Improving Sustainable Field-Grown Wheat Production With Azospirillum brasilense Under Tropical Conditions: A Potential Tool for Improving Nitrogen Management
- 1Center for Nuclear Energy in Agriculture, University of São Paulo, Piracicaba, Brazil
- 2Department of Soil, Water and Climate, Southwest Research and Outreach Center, University of Minnesota, St. Paul, MN, United States
- 3Department of Plant Health, Rural Engineering, and Soils, São Paulo State University, Ilha Solteira, Brazil
Sustainable intensification of cropping systems requires to increase productivity and nutrients use efficiency while reducing negative impacts of agricultural management practices on ecosystem and environment. Plant growth-promoting rhizobacteria (PGPR) inoculations are considered one of the most promising and safe strategy to alleviate environmental alterations in context of climatic extremes to improve plant nutrition while reducing dependency of nitrogen (N) fertilizer application. This study investigated the interactive effects of N levels and inoculation with A. brasilense on plant biomass, grain yield, agronomic efficiency (AE) of applied N, apparent N-fertilizer recovery (AFR) and N content in plant targeting economic feasibility of wheat production system. The field trial tested 4 N application levels applied in side-dressing (control, low, average and high; named 0, 50, 100 and 200 kg N ha−1) and two inoculations (without and with A. brasilense seed inoculation). The results exhibited that inoculation with A. brasilense enhanced AE, AFR and N uptake in wheat plants with increased root and shoot N accumulation and grain N accumulation under average and high N application levels. In addition, inoculation increased root and shoot biomass, leading to a yield increase of 10.3% compared with non-inoculated plants. Wheat plant inoculation associated with application of the average N level provided the greatest profitability. Furthermore, results showed that reducing N fertilization from 100 to 50 kg N ha−1 along A. brasilense inoculation led to an increase in operating profit of 10.5%. In view of low economic cost, ease of application, and high probability of a positive response by wheat crops, even associated with different N application levels, the inoculation with A. brasilense prone to be a key sustainable management practice to improve wheat production under tropical conditions. This practice has the potential to increase wheat grain yield, N use and uptake, and overall farm profitability.
Introduction
Wheat (Triticum aestivum L.) is one of the essential global staple foods that contributes around 20% dietary protein for human nutrition on daily basis (Zheng et al., 2021). Although Brazil is one the world’s largest cereal producers but still importing more than half of wheat from other countries for its consumption (Conab, 2021). The growing demand of wheat consumption has become a threatened to food security in many South American, African, Caribbean and Pacific countries. Increasing population growth, rise and distribution of income, low prices and alteration in food preference are the contributory factors that increase cereal import in tropical countries faster than the ability to pay. Major cereal producers are North America and Europe, and Asia in the developing world, particularly India and China (Grote et al., 2021). However, consumption largely outpaces production in Africa and Asia, which are making these two regions major net importers of wheat (Grote et al., 2021). Therefore, crop failure in tropical and sub-tropical regions may cause a great deficit to wheat production. Thus, increasing wheat cultivation to tropical and sub-tropical regions could be handled as a matter of food security around the world.
The global demand for agricultural crops is expected to roughly double by 2050. The higher food security and production demand lead to an over-application of agricultural inputs (Kopittke et al., 2019). However, these overuses of agricultural inputs could increase methane (CH4), nitrous oxide (N2O) and carbon dioxide (CO2) emissions which are a major source of total anthropogenic greenhouse gas (GHG) that lead to global warming and climate changes (Mateo-Marín et al., 2020; Wang et al., 2021). In addition, these GHG emissions are also expected to threaten sustainability of crop production with increasing environmental concerns worldwide (Mateo-Marín et al., 2020). Therefore, sustainable intensification of cropping systems requires increasing yields within optimized use of agricultural inputs.
Nitrogen (N) is considered one of the most limiting resources for potential crop growth and yield (Xu et al., 2020). Although, excessive N supply could directly contribute to soil acidification, ammonia (NH3) and nitrogen oxide (N2O, NO2 and NO) gases emission and N leaching with extended consequences on environmental pollution and global warming (Sainju et al., 2019). The N application to crops has around 60% contribution to global N2O anthropogenic emissions (Mateo-Marín et al., 2020). In addition, the application of N to wheat and corn through side-dressing technique in Brazilian Savannah has reported in almost 15% of their total operational profit (Galindo et al., 2017, 2019). Over the last decades, several management practices have been developed to enable farmers to reduce the application of N-based fertilizers in cereal crops, in order to limit potential environmental negative impacts and cost factors resulting from heavy chemical N fertilization (Brusamarello-Santos et al., 2017). The integrated N management techniques should imperatively contribute to greater N agronomic efficiency (AE) and recovery in tropical regions, especially for wheat cycle which is highly N demanding crop (Tsujimoto et al., 2019; Galindo et al., 2021).
The inoculation of plant growth-promoting rhizobacteria (PGPR) has quite significant role in alleviation of environmental changes under the context of climate extremes and excessive use of fertilizer in agricultural soils (Backer et al., 2018). The inoculation these microbes is recognized one of the best and alternative strategy for ecofriendly crop-management techniques as it could improve plant nutrition while reducing the dependance of N-fertilizer application (Koskey et al., 2021). The PGPR with several growth promoting attributes can improve AE, plant growth with greater grain yield of cereals in tropical environments (Hungria et al., 2010, 2018; Fukami et al., 2018; Galindo et al., 2019, 2020; Cassán et al., 2020). The free-living rhizobacteria (genus Azospirillum) are able to colonize more than one hundred plant species in almost every living soil on earth for being enhancing crop growth, development and production (Pedrosa et al., 2020). The Azospirillum brasilense Ab-V5 and Ab-V6 strains are officially authorized inoculants for increasing wheat and maize productivity under field conditions in Brazil (Hungria et al., 2010; Santos et al., 2021). To date, the additive hypothesis best addresses A. brasilense operating principle, in which multiple mechanisms of plant growth-promotion works in convergence or in sequence (Bashan and de-Bashan 2010; Cassán and Diaz-Zorita 2016; Cassán et al., 2020). For example, A. brasilense has been reported to promote plant growth by increasing the production of phytohormones like gibberellins, auxins and cytokinins (Fukami et al., 2018), increasing root development leading to greater nutrients and water acquisition (Caires et al., 2020), increasing N use efficiency from applied N-fertilizer (Galindo et al., 2020), enhanced nitrate reductase activity (Ferreira et al., 1987; Pereira-Defilippi et al., 2017), solubilization of phosphates (Rafi et al., 2019), among others. Although the adoption of A. brasilense inoculation by Brazilian farmers has increased in recent years, it is not yet a consolidated management practice in agricultural production systems such as Bradyrhizobium sp. inoculation in soybean [Glycine max (L.) Merr.].
The hypothesis is that AE and apparent N-fertilizer recovery (AFR) in wheat plant could be significantly improved by inoculation with A. brasilense. The increased AE and AFR may provide increased N plant accumulation and shoot and root development, which may lead to greater grain yield when compared with non-inoculated plants. This research provides a novel view for fomenting wheat cultivation under tropical conditions by allowing reduce N-fertilizer application rates without compromising grain yield. In addition, it would favor sustainable cereal production under tropical conditions with an increase in farming profitability. Therefore, the objective of this study was to investigate the combined effects of N application levels (control, low, average and high levels) and seed inoculation with A. brasilense on: 1) plant biomass, grain yield, AE and AFR; 2) inorganic (N-NH4+ and N-NO3−) and total N content in wheat plant; and 3) economic analysis and operating profit for each individual treatment.
Materials and Methods
Field Experimental Characterization
The field experiment was conducted at the São Paulo State University Experimental Station (20°22′S and 51°22′W, 335 m above sea level) in Selvíria, Mato Grosso do Sul- Brazil, in the wheat growth seasons of 2016 and 2017. The site of experiment was being kept no-till for 15 years with annual cultivation of cereal and legume crops. The crop sequence prior to wheat was corn in both growth seasons. Climate classification was Aw according to Köppen-Geiger classification - tropical Savannah climate with dry winter. Daily temperature and rainfall measurements during the experiment were obtained from a meteorological station, located at experimental station (Supplementary Figure S1). Soil was classified as clayey, Rhodic Haplustox (Soil Survey Staff, 2014) with 47% sand, 9% silt and 44% clay, respectively. Chemical attributes of the 0–0.20 m soil depth were determined according to van Raij et al. (2001) and are presented in Supplementary Table S1. The N total was evaluated with semi-micro Kjeldahl method (Bremner and Breitenbeck, 1983) (Supplementary Table S1).
