Safety and Transcriptome Analysis of Live Attenuated Brucella Vaccine Strain S2 on Non-pregnant Cynomolgus Monkeys Without Abortive Effect on Pregnant Cynomolgus Monkeys
- 1National/OIE Reference Laboratory for Animal Brucellosis, China Institute of Veterinary Drug Control (IVDC), Beijing, China
- 2Academy of Agriculture and Animal Husbandry Sciences, Hohhot, China
Brucellosis, caused by Brucella spp., is an important zoonotic disease leading to enormous economic losses in livestock, posing a great threat to public health worldwide. The live attenuated Brucella suis (B. suis) strain S2, a safe and effective vaccine, is widely used in animals in China. However, S2 vaccination in animals may raise debates and concerns in terms of safety to primates, particularly humans. In this study, we used cynomolgus monkey as an animal model to evaluate the safety of the S2 vaccine strain on primates. In addition, we performed transcriptome analysis to determine gene expression profiling on cynomolgus monkeys immunized with the S2 vaccine. Our results suggested that the S2 vaccine was safe for cynomolgus monkeys. The transcriptome analysis identified 663 differentially expressed genes (DEGs), of which 348 were significantly upregulated and 315 were remarkably downregulated. The Gene Ontology (GO) classification and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated that these DEGs were involved in various biological processes (BPs), including the chemokine signaling pathway, actin cytoskeleton regulation, the defense response, immune system processing, and the type-I interferon signaling pathway. The molecular functions of the DEGs were mainly comprised of 2'-5'-oligoadenylate synthetase activity, double-stranded RNA binding, and actin-binding. Moreover, the cellular components of these DEGs included integrin complex, myosin II complex, and blood microparticle. Our findings alleviate the concerns over the safety of the S2 vaccine on primates and provide a genetic basis for the response from a mammalian host following vaccination with the S2 vaccine.
Introduction
Brucellosis, also known as “undulant fever,” “Mediterranean fever,” or “Malta fever,” is a significant zoonotic disease caused by Brucella spp. that is almost invariably transmitted by direct or indirect contact with infected animals or their products (1). Brucellosis remains as an important economic and public health problem in many countries and regions across the world (1, 2). Approximately 500,000 cases of human brucellosis are reported every year globally. However, the estimated incidence was believed to range from 5,000,000 to 12,500,000 annually worldwide (3, 4). Surprisingly, in the recent years, human brucellosis incidences dramatically increased in China (5–8). From April 1, 2007 to March 31, 2017, a total of 435,108 cases of human brucellosis were reported in the Mainland China, with an average of 3,626 cases per month and an SD of 1,834 cases (9). A very recent report from 2013 to 2017 showed that the positive rate of the antibody against Brucella abortus was 44.2% in 23,381 serum samples collected from dairy cows with the clinical sign of abortion (10). Interestingly, the incidence rate was strongly correlated with the epidemic kinetics of human brucellosis (r = 0.806) (10), which is not too surprising as the cases of human infection with Brucella mainly occurred through contact with the Brucella-infected animal(s) and/or animal product(s) (11, 12). Consistently, it is generally believed that the prevention and control of human brucellosis can be satisfactorily achieved by control and/or eradication of the disease in animals (2, 11, 12).
Vaccination against brucellosis remains as the most effective control measure for preventing the disease spread in the high-prevalence regions (12). The WHO and the World Organization for Animal Health (OIE) recommended the live attenuated vaccines Rev.1 (smooth type), S19 (smooth type), and RB51 (rough type), to fight against Brucella melitensis, B. abortus, and B. abortus, respectively. The abovementioned vaccines are currently extensively used to control animal brucellosis in many countries around the world (11, 13, 14). As for human brucellosis, many groups have made great efforts to develop vaccines for use in the human population; however, thus far, no safe and effective vaccine has been successfully developed and licensed for human brucellosis (14).
In China, the major vaccines used for animal brucellosis include B. melitensis strain M5, Brucella suis strain S2, and B. abortus strain A19. The S2 strain is a live, attenuated smooth phenotype strain that was isolated from the fetus of aborted swine by the China Institute of Veterinary Drug Control (IVDC) in 1952, and it was naturally attenuated to B. suis vaccine by a serial passage (15). The advantages of the S2 vaccine include, but are not limited to, lower virulence compared to S19, M5, and Rev.1 in a wider target animal species (pig, cattle, sheep, and goat), and by convenient administration via the oral route (15, 16). In China, the S2 vaccine used in the recent decades has shown to be robust, efficacious, and safe in the vaccination of animals. Previously, our studies have shown that the S2 vaccine protected mice from challenges faced by heterologous virulent species, including M28 (B. melitensis), 2308 (B. abortus), and S1330 (B. suis), as evidenced by observations which showed that the immunized mice did not develop any clinical sign or tissue damage (17). Nonetheless, the safety of S2 in primates (e.g., humans) has remained elusive. Given that health workers (e.g., veterinarian) might be accidentally exposed to the vaccine during the vaccination of animals (18), there is a necessity to evaluate the safety of the S2 vaccine strain in human beings. To address this problem, in this study, we examined the safety of the S2 vaccine as well as transcriptome analysis using cynomolgus monkeys as an alternative animal infection model. Our results suggest that the S2 vaccine strain was safe to cynomolgus monkeys, and immunization with S2 resulted in an altered expression of various genes related to the biological function and the molecular activity of the host.
Materials and Methods
Ethical Statement
All animals used in this study were kept and handled in compliance with the Experimental Animal Regulation Ordinances defined by the China National Science and Technology Commission. The study was approved by the Animal Ethical Committee of the China Institute of Veterinary Drug Control under permit number, General Requirements for Laboratory Biological Safety of China, GB19489 -2008.
