- 1Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 2Division of Infectious Diseases, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
The intimate relationship between humans and Neisseria gonorrhoeae infections span centuries, which is evidenced in case reports from studies dating back to the late 1700s and historical references that predate medical literature. N. gonorrhoeae is an exclusive human pathogen that infects the genital tract of both men and women as well as other mucosal surfaces including the oropharynx and rectum. In symptomatic infections, N. gonorrhoeae induces a robust inflammatory response at the site of infection. However, infections can also present asymptomatically complicating efforts to reduce transmission. N. gonorrhoeae infections have been effectively treated with antibiotics since their use was introduced in humans. Despite the existence of effective antibiotic treatments, N. gonorrhoeae remains one of the most common sexually transmitted pathogens and antibiotic resistant strains have arisen that limit treatment options. Development of a vaccine to prevent infection is considered a critical element of controlling this pathogen. The efforts to generate an effective gonococcal vaccine is limited by our poor understanding of the natural immunologic responses to infection. It is largely accepted that natural protective immunity to N. gonorrhoeae infections in humans does not occur or is very rare. Previous studies of the natural history of infection as well as some of the humoral and cellular immune responses to infection offer a window into the issues surrounding N. gonorrhoeae vaccine development. In this review, we summarize the current body of knowledge pertaining to human immune responses to gonococcal infections and the role of these responses in mediating protection from N. gonorrhoeae.
Introduction
Neisseria gonorrhoeae is a bacterial sexually transmitted pathogen that most commonly infects the lower genital tract, the cervix in women, and anterior urethra in men. N. gonorrhoeae can also infect other mucosal surfaces, particularly the pharynx and rectum. Symptoms associated with disease including purulent urethral or cervical discharge and discomfort at the site of infection are due to the pathogens ability to induce robust localized inflammation within the host. However, asymptomatic infections with N. gonorrhoeae are common and may serve as a significant reservoir of transmissible bacteria in the population. In women, untreated infections can ascend to the upper genital tract leading to a number of health complications including pelvic inflammatory disease and infertility. Rarely, infection with N. gonorrhoeae disseminates leading to septic arthritis and skin manifestations. Antibiotic resistance in N. gonorrhoeae strains is on the rise worldwide and effective treatment options have become limited. Although individuals with gonococcal infections are known to produce anti-gonococcal humoral immune responses, it is clear that most of these responses are insufficient for providing protection from future infection. We will review the current state of knowledge regarding immune responses to gonococcal infections and the role of those responses in mediating protection from N. gonorrhoeae in humans. Better understanding of the immune response to natural infection with N. gonorrhoeae is vital for the prevention of disease transmission and the development of an effective gonococcal vaccine.
Natural History of Neisseria gonorrhoeae Infection
Our understanding of the natural history of N. gonorrhoeae infection is hampered by a lack of rigorous scientific studies of microbiologically defined N. gonorrhoeae infection from the pre-antibiotic era. Since the introduction of sulfa-based antibiotics and subsequently penicillin, antibiotic treatment for men with symptomatic N. gonorrhoeae infection, usually urethritis, has been the standard of care. In studies of men seeking care for gonococcal urethritis subjects reported average incubation periods of ~6–8 days between presumed exposure and onset of symptoms (1, 2). However, some individuals reported symptom onset as early as 1–2 days. These studies also indicated that men with symptomatic gonococcal urethritis were symptomatic on average for 7 days before seeking care, though that time ranged from 1 day to 1 year. Because current guidelines indicate men with symptomatic gonorrhea should be treated with antibiotics, there are no prospective studies describing natural clearance or natural progression of symptomatic infection. However, some information about the persistence of symptomatic N. gonorrhoeae infection can be drawn from treatment failures in therapeutic trials of antibiotics for gonococcal urethritis. Svinland et al. examined bacterial clearance after treatment with flumequine in 239 patients with uncomplicated gonorrhea (3). Although multiple dosing regimens with flumequine were effective at curing the vast majority of patients, there was a small number of patients who failed to clear the infection following treatment. N. gonorrhoeae infection was found to persist at the test of cure obtained after 14 days in 10 subjects. Six of those subjects harbored strains with high level flumequine resistance. Those six strains represented all high-level resistant strains found in the study and therefore represented a complete cohort of subjects who received ineffective antibiotic therapy in the study. The persistence of infection in all of these subjects suggests that a large proportion of symptomatic N. gonorrhoeae infections that go without treatment are likely to persist at least 14 days. Treatment failures have also been reported in a number of other therapeutic trials that also support the hypothesis that N. gonorrhoeae can infect the lower genital tract and persist in the face of localized inflammatory response for at least 14 days (4, 5). To characterize the average bacterial load during infection, Isbey et al. analyzed the urine and semen of men with symptomatic urethritis.
Since the treatment of men with asymptomatic gonorrhea has not always been the standard for clinical management of N. gonorrhoeae infection, Handsfield and colleagues conducted a prospective study of the natural history of asymptomatic male infection in the early 1970's. Asymptomatic men were identified via positive N. gonorrhoeae urethral cultures from men requesting STI screening or men that were contacts of women positive for symptomatic gonorrhea at Seattle STD clinics. Of the 28 patients examined weekly, 18 remained asymptomatic until they were treated, which varied from 7 to 165 days. Of the remaining 10 subjects, 5 developed urethritis, and the other 5 spontaneously cleared the infection (6). The determinants of symptomatic vs. asymptomatic infection remain unknown. The total number of N. gonorrhoeae recovered per urine sample (~6*106 CFUs) and from semen (~7*106 CFUs) suggests that N. gonorrhoeae are carried and/or excreted at large quantities during male infection (7). Experimental gonococcal infection with male volunteers mimics much of the clinical features of naturally acquired infections and has provided some insight on the natural history of early symptomatic infection. Infected subjects often develop dysuria and urethritis with the onset of symptoms ranging from ~1 to 6 days post inoculation and N. gonorrhoeae can be recovered from the urine in as little as 2 h following inoculation. The quantity of bacteria recovered (~102-105 CFUs/sample) does not appear to correlate with the severity of infection symptoms (8). Overall, these studies highlight the ability of N. gonorrhoeae to persist for prolonged periods in both symptomatic and asymptomatic men. Additionally, it is clear that asymptomatic infection can progress to symptomatic infection across a broad time spectrum. The determination of the natural rate of clearance of infection is complicated by the need to treat infected individuals with effective antibiotics.
