Relationship between the neutrophil to high-density lipoprotein cholesterol ratio and severity of coronary artery disease in patients with stable coronary artery disease
- 1Department of Endocrinology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Cardiology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Heart Function Examination Room, Tongji Hospital, Tongji University, Shanghai, China
Objective: To evaluate the link between the neutrophil to HDL-C ratio (NHR) and the degree of coronary stenosis in patients with stable coronary artery disease (CAD).
Materials and methods: Totally 766 individuals who attended our clinic for coronary angiography between January 2019 and January 2021 were included in this study. The participants were divided into two groups, including the CAD group and control group. Spearman correlation analysis was used to investigate the association between NHR and Gensini score and logistic regression analysis was performed to determine the influence of NHR on CAD and severe CAD. Receiver operating characteristic (ROC) curve was constructed to analyze the predictive value of NHR for severe CAD.
Results: The CAD group had a substantially higher median NHR than the control group (3.7 vs. 3.2, P < 0.01). There was a positive correlation between NHR and Gensini score, as well as the frequency of coronary artery plaques. Logistic regression demonstrated that NHR was an independent contributor for CAD and severe CAD. In ROC analysis, the area under the ROC curve (AUC) for NHR was larger than that for neutrophil, HDL-C or LDL-C/HDL-C, and the differences were statistically significant (all P < 0.05). The NHR limit that offered the most accurate prediction of severe CAD according to the greatest possible value of the Youden index, was 3.88, with a sensitivity of 62.6% and a specificity of 66.2%.
Conclusion: NHR was not only associated with the occurrence and seriousness of CAD, but also a better predictor of severe CAD than neutrophil, HDL-C or LDL-C/HDL-C.
Introduction
Coronary artery disease (CAD) is a global epidemic disorder with high morbidity and mortality (1). With increasing urbanization, changes in lifestyle and population aging, the incidence of CAD is expected to increase globally in the next decade. The development of atherosclerosis is a primary contributor to the pathophysiology of CAD, and studies have shown that inflammation and lipid metabolic disorders are closely related to atherosclerosis (1–6).
Traditional inflammatory parameters such as White blood cell (WBC) and its subtypes, including neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), platelet to lymphocyte ratio (PLR), monocyte to high density cholesterol ratio (MHR) and mean platelet volume to lymphocyte ratio (MPVLR) (7–13) are commonly used to assess inflammation and calculate the potential for cardiovascular events. Additionally, these parameters are effective indicators of CAD initiation and advancement (7, 9, 10, 14). There is also a significant negative relationship between levels of HDL-C and the risk of CAD, with a better prognosis for CAD related to having a high HDL-C level (15). It has been demonstrated that HDL-C is advantageous in a variety of ways, including its ability to reduce the risk of cardiovascular disease, for example, stimulating cholesterol efflux and reversing cholesterol transport (16, 17), decreasing endothelial activation (18), preventing LDL-C oxidation (19), etc. Furthermore, previous studies indicated that activated neutrophils could not only have an impact on the composition and function of HDL-C (20), but its function could also be regulated by HDL-C (21).
As mentioned above, atherogenesis is facilitated by several factors, including neutrophils and HDL-C, therefore, it was hypothesized that the neutrophil to HDL-C ratio (NHR) is an integrated biomarker more relevant to atherosclerosis as it would offer information about both inflammation and lipid metabolism. For this reason, our study investigated the connection between NHR and CAD and develop the projected value of NHR for the grading of coronary stenosis in individuals with stable CAD.
Materials and methods
Study design and subjects
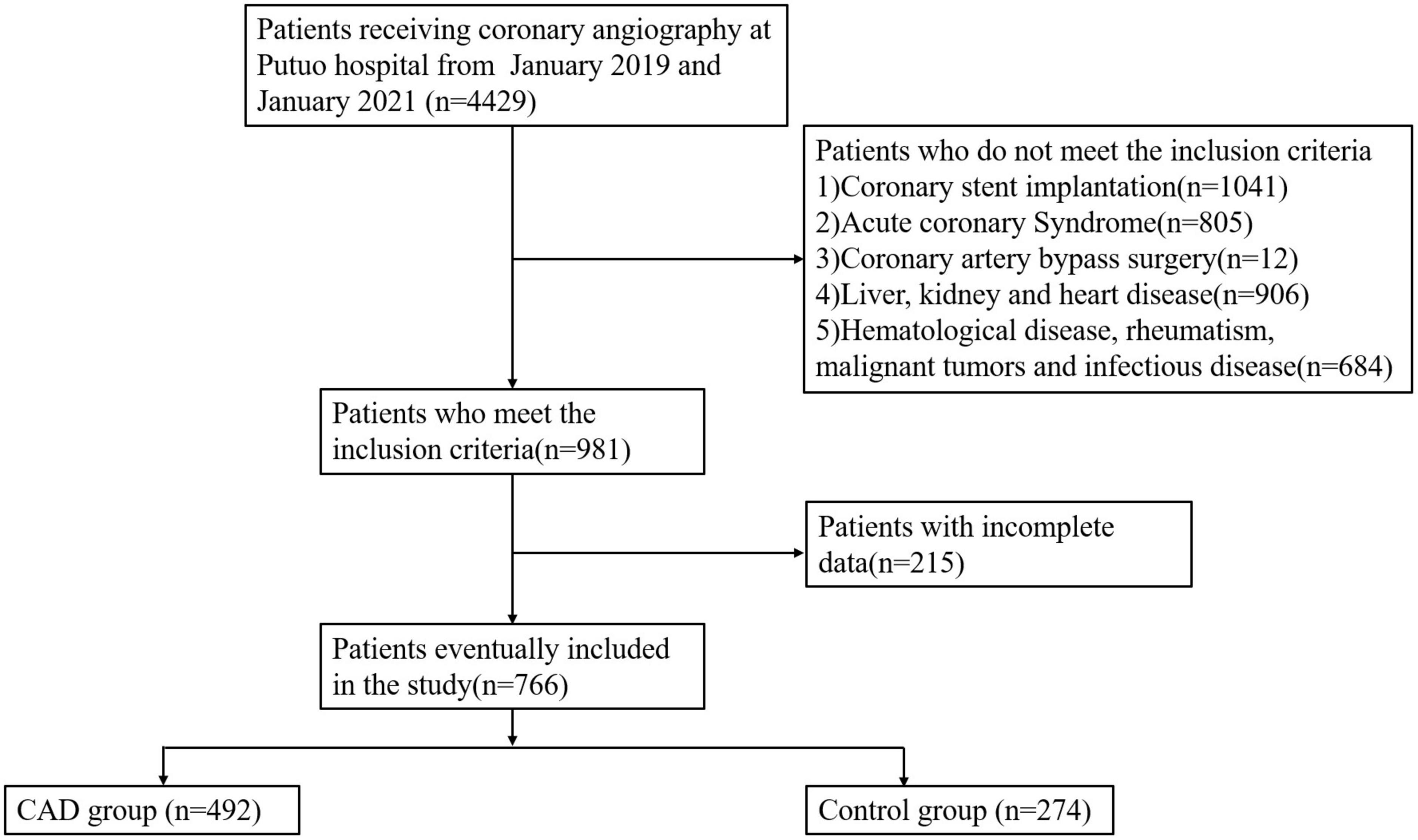
This retrospective study was conducted in a single center at Putuo hospital, which is affiliated to the Shanghai University of Traditional Chinese Medicine. 4,429 participants presented with CAD-related symptoms such as chest tightness or pain between January 2019 and January 2021 underwent coronary angiography. Individuals with acute coronary syndrome, coronary stent implantation, liver and kidney disease, infectious disease, severe heart failure, valvular heart disease, hematological diseases, rheumatic diseases, malignant tumors, who had undergone bypass surgery of their coronary arteries and for whom there were no medical records when the study was conducted were excluded. Finally, 766 patients participated in this study. CAD patients had one of the main coronary arteries or more with greater than 50% lumen stenosis (n = 492), whereas controls had no luminal stenosis or lower than 30% (n = 274) (Figure 1).
