- 1Division of Clinical Immunology and Allergy, Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy
- 2Division of Infectious Diseases, Department of Medicine, Boston Children’s Hospital, Boston, MA, USA
- 3Pathology Unit, Istituto Nazionale Tumori Fondazione “G. Pascale”, Naples, Italy
- 4U.O.C. Immunohematology and Transfusion Medicine, University of Naples Federico II, Naples, Italy
- 5CNRS, Institut de Pharmacologie Moléculaire et Cellulaire, Université Côte d’Azur, Valbonne Sophia Antipolis, France
- 6Department of Medicine, Division of General Pathology, University of Verona, Verona, Italy
- 7CNR Institute of Experimental Endocrinology and Oncology “G. Salvatore”, Naples, Italy
Secreted phospholipases A2 (sPLA2s) are extracellular enzymes that catalyze the release of free fatty acids and lysophospholipids from membrane phospholipids and also bind to different receptors (e.g., PLA2R1 or integrins). To date, 12 mammalian sPLA2s have been identified, which play a critical role in pathophysiological processes including inflammation and cancer. sPLA2s activate immune cells such as human neutrophils (PMNs) by enzymatic activity- or receptor-mediated mechanisms. In addition, human PMNs synthesize and store human group V (hGV) and human group X (hGX) sPLA2s in their granules, but only the former is released upon cellular activation. We investigated the effects of sPLA2s on the release of proangiogenic and antiangiogenic factors by PMNs. We found that exogenous hGV and hGX sPLA2s induce the release of vascular endothelial growth factor (VEGF)-A, angiopoietin 1 (Ang1), and CXCL8/IL-8. Only hGV induces the secretion of the antiangiogenic isoform of VEGF-A, namely, VEGF-A165b. While the release of VEGF-A, Ang1, and CXCL8/IL-8 was likely mediated by hGV enzymatic activity and/or binding to PLA2R1 and heparan sulfate proteoglycans, the release of VEGF-A165b requires the interaction with αVβ3 and α4β1 integrins. We also provide evidence that endogenous hGV released by N-formyl-met-leu-phe (fMLF)-activated PMNs is involved in the release of angiogenic factors. The translational relevance of these data is supported by our findings that hGV expression is increased in human samples of lung cancer which are infiltrated by PMNs. Overall, our results suggest that the hGV–neutrophil axis may play a relevant role in the modulation of cancer-related inflammation and angiogenesis.
Introduction
Secreted phospholipases A2 (sPLA2s) are extracellular enzymes that catalyze the hydrolysis of the sn − 2 position of membrane glycerophospholipids to release free fatty acids and lysophospholipids, thereby regulating several processes including the production of lipid mediators (1). However, sPLA2 effects are not related only to their enzymatic activity but also to their ability to activate target cells through the engagement of different targets [e.g., PLA2R1, heparan sulfate proteoglycans (HSPGs), integrins] (2–9). To date, 12 mammalian sPLA2s, namely, groups IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA, XIIB, and otoconin-95, have been identified (5, 10). They often play critical roles in pathophysiological processes, including inflammation and cancer (1, 5, 11, 12). Indeed, sPLA2s are expressed in inflamed tissues and tumors (1, 12–15). In addition, several immune cells are both sources and/or targets of sPLA2s (16–21). In particular, sPLA2s activate human neutrophils (PMNs) inducing elastase and CXCL8/IL-8 release and activating ERK1/2 and p38 MAP kinases by a receptor-mediated mechanism (8, 21–23). In addition, PMNs store human group V (hGV) and human group X (hGX) in their granules (16, 19), but only the former is released in response to the bacterial N-formylmethionyl peptide, formyl-methionyl-leucylphenylalanine (fMLF) (16, 19).
PMNs are innate immune cells with primary roles in the acute phase of inflammation and resistance against invading pathogens (24). Because of their terminally differentiated phenotype and short half-life, the role of PMNs in tumor development has been considered marginal. Recent evidence changed this point of view. Indeed, tumor-associated neutrophils (TAN) can exert anti-tumoral as well as pro-tumoral functions and findings derived from murine models suggest that PMNs display unsuspected plasticity (25, 26). Epidemiological studies indicate an association between TAN and clinical outcome in several but not all tumors (27–33). In early stages of lung tumors, PMNs exert immunostimulatory properties but acquire immunosuppressive features as the disease progresses (34, 35). There is some evidence that PMNs can modulate tumor initiation and growth through the production of angiogenic factors (36), but further investigations are required to better understand the role of PMNs in modulating tumor angiogenesis.
Angiogenesis, the formation of new blood vessels, and lymphangiogenesis, the formation of new lymphatic vessels, are complex processes that require the coordinated action of several factors, namely, vascular endothelial growth factors (VEGFs) and angiopoietins [angiopoietin 1 (Ang1) and Ang2] (37, 38). Several proangiogenic and antiangiogenic factors have been identified. VEGF-A and VEGF-B are key mitogens for endothelial cells and can act both as pro- and antiangiogenic factors due to the different spliced forms (39–41). For instance, the splicing variant VEGF-A165 exists in two different isoforms: VEGF-A165a is the most potent proangiogenic variant, whereas VEGF-A165b is an antiangiogenic isoform (42–44). Endothelial cell maturation is also promoted by the angiopoietins (Ang1 and Ang2), whose role can be either proangiogenic or antiangiogenic depending on the microenvironment (45, 46). The key regulators of lymphangiogenesis are VEGF-C and VEGF-D (38, 47).
Immune cells are important sources as well as targets of proangiogenic and antiangiogenic factors (48). In particular, PMNs release a variety of proangiogenic and antiangiogenic factors and play important roles in several models of inflammatory and tumor angiogenesis (36, 49–51). Indeed, PMNs release VEGF-A in response to fMLF, LPS, and phorbolmyristate acetate (PMA), while Ang1 is secreted only in response to PMA (50). It is unknown whether sPLA2s can modulate the production of proangiogenic and antiangiogenic factors from PMNs.
Since we demonstrated that sPLA2s induce the production of angiogenic factors from human macrophages (17), in this study, we sought to investigate the production of pro- and antiangiogenic factors by PMNs in response to different forms of sPLA2s.
