Overlooked Parrot Seed Dispersal in Australia and South America: Insights on the Evolution of Dispersal Syndromes and Seed Size in Araucaria Trees
- 1Department of Conservation Biology, Estación Biológica de Doñana, Consejo Superior de Investigaciones Científicas, Sevilla, Spain
- 2Department of Evolutionary Ecology, Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas, Madrid, Spain
- 3Department of Renewable Resources, University of Alberta, Edmonton, AB, Canada
While Psittaciformes (parrots and allies) are well-recognized as highly-mobile seed predators, their role as seed dispersers has been overlooked until very recently. It remains to be determined whether this role is anecdotic or is a key mutualism for some plant species. We recently found that the large nut-like seeds of the two South American Araucaria tree species (Araucaria araucana in Andean forests and Araucaria angustifolia in Atlantic forests, weighing c. 3.5 and 7 g, respectively) are frequently dispersed, and to long distances, by parrots. Moreover, both observational and experimental work demonstrated that dispersed seeds can germinate faster after partial predation by parrots. Here, we hypothesized that a third, even larger-seeded (17.5 g) congeneric Australian species (A. bidwillii) is also dispersed by parrots. We surveyed 52 A. bidwillii and 42 A. cunninghamii (a sympatric species with small winged seeds, c. 0.2 g) during the seeding period. We found that sulfur-crested cockatoos (Cacatua galerita) consumed large amounts of seeds from all of the A. bidwillii trees surveyed. Cockatoos dispersed ca. 30% of the seeds they removed from the mother tree, carrying the seeds to distant perches for handling or dropped them while flying. Dispersal distances ranged between 10 and 153 m (mean = 61 m). Most seeds handled for consumption (93%) were fully eaten but others were dropped intact (3%) or only partially eaten (4%), and germination was confirmed for both intact and partially-eaten dispersed seeds. Moreover, seeds dropped by cockatoos facilitated secondary seed dispersal by conspecifics and another three bird species. We found no evidence of other primary dispersal species for A. bidwillii, while the small, winged seeds of Araucaria cunninghamii were only dispersed through barochory and anemochory. The seed weight of the three Araucaria species dispersed by zoochory is strongly related to the body mass of their main seed-disperser parrot species. These results support a role for parrots as key dispersers of the three large-seeded Araucaria species around the world, and suggest that large seeds may have evolved–at least partially–as an adaptation that allows trees to attract parrots, satiate them, and benefit from their long-distance seed dispersal services.
Introduction
Plant investment in reproductive tissues is shaped by biotic and abiotic factors over evolutionary time. In particular, the variability in seed size among extant plants, which exceeds ten orders of magnitude (Moles et al., 2005), may result from multiple life-history trade-offs combined in complex ways with past and current ecological factors, both being influential (Leishman et al., 2000; Díaz et al., 2016). Conifers, and other non-flowering plants (gymnosperms), provide good models with which to study the evolution of seed size. These plants have several specific features that make them highly suitable for such studies: they bear seed-producing organs as separate, compact structures (seed cones), evolved 225 Myr before flowering plants (angiosperms), and have shown much larger seeds than angiosperms from the time of their origin until the present day (Leslie et al., 2017). Comparative phylogenetic studies combining fossil records and extant plants have identified several drivers in seed size evolution. In conifers, there was an increase in the amount of cone tissue devoted to seed protection (robust, tightly packed scales) without a concomitant increase in seed size, which has been interpreted as an evolutionary response to the diversification of vertebrate and insect seed predators (Leslie, 2011). These antagonistic plant-animal interactions were moreover combined with climatic factors and mutualistic plant-animal interactions, as seeds are generally larger in animal-dispersed than in wind-dispersed conifers (Leslie et al., 2017). However, seed size is expected to be constrained by the gape size of those vertebrates (birds and mammals) that could ingest and disperse them, thus maintaining relatively small propagule sizes (Leslie et al., 2017).
The patterns described above differ in several ways from that found in the family Araucariaceae, an ancient conifer clade with 37 extant species that currently occurs in southern South America, Australia and some Pacific islands (Farjon, 2017; Gleiser et al., 2019). This is the only conifer family in which evolution in seed size was apparently not influenced by climate (Leslie et al., 2017). Moreover, although most species show unspecialized, wind-dispersed, seeds of relatively small size (Leslie et al., 2017), a small clade section composed of Araucaria species (Araucaria araucaria, Araucaria angustifolia, Araucaria bidwillii, and Araucaria hunsteinii) evolved the largest seed cones (Gleiser et al., 2019) and seeds of any living conifer (Farjon, 2017). These large seeds are expected to be part of an animal-mediated dispersal syndrome (Leslie et al., 2017; Gleiser et al., 2019), and thus more detailed information on their seed-dispersing species is needed in order to understand their evolutionary trajectory (Gleiser et al., 2019).
Araucariaceae was the first conifer family to develop large seed cones, where they appear as early as the Jurassic, coinciding with the diversification of large sauropod dinosaurs that could act as their seed predators and dispersers (Leslie, 2011). To our knowledge, however, there is no evidence that these large seeds were dispersed by extinct megafauna and no extant vertebrate can ingest and defecate them intact, although some mammals and birds currently act as seed predators and external seed dispersers (Vieira and Iob, 2009; Shepherd and Ditgen, 2013; Dénes et al., 2018). Recent studies, however, have added new, thus far overlooked actors to this scenario, demonstrating that parrots (Psittaciformes) may not act only as plant antagonists (as pervasive seed predators) but also as legitimate seed dispersers for a variety of plant species (see review in Blanco et al., 2018), as it has been also shown for some Neotropical primates (Barnett et al., 2012). In a recent study, we showed that the only parrot species living in the monkey puzzle (Araucaria araucana) forests of the Andes, the Austral parakeet (Enicognathus ferrugineus), feeds mostly on the large seeds (mean 3.5 g) of this tree during the seeding period, and disperses them at higher rates and for longer distances than do rodents (Tella et al., 2016a). Also in South America, we found that two other, larger parrot species (red-spectacled amazon, Amazona pretrei, and vinaceous amazon, Amazona vinacea) base their diets on the large seeds (7 g) of the Paraná pine (Araucaria angustifolia) in Atlantic forests, dispersing them at high frequencies and over long distances and more efficiently than the previously recognized bird dispersers (Tella et al., 2016b). Moreover, both observational and experimental work on these two Araucaria species has shown that seeds partially eaten by parrots germinate well and even faster than undamaged seeds (Tella et al., 2016b; Speziale et al., 2018). This robust evidence allowed us to hypothesize that large Araucaria seeds could have evolved to attract parrots, satiate them, and benefit from their long-distance and legitimate seed dispersal services (Tella et al., 2016b).