Animals
Twelve adult cynomolgus monkeys, including six pregnant female monkeys (2-month pregnancy, aged 3.5 years and 3.5 kg of weight) and six non-pregnant female monkeys (aged 3 years and 2.5 kg of weight), were purchased from Guangxi Province, China. Upon arrival, all monkeys were serologically tested by the Rose Bengal Plate Agglutination Test (RBPT) (19) and competitive ELISA (Svanova, Switzerland) and were confirmed to be Brucella-negative. All animals and all animal experiments were performed in an absolute negative pressure closed area conforming to BSL-3 or ABSL-3 conditions.
Experiment of Immunization
The lyophilized Brucella live S2 vaccine (with an amount of 1.22 × 1010 CFU and 80 doses/bottle), purchased from Qilu Animal Health Co. Ltd., China, and stored at −20°C in the fridge of the National/OIE Laboratory for Brucellosis of IVDC for no more than 3 months after their arrival, was used for immunization. One bottle of stock S2 vaccine was diluted in 200 ml of phosphate buffered saline (PBS) (pH7.2) to a final concentration of 5 × 109 CFU/ml. Four pregnant and four non-pregnant monkeys were randomly selected. Each monkey was subcutaneously immunized on the thigh with 1 ml (equivalent to 5 × 109 CFU) of the diluted S2 vaccine, according to the Chinese Veterinary Pharmacopeia (Version 2015) (19). Placebo injection was performed using an identical volume of PBS (pH7.2) on two pregnant and two non-pregnant monkeys as negative controls.
Monitoring of Animals
All animals were monitored daily for 14 consecutive days post-immunization (p.i.). The immunization spot of the skin on the thigh was examined for any abnormal signs, such as red swelling, ulceration, and local inflammation. In addition, the behaviors and the mental status of all animals were monitored during activities such as walking, eating, drinking, as well as other similar activities. Starting from 3 days prior to immunization, the body temperature of all monkeys, except for the pregnant animals, was measured and recorded each morning and afternoon. The body temperature of the pregnant monkeys was not tested to minimize the influence on pregnancy. All pregnant monkeys were continuously monitored for abortion or any abortion-like signs after immunization until delivery. The clinical observation period of the test lasted for about 9 months till the weaning of the offspring.
RNA-Sequencing
On day 4 p.i., blood was collected from non-pregnant experimental monkeys. Subsequently, the peripheral blood mononuclear cells (PBMC) were isolated according to the protocol of the manufacturer (Solarbio Life Sciences, Beijing, China). Briefly, Reagents A and D in the kit were gently added into a 15 ml centrifuge tube, resulting in a layered solution. The blood sample with a volume equivalent to that of the layered solution was transferred onto the top of the layered solution and then centrifuged for 25 min at 500 g, resulting in a solution with five visible layers. The solutions in the second and third layers were gently collected and transferred into a new 15 ml centrifuge tube, and then, a 10 ml of wash solution was added and mixed gently. The mixture was centrifuged for 10 min at 250 g, and the supernatant was disposed. The pelleted cells were suspended in a 5 ml wash solution and subjected to centrifugation as above. The cells were again washed three times as above, suspended in a 5 ml wash solution and used for total RNA extraction and purification. The purity, molar concentration, and the integrity of the RNA were determined by Nanodrop (ND 1000 spectrophotometer Thermo Scientific, Wilmington, USA), Qubit 2.0 (Thermo Fisher Scientific, MA, USA), and Aglient 2100 (Agilent Technologies Inc, CA, USA), respectively, to ensure that the samples used for the transcriptomic sequencing were qualified. Subsequently, a cDNA library was constructed using the extracted RNA. Briefly, the eukaryotic mRNA was enriched using magnetic beads and Oligo (dT). A fragmentation buffer was then added to randomly disrupt the mRNA. The first cDNA strand was synthesized utilizing random hexamers as primers and mRNA as a template. The second cDNA strand was synthesized after adding a buffer, dNTPs, RNase H, and a DNA polymerase. The cDNA of each sample was purified by AMPure XP beads (Beckman Coulter, CA, USA) and subjected to end repair, A-tail addition, and adapter sequencing. The fragments with appropriate size were selected by AMPure XP beads. The concentration and size of the fragments inserted in the library were measured using Qubit 2.0 and Agilent 2100, respectively. Finally, PCR enrichment was performed to construct the cDNA library for each sample using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England BioLabs, MA, USA) as recommended by the manufacturer. The accurate concentration of the library was determined by using a real-time PCR. High-throughput sequencing was conducted by the HiSeq X Ten System with a read length of PE150. Sequencing and subsequent bioinformatics analyses were completed at Biomarker Technologies Co., Ltd, Beijing, China.
Identification of Differentially Expressed Genes (DEGs)
Identification and characterization of DEGs were achieved based on the results of differential expression analysis via EBSeq algorithm to filter DEGs with a log-fold expression change (log2FC > 0.585 or < −0.585) using a threshold of false discovery rates (FDR < 0.05), as previously described (17).
Gene Ontology (GO) Analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG)
Pathway Analysis
Comparative transcriptome analyses, employing the GO classification and the KEGG pathway analysis approaches, were performed. The gene products were annotated in terms of a biological function and metabolic pathways. The biological function, signal transduction, and metabolic pathways of the DEGs were analyzed.
