- 1Department of Clinical Psychology, Institute of Psychology, University of Szczecin, Szczecin, Poland
- 2Department of Psychiatry, Pomeranian Medical University, Szczecin, Poland
- 3Independent Clinical Psychology Unit, Department of Psychiatry, Pomeranian Medical University, Szczecin, Poland
- 4Department of Psychiatry, Medical University of Warsaw, Warsaw, Poland
Objectives: Although it has been shown that there are more profound deficits present in deficit schizophrenia (DS) patients than in non-deficit schizophrenia (NDS) patients, there still remain some matters requiring further investigation. In this context, we formulated three research aims: (1) to compare executive functions between the investigated groups, (2) to determine the relationship between particular aspects of executive functions within the groups, and (3) to draw up a neuropsychological profile for executive functions.
Methods: The study involved 148 schizophrenia patients divided into two groups on the basis of the Schedule for the Deficit Syndrome: DS (n = 70) and NDS (n = 78). Patients were matched for sex, age, years of education, and overall cognitive functioning. For assessing executive functions we used the Wisconsin Card Sorting Test (WCST), the Trail Making Test (TMT), the Phonemic Verbal Fluency Test (VFT P), the Stroop Color and Word Test (SCWT), and the Go/No Go task (GNG).
Results: Deficit schizophrenia patients scored lower on the WCST and TMT (relative flexibility) than did the NDS patients. There were no inter-group differences in the VFT P, SCWT (relative inhibition), or GNG. There were significant correlations between WCST and TMT scores in both groups. The general neuropsychological profiles were similar in both groups.
Conclusion: Deficit schizophrenia patients exhibited slightly greater interference with concept formation and non-verbal cognitive flexibility. Therefore, such problems may be specific to this particular type of schizophrenia. These results may be useful for the development of neuropsychological diagnostic methods for patients with schizophrenia.
Introduction
There is an ongoing discussion about whether different types of schizophrenia are associated with specific types of executive dysfunction (Brazo et al., 2002; Simon et al., 2009; Fioravanti et al., 2012; Hegde et al., 2013; Ventura et al., 2013). The heterogeneity of schizophrenia symptoms has led to a distinction between different clinical syndromes within a single disease. The term ‘deficit schizophrenia’ was first suggested by Carpenter et al. (1988) as a type of schizophrenia with dominant negative symptoms persisting for a long time. Among these are persistent and primary negative symptoms such as social withdrawal, poverty of speech, limited content of verbal expression, apathy, and blunting of affect (Strauss et al., 2010). Longitudinal analyses show that these symptoms are stable over time (Tek et al., 2001; Chemerinski et al., 2006; Strauss et al., 2010). There are numerous reports confirming the validity of deficit schizophrenia (DS) diagnoses (Tek et al., 2001; Arango et al., 2004; Messias et al., 2004; Dickerson et al., 2006; Cohen et al., 2007; Galderisi et al., 2008; Kirkpatrick and Galderisi, 2008; Pełka-Wysiecka et al., 2013). However, apart from negative/deficit symptoms, the basic symptomatic dimensions in schizophrenia include also reality distortion and disorganization (Liddle et al., 1992; Schröder et al., 1992). The occurrence of the two latter types of symptoms may also be associated with executive function impairments.
The construct of executive functions has enabled a more insightful understanding of the self-regulatory processes responsible for the management of one’s thoughts, emotions, and behavior (Alvarez and Emory, 2006; Jurado and Rosselli, 2007; Diamond, 2013). In clinical neuropsychology, it has been assumed that they form a superordinate system which allows the implementation of purposeful action, and involves four domains: volition, planning, purposive action, and effective performance (Lezak, 1995; Jodzio, 2008). Many clinical and experimental studies have confirmed that these functions are carried out by a complex central executive network which includes a variety of brain structures, the most important of which are the prefrontal cortex, the anterior cingulate cortex, the subcortical nuclei, and the cerebellum (Stuss, 2011; Niendam et al., 2012; Yuan and Raz, 2014; Mak et al., 2016). Many studies suggest the presence of greater structural and functional disorders of the brain in DS patients than in their NDS counterparts (Liddle et al., 1992; Tamminga et al., 1992; DeQuardo et al., 1998; Heckers et al., 1999; Lahti et al., 2001). Based on these studies, Buchanan et al. (1994) and Kirkpatrick et al. (2001) asserted that malfunctioning of the loop created by the prefrontal cortex, the inferior parietal cortices, and the thalamus is implicated in the pathophysiology and executive dysfunctions of the deficit syndrome in schizophrenia.
As can be seen in Table 1, neuropsychological analyses of the executive functioning of patients with DS and non-deficit schizophrenia (NDS) yield somewhat inconsistent results. Polgár et al. (2010) showed that patients with DS achieved lower scores than those with NDS in specific measures of the Wisconsin Card Sorting Test (WCST). In addition, a factor analysis was performed, showing that there are at least two factors relating to mental processes engaged in this test. The first is concept formation and flexibility, and it includes, inter alia, Perseverative Responses (PR), and Perseverative Errors (PE). The second is unsuccessful problem-solving with an ineffective hypothesis-testing strategy and includes Non-perseverative Errors (NPE). Analysis of the results showed that only some DS patients obtained lower PE scores than did those with NDS (Table 1). Other reports found no inter-group differences (or differences in PR score, see Table 1). These particular scores are not considered at all in some papers. Furthermore, Wang et al. (2008) and Vogel et al. (2013) report some contradictory findings, as their subjects differed in terms of PE scores, but not PR scores. NPE scores were only considered in four papers, and only Réthelyi et al. (2012) found that patients with DS had lower scores than those with NDS. A review of research which used the Trail Making Test (TMT, version B) revealed that, in some papers, patients with DS scored lower than NDS patients. Unfortunately, only two papers reported patient scores for absolute non-verbal cognitive flexibility [time B – A], some independent of the speed of information processing (TMT AF, Chan et al., 2015). In the study of Wang et al. (2008), patients with DS obtained lower scores than those with NDS, while Galderisi et al. (2002) did not report any inter-group differences. A meta-analysis of research which used the Phonemic Verbal Fluency Test (VFT P) to measure verbal cognitive flexibility showed that DS patients scored lower than NDS patients in three studies, while in five others there were no reports of any inter-group differences. A review of studies which used the Stroop Color and Word Test (SCWT) showed that only in the study by Réthelyi et al. (2012) did DS patients score lower than NDS patients in the task of reading the names of colors printed in a color different (incongruent) to that denoted by the name. Cohen et al. (2007) found no inter-group differences. Buchanan et al. (1994) was the only study in which the interference index was applied, where reaction time was controlled for the congruent trial. The authors showed that DS patients exhibited higher (worse) scores than did the NDS patients. We could not find any available research on DS patients performing the Go/No Go task (GNG).
