The development of a decision aid for shared decision making in the Dutch implantable cardioverter defibrillator patient population: A novel approach to patient education
- 1Department of Cardiology, Leiden University Medical Center, Leiden, Netherlands
- 2Department of Cardiology, Sint Maarten Medical Center, Cay Hill, Sint Maarten
- 3Department of Cardiology, Maxima Medical Center, Eindhoven, Netherlands
Background: Counseling of Implantable Cardioverter-defibrillator (ICD) patients with regard to individual risks and benefits is challenging. An evidence-based decision aid tailored to the needs of Dutch ICD patients is not yet available. The objective of this pilot project was to structurally evaluate the current clinical practice in The Netherlands and the ICD patient experience, in order to develop an online decision aid to facilitate shared decision making in ICD procedures.
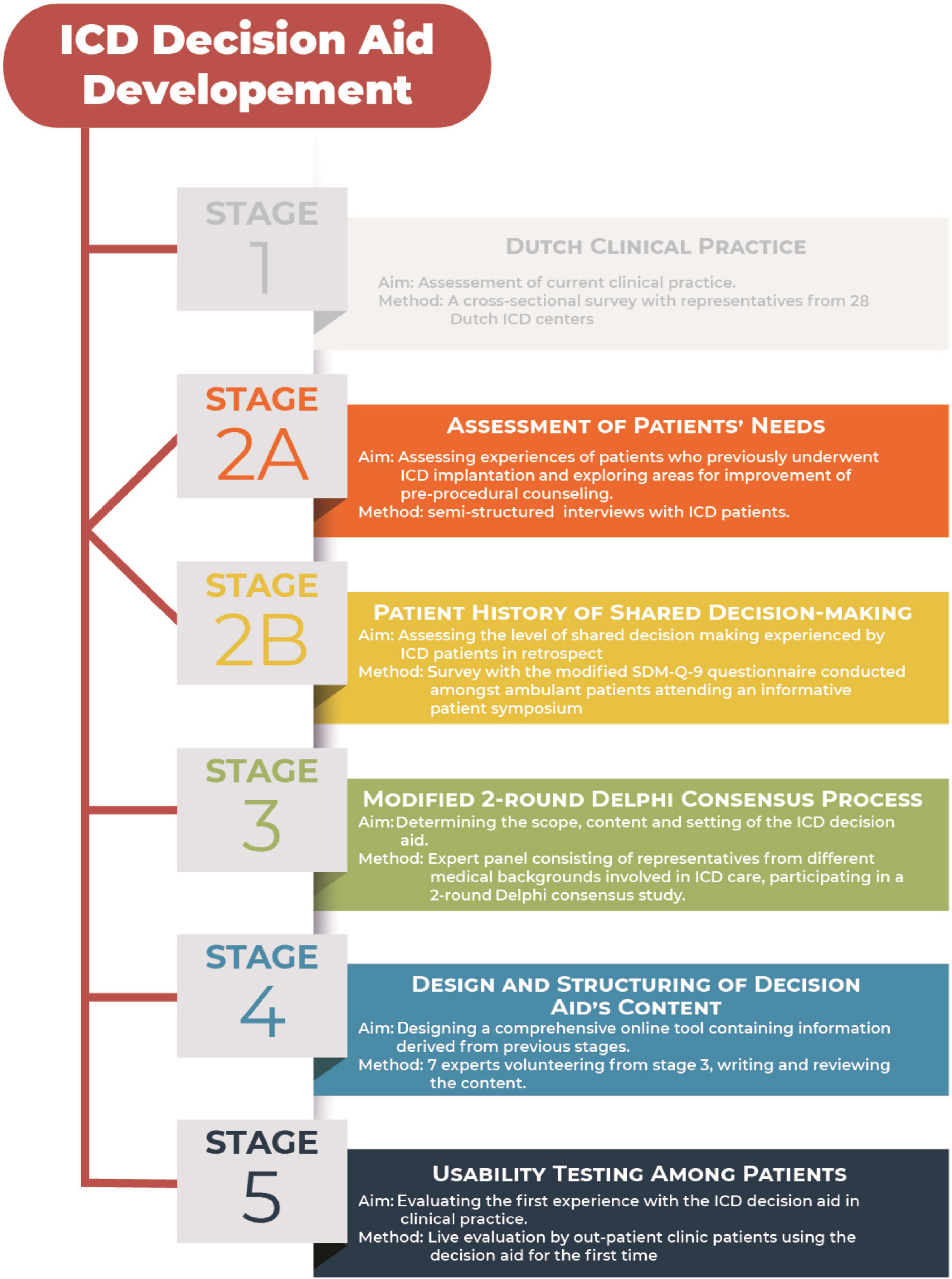
Methods: Between June 2016 and December 2017, a Dutch web-based decision aid was developed according to the Patient Decision Aid Standards (IPDAS) using the RAND-UCLA/multi-stepped Delphi model. Development process consisted of 5 stages in which the Dutch clinical practice was reviewed (stage 1), patients’ needs and their history of decision making was structurally assessed (stages 2A and B) and a modified Delphi consensus process was performed with an expert panel consisting of representatives from different medical fields (stage 3). Results from stages 1–3 were used to design and structure the content of an online-based decision aid (stage 4) which was finally evaluated in a usability testing by patients in stage 5.
Results and conclusion: This study describes the evidence-based approach to the development of the Dutch ICD decision aid. In our population, levels of shared decision-making experience were low. The ICD decision aid was structurally developed for the Dutch ICD patient population. Our upcoming multicenter stepped wedge clustered randomized trial will further evaluate the ICD decision aid in clinical practice.
Introduction
A large body of evidence has shown that Implantable Cardioverter-defibrillators (ICD) play an important role in primary and secondary prevention of sudden cardiac death. For secondary prevention, ICD benefit is more clear (1, 2). Nevertheless, the majority (50–90%) of the ICD patient population receives an ICD for primary prevention (3). Benefit in terms of appropriate tachytherapy varies widely within the latter population: from 50% at 3 years follow-up to only 2.4% in a recent meta-analysis for non-ischemic cardiomyopathy patients (4). Despite the increasing number of trials and scientific literature, it remains challenging for individual patients to perceive the impact of an ICD (5) and for medical professionals to appreciate patient’s values and to translate scientific data into individually applicable advantages (6). In addition to potential periprocedural and later complications, ICDs also impose psychological and social consequences on patients and their family (7, 8). This makes patient counseling challenging. The most recent European guideline (2021) on cardiac pacing and cardiac resynchronization therapy stipulates the importance of patient-centered counseling and shared decision making with regard to device implantations (9). Moreover, the American Medicaid insurance policy has mandated shared decision making in patients undergoing cardiac device implantations with the help of evidence-based decision tools (10). An evidence-based decision aid tailored to the needs of Dutch ICD patients is not yet available.
The objective of this pilot project was to structurally evaluate current clinical practice in Netherlands and ICD patient experience, in order to develop an online decision aid that may improve the levels of shared decision making in ICD implantations and pulse generator exchanges and to decrease decisional conflict.
