Structural and Functional Significance of the Endoplasmic Reticulum Unfolded Protein Response Transducers and Chaperones at the Mitochondria–ER Contacts: A Cancer Perspective
- 1Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana,” University of Salerno, Baronissi, Italy
- 2Department of Pharmacy, University of Salerno, Fisciano, Italy
In the last decades, the endoplasmic reticulum (ER) has emerged as a key coordinator of cellular homeostasis, thanks to its physical interconnection to almost all intracellular organelles. In particular, an intense and mutual crosstalk between the ER and mitochondria occurs at the mitochondria–ER contacts (MERCs). MERCs ensure a fine-tuned regulation of fundamental cellular processes, involving cell fate decision, mitochondria dynamics, metabolism, and proteostasis, which plays a pivotal role in the tumorigenesis and therapeutic response of cancer cells. Intriguingly, recent studies have shown that different components of the unfolded protein response (UPR) machinery, including PERK, IRE1α, and ER chaperones, localize at MERCs. These proteins appear to exhibit multifaceted roles that expand beyond protein folding and UPR transduction and are often related to the control of calcium fluxes to the mitochondria, thus acquiring relevance to cell survival and death. In this review, we highlight the novel functions played by PERK, IRE1α, and ER chaperones at MERCs focusing on their impact on tumor development.
Introduction
The endoplasmic reticulum (ER) presides over the biogenesis and maintenance of almost all the cell compartment and participates in the regulation of their functions throughout exchanges of structural molecules and signaling factors. It is well known that the ER is the place where lipid and steroids are synthesized and, then, delivered to other endo-membranes throughout vesicular carriers or non-vesicular mechanisms that, if out of control, result in several pathologies including cancer (Peretti et al., 2019). It is universally recognized that the largest part of the intracellular calcium is stored within the ER and that different ER integral membrane proteins control Ca2+ homeostasis by modulating either the ion uptake or its delivery to the neighboring compartments (Putney, 2005; Clapham, 2007). Remarkably, calcium is engaged in the managing of several pathophysiological processes having an important impact in human malignancies development (Limia et al., 2019; Reich et al., 2020).
On the other hand, a large portion of the ER is associated to protein synthesis and translocation. The ER lumen is equipped with a molecular machinery that carries out a rigorous chaperone-mediated quality control (QC) of protein folding to ensure that proteins are delivered in a functional state to secretory compartments. QC is under the regulation of the unfolded protein response (UPR): a battery of signaling pathways that, when excessive protein unfolding occurs is able to choose between cell death and survival, this choice affecting either normal or cancer cells. This is why the participation of the UPR in tumorigenesis has long been studied. Moreover, the UPR has always been considered a strategic target for potential cancer therapy for its consequence on neoplastic cell proliferation and survival (Wang and Kaufman, 2014). Between the mitochondria and the ER, signals and molecules are rapidly exchanged throughout the molecular structures known as mitochondria–ER contacts (MERCs), also called mitochondria-associated membranes (MAMs) (Vance, 2014). A growing evidence shows that both UPR transducers and chaperones are structural components involved in various ways in the MERCs functions (Ilacqua et al., 2017). In this review, we aim to outline the role of UPR actors in the MERCs functions and to make a point on the consequence of their presence in MERCs in the fate of cancer cells.
The UPR Signaling Pathway
The control of lipid and protein flux toward the secretory pathway compartments is an essential function of the ER, which is the site of entrance, folding, and departure of luminal and integral membrane proteins destined to the ER itself, Golgi apparatus, endo-lysosomal compartment, the plasma membrane (PM), or the extracellular environment (Walter and Schultz, 1981; Jan et al., 2014; Fasano et al., 2018).
Quantitatively, ER exit relies on an autoregulatory system consisting of a set of signals that, in case of overload of newly synthesized cargo proteins, modulate protein export by regulating their packaging at the ER exit sites (ERESs) into COPII anterograde vesicles and by attenuating protein synthesis to prevent traffic congestion of cargo proteins (Subramanian et al., 2019).
Qualitatively, within the ER, a sophisticated machinery of enzymes and chaperones has evolved to retain unfunctional proteins within the organelle or to direct them to ubiquitin/proteasome degradation (Hartl and Hayer-Hartl, 2009; Ruggiano et al., 2014). QC of protein folding is controlled by the UPR pathways made of a battery of ER transmembrane transducers consisting of the pancreatic ER kinase (PERK), the inositol-requiring enzyme 1 (IRE1), and the activating transcription factor 6 (ATF6) (Schroder and Kaufman, 2005). UPR activation occurs because, normally, each transducer is kept inactive by the chaperone glucose-regulated protein 78-kDa/binding immunoglobulin protein (GRP78/BiP). Instead, when unfolded proteins increase within the ER lumen, GRP78/BiP releases IRE1, PERK, and ATF6 synchronizing their activity (Figure 1). The UPR orchestrates a vigorous expression of ER enzymes and chaperones such as calreticulin (CLR), calnexin (CLNX), GRP78/BiP, and the ER Protein 57-kDa (ERp57) with the purpose to potentiate QC machinery (Sitia and Braakman, 2003; Amodio et al., 2009). Moreover, under ER stress, the UPR activates genes encoding vesicular trafficking factors and limitates the ER exit of secretory proteins (Renna et al., 2006; Amodio et al., 2009, 2011). Furthermore, the UPR boosts up the clearance of uncorrectly folded proteins, which would otherwise compromise cell functions, by fine tuning either the ER-associated protein degradation (ERAD) (Meusser et al., 2005) or autophagy (Simmen and Herrera-Cruz, 2018).
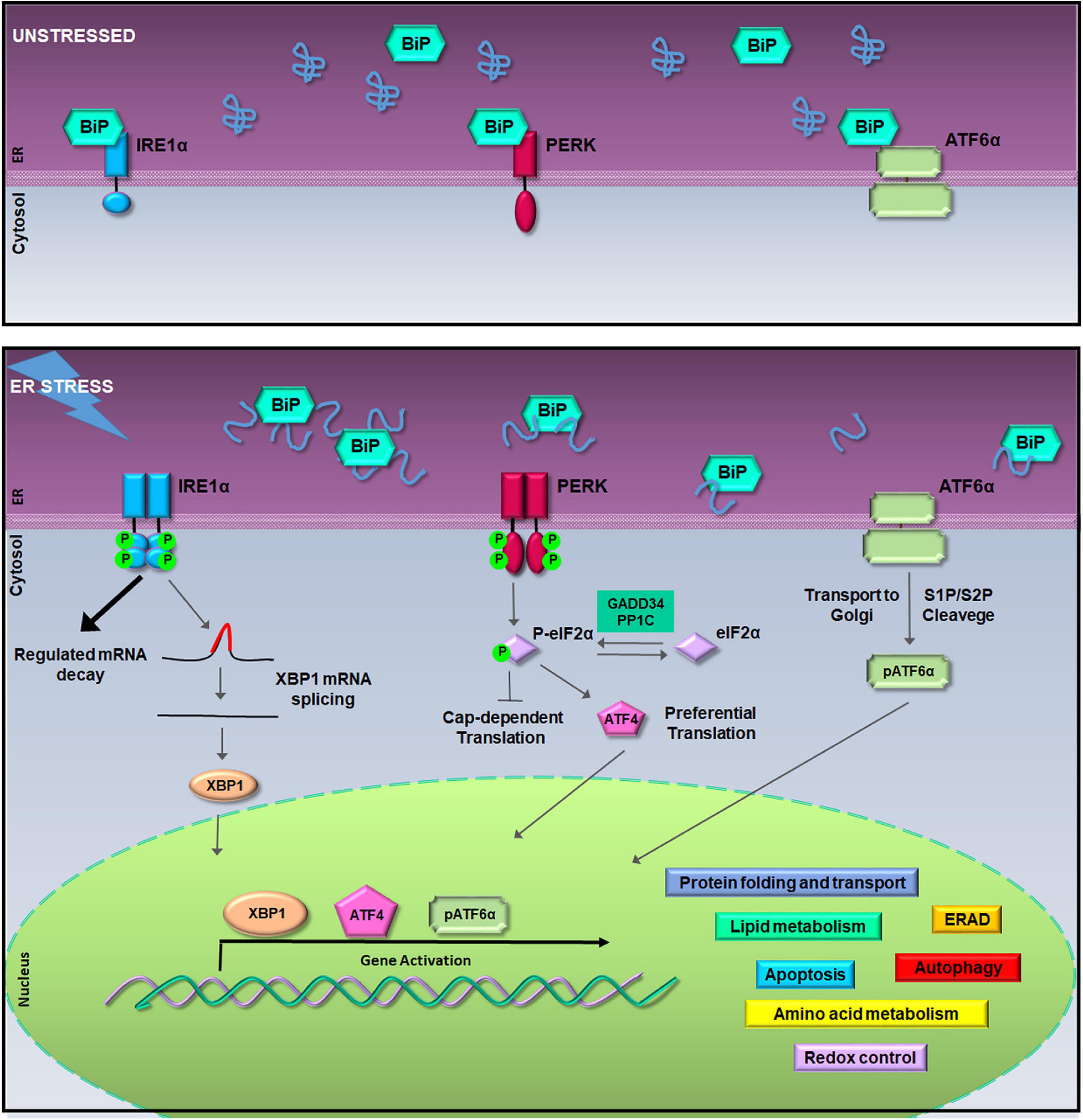
Figure 1. The unfolded protein response (UPR) signaling pathway. In unstressed conditions (upper box), the activation of inositol-requiring enzyme 1a (IRE1α), pancreatic ER kinase (PERK), and activating transcription factor 6a (ATF6α) is inhibited by the binding of Bip/GRP78. During the endoplasmic reticulum (ER) stress, binding of glucose regulated protein 78-kDa/binding immunoglobulin protein (GRP78/BiP) to misfolded proteins allows the activation of IRE1α, PERK, and ATF6α (lower box). Activated IRE1α cleaves 26-nucleotides from the X-box binding protein 1 (XBP1) mRNA allowing the translation of XBP1; hyper-oligomerized IRE1α executes regulated IRE1a-dependent decay (RIDD) activity on selected cytosolic mRNAs (Cox and Walter, 1996; Hollien et al., 2009). Activated PERK phosphorylates the eukaryotic initiation factor 2a (eIF2α) leading to attenuation of protein synthesis and to the preferential translation of activating transcription factor 4 (ATF4) mRNA (Harding et al., 2000). ATF6α activation is achieved in the Golgi complex where it undergoes to intramembrane proteolysis-specific cleavage by site-1 protease (S1P) and S2P to produce a transcriptionally active fragment (pATF6α). XBP1, ATF4, and pATF6α are responsible for the execution of the UPR transcriptional program in the nucleus (Walter and Ron, 2011).
At the same time, to limit the amount of unfolded proteins entering the ER, PERK phosphorylates the eukaryotic initiation factor 2α (eIF2α) producing p-eIF2α that acts as a suppressor of protein translation initiation (Harding, 1999; Zhao and Ackerman, 2006). This event reduces protein translocation into the ER initiating the ER folding machinery. In fact, besides the reduction of protein load, p-eIF2α allows the selective translation of a subset of mRNAs (Ma and Hendershot, 2003). Among them, activating transcription factor 4 (ATF4) is preferentially translated to regulate an adaptive response that activates transcription of ER chaperones and ER stress response genes, such as GRP78/BiP, and of genes involved in the amino acid metabolism, mitochondrial function (Ameri and Harris, 2008), autophagy (Rouschop et al., 2010), and in the oxidative stress response (Harding et al., 2000; Blais et al., 2006; Walter and Ron, 2011) (Figure 1). In particular, the ATF4 antioxidant program culminates with the PERK-dependent activation of Nrf2, a transcription factor whose target genes are involved in the antioxidant defense (Cullinan and Diehl, 2006). However, aside from PERK, eIF2α phosphorylation is carried out by other kinases such as the protein kinase R (PKR), general control non-derepressible 2 (GCN2), and heme-regulated inhibitor (HRI), which work as transducers of the stress signaling known as the integrated stress response (ISR) (Pakos-Zebrucka et al., 2016).
On the other hand, when ER stress is persistent, the UPR survival project turns into the cell death program (Zhang and Kaufman, 2006; Mori, 2009; Diehl et al., 2011; Walter and Ron, 2011) switching the PERK-eIF2α-ATF4 signaling to promote the activation of the transcription factor C/EBP homologous protein (CHOP) (Urra et al., 2013). Under these circumstances, both CHOP and ATF4 cooperate to induce amino acid biosynthetic pathways that revert translational repression, promote oxidative stress, and the expression of pro-apoptotic factors thereby leading to cell death (Marciniak et al., 2004; Han et al., 2013; Urra et al., 2013). Instead, the PERK–CHOP/growth arrest- and DNA damage-inducible gene 153 (GADD153) route is crucial for the apoptosis initiation (Zinszner et al., 1998; Rutkowski et al., 2006). This happens by activating proapoptotic factors that drive apoptosis, such as the death receptor 5 (DR5), the Tribbles ortholog in humans (Trb3), Bcl-2-interacting mediator of cell death (BIM), and P53-upregulated modulator of apoptosis (PUMA) (Yamaguchi and Wang, 2004; Ohoka et al., 2005; Puthalakath et al., 2007; Cazanave et al., 2010). Notably, CHOP also induces the regulatory subunit of GADD34, which dephosphorylates eIF2α and initiates a negative feedback that restores mRNA translation and protein synthesis. Moreover, CHOP and GADD34 knockout preserves tissues from ER stress-mediated damage, intensifies protein misfolding, and leads to apoptosis (Marciniak et al., 2004).
