- 1Department of Psychiatry, Rady Faculty of Health Sciences, Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada
- 2School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 3Centre for Addiction and Mental Health and The Hospital for Sick Children, Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 4Autism Research Centre, Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom
- 5Department of Psychiatry, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan
Immune dysfunction and abnormal immune response may be associated with certain mechanisms underlying autism spectrum disorder (ASD). The early evidence for this link was based on the increased incidence of ASD in children with a history of maternal infection during pregnancy. Observational studies show increased prevalence of immune-related disorders—ranging from atopy, food allergy, viral infections, asthma, primary immunodeficiency, to autoimmune disorders—in individuals with ASD and their families. Evidence of neuroglial activation and focal brain inflammation in individuals with ASD implies that the central nervous system immunity may also be atypical in some individuals with ASD. Also, both peripheral and central inflammatory responses are suggested to be associated with ASD-related behavioral symptoms. Atypical immune responses may be evident in specific ASD subgroups, such as those with significant gastrointestinal symptoms. The present review aimed to evaluate current literature of potential interventions that target inflammatory pathways for individuals with ASD and to summarize whether these interventions were associated with improvement in autism symptoms and adaptation. We found that the current literature on the efficacy of anti-inflammatory interventions in ASD is still limited and large-scale randomized controlled trials are needed to provide robust evidence. We concluded that the role of immune-mediated mechanisms in the emergence of ASD or related challenges may be specific to subsets of individuals (e.g. those with concurrent immunological disorders, developmental regression, or high irritability). These subsets of individuals of ASD might be more likely to benefit from interventions that target immune-mediated mechanisms and with whom next-stage immune-mediated clinical trials could be conducted.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by early onset social-communication challenges, repetitive, stereotypical behaviors, and idiosyncratic sensory responsivity (1). Although several genetic and environmental factors have been linked to ASD, the underlying pathophysiology is still not well-understood (2). To date, although there have been great progress in developing behavioral and psychological support for individuals with ASD, no approved medication therapy exist for the core symptoms; established medication treatment aims at reducing concurrent challenges, such as increased irritability (3). Considering the heterogeneous nature of this condition and the paucity of biomarkers, development of a safe and effective “one-size-fits-all” medical therapy is challenging.
Several lines of evidence suggest a role of inflammation in the underlying developmental mechanisms of ASD (4–6). Epidemiological studies show an association between maternal infection [such as influenza (7) or cytomegalovirus (8)] during pregnancy and increased risk of offspring autism (9, 10). Also, family history of autoimmune diseases is associated with higher rate of ASD (11). In the animal models of ASD, offspring of animals with infection during pregnancy present physiological and behavioral alterations resemble ASD symptoms in human (12,13). People with ASD are suggested to have altered levels of inflammatory markers in which pro-inflammatory markers tend to increase while anti-inflammatory markers decrease (14, 15). Furthermore, postmortem studies of inflammation in the brain suggest alterations of brain immune response in ASD, including increased levels of proinflammatory markers (16) and increased microglial activation (17–19). Suzuki and colleagues showed increased microglial activation in the brain of individuals with ASD (20). Also, multiple studies on CSF and plasma/serum samples of individuals with ASD reported increased levels of proinflammatory markers (4, 21–23). Genetic studies reveal association between genomic variations in the immune-related genes and ASD (24). For example, human leucocyte antigen (HLA) DRB1 alleles are suggested to be associated with a higher risk of autism (25, 26). There is an emerging consensus that a better understanding of inflammation and its relation with the underlying pathophysiology of autism can provide new insight into the underlying mechanisms of the challenges faced by at least a subgroup of people with ASD (27), and help with developing new treatments. Given the current evidence, a growing body of literature has examined the role of medications with anti-inflammatory effects in the treatment of ASD-associated challenges, either alone or as an adjuvant therapy. In this narrative review, we aimed to summarize studies exploring the use of medications that target inflammatory pathways for people with ASD and evaluate the effectiveness and side effect profiles. Both medications with primary anti-inflammatory action (e.g. celecoxib) and those with additional anti-inflammatory properties beside their primary mechanisms of action (e.g. minocycline) have been included. These medications were selected based on the previous evidence indicating their anti-inflammatory properties in other psychiatric or medical disorders. Due to the availability of up-to-date meta-analysis on some of these agents, such as polyunsaturated fatty acids (28–31), they were not included in this review.
Methods
This narrative review aimed to summarize the available evidence on the role of anti-inflammatory medications in the management of challenges associated with ASD. The literature search was performed using PubMed in July 2018. No publication date restriction was applied. We only included peer-reviewed studies on human subjects (with no age limitation) that were published in English language. The following search terms were used: (autism OR autistic OR autism spectrum disorder OR ASD OR pervasive OR pervasive developmental disorder OR pervasive developmental disorders OR Asperger OR Asperger’s) AND (celecoxib OR minocycline OR NAC OR N-acetylcysteine OR ACTH OR prednisone OR prednisolone OR hydrocortisone OR methylprednisolone OR dexamethasone OR cortisone OR triamcinolone OR betamethasone OR flavonoid OR lenalidomide OR pentoxifylline OR pioglitazone OR IVIG OR spironolactone OR topiramate OR memantine OR amantadine OR galantamine OR riluzole OR palmitoylethanolamide). We also identified additional articles by reference list/hand searching.
Studies of Medications Targeting Inflammatory Pathways in People With ASD (In Alphabetical Order)
Amantadine
Amantadine is an antiviral and anti-Parkinson medication that is widely used in the management of central nervous system disorders including multiple sclerosis (32) and traumatic brain injury (33). This medication is shown to be neuroprotective by the following mechanisms: (1) anti-inflammatory properties, mainly by inhibiting the release of proinflammatory factors (34, 35); (2) increasing the level of neurotrophic factors; and (3) inhibiting effect on N-methyl-D-aspartate (NMDA) receptors (34). In a placebo-controlled trial, King and colleagues studied the efficacy of amantadine in the management of ASD (36). They administered amantadine (n = 19) or placebo (n = 20) to individuals with ASD (age range of 5–19 years) over 4 weeks. The efficacy was measured using the Aberrant Behavior Checklist (ABC) and the Clinical Global Impression (CGI) scales. Amantadine group had slightly higher percentage of responders (equal or more than 25% reduction in irritability and/or hyperactivity according to parent-rated ABC). Based on the clinician-rated ABC, the amantadine group had significantly more improvement in absolute scores of hyperactivity and inappropriate speech. Also, amantadine group had higher improvement according to their CGI score. The side effects did not significantly differ between the amantadine and placebo groups.
Mohammadi and colleagues in a randomized controlled trial on children with severe behavioral issues (i.e. disruptive symptoms such as irritability) related to autism (age range of 4–12 years), compared risperidone plus amantadine (n = 20) with risperidone plus placebo (n = 19) over a 10-week period (37). They observed a significant improvement in hyperactivity and irritability as measured by ABC in the amantadine group as compared to placebo. Also, the amantadine group had significantly more improvement than the placebo group based on the CGI scores. Side effects were not significantly different between the groups.
Celecoxib
Celecoxib is a nonsteroidal anti-inflammatory drug that selectively inhibits cyclooxygenase-2 (COX-2) enzyme. It has been widely studied as an adjuvant therapy in several psychiatric disorders including schizophrenia and depression (38, 39). In a randomized and double-blind controlled trial, Asadabadi and colleagues investigated the effect of celecoxib as an adjuvant therapy to risperidone in the treatment of children (age range of 4–12 years) with ASD and severe behavioral issues over 10 weeks. They used ABC rating scale to assess the clinical symptoms (40) (Table 1). Autistic children under treatment with celecoxib as an adjuvant therapy (n = 20) had significantly improved scores in irritability, social withdrawal/lethargy, and stereotypic behavior compared to the placebo group (n = 20). The study groups did not differ in terms of inappropriate speech, hyperactivity/non-compliance, or medications side effects.