Quantitative Reverse-Transcription PCR
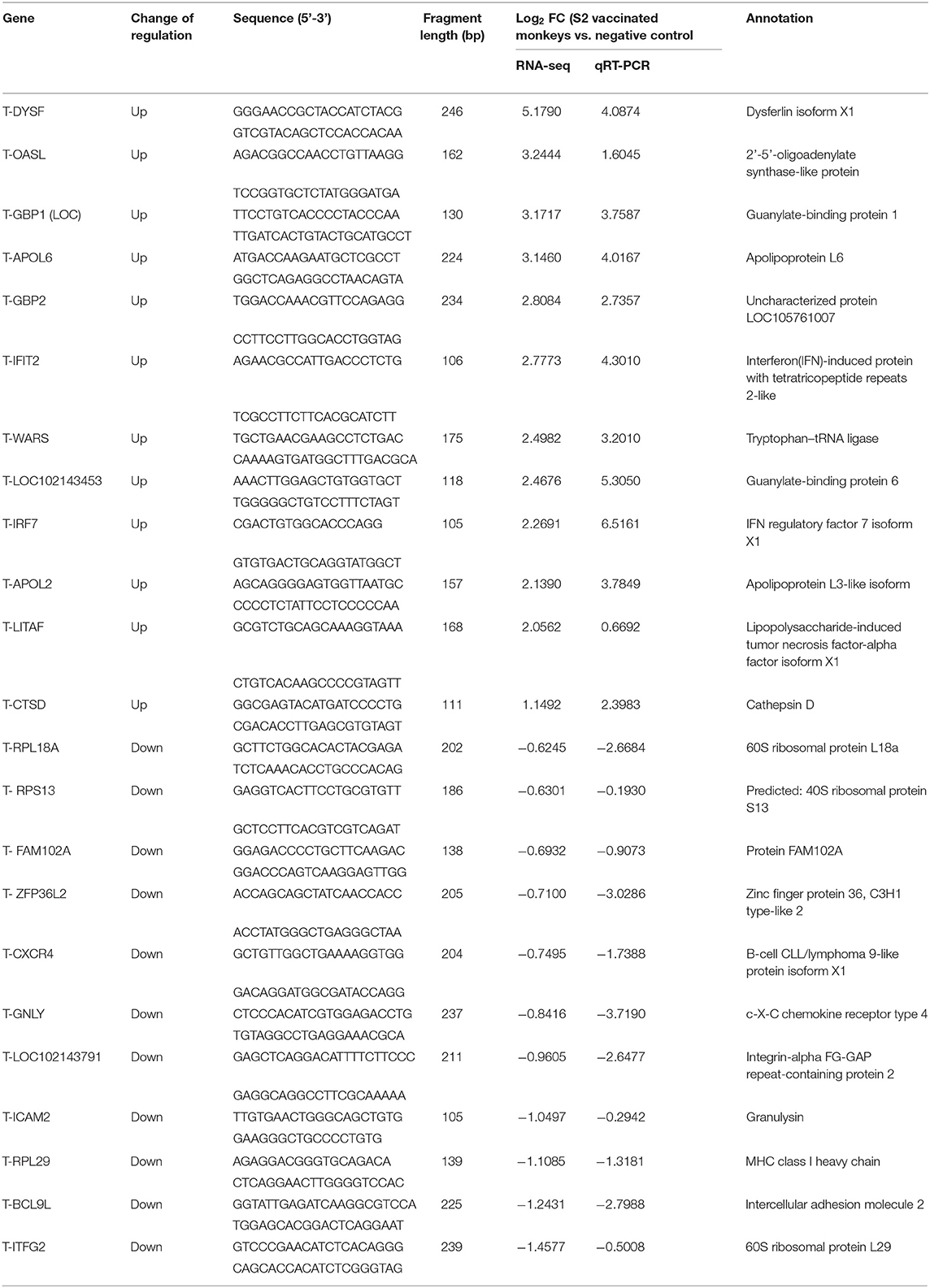
To validate the difference of gene expression profiling, a quantitative reverse-transcription PCR (qRT-PCR) was performed. A total of 23 DEGs were randomly selected, including 12 upregulated and 11 downregulated genes. The targeted genes and the designed primers used for qRT-PCR are shown in Table 1. Briefly, the mRNAs from samples of six non-pregnant monkeys were extracted and used for the following qRT-PCR system. The reaction mixture was prepared for reverse transcription, including 5 × PrimeScriptTM RT Master Mix [containing PrimeScriptRTase, RNase inhibitor, Random Hex Primer, Oligo (dT), and dNTPs] (Takara Bio, Dalian, China) of 2 μl, Total RNA of 5 μl, and sterile RNase-free H2O of 3 μl. The mixture was incubated at 37°C in a water bath for 15 min for reverse transcription, followed by incubation at 85°C in a water bath for 5 s for the inactivation of reverse transcriptase. The cDNA was 10-fold diluted using a sterile RNase-free H2O and used for PCR amplification. The PCR reaction system consisted of 10 μl 2 × TB Green Premix Ex Taq (Tli RNase H Plus), 0.4 μl 50 × ROX Reference Dye II, and 6 μl sterile RNase-free H2O. The mixture was incubated at 95°C for 30 s and then subjected to 40 cycles of incubation at 95°C for 5 s and 60°C for 34 s in the LightCycler 2.0 system (Roche Life Sciences, Basel, Switzerland). To normalize the expression, the β-actin of cynomolgus monkey was used as an internal amplification control, for which the forward primer ACTB-1 (5′-CGCCATGGATGATGATATCGC-3′C) and the reverse primer ACTB-2 (5′-ATCCTTCTGACCCATGCCCA-3′A) were used, and the preparation of the same mixture as well as thermocycling were performed.
Results
Clinical Assessment
On the day of immunization, very slight swelling was observed at the injection site of the thigh skin of two cynomolgus monkeys (data not shown). However, these observatory signs rapidly vanished on the next day p.i. Afterward, no abnormal clinical manifestation was observed on the skin of the immunized monkeys until the end of the experiment, indicating that S2 immunization did not result in deleterious clinical effects at the injection spot of the monkeys.
The body temperature of all non-pregnant cynomolgus monkeys was detected daily during the whole experimental period, starting from 3 days before immunization until 14 days p.i. The results indicated that the temperature of all non-pregnant monkeys monitored throughout the entire experimental period fell into a normal level, ranging from 35.5°C to 37.5°C, and no difference was observed between monkeys immunized with S2 and those inoculated with PBS (Figure 1). These results demonstrated that immunization of S2 and PBS did not lead to abnormal change in the body temperature of the non-pregnant monkeys.

