- 1Department of Psychological Medicine, University of Otago, Christchurch, New Zealand
- 2Specialist Mental Health Services, Canterbury District Health Board, Christchurch, New Zealand
Background: Research suggests that only 50% of patients with major depression respond to psychotherapy or pharmacological treatment, and relapse is common. Therefore, there is interest in elucidating factors that help predict clinical response. Cognitive impairment is a key feature of depression, which often persists beyond remission; thus, the aim of this systematic review was to determine whether baseline cognitive functioning can predict treatment outcomes in individuals with depression.
Method: Studies examining cognitive predictors of treatment response in depression were identified using Pub Med and Web of Science databases. Given the heterogeneity of outcome measures, the variety of treatment protocols, and the differing ways in which data was presented and analyzed, a narrative rather than meta-analytic review technique was used.
Results: 39 studies met inclusion criteria. Findings in younger adult samples were inconclusive. There was some evidence for a predictive effect of executive function and to a lesser extent, psychomotor speed, on treatment response. There was no evidence of learning or memory being associated with treatment response. In older-aged samples, the evidence was much more consistent, suggesting that poor executive function predicts poor response to SSRIs.
Conclusions: Findings from the present review suggest that certain aspects of cognitive functioning, particularly executive function, may be useful in predicting treatment response in depression. This is certainly the case in elderly samples, with evidence suggesting that poor executive functioning predicts poor response to SSRIs. With further research, baseline cognitive functioning may serve as a factor which helps guide clinical decision making. Moreover, cognitive deficits may become targets for specific pharmacological or psychological treatments, with the hope of improving overall outcome.
Introduction
Major depression is among the leading causes of global disability (1) and although our understanding of the disorder is growing, treatment outcomes remain unsatisfactory. Research indicates that only 50% of patients respond to psychotherapy or pharmacological treatment and relapse is common (2, 3). Clinical factors predict differential response to treatments to a limited extent, leaving clinicians to choose first-line treatment on the basis of likely side effects, availability and their own clinical experience (4). With each treatment failure, there is an increased risk of both longer-term failure to respond to treatment and of relapse (5). In this context, there has been increased interest in elucidating factors that help predict clinical response in depression, including cognitive factors, hormonal measures, and neural markers.
Cognitive functioning is relatively easy to measure in clinical practice, and if found to be predictive of treatment response, it has the potential to be widely used. Evidence indicates that depression is associated with widespread cognitive deficit, including impairments in executive functioning, attention, verbal learning and memory, visual learning and memory, emotional processing and psychomotor speed (6). Although aspects of these cognitive deficits may resolve following successful treatment for some individuals, it is often the case that they persist beyond remission (7, 8).
If baseline cognitive deficits are predictive of eventual response, then such deficits could be targeted by specific pharmacological or psychological treatments, in the hope of improving overall outcome. For example, the antidepressant Vortioxetine has been shown to improve psychomotor and verbal memory function in moderate to severe depression (9), while RU486 has been shown to improve spatial working memory in the depressed phase of bipolar disorder (10). Considerable research is currently occurring into psychological techniques that aim to improve cognitive function in depression (11). Indeed, studies that have specifically targeted executive dysfunction in elderly depressed patients, have found positive effects (12). However, due to the intensive nature of such psychological treatments, it is likely that these techniques need to be aimed at those who would have otherwise experienced a more difficult and prolonged recovery. A further implication of finding cognitive predictors of treatment response is that if cognitive impairment is known to predict poorer outcomes, then this may prompt a more aggressive approach in the initial stages of treatment. For example, a clinician may use a combination of psychotherapy and pharmacotherapy in situations where only one of these modalities would have been typically used.
The aims of the present review were therefore as follows: (i) to examine findings from studies investigating cognitive predictors of treatment response in depression, and (ii) to examine the methodological issues arising from the studies that have examined this. We reviewed all the available literature in which cognitive testing was conducted at baseline, to determine whether aspects of cognitive functioning would impact on treatment outcomes.
Research Questions
1. Does baseline cognitive functioning predict treatment outcomes in major depression?
2. Is the predictive relationship dependent on treatment modality?
Methods
Protocol and Registration
Details of the protocol for this systematic review were registered on PROSPERO (42018081980) and can be accessed at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42018081980.
Search Strategy
Up to 1 December 2017, a systematic review of electronic databases was carried out for relevant papers using Pub Med and Web of Science. In the initial search, the following search items were used “major depression” or “depression” and “neuropsychological predictors” or “cognitive predictors” and “treatment response.” To ensure inclusion of all available articles, reference lists of all relevant papers were checked. Further, Web of Science was used to review articles that had cited the relevant articles found using the aforementioned search strategies, enabling the inclusion of more recent publications.
Inclusion Criteria
Any peer-reviewed article involving baseline assessment of cognitive functioning, a proposed active treatment of depression and a follow up measure of depression severity, were included in the present review. All subtypes of depression were also included (unipolar or bipolar - depressed phase, psychotic or non-psychotic). “Treatment” could be pharmacotherapy, electroconvulsive therapy (ECT), transcranial stimulation (direct current or magnetic), psychotherapy, or cognitive remediation (CR). Studies were required to use adult samples, with all participants 18 years of age or older.
Exclusion Criteria
Reasons for exclusion were: (i) use of a depressed sample with comorbid major medical, neurological or endocrinological conditions, (ii) inclusion of individuals scoring <24 on a Mini Mental Status Exam (n = 6), and (iii) not presenting data on baseline depression severity (n = 1). All studies were limited to English-language publications.
Full Study Review
Articles were initially screened by two of the reviewers who independently reviewed the titles and abstracts of studies, to accept or reject for full text review. The same two reviewers then examined the full texts of the studies that had passed initial screening, to determine if they still met inclusion criteria. If inclusion of a paper was unclear, then all three co-authors discussed in order to achieve a consensus. Data was extracted from eligible studies into a spreadsheet. For each study, we extracted the following data: (1) characteristics of the sample, including sample size, average age and baseline depression severity, (2) study design, (3) cognitive tests used during assessment, (4) response/remission criteria, and (5) study outcomes.