Given the above hypothesis, we expected that the Bunya pine (Araucaria bidwillii), which is distributed throughout eastern Australia and has the largest seeds of any extant congener (averaging 17.5 g), would also be dispersed by a large parrot species. Here, we present the results of a field work expedition designed to test this prediction. The little information available suggested that A. bidwillii seeds mainly disperse by gravity (barochory), with poorly-known secondary dispersal by water and small mammals (Smith and Butler, 2002; Smith et al., 2005, 2007; Picone, 2014). We investigated seed predation and seed dispersal in A. bidwillii and, as a control, in the coexisting hoop pine (Araucaria cunninghamii), whose small (0.2 g) winged seeds are considered to be wind-dispersed (anemochory) (Leslie et al., 2017). Following this rationale, we predicted a positive relationship between Araucaria species seed weights and the body mass of their main seed-disperser parrot species, supporting a role for animal dispersal as a driver in the evolution of large seeds and seed cones in this Araucariaceae clade (Gleiser et al., 2019).
Materials and Methods
Field Work Procedures
Field work was conducted between 12 and 25 May 2017, coinciding with the end of the seeding period of Araucaria bidwillii that year. This sampling timing facilitated the detection of seed predators and dispersers (Tella et al., 2016a,b). We traveled throughout the distribution range of these species in Queensland, Australia (Thomas, 2011a,b), but concentrated our surveys in three areas: (1) the region between Lamington National Park and Canungra, southern Queensland, inhabited by A. cunninghamii, (2) Bunya Mountains, occupied by the latter species but also host to one of the largest populations of A. bidwillii, and (3) the surroundings of Cannabullen Falls, about 1,000 km north of the Bunya Mountains, where a relict population of <100 mature A. bidwillii persists (Thomas, 2011b).
Surveys replicated our previous work on seed dispersal of Araucaria species in South America see (Tella et al., 2016a,b). Briefly, we drove a car at low speed through unpaved and secondary roads to increase the chances of finding highly mobile flocks of foraging parrots. When we located parrots feeding on Araucaria trees, we stopped and observed them with telescopes from a distance to avoid disturbance. When good visibility allowed, we recorded dispersal rates by counting the number of seeds consumed in the mother tree and the number of seeds transported in flight to distant perches. In many other instances, we could only see parrots flying with seeds in their beaks, without evaluating the actual dispersal rates. Dispersal distances were measured with a laser rangefinder (Leica Geovid 10x42x, range: 10–1,300 m) as the distance from the mother tree to the perching site (exact distance) or up to where the seed-carrying flying parrot went out of sight (minimum distance). Once perching sites were identified, we looked underneath them for additional dispersed seeds and measured the distance to the nearest seeding tree (i.e., we conservatively recorded a minimum dispersal distance). These perching sites were repeatedly used by parrots (cockatoos), so we can satisfactorily assume that seeds found there were moved by them but not by secondary disperser species. We also recorded whether the dispersed seeds were fully consumed, dropped intact, or partially eaten. Intact and partially-eaten seeds were apparently viable (i.e., they were not fungal-infested or rotten).
The proportion of seeding trees on whose seeds parrots fed was also obtained following our previous work (Tella et al., 2016a,b; Dénes et al., 2018). Briefly, we selected well-spaced seeding trees (separated > 40 m from the closest seeding tree) and well spatially distributed across the study areas to cover potential effects of spatial heterogeneity in parrots' distributions and movements. We then looked below their canopies for signals of seed predation, the forms of which allowed unambiguous distinction between the different bird and mammal seed predators (Tella et al., 2016a,b; Dénes et al., 2018). In this study, fieldwork was facilitated by the low number of predator species found (see Results) and by the relatively low number of seeds produced by A. bidwillii. Therefore, we were able to count all the seeds found under A. bidwillii canopies and recorded whether they were preyed upon, dropped intact, or partially eaten by cockatoos (after observing the characteristic way cockatoos opened the seeds, see Results). Dropped seeds further consumed by non-native wild boars (Sus scrofa) and native rodents were easily identified, as described in our previous studies (Tella et al., 2016a,b). Only a few seeds of A. bidwillii were consumed by unidentified mammals. The number of seeds of A. cunninghamii was much greater, so we restricted our sampling to 50–100 seeds per tree.
Data Analyses
Contingency tables and Chi-square tests were used for testing differences in proportions. Dispersal distance distributions were right-censored as they included a number of minimum distances, so we employed an adaptation of Kaplan-Meier estimators for survival functions (Klein and Moeschberger, 2003) to estimate dispersal functions, D(d) see (Tella et al., 2016b) for the same approach. Mean and median dispersal distances were obtained from the estimated function, as the integral of the dispersal curve, conservatively restricting the mean to the larger distance recorded, and as the intersection of the curve with a horizontal line drawn at 0.5, respectively (Therneau, 2015). We used the package survival (Therneau, 2015) in R (R Core Team, 2015) to estimate the dispersal function.
Data Compilation
For comparative purposes, information on seed dispersal rates and dispersal distances of A. araucana and A. angustifolia was obtained from Tella et al. (2016a) and Tella et al. (2016b), respectively. Body masses of the parrot species recorded in these previous studies, and in the current one, independent of whether they acted as seed dispersers, were obtained from Forshaw (2006). Seed weights of each Araucaria species were also obtained from the literature (Ntima, 1968; Henderson, 1979; Muñoz, 1984; Mantovani et al., 2004; Burrows et al., 2017). In all cases, the midpoint was used when a range of weights was provided for a given species.
Results
Seed Predation and Dispersal of Araucaria bidwillii
During field work in the Bunya Mountains, we only observed sulfur-crested cockatoos (Cacatua galerita) in a highly mobile flock of ca. 70 individuals, preying upon A. bidwillii seeds (Figure 1A). Cockatoos perched on the large female cones to extract the seeds (Figure 1B), and opened the pericarp in a characteristic way to gain access to the seed content and consume it (Figure 1C). This allowed us to clearly identify the seeds preyed upon by this species. Individuals from another four parrots species (12 galahs Eolophus roseicapilla, 10 Australian king parrots Alisterus scapularis, 8 crimson rosellas Platycercus elegans, and 3 red-winged parrots Aprosmictus erythropterus) were also observed, but none of them were seen preying upon A. bidwillii seeds. We observed Torresian crows (Corvus orru) obtaining the remainders of seeds discarded by sulfur-crested cockatoos in the same tree canopy, and Australian ravens Corvus coronoides and Australian brush-turkeys (Alectura lathami) taking seeds dropped by cockatoos under the trees. The latter two species repeatedly pecked the seeds but seemed unable to open the pericarp for obtaining seed fragments to ingest them, so they acted as secondary seed dispersers when moving the seeds to distant sites.