Materials and methods
Between June 2016 and December 2017, a Dutch web-based decision aid was developed according to the Patient Decision Aid Standards (IPDAS) (11) using the RAND-UCLA/multi-stepped Delphi model (12, 13). Development process consisted of 5 stages, illustrated in Figure 1.
Stage 1: Interview-based evaluation of the Dutch Clinical Practice on Implantable Cardioverter-defibrillators.
All centers in Netherlands qualified to implant ICDs were contacted (n = 28). Representative cardiologists of Dutch ICD implanting centers were interviewed. The results of this study have been recently published (14).
Stage 2A: Assessment of patients’ needs.
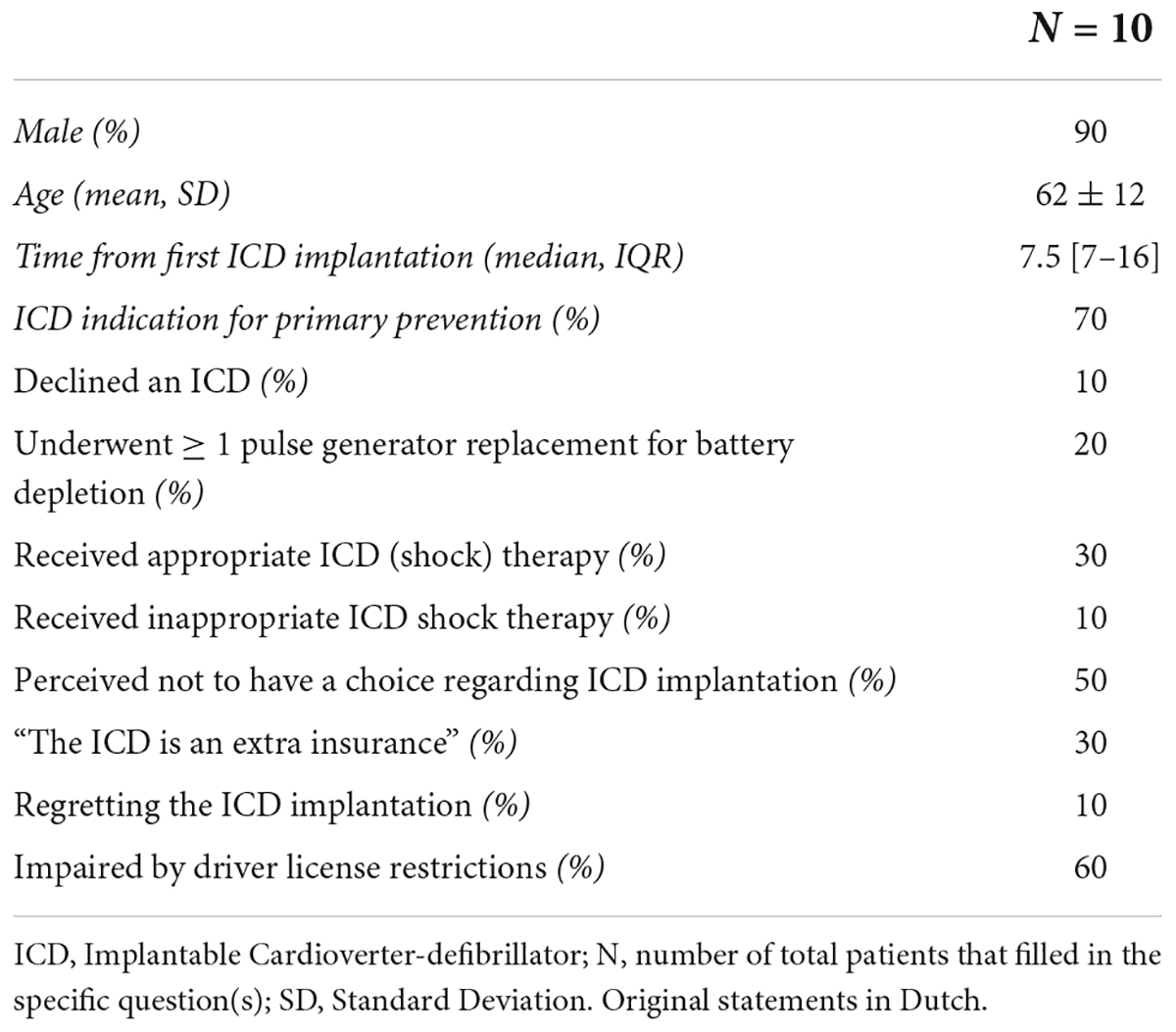
Ten (10) patients [median age 66 (IQR 52–77) years, 30% female, 50% ICD for primary prevention, 10% previously declined a device, 20% CRT-D and 20% ICD] were interviewed between March and April 2017. Patients were selected at the cardiology outpatient clinic of the Leiden University Medical Center and represented the following categories: patients with an ICD for primary and secondary prevention, patients with a Cardiac Resynchronization Therapy-defibrillator (CRT-D) device and patients who previously declined an ICD implantation. To avoid the potential impact of a medical environment on the in-depth interviews, patients were interviewed at a neutral office outside of the hospital. Semi-structured interviews with questions on their decision making process were performed, inquiring about their reasons for choosing or refraining from an ICD, pre-operative counseling by caregivers and current experiences and needs as an ICD patient. Questions on the survey were designed based on clinical experience and outcome of interest. Desired outcome parameters were predefined. Responses were recorded on audiotape with permission from the participants and transcribed as text. Answers were analyzed by the primary investigator and matched and scored accordingly to the predefined outcome parameter. Participants were invited to propose topics and items which they considered to be valuable for peers to be included in a decision aid.
Stage 2B: Patient history of shared decision-making.
