Variation in Female Leverage: The Influence of Kinship and Market Effects on the Extent of Female Power Over Males in Verreaux’s Sifaka
- 1Department of Anthropology, University of Texas, Austin, TX, United States
- 2Tiputini Biodiversity Station, College of Biological and Environmental Sciences, Universidad San Francisco de Quito, Cumbayá, Ecuador
Female mammals employ reproductive strategies (e.g., internal gestation) that result in power asymmetries specific to intersexual dyads. Because the number of eggs available for fertilization at any given time for most mammals is quite limited, having a fertilizable egg is potentially an important source of economic power for females. Control over mating opportunities is a source of intersexual leverage for female Verreaux’s sifaka (Propithecus verreauxi). We examined economic factors thought to influence the value of mating opportunities, and, thus, the extent of female leverage: kinship and market effects. Using a longitudinal dataset of agonistic interactions collected during focal animal sampling of all adult individuals in 10 social groups from 2008 to 2019, we tested the effects of relatedness, female parity, reproductive season, and adult sex ratio (population and group) on (1) the direction of submissive signaling and (2) which sex won a contested resource. While 96% of the acts of submission were directed from males toward females, females only won a third of their conflicts with males. Thus, our study has implications for evolutionary explanations of female-biased power. If female power evolved due to their greater need for food and other resources, then intersexual conflicts would be expected to result in males more consistently relinquishing control of resources. As expected, males were more likely to chatter submissively toward successful mothers, during the mating season, and when the sex ratio was male-biased. Although females generally had less power to win a conflict when their fertilizable egg was less valuable (when they were nulliparous or unsuccessful mothers or when interacting with male kin) and with an increasing female-bias in the sex ratio, this ability to win additionally was influenced by which sex initiated the conflict. Our study demonstrates that female leverage can be influenced by the supply and demand for mating opportunities, but evoking submission does not translate into winning a resource. Indeed, intersexual power is dynamic, contextual, and dependent on the individuals in the dyad.
Introduction
In some animal societies, females are more powerful than males. This phenomenon is often called “female dominance,” but both theoretical (Hand, 1986; Smuts, 1987; Lewis, 2002, 2018, 2020) and empirical (Lewis, 2004; Surbeck and Hohmann, 2013; Young et al., 2017; Voyt et al., 2019) work suggests that female power over males can also be leverage, i.e., where female power over males arises from an asymmetry in intrinsic economic resources rather than an asymmetry in fighting abilities (Hand, 1986; Lewis, 2002, 2018, 2020). For example, the reproductive cycles, internal gestation, and lactation of female mammals can result in operational sex ratios that are highly male skewed (Emlen and Oring, 1977), i.e., where the number of sexually active males in a population greatly exceeds the number of sexually available females. Under this situation, having a fertilizable egg can be an important source of economic power (i.e., leverage) for females (Noë et al., 1991; Lewis, 2002, 2004, 2018, 2020). Indeed, fertilization potential (a source of leverage) predicts intersexual status in sifaka, but sexual size dimorphism within a dyad (a source of dominance) does not (Voyt et al., 2019). Similarly, mating opportunities are a source of female leverage in bonobos that results in reduced intersexual aggression by males (Surbeck and Hohmann, 2013). Thus, species exhibiting a female-biased power structure can exhibit female dominance, such as in spotted hyenas where intersexual power is determined by the combined fighting abilities of coalitions (Vullioud et al., 2019), female leverage, such as in bonobos and sifaka, or a combination of both types of female power. In fact, these and other species likely exhibit multiple types of power simultaneously.
Empirical research on leverage suggests that it may be central to understanding female-biased power in these societies, especially if the extent of female economic power varies with the value of the commodity being offered (Noë and Hammerstein, 1994, 1995; Lewis, 2002). For example, bonobo males do not exhibit aggression toward females when mating has a high probability of resulting in a conception (Surbeck and Hohmann, 2013), suggesting that the value of a female as a mating partner is discounted at other times. Consistent with the hypothesis that the value of a female’s fertilizable egg varies with her experience and success at mothering, male sifaka are more submissive toward successful mothers than nulliparous females or parous females who have not yet successfully reared an infant, indicating that successful mothers have more leverage than other females in their relationships with males (Voyt et al., 2019). In other words, intersexual power can be highly dynamic, contextual, and dependent on the individuals in the dyad (Lewis, 2002).
Hypotheses for the evolution of female-biased power in animal societies often focus on the importance of females winning resources (e.g., Jolly, 1984; Young et al., 1990; Wright, 1999). A winner is the “contestant that expressed consummatory behavior according to its initial goal” (Drews, 1993, 285). Despite this emphasis on resources, “winning” is often assessed by whether a male is submissive toward females (Pereira et al., 1990; Pochron et al., 2003; Bolt, 2013; Hohenbrink et al., 2016; Voyt et al., 2019), with the assumption that the ability to evoke submission and the ability to command priority of access to resources are equivalent. However, eliciting a submissive signal and usurping resources are different scopes of power (sensu Lewis, 2002: the outcomes that can be achieved due to the power asymmetry in the dyadic relationship). Additionally, winning a particular contest may be determined by either a power asymmetry (difference in fighting ability or difference in inalienable resources) or an asymmetry in motivational state independent of the power relationship (Schjelderup-Ebbe, 1922; Lewis, 2002, 2022; Allen et al., 2016). Some authors do consider motivation along with dominance and leverage to explain power (e.g., Surbeck and Hohmann, 2013; Vermande and Sterck, 2020), however, we consider motivation to be an orthogonal axis relevant to explaining the outcome of particular interactions but not a source of power in a relationship (Lewis, 2002, 2022). While the term “motivation” can be used to describe how an individual might be driven to seek resources due to evolutionary strategies (Surbeck and Hohmann, 2013), we limit our use of the term “motivation” to refer to ephemeral physiological states such as hunger, thirst, or exhaustion (see also Allen et al., 2016). Hypotheses about the evolution of female-biased power endeavor to explain the pattern of asymmetries in intersexual relationships (sensu Hinde, 1976) rather than the outcomes of any single conflict.
The aim of this study was to test the hypothesis that female leverage in intersexual dyads varies with the value of mating opportunities in Verreaux’s sifaka using novel factors that investigate different levels of commodity value. First, female leverage over males may vary with kinship. The value of a mating opportunity with close kin is expected to be lower than the value of an opportunity with an unrelated individual because inbreeding can increase the chance of offspring being homozygous for deleterious alleles (Charlesworth and Charlesworth, 1999). Indeed, animals often have strategies to avoid mating with close kin (Pusey and Wolf, 1996). If intersexual power is based primarily on female control over mating opportunities (i.e., access to her fertilizable egg), then females are expected to have more power over unrelated males than male kin. Nevertheless, inbred offspring can potentially contribute to fitness and can even be preferred due to the inclusive fitness benefits (Kokko and Ots, 2006; Puurtinen, 2011). Hence mating with close kin may still occur (Szulkin et al., 2013). Consequently, control over mating opportunities may be a source of female leverage in dyads with male kin, albeit to a lesser extent than with unrelated males. In other words, females may only have leverage over some males and not others because females may be low-value mates for a subset of the male population.
Second, the proportion of females in a population may influence the extent of female leverage (Lewis, 2004; Norscia et al., 2009; Noë, 2017) because the supply and demand of estrous females can potentially affect the value of mating opportunities (Noë et al., 1991). Female power increases as the proportion of males in the group increases in simulated and wild monkey studies (Hemelrijk et al., 2008; Izar et al., 2021). If female power is based on control over mating opportunities, then females are expected to have greater power when fewer other fertilizable females and more reproductively available males are present in a population (Noë et al., 1991; Noë, 2017). When mammals live in permanent social groups, their mating options also may be mostly limited to the members of their social group (Isvaran and Clutton-Brock, 2007). Thus, the ratio of fertilizable females to males within the social group (as opposed to in the population at large) may also influence female leverage in intersexual dyads.
Sifaka are folivorous lemurs (Richard, 1978; Lewis and Lawler, 2013) that live in small, cohesive social groups containing 0 to 3 adult individuals of each sex (Richard et al., 1991; Lewis and van Schaik, 2007). Sex ratios can be highly variable between groups and in the same group across time (Richard et al., 1991; Lewis and van Schaik, 2007; Leimberger and Lewis, 2017). While both sexes can disperse, dispersal is male-biased (Richard et al., 1993; Leimberger and Lewis, 2017). Sifaka societies are characterized by a female-biased power structure (sensu Lewis, 2018), often referred to as “female dominance” (Richard, 1987; Brockman, 1994, 1999). Their highly seasonal reproduction and short estrus duration (0.5–96 h/year: Brockman, 1999), combined with little to no sexual size dimorphism (Lewis and Kappeler, 2005), leads to females having leverage over males (Lewis, 2004, 2020; Voyt et al., 2019). Sifaka chatter vocalizations can be an immediate signal of submission in response to aggression or a spontaneous signal about the general power status in the relationship (Lewis, 2019), depending on whether it is provoked (Flack and de Waal, 2007). Interestingly, male sifaka frequently chatter without provocation to other males but chatter submissively to females often after receiving aggression (Lewis, 2019). Moreover, female sifaka are less likely to usurp a male’s resources if he chatters without provocation (Lewis, 2019). The combination of dynamic grouping patterns and power relationships in Verreaux’s sifaka provide an opportunity to study how the scope (i.e., the outcomes that can be evoked) of female power (sensu Lewis, 2002, 2020, 2022) fluctuates with the value of their fertilizable eggs and the concomitant leverage that extends.
Using more than a decade of longitudinal behavioral, demographic, and genetic data collected for Verreaux’s sifaka living in multiple social groups in the Kirindy Mitea National Park of western Madagascar, we tested the hypotheses that kinship and female scarcity influence the extent of female leverage over males. The natural variation within and across social groups in our longitudinal dataset facilitates an examination of the relational aspect of power and how it varies across dyads. We examined dyadic social interactions involving male-female dyads and predicted that (1) males are less likely to be submissive to female kin than to other females and that (2) males are more likely to win conflicts with female kin than other females because mating opportunities with related females should be less valuable. We further predicted that males are more likely to be submissive and less likely to win conflicts with females when (3) population and (4) group adult sex ratios are male-biased because the supply of mating opportunities is lower when fewer reproductively mature females are available. Similar to previous studies (Voyt et al., 2019), we further predicted that (5) males submit most to parous females who have successfully reared an offspring and that (6) males win less when interacting with successful mothers than with nulliparous or unsuccessful parous females. Finally, because the value of a mating opportunity might be discounted when a female is unlikely to be in estrus, we also predicted that (7) males are less likely to be submissive and (8) more likely to win encounters outside of the mating season than during the mating season.
Materials and Methods
Study Population
We studied Verreaux’s sifaka at the Ankoatsifaka Research Station (20°47’17”S, 44°10’0”E) in the dry, deciduous forest of Kirindy Mitea National Park in western Madagascar. This highly seasonal forest experiences substantial variation in rainfall across years (range: 374–1,577 mm), but averages 850 mm annually, mostly in January and February (Lewis and Axel, 2019). A grid system of trails every 25 m is maintained within the 1-km2 study area to facilitate observations. Because the forest is not very tall (emergent trees are 8–18 m tall: Lewis and Bannar-Martin, 2012) and sifaka spend a substantial portion of their time in the understory or canopy, detailed social interactions can be observed easily.
All residents in multiple social groups within the study area and some individuals residing in neighboring groups were identifiable with unique nylon collars and tags or radio collars (Rasambainarivo et al., 2014) and/or using natural markings. Ages either were known based on when an individual was born into a group or estimated using dental development and wear, body size, and nipple shape (for females) assessed during annual captures (Rasambainarivo et al., 2014). We studied intersexual social interactions involving all adult (≥5 years) and subadult (3 and 4 years) individuals for which we had kinship data and that resided in 10 different social groups (Groups I-VI, XI-XII, Bella, Albert). Verreaux’s sifaka groups can be impermanent, and focal groups were observed an average of 6.2 years (SD = 4.9) across the 12-year study period. We tested our predictions using two age groupings, one that included only adults (N = 46 individuals) and one that included both adults and subadults (aged ≥ 3 years, N = 22 additional individuals). We did this because sifaka are reported to sometimes be sexually active as subadults and because the age of adulthood is inconsistely applied across studies in Verreaux’s sifaka (Lewis, 2008). For simplicity, we only present analysis of “adults” in the main manuscript and include our analysis of “adults + subadults” (individuals ages ≥ 3 years) in the Supplementary File.
