Bat Flies and Their Microparasites: Current Knowledge and Distribution
- 1Department of Ecology and Evolution, University of Lausanne, Lausanne, Switzerland
- 2Museum of Zoology, Lausanne, Switzerland
Bats are the second most diverse mammalian group, playing keystone roles in ecosystems but also act as reservoir hosts for numerous pathogens. Due to their colonial habits which implies close contacts between individuals, bats are often parasitized by multiple species of micro- and macroparasites. The particular ecology, behavior, and environment of bat species may shape patterns of intra- and interspecific pathogen transmission, as well as the presence of specific vectorial organisms. This review synthetizes information on a multi-level parasitic system: bats, bat flies and their microparasites. Bat flies (Diptera: Nycteribiidae and Streblidae) are obligate, hematophagous ectoparasites of bats consisting of ~500 described species. Diverse parasitic organisms have been detected in bat flies including bacteria, blood parasites, fungi, and viruses, which suggest their vectorial potential. We discuss the ecological epidemiology of microparasites, their potential physiological effects on both bats and bat flies, and potential research perspectives in the domain of bat pathogens. For simplicity, we use the term microparasite throughout this review, yet it remains unclear whether some bacteria are parasites or symbionts of their bat fly hosts.
Introduction
Bats are the second most diverse mammalian group after rodents, with ~1,390 recognized species across 227 genera (1). Many bat species play keystone roles in ecosystems, where they are essential to pollination, seed dispersal, and pest control (2). Several studies have also highlighted their prominent role as pathogen-reservoirs (3, 4); viruses being the best studied due to their potential as human pathogens (3, 5–8). Bats host more viruses per species than rodents, making them an interesting system for both disease ecology and public health research (4, 9).
Bacteria (such as Bartonella spp. and Borrelia spp.) and protozoans (such as Trypanosoma spp. and Plasmodium spp.) have also been detected in bats (8, 10, 11). In recent years, bat-associated Bartonella genotypes have been found in humans, indicating the public health importance of this parasite in bats (12–14). Bartonella and other pathogen transmission from bats to humans may occur through religious activities in caves, bat consumption or contact with contaminated products (12, 15). There are documented cases of bat-specific ectoparasites biting humans (16, 17), increasing the potential of bat-born pathogen transmission. Additionally, bat-associated pathogen, such as Trypanosoma cruzi genotype has also been found in humans (18).
Bats host numerous ectoparasitic groups, such as bat flies (Diptera: Nycteribiidae and Streblidae), bugs (Hemiptera: Cimicidae and Polyctenidae), fleas (Siphonaptera: Ischnopsyllidae), and several bat specialized arachnids, such as mites (Mesostigmata: Spinturnicidae and Macronyssidae) and ticks (e.g., Argas spp., Carios spp., Ixodes spp., and Ornithodoros spp.) (19–25).
Bat flies (Nycteribiidae and Streblidae) are the most common bat ectoparasites (Figure 1). Both families, along with Hippoboscidae (louse and ked flies) and Glossinidae (tsetse flies) belong to the Hippoboscoidea superfamily. Currently 275 species across 21 genera of nycteribiids and 227 species across 31 genera of streblids are recognized. Nycteribiids have a higher diversity in the Eastern Hemisphere, while streblids are mainly found in the Western Hemisphere (17).
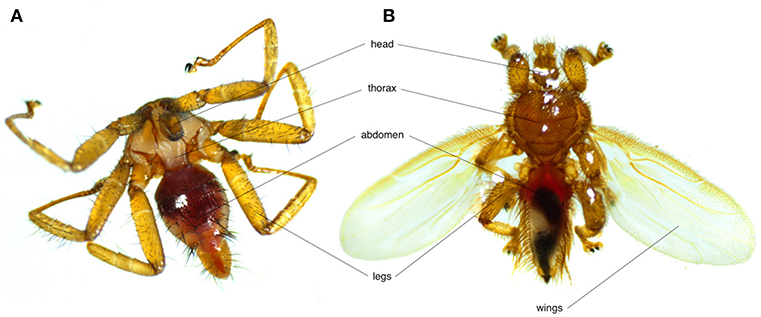
Figure 1. Photos showing the morphological differences between (A) a wingless nycteribiid and (B) a streblid bat fly.
Members of Hippoboscoidea have developed a unique reproductive strategy. A single larva develops within a female, feeding on the secretion of the so-called milk glands. Larviposition occurs at the third instar stage and the larva immediately pupates. The four families have thus been previously referred as “Pupipara” (an obsolete clade). This unique reproductive strategy necessitates milk gland secretion transfer for larval development (26–28), which may shape the community of certain bacteria such as Arsenophonus, Bartonella, or Wolbachia by vertical transmission (26, 27, 29, 30). Horizontal transmission may occur through parasitoids or individuals contacting contaminated saliva, as in plant consuming insect communities (31, 32).
Bat flies deposit their larva on substrates such as the host roost wall. After larviposition, females return to their host. When the offspring emerge, they actively search for bat hosts. Emergence time depends on several factors including temperature and host presence (33, 34). Regarding their reproductive strategy, bat flies also show strong morphological adaptations to their parasitic life style. Some species are eyeless or have reduced facets (35). Nycteribiids are wingless, while most streblid species have partly or fully developed wings.
Early studies assumed that bat flies show no strong host specificity (36, 37); nevertheless more comprehensive recent works showed that the majority of bat fly species exhibit high specificity to a single or closely related bat species when collection is controlled and contamination avoided (25, 38–41).
Bats' ectoparasites may have vectorial potential. For example, Polychromophilus spp. are transmitted by nycteribiids (42) and Trypanosoma spp. by cimicids (43). Although, the transmission route of Bartonella has not been experimentally tested, this bacteria has been detected in a wide range of bat ectoparasites, such as bat flies (44–46), tick, and mites (47–51). In a recent study, ectoparasite burden was shown to positively correlate with Bartonella infection, suggesting their potential role as vectors (52). Furthermore, Bartonella was detected in bat flies and their host in the Madagascan fruit bat (Eidolon dupreanum), but not in fleas, indicating the potentially crucial role of bat flies in Bartonella transmission (53). Additionally, ectoparasite and virus species richness positively correlate, suggesting a vectorial role of ectoparasites for viruses (54).
In this review we focus on bat flies, the most diverse and prevalent group of bat ectoparasites. Bat flies are common on most species and since they are obligate hematophagous dipterans, they may play an important role in the transmission and maintenance of bat pathogens. The exact nature of the interaction between some bacteria and their bat fly hosts is unknown: Wolbachia and Arsenophonus may act as parasites and/or as mutualists (55, 56) (we consider them as potential microparasites in this review).
Here we review the presence of microparasites in bat flies and their geographical distribution. We consider the following organisms as microparasites: blood parasites, represented by Polychromophilus spp. and the extinct genus Vetufebrus sp. (Haemosporidia: Plasmodiidae); bacteria, such as Arsenophonus and Providencia (Enterobacteriales: Enterobacteriaceae), Bartonella (Rhizobiales: Bartonellaceae), Wolbachia and Rickettsia (Rickettsiales: Anaplasmataceae and Rickettsiaceae); viruses, such as Kanyawara virus (Mononegavirales: Rhabdoviridae), Mahlapitsi virus (Reoviridae), Wolkberg virus and Kaeng Khoi virus (Bunyavirales: Bunyaviridae and Peribunyaviridae), dengue virus (Flaviviridae); hyperparasites, such as fungi (Ascomycota: Laboulbeniaceae) and finally parasitoids (Hymenoptera: Eupelmidae). We test whether bat host phylogenetic origin effects the presence of different microparasitic groups of bat flies. We discuss the potential physiological effects of microparasites on both bats and bat flies, and future research perspectives related to bat-associated ectoparasites and microparasites.
Materials and Methods
We present microparasite data collected from various literature source (Supplementary Data Sheet 1). We searched Google Scholar and ISI Web of Science, using all combinations of the following terms in English and French: Chiroptera or bat*; ectoparasite, bat fly, Nycteribiidae, Streblidae or Hippoboscidae*; and pathogen, parasitoid, parasite, microparasite, fungi, protozoa, haemosporidians, bacteria or virus.
