Retroperitoneal laparoscopic radical nephrectomy (RLRN) is associated with poor integrity of Gerota's fascia and perirenal fat: A prospective comparative study
- Department of Urology, Lanzhou University Second Hospital, Lanzhou, China
Purpose: To figure out the difference of integrity of Gerota's fascia and perirenal fat between Retroperitoneal Laparoscopic Radical Nephrectomy (RLRN) and Transperitoneal Laparoscopic Radical Nephrectomy (TLRN).
Methods: This is a prospective comparative study of patients with Renal Cell Carcinoma (RCC) from a designated tertiary center in Lanzhou, China. We have developed and propose a scoring tool to quantify the integrity of nephrectomy specimens from both approaches. The integrity score is based on 6 common conditions of nephrectomy specimens. Specimens are scored on a 1 to 6-point scale according to the integrity of Gerota's fascia and perirenal fat. We applied the integrity score to 142 consecutive patients. Integrity scores were compared between RLRN and TLRN groups. Factors associated with low integrity score were assessed by logistic regression.
Results: Among 142 patients, 79 (55.6%) patients and 63 (44.4%) patients, respectively, underwent RLRN and TLRN. There was a significant difference in the distribution of integrity score between the two groups (P < 0.001). RLRN (odds ratio 10.65, 95%CI 4.29–26.45, P < 0.001), tumor size (odds ratio 1.22, 95%CI 1.04–1.42, P = 0.015) and Body Mass Index (BMI) (odds ratio 0.83, 95%CI 0.72–0.96, P = 0.010) were significantly associated with low integrity score. The logistic regression equation showed good power to predict low integrity score.
Conclusion: RLRN has poor integrity of Gerota's fascia and the perirenal fat. The integrity score can be used to evaluate the extent of resection and specimen completeness in LRN. Postoperative evaluation of the integrity score is of great value for urologists to evaluate the risk of tumor residue.
Introduction
Renal cell carcinoma (RCC) accounts for about 3% of all cancers, with the highest incidence in western countries (1). In most countries, the incidence of RCC has been steadily increasing (2). Possible reasons are related to the increased application of tomographic imaging and longer life expectancies (2, 3).
Radical nephrectomy has been an effective treatment for localized RCC. In the past, radical nephrectomy was usually performed via open technique. However, with the widespread uptake of minimally invasive surgery, open radical nephrectomy has been replaced by laparoscopic radical nephrectomy (LRN) which is of minimal trauma and rapid postoperative recovery. LRN includes both retroperitoneal and transperitoneal approaches. Regardless of approach, complete surgical resection to maximize tumor control is important.
In pervious clinical practice, we found that there seems to be a difference in the degree of specimen integrity between the two approaches, however no literature has been reported so far. In the current study, we proposed a novel integrity score related to Gerota's fascia and the perirenal fat. Then we evaluated the difference in integrity score and compared between the two groups. Finally we analyzed the predictive factors of low integrity score and intended to find associations of integrity score with clinically meaningful outcomes.
Methods
A prospective comparative study was performed on 142 patients with RCC in the Department of Urology, Lanzhou University Second Hospital from November 2018 to December 2021. Patients diagnosed as having localized renal cell carcinoma were screened for inclusion. Exclusion criteria were patients with renal vein and inferior vena cava invasion, patients with radiologically proven distant metastasis, history of other malignant tumors and/or chemotherapy, previous abdominal surgery, pregnancy and/or lactation, patients who were incapacitated, and refusal of the patient to sign the informed consent form. The Ethics Committee of Lanzhou University Second Hospital approved the collection of clinical data from the included patients with RCC (2017A-054). All subjects provided informed consent.
Interventions
Our RLRN technique had been reported before (4). Details of RLRN will not be discussed in this study because they are well-known. We will describe our technique for TLRN which is also well described in literature (5). Modifications to the procedure are discussed below. All procedures were performed by expert senior surgeons with at least 5 years of experience of both TLRN and RLRN techniques.