Results
Study Characteristics
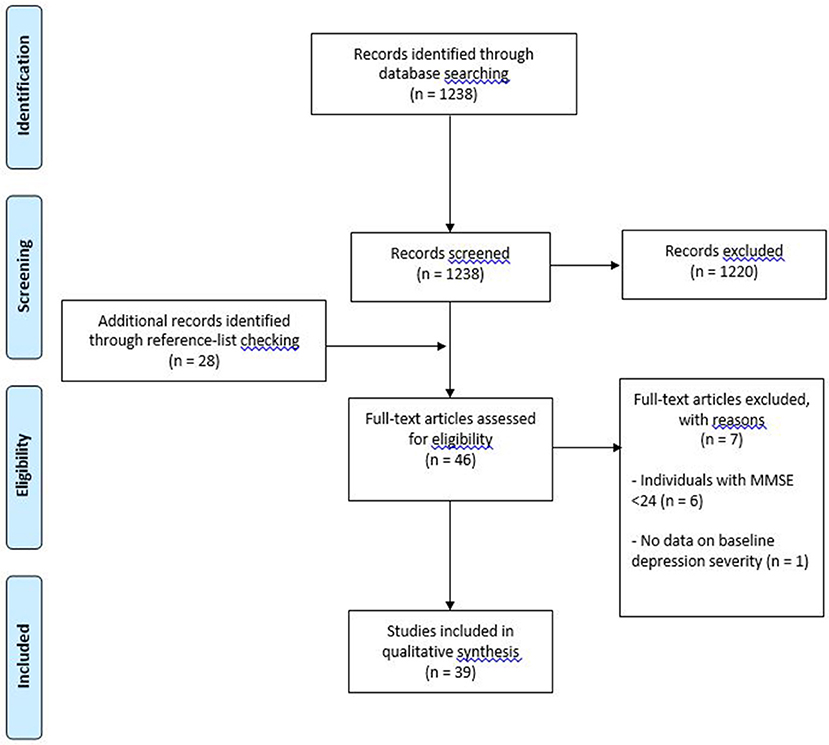
Thirty-nine studies met inclusion criteria (see Figure 1 for flow diagram of studies retrieved for review). Of these studies, 32 used pharmacotherapy as the primary treatment (18 single antidepressant, 14 mixed antidepressant treatment); 1 study used pharmacotherapy, psychotherapy and a combination thereof; 1 study used unrestricted pharmacotherapy treatment in addition to ECT (the latter being the final treatment option); 1 study used transcranial direct current stimulation; 1 study used deep brain stimulation, and 3 studies used psychosocial interventions (see Tables 1, 2). Regarding the term “mixed antidepressant treatment,” we refer to a number of possible situations. Firstly, open label treatment with a specific type of antidepressant but with the option to use various antidepressants within that class; secondly, open label treatment with any type of antidepressant; and thirdly, a standardized treatment algorithm allowing for treatment changes according to response. In one of the pharmacological studies (47), a small proportion of the sample received ECT in conjunction with pharmacotherapy (4 out of 100 participants). Given the small number of participants receiving adjunctive ECT, it was decided to group this study with others involving mixed antidepressant treatment. An additional pharmacological study (55) utilized ECT as a final treatment option. Given that 48% of the participants were treated with ECT, it was decided to group this study with the “other biological” treatment studies. One study utilized a naturalistic treatment protocol (38), which meant that some of the participants received no recognized treatment (n = 4). Because most participants received some form of antidepressant medication, it was decided to include the study in this review.
Studies used a range of cognitive tests and the clinical characteristics of the depressed samples varied substantially across studies. In this review, more emphasis is placed on those studies with the greatest number of participants, as they have more statistical power. While we did not formally rate the quality of studies, we have discussed methodological strengths and weaknesses and taken this into account in synthesizing the evidence. In the sections that follow, the studies will be briefly discussed according to treatment type, and findings will be further divided into different cognitive domains. It is important to note that the way in which tests have been categorized in this review may not align with the cognitive domains described in the original studies; however, it was imperative to organize tests in a standardized way. Given there is considerable overlap in tasks assessing executive function and attention, it was decided to combine both of these cognitive functions together. Further, although working memory is sometimes classified under learning and memory, in this review, it has been classified under the umbrella term of executive functioning. For the purposes of this review, samples containing participants ranging from 18 to 65 years will be referred to as adult samples, and those containing individuals aged 65 and above, will be referred to as older-aged samples.
Single Antidepressant Trials
Executive Function/Attention
Eleven studies examined the relationship between executive function/attention and treatment outcomes in 13 adult samples receiving antidepressant monotherapy. Four shorter treatment trials (five samples), examined predictors of response to selective serotonin reuptake inhibitors (SSRIs). Three samples showed an association between poorer executive function/attention performance and poor overall treatment response (total n = 268) (13, 14, 20). Conversely, two samples showed no evidence of an association (total n = 306) (17, 20). Etkin et al. (20) examined predictors of response to Escitalopram (n = 217) and Sertraline (n = 234). They found that impairment in attention and working memory was associated with non-remission on Escitalopram; however, this relationship was not seen with Sertraline (20). Of the negative studies, Gudayol- Ferré et al. found no evidence of an association between executive function or attention and overall response to Fluoxetine (17); however, they did find an association with early treatment response and time to remission (16, 18). In their sample of 72 depressed patients, poorer attention and spatial working memory were associated with poorer response at 4 weeks, but the opposite relationship was seen with “subsequent thinking time” on the Stockings of Cambridge (16). Additionally, the authors also found that those with poorer spatial working memory performance were slower to remit at treatment-end; thus, their findings with respect to attention and spatial working memory are in line with the other positive studies (18).
In a longer treatment trial, Bastos et al. (25) examined the relationship between executive function performance and response to 24 months of treatment with Fluoxetine (n = 91), psychodynamic psychotherapy (n = 90) or a combination thereof (n = 90). The largely negative results were complex. Of 14 cognitive variables, higher scores on two (WAIS-III, Letter Number Sequencing and Matrix Reasoning) were associated with better response across all three treatments (Fluoxetine, psychodynamic psychotherapy and the combination) and higher scores on one (WAIS-III, Similarities) was associated with poorer response (25).
Whilst one small study has found an association between poorer executive function/attention performance and poor response to SNRIs (n = 25) (21), a much larger study has found no evidence of a relationship (n = 204) (20).
In a naturalistic multi-center trial of 6–8 weeks of Agomelatine (an antidepressant with a primarily melatonergic action) treatment (n = 508), the number of omissions on the D2 Cancellation Task (a measure of attention) predicted clinical and functional remission in patients with moderate to severe depression. Moreover, a dose-response relationship was observed, whereby treatment outcomes were increasingly more positive as less omission errors were made on the task (24).
One study has examined the relationship between baseline executive measures and response to the combined dopamine and noradrenaline re-uptake inhibitor, Bupropion. Herrera-Guzmán et al. (n = 26) found that poorer performance on the Stockings of Cambridge at baseline predicted poorer response to 8 weeks of Bupropion treatment (15).
Three studies have examined cognitive predictors of Ketamine response, with two (total n = 38) finding evidence of an association between poorer executive function/attention performance and better treatment response (19, 22). Murrough et al. (22) (n = 25) found that responders to a single infusion of Ketamine Hydrochloride performed significantly worse on tests assessing working memory, than non-responders (22). In line with this finding, Shiroma et al. (19) found that the likelihood of responding to six infusions of Ketamine was greater in those who demonstrated poorer attentional abilities at baseline (19). In contrast, a second study by Murrough et al. (n = 43), showed no association between executive function/attention performance and treatment response (23).
Seven studies examined the relationship between executive function/attention and treatment-related outcomes in response to antidepressant (SSRI) monotherapy in older-aged samples. Five of the studies found that deficits in executive functioning were associated with poor remission rates/antidepressant response (combined n = 341) (26, 28, 29, 32, 34, 35). In contrast, one small study (n = 12) found no difference in executive function performance between remitters and non-remitters (30). One study (n = 13) examined the relationship between executive function/attention and time to remission and found that individuals with impaired executive functioning, as shown by greater conflict scores on the Attention Network Test, took longer to remit (31).
Psychomotor Speed
Ten studies examined the relationship between psychomotor speed and response to antidepressant monotherapy, in 12 adult samples. Four shorter treatment trials examined predictors of response to SSRIs. In two samples (14, 20), slower psychomotor speed was associated with poorer response to treatment (total n = 254). In contrast, three samples showed no association between SSRI treatment and psychomotor speed (n = 320) (13, 17, 20). In the large study by Etkin et al. slower psychomotor speed was associated with non-remission to Escitalopram, but not to Sertraline (20). One study examined the relationship between psychomotor speed and 24 months of treatment with an SSRI (25). The study found that slower psychomotor speed was associated with poorer response to Fluoxetine treatment (n = 91).
No association was found between psychomotor speed and response to SNRIs (n = 204) (20), Bupropion (n = 26) (15) and Agomelatine (n = 508) (24). Two studies (total n = 68) (22, 23) examining the relationship between psychomotor speed and response to Ketamine found that slower psychomotor speed at baseline predicted greater improvement in depressive symptoms following treatment. Conversely, one Ketamine study (n = 13) found no association (19).