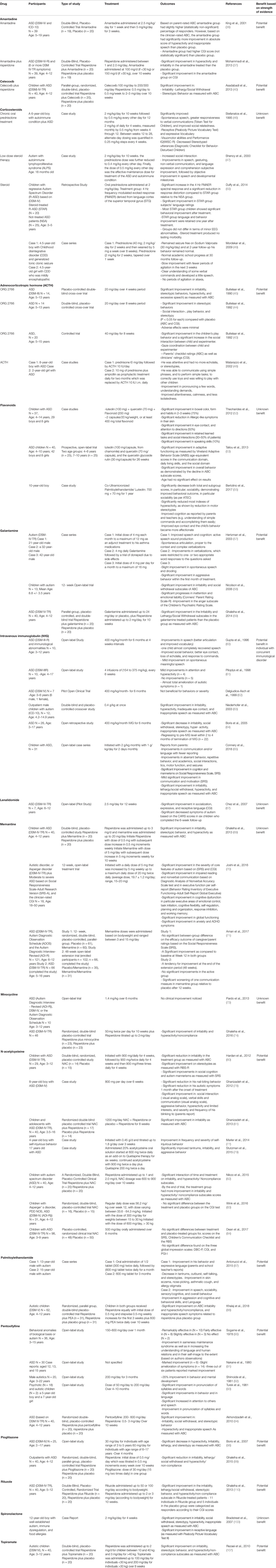
Table 1 Summary of the studies on the role of anti-inflammatory medications in the management of ASD.
Corticosteroids and ACTH
Corticosteroids have been effectively used in the management of neurological disorders, such as Landau–Kleffner syndrome (LKS) (41). Due to similarities in the progression and development between LKS and regressive ASD, and considering the growing evidence on the link between inflammation and ASD, a number of studies have investigated the effects of corticosteroids or adrenocorticotrophic hormone (ACTH) in regressive ASD (42). In a series of trials, Buitelaar and colleagues studied the role of ORG 2766, an ACTH analog, in the management of children with ASD (43–45). In the first trial, they enrolled 14 children (age range of 5–13 years) with ASD in a double-blind crossover study (45). ORG 2766 was administered over a 4-week period. They reported significant improvement in clinical symptoms (i.e. irritability, stereotypic behaviors, hyperactivity, and excessive speech) as measured by the parent-reported ABC and playroom data. In the second crossover trial, they reported positive effects of ORG 2766 (administered over an 8-week period) on symptoms of 20 children (age range of 5–15 years) with ASD as measured by ABC and CGI (43). In their third study, they reported the effect of ORG 2766 on social interactions of autistic children enrolled in their first trial (44). They found that ORG 2766 therapy resulted in a significant increase in the quality and quantity of social interactions in the participants.
In a single-case study, Stefanatos and colleagues administered corticosteroid (i.e. prednisone) to a 6-year-old boy with regressive ASD over a 28-month period (46). The patient started losing his language abilities at age 22 months. The medical and neurological assessments were mostly unremarkable except for hypoperfusion of perisylvian cortical region in SPECT and abnormal steady-state auditory evoked potentials. Corticosteroid therapy resulted in significant improvement in language, social abilities, and stereotypic behaviors.
More recently, Shenoy and colleagues reported a case of regressive ASD that was diagnosed at the age of 18 months (47). He presented with progressive lymphadenopathy, microcytic anemia, mild thrombocytopenia, and low white blood cells count. He was started on corticosteroid at the age of 33 months. After about a month of steroid therapy, the patient started regaining his language and communication abilities. After 26 months of therapy, all the laboratory values returned back to normal.
Matarazzo described two cases of regressive ASD with histories of recurrent bacterial infections (48). Both individuals were initially started on corticosteroid therapy and later due to the side effects, were switched to ACTH. In both cases, corticosteroid therapy led to improvement in language and communication skills, as well as behavioral symptoms, such as stereotypic behaviors.
Mordekar and colleagues reported two cases of regressive ASD treated with corticosteroid (49). The first case was a 4.5-year-old boy with ASD and generalized tonic–clonic seizure that regained his personality and language abilities after treatment with corticosteroid. The second case was a 4-year-old girl with ASD who lost her language and communication abilities after experiencing neurological symptoms associated with ataxia and fluctuation of consciousness. Her symptoms started to improve after three weeks of treatment with prednisolone. Forty-eight months after treatment, she was back to her normal function and personality.
Most recently, a retrospective study (age range of participants, 3–5 years) compared 20 children with regressive ASD treated with corticosteroids (for a maximum period of 4 months) with 24 autistic children without corticosteroid treatment; the authors observed significant improvement in the frequency modulated auditory evoked response, language function, and ASD symptom score according to DSM-IV criteria after corticosteroid therapy (50).
Taken together, there is a lack of randomized controlled trials to inform the clinical utility of corticosteroid in individuals with ASD. Single case, case series, and observational studies indicate the potential of corticosteroid or ACTH as a treatment for regressive ASD, but this awaits rigorous controlled trials to validate and to investigate the potential underlying mechanisms of action.
Flavonoids
Flavonoids are a large group of nutrients found in vegetables and fruits. They are shown to have anti-inflammatory effects through inhibition of inflammatory pathways, including the synthesis/activity of c-reactive protein, proinflammatory cytokines, and microglia (51). Theoharides and colleagues studied the effects of a flavonoids mixture (luteolin, quercetin, and rutin) in an open-label case-series of 37 children (age range of 4–14 years) with ASD over a period of 4 months (52). They reported improvement in attention and eye contact (50%), allergy and gastrointestinal symptoms (75%), and social interaction (25%). No side effects due to the treatment were noted. More recently, using similar supplement, Taliou and colleagues in an open-label trial studied 40 children (age range of 4–10 years) with ASD over a 26-week period (53). They reported significant improvement in communication, daily living skills, and social skills as measured by the Vineland Adaptive Behavior Scales (VABS) and also in hyperactivity, irritability, lethargy, and stereotypical behavior as measured by the ABC. More recently, the same group using the same database reported that children with ASD that had the most behavioral improvement were the ones that had reduction in the serum levels of IL-6 and TNF following treatment with luteolin (54). Most recently, Bertolino and colleagues reported a single case of a 10-year-old boy with regressive ASD and history of recurrent febrile seizure who underwent treatment with a flavonoid supplement (Palmitoylethanolamide/Luteolin) over a 12-month period (55). Treatment with the flavonoid supplement led to improvement in ASD symptoms as measured by ASD treatment evaluation checklist (ATEC) and a scale for stereotypic behavior and the frequency of enuresis. No side effects were reported.
Overall, there may be a potential for the use of flavonoid in the management of individuals with ASD that need to be further examined in robust clinical trials. Due to insufficient evidence, no clinical recommendations can be made so far with regard to the use of flavonoids.
