- 1Andrology Laboratory, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
Background: 2, 4-dichlorophenoxyacetic acid (2,4-D) is one of the most frequently used herbicides in the world, and it has been linked with low testosterone; however, studies regarding its effect on erectile function are limited. The current study aimed to determine the association between the 2,4-D exposure and erectile dysfunction (ED) in men from the National Health and Nutrition Examination Survey (NHANES).
Methods: We analyzed data for urinary 2,4-D levels from 1,311 men (>20 years of age) in the NHANES 2001–2004. ED was assessed by a single, validated survey question. Multivariable logistic regression analysis utilizing sampling weights was performed to determine the relationship between 2,4-D exposure and ED.
Results: Multivariable logistic regression models demonstrated no statistically significant association between 2,4-D exposure and ED after full adjustment [odds ratio (OR) 1.02; 95% CI 0.77–1.36; P = 0.882)]. Men in the 2,4-D quartile 4 groups were not associated with an increased risk of ED (OR 1.13; 95% CI 0.74–1.75; P for trend = 0.481). Furthermore, the association between urinary 2,4-D level and ED was not significant in the subgroup analysis stratified by age, BMI, cardiovascular disease, hypertension, diabetes, and high cholesterol.
Conclusion: We demonstrated that there was no association between 2,4-D exposure and ED. Further studies are warranted to corroborate our results.
Introduction
Erectile dysfunction (ED), defined as the persistent inability to achieve or maintain a penile erection sufficient for successful vaginal intercourse, is the most common disorder affecting middle-aged and older men (1). The prevalence rate of ED ranges from 1 to 10% in men younger than 40 years, and this number increases to 70.2% in men aged ≥70 years (1, 2). Furthermore, it is estimated that more than 322 million men globally experience ED by 2025 (3). The trends pose a great challenge for public health systems to develop policies to prevent or alleviate ED.
Herbicides are a large class of synthetic compounds that have been widely utilized to control broadleaf weeds. The use of herbicides is increasing around the world. It was estimated that the value of the worldwide herbicide market increased by 11% between 2011 and 2016 (4). 2,4-dichlorophenoxyacetic acid (2,4-D) is one of the most frequently used herbicides in the world because of its selectivity, efficiency, and low cost. In the US, the annual cost of markets for 2,4-D is nearly 57 million dollars (5). Liu et al. reported that the production of 2,4-D reached 40,000 tons in 2010 in China (6). Given the extensive application of 2,4-D, the possibility of human exposure is increasing via household and agricultural use, and consumption of food and water contaminated with 2,4-D (7). The Farm Family Exposure Study revealed that 71% of applicators had detectable 2,4-D in their urine before the application, and 100% had it in their urine (8).
Increasing evidence showed that 2,4-D exposure had adverse effects on the production and function of testosterone, which plays a critical role in male erection (9–11). Additionally, exposure to 2,4-D might increase the risks of dyslipidemia and impaired glucose metabolism that are linked to the pathogenesis of ED (12). However, literature regarding the effects of 2,4-D exposure on ED is scarce. Accordingly, in this study, we determined the association between 2,4-D exposure and ED utilizing 1,311 samples from the National Health and Nutrition Examination Survey (NHANES) 2001–2004. We hypothesized that 2,4-D exposure would be associated with the increased risks of ED.
Methods
Study Population
As a nationwide survey, the NHANES has been conducted every 2 years to obtain health and diet information from a representative, non-institutionalized U.S. population since 1999. This survey combines detailed in-person interviews, physical examinations, computer-based questionnaires, and laboratory tests to collect a large amount of quantitative and qualitative data. Detailed information regarding NHANES survey methods can be found at http://www.cdc.gov/nchs/nhanes/index.htm. All study protocols were approved by the ethics review board of the National Center for Health Statistics and written informed consent was obtained from all participants before any data collection.
In our analysis, we utilized NHANES data from two independent waves (2001–2002 and 2003–2004) because questionnaire information regarding ED was available only in those years. The study population was limited to those >20 years of age as the age under 20 years was not interviewed about erectile function. We excluded those participants with a history of prostate cancer because treatment for prostate cancer might be a potential iatrogenic cause of ED.