Each bat fly—microparasite association (genus or species, depending on the taxonomic level provided by the authors) is an entry of the dataset, and is characterized by its geographical origin and bat host species.
We use currently valid taxonomical names for both bats and bat flies in our database (57–59). Statistics are conducted using R 3.5.1 (60). Bat fly-microparasite networks were visualized using the R package bipartite (61). Map of reported bat fly-microparasite associations were made in QGIS 2.16 (62).
Results
Effect of Bat Host Family on Detected Microparasite Distribution in Bat Flies
Bat flies infected with microparasites were observed on 75 bat species comprising 33 bat genera, with most in Vespertilionidae (16/505 known species), Phyllostomidae (21/216), Pteropodidae (13/196), Miniopteridae (10/38, the highest observed ratio), and Rhinolophidae (8/103). Bat flies with microparasite observations were also found in only a few species of Emballonuridae, Hipposideridae, Noctilionidae, and Mormoopidae.
Microparasite distribution in bat flies is dominated by bacterial and fungal parasites (Figure 2). Viruses detected in bat flies are only known from the family Phyllostomidae (n = 2) and Pteropodidae (n = 4). Blood parasites were mostly in flies from Miniopteridae (n = 7), but were also found in Pteropodidae (n = 1) and Vespertilionidae (n = 2) (Figure 2).
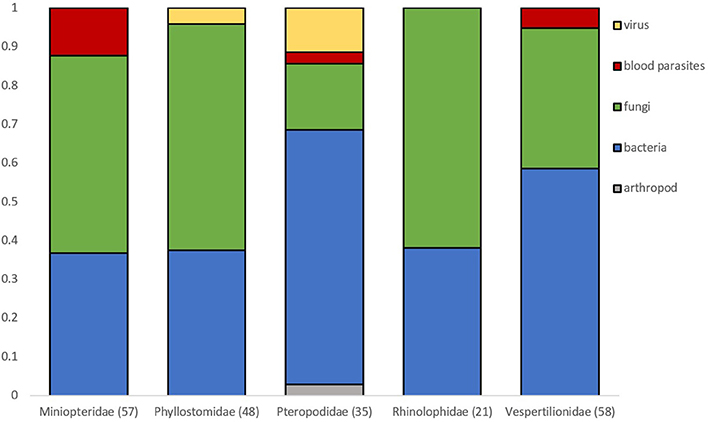
Figure 2. Proportion of microparasite groups observed in bat flies collected from different bat host families. Numbers in brackets are sample sizes. Families with <20 observations are not represented.
Diversity Within Nycteribiidae and Streblidae
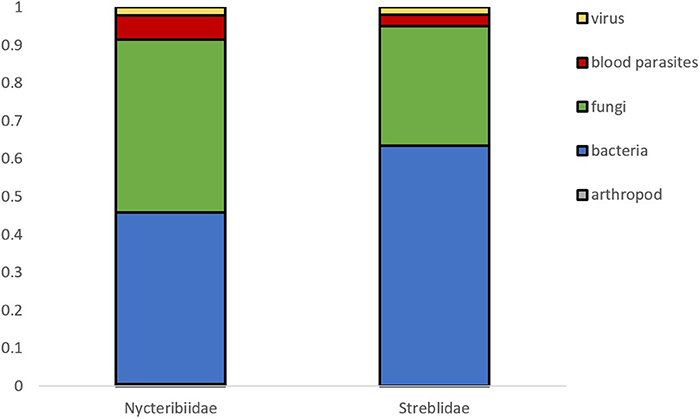
A total of 188 and 101 microparasite observations are reported in bat fly families Nycteribiidae and Streblidae respectively, belonging to 27 bat fly genera (Figure 3). The most frequently reported infected bat fly genera are Penicillidia (n = 67), Nycteribia (n = 51), Trichobius (n = 44), Eucampsipoda (n = 20), and Basilia (n = 15); all of them Nycteribiidae, with the exception of the streblid genus Trichobius. Both host fly families displayed a similar distribution of microparasite taxa (Figure 4).
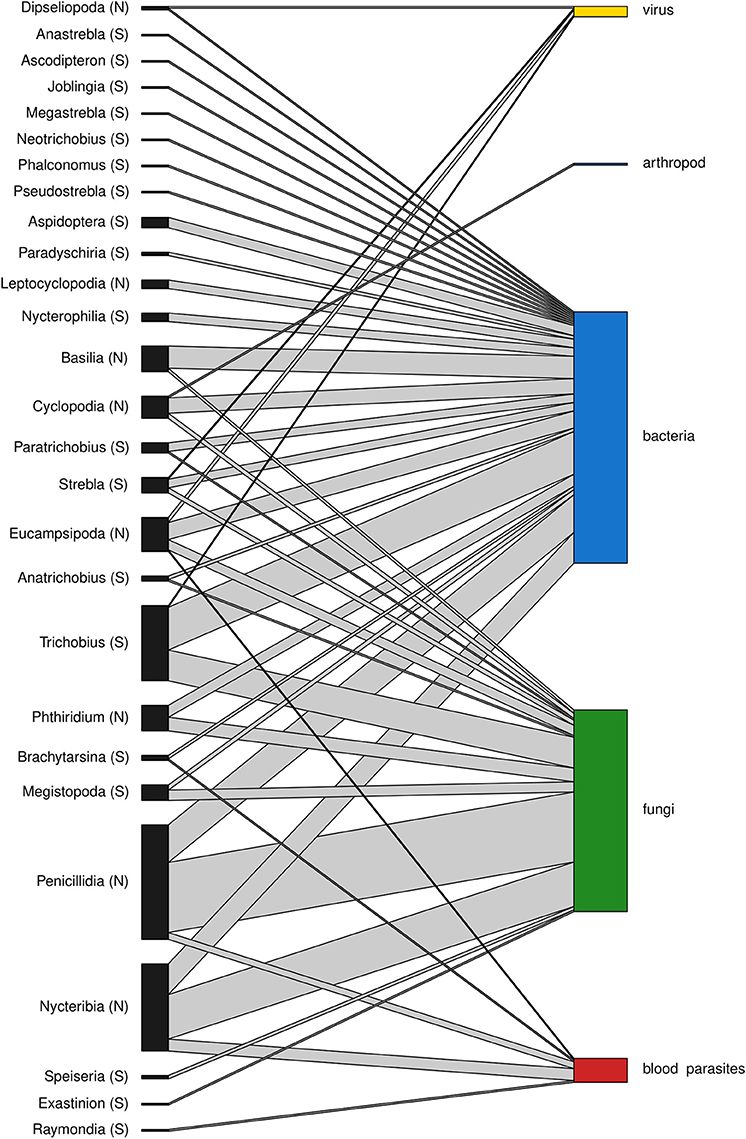
Figure 3. Association between bat fly genera of Nycteribiidae (N) and Streblidae (S) families and microparasitic groups. The height of the bars represents the relative abundance of the groups within each network level.
The most commonly reported microparasites in bat flies are bacteria (n = 149), followed by fungi (n = 118), blood parasites (n = 15), viruses (n = 6), and arthropods (n = 1) (Table 1). Within bacteria, the three most frequently detected microparasites are Bartonella sp. (Alphaproteobacteria: Bartonellaceae) (n = 91, 61%), Arsenophonus sp. (Gammaproteobacteria: Enterobacteriaceae) (n = 30, 20.1%) and Wolbachia sp. (Alphaproteobacteria: Anaplasmataceae) (n = 8, 5.4%). All observed fungi are Laboulbeniaceae (Ascomycota: Laboulbeniales) and belong to three genera, Arthrorhynchus (n = 80, 67.8%), Gloeandromyces (n = 16, 13.6%), and Nycteromyces (n = 5, 4.2%), as well as 17 (14.4%) unidentified or undescribed observations. Polychromophilus species (Haemosporida: Plasmodiidae) represent 93.3% (n = 14) of blood parasite observations in bat flies. Virus and parasitoid arthropod represent a much smaller proportion of all microparasitic observations in bat flies, with only six and one published record, respectively.