The study was approved by the institutional review board of Shanghai University of Traditional Chinese Medicine’s Putuo hospital and was conducted according to the principles articulated in the second version of the Helsinki Declaration. All participants provided written informed consent and their individual information was kept strictly confidential.
Anthropometric and biochemical measurements
At the time of admission, the patient’s anthropometric measurements and biochemical variables were recorded. After subjects had rested for 10 min and been seated, their blood pressure was measured twice. The mean blood pressure was then calculated. All patients’ height and weight were measured to calculated the body mass index (BMI) by the formula of weight/height2 (kg/m2). All patients were required to fast the previous night before having peripheral venous blood samples taken for measurement of CRP, FBG, PBG, TC, TG, HDL-C, LDL-C, WBC, neutrophils, and serum creatinine automatically using a Beckman Colter AU5800 biochemical analyzer (Brea, CA, USA). The CKD-EPI formula was used to derive estimated Glomerular Filtration Rate (eGFR) (22). HbA1c was measured by high-performance liquid chromatography on a Tosoh Automated Glycohemoglobin Analyzer HLC-723G11(Shunan, Yamaguchi, Japan). LDL-C/HDL-C was calculated by taking LDL-C (mmol/l) and dividing it by HDL-C (mmol/l), while NHR was determined by dividing the neutrophil counts (in 109/l) by HDL-C (in mmol/l).
Systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg (23) were used as the criteria for the diagnosis of hypertension and/or the need for the treatment with antihypertensive drugs. Type 2 diabetes mellitus(T2DM) was defined by the American Diabetes Association in 2021 (24) as follows: FBG ≥ 7.0 mM, 2-h PBG ≥ 11.1 mM and/or HbA1c ≥ 6.5% or the subjects who are using glucose-controlling medicines.
Coronary angiography and assessment of severity of coronary artery disease
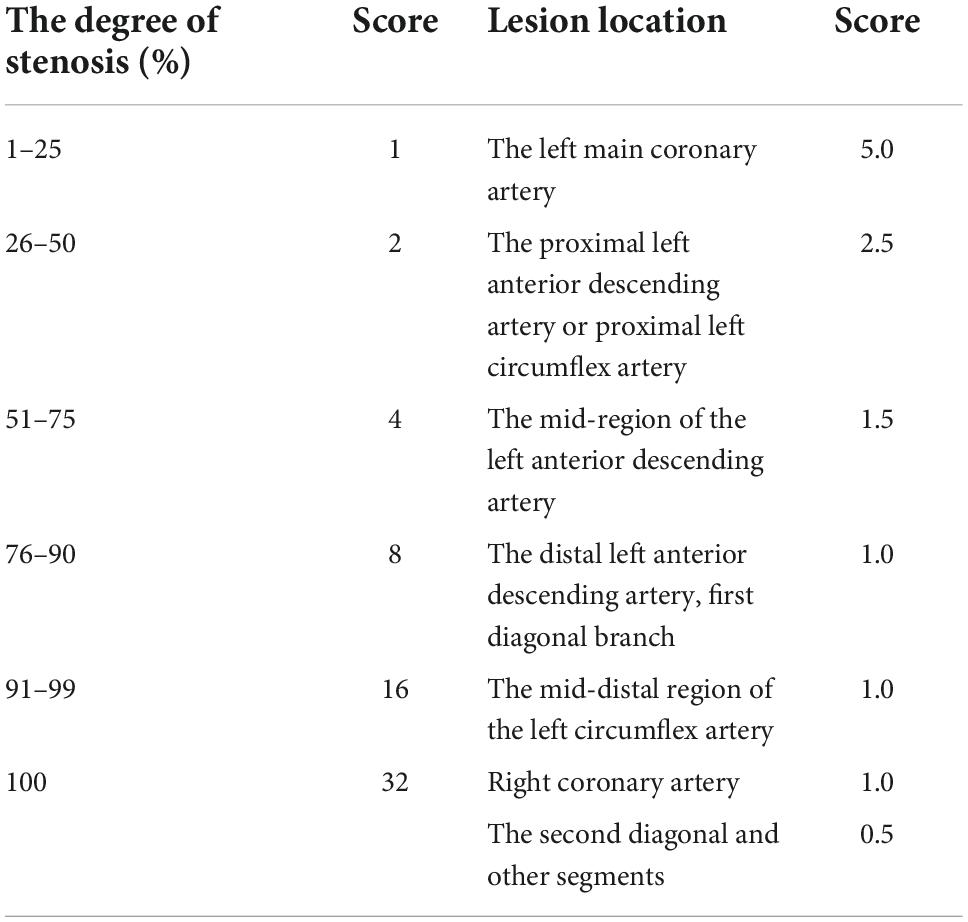
All participants underwent coronary angiography with the cardiologist blinded to the patient’s details. At least 50% stenosis in one of the four main coronary arteries (left main, anterior descending, left circumflex, and right coronary) was required to diagnose CAD. The coronary artery stenosis disorders were classified as single-vessel (n = 178), double-vessel (n = 154), and triple-vessel (n = 160) forms based on the number of affected coronary arteries. The Gensini score was utilized to determine the degree of