Assessment of ED
We assessed erectile function with the following question from the Massachusetts Male Aging Study (13): “Many men experience problems with sexual intercourse. How would you describe your ability to get and keep an erection adequate for satisfactory intercourse?” Response options were available as “always or almost always able,” “usually able,” “sometimes able” and “never able.” ED was defined as “sometimes able” or “never able” to keep an erection adequate for satisfactory intercourse as previously validated (14). Participants who responded “almost always able” or “usually able” to maintain an erection were defined as not having ED.
Assessment of 2,4-D Exposure
In humans, 2,4-D is primarily excreted by the kidneys with minimal metabolism, which retains its parent form in urine (15). Therefore, urine measurements can be utilized as a good indicator for 2,4-D exposure. The urinary level of 2,4-D was measured and quantified from the urine matrix of participants using an automated solid-phase extraction system. Samples were analyzed by high-performance liquid chromatography and triple quadrupole mass spectrometer with a heated electrospray ionization source (16). For 2,4-D concentrations below the limit of detection (LOD), a value of 0.15 μg/L, they were replaced with LOD divided by the square root of 2 for the purpose of increasing statistical power and precision of effects estimates (13). Additionally, we adjusted the urinary dilution by dividing each metal by the grams of creatinine per liter of urine (μg/g creatinine). All urinary metal concentrations were adjusted for log2-transformed to approximate a normal distribution.
Covariates
Based on previous literature results, potential variables confounding the association between 2,4-D exposure and ED were included in multivariable models (17, 18). The confounders included age, race/ethnicity, body mass index (BMI), the ratio of family income to poverty, education level, marital, alcohol intake, smoking status, physical activity, cardiovascular disease, diabetes, hypertension, and high cholesterol. The ratio of family income to poverty is an index aiming to evaluate household socioeconomic status. The ratio was classified as <1.5, 1.5–3.5, and over 3.5 (19). Alcohol intake was defined as none (<1 drink per week), light (1–3 drinks per week), and heavy (≥4 drinks per week) (20). Men who smoked more than 100 cigarettes in their entire life and reported smoking every day or some days at the time of the interview were regarded as current smokers. In addition, we considered those who smoked more than 100 cigarettes in their entire life but were not smoking at the time of the interview as former smokers. Non-smokers are those who smoked <100 cigarettes during their lifetime. Physical activity status was evaluated based on responses to questions regarding whether the participants engaged in moderate or vigorous activity during the past 30 days. Participants were classified as having a history of cardiovascular disease if they reported a previous diagnosis of angina, heart attack, or coronary heart disease. Men who had a prior diagnosis of diabetes or fasting plasma glucose ≥126 mg/dl were defined as diagnosed diabetics. Four times of systolic and diastolic blood pressure on 2 separate occasions were measured from individuals. The average of those 4 measurements (≥140/90 mmHg), reporting a physician diagnosis of high blood pressure, or self-reported taking antihypertensive medication was considered hypertension. Hypercholesterolemia was defined as a total cholesterol level of 240 mg/dL or higher, a prior diagnosis of “high cholesterol,” or self-reported taking a cholesterol-lowering medication.
Statistical Analysis
Sampling weights, strata, and primary sampling units were utilized to account for the selective bias, non-response, and over-sampling of certain subpopulations. Demographic variables were described utilizing weighted means, standard deviation, and weighted proportions. One-way ANOVA (continuous variables) and chi-square (categorical variables) tests were utilized to evaluate between-group differences. Three models were built to determine the independent association between urinary levels of 2,4-D and ED: model I, no adjustment for covariates; model II, adjusted for age, race, and BMI; model III, adjusted for age, race, the ratio of family income to poverty, education level, marital status, BMI, alcohol intake, smoking, physical activity, cardiovascular disease, diabetes, hypertension, and high cholesterol. Strengths of the association for the multivariate models were estimated by the odds ratio (OR) and their associated 95% CI.
