The Clinical Value of Pulmonary Rehabilitation in Reducing Postoperative Complications and Mortality of Lung Cancer Resection: A Systematic Review and Meta-Analysis
- Pulmonary and Critical Care Medicine, Shanghai Jiao Tong University, Shanghai Chest Hospital, Shanghai, China
Background: Pulmonary rehabilitation is one meaningful way of improving exercise tolerance and pulmonary function. Thus, it may reduce the postoperative complications and mortality of pulmonary resection. Hence, we refreshed the data and conducted this systemic analysis.
Method: We searched Pubmed, Web of Science, and EMBASE using “lung OR pulmonary” AND “operation OR resection OR surgery” AND “rehabilitation or exercise.” The cut-off date was September 30, 2020. The publications were filtrated, and data were extracted from all selected studies by two reviewers. Review Manger 5.1 and the fixed or random regression model were used for calculating the pooled odds ratio (OR).
Result: Finally, 13 publications were enrolled in this study. Among them, five publications reported mortality, nine reported postoperative complications, and seven reported postoperative pulmonary complications. The pooled OR of mortality was 1.32 [95% confidence interval (CI): 0.54–3.23] for the pulmonary rehabilitation group, the pooled OR of postoperative complications was 0.62 (95% CI: 0.49–0.79) for the pulmonary rehabilitation group, and the pooled OR of postoperative pulmonary complications was 0.39 (95% CI: 0.27–0.56) for the pulmonary rehabilitation group. Subgroup analysis revealed the perioperative pulmonary rehabilitation was the most important part.
Conclusion: Pulmonary rehabilitation may not affect the mortality of pulmonary resection patients, however, it could decrease the number of postoperative complications, especially pulmonary complications. Perioperative pulmonary rehabilitation was the most important part of the program.
Lung cancer was the most leading cause of cancer-related deaths in China and even around the World (1, 2). Among all cases of lung cancer, 80% were non-small cell lung cancer (NSCLC) (3). Radical operation was a valuable way for early-stage NSCLC patients in multidisciplinary team (4). Usually, lung cancer patient characteristics include old age (5), having a history of smoking, and suffering from cardiovascular or respiratory comorbidities (6). These characteristics were also known as negative impactors in surgical tolerability, and they increase the perioperative risk (7). Under current surgical techniques and nursing skills, postoperative pulmonary complications (PPCs) occurred in 20–30% of patients (8). PPCs were regarded as the main causes of prolonged length of hospital stay, increased hospitalization cost, and poor life quality.
Pulmonary rehabilitation was a meaningful intervention in the management of chronic obstructive pulmonary disease or other chronic respiratory diseases (9). In 2015, “An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Respiratory rehabilitation” defined pulmonary rehabilitation as “comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors” (10). So, a well-designed pulmonary rehabilitation program should include exercise training, pharmacotherapy, smoking cessation, nutritional support, behavior change, health education, etc. (11). The National Institute of Health and Clinical Excellence guidelines on lung cancer also emphasized the need for rehabilitation programs before and after surgery, stating that the outcomes should include mortality, pulmonary complications, pulmonary function, etc. (12). This topic was frequently studied. Several studies had reported the clinical value of pulmonary rehabilitation in shortening the length of hospital stay and improving exercise tolerance (13–15). At the same time, there had been other studies not showing positive effects of pulmonary rehabilitation program (16, 17). Also, some systemic analyses tried to answer the question of the clinical significance of pulmonary rehabilitation during the peri-operative period (18–22). However, some studies only included a randomized controlled trial (RCT) for future calculation (22). In addition, the newest one was published in 2019, and it only enrolled the publications before June 2017 (21). In the last few years, some new pulmonary rehabilitation clinical trials have been reported, including some non-RCT trials.
Thus, in this study, we aim to update the records and conduct this systemic analysis to explore the clinical value of pulmonary rehabilitation in decreasing postoperative complications and mortality of pulmonary resection.
Methods
Literature Search
We carried out a computerized search of published research studies in the Medline, Embase, and Web of Science databases and the Cochrane Library with the following: “lung OR pulmonary” AND “operation OR resection OR surgery” AND “rehabilitation or exercise.” Alternative spellings and abbreviations were also considered. Reference lists of included studies and relevant reviews were also manually searched. The literature search was conducted without any limitations. The publication date boundaries were January 1, 2005, and September 30, 2020.
All publications in English were considered. Conference abstracts or letters to editors were excluded due to their limited data. No minimum number of patients for a study was required to be included in our meta-analysis.
Inclusion Criteria and Exclusion Criteria
All potentially relevant studies that met the following criteria were retrieved and assessed for inclusion: (1) the study should include the pulmonary rehabilitation and control group; (2) the outcome of the study should be one of the last items (postoperative complications, post-operative pulmonary complications, and mortality); (3) the study should include sufficient data for calculation. The exclusion criteria were as follows: (1) part of patients enrolled in the study not having received surgery.
If the same study cohort appeared in several articles, only the latest article was selected. Disagreements were resolved by discussion.
Data Extraction
Data were extracted from all selected studies by two reviewers who worked independently, using a standardized form to ensure that all relevant information was captured. The following data were extracted from each publication: author, publication year, country, study design, pre- or post-operation, number of each group, the pulmonary rehabilitation program, the frequency of pulmonary rehabilitation, the time of pulmonary rehabilitation, the choice of operation, tumor stage of the patients enrolled, post-pulmonary operation complications, postoperative pulmonary complications, and mortality. If data of the items mentioned above were not reported in the study, the item was treated as “not reported.” Two reviewers assessed the Quality Rating Scheme for Studies (23). The third author assessed the data and resolved the disagreement.