Three studies have examined the relationship between psychomotor speed and overall treatment response in older-aged adults. None of the studies (total n = 594) found an association between psychomotor speed and treatment response (27, 33, 34). However, one study (n = 84) did find that slower psychomotor speed was associated with slower response to treatment. Sneed et al. (33) found that individuals with slower psychomotor speed took longer to respond to Citalopram than those with faster psychomotor speed; however, by the end of treatment (week 8), both groups were equal in their level of response (33).
Verbal Learning and Memory
Eight studies examined the relationship between verbal learning and memory, and treatment-related outcomes in response to antidepressant monotherapy in adult samples. Seven shorter treatment trials (total n = 885) (13, 15, 17, 19, 22, 23), plus one long-term treatment trial (n = 91) (25), found no evidence of an association between the two. Likewise, the four studies that examined verbal learning and memory in older-aged samples (total n = 616) found no relationship between verbal learning and memory performance and treatment-related outcomes (26, 27, 33, 34).
Non-verbal Learning and Memory
Seven studies examined the relationship between non-verbal learning and memory performance and treatment-related outcomes in response to antidepressant monotherapy in adult samples. Six shorter studies (total n = 204) (13, 14, 17, 19, 22, 23) and one longer-term study (n = 91) (25) found no relationship between non-verbal learning and memory, and treatment response. One study (n = 26) found that poorer non-verbal memory performance was associated with better response to a combined dopamine and noradrenaline re-uptake inhibitor (26). In their 8-week trial of Bupropion, Herrera-Guzmán et al. found that responders performed significantly worse on a measure of visual memory (Paired Associates Learning) than non-responders (15). One study has examined the association between non-verbal learning and treatment response in older-aged depression (n = 22); however, they found no evidence of a relationship between the two (26).
Emotional Processing
Only two studies have examined the predictive nature of emotional processing in relation to treatment response in depression. In a younger adult sample, Etkin et al. (20) found that slower emotion identification speed was associated with non-remission to Escitalopram (n = 217), but not Sertraline (n = 234) or Venlafaxine (n = 204) (20). In an older-aged sample (n = 12), Alexopoulos et al. (30) found no differences between remitters and non-remitters in terms of their performance on an emotional go/no-go task following 8 weeks of treatment with Escitalopram (30).
Mixed Antidepressant Treatment
Executive Function/Attention
Ten studies examined the relationship between executive function/attention and response to mixed antidepressant treatment in 11 adult samples with depression. In five of the samples (total n = 291), poorer executive function/attention was associated with poorer response to various pharmacological treatments (37, 39, 40, 42, 43). In a sample of particularly severely depressed inpatients, Whithall et al. (40) found that poorer executive function performance was associated with negative clinical and functional outcomes in inpatients treated with SSRIs or SNRIs. More perseverative errors on the shortened Wisconsin Card Sorting Test (WCST) at baseline, was associated with greater depression severity at follow-up. Further, more perseverative errors on the WCST in addition to poorer event-based prospective memory, was associated with poorer social and occupational outcomes in their sample (40). In contrast, one small study (n = 36) found the opposite relationship between executive function performance and treatment response. Crane et al. (45) found that more commission errors on the Parametric Go/No-Go test predicted better treatment response to Escitalopram or Duloxetine (45). Four samples (total n = 228) showed no association between executive function/attention performance and overall treatment response (36, 38, 41, 43, 44).
Four studies examined the relationship between executive function/attention and treatment response in older-aged samples. Two studies (total n = 159) found an association between executive dysfunction and poor treatment response (46, 47). In the largest positive study, Potter et al. (47) (n = 110) found that remitters to a standardized treatment algorithm over 3 months had significantly fewer perseverative errors on the Controlled Oral Word Association Task (COWAT) and better performance on Digit Span Forward, than non-remitters (47). Story et al. (48) (n = 177) examined response to a standardized treatment algorithm over one year and found no association with executive function performance (48). Additionally, in a 12-week randomized controlled trial (RCT) comparing Olanzapine plus Sertraline with Olanzapine plus placebo (n = 142), Bingham et al. (49) found no association between baseline executive functioning and depression scores post treatment (49). This study did not present data separately for the two treatment groups.
Psychomotor Speed
Eight studies examined the relationship between psychomotor speed and treatment outcomes with mixed antidepressant treatment in nine adult samples. Three samples (total n = 159) showed an association between slower psychomotor speed and poorer response to treatment (38, 42, 43). In the largest positive study (n = 86), slower performance on Part A of the Trail Making Test (TMT) was associated with greater depressive symptomatology following 8 weeks of SSRI treatment in a sample of adults with severe depression (42). In contrast, six samples (total n = 283) showed no association between psychomotor speed and treatment response (39, 41, 43, 44). In a sample of 104 individuals with depression, Lin et al. (44) found no association between psychomotor speed and improvement in HDRS scores following 6 weeks of treatment (44).
Four studies examined the relationship between psychomotor speed and treatment response in older-aged samples. One 6-week study (n = 49) and one 12-month study (n = 177) found an association between poorer psychomotor speed and poor treatment response (46, 48), whilst two studies (n = 252) did not (47). In the larger positive study (n = 177), Story et al. (48) found that depressed older persons with better baseline performance on the Symbol Digit Modalities Test, showed the greatest improvement in depressive symptomatology at one-year follow-up (48).
Verbal Learning and Memory
Five studies have examined the predictive value of verbal learning and memory in relation to mixed antidepressant treatment in adult samples. Spronk et al. (41) found that higher pre-treatment verbal memory performance was associated with a greater reduction in depressive symptoms, in a sample of 25 individuals with major depressive disorder (MDD) (41). Four other studies found no association between verbal memory and treatment response (total n = 157) (38–40).
Two studies have examined the relationship between verbal learning and memory, and treatment response in older-aged adults. Story et al. (48) (n = 177) found that depressed older adults who performed well on verbal memory tasks prior to being treated with a stepped approach, showed the greatest improvement in depressive symptomatology at 1-year follow-up (48). However, another study (n = 110) found no relationship at 3-month follow-up (47).
Non-verbal Learning and Memory
Three studies examined the relationship between non-verbal learning and memory and treatment outcomes with mixed antidepressant treatment in adults (38, 44), and none of the studies found evidence that non-verbal learning and memory predicts treatment response (total n = 181). Likewise, the single study that examined non-verbal learning and memory in older-aged samples found no association between the two (n = 110) (47).
Emotional Processing
Only one study has examined the relationship between emotional processing and treatment outcomes with mixed antidepressant treatment in adults with depression. In the study by de Groot et al. (36), there was no significant difference between responders and non-responders in terms of their performance on a facial expression recognition task at baseline (36).