Global Geographical Distribution of Bat Fly—Microparasite Associations
Bat fly -microparasite associations originated from 61 countries (Figure 5) with a total of 269 reports (excluding those with unspecified or unknown geographical locations). Associations reported from countries were most commonly from Europe (n = 89, 33%), North America (n = 69, 25.7%), and Africa (n = 61, 22.7%). Observations in Asia (n = 33, 12.3%), South America (n = 21, 7.8%), and Oceania (n = 5, 1.9%) were represented less frequently. The highest number of microparasite—bat fly species associations are reported from Madagascar (n = 33).
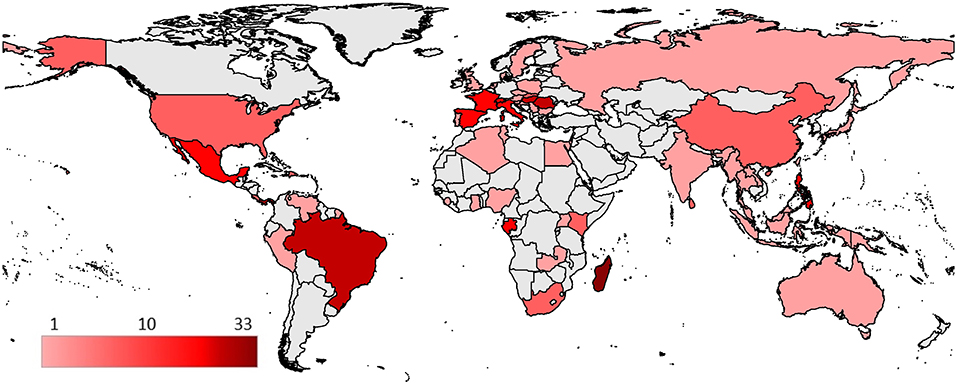
Figure 5. Geographical distribution of reported bat fly—microparasite species associations. Countries are colored according to the number of different described species associations.
Sampling Effort on Microparasite Diversity in Bat Flies
We tested the number of published studies by bat fly genera and number of microparasite associations reported (including same species associations but different bat hosts and countries). Spearman rank correlation showed that sampling effort strongly predicts the number of detected microparasites in different bat fly genera (n = 27, r = 0.68, p = 0.0001; Figure 6).
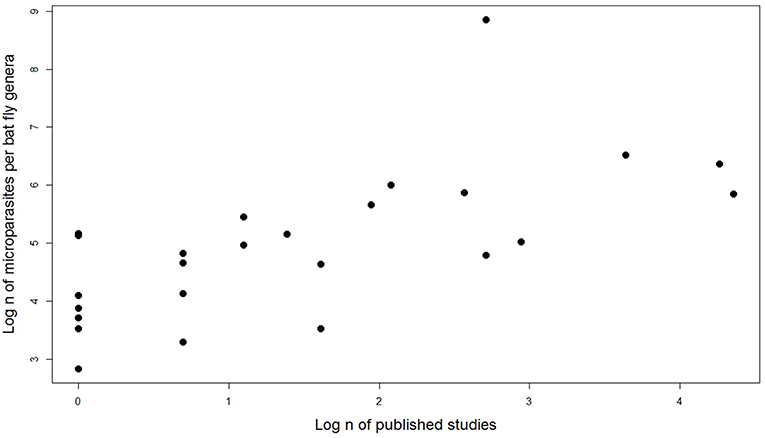
Figure 6. Effect of sampling effort on the number of microparasite associations in different bat fly genera.
Discussion
Microparasite Diversity in Bat Flies
Based on literature data, we have identified five main groups of microparasites in parasitic bat flies. Bacteria are the most frequently observed group in both Nycteribiidae and Streblidae and within bacteria, Bartonella is the most prevalent microorganism. Some species of Bartonella are blood-borne parasites, transmitted by blood-sucking arthropods (104) found in a wide range of mammalian groups and several arthropod ectoparasites (14). For example, Bartonella quintana, a louse-borne parasite, was responsible for trench fever, which affected over one million soldiers during World War 1 (105). The presence of identical Bartonella genotypes in bats and bat flies suggests that bat flies may serve as vectors (44, 53, 80, 81). Host specific bat flies show higher prevalence but lower diversity of Bartonella infection than polyxenous species (46). However, the generally high prevalence and diversity of Bartonella suggests their long co-evolutionary history with bats.
The second most frequently observed microparasites in bat flies are fungi. All species recognized here belong to the order Laboulbeniales. Three genera of Laboulbeniales are known to parasite bat flies, Arthrorhynchus spp. (the most frequently reported genus), Gloeandromyces spp., and Nycteromyces spp. The distribution, specificity and diversity of these microparasites have recently been uncovered. Locally (e.g., in Europe) these species show some degree of high specificity (with occasional “accidental” transfers) (64, 69), although at a larger geographical scale, they do not show strict specificity to host species or genera (65).
While blood parasites are frequently found in bats (77, 106–108), observations in bat flies are much less common. Polychromophilus species are vectored by nycteribiids (102), and one haemosporidian report is known from a single fossil streblid specimen but observations from extant streblids are still missing (109). Other blood parasites, such as Trypanosoma is transmitted to bats by hemipterans including Cimex species (42). Trypanosoma cruzi cruzi, the causative agent of Chagas disease in humans and other mammal species, is transmitted by triatomine bugs (110). Bat flies have not yet been reported as vectors of Trypanosoma species. Nevertheless, Glossina tsetse flies (members of the Hippoboscoidea superfamily along with bat flies) are known to transmit T. brucei. Therefore, it remains possible that bat flies transmit other blood parasites besides Polychromophilus (e.g., trypanosomatids). Additionally, nycteribiids may serve as vectors in the transmission of other protozoans, such as Nycteria spp. (Haemosporida: Plasmodiidae), infecting Afrotropical insectivorous bats; but their vectorial potential has not yet been clarified (107). More work is needed to address these questions.
Most of the reports on viruses in bat flies are relatively recent (87, 92–96). As such, it is possible that the number of isolated viruses in bat ectoparasites might thus rise in the future with improvement in diagnostic methods.
There is only one report of a parasitoid wasp using nycteribiids as host (88). Parasitoid wasps are extremely diverse groups with about 100,000 described species. However, host species information is missing for many species. We expected that other parasitoids use bat flies as hosts during their development, but data collection is challenging due to the ecology of these flies. Furthermore, it has been observed that mite species can have phoretic relationships with bat flies (111–113), but their effect on bat flies is not clear. Nonetheless, some phoretic mites which were previously assumed to have no effect on their invertebrate hosts, have now been shown to negatively affect their fecundity and/or survival rate (114, 115).
Studies have previously suggested that microfilaria might be transmitted by hippoboscid louse flies to their vertebrate hosts, such as dogs (116). Filarial nematode DNA has also been observed in streblid bat flies and bat mites (117). It is not clear if these microfilaria are transmitted by bat flies or if the detected microfilaria DNA was only present in the last blood meal (117).
Microparasite diversity is similar between nycteribiids and streblids flies, although nycteribiids have 2.5 times more reported cases of microparasites. The reason behind this is more likely due to biased sampling efforts in different geographical regions. For example, in Europe where most of the studies were performed, 16 species of nycteribiids are present, whereas only one streblid species have been recorded.
Geographical Distribution
All major groups of microparasites have been reported widely, though our knowledge of the diversity and distribution of many groups remains scarce. Bacteria such as Bartonella show a high molecular and geographic diversity in bats and bat flies, at global and regional scales (44, 46, 118). Six major bat associated Bartonella clades have been reported so far from bats and bat flies (118). Clade I, II, IV, and V are represented in both Old and New World areas while clade III seems to be restricted to the Old World (Africa, Asia, and Europe) and clade VI to some parts of the New World (Central America) (118).