The patient is placed in a 70–90° healthy side-lying position. The Veress needle is inserted via an incision of the lateral margin of the rectus abdominis at the level of the umbilicus, and the pressure is controlled at 13–15 mmHg. After the pneumoperitoneum is established, a 10 mm trocar is inserted as the camera port. Two further trocars are placed under direct laparoscopic vision in the lateral margin of the rectus abdominis (8 cm above the umbilicus) and mid-axillary line at the level of the umbilicus. An additional 5 mm trocar is placed for liver or spleen retraction over the subxiphoid incision. The lateral peritoneum is dissected along the paracolic sulcus, and the bowel, extraperitoneal fat, and abdominal contents are pushed to the contralateral side. In the right kidney, the first step is identification of the vena cava. The right renal vein can be found at the right side of the vena cava. In the left kidney, the left renal vein can be found along the left genital vein. The renal artery usually lies posterosuperior to the renal vein. The renal artery is first ligated with Hem-o-lok and finally the renal vein is transected after all arterial blood supply is controlled. The ureter is then dissected and divided. En bloc removal of the entire kidney including Gerota's fascia and the perirenal fat capsule and, if necessary the adrenal gland, is performed. The pressure of the pneumoperitoneum is decreased, and the whole abdomen is checked for any hemorrhage. Finally, the specimen is placed into an ENDO-bag and extracted through 6 cm pararectus abdominis incision.
Integrity scoring
Resected kidney specimens were photographed by a specially-assigned person, and then scored by an independent rater based on these photographs. The rater was completely blind to the surgical approach. Rater training was conducted with case vignettes including 12 cases for each integrity score. Finally, a senior physician assessed the rater's scoring accuracy by randomly selected specimen photos (about 20), and a reliability of 80% or greater was established. Scoring criteria used to assess the integrity of specimen were as follows (Figures 1A–F):
1. point: Gerota's fascia and perirenal fat capsule at the pole of tumor were incised; renal parenchyma and tumor surface were visible, and tumor capsule was incomplete or partially ruptured.
2. points: Gerota's fascia and perirenal fat capsule at the pole of tumor were incised; renal parenchyma and tumor surface were visible, but tumor capsule was intact.
3. points: Gerota's fascia at the pole of tumor was incised, but perirenal fat capsule was intact and the renal parenchyma and tumor surface were not visible.
4. points: Gerota's fascia and perirenal fat capsule at the other pole of tumor were incised, and renal parenchyma was visible; Gerota's fascia at the pole of tumor was not opened.
5. points: Gerota's fascia at the other pole of tumor was incised, perirenal fat capsule was intact, and renal parenchyma was not visible; Gerota's fascia at the pole of tumor was not incised.
6. points: Gerota's fascia was not incised; perirenal fat capsule and renal parenchyma were not visible.
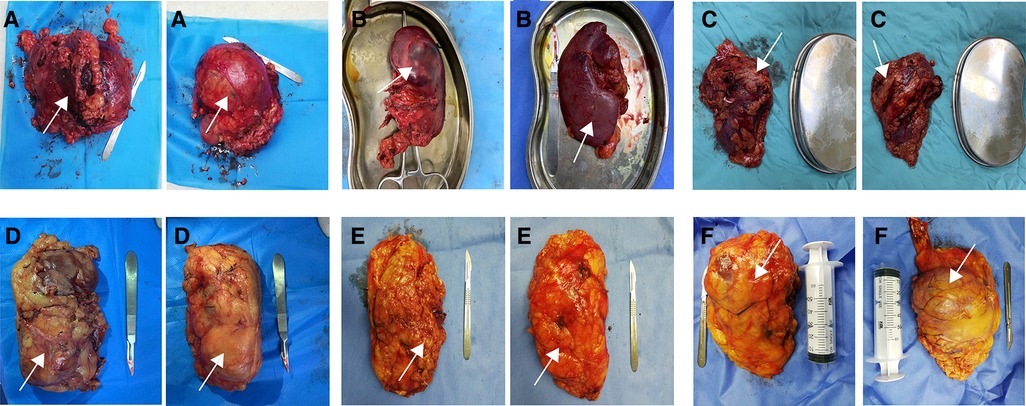
Figure 1. Diagram of the scoring criteria. A-1 point; B-2 point; C-3 points; D-4 points; E-5 points; F-6 points. Arrows in the figure indicate the location of the tumor.
Statistical analysis
Baseline characteristics were presented as frequencies (percentages) for categorical variables and mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables. Differences in distributions were compared between RLRN and TLRN groups using t-test or Wilcoxon rank-sum test for continuous variables and Fisher's exact test or Pearson's chi-square test for categorical variables as appropriate. Univariate and multivariate analyses were performed by logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were summarized. The optimal cutoff value was determined by the receiver operating characteristic (ROC) curve and maximum Youden index. The area under the curve (AUC) was calculated to evaluate the logistic regression equation. All results were considered statistically significant when two-sided P-values were <0.05. Statistical analysis was performed using SPSS 25.0 software (IBM Institute, Inc., Armonk, NY, USA).