A similar shift from pro-survival to pro-apoptotic activity happens to IRE1α transducer, which owns both kinase and RNAse activity (Wang et al., 1998). During ER stress, freed from GRP78/BiP, IRE1α undergoes sequential autophosphorylation, conformational change, and higher order oligomerization that activates its RNase domain (Korennykh et al., 2009; Gardner and Walter, 2011). The main target of IRE1α RNase activity is the X-box-binding protein 1 (XBP1) mRNA, whose splicing allows translation of the XBP1 transcription factor that, once in the nucleus, upregulates genes implicated in the QC and cell survival, including ER chaperones and ERAD factors (Cox and Walter, 1996). However, during persistent ER stress, IRE1α hyper-oligomerization enhances RNase activity of specific mRNAs encoding ER resident proteins, an event known as regulated IRE1α-dependent decay (RIDD) (Hollien et al., 2009; Ghosh et al., 2014) (Figure 1). Therefore, RIDD activity first promotes cell survival by limiting the number of proteins entering the ER, but then, during irresolvable ER stress, it favors apoptosis through the degradation of anti-apoptotic microRNAs, for instance, those that control TXNIP and caspase-2 expression (Lerner et al., 2012; Upton et al., 2012). Thus, RIDD activity along with the TRAF2–ASK1–JNK pathway activation, also due to enhanced kinase activity of hyper-oligomerized IRE1α, switches the IRE1α signaling from survival to apoptosis during unresolved ER stress (Urano et al., 2000; Nishitoh et al., 2002).
It is universally acknowledged that the UPR plays critical roles in tumor progression, survival, and metastasis (Romero-Ramirez et al., 2004; Lee, 2007; Moenner et al., 2007). Moreover, in cancer cells, the UPR is often activated, and ER chaperones are overexpressed in response to various environmental conditions including hypoxia, oxidative stress, and nutrient starvation (Blais et al., 2006; Luo and Lee, 2013). On these grounds, and based on growing evidences showing that UPR transducers and their related chaperones are involved in various ways in the MERCs structure, we will discuss the functional significance of their residence at MERCs and their role in the pathogenesis of cancer.
The Membrane Contact Sites
The ER shows a complex membrane architecture consisting of plane sheets in continuity with the nuclear envelope (NE) and peripheral membranes made of tubules that owe their shape to endogenous, or also exogenous, proteins that form and stabilize tubules at the ER sheet boundaries (Shibata et al., 2010; Friedman et al., 2011; Voeltz and Barr, 2013; Westrate et al., 2015; Grimaldi et al., 2018). While ER sheets are involved in protein synthesis, the ER tubular network is free of ribosomes and extremely dynamic, as it rapidly expands and eventually re-modulates by repetitive fission and fusion events (Lee and Chen, 1993). Tubules may efficiently move since they are interconnected with microtubules throughout motor proteins (namely, kinesins and dyneins) and, thereof, they can reach almost all the cell components thus facilitating the development of several connections known as membrane contact sites (MCS) (Phillips and Voeltz, 2016). MERCs were the first inter-organelle connections discovered (Bernhard and Rouiller, 1956; Vance and Shiao, 1996) followed by ER–PM (Stefan et al., 2013), ER–Golgi (Hanada et al., 2003; Peretti et al., 2008), ER–peroxisomes (Costello et al., 2017), and ER–lipid droplets (LDs) (Walther et al., 2017) disclosing that organelles rely on MCSs for many interdependent functions. MCSs are distributed along ER membrane tubules and are overall stable, since they are preserved during trafficking and membrane fusion and fission (Wu et al., 2018).
Findings on MCSs overturned the view of the cell seen like a complex of isolated compartments and acknowledged the ER, the role of intermembrane network that homeostatically controls exchange of several structural and bioactive molecules (Jan et al., 2014; Phillips and Voeltz, 2016). Despite this, so far, little is still known about the role of MCSs in pathogenic processes including cancer.
Mitochondria–ER contacts are spaced apart from 10 to 80 nm (Krols et al., 2016; Sezgin et al., 2017; Chakkarapani et al., 2018; Moltedo et al., 2019) suggesting that the two organelles are kept together by tethering factors, holding the two opposite membranes in close proximity. Among them is Mitofusin 2 (Mfn2), a large GTPase usually involved in outer mitochondrial membrane (OMM) fusion events. Mnf2 interacts in trans with Mfn1 or Mfn2 forming hetero- or homo-dimers that hook mitochondria to the ER. Another tethering complex is formed by the vesicle-associated protein B (VAPB), an ER membrane protein that interacts with the OMM protein tyrosine phosphatase-interacting protein-51 (PTPIP51). In addition, at MERCs, the ER calcium-release channel inositol 1,4,5-trisphosphate receptor (IP3R) interacts with the voltage-dependent anion channel (VDAC) located at the OMM, through the molecular chaperone glucose-regulated protein 75-kDa (GRP75), and such a complex regulates Ca2+ flux from the ER to the mitochondria (Moltedo et al., 2019).
In this context, MERCs provide fundamental platforms for several cellular functions, such as phospholipid synthesis and exchange, Ca2+ flows, mitochondrial fission, and apoptosis. The homeostatic control of Ca2+ occurring at these sites has constantly attracted particular interest, and MERCs are growingly emerging as the residence of the regulation of cancer cell onset, growth, progression, and metabolism (Herrera-Cruz and Simmen, 2017). Indeed, altered Ca2+ signaling at the MERCs is considered a characteristic of cancer cells because it can shift cell metabolism to glycolysis and increases cancer cell resistance to cell death (Herrera-Cruz and Simmen, 2017). Cancer cells fundamentally require basal mitochondrial Ca2+ uptake for survival, thus lowering ER-to-mitochondria Ca2+ transfer causing cancer cell death (Cardenas et al., 2016). In contrast, mitochondrial Ca2+ overload can lead to the opening of mitochondrial permeability transition pore (PTP). As a consequence, PTP induction leads to the increase of IMM permeability and the loss of mitochondrial membrane potential, which, in turn, induces mitochondrial swelling, OMM break, cytochrome c release, and cell death (Rizzuto et al., 2012). For this reason, Ca2+ accumulation in the mitochondrial matrix has important implications also in other processes, including autophagy, metabolism, and apoptosis (Morciano et al., 2018). In particular, apoptosis is closely connected to calcium status of mitochondria (Csordas et al., 2006) and tumorigenesis. In addition, it has been noted that conditions that prevent massive Ca2+ transfer from the ER to the mitochondria through the regulation of Ca2+ channel activity, protect from cell death normal cells, but can lead to neoplastic transformation (Rizzuto et al., 2012). It is also worth noting that numerous regulators of Ca2+ flux acting as tumor suppressors and oncogenes have been recently identified as MERC proteins (Marchi and Pinton, 2014; Bittremieux et al., 2016).
Mitochondrial Ca2+ flux is also regulated by the mitochondrial calcium uniporter (MCU) complex (Raffaello et al., 2012; Marchi and Pinton, 2014). This channel is located at the inner mitochondrial membrane (IMM) and is composed of uniporter pore-forming subunits MCU, MCUb, and the essential MCU regulator (EMRE). Two regulatory proteins (MICU1 and MICU2) act as a gatekeeper. Normally, MICU2 keeps the channel closed, but when the Ca2+ level is higher, Ca2+ MICU2 undergoes to a conformational change and dissociates from the complex. MICU2 release produces the loss of the gatekeeping function and formation of the MICU1–MICU1 homodimer that improves mitochondrial calcium uptake (Patron et al., 2014). Interestingly, dysfunction in one or more MCU complex subunits has been associated to different cancers (Marchi et al., 2019).
Pancreatic ER Kinase at Mitochondria–ER Contacts: Structural Role and Modulation of Cancer Progression
Since its discovery, PERK localization at MERCs has suggested a non-canonical mechanism through which this protein could communicate with mitochondria to drive cell death. In an exemplary work, it has been shown that PERK localizes at MERCs to establish structural and functional connection with mitochondria thus facilitating the propagation of ROS-based stress signals and apoptotic signals from the ER (Verfaillie et al., 2012) (Figure 2A,i). Instead, cells lacking PERK show less solid ER–mitochondria contacts, and as a consequence, cells are less sensitive to cell death in response to ER-derived ROS. In addition, these studies show that since a kinase mutant of PERK is still maintained at MERCs, the tethering function of MERC-localized PERK is independent of its UPR transducer activity (Verfaillie et al., 2012; Cao and Kaufman, 2014).
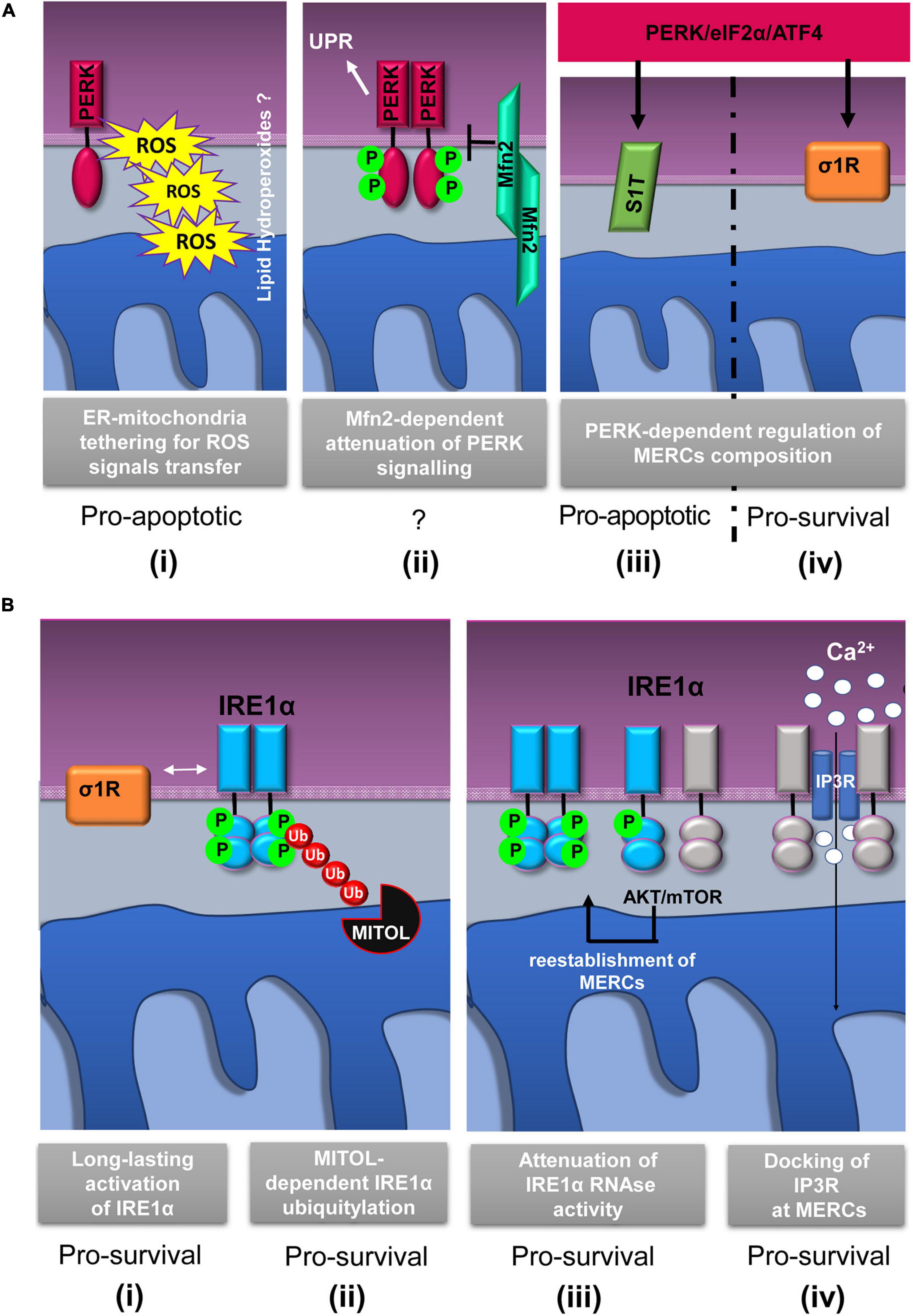
Figure 2. Schematic representation of relevant events involving IRE1α and PERK proteins at mitochondria–ER contacts (MERCs). (A) PERK-involving events at MERCs: (i) PERK tethers the ER to mitochondria promoting the rapid transfer of reactive oxygen species (ROS) signals, likely under the form of lipid hydroperoxides (Verfaillie et al., 2012); (ii) Mitofusin 2 (Mfn2) lies upstream of PERK and under basal conditions maintains PERK inactive (Munoz et al., 2013); the PERK/eIF2α/ATF4 signaling pathway upregulates the expression of SERCA1 truncated proteins (S1T) (iii) and sigma-1 receptor (σ1R) (iv) at MERCs (Chami et al., 2008; Mitsuda et al., 2011). (B) Events involving IRE1α at MERCs: (i) σ1R-dependent stabilization of IRE1α oligomerization at MERCs (Mori et al., 2013); (ii) mitochondrial ubiquitin ligase (MITOL)-dependent ubiquitylation of IRE1α at MERCs prevents ER stress induced apoptosis (Takeda et al., 2019); (iii) the AKT-mammalian target of rapamycin (mTOR) signaling attenuates IRE1 RNase activity by promoting the re-establishment of ER-mitochondria contacts (Sanchez-Alvarez et al., 2017); (iv) IRE1α scaffolds IP3R at MERCs to sustain calcium transfer and mitochondrial bioenergetics (Carreras-Sureda et al., 2019). The putative pro-survival or pro-apoptotic outputs of the depicted events are reported.