Other Biological Treatments
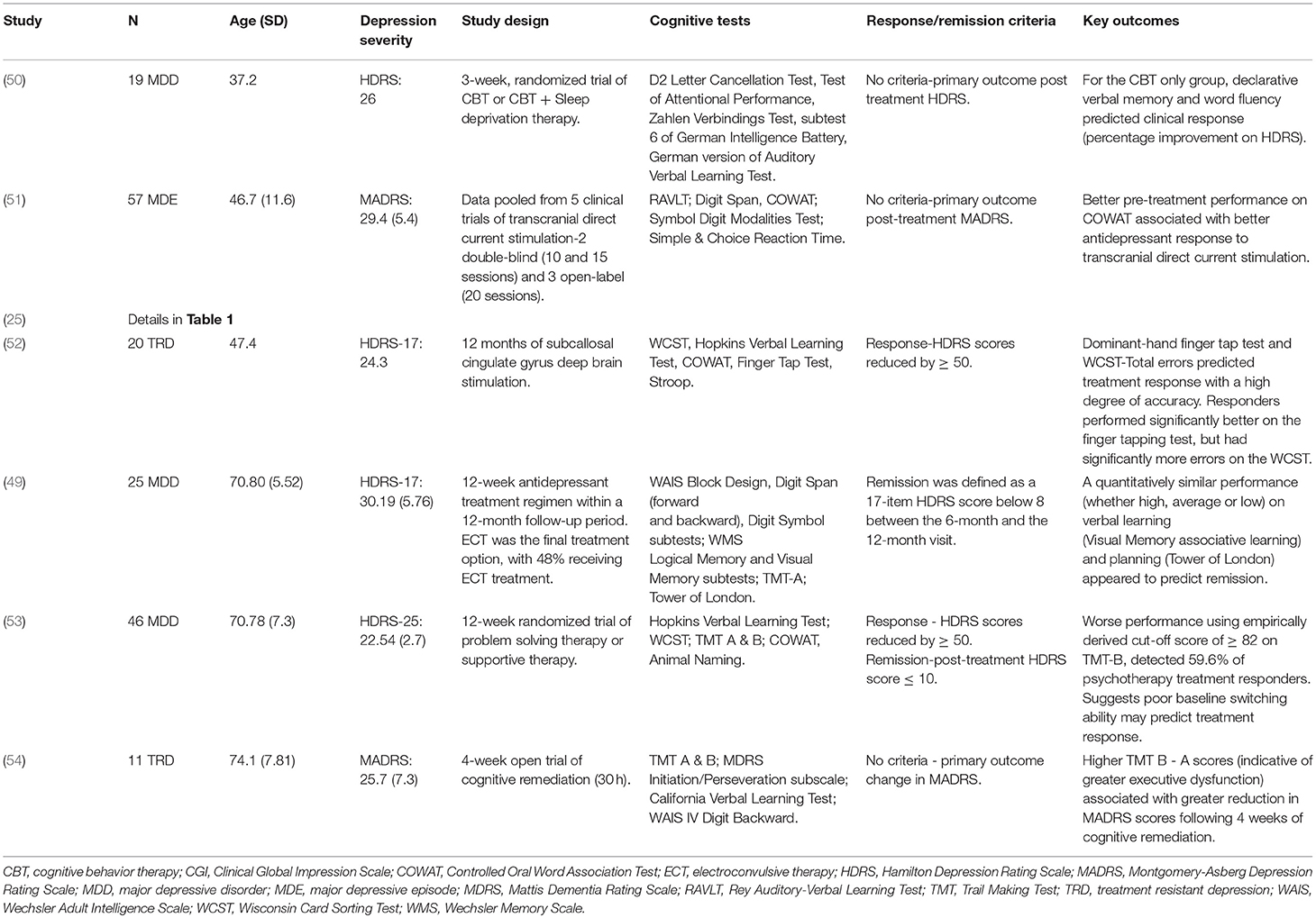
Two studies have examined cognitive predictors (executive function, verbal learning and memory, and psychomotor speed) of treatment response to other biological treatments in younger adult populations. Martin et al. (51) pooled data (total sample, n = 57) from five clinical trials of anodal transcranial direct current stimulation and found that better baseline performance on the COWAT (a measure of executive functioning), was associated with better response to transcranial stimulation (51). McInerney et al. (52) examined cognitive predictors of 12 months of subcallosal cingulate gyrus deep brain stimulation (n = 20) and found that better psychomotor speed, but greater executive dysfunction, predicted better response to treatment (52).
One study examined cognitive predictors of treatment response to other biological treatments in older-aged individuals. Marcos et al. (55) assessed the predictive value of executive functioning/attention, verbal learning and memory, non-verbal learning and memory, and psychomotor speed, in a 12-week antidepressant trial (n = 25). ECT was available if patients failed to respond to pharmacological treatment, with 48% of the sample receiving ECT at some point during the trial. The authors found no association between cognitive function and response to treatment (55).
Psychosocial Treatments
Two studies have examined cognitive predictors of psychosocial treatment response in adults with depression (25, 50). Kundermann (50) examined the predictive value of executive function/attention, verbal learning and memory and psychomotor speed, in a 3-week trial of Cognitive Behavior Therapy (CBT) vs. CBT + sleep deprivation therapy (n = 19). The authors found that better verbal fluency and declarative verbal memory were associated with better clinical response (percentage improvement on the HDRS) in those receiving CBT alone. However, this relationship was not seen in the group receiving CBT + sleep deprivation therapy. No association was found between psychomotor speed and treatment response in either of the two groups (50).
Bastos et al. (25) examined the relationship between executive function, verbal learning and memory, and processing speed, and response to 24 months of psychodynamic therapy (n = 90), Fluoxetine (n = 91) or psychodynamic therapy with adjunctive Fluoxetine treatment (n = 90). The authors found mixed findings in relation to the predictive value of executive functioning. Across all three treatment groups, better Letter-Number Sequencing and Matrix Reasoning scores, predicted lower depression symptoms (BDI) at 24 months. Conversely, better Similarities scores were associated with greater depression symptoms following treatment. The authors also found that in those receiving psychodynamic therapy or both treatments combined, better Digit-Symbol Coding scores (i.e., faster psychomotor speed) were associated with greater depression severity at follow-up (25).
Two studies have examined the association between cognitive function and response to psychotherapy in older-aged samples with depression (53, 54). Both examined executive functioning/attention, verbal learning and memory, and psychomotor speed. Both studies found executive functioning to be the only domain associated with treatment-related outcomes. Beaudreau et al. (53) (n = 46) found that poor baseline performance on Part B of the TMT (a measure of cognitive flexibility), detected 59.6% of individuals who responded to 12 weeks of either problem-solving therapy or supportive therapy. In a 4-week trial of cognitive remediation, Morimoto et al. (54) found that higher TMT B-TMT A scores (indicative of greater executive dysfunction) was associated with greater reduction in Montgomery-Asberg Depression Rating Scale (MADRS) scores following treatment (54).
Discussion
Summary of Results
Since different treatments may act differently on brain circuitry and there is evidence that modulation of specific receptors or circuits may differentially affect cognitive function, it is important in the first instance to divide the results of the review into studies examining response to different treatment modalities. In addition, whilst this review excluded studies that included patients with likely onset of dementia (MMSE <24), changes associated with aging, multiple episodes of depression or late onset depression may result in a different pattern of association in older samples. Therefore, we have separated the results into adult and older-aged samples. In summary, the results are as follows:
Executive Function/Attention
There was some consistency in findings from studies examining response to SSRIs in adult samples. Three samples treated with a single SSRI (13, 14, 20) (total n = 268) and two samples openly treated with any SSRI (n = 123) (39, 42) showed that reduced executive function was associated with poorer response. In contrast, two single SSRI studies (total n = 306) showed no association (17, 20). In older-aged samples, the findings were much more consistent. Five studies showed an association between executive function and overall response to SSRIs (total n = 341) (26, 28, 29, 32, 34, 35), and one found an association between executive function performance and time to remission (31). Only one small study (n = 12) found no differences in executive functioning between remitters and non-remitters (30).
In terms of other agents, one large study found that poorer attention was associated with poorer response to a melatonergic agent in an adult sample (n = 508) (24). There was limited, or no evidence, of an association between executive function/attention and response to SNRIs, Bupropion, Ketamine, ECT or psychosocial treatments.
Psychomotor Speed
There was some evidence of a relationship between psychomotor function and response to SSRIs in adult samples. Two samples (total n = 254) showed that slower psychomotor speed was associated with poorer response (14, 20). Additionally, one longer-term SSRI study (n = 91) (25) and one sample openly treated with any SSRI (n = 86) (42), showed the same association. In contrast, three adult samples (n = 320) treated with a single SSRI (13, 17, 20) and one sample openly treated with any SSRI (39), showed no association. There was no association in older-aged adults.
There was limited, or no evidence, of an association between psychomotor speed and response to SNRIs, Bupropion, Ketamine, Agomelatine, ECT or psychosocial treatments in adult or older-aged samples.