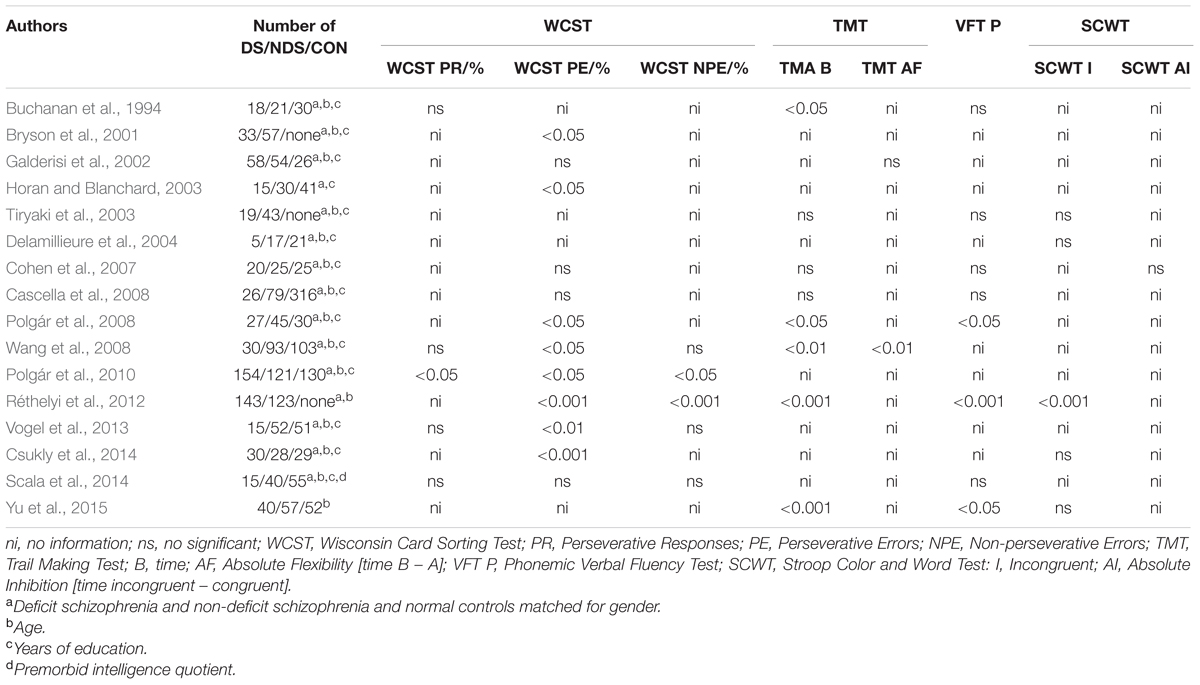
TABLE 1. Survey of studies on PubMed which test executive functions in deficit schizophrenia (DS) and non-deficit schizophrenia (NDS) patients, and normal controls (CON).
Furthermore, the specific relationship between the particular aspects of DS and NDS may prove important for understanding the nature of executive functions in DS/NDS patients, as demonstrated in research conducted on healthy persons (Miyake et al., 2000) and older subjects (McCabe et al., 2010; Brown et al., 2012). Unfortunately, such relationships have been very rarely examined in this group of patients. Only Yu et al. (2015) managed to demonstrate a significant correlation between scores on the TMT and VFT P in patients with DS. It may also be important to identify which aspects of executive function are most impaired in patients with DS and NDS. This is made possible by a profile analysis of neuropsychological function (Lezak et al., 2004; Voglmaier et al., 2005). Brazo et al. (2002) found the greatest disturbance in patients with DS in areas of concept formation (Modified Card Sorting Test, MCST) and verbal cognitive flexibility (VFT P), and their non-verbal cognitive flexibility (TMT) and cognitive inhibition (SCWT) were least affected. The aforementioned functions remained on a similar level in NDS patients. In turn, Cascella et al. (2008) demonstrated that DS patients exhibit the greatest difficulty with speed of information processing and verbal cognitive flexibility (VFT P), and they tend to do slightly better in concept formation (MCST), with a similar profile observed in both DS and NDS patients. However, Réthelyi et al. (2012) and Yu et al. (2015) showed that patients with DS and NDS have greater problems with regards to non-verbal flexibility (TMT), than with verbal cognitive flexibility (VFT P).
As can be seen in the above results, there are still a few unresolved issues concerning executive function in patients with DS. First of all, the precise nature of executive dysfunction in this group of patients has not been established. Secondly, it is not clear what is the relationship between various aspects of the executive function in those patients or whether there exists any at all. Also, it is not fully known which domains of the described processes suffer the greatest impairment within the group. Therefore, both the inconclusiveness of findings and the importance of executive functions for the performance of complex actions have led to formulation of three research aims: (1) to compare executive function performance between the investigated groups, (2) to determine the relationship between the particular aspects of executive functions within the groups, and (3) to draw up a neuropsychological profile for executive functions which takes into account the diversity of the different aspects of these processes.
Materials and Methods
Participants
The patient group consisted of 148 right-handed Caucasians (74 female and 74 male) who had been diagnosed with schizophrenia according to ICD-10 (World Health Organization [WHO], 1992) for a minimum of 18 months. Patient interviews were done by properly licensed psychiatrists. Among the inclusion criteria were the ability to understand the research procedure, being aged between 20 and 60, and having given informed consent. Exclusion criteria were other mental diseases, neurological diseases, dementia, a history of traumatic brain injury, and severe diseases of the parenchymal organs, a history of alcohol or drug misuse, or intellectual disability. With the construction of the study in mind, patients who exhibited clear symptoms of disorganization were also excluded. The patients were recruited from inpatient psychiatric wards, psychiatric daycare wards, and outpatient clinics in the Western Pomerania district of Poland. All subjects were fully informed about the aims and the protocol of the study and all gave written informed consent. The protocol was approved by the local bioethics committee.
Measures
Clinical Assessment
The presence of psychopathological symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987), and the Clinical Global Impression – Schizophrenia scale (CGI-SCH, Haro et al., 2003), which assessed four groups of symptoms (positive, negative, depressive, and cognitive) during a psychiatric examination. To describe the severity and type of deficit symptoms, we used a Polish translation of the Schedule for the Deficit Syndrome (SDS, Kirkpatrick et al., 1989). DS was diagnosed by the presence of the following negative symptoms: restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose, and diminished social drive. All the above symptoms had to be primary, i.e., not caused by positive symptoms such as depression, cognitive dysfunction, psychopharmacotherapy, or poor general health, and had to have been present for the preceding 12 months.
The patients were in symptomatic remission, not acute psychosis. All subjects were treated according to the guidelines for the psychopharmacological treatment of schizophrenia. In both groups the patients received typical (perazine, zuclopenthixol, haloperidol) or atypical (risperidone, olanzapine, clozapine, quetiapine, aripiprazole, amisulpride) antipsychotics. The DS and NDS groups did not differ in terms of type of neuroleptics used.