It is important to consider that ER stress can be communicated to the mitochondria through MERCs and in particular by the PERK-mediated branch of the UPR (Chami et al., 2008; Mitsuda et al., 2011; Munoz et al., 2013; van Vliet and Agostinis, 2016; Saito and Imaizumi, 2018) thanks to the PERK-induced overexpression of a truncated variant of the ER Ca2+ ATPase (SERCA1), known as S1T, which is located at MERCs (Figure 2A,iii). S1T upregulation at MERCs is concomitant to mitochondrial Ca2+ overload, increased number of MERCs, inhibition of mitochondrial dynamics, and, in the end, apoptosis (Chami et al., 2008).
Similarly, further research has shown that, under basal conditions, PERK signaling could be inhibited by the interaction to Mfn2 at MERCs (Munoz et al., 2013) (Figure 2A,ii). As proof of this, it has been observed that Mfn2 ablation causes a sustained activation of PERK in unstressed cells and that, unexpectedly, PERK silencing rescues ROS production, mitochondrial Ca2+ overload, and defective mitochondrial morphology in Mfn2-deficient cells. On these grounds, it is very likely that Mfn2 could act as an upstream regulator of the PERK signaling and that, strikingly, such a regulation occurs at MERCs suggesting that MERCs can be seen as hot spots for the stress signaling transmission and modulation.
Interestingly, more recently, an additional role of PERK in the control of mitochondrial functions has emerged that strengthens the importance of its localization at MERCs. Indeed, PERK is required to modulate mitochondrial morphology (Munoz et al., 2013). In particular, PERK promotes protective stress-induced mitochondrial hyperfusion (SIMH) in order to prevent mitochondrial fragmentation and favor mitochondrial metabolism in response to ER stress (Lebeau et al., 2018). Furthermore, in line with these evidence, it is reported that PERK is involved in the remodeling of mitochondrial inner membranes and in the ATF4-mediated upregulation of the super complex assembly factor (SCAF), a protein responsible for the anchoring of the respiratory chain complexes to the IMM with the effect of enhancing ATP production during ER stress (Balsa et al., 2019).
To sum up, these data suggest that PERK is involved in the control of mitochondrial bioenergetics and morphology occurring at MERCs. However, since these effects have been described in ER stress conditions or nutrient deprivation, additional studies need to be carried out in order to understand whether these effects are independent by the PERK role in the UPR.
As promoter of the antioxidant defense, the PERK pathway is widely involved in cancer initiation and progression. Indeed, the higher rate of metabolism required to support malignant expansion exposes cancer cells to excessive production of ROS and self-cytotoxic stimuli, which, for cancer cell survival, is the main obstacle to deal with. In this harmful microenvironment, cancer cells develop adaptive mechanisms to escape apoptosis, among them, the utilization of the PERK pro-survival signaling (Ma and Hendershot, 2004; Bi et al., 2005). In particular, PERK-driven Nrf2 activation runs regeneration of intracellular antioxidants, neutralization of ROS, defense from oxidative DNA damage, cell cycle checkpoint inhibition, and significant improvement of tumor growth (Luo and Lee, 2013). In most cases, cancer cells show a higher level of phospho-PERK and phospho-eIF2α (Avivar-Valderas et al., 2011; Cubillos-Ruiz et al., 2017; Feng et al., 2017; Li et al., 2018). For example, elevated levels of p-eIF2α are reported in bronchioloalveolar and gastrointestinal carcinoma, Hodgkin’s lymphoma, as well as in benign and malignant melanocytic and colon epithelial neoplasms (Lobo et al., 2000; Rosenwald et al., 2001, 2003, 2008). It has been remarked that the p-eIF2α level is correlated to a disease-free survival and better prognosis in triple negative breast cancer (TNBC) patients (Guo et al., 2017). Additionally, as demonstrated in K-Ras-transformed tumor cells, PERK can induce the expression of different pro-angiogenic factors in cells subjected to hypoxia. This event favors the formation of microvessels, hence, the adaptation of cancer cells to hypoxic stress (Blais et al., 2006). Accordingly, the PERK/ATF4 axis induces VEGF expression and, thereof, stimulates endothelial cells survival and angiogenesis in hostile microenvironments (Ghosh et al., 2010). Moreover, PERK activity is required for epithelial-to-mesenchymal transition (EMT) usually employed by cancer cells to migrate and metastasize (Denard et al., 2012; Feng et al., 2017). In particular, the PERK pathway axis mediates the upregulation of CREB3L1 in the mesenchymal subtype of TNBC resulting in enhanced metastasis and poor prognosis. In this regard, it is important to underline that the inhibition of CREB3L1 was proposed as a better therapeutic strategy since the pharmacological inhibition of PERK is known to cause severe side effects on pancreas metabolism (Feng et al., 2017).
On these grounds, by both enhancing angiogenesis and EMT, PERK is strictly correlated to cancer invasion and metastasis. Accordingly, PERK knockout reduces tumor growth and impairs angiogenesis and metastasis spread (Gupta et al., 2009). In addition, it has been shown that in de-differentiated malignant cells, the PERK/Nrf2 signaling is constitutively activated to protect from chemotherapy by reducing ROS levels and increasing drug efflux, thus implying PERK in the mechanisms of multidrug resistance (MDR) (Del Vecchio et al., 2014). Research also reports that resistant phenotypes are due to PERK/Nrf2-induced expression of the plasma-membrane transporter MDR-related protein 1 (MRP1), while disrupted PERK-to-Nrf2 axis reverses resistance to chemotherapy (Salaroglio et al., 2017). However, a large body of literature also correlates PERK signaling to antitumor activity since PERK can direct the UPR toward activation of death-triggering pathways. For an example, in human osteosarcoma (OS), one of the most common malignant tumors in children, decreased level of p-eIF2α is found, as in a recent work by Wang et al. showing that PERK enhances apoptotic OS cell death in response to lexibulin (Wimbauer et al., 2012; Wang et al., 2019). Moreover, in hepatocellular carcinomas (HCC), the induction of PERK/eIF2α/ATF4 signaling following pterostilbene treatment is associated with HCC cell death and reduced tumor growth (Yu et al., 2019). Accordingly, in human lung adenocarcinomas, paraquat induces PERK/eIF2α/ATF4 signaling leading to cancer cells apoptosis (Wang et al., 2018), and similarly, the use of PERK kinase inhibitors in vivo potentiates the chemioterapics in colon cancer cells (Shi et al., 2019; Wu et al., 2019). Thereby, PERK signaling, as also seen for the IRE1α pathway (see further), seems to have both pro-survival and antitumor effects in different carcinogenesis stages and types with paradoxical outcomes in cancer cells. One interesting theory has been recently proposed in melanoma cells to explain the conflicting role exerted by PERK in tumor initiation and progression (Pytel et al., 2016), whereby PERK is a “haplo-insufficient tumor suppressor,” and gene dose determines tumor-suppressive versus tumor-promoting properties in melanoma, where retention of one allele of PERK seems essential for tumor progression, opposed to the deletion of two alleles, which generates diametrically opposing results (Pytel et al., 2016).
Conclusively, the accumulating evidence of the potential therapeutic use of novel UPR inhibitors is currently paving the way for clinical trials (Cubillos-Ruiz et al., 2017; Hsu et al., 2019; Raymundo et al., 2020). In particular, since the inhibition of PERK sensitizes cancer stem cells to apoptosis (Fujimoto et al., 2016), an important aspect of these promising therapies could be the inhibition of cancer stem cell proliferation, which is responsible for post-therapy tumor relapse in oncological patients (Li et al., 2008).
However, the contribution of PERK to cancer development is still contradictory, and it is still unclear whether it promotes or, conversely, suppresses tumorigenesis. Currently, the involvement of MERC-localized PERK to the mechanisms of cancer initiation, progression, and metastasis is not as well documented in the literature, as is for MERCs-localized IRE1α (see below). Nevertheless, it can be assumed that the role played by PERK at MERCs in the transmission of ROS-derived signals between ER and mitochondria is more significant for cancer cells in the mechanism of adaptation to oxidative stress and resistance to apoptosis. The modulation of PERK activity by Mfn2 at MERCs, in basal conditions, should be further investigated in cancer cells to understand whether Mfn2-dependent deregulation of PERK activity occurs at MERCs during carcinogenesis. At the same time, the non-canonical role performed by PERK at MERCs in the control of mitochondria bioenergetics could be strategic for cancer cells to overcome nutrient deprivation and adapt to hypoxic conditions. Altogether, increased knowledge about the contribution of MERC-localized PERK in cancer initiation and progression could considerably impact human studies and clinical therapies not only for cancer cell elimination, but for many other disorders correlated to PERK such as diabetes, neurodegenerative, or heart diseases (Amodio et al., 2019; Poplawski et al., 2019).
Dynamics of the Ire1α Activity at Mitochondria–ER Contacts and Deregulation in Cancer
Since the discovery of the IRE1α presence at MERCs (Mori et al., 2013), it is clear that crucial events of the IRE1α regulation occur at MERCs. Initial works demonstrated that the Sigma-1 receptor (σ1R) stabilizes IRE1α at MERCs allowing a long-lasting dimeric conformation to efficiently splice the XBP1 mRNA and promote cell survival in response to mitochondria-derived ROS (Mori et al., 2013) (Figure 2B,i). In addition, more recently, the MERCs’ contribution in promoting IRE1α- pro-survival signaling has been reinforced by the finding that, at MERCs, mitochondrial ubiquitin ligase (MITOL/MARCH5) (Figure 2B,ii) prevents ER stress-induced apoptosis through IRE1α ubiquitylation and degradation (Takeda et al., 2019). Previous work showed that restoration of ER–mitochondria contacts by the AKT–mTOR signaling attenuates IRE1 RNase activity, thereby favoring the recovery from ER stress (Sanchez-Alvarez et al., 2017) (Figure 2B,iii).
In the light of these results, it was proposed that MERC stabilization limits IRE1α RNase activity that could lead to cell damage and apoptosis. It is assumed that this event could occur by a “timing mechanism” and throughout different ways. First, MERC reinstallation could destabilize IRE1α oligomers by modifying ER membrane fluidity; second, localization of IRE1α at MERCs could change its redox state and alter its endonuclease activity, and third, MERC-localized PPM1L and PP2A phosphatases could dephosphorylate and deactivate IRE1α (Sanchez-Alvarez et al., 2017).
Taken together, these data highlight that IRE1α activity modulation during ER stress occurs at MERCs. At MERCs, another IRE1α function, independent of its role in the UPR, has emerged (Carreras-Sureda et al., 2019), and recently, it was suggested that MERC-located IRE1α could act as a scaffolding protein for the IP3Rs to support Ca2+ transfer and mitochondrial bioenergetics (Figure 2B,iv). This idea is in agreement with previous evidences showing that, in non-stressed condition, reduced IRE1α activity appears to induce mitochondrial ROS production and cell death by accelerating ER-to-cytosolic efflux of Ca2+ through IP3Rs (Son et al., 2014). However, the observations just discussed confirm that IRE1 function at MERCs could be independent of its classic activity of UPR transducer.
Given its role in the control of cell death or survival, IRE1α involvement in cancer initiation and progression is not surprising. Different cancers are associated to IRE1α deregulation including leukemia, glioblastoma, myeloma, prostate, and breast cancers (Lhomond et al., 2018; Logue et al., 2018; Zhao et al., 2018; Sheng et al., 2019). Altered IRE1α expression is reported in different cancer types including bladder urothelial carcinoma, glioblastoma, cervical squamous cell carcinoma, renal clear cell carcinoma, and mesothelioma (Chandrashekar et al., 2017; Tang et al., 2017). In many tumors, IRE1 activation is independent on its expression level, especially considering the opposite consequence of XBP1 mRNA splicing and RIDD activity. In fact, altered XBP1 expression and RIDD activity has been extensively analyzed in several human cancers with controversial results since both pro-tumorigenic and tumor-suppressor activity of IRE1α have been reported (Blazanin et al., 2017; Bujisic et al., 2017; Logue et al., 2018). As an example, in glioblastoma multiform (GM), higher IRE1α activity is associated to poor prognosis due to XBP1-dependent promotion of angiogenesis and macrophage recruitment (Lhomond et al., 2018). Accordingly, higher XBP1 splicing has been found in several hematological malignancies and solid tumors as a negative prognostic factor (Chen et al., 2014; Yang et al., 2015; Xia et al., 2016; Sun et al., 2018). In TNBC, XBP1 hyperactivation promotes an adaptive response to ER and hypoxic stress through the regulation of hypoxia-inducible factor 1α (HIF1α) leading to enhanced cancer cell survival and decreased patient survival, as well as promoting TNBC resistance to paclitaxel and doxorubicin treatments (Chen et al., 2014; Hu et al., 2015). In addition, XBP1 was found to contribute to oncogenicity in prostate cancer and TNBC through the regulation of c-myc expression (Zhao et al., 2018; Sheng et al., 2019).