Galantamine
Galantamine is an acetylcholinesterase inhibitor approved for the treatment of Alzheimer’s disease. It also has anti-inflammatory properties by inhibiting the pro-inflammatory markers, such as TNF and cytokines (56). Hertzman studied the effect of galantamine on three adults with autism (57). The first patient was a 21-year-old male. Treatment with 4 mg of galantamine for a month led to improvement in verbal communication and the patient started making sound and responding. The second case was a 32-year-old male that after treatment with 4 mg of galantamine presented with improvement in verbalization; however, he experienced allergic reactions and the medication was discontinued. The third case was a 42-year-old male. He presented aggressive behaviors and never had verbal communication. Treatment with 4 mg of galantamine within a month led to reduction of aggressive behaviors. In a more recent study, Nicolson and colleagues studied galantamine effects in an open-label trial on 13 children (mean age, 8.8 ± 3.5 years) with autism over a 12-week period (58). Children presented significant improvement in irritability and social withdrawal as measured by ABC and emotional liability and attention as measured by Conners’ parent-rating scale. Moreover, children presented improvement in aggression as measured by the children’s psychiatric rating scale. Except for headache in one child, galantamine treatment was well tolerated. In the only randomized controlled trial available, Ghaleiha and colleagues compared risperidone plus galantamine (n = 20) with risperidone plus placebo (n = 20) over a 10-week period in children (age range of 4–12 years) with ASD (59). They observed a significant improvement in irritability and lethargy/social withdrawal subscales of ABC with galantamine as compared to placebo. The side effects were not significantly different between the groups. These initial findings indicate the potential to further evaluate the benefit and safety of galantamine for autistic individuals in robust clinical trials.
Intravenous Immunoglobulin
Several open-label trials have investigated intravenous immunoglobulin (IVIG) therapy in ASD, particularly those with concomitant immunological deficits. Gupta and colleagues administered IVIG to 10 children (age range of 3–6 years) with ASD and IgG deficiency and/or high levels of maternal rubella antibody at 4 weeks interval for a minimum period of 6 months (60). Behavior, cognition, and developmental characteristics of these children were evaluated using Peabody picture vocabulary test, the VBAS, skill evaluation, and preschool language test. Treatment with IVIG resulted in improvement in social behavior, eye contact, echolalia, response to commands, and speech.
Later, Plioplys and colleagues studied IVIG administration in 10 children (age range of 4–17 years) with ASD and immunologic abnormalities (61). The IVIG infusions were administered every 6 weeks. Six children received four infusions and one individual each received 1, 3, 5, and 6 infusions. While five individuals did not experience any changes in their symptoms, four children were reported by their parents to have mild improvement in attention and hyperactivity. One individual had a significant improvement in social behavior and language in a step-wise fashion after each infusion. However, the clinical improvement reversed 2 months after the last (6th) infusion, and he again presented with the same severe autistic symptoms as he presented before participating in the study.
DelGiudice-Asch and colleagues administered IVIG to seven children (age range of 3.5–6 years) with ASD without known immunological anomalies at monthly intervals over a 6-month period (62). Five children received the six infusions and finished the study. Overall there were no significant improvement in any of the clinical symptoms as measured by Ritvo-Freeman Real Life rating scale, the children Yale-Brown Obsessive-Compulsive Scale, CGI scale for autistic disorder, and the ASD modification of the NIMH global obsessive-compulsive scale.
In a double-blind, placebo-controlled crossover study, Niederhofer and colleagues studied the effects of a single dose of IVIG on clinical symptoms of 12 children (age range of 4.2–14.9 years) with ASD as measured by ABC (63). The participants were medication free and had no immunological disorders. They found a significant improvement in parent-reported irritability, hyperactivity, inadequate eye contact, and inappropriate speech as measured by ABC and drowsiness and decreased activity as measured by the symptom checklist. However, none of the clinician ratings showed significant differences between placebo and IVIG groups. No remarkable side effects were noted.
In an open-label study, Boris and colleagues administered IVIG to 26 children (age range of 3–17 years) with ASD (with history of developmental regression) at a monthly basis for 6 months (64). They observed a significant improvement in total aberrant score (37%), hyperactivity (39%), inappropriate speech (25%), irritability (42%), lethargy (35%), and stereotypic behavior (28%) on the ABC. Only a small number of the participants experienced side effects. However, most children’s behavior returned to their pre‐IVIG status within 2–4 months of discontinuing the IVIG.
In an open-label case-series study, Connery and colleagues administered IVIG to 31 children (mean age of 9 years and 9 months with standard deviation of 4 years and 5 months) with ASD and autoimmune encephalopathy on a monthly basis (65). Clinical outcomes were measured using ABC and the Social Responsiveness Scale (SRS) as completed by the caretakers. IVIG treatment resulted in significant improvement in cognition and mannerism sub-scales and the total score of SRS. Moreover, they reported a significant improvement in irritability, lethargy, hyperactivity, and speech sub-scores as well as the total score of ABC. The most common side effects were headache and vomiting, which were common but limited to the infusion period.
Taken together, current evidence suggests potential benefit of IVIG in children with ASD and concomitant immunological disorders, such as autoimmune encephalopathy; however, given the common side effects and the invasiveness of the intervention, IVIG treatment needs to be considered cautiously, weighing the risks and benefits. More robust clinical trials in large cohort are required to further establish the evidence. For children with ASD without known immunological deficits, current evidence is inconsistent and insufficient, and therefore, there is no clear rationale so far to recommend IVIG treatment for this subpopulation of ASD.
Lenalidomide
Lenalidomide, a derivative of thalidomide, is an immunomodulatory medication widely used in the treatment of hematologic disorders and malignancies (66). In an open-label study, Chez and colleagues administered lenalidomide to seven male children aged 6 to 12 years with ASD and history of developmental regression and elevated levels of TNF-alpha, for a 12-week period (67). They observed a significant improvement in ASD symptoms as measured by the Childhood Autism Rating Scale (CARS) and also in expressive language as measured by CGI. Moreover, although not significant, treatment with lenalidomide decreased the levels of TNF-alpha in both serum and CSF. However, it is important to note that among the seven children enrolled, two developed a rash and discontinued the study, and an additional one discontinued from the study after eight weeks due to transient drop of absolute neutrophil count to <1500.
Memantine
Memantine is an NMDA receptor blocker with suggested neuroprotective and anti-inflammatory effects (68). Ghaleiha and colleagues in a randomized controlled trial compared treatment with memantine plus risperidone (n = 20) with risperidone plus placebo (n = 20) in children (age range of 4–12 years) with ASD (69). They reported significant improvements in irritability, stereotypic behavior, and hyperactivity/non-compliance as measured by ABC in the memantine group compared to the placebo group. They did not find any significant difference in the side effects between groups. More recently, an open-label study evaluated the efficacy and tolerability of memantine on 18 adults (age range of 18–50 years) with autism over a 12-week period (70). Treatment with memantine was associated with significant improvement in autism severity as measured by the SRS, CGI, MGH ASD rating scale, and brief psychiatric rating scale as well as anxiety, ADHD symptoms, nonverbal communication (measured by Diagnostic Analysis of Nonverbal Accuracy Scale test) and in executive function (measured by the Behavioral Rating Inventory of Executive Functioning and Cambridge neuropsychological test automated battery). Treatment with memantine was not associated with any serious adverse event. Most recently, in the largest randomized controlled trials so far on the efficacy of memantine in children (age range of 6–12 years) with autism, Aman and colleagues compared treatment with memantine (n = 60) with placebo (n = 61) over a 12-week period (71). They did not observe any significant clinical improvement as measured by the SRS, although in the 48-week open-label follow up, a trend of improvement was observed. They reported two serious adverse events during the study that both were recognized to be unrelated to the treatment.
Taken together, the current evidence does not support a role for memantine in the treatment of core autism characteristics and associated challenges. It is notable that some large-scale randomized controlled trials are still ongoing (e.g. https://clinicaltrials.gov/ct2/show/NCT01972074).
Minocycline
Minocycline is a tetracycline-class antibiotic with anti-inflammatory effects (72). In an open-label trial, Pardo and colleagues investigated the effect of minocycline on clinical measures (as measured by CGI and VABS) and blood/cerebrospinal fluid (CSF) growth factors and markers of inflammation in autistic children (age range of 3–12 years) with history of regression (n = 10) over a 6-month period (73). While minocycline did not have a significant effect on core autism symptoms and adaptive functioning, it significantly reduced IL-8, an anti-inflammatory cytokine, and brain-derived neurotrophic factor (BDNF) in the CSF, and BDNF level (as normalized by alpha-2 macroglobulin level) in serum. In contrast to BDNF, hepatic growth factor (HGF) in the CSF significantly increased after treatment with minocycline.