Materials and Methods
Reagents
The following were purchased: l-glutamine, antibiotic–antimycotic solution (10,000 IU/ml penicillin, 10 mg/ml streptomycin, and 25 µg/ml amphotericin B), Triton X-100, Histopaque®-1077, bovin serum albumin (BSA), Heparinase I and III Blend from Flavobacterium heparinum, N-formyl-met-leu-phe (fMLF), phenolphthalein β-d-glucuronide sodium salt, detoxified LPS (from E. coli serotype 0111:B4), PMA, brefeldin A, and cycloheximide (Sigma-Aldrich, St. Louis, MO, USA); RPMI and fetal calf serum (FCS, endotoxin level <0.1 EU/ml) (MP Biomedicals Europe, Illkirch, France); P11, TCS 2314 (Tocris Bioscience, UK); anti-human VEGF-A165 (monoclonal mouse IgG2B; Clone 26603) and anti-human VEGF165b (monoclonal mouse IgG1; Clone 56-1) (R&D System, Minneapolis, MN, USA). Target-specific primers for VEGFA165a, VEGFA165b, VEGFB, VEGFC, VEGFD, Ang1, Ang2, and β-actin were produced and purified by Custom Primers (Life Technologies, Milan, Italy). The recombinant sPLA2s human group IB, hGIIA, hGIIE, hGIIF, hGV, hGX, and hGXIIA and the inhibitors Me-Indoxam and RO092906A were prepared in the laboratory of Gerard Lambeau and were a generous gift from and Michael H. Gelb (Departments of Chemistry and Biochemistry, University of Washington, Seattle, WA, USA). sPLA2 preparations were routinely checked for LPS contamination (Limulus amebocyte Test, MP Biomedicals) and discarded if the LPS concentration was above the detection limit of the assay (0.125 EU/ml). All other reagents were from Carlo Erba (Milan, Italy).
Isolation and Purification of Human Neutrophils
Granulocytes were isolated from buffy coats of healthy donors obtained from the Leukapheresis Unit. After dextran sedimentation, PMNs were obtained by centrifugation over Histopaque®-1077 at 400 × g for 30 min, at 22°C, at a 1:1 ratio. Finally, PMNs were isolated by negatively removing all contaminating cells using the MACSxpress Neutrophil Isolation Kit and MACSxpress Erythrocyte Depletion Kit (Miltenyi Biotec, Bologna, Italy). This procedure yields a population of CD66b+ cells with a purity greater than 99% as assessed by flow cytometry. PMNs were suspended (5 × 106 cells/ml) in complete medium (RPMI 1640 containing 5% FCS, 2 mM l-glutamine, and 1% antibiotic–antimycotic solution) and incubated in different plates (Falcon, Becton Dickinson, Franklin Lakes, NJ, USA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After 30 min of rest, the cells were used for the experiments.
Cell Incubations
PMNs were incubated (37°C, 5 min–3 h) in RPMI 1640 containing 5% FCS, 2 mM l-glutamine, and 1% antibiotic–antimycotic solution and stimulated with various concentrations (0.3–10 µg/ml) of human GIB, GIIA, GIIE, GIIF, GIII, GV, GX, GXIIA, fMLF (50 nM), detoxified LPS (100 ng/ml), and PMA (80 nM). In selected experiments, hGV and hGX (3 µg/ml) were preincubated (37°C, 20 min) with increasing concentrations (0.01–10 µM) of their inhibitors Me-Indoxam or RO092906A. In other experiments, PMNs were preincubated (37°C, 1 h) with heparinase (0.4 U/ml) or (37°C, 30 min) with P11 (100 nM) and/or TCS 2314 (100 nM), brefeldin A (10 µg/ml), cycloheximide (10 µg/ml) and then stimulated (37°C, 30 min) with hGV (3 µg/ml). In selected experiments, PMNs were preincubated (37°C, 15 min) with Me-Indoxam or RO092906A and then stimulated with fMLF (37°C, 10 min). At the end of the experiment, the supernatants were removed, centrifuged (1,000 × g, 4°C, 5 min) and stored at −80°C for subsequent determination of mediator release.
RT-PCR
Total cellular RNA was isolated from PMNs using the SV RNA isolation system (Promega, Madison, WI, USA), treated with RNase-free DNase I and resuspended in DEPC water. RNA concentration and quality were assessed by spectrophotometry. Total mRNA was reverse-transcribed (Superscript III Reverse Transcriptase 200 U, Life Technologies) and quantitative PCR (qPCR) was carried out in iCycler-iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using SYBR Green Master Mix (Bio-Rad). Target-specific primers for VEGF165a, VEGFA165b, VEGFB, VEGFC, VEGFD, Ang1, Ang2, and β-actin suitable for qPCR were produced and purified by Custom Primers (Life Technologies, Milan, Italy) and are reported in Table 1. β-Actin was used as housekeeping gene to normalize cycle threshold (Ct) values using the 2−ΔCt formula. The data were analyzed with iCycler iQ analysis software (Bio-Rad).
Mediator Release Assays
The concentration of VEGF-A, VEGFA165b, VEGF-B, VEGF-C, VEGF-D, Ang1, Ang2, and CXCL8/IL-8 in the supernatants, lysed or freshly isolated PMNs (lysed in Tryton 0.1%) was measured in duplicate determinations using commercially available ELISA kits (R&D System). The ELISA sensitivity is 31.1–2,000 pg/ml for VEGF-A, 31.1–4,000 pg/ml for VEGF-A165b, 9.4–300 pg/ml for VEGF-B, 62–4,000 pg/ml for VEGF-C, 31.1–2,000 pg/ml for VEGF-D, 156.25–10,000 pg/ml for Ang1, 31.1–4,000 pg/ml for Ang2, and 31.1–2,000 pg/ml for CXCL8/IL-8. β-Glucuronidase release was measured with a colorimetric assay.
PLA2 Activity Assay
A modified liposomal-based fluorescent assay was used to measure PLA2 activity in neutrophil supernatants. Briefly, a PLA2 substrate cocktail consisting of 7-hydroxycoumarinyl-arachidonate (0.3 mM), 7-hydroxycoumarinyl-linolenate (0.3 mM), hydroxycoumarinyl-6 heptenoate (0.3 mM), 10 mM dioleoylphosphatidylcholine (DOPC), and 10 mM dioleoylphosphatidylglycerol (DOPG) was prepared in ethanol. Liposomes were formed by gradually adding 77 µl substrate/lipid cocktail to 10 ml PLA2 buffer (50 mM Tris–HCl at pH 8.9, 100 mM NaCl, 1 mM CaCl2) while stirring rapidly over 1 min using a magnetic stirrer (Invitrogen EnzChek® phospholipase A2 assay). Neutrophil supernatants (50 µl) was added to 96-well plates, and PLA2 activity was initiated by adding 50 µl substrate cocktail. Fluorescence (excitation at 360 nm and emission at 460 nm) was measured, and specific activity (relative fluorescent units/μg protein/min) for each sample was calculated.