Experimental Design and Treatments
Four N application levels × 2 A. brasilense inoculations were factorially arranged in a randomized complete block experimental design with four replications. Each experimental plot was consisted of twelve 0.17 m wide rows with length of 6 m long. The useful area was the eight central rows, excluding 0.5 m at the end of each wheat row.
Nitrogen levels refers to side-dress N application as a control (0 kg N ha−1), low (50 kg N ha−1), average (100 kg N ha−1) and high (200 kg N ha−1) levels. These levels were selected based on previous studies with N management in wheat crop under Brazilian tropical Savannah conditions (Teixeira Filho et al., 2010). The applied N source was urea (45% of N) at wheat tillering decimal growth stage GS21 (Zadoks et al., 1974).
The two inoculations treatments consisted of with A. brasilense and absence of inoculation. The seeds of wheat were treated with A. brasilense strains (Ab-V5 and Ab-V6) selected from the Collection of Diazotrophic and Plant Growth Promoting Bacteria of Embrapa Soja (WFCC #1213 and WDCM #1054 with guarantee of 2 × 108 colony forming units mL−1). A 300 ml liquid inoculant was manually coated and mixed per 50 kg of wheat seeds in plastic bags an hour before sowing. To check if inoculation was well succeeded (presence of at least 1× 106 colony forming units g−1 soil), rhizosphere soil was randomly collected from wheat cultivated area at decimal growth stage GS619 (Zadoks et al., 1974) to analyze Azospirillum sp. colonization. The most probable number (MPN) mechanism with dilutions and inoculations in flasks having a medium of semisolid NFb without N addition and followed by growth at 35°C for 48 h (Döbereiner et al., 1995) was used and the obtained results were presented in Supplementary Table S2.
Wheat Management
The wheat cultivar CD 1104 was sown in both cultivated years (2016 and 2017) at a density of 412 viable seeds m−2 with a no-till drill. Also, basal fertilization application was performed at sowing for all treatments with 275 kg ha−1 of the granular fertilizer 08-28-16 (N-P2O5-K2O) in the sowing furrows based on soil analysis and crop requirements (Cantarella et al., 1997). During this nutrient application, 22 kg N ha−1 was applied to the entire experimental area. Therefore, the total amount of N applied in each treatment was the amount of N applied at the sidedress (0–200 kg N ha-1, as indicated above) in addition to the basal application of 22 kg N ha−1. As mentioned before, seed inoculation for the treatments with A. brasilense was performed at sowing. In addition, N treatment application in side-dressing (N application levels) was performed manually to uniformly distribute fertilizer on soil surface without incorporation during wheat tillering. The crop was irrigated with a center pivot sprinkling system with water depth of 14 mm depending on crop moisture requirement. The crop was cultivated during third May 2016 to 8 September 2016 (harvested 120 days after emergence, DAE) and 10 May 2017 to 12 September 2017 (harvested 117 DAE). The pre- and post-emergence herbicides were used for weeds control during crop cycle.
Samplings and Analysis
Wheat plants from an area of 0.17 m2 (0.17 m—width of the row × 1.0 m) were manually harvested by cutting plants at the ground level during flowering for shoot collection. At the same time, a side trench of approximately 0.40 m depth was dug opened for roots collection which were then washed with deionized water. In addition, 30 wheat flag leaves per plot were collected for nutritional analysis according to Cantarella et al. (1997). Shoot, root, and leaves were dried for 90 h in a forced-air oven at 60°C. Then, shoot and root were weighed for dry mass (kg ha−1). The N concentration was determined following the methodology of Malavolta et al. (1997), with sulfuric digestion and semi-micro Kjeldahl analysis method. Inorganic N concentration (N-NO3- and N-NH4+) in plant tissue was determined following Tedesco et al. (1995). Briefly, 1 g of plant tissue was extracted with 1 mol KCl L−1 (ratio of 1:15, plant tissue: solution, w/v), distilled with MgO (N-NH4+) and Devarda’s alloy and titrated with 2.5 mmol H2SO4 L−1 (N-NO3-). The accumulated total N, NH4+ and NO3− in shoot and root was obtained by the product of total N and inorganic N concentrations in tissue and produced dry biomass. The grain yield was obtained by collecting spike from the useful area of each plot which were mechanically harvested and grains were weighted (kg ha−1 and adjusted to a 13% moisture content in wet basis). Grain N accumulation was determined following the same procedures as shoot and root N accumulation. Agronomic efficiency of applied N-fertilizer (AE) was calculated following the methodology of Fageria et al. (2005) according to equation :
Where Nx is the N level applied and N0 is the control treatment (without N applied in side-dressing).
Apparent N-fertilizer recovery (AFR) was calculated following Sieling and Kage, (2021) and presented in equation :
Where Nx is the N level applied and N0 is the control treatment (without N applied in side-dressing).
Statistical Analysis
All data were initially tested for Levene’s homoscedasticity test (p ≤ 0.05) and normality using the Shapiro and Wilk test, which showed the data to be normally distributed (W ≥ 0.90). Data were submitted to analysis of variance (F test) using repeated measures (with year as the repeated variable) using a compound symmetry model for the covariance parameter. When a significant main effect or interaction was observed by the F test (p ≤ 0.05), then Tukey test (p ≤ 0.05) was used for comparison of means of N application levels, inoculation, years of study and their interactions using the ExpDes package in R software (R Core Team, 2015).
Principal Component Analysis
Principal component analysis (PCA) was used to evaluate biomass, grain yield, AE, inorganic and total N content in wheat plant. The PCA was carried out with the help of FactoMineR and factoextra packages in R software (R Development Core Team, 2015). The selection of PCs were based on eigenvalue such as PCs with eigenvalues ≥1 were reserved while the rest were excluded. The selected PCs indicated a total variability of 70% or greater where the correlations between chosen PCs and observed variables were elaborated with factor loading which was estimated via equation:
A factor loading of >0.30 was considered significant according to Lawley and Maxwell (1962). The biplot graphics indicated that PC1 (axis x) and PC2 (axis y) were plotted to separate inoculated and non-inoculated treatments.
Economic Analysis and Operating Profit
The structure of economic analysis was based on total operating expenses (TOE) that was adopted from Brazilian Institute of Agricultural Economics (BIEA), following Matsunaga et al. (1976). The cost-effectiveness of applied treatments were determined by profitability analyses, following Martin et al. (1998) and Galindo et al. (2019). Gross revenue, operating profit and average operating profit (average profit in both cropping years) were determined (in US$ ha−1). The mean expenses were estimated in Savannah region (Midwest region of Brazil) (average of the last 3 years—2019–2021). The selling price of wheat 60-kg sack was US$ 13.09 per unit. The cost of urea fertilizer was US$ 381.94 per Mg. The A. brasilense inoculant was priced for US$ 1.88 per rate (100 ml). The average dollar exchange rate was R$5.33 = US$1.00 according to Central Bank of Brazil.
Results
Wheat Biomass, Grain Yield, Agronomic Efficiency and Apparent N-Fertilizer Recovery
Root and shoot biomass were greater in 2016 than 2017 (Supplementary Table S3, Figures 1A,B). Interaction between inoculation and N levels significantly affected root biomass (Supplementary Table S3, Figure 1A). In the absence of N application and under low and average N applications, inoculated plots showed 15.9, 37.6 and 12.7% greater root dry mass (ctl: 494 kg ha−1; low: 685 kg ha−1; avg: 619 kg ha−1) compared to non-inoculated plots (ctl: 426 kg ha−1; low: 498 kg ha−1; avg: 549 kg ha−1) (Supplementary Table S3, Figure 1A). In addition, root biomass response to N application was different between inoculated and non-inoculated treatments (Supplementary Table S3, Figure 1A). When inoculation was performed, root biomass was greater when coupled with low and average N levels. Whereas, without A. brasilense inoculation, root biomass was greater when the average and high N levels were applied (Supplementary Table S3, Figure 1A).