Statistical Analysis
All calculations were carried out with Review Manger 5.1 statistical software. All the analysis was conducted according to the standard methods recommended for a meta-analysis of. For each study, we calculated the odds ratio (OR) with 95% confidence interval (CI) to summarize the effects of pulmonary rehabilitation programs on postoperative morbidity and mortality. The fixed or random regression model was applied, and P < 0.05 was regarded as statistically significant. I2 statistics were used to detect statistically significant heterogeneity across the studies. Heterogeneity was evaluated by I2: if I2 > 50%, an article was considered to display substantial heterogeneity, requiring subgroup analysis. The potential publication bias was estimated by Deeks' funnel plots. A statistically significant publication bias existed if the P-value was <0.1 (24).
Begg's tests were used to detect any potential publication bias within the meta-analyses. The Begg's funnel plot showed the presence of bias visually.
Results
Study Selection
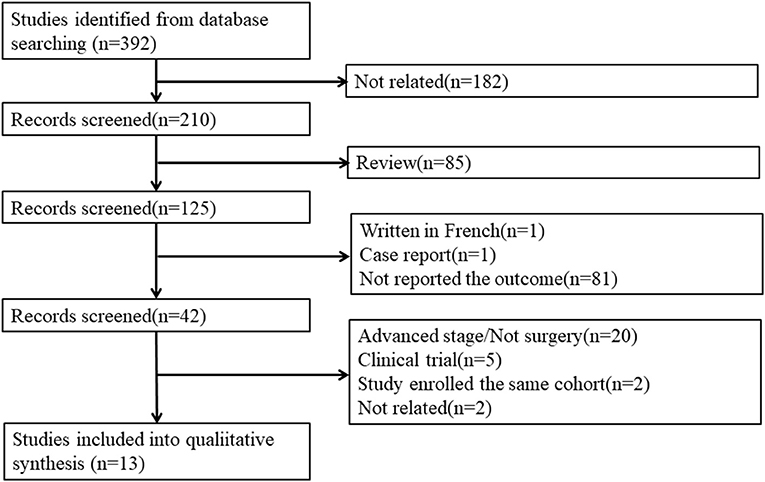
Our search strategy identified 392 publications for consideration. Among these, 182 were irrelevant studies, and 85 reviews were removed. Then, the abstracts were reviewed: 81 studies were excluded because they did not report the three outcomes, 1 was written in French, and 1 was a case report. Of the 42 remaining publications, the full articles were obtained and reviewed, and another 29 studies were excluded for the following reasons: 20 studies were excluded because they enrolled advanced stage patients or not all