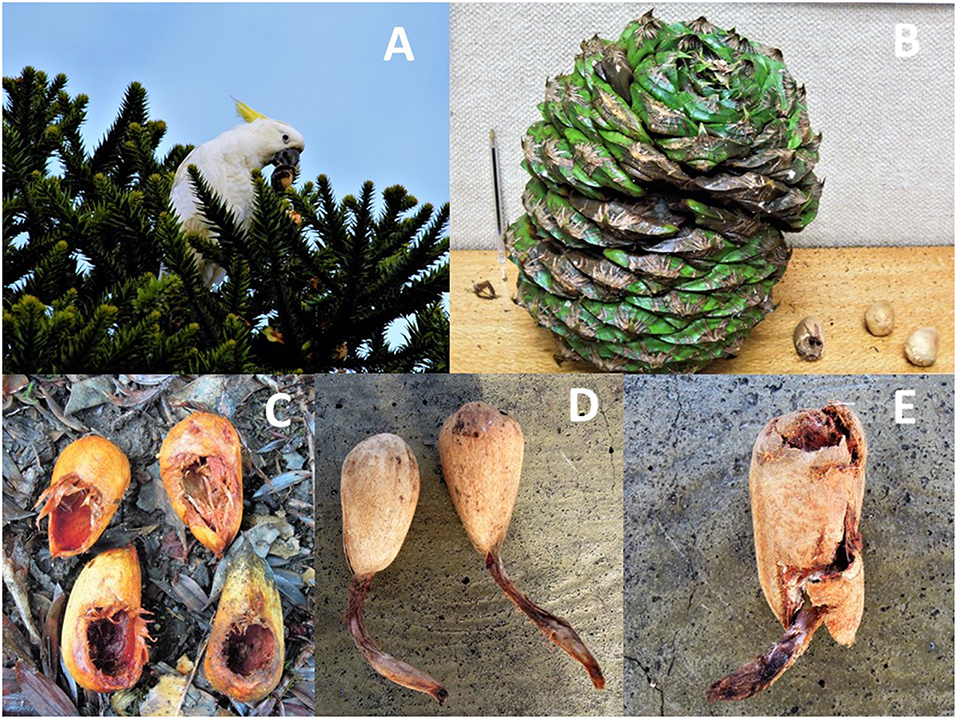
Figure 1. A sulfur-crested cockatoo handling a Bunya pine (A. bidwillii) seed (A) after extracting it from the large and strong female cone (B). Note the size of the cone related to the size of the seeds, in the bottom right corner of the picture. Cockatoos open the pericarp to consume the endosperm in a characteristic way (C). Entire (D) and partially-eaten (E) seeds germinating after dispersal by cockatoos (Pictures: GB and JT).
The detailed observations of cockatoos foraging on female cones, totaling 2.15 h, gave a seed dispersal rate of 29.63% (n = 27); 19 seeds were picked and consumed in the mother tree while 8 seeds were transported with the beak, flying to distant perches to handle and consume them. Only five cockatoos were observed looking for seeds dropped by others, despite the fact that this species often forages on the ground. Four of these cockatoos consumed the seeds on the ground, while the other (20%) dispersed one seed by flying to a distant perch. Dispersal distances, including exact measurements (n = 115) and minimum distances (i.e., from birds lost in flight or from the perching site to the closest seeding female tree, n = 126) ranged from 10 to 153 m (n = 241) (Figure 2A). Kaplan-Meyer analysis for right-censored data allowed us to estimate a mean dispersal distance of 60.86 m (SE = 4.57) and a median dispersal distance of 40 m (95% CI = 40–72 m) (Figure 2B).
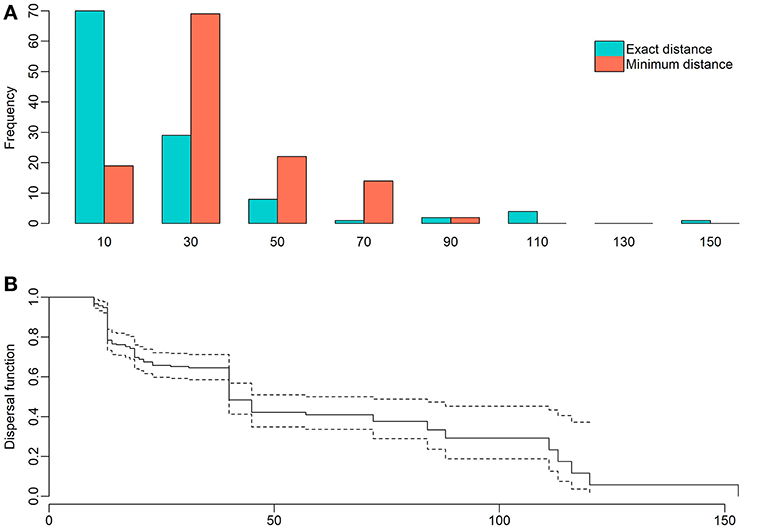
Figure 2. Dispersal distances (in m) of A. bidwillii seeds transported by sulfur-crested cockatoos. (A) Distribution of the exact and minimum dispersal distances recorded (n = 241). (B) Kaplan-Meyer estimate of the dispersal function. Dashed lines show 95% confidence bounds.
In three instances (1.2%) cockatoos dropped seeds in flight, while the rest were handled for consumption at distant perches. Perching sites (n = 48) included branches of non-seeding A. bidwillii trees (89.6%), Eucalyptus spp. trees (2.1%), other species of trees (6.2%) and electricity poles (2.1%). Most dispersed seeds (92.9%, n = 241) were fully eaten but others were dropped intact (3.3%) or only partially eaten (3.7%). Two undamaged seeds and one partially-eaten seed were already germinating under perches after dispersed by cockatoos (Figures 1D,E).
About half (52 out of 109) of the well-spaced A. bidwillii trees inspected produced seeds during the study season. All of these seeding trees were previously visited by cockatoos, as indicated by the characteristically predated seeds (Figure 1C) we found below their canopies. We only found the remainders of a single case of barochory, i.e., when the entire mature female cone falls to the ground and the contained seeds disaggregate due to strong impact. Therefore, almost all the seeds produced by the 52 trees were preyed upon, dropped (intact or partially eaten) in situ or dispersed by cockatoos before naturally falling to the ground. The proportion of seeds preyed upon (97.8%), undamaged (1.9%), or partially eaten by cockatoos (0.3%) found under the canopies (n = 1,283 seeds) differed from those regarding dispersed seeds (see above, χ2 = 30.4, df = 2, p < 0.001), due to a lower proportion of undamaged and partially-eaten seeds. This was probably due to the predation of the seeds dropped by cockatoos by terrestrial mammals attracted by the residual material they left. In fact, we found clear evidence of seed predation by wild boars (Sus scrofa), rodents and unidentified mammals under 15.5% (5.8, 1.9, and 7.7%, respectively) of inspected trees.
