Multimodal Aposematic Defenses Through the Predation Sequence
- 1Department of Biological and Environmental Science, University of Jyväskylä, Jyväskylä, Finland
- 2Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Penryn, United Kingdom
- 3Organismal and Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Aposematic organisms warn predators of their unprofitability using a combination of defenses, including visual warning signals, startling sounds, noxious odors, or aversive tastes. Using multiple lines of defense can help prey avoid predators by stimulating multiple senses and/or by acting at different stages of predation. We tested the efficacy of three lines of defense (color, smell, taste) during the predation sequence of aposematic wood tiger moths (Arctia plantaginis) using blue tit (Cyanistes caeruleus) predators. Moths with two hindwing phenotypes (genotypes: WW/Wy = white, yy = yellow) were manipulated to have defense fluid with aversive smell (methoxypyrazines), body tissues with aversive taste (pyrrolizidine alkaloids) or both. In early predation stages, moth color and smell had additive effects on bird approach latency and dropping the prey, with the strongest effect for moths of the white morph with defense fluids. Pyrrolizidine alkaloid sequestration was detrimental in early attack stages, suggesting a trade-off between pyrrolizidine alkaloid sequestration and investment in other defenses. In addition, pyrrolizidine alkaloid taste alone did not deter bird predators. Birds could only effectively discriminate toxic moths from non-toxic moths when neck fluids containing methoxypyrazines were present, at which point they abandoned attack at the consumption stage. As a result, moths of the white morph with an aversive methoxypyrazine smell and moths in the treatment with both chemical defenses had the greatest chance of survival. We suggest that methoxypyrazines act as context setting signals for warning colors and as attention alerting or “go-slow” signals for distasteful toxins, thereby mediating the relationship between warning signal and toxicity. Furthermore, we found that moths that were heterozygous for hindwing coloration had more effective defense fluids compared to other genotypes in terms of delaying approach and reducing the latency to drop the moth, suggesting a genetic link between coloration and defense that could help to explain the color polymorphism. Conclusively, these results indicate that color, smell, and taste constitute a multimodal warning signal that impedes predator attack and improves prey survival. This work highlights the importance of understanding the separate roles of color, smell and taste through the predation sequence and also within-species variation in chemical defenses.
Introduction
Predation is one of the main threats to an organism’s survival. As a result, there are many different traits that have evolved to help organisms avoid predation and most organisms use more than one line of defense. In some cases, these multiple defenses can act simultaneously (Ruxton et al., 2018). For example, prey may evolve behaviors such as background choice (Sargent, 1966; Kang et al., 2012; Kjernsmo and Merilaita, 2012; Green et al., 2019) or body orientation (Kang et al., 2012; Rowland et al., 2020) and also to have a color pattern that is camouflaged against their surroundings, all of which help avoid detection by predators (Stevens and Ruxton, 2018). However, many animals have defense mechanisms that act sequentially by impeding different stages of attack (Endler, 1991; Caro, 2005; Ruxton et al., 2018). Primary defenses act to prevent physical contact between predator and prey (i.e., at the encounter, detection, identification and approach stages of an attack), whereas secondary defenses deter attack after or just before the predator has made physical contact with the prey (i.e., at the subjugation and consumption stages of attack) (Ruxton et al., 2018). Whether selection favors investment in primary and/or secondary defenses depends on the relative properties of those defenses such as their energetic cost and efficacy against predators (Broom et al., 2010).
Aposematism is a defense strategy that relies on communication to signal unprofitability to predators (Poulton, 1887, Poulton, 1890; Cott, 1940; Stevens, 2013). Aposematic prey (the signaler) use a warning signal to inform predators (the receiver) of unpleasant or harmful defenses to reduce the likelihood or extent of attack by the predator and to promote, enhance, or maintain learned avoidance of that prey type in future encounters (Poulton, 1887, Poulton, 1890; Cott, 1940). Such warning signals may act as a primary defense if the predator has an innate color bias (Smith, 1975; Roper, 1990; Schuler and Roper, 1992; Mastrota and Mench, 1995; Lindström et al., 1999) or has learned to avoid the warning signal through prior experience (Gittleman and Harvey, 1980; Roper and Wistow, 1986; Alatalo and Mappes, 1996; Ham et al., 2006; Green et al., 2018). Conversely, warning signals may act as a secondary defense if increased predator wariness improves the chance that prey will escape or reduces harm to prey after subjugation (Halpin et al., 2008; Ruxton et al., 2018) or if the warning signal is “switchable” and only becomes apparent after the predator has engaged with the prey (Blest, 1964; Sivinski, 1981; Grober, 1988; Broom et al., 2010; Umbers and Mappes, 2015; Kang et al., 2016; Umbers et al., 2017; Song and Jablonski, 2020). Often, visual or auditory warning signals are combined with chemical defenses, which deter predators through some combination of taste (Marples et al., 1994; Skelhorn and Rowe, 2006, 2010), smell (Rowe and Guilford, 1996, 1999; Lindström et al., 2001; Jetz et al., 2001; Kelly and Marples, 2004; Rojas et al., 2019), or toxicity (Cortesi and Cheney, 2010; Arenas et al., 2015). These chemical defenses are typically considered secondary defenses, which act to prevent consumption after subjugation has occurred or to dissuade predators from attacking such prey in the future (Ruxton et al., 2018). However, chemical defenses may also be detected before subjugation and influence the predator’s likelihood or latency to approach or attack the prey (Guilford et al., 1987; Rowe and Halpin, 2013; Rojas et al., 2017, 2019). Therefore, the dichotomy between primary and secondary defenses is not perfect, and it is possible for a single defense mechanism to protect prey across multiple stages of a predator’s attack.
Aposematism is formed by multimodal signaling (Rowe and Halpin, 2013). That is, it involves the use of signal components that are received through two or more sensory modalities by a single receiver (Stevens, 2013). Warning signals are usually conspicuous visual or auditory signals that are combined with some form of chemical defense (either sequentially or simultaneously), which predators perceive through certain smell or taste receptors. However, smell and taste reception are thought to have evolved largely to help animals avoid the inadvertent consumption of harmful, toxic food (Shi et al., 2003; Fischer et al., 2005; Chandrashekar et al., 2006; Reed and Knaapila, 2010). In this way, smell and taste can also be considered signals that warn predators of toxicity (Eisner and Grant, 1981; Weldon, 2013). However, as with Batesian mimics, which imitate visual aposematic signals, the information content of such chemical signals may not always be truthful, as not all chemicals that are perceived to have an unpleasant smell or taste are toxic (Ruxton and Kennedy, 2006; Nissim et al., 2017; Winters et al., 2018; Lawrence et al., 2019). In addition, there is evidence that defensive smell and bitter taste alone are not necessarily sufficient to prevent successful attack from predators (Eisner and Grant, 1981; Guilford et al., 1987; Moore et al., 1990; Rowe and Guilford, 1996; Kelly and Marples, 2004; Siddall and Marples, 2011), therefore the function of defense chemicals as honest signals of toxicity within aposematic systems requires further study (Holen, 2013).
Many chemically defended species use complex chemical mixtures that contain different types of chemicals, and utilizing multiple defensive compounds can be an adaptive strategy in a number of different ways. Chemical diversity may help prey defend themselves against multiple enemies, whereby different compound types are used to target different predators. For example, in A. plantaginis neck fluids defend against bird predators (but not invertebrates) and abdominal fluids defend against invertebrates (but not birds) (Rojas et al., 2017). It may also be more difficult for predators to evolve immunity to a suite of toxins compared to just one (Zhao et al., 2003). In addition, multiple defense compounds may be used as a multimodal signal if a single predator uses both smell (of volatile compounds) and taste (of non-volatile, bitter compounds) to assess the toxicity of chemically defended prey (Marples et al., 1994). Smell and taste may also act at different stages of attack. Smell can be used to detect volatile odorants from a distance, potentially allowing predators to perceive chemical defenses before prey capture (Rowe and Halpin, 2013; Rojas et al., 2017, 2019). Whereas non-volatile compounds require predators to first capture prey before the chemical defense can be perceived via taste receptors. Despite this, the effect of smell and taste is rarely differentiated in studies of multimodal aposematic displays, but see Marples et al. (1994), and therefore it remains unclear how interactions between smell and taste fit within the theoretical framework of multimodal aposematic signals (Rowe and Halpin, 2013). Thus, measuring the individual and combined effects of smell, taste, and warning coloration is essential for understanding the evolution and maintenance of both chemical diversity and multimodal warning signals.
Here, we investigate a multi-modal aposematic defense (visual warning signal, smell, taste) in the polymorphic wood tiger moth Arctia plantaginis to blue tit (Cyanistes caeruleus) predators. In a recent study, blue tits used the potent smell of methoxypyrazines as context-setting signals for the aposematic colors of A. plantaginis (Rojas et al., 2019). Birds only delayed attack in response to the white model color when the methoxypyrazine smell was present. This finding differed from previous studies that found the yellow morph to be better protected when live moths were offered to blue tits in the lab (Nokelainen et al., 2012), when dead moths were placed in the field (Nokelainen et al., 2012) and when moth models (i.e., dummies) were placed in the field (Nokelainen et al., 2014). One explanation for the difference between the response of blue tits to yellow and white morphs under laboratory conditions could be the use of models (Rojas et al., 2019) rather than live prey (Nokelainen et al., 2012). To address this issue, we use live moths in this study. In addition, Rojas et al. (2019) found that chemically treated models differed to controls in terms of proportion of moth’s body eaten and beak wiping behavior (a common disgust response), suggesting the presence of both an aversive smell and taste in this species and highlighting the need to disentangle these two modalities from the defense fluid (Rojas et al., 2019). A. plantaginis also have the ability to sequester pyrrolizidine alkaloids, which they distribute to all tissues including neck fluids (Anne Winters unpublished data), which might explain the aversive taste. Pyrrolizidine alkaloids are well documented to defend against invertebrate predators (Brown, 1984; Dussourd et al., 1988; Masters, 1990; Eisner and Eisner, 1991; Conner et al., 2000; Eisner et al., 2000). However, evidence for their defense against vertebrates is less robust (Ritland, 1991; Rowell-Rahier et al., 1995; Yosef et al., 1996; Cardoso, 1997). Therefore, while lepidopterans that sequester pyrrolizidine alkaloids widely exhibit conspicuous coloration (Nishida, 2002), further evidence is needed to support the role of pyrrolizidine alkaloids in aposematic defenses against vertebrates (Nishida, 2002). In the present study, live moths of each color morph that were manipulated to have only the smell (methoxypyrazines), only the bitter taste (pyrrolizine alkaloids) or with both present, were offered to birds to test whether color, smell and taste constitute a multimodal warning signal in A. plantaginis. We test whether combined modalities improve discrimination of toxic prey by predators and enhance aversion learning. We also investigate at which stage of attack (approach, attack, subjugation, consumption) each defense modality is effective at influencing predator behavior and whether multimodality improves prey survival.
Materials and Methods
Study Species A. plantaginis
The aposematic wood tiger moth (Arctia plantaginis, formerly Parasemia plantaginis) is a member of the Erebidae family (Rönkä et al., 2016) and widely distributed across the Northern hemisphere (Hegna et al., 2015). There is geographical variation in warning coloration (Hegna et al., 2015). In Europe, male hindwings are either yellow or white and female hindwings vary continuously from yellow to red (Lindstedt et al., 2011; Nokelainen et al., 2012; Hegna et al., 2015). Hindwings are often exposed at rest in this species, particularly if moths are alerted, preparing to fly, or if the weather is cool. The discrete variation in male hindwing coloration follows a one-locus two allele model, where the yellow allele (y) is recessive to the white (W), resulting in three genotypes (WW, Wy, yy). Both homozygous white (WW) and heterozygous (Wy) genotypes have white hindwing coloration, while the homozygous recessive genotype (yy) has yellow (Suomalainen, 1938; Nokelainen et al., in prep a). Therea are also differences between genotypes in the white hue of the forewings, which is perceptible to birds (Nokelainen et al., in prep a). The color polymorphism is under selection by bird predators in the wild (Rönkä et al., 2020). In predation experiments, birds respond differently toward the hindwing morphs, avoiding either yellow (Nokelainen et al., 2012, 2014) or white (Rojas et al., 2019), but see Rönkä et al. (2018). Rojas et al. (2019) speculate that the variable response by predators could be due to differences in cues between the moths and their model stimuli, differences in light environment between experiments (Nokelainen in prep b), or the presence or absence of methoxypyrazine odor.
Arctia plantaginis is chemically defended, with two uniqe defense secretions that target different predators (Rojas et al., 2017). One secretion is released between the head and thorax when the thorax is grabbed or pinched and deters birds (neck fluid), and a second secretion is released from the abdomen when the moth is disturbed and deters ants (abdominal fluid) (Rojas et al., 2017). Two main methoxypyrazine compounds are released from the neck fluids: 2-sec-butyl-3-methoxypyrazine (SBMP) and 2-isobutyl-3-methoxypyrazine (IBMP) (Burdfield-Steel et al., 2018). These are produced de novo by the moth when raised on an artificial diet (Burdfield-Steel et al., 2018). These methoxypyrazines emit a potent odor that is aversive to blue tit predators, causing delayed attack, increasing disgust behaviors such as beak wiping, and reducing the amount or likelihood of consumption (Rojas et al., 2017, 2019). In addition, A. plantaginis is efficient at sequestering pyrrolizidine alkaloids from their diet (Table 1). These alkaloids are present in wild-caught moths, and distributed to all body parts of the moths including both neck and abdominal defense fluids of moths (Anne Winters, unpublished data). The efficacy of pyrrolizidine alkaloids sequestered by A. plantaginis in defense against predation has not yet been tested.

Table 1. Quantification of pyrrolizidine alkaloids (PA) seneciphylline and senecionine in the larvae, food, and feces of A. plantaginis raised on an artificial diet with 10% freeze-dried Senecio vulgaris.
Manipulation of Color, Smell, and Taste of A. plantaginis
The wood tiger moth (Arctia plantaginis) is well suited to examine the role of color, smell and taste in the multimodal aposematic display of a live insect because each of these three components can be independently manipulated (Figure 1). Importantly, the use of live prey accounts for the actual nutritional value of prey (Halpin et al., 2014) and natural delivery mechanism(s) of the chemical defense secretions (Eisner and Meinwald, 1966), both of which improve the ecological significance of results compared experiments using models as stimuli (Rowe and Halpin, 2013).