We also conducted additional analyses. First, we categorized the urinary level of 2,4-D into four clinically relevant quartiles as ordinal categorical variables (first to fourth, setting the first as reference) to assess potential trends in the association. Second, subgroup analysis was conducted stratified by age, BMI, cardiovascular disease, hypertension, diabetes, and high cholesterol. We utilized an interaction test to assess the heterogeneity of associations between different subgroups. Third, because only 25.7 and 63% of the values were above the LOD in 2001–2002 and 2003–2004, respectively, we chose to further dichotomized urinary 2,4-D to above LOD or below LOD. Missing values were input by median (continuous) or mode (categorical) of existing cases of that variable. All statistical analyses were conducted utilizing the R-3.4.3 (http://www.R-project.org; The R Foundation, Vienna, Austria) and Empower (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A P-value ≤ 0.05 was considered statistically significant.
Results
Baseline characteristics of 1,311 participants included in this study, classified by quartile of urinary 2,4-D level, were presented in Table 1. Men in the quartile 4 group tend to be older, Mexican American or Non-Hispanic White, married or living with a partner, non-drinker, engaging in moderate physical activity, and having diabetes. In addition, compared to the Q1 group, men with higher urinary 2,4-D levels are more likely to have ED although did not meet the level of statistical significance (P = 0.064, Table 1).
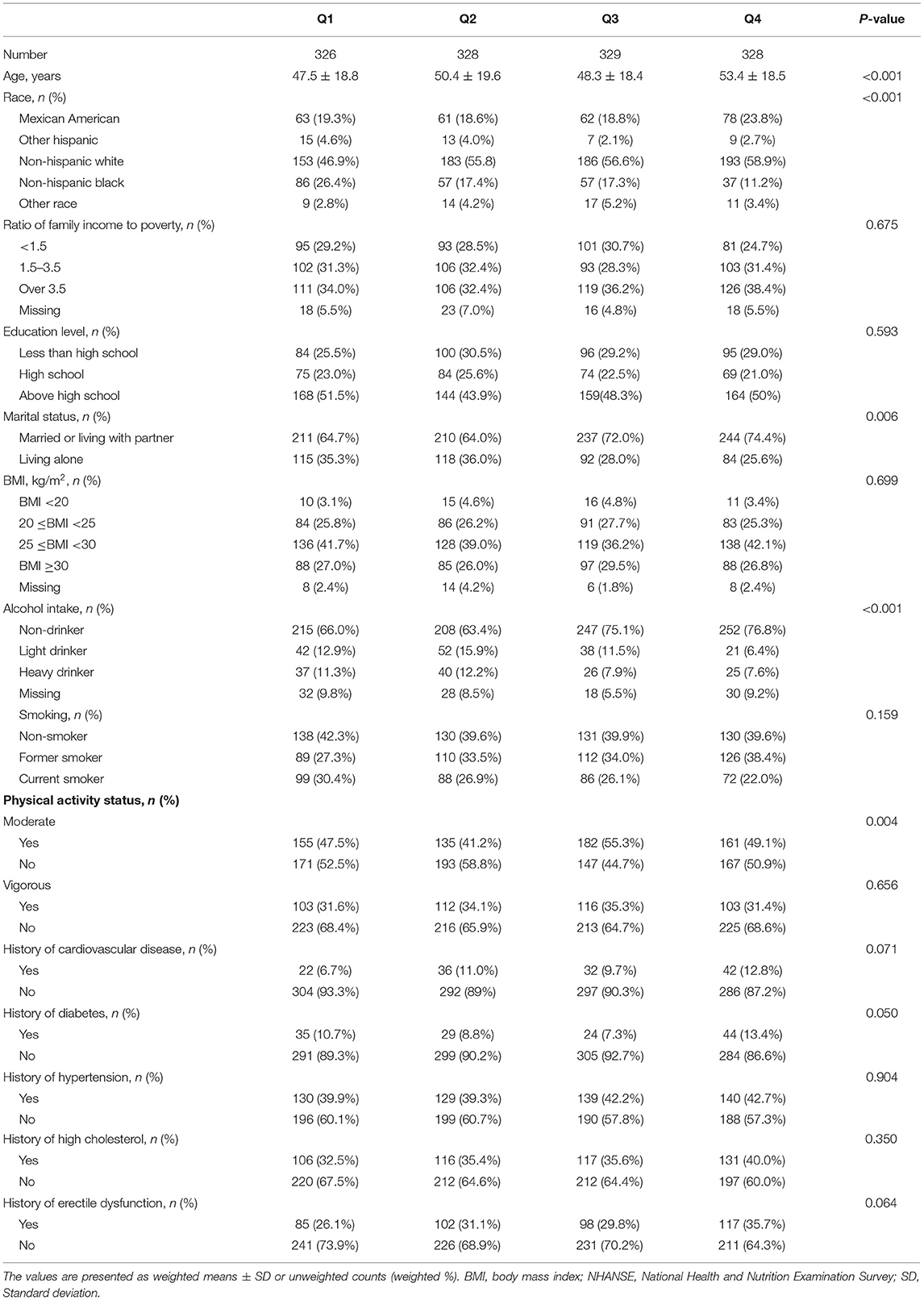
Table 1. Baseline characteristics of the participants in the National Health and Nutrition Examination Survey (NHANES) 2001–2004.