All these data indicate an oncogenic role for XBP1, though evidence shows a tumor-suppressor function of RIDD activity. De facto, RIDD activation in GM is associated with inhibition of angiogenesis and cell migration, and a longer survival of patients compared to tumors with higher XBP1 and lower RIDD activity showing more aggressiveness and a mesenchymal phenotype (Lhomond et al., 2018). Moreover, in another in vitro model of Ras-driven cancer, XBP1 was found to promote survival, whereas RIDD was found to suppress either survival or transformation of keratinocytes (Blazanin et al., 2017).
Altogether, these data suggest a dual role of IRE1 RNase function in tumor development depending on the prevalence of XBP1 splicing or RIDD activity. Currently, no works have analyzed the localization of IRE1 in cancer cells, in particular at MERCs, although as we have previously discussed, MERCs play an essential role in the regulation of the pro-survival or pro-apoptotic outputs of IRE1 RNase activity. In this scenario, we consider it very plausible that in cancer cells, MERCs could influence IRE1 oncogenic or tumor-suppressor activity. Moreover, the MERC modulation of IRE1α activity could be an important strategy to develop anticancer therapy. Indeed, the dual opposite outputs of IRE1 RNase activity represent a great challenge in defining the right therapy for XBP1 splicing inhibition and to promote RIDD activity, depending on the cancer type. In this view, the control of IRE1 localization at MERCs in cancer cells could represent an additional therapeutic approach. An important work showed that the IRE1α RNase inhibitor MKC8866 reduced the synthesis and secretion of protumorigenic cytokines in TNBC cells and increased paclitaxel-mediated tumor suppression in xenograft mouse models of TNBC (Logue et al., 2018). In these circumstances, in vitro mammosphere formation was also reduced, suggesting an important inhibitory effect of MKC8866/paclitaxel therapy on the proliferation of cancer stem cells. Accordingly, XBP1 expression was correlated to cancer stem cell expansion and tumor relapse post therapy (Chen et al., 2014).
Considerable evidence has revealed that ER chaperones such as GRP78/BiP, GRP75, CLNX, and ER oxidoreductases, such as σ1R or Ero1α, are components of MERCs (Anelli et al., 2012; Lynes et al., 2012; Rizzuto et al., 2012; Sassano et al., 2017), and all these ER stress factors are central players in the control ER–mitochondria calcium flux (Simmen et al., 2010). In the following sections, we discuss the role of MERC-located chaperones and their impact in tumorigenesis.
Pro-Tumorigenic Function of σ1R During Regulation of Ca2+ Homeostasis at Mitochondria–ER Contacts
The sigma-1 receptor, σ1R, is a 26-kDa integral ER membrane protein found in several tissues (Tesei et al., 2018). Many studies showed that σ1R is located at MERCs where it forms a complex with GRP78/BiP to modulate Ca2+ signaling between the two organelles (Hayashi and Su, 2007; Tesei et al., 2018). Following ER Ca2+ release, σ1R dissociates from GRP78/BiP and binds to IP3Rs (Figure 3A), which is thus stabilized because this interaction prevents IP3Rs degradation and prolongs mitochondria Ca2+ uptake (Hayashi and Su, 2007; Rizzuto et al., 2012).
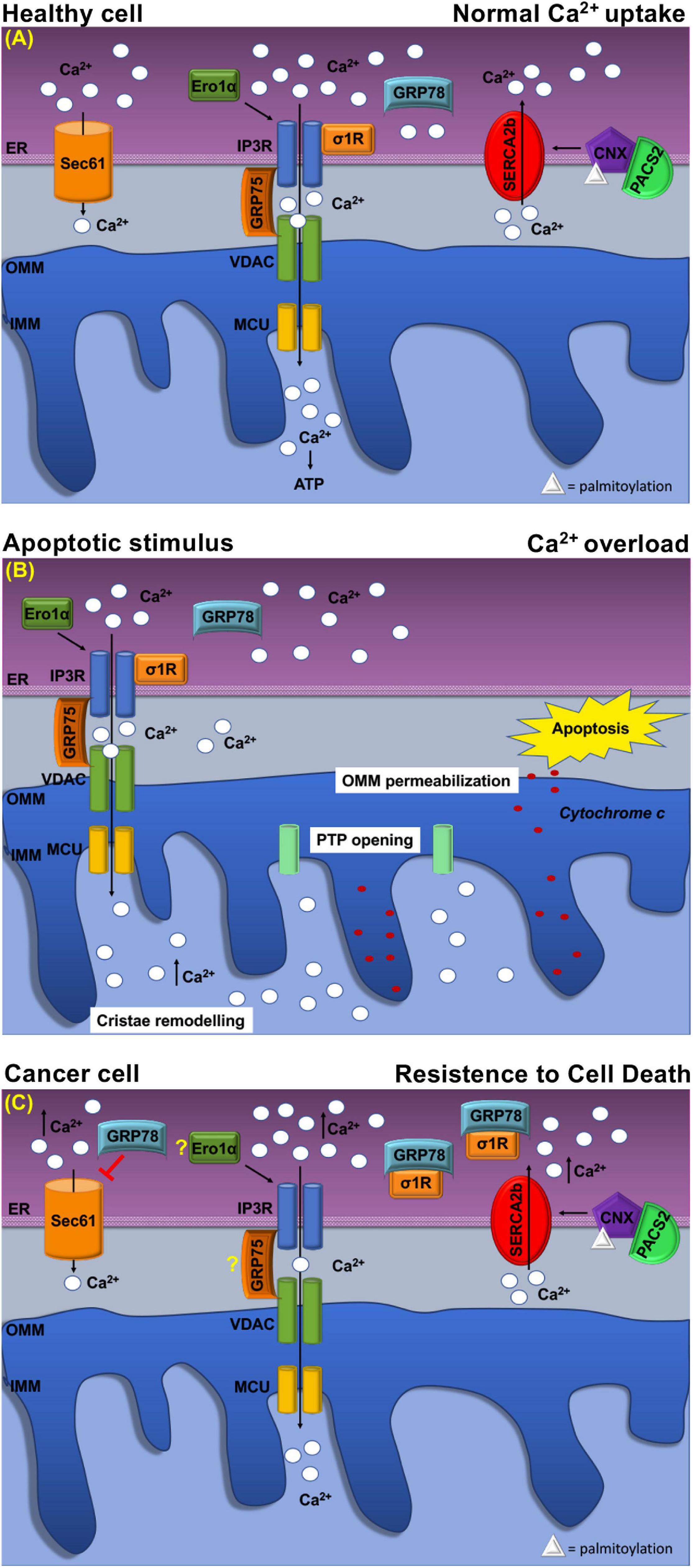
Figure 3. Schematic representation of Ca2+ handling at MERCs in healthy cell and cancer cell. (A) Healthy cell: The ER Ca2+ load is determined by ER Ca2+ uptake systems sarco-ER Ca2 + transport ATPases (SERCAs) and Ca2+-binding protein calnexin (CLNX), which is targeted to MERCs following a phosphofurin-acidic cluster sorting protein 2 (PACS2)-dependent palmitoylation to maintain high Ca2+ levels with the ER. Ca2+ is released from the ER through the activation of inositol 1,4,5-tris phosphate receptors (IP3Rs) Ca2+ channels that are physically connected to voltage-dependent anion channel (VDAC) located at the outer mitochondrial membrane (OMM) via glucose-regulated protein 75-kDa (GRP75). The Ca2+ ions transferred through the IP3Rs–GRP75–VDAC complex are imported into the mitochondrial matrix via the mitochondrial Ca2+ uniporter (MCU) complex. The activity of IP3Rs is controlled by several chaperones. The oxidoreductase ER oxidoreductin 1a (Ero1α) interacts with IP3Rs and modulates Ca2+ flux in a redox-sensitive manner. In addition, the σ1R is released from the Ca2+-dependent chaperone GRP78/BiP and promotes prolonged ER calcium release by stabilizing IP3R3. Regulation of Ca2 + efflux from ER is regulated, also, through the Sec61 channel that acts as a passive ER calcium leak channel. (B) Mitochondrial Ca2+ uptake affects cell death pathways. Following apoptotic stimuli, at MERCs, calcium uptake by the IP3R–GRP75–VDAC complex favors Ca2+ overload that promotes OMM permeabilization, inner mitochondrial membrane (IMM) cristae remodeling, and the opening of the permeability transition pore (PTP). This causes a rapid collapse of the membrane potential and the swelling of mitochondria, with consequent loss of cytochrome c that is released into the cytosol to trigger apoptosis. (C) At MERCs, ER chaperones regulate the Ca2+ homeostasis. Overexpression of GRP78/BiP at MERCs increases the Ca2+ storage capacity of the ER and attenuates apoptosis. Red arrows indicate inhibitory effect of GRP78/78 on the passive Ca2+ efflux through the Sec61 channel. Under chronic ER stress, involving prolonged ER Ca2+ depletion, σ1R translocates to the peripheral ER and attenuates cellular damage; in this way, σ1R is no more able to stabilize IP3R3, thereby preventing cell death. CLNX could be considered as a novel biomarker as its upregulation is related to the increased activity of sarco/endoplasmic reticulum Ca2+ATPase (SERCA) pump, that concentrates Ca2+ into the ER lumen, allowing resistance to cell death. Ero1-α and GRP75 contribution to the tumorigenesis is still unclear.
Evidence suggests that deregulation of σ1R may be involved in several events including apoptosis resistance, tumor growth, migration, invasive potency, angiogenesis, and cell response to the microenvironment (Crottes et al., 2013). Indeed, σ1R overexpression occurs in distinct types of cancer (Renaudo et al., 2004; Crottes et al., 2013; Soriani and Kourrich, 2019). In breast and colon cancers, σ1R overexpression occurs predominantly at the ER–mitochondria interface and correlates with an invasive and metastatic phenotype, whereas low expression levels are found in normal cells (Aydar et al., 2006; Gueguinou et al., 2017). In these cancer models, σ1R forms a molecular platform with calcium-activated K+ channel SK3 and Orai1, which is responsible for increased Ca2+ entry that finally enhances cancer cell migration (Gueguinou et al., 2017). These finding supports the pro-tumorigenic function of σ1R, which is related to the regulation of Ca2+ dynamics at the ER–mitochondria interface. Instead, under prolonged ER stress and Ca2+ depletion, σ1R translocates from MERCs to the peripheral ER (Figures 3A,C) and attenuates cellular damage, thereby preventing cell death (Hayashi and Su, 2003). Furthermore, σ1R expression is under the transcriptional control, the PERK pathway of the UPR. Indeed, ATF4 binds σ1R gene promoter, while its silencing reduced σ1R expression (Mitsuda et al., 2011) (Figure 2A,iv). In addition, σ1R itself can modulate the UPR through the modulation of IRE1α stability at MERCs (Mori et al., 2013) (Figure 2B,i) confirming the importance of the UPR for the MERC function.
Glucose-Regulated Protein 78-kDa/Binding Immunoglobulin Protein: Ca2+ Handling at Mitochondria–ER Contacts and Resistance to Cell Death
GRP78/BiP has a key role in several cancers where it is often upregulated (Ibrahim et al., 2019). Higher GRP78/BiP expression is described in colon cancer, and GRP78/BiP downregulation increases epirubicin-induced apoptosis and cell migration (Xing et al., 2006; Chang et al., 2015). Similarly, in breast cancer, higher GRP78/BiP expression promotes drug resistance reducing the sensitivity of gemcitabine and causing apoptosis inhibition (Xie et al., 2016). GRP78/BiP activity depends on its intracellular localization (Casas, 2017) as sub-mitochondrial fractionation revealed that GRP78/BiP is associated with co-chaperones known to be involved in Ca2+-mediated signaling between the ER and mitochondria (Sun et al., 2006). Therefore, at MERCs, GRP78/BiP controls the efflux of Ca2+ ions from the ER by closing the Sec61 channel (Figure 3A) in the absence of translocation (Casas, 2017). However, GRP78/BiP overexpression can increase ER Ca2+ storage capacity and attenuate oxidant-induced Ca2+ fluctuations and subsequent apoptosis (Figure 3B). Additionally, GRP78/BiP silencing increases intracellular free Ca2+ in response to hydrogen peroxide, thereby supporting the role of GRP78/BiP in maintaining cellular Ca2+ homeostasis and preventing Ca2+-induced apoptosis (Aoki et al., 2001; Lamb et al., 2006). Finally, these considerations highlight that GRP78/BiP, at MERCs, is subjected to cancer-specific variations, but further studies are necessary to unveil the exact link among this chaperone in MERCs and tumorigenesis.