Immunohistochemistry Analysis of Non-Tumor and Tumor Lung Tissues for hGV and CD66b
Non-tumor and tumor lung tissues were obtained from patients affected by lung adenocarcinoma (HCV−, HBsAg−, and HIV-1−) undergoing lung resection. Immunohistochemical staining has been carried out on 4-µm lung cancer serial sections from formalin-fixed, paraffin-embedded tissues, in order to evaluate the expression of hGV and CD66b. Negative control slides without primary antibody were included for each staining (not shown). Paraffin slides were deparaffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed with slides heated in 1 mM EDTA buffer (pH 9.0) for hGV and 0.01 M citrate buffer (pH 6.0) for CD66b, in a bath for 20 min at 97°C. After antigen retrieval, the slides were allowed to cool and rinsed with TBS. The endogenous peroxidase activity was inactivated with 3% hydrogen peroxide. Following the protein block (BSA 5% in PBS 1×), the slides were incubated with a polyclonal rabbit antibody against hGV (NBP2-31558, Novus Biologicals, Littleton, CO, USA, dilution 1:100) or a monoclonal mouse anti-human CD66b antibody (555723, BD Pharmingen, San Jose, CA, USA, dilution 1:400 at 4°C overnight). The sections were rinsed in TBS and incubated with biotinylated anti-rabbit or anti-mouse antibodies, respectively, for 1 h at room temperature. Immunoreactivity was visualized using 3,3′-diaminobenzidine (DAB) and avidin–biotin–peroxidase complex. Finally, sections were weakly counterstained with hematoxylin, mounted, and interpreted using light microscope.
Statistical Analysis
The data are expressed as mean values ±SD of the indicated number of experiments. Statistical analysis was performed with Prism 6 (GraphPad Software) by one-way analysis of variance followed by Dunnett’s test (when comparison was made against a control) or Bonferroni’s test (when comparison was made between each pair of groups). Statistically significant differences were accepted when the p value was at least ≤0.05.
Results
sPLA2s Induce the Release of Proangiogenic and Antiangiogenic Factors by PMNs
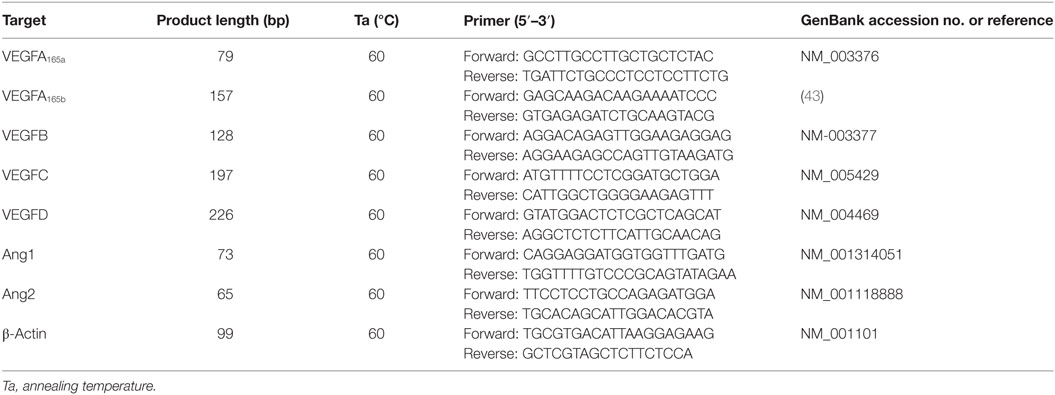
To evaluate the impact of sPLA2 on the production of angiogenic and lymphangiogenic factors by human PMNs, we first assessed the basal expression of VEGFs and Angs. Freshly isolated PMNs constitutively expressed VEGF-A, VEGF-B, and Ang1 at both mRNA and protein levels. By contrast, no expression of VEGF-C and VEGF-D (lymphangiogenic factors) and Ang2 could be detected (Figures 1A,B). Interestingly, PMNs constitutively expressed VEGF-A165b (Figures 1A,B), the antiangiogenic splice variant of VEGF-A165 (42).
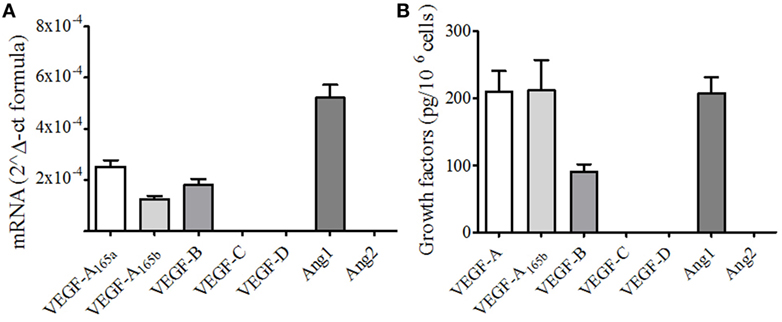
Figure 1. Human neutrophils (PMNs) constitutively express different forms of vascular endothelial growth factors (VEGF) and angiopoietins. (A) VEGFA165a, VEGFA165b, VEGFB, VEGFC, VEGFD, Ang1, and Ang2 mRNA expression in PMNs. The results are the mean ± SD of four different preparations of PMNs. RNA extraction from resting PMNs and RT-PCR was performed as described under Section “Materials and Methods.” (B) Detection of VEGF and Ang proteins. Freshly isolated PMNs were lysed in Tryton 0.1%, and the concentrations of VEGFs and Angs were determined by ELISA. The results are the mean ± SD of six different preparations of PMNs.
We then evaluated the effects of several human recombinant sPLA2s on the secretion of VEGF-A, VEGF-A165b, VEGF-B, and Ang1 as well as the proangiogenic chemokine CXCL8/IL-8 from PMNs. Several sPLA2s induced the release of VEGF-A (Figure 2A) and CXCL8/IL-8 (Figure 2B) and promoted the release of Ang1 (Figure 2C). However, at the concentration used (5 µg/ml), the release of VEGFA and CXCL8 was significant upon stimulation with hGV and hGX, and the release of Ang1 was significant upon stimulation with hGIIF, hGV, and hGX. These results were paralleled by the highest β-glucuronidase release used as a marker of exocytosis (Figure S1A in Supplementary Material) suggesting the release of preformed mediators rather than de novo synthesis. By contrast, no secretion of VEGF-B (Figure S1B in Supplementary Material) could be observed in any of the tested conditions. Interestingly, hGV and, to a lesser extent, hGIIA were the only sPLA2s to induce the secretion VEGF-A165b (Figure 2D).