Data Collection
Behavioral Data
We collected all occurrences of intragroup agonistic intersexual interactions during 1-h focal animal sampling sessions (Altmann, 1974) of all adult and subadult sifaka, for a total of approximately 14,000 h of observation from 2008 through 2019. Insufficient behavioral and demographic data were available for 2009–2010 because Cyclone Fanele interrupted data collection and thus were excluded from our analysis. The identity of the initiator and of the receiver was recorded for each interaction, and all behaviors occurring during the interaction were recorded as occurring either in isolation or as part of a sequence. Agonistic behaviors were defined according to the Brockman (1994) ethogram with the following additions: “food rob +” (X tries to take the food away from Y and is successful), “food rob –” (X tries to take the food away from Y and is unsuccessful), and “snap at” (X bites in the direction of Y but does not make contact). In addition, “proximity” (a concept implicit to the definition of certain behaviors) was defined as occurring when individuals were within 1 m of each other (Lewis, 2019).
For all agonistic encounters, we scored an individual as “winning” a conflict if the other individual in the dyad moved at least 1 m away from the “winner” within 10 s of the agonistic interaction. Note that we did not limit our analysis of “winning” to the feeding context because sifaka compete for other resources in addition to food (e.g., water, space, sun, shade, grooming partners, and huddling partners). Moreover, our definition of a “win” included a broader set a behaviors than merely “supplant” [X moves toward Y, Y immediately changes location (within 5 s), X occupies the location previously held by Y: cf. Brockman, 1994)]. If neither individual withdrew after the agonistic interaction, neither individual was considered the winner and the outcome was scored as “neutral.” The one exception to this rule was for the “food rob” behaviors because “food rob +” is defined as an initiator successfully gaining control of the food resource, while “food rob –” necessarily means that the initiator was not successful. Therefore, the identity of the sifaka that had control of the food resource at the end of the “food rob” behavior was scored as the winner.
Sifaka often exhibit multiple aggressive and/or submissive behaviors within an agonistic interaction. We thus used the following rules for scoring an interaction as “win” vs. “neutral” when multiple behaviors occurred in a sequence. If a social interaction began with an approach, the 10 s rule started with the time of the first non-approach agonistic behavior. If an individual used multiple types of aggressive behaviors essentially simultaneously (e.g., lunge and cuff), then we only scored the first aggressive act. However, if an individual used repeated, successive acts of aggression toward another individual (e.g., three cuffs within 10 s) and the receiver chattered submissively immediately after each individual aggressive act (e.g., cuff then chatter response, cuff then chatter response, and cuff then chatter response), we scored each aggressive act independently, with the assumption that the additional acts of aggression were needed because the first aggressive act was not successful. When animals repeatedly made a submissive chatter vocalization spontaneously (i.e., without receiving aggression within 10 s beforehand), we scored the agonistic events as independent when there was at least 5 s between the end of the first chatter and the beginning of the second chatter. To address the issue that these repeated aggressive or submissive acts are not entirely independent of one another, we assigned a corresponding proximity “bout identity” to each agonistic act and then used bout ID as a random factor in our statistical models. A proximity bout was defined as a period of time in which the members of a dyad were continuously within 1 m of each other within a given 1-h focal sample, and all behaviors occurring during this period were assigned with the same bout ID. Finally, for each agonistic interaction, we also scored the identity of the initiator of the interaction. Note that by definition, the winner of an interaction was always scored as the initiator for supplants.
Demographic Data
We conducted monthly censuses of the population in the Ankoatsifaka grid system of trails (Leimberger and Lewis, 2017; Lewis et al., 2020). In addition to locating all groups with radio collars and recording the identity of each individual present in the group, we located unmarked groups and solitary individuals by walking the trail system. While sifaka live in cohesive groups, they sometimes visit other groups, and males occasionally roam independently during the mating season (Richard et al., 1993; Brockman, 1999; Leimberger and Lewis, 2017). On the rare occasions when a known individual was not observed on the day of the census, we nonetheless retroactively added them to the census data for that month if the individual was observed during behavioral data collection within 7 days of the census.
Analyses
Predictors
We examined several key factors that we predicted might influence female intersexual leverage based on control of mating opportunities: relatedness between members of the dyad, female parity status, reproductive season, and the population and social group sex ratios. We considered dyads as “related” if the two individuals involved were either parent and offspring, full-siblings, or half-siblings and “unrelated” otherwise. We used multilocus microsatellite marker genotypes derived from either fecal or tissue DNA for 56 of the 68 individuals (aged ≥ 3 years) included in this study to conduct genetic assessment of parentage and relatedness between dyads. Details of the procedures used for genotyping and for evaluating parentage and estimated relatedness are discussed in Abondano (2014) and Perofsky et al. (2021). Briefly, we used DNA extracted from either fecal samples or tissue biopsies collected during captures to genotype all individuals at a set of 14 loci known to be variable in other populations of wild sifaka (Lawler et al., 2001; Rakotoarisoa et al., 2006). The average allelic diversity across loci was 10.2 ± 3.1 SD, and the average He across loci was 0.79 ± 0.06. We used the software Cervus (Marshall et al., 1998; Kalinowski et al., 2007) to conduct likelihood-based maternity and paternity analyses for all younger individuals, using all adult males and females sampled in the population as candidate sires and dams, respectively. For these analyses, we assumed a genotyping error rate of 1% and assumed that we had sampled 90 and 75% of candidate dams and sires, respectively. The average proportion of loci typed in our dataset was >99%. For the panel of loci, the combined PI and PIsib values were 6.3 × 10–11 and 8.4 × 10–7, respectively, indicating a very low probability that any two individuals or two full siblings could be expected to share the same multilocus genotype by chance. Based on the distribution of likelihood scores across candidate parents, the estimated confidence in all of our assignments of maternity and paternity was ≥95%.
We also used the software Kingroup2 (Konovalov et al., 2004) to evaluate whether, given their particular genotypes and allele frequencies in the population at each locus, the individuals comprising each dyad were more likely to be “related” or “unrelated” using likelihood ratio tests (see Perofsky et al., 2021 for further details). Briefly, this involved generating distributions of pairwise relatedness estimates for simulated dyads of three different levels of close kinship (parent and offspring, full-siblings, or half-siblings) and for simulated unrelated pairs and then examining the relative likelihood that a given dyad is drawn from one of the close kinship categories relative to the unrelated category. We scored a pair as “related” if the magnitude of the likelihood ratio for one or more of the close kin-unrelated comparisons was associated with a p value of <0.05 across 10,000 permutations.
For 16 individuals in this study, no fecal or tissue samples were available, thus “relatedness” between these individuals and others with whom they interacted could not be assigned. After excluding these individuals, our final sample included behavioral and relatedness data for 38 adults (and 18 additional subadults; see Supplementary Material). We scored three levels of female parity status, building on Voyt et al. (2019): “nulliparous” (never given birth), “parous unsuccessful” (given birth but never successfully reared an infant to age 1 year), and “parous successful” (given birth and had an infant who survived to at least 1 year). Eighteen adult females could be categorized as nulliparous because we defined adult as age 5 years. The year was divided into the mating season (January–March) and non-mating season (April–December) because the value of a potential mating opportunity might be discounted when a female is unlikely to be in estrus.
Adult sex ratio was used as an estimate of supply/demand and was defined as the proportion of adult females in the group or population (cf. Richard et al., 2002) using monthly census data. The population was defined as all individuals with the Ankoatsifaka Research Station trail system and included both marked and unmarked individuals that were solitary, roaming, and group-living. We calculated population and group adult sex ratios as the number of females divided by the total number of adults. For example, the group sex ratio was calculated as the number of adult females in a group divided by the number of total adult females and males in the group, and the population sex ratio was calculated as the number of adult females in the population divided by the total number of adults in the population. Because age class of four unmarked, transient immigrants could not be determined with certainty, we calculated group and population sex ratios with all individuals of unknown age scored as adults and, again, with these individuals scored as subadults. While we ran all statistical models with individuals of unknown age scored as adults in the sex ratio calculations and then ran the same models again with unknown individuals scored as subadults, the results were essentially the same, and thus we only present the models where these individuals were coded as adults.
Because prior work suggests that the outcome of agonistic contests may be determined, in part, by who initiates an interaction (e.g., bison: Lott, 1979; chimpanzees: Wittig and Boesch, 2003), we initially included initiator sex as an additional predictor in our models focusing on who wins contests to, in effect, control for this variation. However, we found that initiator sex interacted in complex ways with several of our predictors of interest, and, thus, we ultimately decided to address the issue of initiator sex interacting with our predictors of interest by conducting separate analyses for when females initiated conflicts and when males initiated conflicts.
Statistical Analysis
Submissive Chatters
We first examined the direction of submissive chatters in intersexual dyads to assess the effects of the above factors on the scope (i.e., consequence) of female power. We ran a set of Bayesian binomial generalized linear mixed models (GLMMs) using the brms package (Bürkner, 2021) for the statistical programming R version 4.1.2 (RStudio Team, 2020; R Core Team, 2020). We used the direction of submissive chatter [female chatter directed at a male (0) vs. male chatter directed at a female (1)] as the binary response variable. Relatedness, female parity status, reproductive season, and either the population (Model 1a) or group (Model 1b) sex ratio were included as fixed effects of interest, and male identity, female identity, and bout ID were included as random effects. Note that for the model examining the group sex ratio (Model 1b), we included only the dyadic interactions for which the group membership was the same for both individuals. Interactions occurring during intergroup encounters or short visits were excluded. For each model we ran four independent MCMC chains for 10,000 iterations, sampling from the posterior distribution using the No-U-Turn sampler (NUTS) after a warmup period of 50% of the run. This yielded a total of 20,000 post-warmup draws, resulting in ESSs of >2,400 for all parameters.
Wins
We next examined whether and how who wins dyadic agonistic interactions is associated with factors potentially associated with female leverage, considering datasets of incidents initiated by males and those initiated by females separately, as noted above. For each of these datasets, we ran two sets of models using different binary response variables. The first considered whether the male in the interaction won the encounter (1) vs. either the female winning or the outcome being neutral (0). However, we also wanted to address the following question: when the conflict has a clear winner, was it the male or the female? Thus, the response variable for the second set of models was whether the male (1) or the female (0) in the interaction won the encounter, excluding all interactions where the outcome was neutral. Note that this second analysis utilized a reduced dataset.
Thus, we again ran a set binomial Bayesian GLMMs for each of the two response variables (Model 2 for male win vs. female win or neutral and Model 3 for male win vs. female win) using the same datasets and model variations as described above for submissive chatters, except that female initiated and male initiated interactions were analyzed separately. Male identity, female identity, and bout ID were included as random effects in all models. Again, for each model (with two exceptions) we ran four independent MCMC chains for 10,000 iterations, sampling from the posterior distribution using the NUTS sampler after a warmup period of 50% of the iterations. This process yielded a total of 20,000 post-warmup draws, ESSs of >800 for all model parameters. For two models (6a and 6b) involving male-initiated contests and including data from subadults (see Supplementary Material) we ran our MCMC chains for 20,000 iterations after we found that initial runs of 10,000 iterations yielded low ESS values. With these longer runs, the total number of post-warmup draws across chains in each of these two models was 40,000, ESSs for all parameters were >490. Finally, for male-initiated contests with a winner and involving adults only (Models 5a and 5b), we excluded relatedness as a predictor variable because there were no cases in our dataset of a male initiating a contest that he won with a close female relative.