patients received surgery, five studies were clinical trial protocol reports, two studies enrolled the same cohort, and two studies were not related to our study (Figure 1). Finally, 13 publications meeting all of the inclusion criteria were considered for the meta-analysis. Among them, five publications reported mortality, nine reported postoperative complications, and seven reported postoperative pulmonary complications.
Study Descriptions and Quality Assessment
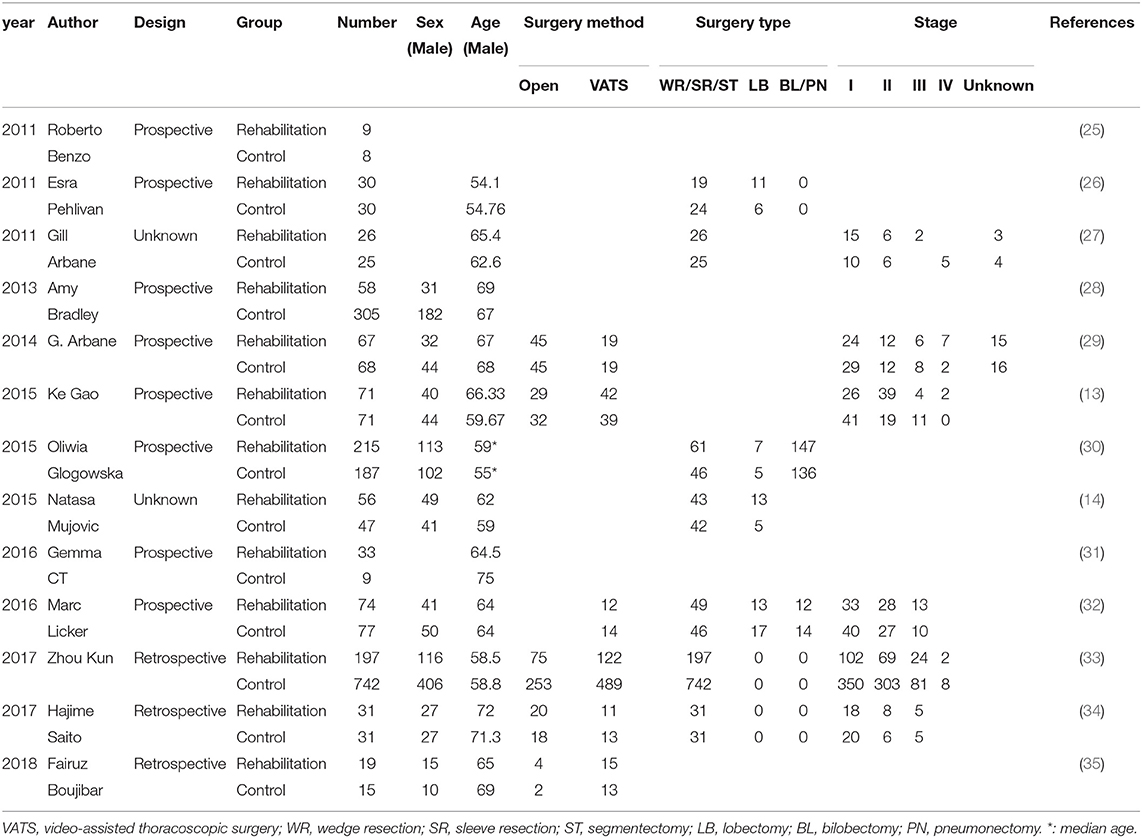
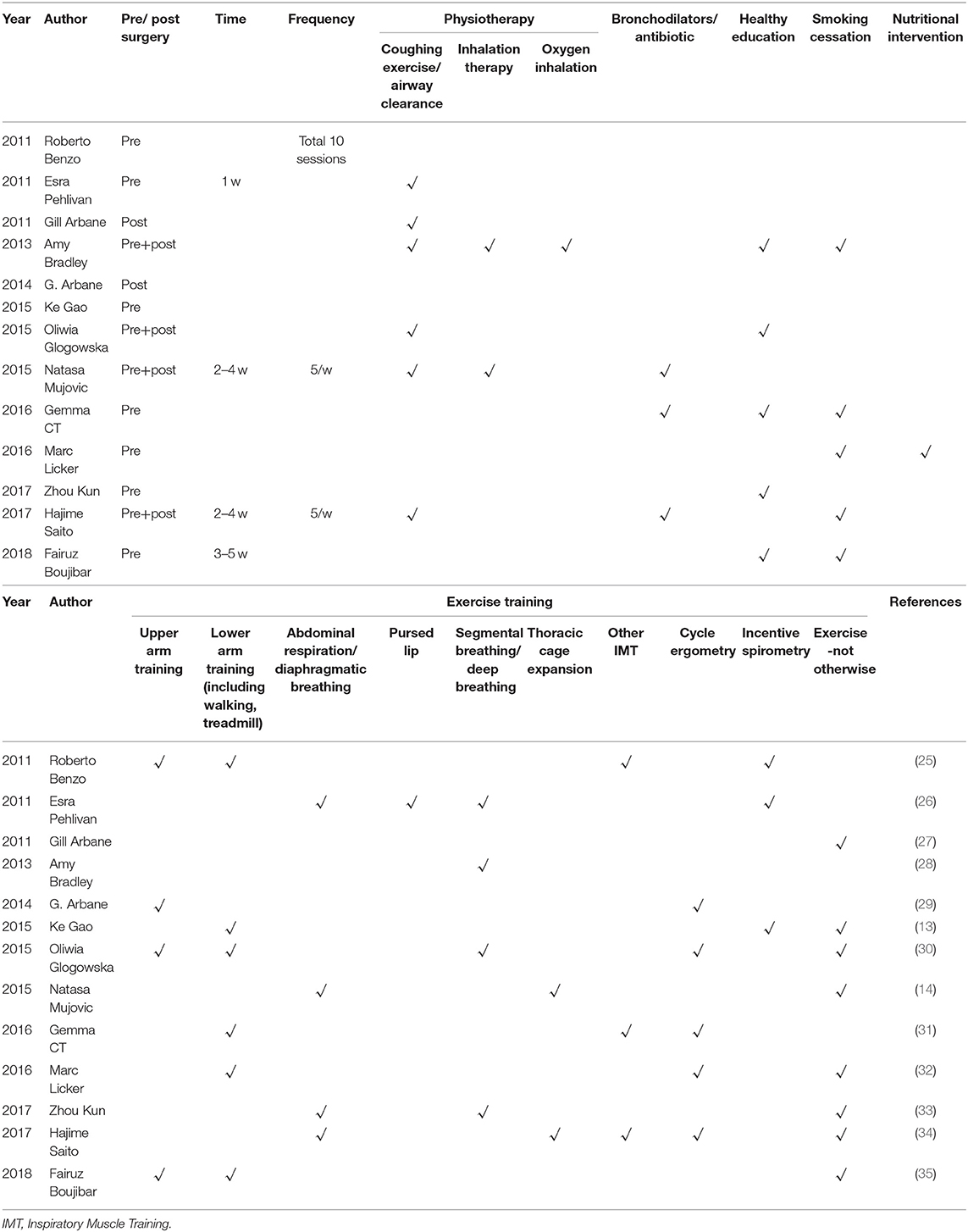
The 13 publications enrolled 2,501 patients totally. Among them, eight studies were prospective designs, three were retrospective, and the remaining two did not report. Six studies reported surgery method (video-assisted thoracoscopic surgery (VATS) or open), seven reported the surgery type (wedge resection, sleeve resection, segmentectomy, lobectomy, bilobectomy, and pneumonectomy), and six studies reported the cancer stage. In seven studies, the pulmonary rehabilitation was conducted before surgery, in two studies it was conducted after surgery, and in the remaining four studies, it was performed both pre- and post-operation. All the 13 studies adopted at least one exercise training, six studies adopted physiotherapy, and three studies adopted bronchodilators or antibiotics. Besides, healthy education was added to five studies, and nutritional intervention was used in one study. Smoking cessation was emphasized in five studies. The details were showed in Tables 1–3 and Supplement Table 1.
The data of quality assessment was showed in Supplementary Figure 1.