Results
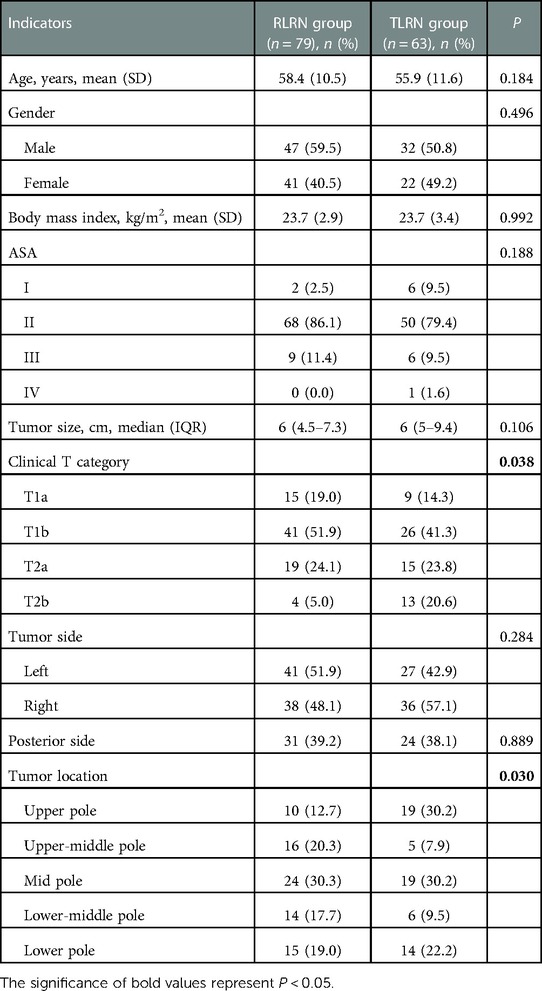
Between 2018 and 2021, a total of 203 RCC patients were assessed for eligibility. Among 196 eligible patients, 6 did not undergo surgery, 48 had missing a integrity score. Finally, we carried out analysis in the remaining 142 patients. 79 underwent RLRN (58.4 [10.5] years and 47 [59%] male), 63 underwent TLRN (55.9 [11.6] years and 32 [51%] male). Demographic details are shown in Table 1.
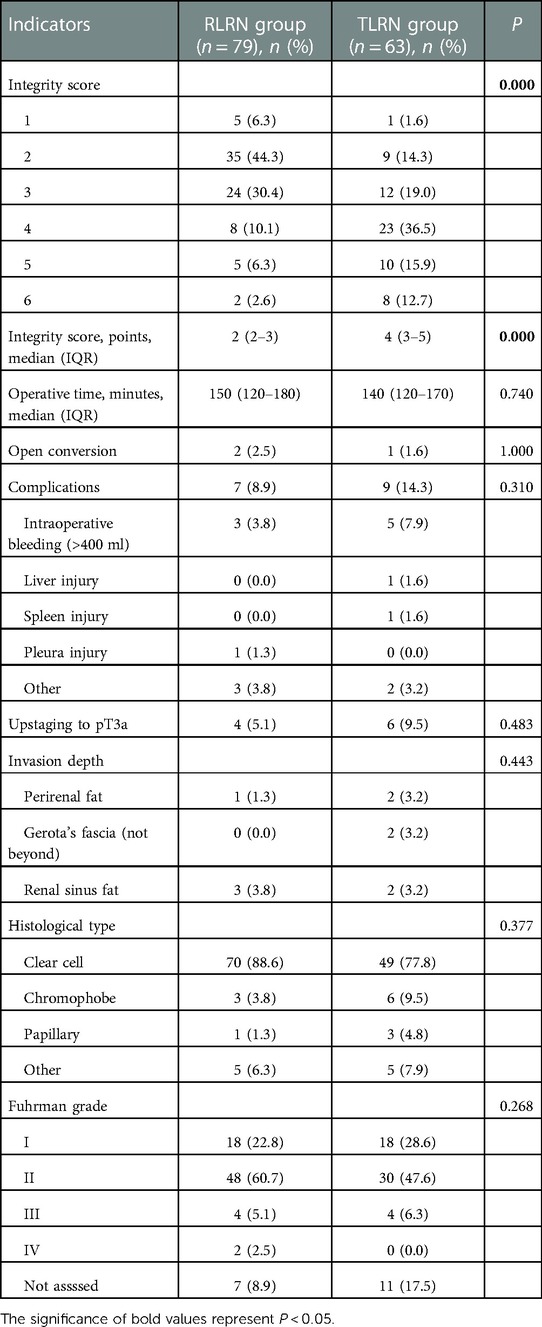
The median operative time was similar between the two groups (150 [120–180] vs. 140 [120–170] mins; P = 0.740). No difference were appreciated in either groups for pathological types (P = 0.377), and Fuhrman grade (P = 0.268). Further details are presented in Table 2. There was a significant difference in the distribution of integrity score between the two groups (Figure 2). In RLRN group, most of the specimens were concentrated in 2–3 points, while in TLRN group, most of the specimens were concentrated in 3–5 points. There was also a significant difference in median point between the two groups (P < 0.001) (Table 2).