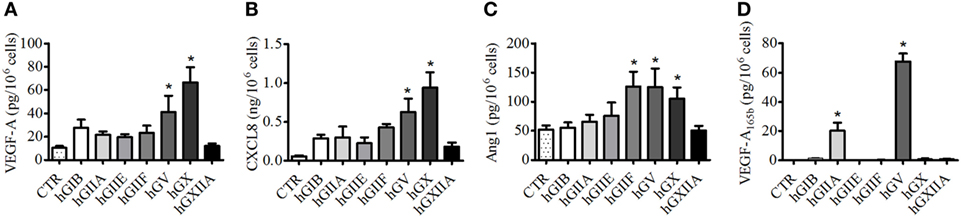
Figure 2. Human secreted phospholipases A2 (sPLA2s) induce the release of vascular endothelial growth factors (VEGFs), Ang1, and CXCL8/IL-8 from PMNs. PMNs were incubated (37°C, 3 h) with sPLA2 [5 µg/ml, human group IB (hGIB), hGIIA, hGIIE, hGIIF, hGV, hGX, and hGXIIA] or control medium (A–D). At the end of incubation, the supernatants were collected and centrifuged (1,000 × g, 4°C, 5 min). VEGF-A (A), CXCL8/IL-8 (B), Ang1 (C), and VEGF-A165b (D) were determined by ELISA. The values are expressed as picograms or nanograms of mediators per 106 cells. The results are the mean ± SD of eight different preparations of PMNs. *p < 0.05 vs. control.
To better understand the mechanisms of sPLA2 neutrophil stimulation, we performed dose–response and time-dependent experiments with hGV and hGX sPLA2s, which are more effective in stimulating human PMNs and are the only sPLA2s expressed by these cells (16). Figure 3 shows that the release of VEGF-A (Figure 3A), CXCL8/IL-8 (Figure 3B), and Ang1 (Figure 3C) in response to hGV and hGX was induced by concentrations as low as 1 µg/ml. By contrast, Figure 3D shows that VEGF-A165b secretion was observed only with hGV, but not with hGX, at all tested concentrations. Moreover, hGV induced the release of these mediators as early as after 5 min of stimulation (Figures 3E,H).
Figure 3. Effect of increasing concentrations of human group V (hGV) and human group X (hGX) on vascular endothelial growth factor (VEGF)-A (A), CXCL8/IL-8 (B), angiopoietin 1 (Ang1) (C), and VEGF-A165b (D) release from PMNs. PMNs were incubated (37°C, 3 h) with hGV and hGX (0.3–10 µg/ml) or control medium. (E–H) Kinetics of hGV-induced release of VEGFs, Ang1, and CXCL8/IL-8 from PMNs. The cells were incubated (37°C, 5–30 min) with hGV (3 µg/ml). At the end of incubations, the supernatants were collected and centrifuged (1,000 × g, 4°C, 5 min). Data are the mean ± SD of different eight preparations of PMNs *(for hGV) and §(for hGX) p < 0.05 vs. control.
To verify whether hGV-induced secretion of proangiogenic and antiangiogenic mediators requires de novo protein synthesis, neutrophils were stimulated with hGV in the presence or absence of brefeldin A (an inhibitor of anterograde cellular transport and protein secretion) or cycloheximide (an inhibitor of protein synthesis). Neither brefeldin A nor cycloheximide affected the spontaneous release of VEGF-A, CXCL8/IL-8, Ang1, and VEGF-A165b (data not shown). However, brefeldin A but not cycloheximide significantly inhibited the release of these mediators induced by hGV (Table S1 in Supplementary Material). Moreover, we measured VEGF-A, Ang1, and VEGF-A165b protein levels in supernatants and cellular lysates of unstimulated and hGV-stimulated PMNs and found that total protein levels (supernatants plus cellular lysates) of angiogenic mediators were not significantly modulated by hGV stimulation (Table S2 in Supplementary Material).
To corroborate these findings and to exclude a non-specific PMN activation by sPLA2, we assessed the secretion of VEGF-A, VEGF-A165b, Ang1, and CXCL8/IL-8 following the stimulation of PMNs with well-known neutrophil stimuli, such as fMLF, PMA, and LPS. Even though all these stimuli induced the release of VEGF-A (Figure 4A) and CXCL8/IL-8 (Figure 4B), the secretion of Ang1 (Figure 4C) was only induced by PMA. By contrast, none of the tested stimuli induced the release of VEGF-A165b (Figure 4D). Taken together, these results indicate that hGV was the only sPLA2 able to induce the release of preformed pro- and antiangiogenic molecules, whereas the effect of hGX was limited to proangiogenic factors.
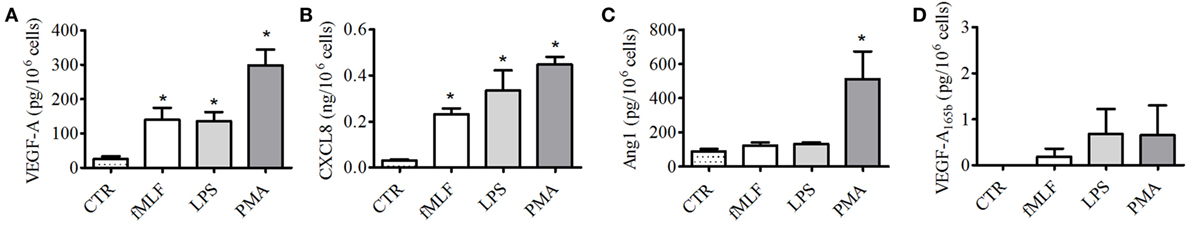
Figure 4. Effect of fMLF, LPS, and phorbolmyristate acetate (PMA) on vascular endothelial growth factors (VEGFs), angiopoietin 1 (Ang1), and CXCL8/IL-8 release from PMNs. PMNs were incubated (37°C, 3 h) with fMLF (50 nM), LPS (100 ng/ml), PMA (80 nM), or control medium (A–D). At the end of incubation, the supernatants were collected and centrifuged (1,000 × g, 4°C, 5 min). VEGF-A (A), CXCL8/IL-8 (B), Ang1 (C), and VEGF-A165b (D) were determined by ELISA. The values are expressed as picograms or nanograms of mediators per 106 cells. The results are the mean ± SD of eight different preparations of PMNs. *p < 0.05 vs. control.
hGV-Induced Secretion of Angiogenic and Antiangiogenic Factors Requires the Interaction with Different Targets
Several evidences demonstrate that PMNs express PLA2R1 that is involved in sPLA2-induced neutrophil activation (8, 21–23). To verify whether hGV enzymatic activity and/or PLA2R1 were involved in the production of pro- and antiangiogenic factors induced by hGV, we stimulated PMNs in the presence of Me-Indoxam. This inhibitor protrudes out of the catalytic groove when bound to sPLA2s, thereby leading to steric hindrance and hence interfering with the sPLA2–receptor interaction (52). We have previously shown that Me-Indoxam prevents receptor-mediated activation of HLMs and PMNs stimulated with sPLA2s (17, 18, 21, 53). hGV also binds HSPGs that mediate its internalization (8). To verify a possible role for HSPGs, PMNs were pre-treated with heparinase to eliminate surface HSPGs before stimulation with hGV. Both Me-indoxam and heparinase pre-treatment markedly reduced the secretion of VEGF-A (Figure 5A), CXCL8/IL-8 (Figure 5B), and Ang1 (Figure 5C). Surprisingly, the release of VEGF-A165b (Figure 5D) was not inhibited, instead it was significantly increased by Me-Indoxam and to a lesser extent also by heparinase pre-treatment. To explain these findings, we reasoned that Me-Indoxam and heparinase pre-treatment could enhance hGV binding to a different cell surface target on PMNs.