Fungal microparasites (Laboulbeniales) show a rather divided Eastern (Arthrorhynchus spp.) and Western Hemisphere (Nycteromyces spp. Gloeandromyces spp.) distribution and diversity (65). Similar patterns have been demonstrated regarding nycteribiids (Eastern) and streblids (Western) (17). These diversity and distribution patterns suggest a long evolutionary history between bat flies and these fungal microparasites.
It is important to highlight that these distribution patterns might be strongly influenced by biased sampling efforts rather than actual geographical patterns. Therefore, the distribution map helps to recognize well studied areas on a global scale, however it does not necessarily reflects actual distributional patterns of these microparasites detected in bat flies. It is our hope that it will be useful for further studies.
Effects of Bat Host Ecology on Microparasites
Previous work showed that viral richness in bats correlates with IUCN threat status, with near-threatened and vulnerable hosts having higher viral richness. In addition, population genetic structure positively correlates with viral richness (119). Host longevity, reproductive strategy and distribution pattern may also play an important role in viral richness (9, 54, 120).
In general, the bat host family does not affect the distribution of microparasites in their bat flies. The bent-winged bats, family Miniopteridae, have the highest observed ratio of bat species infected by bat flies parasitized by microparasites. Miniopteridae are insectivorous, cave-dwelling species occurring in dense and multi-species colonies. From a disease ecology and parasitology point of view, it is a unique family hosting many highly specific ecto- and endoparasites such as mites, bat flies and malarial parasites (21, 121, 122). It is still unclear whether the ecology and/or the immune system of Miniopteridae species is responsible for such a high parasite diversity compared to other bat families. Moreover, Miniopteridae is considered as underrepresented in viral research so more parasites and pathogens likely remain undiscovered in these species (123).
Bacteria and fungi are the most abundant group of microparasites in all bat flies from different host families. The occurrence of Bartonella infection in bats is associated with host diet; hematophagous and carnivorous species are more frequently infected than species with other diets (124). Hematophagous and carnivorous bat species also show higher white-blood cell count, suggesting a higher risk of pathogen exposure, probably due to the fact that these bat species are more exposed to vertebrate specific pathogens (125). Therefore, we might expect a higher microparasite occurrence in bat flies collected from bat species that feed on vertebrates or blood. Nevertheless, there are only a few studies that have focused on microparasites in parasitic bat flies collected from these host species (44, 80, 87).
Viruses are only known from bat flies infecting the New World leaf-nosed bats Phyllostomidae and the Old World fruit bats Pteropodidae, but observations are still scarce. These observed viruses represent distant groups, such as Dengue virus (family Flaviviridae) isolated from the bat flies of the common vampire bat, Desmodus rotundus (87); Kaeng Khoi virus (Peribunyaviridae), Kanyawara virus (Rhabdoviridae), Mahlapitsi virus (Reoviridae), and Wolkberg virus (Bunyaviridae), isolated from Myonycteris and Rousettus species (92–96).
There are great ecological differences between bat families. Bat host ecology and physiology, such as roosting habits, body size, and sex can affect bat fly burden and species richness (126–129). More studies are again needed to clarify how host traits affect the distribution of microparasite communities of bat flies.
Potential Physiological Effects on Flies and Bats
We still know little about the physiological effects of microparasites on bat flies and on their bat host. Viruses such as Lyssavirus spp. are known to cause mortality in bats (130, 131). The bacterial parasite Borellia sp. (from the relapsing fever group) has been documented causing fatal borreliosis in a single bat individual (Pipistrellus sp.) (132). The haemosporidian parasite Polychromophilus murinus has a well-documented impact on both bat and bat fly life-history traits (103, 106). In the Daubenton's bats (Myotis daubentonii), it has a strong negative effect on the body condition of subadults (106). Additionally, it negatively affects the life span of infected bat flies (103).
The relationship between bat flies and some bacterial species such as Wolbachia and Arsenophonus has not yet been clarified. It is suspected that they are either parasitic and/or symbiotic of bat flies. In some cases, Wolbachia is considered as a nutritional mutualist, due to its ability to produce vitamin B in certain hematophagous arthropod species, such as Cimex spp. (133). Arsenophonus is a highly diverse group of bacteria found mainly in insects, including bat flies (134–138). Arsenophonus species have been suggested to be primary or secondary symbionts in other taxa (134, 138, 139). Here, we categorize Arsenophonus and Wolbachia as microparasitic organisms in bat flies, since it is unclear how they affect their hosts (35). Furthermore, Wolbachia DNA has been also detected in mammalian blood due to the presence of infected nematodes in host blood (140). It has been observed once in an avian blood system, with the strain being more closely related to the arthropod-associated Wolbachia group (141), and likely having no direct effect on their vertebrate hosts.
The presence of the fungal parasite Laboulbeniales has an effect on bat fly mortality in some species (Szentiványi et al., Unpublished), as an arthropod specialized microparasite. Nevertheless, it is unclear if it has any direct or indirect effect on the bat host.
Additionally, and as mentioned above, the potential effect of phoretic mite infestation on bat flies has never been tested. Therefore, it remains possible that these mites have direct or indirect negative effects on host behavior, survival rate, and/or fecundity.
Perspectives for Additional Research, Sampling Effort
Our knowledge of the microparasites of bat flies is strongly biased by sampling effort, which may also strongly reflect the currently known geographical distribution patterns of these parasites. We suggest to balance these biases by increasing sampling effort in less prospected countries as well as areas where human exposure to pathogen transmission is more likely to occur, due to cultural or touristic reasons (e.g., visiting caves) (15, 142). Additionally, we have little knowledge on the microparasites of other bat ectoparasitic groups, such as fleas, bugs, and mites. Future studies should focus on how microparasite and pathogen communities interact on the intra- and interspecific levels. For example, Wolbachia infection is known to inhibit malarial infection in mosquitos (143). Additionally, it is important to understand how bat host traits such as sex, geographical distribution and/or host group size [which are known to shape the distribution of bat fly populations (17, 128, 129)] may affect the occurrence of microparasitic communities in these ectoparasites. Lastly, experimental studies are needed to understand the relationship between bat hosts and ectoparasites, including the transmission and the distribution of microparasites.
Author Contributions
PC and OG initiated the study. TS performed data collection and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Orsolya Vincze for her suggestions during the preparation of the figures. We are thankful to Tomas Kay and Eric Tremblay for their help with the grammatical revision and for their suggestions. We are also grateful to the reviewers for their helpful and constructive comments, which greatly increased the quality of our work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00115/full#supplementary-material
References
1. Burgin CJ, Colella JP, Kahn PL, Upham NS. How many species of mammals are there? J Mammal. (2018) 99:1–14. doi: 10.1093/jmammal/gyx147
2. Kunz TH, de Torrez EB, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Ann N Y Acad Sci. (2011) 1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x
3. Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. (2006) 19:531–45. doi: 10.1128/CMR.00017-06
4. Dobson AP: What links bats to emerging infectious diseases? Science. (2005) 310:628–9. doi: 10.1126/science.1120872
5. Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. Fruit bats as reservoirs of Ebola virus. Nature. (2005) 438:575–6. doi: 10.1038/438575a
6. Tong S, Li Y, Rivailler P, Conrardy C, Castillo DAA, Chen L-M, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci. (2012) 109:4269–74. doi: 10.1073/pnas.1116200109
7. Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. (2005) 310:676–9. doi: 10.1126/science.1118391
8. Brook CE, Dobson AP. Bats as “special” reservoirs for emerging zoonotic pathogens. Trends Microbiol. (2015) 23:172–80. doi: 10.1016/j.tim.2014.12.004
9. Luis AD, Hayman DTS, O'Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc B Biol Sci. (2013) 280:1756. doi: 10.1098/rspb.2012.2753
10. Veikkolainen V, Vesterinen EJ, Lilley TM, Pulliainen AT. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg Infect Dis. (2014) 20:960–7. doi: 10.3201/eid2006.130956
11. Schaer J, Perkins SL, Decher J, Leendertz FH, Fahr J, Weber N, et al. High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc Natl Acad Sci. (2013) 110:17415–9. doi: 10.1073/pnas.1311016110
12. Bai Y, Osinubi MOV, Osikowicz L, McKee C, Vora NM, Rizzo MR, et al. Human exposure to novel Bartonella species from contact with fruit bats. Emerg Infect Dis. (2018) 24:2317–23. doi: 10.3201/eid2412.181204
13. Urushadze L, Bai Y, Osikowicz L, McKee C, Sidamonidze K, Putkaradze D, et al. Prevalence, diversity, and host associations of Bartonella strains in bats from Georgia (Caucasus). PLoS Negl Trop Dis. (2017) 11:1–19. doi: 10.1371/journal.pntd.0005428
14. Frank HK, Hadly EA. Global fingerprint of humans on the distribution of Bartonella bacteria in mammals. PLoS Negl Trop Dis. (2018) 12:1–17. doi: 10.1101/249276
15. Obame-Nkoghe J, Rahola N, Ayala D, Yangari P, Jiolle D, Allene X, et al. Exploring the diversity of bloodsucking Diptera in caves of Central Africa. Sci Rep. (2017) 7:1–11. doi: 10.1038/s41598-017-00328-z
16. Piksa K, Nowak-Chmura M, Siuda K. First case of human infestation by the tick Ixodes vespertilionis (Acari: Ixodidae). Int J Acarol. (2013) 39:1–2. doi: 10.1080/01647954.2012.737831
17. Dick CW, Patterson BD. Bat flies-obligate ectoparasites of bats. In: Morand S, Krasnov BR, Poulin R, editors. Micromammals Macroparasites. Tokyo: Springer-Verlag (2006). p. 179–94.