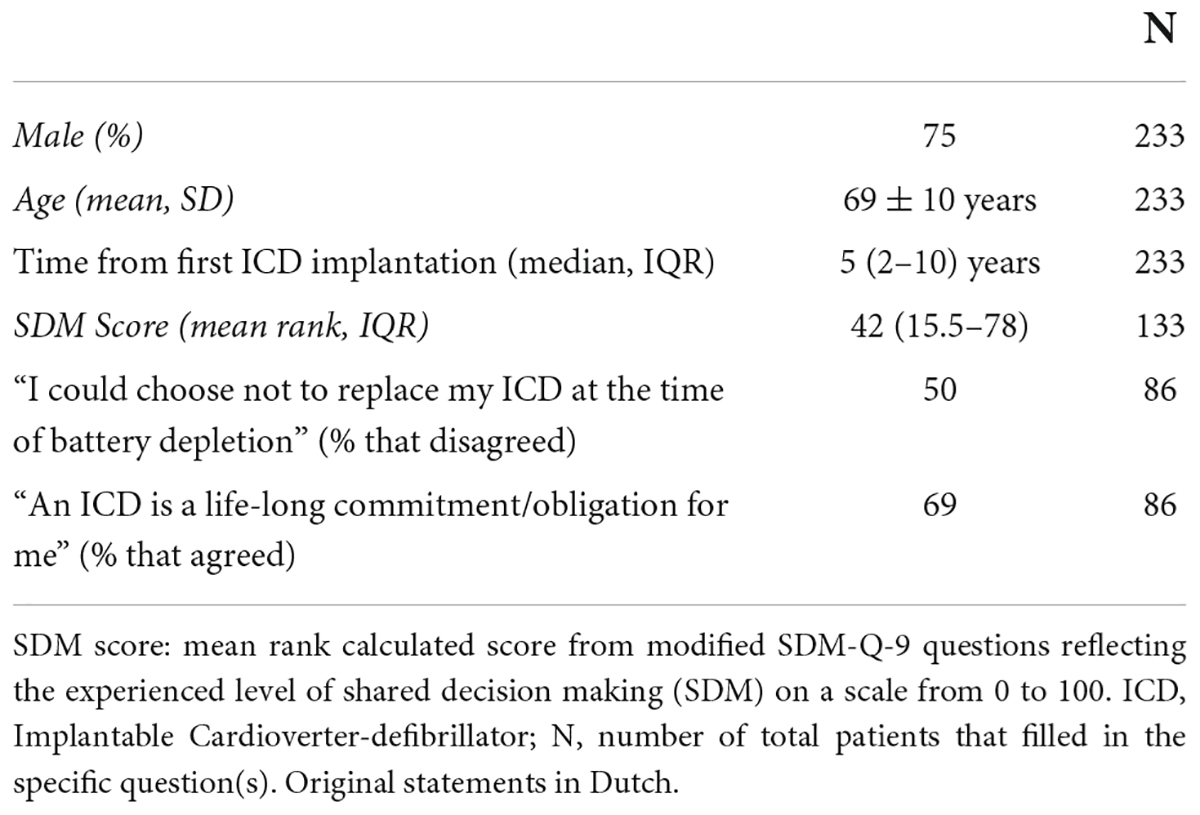
A cross-sectional assessment of shared decision-making experience levels was performed in ICD patients attending a biannual ICD patient conference in the Leiden area. All the attending patients (n = 245) received questionnaires comprising of questions based on the Dutch SDM-Q-9 (15) (Table 1, questions 5–13). In addition, questions regarding patient demographics, together with two statements of interest for patients who previously had undergone a pulse-generator exchange at the time of battery depletion, were added. Patients indicated their level of agreement on a 5-point Likert Scale. The outcomes were analyzed according to the SDM-Q-9 user manual (15). Questionnaires missing answers to more than 2 questions were excluded from the analysis. In the case of 1 or 2 missing values, these were corrected by imputation: the imputed score was the mean score of the present variables (15). We evaluated the additional questions (questions 14–16) as a percentage by grouping the agreement and disagreement answers.

Table 1. Modified 9-item Shared Decision-Making Questionnaire (SDM-Q-9) with additional questions for regional ICD patient conference.
Stage 3: Modified 2-round Delphi Consensus Process.
For determining the content and setting of the decision aid, a modification of the RAND Corporation/University of California, Los Angeles consensus methodology on appropriateness ratings was used as described below (16). A total of 19 experts from different medical centers over the country were to participate in the expert panel for determining the setting and content of the decision aid. The panel consisted of 7 cardiologists, 1 ICD nurse, 2 general practitioners/family medicine doctors, 1 dedicated MD PhD-fellow focusing on ICD patient care, 3 specialists in elderly care medicine/geriatric specialist, 2 internal medicine physicians specialized in elderly medicine, 1 lawyer specialized in medical ethics, 1 psychologist, the chairman of the ICD patient federation and 1 expert on decision aid development. Statements for the experts to evaluate were formulated to determine the content and setting of the decision aid based on information from literature, guidelines and findings from the previous stages (1, 2, 4, 17–24).
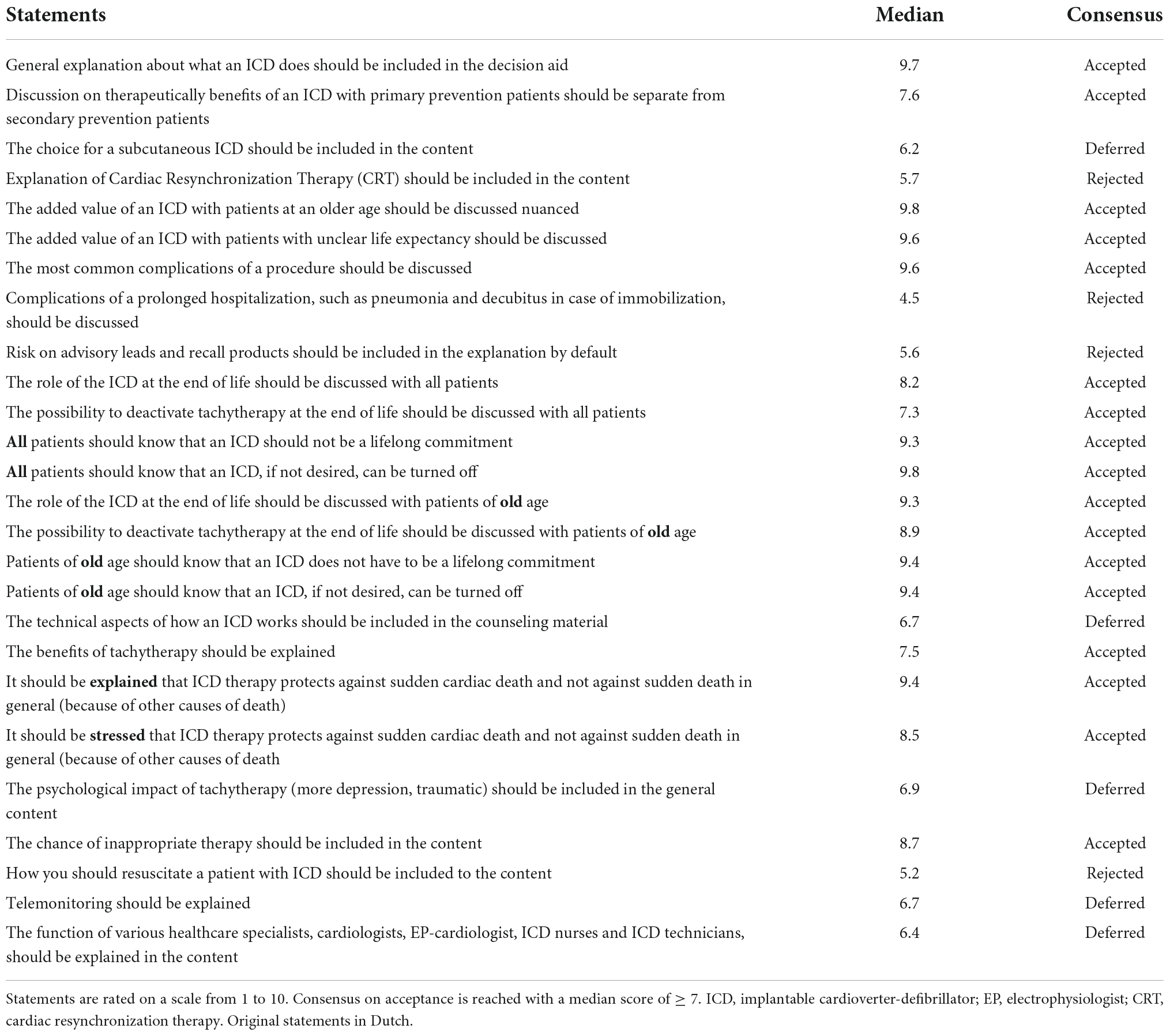
Round 1: Participants received an online questionnaire with 84 items divided into 5 categories (1-target group and setting, 2-content, 3-to be included patient preferences, 4-screening and tools, and 5-format of the decision aid) (Supplementary Appendix 2). Nineteen (19) items consisted of yes or no questions and 64 items were statements for which the experts indicated their level of agreement on a 10-point Likert scale (12, 13). Consensus outcomes were classified as median scores. A median score > 7 was considered as positive consensus and the statement was accepted. A median score < 5 was resulted in the rejection of the statement. Scores between 5 and 7 were discussed in the second round to seek consensus. Participants also had the opportunity to add on items they felt were missing from the questionnaire, which could be discussed in the second round.