Role of Glucose-Regulated Protein 75-kDa in Ca2+ Homeostasis at Mitochondria–ER Contacts
The interaction between the IP3Rs, VDAC, and GRP75 has long been proven. At the MERCs, the IP3R/VDAC1 interaction is modulated by GRP75 acting as a bridge to allow Ca2+ transfer from the ER to the OMM, from which the ions can easily reach the mitochondrial matrix through the mitochondrial Ca2+ uniporter MCU (Figure 3A). Silencing of GRP75 abolishes the IP3Rs/VDAC1 coupling, thereby reducing mitochondrial Ca2+ uptake in response to agonist stimulation (Rizzuto et al., 2012; Sassano et al., 2017). IP3Rs/VDAC1 interaction directly enhances Ca2+ accumulation in the mitochondria, while GRP75 gene silencing inhibits Ca2+ flux signaling at MERCs (Szabadkai et al., 2006). With this regard, GRP75 represents an important player in the control of cell fate and pathogenesis, as it is often overexpressed in different tumor types (Dundas et al., 2005; Wadhwa et al., 2006) in which it is an important regulator of tumor growth and survival. It has been remarked that, several cancer cells, in which GRP75 is increased, acquire the ability to form tumors in Balb/c nude mice, while GRP75 overexpression is sufficient to increase malignancy of breast cancer cells (Wadhwa et al., 2006). Similarly, upregulated GRP75 in human medullary thyroid cancer (MTC) tissues is essential for MTC cell survival and proliferation (Starenki et al., 2015). On these grounds, GRP75 upregulation contributes significantly to tumorigenesis, but whether this function depends on its ability to regulate Ca2 + homeostasis at MERCs requires to be further investigated. However, both the location and the function at MERCs pinpoint GRP75 as a promising target in cancer therapy.
Role of Calnexin as Modulator of Endoplasmic Reticulum–Mitochondria Ca2+ Signaling and Apoptosis
Calnexin participates to the ER QC by retaining newly synthesized glycoproteins until they reach their correct conformation. At MERCs, CLNX enrichment depends on the interaction with cytosolic phosphofurin-acidic cluster-sorting protein 2 (PACS-2), which binds non-phosphorylated CLNX to the cytosolic cluster of acidic amino acids and places CLNX at MERCs (Figure 3A) (Myhill et al., 2008). Moreover, to be localized at MERCs, CLNX needs to be palmitoylated, suggesting that special lipid constitution determines MERC targeting (Raturi and Simmen, 2013).
However, the task of MERCs-localized CLNX is to regulate the activity of SERCA2b pump (Rizzuto et al., 2012). As shown in Xenopus oocytes (Roderick et al., 2000), CLNX/SERCA2b interaction requires CLNX phosphorylation on S562 in the cytosolic domain, by ERK and PKC kinases. In this state, CLNX is free to interact with the COOH terminus of SERCA2b and promotes the SERCA2b pump activity (Roderick et al., 2000).
Another role of CLNX concerns the modulation of cell sensitivity to apoptosis, which occurs differently from the other ER resident chaperones, since CLNX deficiency lowers ER luminal Ca2+ concentrations and protects cells from apoptosis (Figures 3B,C), while overexpression of CLNX sensitizes cells to apoptosis (Nakamura et al., 2000; Arnaudeau et al., 2002). For this reason, since its deficiency could enhance responsiveness to 5-FU-based chemotherapy, CLNX was indicated as a new prognostic biomarker of low onset in colorectal cancer patients (Nakamura et al., 2000). Moreover, other in vitro studies showed the importance of CLNX for HTC116 colon cancer cell growth and proliferation (Ryan et al., 2016).
Altogether, the above considerations suggest a possible role for CLNX as modulator of ER–mitochondria Ca2+ signaling, and therefore of apoptosis, and therefore indicating this lectin as a potential therapeutic target for cancer therapy.
Ero1α-Mediated Ca2+ Flux at Mitochondria–ER Contacts and Cancer Drug Resistance
Within the ER, Ero1-α is an oxidizing enzyme that, in concert with protein disulfide isomerase (PDI), promotes proper protein folding and the maintenance of the redox state.
Instead, recent findings have shown that, at MERCs, Ero1-α regulates Ca2+ fluxes toward the mitochondria (Figure 3A) by interacting with the ERp44 to control IP3Rs activity (Anelli et al., 2012). Furthermore, IP3Rs-assisted Ca2+-transport is controlled by Ero1α oxidoreductase in concert with ERp44, another multitask chaperone, which interacts with IP3Rs in order to modulate its Ca2+-flux properties in a redox-sensitive manner (Higo et al., 2005; Anelli et al., 2012). Therefore, the Ero1α–ERp44 axis plays an important role in regulating Ca2+ homeostasis at MERCs (Anelli et al., 2012). Moreover, Ero1-α is a key regulator of oxidative stress not only within the ER but also at MERCs, where it regulates calcium flux via diffusible H2O2. Indeed, ROS generated by either ER or mitochondria in the H2O2 nanodomains generated by cristae can also localize at MERCs and perturb calcium signaling (Booth et al., 2016). Moreover, overexpression of Ero1-α is frequent in various types of tumors (Shergalis et al., 2020), in particular in the aggressive and/or drug-resistant ones with a poor prognosis. It has been proven that Ero1-α-mediated functions are key events in the cell death induced by the procaspase-activating compound-1 (PAC-1), which promotes apoptosis in a variety of cancer cell types (Seervi et al., 2013). Nevertheless, as observed in breast cancer cells, Ero1-α expression correlates with the expression of programmed cell death-ligand 1 (PD-L1), while Ero1-α knockdown results in a significant attenuation of PD-L1-mediated T-cell apoptosis, suggesting a role of Ero1-α in tumor-mediated immunosuppression (Tanaka et al., 2017).
To conclude, the mechanisms by which Ero1-α expression affects the poor prognosis of tumors are still to be elucidated and should be explored in depth, particularly by evaluating the involvement of Ero1-α-mediated Ca2+ flux at MERCs.
Conclusion
In the last decades, an increasing number of studies have shed light on the role of ER MCS in coordinating key cellular functions and the maintenance of cell physiology. Along with the complex analysis of MCS composition and function, the study of MERCs has emerged as a fundamental topic in the cell biology of MCSs. Among the multiple aspects analyzed, the literature discussed hereby has unveiled an additional role played at MERCs by the UPR transducers and chaperones in the orchestration of ER-to-mitochondria calcium flux, mitochondria bioenergetics, and apoptosis, and how these functions are deregulated in specific cancer settings. Taken together, the localization of PERK, IRE1α, and ER chaperones at MERCs appears to be relevant for cancer initiation and progression, and we think that it may represent one of the aspects to be deeply explored in the upcoming cancer research. Ultimately, many outstanding questions have to be answered: Which of the effects showed by UPR transducers on cancer development are dependent on their localization at MERCs? In particular, can the dual role played by the UPR transducers as tumor suppressor versus tumor promoter be specifically attributed to the ER-localized actors rather than to the MERC-localized one? Are there any properties of the MERC-localized proteins that could be related to one specific type of cancer rather than another? How do the additional functions performed by the UPR transducers and ER chaperones at MERCs affect the strength and the outcome of UPR signaling?
Future studies defining the significance of UPR protein residence at MERCs will allow to gain further insight into their role in cellular physiology and cancer development and, auspicably, to lead to breakthroughs in therapeutic strategies.
Nomenclature: ASK1, apoptosis signal-regulating kinase 1; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; BAP31, B-cell receptor-associated protein 31; BIM, B-cell lymphoma 2; BIP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; CLNX, calnexin; CLR, calreticulin; COPII, coat protein complex II; CREB3L1, CAMP responsive element binding protein 3 like 1; DR5, death receptor 5; eIF2α, eukaryotic initiation factor 2α; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; ERAD, ER-associated protein degradation; ERESs, ER exit sites; ERK, extracellular signal-regulated kinases; Ero1-α, ER oxidoreductin 1; ERp44, endoplasmic reticulum resident protein 44; ERp57, ER resident protein 57; Fis1, fission 1 protein; GADD153, growth arrest- and DNA damage-inducible gene 153; GADD34, growth arrest and DNA damage-inducible gene 34; GCN2, general control non-derepressible 2; GM, glioblastoma multiform; GRP75, glucose-regulated protein 75-kDa; GRP78, glucose-regulated protein 78-kDa; HCC, hepatocellular carcinoma cell; HIF1α, hypoxia-inducible factor 1α; HRI, heme-regulated eIF2α kinase inhibitor; IMM, inner mitochondrial membrane; IP3Rs, inositol trisphosphate receptor; IRE1, inositol-requiring enzyme 1; JNK, c-Jun N-terminal kinases; LDs, lipid droplets; MAMs, mitochondria-associated membranes; MCS, membrane contact sites; MCU, mitochondrial Ca2+ uniporter; MDR, mechanisms of multidrug resistance; MERCs, mitochondria–ER contacts; Mfn2, Mitofusin 2; MITOL, mitochondrial ubiquitin ligase; MRP1, multidrug resistance-associated protein 1; MTC, medullary thyroid cancer; mTOR, aammalian target of rapamycin; NE, nuclear envelope; Nrf2, nuclear factor erythroid 2-related factor 2; OMM, mitochondrial outer membrane; PACS-2, phosphofurin-acidic cluster sorting protein 2; PD-L1, programmed cell death-ligand 1; PDI, protein disulfide isomerase; PERK, protein kinase RNA-like endoplasmic reticulum kinase; PKC, protein kinase C; PP2A, protein phosphatase 2; PPM1L, protein phosphatase, Mg2+/Mn2+-dependent 1L; PTP, permeability transition pore; PTPIP51, protein tyrosine phosphatase-interacting protein-51; PUMA, p53 upregulated modulator of apoptosis; QC, quality control; RIDD, regulated Ire1-dependent decay; ROS, reactive oxygen species; S1P, site-1 protease; S1T, SERCA1 truncated proteins; SCAF, super complex assembly factor; SERCA1, sarco/endoplasmic reticulum Ca2+ ATPase1; SERCA2b, sarco/endoplasmic reticulum Ca2+ ATPase2b; σ1R, sigma-1 receptor; SIMH, stress-induced mitochondrial hyperfusion; SK3, small conductance calcium-activated potassium channel 3; TNBC, triple negative breast cancer; TRAF2, TNF receptor-associated factor 2; Trb3, Tribbles ortholog 1; TXNIP, thioredoxin-interacting protein; UPR, unfolded protein response; VDAC, voltage-dependent anion channel; VEGF, vascular endothelial growth factor; XBP1, X-box binding protein 1.
Author Contributions
GA and VP performed the literature searches and wrote the manuscript. OM gave suggestions and significantly refined the manuscript. PR contributed to the conceptual idea, supervised the writing, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PR acknowledges the support of POR FERS Regione Campania 2014-2020 grant System Innovation for Cancer Early Diagnosis (SICED).
References
Ameri, K., and Harris, A. L. (2008). Activating transcription factor 4. Int. J. Biochem. Cell Biol. 40, 14–21. doi: 10.1016/j.biocel.2007.01.020.
Amodio, G., Moltedo, O., Fasano, D., Zerillo, L., Oliveti, M., Di Pietro, P., et al. (2019). PERK-Mediated unfolded protein response activation and oxidative stress in PARK20 fibroblasts. Front. Neurosci. 13:673. doi: 10.3389/fnins.2019.00673.
Amodio, G., Moltedo, O., Monteleone, F., D’Ambrosio, C., Scaloni, A., Remondelli, P., et al. (2011). Proteomic signatures in thapsigargin-treated hepatoma cells. Chem. Res. Toxicol. 24, 1215–1222. doi: 10.1021/tx200109y.
Amodio, G., Renna, M., Paladino, S., Venturi, C., Tacchetti, C., Moltedo, O., et al. (2009). Endoplasmic reticulum stress reduces the export from the ER and alters the architecture of post-ER compartments. Int. J. Biochem. Cell Biol. 41, 2511–2521. doi: 10.1016/j.biocel.2009.08.006.
Anelli, T., Bergamelli, L., Margittai, E., Rimessi, A., Fagioli, C., Malgaroli, A., et al. (2012). Ero1alpha regulates Ca(2+) fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal. 16, 1077–1087. doi: 10.1089/ars.2011.4004.
Aoki, M., Tamatani, M., Taniguchi, M., Yamaguchi, A., Bando, Y., Kasai, K., et al. (2001). Hypothermic treatment restores glucose regulated protein 78 (GRP78) expression in ischemic brain. Brain Res. Mol. Brain Res. 95, 117–128. doi: 10.1016/s0169-328x00255-258.
Arnaudeau, S., Frieden, M., Nakamura, K., Castelbou, C., Michalak, M., and Demaurex, N. (2002). Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J. Biol. Chem. 277, 46696–46705. doi: 10.1074/jbc.M202395200.
