- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, Kurdistan Region, Erbil, Iraq
- 3Center of Research and Strategic Studies, Lebanese French University, Kurdistan Region, Erbil, Iraq
- 4Department of Medical Biotechnology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
- 5Dietary Supplements and Probiotic Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 6Institute of Human Genetics, Jena University Hospital, Jena, Germany
- 7Skull Base Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Long non-coding RNAs (lncRNAs) have been demonstrated to in the pathophysiology of multiple sclerosis (MS). In order to appraise the role of T cell-related lncRNAs in this disorder, we assessed expressions of NEST, RMRP, TH2-LCR, MAFTRR and FLICR in MS patients and healthy individuals. We detected significant difference in the expression of RMRP and FLICR between cases and controls. There were substantial correlations between expressions of NEST, RMRP, TH2-LCR, MAFTRR and FLICR lncRNAs among patients, but not controls. The strongest correlations were found between RMRP and TH2-LCR, and between MAFTRR and RMRP with correlation coefficients of 0.69 and 0.59, respectively. ROC curve analysis revealed appropriate power of FLICR in differentiating between MS patients and healthy controls (AUC value = 0.84). Expression of NEST lncRNA was positively correlated with disease duration in MS patients, but negatively correlated with age at onset. In brief, we reported dysregulation of two T cell-related lncRNAs in MS patients and proposed FLICR as a putative marker for this disorder.
Introduction
Multiple sclerosis (MS) is an autoimmune condition in which the underlying cause is not completely understood. Autoimmune reactions may occur as a result of inappropriate function of regulatory T cells (Tregs). This population of T cells expresses Foxp3 and low level of CD127 (Seddiki et al., 2006). Several genes associated with MS such as CD25, CTLA4, CD127, IL-10 have been shown to be related to Treg function or differentiation (ANZgene, 2009; Rioux et al., 2009). Numerous studies have reported abnormal function or quantities of Tregs in blood (Viglietta et al., 2004; Venken et al., 2008; Dalla Libera et al., 2011; Rodi et al., 2016), central nervous system (CNS) lesions and cerebrospinal fluid (Fritzsching et al., 2011) of MS patients. Notably, expression levels of Foxp3 have been revealed to be diminished in Tregs of MS patients (Venken et al., 2008; Frisullo et al., 2009). Moreover, their ability in inhibition of T cell response to myelin basic protein has been diminished (Haas et al., 2005; Venken et al., 2007). A recent study has reported decline in the number of resting and increase in activated CD4+CD25+FOXP3+Tregs in MS patients (Verma et al., 2021). Cumulatively, abnormal function of Tregs is a predominant finding in MS patients.
Recent studies have indicated the importance of non-coding RNAs in the regulation of Treg function and their plasticity as well as commitment of Treg lineage (Ghafouri-Fard and Taheri, 2020; Luo and Wang, 2020; Jalaiei et al., 2021; Taheri et al., 2021). A number of newly identified long non-coding RNAs (lncRNAs), namely FLICR (FOXP3 Regulating Long Intergenic Non-Coding RNA), MAFTRR (MAF Transcriptional Regulator RNA), NEST (IFNG-AS1), RMRP (RNA Component Of Mitochondrial RNA Processing Endoribonuclease) and TH2-LCR (Th2 Cytokine Locus Control Region) are supposed to affect function of Tregs. However, experimental data for confirmation of their effect in the pathogenesis of MS is missing. We conducted this study to evaluate expression of these lncRNAs in the peripheral blood of MS patients in comparison with healthy persons.
Materials and methods
Study participants
The study included 12 male MS patients and 38 female MS patients. Additionally, 50 age and sex matched healthy individuals were recruited as controls. The latter group of individuals had no history of neuropsychiatric or immune-mediated diseases. MS patients were diagnosed based on the revised McDonald criteria (Polman et al., 2011). Relapsing-remitting MS was diagnosed based on the presence of at least two separate areas of damage in the CNS that have happened at dissimilar points in time. MRI results, history of symptoms and findings on the neurological examination were used for diagnosis. Informed consent was obtained from all MS patients and healthy controls. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1400.562). General data of MS patients and controls is summarized in Table 1.