The association between urinary 2,4-D level and ED was positive in the unadjusted model (OR 1.27; 95% CI 1.03–1.6; P = 0.029, Table 2). However, this association was no longer significant after adjusting for covariates in the model I (OR 1.06; 95% CI 0.81–1.4; P = 0.652) and model II (OR 1.02; 95% CI 0.77–1.36; P = 0.882). Furthermore, we categorized the urinary level of 2,4-D into four clinically relevant quartiles as ordinal categorical variables. We found that men in the quartile 4 group were not associated with an increased risk of ED (OR 1.13; 95% CI 0.74–1.75; P for trend = 0.481, Table 2). As only 25.7 and 63% of the 2, 4-D values were above the LOD in 2001–2002 and 2003–2004, respectively, we chose to further dichotomize urinary 2,4-D to above LOD or below LOD. After full adjustment, no statistically significant association was observed between 2,4-D exposure and ED (OR 1.01; 95% CI 0.74–1.38; P = 0.943, Table 2).
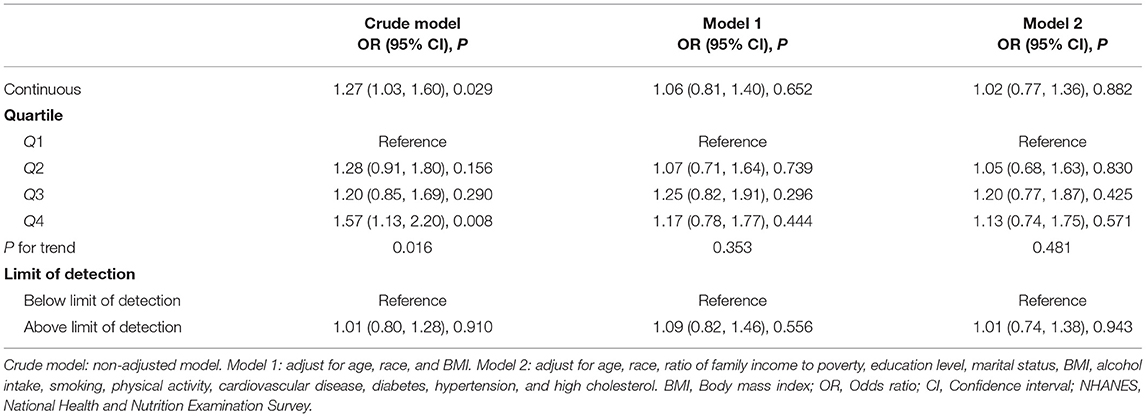
Table 2. Association between urinary 2, 4-dichlorophenoxyacetic acid (2,4-D) and erectile dysfunction among U.S. men in the NHANES 2001–2004.
No significant difference was indicated by the interaction test between urinary 2,4-D level and ED in subgroup analysis (all P for interaction > 0.05, Table 3). Our results demonstrated that after full adjustment, the association between urinary 2,4-D level and ED was not significant in the subgroup analysis stratified by age, BMI, cardiovascular disease, hypertension, diabetes, and high cholesterol (Table 3).