Model Diagnostics and Interpretation
For all models, we evaluated convergence using several standard methods implemented in the R package {shinystan} (Gabry and Veen, 2022), including visual examination of MCMC trace plots, graphical posterior predictive checks, and calculation of Brooks-Gelman-Rubin convergence diagnostic (“Rhat”) values (Gelman and Rubin, 1992; Brooks and Gelman, 1998), as well as checks for multicollinearity among predictors of interest using variance inflation factors. Rhat values for all parameters in all “submissive chatter” models was ≤1.004 and was ≤1.008 in all “win” models. VIF values for all variables in all models were low (between 1.0 and 1.1 for all “submissive chatter” models, between 1.0 and 1.6 for all “win” models initiated by females, and between 1.0 and 3.6 for all “win” models initiated by males).
Finally, we used an HDI + ROPE approach (Kruschke, 2015, 2018; Kruschke and Liddell, 2018) to evaluate which of our variables of interest might be considered important predictors in all of our models. This approach evaluates how much of the credible interval for the posterior distribution around each parameter estimate (operationalized as the 95% HDI interval) falls within vs. outside a “region of practical equivalence” (or ROPE). The ROPE range is defined as an area around a null parameter value of zero within which, for practical purposes, values are equivalent to that null (Kruschke, 2015, 2018; Kruschke and Liddell, 2018). We calculated % in ROPE values using the rope() function from the R package {bayestestR} (Makowski et al., 2019). Below, we highlight as being potentially important those predictors for which <10% of the 95% HDI posterior distribution fell within the ROPE range. We note that we are explicitly not making dichotomous decisions about whether these predictors are “significant”; rather, we call attention to those predictors for which the bulk of the posterior probability distribution for their coefficient estimate under the model falls outside the ROPE range and discuss those in relation to our motivating hypotheses.
Results
Submissive Chatters
Out of our initial 1,931 observations of submissive chatters, we identified 1,437 instances of submissive chatters for 35 adults (Nfemales = 17, Nmales = 18) resulting in 39 dyads for which the relatedness between the initiator and the receiver could be estimated (Nrelated = 2 dyads, Nunrelated = 37). Females rarely chattered at males (only 3.9% of the total observations of chatters; Figure 1).
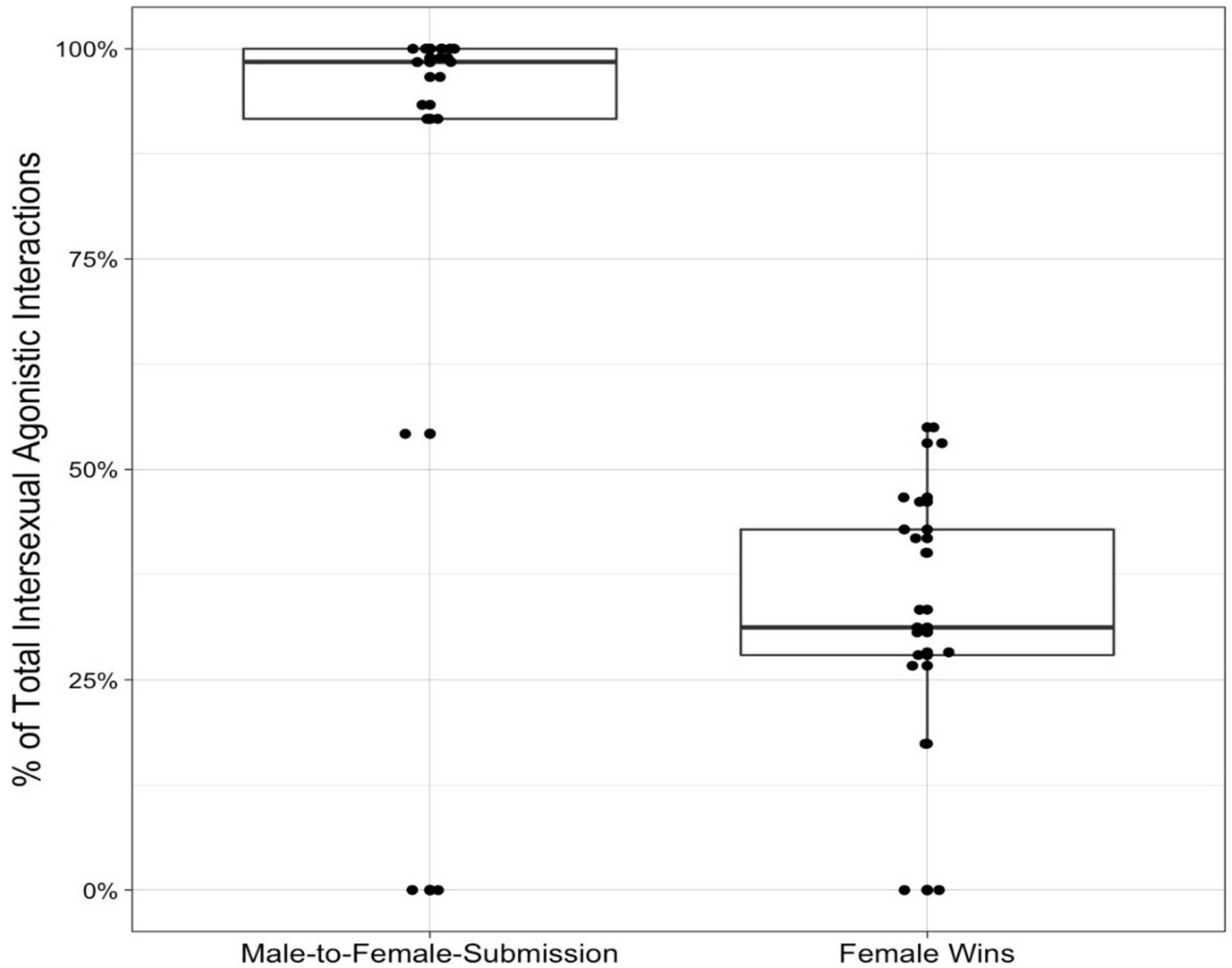
Figure 1. Outcomes of agonistic interactions in intersexual Verreaux’s sifaka dyads. When one individual chattered submissively, males more often chattered at females (N = 1,381) than females chattered at males (N = 56). Percent of conflicts for which females won (N = 639 interactions) out of the total intersexual agonistic interactions (N = 1,850 interactions). Lines represent medians, boxes represent 25th/75th percentiles, and whiskers represent the smallest/largest value within 1.5 times the interquartile range. Each dot represents an individual female’s percentage of total submissive chatters received out of all her submissive chatter interactions, and percentage of total agonistic encounters won out of all her agonistic encounters.
In the model including population sex ratio as a fixed effect (Model 1a), relatedness, female parity, reproductive season, and sex ratio were all potential predictors of the direction of submissive chatters (Table 1 and Figure 2). The odds that a male submitted to a female were greater when the female was a close relative, when she was parous successful (as compared to nulliparous), but not when the female was parous unsuccessful. The odds that a male submitted to a female were lower outside of the mating season than during the mating season and decreased as the population sex ratio became more female-biased.

Table 1. Summary of Bayesian binomial GLMMs modeling the direction of submissive chatters among intersexual adult sifaka dyads.
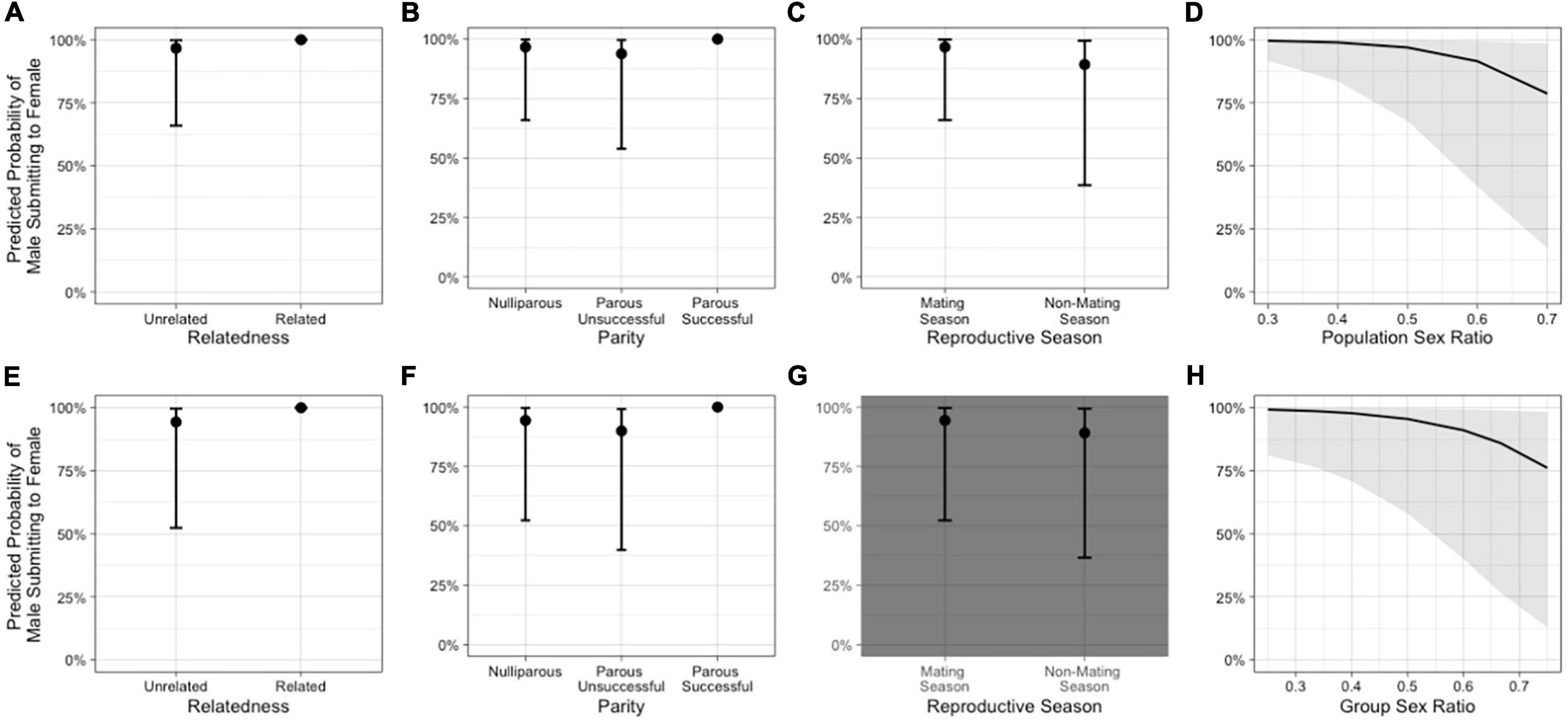
Figure 2. Illustration of the predicted probability of a male submissively chattering to a female based on (A) relatedness (NRelated = 2 dyads, NUnrelated = 37 dyads), (B) female parity, (C) reproductive season, and (D) population sex ratio for Model 1a, which included population sex ratio as a fixed effect, and based on (E) relatedness (NRelated = 2 dyads, NUnrelated = 37 dyads), (F) female parity, (G) reproductive season, and (H) group sex ratio for Model 1b, which included group sex ratio as a fixed effect. The gray background in a panel indicates that the variable was not an important predictor in the model.
In the model including group sex ratio as a fixed effect (Model 1b), relatedness, female parity, and sex ratio were again potential predictors of the direction of submissive chatters, but reproductive season was not (Table 1). The odds that a male submitted to a female were greater when the female was a close relative and when the female was parous successful as compared to nulliparous females. The odds that a male submitted to a female again decreased as the group sex ratio became more female-biased (Supplementary Table 1).
Wins
Of the 2,530 agonistic interactions involving adults where the outcome could clearly be scored, the relatedness between the initiator and the receiver could be estimated for 35 adults (Nfemales = 17, Nmales = 18) in 1,850 of the agonistic interactions, resulting in 41 dyads (Nrelated = 2 dyads, Nunrelated = 39 dyads). Females won 34.5% of the interactions, males won 17.6%, and 47.9% of the interactions were neutral (Figure 1).