Round 2: All participants from round 1 were invited for a face-to-face meeting. Statements from the previous questionnaire on which no consensus was yet reached and items added on by individual experts, were put up for discussion one by one. At the end of each discussion, consensus on agreeing or rejecting the statement was reached by popular vote with a 2/3 majority.
Stage 4: Design and Structuring of decision aid’s content.
Members of the working group were recruited from the previous expert panel (described in stage 3) in order to form a dedicated team for the materialization of the actual decision aid. Members of the working group consisted of three cardiologists from different hospitals, one decision aid development expert, 1 general practitioner/family medicine physician, one Internist-geriatrician and one dedicated MD PhD-fellow focusing on ICD patient care.
The working group formulated the factual content of the decision aid based on the findings and recommendations from previous stages. Engineers and designers from ZorgKeuzeLab (Delft, Netherlands) designed a functioning web-based tool encasing the information provided by the working group.
Stage 5: Usability testing of the prototype among patients.
The four patients from the outpatient clinic with an ICD device were randomly selected and invited to undergo in-depth interviews while testing and analyzing the usability of the prototype of the ICD decision aid. These patients were not involved in the previous stages of the study. Patients were invited for participation by the device technician during their regular semi-annual check-ups. Patients were encouraged to provide live commentary on their experience as they navigated through the decision aid. Patients received open questions addressing whether the decision aid was easy to navigate though, whether they understood the images and animations, whether explanations were clear and easy to read and if they had suggestions for improvement.
Statistics
Categorical variables were presented as numbers and percentages. Based on their distributions, continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR) [25th to 75th percentile].
Results
Stage 1: Interview-based evaluation of the Dutch Clinical Practice on Implantable Cardioverter-defibrillators.
Results have recently been published (14).
Stage 2A: Assessment of patients’ needs.
The patients’ response rate was 100% (n = 10). The mean age was 62 ± 12 years, 90% male, 90% underwent an ICD implantation (70% for primary prevention) and the median time from first ICD implantation to interview was 7.5 [7–16] years. One patient (10%) with an indication for ICD implantation had declined this. Three (30%) patients experienced appropriate shock therapy and two (20%) had received one or more pulse-generator exchanges for battery depletion. Patients reported shocks as unpleasant and painful, however, they were also well accepted. One patient (10%) had experienced inappropriate shock therapy. Patients frequently reported that they experienced not to have had a choice or to have trusted their doctor’s judgment and (strong) recommendations (50%). In addition, all three patients with an ICD for secondary prevention referred to their choice as “choosing between life or death,” whereas primary prevention patients mostly deemed their ICD as an extra insurance (4 out of 7, 57%). One patient (with an ICD for primary prevention) reported to regret the decision, due to limitations in (life-)insurance and traveling opportunities. Furthermore, patients reported implications for their driver’s license as an important downside to having an ICD (60%) (Table 2A).
Stage 2B: Patient history of shared decision-making.
A total of 233 patients completed the modified SDM-Q-9 questionnaire (95% response rate). The mean age was 69 ± 10 years, 75% male, the median time from first ICD implantation to interview was 5 (IQR 2–10) years and 56% had a CRT-D. Eighty-six respondents (40%) had previously undergone at least once a pulse-generator exchange due to battery depletion. Scores from the modified SDM-Q-9 questionnaires on the level of decision-making could be calculated for 133 respondents (57%). The remaining questionnaires were excluded from analysis due to missing data in accordance with the SDM-Q-9 manual (25). Patients reported to be satisfied with the pre-operative information, however, on a scale of 0 (no shared decision experienced) to 100 (strong shared decision experienced) levels of shared decision were marked at a mean ranked score of 42 (IQR 15.5–78). Furthermore, most of the patients perceived the ICD to be a “lifelong commitment” (69%). Remarkably, 21 (10%) of the respondents wrote an extra note stating: “I did not have a choice” (Table 2B).
Stage 3: Modified 2-round Delphi Consensus Process.
Round 1
The panel of experts consisted of 7 cardiologists, 1 ICD nurse, 2 general practitioners/family medicine doctors, 1 dedicated MD PhD-fellow focusing on ICD patient care, 3 specialists in elderly care medicine/geriatrics, 2 internal medicine physicians specialized in elderly medicine, 1 lawyer specialized in medical ethics, 1 psychologist, the chairman of the ICD patient federation and 1 expert on decision aid development. Of these experts, 6 were female (32%). The median age was 55 (IQR 43.5–59.5) years and the median clinical experience was 27 (IQR 15.5–30) years. Response rate of the experts on the panel was 100% (n = 19). The experts reached a consensus on 56 (86%) statements in the first round. Experts decided that the ICD decision aid was not limited to one category of ICD patients, but should be made available to all patients receiving a first ICD device (de novo implants), or who were up for pulse-generator replacement due to battery depletion. In addition, it was agreed that the decision aid would include a tool enabling patients to review their personal preferences. Questions and the corresponding results are found in Tables 3A–E and Figure 2.

Table 3A. Statements from Delphi round 1 on who should be the target group of the decision aid should be.