Studies of the natural history of N. gonorrhoeae infection in women are also limited due to a standard of care that requires the use of antibiotics to treat known N. gonorrhoeae infection. In a case survey comparing cure rates of N. gonorrhoeae infection after treatment with sulfathiazole or penicillin, patients were followed for 3 months before being declared cured. The majority of the observed patients were female in this report. Sulfathiazole treatment had a 21% failure rate, with some patients found to have positive cultures 3 months or longer after initial therapy (9). A study of women determined to be recently exposed (average of 11 days after exposure) to N. gonorrhoeae provided some insight into the acquisition and presentation of N. gonorrhoeae infection. Twenty-six women were identified through contact tracing of partners of men with gonococcal urethritis. Of the 26 subjects, 19 were found to be infected with N. gonorrhoeae. Risk of infection after exposure in these women increased with number of exposures to the infection (sexual encounters with the infected contact): 6/12 women with one exposure were found to be infected while 6/7 women with two exposures and 7/7 women with more than two exposures were found to be infected. Of the 19 infected subjects, 9 subjects had clinically defined pelvic inflammatory disease or tenderness of the adnexa suggestive of inflammation in the upper reproductive tract (10). Because of the risk for ascending infection, study of the natural history of asymptomatic infection in women, once identified is not considered ethically acceptable. However, Stupiansky et al. performed a study using self-collected vaginal swabs samples collected in a prospective cohort designed to study sexual health in adolescent women. In this study, subjects underwent examination and clinic-based STD screening every 3 months, 4 times more frequently than recommended for asymptomatic adolescent women. In between screenings, the subjects self-collected cervicovaginal swabs and kept diaries of sexual activity and urogenital symptoms. N. gonorrhoeae DNA was identified in cervicovaginal samples collected prior to the identification of N. gonorrhoeae infection at a quarterly visit in 18 women. Although the quantity of N. gonorrhoeae DNA found in self-collected swabs from individual women varied greatly, the mean bacterial load (~103-105 CFUs/ sample) was similar regardless of the length of time of infection. Additionally, women with Chlamydia trachomatis coinfections displayed higher mean bacterial load, though the difference in N. gonorrhoeae DNA levels between C. trachomatis co-infected and uninfected subjects was not statistically significant. Because the longest period between clinic-based STD screens in this was 12 weeks, persistent infection longer than 12 weeks could not be identified in this group. Interestingly, vaginal discharge and dysuria were reported in the diaries of 3 of the 18 women. However, the presence of symptoms did not correlate with bacterial load (11). These studies suggest that both symptomatic and asymptomatic N. gonorrhoeae infections can persist in women for at least 12 weeks. Further, the frequency of natural clearance in the setting of prolonged infection was not reported for these 18 subjects, but it at least some infections persisted from initial onset up to the quarterly in person visit. Taken together with evidence of persistent symptomatic and asymptomatic infections in men, this report suggests that N. gonorrhoeae is capable of evading or resisting host immune responses to infection.
Historical writings like those from James Boswell, an eighteenth century biographer who reported 20 episodes of symptoms consistent with gonococcal infection, have long been offered as anecdotal evidence that N. gonorrhoeae infection does not induce protective immunity (12). Studies that provide insight into the natural history of N. gonorrhoeae infection suggest that prior infection with N. gonorrhoeae induces little protective immune responses to the pathogen. Platt's study of N. gonorrhoeae acquisition by women exposed to men with gonococcal urethritis demonstrated that 1–7 women exposed and uninfected had prior history of N. gonorrhoeae infection while 10 of 19 exposed and infected individuals had prior history of N. gonorrhoeae, which is consistent with prior infection not leading to protective immune response (10). An epidemiologic study of N. gonorrhoeae infections in rural North Carolina found 14.8% of N. gonorrhoeae infected individuals experienced a second infection during the study period. The N. gonorrhoeae strain recovered from those repeatedly infected individuals was more likely to be the same serovar as the strain recovered from their initial infection than an alternative serovar, suggesting that strain specific immune responses were inadequate to provide protection from N. gonorrhoeae (13). In the prospective cohort study of adolescent women that described the presence of N. gonorrhoeae DNA in self-collected cervicovaginal swabs, no difference in the quantity of N. gonorrhoeae DNA was observed between individuals with a history of prior infection (11). Overall, the data from clinical studies of N. gonorrhoeae infection suggest that immune clearance of infection and protection from repeated infection are largely inadequate leading to prolonged symptomatic and asymptomatic infections (Figure 1).
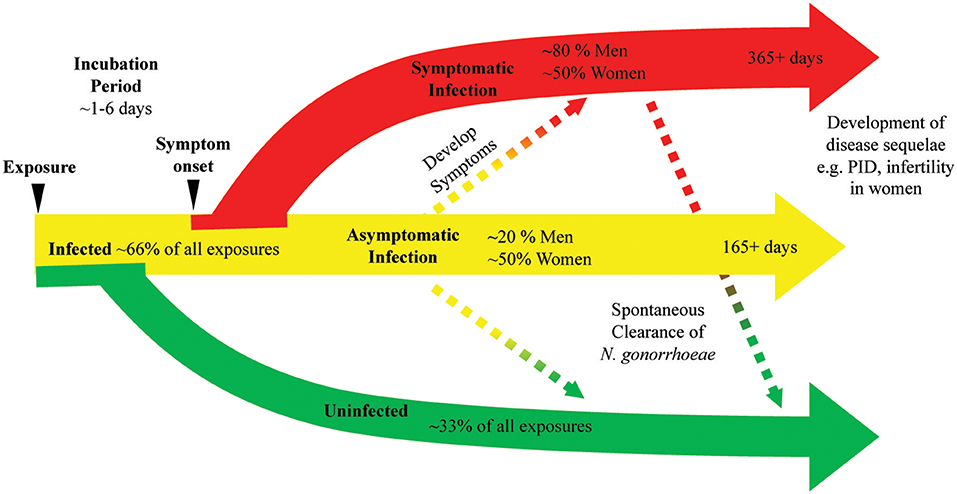
Figure 1. N. gonorrhoeae exposure can lead to bacterial clearance or prolonged infection with or without symptoms. A diagram of the natural history of N. gonorrhoeae infection after exposure demonstrates as many as 33% of exposed individuals will not develop infection. Infected individuals can have symptoms or remain asymptomatic. Asymptomatic infection can eventually progress to symptomatic infection, with studies indicating this progression may occur in as many as 25% of asymptomatic infections. Symptomatic infection can persist at least 14 days and infection is documented to last as long as 1 year. Asymptomatic infection is documented to persist as long as 165 days with as many as 25% of asymptomatic infections clearing over that time frame. The use of antibiotic therapy in all studies generating these data limit our knowledge of the actual rates of bacterial clearance and length of time chronic infection can persist.