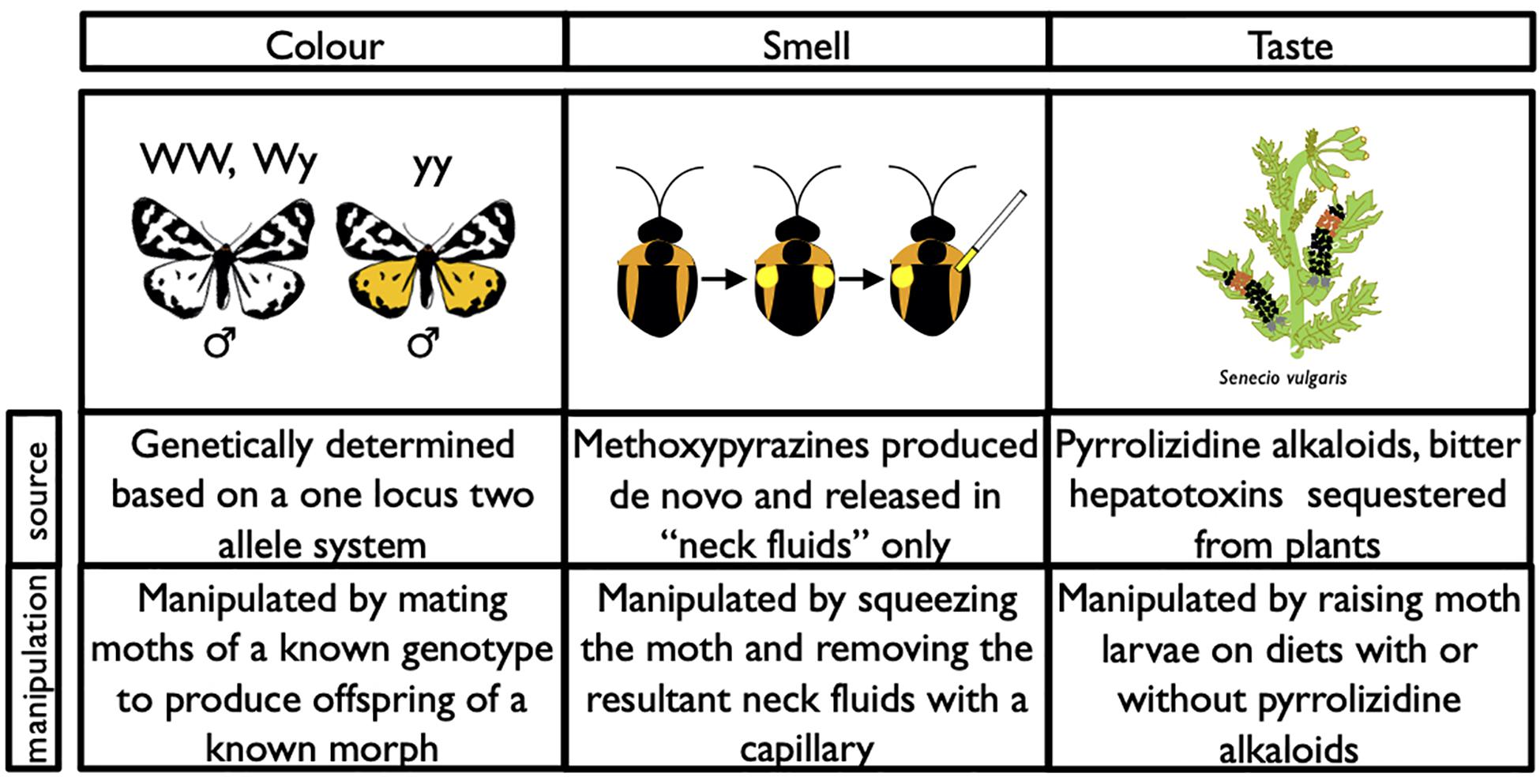
Figure 1. Illustration of the source for each component (color, smell, and taste) in the multimodal warning signal of A. plantaginis and brief description of the method used to manipulate that component in live moths for this experiment.
Color Manipulation
To control the color morph of male A. plantaginis used in this experiment, moth families were purpose bred from 3rd generation 2019 lab stock of known (color morph) genotype at the University of Jyväskylä. Moths were paired to produce offspring of WW, Wy, and yy genotypes and mate pairings were staggered so that adults would continuously emerge from November-March, providing a sufficient time period to conduct the behavioral experiment.
Taste Manipulation
To control the pyrrolizidine alkaloid “taste” of the moth, each clutch was then split between two artificial diet treatments: a control diet with no dietary source of pyrrolizidine alkaloids (4.6 agar, 8.58 g yeast, 32.1 g semolina, 8.3 g wheat germ, 150 ml boiling water, 1.76 g Vanderzant vitamin mix, 1800 μl nipagen and 180 μl acetic acid) and an artificial diet with 10% freeze-dried Senecio vulgaris, as a dietary source of pyrrolizidine alkaloids (4.6 g agar, 8.15 g yeast, 30.5 g semolina, 7.89 g wheat germ, 150 ml boiling water, 2.5 g freeze-dried Senecio vulgaris, 1.76 g Vanderzant vitamin mix, 1800 μl nipagen and 180 μl acetic acid). Larvae of each family/diet treatment were housed together in plastic containers until pupation and fed daily with fresh food spooned onto small squares of baking paper. To confirm the sequestration of pyrrolizidine alkaloids from the second diet, six larvae were selected and subject to chemical analysis along with 2 samples of their diet treatment, food and feces. Briefly, the samples were first freeze-dried and then weighed to the nearest 0.1 mg. Samples were then homogenized, extracted and processed through LC-MS/MS following the protocol outlined in Reinwaldt et al. (2017). Seneciphylline and Senecionine were identified as major compounds and quantified using a stock solution of standards (2 mg each of Monocrotaline, Monocrotaline N-oxide, Jacobine, Jacobine N-oxide, Intermedine, Intermedine N-oxide, Retrorsine, Seneciphylline, Seneciphylline N-oxide, Senecionine, Senecionine N-oxide, and Senkirkine, in 20 ml of 5% methanol solution). A. plantaginis efficiently sequestered pyrrolizidine alkaloids. Both major compounds identified were accumulated, rather than excreted by the larvae, resulting in a greater concentration of pyrrolizidine alkaloid in the moth compared to their dietary source. Seneciphylline was 10× as concentrated in the larvae compared to their food, while only trace amounts were excreted in the feces. Senecionine was 3× as concentrated in the larvae compared to their food, while only trace amounts were excreted in the feces (Table 1).
Smell Manipulation
After pupation, individuals were placed singly in vials with a sponge cap, which was sprayed daily with water to prevent desiccation until they eclosed. After the moth eclosed, it was stored in a refrigerator at ∼4°C until use in the experiment (12 days ± 0.5 SE). To manipulate the methoxypyrazine “smell” of the moth, neck fluids were removed from a subset of the emerging adults by squeezing the thorax between the fingers and collecting the resultant fluid using a microcapillary. Moths were squeezed the day before they were used in the experiment so that the majority of the methoxypyrazine smell could be released and dissipated and then again 15 min before the experiment on the day of the trial (see below for further details), to remove any remaining methoxypyrazines. The moth was removed from the refrigerator 30 min before each sampling and the sponge cap was sprayed with water to allow the moth to warm up and hydrate for 30 min. Moths that retained their neck fluids for the experiment underwent the same protocol except the neck fluids were not collected. Neck fluids were sampled in a separate room with closed doors so that the odor was not pervasive in the bird housing or experimental enclosures.
From these manipulations, 251 adult male moths were spread between 12 treatment groups with at least n = 9 moths per treatment (Table 2). Treatments with moths of the yellow morph have lower sample size for two reasons: (1) Moths of the yellow morph have poor fecundity compared to other genotypes (Nokelainen et al., 2012; Gordon et al., 2018); poor mating success and small clutch sizes resulted in fewer offspring with yellow hindwings. (2) The experiment was ended prematurely due to COVID-19, and the recently eclosed and yet to eclose yellow males that could have increased the sample size had to be discarded.
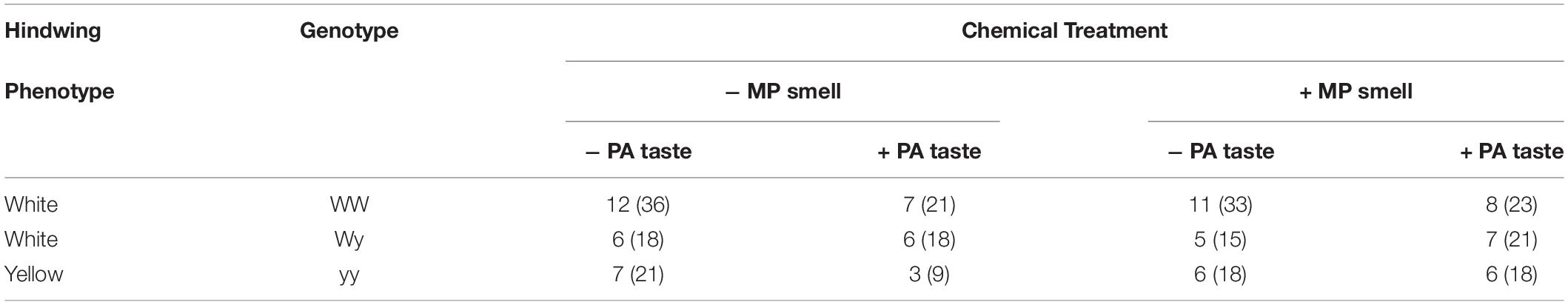
Table 2. Number of birds (and moths) in each of treatment group including those of each hindwing phenotype: white and yellow, genotype: WW, Wy, or yy, those with (+) and without (−) methoxypyrazine smell (MP), and those with (+) and without (−) pyrrolizidine alkaloid taste (PA).
C. caeruleus Capture and Husbandry
In total, 84 wild blue tits (C. caeruleus) were trapped from a feeding station at Konnevesi Research Station in Central Finland between November 2019 and March 2020. Birds were weighed on the day of capture and then individually housed in plywood enclosures (65 cm × 50 cm × 80 cm) on a 11 h : 13 h (light : dark) cycle for at least one day (8 days ± 0.5 SE) before the experiment started so that they acclimatized to captive conditions. During this time, birds had ad libitum access to sunflower seeds, peanuts, a vitamin enriched food supplement and water. After the experiment, birds were ringed for identification purposes, aged and sexed according to established methods published in: “Svensson (1992) Identification Guide to European Passerines ISBN: 9789163011184 Publisher: British Trust for Ornithology,” and then released at their site of capture. Birds were captured and housed with permission of Central Finland Centre for Economic Development, Transport and Environment (VARELY/294/2015) and a license from the National Animal Experiment Board (ESAVI/9114/04.10.07/2014).
Behavioral Experiment
Birds were transported to a separate experimental room and placed inside masonite enclosures (50 cm × 50 cm × 70 cm), which were equipped with a perch and water bowl and lit with an Exo Terra Repti Glo 25 W 5.0 UVB compact light bulb (see Waldron et al., 2017Supplementary Material for irradiance measurements). Spectral reflectance measurements of the masonite background along with the forewings and hindwings for each genotype are included in the Supplementary Material (Supplementary Figure 1). Birds were observed through a mesh-covered opening at the front of the cage and by a video camera (Sony DSC-HX1) at the top of the cage (Figure 2). During an acclimation/training period, birds were offered two sunflower seeds through a hatch behind a visual barrier, which was used to accurately measure when the moth was seen, approached, and attacked. The first sunflower seed was offered immediately after the bird was placed in the cage. After 1 h, if the bird ate the first sunflower seed, it was then offered a second sunflower seed and monitored every 15 min until the second seed was eaten. If after 1 h the bird had not eaten the first seed, it was monitored every 15 min and offered the second seed only after the first was eaten. To ensure the birds were sufficiently hungry, the behavioral experiment was initiated 1 h after the bird ate the second sunflower seed. To measure predator avoidance learning across trials, each bird was presented with 3 moths (one moth per day for three consecutive days) from one of the treatment groups (Table 2).