Neuropsychological Assessment
In this study we used the WCST in its original computerized form (Heaton et al., 1993; Jaworowska, 2002). Based on data collected by Polgár et al. (2010), we decided to measure concept formation using two scores: PE and PR, and to assess problem-solving using NPE. The subject’s task was to discover the rule that is currently in place (color, shape or number) and answer by pressing the right key on the keyboard, from 1 to 4 based on the feedback (correct or incorrect) displayed on a 15″ screen. Before the test, each participant received instructions from a psychologist. For the assessment of non-verbal cognitive flexibility, we used the TMT (Reitan, 1958). However, bearing in mind that DS and NDS patients’ speed of information processing is generally slower (Morrens et al., 2007), we decided to use the Relative Flexibility indicator (TMT), applying the formula: [(time B - A/time B) × 100] (Stuss et al., 2001; Perianez et al., 2007). In TMT A, subjects had to connect 25 circles containing numbers from 1 to 25, which were irregularly placed on a white, A4 sheet, with a continuous line, as quickly as possible. TMT B consisted of connecting circles, going by turns from number to letter, while preserving the order of numbers and following alphabet (from 1 to A, from A to 2, etc.), finishing at number “13” and the letter “L.” A practice trial was done before each task so that the investigator could be sure that the patient understood the instructions. Instructions were provided verbally by the investigator (psychologist) both before the practice task and the actual task. In turn, to assess verbal cognitive flexibility, we administered the VFT P (Lezak, 1995; Tyburski et al., 2015). Each individual was asked to list as many words as they can, as fast as possible, according to the given criterion (words beginning with k or p). The time for completing each trial was 60 s. The researcher wrote down each word on an answer sheet. Since it has been demonstrated that the number of correctly spoken words strongly correlates with the number of word switches, this indicator was considered to be a good measure of verbal cognitive flexibility (Ross, 2003). We also assessed cognitive inhibition (dominant verbal response) by means of the SCWT. However, because patients with DS exhibit slowing of information processing (Morrens et al., 2007), we decided to use the Relative Inhibition Indicator (SCWT RI) in the formula: [(time incongruent - congruent/time congruent) × 100] (Denney and Lynch, 2009). In the first task, the subject had to read aloud as fast as possible the names of colors printed in a black font on a white A4 sheet. In the second task, the subject had to name the colors of words printed in a colored font, where the font color was incongruent with the word’s meaning (e.g., the word “green” printed in red). Instructions were provided verbally each time by the investigator (psychologist) before the task. The computer version of the GNG was also used and motor inhibition was measured with the number of No Go type errors (Strauss et al., 2006; Wright et al., 2014). The subject’s task was to press the spacebar on the keyboard when a green square appeared on the computer screen (15″), and to refrain from pressing the spacebar when a blue square appeared on the screen. Instructions were presented on the computer screen before the task.
Procedure
At their first appointment, all patients were examined by one of four psychiatrists who carried out a structured interview and assessment based on clinical scales (each patient was evaluated using the PANSS, CGI-SCH, and SDS). The psychiatrists were members of the research team and had been trained in the research procedure, including the use of the psychiatric scales. The next appointment involved neuropsychological assessment, carried out by one of three trained psychologists. All patients were examined with the same neuropsychological battery. Administration of each tool was preceded by the standard instructions.
Statistical Analysis
Statistical analysis of the results was done using the IBM SPSS 21 Statistical package. Continuous variables were presented as means (M) and standard deviations (SD) or standard errors (SE). The normality of the distribution was tested with the Shapiro–Wilk test. Before any analyses were conducted, square root transformation was used to transform the raw results of variables which were not normally distributed. Then selected scores were transformed into unitarized results using the formula xu = [(xi - min)/(max - min) × 100] (ranges from 0 to 100, the higher the score, the more difficult the task). To check for differences between the groups, the non-parametric Mann–Whitney U-test (for demographic and clinical variables) or parametric Student’s t-tests were used (for neuropsychological variables). The Wendt rU rank-biserial correlation method (Wendt, 1972; Rosenthal and Rubin, 2003) was used to determine the magnitude of effect size measures for the non-parametric tests and Cohen’s d or η2 effect size (Cohen, 1992) was used to determine the magnitude of effect size measures for the parametric test and analysis of variance (ANOVA). For multiple comparisons the Bonferroni correction was used. To assess the strength of the relationship between different aspects of executive functioning, Pearson’s r correlation coefficient was used. To draw up the executive function profile and compare the results from different neuropsychological tests, we used a repeated measures/mixed model ANOVA. We assumed the group type (DS or NDS) as the inter-object factor, and the aspect of executive function (the type of measure) as an intra-object 7-level factor scale.
Results
Subjects’ Characteristics
The patients’ socio-demographic and clinical characteristics are shown in Table 2. Neither investigated group differed in terms of number of years of education, gender, length of time since diagnosis, level of general mental functioning (assessed with MMSE), or number of hospitalizations at psychiatric wards. DS patients had higher scores than non-deficit patients on all PANSS (p < 0.001) and SGI-SCH subscales (p < 0.001). The effect size (rU) was found to be 0.25–0.74, i.e., a small to large effect size.
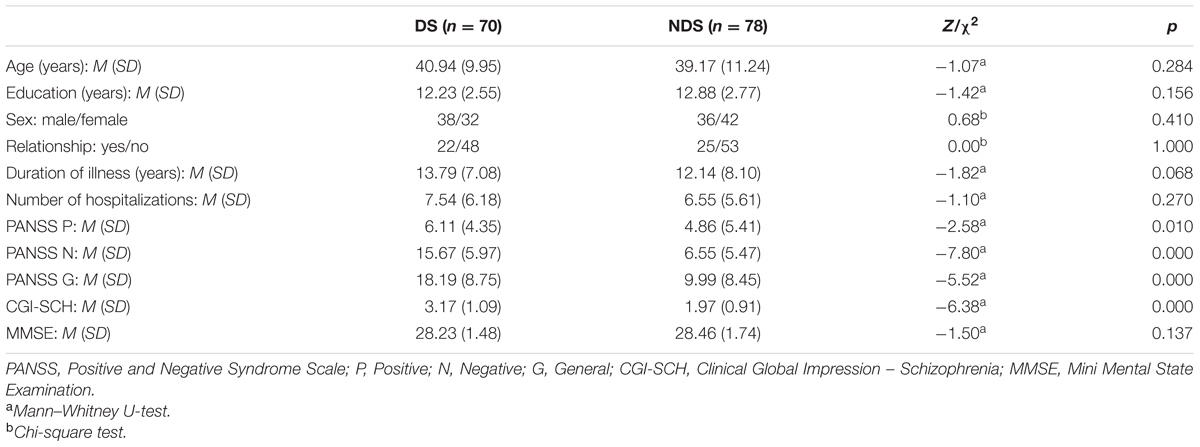
TABLE 2. Demographic and clinical characteristics of deficit schizophrenia (DS) and non-deficit schizophrenia (NDS) patients.
Performance in Specific Aspects of Executive Functions
As shown in Table 3, DS patients scored lower in concept formation (WCST PR: p < 0.05; WCST PE: p < 0.05) and non-verbal cognitive flexibility (TMT RF: p < 0.05) in comparison to NDS patients. The effect size (d) of executive dysfunctions in WCST and TMT was found to be 0.38–0.39, indicating a small effect size. No differences were observed in verbal cognitive flexibility (VFT P) and cognitive (SCWT RI) or motor inhibition (GNG).