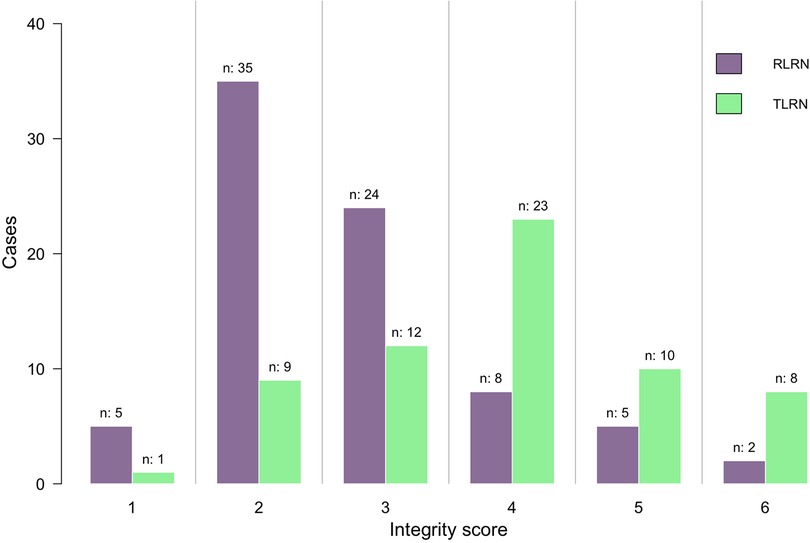
Figure 2. Distribution of the integrity score between RLRN group and TLRN group. RLRN retroperitoneal radical nephrectomy, TLRN transperitoneal radical nephrectomy.
Variables associated with low integrity score in the univariate analysis are shown in Table 3. Surgical approach, BMI, and tumor location were significantly associated with low integrity score. In multivariate analysis, RLRN (OR: 10.65; 95%CI: 4.29–26.45; P < 0.001) and tumor size (OR: 1.22; 95%CI: 1.04–1.42; P = 0.015) were independent risk factors for low integrity score, while BMI (OR: 0.83; 95%CI: 0.72–0.96; P = 0.010) was an independent protective factor (Table 3).