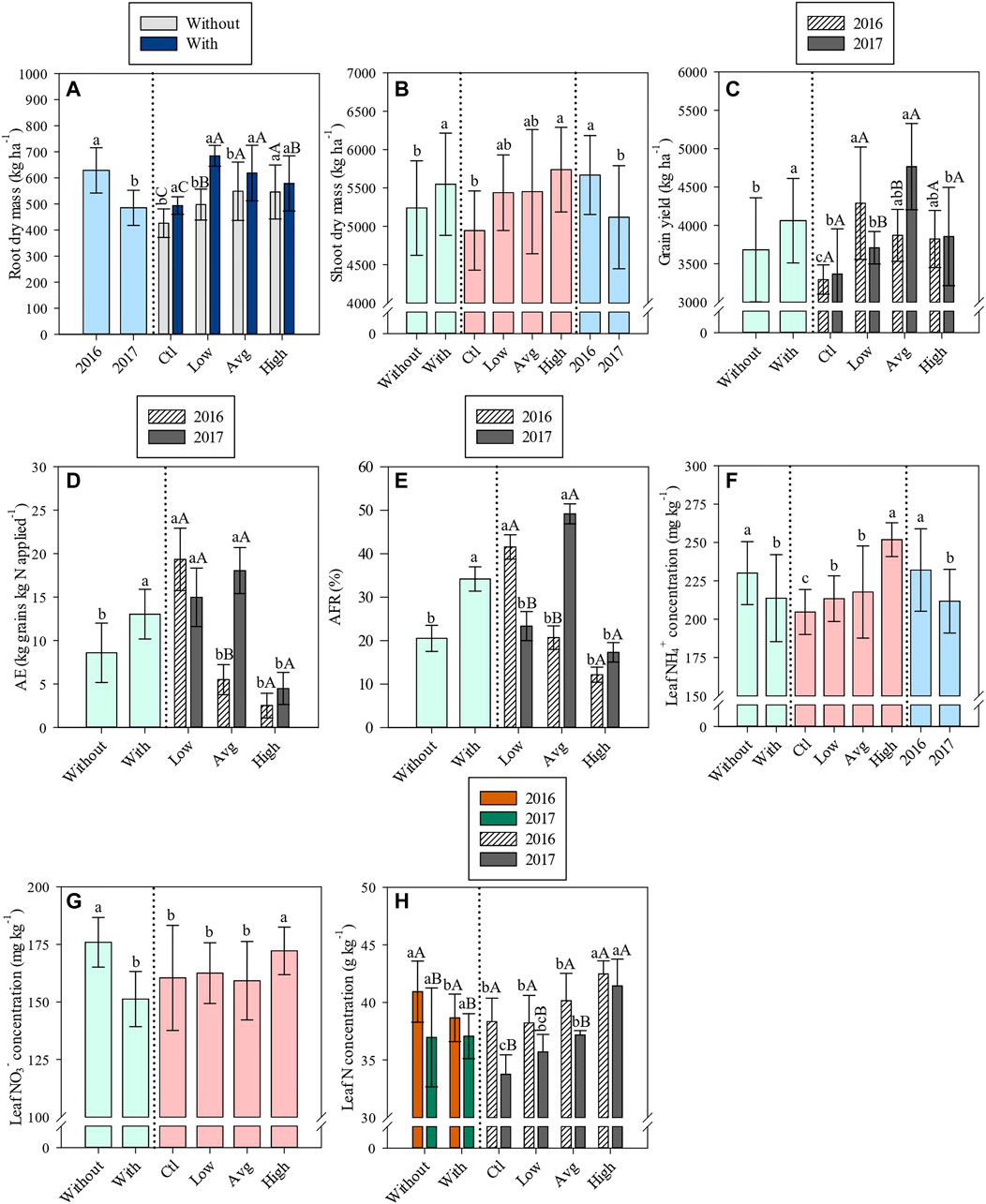
FIGURE 1. Root dry mass (A), shoot dry mass (B), grain yield (C), agronomic efficiency (D), apparent N-fertilizer recovery (E), leaf NH4+ concentration (F), leaf NO3− concentration (G) and leaf N concentration (H) affected by inoculation with A. brasilense, N levels, years of study and/or their interactions.
Shoot biomass was 5.9% greater in inoculated plots (5,549 kg ha−1) in comparison to without inoculation plots (5,239 kg ha−1) (Figure 1B). The application of high N level provided greater shoot biomass compared with control treatment (absence of side-dressing N application) (Supplementary Table S3, Figure 1B).
Similarly, as verified in shoot biomass data, inoculated plots (4,062 kg ha−1) showed 10.3% greater grain yield compared to without inoculation plots (3,682 kg ha−1) (Supplementary Table S3, Figure 1C). In 2016, grain yield was greater under low and average N levels while in 2017, grain yield was greater under average N application level (Supplementary Table S3, Figure 1C). Under low N application, grain yield was greater in 2016 than 2017 however, grain yield under average N application was greater in 2017 than 2016 (Supplementary Table S3, Figure 1C).
Agronomic efficiency (AE) was 51.2% greater in inoculated plots (13.0 kg grain kg−1 N applied) in comparison to non-inoculated plots (8.6 kg grain kg N applied−1) (Supplementary Table S3, Figure 1D). In 2016, AE was greater under low N application while in 2017, AE was decreased under high N application (Supplementary Table S3, Figure 1D). Agronomic efficiency under average N application was greater in 2017 than 2016 (Supplementary Table S3, Figure 1D). Similar trend was verified for apparent N-fertilizer recovery (AFR) (Supplementary Table S3, Figure 1E). Apparent N-fertilizer recovery was 66.7% higher in inoculated plots (34.2%) in comparison to without inoculation plots (20.5%) (Supplementary Table S3, Figure 1E). In 2016, AFR was greater under low N application while in 2017, AFR was greater under average N application (Supplementary Table S3, Figure 1E). Apparent N-fertilizer recovery under low N application was greater in 2016 than 2017, whereas average N application was greater in 2017 than 2016 (Supplementary Table S3, Figure 1E).
Inorganic (N-NH4+ and N-NO3−) and Total N Content in Wheat Plants
The treatments without inoculation were observed with higher leaf NH4+ and NO3− concentration as compared to inoculated plots (Supplementary Table S3, Figures 1E,F). Both NH4+, NO3− and total N in leaf tissue tended to be greater when high N level was applied (Supplementary Table S3, Figures 1E–G). In addition, leaf NH4+ was greater in 2016 than 2017 (Supplementary Table S3, Figure 1E). Leaf total N concentration in 2016 was greater in non-inoculated plots compared to A. brasilense inoculated plots (Supplementary Table S3, Figure 1G). Leaf total N concentration was greater in 2016 than 2017, regardless of inoculations (Supplementary Table S3, Figure 1G).
In 2017, root NH4+ accumulation was 38.4% greater in inoculated plots (100.2 g ha−1) compared to non-inoculated plots (72.4 g ha−1) (Supplementary Table S3, Figure 2A). In the absence of inoculation, root NH4+ was greater in 2016 than 2017 while no significant differences were observed for inoculated plots in both 2016 and 2017 (Supplementary Table S3, Figure 2A).
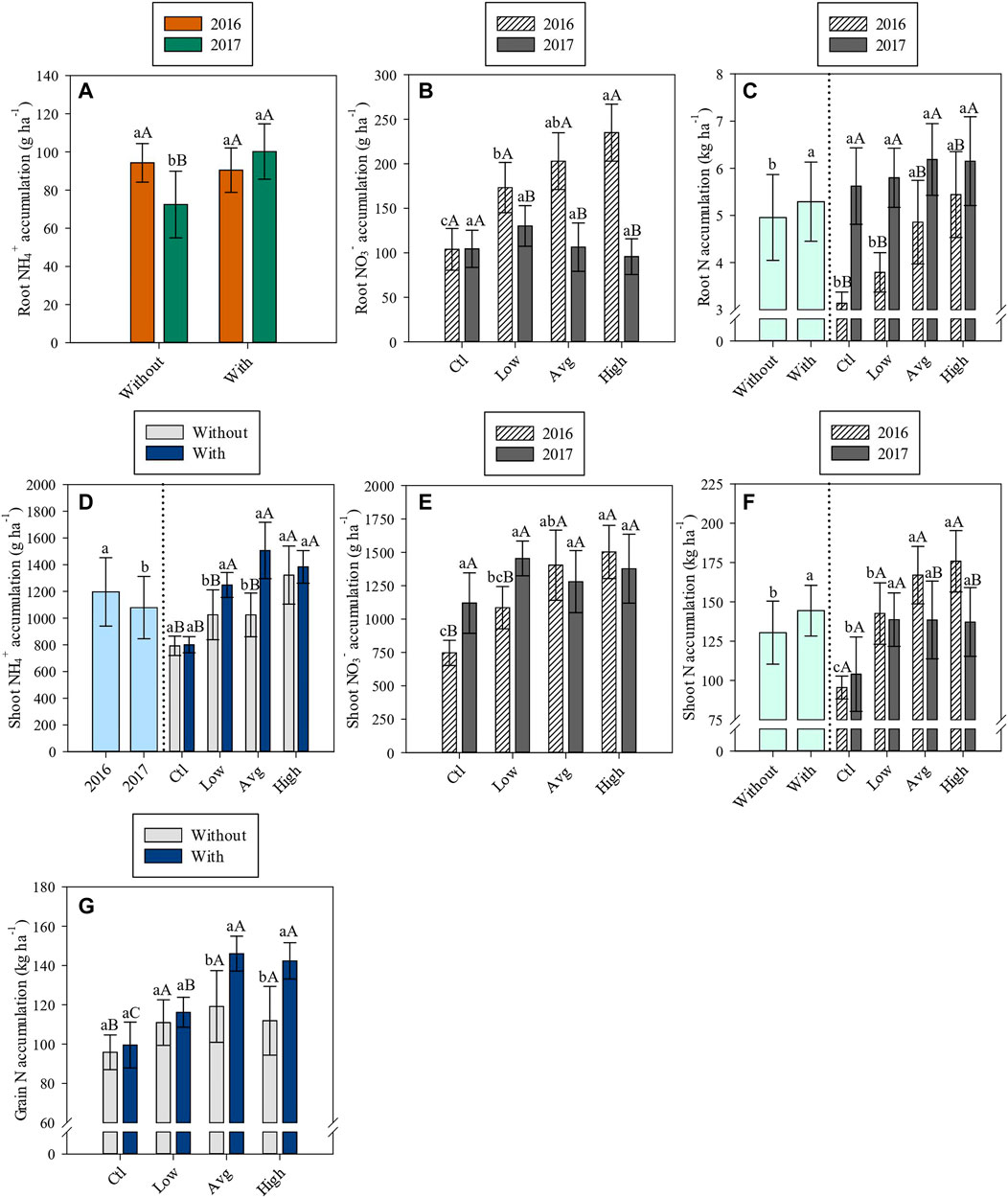
FIGURE 2. Root NH4+ accumulation (A), root NO3− accumulation (B), root N accumulation (C), shoot NH4+ accumulation (D), shoot NO3− accumulation (E), shoot N accumulation (F) and grain N accumulation (G) affected by inoculation with A. brasilense, N levels, years of study and/or their interactions.