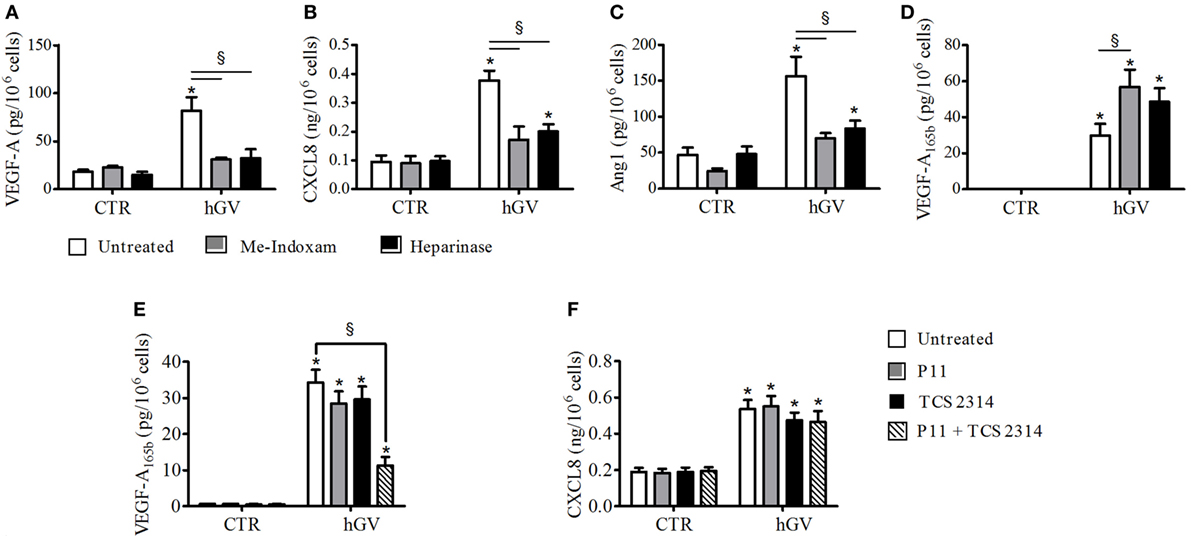
Figure 5. (A–D) Effect of Me-Indoxam or with Heparinase on human group V (hGV)-induced vascular endothelial growth factor (VEGF)-A, CXCL8/IL-8, Ang1, and VEGF-A165b release from PMNs. hGV (3 µg/ml) was preincubated (37°C, 20 min) with Me-Indoxam (0.1 µM) or control medium. PMNs were then incubated (37°C, 1 h) with heparinase (0.4 U/ml) or control medium and then stimulated (37°C, 30 min) with hGV alone or with the combination of hGV with Me-Indoxam. (E,F) αVβ3 (P11) and α4β1 (TCS2314) receptor antagonists inhibit GV-induced PMNs production of antiangiogenic factors. PMNs were preincubated (37°C, 30 min) with or without P11 and TCS 2314 (100 nM) and then stimulated (37°C, 30 min) with hGV (3 µg/ml). Data are the mean ± SD of eight different preparations of PMNs. *p < 0.05 vs. respective control. §p < 0.05 vs. hGV alone.
Two independent studies have shown that sPLA2s can activate human monocytes by binding to αVβ3 and α4β1 integrins (3, 7), which are also expressed on PMNs (54, 55). hGIIA is structurally related to hGV (8, 56) and binds to αVβ3 and α4β1 integrins through a specific domain, different from the catalytic center or the PLA2R1-binding site (3, 7). To investigate whether integrins could be involved in hGV-mediated release of VEGF-A165b, we stimulated PMNs with hGV in the presence of the two integrin antagonists P11 and TCS 2314, which inhibit αVβ3 and α4β1, respectively (57, 58). The combination of these inhibitors markedly reduced the release of VEGF-A165b (Figure 5E). The specificity of this finding is supported by the observation that the secretion of CXCL8/IL-8 was not affected (Figure 5F).
Endogenous sPLA2s Modulate fMLF-Induced Neutrophil Activation in an Autocrine Fashion
fMLF induces the release of hGV by human PMNs (16, 19). We confirmed these results, showing an increased sPLA2 activity in supernatants from fMLF-stimulated PMNs which was inhibited by Me-Indoxam but not by the hGX-specific inhibitor RO092906A (Figure S2 in Supplementary Material) (59). Since we found that hGV induces VEGF-A, CXCL8/IL-8, Ang1, and VEGF-A165b (Figure 3) release, it would be conceivable that also fMLF induced the release of these mediators in an hGV-dependent manner. However, in our model, fMLF only promoted the secretion of VEGF-A and CXCL8/IL-8 (Figure 4). Figure 3 shows that the concentration of hGV required for the release of VEGF-A (Figure 3A) and CXCL8/IL-8 (Figure 3B) was 0.3 µg/ml whereas that for the release of Ang1 (Figure 3C) and VEGF-A165b (Figure 3D) was at least 1 µg/ml. Therefore, we measured the enzymatic activity of several concentrations of recombinant hGV and found that the sPLA2 activity in supernatants from fMLF-stimulated PMNs corresponded to a concentration of recombinant hGV ≤0.3 µg/ml (data not shown). Thus, it is likely that the release of hGV in response to fMLF is in our conditions insufficient to induce VEGF-A165b and Ang1 release. Nevertheless, we asked whether fMLF-induced secretion of VEGF-A and CXCL8/IL-8 was mediated by the release of endogenous hGV. To address this question, PMNs were stimulated with fMLF in the presence or absence of Me-Indoxam or RO092906A. As expected, Me-indoxam reduced VEGF-A (Figure 6A) and CXCL8/IL-8 (Figure 6B) secretion while RO092906A had no effect (Figures 6C,D), suggesting an involvement of hGV but not hGX in fMLF-mediated neutrophil activation.