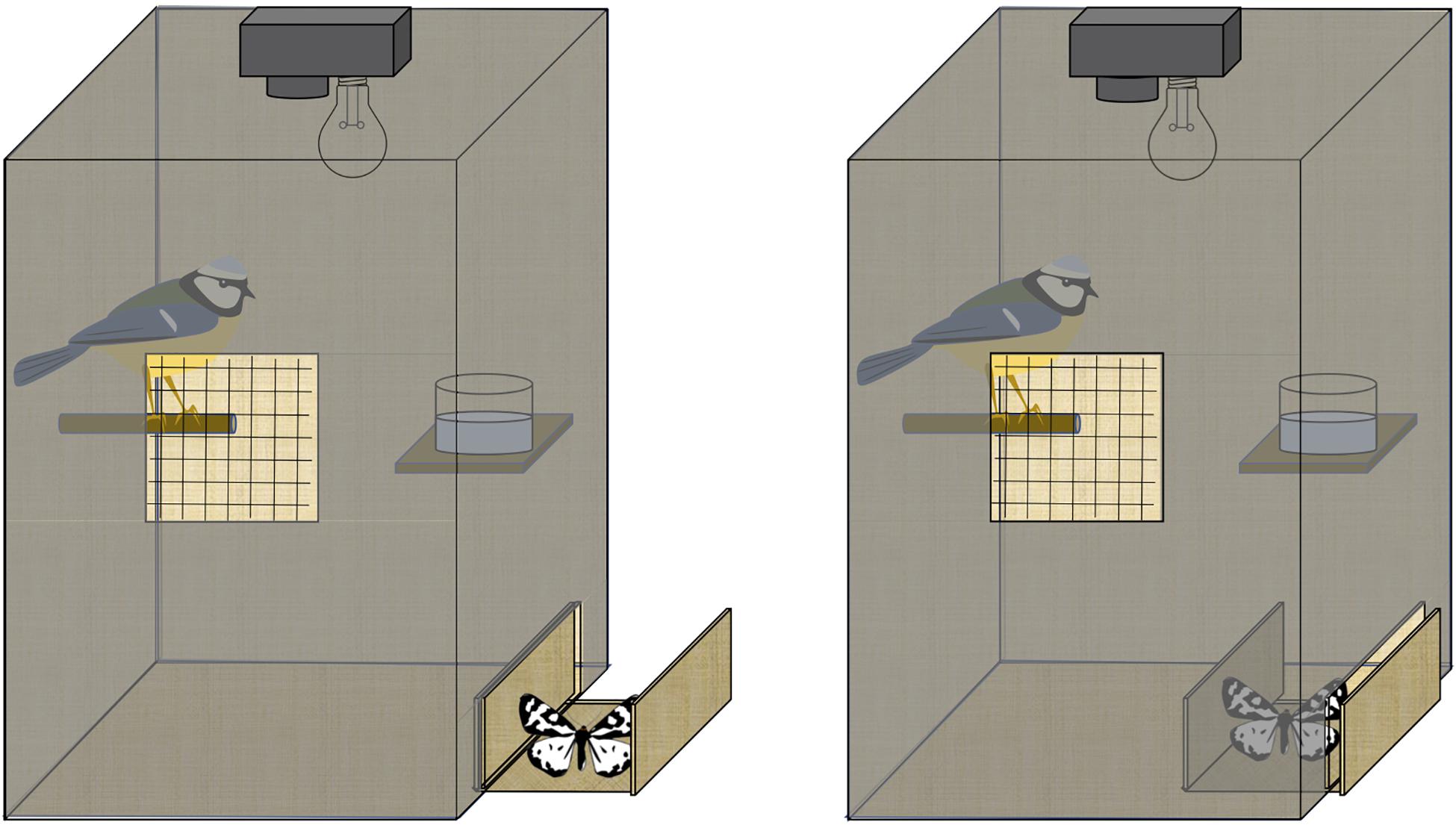
Figure 2. Experimental setup of the behavioral experiment demonstrating the placement of perch, water, camera, light source, mesh opening for observation, and hatch for inserting moths into the enclosure.
During the experiment, the observing room was kept dark and silent to reduce the effect of the researcher on bird behavior. Moths were held with forceps by the forewing and then placed into the experimental enclosure using the same hatch used for the sunflower seeds (Figure 2). We observed moths to hold their wings slightly open, partially exposing their polymorphic hindwings, which is a common natural resting position for the moth. After the bird saw the moth, it had 15 min to attack, if it did not attack, the experiment ended. After the bird attacked the moth, the experiment ended when the bird showed no further interest in any part of the moth for one full minute. During the assay, birds and moths were observed by two authors (JL and AEW) and data was recorded to measure the following 16 variables (Supplementary Table 1): (1) approach probability measured whether or not the bird approached the moth (yes/no, for all moths) (2) approach latency was measured as the time (in seconds) from seeing the moth (tilts head to look down at it) to approaching it (landing beside the moth, usually on the movable platform the moth was placed unless the moth moved) (3) attack probability measured whether or not the bird attacked the moth (yes/no, for moths that were approached) (4) attack latency measured the time (in seconds) from approaching the moth to grabbing it (5) drop probability measured whether or not the bird dropped the moth at least once before eating it (yes/no, for moths that were attacked) (6) prey drop latency measured the time (in seconds) from grabbing the moth to dropping it for the first time for birds that ate <50% of the moth (7) prey dropping counted the number of times the bird dropped the moth before beginning to eat it (8) handing duration was a sum of the time (in seconds) the bird spent holding the moth (grabbing the moth until dropping the moth). Includes each occurrence the moth was held and includes eating duration (9) eating probability measured whether or not the bird ate at least part of the moth (yes/no, for moths that were attacked) (10) eating duration was a sum of the time (in seconds) the bird spent eating the moth (started eating the moth until stopped eating the moth). Includes each occurrence the moth was eaten. (11) proportion eaten was calculated by adding together the proportion of each of six body parts eaten (antennae, head, thorax, abdomen, legs, wings), as estimated by eye, and dividing by six to calculate the total proportion of the moth that was eaten by the bird (12) kill latency was measured as the time (in seconds) from seeing the moth to killing it (usually by eating or removing the head) (13) beak wiping, which is a common disgust behavior (Evans and Waldbauer, 1982; Skelhorn and Rowe, 2009; Rowland et al., 2015; Rojas et al., 2017, 2019) was measured as the number of bouts of beak wiping the bird performed after grabbing the moth until the end of the trial, (14) water drinking, which may increase after the bird has consumed something distasteful (Burdfield-Steel et al., 2019), was measured as the number of “sips” taken from the water bowl after grabbing the moth until the end of the trial. In addition, (15) moth activity, sum of the time (in seconds) the moth spent crawling, flying, or flexing which includes each occurrence the moth was active, and (16) moth survival (yes/no, for all moths) were also recorded. These behaviors were first recorded on datasheets during the experiment using a stopwatch (to nearest second) and then confirmed by JL watching the video afterward. If there was a discrepancy between the video and the original observation in terms of the timing or counts of a behavior, the video observation was used because these behaviors could be measured more accurately using the video. However, kill latency was always measured using the original observation because it is difficult to ascertain the time of death from the video. Birds remained under observation for 30 min following the experiment to monitor for ill effects from moth consumption, but none were observed. After the observation period, birds were offered 8 g of meal worms. The weight of mealworms eaten within 10 min was used as a measure of the bird’s hunger level (Stevens et al., 2010). If the bird did not eat the moth or the mealworms (2 individuals), it was excluded from the experiment.
Statistical Analysis
All analyses were conducted using R version 4.0.3 (R Core Team, 2011). All models include the fixed effects of moth morph (white, yellow), methoxypyrazine smell (present, absent), pyrrolizidine alkaloid taste (present, absent), and trial number (1, 2, 3). We then used a forward stepwise selection process to include interactions and co-variates based on AICc and a threshold of Δ 2. As there are a large number of interactions in our multimodality study to consider that may have potential to be biologically meaningful, we used the dredge function in the MuMin Package (Barton and Barton, 2015) for this step. In all cases the simplest model within Δ 2 of the top model was selected. Then, we compared models using genotype (WW, Wy, yy) or color morph (w, y). If genotype improved the AICc score of the model by greater than Δ 2, moth genotype was used instead. Finally, after selecting interactions and morph or genotype, additional relevant co-variates were selected to be included in the model if they improved the AICc score of the model by greater than Δ 2. These co-variates that have potential to influence the predation sequence include: moth activity which can influence the bird’s required effort, hunger level which can influence the bird’s motivation, bird age which may relate to experience, bird sex where physiological differences may influence behavior and motivation, and bird weight which may relate to body condition and motivation. In all models, except for moth survival probability, bird ID was included as a random factor to account for multiple trials per bird. Model assumptions were checked and distributions were chosen accordingly. Follow-up analyses were conducted to determine which treatments differed from the control. Tables detailing model selection (Supplementary Table 2) and model summaries (Supplementary Table 3) are provided in the Supplementary Material.
First, we tested whether the probability that blue tits would progress through the predation sequence (binomial response variables = approach probability, attack probability, drop probability, eating probability, or moth survival) differed among treatments. To do this, we used generalized linear mixed-effects models (GLMM) with binomial distributions using the package lme4 (Bates et al., 2015). Bird weight improved the AICc score for the models of drop probability, eating probability, and moth survival by more than Δ 2, so it was included as a co-variate in those models (Supplementary Table 2).
Next, we tested whether timed bird behaviors (approach and attack latencies, eating and handling durations, drop latency, and kill latency) differ among treatments to using cox proportional hazards models (Therneau and Therneau, 2015). Moth genotype improved the AICc score for the models of approach latency, attack latency, drop latency, and eating duration by more than Δ 2 AICc, so moth genotype was used instead of moth morph for these models (Supplementary Table 2). For the model of attack latency, the interaction between moth genotype and pyrrolizidine alkaloid taste improved the AICc score by more than Δ 2, and for kill latency the interaction between methoxypyrazine smell and pyrrolizidine alkaloid taste improved the AICc score by more than Δ 2, so these interactions were included in the models (Supplementary Table 2). Based on AICc comparison, bird age was selected to be included as a co-variate in the model of attack latency, and bird weight was included in the models of drop latency, handling duration, and eating duration, while bird hunger level was included in the model of kill latency (Supplementary Table 2).
Then, we tested whether counts of bird disgust behaviors after attacking the moth (prey dropping, beak wiping, water drinking) differed among our treatments using GLMM with poisson distributions (except for beak wiping). The sum of squared Pearson residuals indicated that the model for beak wiping behavior was overdispersed, so a negative binomial distribution was used instead. For the model of water drinking, the interaction between methoxypyrazine smell and trial number improved the AICc score by more than Δ 2, so it was included in the model (Supplementary Table 2). Bird weight improved the AICc score for the model of prey dropping by more than Δ 2, so it was included as a co-variate in that model (Supplementary Table 2).
Finally, we tested whether the proportion eaten differed among treatments. Model residuals were normally distributed, therefore we used a linear mixed effects model with a Gaussian distribution. Bird weight improved the AICc score by more than Δ 2, so it was included as a co-variate in the model (Supplementary Table 2).
Results
Approach
Birds approached the moths in each of the 251 trials (except for one case, trial 2, Wy, white morph, both chemical defenses). Independent of neck fluids, approach latency was longer for moths of the WW genotype, but not the Wy genotype, compared to moths of the yy genotype (estimate ± SE = −0.6958 ± 0.2143, z = −3.25, p = 0.001; Figure 3A and Supplementary Table 3). However, when neck fluids with methoxypyrazine smell were present, birds approached both white morph genotypes more slowly compared to yy moths (WW estimate ± SE = −0.8214 ± 0.2998, z = −2.4, p = 0.006; Wy estimate ± SE = −0.6445 ± 0.3324, z = −1.94, p = 0.052, Figure 3A and Supplementary Table 3) suggesting that the methoxypyrazine smell of heterozygote moths is especially important at this stage of attack. Approach latency was longer for juvenile birds compared to adults (estimate ± SE = −0.4321 ± 0.2054, z = −2.10, p = 0.035; Supplementary Figure 2A and Supplementary Table 3). Overall, approach latency decreased as the trials progressed (estimate ± SE = 0.2899 ± 0.0873, z = 3.32, p = 0.001; Supplementary Figure 3A and Supplementary Table 3). Approach latency was not affected by the presence of neck fluids or pyrrolizidine alkaloids alone (Supplementary Table 3).
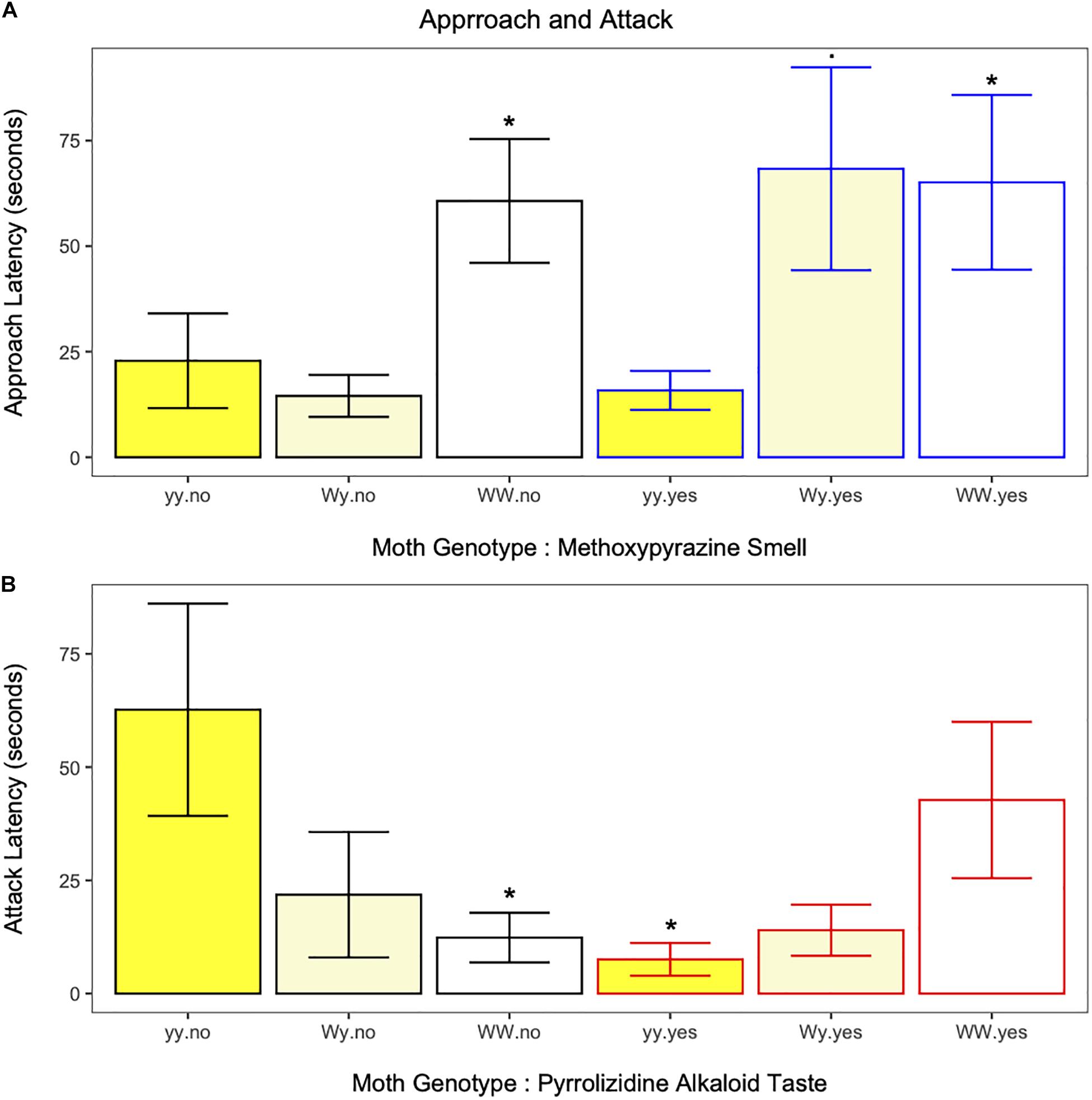
Figure 3. (A) Approach latency in response to each moth genotype with (blue outline) and without (black outline) methoxypyrazine smell. Latency to approach moths of the Wy genotype (white hindwings) morph was dependent on the presence of methoxypyrazine smell. Whereas, birds hesitated longer to approach moths of the WW genotype (white hindwings) compared to moths of the yy genotype (yellow hindwings) irrespective of methoxypyrazine smell. *Indicates significant differences (. = trend) from yy moths without methoxypyrazine smell. (B) Attack latency in response to each moth genotype with (red outline) and without (black outline) pyrrolizidine alkaloid taste. Latency to attack moths of the yy genotype (yellow hindwings) was lower for moths on the pyrrolizidine alkaloid diet, while latency to attack moths of the WW genotype (white hindwings) was higher for moths on the pyrrolizidine alkaloid diet. Pyrrolizidine alkaloid diet did not impact attack latency for moths of the Wy genotype (white hindwings) *Indicates significant differences from yy moths without pyrrolizidine alkaloid taste.
