- 1Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Statistical Office, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
- 3Department of Neurosurgery, The Affiliated People’s Hospital of Ningbo University, Ningbo, China
- 4Department of Neurosurgery, The Affiliated Quzhou People’s Hospital of Wenzhou University, Quzhou, China
Background: Pituitary adenoma (PA) is a benign neuroendocrine tumor caused by adenohypophysial cells, and accounts for 10%-20% of all primary intracranial tumors. The surgical outcomes and prognosis of giant pituitary adenomas measuring ≥3 cm in diameter differ significantly due to the influence of multiple factors such as tumor morphology, invasion site, pathological characteristics and so on. The aim of this study was to explore the risk factors related to the recurrence or progression of giant and large PAs after transnasal sphenoidal surgery, and develop a predictive model for tumor prognosis.
Methods: The clinical and follow-up data of 172 patients with large or giant PA who underwent sphenoidal surgery at the Second Affiliated Hospital of Zhejiang University School of Medicine from January 2011 to December 2017 were retrospectively analyzed. The basic clinical information (age, gender, past medical history etc.), imaging features (tumor size, invasion characteristics, extent of resection etc.), and histopathological characteristics (pathological results, Ki-67, P53 etc.) were retrieved. SPSS 21.0 software was used for statistical analysis, and the R software was used to establish the predictive nomogram.
Results: Seventy out of the 172 examined cases (40.7%) had tumor recurrence or progression. The overall progress free survival (PFS) rates of the patients at 1, 3 and 5 years after surgery were 90.70%, 79.65% and 59.30% respectively. Log-rank test indicated that BMI (P < 0.001), Knosp classification (P < 0.001), extent of resection (P < 0.001), Ki-67 (P < 0.001), sphenoidal sinus invasion (P = 0.001), Hardy classification (P = 0.003) and smoking history (P = 0.018) were significantly associated with post-surgery recurrence or progression. Cox regression analysis further indicated that smoking history, BMI ≥25 kg/m2, Knosp classification grade 4, partial resection and ≥3% Ki-67 positive rate were independent risk factors of tumor recurrence or progression (P < 0.05). In addition, the nomogram and ROC curve based on the above results indicated significant clinical value.
Conclusion: The postoperative recurrence or progression of large and giant PAs is related to multiple factors and a prognostic nomogram based on BMI (≥25 kg/m2), Knosp classification (grade 4), extent of resection (partial resection) and Ki-67 (≥3%) can predict the recurrence or progression of large and giant PAs after transnasal sphenoidal surgery.
Introduction
Pituitary adenoma (PA) is a benign neuroendocrine tumor that originates from adenohypophysial cells, and accounts for 10%-20% of all primary intracranial tumors (1, 2). The global prevalence of PA ranges from 80 to 100 cases per 100,000 (3). Pituitary adenomas disrupt the endocrine function of the pituitary gland, and the growing tumor mass can lead to headaches, vision problems and visual field changes. Giant PA is defined as tumors with the largest diameter ≥4 cm (4–6), and some investigators have defined tumors with the largest diameter ≥3 cm as large PA (5, 7, 8). Large and giant PAs tend to invade the region surrounding the saddle, starting from the bone of saddle bottom and then progressing to the sphenoid sinus. Tumor growth through the diaphragm sella compresses the optic chiasm and third ventricle, and involvement of the cavernous sinus leads to encasement of the internal carotid artery. Therefore, the internal carotid artery and peripheral nerves can be easily damaged during the operation. In addition, some lesions are tough in texture or accompanied by spontaneous stroke, which further increases the difficulty of surgery and increases the risk of recurrence or progression.
Surgical removal is the first-line treatment for most large and giant PAs, except for prolactinoma (9, 10). Since craniotomy causes greater damage to normal brain tissues and results in more postoperative complications, it is now gradually being replaced with the transnasal sphenoidal approach (11). Jankowski et al. were the first to successfully perform neuroendoscopy-guided transsphenoidal resection of PA (12). Nevertheless, regardless of whether the surgery is performed using neuroendoscopy or microscopy, or via the transnasal sphenoidal or transcranial approach, the surgical outcomes are still not satisfactory. Over 50%-72% of the cases have postoperative residual (13–16), and the recurrence rates at 5- and 10 years after surgery are 40% and 50% respectively (17). Even with gross total resection, at least 10-20% of the patients relapse within 5 to 10 years (2, 14), which greatly affects their quality of life.
Apart from postoperative residual, the recurrence of PA also depends on the tumor size and cavernous sinus invasion, which are risk factors for poor prognosis (14, 18, 19). In addition, the pathological subtype is also a factor influencing tumor recurrence and progression (20). It was reported that sparsely granulated GH adenomas and prolactin PAs in male patients are highly aggressive and have poor prognosis (21, 22).
Recurrence and progression of residual PA lesions impair the function of the pituitary gland. Therefore, long-term hormone replacement therapy is often required after surgery, which greatly reduces the quality of life of patients. In addition, surgical resection of the recurrent or evolved tumors increases the risk of vascular rupture, nerve injury and other adverse complications, which further worsens patient prognosis. In this study, we retrospectively analyzed the clinical, pathological, surgical and imaging data of 172 patients with large or giant PA to identify the risk factors of tumor recurrence and progression. In addition, we developed a nomogram to predict tumor recurrence or progression and assess postoperative prognosis.
Materials and Methods
Study Population
After obtaining institutional review board approval, the clinical data of 172 patients with large (>3 cm) or giant (>4 cm) PA that underwent surgery at The Second Affiliated Hospital Zhejiang University School of Medicine between January 2011 and December 2017 were retrospectively analyzed. The inclusion criteria were as follows: 1) histologically confirmed PAs with maximal diameter >3–4 cm (large) or >4 cm (giant), 2) underwent tumor resection through transnasal sphenoidal surgery, 3) no radiotherapy during the period of treatment, and 4) regular follow-up for a minimum of 6 months. Patients with serious intraoperative complications (such as internal carotid artery rupture or anesthesia accident), severe cardiopulmonary insufficiency, organ failure or other serious underlying diseases, anatomical variation or complicated with vascular disease (such as aneurysm), and those who underwent craniotomy for PA were excluded. The patients were re-examined 3-, 6- and 12 months after surgery, and yearly thereafter. Images of the pituitary were assessed by a neurosurgeon and a neuroradiologist during the follow-up period. Recurrence or progression was defined as significant enlargement (>2 mm in any direction) of the tumor remnants or the appearance of new masses detected by MRI during the follow-up (14).
Data Collection
The patient data was divided into basic, radiological and pathological categories. Basic information included gender, age, history of hypertension, diabetes, smoking [patients who have smoked more than 100 cigarettes in lifetime were defined as having smoking history (23)], drinking, body mass index (overweight≥25kg/m2, not overweight<25kg/m2), function and operation methods (microscopy or neuroendoscopy). Radiological characteristics included cavernous sinus invasion, sphenoid sinus invasion, sella invasion, Hardy classification, Knosp classification, extent of resection [gross total resection (GTR), the extent of resection is greater than 95%; near total resection, NTR, the extent of resection is between 90% and 95%; subtotal resection, STR, the extent of resection is between 70%-90%; partial resection, PR, the extent of resection is less than 70% (8)]. The pathological classification, and P53 and Ki-67 positive rates were also collected.