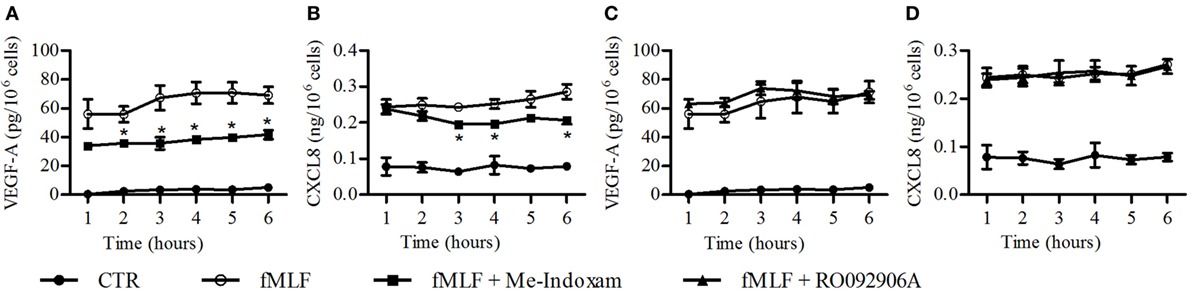
Figure 6. Effect of Me-Indoxam (A,B) and RO092906A (C,D) on fMLF-induced vascular endothelial growth factor (VEGF)-A and CXCL8/IL-8 release from PMNs. Cells were preincubated (37°C, 20 min) with or without Me-Indoxam and RO092906A (0.1 µM) and then stimulated (37°C, 1–6 h,) with fMLF (50 nM). VEGF-A (A–C) and CXCL8/IL-8 (B–D) release was determined by ELISA. Data are the mean ± SD of eight different preparations of PMNs. *p < 0.05 vs. fMLF alone.
Expression of hGV and Neutrophils in Lung Cancers
Our results show that hGV induces the release of proangiogenic and antiangiogenic factors by PMNs. Since angiogenesis is a hallmark of cancer-related inflammation (60) and PMNs infiltrate several human tumors (61), we assessed the expression of hGV and CD66b+ neutrophils by immunohistochemistry in neoplastic lung tissue samples. Figure 7 shows that hGV (Figures 7G,H) and CD66b (Figures 7E,F) were expressed in lung cancer samples but not in non-tumor areas (Figures 7A–D, respectively). These data suggest that hGV, expressed in lung adenocarcinoma microenvironment, can modulate tumor angiogenesis through the activation of TAN.
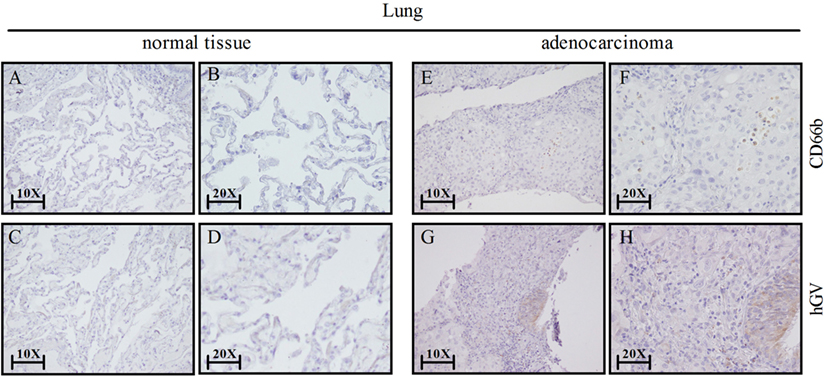
Figure 7. Lung cancer express human group V (hGV) and neutrophils (CD66b+ cells). Sections of non-tumor (A–D) and tumor lung tissues (E–H) were stained for hGV [(C,D,G,H); brown] or CD66b [(A,B,E,F); brown]. Panels (B,D,F,H) display higher magnification (20×) of panels (A,C,E,G) (10×), respectively.
Discussion
This study demonstrates that human PMNs constitutively express and contain several proangiogenic (VEGF-A165, VEGF-B, and Ang1) and antiangiogenic (VEGF-A165b) factors. Interestingly, human PMNs, similar to other circulating immune cells (e.g., basophils) (62), do not express lymphangiogenic factors (VEGF-C and VEGF-D). We observed that several human recombinant sPLA2s can selectively induce the release of pro- and antiangiogenic factors from PMNs. In particular, hGV and hGX, which are the most effective sPLA2s in activating human PMNs, stimulate the secretion of VEGF-A, Ang1, and CXCL8/IL-8, while hGV is unique in inducing VEGF-A165b secretion. Critically and in contrast with VEGF-A, Ang1, and CXCL8/IL-8, the binding of hGV to integrins appears to be required for VEGF-A165b secretion. Indeed, the effect of hGV on VEGF-A165b secretion was abrogated by the addition of known inhibitors of αVβ3 and α4β1 integrins, suggesting that hGV physically interacts with these integrins. Endogenous hGV is released by fMLF-stimulated human neutrophils and acts in an autocrine/paracrine fashion to modulate VEGF-A and CXCL8/IL-8 release. The translational relevance of these findings is supported by the increased expression of hGV in neutrophil-infiltrated lung cancer samples compared to non-tumor lung tissue.
On the one hand, neutrophils are modulators of inflammatory and tumor angiogenesis (36, 51). On the other hand, sPLA2s have been implicated in cancer (13, 15). In this study, we sought to investigate whether sPLA2s induce PMN secretion of angiogenic factors. Strikingly, we found a remarkable heterogeneity in the response to sPLA2s. hGV and hGX induced the highest levels of VEGF-A, Ang1, and CXCL8/IL-8. hGV and, to a lesser extent, hGIIA are the only tested sPLA2s to promote the secretion of the antiangiogenic factor VEGF-A165b. This molecule arises from an alternative splicing at the exon 8 distal site of VEGF-A mRNA and binds to both VEGFR-1 and VEGFR-2 (but not to co-receptor neuropilin-1) (42). However, VEGF-A165b fails to induce VEGFR-2 tyrosine phosphorylation and to activate the downstream signaling pathway that characterizes the proangiogenic isoform VEGF-A165a (42–44). As such, VEGF-A165b restrains tumor growth and impairs angiogenesis in systemic sclerosis and peripheral artery disease (63–66). Our results support a model in which human neutrophil stimulation with hGV induces a mixed secretion profile whose functional outcome likely depends on the balance between proangiogenic and antiangiogenic factors. Further in vitro (e.g., endothelial cell tube formation assays) and ex vivo (e.g., immunohistochemistry analysis of neutrophil infiltration, hGV expression, and vascular patter in tumor biopsies) studies are required to define the role of hGV-induced neutrophil release of proangiogenic and antiangiogenic factors in inflammatory and tumor angiogenesis.
Human group V, an sPLA2 expressed by PMNs, induces VEGF-A, Ang1, and VEGF-A165b secretion as early as 5 min after stimulation and independently of de novo protein synthesis. Indeed, freshly isolated PMNs contain these mediators as assessed in protein lysates. These results suggest that pro- and antiangiogenic factors are preformed and rapidly released upon activation. We also found that hGV stimulation does not increase VEGF-A, Ang1, and VEGF-A165b mRNA levels (data not shown), indicating that hGV preferentially acts by modulating the release of these factors rather than by acting at the level of transcription or alternative splicing. The signals responsible for the constitutive expression of angiogenic mediators, in particular VEGF-A165b, in PMNs are not known. VEGF-A is stored almost exclusively in the specific (β) granules (49), by contrast, Ang1 is predominantly located in the cytosolic fraction (50). Further studies are needed to understand the molecular details of VEGF-A165b expression in resting and hGV-stimulated PMNs.