18. Ramírez JD, Hernández C, Montilla M, Zambrano P, Flórez AC, Parra E, et al. First report of human Trypanosoma cruzi infection attributed to TcBat genotype. Zoonoses Public Health. (2014) 61:477–9. doi: 10.1111/zph.12094
19. Sonenshine DE, Anastos G. Observations on the life history of the bat tick Ornithodoros kelleyi (Acarina: Argasidae). J Parasitol. (1960) 46:449–54.
20. Marshall AG. Ecology of insects ectoparasitic on bats. In: Kunz TH, editors. Ecology of Bats. New York, NY: Springer US (1982). p. 369–401.
21. Bruyndonckx N, Dubey S, Ruedi M, Christe P. Molecular cophylogenetic relationships between European bats and their ectoparasitic mites (Acari, Spinturnicidae). Mol Phylogenet Evol. (2009) 51:227–37. doi: 10.1016/j.ympev.2009.02.005
22. Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae), a bat tick from the central-western region of Brazil. Acta Parasitol. (2009) 54:355–63. doi: 10.2478/s11686-009-0051-1
23. Balvín O, Bartonička T, Simov N, Paunović M, Vilímová J. Distribution and host relations of species of the genus Cimex on bats in Europe. Folia Zool. (2014) 63:281–9. doi: 10.25225/fozo.v63.i4.a7.2014
24. Hornok S, Kontschán J, Estrada-Peña A, De Mera IGF, Tomanović S, De La Fuente J. Contributions to the morphology and phylogeny of the newly discovered bat tick species, Ixodes ariadnae in comparison with I. vespertilionis and I. simplex. Parasit Vectors. (2015) 8:1–7. doi: 10.1186/s13071-015-0665-0
25. Szentiványi T, Estók P, Földvàri M. Checklist of host associations of European bat flies (Diptera: Nycteribiidae, Streblidae). Zootaxa. (2016) 4205:101. doi: 10.11646/zootaxa.4205.2.1
26. Hosokawa T, Nikoh N, Koga R, Satô M, Tanahashi M, Meng XY, et al. Reductive genome evolution, host-symbiont co-speciation and uterine transmission of endosymbiotic bacteria in bat flies. ISME J. (2012) 6:577–87. doi: 10.1038/ismej.2011.125
27. Morse SF, Dick CW, Patterson BD, Dittmard K. Some like it hot: evolution and ecology of novel endosymbionts in bat flies of cave-roosting bats (Hippoboscoidea, Nycterophiliinae). Appl Environ Microbiol. (2012) 78:8639–49. doi: 10.1128/AEM.02455-12
28. Morse SF, Bush SE, Patterson BD, Dick CW, Gruwell ME, Dittmar K. Evolution, multiple acquisition, and localization of endosymbionts in bat flies (Diptera: Hippoboscoidea: Streblidae and Nycteribiidae). Appl Environ Microbiol. (2013) 79:2952–61. doi: 10.1128/AEM.03814-12
29. De Bruin A, Van Leeuwen AD, Jahfari S, Takken W, Földvári M, Dremmel L, et al. Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi. Parasit Vectors. (2015) 8:4–9. doi: 10.1186/s13071-015-0764-y
30. Duron O, Schneppat UE, Berthomieu A, Goodman SM, Droz B, Paupy C, et al. Origin, acquisition and diversification of heritable bacterial endosymbionts in louse flies and bat flies. Mol Ecol. (2014) 23:2105–17. doi: 10.1111/mec.12704
31. Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biol. (1999) 9:313–6. doi: 10.1016/S0960-9822(99)80139-0
32. Gonella E, Pajoro M, Marzorati M, Crotti E, Mandrioli M, Pontini M, et al. Plant-mediated interspecific horizontal transmission of an intracellular symbiont in insects. Sci Rep. (2015) 5:1–10. doi: 10.1038/srep15811
33. Marshall AG. The life cycle of Basilia hipsida Theodor 1967 (Diptera: Nycteribiidae) in Malaysia. Parasitology. (1970) 61:1–18.
35. Dittmar K, Morse SF, Dick CW, Patterson BD. Bat fly evolution from the Eocene to the present (Hippoboscoidea, Streblidae and Nycteribiidae). In: Morand S, Krasnov BR, Littlewood DTJ, editors. Parasite Diversity and Diversification. Cambridge: Cambridge University Press (2015). p. 246–64. doi: 10.1017/CBO9781139794749.015
36. Jobling B. Host-parasite relationship between the American Streblidae and the bats, with a new key to the American genera and a record of the Streblidae from Trinidad, British West Indies (Diptera). Parasitology. (1949) 39:315–29.
37. Theodor O. Parasitic adaptation and host-parasite specificity in the pupiparous Diptera. In: Mayr R, editor. First Symposium on Host Specificity Among Parasites of Vertebrates. Neuchâtel: Institut de Zoologie, Université de Neuchâtel (1957). p. 50–63.
38. Marshall AG. The comparative ecology of insects ectoparasitic upon bats in west Malaysia. In: Wilson DE, Gardner AL, editors. Proceedings. Fifth International Bat Research Conference. Lubbock, TX: Texas Tech Press (1957). p. 135–42.
39. Dick CW, Gettingert D. A faunal survey of streblid flies (Diptera: Streblidae) associated with bats in Paraguay. J Parasitol. (2005) 91:1015–24. doi: 10.1645/GE-536R.1
40. Dick CW. High host specificity of obligate ectoparasites. Ecol Entomol. (2007) 32:446–50. doi: 10.1111/j.1365-2311.2006.00836.x
41. ter Hofstede HM, Fenton MB, Whitaker JO, Jr. Host and host-site specificity of bat flies (Diptera: Streblidae and Nycteribiidae) on Neotropical bats (Chiroptera). Can J Zool. (2004) 82:616–26. doi: 10.1139/z04-030
42. Gardner RA, Molyneux DH. Trypanosoma (Megatrypanum) incertum from Pipistrellus pipistrellus: development and transmission by cimicid bugs. Parasitology. (1988) 96:433–47. doi: 10.1017/S0031182000080082
43. Paterson WB, Woo PT. The development of the culture and bloodstream forms of three Trypanosoma (Schizotrypanum) spp. (Protista: Zoomastigophorea) from bats in Cimex lectularius (Hemiptera: Cimicidae). Can J Zool. (1984) 62:1581–7.
44. Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, et al. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infect Genet Evol. (2012) 12:1717–23. doi: 10.1016/j.meegid.2012.06.009
45. Do Amaral RB, Lourenço EC, Famadas KM, Garcia AB, Machado RZ, André MR. Molecular detection of Bartonella spp. and Rickettsia spp. in bat ectoparasites in Brazil. PLoS ONE. (2018) 13:e0198629. doi: 10.1371/journal.pone.0198629
46. Sándor AD, Földvári M, Krawczyk AI, Sprong H, Corduneanu A, Barti L, et al. Eco-epidemiology of novel Bartonella genotypes from parasitic flies of insectivorous bats. Microb Ecol. (2018) 76:1076–88. doi: 10.1007/s00248-018-1195-z
47. Hornok S, Kovács R, Meli ML, Gönczi E, Hofmann-Lehmann R, Kontschán J, et al. First detection of bartonellae in a broad range of bat ectoparasites. Vet Microbiol. (2012) 159:541–3. doi: 10.1016/j.vetmic.2012.04.003
48. Leulmi H, Aouadi A, Bitam I, Bessas A, Benakhla A, Raoult D, et al. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasit Vectors. (2016) 9:1–8. doi: 10.1186/s13071-016-1316-9
49. Loftis AD, Gill JS, Schriefer ME, Levin ML, Eremeeva ME, Gilchrist MJR, et al. Detection of Rickettsia, Borrelia, and Bartonella in Carios kelleyi (Acari: Argasidae). J Med Entomol. (2005) 42:473–80. doi: 10.1603/0022-2585(2005)042[0473:DORBAB]2.0.CO;2
50. Reeves WK, Loftis AD, Gore JA, Dasch GA. Molecular evidence for novel Bartonella species in Trichobius major (Diptera: Streblidae) and Cimex adjunctus (Hemiptera: Cimicidae) from two southeastern bat caves, U.S.A. J Vector Ecol. (2005) 30:339–41.
51. Szubert-Kruszynska A, Stanczak J, Cieniuch S, Podsiadły E, Postawa T, Michalik J. Bartonella and Rickettsia infections in haematophagous Spinturnix myoti mites (Acari: Mesostigmata) and their bat host, Myotis myotis (Yangochiroptera: Vespertilionidae), from Poland. Microb Ecol. (2018) 2018:1–10. doi: 10.1007/s00248-018-1259-0
52. Stuckey MJ, Chomel BB, Galvez-Romero G, Olave-Leyva JI, Obregón-Morales C, Moreno-Sandoval H, et al. Bartonella infection in hematophagous, insectivorous, and phytophagous bat populations of Central Mexico and the Yucatan Peninsula. Am J Trop Med Hyg. (2017) 97:413–22. doi: 10.4269/ajtmh.16-0680
53. Brook CE, Bai Y, Dobson AP, Osikowicz LM, Ranaivoson HC, Zhu Q, et al. Bartonella spp. in fruit bats and blood-feeding ectoparasites in Madagascar. PLoS Negl Trop Dis. (2015) 9:1–9. doi: 10.1371/journal.pntd.0003532
54. Gay N, Olival KJ, Bumrungsri S, Siriaroonrat B, Bourgarel M, Morand S. Parasite and viral species richness of Southeast Asian bats: fragmentation of area distribution matters. Int J Parasitol Parasites Wildl. (2014) 3:161–70. doi: 10.1016/j.ijppaw.2014.06.003
55. Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. (2008) 6:1–12. doi: 10.1186/1741-7007-6-27
56. Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. (2012) 7:e0038544. doi: 10.1371/journal.pone.0038544
57. Simmons NB. Mammal species of the World: a taxonomic and geographic reference, eds. Wilson DE, Reeder DAM, Vols. 1 and 2 (2005).
58. Graciolli G, Dick C. Checklist of World Nycteribiidae (Diptera: Hippoboscoidea). Chicago: Field Museum of Natural History (2013).
59. Dick C, Graciolli G. Checklist of World Streblidae (Diptera: Hippoboscoidea). Chicago: Field Museum of Natural History (2013).
60. R Core Team. R: A Language and Environment for Statistical Computing. (2018). Available online at: https://www.r-project.org/
61. Dormann CF, Gruber B, Fründ J. Introducing the bipartite Package: analysing ecological networks. R News. (2010) 3:343–4. doi: 10.1159/000265935
62. TEAM QD. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. (2014). Available online at: qgis.org
63. Obame-Nkoghe J, Rahola N, Bourgarel M, Yangari P, Prugnolle F, Maganga GD, et al. Bat flies (Diptera: Nycteribiidae and Streblidae) infesting cave-dwelling bats in Gabon: diversity, dynamics and potential role in Polychromophilus melanipherus transmission. Parasit Vectors. (2016) 9:1–12. doi: 10.1186/s13071-016-1625-z
64. Haelewaters D, Pfliegler WP, Szentiványi T, Földvári M, Sándor AD, Barti L, et al. Parasites of parasites of bats: laboulbeniales (Fungi: Ascomycota) on bat flies (Diptera: Nycteribiidae) in central Europe. Parasit Vectors. (2017) 10:96. doi: 10.1186/s13071-017-2022-y
65. Haelewaters D, Hiller T, Dick CW. Bats, bat flies, and fungi: a case of hyperparasitism. Trends Parasitol. (2018) 34:784–99. doi: 10.1016/j.pt.2018.06.006
66. Blackwell M. Incidence, host specificity, distribution, and morphological variation in Arthrorhynchus nycteribiae and A. eucampsipodae (l^Laboulbeniomycetes). Mycolo. (1980) 72:143–58.
67. Scott H. Descriptions and records of Nycteribiidae (Diptera Pupipara), with a discussion of the genus Basilia. J Linn Soc London Zool. (1936) 39:479–505.
68. Wilkinson DA, Duron O, Cordonin C, Gomard Y, Ramasindrazana B, Mavingui P, et al. The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. Appl Environ Microbiol. (2016) 82:1778–88. doi: 10.1128/AEM.03505-15
69. Szentiványi T, Haelewaters D, Pfliegler WP, Clément L, Christe P, Glaizot O. Laboulbeniales (Fungi: Ascomycota) infection of bat flies (Diptera: Nycteribiidae) from Miniopterus schreibersii across Europe. Parasit Vectors. (2018) 11:395. doi: 10.1186/s13071-018-2921-6
70. Samsinakova A. Prispevek k poznáni entomofytnich hub na muchulovitych (Nycteribiidae). Beiträge zur Kenntnis der entomophagen Pilze auf den Nycteribiiden. Zool List. (1960) 9:237–8.
71. Balazuc J. Notes sur les Laboulbéniales. III. Rectifications, synonymies et mises au point (suite). Bull Mens Soc Linnéenne Lyon. (1971) 40:168–71.