Expression studies
Four milliliters of whole peripheral blood were gathered from cases and controls. Blood specimens were used for RNA extraction using the Hybrid-RTM blood RNA extraction kit (GeneAll Biotechnology Co Ltd., South Koera). Then, cDNA was produced using the extracted RNA and a commercial Reverse Transcription kit (Applied Biosystems). Expressions of NEST, RMRP, TH2-LCR, MAFTRR and FLICR were quantified using the primers listed in Table 2. B2M was used as the reference gene for qPCR.
Statistical analysis
GraphPad Prism version 9.0 (La Jolla, CA, United States) was used for data assessment. Expression levels of five lncRNAs, namely NEST, RMRP, TH2-LCR, MAFTRR, and FLICR were compared between MS patients and healthy controls using the comparative–delta Ct method. The normal/gaussian distribution of the values was assessed using the Shapiro-Wilk test. Mann-Whitney U test was applied to recognize differentially expressed genes between MS patients and controls. Two-way ANOVA test was applied to examine the effects of disease and gender on expressions of lncRNAs.
Correlations between expression levels NEST, RMRP, TH2-LCR, MAFTRR and FLICR were measured using Spearman’s rank correlation coefficient since expression data was not normally distributed. Correlations between expressions of lncRNAs and demographic/clinical data such as age, disease duration, age at onset and EDSS were assessed using Spearman’s rank correlation coefficients.
Receiver operating characteristic (ROC) curve was illustrated to value the diagnostic power of expression levels of differentially expressed genes. p value < 0.05 was considered as significant.
Results
Expression data
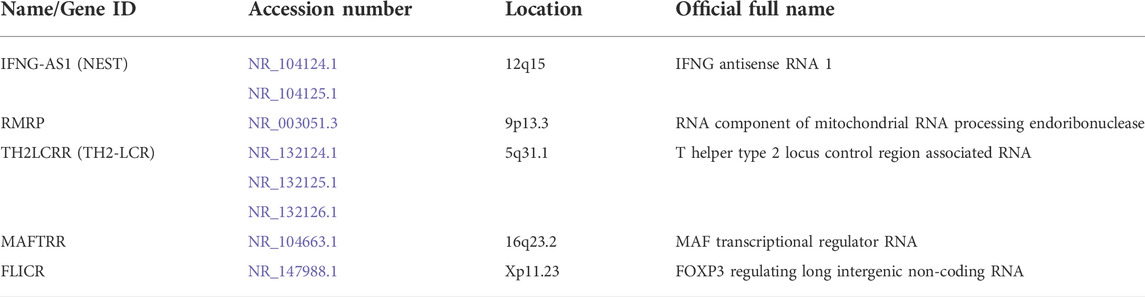
In the current study, we assessed expression of five lncRNAs, namely NEST, RMRP, TH2-LCR, MAFTRR and FLICR. Table 3 shows the information about selected lncRNAs.
Expression levels of RMRP and FLICR were different between MS patients and controls (Figure 1).
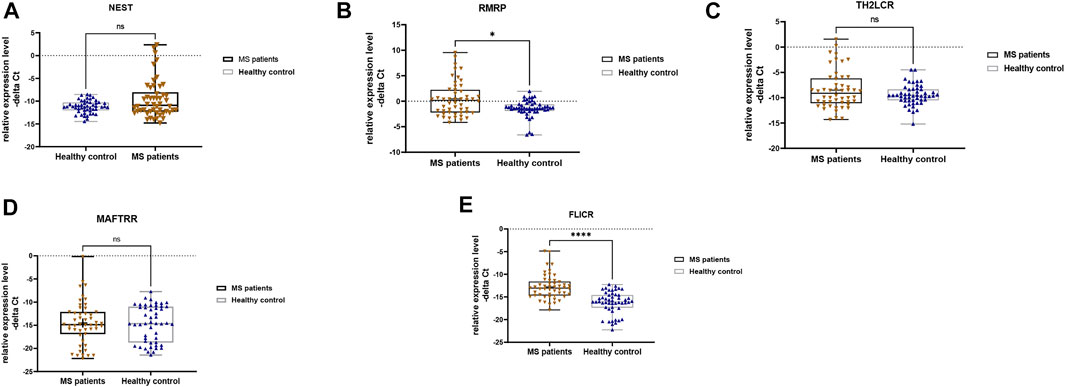
FIGURE 1. Relative expression levels of five lncRNAs in total multiple sclerosis (MS) patients and controls as measured by–delta Ct values (A–E). Mann-Whitney U test was applied to find differentially expressed genes between cases and controls (∗∗p value <0.01 and ∗∗∗∗p value <0.0001).