In a randomized placebo-controlled trial, Ghaleiha and colleagues evaluated the effect of minocycline as an adjunctive therapy to risperidone in autistic children over a 10-week period (74). Minocycline significantly improved scores on irritability and hyperactivity/noncompliance on the ABC; however, it did not have any significant effect on lethargy/social withdrawal, stereotypic behavior, and inappropriate speech. The authors did not report any significant difference in side effects between groups. The observed difference in the effects of minocycline on clinical presentations of the participants in the above studies might be due to the difference in the administered dosage, 1.4 mg/kg/day in the Pardo et al. study (73) versus 100 mg/day in the Ghaleiha et al. study (74).
Taken together, there is still insufficient evidence to support the use of minocycline in the treatment of core autism symptoms or associated challenges.
N-Acetylcysteine
There is a growing interest in the potential use of N-acetylcysteine (NAC) in the treatment of psychiatric disorders (75). NAC acts as a precursor for glutathione, the most abundant antioxidant in the brain and is shown to have both anti-oxidant and anti-inflammatory properties in the body (76). Several case reports and trials have investigated the effect of NAC in individuals with ASD.
In a case study, Marler and colleagues administered NAC to a 4-year-old autistic child with self-injurious behavior over a 3-week period and observed improvement in frequency and severity of self-injurious behavior (77). In another case study, Ghanizadeh and Derakhshan examined NAC effects on clinical symptoms of an 8-year-old boy with ASD over a 6-week period (78). They observed significant improvement in social impairment, social interaction, nail-biting behavior, verbal skills and communication, tics, and aggression as measured with visual analog scale by the parents. Stutzman and Dophiede studied a 17-year-old boy with ASD and intellectual disability who was treated with NAC as an adjuvant therapy to quetiapine over 6 weeks (79). Treatment with NAC led to a reduction in irritability and aggressive behaviors.
Several small-scale clinical trials suggest a potentially positive role for NAC. Hardan and colleagues in a 12-week randomized controlled trial treated children (age range of 3.2–10.7 years) with ASD with NAC (n = 14) or placebo (n = 15) (80). They observed a significant improvement in irritability (as measured by the ABC) and a trend toward significance on stereotypic/repetitive behaviors (as measured by ABC and Repetitive Behavior Scale-Revised) in the NAC compared to the placebo group. Additionally, NAC significantly improved mannerism score (as measured by the SRS). Groups did not significantly differ in terms of medication side effects. In a more recent randomized controlled trial, Ghanizadeh and colleague observed a significant improvement in irritability score (as measured by ABC), but not the core autism symptoms, in children (age range of 3.5–16 years) with ASD treated with risperidone and NAC (n = 17) as compared to risperidone and placebo (n = 14) over an 8-week period (81). In this trial, the most commonly reported side effects were constipation, increased appetite, fatigue, nervousness, and daytime drowsiness. In a similar study, Nikoo and colleagues compared autistic children (age range of 4–12 years) under treatment with risperidone and NAC (n = 20) with those treated with risperidone and placebo (n = 20) over a 10-week period (82). They observed a significant improvement in irritability and hyperactivity/noncompliance as measured by the ABC. No significant differences in side effects were found between the groups. Wink and colleagues in a 12-week randomized controlled trial compared autistic children (age range of 4–12 years) on NAC (n = 16) versus placebo (n = 15) (6 patients were later excluded due to adverse effects or losing follow up) in terms of their social impairment as measured by CGI scale, and also oxidative stress markers in the blood (83). They did not find any significant difference between groups with regard to their CGI, ABC, SRS, or VABS-II scores. However, they observed a significant increase in the level of glutathione and a trend toward significance for increase in the level of oxidized glutathione (GSSG) in the NAC group.
Dean and colleagues in a randomized controlled trial compared treatment with NAC versus placebo in 98 children (age range of 3.1–9.9 years) with ASD (50 in the placebo group, 48 in the NAC group) (84) and observed no significant effect of NAC on their primary (i.e. SRS, RBS-R, and children’s communication checklist (CCC-2)) or secondary (i.e. CGI or developmental behavior checklist) outcomes. There was no significant difference in the frequency or severity of adverse events between groups.
Overall, although initial small-scale clinical trials show the potential of NAC in reducing irritability in children with ASD (and possibly some aspects of autistic characteristics in the repetitiveness domain), follow-up larger-scale trials so far fail to show impact on ASD core characteristics. Considering the relatively safe side effect profile, NAC may have a positive role in the treatment of irritability in autistic children (85), but so far there is no evidence supporting its use in targeting ASD core symptoms.
Palmitoylethanolamide
Palmitoylethanolamide is a fatty acid amide with neuroprotective, anti-inflammatory, and anti-nociceptive properties that exert its effect through the peroxisome proliferator-activated receptors (PPAR)-dependent pathway (86). In a case report, Antonucci and colleagues studied efficacy of palmitoylethanolamide on two adolescents with autism. The first was a 13-year-old male with regressive autism and history of eczema and allergy. After treatment with palmitoylethanolamide (Normast®; initially 600 mg daily and then 1200 mg daily) for 1 month, they observed a significant improvement in the CARS-2 score, behavior (i.e. tantrums, stereotyped behaviors, self-talking, outbursts), language abilities, and allergic symptoms (87). The second child was a 15-year-old male with a history of epilepsy. Treatment with Normast for three months led to significant improvement in ATEC score, aggression, cognition, and behavioral skills as well as blood IgE levels. In a randomized controlled trial, Khalaj and colleagues compared risperidone plus palmitoylethanolamide versus risperidone plus placebo over a 10-week period in children (age range of 4–12 years) with ASD (88). Treatment with palmitoylethanolamide resulted in significant improvement in ABC irritability and hyperactivity/noncompliance compared to placebo. The groups did not differ with regard to the side effects.
Taken together, more randomized controlled trials are needed to evaluate the use of palmitoylethanolamide in the treatment of core autism characteristics or associated challenges, as current evidence is still too limited to support its clinical use.
Pentoxifylline
Pentoxifylline is a xanthine derivative which has an inhibitory effect on inflammatory responses in the body (e.g. by interfering with TNF-alpha effect) (89). In an early open-label trial, Sogame administered pentoxifylline to 36 children (age range of 3–15 years) with ASD or behavioral disorders with organic etiology (90). He observed improvement in maintenance of sameness, language, and relation with others in the majority of the participants. He observed side effects, such as nausea, vomiting, headache, and low blood pressure in a small number of the participants. Nakane reported results of an open-label study of pentoxifylline on 30 children with ASD (91). Children were also on haloperidol. The assessment was based on the parents’ observation. While six children had significant improvement of symptoms, 14 children presented minor improvements. Shimoide administered pentoxifylline to 20 male individuals (age range of 3–22 years) with ASD over a 3-month period and observed improvement in at least two of their three assessments (i.e. patients’ behavior in specific situations, mental developmental scale for young children, Seiken’s critical list for autistic children) (92). Side effects were related to gastrointestinal symptoms. Turek studied the effects of pentoxifylline on 2 children (a 5-year-old boy and a 7-year-old girl) with ASD and 18 with psychosis (93). He observed significant improvement in syllables and words pronunciation of the autistic children. Observed side effects were limited to excitement and sleep disturbances. In the only randomized controlled trial, Akhondzadeh and colleagues compared treatment with risperidone plus pentoxifylline (n = 20) versus risperidone plus placebo (n = 20) (94). Treatment with pentoxifylline led to a significant improvement on ABC in irritability, lethargy/social withdrawal, stereotypic behavior, hyperactivity/noncompliance, and inappropriate speech. Side effects were not significantly different between groups.