Data Analysis
Differences between subgroups were analyzed using Student’s t-test for the measurement data and the X2 test was used for categorical variables. The Kaplan-Meier survival curves were plotted and compared by log-rank tests using SPSS 21.0. Multivariate Cox regression analysis was performed to assess the independent predictive risk factors for tumor relapse, and a nomogram model was established with the R software version 4.0.3 (https://www.r-project.org, R package “rms”, “survival”.) to predict outcomes at the 1-, 3-, and 5-year follow-up. The performance of the nomogram was determined with ROC curve and calibration curve. Two-sided P values below 0.05 were considered statistically significant.
Results
General Characteristics
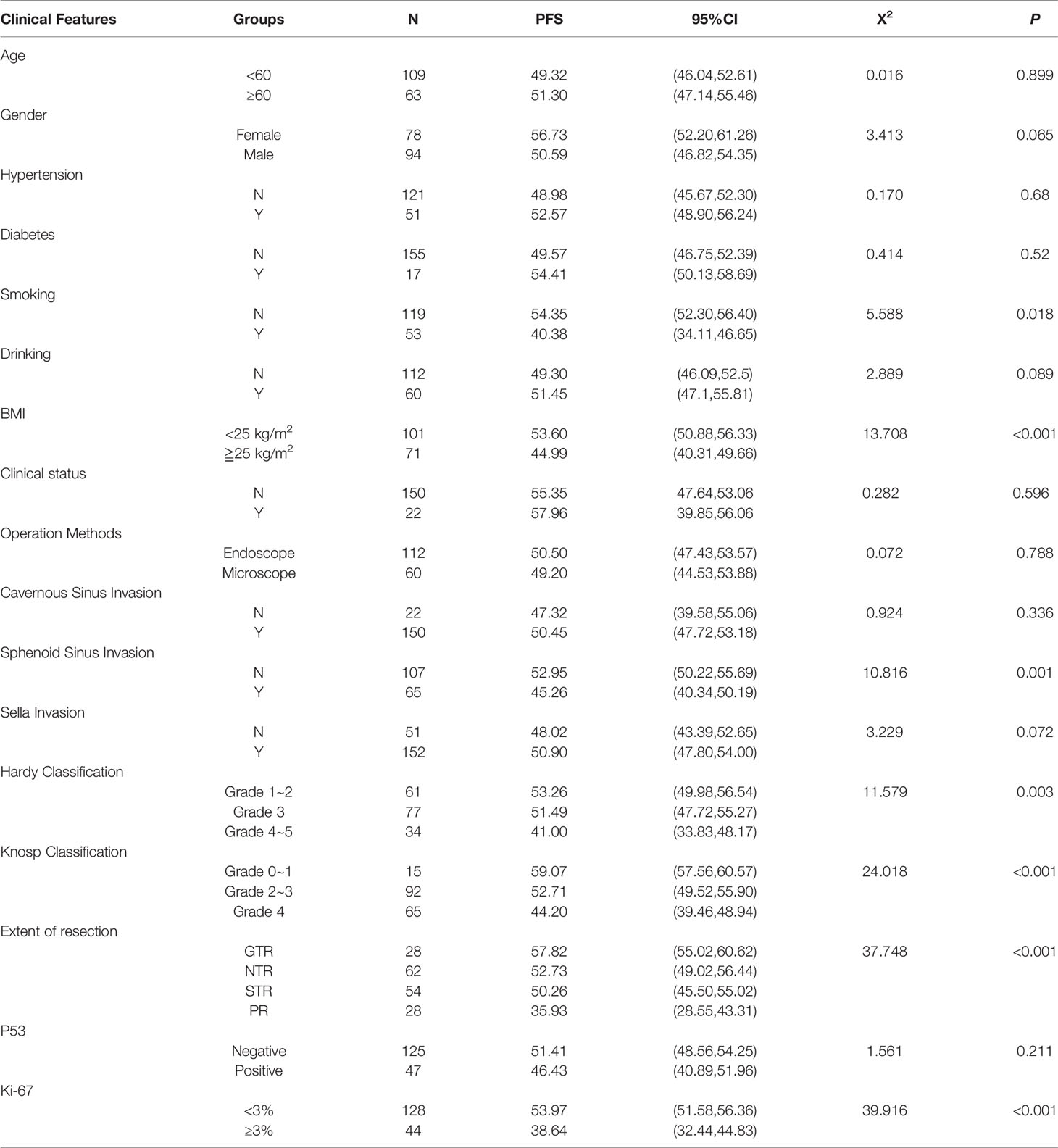
A total of 172 patients (83 females and 89 males) with pathologically confirmed PA were included. The mean age was 53.7 ± 12.7 years (range, 18-83 years), of which 109 patients (63.4%) were younger than 60 years and 63 patients (36.6%) were older than 60 years. Non-functional PA was detected in 150 patients (87.2%) and 22 patients (12.8%) had functional PAs. In addition, 101 patients (58.7%) had a BMI<25 kg/m2 and 71 patients (41.3%) had BMI≥25 kg/m2, 53 patients (30.8%) had a history of smoking, 60 patients (34.9%) had a history of alcohol consumption, 51 patients (29.7%) had a history of hypertension, and 17 patients (9.9%) had a history of diabetes. Based on the preoperative results of MRI and intraoperative observation, there were 150 cases of cavernous sinus invasion, 65 of sphenoid sinus invasion, and 152 cases of sella invasion. Due to the low number of patients in each group, the subgroups made on the basis of Knosp and Hardy classifications were pooled into several larger groups. For instance, the patients were divided into Knosp classification 0-1 (15 patients, 8.7%), 2-3 (92 patients, 53.5%) and 4 (65 patients, 37.8%), Hardy classification 1-2 (61 patients, 8.7%), 3 (77 patients, 55.3%) and 4-5 (34 patients, 37.8%). All patients underwent transnasal sphenoidal surgery, of which 112 patients (65.1%) were treated with neuroendoscopy and 60 (34.9%) with microscopy. GTR was performed in 28 cases (16.3%), NTR in 62 cases (36%), STR in 54 cases (31.4%) and PR in 28 cases (16.3%). According to the 2017 WHO classification of PA (some patients were not classified according to this standard, so this part of the results could not be included in the study), there was 1 case of somatotroph adenoma, 1 of lactotroph adenoma, 5 corticotroph adenoma (sparsely granulated corticotroph adenoma in 2 cases), 4 gonadotroph adenoma, 5 null cell adenoma, and 3 plurihormonal adenoma. Furthermore, 47 cases (27.3%) were P53 positive and 44 cases (25.6%) were Ki-67≥3% (Table 1).
Tumor Recurrence and Progression
Seventy patients (40.7%) had recurrence or progression, and the overall progress free survival (PFS) rates 1-, 3-, and 5 years after surgery was 90.70%, 79.65% and 59.30% respectively (Figure 1).