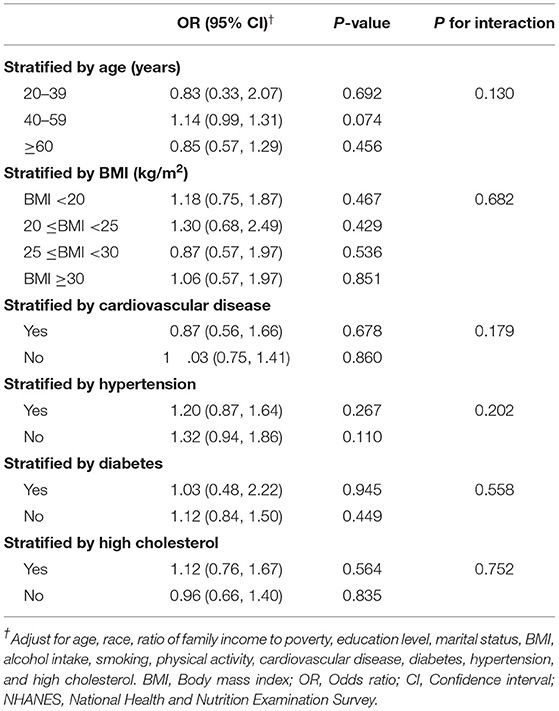
Table 3. Subgroup analysis of the association between urinary 2,4-D and erectile dysfunction among U.S. men in the NHANES 2001–2004.
Discussion
This is the first epidemiologic study to determine the independent associations between 2,4-D exposure and ED. Our results demonstrated that no statistically significant association was observed between 2,4-D exposure and ED after full adjustment. Furthermore, the association between urinary 2,4-D level and ED was not significant in the subgroup analysis stratified by age, BMI, cardiovascular disease, diabetes, and high cholesterol.
In general, the application of 2,4-D in agriculture increased by 67% between 2012 and 2020, and by more than 240% between 1991 and 2020 (21). This trend continues because of unabated weed resistance. Since its first introduction on the market in the 1940 s, the 2,4-D application has dramatically increased over the next 4 decades (5). In 1996, 2,4-D consumption began to decline because of the emergence of genetically engineered glyphosate-tolerant Roundup™ soybeans and cotton (21). By the mid-2000 s, glyphosate-resistant weeds had grown and spread rapidly from farm to farm, which forced growers to add 2,4-D for weed management (21).
The increased usage of 2,4-D raises concerns regarding acute and chronic toxicity of 2,4-D exposure. Freisthler et al. revealed that the frequency of participants with high urinary 2,4-D levels increased from 17.1% in 2001–2002 to 39.6% in 2011–2012 (21). Some studies indicated that 2,4-D was associated with Parkinson's disease, autism, and non-Hodgkin lymphoma (5). Moreover, 2,4-D exposure has been linked with endocrine disruption, reproductive disorders, genetic alterations, and cancer (5). Glover et al. utilized data from 456 participants in the 2013–2014 NHANES cycle, they found a negative association between urinary 2,4-D and mean serum testosterone (β = −11.4 ng/dL, P = 0.02) (22). Similarly, Panuwet et al. analyzed urine and serum samples of 133 male Thai farmers and reported significant negative relationships between total testosterone and 2,4-D after controlling for covariates (11). As the principal male sex hormone, testosterone is responsible for reproduction and sexual function. Low testosterone can cause symptoms including decreased libido, fatigue, coronary artery disease, and ED (23). Nevertheless, the effects of 2,4-D exposure on ED have never been determined before. In our study, we found that the association between urinary 2,4-D level and ED was not significant after full adjustment (OR 1.02; 95% CI 0.77–1.36; P = 0.882). Furthermore, men in the quartile 4 group were not associated with an increased risk of ED (OR 1.13; 95% CI 0.74–1.75; P for trend = 0.481). These results should be interpreted with much caution as it is not generalizable for special populations exposed to higher than normal 2,4-D level. Therefore, we further performed a subgroup analysis and found that the association remained not significant in the subgroup analysis stratified by age, BMI, cardiovascular disease, diabetes, and high cholesterol.
