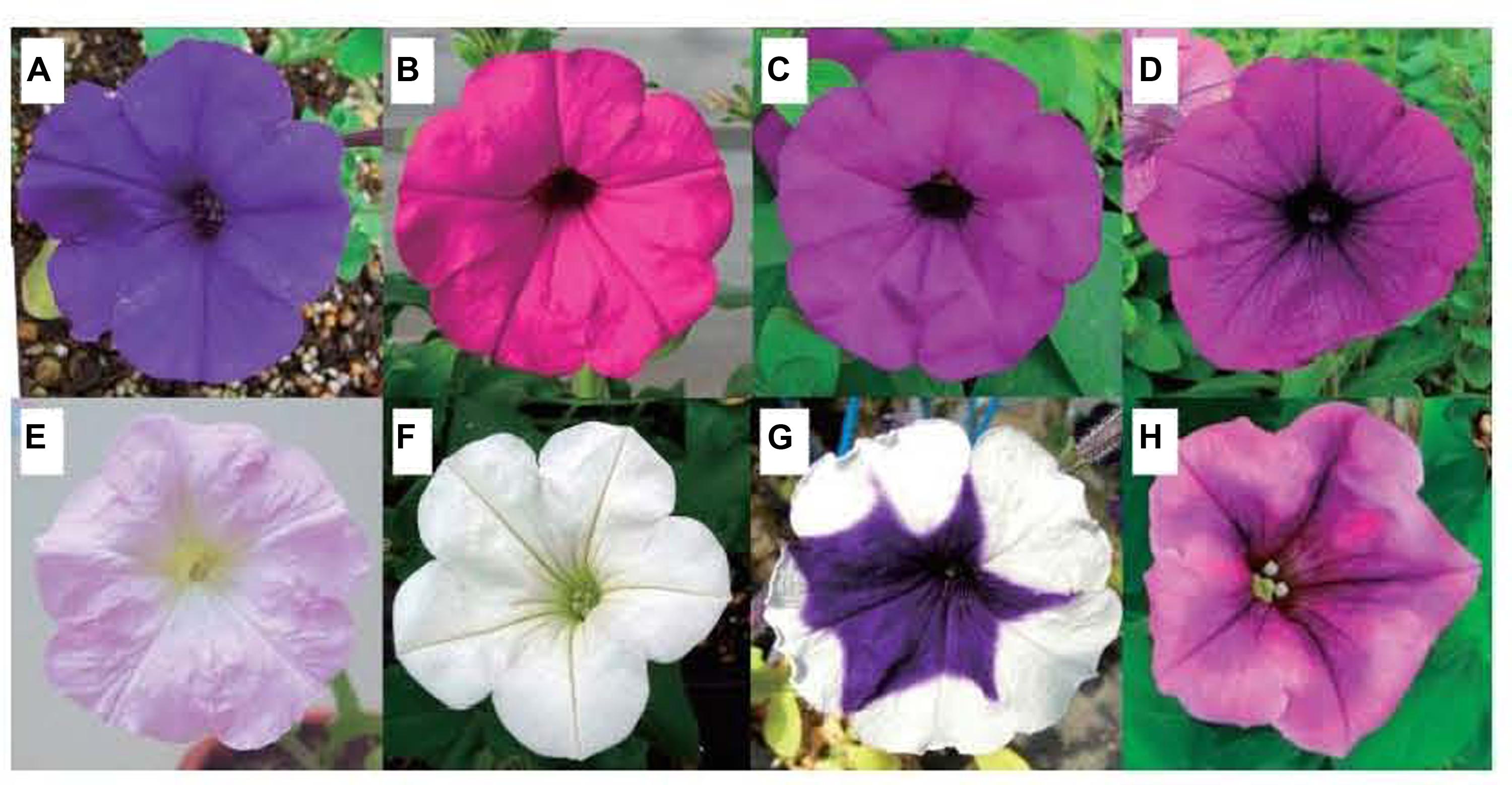
FIGURE 6. Parental line of petunia and flower-color mutants by ion beam irradiation (Hase et al., 2010). (A) Parental line with violet flower color; (B–H) Flower-color mutants; (B) Magenta; (C) purple; (D) purple vein; (E) light pink; (F) white; (G) blue picotee; (H) burgundy.
Chemical Factors
Environmental pH plays an important role in plant color. When Acer pseudosieboldianum was planted in acidic soil, autumn leaf coloration occurred early, with a prolonged period of full-color and more splendid leaf color (Han and Gong, 2010). Acidification of the soil was found to affect anthocyanin synthesis in the leaves and enhance leaf color (Sun et al., 2008). Moreover, Liu et al. (2011) found that soil pH did not affect the types of anthocyanins in the petals of lupine. In addition to soil pH, we also examined the effects of pH in irrigation water on flower color (Zhao et al., 2013). When irrigation water pH was at 4.0, herbaceous peony exhibited a lighter flower color (Figure 7) with significantly reduced anthocyanin content and markedly increased petal pH. The large decline in the expression level of the anthocyanin biosynthesis structural gene PlDFR and the increased expression level of the pH-regulating gene vacuolar Na+/H+ antiporter1 (NHX1) in the petals played vital roles in flower color fading in herbaceous peony.

FIGURE 7. Herbaceous peony flowers in the blooming stage at pH 7.0 and 4.0 (Zhao et al., 2013).