CAD intensity, which was calculated by summing the values for the location and luminal narrowing of each lesion (Table 1). The participants were divided into three categories (tertiles) based on their Gensini scores: tertile 1 ≤ 24 points (n = 180), tertile 2 from 24–45 points (n = 149), and tertile 3 had a score ≥ 45 points (n = 163). Patients with a Gensini score ≥ 45 points were considered to have severe CAD.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0. The Kolmogorov–Smirnov test was used to validate the distribution of continuous variables. The mean and SD of regularly distributed variables are presented, whereas the median is provided for non-normally distributed data (interquartile range). Categorical variables are represented numerically and statistically in numbers and percentages. The independent samples t-test and the Mann–Whitney U test were used to assess the data, depending on whether or not they followed a normal distribution. For comparison among three groups, ANOVA or Kruskal–Wallis (KW) test was performed according to the nature of the variables. Chi–square test was used to analyse the statistical significance of differences between groups of categorical variables. The relationship between NHR and CAD was investigated using multivariable logistic regression and a ROC curve was used to evaluate the sensitivity and specificity of NHR’s ability to predict severe CAD. The maximum Youden index was utilized to establish the optimal cut-off for the NHR. MedCalc was used to compare the AUCs for NHR, neutrophil, HDL-C and LDL-C/HDL-C. The level of statistical significance was determined to be a two-tailed P < 0.05.
Results
Baseline patient characteristics
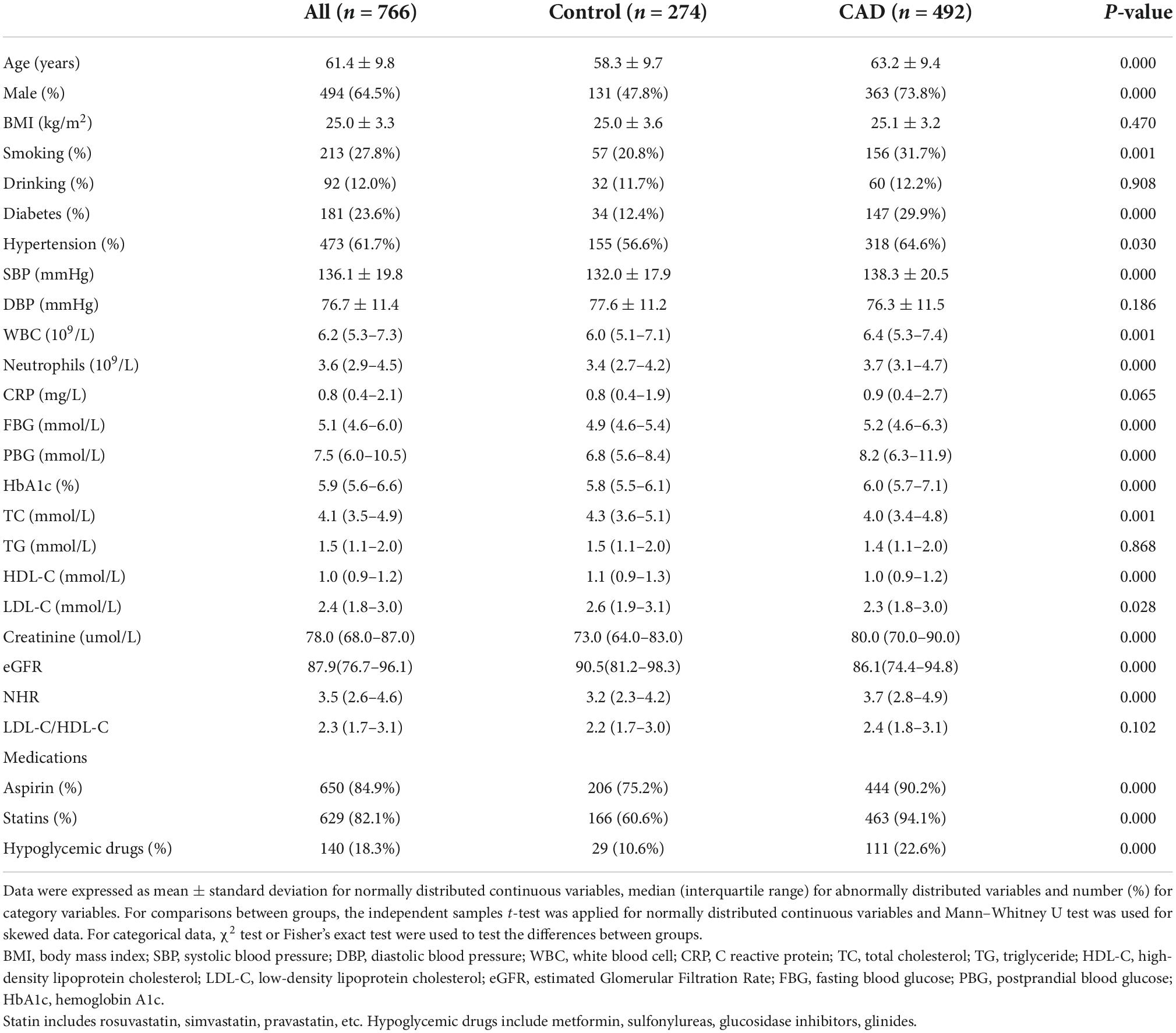
Table 2 displays the demographic and biochemical variables for the 766 patients. Compared to the control group, the prevalence of males, hypertension, diabetes, smoking, and the use of medication was considerably greater among those with CAD (all P < 0.01). Besides, subjects with CAD were of older age and had higher SBP, serum creatinine and poorer glycemic management than their counterparts (all P < 0.01). When comparing CAD patients with non-CAD patients, the total WBCs and neutrophil counts were higher, whereas levels of TC, TG, HDL-C, and LDL-C were lower in the former group. The CAD group also had higher median NHR levels (3.7 vs. 3.2, P < 0.01). BMI, DBP, CRP, LDL-C/HDL-C, and frequency of alcohol consumption were not different between the two groups.