Figure 1. Results of body temperature monitoring of cynomolgus monkeys immunized with the S2 vaccine and inoculated with phosphate buffered saline (PBS) (negative control). The body temperature of all non-pregnant cynomolgus monkeys, including the animals immunized with the S2 vaccine and those inoculated with PBS, was detected daily during the entire experimental period, starting from 3 days prior to injection till 14 days post-immunization (p.i.). (A) Indicated the results of body temperature of all the animals detected in the morning, and (B) showed the results obtained in the afternoon. The bottom line of normal body temperature (35.5°C) and the top line of normal body temperature (35.7°C) were indicated.
The behavior of all monkeys was monitored daily during the whole experimental period. Our results showed that none of the animals exhibited any abnormal behavior (data not shown). No difference was observed between the S2-vaccinated and PBS-inoculated animals in terms of behavior over the whole experimental period, including activities such as drinking and eating. These results suggested that the S2 vaccination did not alter the behavior of any cynomolgus monkeys.
Monitoring of Abortion
A previous study reported that natural infection of Brucella spp. frequently resulted in abortion (1). Therefore, the clinical sign of abortion is an important indicator for the infection of Brucella spp. In order to understand whether immunization of the S2 vaccine leads to abortion, we inoculated four pregnant cynomolgus monkeys with S2 and then continuously monitored them on a daily basis for abortion till delivery. None of the immunized monkeys got aborted after vaccination. Moreover, the offspring of monkeys did not exhibit abnormal behaviors or clinical signs (data not shown), thus suggesting that the S2 vaccination did not cause abortion in the cynomolgus monkeys.
Transcriptomic Analysis of S2-Vaccinated and PBS-Inoculated Cynomolgus Monkeys
To identify the genetic factors correlating with S2 immunization, changes of the transcriptomic profile of gene expression in cynomolgus monkeys immunized with S2 were examined by a transcriptomic analysis. The same analysis was also performed on the negative control samples collected from cynomolgus monkeys inoculated with PBS. RNA extracted from the blood of non-pregnant monkeys was used as a template for the construction of a library, followed by a transcriptomic RNA-seq. We did not collect blood from pregnant cynomolgus monkeys, considering that the fixation of animal and blood collection may lead to unexpected abortion. Identification and analysis of DEGs were performed based on the results of EBSeq algorithm analyses. On day 4 p.i., transcriptomic sequencing of the mRNA of 15,009 genes derived from the S2-immunized group identified 663 DEGs (log2FC > 0.585 or < −0.585 and FDR < 0.05), compared to the negative control group (in which animals were inoculated with PBS), including 348 significantly upregulated genes and 315 significantly downregulated genes (Figure 2). Among these DEGs, the highest-ranked upregulated genes included DEXH (Asp-Glu-X-His) box polypeptide 58 (DHX58), integrin-alpha 2 (ITGA2), and 2'-5'-oligoadenylate synthetase 2 (OAS2), whereas the lowest downregulated genes comprised of myosin VIIA (MYO7A), Usher syndrome 1C (USH1C), and chemokine (C-X-C motif) receptor 6 (CXCR6) (Figure 2).
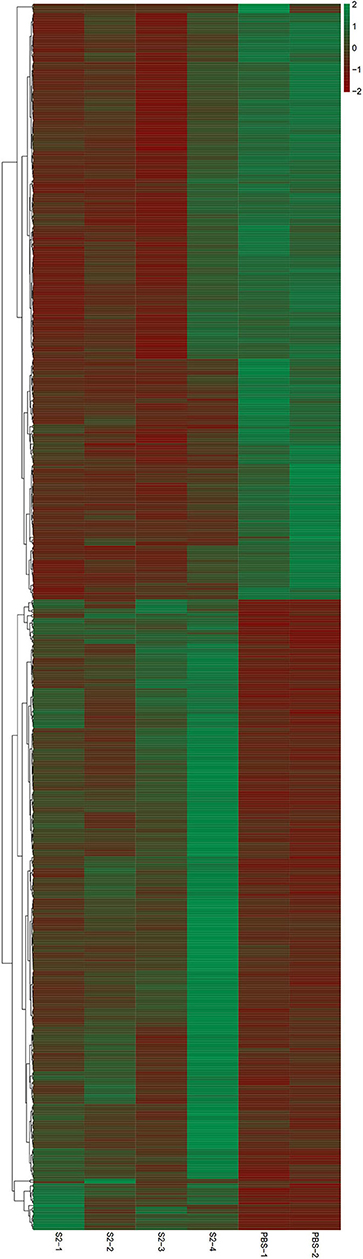
Figure 2. Heatmap for RNA-sequencing (RNA-seq) data. Blood of four cynomolgus monkeys immunized with the S2 vaccine and that of two cynomolgus monkeys inoculated with PBS (negative control) were collected and used for RNA extraction. A cDNA library was constructed and then transcriptomic RNA-seq was performed. Red and blue pixels indicate upregulated and downregulated genes, respectively. Genes were categorized into different clusters based on the generated dendrogram.
Gene Ontology Category
The term GO has three ontologies, including the biological process (BP), molecular function (MF), and cellular component (CC). The sequences of these aforementioned DEGs were analyzed employing a GO analytical approach. Our investigations based on the GO functional enrichment analysis revealed that the 663 DEGs were mainly involved in various BPs, including defense response, immune system processing, and the type-I interferon signaling pathway (Figure 3). The MF of these DEGs included 2'-5'-oligoadenylate synthetase activity, double-stranded RNA (dsRNA) binding, and actin-binding (Figure 3). In addition, these genes were correlated with C, including integrin complex, myosin II complex, and blood microparticle (Figure 3).