Male Winner vs. Female Winner or Neutral Outcome
In the model including population sex ratio as a fixed effect with the female-initiated subset of data (Model 2a – females), relatedness, female parity status, and sex ratio were potentially important predictors of whether the male won the interaction, but reproductive season was not (Table 2 and Figure 3). While the odds that a male won were much greater when the female was a close relative, relatedness results should be interpreted with caution because we had very few related dyads. The odds that a male won were lower when the female was parous (successful or unsuccessful) as compared to nulliparous and decreased as the population sex ratio became more female-biased. For the same model including population sex ratio as a fixed effect but using the male-initiated subset of data (Model 2a – males), the results were rather different. Relatedness, female parity status, reproductive season, and sex ratio were potentially important predictors of whether the male won the interaction (Table 2 and Figure 3). The odds that a male won were greater when the female was parous unsuccessful (compared with nulliparous) and increased (rather than decreased) as the population sex ratio became more female-biased. The odds that a male won were lower when the female was a close relative (in contrast to the direction of the effect when females initiated the conflict), were lower when the female was parous successful (as compared to nulliparous), and outside of the mating season (Supplementary Table 1).
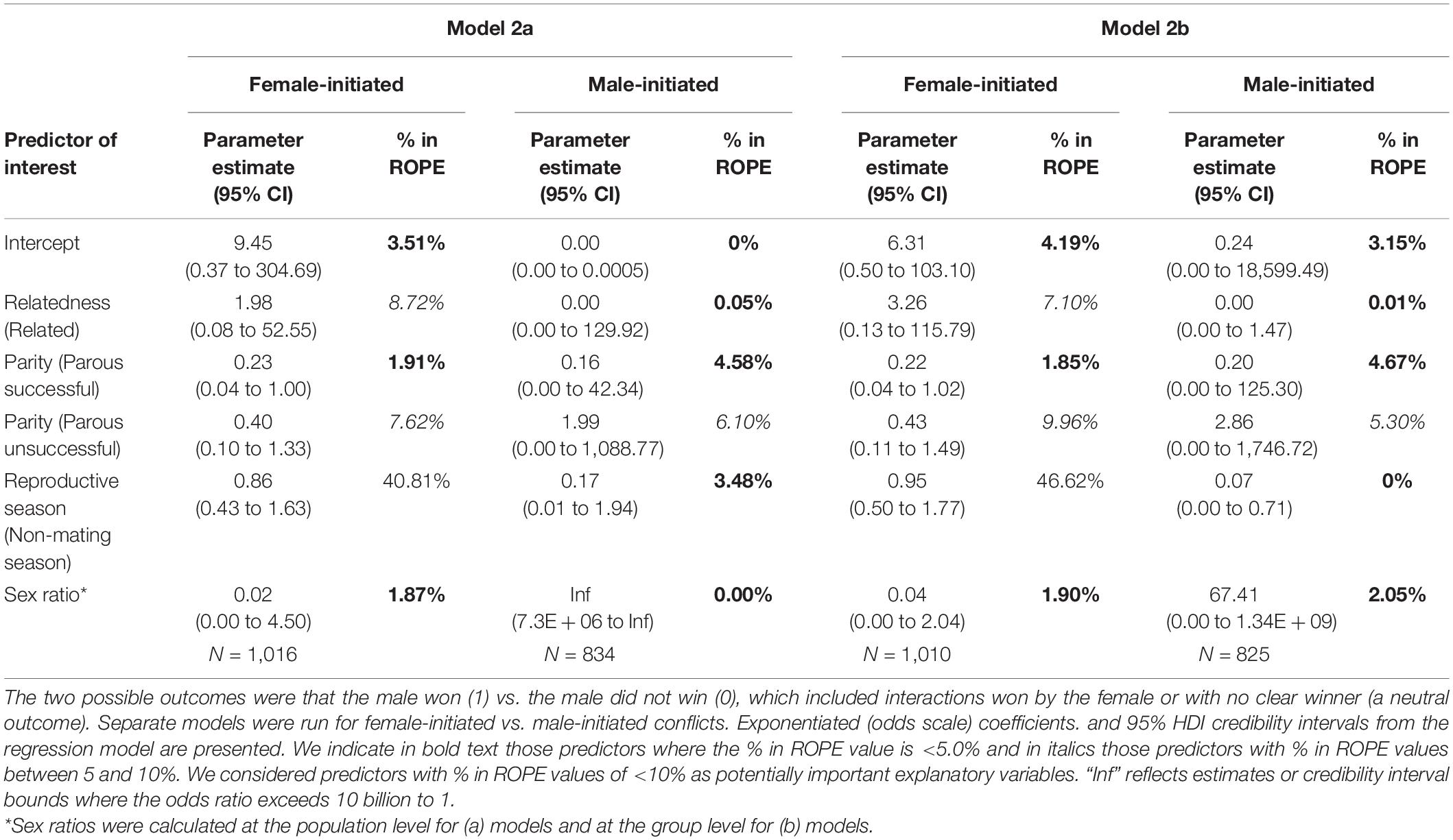
Table 2. Summary of Bayesian binomial GLMMs modeling whether the male in the interaction won the encounter, examining only adult dyads.

Figure 3. Predicted probabilities from Model 2a of the male in an intersexual agonistic encounter winning a resource (1) vs. the female winning or a neutral outcome (0) when the female initiates the encounter, based on (A) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (B) female parity, (C) reproductive season, and (D) population sex ratio. The second row depicts the predicted probabilities from Model 2a of the male winning a resource vs. the female winning or a neutral outcome when the male initiates the encounter, based on (E) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (F) female parity, (G) reproductive season, and (H) group sex ratio. The gray background in a panel indicates that the variable was not an important predictor in the model (see Supplementary Table 2).
In the model including group sex ratio with the female-initiated subset of data (Model 2b – females), relatedness, female parity, and sex ratio were potentially important predictors of whether the male won the interaction but reproductive season was not (Table 2 and Figure 4). The odds that a male won were greater if the female was a close relative but we had few related dyads in our dataset. The odds that a male won were lower when the female was parous (successful or unsuccessful) compared to nulliparous and decreased as the group sex ratio became more female-biased. In the same model including group sex ratio, but using the male-initiated subset of data (Model 2b – males), relatedness, female parity, reproductive season, and sex ratio were predictors of whether the male won the interaction (Table 2 and Figure 4). The odds that the male won were greater when the female was parous unsuccessful (compared to nulliparous) and increased as the group sex ratio became more female-biased. The odds that a male won were lower when the female was a close relative, when the female was parous successful (compared to nulliparous), and when the conflict occurred outside of the mating season (Supplementary Table 1).
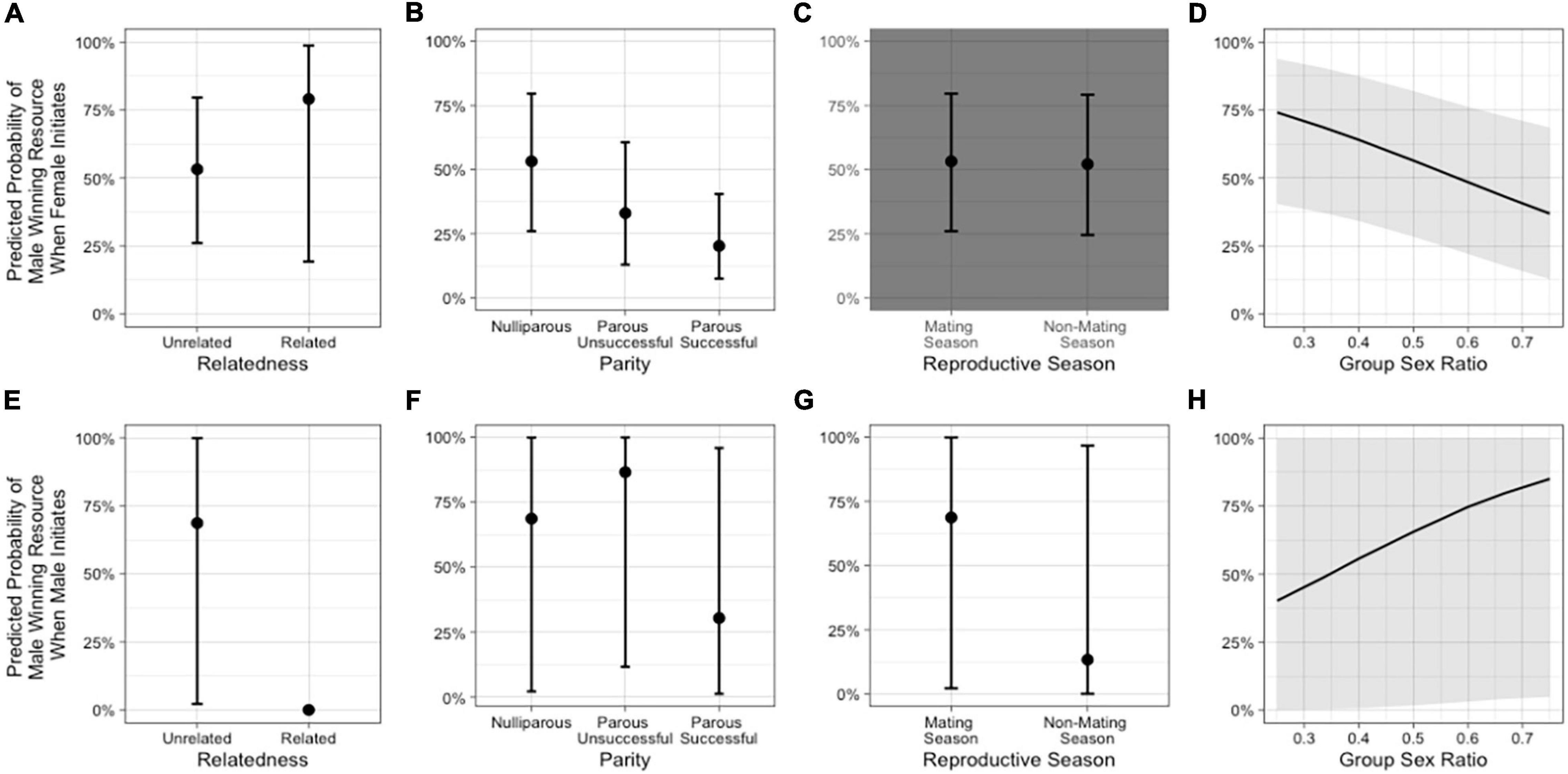
Figure 4. Predicted probabilities from Model 2b of the male in an intersexual agonistic encounter winning a resource (1) vs. the female winning or a neutral outcome (0) when the female initiates the encounter, based on (A) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (B) female parity, (C) reproductive season, and (D) group sex ratio. The second row depicts the predicted probabilities from Model 2b of the male winning a resource vs. the female winning or a neutral outcome when the male initiates the encounter, based on (E) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (F) female parity, (G) reproductive season, and (H) group sex ratio. The gray background in a panel indicates that the variable was not an important predictor in the model (see Supplementary Table 3).
Male Winner vs. Female Winner
We next excluded neutral outcomes and only examined conflicts with a “winner” to explore predictors of whether the male won or not. In the model including population sex ratio with the female-initiated subset of data (Model 3a – females), relatedness, female parity status, reproductive season, and sex ratio were potentially important predictors (Table 3 and Figure 5). The odds that the male won were higher when the female was a close relative and increased as the population sex ratio became more female-biased. The odds that a male won were lower when the female was parous (successful or unsuccessful) compared with nulliparous and when the conflict occurred outside of the mating season. In the same model including population sex ratio, but looking at the male-initiated subset of data (Model 3a – males), female parity status, reproductive season, and sex ratio were potentially important predictors (Table 4 and Figure 5). As noted above, relatedness was excluded as a predictor in this model because we observed no cases of males initiating and winning an interaction against a female relative, likely due, in part, to the small number of related dyads in our sample. The odds that the male won were higher when the female was parous (successful or unsuccessful) compared with nulliparous and increased as the population sex ratio became more female-biased. The odds that a male won were lower when the conflict occurred outside of the mating season (Supplementary Table 1).