Attack
Birds attacked the moths in 94% of the trials (236 out of 251), with a trend for attack probability to increase with trial number (estimate ± SE = 0.8207 ± 0.4833, z = 1.698, p = 0.089, Supplementary Figure 3B and Supplementary Table 3). There was no effect of moth morph, methoxypyrazine smell, or pyrrolizidine alkaloid taste on attack probability (Supplementary Table 3).
The interaction between moth genotype and pyrrolizidine alkaloid taste influenced bird attack latency (estimate ± SE = −1.1902 ± 0.3563, z = −3.34, p = 0.001, Figure 3B and Supplementary Table 3). The pyrrolizidine alkaloid diet increased bird attack latency for moths of the WW genotype, but decreased bird attack latency for moths of the yy genotype (Figure 3B), suggesting the diet treatments affect the primary anti-predator defenses of genotypes in different ways. Bird attack latency decreased as the trials progressed (estimate ± SE = 0.3244986 ± 0.08131366, z = 3.99, p < 0.001, Supplementary Figure 3C and Supplementary Table 3). Attack latency was not affected by methoxypyrazine smell.
Subjugation
Following attack, birds dropped the moth at least once in 28% of the trials (65 out of 236), and independent of moth morph, bird drop probability was higher for moths that had neck fluids than those that did not (estimate ± SE = 1.2688 ± 0.5532, z = 2.294, p = 0.0218; Figure 4A and Supplementary Table 3). However, when investigated separately, it was only white moths with methoxypyrazine smell that significantly differed from yellow moths with none (estimate ± SE = 2.4375 ± 1.0445, z = 2.334, p = 0.0196; Figure 4A and Supplementary Table 3). Drop probability was positively associated with bird weight, heavier birds were more likely to drop the moth (estimate ± SE = 1.4527 ± 0.4463, z = 3.255, p = 0.0011, Supplementary Figure 2B and Supplementary Table 3). Independently, there was no effect of moth morph, pyrrolizidine alkaloid taste, or trial number on drop probability (Supplementary Table 3).
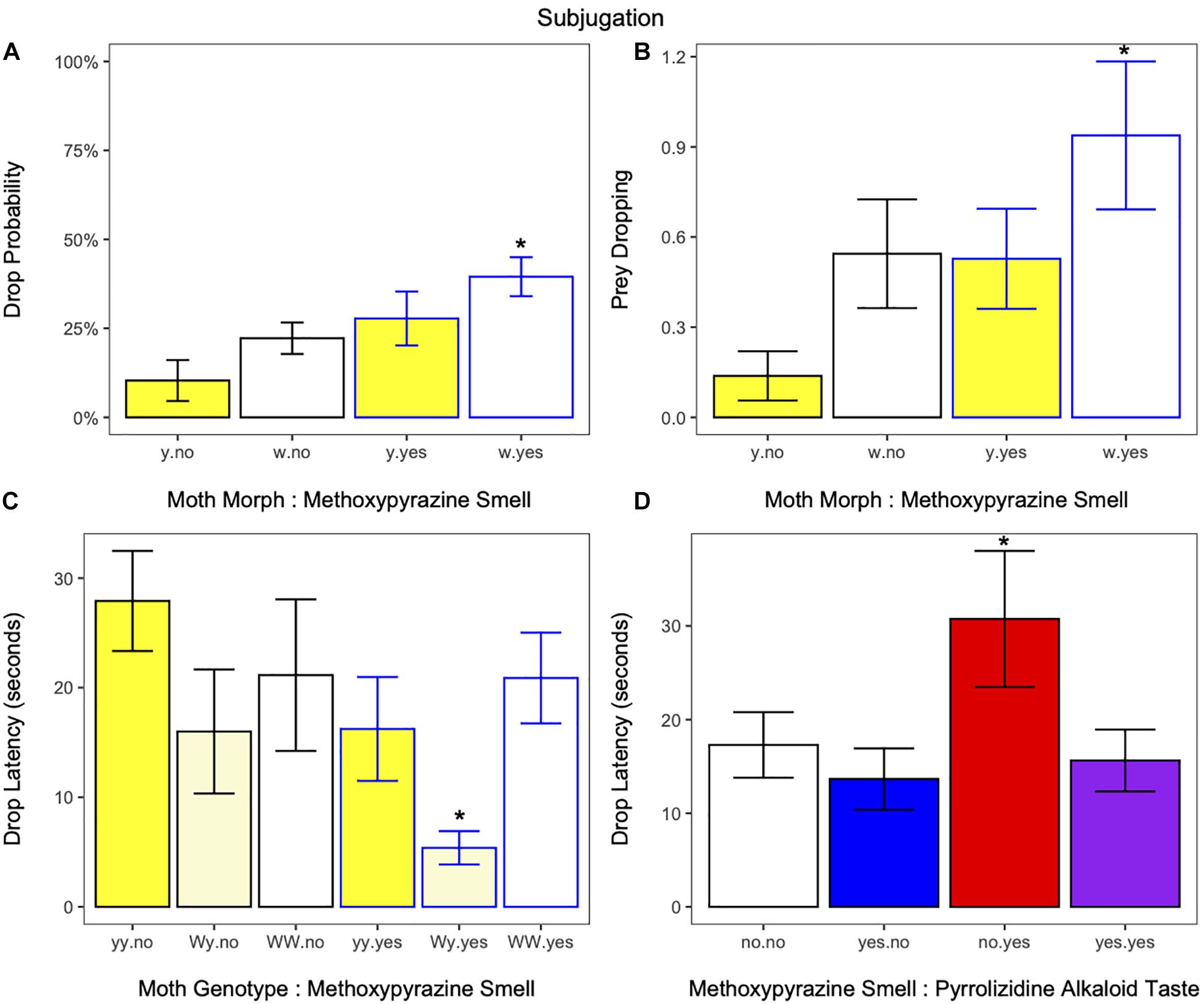
Figure 4. (A) % of moths that were dropped at least once after being grabbed in response to moth morph (yellow or white) and methoxypyrazine smell. Moths with white hindwings and methoxypyrazine smell were more likely to be dropped and with (blue outline) or without (black outline) methoxypyrazine smell. Panel (B) were also dropped a greater number of times compared to yellow moths without methoxypyrazine smell. *Indicates significant differences from yellow moths without methoxypyrazine smell. (C) Drop latency (time between grabbing and dropping the moth for the first time for birds that ate<% 50 of the moth) was quicker for moths of the Wy genotype with methoxypyrazine smell compared to yellow moths without (*indicates significant differences from moths of the yy genotype without methoxypyrazine smell), suggesting a more potent defense upon contact and (D) was slower for moths that were raised on a pyrrolizidine alkaloid diet compared to moths with no chemical defenses, suggesting toxin sequestration is costly to other (primary) defenses (*indicates significant difference from moths without methoxypyrazine smell or pyrrolizidine alkaloid taste). Bar graphs show mean ± SE [or percent (Dropped = yes)].
In addition, prey dropping behavior (number of times the bird dropped the moth before eating it) increased if the moth had methoxypyrazine smell (estimate ± SE = 0.9679, ± 0.4286, z = 2.258, p = 0.0239, Figure 4B and Supplementary Table 3) and heavier birds exhibited more prey dropping behavior (estimate ± SE = 0.8179 ± 0.3261, z = 2.508, p = 0.0121, Supplementary Figure 2D). However, again it seems likely this effect is driven by moths of the white morph, as this was the only treatment to independently differ from yellow moths without methoxypyrazine smell (estimate ± SE = 1.9527 ± 0.8343, z = 2.341, p = 0.0193, Figure 4B and Supplementary Table 3) in a separate analysis. Independently, there was no effect of moth morph, pyrrolizidine alkaloid taste, or trial number on prey dropping behavior.
Of the birds that ate less than half of the moth, birds had a shorter drop latency for moths with methoxypyrazine smell compared those without (estimate ± SE = 0.6173 ± 0.2360, z = 2.62, p = 0.0089, Figure 4C and Supplementary Table 3), and moths of the Wy genotype compared to those of the WW (estimate ± SE = 0.9142 ± 0.2886, z = 3.17, p = 0.0015) or yy (estimate ± SE = 0.7022 ± 0.3040, z = 2.31, p = 0.0210, Supplementary Table 3) genotypes, with a shorter period of time between grabbing and abandoning Wy moths (Figure 4C). This effect is likely being driven by the drop latency for Wy moths with methoxypyrazine smell, which was the only treatment to significantly differ from yellow moths without methoxypyrazine smell (estimate ± SE = 1.5433, 0.4460, z = 3.46, p = 0.0005, Figure 4C and Supplementary Table 3). Surprisingly, drop latency was quicker for moths raised on the control diet compared to those raised on a diet with pyrrolizidine alkaloids (estimate ± SE = −0.5087 ± 0.2288, z = −2.2, p = 0.0260, Figure 4D and Supplementary Table 3), which is likely being driven by drop latency for moths with pyrrolizidine alkaloids but without methoxypyrazine smell, which was the only treatment to significantly differ from moths with no chemical defenses (estimate ± SE = −0.8317 ± 0.3934, z = −2.11, p = 0.0350), suggesting a trade-off between pyrrolizidine alkaloid sequestration and synthesis of methoxypyrazine reserves. Heavier birds had quicker drop latency (estimate ± SE = 0.6337 ± 0.1748, z = 3.63, p = 0.0003, Supplementary Figure 2C). There was no effect of trial number on drop latency (Supplementary Table 3).
Handling duration decreased as the trials progressed (estimate ± SE = 0.6538 ± 0.0978, z = 6.68, p < 0.001; Supplementary Figure 3D and Supplementary Table 3). Heavier birds had a shorter handling duration (estimate ± SE = 0.62076875 ± 0.20082539, z = 3.09, p = 0.002, Supplementary Figure 2E and Supplementary Table 3). Moth morph, methoxypyrazine smell, and pyrrolizidine alkaloid taste did not influence handling duration (Supplementary Table 3).
Consumption
Birds ate at least part of the moth in 81% of the trials (48 out of 251), and were less likely to eat moths that had methoxypyrazine smell than those that did not (estimate ± SE = −2.2490 ± 0.9158, z = −2.456, p = 0.0141; Figure 5A and Supplementary Table 3). There was no effect of moth morph, pyrrolizidine alkaloid taste, or trial number on eating probability (Supplementary Table 3).
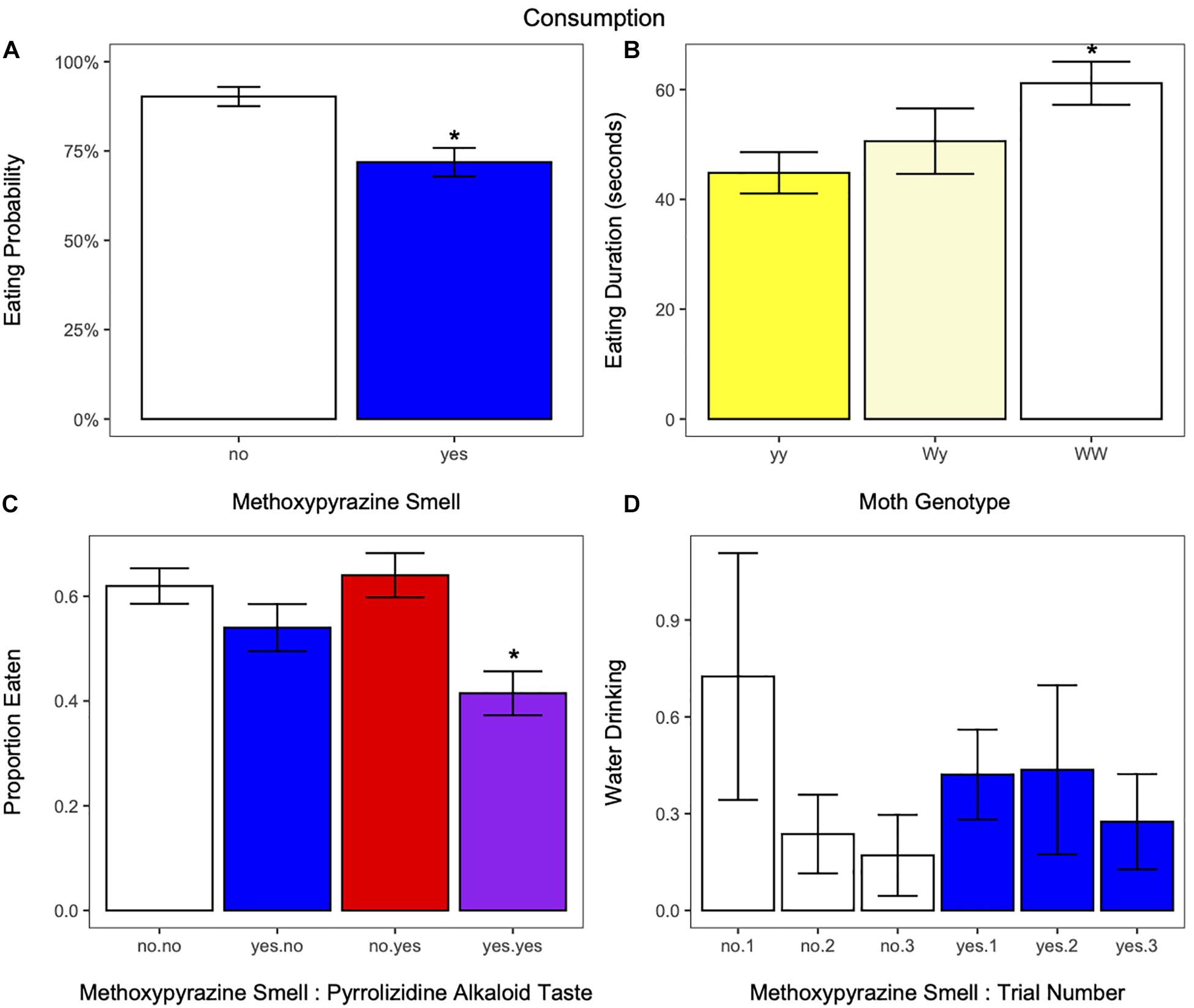
Figure 5. (A) The probability for birds to eat at least part of the moth in response to the presence (blue) or absence (white) of methoxypyrazine smell. Birds were more likely to eat moths without methoxypyrazine smell. (B) The amount of time birds spent eating moths of each genotype. Birds ate moths of the WW (white hindwings) genotype more slowly compared to moths of the yy genotype (yellow hindwings). *Indicates significant differences from the yy genotype. (C) The proportion of the moth’s body eaten in response to methoxypyrazine smell and pyrrolizidine alkaloid taste. The proportion eaten was smaller if the moth had both types of chemical defense. *Indicates significant difference from moths without methoxypyrazine smell or pyrrolizidine alkaloid taste. (D) The number of times birds took a drink of water in response to trial number and methoxypyrazine smell. Birds reduced their water drinking as trials progressed to a greater degree for moths that did not have methoxypyrazine smell compared to those that did, suggesting that moths with methoxypyrazine smell maintain aversion across trials. Bar graphs show mean ± SE [or percent (Eaten = yes)].