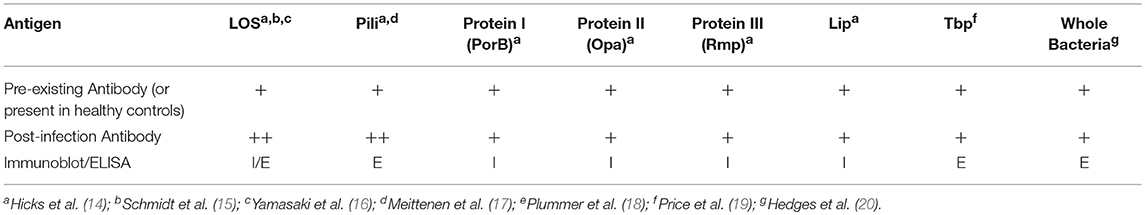
Humoral Immune Responses to N. gonorrhoeae Infection (Table 1)
Individuals infected with N. gonorrhoeae have been shown to produce anti-gonococcal antibodies in sera, seminal plasma, and cervical secretions. Although increases in the different immunoglobulin classes are detected in the serum and secretions of infected individuals, the difference between infected and uninfected individuals have often been reported as modest. Hedges et al. tested both serum and secretions of infected and uninfected male and female attendees of an STD clinic for antibodies against the MS11 strain and homologous infecting strains. When compared to uninfected patients, slight increases in serum IgA1 from female patients and serum IgG from male patients against the MS11 strain were observed. The level of serum IgA and IgG against infecting isolates did not drastically change over the 6-week observation period. Additionally, no difference in anti-gonococcal antibody levels was noted between individuals with no prior history of infection and previously infected individuals regardless of their current infection status (20). However, this study focused on antibody directed against fixed whole bacteria and not specific antigens, suggesting that the bulk of the reactive antibodies measured in this assay resulted from cross reactive immunoglobulins directed against commensal bacterial targets or that the assay was performed in a way that detected high level non-specific antibody binding that masked the ability to detect specific immune responses to gonococcal infection. Antigen specific antibody responses have been reported for a number of gonococcal polysaccharide and protein antigens, in some studies preadsorption of sera with other bacterial species may have contributed to the ability to detect antibodies that developed specifically during N. gonorrhoeae infection.
Polysaccharides are a major source of microbial antigens from pathogenic Neisseria species, as well as many other bacteria. Unlike Neisseria meningitidis, N. gonorrhoeae does not produce a polysaccharide capsule, which is a major immunogenic, saccharide-based antigen for N. meningitidis. However, both N. gonorrhoeae and N. meningitidis produce lipooligosaccharide (LOS) which makes up roughly 50% of the mass of the outer membrane of the bacteria. LOS from N. gonorrhoeae and N. meningitidis share a common core oligosaccharide along with lipid A structure (21). The structure of the oligosaccharide of LOS is determined by the expression of multiple phase variable saccharide transferases (22). Thus, even within a single strain there can be substantial LOS heterogeneity. The ability of these variations in structure to lead to heterogeneity in antigenic epitopes that can be recognized by antibodies was demonstrated clearly by the characterization of N. gonorrhoeae LOS from 20 different strains with a panel of murine monoclonal antibodies (23). Despite these variations in LOS structure, almost all N. gonorrhoeae strains examined by Gulati and colleagues maintain expression of the LOS structure recognized by monoclonal antibody 2C7 (24). Antibodies to LOS have been detected in both normal human sera and in sera from N. gonorrhoeae infected individuals. Sera collected from a small subset of male subjects in a gonococcal pilus-based vaccine trial were used to characterize antigen specific antibody responses during acute gonococcal infection. These subjects who received the placebo vaccination, reported no previous history of STIs, had a negative culture at the start of the trial and developed gonococcal urethritis during the trial period. Sera were collected bi-weekly over the course of 8 weeks. This study found 4 of 13 subjects carried pre-existing LOS antibodies that presumably arose from cross reactivity with polysaccharides from other commensal bacteria species. Of the remaining 9 subjects who did not have preexisting antibodies that recognize N. gonorrhoeae LOS, 6 developed LOS antibodies after developing N. gonorrhoeae infection (14). In another study, sera collected from patients with complicated gonococcal infections [8 disseminated gonococcal infections, 4 cases of pelvic inflammatory disease (PID), and 1 case of epididymitis] was used to characterize antibody responses to N. gonorrhoeae outer membrane proteins. Sera obtained from a majority of patients (9/13) exhibited antibody responses to LOS (4). Antibodies against LOS were also studied by researchers using an experimental human male urethral inoculation to test whether recent gonococcal urethritis led to protection from reinfection. Some immunoreactivity toward N. gonorrhoeae LOS was detected in serum from 21 of 24 subjects prior to inoculation with N. gonorrhoeae for the first time. Of the 14 subjects in the study who were rechallenged, 6 of 14 demonstrated an increase in anti-LOS antibody demonstrated by immunoblot, and 9 of 14 had at least a 2-fold increase in antibody titer measured by ELISA (15). Consistent with baseline observations of subjects in these studies, the presence of N. gonorrhoeae LOS-directed antibodies was detected from pooled sera taken from eight volunteers with no history of gonococcal infection (16). The predominant antibodies that bound LOS in this study were of the IgG class. Affinity purified anti-oligosaccharide IgG isolated from normal human serum was found to contain bactericidal activity toward a serum sensitive strain of N. gonorrhoeae. In a separate study, Yamasaki and colleagues found that N. gonorrhoeae LOS-binding IgG2 from NHS recognized at least 3 different oligosaccharide (25). Thus, numerous studies indicate that the majority, but not all, humans mount antibody responses to a variety of saccharide epitopes found in the lipooligosaccharide of N. gonorrhoeae in response to mucosal or invasive infection with the bacteria.