Taken together, pentoxifylline may be a beneficial adjuvant therapy for ASD when combined with risperidone, but the evidence is still insufficient, and robust clinical trials are needed.
Pioglitazone
Pioglitazone is an antidiabetic medication of the thiazolidinedione class that is commonly used for reducing blood glucose in patients with type 2 diabetes mellitus. It exerts its effect mainly through the peroxisome proliferator-activated receptors (PPAR) pathway. Due to the anti-inflammatory properties of this medication, it is suggested to be potentially beneficial in the management of psychiatric disorders (95–97). In an open-label trial, Boris and colleagues administered pioglitazone to 25 children (age range of 3–17 years) with ASD over a 3- to 4-month period and observed a significant improvement in irritability, lethargy, stereotypy behaviors, and hyperactivity as measured by the ABC (98). Improvements in irritability, lethargy, and hyperactivity were significantly associated with age, suggesting younger participants may benefit more from pioglitazone than older children. No significant side effects were observed among the participants.
Most recently, a 10-week randomized controlled trial compared the efficacy of risperidone plus pioglitazone (n = 20) versus risperidone plus placebo (n = 20) (99). This study found a significant improvement in ABC irritability, lethargy/social withdrawal, and hyperactivity/non-compliance after treatment with pioglitazone as compared to placebo. There was no significant difference in the side effects (mostly vomiting and headache) between pioglitazone and placebo groups.
Collectively, initial findings suggest a potential beneficial role for pioglitazone in the management of ASD in children, yet robust clinical trials are needed to provide adequate evidence.
Riluzole
Riluzole is known as the only beneficial medication for increasing survival in patients with amyotrophic lateral sclerosis (ALS). It mainly exerts its effect by inhibiting presynaptic release of glutamate. It is also shown to have anti-inflammatory effects (100). In a randomized controlled trial, Ghaleiha and colleagues (110) compared risperidone plus riluzole (n = 20) versus risperidone plus placebo (n = 20) over a 10-week period in children (age range of 5–12 years) with ASD. They observed significant improvement in ABC irritability, lethargy/social withdrawal, stereotypic behavior, and hyperactivity/noncompliance. Regarding the side effects, the riluzole group had significantly higher increase in appetite and weight gain. It is noteworthy that a large phase 2 trial studying riluzole effects on 58 participants has been conducted recently (https://clinicaltrials.gov/ct2/show/NCT01661855).
Spironolactone
Spironolactone as an antagonist for aldosterone receptor has a primary use in the management of condition associated with elevated levels of aldosterone. It is shown to have anti-inflammatory properties, potentially due to its affinity for other steroid receptors (101, 102). In a single case report and hypothesis paper, Bradstreet and colleagues administered spironolactone to a 12-year-old child with ASD and elevated testosterone level, over a 4-week period and observed improvement in irritability (79%), lethargy (83%), stereotypic behavior (60%), hyperactivity (72%), and inappropriate speech (67%) as measured by the ABC (103). They also noted improvement in receptive language.
Topiramate
Topiramate is an anti-epilepsy medication which is known to have neuroprotective effects (104), exerting through anti-inflammatory and antioxidant effects (105, 106). In the only study in autistic individuals, Rezaei and colleagues compared risperidone plus topiramate (n = 20) with risperidone plus placebo (n = 20) over an 8-week period in children (age range of 3–12 years) with ASD and observed a significant improvement in ABC irritability, stereotypic behavior, and hyperactivity/noncompliance (107). The topiramate group had significantly higher number of somnolence and decreased appetite than the placebo group.
Although initial evidence implies a potentially positive adjuvant role for topiramate, more robust clinical trials are required to determine whether the benefits are consistent and outweigh the side effects.
Conclusions and Future Direction
While pharmacological interventions are frequently used in the management of concomitant psychiatric and behavior challenges in individuals with ASD, they have limited effect on the core symptoms of ASD. Current evidence on the role of inflammation in the underlying mechanisms leading to autism, or more importantly, to specific subgroups/subtypes of autism (e.g. those with concurrent immunological disorders) (27), has fueled the research on the potential use of agents with anti-inflammatory effects in the management of this condition (108) [as in a recent example of the treatment for a subgroup of individuals with depression (109)]. The literature on anti-inflammatory medications in the management of ASD is still in its infancy, dominated by case reports and open-label studies, with only a small portion of double-blind randomized controlled trials (in which many acted as adjuvant agents combined with risperidone) for which the findings are often not replicated yet. Nevertheless, certain findings imply potential for future investigation with more robustly conducted trials, especially in ASD subgroups such as those with concurrent immunological disorders, developmental regression, or high irritability/behavioral challenges.
This literature implies potential benefits of the following medications for ASD children with severe behavioral challenges (alone or especially when acting as an adjuvant agent with risperidone): amantadine, celecoxib, galantamine, N-acetylcysteine, palmitoylethanolamide, pentoxifylline, pioglitazone, riluzole, and topiramate. A small number of case report and case-series studies suggests a potential role for corticosteroids and ACTH in the management of regressive autism. Flavonoids are suggested by a few open-label studies to be a safe medication to potentially improve behavioral symptoms such as irritability. IVIG might be beneficial in ASD individuals with concurrent immunological disorders. Lenalidomide appeared to be beneficial to ASD individuals with developmental regression and with elevated TNF-alpha. However, it is important to note that the current evidence for efficacy and safety of all these medications in individuals with ASD is still very preliminary and inconclusive. For clinical decision making, safety should be the primary concern. These medications should be considered only when 1) the medication is indicated for the co-occurring medical disorder (e.g. immunological disorders); or 2) standard treatments have been depleted or are insufficient, and the potential benefits are judged to outweigh potential harms [note: N-acetylcysteine has been recommended in the latest clinical care pathway for reducing irritability in autistic individuals (85)]. Current evidence on the role of anti-inflammatory agents in ASD should be interpreted considering the following limitations. First, across all medications reviewed here, there is a lack of rigorous randomized double-blind placebo-controlled trials and very few successful replications so far. The available literature is enriched with case reports and open-label studies. There are a few randomized controlled trials, but they are often of small sample sizes and are being tested in the context of an adjuvant agent (i.e., with risperidone). Therefore, based on the studies that evaluate anti-inflammatory medications as adjuvant therapy, it is unclear whether the effect of an anti-inflammatory medication comes from the medication (or the anti-inflammatory action) itself. Secondly, most clinical trials have focused on short-term effects (about 12 weeks) of the medications. It can be difficult to detect significant changes in a short period of time, and the evidence for long-term efficacy or safety is still lacking. Finally, for most of the medications reviewed here, the exact mechanisms of action (including the biological link between their anti-inflammatory mechanisms and behavior changes of the individual) are not clear, so the interpretation of the findings is challenging, especially when there are other potentially non-immune-mediated mechanisms of action that may contribute to the neurobiological basis of ASD (e.g. the glutamatergic system).
In conclusion, current evidence supporting the efficacy and safety of anti-inflammatory interventions in ASD is still limited. Robust large-scale clinical trials are much needed. Nevertheless, some findings imply that the role for immune-mediated mechanisms in the emergence of autism or autism-related challenges may be specific to a subset of people with autism (27, 111). It may be the case that the greatest potential of anti-inflammatory agents lies in this aspect, in the light of stratified psychiatry and precision medicine.