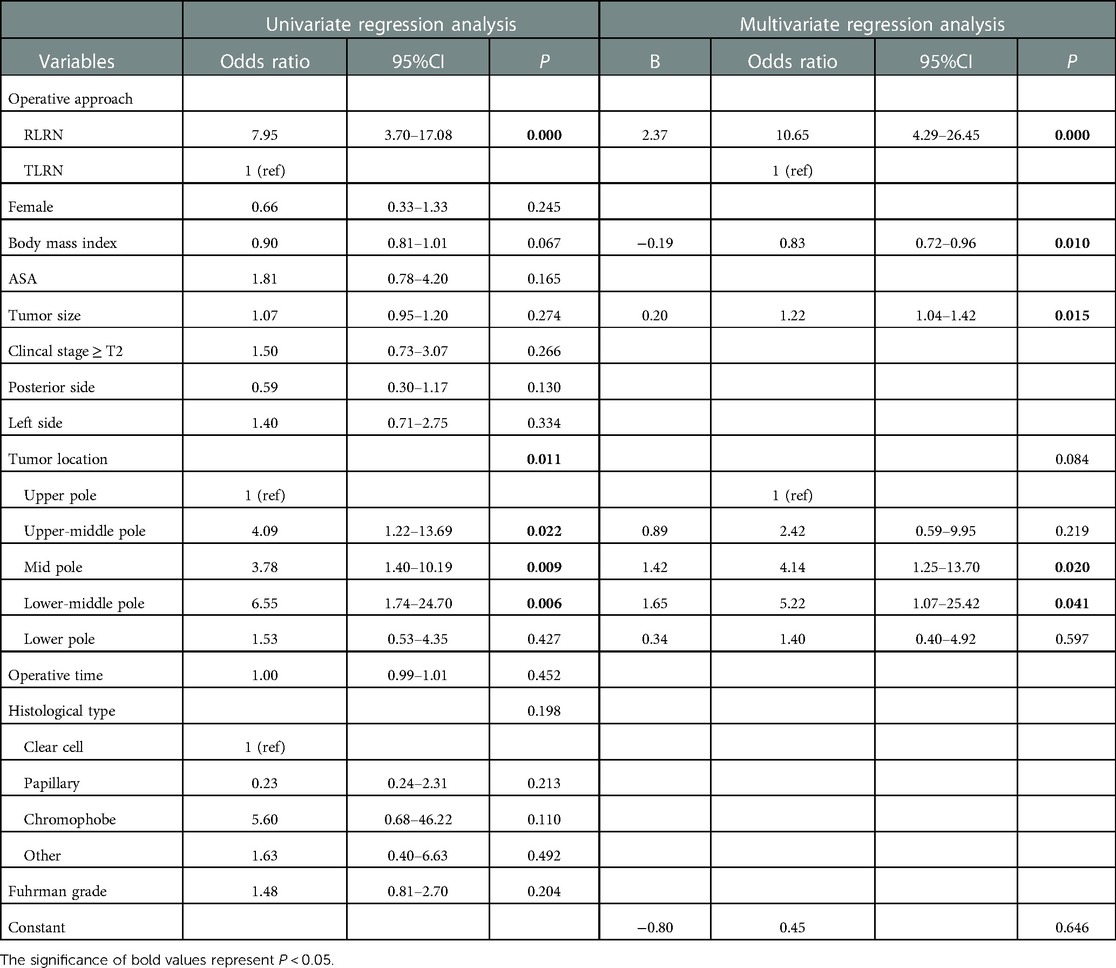
Table 3. Variables associated with poor integrity score (integrity score ≤ 3) in univariate and multivariate analysis.
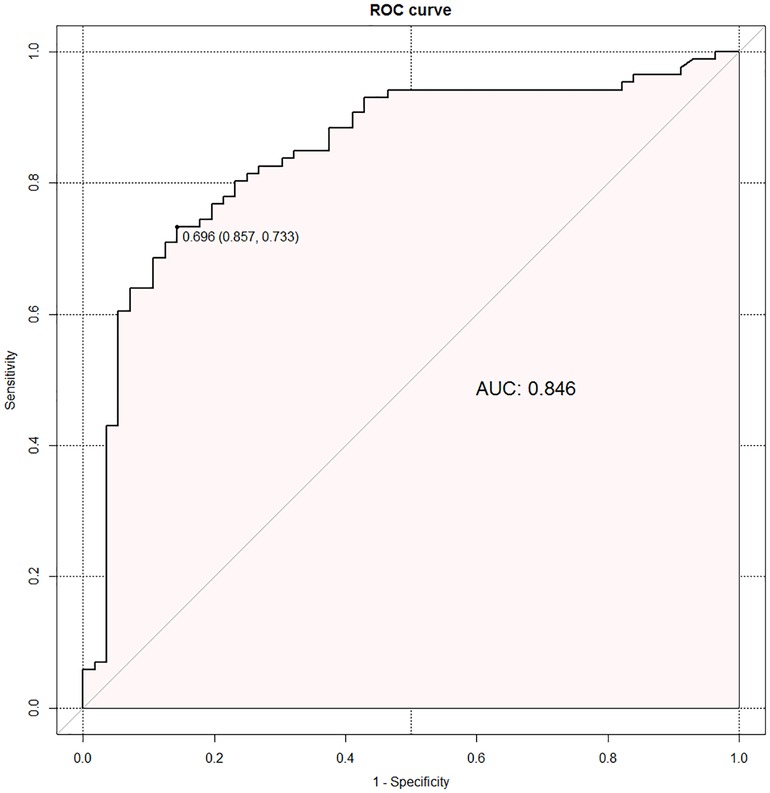
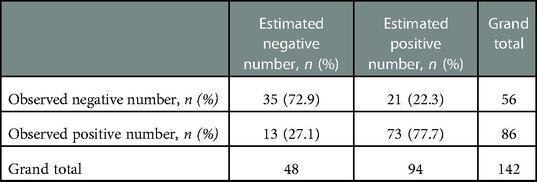
The regression equation was as follows: Ln (P/1—P) = β0 + β1X1 +β2X2 + β3X3 + β4X4–1 + β5X4–2 + β6X4–3 + β7X4–4 = −0.799 + 2.366X1—0.189X2 + 0.196X3 + 0.885X4–1 + 1.421X4–2 + 1.653X4–3 + 0.338X4–4. X1 is RLRN, X2 is body mass index, X3 is tumor size, X4–1 is upper-middle-pole tumor, X4–2 is mid-pole tumor, X4–3 is lower-middle-pole tumor, and X4–4 is lower-pole tumor. Hosmer–Lemeshow goodness-of-fit test of regression equation showed that the model was reasonable (χ2 = 13.353, P = 0.100). The optimal cutoff value of the logistic regression equation in this study was the predicted value = 0.696, which was determined by calculating the point on the ROC curve with the maximum Youden index. P ≥ 0.696 was considered as estimated positive, and P < 0.696 was considered as estimated negative, and a cross classification table was made (Table 4). The results showed that the sensitivity, specificity, positive predictive value, negative predictive value, false-positive rate, and false-negative rate of the regression equation were 73.3%, 85.7%, 77.7%, 72.9%, 37.5%, and 15.1%, respectively. The AUC of ROC curve was 0.846 (95%CI: 0.778–0.914) (Figure 3), which indicates that the equation has a good power to predict low integrity score.
Discussion
Traditional radical nephrectomy involves removing the entire kidney, including the surrounding Gerota's fascia, the perirenal fat and the ipsilateral adrenal gland. Modern radical nephrectomy definitions allow adrenal-sparing if there is no clinical evidence of adrenal gland invasion or metastases (6). The evolution of the extent of resection for radical nephrectomy reflects the evolution in surgical philosophy over time. However, the principle of removing Gerota's fascia incorporating the perirenal fat remains unchanged.