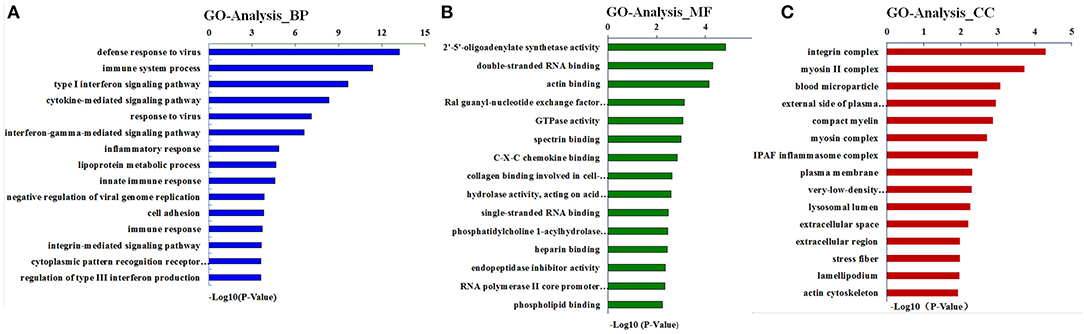
Figure 3. Gene ontology (GO) analysis for differentially expressed genes (DEGs). Results are grouped by three main functional categories, including (A) the biological process (BP), (B) molecular function (MF), and (C) cellular component (CC).
Pathway Analysis
Next, we sought to analyze the above DEGs via the KEGG pathway analysis. As shown in Figure 4, the DEGs were mainly related to the chemokine signaling pathways, signaling pathways after measles virus, and the infection of influenza A virus. Interestingly, the upregulated genes were enriched in pathways relating to the regulation of actin cytoskeleton, as well as the infection by measles virus and influenza A virus, whereas the enriched downregulated genes were relevant to cell adhesion molecules, cytokine–cytokine receptor interaction, and chemokine signaling pathway.
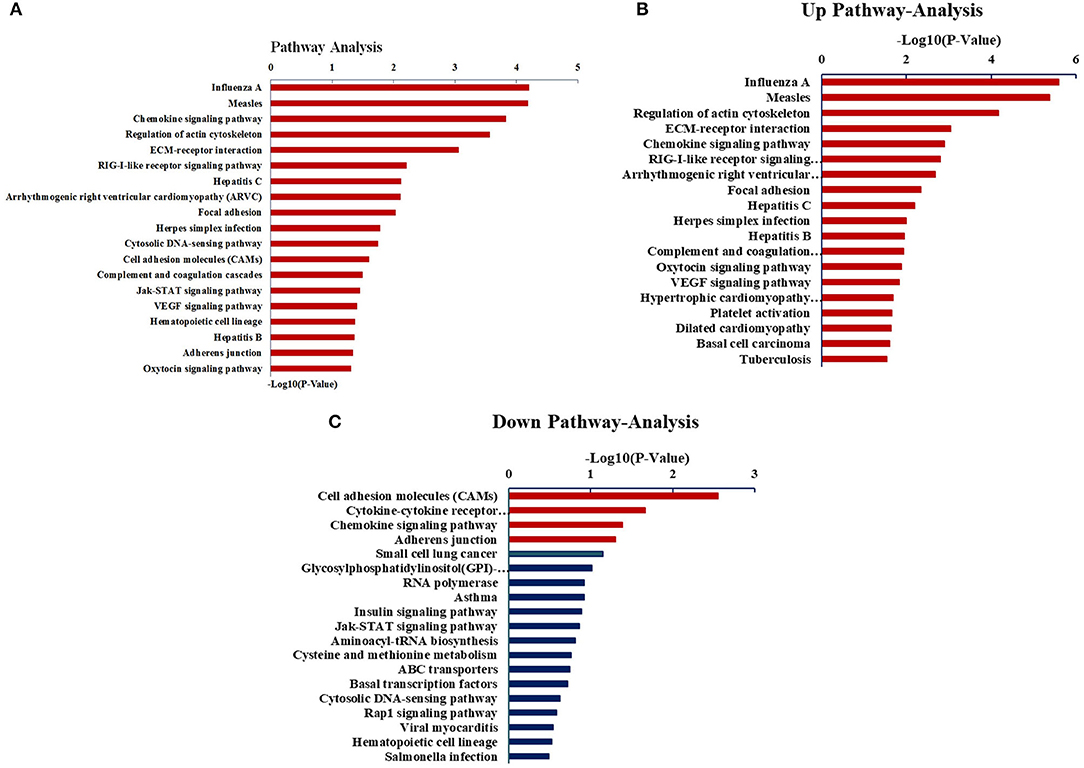
Figure 4. Analysis of DEGs through the KEGG pathway. (A) The KEGG pathway analysis of all DEGs. (B) The KEGG pathway analysis of upregulated expressed genes. (C) The KEGG pathway analysis of downregulated expressed genes.
Quantitative Reverse-Transcription Polymerase Chain Reaction
To validate the RNA-seq results, a total of 23 DEGs, including 12 significantly upregulated genes and 11 significantly downregulated genes, were randomly selected and quantified by qRT-PCR. The β-actin gene was simultaneously detected and analyzed as an internal control. As outlined in Table 1, the expression levels of the 23 selected genes in the qRT-PCR exhibited a very similar profile to that of the RNA-seq, confirming the consistency of results obtained in the RNA-seq and the qRT-PCR.