Mineral nutrients have been widely employed in the regulation of plant color. In the study by Yang et al. (2012), the foliar application of urea, monopotassium phosphate, diammonium phosphate or a combination in climbing rose ‘Angela’ resulted in copious flowers and brighter flower colors. Liu et al. (2009) found that the foliar application of Fe2+ improved flower color to different extents. Previous studies reported that Impatiens hawkerii exhibited darker flowers under sand culture conditions with 7.41 × 10-6 mol/L of aluminum or 3.2 × 10-7 mol/L of copper (Li et al., 2005; Li and Fang, 2006), which was due to an increase in soluble sugars and anthocyanins. The effect of the same elements is different in different color varieties. Flower color was significantly improved in the red and orange varieties of lily after the application of a potassium spray, but no effect was observed in yellow lily. The specific mechanism underlying the increased pigment concentrations is still unclear (Burchi et al., 2010).
Plant hormones are closely related to flower color, and their effects on color have been examined in a number of studies. Generally, plant growth retardants can effectively improve the color of plants. Currently, this effect has been confirmed for prohexadione-calcium (Pro-Ca; Schmitzer et al., 2012). The petal color of China rose changed from red to light pink and eventually to white after the application of Pro-Ca (Figure 8). In addition to anthocyanin content, this phenomenon was found to be directly related to the induction of 3-deoxyflavonoids synthesis (Schmitzer et al., 2012). Furthermore, a study reported that the inhibition of anthocyanidin synthase resulted in red color loss in the ray florets of bronze chrysanthemum after daminozide application which was a well-known chemical inhibitor of the gibberellin biosynthesis (Roepke et al., 2013). In addition, Weiss et al. (1995) found that gibberellin produced in anthers was transported to petals to take effect, where it directly induced the expression of genes, including CHS, CHI, DFR and UF3GT.

FIGURE 8. Flower color changes of two China rose cultivars due to Pro-Ca application (Schmitzer et al., 2012). (A) Flowers prior to the application of Pro-Ca; (B) Flowers 9 days after Pro-Ca application turned light pink; (C) Flowers 15 days after Pro-Ca application turned white.
As a moiety in anthocyanins, carbohydrates provide a precursor substance and energy for anthocyanin synthesis. Therefore, their content directly affects the accumulation of anthocyanins. At present, sucrose, glucose and fructose have been demonstrated to be the main carbohydrates that are effective in promoting anthocyanin accumulation (Neta et al., 2000; Hara et al., 2003; Solfanelli et al., 2006; Zheng et al., 2009; Zhang et al., 2015). In addition, carbohydrates can serve as signaling molecules in the regulation of anthocyanin synthesis-related gene expression and the induction of anthocyanin synthesis via specific signal transduction pathways. A study by Zhang et al. (2015) showed that glucose treatment was found to greatly enhance anthocyanin content and induced the expression of WD40-2, MYB2, CHS1, CHI1 and F3′H1 through glucose signaling in tree peony. Neta et al. (2000) found that carbohydrates also regulated anthocyanin synthesis and the expression of genes encoding related enzymes in the corollas of petunia through signaling transduction pathways associated with phosphorylation by hexokinase.
From the perspective of biochemistry and genetics, flower color development is an extremely complex process. Thus, breeding for varieties of different flower colors seems to be outside the scope of traditional breeding techniques. The blooming genetic engineering field brings new ideas and approaches to basic research and variety breeding for flower color in ornamental plants. The identification and characterization of genes encoding key enzymes involved in plant anthocyanin biosynthesis and other genes that influence petal color makes the regulation of plant flower color possible through genetic engineering.
Currently, there are two main strategies for the regulation of flower color through transgenic methods. One strategy is to regulate the intrinsic pigment composition and content in petals, while the other is to introduce new pigments into petals. The effects of these two strategies have been confirmed in recent studies (Nishihara and Nakatsuka, 2011). Boase et al. (2010) suppressed the F3′5′H gene in cyclamen via antisense inhibition, which led to reduced delphinidin content and elevated cyanidin content, resulting in the petal color changing from purple to red to pink. Takashi et al. (2010) regulated flower color in blue gentian using RNA interference technology. When the anthocyanin 5,3′-aromatic acyltransferase gene (5/3′AT) was inhibited, the petals became lilac. However, when 5/3′AT and F3′5′H were co-suppressed, the petals were pale blue (Figure 9). Meanwhile, the anthocyanin of the petals contents were changed in all transgenic plants. Katsumoto et al. (2007) generated transgenic roses by introducing F3′5′H from violet and DFR from iris into rose for overexpression. The resulting flowers showed the accumulation of a large amount of delphinidin and a novel blue color in the petals. Zhou et al. (2014) overexpressed CHI1 from tree peony in tobacco, and the transgenic tobacco petals produced up to three-fold the flavonols and flavones compared to the wild-type. They showed a remarkable reduction in anthocyanin content and flower color intensity.

FIGURE 9. Flower phenotypes of transgenic gentian plants (Takashi et al., 2010). (A) The typical flowers of wild-type gentian; (B) 5/3′AT-suppressed transgenic gentian; (C) 5/3′AT and F3′5′H double-suppressed transgenic gentian.
In addition, Momonoi et al. (2009) identified the vacuole ion transporter Vit1 in tulips, which made petal cells blue through regulating the accumulation of ions. Verweij et al. (2008) discovered that the PH5 gene of the petunia generated the blue color by reducing the acidification in vacuoles. In addition, the MYB transcription factor affects flower color through regulating petal cell morphology (Noda et al., 1994; Baumann et al., 2007; Di et al., 2009). All these genes can be used to improve flower color via genetic engineering.