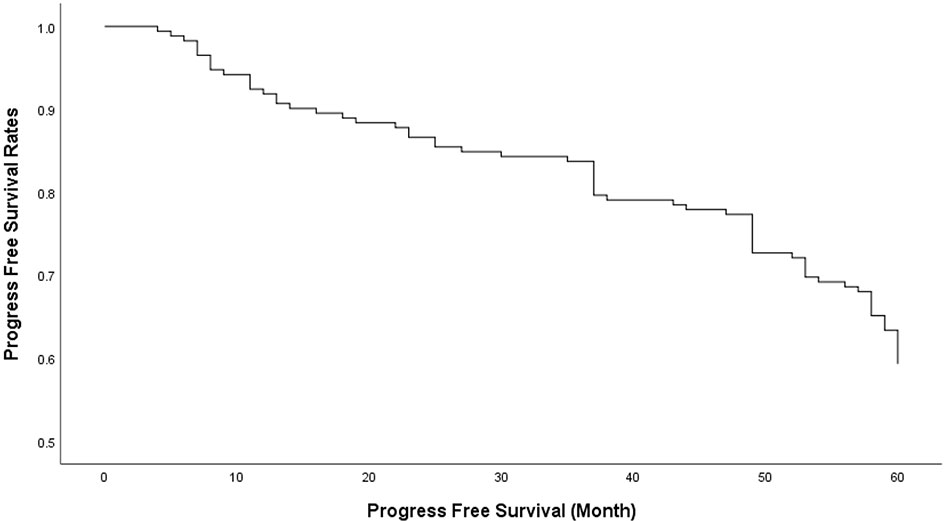
Figure 1 The overall progress free survival (PFS) rates of 1, 3 and 5 years after transnasal sphenoidal surgery was 90.70%, 79.65% and 59.30% respectively.
Univariate Analysis of Potential Risk Factors
The following factors were subjected to univariate analysis to identify those significantly associated with postoperative recurrence or progression of large and giant PAs: age, gender, history of hypertension and diabetes, smoking history, drinking history, BMI (<25 kg/m2/≧25 kg/m2), clinical function, surgical method (endoscopic/microscope), cavernous sinus invasion, spinous sinus invasion, suprasellar invasion, Hardy classification, Knosp classification, extent of resection (GTR >95%, NTR 90%-95%, STR 70%-90% and PR <70%), P53 and Ki-67 (<3%/≥3%). BMI (P <0.001), Knosp classification (P <0.001), extent of resection (P <0.001), Ki-67 (P <0.001), sphenoid sinus invasion (P =0.001), Hardy classification (P =0.003) and smoking history (P =0.018) were the significant risk factors of tumor recurrence or progression (Table 2).
Studies show that tumor size and the extent of invasion into the sphenoid sinus significantly affect post-operative recurrence or progression of PA (14, 24, 25). The mean PFS duration (Table 2) and survival rate (Figure 2) of patients with Grade 4 Knosp classification were significantly less compared to that of the other groups (P < 0.001). Likewise, the mean PFS of Grade 4-5 Hardy classification was only 41 months, and the survival rate was significantly lower compared to that of grades 1-2 and 3 (P = 0.003). The mean PFS of patients with sphenoid sinus invasion was 45.26 months compared to 52.59 months in patients without sphenoid sinus invasion (P = 0.001), and the survival rates were significantly different (Figure 2). Ki-67 expression is an indicator of tumor cell proliferation and invasion (26). The mean PFS of patients with highly proliferative tumors (Ki-67 ≥3%) was only 38.64 months compared to the 53.97 months in patients with less active tumors (Ki-67 <3%) (P < 0.001). The extent of surgical resection was determined on the basis of surgical records and postoperative imaging results, and the patients were divided into GTR, NTR, STR and PR groups. The shortest mean PFS and survival rate was seen in the PR group (P < 0.001 versus all).
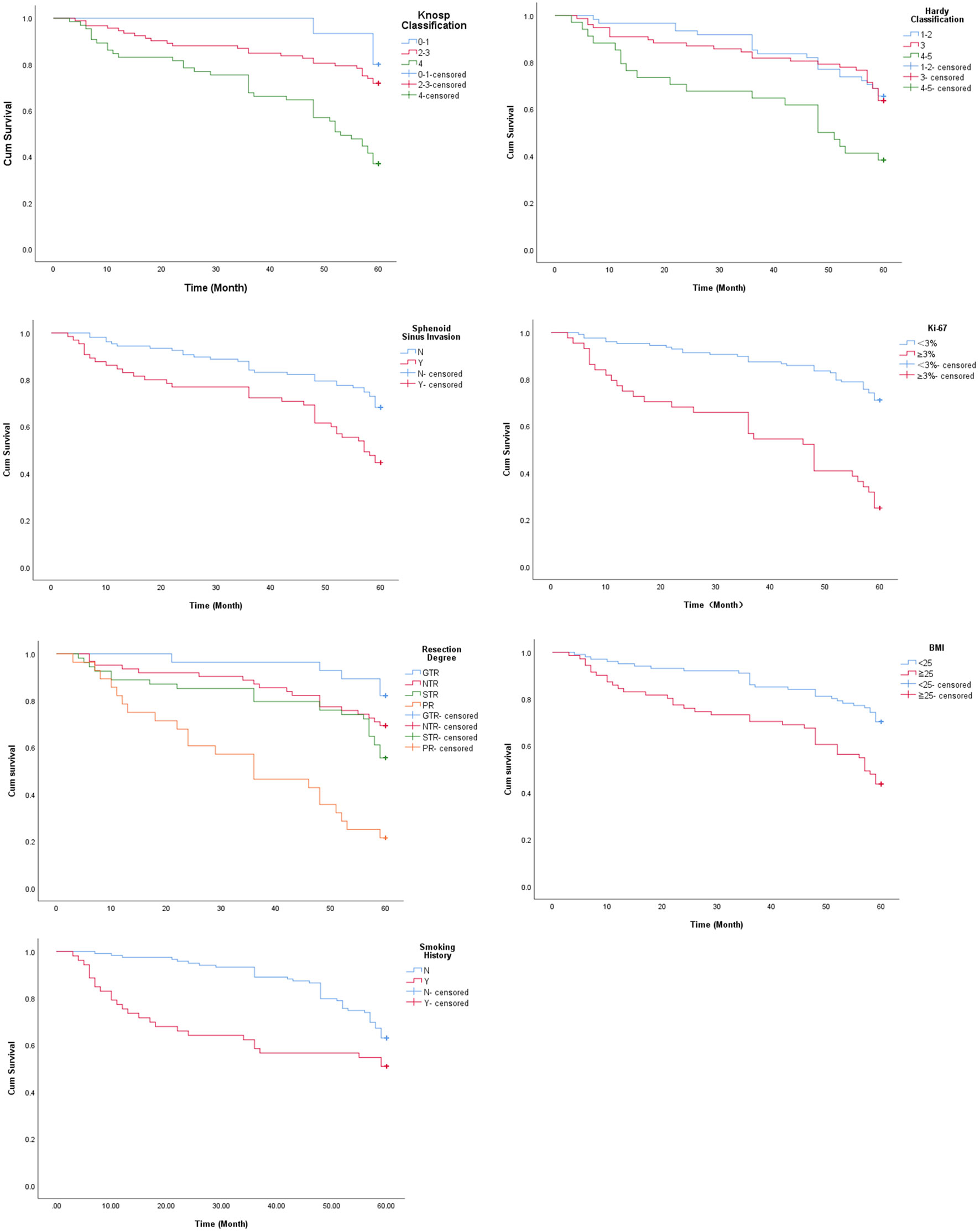
Figure 2 Survival curves of patients stratified on the basis of Knosp classification, Hardy classification, sphenoid sinus invasion, Ki-67, extent of resection, BMI and smoking history.