Discussion
Brucellosis is a worldwide zoonotic infectious disease caused by the members of the genus, Brucellae, which are circulating in many countries, especially in the Mediterranean basin, Asia, the Middle East, and in the Central and South America (20). Brucellosis, which leads to substantial economic losses in the animal industry, has a significant impact on the health of human populations, as well as has an influence on the structure of the whole society around the world, e.g., public safety, food hygiene, and foreign trade (21, 22). A recent report described the analyses of the species distribution, host lineage profiles, genetic relatedness, and the epidemiological correlation of the Brucella species in countries along the Silk Road. The authors concluded that numerous animal reservoirs were a potential reason for the continuous circulation of the brucellosis in the examined countries, while the B. abortus strains were dominated in these countries and spreading within the national borders (23). In addition, using descriptive analysis and meta-analysis approaches, Zhou et al. reported very recently that dogs and yaks were the leading reservoirs and emerging hosts for Brucella transmission in China (24). Meanwhile, the authors inferred that humans probably get infected along with different kinds of animal species, among which the sheep and goats were likely at the highest risks (24). The aforementioned studies, as well as the ligatures, e.g., a recent report from our collaborators (25), consistently demonstrated the significance of the One Health concept and recommended implementing it to disrupt the Brucella transmission chain between animals and humans, as well as to strengthen the close collaboration between the local and public partnerships.
It has been well-documented that vaccination plays a critical role in the prevention and control of brucellosis (11, 14, 17). However, so far, there has been no licensed vaccine for brucellosis in human, and currently, the available animal vaccines may cause huge concern and disease; therefore, they were considered unsuitable for use in human populations (26). Therefore, the search for brucellosis vaccine for humans has continued to raise the interests of the researchers. As far as vaccines against animal brucellosis are concerned, there are currently a variety of vaccines available in the world. The Brucella live vaccines, S19, Rev.1, and RB51, which are widely used in different regions of the world, have been demonstrated to effectively offer immune protection against brucellosis (11, 14, 17). Nevertheless, the negative implications of these vaccines, such as the residual virulence in the host, diagnostic interference, and the pathogenic effect to human health, in particular, have remained poorly understood, thus raising doubts and concerns in the implementation of these vaccines in the field conditions (11, 14, 17, 27). For instance, a previous study showed that although the vaccine strain S19 was of low virulence for cattle, the vaccination of pregnant cows resulted in abortion with incidence rate ranging from <1–2.5% under field conditions (12). These concerns and problems prompted us to evaluate the safety of live attenuated Brucella vaccine strain in primates. Given that the virulence of the S2 strain was the lowest compared to other Brucella vaccine strains (17, 28), we explored it to determine the safety of S2 on cynomolgus monkeys.
The S2 vaccine is extensively used in China to control brucellosis in goats, sheep, and cattle. In our previous study, we demonstrated that the S2 vaccine could protect mice from virulent challenge by heterologous strains, including B. militensis M28, B. abortus 2308, and B. suis 1,330 (17). However, the safety of S2 in primates, especially humans, has remained undetermined. The possibility of the infection of S2 vaccine strain in a group of veterinary professionals due to unexpected reasons, such as accident exposure while handling the vaccine, should not be ignored, considering the fact that S2 is a live vaccine. Accordingly, the safety of the S2 vaccine to humans needs to be comprehensively evaluated. In addition, it has been well-documented that cases of Brucella infection in human populations were predominantly caused by animals or animal products infected with Brucella (11, 12). Thus, prevention, control, and elimination of Brucella in animals would be extremely beneficial to the control of human brucellosis. Therefore, in the present study, we sought to determine the safety of the S2 strain to humans using the cynomolgus monkey infection model. Our results clearly showed that the S2 vaccine was indeed safe to cynomolgus monkeys, as evidenced by observations that all monkeys immunized with the S2 strain neither showed any abnormal behaviors nor suffered abortion. This evidence might alleviate concerns of grass-root veterinarians over the accidental exposure to the S2 vaccine strain when using it to immunize animals in practice. In other words, our data would enhance the confidence of professionals in the use of the S2 vaccine in the field conditions. To the best of our knowledge, this is the first report of evaluation on live attenuated Brucella spp. vaccine in cynomolgus monkeys, which is an ideal primate species as it is genetically close to human beings. Furthermore, given that the S2 strain was highly attenuated in mice (17, 28) and was safe to cynomolgus monkeys as described here, it might be feasible to further attenuate the S2 strain while keeping its immunogenicity unchanged using genetic engineering, thus making it a potential vaccine candidate for use in human populations.
Very recently, a total of 1,205 DEGs were identified between artificially induced rough-mutant Brucella strain RM57 and its parent strain, M1981, via the transcriptome analysis (29). The goal of this study was to evaluate the safety of the live vaccine S2 in human utilizing cynomolgus monkeys as an alternative model, as well as performing the transcriptome analysis of the peripheral blood collected from S2-immunized cynomolgus monkeys to obtain information of the immune response relevant to the safety of the S2 vaccine at an early stage. Toward this end, instead of using the lymph tissues or organs, in this study, we took an alternative way, i.e., using the peripheral blood to isolate lymphocytes for transcriptomic analysis. We did not examine the lymph nodes for the innate immune response due to our concern that the fixation of animals and the collection of blood may lead to unexpected abortion, and as a consequence, it may interfere with our evaluations on the safety of the S2 vaccine, since abortion is an important parameter in our evaluation in this study. The use of the peripheral blood for the isolation of lymphocytes for transcriptomic analysis is not new, as the use of the peripheral blood from pigs (30), dogs (31), Chinese geese (32), and humans (33–35), including patients with COVID-19 (36), have been well-documented.