72. Moesz G. Mykológiai közlemények VIII. Mykologische Mitteilungen VIII. Bot Közlemények. (1931) 161–,75.
74. Thaxter R. Contribution towards a monograph of the Laboulbeniaceae. Part V. Mem Am Acad Arts Sci. (1931) 16:1–435.
75. Falcoz L. Pupipara (Dipteres) (première série). Biospeologica XLIX. Arch Zool. (1923) 61:521–52.
76. Jensen K, Rodrigues L, Pape T, Garm A, Santamaria S, Reboleira AS. Hyperparasitism in caves: bats, bat flies and ectoparasitic fungus. ARPHA Conf Abstr. (2018) 1:1–2. doi: 10.3897/aca.1.e29967
77. Witsenburg F, Clément L, López-Baucells A, Palmeirim J, Pavlinić I, Scaravelli D, et al. How a haemosporidian parasite of bats gets around: the genetic structure of a parasite, vector and host compared. Mol Ecol. (2015) 24:926–40. doi: 10.1111/mec.13071
78. Ramasindrazana B, Goodman SM, Dsouli N, Gomard Y, Lagadec E, Randrianarivelojosia M, et al. Polychromophilus spp. (Haemosporida) in Malagasy bats: host specificity and insights on invertebrate vectors. Malar J. (2018) 17:1–11. doi: 10.1186/s12936-018-2461-8
79. Haelewaters D, Vergaeghen SJC, Gonzàles TAR, Vega JAB, Saucedo RVV. New and interesting Laboulbeniales from Panama and neighboring areas. Nov Hedwigia. (2017) 105:267–99. doi: 10.1127/nova_hedwigia/2017/0410
80. Moskaluk AE, Stuckey MJ, Jaffe DA, Kasten RW, Aguilar-Setién A, Olave-Leyva JI, et al. Molecular detection of Bartonella species in blood-feeding bat flies from Mexico. Vector-Borne Zoonotic Dis. (2018) 18:258–65. doi: 10.1089/vbz.2017.2213
81. Judson SD, Frank HK, Hadly EA. Bartonellae are prevalent and diverse in Costa Rican bats and bat flies. Zoonoses Public Health. (2015) 62:609–17. doi: 10.1111/zph.12188
82. Walker MJ, Dorrestein A, Camacho JJ, Meckler LA, Silas KA, Hiller T, et al. A tripartite survey of hyperparasitic fungi associated with ectoparasitic flies on bats (Mammalia: Chiroptera) in a neotropical cloud forest in Panama. Parasite. (2018) 25:17. doi: 10.1051/parasite/2018017
83. Bertola PB, Aires CC, Favorito SE, Graciolli G, Amaku M, Pinto-da-rocha R. Bat flies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: Chiroptera) at Parque Estadual da Cantareira, São Paulo, Brazil: parasitism rates and host-parasite associations. Mem Inst Oswaldo Cruz. (2005) 100:25–32.doi: 10.1590/S0074-02762005000100005
84. Fritz GN. Biology and ecology of bat flies (Diptera: Streblidae) on bats in the genus Carollia. J Med Entomol. (1983) 20:1–10. doi: 10.1093/jmedent/20.1.1
85. Thaxter R. New Laboulbeniales, chiefly dipterophilous American species. Proc Am Acad Arts Sci. (1917) 52:649–721.
86. Graciolli G, Coelho DC. Streblidae (Diptera, Hippoboscoidea) sobre morcegos filomídos (Chiroptera, Phyllostomidae) em cavernas do Distrito Federal Brasil. Rev Bras Zool. (2001) 18:965–70. doi: 10.1590/S0101-81752001000300028
87. Abundes-Gallegos J, Salas-Rojas M, Galvez-Romero G, Perea-Martínez L, Obregón-Morales CY, Morales-Malacara JB, et al. Detection of dengue virus in bat flies (Diptera: Streblidae) of common vampire bats, Desmodus rotundus, in Progreso, Hidalgo, Mexico. Vector-Borne Zoonotic Dis. (2018) 18:70–3. doi: 10.1089/vbz.2017.2163
88. Urich FW. Note on the dipterous bat-parasite Cyclopodia greeffi Karsch, and on a new Species of Hymenopterous (Chalcid) Parasite bred from it. Proc Zool Soc L. (1922) 92:471–7.
89. Billeter SA, Hayman DTS, Peel AJ, Baker K, Wood JLN, Cunningham A, et al. Bartonella species in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology. (2012) 139:324–9. doi: 10.1017/S0031182011002113
90. Maa TC. Records and descriptions of Nycteribiidae and Streblidae (Diptera). Pacific Insects. (1962) 4:417–36.
91. Speiser P. Zur Kenntniss der geographischen Verbreitung der Ascomyceten-Gattung Helminthophana Peyritsch. Ber Dtsch Bot Ges. (1901) 18:498–500.
92. Xu Z, Yang W, Feng Y, Li Y, Fu S, Li X, et al. Isolation and identification of a highly divergent kaeng khoi virus from bat flies (Eucampsipoda sundaica) in China. Vector-Borne Zoonotic Dis. (2019) 19:73–80. doi: 10.1089/vbz.2018.2350
93. Van Vuren PJ, Wiley M, Palacios G, Storm N, McCulloch S, Markotter W, et al. Isolation of a novel fusogenic orthoreovirus from Eucampsipoda africana bat flies in South Africa. Viruses. (2016) 8:1–25. doi: 10.3390/v8030065
94. Feng Y, Li Y, Fu S, Li X, Song J, Zhang H, et al. Isolation of Kaeng Khoi virus (KKV) from Eucampsipoda sundaica bat flies in China. Virus Res. (2017) 238:94–100. doi: 10.1016/j.virusres.2017.06.007
95. Goldberg TL, Bennett AJ, Kityo R, Kuhn JH, Chapman CA. Kanyawara virus: a novel rhabdovirus infecting newly discovered nycteribiid bat flies infesting previously unknown pteropodid bats in Uganda. Sci Rep. (2017) 7:1–8. doi: 10.1038/s41598-017-05236-w
96. Jansen Van Vuren P, Wiley MR, Palacios G, Storm N, Markotter W, Birkhead M, et al. Isolation of a novel orthobunyavirus from bat flies (Eucampsipoda africana). J Gen Virol. (2017) 98:935–45. doi: 10.1099/jgv.0.000753
98. Lack JB, Nichols RD, Wilson GM, Van Den Bussche RA. Genetic signature of reproductive manipulation in the phylogeography of the bat fly, Trichobius major. J Hered. (2011) 102:705–18. doi: 10.1093/jhered/esr090
99. Peyritsch J. Über einige pilze aus der familie der laboulbenien. Sitzungsber Kaiserl Akad Wiss Wien, Math-Naturwiss Cl. (1871) 64:441–58.
100. Nowosad A. Arthrorhynchus nycteribiae (Peyritsch) Thaxter (Ascomycetes, Laboulbeniales) w Polsce. Bull Entomol Pologne. (1973) 43:423–30.
101. Santamaria S. New or interesting Laboulbeniales (Fungi, Ascomycota) from Spain, V. Nov Hedwigia. (2006) 82:349–63. doi: 10.1127/0029-5035/2006/0082-0349
102. Gardner RA, Molyneux DH. Polychromophilus murinus: a malarial parasite of bats: life-history and ultrastructural studies. Parasitology. (1988) 96:591–605. doi: 10.1017/S0031182000080215
103. Witsenburg F, Schneider F, Christe P. Signs of a vector's adaptive choice: on the evasion of infectious hosts and parasite-induced mortality. Oikos. (2015) 124:668–76. doi: 10.1111/oik.01785
104. Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. (2008) 22:1–15. doi: 10.1111/j.1365-2915.2008.00713.x
105. Anstead GM. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis. (2016) 16:e164–72. doi: 10.1016/S1473-3099(16)30003-2
106. Witsenburg F, Schneider F, Christe P. Epidemiological traits of the malaria-like parasite Polychromophilus murinus in the Daubenton's bat Myotis daubentonii. Parasit Vectors. (2014) 7:1–8. doi: 10.1186/s13071-014-0566-7
107. Schaer J, Reeder DAM, Vodzak ME, Olival KJ, Weber N, Mayer F, et al. Nycteria parasites of Afrotropical insectivorous bats. Int J Parasitol. (2015) 45:375–84. doi: 10.1016/j.ijpara.2015.01.008
108. Maia da Silva F, Marcili A, Lima L, Cavazzana M, Ortiz PA, Campaner M, et al. Trypanosoma rangeli isolates of bats from Central Brazil: genotyping and phylogenetic analysis enable description of a new lineage using spliced-leader gene sequences. Acta Trop. (2009) 109:199–207. doi: 10.1016/j.actatropica.2008.11.005
109. Poinar GO. Vetufebrus ovatus n. gen., n. sp. (Haemospororida: Plasmodiidae) vectored by a streblid bat fly (Diptera: Streblidae) in Dominican amber. Parasit Vectors. (2011) 4:2–6. doi: 10.1186/1756-3305-4-229
110. Miles MA, Llewellyn M, Lewis MD, Yeo M, Baleela R, Fitzpatrick S, et al. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology. (2009) 136:1509–28. doi: 10.1017/S0031182009990977
111. Ratcliffe FN. The flying fox (Pteropus) in Australia. Commonw Aust Counc Sci Ind Res Bull. (1931) 53:1–81.
112. Domrow R. New and little known Laelaptidae, Trombiculidae and Listrophoridae (Acarina) from Australasian mammals. Proc Linn Soc New South Wales. (1961) 86:60–95.