Concluding Remarks
Flower color in ornamental plants is the result of the joint actions of many factors. To date, a certain understanding of the mechanisms underlying flower color development have been achieved, with in-depth studies on the anthocyanin components, contents, biosynthetic pathways and key genes. In addition, a basic understanding of the types of anthocyanins and their biosynthetic pathways in different ornamental plants has been reached, the regulatory of physical and chemical factors has been explored and their regulative mechanisms are clarified preliminarily. Along with the deepening of research on the functional genomics, proteomics, metabolomics and epigenetics in model plants and the rapid development of high throughput sequencing technology, new opportunities and challenges are brought for researches on the development and regulation of flower color in ornamental plants. And some difficult questions could be solved by drawing on research results in model plants as well as making full use of high-throughput sequencing technology. For example, the complete regulatory mechanisms of flower colors affecting by physical and chemical factors, the interactions among the regulatory factors that can be used for the regulation of flower color and the mechanisms of rare flower color development and directed breeding. However, the huge amounts of data produced in the researches of the development and regulation of flower color in ornamental plants by high-throughput sequencing technology also poses a challenge for our analysis. In order to make great progress in the researches of the development and regulation of flower color in ornamental plants, we must be skilled in bioinformatics, as well as need the infiltration and mergence of multiple subjects.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31372097, 31400592), the Major Project of College Natural Science Research of Jiangsu Province (13KJA210005), the Agricultural Science and Technology Independent Innovation Fund of Jiangsu Province (CX[14]4098) and the Priority Academic Program Development from Jiangsu Government.
References
Adriana, C. P., Juliana, B. S., Renato, G., Gabriel, A. R., and Melo, I. G. V. (2011). Flower color change accelerated by bee pollination in Tibouchina. Flora 206, 491–497. doi: 10.1016/j.flora.2011.01.004
Allan, A. C., Hellens, R. P., and Laing, W. A. (2008). MYB transcription factors that colour our fruit. Trends Plant Sci. 13, 99–102. doi: 10.1016/j.tplants.2007.11.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bala, M., and Singh, K. P. (2013). In vitro mutagenesis of rose (Rosa hybrida L.) explants using gamma-radiation to induce novel flower colour mutations. J. Hortic. Sci. Biotech. 88, 462–468.
Ballester, A. R., Molthoff, J., deVos, R., Hekkert, B., Orzaez, D., Fernández-Moreno, J. P., et al. (2010). Biochemical and molecular analysis of pink tomatoes: deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 152, 71–84. doi: 10.1104/pp.109.147322
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baumann, K., Perez-Rodriguez, M., Bradley, D., Venail, J., Bailey, P., Jin, H., et al. (2007). Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development 134, 1691–1701. doi: 10.1242/dev.02836
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beerhues, L., and Wiermann, R. (1988). Chalcone synthases from spinach (Spinacia oleracea L.). Planta 173, 544–553. doi: 10.1007/BF00958968
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Boase, M. R., Lewis, D. H., Davies, K. M., Marshall, G. B., Patel, D., Schwinn, K. E., et al. (2010). Isolation and antisense suppression of flavonoid 3’5’-hydroxylase modifies flower pigments and colour in Cyclamen. BMC Plant Biol. 10:107. doi: 10.1186/1471-2229-10-107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bordignon-Luiz, M. T., Gauche, C., Gris, E. F., and Falcão, L. D. (2007). Colour stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. LWT Food Sci. Technol. 40, 594–599. doi: 10.1016/j.lwt.2006.02.022
Britton, G., Liaaen-Jensen, S., and Pfander, H. (2004). Carotenoids Handbook. Basel: Birkhäuser. doi: 10.1007/978-3-0348-7836-4
Burchi, G., Prisa, D., Ballarin, A., and Menesatti, P. (2010). Improvement of flower color by means of leaf treatments in lily. Sci. Hortic. 125, 456–460. doi: 10.1016/j.scienta.2010.04.028
Castillo, R., Fernandez, J. A., and Gomez-Gomez, L. (2005). Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 139, 674–689. doi: 10.1104/pp.105.067827
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, J. (2012). Studies on the Effects of Structure and GA3 Treatments on Flower Coloration of Gerbera Jamesonii. MS thesis, Agricultural University of Hunan, Changsha.
Chen, L., Sun, Z. F., Li, M., Xu, K., and Yang, X. (2000). Quality evaluation standard of cut flower and the influence of growth conditions before harvest on cut flower. North. Hortic. 1, 40–42.
Chen, S. M., Li, C. H., Zhu, X. R., Deng, Y. M., Sun, W., Wang, L. S., et al. (2012). The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 56, 458–464. doi: 10.1007/s10535-012-0069-3
Cheynier, V., Comte, G., Davies, K. M., Lattanzio, V., and Martens, S. (2013). Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Bioch. 72, 1–20. doi: 10.1016/j.plaphy.2013.05.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chmiel, E., Sutfeld, R., and Wiermann, R. (1983). Conversion of phloroglucinol-type chalcones by purified chalcone isomerase from Tulip anthers and from cosmos petals. Biochem. Physiol. Pflanz. 178, 139–146. doi: 10.1016/S0015-3796(83)80027-4
Cocciolone, S. M., Chopra, S., Flint-Garcia, S. A., McMullen, M. D., and Peterson, T. (2001). Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant J. 27, 467–478. doi: 10.1046/j.1365-313X.2001.01124.x
Das, O. P., and Messing, J. (1994). Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics 136, 1121–1141.
Davies, K. M., Albert, N. W., and Schwinn, K. E. (2012). From landing lights to mimicry: the molecular regulation of flower colouration and mechanisms for pigmentation patterning. Func. Plant Biol. 39, 619–638. doi: 10.1071/FP12195
Dela, G., Or, E., Ovadia, R., Nissim-Levi, A., Weiss, D., and Oren-Shamir, M. (2003). Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high-temperature conditions. Plant Sci. 164, 333–340. doi: 10.1016/S0168-9452(02)00417-X
Dehghan, S., Sadeghi, M., Poppel, A., Fischer, R., Lakes-Harlan, R., Kavousi, H. R., et al. (2014). Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci. Rep. 34:e00114. doi: 10.1042/BSR20140026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Di, S., Martin, C., Schulfer, A. F., and Connelly, C. F. (2009). An ortholog of MIXTA-like2 controls epidermal cell shape in flowers of Thalictrum. New Phytol. 183, 718–728. doi: 10.1111/j.1469-8137.2009.02945.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dwivedi, A. K., Banerji, B. K., Chakrabarty, D., Mandal, A. K. A., and Datta, S. K. (2000). Gamma ray induced new flower colour chimera and its management through tissue culture. Indian J. Agric. Sci. 70, 853–855.