Although body mass index (BMI) and smoking history are not directly related to PA, univariate analysis suggested that both factors have a significant impact on postoperative recurrence or progression of large and giant PAs. Based on the pre-operative BMI, the patients were divided into overweight (BMI≧25 kg/m2) and non-overweight (BMI < 25 kg/m2) groups. The mean PFS of the overweight patients was 44.99 months as opposed to 53.6 in patients with healthy BMI (P< 0.001). Furthermore, patients with a history of smoking had a shorter mean PFS compared to the non-smokers (40.38 months vs 54.35 months). As shown in Figure 2, there was a significant difference in PFS between the two groups (P = 0.018).
Independent Risk Factors for Postoperative Recurrence or Progression of Large and Giant Pas
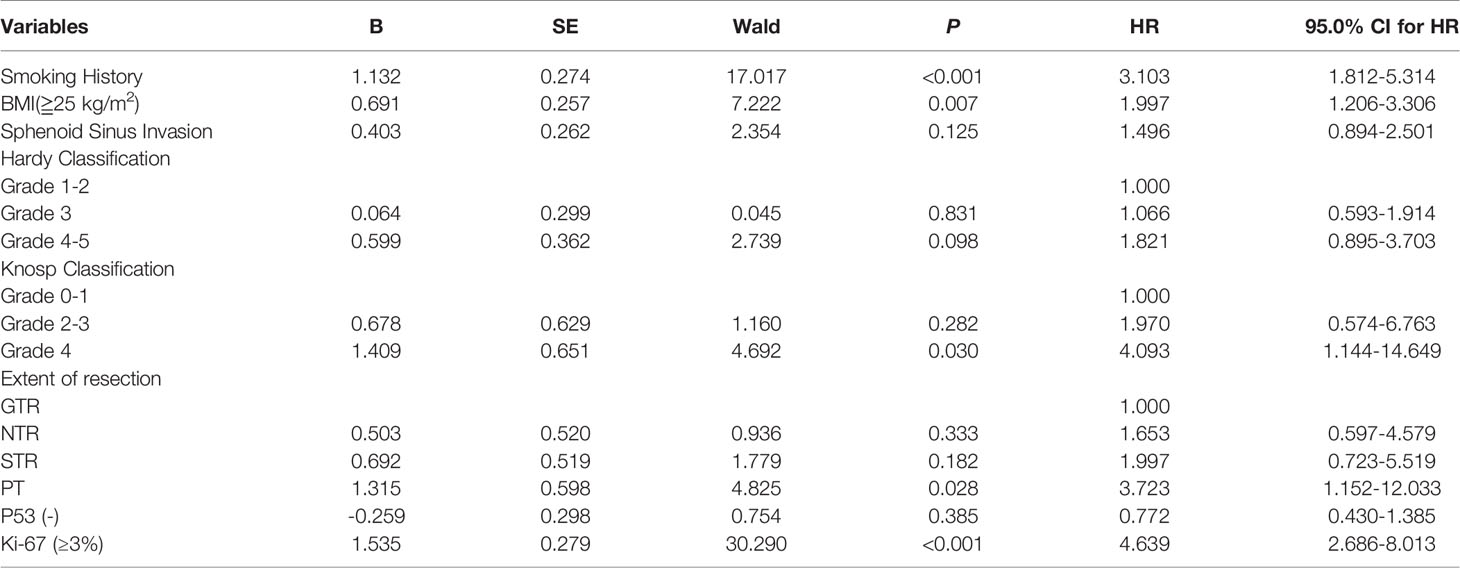
The factors identified in the univariate analysis were incorporated into the multivariate Cox regression model. Smoking history (HR=3.103, 95% CI: 1.812-5.314), BMI (≧25 kg/m2) (HR=1.997, 95% CI:1.206-3.306), Knosp classification (grade 4) (HR=4.093, 95% CI:1.144-14.649), extent of resection (PR) (HR=3.723, 95% CI:1.152-12.033) and Ki-67 positivity (≥3%) (HR=4.639, 95% CI:2.686-8.013) were identified as the independent risk factors (Table 3).
Establishment and Validation of Predictive Nomogram
We next established a nomogram based on the extent of resection, BMI, Ki-67, Knosp classification and smoking to predict the 1-, 3-, and 5-year prognosis of patients (Figure 3). Each risk factor was designated a score, and the sum of the five scores in the individual patients corresponded to the probability of PFS 1, 3 and 5 years after surgery. The calibration curve and ROC were used to confirm the clinical value of this model, and indicated good agreement between the predicted and observed values (Figure 4A). As shown in Figure 4B, the area under the ROC curves (AUC) for 1-, 2-, and 3-year survival were 0.889, 0.885 and 0.832 respectively, indicating satisfactory accuracy.
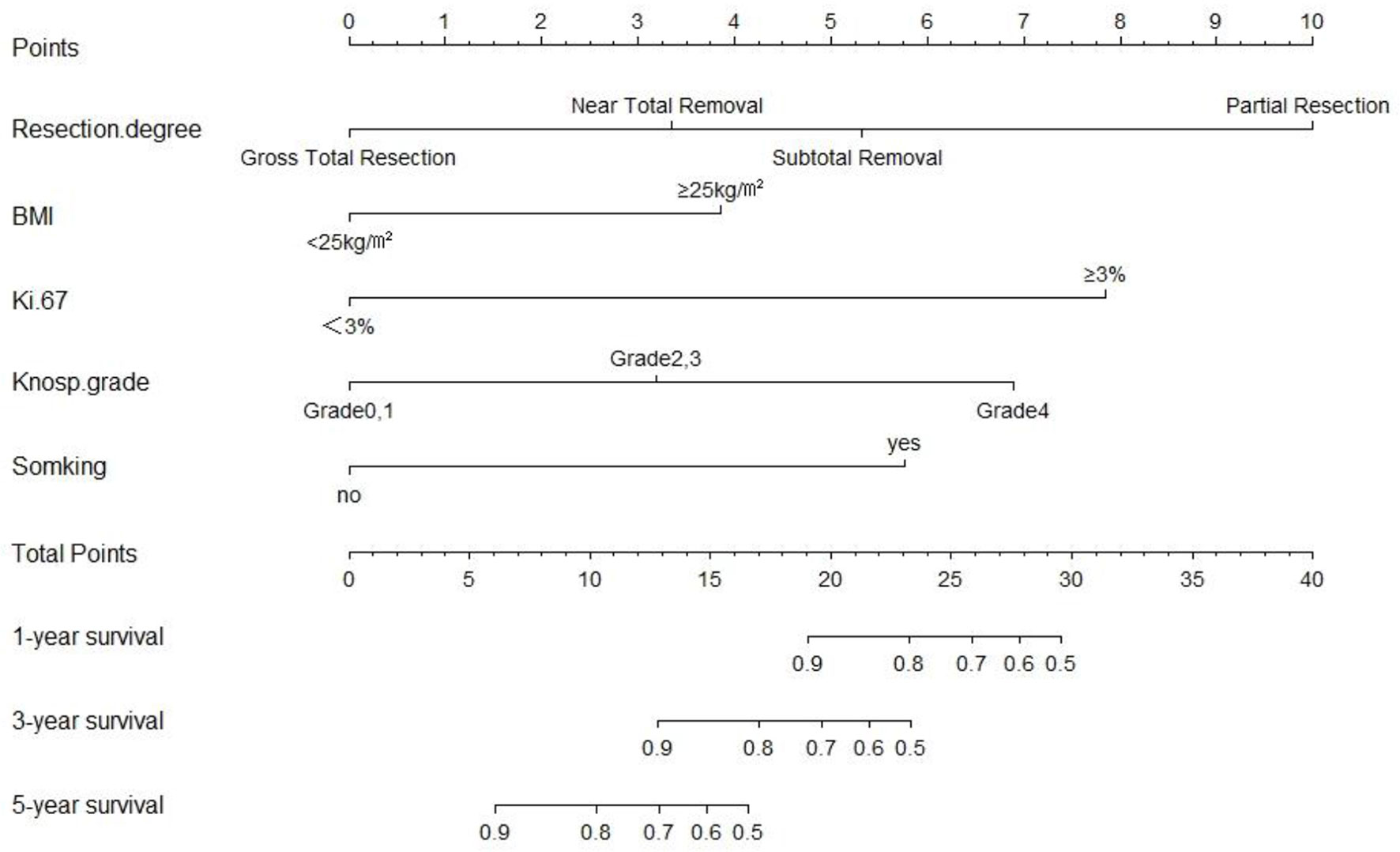
Figure 3 The predictive nomogram based on resection degree, BMI, Ki-67, Knosp classification and smoking history.