Learning and Memory
There was limited evidence of a relationship between learning and memory (verbal or non-verbal) and treatment response in adult or older-aged samples.
Prediction of Response to Monoamine Reuptake Inhibitors
As noted in the summary above, the data in younger participants is dominated by the large study of Etkin et al. (20). The sample randomly assigned to Escitalopram showed that executive dysfunction was associated with poorer response, while there was no such association for the other SSRI, Sertraline (20). The authors speculated that the difference between Escitalopram and Sertraline may relate to the exact pharmacodynamic properties of the agents, the suggestion being that Escitalopram is a more specific SSRI while Sertraline has more noradrenergic and dopaminergic reuptake inhibition. The other related possibility is that their findings were related to dose; however, the validity of such explanations is not clear. The doses of all three antidepressants in the international Study to Predict Optimized Treatment in Depression (iSPOT) study were low (Escitalopram 12 mg, Sertraline 62 mg, Venlafaxine 83 mg) (56). Evidence does not suggest that Venlafaxine has significant effects on noradrenaline re-uptake at this dose (57, 58). In vitro, Sertraline has been shown to inhibit noradrenaline and dopamine reuptake (59), but the extent to which this occurs at the doses used in this study in vivo is unclear. Furthermore, neither of these factors can explain the differential response whereby those with poorer cognitive function were more likely to remit with Sertraline/Venlafaxine and those with better cognitive function were more likely to remit with Escitalopram. In the iSPOT study, and in meta-analyses, there was no difference in overall efficacy between these three antidepressants (56, 60). The analysis used in this study was different from that used in all other studies, using a cross-validated multivariate pattern classification approach. This allows different variables to be weighted differentially in order to obtain the best predictive model. As such, it is significantly different from the simpler methods of examining association and less comparable than the results from other studies.
Results for processing speed are similar to those for executive function, with the largest study showing a relationship between processing speed and response to treatment with Escitalopram but not Sertraline or Venlafaxine (20). It has been suggested that reduced psychomotor function indicates a particular subtype of depression; melancholic depression (61). Further, it is suggested that this responds preferentially to dual action drugs compared with SSRIs or psychotherapy (62, 63). The association with response to Escitalopram but not Venlafaxine could therefore relate to a poor response of “melancholic” patients—in this context indicated by psychomotor impairment—to Escitalopram but not Venlafaxine. However, as noted, Venlafaxine at this dose may vary little from a standard SSRI. It has been suggested that measuring psychomotor function either by testing or observation, is a better way of assessing a measurably different (“melancholic”) group.
Response to Other Agents and Biological Treatments
A large study examining predictors of response to Agomelatine (an antidepressant with a primarily melatonergic action), showed that those who performed better on an attentional task were more likely to achieve both clinical and functional remission (24). The authors postulated that the ability to direct attentional resources toward, and away from, emotionally-laden stimuli, is critical for effective emotional regulation. Thus, attentional deficits would likely impact one's ability to regulate emotion; thereby, contributing to persistent negative affect (24).
Interestingly, studies examining Ketamine found the opposite relationship between cognitive functioning and treatment response, with poorer neurocognitive performance, particularly slower psychomotor speed, predicting greater improvement in depressive symptoms (19, 22, 23). While preliminary, the findings suggest that responders to Ketamine may show a distinct cognitive profile compared with those who respond to other types of antidepressants, such as SSRIs. Dopaminergic transmission within prefrontal-subcortical circuits has been implicated in several cognitive processes, including psychomotor function (64); further, Ketamine has been shown to modulate dopamine transmission within these brain regions (65, 66). Although Ketamine's exact mechanism of action is yet to be fully elucidated, it is possible that its antidepressant effects are through modulation of dopaminergic signaling. In line with this, Bupropion is a relatively specific dopamine and noradrenaline reuptake inhibitor, and Herrera-Guzmán et al. (15) found that poorer executive function performance predicted better response to Bupropion (n = 26).
Response to Psychotherapy
Few studies have examined cognitive predictors of response to psychotherapy. Focusing on the short-term studies, one study found that better executive functioning and verbal memory were associated with better response to treatment with CBT (50). Conversely, two studies found that executive dysfunction predicted better response to Problem Solving Therapy (PST) and supportive therapy (67), and cognitive remediation (54). The discrepancy in findings may be due to the nature of the psychosocial treatments being used. The latter studies incorporated treatments that either targeted executive dysfunction and its underlying pathophysiology (PST and cognitive remediation) or did not rely heavily on executive processes (supportive therapy). Therefore, it is possible that treatments which support or improve executive functioning, may facilitate clinical improvement in those experiencing such cognitive deficits.
Data in Older-Age Samples
Particularly for executive function, data in older-age samples are remarkably consistent. Most studies showed that impaired executive function was associated with poorer response to treatment. It is possible that this may be related to the number of previous episodes, as both cognitive functioning and treatment response decline with increasing depressive episodes (68, 69). Some authors have suggested that executive dysfunction is particularly prominent in the older-age individuals (70), although not all studies have agreed (71). If executive function is particularly prominent or frequent in these samples, then this would reduce the likely dilution effect of including patients with minimal deficit. One large study which illustrates this effect was excluded from this review based on their use of a different measure of response (functional measure) (12). A treatment specifically designed to counteract the negative prognostic effect of executive deficit, Problem Solving Therapy (PST), was compared with supportive therapy in a sample of old-age depressed patients. An advantage was seen for PST, particularly in those patients with greater executive deficit. This study is unique because it is enriched specifically for executive impairment. It therefore addresses an issue which is particularly important in this area—the dilution of findings by inclusion of patients without cognitive impairment (72).
Neurobiological Underpinnings
As mentioned above, there appears to be some support for the notion that executive dysfunction can predict treatment-related outcomes, particularly in the elderly depressed; with deficits in executive functioning/attention predicting poorer or slower response to treatment. One reason for this finding may be that impaired executive function performance serves as a marker for dysfunction within the fronto-limbic circuits. Executive functioning is sub-served by areas within the prefrontal cortex and there is consistent evidence that depression is associated with aberrant neural activity in these brain regions (73, 74). It has been proposed that reduced prefrontal control over limbic activity leads to impaired emotional regulation and maladaptive thinking patterns, such as rumination and worry, all of which are believed to contribute to the development and maintenance of depression (75, 76). Thus, poor performance on executive function tasks may highlight key pathological processes that serve to not only maintain depression but preclude response to treatment.
Methodological Issues
Although not an exhaustive list, the following section will discuss the most pertinent methodological considerations related to the studies included in the current review.
1. Standardized monotherapy vs. open label trials and algorithm-based treatment—We have dealt with the results based on the treatment used. It is important to note that there is a fundamental difference between a trial of a single agent and one which allows changes in treatment based on tolerance and response. In monotherapy, for example, patients may not respond because they cannot tolerate the treatment; something which is unlikely to be directly related to cognitive function. In contrast, open and algorithm-based treatment trials permit changes to treatment if an agent is not tolerated, and the patient may still be classified as a responder. These trials have greater ecological validity and may more accurately reflect the clinical implications of cognitive impairment in determining real-life response. However, they do not give accurate information regarding likelihood of response to one agent compared with another.
2. Length of trial–There is a marked difference between the timing of the clinical outcome between trials (in this review ranging from 24 h to 1 year). On one hand, a short outcome may constrain the time available to respond, making differentiation between patients less likely. However, longer outcome times (e.g., 1 year) allow various other factors to operate, thereby reducing the likelihood of finding a clear association. For example, patients in such studies may respond and then relapse within the time frame of the study. In older-age samples, a longer outcome may also involve development or progression of a neurodegenerative process, meaning that cognitive impairment is in fact associated with further cognitive decline.