Apart from the A. bidwillii population in the Bunya Mountains, we could only inspect 14 adult trees in the northern relict population, and none of them produced seeds successfully.
Seed Predation and Dispersal of Araucaria cunninghamii
We did not observe predation of A. cunninghamii seeds by any of the species of parrots recorded in the Bunya Mountains (see above), or in the Lamington-Canungra area where we also recorded a number of foraging granivorous parrots (140 sulfur-crested cockatoos, 113 galahs, 24 Australian king parrots, 4 crimson rosellas, 3 pale-headed rosellas Platycercus adscitus, and 2 yellow-tailed black cockatoos Calyptorhynchus funereus). We observed sulfur-crested cockatoos feeding on fresh and dry branches and on gum of several A. cunninghamii trees, but not on their abundant seeds.
We inspected a sample of 2,550 seeds from 42 seeding trees (13 in Lamington–Canungra area and 29 in the Bunya Mountains). No seeds showed signs of predation by parrots, and only 0.8% of them had been preyed upon by rodents, with the rest being undamaged. These trees were isolated or separated >40 m from the closest seeding tree, thus avoiding the possibility that seeds found under a particular tree came from another tree. Most seeds were dispersed by barochory (i.e., they were found just below the mother canopy tree), while in a few cases the wind seemed responsible of dispersing seeds in a radius of up to 30 m (anemochory).
Comparison of Parrot-Mediated Seed Dispersal in Araucaria Species
Table 1 summarizes the available information on seed dispersal of Araucaria species by parrots. The Austral parakeet (E. ferrugineus) was the only bird species recorded dispersing seeds of A. araucana, which constitutes the main food resource for this species during the seeding period (Tella et al., 2016a). Primary dispersal rate performed by this species was extremely low, while secondary dispersal (i.e., after mature seeds fall to the ground) showed the highest dispersal rate for the three Araucaria species. Maximum and mean dispersal distances are the lowest, although they were clearly underestimated since only minimum distances (i.e., right-censored) could be recorded. In the case of A. angustifolia, two amazon species (A. pretrei and A. vinacea) are strongly linked to this species, as its seeds constitute the bulk of their winter diet (Tella et al., 2016b). Other parrot species play a minor role as dispersers and/or their distributions barely overlap with the distribution of A. angustifolia forests (Tella et al., 2016b). Amazons exclusively act as primary seed dispersers, with moderate dispersal rates and the largest dispersal distances recorded for Araucaria species. Finally, based on the present study, C. galerita seems to be the only primary seed-disperser parrot of A. bidwillii, showing moderate dispersal rates and dispersal distances compared to the rest of parrot species. This cockatoo also acts, although at a much lower frequency, as a secondary seed disperser.
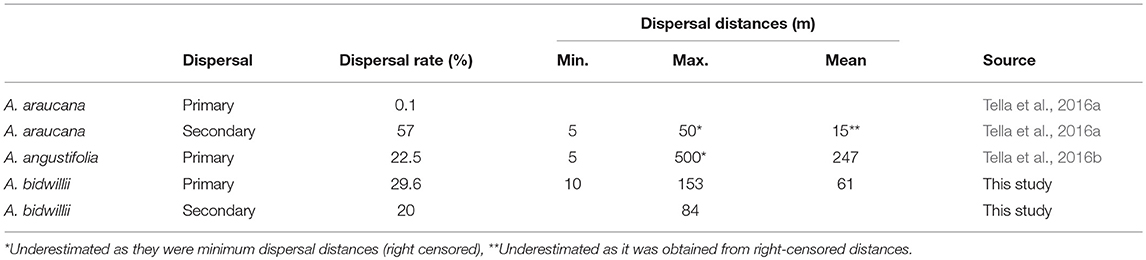
Table 1. Comparison of seed dispersal traits by parrots of the three Araucaria species dispersed by zoochory.
Figure 3 shows the variability in seed weight between the three zoochoraceous species of Araucaria (A. araucana, A. anfustifolia, and A. bidwillii) compared to those exclusively dispersed by barochory and anemochory (A. cunninghamii). As expected from a potential role of parrots in the evolution of seed size, the relationship between the seed weights of the three zoochorous Araucaria species and of their main parrot-disperser species (Austral parakeet, amazons and sulfur-crested cockatoo) closely matches the diagonal line between plant and disperser traits (i.e., the line depicting a theoretical full correlation, r = 1; Figure 3). The body mass of most parrot species that contribute little or nothing to Araucaria seed dispersal largely departs from the diagonal line (Figure 3).
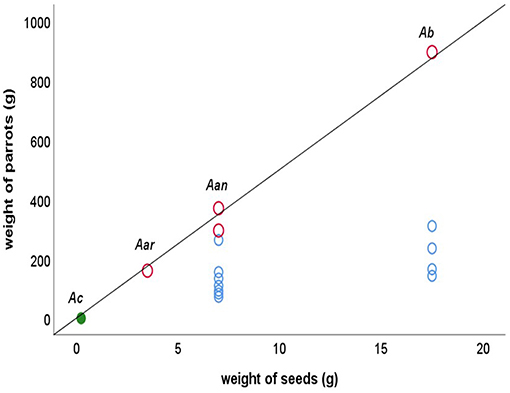
Figure 3. Relationship between the seed weight of three Araucaria species (Aar, A. araucaria; Aan, A. angustifolia; Ab, A. bidwillii) and the body mass of parrot species that disperse them (red circles). Blue circles represent parrot species that have a small (for Aan) or null seed dispersal role (for Ab). The diagonal line represents the expected perfect correlation between seed size and parrot size. A. cunninghamii (Ac) is depicted (green dot) as a reference, since no parrots disperse its seeds (they are only dispersed by barochory and anemochory).
Discussion
Seed Dispersal in Araucaria bidwillii
Little information is available on seed dispersal strategies of A. bidwillii. Although some authors have argued that dinosaurs and large mammals may have dispersed its seeds in the past (Smith et al., 2007), the prevailing view is that the species is now mostly gravity-dispersed (Smith and Butler, 2002; Pye, 2005; but see Farjon, 2017). The large seeds are retained in similarly large female cones (which can weigh in excess of 10 kg) until they mature and fall intact to the ground; therefore, excluding instances of rolling down slopes or falling into watercourses, the majority of seeds are expected to initiate an intra-cone competition to germinate beneath the parent tree (Pye, 2005). It has been suggested that this limited dispersal capacity explains the poor ability of the species to recolonize areas following its past range contraction (Smith and Butler, 2002). However, observations of rodents caching seeds suggested that additional mechanisms might be available for dispersal (Pye, 2005). Smith et al. (2005) tagged seeds and placed automatic cameras for monitoring seed predation and secondary dispersal by mammals, showing that a small proportion of seeds were handled and eaten by rodents and that some seeds were dispersed at least 16 m. Additional work by the same authors (Smith et al., 2007) showed that some seeds were carried up to 8 m outside the parent tree canopy, and that the short-eared possum (Trichosurus caninus) is able to disperse the seeds. Moreover, Picone (2014) observed cockatoos, though not indicating the species, feeding on seeds and suggested that they could also act as dispersal vectors.