TLRN and RLRN are the most commonly used approaches, and each has its own advantages and limitations. Retrospective studies with large sample sizes compared both methods in detail and demonstrated similar perioperative and oncologic outcomes between the two methods (7, 8). Current guidelines also do not specify the application scenarios for either approach (9, 10). In China, RLRN is more prevalent. Kim et al. previously showed that RLRN achieved better perioperative outcomes than TLRN on the premise that the mean tumor volume was larger, suggesting that RLRN was feasible in patients with >7 cm tumor (8).
Although this could be achievable, it should be pointed out that Kim et al. only contrasted the perioperative results (rather than survival data) between the two groups. In clinical practice, we found that the disadvantage of RLRN in working space would be amplified, especially in cases with large tumor volumes. In addition, tumors with high clinical stage, especially with T3 upstaging, are often characterized by wide intertissue adhesion and increased vascular branches. Thus, achieving the ideal extent of resection is a challenge in such cases. In order to maintain the integrity of the peritoneum and reduce bleeding, surgeon often incises Gerota's fascia when performing RLRN and mobilizes the kidney in the avascular space between the perirenal fat and the anterior renal fascia. Thus, resulting in difficulty in removing part of Gerota's fascia, especially the anterior renal fascia. Deger et al. found that removal of the entire tumor including the perirenal tissue covered by Gerota's fascia can be difficult by retroperitoneoscopic approach, especially in T2 and T3a tumors (11). Moreover, in the study by Taue et al., most patients underwent RLRN, and the kidney was dissected and removed within Gerota's fascia (12). This does not meet the resection principles of radical nephrectomy and is likely to affect tumor control, especially for the prognosis of stage T3a renal cancer with tumor invasion of Gerota's fascia. It has been pointed out that the performance of a perifascial nephrectomy is undoubtedly important for preventing local recurrence after surgery because approximately 25% of clinical T1b/T2 RCCs manifest perirenal fat involvement (13, 14). If intrafascial resection of RLRN is prevalent in institutions, this may increase the risk of local residual, thereby converting a proportion of patients to advanced renal cell carcinoma.