Root NO3− accumulation tended to be greater with application of high N level in 2016 however, no significant differences in root NO3− due to N levels were observed in 2017 (Supplementary Table S3, Figure 2B). Under low, average, and high N application, root NO3− was greater in 2016 than 2017 (Supplementary Table S3, Figure 2B).
Root total N accumulation was 6.9% greater in inoculated plots (5.3 kg ha−1) compared to non-inoculated plots (4.96 kg ha−1) (Supplementary Table S3, Figure 2C). Root total N accumulation tended to be greater with application of high N level in 2016 however, it was not significantly influenced by N levels in 2017 (Supplementary Table S3, Figure 2C). In addition, root total N accumulation was greater in 2017 than 2016 (Supplementary Table S3, Figure 2C).
Shoot NH4+ accumulation was greater in 2016 than 2017 (Supplementary Table S3, Figure 2D). Under low and average N application, shoot NH4+ was 21.9 and 47.1% greater in inoculated plots (1,249 g ha−1 and 1,506 g ha−1) compared to non-inoculated plots (1,025 g ha−1 and 1,024 g ha−1) respectively (Supplementary Table S3, Figure 2D). In the absence of inoculation, the high N application level provided greater shoot NH4+ (Supplementary Table S3, Figure 2D). Whereas, when inoculation was performed, the low, average, and high N levels provided similar shoot NH4+ accumulation, being greater as compared with control (absence of side-dressing N application) (Supplementary Table S3, Figure 2D).
Shoot NO3− accumulation tended to be greater with high N application level in 2016 (Supplementary Table S3, Figure 2E), however, it was not significantly influenced by N levels in 2017 (Supplementary Table S3, Figure 2E). Without (control) and under low N application, shoot NO3− was greater in 2017 than 2016 (Supplementary Table S3, Figure 2E).
Shoot total N accumulation was 10.8% greater in inoculated plots (144 kg ha−1) compared to non-inoculated plots (130 kg ha−1) (Supplementary Table S3, Figure 2F). In 2016, application of the average and high N levels provided greater shoot N accumulation (Supplementary Table S3, Figure 2F). Whereas in 2017, all N levels provided similar shoot N accumulation, but still greater compared with control (Supplementary Table S3, Figure 2F). Under average and high N application levels, shoot total N was greater in 2016 than 2017 (Supplementary Table S3, Figure 2F).
Grain N accumulation when coupled with average and high N application levels was 22.7 and 26.8% greater in inoculated plots (146 kg ha−1 and 142 kg ha−1) compared with non-inoculated plots (119 kg ha−1 and 112 kg ha−1) (Supplementary Table S3, Figure 2G). In the absence of inoculation, all 3 N application levels provided similar grain N accumulation, being greater only when compared with control (absence of side-dressing N application) (Supplementary Table S3, Figure 2G).
Principal Component Analysis
The eigenvalues of extracted PCs were <1 which were grouped into a four-component model that accounts for 87 and 82% of data variation in plots without and with A. brasilense inoculation respectively (Supplementary Table S4).
In non-inoculated plots, PC1 represented 42% of variance which indicated shoot and root dry mass, leaf N and NH4+ concentration, shoot N and NH4+ accumulation, root NO3− accumulation and AFR as positively concordant (Supplementary Table S4). The PC2 exhibited a positive correlation between shoot N, NH4+ and NO3− accumulation, root N accumulation and AFR (Supplementary Table S4). Conversely, leaf NO3− concentration was negatively correlated with the PC2 components (Supplementary Table S4). There was a cumulative variance of 61.4% between PC1 and PC2 (Supplementary Table S4). The rest of two extracted factors were least important in terms of eigenvalues and variability (Supplementary Table S4). Principal component 3 showed a positive correlation between grain yield and grain N accumulation and AE however, a negative correlation between root NH4+ accumulation and the PC3 components were verified (Supplementary Table S4).
In A. brasilense inoculated plots, PC1 represented 39.3% of the variance and indicated positive correlation between shoot and root dry mass, shoot and grain N, NH4+ and NO3− accumulation and AFR (Supplementary Table S4). Principal component 2 exhibited that leaf N, NH4+ and NO3− concentration and root N accumulation positively correlated however, root dry mass was negatively correlated with both leaf total N and inorganic N forms concentration and root N accumulation (Supplementary Table S4). Both PC1 and PC2 indicated 57.9% of the cumulative variance (Supplementary Table S4. Similarly, as observed in non-inoculated plots, the other two extracted factors are of minor importance in terms of both eigenvalues and explained variability (Supplementary Table S4). Principal component 3 showed grain yield, root N accumulation and AE as positively concordant (Supplementary Table S4). In contrast, leaf NH4+ concentration was negatively correlated with all previously reported parameters (Supplementary Table S4).
Analyzing the grouped PCA biplot graph (PC1 and PC2) in non-inoculated plots, it was verified that group formed by the high N application level best comprised most of the analyzed parameters (Figure 3A). However, the group formed by low N application level best comprised grain yield, AE, grain N accumulation and leaf NO3− concentration (Figure 3A). Whereas, when inoculation was performed, the group formed by average N application level was found to comprised better the total and inorganic N content in wheat plant, biomass, grain yield, AE and AFR (Figure 3B).