A few limitations are present in the current study. First, the causal association between 2,4-exposure and ED cannot be examined because of the cross-sectional design of the NHANES database. Second, the diagnosis of ED was on the basis of patients' self-report, which might underestimate the actual prevalence rate of ED. However, the validity of a single-question self-report of ED has been validated previously (14). Third, a single measurement of urinary 2,4-D level might deviate from the long-term chronic exposure of 2,4-D, but Suzuki et al. suggested that metabolite levels in single spot urine could reflect longer-term exposure of subjects (24). Lastly, our study conclusions cannot be generalized because all analysis was performed only based on U. S. population, and those participants with a history of prostate cancer were excluded. Nevertheless, our study included a large-scale sample with the NHANES's rigorous quality control of the procedures. This is the first study to evaluate the relationship between 2,4-D exposure and ED. We also conducted a subgroup analysis for the sensitivity test.
Conclusion
Utilizing a large-scale national database with rigorous quality control, our findings revealed that no statistically significant association was observed between 2,4-D exposure and ED after full adjustment. Future prospective studies are needed to validate our results.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES.
Ethics Statement
The studies involving human participants were reviewed and approved by National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WW and JY proposed the conception and design. YM and JC provided administrative support. LP, XG, and LL supplied the study materials. FZ and YX collected and assessed the data. FQ analyzed the data. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of China (Nos. 81871147 and 82071639) and the Sichuan Science and Technology Program (Nos. 2022YFS0028 and 2022YFS0134).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
2, 4-D, 2, 4-dichlorophenoxyacetic acid; ED, erectile dysfunction; NHANES, national health and nutrition examination survey; LOD, limit of detection; BMI, body mass index; OR, odds ratio; CI, confidence interval.
References
1. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. (2013) 381:153–65. doi: 10.1016/S0140-6736(12)60520-0
2. Xiong Y, Zhang Y, Zhang F, Wu C, Qin F, Yuan J. Applications of artificial intelligence in the diagnosis and prediction of erectile dysfunction: a narrative review. Int J Impot Res. (2022). doi: 10.1038/s41443-022-00528-w [Epub ahead of print].
3. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. (1999) 84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x
4. Gianessi LP. The increasing importance of herbicides in worldwide crop production. Pest Manag Sci. (2013) 69:1099–105. doi: 10.1002/ps.3598
5. Zuanazzi NR, Ghisi NC, Oliveira EC. Analysis of global trends and gaps for studies about 2,4-D herbicide toxicity: a scientometric review. Chemosphere. (2020) 241:125016. doi: 10.1016/j.chemosphere.2019.125016
6. Liu W, Li H, Tao F, Li S, Tian Z, Xie H. Formation and contamination of PCDD/Fs, PCBs, PeCBz, HxCBz and polychlorophenols in the production of 2,4-D products. Chemosphere. (2013) 92:304–8. doi: 10.1016/j.chemosphere.2013.03.031
7. LaKind JS, Burns CJ, Naiman DQ, O'Mahony C, Vilone G, Burns AJ, et al. Critical and systematic evaluation of data for estimating human exposures to 2,4-dichlorophenoxyacetic acid (2,4-D) - quality and generalizability. J Toxicol Environ Health Part B Crit Rev. (2017) 20:423–46. doi: 10.1080/10937404.2017.1396704
8. Mandel JS, Alexander BH, Baker BA, Acquavella JF, Chapman P, Honeycutt R. Biomonitoring for farm families in the farm family exposure study. Scand J Work Environ Health. (2005) 31(Suppl. 1):98–104; discussion 63-5
9. Marouani N, Tebourbi O, Cherif D, Hallegue D, Yacoubi MT, Sakly M, et al. Effects of oral administration of 2,4-dichlorophenoxyacetic acid (2,4-D) on reproductive parameters in male Wistar rats. Environ Sci Pollut Res Int. (2017) 24:519–26. doi: 10.1007/s11356-016-7656-3