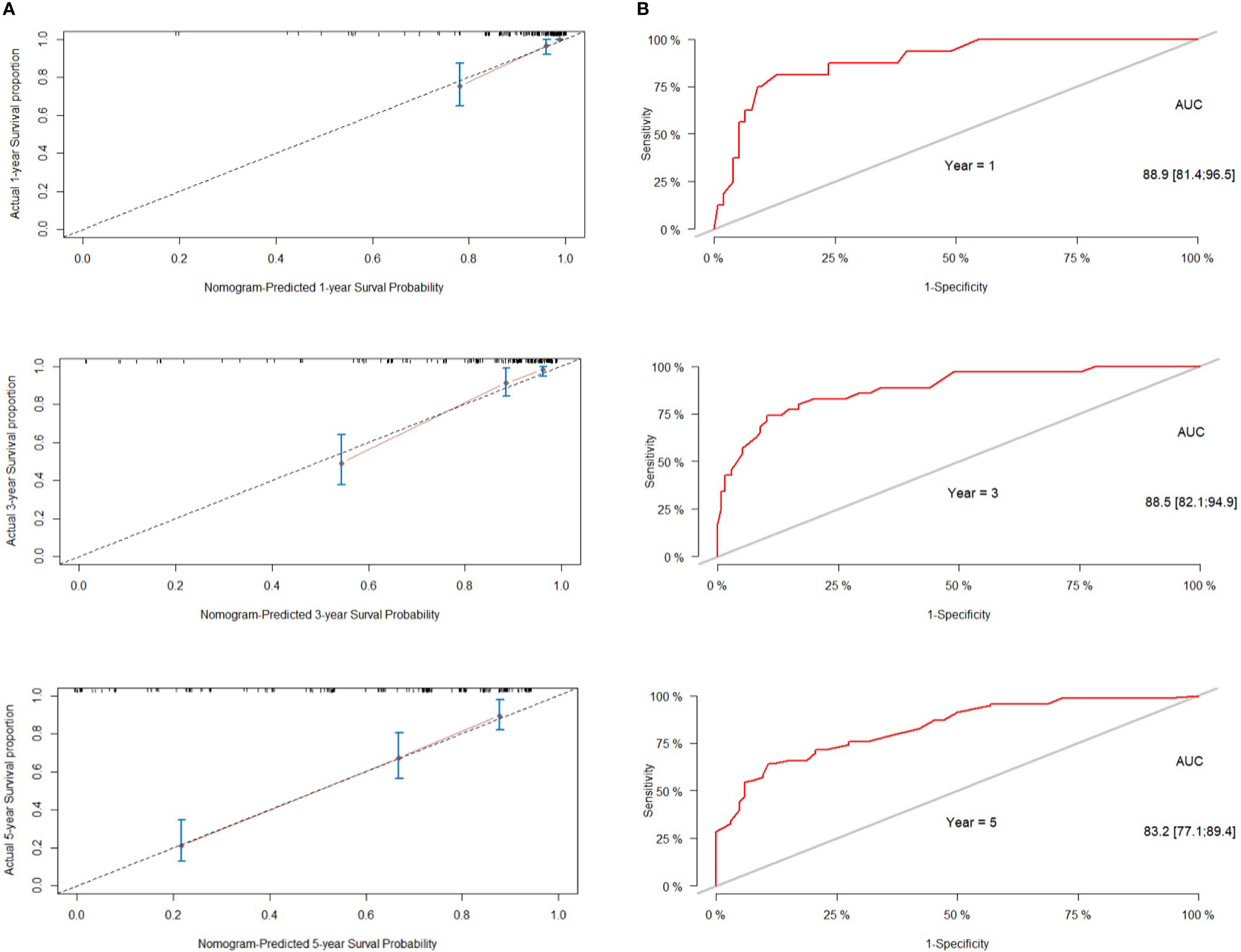
Figure 4 Performance of the nomogram for predicting tumor recurrence or progression. (A) calibration curve of the nomogram. (B) The predictive performance of the nomogram was assessed by receiver operator characteristics (ROC) analysis and area under the curve (AUC).
Discussion
In this study, we retrospectively analyzed the clinical data of 172 patients with large or giant PA, and identified multiple risk factors for postoperative recurrence or progression. A prognostic nomogram was established based on these factors, which could predict survival with satisfactory accuracy.
The extent of surgical resection is the main factor influencing tumor recurrence or progression. In this study, we defined GTR (>95%), NTR (90%-95%), STR (70%-90%) and PR (<70%) on the basis of postoperative imaging results and surgical records. PR was significantly associated with tumor recurrence or progression (HR: 3.302; P=0.048). The transsphenoidal approach with neuroendoscopy can improve the extent of surgical resection (19, 27). Fathalla et al. reviewed 65 patients with acromegaly, and found that the extent of resection was significantly greater in patients who underwent endoscopy as opposed to the microscopy group (61% vs 42%, P=0.05). Furthermore, endoscopy also improved the extent of resection (48% vs 14.2%, P=0.09) when the tumor invaded the cavernous sinus (27). In our cohort however, the mean PFS of patients who were treated with neuroendoscopy and microscopy were 50.50 and 49.2 months respectively, indicating that the surgical method did not have any impact on the postoperative recurrence and progression.