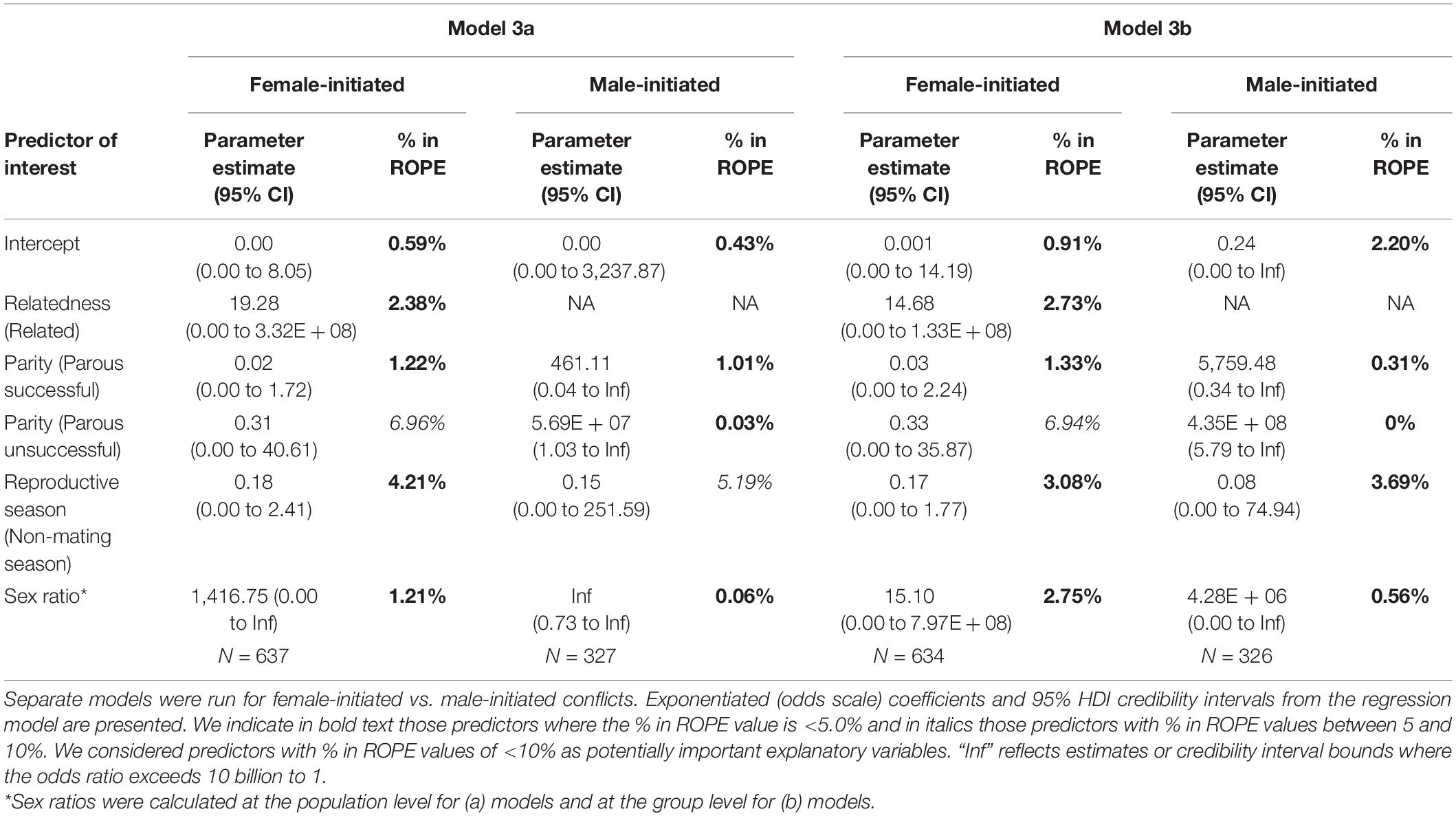
Table 3. Summary of Bayesian binomial GLMMs modeling whether the male in the interaction won (1) or the female in the interaction won (0) examining adult only dyads.
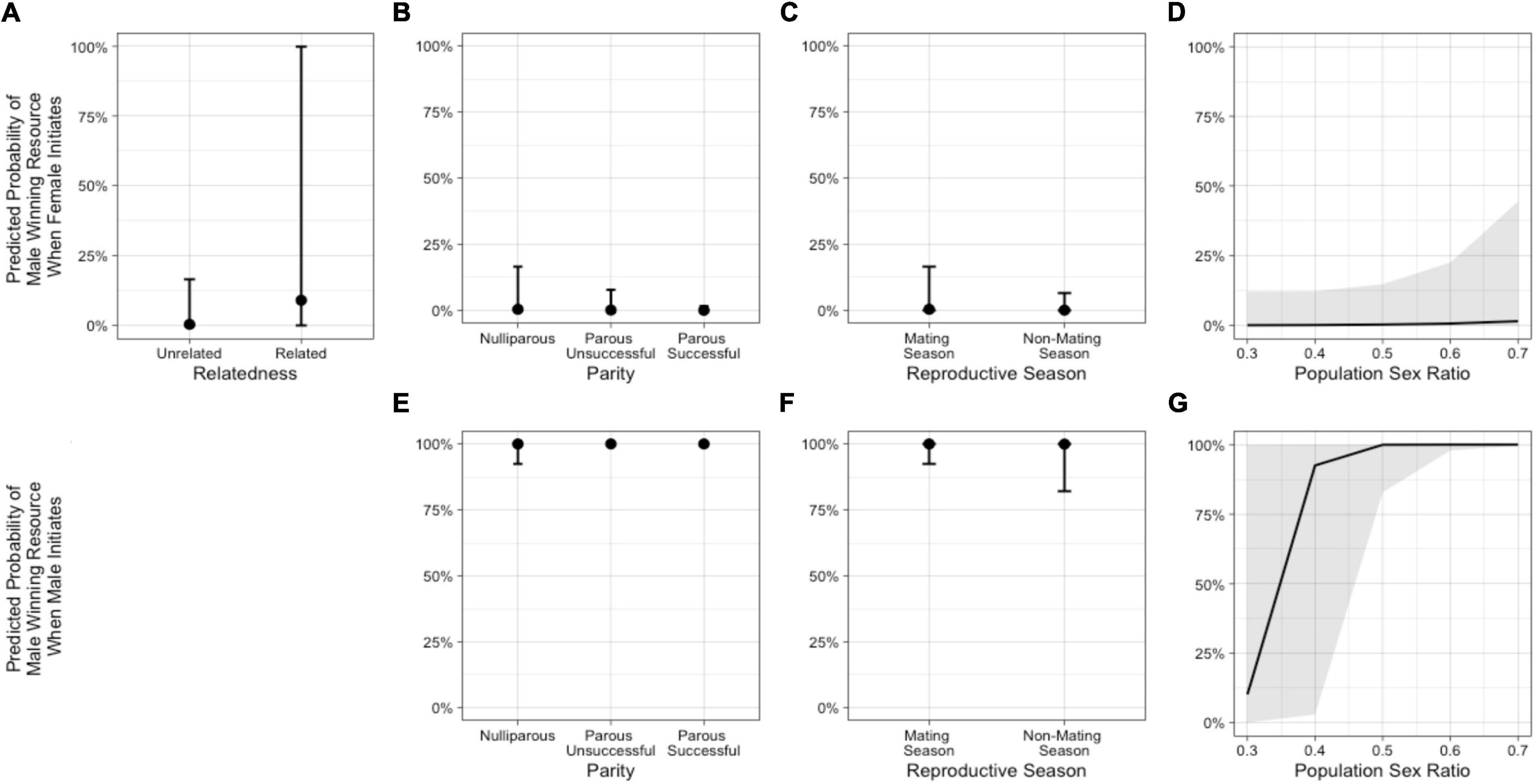
Figure 5. Predicted probabilities from Model 3a of the male in an intersexual agonistic encounter winning a resource (1) vs. the female winning (0) when the female initiates the encounter, based on (A) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (B) female parity, (C) reproductive season, and (D) population sex ratio. The second row illustrates the predicted probabilities from Model 3a of the male winning a resource vs. the female winning when the male initiates the encounter, based on (E) female parity, (F) reproductive season, and (G) population sex ratio. (See Supplementary Table 4).
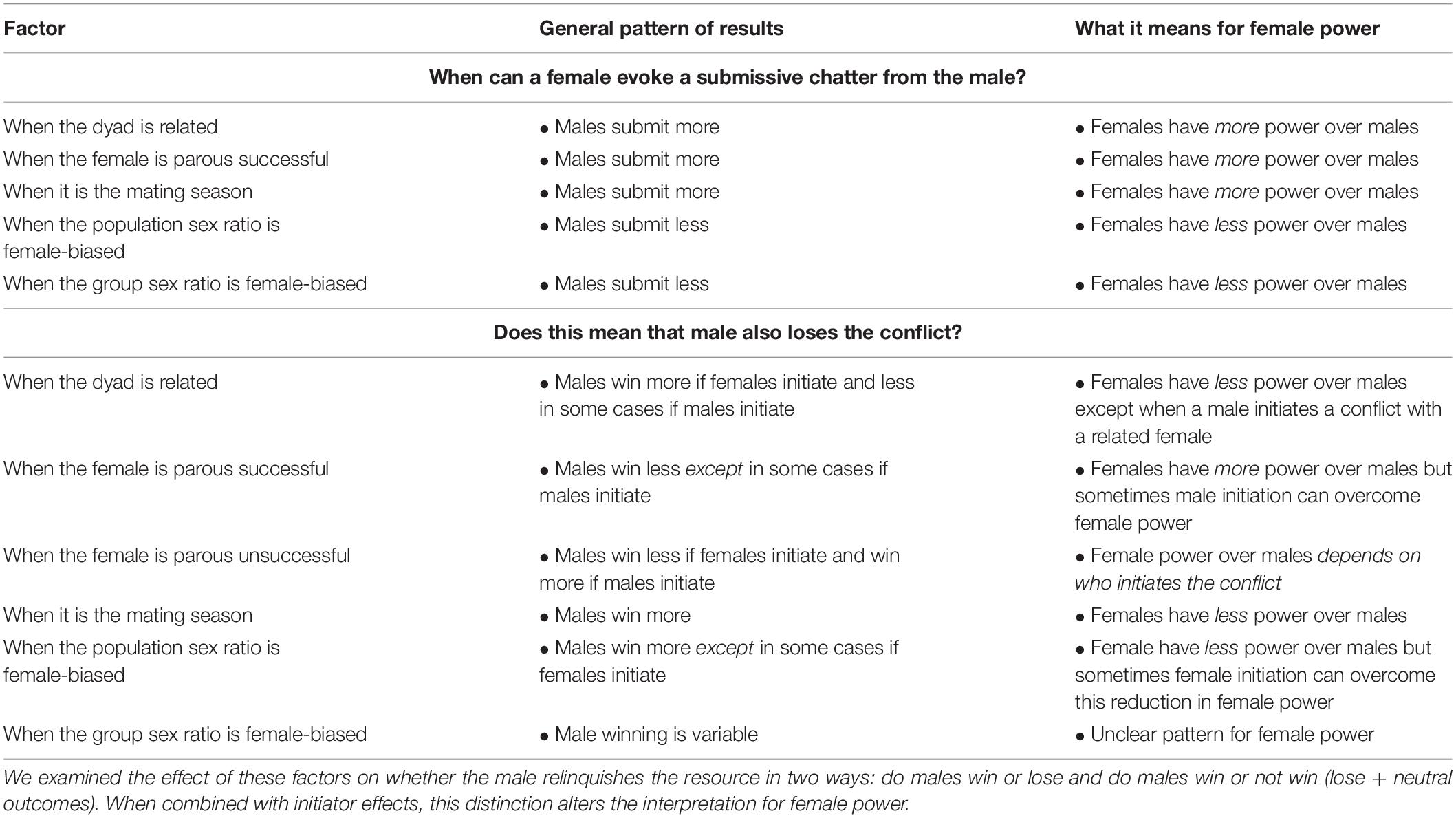
Table 4. Factors thought to affect the value of mating opportunities with females and pattern of their effects on female leverage in intersexual dyads.