Author Contributions
SH, DT, and MC-L designed the study, ran the literature review, prepared the manuscript, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Takumi T, Tamada K, Hatanaka F, Nakai N, Bolton PF. Behavioral neuroscience of autism. Neurosci Biobehav Rev (2019). doi: 10.1016/j.neubiorev.2019.04.012
2. Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Curr Neurol Neurosci Rep (2009) 9(2):129–36. doi: 10.1007/s11910-009-0021-x
3. Anagnostou E. Clinical trials in autism spectrum disorder: evidence, challenges and future directions. Curr Opin Neurol (2018) 31(2):119–25. doi: 10.1097/WCO.0000000000000542
4. Zimmerman AW., et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol (2005) 33(3):195–201. doi: 10.1016/j.pediatrneurol.2005.03.014
5. Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry (2012) 17(4):389–401. doi: 10.1038/mp.2011.165
6. Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science (2016) 353(6301):772–7. doi: 10.1126/science.aag3194
7. Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics (2012) 130: (6):e1447. doi: 10.1542/peds.2012-1107
8. Slawinski BL, Talge N, Ingersoll B, Smith A, Glazier A, Kerver J, et al. Maternal cytomegalovirus sero-positivity and autism symptoms in children. Am J Reprod Immunol (2018) 79(5):e12840. doi: 10.1111/aji.12840
9. Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun (2016) 58:165–72. doi: 10.1016/j.bbi.2016.06.005
10. Lombardo MV, Moon HM, Su J, Palmer TD, Courchesne E, Pramparo T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry (2018) 23(4):1001–13. doi: 10.1038/mp.2017.15
11. Atladóttir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatr (2009) 124(2):687–94. doi: 10.1542/peds.2008-2445
12. Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry (2014) 75(4):307–15. doi: 10.1016/j.biopsych.2013.07.011
13. Jones KL, Pride MC, Edmiston E, Yang M, Silverman JL, Crawley JN, et al. Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol Psychiatry (2018) 1. doi: 10.1038/s41380-018-0126-1
14. Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med (2011) 17(7):389–94. doi: 10.1016/j.molmed.2011.03.001
15. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry (2015) 20(4):440–6. doi: 10.1038/mp.2014.59
16. Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol (2009) 207(1-2):111–6. doi: 10.1016/j.jneuroim.2008.12.002
17. Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry (2010) 68(4):368–76. doi: 10.1016/j.biopsych.2010.05.024
18. Edmonson C, Ziats MN, Rennert OM. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism (2014) 5(1):3. doi: 10.1186/2040-2392-5-3
19. Young AM, Chakrabarti B, Roberts D, Lai MC, Suckling J, Baron-Cohen S. From molecules to neural morphology: understanding neuroinflammation in autism spectrum condition. Mol Autism (2016) 7(1):9. doi: 10.1186/s13229-016-0068-x
20. Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry (2013)70(1):49–58. doi: 10.1001/jamapsychiatry.2013.272
21. Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol (2007) 36(6):361–5. doi: 10.1016/j.pediatrneurol.2007.01.012
22. Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PloS One (2011) 6(5):e20470. doi: 10.1371/journal.pone.0020470
23. Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun (2011) 25(1):40–5. doi: 10.1016/j.bbi.2010.08.003
24. Michel M, Schmidt MJ, Mirnics K. Immune system gene dysregulation in autism and schizophrenia. Dev Neurobiol (2012) 72(10):1277–87. doi: 10.1002/dneu.22044
25. Chien YL, Wu YY, Chen CH, Gau SS, Huang YS, Chien WH, et al. Association of HLA-DRB1 alleles and neuropsychological function in autism. Psychiatr Genet (2012) 22(1):46–9. doi: 10.1097/YPG.0b013e32834915ae
26. Mostafa GA, Shehab AA, Al-Ayadhi LY. The link between some alleles on human leukocyte antigen system and autism in children. J Neuroimmunol (2013) 255: (1-2):70–4. doi: 10.1016/j.jneuroim.2012.10.002
27. Thom RP, Keary CJ, Palumbo ML, Ravichandran CT, Mullett JE, Hazen EP, et al. Beyond the brain: a multi-system inflammatory subtype of autism spectrum disorder. Psychopharmacol (Berl) (2019) 236:1–17. doi: 10.1007/s00213-019-05280-6
28. Cheng Y-S, Tseng P-T, Chen Y-W, Stubbs B, Yang W-C, Chen T-Y, et al. Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat (2017)13:2531. doi: 10.2147/NDT.S147305
29. Rahimloua M, Rayyanib E, Hosseinia SA, Husaina D. An updated systematic review of the effects of n-3 long-chain polyunsaturated fatty acids on Autistic Spectrum Disorder. J Nutr Sci Diet(2018) 4(1):15–19.
30. Agostoni C, Nobile M, Ciappolino V, Delvecchio G, Tesei A, Turolo S, et al. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: a Systematic Review on Early Psychosis, Autism, and ADHD. Int J Mol Sci (2017) 18(12):2608. doi: 10.3390/ijms18122608
31. Horvath A, Łukasik J, Szajewska H. ω-3 fatty acid supplementation does not affect autism spectrum disorder in children: a systematic review and meta-analysis-3. J Nutr (2017) 147(3):367–76. doi: 10.3945/jn.116.242354
32. Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, et al. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology (1995) 45(11):1956–61. doi: 10.1212/WNL.45.11.1956
33. Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med (2012) 366(9):819–26. doi: 10.1056/NEJMoa1102609
34. Ossola B, Schendzielorz N, Chen S-H, Bird GS, Tuominen RK, Männistö PT, et al. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GNDF in astroglia.Neuropharmacol (2011)61(4):574–82. doi: 10.1016/j.neuropharm.2011.04.030
35. Kubera M, Maes M, Budziszewska B, Basta-Kaim A, Leskiewicz M, Grygier B, et al. Inhibitory effects of amantadine on the production of pro-inflammatory cytokines by stimulated in vitro human blood. Pharmacol Rep (2009) 61(6):1105–12. doi: 10.1016/S1734-1140(09)70173-2
36. King BH, Wright DM, Handen BL, Sikich L, Zimmerman AW, McMahon W, et al. Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J Am Acad Child Adolesc Psychiatry (2001) 40(6):658–65. doi: 10.1097/00004583-200106000-00010
37. Mohammadi MR, Yadegari N, Hassanzadeh E, Farokhnia M, Yekehtaz H, Mirshafiee O, et al. Double-blind, placebo-controlled trial of risperidone plus amantadine in children with autism: a 10-week randomized study. Clin Neuropharmacol (2013) 36(6):179–84. doi: 10.1097/WNF.0b013e3182a9339d
38. Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry (2006) 11: (7):680. doi: 10.1038/sj.mp.4001805
39. Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry (2002)159(6):1029–34. doi: 10.1176/appi.ajp.159.6.1029
40. Asadabadi M, Mohammadi M-R, Ghanizadeh A, Modabbernia A, Ashrafi M, Hassanzadeh E, et al. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacol (2013) 225 (1):51–9. doi: 10.1007/s00213-012-2796-8
41. Sinclair DB, Snyder TJ. Corticosteroids for the treatment of Landau-Kleffner syndrome and continuous spike-wave discharge during sleep. Pediatr Neurol (2005) 32(5):300–6. doi: 10.1016/j.pediatrneurol.2004.12.006
42. Golla S, Sweeney JA. Corticosteroid therapy in regressive autism: Preliminary findings from a retrospective study. BMC Med (2014) 12(1):79. doi: 10.1186/1741-7015-12-79