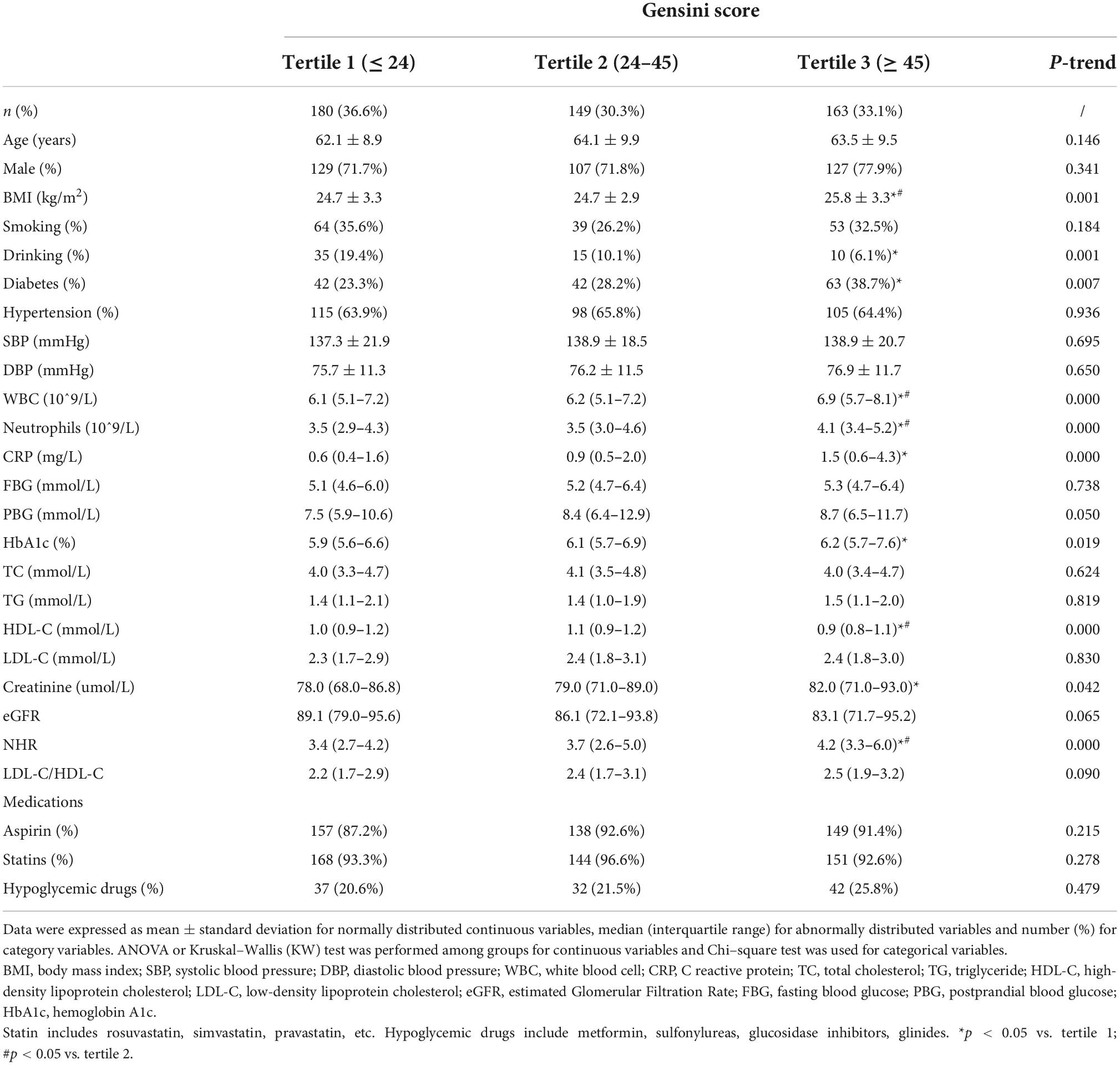
Table 3 summarized the baseline characteristics of the population stratified by tertiles of Gensini score. P for trend was calculated with each tertile of Gensini score taken as a unit. The participants with the highest tertile of Gensini score had higher levels of BMI, WBCs, neutrophils, CRP, HbA1c, Cr, NHR, and higher prevalence of diabetes, but lower levels of HDL-C and less alcohol drinkers, compared to those with the lowest tertile of Gensini score (all P for trend < 0.05).
NHR is associated with CAD and severe CAD
It was established that NHR is a separate risk factor for CAD and severe CAD. Table 4 shows that in the unadjusted model, total NHR was positively correlated to CAD (OR = 1.37, 95% CI 1.23–1.52) and severe CAD (OR = 1.42, 95% CI 1.27–1.59). These significant links were maintained (all P < 0.01) after controlling for characteristics such as gender, age, BMI, smoking status, diabetes and hypertension (Model 2), or after accounting for LDL-C, creatinine, HbA1c, and CRP levels (Model 3), and after accounting for medications (Model 4). In the unadjusted model, each 1SD increase in NHR level was significantly associated with 1.73-folds (95% CI 1.44–2.08) increased risk of CAD and 1.85-folds (95% CI 1.52–2.25) increased risk of severe CAD. The adjusted ORs (95% CI) for CAD and severe CAD were 1.55 (1.27–1.91) and 1.84 (1.50–2.26) in Model 2, 1.50 (1.21–1.86) and 1.86 (1.50–2.30) in Model 3, 1.41 (1.12–1.77) and 1.85 (1.50–2.29) in Model 4 (all P < 0.01).