114. Mazza G, Cini A, Cervo R, Longo S. Just phoresy? reduced lifespan in red palm weevils Rhynchophorus ferrugineus (Coleoptera: Curculionidae) infested by the mite Centrouropoda almerodai (Uroactiniinae: Uropodina). Ital J Zool. (2011) 78:101–5. doi: 10.1080/11250003.2010.509135
115. Polak M. Ectoparasitic effects on host survival and reproduction: the Drosophila-Macrocheles association. Ecology. (1996) 77:1379–89. doi: 10.2307/2265535
116. Rani PAMA, Coleman GT, Irwin PJ, Traub RJ. Hippobosca longipennis - a potential intermediate host of a species of Acanthocheilonema in dogs in northern India. Parasit Vectors. (2011) 4:2–8. doi: 10.1186/1756-3305-4-143
117. Reeves WK, Beck J, Orlova MV, Daly JL, Pippin K, Revan F, et al. Ecology of bats, their ectoparasites, and associated pathogens on saint kitts island. J Med Entomol. (2016) 53:1218–25. doi: 10.1093/jme/tjw078
118. Corduneanu A, Sándor AD, Ionicǎ AM, Hornok S, Leitner N, Bagó Z, et al. Bartonella DNA in heart tissues of bats in central and eastern Europe and a review of phylogenetic relations of bat-associated bartonellae. Parasit Vectors. (2018) 11:1–7. doi: 10.1186/s13071-018-3070-7
119. Turmelle AS, Olival KJ. Correlates of viral richness in bats (Order Chiroptera). Ecohealth. (2009) 6:522–39. doi: 10.1007/s10393-009-0263-8
120. Maganga GD, Bourgarel M, Vallo P, Dallo TD, Ngoagouni C, Drexler JF, et al. Bat distribution size or shape as determinant of viral richness in African bats. PLoS ONE. (2014) 9:e0100172. doi: 10.1371/journal.pone.0100172
121. Duval L, Mejean C, Maganga GD, Makanga BK, Mangama Koumba LB, Peirce MA, et al. The chiropteran haemosporidian Polychromophilus melanipherus: a worldwide species complex restricted to the family Miniopteridae. Infect Genet Evol. (2012) 12:1558–66. doi: 10.1016/j.meegid.2012.06.006
122. Theodor O. An Illustrated Catalogue of the Rothschild Collection of Nycteribiidae in the British Museum (Natural History), With Keys and Short Descriptions for the Identification of Subfamilies, Genera, Species and Subspecies. London: British Museum (Natural History) (1967).
123. Hayman DTS. Bats as viral reservoirs. Annu Rev Virol. (2016) 3:77–99. doi: 10.1146/annurev-virology-110615-042203
124. Mühldorfer K. Bats and bacterial pathogens: a review. Zoonoses Public Health. (2013) 60:93–103. doi: 10.1111/j.1863-2378.2012.01536.x
125. Schneeberger K, Czirják GÁ, Voigt CC. Measures of the constitutive immune system are linked to diet and roosting habits of neotropical bats. PLoS ONE. (2013) 8:e0054023. doi: 10.1371/journal.pone.0054023
126. Bordes F, Morand S, Ricardo G. Bat fly species richness in Neotropical bats: correlations with host ecology and host brain. Oecologia. (2008) 158:109–16. doi: 10.1007/s00442-008-1115-x
127. Patterson BD, Dick CW, Dittmar K. Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). J Trop Ecol. (2007) 23:177–89. doi: 10.1017/S0266467406003816
128. Szentiványi T, Vincze O, Estók P. Density-dependent sex ratio and sex-specific preference for host traits in parasitic bat flies. Parasit Vectors. (2017) 10:1–9. doi: 10.1186/s13071-017-2340-0
129. Patterson BD, Dick CW, Dittmar K. Parasitism by bat flies (Diptera: Streblidae) on neotropical bats: effects of host body size, distribution, and abundance. Parasitol Res. (2008) 103:1091–100. doi: 10.1007/s00436-008-1097-y
130. Turmelle AS, Jackson FR, Green D, McCracken GF, Rupprecht CE. Host immunity to repeated rabies virus infection in big brown bats. J Gen Virol. (2010) 91:2360–6. doi: 10.1099/vir.0.020073-0
131. Wang ZW, Sarmento L, Wang Y, Li X-q, Dhingra V, Tseggai T, et al. Attenuated Rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol. (2005) 79:12554–65. doi: 10.1128/JVI.79.19.12554-12565.2005
132. Evans NJ, Bown K, Timofte D, Simpson VR, Birtles RJ. Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg Infect Dis. (2009) 15:1331–3. doi: 10.3201/eid1508.090475
133. Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, Fukatsu T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci. (2014) 111:10257–62. doi: 10.1073/pnas.1409284111
134. Gherna RL, Werren JH, Weisburg W, Cote R, Woese CR, Mandelco L, et al. Arsenophonus nasoniae gen. nov., sp. nov., the causative agent of the son-killer trait in the parasitic Wasp Nasonia vitripennis. Int J Syst Bacteriol. (1991) 41:563–5. doi: 10.1099/00207713-41-4-563
135. Russell JA, Latorre A, Sabater-Muñoz B, Moya A, Moran NA. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol. (2003) 12:1061–75. doi: 10.1046/j.1365-294X.2003.01780.x
136. Dunn AK, Stabb E V. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera : Myrmeleontidae). Appl Environ Microbiol. (2005) 71:8784–94. doi: 10.1128/AEM.71.12.8784
137. Trowbridge RE, Dittmar K, Whiting MF. Identification and phylogenetic analysis of Arsenophonus- and Photorhabdus-type bacteria from adultHippoboscidae and Streblidae (Hippoboscoidea). J Invertebr Pathol. (2006) 91:64–8. doi: 10.1016/j.jip.2005.08.009
138. Nováková E, Hypša V, Moran NA. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol. (2009) 9:1–14. doi: 10.1186/1471-2180-9-143
139. Bohacsova M, Mediannikov O, Kazimirova M, Raoult D, Sekeyova Z. Arsenophonus nasoniae and Rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS ONE. (2016) 11:e0149950. doi: 10.1371/journal.pone.0149950
140. Rossi MID, Aguiar-Alves F, Santos S, Paiva J, Bendas A, Fernandes O, et al. Detection of Wolbachia DNA in blood from dogs infected with Dirofilaria immitis. Exp Parasitol. (2010) 126:270–2. doi: 10.1016/j.exppara.2010.05.002
141. Hornok S, Ágh N, Takács N, Kontschán J, Hofmann-Lehmann R. Haematospirillum and insect Wolbachia DNA in avian blood. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol. (2018) 111:479–83. doi: 10.1007/s10482-017-0961-0
142. Obame-Nkoghe J, Leroy EM, Paupy C. Diversity and role of cave-dwelling hematophagous insects in pathogen transmission in the Afrotropical region. Emerg Microbes Infect. (2017) 6:1–6. doi: 10.1038/emi.2017.6
Keywords: bat flies, microparasite, chiroptera, pathogen, distribution
Citation: Szentiványi T, Christe P and Glaizot O (2019) Bat Flies and Their Microparasites: Current Knowledge and Distribution. Front. Vet. Sci. 6:115. doi: 10.3389/fvets.2019.00115
Received: 23 November 2018; Accepted: 27 March 2019;
Published: 24 April 2019.
Edited by:
Michael Kosoy, Centers for Disease Control and Prevention (CDC), United StatesReviewed by:
Xiangye Liu, Xuzhou Medical University, ChinaCarl Dick, Western Kentucky University, United States
Copyright © 2019 Szentiványi, Christe and Glaizot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara Szentiványi, tamara.szentivanyi@unil.ch
orcid.org/0000-0001-8123-0374
†These authors have contributed equally to this work
‡Philippe Christe orcid.org/0000-0002-8605-7002
Olivier Glaizot orcid.org/0000-0001-9116-3355
 Tamara Szentiványi
Tamara Szentiványi Philippe Christe
Philippe Christe Olivier Glaizot
Olivier Glaizot