A number of studies have surveyed whether different gonococcal protein antigens induce humoral immune responses during infection. Sera isolated from 68 patients with uncomplicated and 35 women with PID were used to characterize antibody responses to gonococcal pili (17). The mean antibody levels of IgG, IgA, and IgM against N. gonorrhoeae pilus was significantly higher in men with urethritis (~2-fold increase) and women with cervicitis (~4-fold increase) when compared to sera from uninfected patients. Anti-pilus antibodies were also significantly higher in sera from women with confirmed gonococcal PID when compared to sera from patients with non-gonococcal PID. In addition to testing for anti-LOS antibodies, sera isolated from male volunteers participating in pilus vaccine trial who acquired gonorrhea and who reported no previous history of gonococcal infection were analyzed for the presence on anti-gonococcal protein antibodies using immunoblot analysis. Pre-existing antibodies were detected in 12 of 13 subjects by western blot analysis using pre-infection serum. Not only were preexisting antibodies against LOS detected but antibodies against a number of abundant outer-membrane proteins were observed including: Protein I (PorB), Protein III (Rmp), Pili, and Lip. The authors suggested that the preexisting antibodies to LOS and N. gonorrhoeae proteins may be a result of cross reactivity to antigens from nasal carriage of N. meningitidis. After acquisition of N. gonorrhoeae infection, 9 of 13 subjects developed IgG serum antibody responses with antibodies against all 6 major outer membrane antigens analyzed in this study (LOS as well as Proteins I, II, III, Pili, and Lip). In the aforementioned study by Hook et al. in which sera collected from 8 patients with complicated N. gonorrhoeae infections, Protein I antibodies were found in sera collected from all 13 patients with complicated gonococcal infections (4). Antibodies against both PorB (Protein I) and Opa (Protein II) were also detected in the sera of almost all commercial sex workers in Nairobi, Kenya, that were enrolled in a prospective cohort study of sexually transmitted infections (18, 26). The combination of increased levels of gonococcal antigen-directed antibodies in infected humans combined with prevalent antibodies in a highly exposed cohort, suggest that infection with N. gonorrhoeae can cause the development of antibodies directed toward the pathogen. A study of antibody titer or development of specific pathogen-directed antibodies during prolonged infection or after resolution of infection would be required to demonstrate the antibodies develop in response to infection.
Immune responses to gonococcal transferrin binding proteins were analyzed by ELISA using sera and secretions collected from patients attending STD clinics who had confirmed positive N. gonorrhoeae cultures. Sera from healthy volunteers were used as controls. Antibodies to both transferrin binding proteins, TbpA and TbpB, were detected in sera from men and women. However, only IgA and IgM concentrations against TbpB in women was significantly higher than the levels of anti-TBP antibodies in uninfected control sera. One male subject's serum antibody levels to TbpA and TbpB were followed for 6 months. A slight increase in IgG, IgM, and IgA was observed 1 month after infection and the levels returned to baseline for the following 5 months of the study, suggesting that the antibody titers increased in response to infection (19). In the previously mentioned two phase study utilizing urethral challenge and rechallenge, with N. gonorrhoeae in male volunteers with no previous history of gonococcal infection, pre-infection sera from each volunteer contained IgG to at least one of the major gonococcal Outer Membrane Proteins. A majority (18/24) of the volunteers had pre-existing anti-pilus antibodies. Immunoblots conducted with pre- and post-infection sera were reported from a subset of these subjects. Increases in sera antibodies to outer membrane proteins, particularly IgG, was detected following infection in most subjects however the pattern of recognized proteins varied from subject to subject. Though N. gonorrhoeae exposure or infection prior to enrollment was thought to explain the preexisting detectable antibodies to N. gonorrhoeae antigens in the Kenyan commercial sex-worker cohort study, the number of different serogroups of PorB recognized by sera from subjects in this cohort rose with the number of study visits, suggesting that ongoing exposure to new strains of N. gonorrhoeae was resulting in additional antibody responses to that gonococcal antigen (26). Overall, these studies also support the finding that as with lipooligosaccharide, pre-existing antibodies to gonococcal protein antigens are common in humans but antibodies to some antigens do appear to increase in response to infection.