Relationship between NHR and the severity of coronary artery disease
As shown in Figure 2A, an incremental increase in NHR was found with the increasing number of coronary artery lesions. Median NHR levels in the single, double, and multiple vessel disease groups were 3.55 (2.74–4.64), 3.76 (2.79–4.94) and 3.91 (3.00–5.63), respectively, indicating significant differences (P < 0.05). According to the tertiles of Gensini score, median NHR levels from low tertile to high tertile groups were 3.42 (2.75–4.25), 3.64 (2.64–5.05), and 4.28 (3.28–6.03), respectively. The high tertile group also had NHR levels that were noticeably higher than the middle tertile group as well as the low tertile group (Figure 2B). In addition, Spearman correlation analysis revealed that NHR exhibited moderate correlation with Gensini scores (r = 0.287, P = 0.000). The scatter diagram was shown in Figure 2C. And a weak correlation was found between neutrophil (r = 0.200, P = 0.000), LDL-C/HDL-C (r = 0.091, P = 0.044) and HDL-C (r = –0.168, P = 0.000), with the Gensini score (Figures 2D–F).
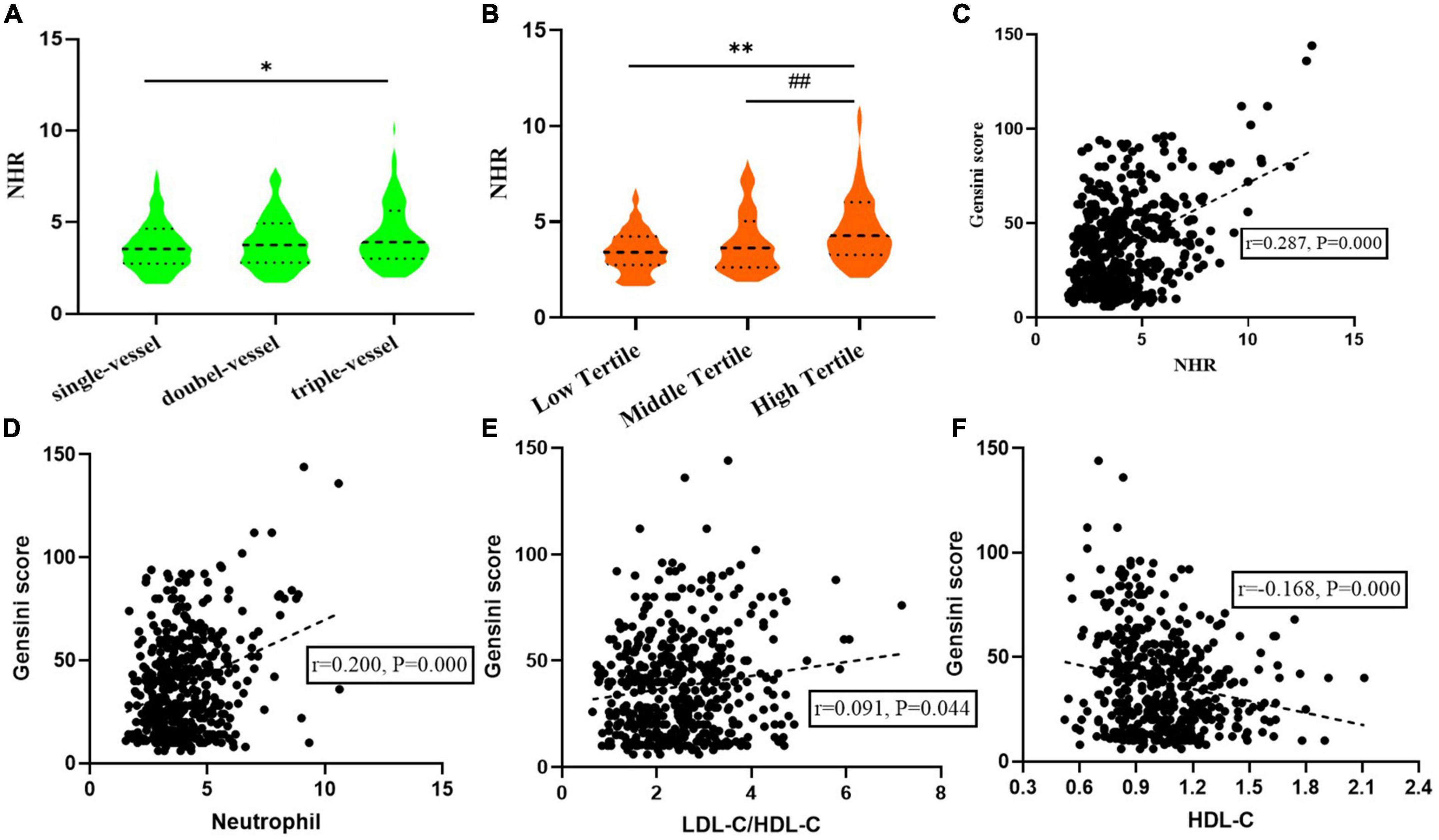
Figure 2. Relationship between neutrophil to HDL-C ratio (NHR) and the severity of coronary artery disease. (A) Relationship between NHR and the number of coronary artery lesions. (B) NHR levels from low to high tertile groups according to Gensini score. (C–F) Correction between NHR, Neutrophil, LDL-C/HDL-C, HDL-C, and Gensini score. *P < 0.01 compared with single-vessel group. **P < 0.01 compared with low tertile group. ##P < 0.01 compared with middle tertile group.
Predictive value of neutrophil to HDL-C ratio (NHR) for the severity of coronary artery disease
Figure 3 shows the ROC curves of NHR, neutrophil, HDL-C and LDL-C/HDL-C for predicting severe CAD. Comparing NHR with neutrophil, HDL-C and LDL-C/HDL-C based ROC curves, the AUC for NHR was greater, and the differences was statistically significant (all P < 0.05). Analysis of ROC curve indicated that 3.88 was the optimal threshold of NHR in detecting severe CAD, which accorded with 62.6% sensitivity and 66.2% specificity.