10. Orton F, Lutz I, Kloas W, Routledge EJ. Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ Sci Technol. (2009) 43:2144–50. doi: 10.1021/es8028928
11. Panuwet P, Ladva C, Barr DB, Prapamontol T, Meeker JD, D'Souza PE, et al. Investigation of associations between exposures to pesticides and testosterone levels in Thai farmers. Arch Environ Occup Health. (2018) 73:205–18. doi: 10.1080/19338244.2017.1378606
12. Schreinemachers DM. Perturbation of lipids and glucose metabolism associated with previous 2,4-D exposure: a cross-sectional study of NHANES III data, 1988-1994. Environ Health Global Access Sci Source. (2010) 9:11. doi: 10.1186/1476-069X-9-11
13. Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol. (2009) 38:1674–80. doi: 10.1093/ije/dyp269
14. O'Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the massachusetts male aging study. J Gen Int Med. (2005) 20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x
15. Sauerhoff MW, Braun WH, Blau GE, Gehring PJ. The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral administration to man. Toxicology. (1977) 8:3–11. doi: 10.1016/0300-483X(77)90018-X
16. Hill RH Jr, Shealy DB, Head SL, Williams CC, Bailey SL, et al. Determination of pesticide metabolites in human urine using an isotope dilution technique and tandem mass spectrometry. J Anal Toxicol. (1995) 19:323–9. doi: 10.1093/jat/19.5.323
17. Loprinzi PD, Edwards M. Association between objectively measured physical activity and erectile dysfunction among a nationally representative sample of American men. J Sex Med. (2015) 12:1862–4. doi: 10.1111/jsm.12977
18. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. (2007) 120:151–7. doi: 10.1016/j.amjmed.2006.06.010
19. Rahman HH, Niemann D, Munson-McGee SH. Association of albumin to creatinine ratio with urinary arsenic and metal exposure: evidence from NHANES 2015-2016. Int Urol Nephrol. (2021) 54:1343–53. doi: 10.1007/s11255-021-03018-y
20. Janiszewski PM, Janssen I, Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med. (2009) 6:1990–8. doi: 10.1111/j.1743-6109.2009.01302.x
21. Freisthler MS, Robbins CR, Benbrook CM, Young HA, Haas DM, Winchester PD, et al. Association between increasing agricultural use of 2,4-D and population biomarkers of exposure: findings from the national health and nutrition examination survey, 2001-2014. Environ Health Global Access Sci Source. (2022) 21:23. doi: 10.1186/s12940-021-00815-x
22. Glover FE, Del Giudice F, Belladelli F, Ryan PB, Chen T, Eisenberg ML, et al. The association between 2,4-D and serum testosterone levels: NHANES 2013-2014. J Endocrinol Invest. (2022) 45:787–96. doi: 10.1007/s40618-021-01709-y
23. Kloner RA, Carson C 3rd, Dobs A, Kopecky S, Mohler ER 3rd. Testosterone and cardiovascular disease. J Am Coll Cardiol. (2016) 67:545–57. doi: 10.1016/j.jacc.2015.12.005
Keywords: erectile dysfunction, 2, 4-dichlorophenoxyacetic acid, NHANES, herbicides, men's health
Citation: Wang W, Ma Y, Chen J, Peng L, Gao X, Lin L, Zhang F, Xiong Y, Qin F and Yuan J (2022) The Association Between 2, 4-Dichlorophenoxyacetic Acid and Erectile Dysfunction. Front. Public Health 10:910251. doi: 10.3389/fpubh.2022.910251
Received: 01 April 2022; Accepted: 23 May 2022;
Published: 24 June 2022.
Edited by:
Ethel Eljarrat, Institute of Environmental Assessment and Water Research, Spanish National Research Council (CSIC), SpainReviewed by:
R. Clinton Webb, University of South Carolina, United StatesJianhuai Chen, Jiangsu Provincial Hospital of Traditional Chinese Medicine, China
Copyright © 2022 Wang, Ma, Chen, Peng, Gao, Lin, Zhang, Xiong, Qin and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiuhong Yuan, jiuhongyuan2107@163.com
†These authors have contributed equally to this work and share first authorship
 Wei Wang
Wei Wang Yucheng Ma
Yucheng Ma Jiawei Chen
Jiawei Chen Liao Peng
Liao Peng Xiaoshuai Gao
Xiaoshuai Gao Lede Lin
Lede Lin Fuxun Zhang
Fuxun Zhang Yang Xiong
Yang Xiong Feng Qin
Feng Qin Jiuhong Yuan
Jiuhong Yuan