While there is clear in vitro evidence that some antibodies directed toward N. gonorrhoeae antigens can promote either complement mediated killing or opsonophagocytic killing of the bacteria, whether these antibodies can prevent human infection or play a role in immunologic clearance of the pathogen remains an open question. In a study of experimental human infection followed by rechallenge with N. gonorrhoeae, 7 of 8 subjects who experienced at least a 2-fold increase in anti-LOS antibody resisted reinfection while only 1 of the 6 subjects who had less than a 2-fold increase in anti LOS were susceptible to reinfection (15). Many human IgG and mouse monoclonal antibodies that recognize N. gonorrhoeae LOS epitopes are bactericidal (16, 27). Passive immunization with an antibody directed against the common 2c7 epitopes of N. gonorrhoeae is protective in a mouse model of vaginal N. gonorrhoeae infection (28). In a prospective study of a cohort of commercial sex workers in Kenya, a strong association between the number of different serogroups of N. gonorrhoeae Opa protein (protein II) recognized by antibodies in subject sera and reduced relative risk of gonococcal salpingitis was observed (18). However, in the same cohort, detectable antibody to Rmp (protein III) was associated with increased risk of gonococcal infection during the study as well as increased rate of gonococcal salpingitis (26). Although antibodies against Rmp are detected during infection, there is substantial evidence indicating that many of the antibodies against N. gonorrhoeae Rmp can block bactericidal activity of other N. gonorrhoeae-directed antibodies. Serum isolated from patients with disseminated gonococcal infection was shown interfere with in vitro killing of N. gonorrhoeae by normal human sera. Early studies aimed at identifying the mediators responsible for this blocking of normal human sera bactericidal activity demonstrated that IgG was the major source of blocking activity in sera from subjects with disseminated gonococcal infection (29, 30). IgG reactive with gonococcal outer membrane proteins was more effective at inhibiting bactericidal activity when compared to normal IgG and depletion of Outer Membrane Protein-binding IgG restored the bactericidal activity of serum. Further examination into specific antigens that elicit blocking antibody responses found that RMP-binding IgG contributed to the majority of the blocking activity observed in vitro (31). Price and colleagues were able to demonstrate that by preincubating N. gonorrhoeae with Rmp-directed IgG from immune sera decreased the binding of bactericidal antibodies from normal human sera to N. gonorrhoeae and inhibited killing of the bacteria by normal human sera (31, 32). Some mouse monoclonal antibodies against N. gonorrhoeae Rmp generated through immunization have been found to have bactericidal activity while others have been shown to have blocking activity similar to that of human sera containing anti-Rmp IgG. Analysis of two Rmp-directed monoclonal antibodies with blocking activity revealed they ound an overlapping linear epitope of the protein between amino acids 24–33, an epitope with substantial homology to an outermembrane protein from Escherichia coli, OmpA (33–35). Another study examining the blocking epitope of Rmp bound by antibodies in sera from humans with disseminated gonococcal infection demonstrated that antibodies directed to residues in disulfide-containing loop near the OmpA homologous region (amino acids 47–64) also had blocking activity (36). In a recent study, Gulati et al. demonstrated that passive immunization with an anti-Rmp monoclonal antibody abrogated the protective effects of the 2c7 monoclonal antibody administration in a female mouse N. gonorrhoeae infection model, causing both increased bacterial burden and duration of infection (28, 37). Although the Rmp protein is conserved and immunogenic, the production of anti-Rmp antibodies can inhibit the development of protective humoral responses. Overall, despite clear evidence that humans develop anti-gonococcal antibodies in response to infection, repeat infections with N. gonorrhoeae are common. These data indicate that the humoral immune responses induced by N. gonorrhoeae infection are complex and in most cases are insufficient to provide protection from infection.
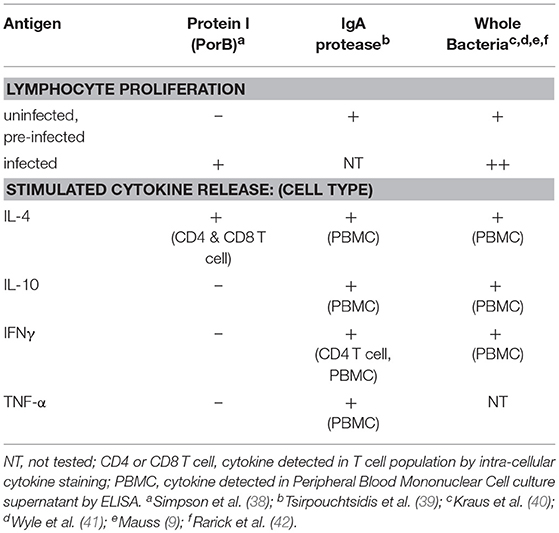
Cell Mediated Immune Responses to N. gonorrhoeae (Table 2)
In addition to humoral immune responses to N. gonorrhoeae, there is also evidence that humans with N. gonorrhoeae develop adaptive cellular immune responses specific to N. gonorrhoeae antigens during infection. Early studies of N. gonorrhoeae cellular immunity were conducted by measuring proliferation in response to antigen stimulation as demonstrated by radioactive adenine incorporation into DNA in cultured primary lymphocytes. Mauss and colleagues studied proliferation of lymphocytes obtained from patients with N. gonorrhoeae infection as well as a set uninfected male controls in response to a variety of N. gonorrhoeae antigen preparations. For the best characterized antigen preparation 3 of 6 female subjects and 4/4 male subjects with N. gonorrhoeae infection had a positive proliferative response while 1 of 7 uninfected men had a positive response (9). Two similar but larger and better controlled studies confirmed these findings, Kraus and colleagues studied lymphocyte proliferation in response to gonococcal antigens in men with gonorrhea and control male subjects with no reported history of gonorrhea (40). Lymphocyte proliferation was higher in lymphocytes from men with two or more gonococcal infections while lymphocytes from those subjects with their initial case of gonorrhea did not exhibit antigen induced proliferation. Similarly, Wyle et al. found that 21 women and 29 men with culture proven N. gonorrhoeae infection were found to have significantly higher proliferative indexes in response to N. gonorrhoeae antigen than lymphocytes from uninfected men and women who denied prior history of N. gonorrhoeae infection (41). Proliferative responses observed in lymphocytes from N. gonorrhoeae infected individuals fell below the level of detection in most individuals within 5 weeks of treatment (43). The specific N. gonorrhoeae antigens that are recognized by cellular immune responses in humans are poorly studied. One study demonstrated that the abundant membrane protein PorB could provide antigenic stimulation to lymphocytes from 20 and 24 of 30 N. gonorrhoeae infected subjects (38). In that study, a lack of PorB-induced proliferation was reported for lymphocytes from uninfected control subjects, suggesting PorB-directed lymphocytes that might arise from cross-reactive bacterial antigens are not highly represented in humans. In a separate study, lymphocytes from 8 of 8 healthy individuals demonstrated proliferative responses to treatment with N. gonorrhoeae IgA protease (39). It is possible these findings result from cross-reactive lymphocyte responses in lymphocytes that recognize homologous IgA proteases from commensal oral pharyngeal bacteria or even N. meningitidis. Genomic analysis of 4 gonococcal strains identified 23 conserved proteins with predicted T and B cell epitopes that could serve as universal antigens (44). However, the gonococcal antigens that elicit natural immune responses during infection remains largely unstudied, or at least unreported in the medical literature, at this time.