We detected significant effect of group (disease) factor on expression levels of RMRP and FLICR. However, gender factor had no significant effect on expressions of these lncRNAs, which means that there is no significant difference in the expression of these lncRNAs between males and females. Moreover, the interaction of gender and group had no effect on expressions of any of the studied genes (Table 4). Therefore, we did not perform post hoc tests for multiple comparisons.
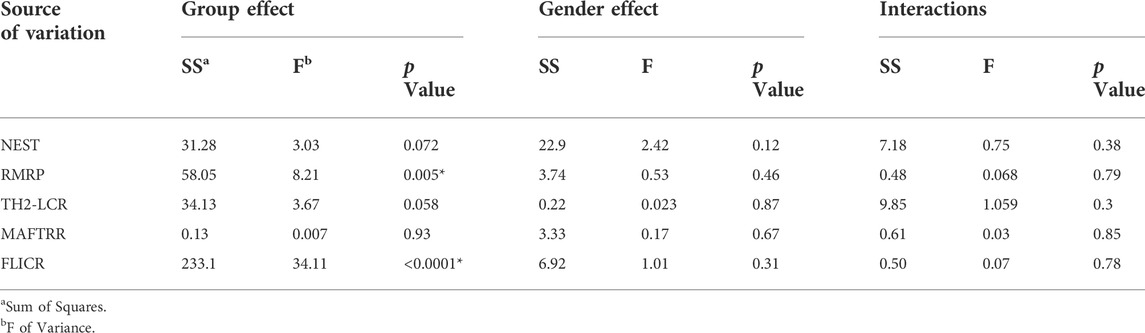
TABLE 4. Graphpad prism output from analysis of effect of Group and Gender (Tests of Between-Subjects Effects) on expression of studied genes in cases compared to healthy controls.
Expression levels of RMRP and FLICR were significantly elevated in MS patients compared with controls (Figure 2).
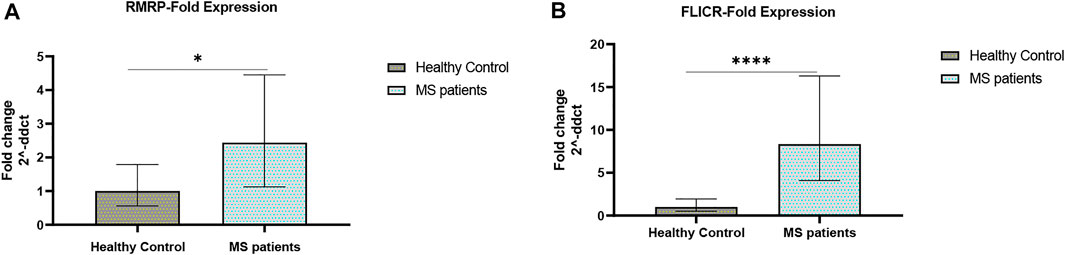
FIGURE 2. Fold changes of RMRP and FLICR expression (A,B) in MS patients versus healthy controls (∗p value <0.05, and ∗∗∗∗p value <0.0001).
Our data revealed significant correlations between expression levels of NEST, RMRP, TH2-LCR, MAFTRR and FLICR lncRNAs among patients, but not controls. The strongest correlations were found between RMRP and TH2-LCR, and between MAFTRR and RMRP with correlation coefficients of 0.69 and 0.59, respectively (Tables 5, 6).