The invasiveness of PA is also a risk factor for postoperative recurrence or progression. Similar to the diaphragm sella, the wall of the cavernous sinus is a thin dural bag (28), and most patients in our cohort had cavernous sinus invasion (87.2%) and/or suprasellar invasion (88.4%). However, only sphenoid sinus invasion was identified as a risk factor of postoperative tumor recurrence or progression. This is consistent with the views of some researchers that sphenoid sinus invasion rather than cavernous sinus invasion is a more indicative of “invasiveness” (29, 30). In one case of a particularly aggressive tumor, the growing mass passed through the bone of sellar floor to invade the sphenoid sinus in a cathepsin K-dependent manner (31). The size and invasion of PA are graded by Knosp classification and Hardy classification that are based on preoperative imaging data, and are used by neurosurgeons in preoperative evaluation and prediction of surgical. We found that both were significantly associated with the recurrence or progression of large and giant PAs after surgery, and Knosp 4 was an independent risk factor, which is highly associated with tumor recurrence. Thus, Knosp classification may play an important role in predicting tumor recurrence as reported in previous studies (32–34).
The invasiveness of adenomas and other tumors is also evaluated in terms of pathological parameters, such as mitotic figures, Ki-67 index (35, 36) and p53 expression in the malignant tissues (26). Petry et al. conducted a retrospective analysis of 52 patients with PA and divided them into Ki-67 ≥3% and Ki-67 <3% groups, and found that high Ki-67 expression was correlated with more aggressive tumor growth and higher recurrence rate (67%vs 17%, P=0.03) (37). Consistent with this, we found that the mean PFS of patients with higher Ki-67 index tumors (≥3%) was significantly lower compared to those with < 3% Ki-67 positivity. Furthermore, Ki-67 ≥ 3% was identified an independent risk factor of postoperative recurrence or progression. However, P53 expression did not have any impact on the mean PFS. According to WHO 2017 classification, the pathological subtypes of PA include sparsely granulated somatotroph adenoma, lactotroph adenoma (in men), pluri-hormonal PIT-1 positive adenoma, silent corticotroph adenoma and crooke cell adenoma, all of which are considered high-risk (38). Due to limitations of specific markers and staining techniques, we were unable to include this pathological classification in our study. Therefore, predicting the proliferative potential of large and giant PAs classified on the basis of more rigorous pathological criteria remains to be elucidated.
The prognosis of PA is evaluated according to imaging features (tumor site, Knosp classification etc.) and pathological results (pathological classification, Ki-67, P53 etc.) (39), which do not take into account other clinical factors. In this study, we found that BMI ≧ 25 kg/m2 and a history of smoking were independent risk factors of postoperative recurrence or progression. Obesity is an established risk factor for intracranial tumors (40, 41), and promotes tumor development through chronic insulin resistance, hyperinsulinemia and enhanced IGF-1 activity. In fact, more than half of meningiomas overexpress IGF-1 receptors, and IGF-1 can promote the growth of meningioma cells in vitro (42). Furthermore, overexpression of IGF-1 receptors in glioma cells promotes their proliferation and inhibits apoptosis (43, 44). In this study, the mean PFS of overweight patients (BMI≥25 kg/m2) was significantly shorter than that of patients with a healthy weight (BMI < 25 kg/m2). All these evidences provide new insights into the molecular mechanisms underlying recurrence or progression of large and giant PAs after surgery. Several studies have shown that smoking is a high-risk factor for lung cancer, bladder cancer, head and neck cancer and other cancers (45–47). Taken together, the correlation of general health and smoking with PA progression warrants further study.
Although the association between PA recurrence or progression and clinical variables has been reported previously (48–50), multiple variables are rarely incorporated to assess prognosis. Nomograms are now widely used to predict the prognosis of other tumors, since they can simplify the statistical model, and estimate the probability of an event (such as death or recurrence) with a single value (51). In addition, a nomogram can integrate multiple prognostic variables and determinants that simulate complex biological and clinical scenarios for personalized medicine (52). On the basis of the univariate and multivariate models, we developed a nomogram to predict the recurrence or progression of PA after transnasal sphenoidal surgery by integrating the independent risk factors, and found that the model can predict tumor recurrence 1-, 3- and 5 years after surgery with satisfactory accuracy. This nomogram provided probability estimates that may be useful for individual patients, and help predict tumor recurrence or progression after surgery. We can use this scoring system after surgery by adding up the scores for all factors, objectively evaluate the possibility of tumor recurrence or progression, and provide a reference for clinical treatment to develop an individual follow-up plan.
However, this study has certain limitations that should be considered. First, this was a single-center retrospective study, which warrants further validation of the predictive model on a larger, multicenter cohort. Second, the association of the pathological subtypes of PA with tumor recurrence or progression should be further explored with a uniform and strict standard. In conclusion, partial resection, BMI ≧25 kg/m2, Ki-67 ≥3%, Knosp classification grade 4 and smoking increase the risk of the recurrence or progression of large and giant PAs. The nomogram model incorporating these risk factors can facilitate prediction of tumor recurrence or progression after transnasal sphenoidal surgery in individual patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was reviewed and approved by Ethics Committee of Second Affiliated Hospital, School of Medicine, Zhejiang University.
Author Contributions
YC and FC performed the analysis and co-wrote the manuscript. AZ, XH, YT, FG, YL, and JC collected the patient information. WY, YW, SC, and XD revised paper. QW and JZ supervised the project, conceived the study, and guided the editing of the manuscript. YC and FC contributed equally to the manuscript. QW and JZ are corresponding authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol (2018) 20(suppl_4):iv1–iv86. doi: 10.1093/neuonc/noy131
2. Chen Y, Wang CD, Su ZP, Chen YX, Cai L, Zhuge QC, et al. Natural History of Postoperative Nonfunctioning Pituitary Adenomas: A Systematic Review and Meta-Analysis. Neuroendocrinology (2012) 96(4):333–42. doi: 10.1159/000339823
3. Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of Pituitary Adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab (2010) 95(9):4268–75. doi: 10.1210/jc.2010-0537
4. Raverot G, Jouanneau E, Trouillas J. Management of Endocrine Disease: Clinicopathological Classification and Molecular Markers of Pituitary Tumours for Personalized Therapeutic Strategies. Eur J Endocrinol (2014) 170(4):R121–32. doi: 10.1530/EJE-13-1031
5. Müslüman AM, Cansever T, Yılmaz A, Kanat A, Oba E, Çavuşoğlu H, et al. Surgical Results of Large and Giant Pituitary Adenomas With Special Consideration of Ophthalmologic Outcomes. World Neurosurg (2011) 76(1-2):141–8; discussion 63-6. doi: 10.1016/j.wneu.2011.02.009