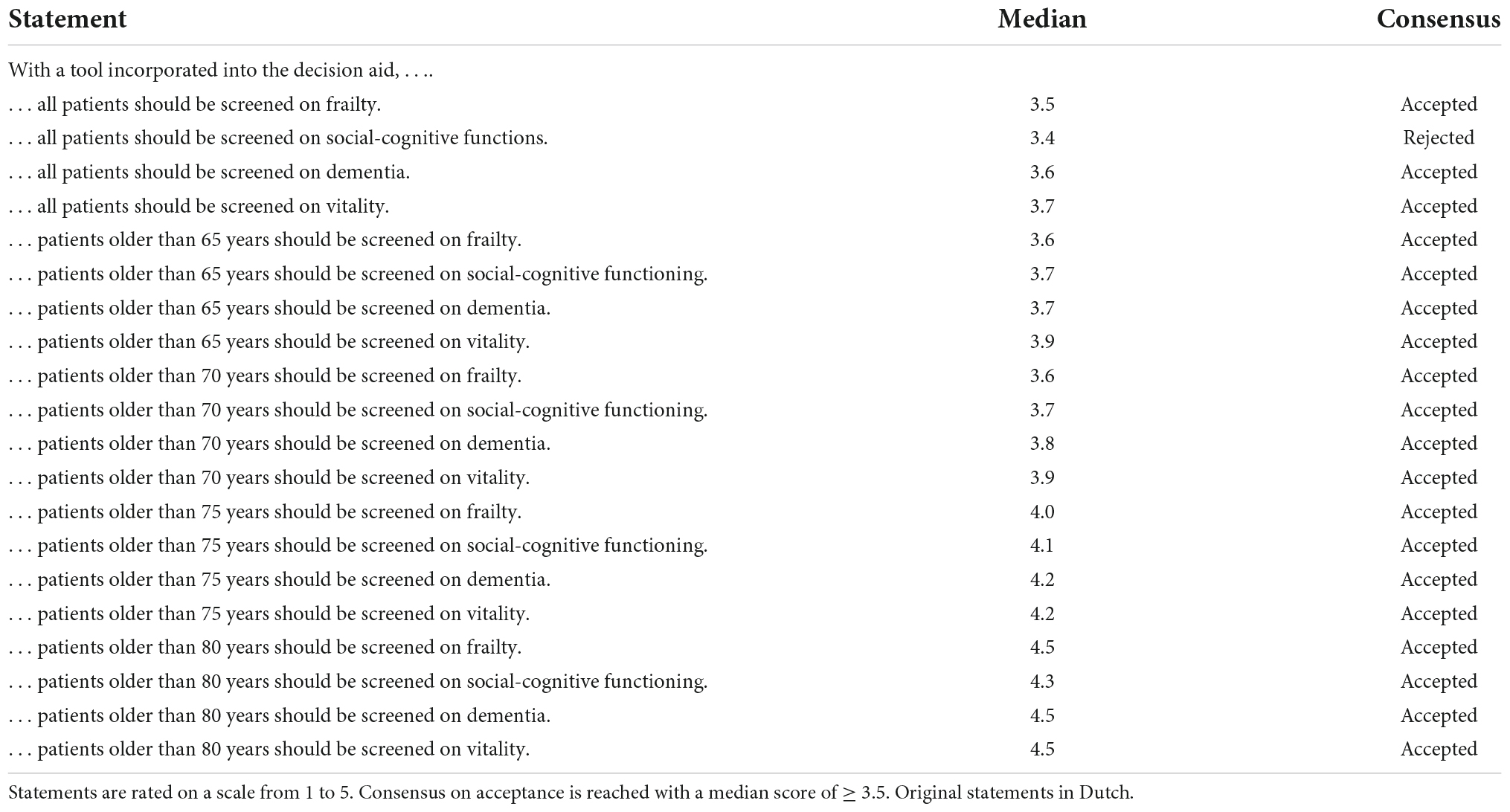
Table 3D. Statements on which patients should be screened on what aspects, by tools integrated into the decision aid.
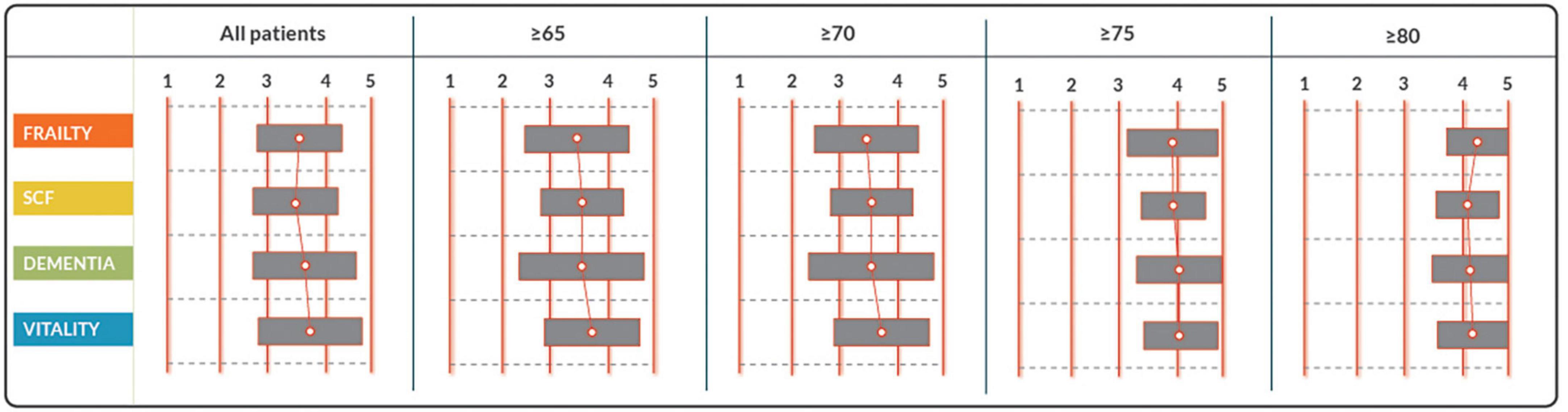
Figure 2. Response to statements on which patients should be screened on what aspects, by tools integrated into the decision aid. Statements are rated on a scale from 1 to 5. Consensus on acceptance is reached with a median score of ≥ 3.5. SCF, social cognitive functioning.
Round 2
With the exception of 1 expert, all experts from round 1 were available for participation in round 2. The panelists reviewed all statements and results from round 1 and proceeded in discussions on only the statements on which no consensus had yet been reached. In all cases, this resulted in the unanimous rejection of all items that were up for discussion. Rejected statements resulted in the exclusion of content on subcutaneous ICDs, information on resuscitation with an ICD and the risk of anxiety after receiving shock therapy from the ICD.
Stage 4: Design and structure of Decision Aid content.
The working group of the fourth stage consisted of 7 members recruited from the expert panel: 3 cardiologists from different hospitals, 1 decision aid development expert, 1 general practitioner/family medicine physician, 1 internist-geriatrician and 1 dedicated MD PhD-fellow focused on ICD patient care. Of the working group members, 5 (71%) were female. The median age was 55 (IQR 37–58) years and the median clinical experience was 22 (IQR 6–31) years. Working group members together formulated the content of the decision aid, based on current guidelines and literature and tailored it to patient preferences and the Dutch clinical practice based on data gathered in the previous stages.
Stage 5: Usability testing among patients.
Four patients participated in the usability testing, of which three were ≥ 70 years. All patients completed the decision aid within half an hour. First impressions of the decision aid were stated to be inviting, clear and of additional value. Three patients appreciated that they could have the opportunity to walk through all the information in a calm home setting. They commented that “it had been impossible to remember all of what the doctor told in the consultation room.” One patient admitted to listen to specific advice from the doctor and read as little as possible about potential complications. Nevertheless, also this patient agreed that the opportunity to be informed on all aspects is beneficial for the general patient group.
Discussion
This study was designed in order to create a decision aid for ICD patients. Information was gathered systematically on the Dutch clinical practice in ICD patients, patient preferences and insight in ICD therapy, incorporating expert opinions and levels of shared decision making as experienced by patients. Findings were incorporated into the design of a decision aid to support patients and caregivers to make well-informed choices regarding ICD therapy. This evidence-based decision aid was developed for all ICD patients facing the choice of receiving a new ICD or replacing one, according to the Patient Decision Aid Standards (IPDAS) (11) using the RAND-UCLA/multi-stepped Delphi model (12, 13). A previous evidence-based developed ICD decision aid was centered around the health care providers. The main findings in the stages of our study are that: [1] patients in retrospect reported they were not aware of having a choice, [2] levels of shared decision making perceived by our ambulatory ICD population were low and [3] the first patient experience with our decision aid was positive and promising.