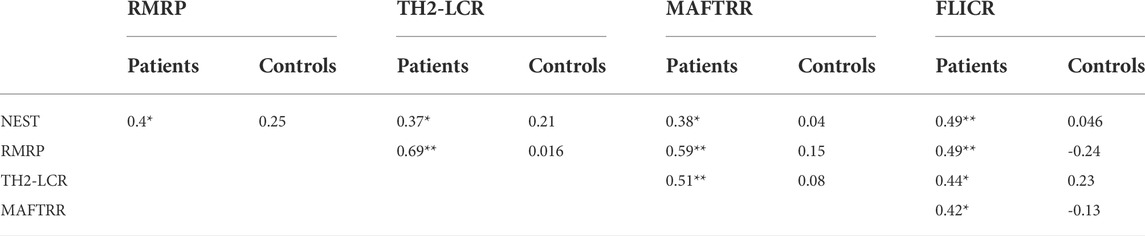
TABLE 5. Spearman’s correlation between expression levels of lncRNAs among MS patients and healthy subjects (* p-Value at a significance level of p < 0.05.** p-Value at a significance level of p < 0.001).
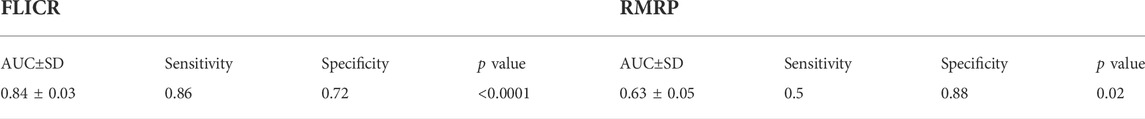
TABLE 6. The results of ROC curve analyses for two differentially expressed lncRNAs in patients with MS disease.
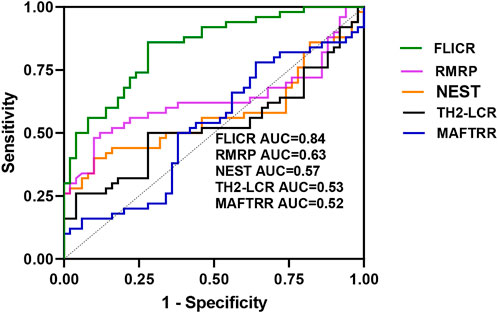
ROC curve analysis revealed appropriate power of FLICR in differentiating between MS patients and healthy controls (AUC value=0.84). RMRP, NEST, TH2-LCR and MAFTRR had AUC values of 0.63, 0.57, 0.53 and 0.52, respectively (Figure 3).
Sensitivity and specificity values for two differentially expressed genes between cases and controls, i.e., FLICR and RMRP are shown in Tables 5, 6.
Expression of NEST lncRNA was positively correlated with disease duration in MS patients, but negatively correlated with age at onset (Table 7).
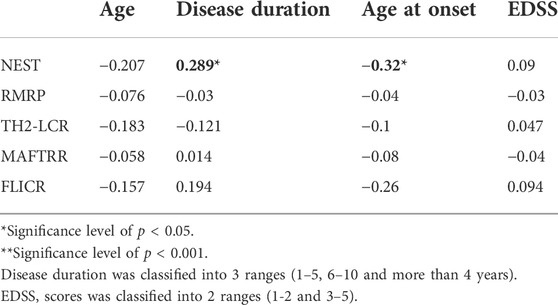
TABLE 7. The results of Spearman’s rank correlation between expressions of five lncRNAs and clinical data.
Discussion
Tregs have important functions in the pathoetiology of MS. Alterations in the frequency and suppressive effects of Tregs in MS patients is implicated with the evolution and exacerbation of MS (Kimura, 2020). Therefore, genes that regulate function of Tregs are potentially involved in the pathoetiology of this disorder. We compared expression of five Treg-associated lncRNAs between MS patients and healthy persons. We noticed higher expression levels of RMRP and FLICR in MS patients compared with controls. Consistent with our findings, a recent investigation has reported significant up-regulation of RMRP in drug-naïve relapsing remitting MS patients compared to healthy persons (Rahmani et al., 2021). However, expression of this lncRNA has been lower in IFNβ-1α-treated patients compared with drug-naïve patients (Rahmani et al., 2021). FLICR has a role as negative regulator of FOXP3 in cis. Notably, it is expressed in Tregs (Zemmour et al., 2017). Its role in the regulation of FOXP3 expression is exerted through cooperation with TGFβ and IL-2 signaling (Zemmour et al., 2017). Up-regulation of this lncRNA in MS patients might reflect abnormal activity of at least a subgroup of Tregs in these patients.