6. de Paiva Neto MA, Vandergrift A, Fatemi N, Gorgulho AA, Desalles AA, Cohan P, et al. Endonasal Transsphenoidal Surgery and Multimodality Treatment for Giant Pituitary Adenomas. Clin Endocrinol (Oxf) (2010) 72(4):512–9. doi: 10.1111/j.1365-2265.2009.03665.x
7. Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric Classification of Pituitary Macroadenomas Predicts Outcome and Morbidity Following Endoscopic Endonasal Transsphenoidal Surgery. Pituitary (2012) 15(3):450–63. doi: 10.1007/s11102-011-0350-z
8. Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B, et al. Endoscopic Endonasal Transsphenoidal Approach to Large and Giant Pituitary Adenomas: Institutional Experienceand Predictors of Extent of Resection. J Neurosurg (2014) 121(1):75–83. doi: 10.3171/2014.3.JNS131679
9. Shimon I, Jallad RS, Fleseriu M, Yedinak CG, Greenman Y, Bronstein MD. Giant GH-Secreting Pituitary Adenomas: Management of Rare and Aggressive Pituitary Tumors. Eur J Endocrinol (2015) 172(6):707–13. doi: 10.1530/EJE-14-1117
10. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the Treatment of Prolactinomas. Endocr Rev (2006) 27(5):485–534. doi: 10.1210/er.2005-9998
11. Pratheesh R, Rajaratnam S, Prabhu K, Mani SE, Chacko G, Chacko AG. The Current Role Oftranscranial Surgery in the Management of Pituitary Adenomas. Pituitary (2013) 16(4):419–34. doi: 10.1007/s11102-012-0439-z
12. Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff MJL. Endoscopic Pituitarytumor Surgery. Laryngoscope (1992) 102(2):198–202. doi: 10.1288/00005537-199202000-00016
13. Dekkers OM, Pereira AM, Roelfsema F, Voormolen JHC, Neelis KJ, Schroijen MA, et al. Observation Alone After Transsphenoidal Surgery for Nonfunctioning Pituitary Macroadenoma. J Clin Endocrinol Metab (2006) 91(5):1796–801. doi: 10.1210/jc.2005-2552
14. Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C, et al. Factors Predicting Relapse of Nonfunctioning Pituitary Macroadenomas After Neurosurgery: A Study of 142 Patients. Eur J Endocrinol (2010) 163(2):193–200. doi: 10.1530/EJE-10-0255
15. Ferrante E, Ferraroni M, Castrignano T, Menicatti L, Anagni M, Reimondo G, et al. Non-Functioning Pituitary Adenoma Database: A Useful Resource to Improve the Clinical Management of Pituitary Tumors. Eur J Endocrinol (2006) 155(6):823–9. doi: 10.1530/eje.1.02298
16. Sassolas G, Trouillas J, Treluyer C, Perrin GJAE. Management of Nonfunctioning Pituitaryadenomas. Acta Endocrinol (Copenh) (1993) 129(Suppl 1):21–6.
17. Sadik ZHA, Voormolen EHJ, Depauw PRAM, Burhani B, Nieuwlaat WA, Verheul J, et al. Treatment of Nonfunctional Pituitary Adenoma Postoperative Remnants: Adjuvant or Delayed Gamma Knife Radiosurgery? World Neurosurg (2017) 100:361–8. doi: 10.1016/j.wneu.2017.01.028
18. Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, et al. Early Results of Surgery in Patients With Nonfunctioning Pituitary Adenoma and Analysis of the Risk of Tumor Recurrence. J Neurosurg (2008) 108(3):525–32. doi: 10.3171/JNS/2008/108/3/0525
19. Micko AS, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the Cavernous Sinus Space Inpituitary Adenomas: Endoscopic Verification and its Correlation With an MRI-Based Classification. J Neurosurg (2015) 122(4):803–11. doi: 10.3171/2014.12.JNS141083
20. Witek P, Zieliński G, Szamotulska K, Maksymowicz M, Kamiński G. Clinicopathological Predictive Factors in the Early Remission of Corticotroph Pituitary Macroadenomas in a Tertiary Referral Centre. Eur J Endocrinol (2016) 174(4):539–49. doi: 10.1530/EJE-15-1226
21. Kato M, Inoshita N, Sugiyama T, Tani Y, Shichiri M, Sano T, et al. Differential Expression of Genes Related to Drug Responsiveness Between Sparsely and Densely Granulated Somatotroph Adenomas. Endocr J (2012) 59(3):221–8. doi: 10.1507/endocrj.ej11-0177
22. Delgrange E, Vasiljevic A, Wierinckx A, François P, Jouanneau E, Raverot G, et al. Expression of Estrogen Receptor Alpha Is Associated With Prolactin Pituitary Tumor Prognosis and Supports the Sex-Related Difference in Tumor Growth. Eur J Endocrinol (2015) 172(6):791–801. doi: 10.1530/EJE-14-0990
23. Williamson TJ, Kwon DM, Riley KE, Shen MJ, Hamann HA, Ostroff JS. Lung Cancer Stigma: Does Smoking History Matter? Ann Behav Med Publ Soc Behav Med (2020) 54(7):535–40. doi: 10.1093/abm/kaz063
24. Lelotte J, Mourin A, Fomekong E, Michotte A, Raftopoulos C, Maiter D. Both Invasivenessand Proliferation Criteria Predict Recurrence of Non-Functioning Pituitary Macroadenomas After Surgery: A Retrospective Analysis of a Monocentric Cohort of 120 Patients. Eur J Endocrinol (2018) 178(3):237–46. doi: 10.1530/EJE-17-0965
25. Lv L, Yin S, Zhou P, Hu Y, Chen C, Ma W, et al. Clinical Andpathologic Characteristics Predicted the Postoperative Recurrence and Progression of Pituitary Adenoma: A Retrospective Study With 10 Years Follow-Up. World Neurosurg (2018) 118:e428–35. doi: 10.1016/j.wneu.2018.06.210
26. Criscitiello C, Disalvatore D, De Laurentiis M, Gelao L, Fumagalli L, Locatelli M, et al. High Ki-67 Score Is Indicative of a Greater Benefit From Adjuvant Chemotherapy When Added to Endocrine Therapy in Luminal B HER2 Negative and Node-Positive Breast Cancer. Breast (2014) 23(1):69–75. doi: 10.1016/j.breast.2013.11.007
27. Fathalla H, Cusimano MD, Di Ieva A, Lee J, Alsharif O, Goguen J, et al. Endoscopic Versus Microscopic Approach for Surgical Treatment of Acromegaly. Neurosurg Rev (2015) 38(3):541–8; discussion 548-9. doi: 10.1007/s10143-015-0613-7
28. Harris FS, Rhoton AL. Anatomy of the Cavernous Sinus. A Microsurgical Study. J Neurosurg (1976) 45(2):169–80. doi: 10.3171/jns.1976.45.2.0169
29. Heaney A. Management of Aggressive Pituitary Adenomas and Pituitary Carcinomas. JNeurooncol (2014) 117(3):459–68. doi: 10.1007/s11060-014-1413-6
30. Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu JI. Are Nonfunctioningpituitary Adenomas Extending Into the Cavernous Sinus Aggressive and/or Invasive? Neurosurgery (2001) 49(4):857–62; discussion 862-3. doi: 10.1097/00006123-200110000-00014
31. Liu H, Wang G, Gu W, Mu Y, Cathepsin K. The Association Between Cathepsin K Expressionand Sphenoid Sinus Invasion of Pituitary Adenomas. Med Hypotheses (2016) 97:88–9. doi: 10.1016/j.mehy.2016.10.013
32. Araujo-Castro M, Pascual-Corrales E, Martínez-Vaello V, Baonza Saiz G, Quiñones de Silva J, Acitores Cancela A, et al. Predictive Model of Surgical Remission in Acromegaly: Age, Presurgical GH Levels and Knosp Grade as the Best Predictors of Surgical Remission. J Endocrinol Invest (2021) 44(1):183–93. doi: 10.1007/s40618-020-01296-4
33. Buchy M, Lapras V, Rabilloud M, Vasiljevic A, Borson-Chazot F, Jouanneau E, et al. Predicting Early Post-Operative Remission in Pituitary Adenomas: Evaluation of the Modified Knosp Classification. Pituitary (2019) 22(5):467–75. doi: 10.1007/s11102-019-00976-6
34. Yuhan L, Zhiqun W, Jihui T, Renlong P. Ki-67 Labeling Index and Knosp Classification Ofpituitary Adenomas. Br J Neurosurg (2021) 27:1–5. doi: 10.1080/02688697.2021.1884186
35. Mete O, Lopes MB. Overview of the 2017 WHO Classification of Pituitary Tumors. EndocrPathol (2017) 28(3):228–43. doi: 10.1007/s12022-017-9498-z
36. Glebauskiene B, Liutkeviciene R, Vilkeviciute A, Gudinaviciene I, Rocyte A, Simonaviciute D, et al. Association of Ki-67 Labelling Index and IL-17A With Pituitary Adenoma. BioMed Res Int (2018) 2018:7490585. doi: 10.1155/2018/7490585
37. Petry C, Poli JHZ, de Azevedo Dossin I, Rech CGSL, Pereira Lima JFS, Ferreira NP, et al. Evaluation of the Potential of the Ki67 Index to Predict Tumor Evolution in Patients With Pituitary Adenoma. Int J Clin Exp Pathol (2019) 12(1):320–6.