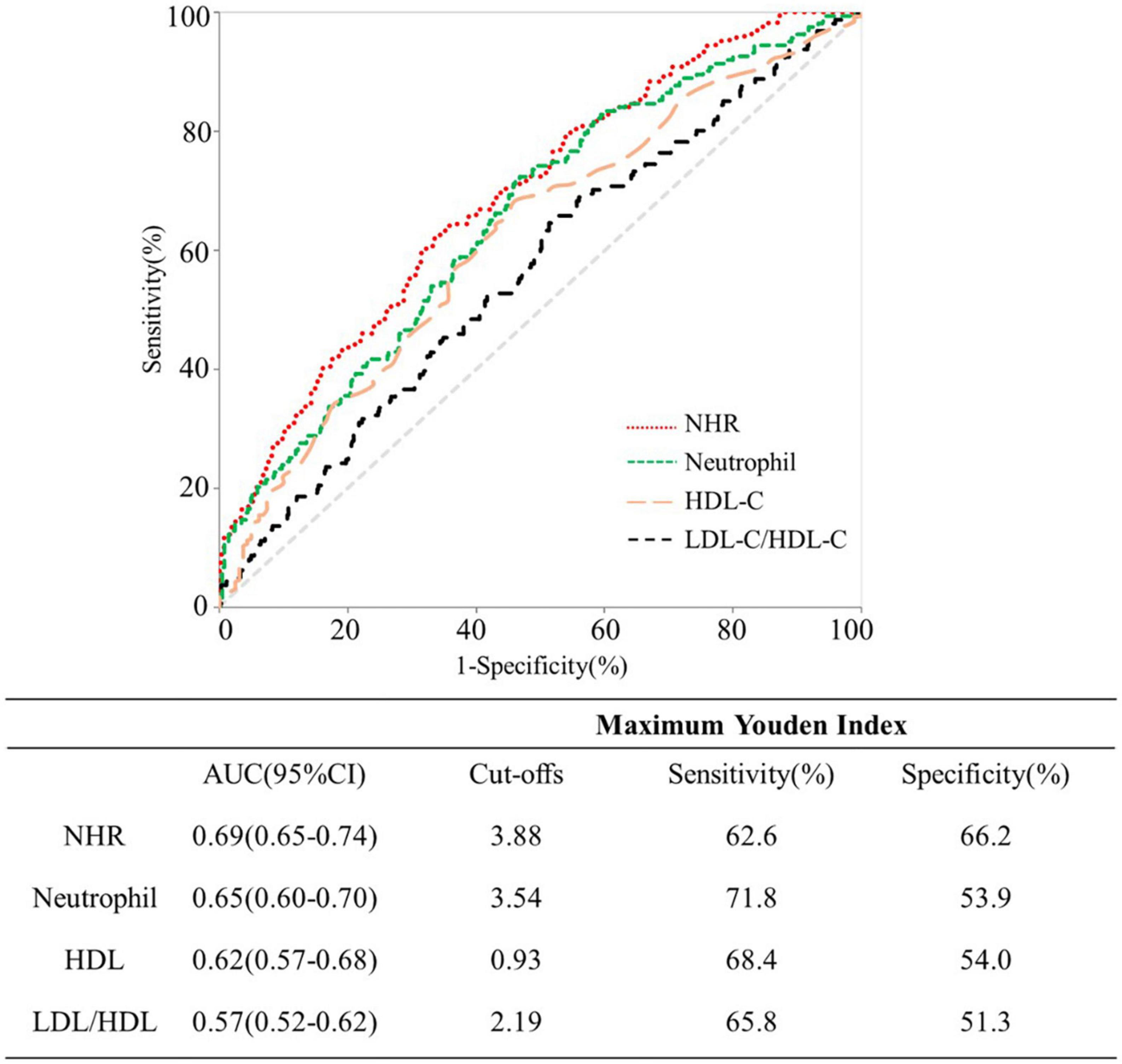
Figure 3. Receiver operating characteristic curves for predicting severe coronary artery disease (CAD).
Discussion
According to the reports, CAD accounts for 44.60% of all deaths in rural areas and 42.51% in urban areas, respectively (25), leading to the major cause of mortality worldwide (26). Plasma inflammatory biomarkers aid in predicting a future CAD event and are involved in the onset, development, and instability of atherosclerotic plaques (27–29). Recent studies also focused more on the association between lipid-related biomarkers and CAD (30). Our study suggested that the integrated biomarker NHR (the combination of inflammation and lipid metabolism level) may be more comprehensive and practical in predicting the initiation, development, and prognosis of CAD. Adjusting for potential confounders, NHR was a significant risk factor for CAD and severe CAD. Furthermore, the predicative value of NHR was superior to neutrophil, HDL-C or LDL-C/HDL-C for severe CAD, with a plasma NHR > 3.88 predicting severe CAD with a sensitivity of 62.6% and a specificity of 66.2%.
There was a correlation between high NHR and severe CAD, possibly because inflammation is involved in the pathogenesis of atherosclerosis, which is largely mediated by circulating neutrophils (31). The role of neutrophils in the process of atherosclerosis has been reported (32) and they were considered as a marker of inflammation as well as a predictor of cardiovascular risk (33). Neutrophils were abundant in coronary artery lesions (34) and increased neutrophil counts were associated with an elevated risk of cardiac complications (35). Furthermore, a previous study confirmed that neutrophil count was associated with the complex coronary stenosis and was an independent predictor of multiple complex stenosis (33). In addition, we found that the neutrophil level in the CAD group was significantly higher than that in the control group and the neutrophil correlated positively with Gensini score. However, our research did not find the statistical difference in CRP levels between the two groups. This could reasonably be associated with the use of statins. Studies have reported that statins could suppress the production of CRP in the liver by interrupting the production of IL-6; decrease the CRP levels produced in the atherosclerotic plaques; reduce the CRP-stimulating factor such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) on the activated endothelial cells (36, 37).
A negative correlation between both myocardial infarction (MI) and CAD with HDL-C was observed (38). A moderate increase in HDL-C may even reduce cardiovascular risk to a certain extent (39–41). HDL-C exerted its atheroprotective effect in multiple ways, such as reverse cholesterol transport (RCT) activity, anti-oxidant and anti-inflammatory properties, endothelial protection, etc., (42, 43). Crucially, HDL-C may be related to the lipid raft abundance by inhibiting neutrophils activation, attachment, diffusion, and migration (31). In our study, we also found that HDL-C was negatively associated with Gensini score, but the lipid levels in CAD group were lower than that in control group. A possible explanation for this is that people with CAD are more likely to take statins than those without CAD (94.1% vs. 60.6%, P < 0.01).