Espley, R. V., Brendolise, C., Chagne, D., Kutty-Amma, S., Green, S., Volz, R., et al. (2009). Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21, 168–183. doi: 10.1105/tpc.108.059329
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Felker, P., Stintzing, F. C., Müssig, E., Leitenberger, M., Carle, R., Vogt, T., et al. (2008). Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 152, 307–318. doi: 10.1111/j.1744-7348.2008.00222.x
Feng, S. Q., Wang, Y. L., Yang, S., Xu, Y., and Chen, X. (2010). Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 232, 45–255. doi: 10.1007/s00425-010-1170-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fumi, T., Norio, S., Kenjiro, T., and Koichi, S., and Toshio, H. (2012). Flower colors and their anthocyanins in Matthiola incana cultivars (Brassicaceae). J. Jpn. Soc. Hortic. Sci. 81, 91–100. doi: 10.2503/jjshs1.81.91
Gandía-Herrero, F., and García-Carmona, F. (2013). Biosynthesis of betalains: yellow and violet plant pigments. Trends Plant Sci. 18, 334–343. doi: 10.1016/j.tplants.2013.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gandía-Herrero, F., García-Carmona, F., and Escribano, J. (2005). Botany: floral fluorescence effect. Nature 437, 334. doi: 10.1038/437334a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Han, H., and Gong, W. (2010). Effect of different soil pH on autumn leaf color change of Acer pseudosieboldianum. Jilin Agric. 6, 76–80.
Han, Y. J., Wang, X. H., Chen, W. C., Dong, M. F., Yuan, W. J., Liu, X., et al. (2014). Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes 10, 329–338. doi: 10.1007/s11295-013-0687-8
Han, Y. Y., Ming, F., Wang, J. W., Wen, J. G., Ye, M. M., and Shen, D. L. (2006). Cloning and characterization of a novel chalcone synthase gene from Phalaenopsis hybrida orchid flowers. Russ. J. Plant Physiol. 53, 223–230. doi: 10.1134/S1021443706020129
Hara, M., Oki, K., Hoshino, K., and Kuboi, T. (2003). Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyls. Plant Sci. 164, 259–265. doi: 10.1016/S0168-9452(02)00408-9
Hase, Y., Okamura, M., Takeshita, D., Narumi, I., and Tanaka, A. (2010). Efficient induction of flower-color mutants by ion beam irradiation in petunia seedlings treated with high sucrose concentration. Plant Biotechnol. 27, 99–103. doi: 10.5511/plantbiotechnology.27.99
He, Q., Shen, Y., Wang, M., Huang, M., Yang, R., Zhu, S., et al. (2011). Natural variation in petal color in Lycoris longituba revealed by anthocyanin components. PLoS ONE:6e22098. doi: 10.1371/journal.pone.0022098
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heller, W., Forkmann, G., Britsch, L., and Grisebach, H. (1985). Enzymatic reduction of (+)-dihydroflavonols to flavan-3 4-cis-diols with flower extracts from Matthiola incana and its role in anthocyanin biosynthesis. Planta 165, 284–287. doi: 10.1007/BF00395052
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hieber, A. D., Mudalige-Jayawickrama, R. G., and Kuehnle, A. R. (2006). Color genes in the orchid Oncidium gower ramsey: identification expression and potential genetic instability in an interspecific cross. Planta 223, 521–531. doi: 10.1007/s00425-005-0113-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, K., Meng, L., Han, K., Sun, Y., and Dai, S. (2009). Isolation and expression analysis of key genes involved in anthocyanin biosynthesis of cineraria. Acta Hortic. Sin. 36, 1013–1022.
Huang, K. L., Miyajima, I., and Okubo, H. (2000). Effects of temperature and shade treatment on flower colors and characteristics in newly established reddish-purple tuberose (Polianthes). J. Fac. Agric. Kyushu Univ. 45, 57–63.
Jin, Z. P., Grotewold, E., Qu, W. Q., Fu, G., and Zhao, D. (2005). Cloning and characterization of a flavanone 3-hydroxylase gene from Saussurea medusa. DNA Seq. 16, 21–129. doi: 10.1080/10425170500050742
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
KangMo, L., TaeYong, J., JuYeon, S., and ByoungRyong, J. (2007). Flower color change of Boronia heterophylla as affected by light intensity and preservative chemicals. Korean J. Hortic. Sci. Technol. 25, 458–462.
Katsumoto, Y., Fukuchi-Mizutani, M., Fukui, Y., Brugliera, F., Holton, T. A., Karan, M., et al. (2007). Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-Hued flowers accumulating delphinidin. Plant Cell Physiol. 48, 1589–1600. doi: 10.1093/pcp/pcm131
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kazuma, K., Noda, N., and Suzuki, M. (2003). Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 64, 1133–1139. doi: 10.1016/S0031-9422(03)00504-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kobayashi, S., Goto-Yamamoto, N., and Hirochika, H. (2004). Retrotransposon-induced mutations in grape skin color. Science 304, 982. doi: 10.1126/science.1095011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kugler, F., Stintzing, F. C., and Carle, R. (2007). Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globasa L. and Bougainvillea sp. By HPLC-DAD-ESI-MSn. Anal. Bioanal. Chem. 387, 637–648. doi: 10.1007/s00216-006-0897-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lai, Y. S., Yamagishi, M., and Suzuki, T. (2011). Elevated temperature inhibits anthocyanin biosynthesis in the tepals of an Oriental hybrid lily via the suppression of LhMYB12 transcription. Sci. Hortic. 132, 59–65. doi: 10.1016/j.scienta.2011.09.030
Li, H. Q., and Liu, S. J. (1998). Effects of light intensity and illumination time on leaf colour variations of coloured leaf trees. Bull. Bot. Res. 18, 194–205.