In the model including group sex ratio with the female-initiated subset of data (Model 3b – females), relatedness, female parity status, reproductive season, and sex ratio were important predictors (Table 3 and Figure 6). The odds that the male won were higher when the female was a close relative and increased as the group sex ratio became more female-biased. The odds that a male won were lower when the female was parous (successful or unsuccessful) compared with nulliparous and when the conflict occurred outside of the mating season. In the model including group sex ratio with the male-initiated subset of data (Model 3b – males), female parity status, reproductive season, and sex ratio were potentially important predictors (Table 3 and Figure 6). The odds that the male won were higher when the female was parous (successful or unsuccessful) compared with nulliparous and increased as the group sex ratio became more female-biased. The odds that a male won were lower when the conflict occurred outside of the mating season (Supplementary Table 1).
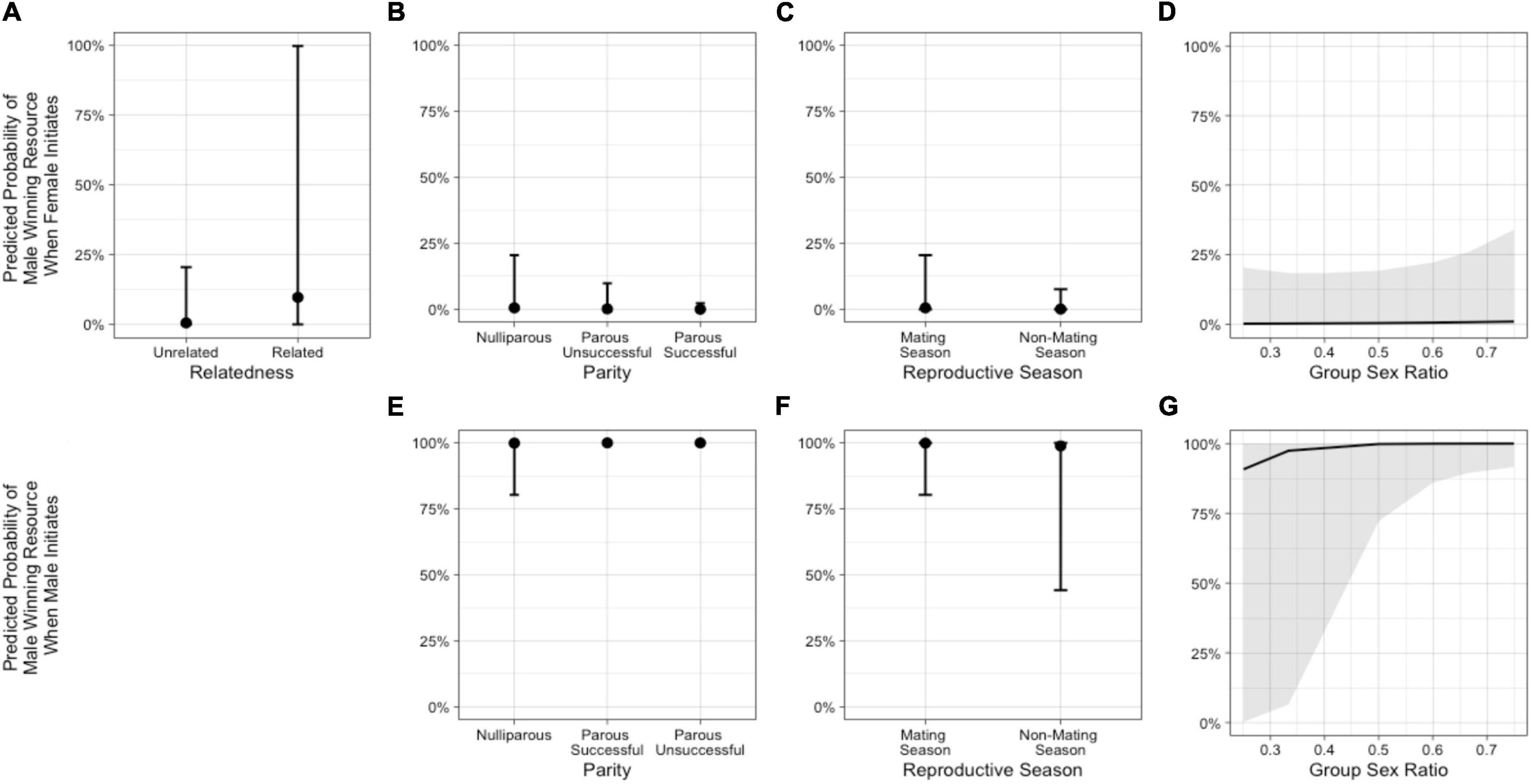
Figure 6. Predicted probabilities from Model 3b of the male in an intersexual agonistic encounter winning a resource (1) vs. the female winning (0) when the female initiates the encounter, based on (A) relatedness (NRelated = 2 dyads, NUnrelated = 39 dyads), (B) female parity, (C) reproductive season, and (D) group sex ratio. The second row illustrates the predicted probabilities from Model 3b of the male winning a resource vs. the female winning when the male initiates the encounter, based on (E) female parity, (F) reproductive season, and (G) group sex ratio. (See Supplementary Table 5).
Discussion
Despite three decades of publications on female economic power (e.g., Hand, 1986; Smuts, 1987; Lewis, 2018, 2020), to date little empirical research has been devoted to examining female leverage over males. We tested the hypothesis that female leverage over males in Verreaux’s sifaka varies with the value of the mating opportunity. Adult females rarely chattered submission toward males, but their ability to evoke submission from males was influenced by sex ratio, parity status, and mating season (Table 4). Consistent with the hypothesis that economic factors shape female intersexal power, females had more leverage over males when their fertilization potential was higher, there were fewer of them, and they had demonstrated successful mothering skills. Interestingly, the strong unidirectionality (male to female) of submission did not correspond with the direction of wins in intersexual conflicts. Both female losses and neutral outcomes were common (Figure 1). Kinship, sex ratio, parity status, and mating season did affect a female’s ability to win an intersexual conflict, and mostly as expected. However, female power to win intersexual conflicts was also seemingly determined, in part, by who initiates the agonistic interaction in question. Together, these findings indicate that female leverage varies with the level of commodity value and is conditional. Females have more power over some males than others, and they are able to make males relinquish contested resources in some situations.
Submissive Chatters
Females rarely chattered at males, but the direction of submission in intersexual dyads was influenced by factors that affect the value of a mating opportunity in important ways. Female parity status had a strong effect on submission. Males were >150 times more likely to chatter submissively to a female who had successfully reared an offspring than to a nulliparous female. This finding is consistent with the hypothesis that female intersexual leverage varies with the potential benefits of mating with a particular female. It is likewise similar to earlier research using a different subset of the data from the same population (Voyt et al., 2019) and research on mouse lemurs (Microcebus murinus, M. lehilahytsara) that found parous females evoke submission from males more than nulliparous females (Hohenbrink et al., 2016). As with other lemurs exhibiting female-biased intersexual power (e.g., Hohenbrink et al., 2016), the potential to reproduce with a female was a less valuable source of leverage outside of the mating season: females were more likely to evoke a submissive chatter from a male during the mating season than outside of it. Additionally, the odds that a male submitted to a female vs. the female submitting to him was lower when the sex ratio was more female-biased, consistent with the idea that female leverage decreases as their supply increases (Noë et al., 1991; Noë, 2017). Previous primate research has also found that sex ratios can have important effects on female power (Hemelrijk et al., 2008; Izar et al., 2021). Taken together, our results suggest that female power over males is influenced by economic factors: female power to evoke submission from males increases with the increasing value of the fertilizable egg and decreases with increasing supply of females.
Contrary to our expectation, however, we did not find that females had greater leverage over unrelated males. Instead, males were more likely to chatter submissively to close female relatives. One possibility is that a different base (sensu Lewis, 2002, 2020) of power, i.e., genes, is a stronger determinant of intersexual power than mating opportunities among kin. Kin selection can mask economic effects (Noë et al., 1991) because, like leverage (Hand, 1986), inclusive fitness adds to the cost of winning in some conflicts. Sex differences in the opportunity costs of inbreeding might also influence intersexual leverage if females exhibit a strong preference to avoid inbreeding (Antfolk et al., 2012). While our findings regarding the effects of relatedness on female leverage should be interpreted with caution because we had very few related dyads in our dataset, our analyses of individuals aged ≥ 3 years that included ≥ 20 related dyads found the same unexpected effect (Supplementary Table 2). More research is clearly needed regarding the effect of kinship on intersexual power in sifaka, but our results suggest that female intersexual leverage may not be consistent across all males.
Wins
Our analysis of whether a male wins an intersexual conflict presents a different and more complicated picture of the factors impacting female leverage. While males readily signaled submission to females (Figure 1), it was not uncommon for females to use repeated aggression to convince a male to relinquish a resource, irrespective of whether he chattered after each aggressive act. Females also sometimes had to use a combination of aggressive acts (e.g., lunge, cuff, and bite) before eventually winning the encounter. Thus, the power to evoke submission is clearly very different from the power needed to usurp a resource from another individual.
For the most part, economic factors had the predicted influence on female power (Table 4), but the sex of the individual that initiated the conflict was an important mitigator. For example, consistent with expectations based on market effects, the odds that a male won a conflict increased as the supply of females increased, but only when males initiated the conflict and only when sex ratio was examined at the population level [(a) models]. Our other results regarding sex ratio effects [i.e., in the group sex ratio (b) models and in population sex ratio models when females initiated the conflict] were inconsistent with expectations based on market effects. Furthermore, reproductive season was not an important determinant of which sex won a conflict unless the male initiated, in which case, contrary to expectations and contrary to the pattern for submissive chatters, males were more likely to win during the mating season. Overall, these findings suggest that economic factors may have some influence on female intersexual power to win agonistic contests, but these affects can be limited.
Our finding that males often are more likely to win when they initiate may be associated with what Flack and de Waal (2007) termed as “subordination signaling” (i.e., when power relationships are fairly institutionalized and individuals spontaneously communicate their lower status to higher ranking groupmates, in contrast with reacting submissively in response to agonism). In a study of power in the different but nearby Kirindy Forest population of Verreaux’s sifaka, Lewis (2019) found that when males emit chatter vocalizations in peaceful contexts (i.e., without provocation), females were less likely to usurp the resource. In our data for the Ankoatsifaka population of sifaka, an interaction could be initiated with an approach, an aggressive act, or an unprovoked submissive act, depending on when the agonistic interaction occurred within a sequence of behaviors (e.g., an approach was only scored as the initiation of the interaction if the agonism began immediately afterward; the approach was not scored as the beginning of the conflict if the dyad members were, for example, in proximity for 20 min prior to the onset of the conflict). Thus, males may be more likely to win an agonistic interaction if they initiated it because (1) they are unlikely to use aggression unless they have a good chance of winning, (2) they may be more likely to win because they started the interaction with an unprovoked, peaceful chatter, or (3) they approached and immediately chattered (cf. “appeasement”: Beisner and McCowan, 2014). In other words, when males start an agonistic interaction by signaling “subordination” (i.e., communicating their lower status without provocation: Flack and de Waal, 2007), they may be more likely to “win.” By providing more information, communication can increase the chance of a peaceful resolution to conflicts (Noë et al., 1991). Given the seemingly importance of initiator sex for determining who wins an intersexual conflict, further research is needed to explore the causes and consequences of different strategies for initiating interactions.