These observations prompted us to quantify the extent of resection for both approaches. We found that the specimen integrity in RLRN was significantly inferior to TLRN. To our knowledge, this is the first time in the literature that we have proposed the association between surgical approaches and specimen integrity. We set six scores based on the integrity of Gerota's fascia and the perirenal fat, which can basically cover common conditions. Theoretically, more score categories can be set for the tumor pole and the other pole according to whether Gerota's fascia and the perirenal fat capsule are incised and according to whether the renal parenchyma and tumor surface are visible, but this may not be conducive to promotion. Another notable aspect from the results in Table 2 is that 10 patients experienced pathologic upstaging. Further analysis found minor between-group differences in tumor invasion depth. Although there were more cases in the RLRN group, tumor extension into perirenal fat or Gerota's fascia was found in only one case. Therefore, we cannot help speculating that specimen integrity might have a potential impact on pathological detection of the actual extent of tumor invasion. Regrettably, we cannot confirm this speculation by the current data, as pathology reports in our center were not standardized.
The standardized nephrometry system, R.E.N.A.L Nephrometry Score, was the first to be proposed. Then followed by the PADUA classification system. R.E.N.A.L. Nephrometry Score is a comprehensive standardized system for quantifying the size, location, and depth of renal tumors (15). PADUA classification is a semi-quantitative anatomical system that can be used to help the surgeon evaluate the risk of perioperative complications (16). However, these nephrometry systems focus on preoperative tumor assessment. In contrast to them, our integrity score focuses on quantifying differences in specimen integrity between approaches and is devised for decision making regarding for the approach for renal tumors. Intact specimens can predict a complete removal of tumor-associated tissue, which reduces contact between the tumor surface and adjacent tissue (protective effect of Gerota's fascia and perirenal fat capsule) and avoids tumor cell dissemination due to excessive extrusion. A study by Chen et al. analyzed the relationship between the R.E.N.A.L score and the Fuhrman grade and concluded that R.E.N.A.L score has a predictive effect on Fuhrman grade and thus further predicts patient prognosis (17). Given that both nephrometry systems have been externally validated and are associated with perioperative outcomes, the integrity score still needs to be modified according to the actual situation to better predict prognosis.
In this study, we also found that RLRN and tumor size were independent risk factors to predicting poor specimen integrity, while BMI was an independent protective factor. As we have previously stated, the disadvantage of RLRN in working space as well as the difficulty in removing the anterior renal fascia do result in poor specimen integrity. As for BMI, we speculate that increased fat thickness decreases tissue adhesion and thus makes extrafascial resection easier.
Our study has several limitations. First, this study was from a single institution and results may therefore not be generalizable to other institutions. Second, our study population of 142 patients is a relatively small sample size and these results need to be validated in a large cohort of patients. Third, our scoring tool cannot cover all situations. Finally, we did not provide survival data. This was because this series included some cases that were not of renal cell cancers. Nonetheless, we believe our study contributes important information regarding specimen integrity of different approaches, indicating that integrity score may be a useful tool for decision making regarding for the approach for renal tumors.
Conclusion