Lymphocytes can elicit a variety of functional responses that are aimed at clearing pathogens from the host upon recognition of pathogen-derived antigens. Differential functions of T lymphocytes are mediated by differential expression of a combination of cell surface proteins and secreted mediators (cytokines and chemokines). Lymphocytes responsible for coordinating immunologic responses known as T helper cells are positive for the CD4 antigen (CD4+) and are generally sub-classified into 4 broad groups that each have a predominant secreted cytokine: 1) Th1 which secrete interferon-gamma (IFNγ), Th2 which secrete interleukin-4 (IL-4), Th17 which secrete interleukin-17 (IL-17), and Treg which secrete interleukin-10 (IL-10). Mucosal infections typically do not induce profound differences in systemic or circulating levels of cytokines. However, sera isolated from patients with gonococcal infection were found to have modestly higher circulating levels of IL-17, IFNγ, and IL-23, when compared to sera from healthy controls (45). An inverse correlation between serum levels of IL-17 and serum levels of IFN-γ was observed suggesting that Th1 responses are blunted as Th17 responses are generated during N. gonorrhoeae infection. In a study of cervical immunologic factors in women undergoing STI screening at primary care clinics in Durbin, South Africa, IL-17 was found to elevated in cervical secretions of women with N. gonorrhoeae infection when compared to women without evidence of bacterial STI (46). IL-17 and other inflammatory cytokines (IL-1α, IL-1β, IL-12p70, TNF-α, RANTES, G-CSF, Flt3L, IL-2, IL-5, IL-15, and IL-17) were also found elevated in cervical lavage of N. gonorrhoeae infected women when compared to women with no detectable STI (47). In a study of women seeking care in a Nairobi STI clinic for acute abdominal pain or vaginal discharge, cervical IL-10, a cytokine produced by both regulatory T cells (Treg), and other immunoregulatory cells, was detected in 19 of 59 women with no detected bacterial STI and in 19 of 36 women with N. gonorrhoeae infection. Overall, published studies suggest that N. gonorrhoeae induces a complex lymphocyte response that is largely driven by an Il-17 or Th17 pro inflammatory response.
While there are a number of studies in which the level of cytokines in serum or site-specific fluids from N. gonorrhoeae infected individuals, few reported studies of the CD4+ T cell functional responses to N. gonorrhoeae in humans also exist. IgA protease-directed CD4 cells from healthy individuals without known N. gonorrhoeae infection have been shown to produce IFNγ using both elispot and intracellular cytokine staining. This finding is consistent with the ability of some gonococcal antigens to elicit Th1 type CD4 responses. In that study, cultured PBMC produced IFNγ, TNF-α, IL-4, and IL-10 in response to IgA protease antigen while only producing IFNγ and TNF-α in response to tetanus toxoid (39). This data suggests that the commensal bacteria colonization that resulted in cross reactive immune response to N. gonorrhoeae IgA protease elicited a polyfunctional immune response different in character from that induced by tetanus toxoid vaccination. In another ex vivo study of PBMC response to N. gonorrhoeae, cultured PBMC from 2 anonymous donors (and unknown N. gonorrhoeae infection history) were exposed to N. gonorrhoeae and CD4+ T cells were found to upregulate CD25, a T cell surface marker that is elevated in T regulatory cells and in activated CD4+ T cells. Further culture supernatant from these N. gonorrhoeae-treated PBMC was found to contain cytokines associated with Th1 (IFNγ), Th2 (IL-4), and Treg (IL-10) responses as well as the chemotactic factors IL-8 and MCP (42). Though the cellular source of these cytokines is unknown, the findings are consistent with the observation that N. gonorrhoeae infection induces a pleotropic immunologic response at the site of infection.
The role of cytotoxic T lymphocytes (CD8+ T cells), as well as cytotoxic innate immune cells like Natural Killer cells (NK cells), in gonococcal infection are unknown. Gonococcal PorB has been shown to induce robust IL-4 production in CD8+ T cells from N. gonorrhoeae infected individuals (38). However, contradicting effects of N. gonorrhoeae infection on CD8+ T cell function in humans are reported. In a longitudinal study of female commercial sex workers, Kaul and colleagues found that CD8+ T cell functional responses to HIV (in HIV infected subjects) and CMV (in HIV infected and uninfected individuals) were reduced during episodes of incident N. gonorrhoeae infection when compared to lymphocytes obtained when subjects were not N. gonorrhoeae infected (48). In contrast, a longitudinal study of HIV acquisition in a cohort of HIV negative female commercial sex workers demonstrated that early HIV-directed CD8+ T cell responses were more robust in subjects who were infected with N. gonorrhoeae at the time of HIV acquisition than in N. gonorrhoeae uninfected individuals (49). Additionally, asymptomatic anorectal N. gonorrhoeae infection in men who have sex with men taking pre-exposure prophylaxis to prevent HIV was found to be associated with increased activation marker in circulating CD8+ T cells (50). The adaptive immune response in cytotoxic T cell populations to N. gonorrhoeae infection remains largely unexplored at this time.
Cytokines from the IL-17 family are important for the recruitment of neutrophils and to induce localized antimicrobial responses. Studies in a mouse model of N. gonorrhoeae infections suggest that the magnitude of Th17 responses to infection are more robust than Th1/Th2 responses (51). These findings are consistent with the neutrophilic inflammatory response commonly associated with N. gonorrhoeae infection in humans and the inverse relationship between systemic IL-17 and IFNγ levels reported in N. gonorrhoeae infected individuals (45). The relative resistance of N. gonorrhoeae to neutrophil mediated killing may render this Th17-skewed immune response less effective at clearing the pathogen than a more robust Th1/Th2-supported immune response might, (52, 53). Further, the ability of N. gonorrhoeae to induce IL-10 secretion and promote or stimulate Treg cells may also reduce humoral and cellular immune responses to infection (54). Supportive of this hypothesis, manipulation of the mouse immune system to block the induction of Treg related activity or to drive more robust Th1/Th2 responses leads to enhanced clearance of N. gonorrhoeae in model infection. Further, stimulation of Th1 responses through intravaginal IL-12 administration during infection in the mouse vaginal infection model resulted in enhanced clearance upon rechallenge when compared to mice initially infected without supplemental IL-12 (55). Because studies of lymphocyte proliferative response demonstrated that prior natural infection was associated with significant increase in proliferative response in subjects with current N. gonorrhoeae infection when compared to subjects without previous infection, it appears the cell mediated immunity being measured was inadequate to provide robust protection from repeat infection. Unfortunately, there are no studies to date that measure cellular immune responses to N. gonorrhoeae either in naïve or infected individuals in which the subjects were subsequently prospectively followed to determine whether these cellular immune responses correlate with any degree of protection from re-infection.