Li, H., Qiu, J., Chen, F., Lv, X., Fu, C., Zhao, D., et al. (2012). Molecular characterization and expression analysis of dihydroflavonol 4-reductase (DFR) gene in Saussurea medusa. Mol. Biol. Rep. 39, 2991–2999. doi: 10.1007/s11033-011-1061-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, M. R., Chen, J. T., Sun, Z. J., Chen, Y. Z., and Li, H. Q. (2003). Advances in molecular breeding of ornamental plants. J. Trop. Subtrop. Bot. 11, 87–92.
Li, R. H., and Fang, Z. (2006). Effects of aluminium on growth and flower color of impatiens hawkeri. J. Agric. Univ. Hebei 29, 32–36.
Li, R. H., Fang, Z., and Bi, S. Q. (2005). Effects of copper on growth and flower color of Impatiens hawkeri. J. Agric. Univ. Hebei 28, 61–64.
Li, Y. F., Li, Y. H., Wang, Z. H., Guan, N., Feng, C. J., and Yang, J. M. (2009). Effect of soil drought stress on leaf coloration-emerging of Prunus cistenena cv. Pissardii. Acta Ecol. Sin. 29, 3678–3684.
Liu, A., Wang, L., Wang, Q., and Pang, C. (2011). Study on the effect of flower color of Lupinus polyphyllus in different soil pH. Chin. Agric. Sci. Bull. 27, 125–129.
Liu, H., Cui, C., Gao, Y., Liu, Y., Yang, F., and Zhang, T. (2009). Effect of Fe2+ on several physiological indices related to the nutrition state in the petals and the color of flowers of chrysanthemum florescence. Hubei Agric. Sci. 48, 1678–1680.
Liu, M., Li, X., Liu, Y., and Cao, B. (2013). Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Bioch. 73, 161–167. doi: 10.1016/j.plaphy.2013.09.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Markham, K. R., Ryan, K. G., Gould, K. S., and Rickards, G. K. (2000). Cell wall sited flavonoids in lisianthus flower petals. Phytochemistry 54, 681–687. doi: 10.1016/S0031-9422(00)00180-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Masayoshi, N., Natsu, T., Yasumasa, M., and Yusuke, B. (2012). Comprehensive analyses of anthocyanin and related compounds to understand flower color change in ion-beam mutants of Cyclamen (Cyclamen spp.) and carnation (Dianthus caryophyllus). Plant Biotechnol. 29, 215–221. doi: 10.5511/plantbiotechnology.12.0102a
Moehs, C. P., Tian, L., Osteryoung, K. W., and Dellapenna, D. (2001). Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 45, 281–293. doi: 10.1023/A:1006417009203
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Momonoi, K., Yoshida, K., Mano, S., Takahashi, H., Nakamori, C., Shoji, K., et al. (2009). A vacuolar iron transporter in Tulip TgVit1 is responsible for blue coloration in petal cells through iron accumulation. Plant J. 59, 437–447. doi: 10.1111/j.1365-313X.2009.03879.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Morgret, M. L., Huang, G. H., and Huang, J. K. (2005). DNA sequence analysis of three clones containing chalcone synthase gene of petunia hybrida. FASEB J. 19, 303–303.
Nakatsuka, A., Izumi, Y., and Yamagishi, M. (2003). Spatial and temporal expression of chalcone synthase and dihydroflavonol 4-reductase genes in the asiatic hybrid lily. Plant Sci. 166, 759–767. doi: 10.1016/S0168-9452(03)00254-1
Nakatsuka, T., Haruta, K. S., Pitaksutheepong, C., Abe, Y., Kakizaki, Y., Yamamoto, K., et al. (2008). Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers. Plant Cell Physiol. 49, 1818–1829. doi: 10.1093/pcp/pcn163
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakatsuka, T., Nishihara, M., Mishiba, K., and Yamamura, S. (2005). Temporal expression of flavonoid biosynthesis related genes regulates flower pigmentation in gentian plants. Plant Sci. 168, 1309–1318. doi: 10.1016/j.plantsci.2005.01.009
Neta, I., Shoseyov, O., and Weiss, D. (2000). Sugars enhance the expression of gibberellin-induced genes in developing petunia flowers. Physiol. Plant. 109, 196–202. doi: 10.1034/j.1399-3054.2000.100212.x
Ngaki, M. N., Louie, G. V., Philippe, R. N., Manning, G., Pojer, F., Bowman, M. E., et al. (2011). Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 7399, 530–533. doi: 10.1038/nature11009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nishihara, M., and Nakatsuka, T. (2011). Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol. Lett. 33, 433–441. doi: 10.1007/s10529-010-0461-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nishihara, M., Nakatsuka, T., and Yamamura, S. (2005). Flavonoid components and flower color change in transgenic tobacco plants by suppression of chalcone isomerase gene. FEBS Lett. 579, 6074–6078. doi: 10.1016/j.febslet.2005.09.073
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Niu, S. S., Xu, C. J., Zhang, W. S., Zhang, B., Li, X., Lin-Wang, K., et al. (2010). Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 231, 887–899. doi: 10.1007/s00425-009-1095-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noda, K., Glover, B. J., Linstead, P., and Martin, C. (1994). Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 369, 661–664. doi: 10.1038/369661a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nozaki, K., Takamura, T., and Fukai, S. (2006). Effects of high temperature on flower colour and anthocyanin content in pink flower genotypes of greenhouse chrysanthemum (Chrysanthemum morifolium Ramat.). J. Hortic. Sci. Biotechnol. 81, 728–734.