Avivar-Valderas, A., Salas, E., Bobrovnikova-Marjon, E., Diehl, J. A., Nagi, C., Debnath, J., et al. (2011). PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol. Cell Biol. 31, 3616–3629. doi: 10.1128/MCB.05164-5111.
Aydar, E., Onganer, P., Perrett, R., Djamgoz, M. B., and Palmer, C. P. (2006). The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 242, 245–257. doi: 10.1016/j.canlet.2005.11.011.
Balsa, E., Soustek, M. S., Thomas, A., Cogliati, S., Garcia-Poyatos, C., Martin-Garcia, E., et al. (2019). ER and nutrient stress promote assembly of respiratory chain supercomplexes through the PERK-eIF2alpha axis. Mol. Cell 74:877–890 e876. doi: 10.1016/j.molcel.2019.03.031.
Bernhard, W., and Rouiller, C. (1956). Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. J. Biophys. Biochem. Cytol. 2(4 Suppl), 73–78. doi: 10.1083/jcb.2.4.73.
Bi, M., Naczki, C., Koritzinsky, M., Fels, D., Blais, J., Hu, N., et al. (2005). ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481. doi: 10.1038/sj.emboj.7600777.
Bittremieux, M., Parys, J. B., Pinton, P., and Bultynck, G. (2016). ER functions of oncogenes and tumor suppressors: modulators of intracellular Ca(2+) signaling. Biochim. Biophys. Acta 1863(6 Pt B), 1364–1378. doi: 10.1016/j.bbamcr.2016.01.002.
Blais, J. D., Addison, C. L., Edge, R., Falls, T., Zhao, H., Wary, K., et al. (2006). Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell Biol. 26, 9517–9532. doi: 10.1128/MCB.01145-1146.
Blazanin, N., Son, J., Craig-Lucas, A. B., John, C. L., Breech, K. J., Podolsky, M. A., et al. (2017). ER stress and distinct outputs of the IRE1alpha RNase control proliferation and senescence in response to oncogenic Ras. Proc. Natl. Acad. Sci. U S A. 114, 9900–9905. doi: 10.1073/pnas.1701757114.
Booth, D. M., Enyedi, B., Geiszt, M., Varnai, P., and Hajnoczky, G. (2016). Redox Nanodomains are induced by and control calcium signaling at the ER-Mitochondrial interface. Mol. Cell 63, 240–248. doi: 10.1016/j.molcel.2016.05.040.
Bujisic, B., De Gassart, A., Tallant, R., Demaria, O., Zaffalon, L., Chelbi, S., et al. (2017). Impairment of both IRE1 expression and XBP1 activation is a hallmark of GCB DLBCL and contributes to tumor growth. Blood 129, 2420–2428. doi: 10.1182/blood-2016-09-741348.
Cao, S. S., and Kaufman, R. J. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21, 396–413. doi: 10.1089/ars.2014.5851.
Cardenas, C., Muller, M., McNeal, A., Lovy, A., Jana, F., Bustos, G., et al. (2016). Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 15, 219–220. doi: 10.1016/j.celrep.2016.03.045.
Carreras-Sureda, A., Jana, F., Urra, H., Durand, S., Mortenson, D. E., Sagredo, A., et al. (2019). Non-canonical function of IRE1alpha determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat. Cell Biol. 21, 755–767. doi: 10.1038/s41556-019-0329-y.
Casas, C. (2017). GRP78 at the centre of the stage in cancer and neuroprotection. Front. Neurosci. 11:177. doi: 10.3389/fnins.2017.00177.
Cazanave, S. C., Elmi, N. A., Akazawa, Y., Bronk, S. F., Mott, J. L., and Gores, G. J. (2010). CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am. J. Physiol. Gastrointest Liver Physiol. 299, G236–243. doi: 10.1152/ajpgi.00091.2010.
Chakkarapani, S. K., Zhang, P., and Kang, S. H. (2018). 3D super-localization of intracellular organelle contacts at live single cell by dual-wavelength synchronized fluorescence-free imaging. Anal. Bioanal. Chem. 410, 1551–1560. doi: 10.1007/s00216-017-0805-809.
Chami, M., Oules, B., Szabadkai, G., Tacine, R., Rizzuto, R., and Paterlini-Brechot, P. (2008). Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol. Cell 32, 641–651. doi: 10.1016/j.molcel.2008.11.014.
Chandrashekar, D. S., Bashel, B., Balasubramanya, S. A. H., Creighton, C. J., Ponce-Rodriguez, I., Chakravarthi, B., et al. (2017). UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19, 649–658. doi: 10.1016/j.neo.2017.05.002.
Chang, Y. J., Chen, W. Y., Huang, C. Y., Liu, H. H., and Wei, P. L. (2015). Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumour Biol. 36, 1859–1869. doi: 10.1007/s13277-014-2788-x.
Chen, X., Iliopoulos, D., Zhang, Q., Tang, Q., Greenblatt, M. B., Hatziapostolou, M., et al. (2014). XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature 508, 103–107. doi: 10.1038/nature13119.
Costello, J. L., Castro, I. G., Hacker, C., Schrader, T. A., Metz, J., Zeuschner, D., et al. (2017). ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. 216, 331–342. doi: 10.1083/jcb.201607055.
Cox, J. S., and Walter, P. (1996). A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404. doi: 10.1016/s0092-867481360-81364.
Crottes, D., Guizouarn, H., Martin, P., Borgese, F., and Soriani, O. (2013). The sigma-1 receptor: a regulator of cancer cell electrical plasticity? Front. Physiol. 4:175. doi: 10.3389/fphys.2013.00175.
Csordas, G., Renken, C., Varnai, P., Walter, L., Weaver, D., Buttle, K. F., et al. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921. doi: 10.1083/jcb.200604016.
Cubillos-Ruiz, J. R., Bettigole, S. E., and Glimcher, L. H. (2017). Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in Cancer. Cell 168, 692–706. doi: 10.1016/j.cell.2016.12.004.
Cullinan, S. B., and Diehl, J. A. (2006). Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 38, 317–332. doi: 10.1016/j.biocel.2005.09.018.
Del Vecchio, C. A., Feng, Y., Sokol, E. S., Tillman, E. J., Sanduja, S., Reinhardt, F., et al. (2014). De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 12:e1001945. doi: 10.1371/journal.pbio.1001945.
Denard, B., Lee, C., and Ye, J. (2012). Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife 1:e00090. doi: 10.7554/eLife.00090.
Diehl, J. A., Fuchs, S. Y., and Koumenis, C. (2011). The cell biology of the unfolded protein response. Gastroenterology 141:38-41, 41.e1-2. doi: 10.1053/j.gastro.2011.05.018.
Dundas, S. R., Lawrie, L. C., Rooney, P. H., and Murray, G. I. (2005). Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J. Pathol. 205, 74–81. doi: 10.1002/path.1672.
Fasano, D., Parisi, S., Pierantoni, G. M., De Rosa, A., Picillo, M., Amodio, G., et al. (2018). Alteration of endosomal trafficking is associated with early-onset parkinsonism caused by SYNJ1 mutations. Cell Death Dis. 9:385. doi: 10.1038/s41419-018-0410-417.
Feng, Y. X., Jin, D. X., Sokol, E. S., Reinhardt, F., Miller, D. H., and Gupta, P. B. (2017). Cancer-specific PERK signaling drives invasion and metastasis through CREB3L1. Nat. Commun. 8:1079. doi: 10.1038/s41467-017-01052-y.
Friedman, J. R., Lackner, L. L., West, M., DiBenedetto, J. R., Nunnari, J., and Voeltz, G. K. (2011). ER tubules mark sites of mitochondrial division. Science 334, 358–362. doi: 10.1126/science.1207385.
Fujimoto, A., Kawana, K., Taguchi, A., Adachi, K., Sato, M., Nakamura, H., et al. (2016). Inhibition of endoplasmic reticulum (ER) stress sensors sensitizes cancer stem-like cells to ER stress-mediated apoptosis. Oncotarget 7, 51854–51864. doi: 10.18632/oncotarget.10126.
Gardner, B. M., and Walter, P. (2011). Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894. doi: 10.1126/science.1209126.
Ghosh, R., Lipson, K. L., Sargent, K. E., Mercurio, A. M., Hunt, J. S., Ron, D., et al. (2010). Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5:e9575. doi: 10.1371/journal.pone.0009575.
Ghosh, R., Wang, L., Wang, E. S., Perera, B. G., Igbaria, A., Morita, S., et al. (2014). Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548. doi: 10.1016/j.cell.2014.07.002.
Grimaldi, M., Stillitano, I., Amodio, G., Santoro, A., Buonocore, M., Moltedo, O., et al. (2018). Structural basis of antiviral activity of peptides from MPER of FIV gp36. PLoS One 13:e0204042. doi: 10.1371/journal.pone.0204042.
Gueguinou, M., Crottes, D., Chantome, A., Rapetti-Mauss, R., Potier-Cartereau, M., Clarysse, L., et al. (2017). The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca(2+) homeostasis. Oncogene 36, 3640–3647. doi: 10.1038/onc.2016.501.
Guo, L., Chi, Y., Xue, J., Ma, L., Shao, Z., and Wu, J. (2017). Phosphorylated eIF2alpha predicts disease-free survival in triple-negative breast cancer patients. Sci. Rep. 7:44674. doi: 10.1038/srep44674.
Gupta, S., McGrath, B., and Cavener, D. R. (2009). PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PLoS One 4:e8008. doi: 10.1371/journal.pone.0008008.
Han, J., Back, S. H., Hur, J., Lin, Y. H., Gildersleeve, R., Shan, J., et al. (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490. doi: 10.1038/ncb2738.
Hanada, K., Kumagai, K., Yasuda, S., Miura, Y., Kawano, M., Fukasawa, M., et al. (2003). Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809. doi: 10.1038/nature02188.
Harding, H. P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., Schapira, M., et al. (2000). Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108. doi: 10.1016/s1097-276500108-108.
Harding, S. M. (1999). Gastroesophageal reflux and asthma: insight into the association. J. Allergy Clin. Immunol. 104(2 Pt 1), 251–259. doi: 10.1016/s0091-674970360-x.
Hartl, F. U., and Hayer-Hartl, M. (2009). Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581. doi: 10.1038/nsmb.1591.
Hayashi, T., and Su, T. P. (2003). Intracellular dynamics of sigma-1 receptors (sigma binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 306, 726–733. doi: 10.1124/jpet.103.051292.
Hayashi, T., and Su, T. P. (2007). Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610. doi: 10.1016/j.cell.2007.08.036.
Herrera-Cruz, M. S., and Simmen, T. (2017). Cancer: untethering mitochondria from the endoplasmic reticulum? Front. Oncol. 7:105. doi: 10.3389/fonc.2017.00105.
Higo, T., Hattori, M., Nakamura, T., Natsume, T., Michikawa, T., and Mikoshiba, K. (2005). Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98. doi: 10.1016/j.cell.2004.11.048.
Hollien, J., Lin, J. H., Li, H., Stevens, N., Walter, P., and Weissman, J. S. (2009). Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331. doi: 10.1083/jcb.200903014.
Hsu, S. K., Chiu, C. C., Dahms, H. U., Chou, C. K., Cheng, C. M., Chang, W. T., et al. (2019). Unfolded Protein Response (UPR) in survival, dormancy, immunosuppression, metastasis, and treatments of Cancer Cells. Int. J. Mol. Sci. 20:2518. doi: 10.3390/ijms20102518.
Hu, R., Warri, A., Jin, L., Zwart, A., Riggins, R. B., Fang, H. B., et al. (2015). NF-kappaB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Mol. Cell Biol. 35, 379–390. doi: 10.1128/MCB.00847-814.
Ibrahim, I. M., Abdelmalek, D. H., and Elfiky, A. A. (2019). GRP78: a cell’s response to stress. Life Sci. 226, 156–163. doi: 10.1016/j.lfs.2019.04.022.
Ilacqua, N., Sanchez-Alvarez, M., Bachmann, M., Costiniti, V., Del Pozo, M. A., and Giacomello, M. (2017). Protein localization at mitochondria-ER contact sites in basal and stress conditions. Front. Cell Dev. Biol. 5:107. doi: 10.3389/fcell.2017.00107.
Jan, C. H., Williams, C. C., and Weissman, J. S. (2014). Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science 346:1257521. doi: 10.1126/science.1257521.
Korennykh, A. V., Egea, P. F., Korostelev, A. A., Finer-Moore, J., Zhang, C., Shokat, K. M., et al. (2009). The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693. doi: 10.1038/nature07661.
Krols, M., Bultynck, G., and Janssens, S. (2016). ER-Mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J. Cell Biol. 214, 367–370. doi: 10.1083/jcb.201607124.
Lamb, H. K., Mee, C., Xu, W., Liu, L., Blond, S., Cooper, A., et al. (2006). The affinity of a major Ca2+ binding site on GRP78 is differentially enhanced by ADP and ATP. J. Biol. Chem. 281, 8796–8805. doi: 10.1074/jbc.M503964200.
Lebeau, J., Saunders, J. M., Moraes, V. W. R., Madhavan, A., Madrazo, N., Anthony, M. C., et al. (2018). The PERK arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep. 22, 2827–2836. doi: 10.1016/j.celrep.2018.02.055.