There was a significant effect of genotype on eating duration, where birds took longer to eat moths of the WW genotype compared to the yy genotype (estimate ± SE = −0.6592 ± 0.2532, z = −2.60, p = 0.0092, Figure 5B and Supplementary Table 3). Eating duration decreased with trial number (estimate ± SE = 0.44751035 ± 0.1026699, z = 4.36, p < 0.001, Supplementary Figure 3E), and heaver birds had a shorter eating duration (estimate ± SE = 0.46967725 ± 0.1540265, z = 3.05, p = 0.0023, Supplementary Figure 2G). Neither methoxypyrazine smell nor pyrrolizidine alkaloid taste affected eating duration (Supplementary Table 3).
In addition, the proportion eaten decreased if the moth had methoxypyrazine smell compared to those without [t(79) = −2.405621, p = 0.0185; Figure 5C and Supplementary Table 3]. However, it seems likely this effect is driven by the treatment where moths that have both methoxypyrazine smell and pyrrolizidine alkaloid taste in their neck fluids. When analyzed separately, the proportion eaten was smaller from moths with both defenses compared to moths with no defenses [t(78) = −2.2654, p = 0.0263; Figure 5C and Supplementary Table 3], but the proportion eaten did not differ between moths with only methoxypyrazine smell and those with no chemical defenses [t(78) = −1.2821, p = 0.2036; Figure 5C and Supplementary Table 3]. The proportion eaten increased with trial number [t(151) = 2.0533, p = 0.0418]; Supplementary Figure 3F and decreased with bird weight [t(78) = −3.6513, p = 0.0005; Supplementary Figure 2H and Supplementary Table 3]. There was no effect of pyrrolizidine alkaloid taste or moth morph on the proportion eaten (Supplementary Table 3).
Disgust
Beak wiping behavior decreased with trial number (estimate ± SE = −0.7963 ± 0.1089, z = −7.311, p < 0.001; Supplementary Figure 3G and Supplementary Table 3). There was no effect of moth morph, methoxypyrazine smell, or pyrrolizidine alkaloid taste on beak wiping (Supplementary Table 3).
The interaction between methoxypyrazine smell and trial number influenced bird water drinking behavior (estimate ± SE = 0.6299 ± 0.2814, z = 2.238, p = 0.0252, Figure 5D and Supplementary Table 3). Water drinking decreased with trial number if the moth did not have neck fluids (Figure 5D). Neither the moth morph nor pyrrolizidine alkaloid taste affected water drinking behavior in the birds (Supplementary Table 3).
Survival
The interaction between methoxypyrazine smell and pyrrolizidine alkaloid taste influenced bird kill latency (estimate ± SE = −1.1483162 ± 0.4830955, z = −2.38, p = 0.017, Figure 6A and Supplementary Table 3). Kill latency was quicker if the moth did not have a methoxypyrazine smell and was raised on a diet with pyrrolizidine alkaloids, suggesting a trade-off between toxin sequestration and methoxypyrazine synthesis. Kill latency decreased with trial number (estimate ± SE = 0.6360943 ± 0.1096281, z = 5.80, p < 0.001; Supplementary Figure 3H and Supplementary Table 3), and with hunger level (estimate ± SE = 1.0167007 ± 0.2755151, z = 3.69, p < 0.001; Supplementary Figure 2J and Supplementary Table 3). There was no effect of moth morph on kill latency (Supplementary Table 3).
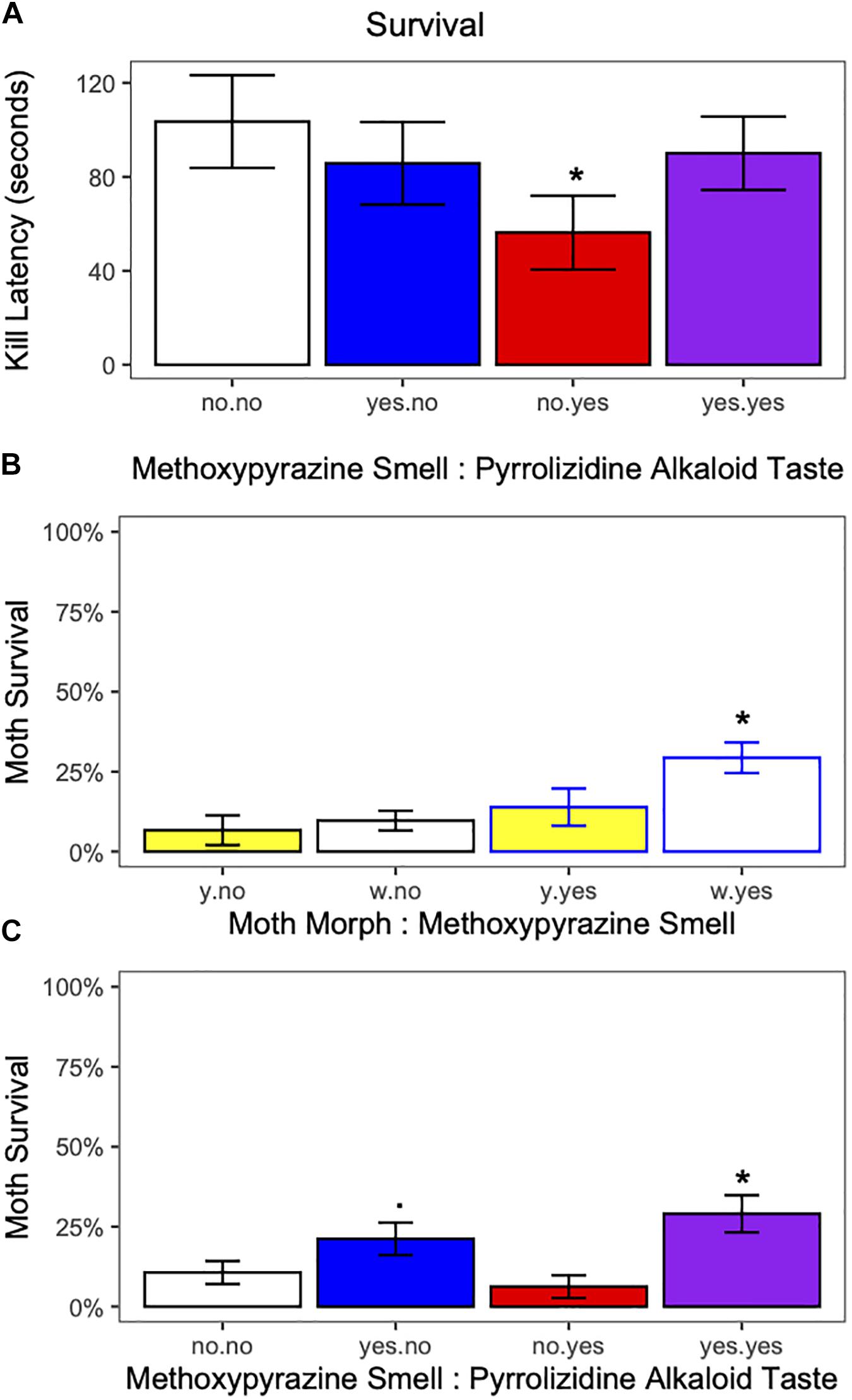
Figure 6. (A) Bird latency to kill the moth in response to methoxypyrazine smell and pyrrolizidine alkaloid taste. Moths raised on a pyrrolizidine alkaloid diet, but without methoxypyrazine smell were killed more quickly than moths without either chemical defense. *Indicates significant difference from moths without either chemical defense. (B) Moth survival in response to moth morph (yellow or white) and methoxypyrazine smell. Moths of the white morph with = blue methoxypyrazine smell had higher survival compared to moths of the yellow morph without = black methoxypyrazine smell. *Indicates significant difference from yellow moths without methoxypyrazine smell. (C) Moth survival in response to methoxypyrazine smell and pyrrolizidine alkaloid taste. Moths with both methoxypyrazine smell and pyrrolizidine alkaloid taste had higher survival than moths without either chemical defense. *Indicates significant differences (. = trend) from moths without either chemical defense. Bar graph shows percent (Survived = yes)±.
Moths survived in only 43 (17%) trials. Moth survival increased if they had methoxypyrazine smell (estimate ± SE = 1.25092 ± 0.40567, z = 3.084, p = 0.0021; Figure 6B and Supplementary Table 3), and moth survival increased with bird weight (estimate ± SE = 1.48241 ± 0.33329, z = 4.448, p < 0.001, Supplementary Figure 2I and Supplementary Table 3). When analyzed separately, white moths with methoxypyrazine smell were the only moths with higher survival compared to yellow moths without (estimate ± SE = 1.6612 ± 0.7887, z = 2.106, p = 0.0352, Figure 6B and Supplementary Table 3), and moths with both methoxypyrazine smell and pyrrolizidine alkaloid taste were the only moths with higher survival compared to moths with no chemical defenses (estimate ± SE = 1.1442 ± 0.4999, z = 2.289, p = 0.0221, Figure 6C and Supplementary Table 3), however, there was a trend for moths with only methoxypyrazine smell to also have higher survival compared to moths with no chemical defenses (estimate ± SE = 0.9899 ± 0.5201, z = 1.903, p = 0.0570). Neither pyrrolizidine alkaloid taste alone nor trial number affected moth survival (Supplementary Table 3).
Discussion
Summary
This study investigated multimodal anti-predator defenses through the predation sequence (Figure 7), and how multimodality impacts predator avoidance learning and moth survival. Bird approach latency toward moths of the Wy (white hindwings) morph was dependent on the presence of neck fluids with methoxypyrazine smell, but approach latency was longer for moths of the WW (white hindwings) compared to moths of the yy genotype (yellow hindwings) irrespective of methoxypyrazine smell. Color and smell had additive effects on dropping behavior, where moths of the white morph with neck fluids were more likely to be dropped and were also dropped a greater number of times. Drop latency (time between grabbing and dropping the moth for the first time) was quickest for moths of the Wy genotype that had neck fluids, suggesting a more potent defense upon contact for this genotype. Furthermore, taste alone did not deter bird predators. Birds were less likely to eat moths with neck fluids compared to those without, but only responded to the presence of pyrrolizidine alkaloids (taste and toxicity) after they had started to eat the moth and when the methoxypyrazine smell was also present, causing them to eat a smaller proportion of the moth’s body with both chemical defenses. Surprisingly, the pyrrolizidine alkaloid diet had a detrimental effect on predator deterrence in the early stages of attack including attack latency, drop latency, and kill latency (Figure 7), suggesting a possible trade-off between secondary (toxin sequestration) and primary (methoxypyrazine synthesis and/or wing pigmentation) defenses. Overall, moths of the white morph with methoxypyrazine smell had the greatest chance of survival. However, even though pyrrolizidine alkaloids had a negative impact on attack stage progression, toxin sequestration did not negatively impact survival. Indeed, moths with both methoxypyrazine smell and pyrrolizidine alkaloid taste had the highest survival overall. We did not find support for predator aversion learning, although birds adjusted their water drinking behavior across trials in response to methoxypyrazine smell. We suggest that methoxypyrazines act as context setting signals for warning colors and as attention alerting or “go-slow” signals for distasteful toxins, thereby mediating the relationship between warning signal and toxicity. The effect of each modality on each stage of attack (Figure 7) is detailed below.