Ohmiya, A., Kishimoto, S., Aida, R., Yoshioka, S., and Sumitomo, K. (2006). Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 142, 1193–1201. doi: 10.1104/pp.106.087130
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Owens, D. K., Crosby, K. C., Runac, J., Howard, B. A., and Winkel, B. S. (2008). Biochemical and genetic characterizat ion of Arabidopsis flavanone 3beta-hydroxylase. Plant Physiol. Biochem. 46, 833–843. doi: 10.1016/j.plaphy.2008.06.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pattanalk, S., Kong, Q., Zaitlin, D., Werkman, J. R., Xie, C. H., Patra, B., et al. (2010). Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta 231, 1061–1076. doi: 10.1007/s00425-010-1108-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Petit, P., Granier, T., d’Estaintot, B. L., Manigand, C., Bathany, K., Schmitter, J. M., et al. (2007). Crystal structure of grape dihydroflavonol 4-reductase a key enzyme in flavonoid biosynthesis. J. Mol. Biol. 368, 1345–1357. doi: 10.1016/j.jmb.2007.02.088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Petroni, K., and Tonelli, C. (2011). Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 181, 3219–229. doi: 10.1016/j.plantsci.2011.05.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pina, F. (2014). Chemical applications of anthocyanins and related compounds. A source of bioinspiration. J. Agr. Food Chem. 62, 6885–6897. doi: 10.1021/jf404869m
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Qi, Y., Lou, Q., Li, H., Yue, J., Liu, Y., and Wang, Y. (2013). Anatomic al and biochem ical studies of bicolored flower development in Muscari latifolium. Protoplasma 250, 1273–1281. doi: 10.1007/s00709-013-0509-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Quattrocchio, F., Wing, J., van der Woude, K., Souer, E., de Vetten, N., Mol, J., et al. (1999). Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 11, 1433–1444. doi: 10.1105/tpc.11.8.1433
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ramsay, N. A., and Glover, B. J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10, 63–70. doi: 10.1016/j.tplants.2004.12.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reimold, U., Kroeger, M., Kreuzaler, F., and Hahlbrock, K. (1983). Coding and 3’ non-coding nucleotide sequence of chalcone synthase messenger RNA and assignment of amino acid sequence of the enzyme. EMBO J. 2, 1801–1806.
Robbins, M. L., Wang, P. H., Sekhon, R. S., and Chopra, S. (2009). Gene structure induced epigenetic modifications of pericarp color1 alleles of maize result in tissue-specific mosaicism. PLoS ONE 4:e8231. doi: 10.1371/journal.pone.0008231
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Roepke, J., Jean, T., Perkel, K. J., Blom, T., and Bozzo, G. G. (2013). Daminozide alters anthocyanin metabolism in ray florets of bronze chrysanthemum (Chrysanthemum morifolium Ramat.). J. Plant Growth Regul. 32, 453–460. doi: 10.1007/s00344-012-9315-3
Roosa, A. E., Laitinen, M., Ainasoja, M., Teeri, T. H., and Elomaa, P. (2008). Identification of target genes for a MYB-type anthocyanin regulator in Gerbera hybrida. J. Exp. Bot. 59, 3691–3703. doi: 10.1093/jxb/ern216
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rosati, C., Cadic, A., Duron, M., Ingouff, M., and Simoneaub, P. (1999). Molecular characterization of the anthocyanidin synthase gene in Forsythia × intermedia reveals organ-specific expression during flower development. Plant Sci. 149, 73–79. doi: 10.1016/S0168-9452(99)00146-6
Sablowski, R. W., Moyano, E., Culianez-Macia, F. A., Schuch, W., Martin, C., and Bevan, M. (1994). A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 13, 128–137.
Schmitzer, V., Veberic, R., Osterc, G., and Stampar, F. (2010). Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 135, 195–202.
Schmitzer, V., Veberic, R., and Stampar, F. (2012). Prohexadione-Ca application modifies flavonoid composition and color characteristics of rose (Rosa hybrida L. flowers). Sci. Hortic. 146, 14–20. doi: 10.1016/j.scienta.2012.07.035
Schroder, J. (1997). A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 10, 373–378. doi: 10.1016/S1360-1385(97)87121-X
Shimizu, K., Ohnishi, N., Morikawa, N., Ishigami, A., Otake, S., Rabah, I. O., et al. (2011). A 94-bp deletion of anthocyanidin synthase gene in acyanic flower lines of lisianthus [Eustoma grandiflorum (Raf.) Shinn.]. J. Jpn. Soc. Hortic. Sci. 80, 434–442. doi: 10.2503/jjshs1.80.434
Shoji, K., Miki, N., Nakajima, N., Momonoi, K., Kato, C., and Yoshida, K. (2007). Perianth bottom-specific blue color development in Tulip cv. murasakizuisho requires ferric ions. Plant Cell Physiol. 48, 243–251. doi: 10.1093/pcp/pcl060
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Solfanelli, C., Poggi, A., Loreti, E., Alpi, A., and Perata, P. (2006). Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 140, 637–646. doi: 10.1104/pp.105.072579
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stiles, E. A., Cech, N. B., Dee, S. M., and Lacey, E. P. (2007). Temperature-sensitive anthocyanin production in flowers of Plantago lanceolata. Physiol. Plant. 129, 56–765. doi: 10.1111/j.1399-3054.2007.00855.x
Strack, D., Vogt, T., and Schliemann, S. (2003). Recent advances in betalain research. Phytochemistry 62, 247–269. doi: 10.1016/S0031-9422(02)00564-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, B., Liu, X. D., and Zhen, D. C. (2008). Response of leaf color change of Acer pseudoo-sieboldianum to soil acidification with FeSO4. J. Northeast Agric. Univ. 36, 51–52, 58.