Besides, our data supported significant correlations between expression levels of NEST, RMRP, TH2-LCR, MAFTRR and FLICR lncRNAs among MS patients, but not controls. This finding indicates the presence of a disease-related regulatory or interactive mechanism among these lncRNAs. Therefore, interruption of this network might influence pathogenesis of MS.
Consistent with substantial up-regulation of FLICR in MS patients, ROC curve analysis revealed appropriate power of FLICR in differentiating between MS patients and healthy controls, suggesting this lncRNA as a possible peripheral marker of MS.
Finally, we detected positive correlation between expression of NEST lncRNA and disease duration in MS patients, but its expression was inversely correlated with age at onset of MS. NEST has been previously reported to participate in the epigenetic activation of the IFNG locus (Gomez et al., 2013). IFN-γ is a key cytokine detected in MS lesions. Moreover, IFN-γ levels are impressively up-regulated during MS activity. Besides, IFN-γ–producing Th1 responses are associated with inflammation both in MS patients and animal models of this disorder (Zamvil and Steinman, 1990; Liblau et al., 1995).
Taken together, we reported up-regulation of two Treg-related lncRNAs in MS patients and suggested one of these lncRNAs as a putative marker for MS. We recommend conduction of additional studies for verification of these results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1400.562). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SG-F wrote the manuscript and revised it. MT and AS designed and supervised the study. SE designed and supervised the study. BH, MD and FE collected the data and performed the experiment. SE analyzed the data. All authors read and approved the submitted manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
ANZgene (2009). Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat. Genet. 41 (7), 824–828. doi:10.1038/ng.396
Dalla Libera, D., Di Mitri, D., Bergami, A., Centonze, D., Gasperini, C., Grasso, M. G., et al. (2011). T regulatory cells are markers of disease activity in multiple sclerosis patients. PloS one 6 (6), e21386. doi:10.1371/journal.pone.0021386
Frisullo, G., Nociti, V., Iorio, R., Patanella, A. K., Caggiula, M., Marti, A., et al. (2009). Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients. Immunology 127 (3), 418–428. doi:10.1111/j.1365-2567.2008.02963.x
Fritzsching, B., Haas, J., König, F., Kunz, P., Fritzsching, E., Pöschl, J., et al. (2011). Intracerebral human regulatory T cells: Analysis of CD4+ CD25+ FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PloS one 6 (3), e17988. doi:10.1371/journal.pone.0017988
Ghafouri-Fard, S., and Taheri, M. (2020). A comprehensive review of non-coding RNAs functions in multiple sclerosis. Eur. J. Pharmacol. 879, 173127. doi:10.1016/j.ejphar.2020.173127
Gomez, J. A., Wapinski, O. L., Yang, Y. W., Bureau, J-F., Gopinath, S., Monack, D. M., et al. (2013). The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell. 152 (4), 743–754. doi:10.1016/j.cell.2013.01.015
Haas, J., Hug, A., Viehöver, A., Fritzsching, B., Falk, C. S., Filser, A., et al. (2005). Reduced suppressive effect of CD4+ CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol. 35 (11), 3343–3352. doi:10.1002/eji.200526065
Jalaiei, A., Asadi, M. R., Sabaie, H., Dehghani, H., Gharesouran, J., Hussen, B. M., et al. (2021). Long non-coding RNAs, novel offenders or guardians in multiple sclerosis: A scoping review. Front. Immunol. 12, 774002. doi:10.3389/fimmu.2021.774002
Kimura, K. (2020). Regulatory T cells in multiple sclerosis. Clin. Exp. Neuroimmunol. 11 (3), 148–155. doi:10.1111/cen3.12591
Liblau, R. S., Singer, S. M., and McDevitt, H. O. (1995). Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 16 (1), 34–38. doi:10.1016/0167-5699(95)80068-9