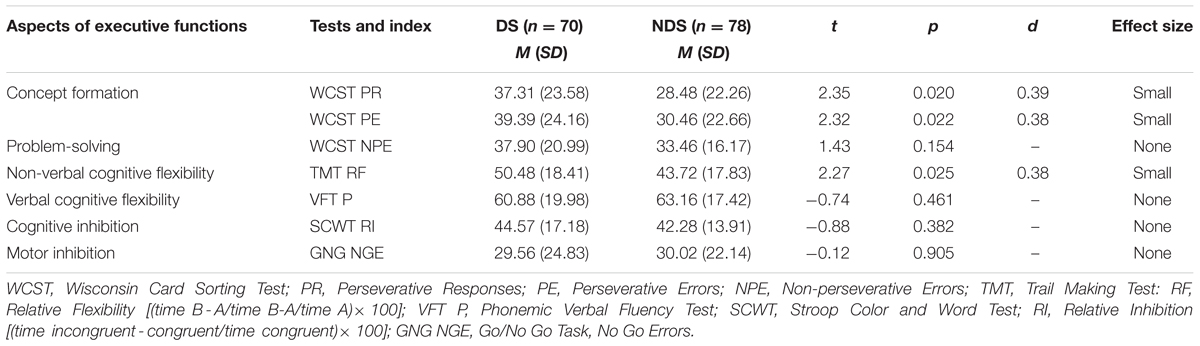
TABLE 3. Comparison of raw scores of executive performance for deficit schizophrenia (DS) versus non-deficit schizophrenia (NDS) patients.
Associations between Particular Aspects of Executive Functions
As can be seen in Table 4, there was a strong positive correlation between the two measures relating to concept formation (WCST PR and PE) in both groups, as well as a weak positive correlation between measures relating to concept formation (WCST PR and PE) and problem-solving (WCST NPE), and a small positive correlation between measures of concept formation (WCST PR and PE) and non-verbal cognitive flexibility (VFT P), as well as problem-solving and cognitive inhibition (SCWT RI). In addition, DS patients showed a slight positive correlation between measures relating to concept formation (WCST PR and PE) and cognitive inhibition (SCWT IR), as well as non-verbal (TMT RI) and verbal cognitive flexibility (VFT P). In turn, in patients with NDS, there was a positive correlation between measures relating to concept formation (WCST PR and PE), problem-solving (WCST NPE), and verbal cognitive flexibility (VFT P).
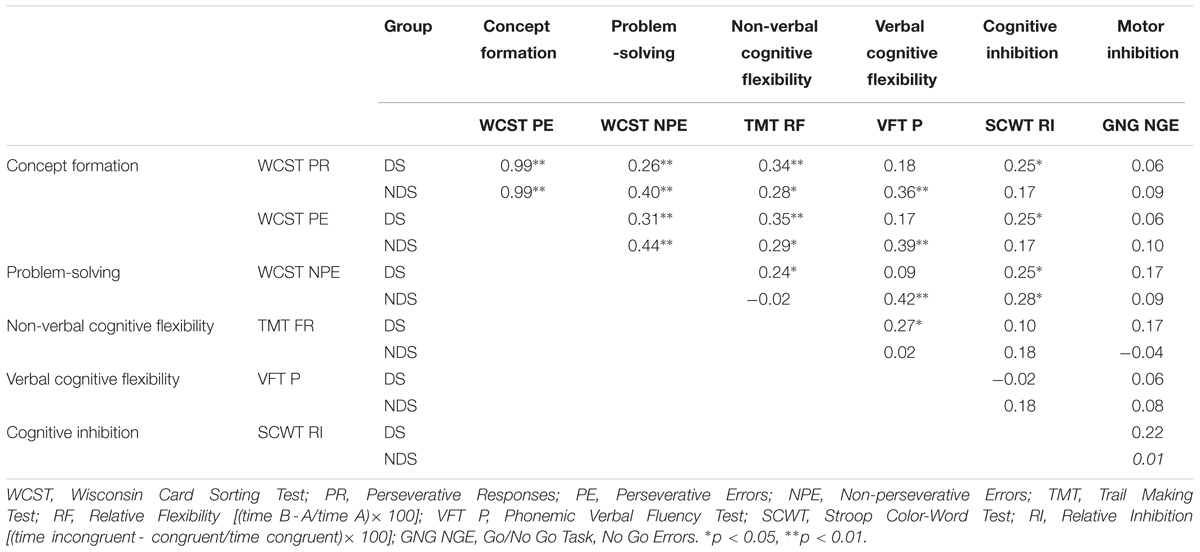
TABLE 4. Correlation (Pearsons’) between particular aspects of executive function for deficits schizophrenia (DS) and non-deficits schizophrenia (NDS) patients.
Neuropsychological Profile of Executive Functions
Figure 1 shows the profile of executive functions for both patient groups. ANOVA with repeated measures/mixed model showed significant differences between the different aspects of executive function in both patient groups [F(6,608) = 57.41; p = 0.000; η2 = 2.82]. There was no statistically significant interaction effect between group type and the nature of the executive domain [F(6,6.08) = 2.28; p = 0.057; η2 = 0.02]. Patients with DS (M = 42.87; SE = 1.43) had higher general scores than patients with NDS, which indicates more severe problems in terms of executive function [F(1,146) = 4.30; p = 0.040; η2 = 0.03]. Pairwise comparison showed that patients with DS scored highest, indicating their greatest difficulties, in the VFT P (M = 60.88; SE = 2.23), and scored lowest in the GNG (M = 29.56; SE = 2.80), WCST NPE (M = 37.90; SE = 2.25), WCST PR (M = 37.31; SE = 2.74) and WCST EP (M = 39.39; SE = 2.80). It was similar in patients with NDS – the greatest problems occurred in the performance of VFT P (M = 63.16; SE = 2.11), and the least problematic were the WCST PR (M = 28.48; SE = 2.59), GNG (M = 30.02; SE = 2.66), WCST EP (M = 30.46; SE = 2.6), and WCST NPE (M = 33.46; SE = 2.13). In addition, patients with DS had similar results in TMT (M = 50.48; SE = 2.16) and SCWT RI (M = 44.57; SE = 1.90), which still differed significantly from the results obtained in the other measures. Patients with NDS also had similar results in TMT RF (M = 43.72; SE = 2.05) and SCWT RI (M = 42.28; SE = 1.80), which were also significantly different from the results in the other factors.