Here, we demonstrate that sulfur-crested cockatoos are both pervasive seed predators and legitimate seed dispersers of A. bidwillii. These cockatoos are able to open the strongly compacted female cones with their strong beaks to extract the seeds, something that other large bird species such as crows and ravens, or smaller parrot species, cannot do. Notably, cockatoos preyed upon seeds on all the well-spaced trees surveyed well before dispersal by gravity, as we only found a single fallen mature female cone at the end of the seeding period, while the rest of the seeds were predated, dropped, or dispersed by cockatoos when cones were still in the canopy. Therefore, at least in the study year, gravity played a marginal role compared to the primary seed dispersal performed by cockatoos, challenging the prevailing idea of barochory being the main dispersal syndrome for this species (Smith and Butler, 2002; Pye, 2005; Picone, 2014). Also notably, cockatoos dispersed seeds at a high rate (30%) and at distances that exceed by one order of magnitude those reported for secondary dispersal by small mammals (Smith et al., 2005, 2007). A relatively high proportion of the dispersed seeds (7%) were dropped intact or partially eaten in flight or under perches, thus having the potential to germinate, as germination was corroborated for both kinds of seeds despite the short-term nature of our survey. Moreover, cockatoos also dropped intact and partially-eaten seeds when extracting them from the cones, making them available under parent trees to other bird species as well as cockatoos (this study) and small mammals (Smith et al., 2005, 2007) that can act as secondary seed dispersers. Overall, our results add to recent findings showing that parrots are not merely seed predators but can be involved in plant-parrot mutualism-antagonism continuums (Montesinos-Navarro et al., 2017) where they can play a key role as seed dispersers (Boehning-Gaese et al., 1999; Blanco et al., 2015, 2016, 2018; Tella et al., 2015, 2016a,b; Baños-Villalba et al., 2017).
Dispersal of Large-Seeded Araucaria Trees by Parrots
The identification of the main seed dispersal syndromes and vectors has been controversial not only for A. bidwillii, but also for the whole Araucariaceae family. In a recent comparative phylogenetic study, Contreras et al. (2017) identified most species of the family as being dispersed by anemochory, with a few species relying on barochory for their seed dispersal. Simultaneously, other analyses suggested that the ancestral seed dispersal strategy for the large-seeded clade of Araucaria (A. araucana, A. angustifolia, A. hunsteini, A. bidwillii) was zoochory, with a reversal to anemochory in A. hunsteini (Leslie et al., 2017), and that animals are still the main dispersal vectors for these species (Gleiser et al., 2019). In fact, there was accumulated evidence supporting current secondary seed dispersal of A. araucana, A. angustifolia, and A. bidwillii by vertebrates (Smith et al., 2005, 2007; Vieira and Iob, 2009; Shepherd and Ditgen, 2013). Our results show that a typical small-winged Araucaria species (A. cunninghamii) is mainly dispersed by barochory and anemochory, as expected given its diaspore morphology (Leslie et al., 2017; Gleiser et al., 2019). On the other hand, parrots seem to play a greater role as seed dispersers than the previously identified animal vectors for the three large-seeded species (Tella et al., 2016a,b; this study). The Austral parakeet is the only bird species dispersing seeds of A. araucana, and over much larger distances (Tella et al., 2016a) than the only rodent species that effectively disperse its seeds (Shepherd and Ditgen, 2013). The same is true for A. bidwillii (see above). A larger number of bird and mammal species were identified as seed dispersers of A. angustifolia (Vieira and Iob, 2009; Dénes et al., 2018). However, large amazon parrots were shown to be more efficient at dispersing seeds than jays, which were previously thought to be the main animal vectors for this species (Tella et al., 2016b). Remarkably, the three large-seeded species are dispersed at high rates by parrots and, perhaps more importantly, to long, underestimated, distances. Several reviews have highlighted the difficulties of measuring long-distance dispersal, as well as its pivotal importance: just a very small proportion of seeds effectively dispersed at long distances is key to maintaining gene flow and facilitating forest regeneration (Cain et al., 2000; Nathan and Muller-Landau, 2000; Howe and Miriti, 2004; Schurr et al., 2009; Jordano, 2017).
Large Araucaria seeds partially eaten by parrots germinate well and even faster than intact seeds, probably because the partial removal of the seed coat eliminates the main barrier to moisture, while favoring subsequent water intake and seedling emergence (Tella et al., 2016a; Speziale et al., 2018). This allowed us to hypothesize that their large seeds evolved to attract parrots—and perhaps also some unknown extinct vertebrate-, satiate them and benefit from their long-distance dispersal services (Tella et al., 2016b). These highly nutritive seeds (Brand et al., 1985; Conforti and Lupano, 2011) are covered by a relatively thin coat, but retained within strongly compacted cones until dispersal by barochory. Since seed size correlates with cone size (Gleiser et al., 2019), larger species of parrots, bearing stronger beaks, would be necessary to open the larger cones, access the seeds and eventually disperse them. Despite the unavoidably small sample size, the strong covariation found between the weight of Araucaria seeds and the body weight of their main seed-disperser parrot species supports our hypothesis of a role for parrots as drivers in the evolution of seed size in Araucaria species. Fossil records suggest that the family Araucariaceae may have originated in the Triassic, achieving its maximum diversity during the Jurassic and Cretaceous periods (Kershaw and Wagstaff, 2001), while molecular studies point to the origin and radiation of parrots in the late Cretaceous-Paleogene periods (Wright et al., 2008). Leslie et al. (2017) showed that dispersal syndromes are good predictors of seed size and cone morphology in conifers, including Araucariaceae. In line with these results, an increase in genome size, which correlates with seed size, may have moved the large-seeded Araucaria clade from the ancestral anemochory/barochory dispersal strategies to zoochory, by making their larger seeds attractive to seed predators (Gleiser et al., 2019). Therefore, there were opportunities during a long geological period for parrots to contribute to the evolution of seed size in Araucaria through seed predation and dispersal. One could also argue that current parrot communities may largely differ from the oldest ones, and thus that the relationship shown in Figure 3 may not result from a long-term but from a contemporary process. This is also a possibility, as seed and cone morphology (e.g., Benkman et al., 2003; Dylewski et al., 2017), and even more complex reproductive strategies such as serotiny (an ecological adaptation exhibited by some plants, in which seed release occurs in response to an environmental trigger such as fire; Talluto and Benkman, 2014), are known to be shaped by seed predators/dispersers at short temporal and small spatial scales in conifers.