Mata-Analysis and Systemic Review
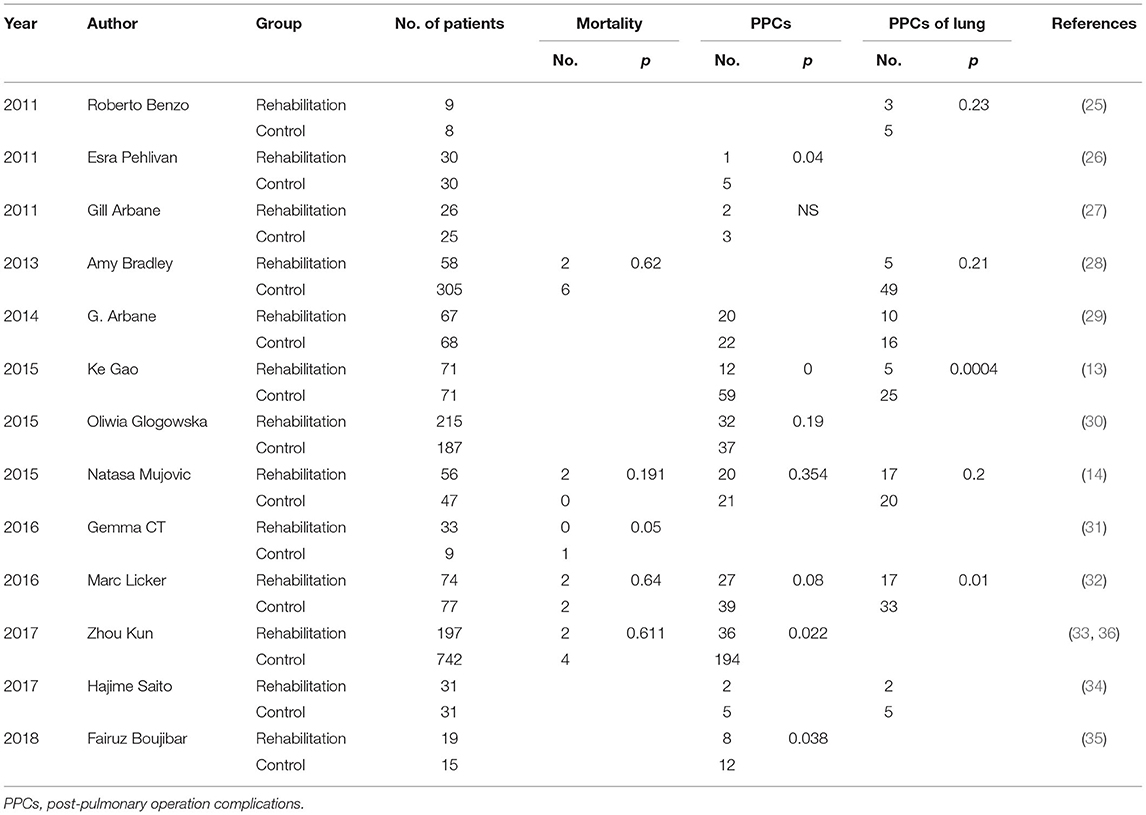
For postoperative complications analysis, nine studies enrolled 1,937 patients. The pooled OR was 0.62 (95% CI: 0.49–0.79), favoring the pulmonary rehabilitation. For subgroup analysis, we found the pre-surgery rehabilitation had a clinical significance, and the post- or pre-+post- only showed tendencies in favor of pulmonary rehabilitation.
For postoperative pulmonary complications analysis, seven studies enrolled 969 patients. The pooled OR was 0.39 (95% CI: 0.27–0.56) favoring the pulmonary rehabilitation. For subgroup analysis, we found the pre-surgery and pre-+post-surgery rehabilitation subgroups had a clinical significance, and the post-surgery subgroup only showed a tendency in favor of pulmonary rehabilitation.
For mortality analysis, five studies enrolled 1,598 patients. The pooled OR was 1.32 (95% CI: 0.54–3.23), no clinical significance was showed in rehabilitation group. For subgroup analysis, both the pre-surgery and pre-+post-surgery rehabilitation subgroups showed no difference in rehabilitation or control group.
The details were showed in Figures 2A–C.
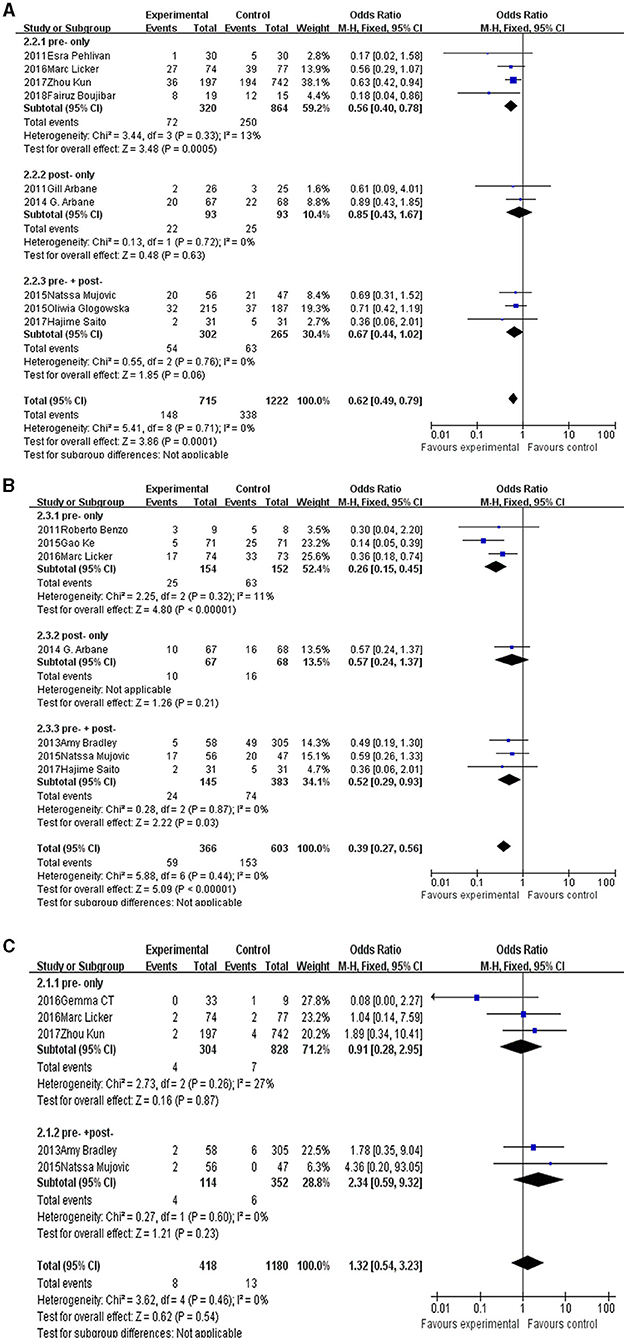
Figure 2. The forest plot of postoperative complications (A), postoperative pulmonary complications (B), and mortality (C).