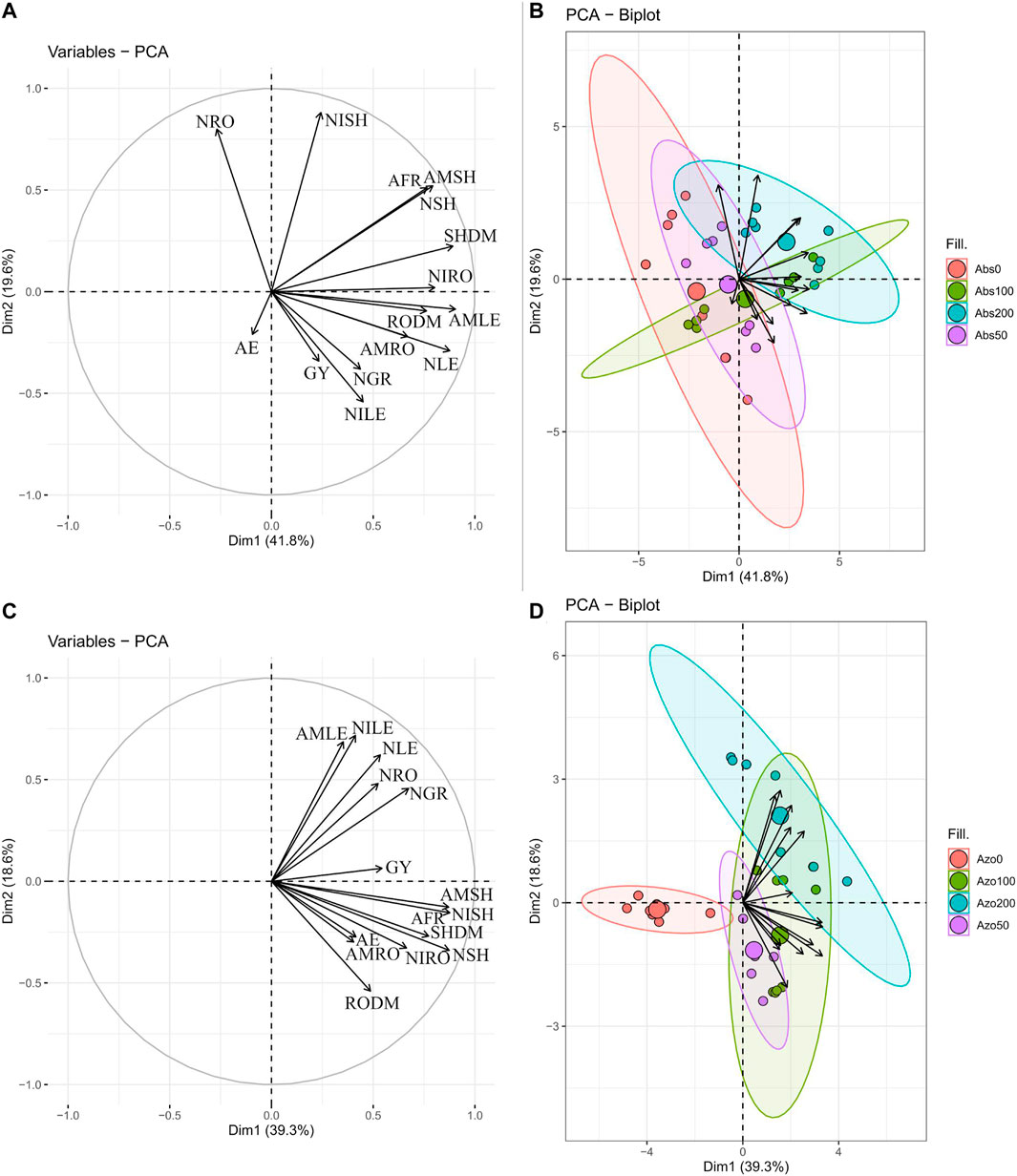
FIGURE 3. Loadings and biplot graphics of principal component analysis among the relationship between wheat shoot dry mass (SHDM), root dry mass (RODM), grain yield (GY), leaf N concentration (NLE), leaf NH4+ concentration (AMLE), Leaf NO3− concentration (NILE), Shoot N accumulation (SHN), Shoot NH4+ accumulation (SHAM), Shoot NO3− accumulation (SHNI), Root N accumulation (RON), Root NH4+ accumulation (ROAM), Root NO3− accumulation (RONI), Grain N accumulation (GRN), agronomic efficiency (AE) and apparent N-fertilizer recovery (AFR) evaluated in non-inoculated (absence) (A,B) and A. brasilense inoculated treatments (C,D).
Economic Analysis and Operating Profit
As expected, TOE tended to increase with increasing N application levels and inoculation, from US$ 385 ha−1 (control) to US$ 569 ha−1 (high N level applied) without inoculation, and from US$ 403 ha−1 (control) to US$ 587 ha−1 (high N level applied) with A. brasilense inoculation (Supplementary Table S5, Table 1). However, the greater wheat grain yield provided by the tested N levels and inoculation lead to an increased gross revenue (Supplementary Table S5, Table 1). The relation between gross revenue and TOE (operating profit) indicated that inoculation of A. brasilense coupled with mean N application level provided greater profit (US$ 493 ha−1), followed by inoculation with the low N application level (US$ 464 ha−1) (Table 1). Similarly, in the absence of inoculation, the highest operating profit was observed with fertilization of an average N level (US$ 420 ha−1) followed by application of the low N level (US$ 401 ha−1) (Table 1).
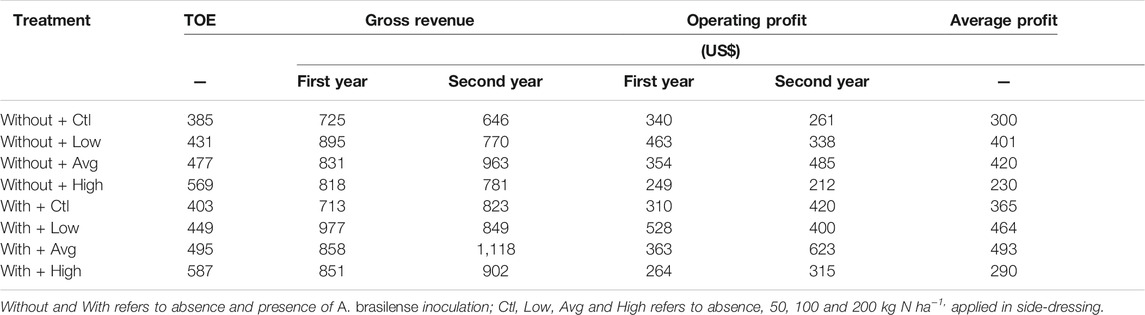
TABLE 1. Wheat total operating expenses (TOE), gross revenue, operating profit and average operating profit (based on the two wheat cropping seasons) affected by inoculation with A. brasilense and N levels.
Discussion
The findings of current study exhibited that inoculation of A. brasilense enhanced agronomic efficiency (AE), apparent N-fertilizer recovery (AFR) and N uptake in wheat plants with increased root and shoot N accumulation and grain N accumulation under the average and high N application levels (Figure 1D; Figure, 2C,F,G). Agronomic efficiency is the product of N recovery efficiency from applied N and the efficiency of plants to use each additional acquired N unit, and usually range from 10 to 30 kg grain kg−1 N applied (Doberman, 2005) as verified in this study. Apparent N-fertilizer recovery indicates directly how well applied fertilizer is removed by the harvest products (Hawkesford and Griffiths, 2019), in this study wheat grains. Several concepts and technologies have been adopted and developed to increase N use efficiency (Sharma and Bali, 2018; Galindo et al., 2021) such as; 1) enhancing crop N requirement and uptake through improvements in genetic and management factors to eliminate restrictions in crop growth and N requirement and 2) adopting such management practices that improve soil health and N supply for plant uptake (Chien et al., 2009). The latter initially include more effective N application methods, site-specific N management and highly effective fertilizers (new N forms, modified fertilizers and inhibitors that lead to slow/controlled release). Based on our results, A. brasilense inoculation could fit in the first above-mentioned group. It is essential to understand but several technological choices had different impacts on crop yield in response to N which is often the combination of such practices that lead to several greater benefit (Doberman, 2005; Chien et al., 2009).
The benefits of A. brasilense inoculation on root development highlighted by the observed increase in root dry mass and N content in roots are likely to be the key mechanism to increase AE and AFR, enhancing wheat growth and grain yield. The positive correlations between root-shoot dry mass, shoot and grain N accumulations had strengthened current hypothesis (Supplementary Table S4). Several previous studies have reported benefits of A. brasilense in root hairs and lateral root growth in different crops (Rondina et al., 2020; Araujo et al., 2021; Barbosa et al., 2021). Studies reported that several rhizobacteria can alter plant physiology with effect on biomass distribution in plant like altering root architecture (Backer et al., 2018; Bavaresco et al., 2020; Araujo et al., 2021) which could influence plant ability to penetrate into soil for greater water and nutrients uptake (Li et al., 2016). The ability of A. brasilense to excrete molecules such as phytohormones and nitric oxide to root uptake or regulate hormone balance in the plants to boost growth has been considered the major factor affecting root architecture (Backer et al., 2018; Cassán et al., 2020; Barbosa et al., 2021). Strong root system is related to higher rhizo-deposition of organic forms of carbon (C) and N from soil that can favor the trophic interactions of rhizosphere biodiversity and benefit overall plants functions (Barbosa et al., 2021).