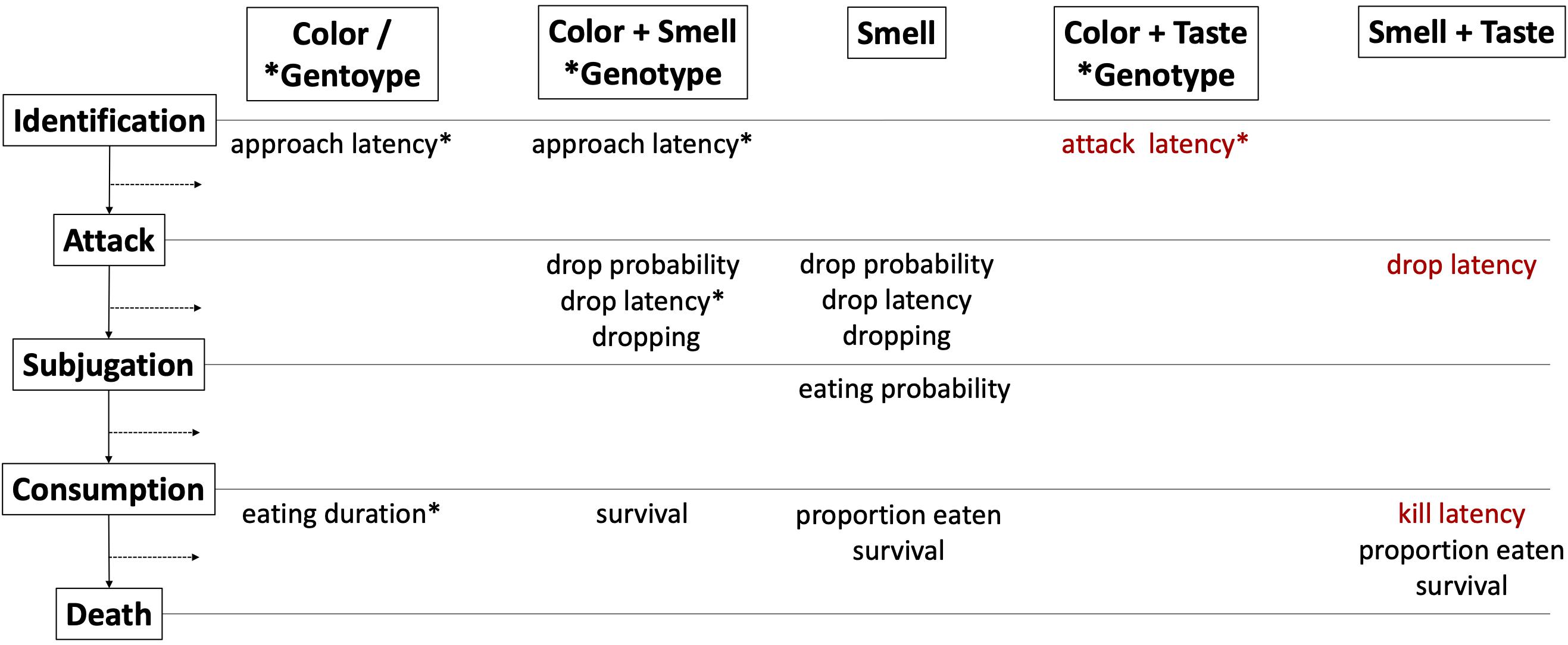
Figure 7. Summary of the results indicating the bird behaviors (approach latency, drop probability, drop latency, prey dropping, eating probability, eating duration, proportion eaten, kill latency, moth survival) that were influenced by each defense modality (color, color + smell, smell, color + taste, smell + taste) and the stage of the attack sequence that was inhibited. *Indicates that genotype rather than moth color morph was included in the model based on AICc. Text in red indicates reduced defense efficacy for that defense treatment/bird behavior (cost of pyrrolizidine alkaloid toxin sequestration may trade-off with primary defenses), while black texts indicates improved efficacy.
Approach
At the approach stage (Figure 7), birds hesitated longer to approach moths of the WW genotype compared to the yy genotype but only hesitated to approach moths of the Wy genotype if they had a methoxypyrazine smell. In addition, adult birds approached moths more quickly than juveniles, which suggests juveniles were more cautious with their prey. In a previous study, Rojas et al. (2019) found that blue tits took longer to approach models with white wings when neck fluids were present, regardless of whether they were coated with fluids from yellow (Y) or white (W) males, suggesting that methoxypyrazine smell did not differ between the morphs. While, neck fluids from Wy and WW males were not differentiated in Rojas et al. (2019), examination of Figure 3 suggests that variation in approach latency is largest for white morphs, which is consistent with the idea that fluid properties may differ between WW and Wy genotypes. Further chemical analysis is necessary to determine genotype differences in the type or quantity of de novo synthesized methoxypyrazines.
The difference in approach latency between WW and Wy moths without neck fluids, both of which have white hindwings, suggests that there is a perceptible visual difference between these two genotypes to blue tits. Ultraviolet (UV) components of the color pattern differ between these two genotypes especially in the forewings (Nokelainen in prep a), and therefore it is possible that birds are responding to UV-reflectance by delaying their attack of prey. Indeed, UV reflective white color is used as a warning signal in other lepidopteran species (Corral-Lopez et al., 2020), although in some earlier experiments UV-reflectance was found to invite rather than deter attacks by birds (Lyytinen et al., 2001, 2004).
Our results support the findings of Rojas et al. (2019) that, in the presence of neck fluid odor, birds take longer to approach A. plantaginis with white hindwings compared to those with yellow hindwings. Rojas et al. (2019) presented moths on a green background, while we presented moths against brown masonite (Supplementary Figure 1), which suggests that the white morph elicits longer approach hesitation even when presented against different colored backgrounds. These findings are at odds with previous studies where the yellow morph was found to be better protected (Nokelainen et al., 2012, 2014), but confirms that this discrepancy is not simply a difference in cues between model stimuli and natural prey. As suggested by Rojas et al. (2019) and experimentally tested by O. Nokelainen et al. (in prep b) there is an interaction between color pattern and light environment on predator response to A. plantaginis hindwing coloration. This could explain differences between experiments. Furthermore, natural prey that are not alive, such as some of those used in the Nokelainen et al. (2012) field experiment, may lack chemical delivery mechanisms to effectively release methoxypyrazine odors, and these volatile compounds may not have been present when the moths were presented to birds. These results highlight the importance of considering the interplay between multiple modalities, but also variation in natural environmental conditions, such as light environment, that can influence predator responses to defended prey.
Attack
Surprisingly, bird attack latency (time from approaching to attacking the moth) depended on the interaction between moth genotype and the pyrrolizidine alkaloid diet treatment (Figure 7), with birds hesitating longest to attack homozygous yellow moths from the control diet and homozygous white moths if they were raised on the pyrrolizidine alkaloid diet. Birds could be more motivated to attack larger prey, however, diet did not impact pupal weight for the WW and yy genotypes and attack latency did not differ between diet treatments for heterozygous moths, which were heavier when raised on the pyrrolizidine alkaloid diet (Supplementary Figure 4). It is puzzling that birds could perceive these non-volatile toxins before they are tasted and that the presence of toxins could cause birds accelerate their attack (for homozygous yellow moths). A more likely explanation is that sequestering toxins from the diet can be costly, limiting investment in other defense mechanisms such as methoxypyrazine synthesis or wing pigmentation, and that this cost differs between the genotypes. For example, it is possible that small reserves of methoxypyrazines are present even in moths that had their neck fluids removed, that the potency of these reserves is impacted by diet, and that the detection of these reserves requires close range (between approach and attack). Similarly, Lindstedt et al. (2010) found that a diet with iridoid glycoside toxins was costly to primary defenses of female A. plantaginis, resulting in hindwings with a lighter hue. The cost of pyrrolizidine alkaloid sequestration, and particularly its impact on other anti-predator defenses in A. plantaginis warrants further study.
Subjugation
After attack, bird likelihood to drop the moth and the number of times they dropped the moth differed in response to the additive effects of methoxypyrazine smell and color morph. Birds increased dropping behavior when moths had methoxypyrazine smell, and even more so when the moth had white hindwings. It is possible that these behaviors vary in response to the combination of visual and chemical signals. Such a relationship between methoxypyrazines and warning coloration is common in the literature, where it is suggested that methoxypyrazines act as context-setting signals (Marples and Roper, 1996; Rowe and Guilford, 1996, 1999; Lindström et al., 2001; Jetz et al., 2001; Kelly and Marples, 2004; Rowe and Halpin, 2013; Vickers and Taylor, 2018, 2020). However, it is also possible that white morph moths, and in particular those that are heterozygous for hindwing coloration, have more potent chemical defense, which causes differences in predator response between the genotypes. These effects are not mutually exclusive. Indeed, Rojas et al. (2019) found that moths of the white morph may have a more aversive taste.
Bird drop latency (the time between grabbing and dropping the moth) was quicker for heterozygous moths and moths with methoxypyrazine smell, but slower for moths from the pyrrolizidine alkaloid diet, and particularly for moths with pyrrolizidine alkaloids but no methoxypyrazines (Figure 7). Again, one explanation for the apparent eagerness for birds to pursue moths that have pyrrolizidine alkaloids is that toxin sequestration may be costly and reduce investment in other defenses, such as methoxypyrazines, which in turn may reduce defense potency in the earlier stages of attack before the bird has encountered the taste of pyrrolizidine alkaloids.
Consumption
Birds were less likely to eat moths with a methoxypyrazine smell than those without. In addition, birds took longer to eat moths of the WW genotype compared to moths of the yy genotype (Figure 7). Bird water drinking, which was correlated with eating duration (Supplementary Figure 5), decreased across trials, but only if the moths did not have neck fluids. However, despite being the only component that is intrinsically linked to the concentration of hepatotoxic pyrrolizidine alkaloids, taste alone did not deter bird predators. Birds only reduced the proportion eaten of the moth’s body with pyrrolizidine alkaloids compared to other treatments when neck fluids containing methoxypyrazines were also present. One possibility is that methoxypyrazines alert predators to the presence of bitter toxins. In the attention-altering hypothesis ‘one signal can increase the degree to which a receiver focuses attention on another sensory field, and by doing so, improves discrimination within that field’ (Hebets and Papaj, 2005). For instance, Guilford (1994) first suggested that visual warning signals might be ‘go-slow’ signals that alert predators to pay better attention in their assessment of prey palatability. Our findings suggest that smell may also provide a ‘go-slow’ signal for taste and toxicity.
Fluid secretion may be an important mechanism for the delivery of chemical defenses, discharging a distasteful chemical cocktail into the bird’s mouth before the bird has had a chance to bite into and taste the more nutritious tissues of the moth (Eisner and Meinwald, 1966). Indeed, in a study of leaf beetles, birds were more likely to reject prey that had their defense secretion intact compared to those that only had pyrrolizidine alkaloids sequestered into their body tissues (Rowell-Rahier et al., 1995). Therefore, it is possible that we have underestimated the effect of pyrrolizidine alkaloid defense, and that pyrrolizidine alkaloids in the neck fluids (Anne Winters Unpublished data) might contribute to moth defense at earlier stages of the attack sequence.
The role of pyrrolizidine alkaloids in defense against invertebrates is well-documented (Brown, 1984; Dussourd et al., 1988; Masters, 1990; Eisner and Eisner, 1991; Hare and Eisner, 1993; Conner et al., 2000; Eisner et al., 2000). Rojas et al. (2017) found that A. plantaginis abdominal fluids were deterrent to ants, but not to birds, but the compounds in the abdominal fluid were not identified. Pyrrolizidine alkaloids are present in the abdominal fluids of A. plantaginis (Anne Winters unpublished data) and it is possible that these contribute toward defense against invertebrates. Birds did not find the abdominal fluids (which do not contain methoxypyrazines) aversive, and this is in line with our finding that birds only reduced consumption of pyrrolizidine alkaloids when methoxypyrazines are also present. Invertebrate predators may also respond to visual aposematic signals. Similar to findings with birds, jumping spiders alter their response to visual signals in response to odor (Vickers and Taylor, 2018, 2020). Therefore, multimodal displays of color, smell, and taste are likely under selection from multiple, taxonomically distinct, predators. Defenses may asymmetrically target these predators, providing marginal protection for some types of predators and strong protection against others. Thus, multiple predators may create different selection pressures that shape the evolution of multimodal aposematic signals.
Survival
Moths raised on the pyrrolizidine alkaloid diet that had their neck fluids (methoxypyrazine smell) removed were killed more quickly than moths with no defenses (Figure 7), which, as mentioned above, suggests a trade-off between toxin sequestration and investment in other anti-predator defenses such as methoxypyrazine reserves and/or wing pigmentation. In addition, while moth activity was not selected to be included in the model based on AICc, moths on the pyrrolizidine alkaloid diet were less active compared to moths raised on the control diet (Supplementary Figure 6), which could reduce the amount of time needed for birds to capture and kill them. Overall, moth survival was highest for moths of the white morph with methoxypyrazine smell (Figure 7) and, despite the detrimental effects of pyrrolizidine alkaloid sequestration in terms of attack latency, drop latency, and kill latency, pyrrolizidine alkaloids did not negatively impact moth survival (Figure 7). Indeed, moths with both methoxypyrazine smell and pyrrolizidine alkaloid taste had the highest survival.
Predator Learning
We did not find evidence for aversion learning in this study. Instead, the time birds took to approach, attack, and handle the moths decreased with trials and birds were more likely to attack the moths as the trials progressed. Birds were quicker and more likely to attack moths in all treatments, including the treatment with no chemical defenses, suggesting a protective benefit of prey novelty that decreases with predator experience.
Birds that are no longer surprised by chemical defenses might still be expected to avoid them if those defenses are toxic or cause harm. However, birds in our experiment did not learn to avoid moths that contained toxic pyrrolizidine alkaloids. There are a number of reasons birds might decide to consume toxic prey, even after they have been warned about it (Barnett et al., 2007, 2012). For example, Hämäläinen et al. (2020) found that great tits differ in taste perception, but that their decision to eat toxic prey depended on the bird’s body condition, and not taste perception. Similarly, we found that bird body weight (as a proxy for condition), impacted behaviors across the predation sequence including dropping, handling, killing and eating the moth. Likewise, decisions about eating chemically defended prey may also relate to the presence and nutritional value of alternative food sources (Brower et al., 1968; Turner and Speed, 1999; Kokko et al., 2003; Sherratt, 2003). It is possible that birds would have learned to avoid toxic moths if they were given the choice of a nutritious and non-toxic alternative. In addition, these experiments took place in the winter, when food, and especially live insects, are scarce and the ambient temperature is cooler compared to summer months, which could influence the choice to consume toxic prey. Indeed, Chatelain et al. (2013) found that starlings increased consumption of prey that they knew to contain toxins when the ambient temperature was cooler. Stevens et al. (2010) found that birds are more likely to eat unpalatable, aposematic prey when they are hungry, and similarly, we found that hungrier birds killed the moths more quickly.