Challenges in patient education and counseling
Patients have a right to be well informed on the various aspects of the proposed intervention, emphasizing the patient’s role in decision making, discussing alternatives and the risks and obtaining the patient’s consent. Moreover, proper counseling of patients is a cornerstone of a prosperous patient-caregiver relationship. The recently updated guidelines of the European Society of Cardiology emphasize the need for patient-centered care and shared decision making (9). Counseling and educating patients on their individual illnesses and therapeutic options can, however, be challenging. This is particularly true for ICD candidates, as not only is it challenging to predict the benefit from an ICD for an individual patient, but an ICD also has its downsides, such as a lower quality of life after receiving ICD therapy (26, 27). This is particularly true in those who have received inappropriate therapy (28). Other important risks include infection, technical failure and receiving shocks in the last moments of life (29–36).
Traditionally, patients are counseled by their caregivers at the outpatient office. These consultations can be supplemented by informative pamphlets filled with information. Or, as a more modern approach, shared decision making with the use of (digital) decision aids can be used.
Consultations with doctors
It has previously been described that doctors have a decisive role in decision making for patients eligible for an ICD (37). Very strong language emphasizing the benefits of an ICD will lead to patients favoring the device implantation (37, 38). These findings reaffirm the necessity for an unbiased decision aid.
Comprehension by patients of mere percentages has been shown to be overall disappointing (5). ICD patients overestimate the potential benefit from ICD therapy and are deficient in their comprehension of device function (39–43). ICD patients have previously reported to have not fully understood the risks and burden of living with an ICD at the time of consent for an ICD implantation (37, 39). In addition, it has appeared that some patients who had previously declined an ICD implantation for primary prevention, in retrospect had not fully understood the benefits for survival (44).
Print-based educational material
Patients desire to have access to comprehensive information that can help them in deciding. Providing patients with comprehensive information and considering their preferences, is important for sustainable decision making. Interestingly, traditional print-based educational material for ICD patients has previously been proven to target the highly literate population (45). For this reason, the expert panel decided for the decision aid in this study to be made available online, be interactive and incorporate illustrative educational videos in simple language. To avoid bias toward patients with lower digital literacy, it was, nevertheless, decided that the content can be printed out and handed to patients by healthcare providers in selected cases. In addition, all text was reviewed by professional content writers to be comprehendible for the lower-literate population.
Shared decision making
The decision making process for the ICD patient is triggered when the risk of sudden cardiac death is discussed (41). However, these ICD patients have reported that the most important factors influencing their final decision were not the odds and numbers, but trust in the advocacy of their treating physician, social influences and their health state (41). Likewise, in stage 2 of the process, patients also reported to have trusted their doctors’ judgment and (strong) recommendations. This illustrates the importance of patient preferences in shared decision making. Moreover, a key factor in shared decision making is helping patients explore preferences and make well thought-out decisions.
In clinical practice, shared decision making is, however, still underutilized. Patients in this study reported relatively low experience of shared decision making. Moreover, most interviewed patients admitted not to have been aware they had a choice. Likewise, patients have previously reported not to recall alternatives for committing to ICD therapy (41). In addition, in a previous study, clinicians have reported that in order to use shared decision making, they needed a hint or trigger from patients, as it was not part of their standard practice (46).
Shared decision making supplemented by decision aids
Shared decision making can be facilitated by the implementation of decision aids. It has been affirmed that a decision aid results in patients playing a more active role in decision making and accurate risk perception improve patient knowledge and decrease decisional conflict (47). Patients have reported to feel more knowledgeable, better informed, and clearer about their values with the use of a decision aid (47). A pilot study with a decision aid for ICD patients showed promising results, with decrease of decisional conflict in patients using the decision aid (48). Medicare and Medicaid Services in the United States of America, even mandated the use of evidence-based decision aids, supporting shared decision making, in patients that were a candidate for cardiovascular device placements, including ICDs (10). Nevertheless, implementation of decision aids in clinical practice is slow (49). American physicians self-reported to engage in shared decision making when obtaining consent prior to an ICD implantation, however, less than half of these physicians used a decision aid in their clinical practice (50). Lewis at al., developed a user-centered ICD decision aid to be used for patients facing and ICD replacement, involving key-users in the development in order to encourage utilization of the product in the future. In our study, we proactively involved not only cardiologists, but also experts from relevant medical fields and patients. Moreover, the opinion of 233 ambulatory ICD patients has been taken into account when designing this decision aid (Stage 2B).
Decision making at the time of battery depletion
The expert panel in this study decided to target the ICD decision aid at not only patients eligible for a first ICD, but also patients facing an ICD replacement as ICD therapy is not a lifelong commitment. However, as our patients stated, the latter is not always information that is clear to patients. Moreover, as illustrated in our previous study, ICD replacement was not always presented as a choice by health care providers (14). Likewise, it has been previously shown that more than half of the patients who had already undergone an ICD replacement at the time of battery depletion, had not been aware that they had a choice (51). This illustrates that ICD replacement at the time of battery depletion goes without saying, whereas patients have been reported to consider non-replacement under certain circumstances such as serious comorbidity and advanced age (51).
Time from first ICD implantation to pulse-generation exchange can easily be longer than 5 years (52). Discussions with the healthcare provider and information provided at the commencement of ICD therapy can be forgotten by the patient. Therefore, at the time of pulse-generator exchange for battery depletion, there is a need for renewed discussions with the patient before deciding on definitely continuing ICD therapy. This is in contrast of continuing ICD therapy regardless of the costs (risk of complications) or patient preferences (e.g., no longer wanting to prevent a sudden cardiac death).
Moreover, patient preferences can change with the progression of age and the development of comorbidities. In addition, the odds of complications increase with every pocket revision/redo procedure (52, 53).
An ICD decision aid can also facilitate decision making in these patients, exploring their current individual preferences and weighing them out against the expected benefits and downsides from ICD therapy. Especially a decision aid that can be reviewed at home, will provide an opportunity for family members to be involved in the decision making resulting in decisions supported by patients and their doctors as well as their families.
Previous endeavors resulted in a healthcare-provider-centered ICD decision aid to be implemented in patients facing an ICD replacement, in order to help healthcare providers step away from the automatism of replacing an ICD at battery depletion instead of discussing the options with their patients first (46). It is expected from the decision aid resulting from this study, to encourage not only healthcare providers but also patients into taking a more active role in the decision making process prior to the definitive continuation of ICD therapy.
Future perspective
The ICD Decision Aid Study is currently being conducted in a multicenter stepped wedge clustered randomized trial at 6 Dutch centers. The study will evaluate the decision aid in a clinical setting and its benefit on shared decision making experienced by both doctors and patients. Shared decision-making levels in our population will be reassessed after implementation, to clarify the benefit of the ICD decision aid.