Future Research Avenues
Clearly, more research is required to confirm the potential role of parrots in the evolution of seed size and seed dispersal strategies in Araucariaceae. A puzzling question is related to the dispersal syndrome of A. hunsteinii, whose range is restricted to New Guinea. This is the only species within the large-seeded Araucaria clade that shows a relatively small and winged seed, suggesting a reversal from zoochory to anemochory dispersal (Leslie et al., 2017). However, its nut-like seed is also highly nutritive and weighs ca. 2 grams (Henderson, 1979), thus being potentially attractive to the large community of parrots, including two cockatoo species inhabiting New Guinea (Forshaw, 2006). In fact, Ntima (1968) vaguely mentioned that cockatoos damage A. hunsteinii seeds. Field work would be needed to assess the relative contribution of wind dispersal and presumably parrot seed dispersal in this species, for a better understanding of the evolution of seed dispersal strategies, seed and cone size in Araucariaceae (Gleiser et al., 2019).
Our results show that parrots currently are frequent, long-distance and legitimate dispersers of the three large-seeded Araucaria species. Future work should assess to what extent parrots may be shaping the spatial recruitment of trees, as has been recently shown for a palm tree species mostly dispersed by large parrots (Baños-Villalba et al., 2017). Moreover, this role for parrots could be translated to the genetic population structure of Araucaria populations. Population genetic studies were carried out on the four species studied here (Bekessy et al., 2002; Pye and Gadek, 2004; Stefenon et al., 2007; Pye et al., 2009; Souza et al., 2009), and results were interpreted under the assumption that only pollen dispersal is responsible for long-range gene flow in these species. This assumption comes from a thorough paternity study conducted on seeds, seedlings and juveniles of A. angustifolia (Bittencourt and Sebbenn, 2007). It contrasts with the fact that pollen grains in Araucariaceae are among the largest non-saccate (i.e., without inflated air bladders) grains of any conifer (Leslie, 2010), raising questions about the effectiveness of wind dispersal for these large, non-floating pollen grains (Sousa and Hattemer, 2003), and its role in long-range gene flow (Pye, 2005). Results indicating that pollen dispersal is more important than seed dispersal for long-distance gene flow were obtained from two small, highly fragmented and isolated patches of A. angustifolia (Bittencourt and Sebbenn, 2007), where seed-disperser parrots could be scarce or even absent. In fact, the two parrot species that act as the main seed dispersers of this species have experienced substantial population declines and range contractions and, as for other parrot-plant (Luna et al., 2018) and plant-animal mutualisms (Valiente-Banuet et al., 2015), the local extinction of these species may have disrupted key seed dispersal processes (Tella et al., 2016b). The fragmented distribution of A. angustifolia thus offers a unique natural experiment for assessing the actual role of parrots as long-distance dispersers of this critically endangered species (Thomas, 2013), by replicating the work of Bittencourt and Sebbenn (2007) in several areas with and without the presence of parrots. Moreover, as Araucaria trees are extremely long-lived (e.g., >1,000 year reported for A. araucana, Aguilera-Betti et al., 2017), the comparison within areas of the genetic arrangement of seedlings and juveniles with that of centenary adults would further deepen the disruption of dispersal processes at long-term temporal scales.
A deeper understanding of the consequences of seed dispersal by parrots could lead to improved design of conservation strategies for these tree species, since A. Araucana and A. angustifolia are listed as Endangered and Critically Endangered species, respectively, by the IUCN (Premoli et al., 2013; Thomas, 2013), while A. bidwillii is listed as Least Concern but has a fragmented distribution and even an isolated, genetically distinct population (Pye and Gadek, 2004; Thomas, 2011b).
Ethics Statement
This study relies on observational data obtained in areas unrestricted to people and thus did not require special permits.
Author Contributions
JT, FH, and GB designed the expedition. GB and JT conducted field work. FD analyzed the data. JT wrote a first draft of the manuscript and all authors contributed to improve it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank R. Heinsohn for his initial advice on preparing the field work expedition, and G. Gleiser and two reviewers for their valuable comments to the manuscript. Loro Parque Fundacion funded the publication of this paper.
References
Aguilera-Betti, I., Muñoz, A. A., Stahle, D., Figueroa, G., Duarte, F., González-Reyes, Á., et al. (2017). The first millennium-age Araucaria Araucana. in Patagonia. Tree Ring Res. 73, 53–56. doi: 10.3959/1536-1098-73.1.53
Baños-Villalba, A., Blanco, G., Díaz-Luque, J. A., Dénes, F. V., Hiraldo, F., and Tella, J. L. (2017). Seed dispersal by macaws shapes the landscape of an Amazonian ecosystem. Sci. Rep. 7:7373. doi: 10.1038/s41598-017-07697-5
Barnett, A. A., Boyle, S. A., Pinto, L. P., Lourenço, W. C., Almeida, T., Silva, W. S., et al. (2012). Primary seed dispersal by three neotropical seed-predating primates (Cacajao melanocephalus ouakary, Chiropotes chiropotes. and Chiropotes albinasus). J. Trop. Ecol. 28, 543–555. doi: 10.1017/S0266467412000600
Bekessy, S. A., Allnutt, T. R., Premoli, A. C., Lara, A., Ennos, R. A., Burgman, M. A., et al. (2002). Genetic variation in the vulnerable and endemic Monkey Puzzle tree, detected using RAPDs. Heredity. 88:243. doi: 10.1038/sj.hdy.6800033
Benkman, C. W., Parchman, T. L., Favis, A., and Siepielski, A. M. (2003). Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. Am. Nat. 162, 182–194. doi: 10.1086/376580
Bittencourt, J. V., and Sebbenn, A. M. (2007). Patterns of pollen and seed dispersal in a small, fragmented population of the wind-pollinated tree Araucaria angustifolia in southern Brazil. Heredity. 99:580–591. doi: 10.1038/sj.hdy.6801019
Blanco, G., Bravo, C., Pacifico, E. C., Chamorro, D., Speziale, K. L., Lambertucci, S. A., et al. (2016). Internal seed dispersal by parrots: an overview of a neglected mutualism. PeerJ. 4:e1688. doi: 10.7717/peerj.1688
Blanco, G., Hiraldo, F., Rojas, A., Dénes, F. V., and Tella, J. L. (2015). Parrots as key multilinkers in ecosystem structure and functioning. Ecol. Evol. 18, 4141–4160. doi: 10.1002/ece3.1663
Blanco, G., Hiraldo, G., and Tella, J. L. (2018). Ecological functions of parrots: an integrative perspective from plant life cycle to ecosystem functioning. Emu. 118, 36–49. doi: 10.1080/01584197.2017.1387031
Boehning-Gaese, K., Gaese, B. H., and Rabemanantsoa, S. B. (1999). Importance of primary and secondary seed dispersal in the Malagasy tree Commiphora guillaumini. Ecology. 80, 821–832. doi: 10.2307/177020
Brand, J. C., Cherikoff, V., and Truswell, A. S. (1985). The nutritional composition of Australian Aboriginal bushfoods. 3. Seeds and nuts. Food Tech. Austr. 37:275.