Intra-Specific Differences in Chemical Defense
As described above, bird approach latency changed based on the presence of methoxypyrazines, but only for the Wy genotype. In addition, birds abandoned Wy moths with methoxypyrazine smell more quickly than other genotypes. And, contrary to our expectation, moth genotype also interacted with pyrrolizidine alkaloid taste at the attack latency stage (the time from approaching to attacking the moth). Together, these results suggest that the neck fluid defenses of moths that are heterozygous for hindwing coloration may be particularly potent, and that the potential cost of toxin sequestration is unequal across the genotypes. Using life history data of moths obtained from this experiment, male moths of the Wy genotype were the only moths that were differentially impacted by the diet manipulation in terms of pupal weight. The pupae of Wy males were heavier when raised on the diet containing pyrrolizidine alkaloids compared to the diet without (Supplementary Figure 4), suggesting the Wy genotype may perform better on this diet compared to the other genotypes. Further research is required to determine whether the Wy genotype differs in the quantity or ratio of de novo synthesized methoxypyrazines SBMP and IBMP and whether the Wy genotype more efficiently sequesters or differentially utilizes pyrrolizidine alkaloids from their diet. However, our findings suggest that heterozygotes may have an advantage when it comes to the dietary sequestration of chemical defenses and in defense against predation, which could help to explain the persistence of color polymorphism in this species (see also Gordon et al., 2018).
Conclusion
Altogether these results suggest that color, smell, and taste function as a multimodal warning signal, and that there may be trade-offs between defense modalities, which impact different stages of attack such that primary defenses may dishonestly signal pyrrolizidine alkaloid content. Color and smell provided protection from a distance and during the initial encounter, while during consumption, methoxypyrazine smell may alert predators to the presence of pyrrolizidine alkaloids, reducing the proportion eaten in the treatment with both chemical defenses compared to the control. Overall, moth survival was highest for moths of the white morph with methoxypyrazine smell and, despite the detrimental effects of pyrrolizidine alkaloid sequestration on defense in the early attack stages, toxin sequestration did not negatively impact moth survival. Indeed, of the chemical defense treatments, moths with both methoxypyrazine smell and pyrrolizidine alkaloid taste had the highest survival. The smell of methoxypyrazines seems to be an especially important signal, facilitating predator responses to both color and taste perception.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by The Central Finland Centre for Economic Development, Transport and Environment, a license from the National Animal Experiment Board (ESAVI/9114/04.10.07/2014) and the Central Finland Regional Environmental Centre (VARELY/294/2015).
Author Contributions
JL participated in the design of the study, lab work, fieldwork, data analysis, and drafting the manuscript. JK participated in the lab work, fieldwork, and drafting the manuscript. ON participated in the design of the study and drafting the manuscript. JM participated in the conception and design of the study and drafting the manuscript. AW conceived, coordinated, and designed the study, participated in lab work, field work, data analysis, and drafting the manuscript. All authors approved the submitted version.
Funding
This work was supported by the Academy of Finland to JM (#320438) and the Grant (#21000038821) to ON and by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant agreement (#840944) to AW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Marjut Mähönen and Teemu Tuomaala for assistance raising moth lab stock, Kaisa Suisto for assistance and advice with moth care, Helinä Nisu for help catching and caring for birds at Konnevesi Research Station, Riccardo Tambornini for assistance with behavioral experiments, and Hannu Pakkanen for quantifying pyrrolizidine alkaloids. We also thank two reviewers for helpful comments on an earlier draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.657740/full#supplementary-material
Supplementary Figure 1 | Spectral reflectance (% reflectance from 300 to 700 nm wavelengths) of the masonite enclosure (background) and for the forewings (FW) and hindwings (HW) and black portion of the wing (BK) of each genotype: WW, white hindwings; Wy, white hindwings; yy, yellow hindwings.
Supplementary Figure 2 | Effect of co-variates: (A) approach latency in response to bird age, (B–I), drop probability, drop latency, prey dropping, handling duration, eating probability, eating duration, proportion eaten, and moth survival in response to bird weight, (J) kill latency in response to hunger level.
Supplementary Figure 3 | Approach latency (A), attack probability (B), attack latency (C), handling duration (D), eating duration (E), proportion eaten (F), beak wiping (G), and kill latency (H) in response to trial number.
Supplementary Figure 4 | Pupal weight in response to diet for male A. plantaginis raised on a diet with (AG) and without (ART) the addition of pyrrolizidine alkaloids (10% freeze dried Senecio vulgaris). Moths of the Wy genotype raised on the pyrrolizidine alkaloid diet were heavier compared to Wy moths raised on the artificial diet.
Supplementary Figure 5 | Bird water drinking (number of sips) increased in response to eating duration.
Supplementary Figure 6 | Moth activity in response to pyrrolizidine alkaloid taste. “no,” moths that were not raised on a pyrrolizidine alkaloid diet; “yes,” moths that were raised on a pyrrolizidine alkaloid diet. Moths raised on a pyrrolizidine alkaloid diet were less active.
Supplementary Table 1 | General definition, operative definition and unit of measure for each type of variable.
Supplementary Table 2 | Model selection using AICc for each response variable. Interactions and co-variates were selected to be included in the model and genotype replaced color morph if it improved the AICc score by more than Δ 2.
Supplementary Table 3 | Model summaries and follow up analyses for each response variable.
References
Alatalo, R. V., and Mappes, J. (1996). Tracking the evolution of warning signals. Nature 382, 708–710. doi: 10.1038/382708a0
Arenas, L. M., Walter, D., and Stevens, M. (2015). Signal honesty and predation risk among a closely related group of aposematic species. Sci. Rep. 5, 1–12.
Barnett, C. A., Bateson, M., and Rowe, C. (2007). State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav. Ecol. 18, 645–651. doi: 10.1093/beheco/arm027
Barnett, C. A., Skelhorn, J., Bateson, M., and Rowe, C. (2012). Educated predators make strategic decisions to eat defended prey according to their toxin content. Behav. Ecol. 23, 418–424. doi: 10.1093/beheco/arr206
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67:1. doi: 10.18637/jss.v067.i01
Blest, A. D. (1964). Protective display and sound production in some New World arctiid and ctenuchid moths. Zoologica 49, 161–181.
Broom, M., Higginson, A. D., and Ruxton, G. D. (2010). Optimal investment across different aspects of anti-predator defences. J. Theor. Biol. 263, 579–586. doi: 10.1016/j.jtbi.2010.01.002
Brower, L. P., Ryerson, W. N., Coppinger, L. L., and Glazier, S. C. (1968). Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. doi: 10.1126/science.161.3848.1349
Brown, K. S. (1984). Adult-obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator. Nature 309, 707–709. doi: 10.1038/309707a0
Burdfield-Steel, E., Pakkanen, H., Rojas, B., Galarza, J. A., and Mappes, J. (2018). De novo synthesis of chemical defenses in an aposematic moth. J. Insect Sci. 18:28.
Burdfield-Steel, E., Brain, M., Rojas, B., and Mappes, J. (2019). The price of safety: food deprivation in early life influences the efficacy of chemical defence in an aposematic moth. Oikos 128, 245–253. doi: 10.1111/oik.05420
Cardoso, M. Z. (1997). Testing chemical defence based on pyrrolizidine alkaloids. Anim. Behav. 54, 985–991. doi: 10.1006/anbe.1997.0505
Chandrashekar, J., Hoon, M. A., Ryba, N. J., and Zuker, C. S. (2006). The receptors and cells for mammalian taste. Nature 444, 288–294.
Chatelain, M., Halpin, C. G., and Rowe, C. (2013). Ambient temperature influences birds’ decisions to eat toxic prey. Anim. Behav. 86, 733–740. doi: 10.1016/j.anbehav.2013.07.007
Conner, W. E., Boada, R., Schroeder, F. C., González, A., Meinwald, J., and Eisner, T. (2000). Chemical defense: bestowal of a nuptial alkaloidal garment by a male moth on its mate. Proc. Natl. Acad. Sci. 97, 14406–14411. doi: 10.1073/pnas.260503797
Corral-Lopez, A., Varg, J. E., Cano-Cobos, Y. P., Losada, R., Realpe, E., and Outomuro, D. (2020). Field evidence for colour mimicry overshadowing morphological mimicry. J. Anim. Ecol. 90, 698–709. doi: 10.1111/1365-2656.13404
Cortesi, F., and Cheney, K. L. (2010). Conspicuousness is correlated with toxicity in marine opisthobranchs. J. Evol. Biol. 23, 1509–1518. doi: 10.1111/j.1420-9101.2010.02018.x
Dussourd, D. E., Ubik, K., Harvis, C., Resch, J., Meinwald, J., and Eisner, T. (1988). Biparental defensive endowment of eggs with acquired plant alkaloid in the moth Utetheisa ornatrix. Proc. Natl. Acad. Sci. 85, 5992–5996. doi: 10.1073/pnas.85.16.5992
Eisner, T., and Eisner, M. (1991). Unpalatability of the pyrrolizidine alkaloid-containing moth, Utetheisa ornatrix, and its larva, to wolf spiders. Psyche 98, 111–118. doi: 10.1155/1991/95350
Eisner, T., Eisner, M., Rossini, C., Iyengar, V. K., Roach, B. L., Benedikt, E., et al. (2000). Chemical defense against predation in an insect egg. Proc. Natl. Acad. Sci. 97, 1634–1639. doi: 10.1073/pnas.030532797
Eisner, T., and Grant, R. P. (1981). Toxicity, odor aversion, and “olfactory aposematism”. Science 213:476. doi: 10.1126/science.7244647
Eisner, T., and Meinwald, J. (1966). Defensive secretions of arthropods. Science 153, 1341–1350. doi: 10.1126/science.153.3742.1341
Evans, D. L., and Waldbauer, G. P. (1982). Behavior of adult and naive birds when presented with a bumblebee and its mimic. Z Tierpsychol. 59, 247–259. doi: 10.1111/j.1439-0310.1982.tb00341.x
Fischer, A., Gilad, Y., Man, O., and Paabo, S. (2005). Evolution of bitter taste receptors in humans and apes. Mol. Biol. Evol. 22, 432–436. doi: 10.1093/molbev/msi027
Gittleman, J. L., and Harvey, P. H. (1980). Why are distasteful prey not cryptic? Nature 286, 149–150. doi: 10.1038/286149a0
Gordon, S. P., Burdillat, S., and Mappes, J. (2018). Phenotype-dependent mate choice and the influence of mixed-morph lineage on the reproductive success of a polymorphic and aposematic moth. Evol. Ecol. 32, 427–441. doi: 10.1007/s10682-018-9944-5
Green, N. F., Urquhart, H. H., van den Berg, C. P., Marshall, N. J., and Cheney, K. L. (2018). Pattern edges improve predator learning of aposematic signals. Behav. Ecol. 29, 1481–1486.
Green, S. D., Duarte, R. C., Kellett, E., Alagaratnam, N., and Stevens, M. (2019). Colour change and behavioural choice facilitate chameleon prawn camouflage against different seaweed backgrounds. Commun. Biol. 2, 1–10.