All three analyses showed no publication bias. The I2 < 50% and P > 0.01 in those three analyses. The funnel plot was showed in Supplementary Figures 2–4.
Discussion
Surgery operation remains the optimal selection for early-stage lung cancer patients, and it was also a crucial part of a multidisciplinary team for advanced lung cancer patients. Lung cancer was related to smoking history, thus the patients always had chronic lung disease, heart disease, and cerebrovascular diseases at the same time (6, 36). Those risk factors may increase the PPCs after pulmonary operation (7). Besides, lung cancer patients suffered deconditioning, muscle weakness, fatigue, cachexia, and anxiety, those sufferings resulted in disability and impaired quality of life among lung cancer individuals (37, 38). Pulmonary rehabilitation was usually applied in chronic obstructive pulmonary disease, and it was significantly associated with a lower risk of death (39, 40). Pulmonary rehabilitation was also recommended for other chronic pulmonary diseases, interstitial lung disease, cystic fibrosis, lung cancer, etc. (10, 11). Several studies have supported the positive effects of rehabilitation in muscle strength, exercise endurance, well-being, and health status (25, 41–43), and it also relieved the discomfort from symptoms (44, 45). In recent years, pulmonary rehabilitation had been advocated by a wide range of surgical specialties, including cardiothoracic surgery. Many single-center-based studies have reported the clinical values of pulmonary rehabilitation. For those who would undergo pulmonary operations, the pulmonary rehabilitation program could apply before surgery, after surgery, or both pre- and post- surgery. For preoperative pulmonary rehabilitation, it can improve individuals' exercise tolerance and overall medical stability before surgery resection (46, 47). Those who received pulmonary rehabilitation after lung cancer resection surgery may gain increasements in walking endurance, peak exercise capacity, and decrease in dyspnea and fatigue (48, 49). At some centers, the pulmonary rehabilitation was applied during hospitalization (14, 28, 30).
Many studies had supported the positive roles of rehabilitation in decreasing postoperative complications and mortality, but the majority of them are based on a single center and a limited number of patients, and they thus could not avoid selection bias. The latest systemic analysis was published in 2019 and only enrolled publications before June 2017 (21). We therefore conducted this study to update the records and explore the clinical value of pulmonary rehabilitation in decreasing postoperative complications and mortality.
After selection, nine studies enrolled 1,937 patients in total and reported postoperative complications, and seven studies enrolled 969 patients in total and reported pulmonary complications. In our study, pulmonary rehabilitation had proved the clinical values in decreasing the postoperative complications for patients, especially pulmonary complications. Previous research suggested that pulmonary function was a good predictor for pulmonary resection, including, for example, the forced expiratory volume in one second (FEV1), forced vital capacity (FVC), carbon monoxide diffusing capacity (DLCO), the cardiopulmonary exercise test (CPET), impulse oscillometry (IOS), etc. (50–54). Pulmonary rehabilitation has been proven to improve the cardiopulmonary function, exercise tolerance, anxiety, depression, etc. (46, 55–61). Lai et al. suggested that pre-surgery pulmonary rehabilitation may improve the FEV1, FVC, and 6-minute walking test (6MWT) (58, 59). Jones's study, apart from pulmonary function, observed an improvement in cardiopulmonary function after presurgical exercise training (46). Stefanelli et al. measured using the BROG scale and found the modified breath in chronic obstructive pulmonary disease (COPD) patients after high-intensity training and cardiopulmonary exercise. In Cavalheri's study, post-surgery pulmonary rehabilitation showed positive values in pulmonary function, cardiopulmonary function, and mental fitness (57). Vagvolgyi's study also demonstrated the clinical value of post-surgery pulmonary rehabilitation (60). Besides, pulmonary rehabilitation may decrease the level of cytokine and inflammation factors. In Messaggi-Sartor's study, after an 8-week training program, an increase of 0.61 μg/mL in the serum IGFBP-3 levels for patients in the intervention group was observed (61). Fiorelli et al. reported a lower level of Serum IL-6 (P = 0.001), IL-10 (P = 0.001), and TNF-α (P = 0.001) in the transcutaneous electrical nerve stimulation group than in the control group (55). In our analyses, we showed a positive result of pulmonary rehabilitation, especially in the pre-surgery subgroup. This may be because the outcome of this study was the main complications after surgery. Pre-surgery rehabilitation improved pulmonary function and cardiopulmonary function before an operation, thus decreasing complications after surgery. For the post-operation rehabilitation subgroup, it showed a favoring of the pulmonary rehabilitation group, but the result was not statistically significant. We inferred that the complications occurred before the pulmonary rehabilitation worked. We suggested the pre-surgery pulmonary rehabilitation should be operated as perioperative interventions, especially for high-risk patients. The main goal of perioperative rehabilitation is to improve pulmonary function, avoiding atelectasia, pneumonia, etc. Herin, apart from calculating the pooled effect of the postoperative complications, we specifically calculated the pooled OR of decreasing postoperative pulmonary complications. We found the pulmonary rehabilitation worked better in decreased PPCs than total complications. This may be because the rehabilitation program focuses on the lung.