Luo, Y., and Wang, H. (2020). Effects of non-coding RNA on regulatory T cells and implications for treatment of immunological diseases. Front. Immunol. 11, 612060. doi:10.3389/fimmu.2020.612060
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69 (2), 292–302. doi:10.1002/ana.22366
Rahmani, S., Noorolyai, S., Ayromlou, H., Khaze Shahgoli, V., Shanehbandi, D., Baghbani, E., et al. (2021). The expression analyses of RMRP, DDX5, and RORC in RRMS patients treated with different drugs versus naïve patients and healthy controls. Gene 769, 145236. doi:10.1016/j.gene.2020.145236
Rioux, J., Goyette, P., Vyse, T., Hammarström, L., Fernando, M., Green, T., et al. (2009). International MHC and Autoimmunity Genetics NetworkMapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc. Natl. Acad. Sci. U. S. A. 106, 18680–18685. doi:10.1073/pnas.0909307106
Rodi, M., Dimisianos, N., De Lastic, A-L., Sakellaraki, P., Deraos, G., Matsoukas, J., et al. (2016). Regulatory cell populations in relapsing-remitting multiple sclerosis (RRMS) patients: Effect of disease activity and treatment regimens. Int. J. Mol. Sci. 17 (9), 1398. doi:10.3390/ijms17091398
Seddiki, N., Santner-Nanan, B., Martinson, J., Zaunders, J., Sasson, S., Landay, A., et al. (2006). Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203 (7), 1693–1700. doi:10.1084/jem.20060468
Taheri, M., Barth, D. A., Kargl, J., Rezaei, O., Ghafouri-Fard, S., and Pichler, M. (2021). Emerging role of non-coding RNAs in regulation of T-lymphocyte function. Front. Immunol. 12, 756042. doi:10.3389/fimmu.2021.756042
Venken, K., Hellings, N., Thewissen, M., Somers, V., Hensen, K., Rummens, J. L., et al. (2008). Compromised CD4+ CD25high regulatory T‐cell function in patients with relapsing‐remitting multiple sclerosis is correlated with a reduced frequency of FOXP3‐positive cells and reduced FOXP3 expression at the single‐cell level. Immunology 123 (1), 79–89. doi:10.1111/j.1365-2567.2007.02690.x
Venken, K., Thewissen, M., Hellings, N., Somers, V., Hensen, K., Rummens, J-L., et al. (2007). A CFSE based assay for measuring CD4+ CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J. Immunol. Methods 322 (1-2), 1–11. doi:10.1016/j.jim.2007.01.025
Verma, N. D., Lam, A. D., Chiu, C., Tran, G. T., Hall, B. M., and Hodgkinson, S. J. (2021). Multiple sclerosis patients have reduced resting and increased activated CD4+ CD25+ FOXP3+ T regulatory cells. Sci. Rep. 11 (1), 10476. doi:10.1038/s41598-021-88448-5
Viglietta, V., Baecher-Allan, C., Weiner, H. L., and Hafler, D. A. (2004). Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199 (7), 971–979. doi:10.1084/jem.20031579
Zamvil, S. S., and Steinman, L. (1990). The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8 (1), 579–621. doi:10.1146/annurev.iy.08.040190.003051
Keywords: FLICR, NEST, RMRP, TH2-LCR, lncRNA, multiple sclerosis, T cell
Citation: Dadyar M, Hussen BM, Eslami S, Taheri M, Emadi F, Ghafouri-Fard S and Sayad A (2022) Expression of T cell-related lncRNAs in multiple sclerosis. Front. Genet. 13:967157. doi: 10.3389/fgene.2022.967157
Received: 12 June 2022; Accepted: 29 July 2022;
Published: 26 August 2022.
Edited by:
Yujing Li, Emory University, United StatesReviewed by:
Rezvan Noroozi, Jagiellonian University, PolandAmin Safa, Complutense University of Madrid, Spain
Copyright © 2022 Dadyar, Hussen, Eslami, Taheri, Emadi, Ghafouri-Fard and Sayad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soudeh Ghafouri-Fard, s.ghafourifard@sbmu.ac.ir; Arezou Sayad, ar.sayad@yahoo.com
 Maryam Dadyar1
Maryam Dadyar1 Bashdar Mahmud Hussen
Bashdar Mahmud Hussen Solat Eslami
Solat Eslami Mohammad Taheri
Mohammad Taheri Farhad Emadi
Farhad Emadi Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Arezou Sayad
Arezou Sayad