Interestingly, the increased shoot and root biomass provided a dilution effect verified by the reduced inorganic (N-NH4+ and N-NO3-) and total N concentration in leaf tissue (Figures 1E–G). Negative correlations among root dry mass and leaf N, NH4+ and NO3− concentrations in PC2 and between grain yield and leaf NH4+ concentration in PC3 when inoculation was performed, support this dilution effect hypothesis (Supplementary Table S4). Nonetheless, the total N accumulation occurred mainly due to the enhanced N-NH4+ accumulation provided by inoculation. It was verified as function of greater root NH4+ accumulation mainly in 2017 and increased shoot NH4+ when inoculation was performed coupled with the low and average N application levels (Figures 2A,D). Plausible explanations for the observed increase in NH4+ content in wheat plant could be related to 1) NH4+ derived from Azospirillum-BNF (Fukami et al., 2018; Pii et al., 2019) and/or 2) improved nitrate reductase activity, mainly due to increased root development provided by inoculation (Fereira et al., 1987; Pereira-Defilippi et al., 2017). Biological N fixation was the first mechanism to be identified that demonstrated the way in which Azospirillum sp. positively affects plant growth (Döbereiner and Day, 1976; Okon et al., 1983; Cassán et al., 2020; Pedrosa et al., 2020). It has been reported that A. brasilense strains (Ab-V5 and Ab-V6) are carrying similar fix and nif genes that are contributing in BNF (Hungria et al., 2018). Despite this, these strains had different capacity of phytohormones production (Fukami et al., 2018) but still sharing similar genes for auxin synthesis. Although evidence that N fixation can contribute to N balance of plants has been reported, the contribution of N fixation by Azospirillum sp. to plants is not the major role in plant growth promotion (Cassán et al., 2020). Additional plant growth promoting mechanisms were proposed, such as the production of phytohormones like gibberellins, auxins and cytokinins (Fukami et al., 2018), increases in root development leading to greater nutrient and water acquisition (Caires et al., 2020), increase in N use efficiency from applied N-fertilizer (Galindo et al., 2020), enhancement of nitrate reductase activity (Fereira et al., 1987; Pereira-Defilippi et al., 2017), solubilization of phosphates (Rafi et al., 2019), among others. Therefore, the additive hypothesis best addresses A. brasilense operating principle, in which multiple mechanisms of plant growth-promotion works in convergence or in sequence (Bashan and de-Bashan 2010; Cassán et al., 2020).
The verified results agreed with previous findings that A. brasilense inoculation associated with N fertilization management could increase wheat grain yield between 3 and 26% compared to N application without inoculation (Galindo et al., 2017; Munareto et al., 2019; Caires et al., 2020; Galindo et al., 2020). It has been broadly explored that multifaceted microbe-environment-plant interactions shifts with expansion of agronomic response to A. brasilense inoculation (Cassán and Díaz-Zorita 2016), including bacteria and plant genotypes (Bashan and de-Bashan, 2010; Cassán et al., 2020). Under field trial evaluations, crop responses to Azospirillum sp. inoculation have been related to harsh environmental conditions (e.g., dryland, limited fertilization and high temperatures), especially during the initial stages of plant growth (Cesari et al., 2019; Caires et al., 2020). In this study, although supplementary irrigation was performed, total rainfall was poor and not well distributed in all the wheat growing seasons (Suppplementary Figures S1A,B). In addition, high temperatures were observed during the field trial (maximum and minimum temperature above 30 and 15°C in most of the cases, respectively). Thus, plant growth response to inoculation with A. brasilense under tropical conditions would be more noticeable, as verified in this study. Nonetheless, considering that Azospirillum sp. are found in almost every living soil on the earth and able to colonize more than 100 plant species (Pedrosa et al., 2020), further investigation should be performed under subtropical and temperate climate conditions.
The higher gross revenue provided by A. brasilense due to the greater wheat grain yield overcame the increased expense in TOE with inoculation. It was verified that inoculation associated with the average N application level provided greater operating profit (US$ 493 ha−1), 17.3% higher than the most profitable treatment in the absence of inoculation (100 kg N ha−1 without inoculation - US$ 420 ha−1) (Table 1). The results showed that it would be possible to reduce N fertilization level to the low when inoculation is performed without compromising profitability. In fact, the operating profit obtained from the low N application level associated with inoculation (US$ 464 ha−1) was greater than the operating profit obtained by applying average N level without inoculation (US$ 420 ha−1). This suggests that it is possible to increase operating profit by 10.5% while reducing N application by 50% (Table 1). Moreover, the grouped PCA biplot graphs indicated that A. brasilense could reduce N application from the high level to average level benefiting N accumulation in shoot and root and biomass production; while enhance the potential for greater AE, grain yield and grain N uptake limited to low N application when inoculation is not performed (Figures 3A–D). It has been reported that inoculation with A. brasilense can generate an economy of 1.2 billion dollars per year with half substitution of N-based fertilizer application required by wheat and maize crops (Hungria et al., 2010). Similarly, Caires et al. (2020) concluded that since wheat seed inoculation A. brasilense has demonstrated economic viability, the strategy of side-dressing N application could be changed and a lower N level may be applied to wheat crops. For instance, it should be also highlighted that the obtained results should be carefully considered for the specific conditions tested (no-till, specific N fertilizer type, environmental conditions, wheat cultivar etc.) and do not allow general recommendations worldwide. However, the use of PGPR has quite significant role in alleviation of environmental changes under the context of climate extremes and excessive use of fertilizer in agricultural soils (Backer et al., 2018). In addition, the use of these beneficial microorganisms is considered one of the most promising alternatives for safe crop-management practices as it could improve plant nutrition while reducing the dependance of N-fertilizer application (Koskey et al., 2021). For these reasons, studies with PGPR such as Azospirillum brasilense should be performed under different production systems (e.g., organic and conventional farming systems) and climatic conditions (tropical, subtropical and temperate environments).
The prospective studies need to evaluate the effects of agronomic practices, climate changes and plant growth-promoting rhizobacteria inoculation under different agroecosystems. The combination of some refined scientific techniques (e.g., metabolomics, isotopic and molecular techniques, among others) are needed to deepen the knowledge of multiple mechanisms and benefits of plant growth-promoting rhizobacteria in soil-plant-environment system.
Conclusion
In view of low economic cost, ease of application and with a high probability of response by wheat crop, even associated with different N application levels, the inoculation with A. brasilense prone to be a key management strategy to improve sustainable wheat production. This practice will also be needed to help fomenting wheat crop expansion under tropical conditions by reducing the dependency of N-fertilizer applications. In addition, A. brasilense inoculation could be a key pillar in combating hunger, promoting food security, and environmental sustainability. The results have revealed that inoculation with A. brasilense enhanced agronomic efficiency and apparent N-fertilizer recovery by wheat plants, benefiting shoot and root development and leading to an increased grain yield by 10.3%). The increased total N accumulation observed occurred mainly as a result of the enhanced N-NH4+ accumulation in wheat root and shoot. Azospirillum brasilense inoculation associated with application of average N application level provided the greatest operating profit compared with any other treatment. However, the results also showed that a reduction in the required amount of N needed for optimum profitability is possible when inoculation is combined with low N application level tested in this study. This represents a reduction of 50% in the amounts of N needed for optimum production.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Design the experiment: FSG and MCMTF. Obtain and process the data: FSG, GCF, WLR, and EHMB. Analyze the data: FSG. Wrote the paper: FSG, PHP, AJ, JL, and MCMTF with contribution of all co-authors. All authors confirm being contributor of this work and has approved it for publication.