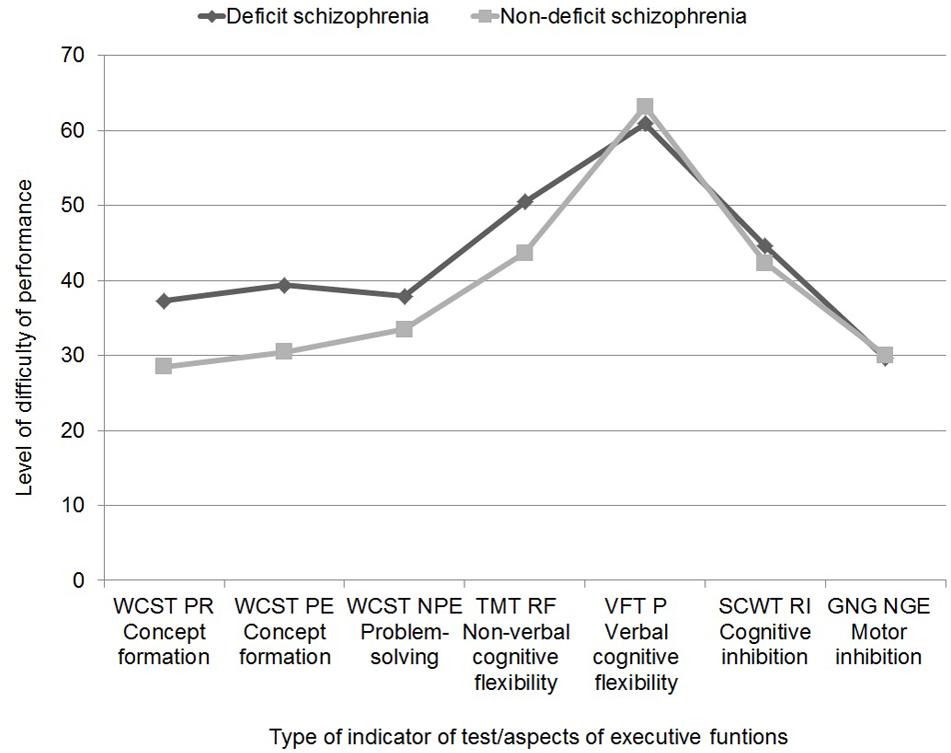
FIGURE 1. Neuropsychological profile of executive functions in patients with deficit and non-deficit schizophrenia (abbreviations in note in Table 3).
Discussion
The results partially confirmed the first hypothesis. It was found that DS patients had lower levels of concept formation than did patients with NDS. Other researchers report similar findings (Table 1). However, in most studies there were only differences in the WCST in the PE score. Only Polgár et al. (2010) report that patients with DS both gave more PR and committed more PE than did NDS patients. Therefore, patients with DS are more likely to have diminished ability to use positive and negative feedback in the learning process and to react optimally to new situations. However, differences in the performance of this test between patients from the two groups could be due to decreased working memory efficiency (working memory is important for holding information in temporary storage, manipulating it, and using it to guide subsequent behavior), which has been noted by, e.g., Park and Gooding (2014). In addition, we have demonstrated that patients with DS have lower levels of non-verbal cognitive flexibility than do NDS patients. However, it was difficult to relate our results to the findings of other researchers, as they did not assess patients’ performance on the TMT (Relative Flexibility Indicator). Wang et al. (2008) found a significant difference between DS/NDS patients regarding their scores on the Absolute Flexibility task, but Galderisi et al. (2002) did not report such a difference. In some studies (Table 1), patients with DS had longer response times in this task (part B), but these results should be interpreted with great caution, as there is a strong dependence between this measure and speed of processing information.
There were no inter-group differences in terms of verbal cognitive flexibility, or cognitive or motor inhibition. Admittedly, there are several studies in which patients with DS got lower results in the VFT P (Polgár et al., 2008; Réthelyi et al., 2012; Yu et al., 2015), but other researchers report no inter-group differences (Table 1). To interpret these results it may be important to note that the ability to generate words is of a complex nature and requires the use of many mental processes, not only set shifting, but also language competence, psychomotor speed, as well as episodic, semantic, and working memory (Szepietowska and Gawda, 2011). Furthermore, its neural correlates include various cooperating brain regions (Amunts et al., 2004). Therefore, the extent to which this task may be useful for differentiating between deficit and NDS remains a matter for further discussion. The SCWT has not been used with Relative Inhibition in previous research. Though, in the work of Buchanan et al. (1994), patients with DS obtained lower scores on the interference index, which was modified using statistical control of the reaction time in the congruent variant, than did patients with NDS. In addition, only the work by Réthelyi et al. (2012) found that patients with DS had longer response times in the incongruent variant of this task than patients with NDS, but these results may reflect a greater slowing of information processing, rather than large deficits in cognitive inhibition (Knowles et al., 2010). It was also observed that DS and NDS patients obtained similar results in motor response, based on their performance of the GNG. The groups did not differ in terms of inhibiting reactions to irrelevant stimuli (No Go). The existence of any larger deficits in patients with DS than those with NDS in the area of cognitive and motor inhibition requires further research, especially in the context of the assessment of brain activity using functional neuroimaging techniques (Egner and Hirsch, 2005; Aron et al., 2007; Gorfein and MacLeod, 2007; Yücel et al., 2014).
A partial confirmation of the second hypothesis was possible, as we have shown the presence of a relationship between certain aspects of executive function, both in patients with DS and NDS. There were, however, some discrepancies between the patient groups. In both groups there were associations between concept formation, problem-solving, and non-verbal cognitive flexibility. Only in patients with DS were there links between concept formation and cognitive inhibition. In turn, significant correlations between concept formation, problem-solving, and verbal cognitive flexibility were only present in patients with NDS. However, it was difficult to relate these results to the findings of other authors, as the relationship between various executive domains in patients with DS and NDS has not been studied very deeply. Admittedly, Yu et al. (2015) reported that there is an important correlation between performance on the TMT and VFT P in patients with DS. A similar relationship was observed in this study, since there was an association between the TMT Relative Flexibility Indicator and the VFT P.
The third hypothesis was confirmed, as we have demonstrated the presence of significant variation in terms of levels of the individual aspects of executive function in patients with DS and NDS. It was found that, in both groups, patients were weaker in the area of verbal cognitive flexibility than in other executive domains. In addition, patients of both groups performed at the same level in terms of concept formation, problem-solving, and motor inhibition. In turn, non-verbal cognitive flexibility and cognitive inhibition remained at a higher level than verbal cognitive flexibility, but still proved significantly more difficult than the rest of the executive domains. The fact that the executive function profiles in both groups were similar was shown by the small effect size (0.03) of differences in the comparison of overall scores in ANOVA, which means that the analysis explained only 3% of the variation of the general results of the two groups. Our results were consistent with the results obtained by Brazo et al. (2002) and Cascella et al. (2008). Réthelyi et al. (2012) and Yu et al. (2015) reported slightly different results – finding that DS patients exhibited greater difficulties with non-verbal than with verbal cognitive flexibility. The obtained results were partially in line with the results of Chen et al. (2014). They found modest differences between the neuropsychological profiles of first-episode drug naive patients with DS and NDS, as well as between medicated patients with DS and NDS. However, only in the case of the first-episode drug naive patients were differences found between particular cognitive domains – i.e., patients with DS scored lower than those with NDS in terms of speed of processing and attention. However, it was difficult to directly compare the results presented in this paper to those of Chen et al. (2014) because the latter authors used different measurement tools (i.e., the CogState battery) for evaluating cognitive functions.