Lee, A. S. (2007). GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 67, 3496–3499. doi: 10.1158/0008-5472.CAN-07-0325.
Lee, C., and Chen, L. B. (1993). Motility and construction of the endoplasmic reticulum in living cells. Subcell Biochem. 21, 343–352. doi: 10.1007/978-1-4615-2912-5_15.
Lerner, A. G., Upton, J. P., Praveen, P. V., Ghosh, R., Nakagawa, Y., Igbaria, A., et al. (2012). IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264. doi: 10.1016/j.cmet.2012.07.007.
Lhomond, S., Avril, T., Dejeans, N., Voutetakis, K., Doultsinos, D., McMahon, M., et al. (2018). Dual IRE1 RNase functions dictate glioblastoma development. EMBO Mol. Med. 10:e7929. doi: 10.15252/emmm.201707929.
Li, C., Fan, Q., Quan, H., Nie, M., Luo, Y., and Wang, L. (2018). The three branches of the unfolded protein response exhibit differential significance in breast cancer growth and stemness. Exp. Cell Res. 367, 170–185. doi: 10.1016/j.yexcr.2018.03.033.
Li, X., Lewis, M. T., Huang, J., Gutierrez, C., Osborne, C. K., Wu, M. F., et al. (2008). Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 100, 672–679. doi: 10.1093/jnci/djn123.
Limia, C. M., Sauzay, C., Urra, H., Hetz, C., Chevet, E., and Avril, T. (2019). Emerging roles of the endoplasmic reticulum associated unfolded protein response in cancer cell migration and invasion. Cancers (Basel) 11:631. doi: 10.3390/cancers11050631.
Lobo, M. V., Martin, M. E., Perez, M. I., Alonso, F. J., Redondo, C., Alvarez, M. I., et al. (2000). Levels, phosphorylation status and cellular localization of translational factor eIF2 in gastrointestinal carcinomas. Histochem. J. 32, 139–150. doi: 10.1023/a:1004091122351.
Logue, S. E., McGrath, E. P., Cleary, P., Greene, S., Mnich, K., Almanza, A., et al. (2018). Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 9:3267. doi: 10.1038/s41467-018-05763-5768.
Luo, B., and Lee, A. S. (2013). The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32, 805–818. doi: 10.1038/onc.2012.130.
Lynes, E. M., Bui, M., Yap, M. C., Benson, M. D., Schneider, B., Ellgaard, L., et al. (2012). Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J 31, 457–470. doi: 10.1038/emboj.2011.384.
Ma, Y., and Hendershot, L. M. (2003). Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J. Biol. Chem. 278, 34864–34873. doi: 10.1074/jbc.M301107200.
Ma, Y., and Hendershot, L. M. (2004). The role of the unfolded protein response in tumour development: friend or foe? Nat. Rev. Cancer 4, 966–977. doi: 10.1038/nrc1505.
Marchi, S., and Pinton, P. (2014). The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J. Physiol. 592, 829–839. doi: 10.1113/jphysiol.2013.268235.
Marchi, S., Vitto, V. A. M., Danese, A., Wieckowski, M. R., Giorgi, C., and Pinton, P. (2019). Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle 18, 1068–1083. doi: 10.1080/15384101.2019.1612698.
Marciniak, S. J., Yun, C. Y., Oyadomari, S., Novoa, I., Zhang, Y., Jungreis, R., et al. (2004). CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077. doi: 10.1101/gad.1250704.
Meusser, B., Hirsch, C., Jarosch, E., and Sommer, T. (2005). ERAD: the long road to destruction. Nat. Cell Biol. 7, 766–772. doi: 10.1038/ncb0805-766.
Mitsuda, T., Omi, T., Tanimukai, H., Sakagami, Y., Tagami, S., Okochi, M., et al. (2011). Sigma-1Rs are upregulated via PERK/eIF2alpha/ATF4 pathway and execute protective function in ER stress. Biochem. Biophys. Res. Commun. 415, 519–525. doi: 10.1016/j.bbrc.2011.10.113.
Moenner, M., Pluquet, O., Bouchecareilh, M., and Chevet, E. (2007). Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 67, 10631–10634. doi: 10.1158/0008-5472.CAN-07-1705.
Moltedo, O., Remondelli, P., and Amodio, G. (2019). The mitochondria-endoplasmic reticulum contacts and their critical role in aging and age-associated diseases. Front. Cell Dev. Biol. 7:172. doi: 10.3389/fcell.2019.00172.
Morciano, G., Marchi, S., Morganti, C., Sbano, L., Bittremieux, M., Kerkhofs, M., et al. (2018). Role of mitochondria-associated ER membranes in calcium regulation in cancer-specific settings. Neoplasia 20, 510–523. doi: 10.1016/j.neo.2018.03.005.
Mori, K. (2009). Signalling pathways in the unfolded protein response: development from yeast to mammals. J. Biochem. 146, 743–750. doi: 10.1093/jb/mvp166.
Mori, T., Hayashi, T., Hayashi, E., and Su, T. P. (2013). Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One 8:e76941. doi: 10.1371/journal.pone.0076941.
Munoz, J. P., Ivanova, S., Sanchez-Wandelmer, J., Martinez-Cristobal, P., Noguera, E., Sancho, A., et al. (2013). Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 32, 2348–2361. doi: 10.1038/emboj.2013.168.
Myhill, N., Lynes, E. M., Nanji, J. A., Blagoveshchenskaya, A. D., Fei, H., Carmine Simmen, K., et al. (2008). The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell 19, 2777–2788. doi: 10.1091/mbc.E07-10-0995.
Nakamura, K., Bossy-Wetzel, E., Burns, K., Fadel, M. P., Lozyk, M., Goping, I. S., et al. (2000). Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J. Cell Biol. 150, 731–740. doi: 10.1083/jcb.150.4.731.
Nishitoh, H., Matsuzawa, A., Tobiume, K., Saegusa, K., Takeda, K., Inoue, K., et al. (2002). ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355. doi: 10.1101/gad.992302.
Ohoka, N., Yoshii, S., Hattori, T., Onozaki, K., and Hayashi, H. (2005). TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 24, 1243–1255. doi: 10.1038/sj.emboj.7600596.
Pakos-Zebrucka, K., Koryga, I., Mnich, K., Ljujic, M., Samali, A., and Gorman, A. M. (2016). The integrated stress response. EMBO Rep. 17, 1374–1395. doi: 10.15252/embr.201642195.
Patron, M., Checchetto, V., Raffaello, A., Teardo, E., Vecellio Reane, D., Mantoan, M., et al. (2014). MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell 53, 726–737. doi: 10.1016/j.molcel.2014.01.013.
Peretti, D., Dahan, N., Shimoni, E., Hirschberg, K., and Lev, S. (2008). Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell 19, 3871–3884. doi: 10.1091/mbc.E08-05-0498.
Peretti, D., Kim, S., Tufi, R., and Lev, S. (2019). Lipid transfer proteins and membrane contact sites in human Cancer. Front. Cell Dev. Biol. 7:371. doi: 10.3389/fcell.2019.00371.
Phillips, M. J., and Voeltz, G. K. (2016). Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82. doi: 10.1038/nrm.2015.8.
Poplawski, T., Pytel, D., Dziadek, J., and Majsterek, I. (2019). Interplay between redox signaling, oxidative stress, and Unfolded Protein Response (UPR) in pathogenesis of human diseases. Oxid Med. Cell Longev. 2019:6949347. doi: 10.1155/2019/6949347.
Puthalakath, H., O’Reilly, L. A., Gunn, P., Lee, L., Kelly, P. N., Huntington, N. D., et al. (2007). ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349. doi: 10.1016/j.cell.2007.04.027.
Putney, J. W. Jr. (2005). Capacitative calcium entry: sensing the calcium stores. J. Cell Biol. 169, 381–382. doi: 10.1083/jcb.200503161.
Pytel, D., Gao, Y., Mackiewicz, K., Katlinskaya, Y. V., Staschke, K. A., Paredes, M. C., et al. (2016). PERK is a haploinsufficient tumor suppressor: gene dose determines tumor-suppressive versus tumor promoting properties of PERK in melanoma. PLoS Genet 12:e1006518. doi: 10.1371/journal.pgen.1006518.
Raffaello, A., De Stefani, D., and Rizzuto, R. (2012). The mitochondrial Ca(2+) uniporter. Cell Calcium 52, 16–21. doi: 10.1016/j.ceca.2012.04.006.
Raturi, A., and Simmen, T. (2013). Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 1833, 213–224. doi: 10.1016/j.bbamcr.2012.04.013.
Raymundo, D. P., Doultsinos, D., Guillory, X., Carlesso, A., Eriksson, L. A., and Chevet, E. (2020). Pharmacological targeting of IRE1 in Cancer. Trends Cancer 6, 1018–1030. doi: 10.1016/j.trecan.2020.07.006.
Reich, S., Nguyen, C. D. L., Has, C., Steltgens, S., Soni, H., Coman, C., et al. (2020). A multi-omics analysis reveals the unfolded protein response regulon and stress-induced resistance to folate-based antimetabolites. Nat. Commun. 11:2936. doi: 10.1038/s41467-020-16747-y.
Renaudo, A., Watry, V., Chassot, A. A., Ponzio, G., Ehrenfeld, J., and Soriani, O. (2004). Inhibition of tumor cell proliferation by sigma ligands is associated with K+ channel inhibition and p27kip1 accumulation. J. Pharmacol. Exp. Ther. 311, 1105–1114. doi: 10.1124/jpet.104.072413.
Renna, M., Faraonio, R., Bonatti, S., De Stefano, D., Carnuccio, R., Tajana, G., et al. (2006). Nitric oxide-induced endoplasmic reticulum stress activates the expression of cargo receptor proteins and alters the glycoprotein transport to the Golgi complex. Int. J. Biochem. Cell Biol. 38, 2040–2048. doi: 10.1016/j.biocel.2006.05.016.
Rizzuto, R., De Stefani, D., Raffaello, A., and Mammucari, C. (2012). Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578. doi: 10.1038/nrm3412.
Roderick, H. L., Lechleiter, J. D., and Camacho, P. (2000). Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J. Cell Biol. 149, 1235–1248. doi: 10.1083/jcb.149.6.1235.
Romero-Ramirez, L., Cao, H., Nelson, D., Hammond, E., Lee, A. H., Yoshida, H., et al. (2004). XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947. doi: 10.1158/0008-5472.CAN-04-1606.
Rosenwald, I. B., Hutzler, M. J., Wang, S., Savas, L., and Fraire, A. E. (2001). Expression of eukaryotic translation initiation factors 4E and 2alpha is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 92, 2164–2171. doi: 10.1002/1097-0142(20011015)92:8<2164::aid-cncr1559>3.0.co;2-a
Rosenwald, I. B., Koifman, L., Savas, L., Chen, J. J., Woda, B. A., and Kadin, M. E. (2008). Expression of the translation initiation factors eIF-4E and eIF-2∗ is frequently increased in neoplastic cells of Hodgkin lymphoma. Hum. Pathol. 39, 910–916. doi: 10.1016/j.humpath.2007.10.021.
Rosenwald, I. B., Wang, S., Savas, L., Woda, B., and Pullman, J. (2003). Expression of translation initiation factor eIF-2alpha is increased in benign and malignant melanocytic and colonic epithelial neoplasms. Cancer 98, 1080–1088. doi: 10.1002/cncr.11619.
Rouschop, K. M., van den Beucken, T., Dubois, L., Niessen, H., Bussink, J., Savelkouls, K., et al. (2010). The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Invest. 120, 127–141. doi: 10.1172/JCI40027.
Ruggiano, A., Foresti, O., and Carvalho, P. (2014). Quality control: ER-associated degradation: protein quality control and beyond. J. Cell Biol. 204, 869–879. doi: 10.1083/jcb.201312042.
Rutkowski, D. T., Arnold, S. M., Miller, C. N., Wu, J., Li, J., Gunnison, K. M., et al. (2006). Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4:e374. doi: 10.1371/journal.pbio.0040374.
Ryan, D., Carberry, S., Murphy, A. C., Lindner, A. U., Fay, J., Hector, S., et al. (2016). Calnexin, an ER stress-induced protein, is a prognostic marker and potential therapeutic target in colorectal cancer. J. Transl. Med. 14:196. doi: 10.1186/s12967-016-0948-z.
Saito, A., and Imaizumi, K. (2018). Unfolded protein response-dependent communication and contact among endoplasmic reticulum, mitochondria, and plasma membrane. Int. J. Mol. Sci. 19:3215;. doi: 10.3390/ijms19103215.
Salaroglio, I. C., Panada, E., Moiso, E., Buondonno, I., Provero, P., Rubinstein, M., et al. (2017). PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol. Cancer 16:91. doi: 10.1186/s12943-017-0657-650.
Sanchez-Alvarez, M., Del Pozo, M. A., and Bakal, C. (2017). AKT-mTOR signaling modulates the dynamics of IRE1 RNAse activity by regulating ER-mitochondria contacts. Sci. Rep. 7:16497. doi: 10.1038/s41598-017-16662-16661.