Some studies have argued for the positive clinical value of pulmonary rehabilitation in long-term survival for those pulmonary resection patients (61, 62). While perioperative rehabilitation would improve lung function, other organs would gain beneficence from this procedure, such as the heart. Mortality related to heart disease and related issues would decrease. But in this study, the pulmonary rehabilitation did not show the clinical value for mortality in those who received pulmonary surgery in either the pre-operative group or pre- +post- group. As mentioned above, pulmonary rehabilitation could improve cardiopulmonary function, exercise tolerance, etc. Those factors also were effective predicted factors for mortality, such as DLCO (63, 64). Both pre- and post-surgery pulmonary rehabilitation showed an improvement in DLCO (56, 65). In our study, no significant value of pulmonary rehabilitation in reducing mortality was observed, several reasons may account for the result. Firstly, only five studies were enrolled in this meta-analysis, limited people were enrolled, especially the rehabilitation group. Secondly, it could be attributed to the development of surgical techniques. Among them, two studies reported on surgical methods. In Licker's study, all patients received VATS. In Zhou's study, more than half of the patients performed VATS. This means low mortality would be observed in those cohorts. Thirdly, some studies were not RCT, so select bias could not be avoided.
Our study also had some limits. Firstly, for defined outcomes, only a few studies were included in the meta-analysis. This may result in publication bias. Secondly, some studies were not randomized controlled trials, and this may cause selected bias when conducted the clinical trial. Thirdly, the studies enrolled were mostly performed in one center, which also resulted in select bias. Forth, it is difficult to divide complications directly related to surgery from those related to comorbidity, and we summarize the complications as PPCs and total complications.
Summarily, pulmonary rehabilitation is meaningful in avoiding postoperative complications of pulmonary resection. We suggested that pulmonary rehabilitation should be included in the perioperative period, and perioperative pulmonary rehabilitation was the most important part of the program. Also, a more well-designed RCT is required to provide proof of our results.
Data Availability Statement
The original contributions generated for the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author Contributions
XM, YNi, and YNiu conducted the literature search and data extraction. XM wrote the manuscript. YNi revised the manuscript. LJ reviewed the manuscript and directed and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China: 2018YFC1313600 and the Chinese Society of Clinical Oncology (CSCO): Y-2019AZZD-0038.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.685485/full#supplementary-material
Supplement Table 1. Main pulmonary rehabilitation protocols.
Supplementary Figure 1. Quality rating scheme for enrolled studies.
Supplementary Figure 2. Funnel plot of postoperative complications.
Supplementary Figure 3. Funnel plot of postoperative pulmonary complications.
Supplementary Figure 4. Funnel plot of mortality.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. (2008) 359:1367–80. doi: 10.1056/NEJMra0802714
4. Baltayiannis N, Chandrinos M, Anagnostopoulos D, Zarogoulidis P, Tsakiridis K, Mpakas A, et al. Lung cancer surgery: an up to date. J Thorac Dis. (2013) 4:S425–39. doi: 10.3978/j.issn.2072-1439.2013.09.17
5. Schulte T, Schniewind B, Dohrmann P, Küchler T, Kurdow R. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest. (2009) 135:322–29. doi: 10.1378/chest.08-1114
6. Jones LW. Physical activity and lung cancer survivorship. Phys Activity Cancer. (2011) 186:255–74. doi: 10.1007/978-3-642-04231-7_11
7. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e166–95. doi: 10.1378/chest.12-2395
8. Thomas PA, Berbis J, Falcoz PE, Pimpec-Barthes LA, Bernard A, Jougon J, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. (2014) 45:652–59. doi: 10.1093/ejcts/ezt452
9. Global Initiative for Chronic Obstructive Lung Disease Pocket Guide to COPD Diagnosis Management and Prevention A Guide for Health Care Professional (2020). Available online at: www.goldcopd.org
10. Carolyn L, Rochester, Ioannis Vogiatzis, Lareau SC, Marciniuk DD, Puhan MA, et al. An official american thoracic society/european respiratory society policy statement: enhancing implementation, use, and delivery of respiratory rehabilitation. Am J Respir Crit Care Med. (2015) 192:1373–86. doi: 10.1164/rccm.201510-1966ST
11. Spruitm A, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An Official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in respiratory rehabilitation. Am J Respir Crit Care Med. (2013) 188:e13–64. doi: 10.1164/rccm.201309-1634ST
12. National Institute for Health and Clinical Excellence. The Diagnosis and Treatment of Lung Cancer (Update). London; England: National Institute for Health and Clinical Excellence (2013). Available online at: http://guidance.nice.org.uk/CG121/Guidance
13. Gao K, Yu PM, Su JH, He CQ, Liu LX, Zhou YB, et al. Cardiopulmonary exercise testing screening and pre-operative respiratory rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. (2015) 6:443–9. doi: 10.1111/1759-7714.12199
14. Mujovic N, Mujovic N, Subotic D, Ercegovac M, Milovanovic A, Nikcevic L, et al. Influence of respiratory rehabilitation on lung function changes after the lung resection for primary lung cancer in patients with chronic obstructive pulmonary disease. Aging Dis. (2015) 6:466–77. doi: 10.14336/AD.2015.0503
15. Morano MT, Araújo AS, Nascimento FB, da Silva GF, Mesquita R, Pinto JS„, et al. Preoperative respiratory rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. (2013) 94:53–8. doi: 10.1016/j.apmr.2012.08.206
16. Bobbio A, Chetta A, Ampollini L, Primomo GL, Internullo E, Carbognani P, et al. Preoperative respiratory rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. (2008) 33:95–8. doi: 10.1016/j.ejcts.2007.10.003