Conclusion
The results in this paper are in line with other research and require further empirical validation. An important strength of this study was the use of a neuropsychological test battery for assessing various aspects of executive function in a large patient group. With this data it was possible to consider a broader diagnostic context, which could inform the work of therapeutic teams (Mak et al., 2013). In particular, the ability to detect deficit patients early on in the course of their disease and identify specific executive domains which are impaired may facilitate the implementation of rehabilitation activities, which can help patients function in society (Semkovska et al., 2004; Zipursky, 2014). One limitation of this study would be the lack of control group (e.g., healthy subjects). However, the main goal was to examine the differences between the two types of schizophrenia, which the authors believe has been achieved. Due to the complex nature of the relationship between brain and behavior, the results of neuropsychological assessment can only suggest a complex neural network dysfunction responsible for performing specific executive functions, which may be another potential limitation of this study (Alexander et al., 2012). Future projects might focus on the assessment of executive function and working memory in deficit patients, based on functional magnetic resonance imaging as well as the assessment of the consequences of impaired executive function on psychosocial functioning in deficit and NDS patients.
Ethics Statement
This study was carried out in accordance with the recommendations of Bioethical Commission of the Pomeranian Medical University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Bioethical Commission of the Pomeranian Medical University.
Author Contributions
All authors contributed to and have approved the final manuscript. JP-W was the principal coordinator of the grant, was involved in the study design, and took part in patient recruitment. ET managed literature searches and analyses, performed statistical analysis, wrote the first draft of the manuscript and took part patient recruitment. JS was involved in conceptualization of the project, study design, and corrected the manuscript. MM took part in patient recruitment. AS took part in patient recruitment. PB took part in patient recruitment.
Funding
This paper was supported by a grant from the Polish Ministry of Science and Higher Education no: NN 402456738. The funding agency had no role in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, M. P., Gillingham, S., Schweizer, T., and Stuss, D. T. (2012). Cognitive impairments due to focal cerebellar injuries in adults. Cortex 48, 980–990. doi: 10.1016/j.cortex.2011.03.012
Alvarez, J. A., and Emory, E. (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42. doi: 10.1007/s11065-006-9002-x
Amunts, K., Weiss, P. H., Mohlberg, H., Pieperhoff, P., Eickhoff, S., Gurd, J. M., et al. (2004). Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space – the roles of Brodmann areas 44 and 45. Neuroimage 22, 42–56. doi: 10.1016/j.neuroimage.2003.12.031
Arango, C., Buchanan, R. W., Kirkpatrick, B., and Carpenter, W. T. (2004). The deficit syndrome in schizophrenia: implications for the treatment of negative symptoms. Eur. Psychiatry 19, 21–26. doi: 10.1016/j.eurpsy.2003.10.004
Aron, A. R., Durston, S., Eagle, D. M., Logan, G. D., Stinear, C. M., and Stuphorn, V. (2007). Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J. Neurosci. 27, 11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007
Brazo, P., Marie, R. M., Halbecq, I., Benali, K., Segard, L., Delamillieure, P., et al. (2002). Cognitive patterns in subtypes of schizophrenia. Eur. Psychiatry 17, 155–162. doi: 10.1001/jamapsychiatry.2013.786
Brown, L. A., Brockmole, J. R., Gow, A. J., and Deary, I. J. (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Exp. Aging Res. 38, 1–9. doi: 10.1080/0361073X.2012.636722
Bryson, G., Whelahan, H. A., and Bell, M. (2001). Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res. 102, 29–37. doi: 10.1016/S165-1781(01)00245-1
Buchanan, R. W., Strauss, M. E., Kirkpatrick, B., Holstein, C., Breier, A., and Carpenter, W. T. (1994). Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch. Gen. Psychiatry 51, 804–811. doi: 10.1001/archpsyc.1994.03950100052005
Carpenter, W. T., Heinrich, D. W., and Wagman, A. M. (1988). Deficit and nondeficit forms of schizophrenia: the concept. Am. J. Psychiatry 145, 578–583. doi: 10.1176/ajp.145.5.578
Cascella, N. G., Testa, S. M., Meyer, S. M., Rao, V. A., Diaz-Asper, C. M., Pearlson, G. D., et al. (2008). Neuropsychological impairment in deficit vs. non-deficit schizophrenia. J. Psychiatric Res. 42, 930–937. doi: 10.1016/j.jpsychires.2007.10.002
Chan, E., MacPherson, S. E., Robinson, G., Turner, M., Lecce, F., Shallice, T., et al. (2015). Limitations of the trail making test part-B in assessing frontal executive dysfunction. J. Int. Neuropsychol. Soc. 21, 169–174. doi: 10.1017/S135561771500003X
Chemerinski, E., Reichenberg, A., Kirkpatrick, B., Bowie, C. R., and Harvey, P. D. (2006). Three dimensions of clinical symptoms in elderly patients with schizophrenia: prediction of six-year cognitive and functional status. Schizophr. Res. 85, 12–19. doi: 10.1016/j.schres.2006.03.002
Chen, C., Jiang, W., Zhong, N., Wu, J., Jiang, H., Du, J., et al. (2014). Impaired processing speed and attention in first-episode drug naive schizophrenia with deficit syndrome. Schizophr. Res. 15, 478–484. doi: 10.1016/j.schres.2014.09.005
Cohen, A. S., Saperstein, A. M., Gold, J. M., Kirkpatrick, B., Carpenter, W. T., and Buchanan, R. W. (2007). Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr. Bull. 33, 1201–1212. doi: 10.1093/schbul/sbl066
Csukly, G., Polgár, P., Tombor, L., Benkovits, J., and Réthelyi, J. (2014). Theory of mind impairments in patients with deficit schizophrenia. Compr. Psychiatry 55, 349–356. doi: 10.1016/j.comppsych.2013.08.025
Delamillieure, P., Constans, J. M., Fernandez, J., Brazo, P., and Dollfus, S. (2004). Relationship between performance on the Stroop test and N-acetylaspartate in the medial prefrontal cortex in deficit and nondeficit schizophrenia: preliminary results. Psychiatry Res. 132, 87–89. doi: 10.1016/j.pscychresns.2004.06.006
Denney, D. R., and Lynch, S. G. (2009). The impact of multiple sclerosis on patients’ performance on the Stroop test: processing speed versus interference. J. Int. Neuropsychol. Soc. 15, 451–458. doi: 10.1017/S1355617709090730
DeQuardo, J. R., Buchanan, R. W., Kirkpatrick, B., Bookstein, F. L., and Tandon, R. (1998). Landmark-based shape analysis of deficit versus non-deficit schizophrenia. Psychiatry Res. 29:77. doi: 10.1016/S0920-9964(97)88489-7
Diamond, A. (2013). Executive functions. Annu. Rev. Psychol. 64, 135–168. doi: 10.1146/annurev-psych-113011-143750
Dickerson, F., Kirkpatrick, B., Boronow, J., Stallings, C., Origoni, A., and Yolken, R. (2006). Deficit schizophrenia: association with serum antibodies to cytomegalovirus. Schizophr. Bull. 32, 396–400. doi: 10.1093/schbul/sbi054
Egner, T., and Hirsch, J. (2005). The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24, 539–547. doi: 10.1016/j.neuroimage.2004.09.007
Fioravanti, M., Bianchi, V., and Cinti, M. E. (2012). Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry 12:64. doi: 10.1186/1471-244X-12-64
Galderisi, S., Maj, M., Mucci, A., Cassano, G. B., Invernizzi, G., Rossi, A., et al. (2002). Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am. J. Psychiatry 159, 983–990. doi: 10.1176/appi.ajp.159.6.983
Galderisi, S., Quarantelli, M., Volpe, U., Mucci, A., Cassano, G. B., Invernizzi, G., et al. (2008). Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophr. Bull. 34, 393–401. doi: 10.1093/schbul/sbm097
Gorfein, D. S., and MacLeod, C. M. (2007). Inhibition in Cognition. Washington, DC: American Psychological Association.