Burrows, G. E., Heady, R. D., and Smith, J. P. (2017). Substantial resource reallocation during germination of Araucaria bidwillii (bunya pine), an Australian rainforest conifer with large seeds and cryptogeal germination. Trees. 31, 115–124. doi: 10.1007/s00468-016-1461-y
Cain, M. L., Milligan, B. G., and Strand, A. E. (2000). Long-distance seed dispersal in plant populations. Am. J. Bot. 87, 1217–1227. doi: 10.2307/2656714
Conforti, P. A., and Lupano, C. E. (2011). Selected properties of Araucaria angustifolia and Araucaria araucana seed protein. Int. J. Food Prop. 14, 84–91 doi: 10.1080/10942910903131431
Contreras, D. L., Duijnstee, I. A. P., Ranks, S., Marshall, C. R., and Looy, C. V. (2017). Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Persp. Plant Ecol. Evol. Syst. 24, 93–117. doi: 10.1016/j.ppees.2016.11.002
Dénes, F., Tella, J. L., Zulian, V., Prestes, N. M., Martínez, J., and Hiraldo, F. (2018). Combined impacts of multiple non-native mammals on two life stages of a critically endangered Neotropical tree. Biol. Invas. 20, 3055–3068. doi: 10.1007/s10530-018-1758-4
Díaz, S., Kattge, J., Cornelissen, J. H., Wright, I. J., Lavorel, S., Dray, S., et al. (2016). The global spectrum of plant form and function. Nature 529, 167–171. doi: 10.1038/nature16489
Dylewski, Ł., Yosef, R., and Myczko, Ł. (2017). Difference on cone size preferences between two coniferous species by Great Spotted Woodpecker (Dendrocopos major). Peer J. 5:e3288. doi: 10.7717/peerj.3288
Forshaw, J. M. (2006). Parrots of the World. An Identification Guide. Princenton: Princeton University Press.
Gleiser, G., Speziale, K. L., Lambertucci, S. A., Hiraldo, F., Tella, J. L., and Aizen, M. A. (2019). Uncoupled evolution of male and female cone sizes in an ancient conifer lineage. Int. J. Plant Sci. 180, 72–80. doi: 10.1086/700580
Henderson, S. (1979). Moisture exchange in Araucaria hunsteinii (Klinki pine seed). J. Stored Prod. Res. 15, 127–129. doi: 10.1016/0022-474X(79)90009-2
Howe, H. F., and Miriti, M. N. (2004). When seed dispersal matters. Bioscience 54, 651–660. doi: 10.1641/0006-3568(2004)054[0651:WSDM]2.0.CO;2
Jordano, P. (2017). What is long-distance dispersal? And a taxonomy of dispersal events. .J. Ecol. 105, 75–84. doi: 10.1111/1365-2745.12690
Kershaw, P., and Wagstaff, B. (2001). The Southern conifer family Araucariaceae: history, status, and value for paleoenvironmental reconstruction. Annu. Rev. Ecol. Syst. 32, 397–414. doi: 10.1146/annurev.ecolsys.32.081501.114059
Klein, J. P., and Moeschberger, M. L. (2003). Survival Analysis: Techniques for Censored and Truncated Data. New York, NY: Springer.
Leishman, M. R., Wright, I. J., Moles, A. T., and Westoby, M. (2000). “The evolutionary ecology of seed size,” in Seeds: the Ecology of Regeneration in Plant Communities, 2nd Edn, ed Fenner M (Oxford: CABI Publishing), 31–57.
Leslie, A. B. (2010). Flotation preferentially selects saccate pollen during conifer pollination. New Phytol. 188, 273–279. doi: 10.1111/j.1469-8137.2010.03356.x
Leslie, A. B. (2011). Predation and protection in the macroevolutionary history of conifer cones. Proc. Biol. Sci. 278, 3003–3008. doi: 10.1098/rspb.2010.2648
Leslie, A. B., Beaulieu, J. M., and Mathews, S. (2017). Variation in seed size is structured by dispersal syndrome and cone morphology in conifers and other nonflowering seed plants. New Phytol. 216, 429–437. doi: 10.1111/nph.14456
Luna, A., Romero-Vidal, P., Hiraldo, F., and Tella, J. L. (2018). Cities may save some threatened species but not their ecological functions. Peer J. 6:e4908. doi: 10.7717/peerj.4908
Mantovani, A., Morellato, L. P. C., and Reis, M. S. D. (2004). Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev. Brasil. Botân. 27, 787–796. doi: 10.1590/S0100-84042004000400017
Moles, A. T., Ackerly, D. D., Webb, C. O., Tweddle, J. C., Dickie, J. B., and Westoby, M. (2005). A brief history of seed size. Science. 307, 576–580. doi: 10.1126/science.1104863
Montesinos-Navarro, A., Hiraldo, F., Tella, J. L., and Blanco, G. (2017). Network structure embracing mutualism-antagonism continuums increases community robustness. Nat. Ecol. Evol. 1, 1661–1669. doi: 10.1038/s41559-017-0320-6
Muñoz, R. (1984). Análisis de la Muñoz, 1984 Cityproductividad de semillas de Araucaria araucana (Mol.) C. Koch. el Area de Lonquimay—IX Región. Tesis para optar al TítulodeIngenieroForestal.FacultaddeCienciasAgrarias,Veterinarias y Forestales. Escuela de Ciencias Forestales, Universidad de Chile, 98.
Nathan, R., and Muller-Landau, H. C. (2000). Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285. doi: 10.1016/S0169-5347(00)01874-7
Ntima, O. O. (1968). Fast Growing Timber Trees of the Lowland Tropics: the Araucarias. Oxford: Commonwealth Forestry Institute.
Picone, A. P. (2014). Habitat, Population Structure and the Conservation Status of Araucaria Bidwillii Hook. The Australian Wet Tropics. Doctoral dissertation, James Cook University.
Premoli, A., Quiroga, P., and Gardner, M. (2013). Araucaria araucana. The IUCN Red List of Threatened Species 2013: e.T31355A2805113.