Conclusion
This study describes the evidence-based approach to the development of the Dutch ICD Decision Aid. In our population, levels of shared decision-making experience were low. Decision aids have previously proven to improve patients’ decision making and facilitate shared decision making. The ICD Decision Aid was developed for the Dutch ICD patient population according to prevailing decision aid development methods. Results from our multicenter stepped wedge clustered randomized trial will further evaluate the ICD decision aid.
Limitations
This study has several limitations. Most importantly, recall bias can be present in the patient groups. Patients reporting experience in Stage 2 are prone to recall bias. However, reported outcomes are accurate for evaluating patient experience as this is what patients eventually actually remember. Moreover, patients from the second round of Stage 2 are a good representation of the average ambulant ICD patients, as hospitalized or patients with end-stage disease would not be able to attend.
With regards to Stage 3, there is a selection bias in patients entering the panel and expert group. Participants have, however, been carefully selected on their roles in the clinical field and experience with ICDs. Using 2 rounds in this stage allowed elaborate discussions of their points of view and consensus was reached on all items.
In Stage 5, the usability of the decision aid was tested amongst a small number of selected patients. The evaluation was, however, performed carefully and with much attention. Patients were not pre-selected on their computer skills and included patients of old age. It is expected that this patient group is a good representation of the whole population. In addition, no health care professionals were included in stage 5. The aim was to assess the usability of the digital tool amongst a diverse group of patients. Health care professionals will, however, be included in the upcoming randomized clinical trial, that will evaluate the decision aid within a broad clinical setting.
Finally, the option of a subcutaneous ICD (without transvenous leads) was not included in the current decision aid. The current ICD decision aid is aimed towards helping patients decide whether or not to protect themselves from sudden cardiac death (i.e., choosing for defibrillator therapy). This decision must be made first, before the option of a subcutaneous ICD can be explored. Development of a future decision aid to help patient explore this subsequent option, could be useful in selected cases suited for both a transvenous and a subcutaneous ICD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Scientific Review Board of the Leiden University Medical Center, Department of Cardiology and Leiden University Medical Center Medical Ethics Committee. All patients and experts involved in panels and interviews for study purposes provided written informed consent to participate in this study.
Author contributions
DY, LE, MS, RV, HS, and AE contributed to conception and design of the study. DY organized the database and wrote the first draft of the manuscript. DY and LE performed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was funded by grants provided by the Dutch Foundation of funding for qualitative research by medical specialists [Stichting Kwaliteitsgelden Medisch Specialisten (SKMS)].
Acknowledgments
We thank the Dutch Society of Cardiology for their support. We also thank the members of the expert panel: Yvonne van Ingen, Robert Verbunt, Richard Braam, Rene Jansen, Maurits Wijffels, Jos van Erp, Han Spierenburg, Gijs Willemsen, Erik Olsman, Erik Moll van Charante, Laurens Tops, Elise Flipse, Cor Spreeuwenberg, Jeanet Blom, and Rinus Split for providing their time and expertise, making this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.946404/full#supplementary-material
References
1. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. (2002) 346:877–83.
2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. (2005) 352:225–37.
3. Haugaa KH, Tilz R, Boveda S, Dobreanu D, Sciaraffia E, Mansourati J, et al. Implantable cardioverter defibrillator use for primary prevention in ischaemic and non-ischaemic heart disease-indications in the post-DANISH trial era: results of the European heart rhythm association survey. Europace. (2017) 19:660–4. doi: 10.1093/europace/eux089
4. Wolff G, Lin Y, Karathanos A, Brockmeyer M, Wolters S, Nowak B, et al. Implantable cardioverter/defibrillators for primary prevention in dilated cardiomyopathy post-DANISH: an updated meta-analysis and systematic review of randomized controlled trials. Clin Res Cardiol. (2017) 106:501–13. doi: 10.1007/s00392-017-1079-0
6. Sapp JL, Parkash R, Wells GA, Yetisir E, Gardner MJ, Healey JS, et al. Cardiac resynchronization therapy reduces ventricular arrhythmias in primary but not secondary prophylactic implantable cardioverter defibrillator patients: insight from the resynchronization in ambulatory heart failure trial. Circ Arrhythm Electrophysiol. (2017) 10:e004875.
7. Rottmann N, Skov O, Andersen CM, Theuns D, Pedersen SS. Psychological distress in patients with an implantable cardioverter defibrillator and their partners. J Psychosom Res. (2018) 113:16–21.
8. Pedersen SS, Sears SF, Burg MM, Van Den Broek KC. Does ICD indication affect quality of life and levels of distress? Pacing Clin Electrophysiol. (2009) 32:153–6.
9. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. (2021) 42:3427–520.
10. Knoepke CE, Allen LA, Kramer DB, Matlock DD. Medicare mandates for shared decision making in cardiovascular device placement. Circ Cardiovasc Qual Outcomes. (2019) 12:e004899. doi: 10.1161/CIRCOUTCOMES.119.004899
11. Elwyn G, O’Connor A, Stacey D, Volk R, Edwards AG, Coulter A, et al. International patient decision aids standards (IPDAS) collaboration. Developing a quality criteria framework for patient decision aid: online international Delphi consensus process. Br Med J. (2006) 333:417–9.
12. Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. (2006) 333:417. doi: 10.1136/bmj.38926.629329.AE
13. Feldman-Stewart D, Brennenstuhl S, McIssac K, Austoker J, Charvet A, Hewitson P, et al. A systematic review of information in decision aids. Health Expect. (2007) 10:46–61.
14. Yilmaz D, Egorova AD, Schalij MJ, van Erven L. Implantable cardioverter-defibrillators and the older patient: the Dutch clinical practice. Eur J Cardiovasc Nurs. (2022) 21:169–73.
15. Rodenburg-Vandenbussche S, Pieterse AH, Kroonenberg PM, Scholl I, van der Weijden T, Luyten GP, et al. Dutch translation and psychometric testing of the 9-item shared decision making questionnaire (SDM-Q-9) and shared decision making questionnaire-physician version (SDM-Q-Doc) in primary and secondary care. PLoS One. (2015) 10:e0132158. doi: 10.1371/journal.pone.0132158
16. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation (2001).
17. Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. (1997) 337:1576–83. doi: 10.1056/NEJM199711273372202
18. Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the cardiac arrest study hamburg (CASH). Circulation. (2000) 102:748–54. doi: 10.1161/01.cir.102.7.748
19. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. (1996) 335:1933–40.
20. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. (2004) 350:2151–8.
21. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology/American heart association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American association for thoracic surgery and society of thoracic surgeons. Circulation. (2008) 117:e350–408.
22. Center Lum. Inwendige Cardioverter Defibrillator (ICD). Leiden: Leiden University Medical Center (2019).
23. Center Lum. Implanteerbare Defibrillator (ICD) en Rijbewijs. Leiden: Leiden University Medical Center (2019).
24. Centraal Bureau voor de Statistiek. CBS StatLine - Overledenen; Doodsoorzaak (uitgebreide lijst), Leeftijd, Geslacht [Dataset]. (2016). Available online at: http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=7233&D1=680&D2=0 &D3=0&D4=a&HDR=G2,G1,G3&STB=T&VW=T (accessed June 15, 2016).
25. Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item shared decision making questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. (2010) 80:94–9. doi: 10.1016/j.pec.2009.09.034
26. Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DB, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: a scientific statement from the American Heart Association. Circulation. (2012) 126:2146–72. doi: 10.1161/CIR.0b013e31825d59fd
27. Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. (2008) 359:999–1008.
28. van Rees JB, Borleffs CJ, de Bie MK, Stijnen T, van Erven L, Bax JJ, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. (2011) 57:556–62.
29. Goldenberg I, Moss AJ, Hall WJ, McNitt S, Zareba W, Andrews ML, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. (2006) 113:2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262
30. Goldstein NE, Kalman J, Kutner JS, Fromme EK, Hutchinson MD, Lipman HI, et al. A study to improve communication between clinicians and patients with advanced heart failure: methods and challenges behind the working to improve discussions about defibrillator management trial. J Pain Symptom Manage. (2014) 48:1236–46. doi: 10.1016/j.jpainsymman.2014.03.005
31. Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation. (2014) 129:422–9.
32. Kirk TW. Implantable cardioverter-defibrillators and hospice care. IEEE Eng Med Biol Magaz. (2007) 26:82–4.
33. Grassman D. EOL considerations in defibrillator deactivation. Am J Hosp Palliat Care. (2005) 22:179; author rely 179–80.
34. Butler K, Puri S. Deathbed shock: causes and cures. JAMA Int Med. (2014) 174:88–9. doi: 10.1001/jamainternmed.2013.11125
35. Bogan C, Kieran T, O’Brien T, Fahy G. Deactivation of an implantable cardioverter defibrillator in a dying patient. Irish Med J. (2006) 99:155–6.
36. Kirk TW. Deactivation of automatic implantable cardioverter-defibrillators in hospice and home care patients at the end of life. Home Healthc Nurse. (2008) 26:431–7. doi: 10.1097/01.NHH.0000326324.09466.97
37. Hauptman PJ, Chibnall JT, Guild C, Armbrecht ES. Patient perceptions, physician communication, and the implantable cardioverter-defibrillator. JAMA Intern Med. (2013) 173:571–7.
38. Matlock DD, Jones J, Nowels CT, Jenkins A, Allen LA, Kutner JS. Evidence of cognitive bias in decision making around implantable-cardioverter defibrillators: a qualitative framework analysis. J Card Fail. (2017) 23:794–9. doi: 10.1016/j.cardfail.2017.03.008
39. Groarke J, Beirne A, Buckley U, O’Dwyer E, Sugrue D, Keelan T, et al. Deficiencies in patients’ comprehension of implantable cardioverter defibrillator therapy. Pacing Clin Electrophysiol. (2012) 35:1097–102.
40. Agård A, Löfmark R, Edvardsson N, Ekman I. Views of patients with heart failure about their role in the decision to start implantable cardioverter defibrillator treatment: prescription rather than participation. J Med Ethics. (2007) 33:514–8. doi: 10.1136/jme.2006.017723
41. Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect. (2013) 16:69–79.
42. Goldstein NE, Mehta D, Siddiqui S, Teitelbaum E, Zeidman J, Singson M, et al. ”That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. (2008) 23(Suppl. 1):7–12. doi: 10.1007/s11606-007-0239-8
43. Stewart GC, Weintraub JR, Pratibhu PP, Semigran MJ, Camuso JM, Brooks K, et al. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail. (2010) 16:106–13.
44. Chan LL, Lim CP, Aung ST, Quetua P, Ho KL, Chong D, et al. Patient barriers to implantable cardioverter defibrillator implantation for the primary prevention of sudden cardiac death in patients with heart failure and reduced ejection fraction. Singapore Med J. (2016) 57:182–7.
45. Strachan PH, de Laat S, Carroll SL, Schwartz L, Vaandering K, Toor GK, et al. Readability and content of patient education material related to implantable cardioverter defibrillators. J Cardiovasc Nurs. (2012) 27:495–504. doi: 10.1097/JCN.0b013e31822ad3dd
46. Lewis KB, Birnie D, Carroll SL, Clark L, Kelly F, Gibson P, et al. User-centered development of a decision aid for patients facing implantable cardioverter-defibrillator replacement: a Mixed-Methods Study. J Cardiovasc Nurs. (2018) 33:481–91. doi: 10.1097/JCN.0000000000000477
47. Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2017) 4:CD001431.
48. Carroll SL, Stacey D, McGillion M, Healey JS, Foster G, Hutchings S, et al. Evaluating the feasibility of conducting a trial using a patient decision aid in implantable cardioverter defibrillator candidates: a randomized controlled feasibility trial. Pilot Feasibility Stud. (2017) 3:49.
49. Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C, et al. ”Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. (2013) 13(Suppl. 2):S14. doi: 10.1186/1472-6947-13-S2-S14
50. Ali-Ahmed F, Matlock D, Zeitler EP, Thomas KL, Haines DE, Al-Khatib SM. Physicians’ perceptions of shared decision-making for implantable cardioverter-defibrillators: results of a physician survey. J Cardiovasc Electrophysiol. (2019) 30:2420–6.
51. Lewis KB, Nery PB, Birnie DH. Decision making at the time of ICD generator change: patients’ perspectives. JAMA Intern Med. (2014) 174:1508–11. doi: 10.1001/jamainternmed.2014.3435
52. Boriani G, Merino J, Wright DJ, Gadler F, Schaer B, Landolina M. Battery longevity of implantable cardioverter-defibrillators and cardiac resynchronization therapy defibrillators: technical, clinical and economic aspects. an expert review paper from EHRA. Europace. (2018) 20:1882–97. doi: 10.1093/europace/euy066
53. Borleffs CJ, Thijssen J, de Bie MK, van Rees JB, van Welsenes GH, van Erven L, et al. Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. Pacing Clin Electrophysiol. (2010) 33:1013–9. doi: 10.1111/j.1540-8159.2010.02780.x
Keywords: cardiac geriatrics, implantable cardioverter-defibrillator, shared decision making, decision aid (DA), decision making
Citation: Yilmaz D, Egorova AD, Schalij MJ, Spierenburg HAM, Verbunt RAM and van Erven L (2022) The development of a decision aid for shared decision making in the Dutch implantable cardioverter defibrillator patient population: A novel approach to patient education. Front. Cardiovasc. Med. 9:946404. doi: 10.3389/fcvm.2022.946404
Received: 17 May 2022; Accepted: 20 September 2022;
Published: 13 October 2022.
Edited by:
Deirdre Lane, University of Liverpool, United KingdomReviewed by:
Susanne Pedersen, University of Southern Denmark, DenmarkPedro Silva Cunha, Hospital de Santa Marta, Portugal
Copyright © 2022 Yilmaz, Egorova, Schalij, Spierenburg, Verbunt and van Erven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lieselot van Erven, l.van_erven@lumc.nl
 Dilek Yilmaz
Dilek Yilmaz Anastasia D. Egorova
Anastasia D. Egorova Martin J. Schalij1
Martin J. Schalij1  Han A. M. Spierenburg
Han A. M. Spierenburg