3. Choice of outcome measure–Trials have used a variety of ways of measuring response, including percentage reduction in mood rating scale scores, rates of response and rates of remission. The former keeps response on a dimension and is likely to be more sensitive than a binary outcome. Especially in short trials, remission is relatively infrequent and reduces the sensitivity of the analysis; however, it is the optimal and arguably most clinically-relevant outcome. Another related issue is the utilization of multiple outcome measures. This increases the number of comparisons being made and the likelihood that an association may be purely due to chance.
4. Severity of depression at baseline–There are several issues regarding depression severity at baseline. Firstly, placebo response tends to be greater in milder depression, possibly indicating less of a biological basis (77). Response in this case is less likely to be biologically determined and therefore, less likely to be influenced by cognitive impairment. Secondly, a related issue is that in mild to moderate depression, the percentage of patients who have cognitive impairment is relatively low (78); hence, using such a sample will likely dilute findings (see below). Thirdly, in milder depression, the range of possible change on depression rating scales is smaller, which ultimately reduces the likelihood of finding an association between baseline cognitive functioning and change in depressive symptomatology.
5. A related issue is the way in which severity of depression has been accounted for in the analysis of the relationship between cognitive variables and outcome. This is clearly important since the relationship between cognitive function and outcome may be mediated wholly or partly by severity of depression. Most large studies accounted for this by some form of covariate analysis. Other studies simply compared the baseline depression rating score of responders compared with non-responders, and if this was not significantly different, concluded that this was not an important mediator of difference in cognitive function. Clearly, this issue should be addressed in future research.
6. Degree of cognitive impairment at baseline–It is less likely that cognitive function will be associated with outcome in patients whom are not classed as “cognitively impaired.” Having such patients in a study will dilute findings, potentially to the point of a genuine association not being demonstrated. Some of the reviewed studies had control participants and indeed found a difference between controls and the group overall (20, 79), but this does not mean that all, or even a high percentage of patients, were impaired (78). It is likely that studies including more severely unwell patients have a higher percentage of patients with significant cognitive impairment and are therefore, more likely to show an association with response. In the study of Etkin et al. (20), the importance of this phenomenon is illustrated, with the predictive effect of cognitive performance only applied in the group who, compared with healthy controls, were significantly impaired.
7. Cognitive battery–Studies using a more extensive battery of cognitive tasks may be more likely to show an association with response simply because they have used multiple tests, and an association with one of these may simply be a feature of multiple comparisons. However, it could be argued that certain aspects of, for example executive function, may be more likely to affect response than others. Pimontel et al. (80), in a meta-analysis of executive function tasks in the elderly, conclude that only planning and organization (as measured by a subtest of the Dementia Rating Scale) was associated with response. Using composite scores for each domain may reduce the problem of multiple comparisons, but as noted, some may argue that this neglects individual aspects of cognitive functioning. Very short batteries may measure cognitive domains inadequately and result in false negative findings. Additionally, cognitive tasks themselves may vary in their sensitivity and suffer from ceiling effects. For example, the Hopkins Verbal Learning Test may not measure verbal learning and memory with adequate sensitivity, and even the Rey Auditory Verbal Learning Test may be subject to ceiling effects; thus, more sensitive tasks are needed (81).
8. Classification of drop-outs–Outcome can be analyzed either by intention-to-treat or using only completers. Most studies in the review classified patients as responders or remitters based on pre-defined criteria and classified drop-outs as non-responders/remitters. A small number, including one of the largest studies in the review (20), examined change in depression rating scale scores and therefore included in the analysis only patients who completed follow-up rating scales. However, they also undertook an intention-to-treat analysis and a sensitivity analysis, showing that the method of analysis made no difference in this case. The advantage of the former is that it includes all patients and gives a potentially more useful clinical result informing what the likely overall outcome is for patients with differing cognitive profiles. However, the relationship between cognitive function and outcome may be altered by the group of patients who are particularly sensitive to side effects which may not relate to cognitive function.
Limitations
There are several limitations of the current review. Firstly, as is usual in English language-based reviews, only peer-reviewed articles in the English language were included, which may have resulted in some useful sources of evidence being missed. Secondly, given the heterogeneity of outcome measures, the variety of treatment protocols, and the differing ways in which data was presented and analyzed, it was not possible to use a meta-analytic technique. This meant that a quantitative result could not be produced. Thirdly, no formal risk of bias methodology was utilized in the present review (e.g., Cochrane risk of bias tool). However, differences in assessment were discussed between the three co-authors and studies of greater quality were given greater weight in synthesizing the evidence. Further, methodological issues related to the reviewed studies are discussed.
Recommendations for future research
1. Studies examining this issue will have low yield unless they have a significant proportion of patients with significant cognitive impairment. Selection of patients may require a strategy to enrich samples, or simply to recruit more severely depressed samples.
2. The issue of chance findings is important. We suggest that studies employ a priori groupings of variables into domains and utilize composite domain scores in analysis. This reduces the number of variables examined. Secondary analyses can examine individual variables to elicit any more specific signals regarding detailed cognitive functions which may be associated with response.
3. The majority of studies have employed outcome measures of response or remission. Although it can be argued that these are more clinically meaningful, examining response on a dimensional scale (i.e., percentage change in mood rating scale scores) is likely to be more sensitive; thereby, increasing the likelihood of detecting an association between cognitive functioning and response. One way to deal with this issue is to be clear regarding which primary outcome is to be related to cognitive function but to report other associations in secondary analyses, thereby making these data easily accessible for meta-analyses, but avoiding the problem of multiple outcomes analyses.
4. It appears that “cold” (i.e., traditional) cognitive functions have been the main focus in this area of research, with only three studies having assessed the predictive nature of emotional processing. There is strong evidence that depressed individuals experience alterations in emotional processing (e.g., negatively interpreting emotionally laden stimuli) (76, 82). Moreover, “hot” (i.e., emotional) cognitive processes are believed to play a role in the development and maintenance of depressive symptoms. Thus, more focus should be placed on assessing the relationship between emotional processing and treatment response.
Summary, Conclusions and Clinical Implications
In younger patients, the data is inconclusive both regarding the association between cognitive function and response to any treatment, and regarding association with response to specific treatments. The best evidence is for a predictive effect of executive function, and with some support for an association with psychomotor function. There is no evidence of learning or memory being associated with treatment response. The main methodological issue we believe is that samples were relatively mildly depressed and therefore, likely contained few patients with significant cognitive impairment. The evidence in older adults is much more consistent and suggests that poor executive function predicts poor response to SSRIs, with little evidence regarding response to other agents. In line with this, one notable study showed that specifically addressing executive dysfunction in the elderly depressed, had positive effects (12).
It is apparent that this area of research is affected by a number of important methodological issues, which need to be addressed in order to help fully elucidate the relationship between cognitive functioning and treatment outcomes in depression. Nevertheless, the findings from the present review do suggest that certain aspects of cognitive functioning, particularly executive function, may be useful in predicting treatment response in depression. This is certainly the case in older-aged samples, with evidence suggesting that executive dysfunction can predict poor response to SSRI treatment. The findings also indicate a possible rationale for specifically targeting cognitive functioning during treatment, as doing so may result in improved treatment outcomes.
Author Contributions
SG conducted the systematic review of papers and prepared the first draft of the manuscript. RP and KD supervised the systematic review and reviewed and updated subsequent drafts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer RS and handling Editor declared their shared affiliation at the time of the review.
Acknowledgments
We are grateful to the University of Otago for their financial support by providing SG with a University of Otago Doctoral Scholarship.