Pye, M. G. (2005). Genetic Diversity and Divergence Within and Among Natural Populations of Araucaria in Eastern Australia. Doctoral dissertation, James Cook University.
Pye, M. G., and Gadek, P. A. (2004). Genetic diversity, differentiation and conservation in Araucaria bidwillii (Araucariaceae), Australia's Bunya pine. Conserv. Gen. 5, 619–629. doi: 10.1007/s10592-003-1857-2
Pye, M. G., Henwood, M. J., and Gadek, P. A. (2009). Differential levels of genetic diversity and divergence among populations of an ancient Australian rainforest conifer, Araucaria cunninghamii. Plant Syst. Evol. 277, 173–185. doi: 10.1007/s,00606-008-0120-1
R Core Team (2015). A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna. Available online at: https://www.R-project.org/
Schurr, F. M., Spiegel, O., Steinitz, O., Trakhtenbrot, A., Tsoar, A., and Nathan, R. (2009). Long-distance seed dispersal. Ann. Plant Rev. 38, 204–237. doi: 10.1002/9781444314557.ch6
Shepherd, J. D., and Ditgen, R. S. (2013). Rodent handling of Araucaria araucana seeds. Austr. Ecol. 38, 23–32. doi: 10.1111/j.1442-9993.2012.02366.x
Smith, I., and Butler, D. W. (2002). The Bunya in Queensland's forests. Qld. Rev. 9, 31–38. doi: 10.1017/S1321816600002932
Smith, I. R., Withers, K., and Billingsley, J. (2005). “The role of native rodents in seed dispersal and seed predation of the Bunya Pine (Araucaria bidwillii),” in: 22nd Annual Conference of the Australian and New Zealand Society for Comparative Physiology and Biochemistry (ANZSCPB 2005), 09-11 Dec 2005 (Dunedin).
Smith, I. R., Withers, K., and Billingsley, J. (2007). “Maintaining the ancient bunya tree (Araucaria bidwillii Hook.) - dispersal and mast years,” in Presentation Given at the 5th Southern Connection Conference (Adelaide, SA).
Sousa, V. A., and Hattemer, H. H. (2003). Pollen dispersal and gene flow by pollen in Araucaria angustifolia. Austr. J. Bot. 51, 309–317. doi: 10.1071/BT02037
Souza, M. I. F. D., Salgueiro, F., Carnavale-Bottino, M., Félix, D. B., Alves-Ferreira, M., Bittencourt, J. V. M., et al. (2009). Patterns of genetic diversity in southern and southeastern Araucaria angustifolia (Bert.) O. Kuntze relict populations. .Gen. Mol. Biol. 32, 546–556. doi: 10.1590/S1415-47572009005000052
Speziale, K. L., Lambertucci, S. A., Gleiser, G., Tella, J. L., Hiraldo, F., and Aizen, M. A. (2018). An overlooked plant-parakeet mutualism counteracts human overharvesting on an endangered tree. R. Soc. Open Sci. 5:171456. doi: 10.1098/rsos.171456
Stefenon, V. M., Gailing, O., and Finkeldey, R. (2007). Genetic structure of Araucaria angustifolia (Araucariaceae) populations in Brazil: implications for the in situ conservation of genetic resources. Plant Biol. 9, 516–525. doi: 10.1055/s-2007-964974
Talluto, M. V., and Benkman, C. W. (2014). Conflicting selection from fire and seed predation drives fine-scaled phenotypic variation in a widespread North American conifer. Proc. Natl. Acad. Sci. U S A. 111, 9543–9548. doi: 10.1073/pnas.1400944111
Tella, J. L., Baños-Villalba, A., Hernández-Brito, D., Rojas, A., Pacífico, E., Díaz-Luque, J. A., et al. (2015). Parrots as overlooked seed dispersers. Front. Ecol. Environ. 13, 338–339. doi: 10.1890/1540-9295-13.6.338
Tella, J. L., Dénes, F. V., Zulian, V., Prestes, N. P., Martínez, J., Blanco, G., et al. (2016b). Endangered plant-parrot mutualisms: seed tolerance to predation makes parrots pervasive dispersers of the Parana pine. Sci. Rep. 6:31709. doi: 10.1038/srep31709
Tella, J. L., Lambertucci, S., Speziale, K., and Hiraldo, F. (2016a). Large-scale impacts of multiple co-occurring invaders on monkey puzzle forest regeneration, native seed predators and their ecological interactions. Glob. Ecol. Conserv. 6, 1–15. doi: 10.1016/j.gecco.2016.01.001
Therneau, T. A. (2015). Package for Survival Analysis in S. version 2.38. Available online at: http://CRAN.R-project.org/package=survival
Thomas, P. (2011a). Araucaria cunninghamii. The IUCN Red List of Threatened Species 2011: e.T32835A9734286.
Thomas, P. (2011b). Araucaria bidwillii. The IUCN Red List of Threatened Species 2011: e.T42195A10660714.
Thomas, P. (2013). Araucaria angustifolia. The IUCN Red List of Threatened Species 2013: e.T32975A2829141.
Valiente-Banuet, A., Aizen, M. A., Alcántara, J. M., Arroyo, J., Cocucci, A., Galetti, M., et al. (2015). Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. doi: 10.1111/1365-2435.12356
Vieira, E. M., and Iob, G. (2009). “Dispersão e predação de sementes da araucária (Araucaria angustifolia),” in Floresta de Araucária: Ecologia, Conservação e Desenvolvimento Sustentável, eds C. R. Fonseca, A. F. Souza, A. M. Leal-Zanchet, T. Dutra, A. Backes, G. GanadeHolos, (Ribeirão Preto: Holos), 85–95.
Keywords: cockatoo, conifers, dispersal syndromes, mutualism, Psittaciformes, seed dispersal, seed predation, seed size
Citation: Tella JL, Blanco G, Dénes FV and Hiraldo F (2019) Overlooked Parrot Seed Dispersal in Australia and South America: Insights on the Evolution of Dispersal Syndromes and Seed Size in Araucaria Trees. Front. Ecol. Evol. 7:82. doi: 10.3389/fevo.2019.00082
Received: 03 October 2018; Accepted: 04 March 2019;
Published: 27 March 2019.
Edited by:
Oana Moldovan, Emil Racovita Institute of Speleology, RomaniaReviewed by:
Adrian Ashton Barnett, National Institute of Amazonian Research (INPA), BrazilWilfredo Falcon, Puerto Rico Department of Natural and Environmental Resources (DRNA), Puerto Rico
Copyright © 2019 Tella, Blanco, Dénes and Hiraldo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José L. Tella, tella@ebd.csic.es
 José L. Tella
José L. Tella Guillermo Blanco
Guillermo Blanco Francisco V. Dénes
Francisco V. Dénes Fernando Hiraldo1
Fernando Hiraldo1