Sassano, M. L., van Vliet, A. R., and Agostinis, P. (2017). Mitochondria-Associated membranes as networking platforms and regulators of Cancer cell fate. Front. Oncol. 7:174. doi: 10.3389/fonc.2017.00174.
Schroder, M., and Kaufman, R. J. (2005). The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789. doi: 10.1146/annurev.biochem.73.011303.074134.
Seervi, M., Sobhan, P. K., Joseph, J., Ann Mathew, K., and Santhoshkumar, T. R. (2013). ERO1alpha-dependent endoplasmic reticulum-mitochondrial calcium flux contributes to ER stress and mitochondrial permeabilization by procaspase-activating compound-1 (PAC-1). Cell Death Dis. 4:e968. doi: 10.1038/cddis.2013.502.
Sezgin, E., Levental, I., Mayor, S., and Eggeling, C. (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374. doi: 10.1038/nrm.2017.16.
Sheng, X., Nenseth, H. Z., Qu, S., Kuzu, O. F., Frahnow, T., Simon, L., et al. (2019). IRE1alpha-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun. 10:323. doi: 10.1038/s41467-018-08152-8153.
Shergalis, A. G., Hu, S., and Bankhead, A. III rd., and Neamati, N. (2020). Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 210:107525. doi: 10.1016/j.pharmthera.2020.107525.
Shi, Z., Yu, X., Yuan, M., Lv, W., Feng, T., Bai, R., et al. (2019). Activation of the PERK-ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci. Rep. 9:3210. doi: 10.1038/s41598-019-39547-x.
Shibata, Y., Shemesh, T., Prinz, W. A., Palazzo, A. F., Kozlov, M. M., and Rapoport, T. A. (2010). Mechanisms determining the morphology of the peripheral ER. Cell 143, 774–788. doi: 10.1016/j.cell.2010.11.007.
Simmen, T., and Herrera-Cruz, M. S. (2018). Plastic mitochondria-endoplasmic reticulum (ER) contacts use chaperones and tethers to mould their structure and signaling. Curr. Opin. Cell Biol. 53, 61–69. doi: 10.1016/j.ceb.2018.04.014.
Simmen, T., Lynes, E. M., Gesson, K., and Thomas, G. (2010). Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 1798, 1465–1473. doi: 10.1016/j.bbamem.2010.04.009.
Sitia, R., and Braakman, I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature 426, 891–894. doi: 10.1038/nature02262.
Son, S. M., Byun, J., Roh, S. E., Kim, S. J., and Mook-Jung, I. (2014). Reduced IRE1alpha mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell Death Dis. 5:e1188. doi: 10.1038/cddis.2014.129.
Soriani, O., and Kourrich, S. (2019). The Sigma-1 receptor: when adaptive regulation of cell electrical activity contributes to stimulant addiction and Cancer. Front. Neurosci. 13:1186. doi: 10.3389/fnins.2019.01186.
Starenki, D., Hong, S. K., Lloyd, R. V., and Park, J. I. (2015). Mortalin (GRP75/HSPA9) upregulation promotes survival and proliferation of medullary thyroid carcinoma cells. Oncogene 34, 4624–4634. doi: 10.1038/onc.2014.392.
Stefan, C. J., Manford, A. G., and Emr, S. D. (2013). ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 25, 434–442. doi: 10.1016/j.ceb.2013.02.020.
Subramanian, A., Capalbo, A., Iyengar, N. R., Rizzo, R., di Campli, A., Di Martino, R., et al. (2019). Auto-regulation of secretory flux by sensing and responding to the folded cargo protein load in the endoplasmic reticulum. Cell 176, 1461–1476 e1423. doi: 10.1016/j.cell.2019.01.035.
Sun, F. C., Wei, S., Li, C. W., Chang, Y. S., Chao, C. C., and Lai, Y. K. (2006). Localization of GRP78 to mitochondria under the unfolded protein response. Biochem. J. 396, 31–39. doi: 10.1042/BJ20051916.
Sun, Y., Jiang, F., Pan, Y., Chen, X., Chen, J., Wang, Y., et al. (2018). XBP1 promotes tumor invasion and is associated with poor prognosis in oral squamous cell carcinoma. Oncol. Rep. 40, 988–998. doi: 10.3892/or.2018.6498.
Szabadkai, G., Bianchi, K., Varnai, P., De Stefani, D., Wieckowski, M. R., Cavagna, D., et al. (2006). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911. doi: 10.1083/jcb.200608073.
Takeda, K., Nagashima, S., Shiiba, I., Uda, A., Tokuyama, T., Ito, N., et al. (2019). MITOL prevents ER stress-induced apoptosis by IRE1alpha ubiquitylation at ER-mitochondria contact sites. EMBO J. 38:e100999. doi: 10.15252/embj.2018100999.
Tanaka, T., Kutomi, G., Kajiwara, T., Kukita, K., Kochin, V., Kanaseki, T., et al. (2017). Cancer-associated oxidoreductase ERO1-alpha promotes immune escape through up-regulation of PD-L1 in human breast cancer. Oncotarget 8, 24706–24718. doi: 10.18632/oncotarget.14960.
Tang, Z., Li, C., Kang, B., Gao, G., and Zhang, Z. (2017). GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102. doi: 10.1093/nar/gkx247.
Tesei, A., Cortesi, M., Zamagni, A., Arienti, C., Pignatta, S., Zanoni, M., et al. (2018). Sigma receptors as endoplasmic reticulum stress “Gatekeepers” and their modulators as emerging new weapons in the fight Against Cancer. Front. Pharmacol. 9:711. doi: 10.3389/fphar.2018.00711.
Upton, J. P., Wang, L., Han, D., Wang, E. S., Huskey, N. E., Lim, L., et al. (2012). IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338, 818–822. doi: 10.1126/science.1226191.
Urano, F., Wang, X., Bertolotti, A., Zhang, Y., Chung, P., Harding, H. P., et al. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666. doi: 10.1126/science.287.5453.664.
Urra, H., Dufey, E., Lisbona, F., Rojas-Rivera, D., and Hetz, C. (2013). When ER stress reaches a dead end. Biochim. Biophys. Acta 1833, 3507–3517. doi: 10.1016/j.bbamcr.2013.07.024.
van Vliet, A. R., and Agostinis, P. (2016). When under pressure, get closer: PERKing up membrane contact sites during ER stress. Biochem. Soc. Trans. 44, 499–504. doi: 10.1042/BST20150272.
Vance, J. E. (2014). MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841, 595–609. doi: 10.1016/j.bbalip.2013.11.014.
Vance, J. E., and Shiao, Y. J. (1996). Intracellular trafficking of phospholipids: import of phosphatidylserine into mitochondria. Anticancer Res. 16, 1333–1339.
Verfaillie, T., Rubio, N., Garg, A. D., Bultynck, G., Rizzuto, R., Decuypere, J. P., et al. (2012). PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 19, 1880–1891. doi: 10.1038/cdd.2012.74.
Voeltz, G. K., and Barr, F. A. (2013). Cell organelles. Curr. Opin. Cell Biol. 25, 403–405. doi: 10.1016/j.ceb.2013.06.001.
Wadhwa, R., Takano, S., Kaur, K., Deocaris, C. C., Pereira-Smith, O. M., Reddel, R. R., et al. (2006). Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int. J. Cancer 118, 2973–2980. doi: 10.1002/ijc.21773.
Walter, M. F., and Schultz, J. E. (1981). Calcium receptor protein calmodulin isolated from cilia and cells of Paramecium tetraurelia. Eur. J. Cell Biol. 24, 97–100.
Walter, P., and Ron, D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086. doi: 10.1126/science.1209038.
Walther, T. C., Chung, J., and Farese, R. V. Jr. (2017). Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 33, 491–510. doi: 10.1146/annurev-cellbio-100616-160608.
Wang, M., and Kaufman, R. J. (2014). The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 14, 581–597. doi: 10.1038/nrc3800.
Wang, R., Sun, D.Z., Song, C.Q., Xu, Y.M., Liu, W., Liu, Z., et al. (2018). Eukaryotic translation initiation factor 2 subunit alpha (eIF2alpha) inhibitor salubrinal attenuates paraquat-induced human lung epithelial-like A549 cell apoptosis by regulating the PERK-eIF2alpha signaling pathway. Toxicol. Vitro 46, 58–65. doi: 10.1016/j.tiv.2017.10.006.
Wang, X. Z., Harding, H. P., Zhang, Y., Jolicoeur, E. M., Kuroda, M., and Ron, D. (1998). Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17, 5708–5717. doi: 10.1093/emboj/17.19.5708.
Wang, Z., Yin, F., Xu, J., Zhang, T., Wang, G., Mao, M., et al. (2019). CYT997(Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J. Exp. Clin. Cancer Res. 38:44. doi: 10.1186/s13046-019-1047-1049.
Westrate, L. M., Lee, J. E., Prinz, W. A., and Voeltz, G. K. (2015). Form follows function: the importance of endoplasmic reticulum shape. Annu. Rev. Biochem. 84, 791–811. doi: 10.1146/annurev-biochem-072711-163501.
Wimbauer, F., Yang, C., Shogren, K. L., Zhang, M., Goyal, R., Riester, S. M., et al. (2012). Regulation of interferon pathway in 2-methoxyestradiol-treated osteosarcoma cells. BMC Cancer 12:93. doi: 10.1186/1471-2407-12-93.
Wu, H., Carvalho, P., and Voeltz, G. K. (2018). Here, there, and everywhere: the importance of ER membrane contact sites. Science 361:eaan5835. doi: 10.1126/science.aan5835.
Wu, M. S., Chien, C. C., Jargalsaikhan, G., Ilsan, N. A., and Chen, Y. C. (2019). Activation of PERK contributes to apoptosis and G2/M arrest by microtubule disruptors in human colorectal carcinoma cells (double dagger). Cancers (Basel) 12:97. doi: 10.3390/cancers12010097.
Xia, T., Tong, S., Fan, K., Zhai, W., Fang, B., Wang, S. H., et al. (2016). XBP1 induces MMP-9 expression to promote proliferation and invasion in human esophageal squamous cell carcinoma. Am. J. Cancer Res. 6, 2031–2040.
Xie, J., Tao, Z. H., Zhao, J., Li, T., Wu, Z. H., Zhang, J. F., et al. (2016). Glucose regulated protein 78 (GRP78) inhibits apoptosis and attentinutes chemosensitivity of gemcitabine in breast cancer cell via AKT/mitochondrial apoptotic pathway. Biochem. Biophys. Res. Commun. 474, 612–619. doi: 10.1016/j.bbrc.2016.03.002.
Xing, X., Lai, M., Wang, Y., Xu, E., and Huang, Q. (2006). Overexpression of glucose-regulated protein 78 in colon cancer. Clin. Chim. Acta 364, 308–315. doi: 10.1016/j.cca.2005.07.016.
Yamaguchi, H., and Wang, H. G. (2004). CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279, 45495–45502. doi: 10.1074/jbc.M406933200.
Yang, J., Cheng, D., Zhou, S., Zhu, B., Hu, T., and Yang, Q. (2015). Overexpression of X-Box Binding Protein 1 (XBP1) correlates to poor prognosis and up-regulation of PI3K/mTOR in Human osteosarcoma. Int. J. Mol. Sci. 16, 28635–28646. doi: 10.3390/ijms161226123.
Yu, C. L., Yang, S. F., Hung, T. W., Lin, C. L., Hsieh, Y. H., and Chiou, H. L. (2019). Inhibition of eIF2alpha dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis 10:418. doi: 10.1038/s41419-019-1639-1635.
Zhang, K., and Kaufman, R. J. (2006). The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66(2 Suppl 1), S102–109. doi: 10.1212/01.wnl.0000192306.98198.ec.
Zhao, L., and Ackerman, S. L. (2006). Endoplasmic reticulum stress in health and disease. Curr. Opin. Cell Biol. 18, 444–452. doi: 10.1016/j.ceb.2006.06.005.
Zhao, N., Cao, J., Xu, L., Tang, Q., Dobrolecki, L. E., Lv, X., et al. (2018). Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. J. Clin. Invest. 128, 1283–1299. doi: 10.1172/JCI95873.
Keywords: unfolded protein response, mitochondria–ER contacts, endoplasmic reticulum, molecular chaperones, cancer
Citation: Amodio G, Pagliara V, Moltedo O and Remondelli P (2021) Structural and Functional Significance of the Endoplasmic Reticulum Unfolded Protein Response Transducers and Chaperones at the Mitochondria–ER Contacts: A Cancer Perspective. Front. Cell Dev. Biol. 9:641194. doi: 10.3389/fcell.2021.641194
Received: 13 December 2020; Accepted: 22 February 2021;
Published: 26 March 2021.
Edited by:
Simone Patergnani, University of Ferrara, ItalyReviewed by:
Claudia Morganti, Albert Einstein College of Medicine, United StatesEsmaa Bouhamida, University of Ferrara, Italy
Copyright © 2021 Amodio, Pagliara, Moltedo and Remondelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Remondelli, premondelli@unisa.it
 Giuseppina Amodio
Giuseppina Amodio Valentina Pagliara
Valentina Pagliara Ornella Moltedo2
Ornella Moltedo2  Paolo Remondelli
Paolo Remondelli