Grober, M. S. (1988). Brittle-star bioluminescence functions as an aposematic signal to deter crustacean predators. Anim. Behav. 36, 493–501. doi: 10.1016/s0003-3472(88)80020-4
Guilford, T. (1994). “Go-slow” signalling and the problem of automimicry. J. Theor. Biol. 170, 311–316. doi: 10.1006/jtbi.1994.1192
Guilford, T., Nicol, C., Rothschild, M., and Moore, B. P. (1987). The biological roles of pyrazines: evidence for a warning odour function. Biol. J. Linn. Soc. 31, 113–128. doi: 10.1111/j.1095-8312.1987.tb01984.x
Halpin, C. G., Skelhorn, J., and Rowe, C. (2008). Being conspicuous and defended: selective benefits for the individual. Behav. Ecol. 19, 1012–1017. doi: 10.1093/beheco/arn069
Halpin, C. G., Skelhorn, J., and Rowe, C. (2014). Increased predation of nutrient-enriched aposematic prey. Proc. Biol. Sci. 281:1781. doi: 10.1098/rspb.2013.3255
Ham, A. D., Ihalainen, E., Lindström, L., and Mappes, J. (2006). Does colour matter? The importance of colour in avoidance learning, memorability and generalisation. Behav. Ecol. Sociobiol. 60, 482–491. doi: 10.1007/s00265-006-0190-4
Hämäläinen, L., Mappes, J., Thorogood, R., Valkonen, J. K., Karttunen, K., Salmi, T., et al. (2020). Predators’ consumption of unpalatable prey does not vary as a function of bitter taste perception. Behav. Ecol. 31, 383–392. doi: 10.1093/beheco/arz199
Hare, J. F., and Eisner, T. (1993). Pyrrolizidine alkaloid deters ant predators of Utetheisa ornatrix eggs: effects of alkaloid concentration, oxidation state, and prior exposure of ants to alkaloid-laden prey. Oecologia 96, 9–18. doi: 10.1007/bf00318024
Hebets, E. A., and Papaj, D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. doi: 10.1007/s00265-004-0865-7
Hegna, R. H., Galarza, J. A., and Mappes, J. (2015). Global phylogeography and geographical variation in warning coloration of the wood tiger moth (Parasemia plantaginis). J. Biogeogr. 42, 1469–1481. doi: 10.1111/jbi.12513
Holen, ØH. (2013). Disentangling taste and toxicity in aposematic prey. Proc. Biol. Sci. 280:1753. doi: 10.1098/rspb.2012.2588
Jetz, W., Rowe, C., and Guilford, T. (2001). Non-warning odors trigger innate color aversions—as long as they are novel. Behav. Ecol. 12, 134–139. doi: 10.1093/beheco/12.2.134
Kang, C., Cho, H. J., Lee, S. I., and Jablonski, P. G. (2016). Post-attack aposematic display in prey facilitates predator avoidance learning. Front. Ecol. Evol. 4:35. doi: 10.3389/fevo.2016.00035
Kang, C. K., Moon, J. Y., Lee, S. I., and Jablonski, P. G. (2012). Camouflage through an active choice of a resting spot and body orientation in moths. J. Evol. Biol. 25, 1695–1702. doi: 10.1111/j.1420-9101.2012.02557.x
Kelly, D. J., and Marples, N. M. (2004). The effects of novel odour and colour cues on food acceptance by the zebra finch (Taeniopygia guttata). Anim. Behav. 68, 1049–1054. doi: 10.1016/j.anbehav.2004.07.001
Kjernsmo, K., and Merilaita, S. (2012). Background choice as an anti-predator strategy: the roles of background matching and visual complexity in the habitat choice of the least killifish. Proc. Biol. Sci. 279, 4192–4198. doi: 10.1098/rspb.2012.1547
Kokko, H., Mappes, J., and Lindström, L. (2003). Alternative prey can change model–mimic dynamics between parasitism and mutualism. Ecol. Lett. 6, 1068–1076. doi: 10.1046/j.1461-0248.2003.00532.x
Lawrence, J. P., Rojas, B., Fouquet, A., Mappes, J., Blanchette, A., Saporito, R. A., et al. (2019). Weak warning signals can persist in the absence of gene flow. Proc. Natl. Acad. Sci. 116, 19037–19045. doi: 10.1073/pnas.1901872116
Lindstedt, C., Eager, H., Ihalainen, E., Kahilainen, A., Stevens, M., and Mappes, J. (2011). Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav. Ecol. 22, 580–587. doi: 10.1093/beheco/arr017
Lindstedt, C., Talsma, J. H. R., Ihalainen, E., Lindström, L., and Mappes, J. (2010). Diet quality affects warning coloration indirectly: excretion costs in a generalist herbivore. Evol. Int. J. Organic Evol. 64, 68–78. doi: 10.1111/j.1558-5646.2009.00796.x
Lindström, L., Alatalo, R. V., and Mappes, J. (1999). Reactions of hand-reared and wild-caught predators toward warningly colored, gregarious, and conspicuous prey. Behav. Ecol. 10, 317–322. doi: 10.1093/beheco/10.3.317
Lindström, L., Rowe, C., and Guilford, T. (2001). Pyrazine odour makes visually conspicuous prey aversive. Proc. Biol. Sci. 268, 159–162. doi: 10.1098/rspb.2000.1344
Lyytinen, A., Alatalo, R. V., Lindström, L., and Mappes, J. (2001). Can ultraviolet cues function as aposematic signals? Behav. Ecol. 12, 65–70. doi: 10.1093/oxfordjournals.beheco.a000380
Lyytinen, A., Lindström, L., and Mappes, J. (2004). Ultraviolet reflection and predation risk in diurnal and nocturnal Lepidoptera. Behav. Ecol. 15, 982–987. doi: 10.1093/beheco/arh102
Marples, N. M., and Roper, T. J. (1996). Effects of novel colour and smell on the response of naive chicks towards food and water. Anim. Behav. 51, 1417–1424. doi: 10.1006/anbe.1996.0145
Marples, N. M., van Veelen, W., and Brakefield, P. M. (1994). The relative importance of colour, taste and smell in the protection of an aposematic insect Coccinella septempunctata. Anim. Behav. 48, 967–974. doi: 10.1006/anbe.1994.1322
Masters, A. R. (1990). Pyrrolizidine alkaloids in artificial nectar protect adult ithomiine butterflies from a spider predator. Biotropica 22, 298–304. doi: 10.2307/2388541
Mastrota, N. F., and Mench, J. A. (1995). Colour avoidance in northern bobwhites: effects of age, sex and previous experience. Anim. Behav. 50, 519–526. doi: 10.1006/anbe.1995.0266
Moore, B. P., Brown, W. V., and Rothschild, M. (1990). Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology 1, 43–51. doi: 10.1007/bf01325227
Nishida, R. (2002). Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 47, 57–92. doi: 10.1146/annurev.ento.47.091201.145121
Nissim, I., Dagan-Wiener, A., and Niv, M. Y. (2017). The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 69, 938–946. doi: 10.1002/iub.1694
Nokelainen, O., Hegna, R. H., Reudler, J. H., Lindstedt, C., and Mappes, J. (2012). Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc. R. Soc. B Biol. Sci. 279, 257–265. doi: 10.1098/rspb.2011.0880
Nokelainen, O., Valkonen, J., Lindstedt, C., and Mappes, J. (2014). Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. J. Anim. Ecol. 83, 598–605. doi: 10.1111/1365-2656.12169
Nokelainen, O., de Moraes Rezende, F., Valkonen, J. K., and Mappes, J. (in prep b). Context dependent coloration of prey and predator decision making in contrasting light environments.
Nokelainen, O., Galarza, J. A., Kirvesoja, J., Suisto, K., and Mappes, J. (in prep a). Detecting cryptic colour polymorphism from phenotypes.
Poulton, E. B. (1887). The experimental proof of the protective value of colour and markings in insects in reference to their vertebrate encmies. Proc. Zool. Soc. Lond. 55, 191–274.
Poulton, E. B. (1890). The Colours of Animals: Their Meaning and Use, Especially Considered in the Case of Insects. New York: D. Appleton and Company.
R Core Team (2011). R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Reed, D. R., and Knaapila, A. (2010). Genetics of taste and smell: poisons and pleasures. Prog. Mol. Biol. Transl. sci. 94, 213–240.
Reinwaldt, C., Bittner, M., and Kempe, G. (2017). Quantitation of Pyrrolizidine Alkaloids in Honey and Herbal Teas by UHPLC/MS/MS Application Note. Waldbronn, Germany: Agilent Technologies.
Ritland, D. B. (1991). Palatability of aposematic queen butterflies (Danaus gilippus) feeding onSarcostemma clausum (Asclepiadaceae) in Florida. J. Chem. Ecol. 17, 1593–1610. doi: 10.1007/bf00984691
Rojas, B., Burdfield-Steel, E., Pakkanen, H., Suisto, K., Maczka, M., Schulz, S., et al. (2017). How to fight multiple enemies: target-specific chemical defences in an aposematic moth. Proc. R. Soc. B Biol. Sci. 284:1863. doi: 10.1098/rspb.2017.1424
Rojas, B., Mappes, J., and Burdfield-Steel, E. (2019). Multiple modalities in insect warning displays have additive effects against wild avian predators. Behav. Ecol. Sociobiol. 73:3. doi: 10.1007/s00265-019-2643-6
Rönkä, K., De Pasqual, C., Mappes, J., Gordon, S., and Rojas, B. (2018). Colour alone matters: no predator generalization among morphs of an aposematic moth. Anim. Behav. 135, 153–163. doi: 10.1016/j.anbehav.2017.11.015
Rönkä, K., Mappes, J., Kaila, L., and Wahlberg, N. (2016). Putting Parasemia in its phylogenetic place: a molecular analysis of the subtribe Arctiina (Lepidoptera). Syst. Entomol. 41, 844–853. doi: 10.1111/syen.12194
Rönkä, K., Valkonen, J. K., Nokelainen, O., Rojas, B., Gordon, S., Burdfield-Steel, E., et al. (2020). Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth. Ecol. Lett. 23, 1654– 1663.
Roper, T. J. (1990). Responses of domestic chicks to artificially coloured insect prey: effects of previous experience and background colour. Anim. Behav. 39, 466–473. doi: 10.1016/s0003-3472(05)80410-5
Roper, T. J., and Wistow, R. (1986). Aposematic colouration and avoidance learning in chicks. Q. J. Exper. Psychol. Sec. B 38, 141–149.
Rowe, C., and Guilford, T. (1996). Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature 383, 520–522. doi: 10.1038/383520a0
Rowe, C., and Guilford, T. (1999). Novelty effects in a multimodal warning signal. Anim. Behav. 57, 341–346. doi: 10.1006/anbe.1998.0974
Rowe, C., and Halpin, C. (2013). Why are warning displays multimodal? Behav. Ecol. Sociobiol. 67, 1425–1439. doi: 10.1007/s00265-013-1515-8
Rowell-Rahier, M., Pasteels, J. M., Alonso-Media, A., and Brower, L. P. (1995). Relative unpalatability of leaf beetles with either biosynthesized or sequestered chemical defence. Anim. Behav. 49, 709–714. doi: 10.1016/0003-3472(95)90045-4
Rowland, H. M., Burriss, R. P., and Skelhorn, J. (2020). The antipredator benefits of postural camouflage in peppered moth caterpillars. Sci. Rep. 10, 1–8.
Rowland, H. M., Parker, M. R., Jiang, P., et al. (2015). “Comparative taste biology with special focus on birds and reptiles,” in Handbook of Olfaction and Gustation, ed. R. L. Doty (Hooken, NJ: Wiley & Sons), 957–982. doi: 10.1002/9781118971758.ch43
Ruxton, G. D., Allen, W. L., Sherratt, T. N., and Speed, M. P. (2018). Avoiding Attack: the Evolutionary Ecology of Crypsis, Aposematism, and Mimicry. Oxford: Oxford University Press.
Ruxton, G. D., and Kennedy, M. W. (2006). Peppers and poisons: the evolutionary ecology of bad taste. J. Anim. Ecol. 75, 1224–1226. doi: 10.1111/j.1365-2656.2006.01133.x
Sargent, T. D. (1966). Background selections of geometrid and noctuid moths. Science 154, 1674–1675. doi: 10.1126/science.154.3757.1674
Schuler, W., and Roper, T. J. (1992). Responses to warning coloration in avian predators. Adv. Stud. Behav. 21, 111–146. doi: 10.1016/s0065-3454(08)60143-6
Sherratt, T. N. (2003). State-dependent risk-taking by predators in systems with defended prey. Oikos 103, 93–100. doi: 10.1034/j.1600-0706.2003.12576.x
Shi, P., Zhang, J., Yang, H., and Zhang, Y. P. (2003). Adaptive diversification of bitter taste receptor genes in Mammalian evolution. Mol. Biol. Evol. 20, 805–814. doi: 10.1093/molbev/msg083
Siddall, E. C., and Marples, N. M. (2011). The effect of pyrazine odor on avoidance learning and memory in wild robins Erithacus rubecula. Curr. Zool. 57, 208–214. doi: 10.1093/czoolo/57.2.208
Sivinski, J. (1981). The nature and possible functions of luminescence in Coleoptera larvae. Coleopts. Bull. 32, 167–179.
Skelhorn, J., and Rowe, C. (2006). Avian predators taste–reject aposematic prey on the basis of their chemical defence. Biol. Lett. 2, 348–350. doi: 10.1098/rsbl.2006.0483
Skelhorn, J., and Rowe, C. (2009). Distastefulness as an antipredator defence strategy. Anim. Behav. 78, 761–766. doi: 10.1016/j.anbehav.2009.07.006
Skelhorn, J., and Rowe, C. (2010). Birds learn to use distastefulness as a signal of toxicity. Proc. R. Soc. B Biol. Sci. 277, 1729–1734. doi: 10.1098/rspb.2009.2092
Smith, S. M. (1975). Innate recognition of coral snake pattern by a possible avian predator. Science 187, 759–760. doi: 10.1126/science.187.4178.759
Song, W., and Jablonski, P. G. (2020). Evolution of switchable aposematism: insights from individual-based simulations. PeerJ 8:e8915. doi: 10.7717/peerj.8915
Stevens, M. (2013). Sensory Ecology, Behaviour, and Evolution. United Kindom: Oxford University Press.
Stevens, M., Mappes, J., and Sandre, S. L. (2010). The effect of predator appetite, prey warning coloration and luminance on predator foraging decisions. Behaviour 147, 1121–1143. doi: 10.1163/000579510x507001
Stevens, M., and Ruxton, G. D. (2018). The key role of behaviour in animal camouflage. Biol. Rev. 94, 116–134.
Suomalainen, E. (1938). Die Erblichkeitsverhältnisse des männlichen Dimorphismus bei Parasemia plantaginis. Hereditas. 24, 386–390.
Svensson, L. (1992). Identification Guide to European Passerines. Norfolk: British Trust for Ornithology.
Therneau, T. M., and Therneau, M. T. M. (2015). Package ‘coxme’. Mixed Effects Cox Models. R package version. 2.
Umbers, K. D., De Bona, S., White, T. E., Lehtonen, J., Mappes, J., and Endler, J. A. (2017). Deimatism: a neglected component of antipredator defence. Biol. Lett. 13:20160936.
Umbers, K. D., and Mappes, J. (2015). Postattack deimatic display in the mountain katydid, Acripeza reticulata. Anim. Behav. 100, 68–73.
Vickers, M. E., and Taylor, L. A. (2018). Odor alters color preference in a foraging jumping spider. Behav. Ecol. 29, 833–839.
Vickers, M. E., and Taylor, L. A. (2020). Hemipteran defensive odors trigger predictable color biases in jumping spider predators. Sci. Rep. 10:21898.
Waldron, S. J., Endler, J. A., Valkonen, J. K., Honma, A., Dobler, S., and Mappes, J. (2017). Experimental evidence suggests that specular reflectance and glossy appearance help amplify warning signals. Sci. Rep. 7, 1–10.
Winters, A. E., Wilson, N. G., van den Berg, C. P., How, M. J., Endler, J. A., Marshall, N. J., et al. (2018). Toxicity and taste: unequal chemical defences in a mimicry ring. Proc. R. Soc. B Biol. Sci. 285:1880. doi: 10.1098/rspb.2018.0457
Yosef, R., Carrel, J. E., and Eisner, T. (1996). Contrasting reactions of loggerhead shrikes to two types of chemically defended insect prey. J. chem. Ecol. 22, 173–181.
Keywords: aposematism, Arctia plantaginis, Cyanistes caeruleus, defense mechanisms, multimodal signaling, predator-prey interactions, chemical defense, warning signals
Citation: Winters AE, Lommi J, Kirvesoja J, Nokelainen O and Mappes J (2021) Multimodal Aposematic Defenses Through the Predation Sequence. Front. Ecol. Evol. 9:657740. doi: 10.3389/fevo.2021.657740
Received: 23 January 2021; Accepted: 14 July 2021;
Published: 16 August 2021.
Edited by:
Julien Terraube, University of the Sunshine Coast, AustraliaReviewed by:
Alice Exnerova, Charles University, CzechiaPiotr Jablonski, Seoul National University, South Korea
Copyright © 2021 Winters, Lommi, Kirvesoja, Nokelainen and Mappes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne E. Winters, a.e.winters@exeter.ac.uk
 Anne E. Winters
Anne E. Winters Jenna Lommi1
Jenna Lommi1  Ossi Nokelainen
Ossi Nokelainen Johanna Mappes
Johanna Mappes