43. Buitelaar JK, van Engeland H, de Kogel K, de Vries H, van Hooff J, van Ree J. The adrenocorticotrophic hormone (4–9) analog ORG 2766 benefits autistic children: report on a second controlled clinical trial. J Am Acad Child Adolesc Psychiatry (1992) 31(6):1149–56. doi: 10.1097/00004583-199211000-00026
44. Buitelaar JK, van Engeland H, de Kogel KH, de Vries H, van Hooff JA, van Ree JM. The use of adrenocorticotrophic hormone (4–9) analog ORG 2766 in autistic children: effects on the organization of behavior. Biol Psychiatry (1992) 31(11):1119–29. doi: 10.1016/0006-3223(92)90156-T
45. Buitelaar JK, van Engeland H, van Ree JM, de Wied D. Behavioral effects of Org 2766, a synthetic analog of the adrenocorticotrophic hormone (4–9), in 14 outpatient autistic children. J Autism Dev Disord (1990) 20(4):467–78. doi: 10.1007/BF02216053
46. Stefanatos GA, Grover W, Geller E. Case study: corticosteroid treatment of language regression in pervasive developmental disorder. J Am Acad Child Adolesc Psychiatry (1995) 34(8):1107–11. doi: 10.1097/00004583-199508000-00022
47. Shenoy S, Arnold S, Chatila T. Response to steroid therapy in autism secondary to autoimmune lymphoproliferative syndrome. J Pediatr (2000) 136(5):682–7. doi: 10.1067/mpd.2000.105355
48. Matarazzo EB. Treatment of late onset autism as a consequence of probable autommune processes related to chronic bacterial infection. World J Biol Psychiatry (2002) 3(3):162–6. doi: 10.3109/15622970209150618
49. Mordekar SR, Prendergast M, Chattopadhyay AK, Baxter PS. Corticosteroid treatment of behaviour, language and motor regression in childhood disintegrative disorder. Eur J Paediatr Neurol (2009) 13(4):367–9. doi: 10.1016/j.ejpn.2008.06.001
50. Duffy FH, Shankardass A, McAnulty GB, Eksioglu YZ, Coulter D, Rotenberg A, et al. Corticosteroid therapy in regressive autism: a retrospective study of effects on the Frequency Modulated Auditory Evoked Response (FMAER), language, and behavior. BMC Neurol (2014) 14(1):70. doi: 10.1186/1471-2377-14-70
51. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc (2010) 69(3):273–8. doi: 10.1017/S002966511000162X
52. Theoharides T, Asadi S, Panagiotidou S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. London, England: SAGE Publications Sage UK (2012). doi: 10.1177/039463201202500201
53. Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther (2013) 35(5):592–602. doi: 10.1016/j.clinthera.2013.04.006
54. Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Trans Psychiatry (2015) 5(9):e647. doi: 10.1038/tp.2015.142
55. Bertolino B, Crupi R, Impellizzeri D, Bruschetta G, Cordaro M, Siracusa R, et al. Beneficial effects of co-ultramicronized Palmitoylethanolamide/Luteolin in a mouse model of autism and in a Case Report of Autism. CNS Neurosci Ther (2017) 23(1):87–98. doi: 10.1111/cns.12648
56. Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun (2009) 23(1):41–5. doi: 10.1016/j.bbi.2008.06.011
57. Hertzman M.Galantamine in the treatment of adult autism: a report of three clinical cases. Int J Psychiatry Med (2003) 33(4):395–8. doi: 10.2190/JE5Q-1NFT-FL40-7PMW
58. Nicolson R, Craven-Thuss B, Smith J. A prospective, open-label trial of galantamine in autistic disorder. J Child Adolesc Psychopharmacol (2006) 16(5):621–9. doi: 10.1089/cap.2006.16.621
59. Ghaleiha A, Ghyasvand M, Mohammadi M-R, Farokhnia M, Yadegari N, Tabrizi M, et al. Galantamine efficacy and tolerability as an augmentative therapy in autistic children: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol (2014) 28(7):677–85. doi: 10.1177/0269881113508830
60. Gupta S, Aggarwal S, Heads C. Brief report: dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. J Autism Dev Disord (1996) 26(4):439–52. doi: 10.1007/BF02172828
61. Plioplys AV. Intravenous immunoglobulin treatment of children with autism. J Child Neurol (1998) 13(2):79–82. doi: 10.1177/088307389801300207
62. Del Giudice-Asch G, Simon L, Schmeidler J, Cunningham-Rundles C, Hollander E. Brief report: a pilot open clinical trial of intravenous immunoglobulin in childhood autism.J Autism Dev Disord (1999) 29(2):157–60. doi: 10.1023/A:1023096728131
63. Niederhofer H, Staffen W, Mair AJN. Immunoglobulins as an alternative strategy of psychopharmacological treatment of children with autistic disorder. Neuropsychopharmacol (2003) 28(5):1014. doi: 10.1038/sj.npp.1300130
64. Boris M, Goldblatt A, Edelson SM. Improvement in children with autism treated with intravenous gamma globulin. J Nutr Environ Med (2005) 15(4):169–76. doi: 10.1080/13590840600681827
65. Connery K, Tippett M, Delhey LM, Rose S, Slattery JC, Kahler SG, et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl Psychiatry (2018) 8(1):148. doi: 10.1038/s41398-018-0214-7
66. List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med (2005) 352(6):549–57. doi: 10.1056/NEJMoa041668
67. Chez M, Low R, Parise C, Donnel T. Safety and observations in a pilot study of lenalidomide for treatment in autism. Autism Res Treat (2012) 2012:291601. doi: 10.1155/2012/291601
68. Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, et al. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacol (2009) 34(10):2344–57. doi: 10.1038/npp.2009.64
69. Ghaleiha A, Asadabadi M, Mohammadi M-R, Shahei M, Tabrizi M, Hajiaghaee R, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol (2013) 16(4):783–9. doi: 10.1017/S1461145712000880
70. Joshi G, Wozniak J, Faraone SV, Fried R, Chan J, Furtak S, et al. A Prospective open-label trial of memantine hydrochloride for the treatment of social deficits in intellectually capable adults with autism spectrum disorder. J Clin Psychopharmacol (2016) 36(3):262–71. doi: 10.1097/JCP.0000000000000499
71. Aman MG, Findling RL, Hardan AY, Hendren RL, Melmed RD, Kehinde-Nelson O, et al. Safety and efficacy of memantine in children with autism: randomized, placebo-controlled study and open-label extension. J Child Adolesc Psychopharmacol (2017) 27(5):403–12. doi: 10.1089/cap.2015.0146
72. Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol (2013) 169(2):337–52. doi: 10.1111/bph.12139
73. Pardo CA, Buckley A, Thurm A, Lee LC, Azhagiri A, Neville DM, et al. A pilot open-label trial of minocycline in patients with autism and regressive features. J Neurodev Disord (2013) 5(1):9. doi: 10.1186/1866-1955-5-9
74. Ghaleiha A, Alikhani R, Kazemi M-R, Mohammadi M-R, Mohammadinejad P, Zeinoddini A, et al. Minocycline as adjunctive treatment to risperidone in children with autistic disorder: A randomized, double-blind placebo-controlled trial. J Child Adolesc Psychopharmacol (2016) 26(9):784–91. doi: 10.1089/cap.2015.0175
75. Minarini A, Ferrari S, Galletti M, Giambalvo N, Perrone D, Rioli G, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol (2017) 13(3):279–92. doi: 10.1080/17425255.2017.1251580
76. Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull (2014) 40(1):181–91. doi: 10.1093/schbul/sbt139
77. Marler S, Sanders KB, Veenstra-VanderWeele J.N-acetylcysteine as treatment for self-injurious behavior in a child with autism. J Child Adolesc Psychopharmacol (2014) 24(4):231–4. doi: 10.1089/cap.2013.0137
78. Ghanizadeh A, Derakhshan N. N-acetylcysteine for treatment of autism, a case report. J Res Med Sci (2012) 17(10):985–7.