The integrity score represents a novel tool to measure anatomical differences between TLRN and RLRN. Based on the results, we believe that it is feasible to evaluate the extent and completeness of surgical resection using the integrity score. To achieve better integrity of Gerota's fascia and the perirenal fat, we recommend TLRN for large tumors. Further studies are needed to evaluate the predictive value of the integrity score in patient prognosis, thus helping urologists to make individualized follow-up plans.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Lanzhou University Second Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization and design: PFS and JYL; data curation: JYL, PQ, and XZ; formal analysis and methodology: JYL and DZ; writing—original draft: JYL; writing—review and editing: PFS, JYL, BZ, YH, and HBW; administrative, technical and material support: ZJY and ZPW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Key research and Development (R&D) Project of Gansu Province (Grant No. 20YF8FA081), Special fund project for doctoral training program of Lanzhou University Second Hospital (Grant No. YJS-BD-14), and Lanzhou Municipal Science and Technology Project (Grant No. 2020-ZD-81).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005
2. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. (2015) 67(3):519–30. doi: 10.1016/j.eururo.2014.10.002
3. Turner RM 2nd, Morgan TM, Jacobs BL. Epidemiology of the small renal mass and the treatment disconnect phenomenon. Urol Clin North Am. (2017) 44(2):147–54. doi: 10.1016/j.ucl.2016.12.001
4. Gill IS, Schweizer D, Hobart MG, Sung GT, Klein EA, Novick AC. Retroperitoneal laparoscopic radical nephrectomy: the Cleveland clinic experience. J Urol. (2000) 163(6):1665–70. doi: 10.1016/S0022-5347(05)67516-7
5. Wille AH, Roigas J, Deger S, Tüllmann M, Türk I, Loeninget SA. Laparoscopic radical nephrectomy: techniques, results and oncological outcome in 125 consecutive cases. Eur Urol. (2004) 45(4):483–8. doi: 10.1016/j.eururo.2003.10.019
6. Ashrafi AN, Gill IS. Minimally invasive radical nephrectomy: a contemporary review. Transl Androl Urol. (2020) 9(6):3112–22. doi: 10.21037/tau-2019-suc-16
7. Ha US, Hwang TK, Kim YJ, Oh TH, Jeon YS, Lee W, et al. Comparison of oncological outcomes of transperitoneal and retroperitoneal laparoscopic radical nephrectomy for the management of clear-cell renal cell carcinoma: a multi-institutional study. BJU Int. (2011) 107(9):1467–72. doi: 10.1111/j.1464-410X.2010.09636.x
8. Kim HY, Lee DS, Yoo JM, Lee JH, Lee SJ. Retroperitoneal laparoscopic radical nephrectomy for large (>7 cm) solid renal tumors: comparison of perioperative outcomes with the transperitoneal approach. J Laparoendosc Adv Surg Tech A. (2017) 27(4):393–7. doi: 10.1089/lap.2016.0689
9. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
10. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. (2017) 198(3):520–9. doi: 10.1016/j.juro.2017.04.100
11. Deger S, Wille A, Roigas J, Lein M, Giessing M, Johannsen M, et al. Laparoscopic and retroperitoneoscopic radical nephrectomy: techniques and outcome. Eur Urol Suppl. (2007) 6(10):630–4. doi: 10.1016/j.eursup.2007.03.007
12. Taue R, Izaki H, Koizumi T, Kishimoto T, Oka N, Fukumori T, et al. Transperitoneal versus retroperitoneal laparoscopic radical nephrectomy: a comparative study. Int J Urol. (2009) 16(3):263–7. doi: 10.1111/j.1442-2042.2008.02219.x
13. Thompson RH, Blute ML, Krambeck AE, Lohse CM, Magera JS, Leibovich BC, et al. Patients with pT1 renal cell carcinoma who die from disease after nephrectomy may have unrecognized renal sinus fat invasion. Am J Surg Pathol. (2007) 31(7):1089–93. doi: 10.1097/PAS.0b013e31802fb4af
14. Lam JS, Klatte T, Patard JJ, Goel RH, Guillè F, Lobel B, et al. Prognostic relevance of tumour size in T3a renal cell carcinoma: a multicentre experience. Eur Urol. (2007) 52(1):155–62. doi: 10.1016/j.eururo.2007.01.106
15. Kutikov A, Uzzo RG. The R.E.N.A.L. Nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. (2009) 182(3):844–53. doi: 10.1016/j.juro.2009.05.035
16. Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De-Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. (2009) 56(5):786–93. doi: 10.1016/j.eururo.2009.07.040
Keywords: renal cell carcinoma, retroperitoneal approach, transperitoneal approach, radical nephrectomy, risk factors
Citation: Liu J, Zheng D, Qi P, Zheng X, Zhang B, He Y, Wang H, Yue Z, Wang Z and Shang P (2023) Retroperitoneal laparoscopic radical nephrectomy (RLRN) is associated with poor integrity of Gerota's fascia and perirenal fat: A prospective comparative study. Front. Surg. 10:1114065. doi: 10.3389/fsurg.2023.1114065
Received: 2 December 2022; Accepted: 31 January 2023;
Published: 16 February 2023.
Edited by:
Yongbao Wei, Fujian Provincial Hospital, ChinaReviewed by:
Luyao Chen, The First Affiliated Hospital of Nanchang University, ChinaKaijie Wu, The First Affiliated Hospital of Xi'an Jiaotong University, China
© 2023 Liu, Zheng, Qi, Zheng, Zhang, He, Wang, Yue, Wang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panfeng Shang shangpf@lzu.edu.cn
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Junyao Liu
Junyao Liu Duo Zheng†
Duo Zheng†  Panfeng Shang
Panfeng Shang