Despite public health efforts, gonorrhea still remains one of the most common sexually transmitted bacterial infections responsible for ~100 million infections per year (56). The development of a gonococcal vaccine had been a focus of the field but no vaccine has been developed to the point of human trials since the failure of the N. gonorrhoeae pilin-based vaccine in clinical trials. The failure of the pilin vaccine was unexpected after it had shown success in preventing infection in a homologous strain challenge in humans and highlights the complex relationship between this human pathogen and its host (57). This review of published literature of human immunologic responses to N. gonorrhoeae largely supports the widely accepted supposition that N. gonorrhoeae manages to infect and re-infect humans using some combination of immunologic evasion and resistance to host mediators of clearance. However, there are some reports that support the possibility that natural clearance or protection from infection may develop in response to gonococcal infection in a small portion of individuals. The lack of a protective immune response observed in most infected humans coupled with the complex nature of antigen specific responses and limited animal infection models have all confounded past vaccine development efforts and stand in stark contrast to successful development of N. meningitidis vaccines. Recently, large scale efforts to develop effective vaccines toward serogroup B N. meningitidis which produces a poorly immunogenic capsule have led to the development of Outer membrane vesicle (OMV)-based vaccines which are effective at raising bactericidal antibodies against group B N. meningitidis in humans (58). Data emerging from Sexually Transmitted Infection surveillance in countries deploying OMV-based vaccine in mass vaccination campaigns suggest that the vaccine may induce some cross-species protection against N. gonorrhoeae infections (59–61). The mechanism by which this vaccine may be protective against N. gonorrhoeae is not fully understood. Further studies that evaluate the cellular and humoral responses directed against N. gonorrhoeae that develop after immunization with N. meningitidis OMV as well as comprehensive studies that combine sophisticated immunologic analysis and prospective monitoring of subjects are needed to understand both natural and the possibility of vaccine mediated immunity to N. gonorrhoeae infection.
Author Contributions
AL and JD conceived and outlined this literature review and subsequently independently conducted literature searches. AL drafted initial manuscript, figures, and tables. JD edited and finalized the manuscript, figures, and tables.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Support for JD and AL was provide by the National Institutes of Health through the Atlantic Coast Sexually Transmitted Infection Cooperative Research Center (U19-AI113170). AL was a trainee of the UNC STD/HIV Training Program supported by the National Institutes of Health through T32-AI007001. Publication content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
1. Schofield CB. Some factors affecting the incubation period and duration of symptoms of urethritis in men. Br J Vener Dis. (1982) 58:184–7. doi: 10.1136/sti.58.3.184
2. Sherrard J, Barlow D. Gonorrhoea in men: clinical and diagnostic aspects. Genitourin Med. (1996) 72:422–6. doi: 10.1136/sti.72.6.422
3. Svindland HB, Svarva PL, Maeland JA. Quinolone derivative, flumequine, as short-term treatment for gonorrhoea. Br J Vener Dis. (1982) 58:317–20. doi: 10.1136/sti.58.5.317
4. Hook EW III, Olsen DA, Buchanan TM. Analysis of the antigen specificity of the human serum immunoglobulin G immune response to complicated gonococcal infection. Infect Immun. (1984) 43: 706–9.
5. Tanphaichitra D, Sahaphong S, Srimuang S. Ofloxacin, a new quinolone in the treatment of genitourinary and enteric infections. Infection (1986) 14 (Suppl. 4):S321–3. doi: 10.1007/BF01661308
6. Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. (1974) 290:117–23. doi: 10.1056/NEJM197401172900301
7. Isbey S.F., Alcorn T. M., Davis R. H., Haizlip J., Leone P. A., Cohen M. S. (1997). Characterisation of Neisseria gonorrhoeae in semen during urethral infection in men. Genitourin Med. 73, 378–382.
8. Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. (1994) 169:532–7. doi: 10.1093/infdis/169.3.532
9. Mauss IH. A comparison of the response of gonorrhea to sulfathiazole and penicillin; analysis of 144 cases. J Lancet (1946) 66:65–7.
10. Platt R, Rice PA, Mccormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA (1983) 250:3205–9. doi: 10.1001/jama.1983.03340230057031
11. Stupiansky NW, Van Der Pol B, Williams JA, Weaver B, Taylor SE, Fortenberry JD. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. (2011) 38:750–4. doi: 10.1097/OLQ.0b013e31820ff9a4
12. Rana K.J. (2012). James Boswell and a History of Gonorrhoea. Available online at: https://www.theguardian.com/commentisfree/2012/jun/13/james-boswell-history-gonorrhoea (Accessed June 13, 2012).
13. Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, Cohen MS. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. (1999) 149:353–8. doi: 10.1093/oxfordjournals.aje.a009820
14. Hicks CB, Boslego JW, Brandt B. Evidence of serum antibodies to Neisseria gonorrhoeae before gonococcal infection. J Infect Dis. (1987) 155:1276–81. doi: 10.1093/infdis/155.6.1276
15. Schmidt KA, Schneider H, Lindstrom JA, Boslego JW, Warren RA, Van De Verg L, et al. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis. (2001) 28:555–64. doi: 10.1097/00007435-200110000-00001
16. Yamasaki R, Maruyama T, Yabe U, Asuka S. Normal human sera contain bactericidal IgG that binds to the oligosaccharide epitope expressed within lipooligosaccharides of Neisseria gonorrhoeae. J Biochem. (2005) 137:487–94. doi: 10.1093/jb/mvi061
17. Miettinen A, Hakkarainen K, Gronroos P, Heinonen P, Teisala K, Aine R, et al. Class specific antibody response to gonococcal infection. J Clin Pathol. (1989) 42:72–6. doi: 10.1136/jcp.42.1.72
18. Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Nagelkerke NJ, et al. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. (1994) 93:1748–55. doi: 10.1172/JCI117159
19. Price GA, Hobbs MM, Cornelissen CN. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect Immun. (2004) 72:277–83. doi: 10.1128/IAI.72.1.277-283.2004
20. Hedges SR, Mayo MS, Mestecky J, Hook EW III, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. (1999) 67:3937–46.