Sun, L. L., Li, Y., Li, S. S., Wu, X. J., Hu, B. Z., and Chang, Y. (2014). Identification and characterisation of DfCHS, a chalcone synthase gene regulated by temperature and ultraviolet in Dryopteris fragrans. Cell. Mol. Biol. 60, 1–7.
Sun, W., Li, C., Wang, L., Dai, S., and Xu, Y. (2009). Anthocyanins present in flowers of Senecio cruentus with different colors. Acta Hortic. Sin. 36, 1775–1782.
Sun, W., Meng, X., Liang, L., Jiang, W., Huang, Y., He, J., et al. (2015). Molecular and biochemical analysis of chalcone synthase from Freesia hybrid in flavonoid biosynthetic pathway. PLoS ONE 10:e0119054. doi: 10.1371/journal.pone.0119054
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, Z., Han, M., Zhai, X., Wu, Z., Yu, Q., Xue, J., et al. (2006). Effect of number of short-day on flowering and visual quality of poinsettia. Acta Hortic. Sin. 33, 583–586.
Suzuki, S., Nishihara, M., Nakatsuka, T., Misawa, N., Ogiwara, I., and Yamamura, S. (2007). Flower color alteration in Lotus japonicus by modification of the carotenoid biosynthetic pathway. Plant Cell Rep. 26, 951–959. doi: 10.1007/s00299-006-0302-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tai, D., Tian, J., Zhang, J., Song, T., and Yao, Y. (2014). A Malus crabapple chalcone synthase gene, McCHS, regulates red petal color and flavonoid biosynthesis. PLoS ONE 9:e110570. doi: 10.1371/journal.pone.0110570
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takashi, N., Kei-ichiro, M., Akiko, K., Yoshiko, A., Saburo, Y., Noriko, N., et al. (2010). Genetic engineering of novel flower colour by suppression of anthocyanin modification genes in gentian. J. Plant Physiol. 167, 231–237. doi: 10.1016/j.jplph.2009.08.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takos, A. M., Jaffé, F. W., Jacob, S. R., Bogs, J., Robinson, S. P., and Walker, A. R. (2006). Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 142, 1216–1232. doi: 10.1104/pp.106.088104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanaka, Y., Brugliera, F., and Chandler, S. (2009). Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 10, 5350–5369. doi: 10.3390/ijms10125350
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thill, J., Miosic, S., Ahmed, R., Schlangen, K., Muster, G., Stich, K., et al. (2012). ‘Le Rouge et le Noir’: a decline in flavone formation correlates with the rare color of black dahlia (Dahlia variabilis hort.) flowers. BMC Plant Biol. 12:225. doi: 10.1186/1471-2229-12-225
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Uddin, A., Hashimoto, F., Kaketani, M., Shimizu, K., and Sakata, Y. (2001). Analysis of light and sucrose potencies on petal coloration and pigmentation of lisianthus cultivars (in vitro). Sci. Hortic. 89, 73–82.
Veitch, N. C., and Grayer, R. J. (2008). Flavonoids and their glycosides including anthocyanins. Nat. Prod. Rep. 25, 555–611. doi: 10.1039/b718040n
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Verweij, W., Spelt, C., Di Sansebastiano, G. P., Vermeer, J., Reale, L., Ferranti, F., et al. (2008). An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat. Cell Biol. 10, 1456–1462. doi: 10.1038/ncb1805
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vignolini, S., Moyroud, E., Hingant, T., Banks, H., Rudall, P. J., Steiner, U., et al. (2015). The flower of Hibiscus trionum is both visibly and measurably iridescent. New Phytologist. 205, 97–101. doi: 10.1111/nph.12958
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Weiss, D., vanderLuit, A., Knegt, E., Vermeer, E., Mol, J., and Kooter, J. M. (1995). Identification of endogenous gibberellins in petunia flowers (induction of anthocyanin biosynthetic gene expression and the antagonistic effect of abscisic acid). Plant Physiol. 107, 695–702.
Wellmann, F., Griesser, M., Schwab, W., Martens, S., Eisenreich, W., Matern, U., et al. (2006). Anthocyanidin synthase from Gerbera hybrida catalyzes the conversion of (+)-catechin to cyanidin and a novel procyanidin. FEBS Lett. 580, 1642–1648. doi: 10.1016/j.febslet.2006.02.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xie, D. Y., Sharma, S. B., Wright, E., Wang, Z. Y., and Dixon, R. A. (2006). Metabolic engineering of proanthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. Plant J. 45, 895–907. doi: 10.1111/j.1365-313X.2006.02655.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, Y., Feng, S., Jiao, Q., Liu, C., Zhang, W., Chen, W., et al. (2012). Comparison of MdMYB1 sequences and expression of anthocyanin biosynthetic and regulatory genes between Malus domestica Borkh. cultivar ‘Ralls’ and its blushed sport. Euphytica 185, 157–170. doi: 10.1007/s10681-011-0494-y
Yamagishi, M., Shimoyamada, Y., Nakatsuka, T., and Masuda, K. (2010). Two R2R3-MYB genes homologs of petunia AN2 regulate anthocyanin biosyntheses in flower tepals tepal spots and leaves of asiatic hybrid lily. Plant Cell Physiol. 51, 463–474. doi: 10.1093/pcp/pcq011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, Y., Zhang, J., Liao, W., and Han, M. (2012). Effects of foliage spray on growth and florescence of climbing rose Anjila. J. Gansu Agric. Univ. 1, 69–72.