Haro, J. M., Kamath, S. A., Ochoa, S. O., Novick, D., Rele, K., Fargas, A., et al. (2003). The clinical global impression–schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr. Scand. 107, 16–23. doi: 10.1034/j.1600-0447.107.s416.5.x
Heaton, R. K., Chelune, G. I., Talley, J. L., Kay, G. G., and Curtiss, G. (1993). Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources.
Heckers, S., Goff, D., Schacter, D. L., Savage, C. R., Fischman, A. J., Alpert, N. M., et al. (1999). Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch. Gen. Psychiatry 56, 1117–1123. doi: 10.1001/archpsyc.56.12.1117
Hegde, S., Thirthalli, J., Rao, S. L., Raguram, A., Philip, M., and Gangadhar, B. N. (2013). Cognitive deficits and its relation with psychopathology and global functioning in first episode schizophrenia. Asian J. Psychiatry 6, 537–543. doi: 10.1016/j.ajp.2013.07.002
Horan, W. P., and Blanchard, J. J. (2003). Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr. Res. 65, 125–137. doi: 10.1016/S0920-9964(02)00410-3
Jaworowska, A. (2002). WCST – Test Sortowania Kart z Wisconsin. Warszawa: Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego.
Jodzio, K. (2008). Neuropsychologia Intencjonalnego Działania. Koncepcje Funkcji Wykonawczych. Warszawa: Wydawnictwo Naukowe Scholar.
Jurado, M. B., and Rosselli, M. (2007). The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 17, 213–233. doi: 10.1007/s11065-007-9040-z
Kay, S. R., Fiszbein, A., and Opfer, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276. doi: 10.1093/schbul/13.2.261
Kirkpatrick, B., Buchanan, R. W., McKenny, P. D., Alphs, L. D., and Carpenter, W. T. (1989). The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 30, 119–123. doi: 10.1016/0165-1781(89)90153-4
Kirkpatrick, B., Buchanan, R. W., Ross, D. E., and Carpenter, W. T. (2001). A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 58, 165–171. doi: 10.1001/archpsyc.58.2.165
Kirkpatrick, B., and Galderisi, S. (2008). Deficit schizophrenia: an update. World Psychiatry 7, 143–147. doi: 10.1002/j.2051-5545.2008.tb00181.x
Knowles, E. E., David, A. S., and Reichenberg, A. (2010). Processing speed deficits in schizophrenia: reexamining the evidence. Am. J. Psychiatry 167, 828–835. doi: 10.1176/appi.ajp.2010.09070937
Lahti, A. C., Holcomb, H. H., Medoff, D. R., Weiler, M. A., Tamminga, C. A., and Carpenter, W. T. Jr. (2001). Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am. J. Psychiatry 158, 1797–1808. doi: 10.1176/appi.ajp.158.11.1797
Lezak, M. D., Howieson, D. B., and Loring, D. W. (2004). Neuropsychological Assesment, 4th Edn. New York, NY: Oxford University Press.
Liddle, P. F., Friston, K. J., Frith, C. D., Hirsch, S. R., Jones, T., and Frackowiak, R. S. (1992). Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry 160, 179–186. doi: 10.1192/bjp.160.2.179
Mak, M., Tybura, P., Bieńikowski, P., Karakiewicz, B., and Samochowiec, J. (2013). The efficacy of cognitive neurorehabilitation with RehaCom program in schizophrenia patients. Psychiatr. Pol. 47, 213–223.
Mak, M., Tyburski, E., Madany,Ł., Sokołowski, A., and Samochowiec, A. (2016). Executive function deficits in patients after cerebellar neurosurgery. J. Int. Neuropsychol. Soc. 22, 47–57. doi: 10.1017/S1355617715001174
McCabe, D. P., Roediger, H. L. III, McDaniel, M. A., Balota, D. A., and Hambrick, D. Z. (2010). The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology 24, 222–243. doi: 10.1037/a0017619
Messias, E., Kirkpatrick, B., Bromet, E., Ross, D., Buchanan, R. W., Carpenter, W. T., et al. (2004). Summer birth and deficit schizophrenia: a pooled analysis from 6 countries. Arch. Gen. Psychiatry 61, 985–989. doi: 10.1001/archpsyc.61.10.985
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit. Psychol. 4, 49–100. doi: 10.1006/cogp.1999.0734
Morrens, M., Hulstijn, W., and Sabbe, B. (2007). Psychomotor slowing in schizophrenia. Schizophr. Bull. 33, 1038–1053. doi: 10.1093/schbul/sbl051
Niendam, T. A., Laird, A. R., Ray, K. L., Dean, Y. M., Glahn, D. C., and Carter, C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 12, 241–268. doi: 10.3758/s13415-011-0083-5
Park, S., and Gooding, D. C. (2014). Working memory impairment as an endophenotypic marker of a schizophrenia diathesis. Schizophr. Res. Cogn. 1, 127–136. doi: 10.1016/j.scog.2014.09.005
Pełka-Wysiecka, J., Wroński, M., Jasiewicz, A., Grzywacz, A., Tybura, P., Kucharska-Mazur, J., et al. (2013). BDNF rs 6265 polymorphism and COMT rs 4680 polymorphism in deficit schizophrenia in Polish sample. Pharmacol. Rep. 65, 1185–1193. doi: 10.1016/S1734-1140(13)71476-2
Perianez, J. A., Rios-Lago, M., Rodriguez-Sanchez, J. M., Adrover-Roig, D., Sanchez-Cubillo, I., Crespo-Facorro, B. E., et al. (2007). Trail making test in traumatic brain injury, schizophrenia, and normal ageing: sample comparisons and normative data. Arch. Clin. Neuropsychol. 22, 433–447. doi: 10.1016/j.acn.2007.01.022
Polgár, P., Farkas, M., Nagy, O., Kelemen, O., Réthelyi, J., Bitter, I., et al. (2008). How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophr. Res. 99, 200–207. doi: 10.1016/j.schres.2007.06.027
Polgár, P., Réthelyi, J. M., Bálint, S., Komlosi, S., Czobor, P., and Bitter, I. (2010). Executive function in deficit schizophrenia: what do the dimensions of the Wisconsin Card Sorting Test tell us? Schizophr. Res. 122, 85–93. doi: 10.1016/j.schres.2010.06.007
Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills 8, 271–276. doi: 10.2466/pms.1958.8.3.271
Réthelyi, J. M., Czobor, P., Polgár, P., Mersich, B., Bálint, S., Jekkel, É, et al. (2012). General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 262, 107–115. doi: 10.1007/s00406-011-0224-4
Rosenthal, R., and Rubin, D. B. (2003). r equivalent: A simple effect size indicator. Psychol. Methods 8, 492–496. doi: 10.1037/1082-989X.8.4.492
Ross, T. P. (2003). The reliability of cluster and switch scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 18, 153–164. doi: 10.1016/S0887-6177(01)00192-5
Scala, S., Lasalvia, A., Seidman, L. J., Cristofalo, D., Bonetto, C., and Ruggeri, M. (2014). Executive functioning and psychopathological profile in relatives of individuals with deficit v. non-deficit schizophrenia: a pilot study. Epidemiol. Psychiatric Sci. 23, 85–97. doi: 10.1017/S2045796013000140
Schröder, J., Geider, F. J., Binkert, M., Reitz, C., Jauss, M., and Sauer, H. (1992). Subsyndromes in chronic schizophrenia: do their psychopathological characteristics correspond to cerebral alterations? Psychiatry Res. 42, 209–220. doi: 10.1016/0165-1781(92)90113-H
Semkovska, M., Bédard, M. A., Godbout, L., Limoge, F., and Stip, E. (2004). Assessment of executive dysfunction during activities of daily living in schizophrenia. Schizophr. Res. 69, 289–300. doi: 10.1016/j.schres.2003.07.005
Simon, V., De Hert, M., Wampers, M., Peuskens, J., and van Winkel, R. (2009). The relation between neurocognitive dysfunction and impaired insight in patients with schizophrenia. Eur. Psychiatry 24, 239–243. doi: 10.1016/j.eurpsy.2008.10.004
Strauss, E., Sherman, E., and Spreen, O. (2006). A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford: Oxford University Press.