According to the above analysis, neutrophil increases and HDL-C level decreases in CAD patients, suggesting that the comprehensive indicator NHR may make more contribution for CAD. Chen et al. (44) found that NHR was a predictive marker for metabolic syndrome. Despite previous research linking NHR to developing CAD (30), the participants in that study were all older people who had suffered an acute myocardial infarction (AMI). In keeping with the earlier study of Tuli Kou et al. (45), we discovered that NHR was not only linked to coronary artery stenosis, but also an independent factor for CAD. We also compared NHR’s predictive power to that of the neutrophil count, HDL-C and the LDL-C/HDL-C ratio. Moreover, our study assessed the predicative value of NHR for severe CAD (Gensini score ≥ 45 points) and had a relatively larger sample size.
The Gensini score is one of the most regularly used CAD severity markers, with varied weight coefficients (46, 47). We also identified a positive correlation between NHR and Gensini scores in the present investigation. Identifying CAD patients at high risk may be improved by using both the NHR and the Gensini score.
In summary, we believe that NHR is an effective serological biomarker to predict CAD and assess the degree of coronary stenosis. Nonetheless, our research was not without its caveats. First, because our study was conducted at a single center, the findings cannot be generalized. Second, it was a retrospective and observational study, so the causal relationship between NHR and CAD could not be determined. Finally, we only collected NHR levels at admission and did not investigate the prognostic value of NHR on adverse cardiovascular outcomes. Further multi-center and prospective studies are needed to confirm these findings.
Conclusion
The high ratio of neutrophils to HDL (NHR) was related to an increased risk of serious CAD. Unlike many other biological parameters, NHR may be inexpensively calculated from a complete blood count on admission, so has the potential as a simple clinical marker for the assessment of CAD.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Shanghai University of Traditional Chinese Medicine’s Putuo hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JG wrote the manuscript. JL and WS performed the statistical analysis. CZ, HW, and JX participated in the data collection and checked the data. BX contributed to the discussion. HZ evaluated the severity of coronary artery disease. TL and WT participated in the design of this study and edited the manuscript. All authors have participated in the work and have reviewed and agreed with the content of the article.
Funding
This study was supported by Clinical Characteristic of Health System in Putuo District, Shanghai (2020tszk01) and Shanghai Key Medical Specialities (ZK2019B16).
Acknowledgments
We would like to thank all the participants and the research team involved in the present project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; CAD, coronary artery disease; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; FBG, fasting blood glucose; PBG, postprandial blood glucose; HbA1c, glycosylated hemoglobin A1c; eGFR, estimated Glomerular Filtration Rate; ROC, receiver operating characteristic curve; AUC, area under the ROC curve; OR, odds ratio; WBC, White blood cell; SD, Standard deviation; NLR, neutrophil to lymphocyte ratio; MLR, monocyte to lymphocyte ratio; PLR, platelet to lymphocyte ratio; MHR, monocyte to high-density cholesterol ratio; RCT, reverse cholesterol transport; MPVLR, mean platelet volume to lymphocyte ratio; MI, myocardial infarction; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
References
1. Akyel A, Yayla Ç, Erat M, Çimen T, Doğan M, Açıkel S, et al. Neutrophil-to-lymphocyte ratio predicts hemodynamic significance of coronary artery stenosis. Anatol J Cardiol. (2015) 15:1002–7. doi: 10.5152/akd.2015.5909
2. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
3. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. (2005) 111:3481–8. doi: 10.1161/circulationaha.105.537878
4. Bartels ED, Christoffersen C, Lindholm MW, Nielsen LB, Bartels ED. Altered metabolism of LDL in the arterial wall precedes atherosclerosis regression. Circ Res. (2015) 117:933–42. doi: 10.1161/circresaha.115.307182
5. Forrester JS. Prevention of plaque rupture: a new paradigm of therapy. Ann Intern Med. (2002) 137:823–33. doi: 10.7326/0003-4819-137-10-200211190-00012
6. Guo TM, Cheng B, Ke L, Guan SM, Qi BL, Li WZ, et al. Prognostic value of neutrophil to lymphocyte ratio for in-hospital mortality in elderly patients with acute myocardial infarction. Curr Med Sci. (2018) 38:354–9. doi: 10.1007/s11596-018-1887-0
7. Verdoia M, Barbieri L, Giovine G Di, Marino P, Suryapranata H, De Luca G, et al. Neutrophil to lymphocyte ratio and the extent of coronary artery disease: results from a large cohort study. Angiology. (2016) 67:75–82. doi: 10.1177/0003319715577529
8. Kose N, Akin F, Yildirim T, Ergun G, Altun I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur Rev Med Pharmacol Sci. (2019) 23:2570–5. doi: 10.26355/eurrev_201903_17406
9. Uysal OK, Sahin DY, Duran M, Turkoglu C, Yildirim A, Elbasan Z, et al. Association between uric acid and coronary collateral circulation in patients with stable coronary artery disease. Angiology. (2014) 65:227–31. doi: 10.1177/0003319713500706
10. Ji H, Li Y, Fan Z, Zuo B, Jian X, Li L, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord. (2017) 17:90. doi: 10.1186/s12872-017-0507-4
11. Ndrepepa G, Braun S, King L, Hadamitzky M, Haase HU, Birkmeier KA, et al. Association of uric acid with mortality in patients with stable coronary artery disease. Metabolism. (2012) 61:1780–6. doi: 10.1016/j.metabol.2012.05.014
12. Akboga MK, Balci KG, Maden O, Ertem AG, Kirbas O, Yayla C, et al. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med. (2016) 10:375–83. doi: 10.2217/bmm-2015-0050
13. Ornek E, Kurtul A. Relationship of mean platelet volume to lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris. Coron Artery Dis. (2017) 28:492–7. doi: 10.1097/mca.0000000000000530
14. Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. (2016) 67:89–95. doi: 10.1177/0003319715583186
15. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. (2017) 16(Suppl. 1):s27–42. doi: 10.5604/01.3001.0010.5495