38. Lopes MBS. The 2017 World Health Organization Classification of Tumors of the Pituitarygland: A Summary. Acta Neuropathol (2017) 134(4):521–35. doi: 10.1007/s00401-017-1769-8
39. Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, Viennet G, et al. Anew Prognostic Clinicopathological Classification of Pituitary Adenomas: A Multicentric Case-Control Study of 410 Patients With 8 Years Post-Operative Follow-Up. Acta Neuropathol (2013) 126(1):123–35. doi: 10.1007/s00401-013-1084-y
40. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N Engl J Med (2016) 375(8):794–8. doi: 10.1056/NEJMsr1606602
41. Wiedmann MKH, Brunborg C, Di Ieva A, Lindemann K, Johannesen TB, Vatten L, et al. Overweight, Obesity and Height as Risk Factors for Meningioma, Glioma, Pituitary Adenoma and Nerve Sheath Tumor: A Large Population-Based Prospective Cohort Study. Acta Oncol (2017) 56(10):1302–9. doi: 10.1080/0284186X.2017.1330554
42. Kurihara M, Tokunaga Y, Tsutsumi K, Kawaguchi T, Shigematsu K, Niwa M, et al. Characterization of Insulin-Like Growth Factor I and Epidermal Growth Factor Receptors in Meningioma. J Neurosurg (1989) 71(4):538–44. doi: 10.3171/jns.1989.71.4.0538
43. Friend KE, Khandwala HM, Flyvbjerg A, Hill H, Li J, McCutcheon IE. Growth Hormone Andinsulin-Like Growth Factor-I: Effects on the Growth of Glioma Cell Lines. Growth Horm IGF Res (2001) 11(2):84–91. doi: 10.1054/ghir.2000.0183
44. Yin D, Tamaki N, Parent AD, Zhang JH. Insulin-Like Growth Factor-I Decreased Etoposide-Induced Apoptosis in Glioma Cells by Increasing Bcl-2 Expression and Decreasing CPP32 Activity. Neurol Res (2005) 27(1):27–35. doi: 10.1179/016164105X18151
45. Hecht SS. Tobacco Smoke Carcinogens and Lung Cancer. J Natl Cancer Inst (1999) 91(14):1194–210. doi: 10.1093/jnci/91.14.1194
46. van Imhoff LC, Kranenburg GG, Macco S, Nijman NL, van Overbeeke EJ, Wegner I, et al. Prognostic Value of Continued Smoking on Survival and Recurrence Rates in Patients With Head and Neck Cancer: A Systematic Review. Head Neck (2016) 38(Suppl 1):E2214–20. doi: 10.1002/hed.24082
47. Li HM, Azhati B, Rexiati M, Wang WG, Li XD, Liu Q, et al. Impact of Smoking Statusand Cumulative Smoking Exposure on Tumor Recurrence of non-Muscle-Invasive Bladder Cancer. Int Urol Nephrol (2017) 49(1):69–76. doi: 10.1007/s11255-016-1441-6
48. Batista RL, Trarbach EB, Marques MD, Cescato VA, da Silva GO, Herkenhoff CGB, et al. Nonfunctioning Pituitary Adenoma Recurrence and Its Relationship With Sex, Size, and Hormonal Immunohistochemical Profile. World Neurosurg (2018) 120:e241–6. doi: 10.1016/j.wneu.2018.08.043
49. Bodhinayake I, Ottenhausen M, Mooney MA, Kesayabhotla K, Christos P, Schwarz JT, et al. Results and Risk Factors for Recurrence Following Endoscopic Endonasal Transsphenoidal Surgery for Pituitary Adenoma. Clin Neurol Neurosurg (2014) 119:75–9. doi: 10.1016/j.clineuro.2014.01.020
50. Lyu W, Fei X, Chen C, Tang YQ. Nomogram Predictive Model of Post-Operative Recurrencein non-Functioning Pituitary Adenoma. Gland Surg (2021) 10(2):807–15. doi: 10.21037/gs-21-47
51. Iasonos A, Schrag D, Raj GV, Panageas KS. How to Build and Interpret a Nomogram Forcancer Prognosis. J Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
Keywords: pituitary adenoma, tumor recurrence, transnasal sphenoidal surgery, Cox regression model, nomogram
Citation: Chen Y, Cai F, Cao J, Gao F, Lv Y, Tang Y, Zhang A, Yan W, Wang Y, Hu X, Chen S, Dong X, Zhang J and Wu Q (2021) Analysis of Related Factors of Tumor Recurrence or Progression After Transnasal Sphenoidal Surgical Treatment of Large and Giant Pituitary Adenomas and Establish a Nomogram to Predict Tumor Prognosis. Front. Endocrinol. 12:793337. doi: 10.3389/fendo.2021.793337
Received: 12 October 2021; Accepted: 23 November 2021;
Published: 14 December 2021.
Edited by:
Congxin Dai, Capital Medical University, ChinaReviewed by:
Zhu Huijuan, Peking Union Medical College Hospital (CAMS), ChinaChangxiang Yan, Capital Medical University, China
Copyright © 2021 Chen, Cai, Cao, Gao, Lv, Tang, Zhang, Yan, Wang, Hu, Chen, Dong, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Wu, 2192010@zju.edu.cn; Jianmin Zhang, zjm135@zju.edu.cn
†These authors have contributed equally to this work and share first authorship
 Yike Chen
Yike Chen Feng Cai
Feng Cai Jing Cao2
Jing Cao2 Yajuan Tang
Yajuan Tang Sheng Chen
Sheng Chen Xiao Dong
Xiao Dong Jianmin Zhang
Jianmin Zhang Qun Wu
Qun Wu