Funding
The authors would like to thank the Fundação de Amparo à Pesquisa do estado de São Paulo (FAPESP) for the first author’s doctoral scholarship (Grant Number 2017/06002-6), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting the sixth author’s doctoral scholarship (CNPq/TWAS Grant Number 166331/2018-0) and the eighth author’s research productivity scholarship (Award Number 312359/2017-9).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.821628/full#supplementary-material
References
Araujo, F. F., Bonifacio, A., Bavaresco, L. G., Mendes, L. W., and Araujo, A. S. F. (2021). Bacillus Subtilis Changes the Root Architecture of Soybean Grown on Nutrient-Poor Substrate. Rhizosphere 18, 100348. doi:10.1016/j.rhisph.2021.100348
Backer, R., Rokem, J. S., Ilangumaran, G., Lamont, J., Praslickova, D., Ricci, E., et al. (2018). Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 9, 1473. doi:10.3389/fpls.2018.01473
Barbosa, J. Z., Hungria, M., Sena, J. V. d. S., Poggere, G., dos Reis, A. R., and Corrêa, R. S. (2021). Meta-analysis Reveals Benefits of Co-inoculation of Soybean with Azospirillum Brasilense and Bradyrhizobium Spp. In Brazil. Appl. Soil Ecol. 163, 103913. doi:10.1016/j.apsoil.2021.103913
Bashan, Y., and De-Bashan, L. E. (2010). How the Plant Growth-Promoting Bacterium Azospirillum Promotes Plant Growth-A Critical Assessment. Adv. Agron. 108, 77–136. doi:10.1016/S0065-2113(10)08002-8
Bavaresco, L. G., Osco, L. P., Araujo, A. S. F., Mendes, L. W., Bonifacio, A., and Araújo, F. F. (2020). Bacillus Subtilis Can Modulate the Growth and Root Architecture in Soybean through Volatile Organic Compounds. Theor. Exp. Plant Physiol. 32, 99–108. doi:10.1007/s40626-020-00173-y
Bremner, J. M., and Breitenbeck, G. A. (1983). A Simple Method for Determination of Ammonium in semimicro‐Kjeldahl Analysis of Soils and Plant Materials Using a Block Digester. Commun. Soil Sci. Plant Anal. 14, 905–913. doi:10.1080/00103628309367418
Brusamarello-Santos, L. C., Gilard, F., Brulé, L., Quilleré, I., Gourion, B., Ratet, P., et al. (2017). Metabolic Profiling of Two maize (Zea mays L.) Inbred Lines Inoculated with the Nitrogen Fixing Plant-Interacting Bacteria Herbaspirillum Seropedicae and Azospirillum brasilenseZea mays L.) Inbred Lines Inoculated with the Nitrogen Fixing Plant-Interacting Bacteria Herbaspirillum Seropedicae and Azospirillum Brasilense. Plos One 12, e0174576. doi:10.1371/journal.pone.0174576
Caires, E. F., Bini, A. R., Barão, L. F. C., Haliski, A., Duart, V. M., and Ricardo, K. d. S. (2020). Seed Inoculation with Azospirillum Brasilense and Nitrogen Fertilization for No‐till Cereal Production. Agron.j. 113, 560–576. doi:10.1002/agj2.20488
Cantarella, H., van Raij, B., and Camargo, C. E. O. (1997). “Cereals,” in Liming And Fertilization Recommendations for the State Of São Paulo (In Portuguese). Editors B. van Raij, H. Cantarella, J. A. Quaggio, and A. M. C. Furlani (Campinas, Brazil: Instituto Agronômico de Campinas), 285.
Cassán, F., Coniglio, A., López, G., Molina, R., Nievas, S., de Carlan, C. L. N., et al. (2020). Everything You Must Know about Azospirillum and its Impact on Agriculture and beyond. Biol. Fertil. Soils 56, 461–479. doi:10.1007/s00374-020-01463-y
Cassán, F., and Diaz-Zorita, M. (2016). Azospirillum Sp. In Current Agriculture: from the Laboratory to the Field. Soil Biol. Biochem. 103, 117–130. doi:10.1016/j.soilbio.2016.08.020
Cesari, A. B., Paulucci, N. S., López-Gómez, M., Hidalgo-Castellanos, J., Lluch Plá, C., and Dardanelli, M. S. (2019). Performance of Bradyrhizobium and Bradyrhizobium-Azospirillum in Alleviating the Effects of Water-Restrictive Conditions during the Early Stages of Arachis hypogaea Growth. J. Plant Growth Regul. 38, 1362–1374. doi:10.1007/s00344-019-09939-4
Chien, S. H., Prochnow, L. I., and Cantarella, H. (2009). Chapter 8 Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts. Adv. Agron. 102, 267–322. doi:10.1016/S0065-2113(09)01008-6
Conab (2021). “Companhia Nacional de Abastecimento,” in Grains Report - April 2021. (In Portuguese. Brasília: Conab. Available at: https://www.conab.gov.br/info-agro/safras.
Döbereiner, J., Baldani, V. L. D., and Baldani, J. I. (1995). “Embrapa-SPI e Seropédica,” in How to Isolate and Identify Diazotrophic Bacteria from Non-leguminous Plants (In Portuguese (Brasília: Embrapa-CNPAB), 60.
Döbereiner, J., and Day, J. M. (1976). “Associative Symbiosis in Tropical Grasses: Characterization of Microorganisms and Dinitrogen-Fixing Sites,” in Proceedings of the international symposium on nitrogen fixation, Pullman. Editors W. E. Newton, and C. T. Nyman (Washington State University Press), 518–538.
Dobermann, A. (2005). “Nitrogen Use Efficiency—State of the Art,” in Proceedings of the International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany (Paris (CD-ROM): International Fertilizer Industry Association).
Fageria, N. K., Baligar, V. C., and Bailey, B. A. (2005). Role of Cover Crops in Improving Soil and Row Crop Productivity. Commun. Soil Sci. Plant Anal. 36, 2733–2757. doi:10.1080/00103620500303939
Ferreira, M. C. B., Fernandes, M. S., and Döbereiner, J. (1987). Role of Azospirillum Brasilense Nitrate Reductase in Nitrate Assimilation by Wheat Plants. Biol. Fert Soils 4, 47–53. doi:10.1007/BF00280350
Fukami, J., Cerezini, P., and Hungria, M. (2018). Azospirillum: Benefits that Go Far beyond Biological Nitrogen Fixation. AMB Expr. 8, 73. doi:10.1186/s13568-018-0608-1
Galindo, F. S., Buzetti, S., Rodrigues, W. L., Boleta, E. H. M., Silva, V. M., Tavanti, R. F. R., et al. (2020). Inoculation of Azospirillum Brasilense Associated with Silicon as a Liming Source to Improve Nitrogen Fertilization in Wheat Crops. Sci. Rep. 10, 6160. doi:10.1038/s41598-020-63095-4
Galindo, F. S., da Silva, E. C., Pagliari, P. H., Fernandes, G. C., Rodrigues, W. L., Biagini, A. L. C., et al. (2021). Nitrogen Use Efficiency and Recovery in a Wheat-Corn Rotation under Tropical savannah Conditions. Nutr. Cycl Agroecosyst 119, 291–305. doi:10.1007/s10705-020-10115-4
Galindo, F. S., Teixeira Filho, M. C. M., Buzetti, S., Pagliari, P. H., Santini, J. M. K., Alves, C. J., et al. (2019). Maize Yield Response to Nitrogen Rates and Sources Associated with Azospirillum Brasilense. Agron.j. 111, 1985–1997. doi:10.2134/agronj2018.07.0481
Galindo, F. S., Teixeira Filho, M. C. M., Buzetti, S., Santini, J. M. K., Alves, C. J., and Ludkiewicz, M. G. Z. (2017). Wheat Yield in the Cerrado as Affected by Nitrogen Fertilization and Inoculation with Azospirillum Brasilense. Pesq. Agropec. Bras. 52, 794–805. doi:10.1590/S0100-204X2017000900012
Grote, U., Fasse, A., Nguyen, T. T., and Erenstein, O. (2021). Food Security and the Dynamics of Wheat and maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 4, 617009. doi:10.3389/fsufs.2020.617009
Hawkesford, M. J., and Griffiths, S. (2019). Exploiting Genetic Variation in Nitrogen Use Efficiency for Cereal Crop Improvement. Curr. Opin. Plant Biol. 49, 35–42. doi:10.1016/j.pbi.2019.05.003
Hungria, M., Campo, R. J., Souza, E. M., and Pedrosa, F. O. (2010). Inoculation with Selected Strains of Azospirillum Brasilense and A. Lipoferum Improves Yields of maize and Wheat in Brazil. Plant Soil 331, 413–425. doi:10.1007/s11104-009-0262-0
Hungria, M., Ribeiro, R. A., and Nogueira, M. A. (2018). Draft Genome Sequences of Azospirillum Brasilense Strains Ab-V5 and Ab-V6, Commercially Used in Inoculants for Grasses and Legumes in Brazil. Genome Announc 6, e00393–18. doi:10.1128/genomeA.00393-18
Kopittke, P. M., Menzies, N. W., Wang, P., McKenna, B. A., and Lombi, E. (2019). Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 132, 105078. doi:10.1016/j.envint.2019.105078
Koskey, G., Mburu, S. W., Awino, R., Njeru, E. M., and Maingi, J. M. (2021). Potential Use of Beneficial Microorganisms for Soil Amelioration, Phytopathogen Biocontrol, and Sustainable Crop Production in Smallholder Agroecosystems. Front. Sustain. Food Syst. 5, 606308. doi:10.3389/fsufs.2021.606308
Lawley, D. N., and Maxwell, A. E. (1962). Factor Analysis as a Statistical Method. The Statistician 12, 209–229. doi:10.2307/2986915
Li, X., Zeng, R., and Liao, H. (2016). Improving Crop Nutrient Efficiency through Root Architecture Modifications. J. Integr. Plant Biol. 58, 193–202. doi:10.1111/jipb.12434
Malavolta, E., Vitti, G. C., and Oliveira, S. A. (1997). Evaluation of the Nutritional Status of Plants: Principles and Applications. (In Portuguese. 2.ed. Potafos: Piracicaba, 319.