References
1. GBD 2016. Disease and injury incidence and prevalence collaborators global regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry (2007) 164:739–52. doi: 10.1176/ajp.2007.164.5.739
3. Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs (2009) 23:627–47. doi: 10.2165/00023210-200923080-00001
4. Malhi GS, Bassett D, Boyce P, Bryant R, Fitzgerald PB, Fritz K, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust New Zealand J Psychiatry (2015) 49:1087–206. doi: 10.1177/0004867415617657
5. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
6. Porter RJ, Robinson LJ, Malhi GS, Gallagher P. The neurocognitive profile of mood disorders - a review of the evidence and methodological issues. Bipolar Disord. (2015) 17(Suppl. 2):21–40. doi: 10.1111/bdi.12342
7. Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust New Zealand J Psychiatry (2009) 43:1105–17. doi: 10.3109/00048670903279887
8. Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. (2013) 43:2017–26. doi: 10.1017/S0033291712002085
9. Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology (2015) 40:2025–37. doi: 10.1038/npp.2015.52
10. Watson S, Gallagher P, Porter RJ, Smith MS, Herron LJ, Bulmer SG, et al. A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biol Psychiatry (2012) 72:943–9. doi: 10.1016/j.biopsych.2012.05.029
11. Porter RJ, Bowie CR, Jordan J, Malhi GS. Cognitive remediation as a treatment for major depression: a rationale, review of evidence and recommendations for future research. Aust New Zealand J Psychiatry (2013) 47:1165–75. doi: 10.1177/0004867413502090
12. Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry (2011) 68:33–41. doi: 10.1001/archgenpsychiatry.2010.177
13. Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. (2000) 60:13–23. doi: 10.1016/S0165-0327(99)00157-3
14. Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, et al. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry (2006) 163:73–8. doi: 10.1176/appi.ajp.163.1.73
15. Herrera-Guzman I, Gudayol-Ferre E, Lira-Mandujano J, Herrera-Abarca J, Herrera-Guzman D, Montoya-Perez K, et al. Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psych Res. (2008) 160:72–82. doi: 10.1016/j.psychres.2007.04.012
16. Gudayol-Ferre E, Herrera-Guzman I, Camarena B, Cortes-Penagos C, Herrera-Abarca JE, Martinez-Medina P, et al. The role of clinical variables, neuropsychological performance and SLC6A4 and COMT gene polymorphisms on the prediction of early response to fluoxetine in major depressive disorder. J Affect Disord. (2010) 127:343–51. doi: 10.1016/j.jad.2010.06.002
17. Gudayol-Ferre E, Herrera-Guzman I, Camarena B, Cortes-Penagos C, Herrera-Abarca JE, Martinez-Medina P, et al. Prediction of remission of depression with clinical variables, neuropsychological performance, and serotonergic/dopaminergic gene polymorphisms. Hum Psychopharmacol. (2012) 27:577–86. doi: 10.1002/hup.2267
18. Gudayol-Ferre E, Guardia-Olmos J, Pero-Cebollero M, Herrera-Guzman I, Camarena B, Cortes-Penagos C, et al. Prediction of the time-course pattern of remission in depression by using clinical, neuropsychological, and genetic variables. J Affect Disord. (2013) 150:1082–90. doi: 10.1016/j.jad.2013.04.024
19. Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. (2014) 17:1805–13. doi: 10.1017/S1461145714001011
20. Etkin A, Patenaude B, Song YJ, Usherwood T, Rekshan W, Schatzberg AF, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology (2015) 40:1332–42. doi: 10.1038/npp.2014.333
21. Mikoteit T, Hemmeter U, Eckert A, Brand S, Bischof R, Delini-Stula A, et al. Improved alertness is associated with early increase in serum brain-derived neurotrophic factor and antidepressant treatment outcome in major depression. Neuropsychobiology (2015) 72:16–28. doi: 10.1159/000437439
22. Murrough JW, Wan LB, Iacoviello B, Collins KA, Solon C, Glicksberg B, et al. Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology (2014). 231:481–8. doi: 10.1007/s00213-013-3255-x
23. Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, Chang LC, et al. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: a randomized controlled trial. Neuropsychopharmacology (2015) 40:1084–90. doi: 10.1038/npp.2014.298
24. Clery-Melin ML, Gorwood P. A simple attention test in the acute phase of a major depressive episode is predictive of later functional remission. Depress Anxiety (2017) 34:159–70. doi: 10.1002/da.22575
25. Bastos AG, Guimarães LS, Trentini CM. Predictors of response in the treatment of moderate depression. Revista Brasileira de Psiquiatria (2017) 39:12–20. doi: 10.1590/1516-4446-2016-1976
26. Kalayam B, Alexopoulos GS. A preliminary study of left frontal region error negativity and symptom improvement in geriatric depression. Am J Psychiatry (2003) 160:2054–6. doi: 10.1176/appi.ajp.160.11.2054
27. Doraiswamy PM, Krishnan KR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol Ser A (2003) 58:1137–44. doi: 10.1093/gerona/58.12.M1137
28. Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology (2004) 29:2278–84. doi: 10.1038/sj.npp.1300557
29. Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry (2005) 58:204–10. doi: 10.1016/j.biopsych.2005.04.024
30. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, et al. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport (2007) 18:217–21. doi: 10.1097/WNR.0b013e328013ceda
31. Murphy CF, Alexopoulos GS. Attention network dysfunction and treatment response of geriatric depression. J Clin Exp Neuropsychol. (2006) 28:96–100. doi: 10.1080/13803390490918101
32. Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry (2007) 15:553–63. doi: 10.1097/JGP.0b013e3180302513
33. Sneed JR, Keilp JG, Brickman AM, Roose SP. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. Int J Geriatr Psychiatry (2008) 23:319–23. doi: 10.1002/gps.1889
34. Morimoto SS, Gunning FM, Murphy CF, Kanellopoulos D, Kelly RE, Alexopoulos GS. Executive function and short-term remission of geriatric depression: the role of semantic strategy. Am J Geriatr Psychiatry (2011) 19:115–22. doi: 10.1097/JGP.0b013e3181e751c4
35. Alexopoulos GS, Manning K, Kanellopoulos D, McGovern A, Seirup JK, Banerjee S, et al. Cognitive control, reward-related decision making and outcomes of late-life depression treated with an antidepressant. Psychol Med. (2015) 45:3111–20. doi: 10.1017/S0033291715001075
36. de Groot MH, Nolen WA, Huijsman AM, Bouvy PF. Lateralized neuropsychological functioning in depressive patients before and after drug therapy. Biol Psychiatry (1996) 40:1282–7. doi: 10.1016/0006-3223(95)00654-0
37. Majer M, Ising M, Kunzel H, Binder EB, Holsboer F, Modell S, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. (2004) 34:1453–63. doi: 10.1017/S0033291704002697
38. Gallagher P, Robinson LJ, Gray JM, Porter RJ, Young AH. Neurocognitive function following remission in major depressive disorder: potential objective marker of response? Aust New Zealand J Psychiatry (2007) 41:54–61. doi: 10.1080/00048670601057734
39. Gorlyn M, Keilp JG, Grunebaum MF, Taylor BP, Oquendo MA, Bruder GE, et al. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transmiss. (2008) 115:1213–9. doi: 10.1007/s00702-008-0084-x
40. Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol. Med. (2009) 39:393–402. doi: 10.1017/S0033291708003620
41. Spronk D, Arns M, Barnett KJ, Cooper NJ, Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: a pilot study. J Affect Disord. (2011) 128:41–8. doi: 10.1016/j.jad.2010.06.021
42. Talarowska M, Zboralski K, Galecki P. Correlations between working memory effectiveness and depression levels after pharmacological therapy. Psychiatria Polska (2013) 47:255–67.
43. Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, et al. Neurocognitive predictors of antidepressant clinical response. J Affect Disord. (2014) 166:108–14. doi: 10.1016/j.jad.2014.04.057
44. Lin K, Xu G, Lu W, Ouyang H, Dang Y, Lorenzo-Seva U, et al. Neuropsychological performance in melancholic, atypical and undifferentiated major depression during depressed and remitted states: a prospective longitudinal study. J Affect Disord. (2014) 168:184–91. doi: 10.1016/j.jad.2014.06.032
45. Crane NA, Jenkins LM, Bhaumik R, Dion C, Gowins JR, Mickey BJ, et al. Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain (2017) 140:472–86. doi: 10.1093/brain/aww326
46. Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch General Psychiatry (1999) 56:713–8. doi: 10.1001/archpsyc.56.8.713
47. Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology (2004) 29:2266–71. doi: 10.1038/sj.npp.1300551
48. Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry (2008) 16:752–9. doi: 10.1097/JGP.0b013e31817e739a
49. Bingham KS, Whyte EM, Meyers BS, Mulsant BH, Rothschild AJ, Banerjee S, et al. Relationship between cerebrovascular risk, cognition, and treatment outcome in late-life psychotic depression. Am J Geriatr Psychiatry (2015) 23:1270–5. doi: 10.1016/j.jagp.2015.08.002
50. Kundermann B, Hemmeter-Spernal J, Strate P, Gebhardt S, Huber M, Krieg JC, et al. Neuropsychological predictors of the clinical response to cognitive-behavioral therapy in patients with major depression. Zeitschrift Neuropsychol. (2015) 26:87–98. doi: 10.1024/1016-264X/a000130
51. Martin DM, Yeung K, Loo CK. Pre-treatment letter fluency performance predicts antidepressant response to transcranial direct current stimulation. J Affect Disord. (2016) 203:130–5. doi: 10.1016/j.jad.2016.05.072
52. McInerney SJ, McNeely HE, Geraci J, Giacobbe P, Rizvi SJ, Ceniti AK, et al. Neurocognitive predictors of response in treatment resistant depression to subcallosal Cingulate gyrus deep brain stimulation. Front Hum Neurosci (2017) 11:74. doi: 10.3389/fnhum.2017.00074
53. Beaudreau SA, Rideaux T, O'Hara R, Arean P. Does cognition predict treatment response and remission in psychotherapy for late-life depression? Am J Geriatr Psychiatry (2015) 23:215–9. doi: 10.1016/j.jagp.2014.09.003
54. Morimoto SS, Gunning FM, Wexler BE, Hu W, Ilieva I, Liu J, et al. Executive dysfunction predicts treatment response to neuroplasticity-based computerized cognitive remediation (nCCR-GD) in elderly patients with major depression. Am J Geriatr Psychiatry (2016) 24:816–20. doi: 10.1016/j.jagp.2016.06.010
55. Marcos T, Portella MJ, Navarro V, Gasto C, Rami L, Lazaro L, et al. Neuropsychological prediction of recovery in late-onset major depression. Int J Geriatr Psychiatry (2005) 20:790–5. doi: 10.1002/gps.1363
56. Saveanu R, Etkin A, Duchemin AM, Goldstein-Piekarski A, Gyurak A, Debattista C, et al. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. (2015) 61:1–12. doi: 10.1016/j.jpsychires.2014.12.018
57. Blier P, Saint-Andre E, Hebert C, de Montigny C, Lavoie N, Debonnel G. Effects of different doses of venlafaxine on serotonin and norepinephrine reuptake in healthy volunteers. Int J Neuropsychopharmacol. (2007) 10:41–50. doi: 10.1017/S1461145705006395
58. Debonnel G, Saint-Andre E, Hebert C, de Montigny C, Lavoie N, Blier P. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. Int J Neuropsychopharmacol. (2007) 10:51–61. doi: 10.1017/S1461145705006413
59. Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Therapeut. (1997). 283:1305–22.
60. Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet (2009) 373:746–58. doi: 10.1016/S0140-6736(09)60046-5
61. Parker G. Classifying depression: should paradigms lost be regained? Am J Psychiatry (2000) 157:1195–203. doi: 10.1176/appi.ajp.157.8.1195
62. Parker G, Blanch B, Paterson A, Hadzi-Pavlovic D, Sheppard E, Manicavasagar V, et al. The superiority of antidepressant medication to cognitive behavior therapy in melancholic depressed patients: a 12-week single-blind randomized study. Acta Psychiatr Scandanavica (2013) 128:271–81. doi: 10.1111/acps.12049
63. Parker G, Bassett D, Outhred T, Morris G, Hamilton A, Das P, et al. Defining melancholia: a core mood disorder. Bipolar Disord. (2017) 19:235–7. doi: 10.1111/bdi.12501
64. Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol. Psychiatry (2006) 59:898–907. doi: 10.1016/j.biopsych.2006.03.004
65. Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry (2015b) 5:e509. doi: 10.1038/tp.2015.10
66. Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry (2000) 48:627–40. doi: 10.1016/S0006-3223(00)00976-8
67. Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, et al. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Res. (2006) 142:139–50. doi: 10.1016/j.psychres.2005.08.010
68. Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. (1998) 28:1027–38. doi: 10.1017/S0033291798006862
69. Gorwood P, Corruble E, Falissard B, Goodwin GM. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry (2008) 165:731–9. doi: 10.1176/appi.ajp.2008.07040574
70. Alexopoulos GS, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan KR. Comorbidity of late life depression: an opportunity for research on mechanisms and treatment. Biol Psychiatry (2002) 52:543–58. doi: 10.1016/S0006-3223(02)01468-3
71. Thomas AJ, Gallagher P, Robinson LJ, Porter RJ, Young AH, Ferrier IN, et al. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychol Med. (2009) 39:725–33. doi: 10.1017/S0033291708004042
72. Miskowiak KW, Burdick KE, Martinez-Aran A, Bonnin CM, Bowie CR, Carvalho AF, et al. Methodological recommendations for cognition trials in bipolar disorder by the International Society for bipolar disorders targeting cognition task force. Bipolar Disord. (2017) 19:614–26. doi: 10.1111/bdi.12534
73. Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. (2002) 53:545–74. doi: 10.1146/annurev.psych.53.100901.135148
74. Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cogn Ther Res. (2007) 31:235–62. doi: 10.1007/s10608-006-9118-6
75. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. (2010) 6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305
76. Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology (2012) 37:117–36. doi: 10.1038/npp.2011.183
77. Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. (2002) 22:40–5. doi: 10.1097/00004714-200202000-00007
78. Douglas KM, Gallagher P, Robinson LJ, Carter JD, McIntosh VV, Frampton CM, et al. Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disord. (2018). doi: 10.1111/bdi.12602
79. Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry (2016) 3:425–35. doi: 10.1016/S2215-0366(16)00012-2
80. Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP, Sneed JR. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psychiatry (2016) 24:31–41. doi: 10.1016/j.jagp.2015.05.010
81. Bourke C, Porter RJ, Carter JD, McIntosh VV, Jordan J, Bell C, et al. Comparison of neuropsychological functioning and emotional processing in major depression and social anxiety disorder subjects, and matched healthy controls. Australian New Zealand J Psychiatry (2012) 46:972–81. doi: 10.1177/0004867412451502
Keywords: major depression, cognitive predictors, cognitive function, treatment response, relapse, remission, executive function
Citation: Groves SJ, Douglas KM and Porter RJ (2018) A Systematic Review of Cognitive Predictors of Treatment Outcome in Major Depression. Front. Psychiatry 9:382. doi: 10.3389/fpsyt.2018.00382
Received: 03 May 2018; Accepted: 30 July 2018;
Published: 28 August 2018.
Edited by:
Allan Young, King's College London, United KingdomReviewed by:
Rebecca Strawbridge, King's College London, United KingdomAndre F. Carvalho, Centre for Addiction and Mental Health, Canada
Copyright © 2018 Groves, Douglas and Porter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard J. Porter, richard.porter@otago.ac.nz
 Samantha J. Groves
Samantha J. Groves Katie M. Douglas
Katie M. Douglas Richard J. Porter
Richard J. Porter