We also demonstrate that the hGV enzymatic activity and/or its binding to PLA2R1 as well as HSPGs are required for VEGF-A, Ang1, and CXCL8/IL-8 but not for VEGF-A165b release that conversely requires the interaction of hGV with the integrins αVβ3 and α4β1. The hGV-activated signaling pathways downstream of integrins required for VEGF-A165b release have yet to be defined. fMLF induces the release of endogenous hGV that modulate the secretion of VEGF-A and CXCL8/IL-8 but not of VEGF-A165b, likely because of the low level of endogenous hGV, insufficient to drive VEGF-A165b secretion. Nevertheless, we cannot exclude that in situations of high density of PMNs (e.g., in the context of acute inflammation), the concentrations of hGV may reach the minimum required to stimulate VEGF-A165b release, possibly driving a switch toward antiangiogenic properties of PMNs.
To evaluate the in vivo relevance of our findings, we assessed the expression of hGV and CD66b (a marker of neutrophils) in human lung cancer and found a higher expression of hGV in neutrophil-infiltrated tumor lung tissue compared to non-tumor lung samples. These results suggest that PMNs may be activated by hGV in the context of lung cancer. Whether this interaction results in the release of proangiogenic and/or antiangiogenic factors has still to be determined. Since VEGF-A165b restrains tumor growth and progression in several experimental models, our results support the exploration of the hGV–neutrophil axis in human lung cancer.
Ethics Statement
The study protocol involving the use of human blood cells was approved by the Ethical Committee of the University of Naples Federico II, and written informed consent was obtained from blood donors undergoing thoracic surgery in according to the principles expressed in the Declaration of Helsinki.
Author Contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: SL, FB, RI, AF, MG, VG, PE, GV, GL, MC, FG, and GM.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grants from the Regione Campania CISI-Lab Project, CRèME Project, and TIMING Project (to GM).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00443/full#supplementary-material.
References
1. Murakami M, Lambeau G. Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie (2013) 95:43–50. doi: 10.1016/j.biochi.2012.09.007
2. Bernard D, Vindrieux D. PLA2R1: expression and function in cancer. Biochim Biophys Acta (2014) 1846:40–4. doi:10.1016/j.bbcan.2014.03.003
3. Fujita M, Zhu K, Fujita CK, Zhao M, Lam KS, Kurth MJ, et al. Proinflammatory secreted phospholipase A2 type IIA (sPLA-IIA) induces integrin activation through direct binding to a newly identified binding site (site 2) in integrins alphavbeta3, alpha4beta1, and alpha5beta1. J Biol Chem (2015) 290:259–71. doi:10.1074/jbc.M114.579946
4. Lambeau G, Ancian P, Barhanin J, Lazdunski M. Cloning and expression of a membrane receptor for secretory phospholipases A2. J Biol Chem (1994) 269:1575–8.
5. Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem (2008) 77:495–520. doi:10.1146/annurev.biochem.76.062405.154007
6. Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol Sci (1999) 20:162–70. doi:10.1016/S0165-6147(99)01300-0
7. Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS, et al. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J Biol Chem (2008) 283:26107–15. doi:10.1074/jbc.M804835200
8. Kim KP, Rafter JD, Bittova L, Han SK, Snitko Y, Munoz NM, et al. Mechanism of human group V phospholipase A2 (PLA2)-induced leukotriene biosynthesis in human neutrophils. A potential role of heparan sulfate binding in PLA2 internalization and degradation. J Biol Chem (2001) 276:11126–34. doi:10.1074/jbc.M004604200
9. Boilard E, Bourgoin SG, Bernatchez C, Poubelle PE, Surette ME. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: role of heparan sulfate proteoglycans. FASEB J (2003) 17:1068–80. doi:10.1096/fj.02-0938com
10. Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K, et al. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res (2011) 50:152–92. doi:10.1016/j.plipres.2010.12.001
11. Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev (2011) 111:6130–85. doi:10.1021/cr200085w
12. Menschikowski M, Hagelgans A, Nacke B, Jandeck C, Mareninova OA, Asatryan L, et al. Epigenetic control of group V phospholipase A2 expression in human malignant cells. Tumour Biol (2016) 37:8097–105. doi:10.1007/s13277-015-4670-x
13. Brglez V, Lambeau G, Petan T. Secreted phospholipases A2 in cancer: diverse mechanisms of action. Biochimie (2014) 107(Pt A):114–23. doi:10.1016/j.biochi.2014.09.023
14. Laye JP, Gill JH. Phospholipase A2 expression in tumours: a target for therapeutic intervention? Drug Discov Today (2003) 8:710–6. doi:10.1016/S1359-6446(03)02754-5
15. Scott KF, Sajinovic M, Hein J, Nixdorf S, Galettis P, Liauw W, et al. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie (2010) 92:601–10. doi:10.1016/j.biochi.2010.03.019
16. Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem (2002) 277:5061–73. doi:10.1074/jbc.M109083200
17. Granata F, Frattini A, Loffredo S, Del Prete A, Sozzani S, Marone G, et al. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur J Immunol (2006) 36:1938–50. doi:10.1002/eji.200535567
18. Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol (2010) 184:5232–41. doi:10.4049/jimmunol.0902501
19. Solodkin-Szaingurten I, Levy R, Hadad N. Differential behavior of sPLA2-V and sPLA2-X in human neutrophils. Biochim Biophys Acta (2007) 1771:155–63. doi:10.1016/j.bbalip.2006.11.013
20. Triggiani M, Giannattasio G, Calabrese C, Loffredo S, Granata F, Fiorello A, et al. Lung mast cells are a source of secreted phospholipases A2. J Allergy Clin Immunol (2009) 124:558–65, 565.e1–3. doi:10.1016/j.jaci.2009.04.035
21. Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta (2006) 1761:1289–300. doi:10.1016/j.bbalip.2006.07.003
22. Jo EJ, Lee HY, Lee YN, Kim JI, Kang HK, Park DW, et al. Group IB secretory phospholipase A2 stimulates CXC chemokine ligand 8 production via ERK and NF-kappa B in human neutrophils. J Immunol (2004) 173:6433–9. doi:10.4049/jimmunol.173.10.6433
23. Silliman CC, Moore EE, Zallen G, Gonzalez R, Johnson JL, Elzi DJ, et al. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiol (2002) 283:C1102–13. doi:10.1152/ajpcell.00608.2001
24. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol (2011) 11:519–31. doi:10.1038/nri3024
25. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell (2009) 16:183–94. doi:10.1016/j.ccr.2009.06.017
26. Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell (2009) 16:173–4. doi:10.1016/j.ccr.2009.08.014
27. Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol (2013) 23:200–7. doi:10.1016/j.semcancer.2013.02.001
28. Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer (2016) 139:446–56. doi:10.1002/ijc.30076
29. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol (2009) 27:4709–17. doi:10.1200/JCO.2008.18.9498
30. Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol (2011) 54:948–55. doi:10.1016/j.jhep.2010.08.041
31. Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One (2012) 7:e30806. doi:10.1371/journal.pone.0030806
32. Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer (2011) 129:2183–93. doi:10.1002/ijc.25892
33. Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res (2003) 63:1405–12.
34. Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest (2014) 124:5466–80. doi:10.1172/JCI77053
35. Saha S, Biswas SK. Tumor-associated neutrophils show phenotypic and functional divergence in human lung cancer. Cancer Cell (2016) 30:11–3. doi:10.1016/j.ccell.2016.06.016
36. Tecchio C, Cassatella MA. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chem Immunol Allergy (2014) 99:123–37. doi:10.1159/000353358
37. Marone G, Granata F. Angiogenesis, lymphangiogenesis and clinical implications. preface. Chem Immunol Allergy (2014) 99:XI–XII. doi:10.1159/000352074
38. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer (2014) 14:159–72. doi:10.1038/nrc3677
39. Carmeliet P. Angiogenesis in health and disease. Nat Med (2003) 9:653–60. doi:10.1038/nm0603-653
40. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med (2003) 9:669–76. doi:10.1038/nm0603-669
41. Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci U S A (1996) 93:2576–81. doi:10.1073/pnas.93.6.2576
42. Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res (2002) 62:4123–31.
43. Bates DO, Mavrou A, Qiu Y, Carter JG, Hamdollah-Zadeh M, Barratt S, et al. Detection of VEGF-A(xxx)b isoforms in human tissues. PLoS One (2013) 8:e68399. doi:10.1371/journal.pone.0068399
44. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer (2008) 8:880–7. doi:10.1038/nrc2505
45. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell (1996) 87:1161–9. doi:10.1016/S0092-8674(00)81812-7
46. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science (1997) 277:55–60. doi:10.1126/science.277.5322.55
47. Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol (2015) 778:146–51. doi:10.1016/j.ejphar.2015.03.088
48. Loffredo S, Staiano RI, Granata F, Genovese A, Marone G. Immune cells as a source and target of angiogenic and lymphangiogenic factors. Chem Immunol Allergy (2014) 99:15–36. doi:10.1159/000353316
49. Gaudry M, Brégerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood (1997) 90:4153–61.
50. Neagoe PE, Brkovic A, Hajjar F, Sirois MG. Expression and release of angiopoietin-1 from human neutrophils: intracellular mechanisms. Growth Factors (2009) 27:335–44. doi:10.3109/08977190903155043
51. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol (2013) 23:159–70. doi:10.1016/j.semcancer.2013.02.004
52. Boilard E, Rouault M, Surrel F, Le Calvez C, Bezzine S, Singer A, et al. Secreted phospholipase A2 inhibitors are also potent blockers of binding to the M-type receptor. Biochemistry (2006) 45:13203–18. doi:10.1021/bi061376d
53. Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, et al. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol (2005) 174:464–74.
54. Johnston B, Kubes P. The alpha4-integrin: an alternative pathway for neutrophil recruitment? Immunol Today (1999) 20:545–50. doi:10.1016/S0167-5699(99)01544-3
55. Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature (1995) 377:75–9. doi:10.1038/377075a0
56. Cho W. Structure, function, and regulation of group V phospholipase A(2). Biochim Biophys Acta (2000) 1488:48–58. doi:10.1016/S1388-1981(00)00109-8
57. Choi Y, Kim E, Lee Y, Han MH, Kang IC. Site-specific inhibition of integrin alpha v beta 3-vitronectin association by a ser-asp-val sequence through an Arg-Gly-Asp-binding site of the integrin. Proteomics (2010) 10:72–80. doi:10.1002/pmic.200900146
58. Muro F, Iimura S, Sugimoto Y, Yoneda Y, Chiba J, Watanabe T, et al. Discovery of trans-4-[1-[[2,5-dichloro-4-(1-methyl-3-indolylcarboxamido)phenyl]acetyl]-(4S)-methoxy-(2S)-pyrrolidinylmethoxy]cyclohexanecarboxylic acid: an orally active, selective very late antigen-4 antagonist. J Med Chem (2009) 52:7974–92. doi:10.1021/jm901154c
59. Oslund RC, Cermak N, Gelb MH. Highly specific and broadly potent inhibitors of mammalian secreted phospholipases A2. J Med Chem (2008) 51:4708–14. doi:10.1021/jm800422v
60. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. doi:10.1016/j.cell.2011.02.013
61. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol (2013) 228:1404–12. doi:10.1002/jcp.24260
62. de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol (2006) 177:7322–31. doi:10.4049/jimmunol.177.10.7322
63. Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Impaired angiogenesis in systemic sclerosis: the emerging role of the antiangiogenic VEGF(165)b splice variant. Trends Cardiovasc Med (2011) 21:204–10. doi:10.1016/j.tcm.2012.05.011
64. Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res (2011) 109:e14–26. doi:10.1161/CIRCRESAHA.111.242057
65. Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med (2014) 20:1464–71. doi:10.1038/nm.3703
Keywords: secreted phospholipase A2, neutrophil, vascular endothelial growth factor, angiopoietin, lung tumor, integrin, PLA2R1
Citation: Loffredo S, Borriello F, Iannone R, Ferrara AL, Galdiero MR, Gigantino V, Esposito P, Varricchi G, Lambeau G, Cassatella MA, Granata F and Marone G (2017) Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 8:443. doi: 10.3389/fimmu.2017.00443
Received: 25 January 2017; Accepted: 30 March 2017;
Published: 19 April 2017
Edited by:
Fabrice Cognasse, The Rhone-Alpes-Auvergne Regional Branch of the French National Blood System, FranceReviewed by:
Nicolas Flamand, Laval University, CanadaSilvano Sozzani, University of Brescia, Italy
Copyright: © 2017 Loffredo, Borriello, Iannone, Ferrara, Galdiero, Gigantino, Esposito, Varricchi, Lambeau, Cassatella, Granata and Marone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Loffredo, stefanialoffredo@hotmail.com;
Gianni Marone, marone@unina.it
 Stefania Loffredo
Stefania Loffredo Francesco Borriello
Francesco Borriello Raffaella Iannone
Raffaella Iannone Anne L. Ferrara
Anne L. Ferrara Maria R. Galdiero
Maria R. Galdiero Vincenzo Gigantino
Vincenzo Gigantino Pasquale Esposito
Pasquale Esposito Gilda Varricchi
Gilda Varricchi Gerard Lambeau
Gerard Lambeau Marco A. Cassatella
Marco A. Cassatella Francescopaolo Granata
Francescopaolo Granata Gianni Marone
Gianni Marone