79. Stutzman D, Dopheide J.Acetylcysteine for treatment of autism spectrum disorder symptoms. Am J Health Syst Pharm (2015) 72(22):1956–9. doi: 10.2146/ajhp150072
80. Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry (2012) 71(11):956–61. doi: 10.1016/j.biopsych.2012.01.014
81. Ghanizadeh A, Moghimi-Sarani E. A randomized double blind placebo controlled clinical trial of N-Acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry (2013) 13:196. doi: 10.1186/1471-244X-13-196
82. Nikoo M, Radnia H, Farokhnia M, Mohammadi M-R, Akhondzadeh S. N-Acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Clin Neuropharmacol (2015) 38(1):11–7. doi: 10.1097/WNF.0000000000000063
83. Wink LK, Adams R, Wang Z, Klaunig JE, Plawecki MH, Posey DJ, et al. A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol Autism (2016) 7(1):26. doi: 10.1186/s13229-016-0088-6
84. Dean OM, Gray KM, Villagonzalo KA, Dodd S, Mohebbi M, Vick T, et al. A randomised, double blind, placebo-controlled trial of a fixed dose of N-acetyl cysteine in children with autistic disorder. Aust N Z J Psychiatry (2017) 51(3):241–9. doi: 10.1177/0004867416652735
85. McGuire K, Fung LK, Hagopian L, Vasa RA, Mahajan R, Bernal P, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatr (2016)137 Suppl 2(Supplement 2):S136–48. doi: 10.1542/peds.2015-2851L
86. Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol (2017) 174(11):1349–65. doi: 10.1111/bph.13580
87. Antonucci N, Cirillo A, Siniscalco D. Beneficial Effects of Palmitoylethanolamide on expressive language, cognition, and behaviors in autism: a report of two cases. Case Rep Psychiatry (2015) 2015:325061. doi: 10.1155/2015/325061
88. Khalaj M, Saghazadeh A, Shirazi E, Shalbafan MR, Alavi K, Shooshtari MH, et al. Palmitoylethanolamide as adjunctive therapy for autism: Efficacy and safety results from a randomized controlled trial. J Psychiatr Res (2018) 103:104–11. doi: 10.1016/j.jpsychires.2018.04.022
89. Sullivan GW, Carper HT, Novick WJ Jr., Mandell GL. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect Immun (1988) 56(7):1722–9.
90. Sogame S. Clinical experiences with administration of pentoxifylline against autism and behaviour abnormalities. Jpn J Child Psych (1978) 19:137–44.
92. Shimoide M. Effect of pentoxifylline (Trental) on infantile autism. Clin Exp Med (1981) 58:285–8.
94. Akhondzadeh S, Fallah J, Mohammadi MR, Imani R, Mohammadi M, Salehi B, et al. Double-blind placebo-controlled trial of pentoxifylline added to risperidone: effects on aberrant behavior in children with autism.Prog Neuropsychopharmacol Biol Psychiatry (2010) 34(1):32–6. doi: 10.1016/j.pnpbp.2009.09.012
95. Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol (2008) 52(10):869–81. doi: 10.1016/j.jacc.2008.04.055
96. Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging (2011) 32(9):1626–33. doi: 10.1016/j.neurobiolaging.2009.10.009
97. Zeinoddini A, Sorayani M, Hassanzadeh E, Arbabi M, Farokhnia M, Salimi S, et al. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial.Depress Anxiety (2015) 32(3):167–73. doi: 10.1002/da.22340
98. Boris M, Kaiser CC, Goldblatt A, Elice MW, Edelson SM, Adams JB, et al. Effect of pioglitazone treatment on behavioral symptoms in autistic children.J Neuroinflammation (2007) 4(1):3. doi: 10.1186/1742-2094-4-3
99. Ghaleiha A, Rasa SM, Nikoo M, Farokhnia M, Mohammadi M-R, Akhondzadeh S. A pilot double-blind placebo-controlled trial of pioglitazone as adjunctive treatment to risperidone: Effects on aberrant behavior in children with autism. J Psychiatry Res (2015) 229(1-2):181–7. doi: 10.1016/j.psychres.2015.07.043
100. Liu BS, Ferreira R, Lively S, Schlichter LC, Spironolactone/Arthritis Study, Group. Microglial SK3 and SK4 currents and activation state are modulated by the neuroprotective drug, riluzole. J Neuroimmune Pharmacol (2013) 8(1):227–37. doi: 10.1007/s11481-012-9365-0
101. Bendtzen K, Hansen PR, Rieneck K, Spironolactone/Arthritis Study, Group. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-alpha and interferon-gamma, and has potential in the treatment of arthritis. Clin Exp Immunol (2003) 134(1):151–8. doi: 10.1046/j.1365-2249.2003.02249.x
102. Young MJ. Mechanisms of mineralocorticoid receptor-mediated cardiac fibrosis and vascular inflammation. Curr Opin Nephrol Hypertens (2008) 17(2):174–80. doi: 10.1097/MNH.0b013e3282f56854
103. Bradstreet JJ, Smith S, Granpeesheh D, El-Dahr JM, Rossignol D. Spironolactone might be a desirable immunologic and hormonal intervention in autism spectrum disorders. Med Hypotheses (2007) 68(5):979–87. doi: 10.1016/j.mehy.2006.10.015
104. Motaghinejad M, Motevalian M. Neuroprotective effects of various doses of topiramate against methylphenidate-induced oxidative stress and inflammation in isolated rat amygdala: the possible role of CREB/BDNF signaling pathway.J Neural Transm (2016) 123(12):1463–77. doi: 10.1007/s00702-016-1619-1
105. Motaghinejad M, Motevalian M. Involvement of AMPA/kainate and GABAA receptors in topiramate neuroprotective effects against methylphenidate abuse sequels involving oxidative stress and inflammation in rat isolated hippocampus.Eur J Pharmacol (2016) 784:181–91. doi: 10.1016/j.ejphar.2016.04.036
106. Tian Y, Guo SX, Li JR, Du HG, Wang CH, Zhang JM, et al. Topiramate attenuates early brain injury following subarachnoid haemorrhage in rats via duplex protection against inflammation and neuronal cell death. Brain Res (2015) 1622:174–85. doi: 10.1016/j.brainres.2015.06.007
107. Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezazadeh SA, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34(7):1269–72. doi: 10.1016/j.pnpbp.2010.07.005
108. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry (2012) 17: (12):1174. doi: 10.1038/mp.2012.105
109. Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry (2019) 214(1):11–9. doi: 10.1192/bjp.2018.66
110. Ghaleiha A, Mohammadi E, Mohammadi M-R, Farokhnia M, Modabbernia A, Yekehtaz H, et al. Riluzole as an adjunctive therapy to risperidone for the treatment of irritability in children with autistic disorder: a double-blind, placebo-controlled, randomized trial.Pediatr Drugs (2013) 15(6):505–14. doi: 10.1007/s40272-013-0036-2
Keywords: autism, inflammation, neuroinflammation, autism spectrum disorder, immune system
Citation: Hafizi S, Tabatabaei D and Lai M-C (2019) Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Front. Psychiatry 10:849. doi: 10.3389/fpsyt.2019.00849
Received: 16 October 2018; Accepted: 28 October 2019;
Published: 25 November 2019.
Edited by:
Richard G. Boles, Center for Neurological and Neurodevelopmental Health (CNNH), United StatesReviewed by:
Herb Lachman, Albert Einstein College of Medicine, United StatesAlvin Wern Howe Loh, University of Toronto, Canada
Copyright © 2019 Hafizi, Tabatabaei and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sina Hafizi, sina.hafizi@gmail.com
†These authors have contributed equally to this work
 Sina Hafizi
Sina Hafizi Dina Tabatabaei2†
Dina Tabatabaei2† Meng-Chuan Lai
Meng-Chuan Lai