Previously, comparative genomic analysis between S2 and the virulent strain 1,330 was reported, and 59 different open reading frames (ORFs) between these two strains were identified, several of which were demonstrated to be related to the virulence of Brucella spp., such as outer membrane autotransport and eryD (16). To further uncover the mechanism underlying the virulence attenuation of S2, our colleagues recently characterized the transcriptional profile of S2 and B. suis 1,330 infected murine macrophages by the transcriptome analysis, showing that the expression of 440 genes was significantly enriched, involving the innate immune response, phagocytosis, recognition, and inflammatory response, as well as the Staphylococcus aureus infection pathway and the NF-kappa B signaling pathway (28). In addition, our findings indicated that the DEGs related to the S2 immunization/infection were different between murine macrophages and monkeys (28). In order to further ascertain the host cellular response and genetic factors associated with S2 vaccination in mammals, in this study, we performed a comparative transcriptome analysis on blood cells collected from S2-immunized cynomolgus monkeys. We demonstrated that S2 vaccination of monkeys resulted in altered levels of the expression of 663 genes relevant to various MFs and biological functions of the host organism, including actin cytoskeleton regulation and binding, defense response, 2'-5'-oligoadenylate synthetase activity, dsRNA binding, the type-I interferon signaling pathway, and the chemokine signaling pathway. These results enhanced our understanding of the immune response of the mammalian species upon S2 vaccination as well as the mechanism of virulence attenuation of the S2 vaccine strain.
The data reported in the present study shed light on the safety of the S2 vaccine. Yet, there are still some limitations in our study. First, only limited numbers of cynomolgus monkeys were used, resulting in insufficient statistical analyses and comparisons. Second, the blood of pregnant cynomolgus monkeys was not collected and tested; thus, we might have missed some important information about the transcriptomic profile of the pregnant cynomolgus monkeys. Third, we did not measure the parameters relevant to the host immune response, such as the humoral immunity and the cell-mediated immune response. Fourth, RT-PCR detecting Brucella in the collected peripheral blood was not performed. These outcomes may suggest that only a partial situation of the safety of the S2 vaccine was truly reflected. Despite these limitations, we believe that our results would enhance the understanding of the safety of the S2 vaccine. Further studies with improved experimental design and larger sample size are warranted to further verify the reported results.
Conclusion
This study provides evidence on the safety of the Brucella S2 vaccine strain on cynomolgus monkeys, enhancing the understanding of the pathogenicity of S2 in human. The work offers insights into the molecular basis of the host gene regulation in response to S2 vaccination.
Data Availability Statement
The datasets presented in this study can be found in online repositories.The names of the repository/repositories and accession number(s) can be found below: NCBI Gene Expression Omnibus, Accession No: GSE165283.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of China Institute of Veterinary Drug Control, China.
Author Contributions
LZ, JD, and YQ conceived and designed the experiments. SS, HJ, QL, and YL performed the experiments, analyzed the data, and prepared figures and tables. QG, YF, XP, GX, QS, and XF performed the experiments and analyzed the data. All authors reviewed the drafts of the paper and approved the final draft.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2017YFD0500901) and Major Science and Technology Projects of Xinjiang Construction Corps (No. 2017AA003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Biomarker Technologies Co., Ltd for their support in the bioinformatics analyses. We also thank NovelBio Bio-Pharm Technology Co., Ltd for their support in the data analyses.
References
2. Roth F, Zinsstag ZJ, Orkhon D, Chimid-Ochir G, Hutton G, Cosivi O, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. (2003) 81:867–76.
3. Godfroid J, Al Dahouk S, Pappas G, Roth F, Matope G, Muma J, et al. A “One Health” surveillance and control of brucellosis in developing countries: moving away from improvisation. Comp Immunol Microbiol Infect Dis. (2013) 36:241–48. doi: 10.1016/j.cimid.2012.09.001
4. Hull NC, Schumaker BA. Comparisons of brucellosis between human and veterinary medicine. Infect Ecol Epidemiol. (2018) 8:1500846. doi: 10.1080/20008686.2018.1500846
5. Lin Y, Xu M, Zhang X, Zhang T. An exploratory study of factors associated with human brucellosis in mainland China based on time-series-cross-section data from 2005 to 2016. PLoS ONE. (2019)14:e0208292. doi: 10.1371/journal.pone.0208292
6. Zhang J. Reasons and prevention and control measures for the high brucellosis incidence. Disease Surveillance. (2010)25:341–2. doi: 10.1007/s10654-010-9446-4
7. Gao GX, Xu J, Ke YH, Chen ZL, Wang YF, Xu XR. Literature analysis of brucellosis epidemic situation and research progress in China from 2000 to 2010. Chinese J Zoonoses. (2012) 2012:1178–84.
8. Lai S, Zhou H, Xiong W, Gilbert M, Huang Z, Yu J, et al. Changing epidemiology of human brucellosis, China, 1955-2014. Emerg Infect Dis. (2017) 23:184–94. doi: 10.3201/eid2302.151710
9. Guan P, Wu W, Huang D. Trends of reported human brucellosis cases in mainland China from 2007 to 2017: an exponential smoothing time series analysis. Environ Health Prev Med. (2018) 23:23. doi: 10.1186/s12199-018-0712-5
10. Bian ZJ, Zhu LQ, Feng Y, Sun SJ, Qin YM, Ding JB. Epidemiological trends and characteristics of Brucellosis in miscarriage dairy cows in China, 2013-2017. Microbiol China. (2019) 46:618–23.
11. Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. (2002) 90:479–96. doi: 10.1016/S0378-1135(02)00255-9
13. Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. (2013) 2013:743509. doi: 10.1155/2013/743509
14. Hou H, Liu X, Peng Q. The advances in brucellosis vaccines. Vaccine. (2019) 37:3981–8. doi: 10.1016/j.vaccine.2019.05.084
15. Xin X. Orally administrable brucellosis vaccine: Brucella suis strain 2 vaccine. Vaccine. (1986) 4:212–6. doi: 10.1016/0264-410X(86)90131-3
16. Di DD, Jiang H, Tian LL, Kang JL, Zhang W, Yi XP, et al. Comparative genomic analysis between newly sequenced Brucella suis Vaccine Strain S2 and the Virulent Brucella suis Strain 1330. BMC Genomics. (2016) 17:741. doi: 10.1186/s12864-016-3076-5
17. Zhu L, Feng Y, Zhang G, Jiang H, Zhang Z, Wang N, et al. Brucella suis strain 2 vaccine is safe and protective against heterologous Brucella spp. infections. Vaccine. (2016)34:395–400. doi: 10.1016/j.vaccine.2015.09.116
18. Global Times News. 3,245 people have been infected by a brucellosis incident at LVRI (2020). Available online at: https://dy.163.com/article/FMJKVI7O05504DPG.html
20. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. (2006) 6:91–99. doi: 10.1016/S1473-3099(06)70382-6
21. Al Dahouk S, Nöckler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti Infect Ther. (2011) 9:833–45. doi: 10.1586/eri.11.55
22. Sun G, Li M, Zhang J, Zhang W, Pei X, Jin Z. Transmission dynamics of brucellosis: mathematical modelling and applications in China. Comput Struct Biotechnol J. (2020) 18:3843–60. doi: 10.1016/j.csbj.2020.11.014
23. Liu Z, Wang C, Wei C, Zhao Z, Wang M, Li D. Investigation of genetic relatedness of Brucella strains in countries along the Silk Road. Front Vet Sci. (2021) 7:539444. doi: 10.3389/fvets.2020.539444
24. Zhou K, Wu B, Pan H, Paudya N, Jiang J, Zhang L, et al. ONE Health approach to address zoonotic brucellosis: a spatiotemporal associations study between animals and humans. Front Vet Sci. (2020) 7:521. doi: 10.3389/fvets.2020.00521
25. Jiang H, O'Callaghan Ding J. Brucellosis in China: history, progress and challenge. Infect Dis Poverty. (2020) 9: 55. doi: 10.1186/s40249-020-00673-8
26. Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. (2010) 34:379–94. doi: 10.1111/j.1574-6976.2010.00211.x
27. Lalsiamthara J, Lee JH. Development and trial of vaccines against Brucella. J Vet Sci. (2017) 18:281–90. doi: 10.4142/jvs.2017.18.S1.281
28. Jiang H, Dong H, Peng X, Feng Y, Zhu L, Niu K, et al. Transcriptome analysis of gene expression profiling of infected macrophages between Brucellasuis 1330 and live attenuated vaccine strain S2 displays mechanistic implication for regulation of virulence. MicrobPathog. (2018) 119:241–47. doi: 10.1016/j.micpath.2018.04.003
29. Peng X, Liu Y, Qin Y, Jiang H, Feng Y, Sun J, et al. Comparative transcriptome analysis of artificially induced rough-mutant brucella strain RM57 and its parent strain Brucella melitensis M1981. Front Vet Sci. (2020) 6:459. doi: 10.3389/fvets.2019.00459
30. Adetula AA, Liu X, Thi TNT, Bhuiyan AA, Du X, Zhu M, et al. Transcriptional profiling of leucocyte count variation from porcine peripheral blood reveals differential gene expression. Biomed Res Int. (2018) 18:1496536. doi: 10.1155/2018/1496536
31. Majewska A, Gajewska M, Dembele K, Maciejewski H, Prostek A, Jank M. Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis. BMC Vet Res. (2016) 12:174. doi: 10.1186/s12917-016-0805-6
32. Tariq M, Chen R, Yuan H, Liu Y, Wu Y, Wang J, et al. De Novo transcriptomic analysis of peripheral blood lymphocytes from the Chinese goose: gene discovery and immune system pathway description. PLoS ONE. (2015) 10:e0121015. doi: 10.1371/journal.pone.0121015
33. Tang X, Yang S, Sheng X, Xing J, Zhan W. Transcriptome analysis of immune response of mIgM+ B lymphocytes in Japanese flounder (paralichthysolivaceus) to lactococcus lactis in vitro revealed that IFN I-3 could enhance their phagocytosis. Front. Immunol. (2019) 10:1622. doi: 10.3389/fimmu.2019.01622
34. Granata S, Santoro G, Signorini L, Malerba G, Patuzzo C, Gambaro G, et al. Comparative transcriptome analysis of peripheral blood mononuclear cells in renal transplant recipients in everolimus- and tacrolimus-based immunosuppressive therapy. Eur J Pharmacol. (2019) 859:172494. doi: 10.1016/j.ejphar.2019.172494
35. Corbi SCT, de Vasconcellos JF, Bastos AS, Bussaneli DG, da Silva BR, Santos RA, et al. Circulating lymphocytes and monocytes transcriptomic analysis of patients with type 2 diabetes mellitus, dyslipidemia and periodontitis. Sci Rep. (2020) 10:8145. doi: 10.1038/s41598-020-65042-9
Keywords: brucellosis, transcriptome analysis, gene expression, cynomolgus monkey, vaccine
Citation: Sun S, Jiang H, Li Q, Liu Y, Gao Q, Liu W, Qin Y, Feng Y, Peng X, Xu G, Shen Q, Fan X, Ding J and Zhu L (2021) Safety and Transcriptome Analysis of Live Attenuated Brucella Vaccine Strain S2 on Non-pregnant Cynomolgus Monkeys Without Abortive Effect on Pregnant Cynomolgus Monkeys. Front. Vet. Sci. 8:641022. doi: 10.3389/fvets.2021.641022
Received: 13 December 2020; Accepted: 05 February 2021;
Published: 09 March 2021.
Edited by:
Min Yue, Zhejiang University, ChinaReviewed by:
Hui Zhang, Shihezi University, ChinaAlaa Sewid, The University of Tennessee, United States
Copyright © 2021 Sun, Jiang, Li, Liu, Gao, Liu, Qin, Feng, Peng, Xu, Shen, Fan, Ding and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiabo Ding, dingjiabo@126.com; Liangquan Zhu, 1367391894@qq.com
 Shijing Sun
Shijing Sun Hui Jiang1
Hui Jiang1  Yu Feng
Yu Feng Xuezheng Fan
Xuezheng Fan Jiabo Ding
Jiabo Ding Liangquan Zhu
Liangquan Zhu