17. Coats V, Maltais F, Simard S, Fréchette E, Tremblay L, Ribeiro F, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J. (2013) 20:e10–6. doi: 10.1155/2013/291059
18. Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: a systematic review and meta analysis. Clin Rehabil. (2015) 29:426–38. doi: 10.1177/0269215514545350
19. Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact CardioVasc Thorac Surg. (2016) 23:486–7. doi: 10.1093/icvts/ivw152
20. Ni HJ, Pudasaini B, Yuan XT, Li HF, Shi L, Yuan P. Exercise training for patients pre- and postsurgically treated for non–small cell lung cancer: a systematic review and meta-analysis. Integr Cancer Ther. (2017) 16:63–73. doi: 10.1177/1534735416645180
21. Li X, Li S, Yan S, Wang Y, Wang X, Sihoe ADL, et al. Impact of preoperative exercise therapy on surgical outcomes in lung cancer patients with or without COPD: a systematic review and meta-analysis. Cancer Manag Res. (2019) 11:1765–77. doi: 10.2147/CMAR.S186432
22. Wang YQ, Liu X, Jia Y, Xie J. Impact of breathing exercises in subjects with lung cancer undergoing surgical resection: a systematic review and meta-analysis. J Clin Nurs. (2019) 28:717–32. doi: 10.1111/jocn.14696
23. Available, online at: https://jamanetwork.com/journals/jamanetworkopen/pages/instructions-for-authors
24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
25. Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. (2011) 74:441–5. doi: 10.1016/j.lungcan.2011.05.011
26. Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg. (2011) 17:461–8. doi: 10.5761/atcs.oa.11.01663
27. Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer. (2011) 71:229–34. doi: 10.1016/j.lungcan.2010.04.025
28. Bradley A, Marshall A, Stonehewer L, Reaper L, Parker K, Bevan-Smith E, et al. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg. (2013) 44:e266–71. doi: 10.1093/ejcts/ezt381
29. Arbane G, Douiri A, Hart N, Hopkinson NS, Singh S, Speed C, et al. Effect of postoperative physical training on activityafter curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. (2014) 100:100–7. doi: 10.1016/j.physio.2013.12.002
30. Glogowska O, Glogowski M, Szmit S. Intensive rehabilitation as an independent determinant of better outcome in patients with lung tumors treated by thoracic surgery. Arch Med Sci. (2017) 13:1442–8. doi: 10.5114/aoms.2016.60706
31. Chesterfield-Thomas G, Goldsmith I. Impact of preoperative pulmonary rehabilitation on the Thoracoscore of patients undergoing lung resection. Interact CardioVasc Thorac Surg. (2016) 23:729–32. doi: 10.1093/icvts/ivw238
32. Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C, et al. Short-Term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. (2017) 12:323–33. doi: 10.1016/j.jtho.2016.09.125
33. Zhou K, Su JH, Lai YT, Li PF, Li SJ, Che GW. Short-term inpatient-based high-intensive pulmonary rehabilitation for lung cancer patients: is it feasible and effective? J Thorac Dis. (2017) 9:4486–93. doi: 10.21037/jtd.2017.10.105
34. Saito H, Hatakeyama K, Konno H, Matsunaga T, Shimada Y, Minamiya Y. Impact of pulmonary rehabilitation on postoperative complications in patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cancer. (2017) 8:451–60. doi: 10.1111/1759-7714.12466
35. Boujibar F, Bonnevie T, Debeaumont D, Bubenheim M, Cuvellier A, Peillon C, et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis. (2018) 10:2240–8. doi: 10.21037/jtd.2018.03.161
36. Gullón JA, Suárez I, Medina A, Martín A, Cabrera C, González IJ. Lung cancer: changes in epidemiology and survival. Rev Clín Española. (2012) 212:18–23. doi: 10.1016/j.rce.2011.06.004
37. Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non–small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. (2005) 23:6865–72. doi: 10.1200/JCO.2005.02.527
38. Ostroff JS, Krebs P, Coups EJ, Burkhalter JE, Feinstein MB, Steingart RM, et al. Health-related quality of life among early-stage, non–small cell, lung cancer survivors. Lung Cancer. (2011) 71:103–8. doi: 10.1016/j.lungcan.2010.04.011
39. Lindenauer PK, Stefan MS, Pekow PS, Mazor KM, Priya A, Spitzer KA, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. (2020) 323:1813–23. doi: 10.1001/jama.2020.4437
40. Rochester CL, Holland AE. Pulmonary rehabilitation and improved survival for patients with COPD. JAMA. (2020) 323:1783–5. doi: 10.1001/jama.2020.4436
41. Dimeo F, Schwartz S, Wesel N, Voigt A, Thiel E. Effects of an endurance and resistance exercise program on persistent cancer related fatigue after treatment. Ann Oncol. (2008) 19:1495–9. doi: 10.1093/annonc/mdn068
42. Benzo RP. Pulmonary rehabilitation in lung cancer: a scientific opportunity. J Cardiopulm Rehabil Prev. (2007) 27:61–4. doi: 10.1097/01.HCR.0000265030.02521.f1
43. Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non–small cell lung cancer: a systematic review. Lung Cancer. (2011) 72:139–53. doi: 10.1016/j.lungcan.2011.01.006
44. Spruit MA, Janssen PP, Willemsen SC, Hochstenbag MM, Wouters EF. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. (2006) 52:257–60. doi: 10.1016/j.lungcan.2006.01.003
45. Riesenberg H, Lübbe AS. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer. (2010) 18:877–82. doi: 10.1007/s00520-009-0727-y
46. Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. (2007) 110:590–8. doi: 10.1002/cncr.22830
47. Shannon VR. Role of pulmonary rehabilitation in the management of patients with lung cancer. Curr Opin Pulm Med. (2010) 16:334–39. doi: 10.1097/MCP.0b013e32833a897d
48. Cesario A, Ferri L, Galetta D, Pasqua F, Bonassi S, Clini E, et al. Post-operative respiratory rehabilitation after lung resection for non–small cell lung cancer. Lung Cancer. (2007) 57:175–80. doi: 10.1016/j.lungcan.2007.02.017
49. Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical non-small cell lung cancer patients: a pilot study. Cancer. (2008) 113:3430–9. doi: 10.1002/cncr.23967
50. Liang Z, Tao J. Risk factors and treatment of acute respiratory distress syndrome after thoracotomy. Chin J Clin Thorac Cardiov Surg. (2010) 17:55–9.
51. Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest. (1995) 108:68–72. doi: 10.1378/chest.108.1.68
52. van Tilburg VPM, Stam H, Hoogsteden HC, van Klaveren RJ. Pre-operative pulmonary evaluation of lung cancer patients: a review of the literature. Eur Respir J. (2009) 33:1206–15. doi: 10.1183/09031936.00020508
53. Stringer WW. Cardiopulmonary exercise testing: current applications. Expert Rev Respir Med. (2010) 4:179–88. doi: 10.1586/ers.10.8
54. Li Q, Cao M, Sun GX, Ruan HY, Wang ZR, Zhang GH. The significance of respiratory impedance for prediction of post-operative respiratory failure in the patients with lung cancer. Chin J Thorac Cardiov Surg. (2002) 18:165–7. doi: 10.3760/cma.j.issn.1001-4497.2002.03.014
55. Fiorelli A, Morgillo F, Milione R, Pace MC, Passavanti MB, Laperuta P, et al. Control of post-thoracotomy pain by transcutaneous electrical nerve stimulation: effect on serum cytokine levels, visual analogue scale, pulmonary function and medication. Eur J Cardiothorac Surg. (2012) 41:861–8. doi: 10.1093/ejcts/ezr108
56. Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. (2013) 44:e260–5. doi: 10.1093/ejcts/ezt375
57. Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips MJ, Sanders LH, et al. Exercise training for people following curative intent treatment for non-small cell lung cancer: a randomized controlled trial. Braz J Phys Ther. (2017) 21:58–68. doi: 10.1016/j.bjpt.2016.12.005
58. Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res. (2017) 209:30–6. doi: 10.1016/j.jss.2016.09.033
59. Lai Y, Su J, Qiu P, Wang M, Zhou K, Tang Y, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interact CardioVasc Thorac Surg. (2017) 25:476–83. doi: 10.1093/icvts/ivx141
60. Vagvolgyi A, Rozgonyi Z, Kerti M, Agathou G, Vadasz P, Varga J. Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: randomized clinical trial. J Thorac Dis. (2018) 10:3519–31. doi: 10.21037/jtd.2018.05.202
61. Messaggi-Sartor M, Marco E, Martínez-Téllez E, Rodriguez-Fuster A, Palomares C, Chiarella S, et al. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med. (2019) 55:113–22. doi: 10.23736/S1973-9087.18.05156-0
62. Solberg Nes L, Liu H, Patten CA, Rausch SM, Sloan JA, Garces YI, et al. Physical activity level and quality of life in long term lung cancer survivors. Lung Cancer. (2012) 77:611–6. doi: 10.1016/j.lungcan.2012.05.096
63. Ferguson MK, Reeder LB, Mick R. Optimizing selection of patients for major lung resection. J Thorac Cardiovasc Surg. (1995) 109:275–83. doi: 10.1016/S0022-5223(95)70389-6
64. Wang J, Olak J, Ferguson MK. Diffusing capacity predicts operative mortality but not long-term survival after resection for lung cancer. J Thorac Cardiovasc Surg. (1999) 117:581–7. doi: 10.1016/S0022-5223(99)70338-7
Keywords: pulmonary rehabilitation, pulmonary resection, postoperative complications, mortality, meta-analysis
Citation: Mao X, Ni Y, Niu Y and Jiang L (2021) The Clinical Value of Pulmonary Rehabilitation in Reducing Postoperative Complications and Mortality of Lung Cancer Resection: A Systematic Review and Meta-Analysis. Front. Surg. 8:685485. doi: 10.3389/fsurg.2021.685485
Received: 25 March 2021; Accepted: 28 June 2021;
Published: 22 September 2021.
Edited by:
Michael Patrick Grocott, University of Southampton, United KingdomReviewed by:
Francesco Petrella, University of Milan, ItalyMarco Massani, ULSS2 Marca Trevigiana, Italy
Copyright © 2021 Mao, Ni, Niu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyan Jiang, jiang_liyan2000@126.com
 Xiaowei Mao
Xiaowei Mao Yiqian Ni
Yiqian Ni  Liyan Jiang
Liyan Jiang