Neutral Outcomes
One of the most surprising results of this study is the finding that half of sifaka agonistic encounters end with both individuals sitting beside one another rather than a withdrawal by one of the interactants. A central dogma of ethology is that winners gain or maintain possession of a resource while losers retreat and avoid further escalation (Parker, 1974). Despite this focus on the binary results of winning and losing, sharing or tolerance is a third possible outcome (Hall et al., 2020). Communication of lower status in the relationship reduces usurpation of resources in sifaka (Lewis, 2019) and may similarly increase the chance that a higher-ranking individual will share a resource. Indeed, sifaka negotiate their relationships using a variety of behaviors (e.g., Lewis, 2005). While it is possible that our operational definition measured in seconds rather than in minutes may partially explain the large number of interactions scored as neutral, the vast majority of outcomes would have been scored the same regardless. Moreover, the speed with which a loser withdraws from a conflict should be indicative of a winner’s power. Supplants involve an immediate displacement of another individual, and exploration of our data indicated that our results did not differ much whether we included (as we do here) or excluded supplants from the dataset. Our study suggests that examining neutral outcomes in more detail may be a fruitful area of future research.
Not all Outcomes Are Equal
Our study also demonstrates the importance of examining multiple scopes of power (sensu Lewis, 2002, 2020) within the full landscape of power (Lewis, 2022). The consequences of female intersexual leverage in sifaka include both evoking submissive chatters and winning resources from males. As noted above, and unlike some other species with female-biased intersexual power (e.g., wooly lemurs: Ramanankirahina et al., 2011) however, female sifaka abilities to achieve these outcomes are not the same. Sifaka chatter vocalizations are formalized signals (Kraus et al., 1999; Lewis, 2019), and, as such, they unambiguously communicate status (de Waal, 1986). These kinds of signals are argued to be associated with a stable layer of power while winning is associated with a more flexible layer of power (e.g., “structure” vs. “surface structure”: Hinde, 1979; “formal dominance” vs. “real dominance”: de Waal, 1986). Hence, when a conflict arises, a male may be able to evoke a win or a neutral outcome with a female if he signals with a chatter vocalization that the stable layer of power is unchanged. Our results are consistent with the hypothesis that a male’s communicating about formal status (de Waal, 1986; cf. “structural power”: Hinde, 1979; cf. “relationship state”: Flack and de Waal, 2007) reduces the chance that a female will usurp contested resources, as was suggested by previous research on a different population of Verreaux’s sifaka (Lewis, 2019). Our study also highlights the value of investigating multiple scopes of power within the same study.
Evolutionary Explanations of Female-Biased Power
Hypotheses about the evolution of female-biased power often point to the importance of resources for female fitness, such as explanations centered around the energetic constraints that females face for supporting reproduction (e.g., Jolly, 1984; Young et al., 1990; Wright, 1999). Researchers then often test these hypotheses by recording whether interactions are “decided” or “undecided” based upon whether one individual exhibits submissive behaviors (Pereira et al., 1990; Pereira and Kappeler, 1997; Pereira, 2006). The implicit assumption in these studies is that if a female can evoke submission from a male, then she wins the interaction. Our study, however, demonstrates that signaling submission and winning access to a resource are not the same. Sifaka males often chatter submissively to a female, but then do not relinquish a resource to her (neutral outcome). Likewise, females also often abandon a resource after the male communicates his subordinate status (Lewis, 2019), effectively the male “wins.” If food and other resources are so critically important to female fitness that it drives the evolution of female-biased power structures, then one would expect intersexual conflicts to result in females more consistently usurping or maintaining control of those resources, but our results suggest that this is not the case, at least among sifaka. Female power in intersexual relationships is known to incorporate a variety of behaviors and outcomes, including aggression, submission, and priority of access to resources (Kappeler, 1990; Radespiel and Zimmermann, 2001; Lewis, 2018, 2020). More research into how these behaviors and outcomes relate with one another is needed if the evolutionary causes of female-biased power are to be determined.
Conclusion
Biological markets result in power asymmetries (Noë et al., 1991; Noë and Hammerstein, 1994, 1995; Lewis, 2002; Noë, 2017). Voyt et al. (2019) demonstrated that female intersexual power in Verreaux’s sifaka is better described as “female leverage” than “female dominance” because females seem to derive power from their control over a resource in high demand – namely an egg that can be fertilized to produce offspring – rather than an asymmetry in fighting ability. Our study builds on this previous research by demonstrating that economic factors affecting the value and supply of reproductive opportunities influence female leverage. The value of mating opportunities is greater when the female has successfully demonstrated that she can translate fertilization into surviving offspring (and presumably higher fitness) and can be discounted outside of the mating season, when the fertilization opportunity that a female represents may not be available for months. However, we also found that factors other than market effects, such as who initiates a conflict, can impact who wins the resource, if anyone wins at all. Finally, our finding that males are less submissive to close female kin highlights the value in conceptualizing the phenomenon often referred to as “female dominance” as an aggregate of multiple social relationships. Rather than all females having power over all males, this power can vary across and within dyads, as well as over time and between contexts. Greater attention needs to be placed on understanding female leverage and this relational aspect of power. The “power framework” (Lewis, 2002, 2020) provides useful tools for standardizing this endeavor. Power is multi-faceted, and more studies will be needed to understand the full power landscape (Lewis, 2002, 2020) in Verreaux’s sifaka and other animals exhibiting female-biased power.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5061/dryad.ngf1vhhwh.
Ethics Statement
The animal study was reviewed and approved by University of Texas at Austin Institutional Animal Care and Use Committee and the CAFF/CORE Committee in Madagascar.
Author Contributions
RL conceived the study, collected the data and paid the research assistants. RL and AD funded for the project. RL and GB scored the data. GB and AD analyzed the data. All authors designed the study, wrote and edited the manuscript.
Funding
Data collection for this project was financed by the University of Texas at Austin, The Leakey Foundation, Primate Conservation, Inc., NSF BES# 1719654, and multiple private donors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Charlotte Hemelrijk, Joey Cheng, Elise Huchard, Peter Kappeler, and Tanja Hentschel for inviting us to participate in this special issue. The manuscript benefited greatly from the suggestions of two reviewers. The majority of the data analyzed here were collected by the Sifaka Research Project research assistants at the Ankoatsifaka Research Station. We would also like to thank the Madagascar government CAFF/CORE, and Madagascar National Parks for permission to conduct this research and the University of Antananarivo and MICET for facilitating research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.851880/full#supplementary-material
References
Abondano, L. (2014). Male Reproductive Skew in Multimale Social Groups of Verreaux’s Sifaka (Propithecus verreauxi) at Kirindy Mitea National Park, Madagascar. Master’s thesis, Austin (TX): The University of Texas at Austin.
Allen, M. L., Wilmers, C. C., Elbroch, L. M., Golla, J. M., and Wittmer, H. U. (2016). The importance of motivation, weapons, and foul odors in driving encounter competition in carnivores. Ecology 97, 1905–1912. doi: 10.1002/ecy.1462
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–267. doi: 10.1163/156853974x00534
Antfolk, J., Lieberman, D., and Santtila, P. (2012). Fitness costs predict inbreeding aversion irrespective of self-involvement: support for hypotheses derived from evolutionary theory. PLoS One 7:e50613. doi: 10.1371/journal.pone.0050613
Beisner, B. A., and McCowan, B. (2014). Signaling context modulates social function of silent bared-teeth displays in rhesus macaques (Macaca mulatta). Am. J. Primatol. 76, 111–121. doi: 10.1002/ajp.22214
Bolt, L. M. (2013). Squealing rate indicates dominance rank in the male ring-tailed lemur (Lemur catta). Am. J. Primatol. 75, 1174–1184. doi: 10.1002/ajp.22179
Brockman, D. (1999). Reproductive behavior of female Propithecus verreauxi at Beza Mahafaly, Madagascar. Int. J. Primatol. 20, 375–398. doi: 10.1023/A:1020500804442
Brockman, D. K. (1994). Reproduction and Mating System of Verreaux’s sifaka, Propithecus verreauxi, at Beza Mahafaly, Madagascar. Dissertation, New Haven, CT: Yale University.
Brooks, S. P., and Gelman, A. (1998). General methods for monitoring convergence of iterative simulations. J. Computat. Graph. Stat. 7, 434–455. doi: 10.2307/1390675
Bürkner, P.-C. (2021). Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 100, 1–54. doi: 10.18637/jss.v100.i05
Charlesworth, B., and Charlesworth, D. (1999). The genetic basis of inbreeding depression. Genet. Res. 74, 329–340. doi: 10.1017/S0016672399004152
de Waal, F. B. M. (1986). The integration of dominance and social bonding in primates. Q. Rev. Biol. 61, 459–479. doi: 10.1086/415144
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour 125, 283–313. doi: 10.1163/156853993x00290
Emlen, S. T., and Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. doi: 10.1126/science.327542
Flack, J. C., and de Waal, F. (2007). Context modulates signal meaning in primate communication. PNAS 104, 1581–1586. doi: 10.1073/pnas.0603565104
Gabry, J., and Veen, D. (2022). shinystan: Interactive Visual and Numerical Diagnostics and Posterior Analysis for Bayesian Models. R Package Version 2.6.0. Available online at: https://CRAN.R-project.org/package=shinystan.
Gelman, A., and Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472.
Hall, C. L., Porter, M. A., and Dawkins, M. S. (2020). Dominance, sharing, and assessment in an iterated Hawk-Dove game. J. Theor. Biol. 493:110101. doi: 10.1016/j.jtbi.2019.110101
Hand, J. L. (1986). Resolution of social conflicts: dominance, egalitarianism, spheres of dominance, and game theory. Q. Rev. Biol. 61, 201–220. doi: 10.1086/414899
Hemelrijk, C. K., Wantia, J., and Isler, K. (2008). Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS One 3:e2678. doi: 10.1371/journal.pone.0002678
Hinde, R. A. (1976). Interactions, Relationships and social structure. Man 11, 1–17. doi: 10.2307/2800384
Hinde, R. A. (1979). “The nature of social structure,” in The Great Apes, eds D. A. Hamburg and E. R. McCown (Menlo Park, CA: Benjamin/Cummings), 295–315.
Hohenbrink, S., Schaarschmidt, F., Bünemann, K., Gerberding, S., Zimmermann, E., and Radespiel, U. (2016). Female dominance in two basal primates, Microcebus murinus and Microcebus lehilahytsara: variation and determinants. Anim. Behav. 122, 145–156. doi: 10.1016/j.anbehav.2016.10.008
Isvaran, K., and Clutton-Brock, T. (2007). Ecological correlates of extra-group paternity in mammals. Proc. Biol. Sci. 274, 219–224. doi: 10.1098/rspb.2006.3723
Izar, P., Fernández-Bolaños, M., Seex, L., Gort, G., Suscke, P., and Tokuda, M. (2021). Female emancipation in a male dominant, sexually dimorphic primate under natural conditions. PLoS One 16:e0249039. doi: 10.1371/journal.pone.0249039
Jolly, A. (1984). “The puzzle of female feeding priority,” in Female Primates: Studies by Women Primatologists, ed. M. Small (New York, NY: Liss), 197–215.
Kalinowski, S. T., Taper, M. L., and Marshall, T. C. (2007). Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x
Kappeler, P. (1990). Female dominance in Lemur catta: more than just female feeding priority? Folia Primatol. 55, 92–95. doi: 10.1159/000156504
Kokko, H., and Ots, I. (2006). When not to avoid inbreeding. Evolution 60, 467–475. doi: 10.1111/j.0014-3820.2006.tb01128.x
Konovalov, D. A., Manning, C., and Henshaw, M. T. (2004). kingroup: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol. Ecol. Notes 4, 779–782. doi: 10.1111/j.1471-8286.2004.00796.x
Kraus, C., Heistermann, M., and Kappeler, P. M. (1999). Physiological suppression of sexual function of subordinate males: a subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiol. Behav. 66, 855–861. doi: 10.1016/S0031-9384(99)00024-24
Kruschke, J. K. (2015). Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan. Amsterdam: Elsevier.