Martin, N. B., Serra, R., Oliveira, M. D. M., Ângelo, J. A., and Okawa, H. (1998). Integrated Farm Costs System– CUSTAGRI (In Portuguese, with English Abstract). Info Econ. 28, 7–28. doi:10.1287/inte.28.4.38
Mateo-Marín, N., Quílez, D., Guillén, M., and Isla, R. (2020). Feasibility of Stabilised Nitrogen Fertilisers Decreasing Greenhouse Gas Emissions under Optimal Management in Sprinkler Irrigated Conditions. Agric. Ecosyst. Environ. 290, 106725. doi:10.1016/j.agee.2019.106725
Matsunaga, M., Bemelmans, P. F., Toledo, P. N. E., Dulley, R. D., Okawa, H., and Pedroso, I. A. (1976). Cost of Production Methodology Utilized by the IEA (In Portuguese, with English Abstract). Agric. São Paulo 23, 123–139.
Munareto, J. D., Martin, T. N., Fipke, G. M., Cunha, V. d. S., and Rosa, G. B. d. (2019). Nitrogen Management Alternatives Using Azospirillum Brasilense in Wheat. Pesq. Agropec. Bras. 54, e00276. doi:10.1590/S1678-3921.pab2019.v54.00276
Okon, Y., Heytler, P. G., and Hardy, R. W. F. (1983). N 2 Fixation by Azospirillum Brasilense and its Incorporation into Host Setaria Italica. Appl. Environ. Microbiol. 46, 694–697. doi:10.1128/aem.46.3.694-697.1983
Pedrosa, F. O., Oliveira, A. L. M., Guimarães, V. F., Etto, R. M., Souza, E. M., Furmam, F. G., et al. (2020). The Ammonium Excreting Azospirillum Brasilense Strain HM053: a New Alternative Inoculant for maize. Plant Soil 451, 45–56. doi:10.1007/s11104-019-04124-8
Pereira-Defilippi, L., Pereira, E. M., Silva, F. M., and Moro, G. V. (2017). Expressed Sequence Tags Related to Nitrogen Metabolism in maize Inoculated with Azospirillum Brasilense. Genet. Mol. Res. 16, gmr16029682. doi:10.4238/gmr16029682
Pii, Y., Aldrighetti, A., Valentinuzzi, F., Mimmo, T., and Cesco, S. (2019). Azospirillum Brasilenseinoculation Counteracts the Induction of Nitrate Uptake in maize Plants. J. Exp. Bot. 70, 1313–1324. doi:10.1093/jxb/ery433
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Disponible in: https://www.R-project.org/.
Rafi, M. M., Krishnaveni, M. S., and Charyulu, P. B. B. N. (2019). “Phosphate-solubilizing Microorganisms and Their Emerging Role in Sustainable Agriculture,” in Recent Developments in Applied Microbiology and Biochemistry. Editor V. Buddolla (Cambridge, MA, USA: Academic PressElsevier: Amsterdam, Netherlands), 223–233. doi:10.1016/b978-0-12-816328-3.00017-9
Rondina, A. B. L., dos Santos Sanzovo, A. W., Guimarães, G. S., Wendling, J. R., Nogueira, M. A., and Hungria, M. (2020). Changes in Root Morphological Traits in Soybean Co-inoculated with Bradyrhizobium Spp. And Azospirillum Brasilense or Treated with A. Brasilense Exudates. Biol. Fertil. Soils 56, 537–549. doi:10.1007/s00374-020-01453-0
Sainju, U. M., Ghimire, R., and Pradhan, G. P. (2019). “Nitrogen Fertilization I: Impact on Crop, Soil, and Environment,” in Nitrogen in Agricultural Systems (London, UK: IntechOpen).
Santos, M. S., Nogueira, M. A., and Hungria, M. (2021). Outstanding Impact of Azospirillum Brasilense Strains Ab-V5 and Ab-V6 on the Brazilian Agriculture: Lessons that Farmers Are Receptive to Adopt New Microbial Inoculants. R. Bras Ci Solo 45, e0200128. doi:10.36783/18069657rbcs20200128
Sharma, L., and Bali, S. (2018). A Review of Methods to Improve Nitrogen Use Efficiency in Agriculture. Sustainability 10, 51. doi:10.3390/su10010051
Sieling, K., and Kage, H. (2021). Apparent Fertilizer N Recovery and the Relationship between Grain Yield and Grain Protein Concentration of Different winter Wheat Varieties in a Long-Term Field Trial. Eur. J. Agron. 124, 126246. doi:10.1016/j.eja.2021.126246
Soil Survey Staff (2014). Keys to Soil Taxonomy. Twelfh ed. Washington, DC: USDA. Natural Resources Conservation Service.
Tedesco, M. J., Gianello, C., Bissani, C. A., Bohnen, H., and Volkweiss, S. J. (1995). “Analysis of Soil, Plants and Other Materials,” in Portuguese (Porto Alegre: Universidade Federal do Rio Grande do Sul), 174p.
Teixeira Filho, M. C. M., Buzetti, S., Andreotti, M., Arf, O., and Benett, C. G. S. (2010). Doses, fontes e épocas de aplicação de nitrogênio em trigo irrigado em plantio direto. Pesq. Agropec. Bras. 45, 797–804. doi:10.1590/S0100-204X2010000800004
Tsujimoto, Y., Rakotoson, T., Tanaka, A., and Saito, K. (2019). Challenges and Opportunities for Improving N Use Efficiency for rice Production in Sub-saharan Africa. Plant Prod. Sci. 22, 413–427. doi:10.1080/1343943X.2019.1617638
van Raij, B., Andrade, J. C., Cantarella, H., and Quaggio, J. A. (2001). Chemical Analysis for Fertility Evaluation of Tropical Soils (In Portuguese). Campinas: IAC, 285.
Wang, H., Ma, S., Shao, G., and Dittert, K. (2021). Use of Urease and Nitrification Inhibitors to Decrease Yield-Scaled N2O Emissions from winter Wheat and Oilseed Rape fields: A Two-Year Field experiment. Agric. Ecosyst. Environ. 319, 107552. doi:10.1016/j.agee.2021.107552
Xu, A., Li, L., Xie, J., Wang, X., Coulter, J. A., Liu, C., et al. (2020). Effect of Long-Term Nitrogen Addition on Wheat Yield, Nitrogen Use Efficiency, and Residual Soil Nitrate in a Semiarid Area of the Loess Plateau of China. Sustainability 12, 1735. doi:10.3390/su12051735,
Zadoks, J. C., Chang, T. T., and Konzak, C. F. (1974). A Decimal Code for the Growth Stages of Cereals. Weed Res. 14, 415–421. doi:10.1111/j.1365-3180.1974.tb01084.x
Keywords: Triticum aestivum, sustainable crop production, tropical agriculture, plant growth promoting rhizobacteria, improved nitrogen management
Citation: Galindo FS, Pagliari PH, Fernandes GC, Rodrigues WL, Boleta EHM, Jalal A, Céu EGO, Lima BHd, Lavres J and Teixeira Filho MCM (2022) Improving Sustainable Field-Grown Wheat Production With Azospirillum brasilense Under Tropical Conditions: A Potential Tool for Improving Nitrogen Management. Front. Environ. Sci. 10:821628. doi: 10.3389/fenvs.2022.821628
Received: 24 November 2021; Accepted: 27 January 2022;
Published: 17 February 2022.
Edited by:
Rosa Francaviglia, Council for Agricultural and Economics Research (CREA), ItalyReviewed by:
Ertan Yildirim, Atatürk University, TurkeyDinesh Kumar Maheshwari, Gurukul Kangri Vishwavidyalaya, India
Copyright © 2022 Galindo, Pagliari, Fernandes, Rodrigues, Boleta, Jalal, Céu, Lima, Lavres and Teixeira Filho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Shintate Galindo, fsgalindo@usp.br
 Fernando Shintate Galindo
Fernando Shintate Galindo Paulo Humberto Pagliari
Paulo Humberto Pagliari Guilherme Carlos Fernandes3
Guilherme Carlos Fernandes3  Eduardo Henrique Marcandalli Boleta
Eduardo Henrique Marcandalli Boleta Arshad Jalal
Arshad Jalal José Lavres
José Lavres Marcelo Carvalho Minhoto Teixeira Filho
Marcelo Carvalho Minhoto Teixeira Filho