Strauss, G. P., Harrow, M., Grossman, L. S., and Rosen, C. (2010). Periods of recovery in deficit syndrome schizophrenia: a 20-year multi–follow-up longitudinal study. Schizophr. Bull. 36, 788–799. doi: 10.1093/schbul/sbn167
Stuss, D. T. (2011). Functions of the frontal lobes: relation to executive functions. J. Int. Neuropsychol. Soc. 17, 759–765. doi: 10.1017/S1355617711000695
Stuss, D. T., Bisschop, S. M., Alexander, M. P., Levine, B., Katz, D., and Izukawa, D. (2001). The trail making test: a study in focal lesion patients. Psychol. Assess. 13, 230–239. doi: 10.1037/1040-3590.13.2.230
Szepietowska, E. M., and Gawda, B. (2011). Ścieżkami Fluencji Werbalnej. Lublin: Wydawnictwo Uniwersytetu Marcii Curie-Skłodowskiej.
Tamminga, C. A., Thaker, G. K., Buchanan, R., Kirkpatrick, B., Alphs, L. D., Chase, T. N., et al. (1992). Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch. Gen. Psychiatry 49, 522–530. doi: 10.1001/archpsyc.1992.01820070016003
Tek, C., Kirkpatrick, B., and Buchanan, R. W. (2001). A five-year followup study of deficit and nondeficit schizophrenia. Schizophr. Res. 49, 253–260. doi: 10.1016/S0920-9964(00)00146-8
Tiryaki, A., Anıl, A. E., Kabakçı, E., Karaağaoğlu, E., and Göğüş, A. (2003). Reexamination of the characteristics of the deficit schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci. 253, 221–227. doi: 10.1007/s00406-003-0434-5
Tyburski, E., Sokołowski, A., Chȩć, M., Pełka-Wysiecka, J., and Samochowiec, A. (2015). Neuropsychological characteristics of verbal and non-verbal fluency in schizophrenia patients. Arch. Psychiatr. Nurs. 29, 33–38. doi: 10.1016/j.apnu.2014.09.009
Ventura, J., Wood, R. C., Jimenez, A. M., and Hellemann, G. S. (2013). Neurocognition and symptoms identify links between facial recognition and emotion processing in schizophrenia: meta-analytic findings. Schizophr. Res. 151, 78–84. doi: 10.1016/j.schres.2013.10.015
Vogel, S. J., Strauss, G. P., and Allen, D. N. (2013). Using negative feedback to guide behavior: Impairments on the first 4 cards of the Wisconsin Card Sorting Test predict negative symptoms of schizophrenia. Schizophr. Res. 151, 97–101. doi: 10.1016/j.schres.2013.07.052
Voglmaier, M. M., Seidman, L. J., Niznikiewicz, M. A., Dickey, C. C., Shenton, M. E., and McCarley, R. W. (2005). A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schizophr. Res. 74, 43–49. doi: 10.1016/j.schres.2004.09.013
Wang, X., Yao, S., Kirkpatrick, B., Shi, C., and Yi, J. (2008). Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Res. 158, 195–205. doi: 10.1016/j.psychres.2006.09.007
Wendt, H. W. (1972). Dealing with a common problem in social science: a simplified rank-biserial coefficient of correlation based on the U statistic. Eur. J. Soc. Psychol. 2, 463–465. doi: 10.1002/ejsp.2420020412
World Health Organization [WHO] (1992). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization.
Wright, L., Lipszyc, J., Dupuis, A., Thayapararajah, S. W., and Schachar, R. (2014). Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. J. Abnorm. Psychol. 123, 429–439. doi: 10.1037/a0036295
Yu, M., Tang, X., Wang, X., Zhang, X., Zhang, X., Sha, W., et al. (2015). Neurocognitive impairments in deficit and non-deficit schizophrenia and their relationships with symptom dimensions and other clinical variables. PLoS ONE 10:e0138357. doi: 10.1371/journal.pone.0138357
Yuan, P., and Raz, N. (2014). Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 42, 180–192. doi: 10.1016/j.neubiorev.2014.02.005
Yücel, M., Pantelis, C., Stuart, G. W., Wood, S. J., Maruff, P., Velakoulis, D., et al. (2014). Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am. J. Psychiatry 159, 251–254. doi: 10.1176/appi.ajp.159.2.251
Keywords: executive functions, concept formation, verbal cognitive flexibility, non-verbal cognitive flexibility, deficit schizophrenia
Citation: Tyburski E, Pełka-Wysiecka J, Mak M, Samochowiec A, Bieńkowski P and Samochowiec J (2017) Neuropsychological Profile of Specific Executive Dysfunctions in Patients with Deficit and Non-deficit Schizophrenia. Front. Psychol. 8:1459. doi: 10.3389/fpsyg.2017.01459
Received: 16 June 2017; Accepted: 14 August 2017;
Published: 30 August 2017.
Edited by:
Kenji Hashimoto, Chiba University, JapanReviewed by:
Diane Carol Gooding, University of Wisconsin–Madison, United StatesMaria Semkovska, University of Limerick, Ireland
Copyright © 2017 Tyburski, Pełka-Wysiecka, Mak, Samochowiec, Bieńkowski and Samochowiec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justyna Pełka-Wysiecka, justyna.pelka@pum.edu.pl
 Ernest Tyburski
Ernest Tyburski Justyna Pełka-Wysiecka2*
Justyna Pełka-Wysiecka2*