Kruschke, J. K. (2018). Rejecting or accepting parameter values in Bayesian estimation. Adv. Meth. Pract. Psychol Sci. 1, 270–280. doi: 10.1177/2515245918771304
Kruschke, J. K., and Liddell, T. M. (2018). The Bayesian New Statistics: hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychonom. Bull. Rev. 25, 178–206. doi: 10.3758/s13423-016-1221-4
Lawler, R. R., Richard, A. F., and Riley, M. A. (2001). Characterization and screening of microsatellite loci in a wild lemur population (Propithecus verreauxi verreauxi). Am. J. Primatol. 55, 253–259. doi: 10.1002/ajp.1058
Leimberger, K. G., and Lewis, R. J. (2017). Patterns of male dispersal in Verreaux’s sifaka (Propithecus verreauxi) at Kirindy Mitea National Park. Am. J. Primatol. 79:e22455. doi: 10.1002/ajp.22455
Lewis, R. J. (2002). Beyond dominance: the importance of leverage. Q. Rev. Biol. 77, 149–164. doi: 10.1086/343899
Lewis, R. J. (2004). Sources of Variation in Male-female Relationships: Power, Conflict, and Cooperation. Dissertation, Durham (NC): Duke University. dissertation
Lewis, R. J. (2005). Sex differences in scent-marking in sifaka: mating conflict or male services? Am. J. Phys. Anthropol. 128, 389–398. doi: 10.1002/ajpa.20206
Lewis, R. J. (2008). Social influences on group membership in Propithecus verreauxi verreauxi. Int. J. Primatol. 29, 1249–1270. doi: 10.1007/s10764-008-9304-3
Lewis, R. J. (2018). Female power in primates and the phenomenon of female dominance. Annu. Rev. Anthropol. 47, 533–551. doi: 10.1146/annurev-anthro-102317-045958
Lewis, R. J. (2019). Subordination signals improve the quality of social relationships in Verreaux’s Sifaka: implications for the evolution of power structures and social complexity. Am. J. Phys. Anthropol. 169, 599–607. doi: 10.1002/ajpa.23876
Lewis, R. J. (2020). Female power: a new framework for understanding “female dominance” in lemurs. Folia Primatol. 91, 48–68. doi: 10.1159/000500443
Lewis, R. J. (2022). Aggression, rank and power: why hens (and other animals) do not always peck according to their strength. Phil. Trans. R. Soc. B 377:20200434. doi: 10.1098/rstb.2020.0434
Lewis, R. J., and Bannar-Martin, K. H. (2012). The impact of Cyclone Fanele on a tropical dry forest in Madagascar: impact of cyclone on dry forest. Biotropica 44, 135–140. doi: 10.1111/j.1744-7429.2011.00799.x
Lewis, R. J., and Kappeler, P. M. (2005). Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest. Am. J. Primatol. 67, 347–364. doi: 10.1002/ajp.20187
Lewis, R. J., and Lawler, R. R. (2013). “Verreaux’s sifaka,” in All the World’s Lemurs, Lorises, Bushbabies, and Pottos: The Primate Suborder Strepsirhini. All the World’s Primates Ebook Series, ed. N. Rowe (Charlestown, RI: Pogonias Press).
Lewis, R. J., Sandel, A. A., Hilty, S., and Barnett, S. E. (2020). The collective action problem but not numerical superiority explains success in intergroup encounters in Verreaux’s sifaka (Propithecus verreauxi): implications for individual participation and free-riding. Int. J. Primatol. 41, 305–324. doi: 10.1007/s10764-020-00155-156
Lewis, R., and Axel, A. (2019). “Using vegetation phenology and long-term demographic data to assess the impact of cyclone fanele on a lemur population in madagascar,” in Primate Research and Conservation in the Anthropocene, eds A. M. Behie, J. A. Teichroeb, and N. Malone (Cambridge: Cambridge University Press), 216–236. doi: 10.1017/9781316662021.013
Lewis, R., and van Schaik, C. (2007). Bimorphism in male Verreaux’s sifaka in the Kirindy Forest of Madagascar. Int. J. Primatol. 28, 159–182. doi: 10.1007/s10764-006-9107-3
Lott, D. F. (1979). Dominance relations and breeding rate in mature male american bison. Z. Tierpsychol. 49, 418–432. doi: 10.1111/j.1439-0310.1979.tb00302.x
Makowski, D., Ben-Shachar, M. S., and Lüdecke, D. (2019). bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Software 4:1541. doi: 10.1371/journal.pmed.1000048
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M. (1998). Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655. doi: 10.1046/j.1365-294x.1998.00374.x
Noë, R. (2017). Local mating markets in humans and non-human animals. Behav. Ecol. Sociobiol. 71:148.
Noë, R., and Hammerstein, P. (1994). Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11. doi: 10.1007/bf00167053
Noë, R., Schaik, C., and van Hooff, J. (1991). The market effect: an explanation for payoff asymmetries among collaborating animals. Ethology 87, 97–118. doi: 10.1111/j.1439-0310.1991.tb01192.x
Norscia, I., Antonacci, D., and Palagi, E. (2009). Mating first, mating more: biological market fluctuation in a wild prosimian. PLoS One 4:e4679. doi: 10.1371/journal.pone.0004679
Parker, G. A. (1974). Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243. doi: 10.1016/0022-5193(74)90111-8
Pereira, M. E. (2006). “Obsession with agonistic power,” in Ringtailed Lemur Biology: Lemur catta in Madagascar, eds A. Jolly, R. W. Sussman, N. Koyama, and H. Rasamimanana (Boston, MA: Springer US), 245–270. doi: 10.1007/978-0-387-34126-2_15
Pereira, M. E., and Kappeler, P. M. (1997). Divergent systems of agonistic behaviour in lemurid primates. Behaviour 134, 225–274. doi: 10.1163/156853997x00467
Pereira, M., Kaufman, R., Kappeler, P., and Overdorff, D. (1990). Female dominance does not characterize all of the lemuridae. Folia Primatol. 55, 96–103. doi: 10.1159/000156505
Perofsky, A. C., Ancel Meyers, L., Abondano, L. A., Di Fiore, A., and Lewis, R. J. (2021). Social groups constrain the spatiotemporal dynamics of wild sifaka gut microbiomes. Mol. Ecol. 30, 6759–6775. doi: 10.1111/mec.16193
Pochron, S. T., Fitzgerald, J., Gilbert, C. C., Lawrence, D., Grgas, M., Rakotonirina, G., et al. (2003). Patterns of female dominance in Propithecus diadema edwardsi of Ranomafana national park, Madagascar. Am. J. Primatol. 61, 173–185. doi: 10.1002/ajp.10119
Pusey, A., and Wolf, M. (1996). Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. doi: 10.1016/0169-5347(96)10028-8
Puurtinen, M. (2011). Mate choice for optimal (k)inbreeding. Evolution 65, 1501–1505. doi: 10.1111/j.1558-5646.2010.01217.x
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radespiel, U., and Zimmermann, E. (2001). Female dominance in captive gray mouse lemurs (Microcebus murinus). Am. J. Primatol. 54, 181–192. doi: 10.1002/ajp.1029
Rakotoarisoa, G., Shore, G. E., Mcguire, S. M., Engberg, S. E., Edward, E., Louis, J., et al. (2006). Characterization of 13 microsatellite marker loci in Verreaux’s sifaka (Propithecus verreauxi). Mol. Ecol. Notes 6, 1122–1125. doi: 10.1111/j.1471-8286.2006.01458.x
Ramanankirahina, R., Joly, M., and Zimmermann, E. (2011). Peaceful primates: affiliation, aggression, and the question of female dominance in a nocturnal pair-living lemur (Avahi occidentalis). Am. J. Primatol. 73, 1261–1268. doi: 10.1002/ajp.20998
Rasambainarivo, F. T., Junge, R. E., and Lewis, R. J. (2014). Biomedical evaluation of Verreaux’s sifaka (Propithecus verreauxi) from Kirindy Mitea National Park in Madagascar. J. Zoo Wildl. Med. 45, 247–255. doi: 10.1638/2013-0038R1.1
Richard, A. F. (1978). Behavioral Variation: Case Study of a Malagasy Lemur. Lewisburg, PA: Bucknell University Press.
Richard, A. F. (1987). “Malagasy prosimians: female dominance,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, and R. W. Wrangham (Chicago, IL: University of Chicago Press), 25–33.
Richard, A. F., Dewar, R. E., Schwartz, M., and Ratsirarson, J. (2002). Life in the slow lane? demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi). J. Zool. 256, 421–436. doi: 10.1017/s0952836902000468
Richard, A. F., Rakotomanga, P., and Schwartz, M. (1991). Demography of Propithecus verreauxi at Beza Mahafaly, Madagascar: sex ratio, survival, and fertility, 1984-1988. Am. J. Phys. Anthropol. 84, 307–322. doi: 10.1002/ajpa.1330840307
Richard, A. F., Rakotomanga, P., and Schwartz, M. (1993). Dispersal by Propithecus verreauxi at Beza Mahafaly, Madagascar: 1984-1991. Am. J. Primatol. 30, 1–20. doi: 10.1002/ajp.1350300102
Schjelderup-Ebbe, T. (1922). Beiträge zur Sozialpsychologie des Haushuhns (trans. M Schleidt, WM Schleidt). Z. Psychol. 88, 225–252.
Smuts, B. B. (1987). “Gender, aggression, and influence,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, and R. W. Wrangham (Chicago, IL: University of Chicago Press). doi: 10.2307/1130189
Surbeck, M., and Hohmann, G. (2013). Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767–1780. doi: 10.1007/s00265-013-1584-8
Szulkin, M., Stopher, K. V., Pemberton, J. M., and Reid, J. M. (2013). Inbreeding avoidance, tolerance, or preference in animals? Trends Ecol. Evol. 28, 205–211. doi: 10.1016/j.tree.2012.10.016
Vermande, M. M., and Sterck, E. H. M. (2020). how to get the biggest slice of the cake. a comparative view of social behaviour and resource access in human children and nonhuman primates. Front. Psychol. 11:584815. doi: 10.3389/fpsyg.2020.584815
Voyt, R. A., Sandel, A. A., Ortiz, K. M., and Lewis, R. J. (2019). Female power in Verreaux’s sifaka (Propithecus verreauxi) is based on maturity, not body size. Int. J. Primatol. 40, 417–434. doi: 10.1007/s10764-019-00096-9
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. Evol. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Wittig, R. M., and Boesch, C. (2003). “Decision-making” in conflicts of wild chimpanzees (Pan troglodytes): an extension of the Relational Model. Behav. Ecol. Sociobiol. 54, 491–504. doi: 10.1007/s00265-003-0654-8
Wright, P. C. (1999). Lemur traits and Madagascar ecology: coping with an island environment. Am. J. Phys. Anthropol. 110, 31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0
Young, A. L., Richard, A. F., and Aiello, L. C. (1990). Female dominance and maternal investment in strepsirhine primates. Am. Nat. 135, 473–488. doi: 10.1086/285057
Keywords: female dominance, intersexual relationships, subordination, primate, lemur
Citation: Lewis RJ, Bueno GL and Di Fiore A (2022) Variation in Female Leverage: The Influence of Kinship and Market Effects on the Extent of Female Power Over Males in Verreaux’s Sifaka. Front. Ecol. Evol. 10:851880. doi: 10.3389/fevo.2022.851880
Received: 10 January 2022; Accepted: 22 April 2022;
Published: 13 May 2022.
Edited by:
Elise Huchard, UMR 5554 Institut des Sciences de l’Evolution de Montpellier (ISEM), FranceReviewed by:
Eve Davidian, Leibniz Institute for Zoo and Wildlife Research, GermanyChristopher Young, Max Planck Institute for Evolutionary Anthropology, Germany
Copyright © 2022 Lewis, Bueno and Di Fiore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca J. Lewis, rjlewis@austin.utexas.edu
 Rebecca J. Lewis
Rebecca J. Lewis Gabrielle L. Bueno
Gabrielle L. Bueno Anthony Di Fiore
Anthony Di Fiore