16. Triolo M, Annema W, Dullaart RP, Tietge UJF. Assessing the functional properties of high-density lipoproteins: an emerging concept in cardiovascular research. Biomark Med. (2013) 7:457–72. doi: 10.2217/bmm.13.35
17. Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. (2014) 55:168–79. doi: 10.1194/jlr.R039297
18. Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. (1995) 15:1987–94. doi: 10.1161/01.atv.15.11.1987
19. Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr Opin Lipidol (2010) 21:312–8. doi: 10.1097/MOL.0b013e32833bcdc1
20. Cogny A, Atger V, Paul JL, Soni T, Moatti N. High-density lipoprotein 3 physicochemical modifications induced by interaction with human polymorphonuclear leucocytes affect their ability to remove cholesterol from cells. Biochem J. (1996) 314(Pt 1):285–92. doi: 10.1042/bj3140285
21. Curcic S, Holzer M, Frei R, Pasterk L, Schicho R, Heinemann A, et al. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim Biophys Acta. (2015) 1851:184–93. doi: 10.1016/j.bbalip.2014.11.010
22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
23. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
24. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44(Suppl. 1):S15–33. doi: 10.2337/dc21-S002
25. Chen WW, Gao RL, Liu LS, Zhu ML, Wang W, Wang YJ, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol. (2017) 14:1–10. doi: 10.11909/j.issn.1671-5411.2017.01.012
26. Guo T, Huang L, Liu C, Shan S, Li Q, Ke L, et al. The clinical value of inflammatory biomarkers in coronary artery disease: PTX3 as a new inflammatory marker. Exp Gerontol. (2017) 97:64–7. doi: 10.1016/j.exger.2017.07.018
27. Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. (2013) 62:329–37. doi: 10.1016/j.jacc.2013.03.072
28. Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. (2016) 109:708–15. doi: 10.1016/j.acvd.2016.04.002
29. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. (2019) 124:315–27. doi: 10.1161/circresaha.118.313591
30. Huang JB, Chen YS, Ji HY, Xie WM, Jiang J, Ran LS, et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: a comparison study. Lipids Health Dis. (2020) 19:59. doi: 10.1186/s12944-020-01238-2
31. Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. (2011) 31:1333–41. doi: 10.1161/atvbaha.111.226258
32. Güven R, Akyol KC, Bayar N, Güngör F, Akça AH, Çelik A. Neutrophil count as a predictor of critical coronary artery stenosis in young patients. Iran J Public Health. (2018) 47:765–7.
33. Avanzas P, Arroyo-Espliguero R, Cosín-Sales J, Quiles J, Zouridakis E, Kaski JC. Multiple complex stenoses, high neutrophil count and C-reactive protein levels in patients with chronic stable angina. Atherosclerosis. (2004) 175:151–7. doi: 10.1016/j.atherosclerosis.2004.03.013
34. Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. (2009) 31:35–47. doi: 10.1007/s00281-009-0141-z
35. Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. (2009) 30:511–2. doi: 10.1016/j.it.2009.07.002
36. Arévalo-Lorido JC. Clinical relevance for lowering C-reactive protein with statins. Ann Med. (2016) 48:516–24. doi: 10.1080/07853890.2016.1197413
37. Kandelouei T, Abbasifard M, Imani D, Aslani S, Razi B, Fasihi M, et al. Effect of statins on serum level of Hs-CRP and CRP in patients with cardiovascular diseases: a systematic review and meta-analysis of randomized controlled trials. Mediat Inflamm. (2022) 2022:8732360. doi: 10.1155/2022/8732360
38. Castelli WP. Cholesterol and lipids in the risk of coronary artery disease–the Framingham Heart Study. Can J Cardiol. (1988) 4(Suppl. A):5a–10a.
39. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. (2009) 302:1993–2000. doi: 10.1001/jama.2009.1619
40. Andersson C, Lyass A, Vasan RS, Massaro JM, D’Agostino RB Sr., Robins SJ. Long-term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham heart study. Am Heart J. (2014) 168:878–83.e1. doi: 10.1016/j.ahj.2014.08.007
41. Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, et al. High-density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory-related events versus cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. (2015) 4:e002295. doi: 10.1161/jaha.115.002295
42. Li Y, Jin P, Hou F, Zhou Y. Association between TG-to-HDL-C ratio and in-stent stenosis under optical coherence tomography guidance. J Med Syst. (2018) 43:4. doi: 10.1007/s10916-018-1119-y
43. Sun T, Hu J, Yin Z, Xu Z, Zhang L, Fan L. Low serum paraoxonase1 activity levels predict coronary artery disease severity. Oncotarget. (2017) 8:19443–54. doi: 10.18632/oncotarget.14305
44. Chen T, Chen H, Xiao H, Tang H, Xiang Z, Wang X, et al. Comparison of the value of neutrophil to high-density lipoprotein cholesterol ratio and lymphocyte to high-density lipoprotein cholesterol ratio for predicting metabolic syndrome among a population in the southern coast of China. Diabetes Metab Syndr Obes. (2020) 13:597–605. doi: 10.2147/dmso.s238990
45. Kou T, Luo H, Yin L. Relationship between neutrophils to HDL-C ratio and severity of coronary stenosis. BMC Cardiovasc Disord. (2021) 21:127. doi: 10.1186/s12872-020-01771-z
46. İşcanlı MD, Aksu N Metin, Evranos B, Aytemir K, Özmen MM. Comparison of TIMI and gensini score in patients admitted to the emergency department with chest pain, who underwent coronary angiography. Med Sci Monit. (2014) 20:343–9. doi: 10.12659/msm.889600
Keywords: neutrophil, inflammation, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), coronary artery disease
Citation: Gao J, Lu J, Sha W, Xu B, Zhang C, Wang H, Xia J, Zhang H, Tang W and Lei T (2022) Relationship between the neutrophil to high-density lipoprotein cholesterol ratio and severity of coronary artery disease in patients with stable coronary artery disease. Front. Cardiovasc. Med. 9:1015398. doi: 10.3389/fcvm.2022.1015398
Received: 09 August 2022; Accepted: 04 November 2022;
Published: 24 November 2022.
Edited by:
Istvan Szokodi, University of Pécs, HungaryReviewed by:
Ming Zhang, Beijing Anzhen Hospital, Capital Medical University, ChinaFederico Vancheri, Presidio Ospedaliero “S .Elia” Di Caltanissetta, Italy
Copyright © 2022 Gao, Lu, Sha, Xu, Zhang, Wang, Xia, Zhang, Tang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Lei, taolei_12@sina.com; Wenjun Tang, junw0627@163.com
†These authors have contributed equally to this work
 Jie Gao
Jie Gao Jun Lu
Jun Lu Wenjun Sha
Wenjun Sha Bilin Xu1
Bilin Xu1