Yoshida, K., Kitahara, S., Ito, D., and Kondo, T. (2006). Ferric ions involved in the flower color development of the himalayan blue poppy Meconopsis grandis. Phytochemistry 67, 992–998. doi: 10.1016/j.phytochem.2006.03.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoshida, K., Kondo, T., Okazaki, Y., and Katou, K. (1995). Cause of blue petal colour. Nature 373, 291. doi: 10.1038/373291a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoshida, K., Miki, N., Momonoi, K., Kawachi, M., Katou, K., Okazaki, Y., et al. (2009). Synchrony between flower opening and petal-color change from red to blue in morning glory Ipomoea tricolor cv. heavenly blue. Proc. Jpn. Acad. Ser. B 85, 187–197. doi: 10.2183/pjab.85.187
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Young, H. K., Hwan, C. J., Sun, K. K., and Yang, H. K. (1997). Effects of light quality on growth and flowering of Hibiscus syriacus L. J. Korean Soc. Horti. Sci. 38, 272–277.
Zhang, C., Fu, J., Wang, Y., Gao, S., Du, D., Wu, F., et al. (2015). Glucose supply improves petal coloration and anthocyanin biosynthesis in Paeonia suffruticosa ‘Luoyang Hong’ cut flowers. Postharvest Biol. Tec. 101, 73–81. doi: 10.1016/j.postharvbio.2014.11.009
Zhang, F., Gonzalez, A., Zhao, M. Z., Payne, C. T., and Lloyd, A. (2003). A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130, 4859–4869. doi: 10.1242/dev.00681
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, J., Wang, L., Gao, J., Li, S., Xu, Y., Li, C., et al. (2011). Identification of anthocyanins involving in petal coloration in Chaenomeles speciosa cultivars. Acta Hortic. Sin. 38, 527–534.
Zhao, D. Q., Hao, Z. J., and Tao, J. (2012a). Effects of shade on plant growth and flower quality in herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Biochem. 61, 187–196. doi: 10.1016/j.plaphy.2012.10.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, D. Q., Tao, J., Han, C. X., and Ge, J. T. (2012b). Flower color diversity revealed by differential expression of flavonoid biosynthetic genes and flavonoid accumulation in herbaceous peony (Paeonia lactiflora Pall.). Mol. Biol. Rep. 39, 11263–11275. doi: 10.1007/s11033-012-2036-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, D. Q., Hao, Z. J., Wang, J., and Tao, J. (2013). Effects of pH in irrigation water on plant growth and flower quality in herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. 154, 45–53. doi: 10.1016/j.scienta.2013.02.023
Zhao, D. Q., Jiang, Y., Ning, C. L., Meng, J. S., Lin, S. S., Ding, W., et al. (2014). Transcriptome sequencing of a chimaera reveals coordinated expression of anthocyanin biosynthetic genes mediating yellow formation in herbaceous peony (Paeonia lactiflora Pall.). BMC Genomics 15:689. doi: 10.1186/1471-2164-15-689
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, X., Sheng, F., Zheng, J., and Liu, R. (2011). Composition and stability of anthocyanins from purple Solanum tuberosum and their protective influence on Cr(VI) targeted to bovine serum albumin. J. Agr. Food Chem. 59, 7902–7909. doi: 10.1021/jf2011408
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zheng, Y., Tian, L., Liu, H., Pan, Q., Zhan, J., and Huang, W. (2009). Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 58, 251–260. doi: 10.1007/s10725-009-9373-0
Zhi, W. T., Zhao, C. L., Chen, Z. J., Miao, K. R., Chen, W. L., and Mao, L. X. (2012). Determination of the higher plant organ colors by anthocyanins and its influence factors. J. Trop. Subtrop. Bot. 20, 303–310.
Zhou, L., Wang, Y., Ren, L., Shi, Q. Q., Zheng, B. Q., Miao, K., et al. (2014). Overexpression of Ps-CHI1 a homologue of the chalcone isomerase gene from tree peony (Paeonia suffruticosa) reduces the intensity of flower pigmentation in transgenic tobacco. Plant Cell Tissue Organ Cult. 116, 285–295. doi: 10.1007/s11240-013-0403-2
Zhu, C., Bai, C., Sanahuja, G., Yuan, D., Farré, G., Naqvi, S., et al. (2010). The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 504, 132–141. doi: 10.1016/j.abb.2010.07.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhu, M., Zheng, X., Shu, Q., Li, H., Zhong, P., Zhang, H., et al. (2012). Relationship between the composition of flavonoids and flower colors variation in tropical water lily (Nymphaea) cultivars. PLoS ONE 7:e34335. doi: 10.1371/journal.pone.0034335
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: flavonoids, anthocyanidins, ornamental plants, physical and chemical factors, genetic engineering
Citation: Zhao D and Tao J (2015) Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 6:261. doi: 10.3389/fpls.2015.00261
Received: 29 January 2015; Accepted: 02 April 2015;
Published online: 27 April 2015
Edited by:
Stefan De Folter, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, MexicoReviewed by:
Kevin Davies, New Zealand Institute for Plant and Food Research, New ZealandMarcelo Carnier Dornelas, Universidade Estadual de Campinas, Brazil
Copyright © 2015 Zhao and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Tao, Key Laboratory of Crop Genetics and Physiology of Jiangsu Province, College of Horticulture and Plant Protection, Yangzhou University, Yangzhou 225009, China taojun@yzu.edu.cn
 Daqiu Zhao
Daqiu Zhao Jun Tao
Jun Tao