21. Mandrell RE, Griffiss JM, Macher BA. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. (1988) 168:107–26. doi: 10.1084/jem.168.1.107
22. Shafer WM, Datta A, Kolli VS, Rahman MM, Balthazar JT, Martin LE, et al. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. (2002) 8:47–58. doi: 10.1177/09680519020080010501
23. Mandrell R, Schneider H, Apicella M, Zollinger W, Rice PA, Griffiss JM. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. (1986) 54:63–9.
24. Gulati S, Mcquillen DP, Sharon J, Rice PA. Experimental immunization with a monoclonal anti-idiotope antibody that mimics the Neisseria gonorrhoeae lipooligosaccharide epitope 2C7. J Infect Dis. (1996) 174:1238–48. doi: 10.1093/infdis/174.6.1238
25. Yamasaki R, Yabe U, Kataoka C, Takeda U, Asuka S. The oligosaccharide of gonococcal lipooligosaccharide contains several epitopes that are recognized by human antibodies. Infect Immun. (2010) 78:3247–57. doi: 10.1128/IAI.01445-09
26. Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Maclean I, et al. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest. (1993) 91:339–43. doi: 10.1172/JCI116190
27. Apicella MA, Westerink MA, Morse SA, Schneider H, Rice PA, Griffiss JM. Bactericidal antibody response of normal human serum to the lipooligosaccharide of Neisseria gonorrhoeae. J Infect Dis. (1986) 153:520–6. doi: 10.1093/infdis/153.3.520
28. Gulati S, Mu X, Zheng B, Reed GW, Ram S, Rice PA. Antibody to reduction modifiable protein increases the bacterial burden and the duration of gonococcal infection in a mouse model. J Infect Dis. (2015) 212:311–5. doi: 10.1093/infdis/jiv024
29. Mccutchan JA, Katzenstein D, Norquist D, Chikami G, Wunderlich A, Braude AI. Role of blocking antibody in disseminated gonococcal infection. J Immunol. (1978) 121:1884–8.
30. Rice PA, Kasper DL. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. (1982) 70:157–67. doi: 10.1172/JCI110589
31. Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. (1986) 164:1735–48. doi: 10.1084/jem.164.5.1735
32. Joiner KA, Scales R, Warren KA, Frank MM, Rice PA. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. (1985) 76:1765–72. doi: 10.1172/JCI112167
33. Virji M, Zak K, Heckels JE. Outer membrane protein III of Neisseria gonorrhoeae: variations in biological properties of antibodies directed against different epitopes. J Gen Microbiol. (1987) 133:3393–401. doi: 10.1099/00221287-133-12-3393
34. Virji M, Heckels JE. Location of a blocking epitope on outer-membrane protein III of Neisseria gonorrhoeae by synthetic peptide analysis. J Gen Microbiol. (1989) 135:1895–9. doi: 10.1099/00221287-135-7-1895
35. De La Paz H, Cooke SJ, Heckels JE. Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer-membrane antigens. Microbiology (1995) 141:913–20. doi: 10.1099/13500872-141-4-913
36. Rice PA, McQuillen DP, Gulati S, Jani DB, Wetzler LM, Blake MS, et al. Serum resistance of Neisseria gonorrhoeae. Does it thwart the inflammatory response and facilitate the transmission of infection? Ann N Y Acad Sci. (1994) 730:7–14. doi: 10.1111/j.1749-6632.1994.tb44234.x
37. Gulati S, Zheng B, Reed GW, Su X, Cox AD, St Michael F, et al. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog. (2013) 9:e1003559. doi: 10.1371/journal.ppat.1003559
38. Simpson SD, Ho Y, Rice PA, Wetzler LM. T lymphocyte response to Neisseria gonorrhoeae porin in individuals with mucosal gonococcal infections. J Infect Dis. (1999) 180:762–73. doi: 10.1086/314969
39. Tsirpouchtsidis A, Hurwitz R, Brinkmann V, Meyer TF, Haas G. Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans. Infect Immun. (2002) 70:335–44. doi: 10.1128/IAI.70.1.335-344.2002
40. Kraus SJ, Perkins GH, Geller RC. Lymphocyte transformation in repeated gonococcal urethritis. Infect Immun. (1970) 2:655–8.
41. Wyle FA, Rowlett C, Blumenthal T. Cell-mediated immune response in gonococcal infections. Br J Vener Dis. (1977) 53:353–9. doi: 10.1136/sti.53.6.353
42. Rarick M, Mcpheeters C, Bright S, Navis A, Skefos J, Sebastiani P, et al. Evidence for cross-regulated cytokine response in human peripheral blood mononuclear cells exposed to whole gonococcal bacteria in vitro. Microb Pathog. (2006) 40:261–70. doi: 10.1016/j.micpath.2006.02.003
43. Esquenazi V, Streitfeld MM. Transformation of lymphocytes in gonorrhea before and after therapy. Infect Immun. (1973) 8:503–9.
44. Jain R, Sonkar SC, Chaudhry U, Bala M, Saluja D. In-silico Hierarchical approach for the identification of potential universal vaccine candidates (PUVCs) from Neisseria gonorrhoeae. J Theor Biol. (2016) 410:36–43. doi: 10.1016/j.jtbi.2016.09.004
45. Gagliardi MC, Starnino S, Teloni R, Mariotti S, Dal Conte I, Di Carlo A, et al. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS Immunol Med Microbiol. (2011) 61:129–32. doi: 10.1111/j.1574-695X.2010.00759.x
46. Masson L, Salkinder AL, Olivier AJ, Mckinnon LR, Gamieldien H, Mlisana K, et al. Relationship between female genital tract infections, mucosal interleukin-17 production and local T helper type 17 cells. Immunology (2015) 146:557–67. doi: 10.1111/imm.12527
47. Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. (2014) 90:580–7. doi: 10.1136/sextrans-2014-051601
48. Kaul R, Rowland-Jones SL, Gillespie G, Kimani J, Dong T, Kiama P, et al. Gonococcal cervicitis is associated with reduced systemic CD8+ T cell responses in human immunodeficiency virus type 1-infected and exposed, uninfected sex workers. J Infect Dis. (2002) 185:1525–9. doi: 10.1086/340214
49. Sheung A, Rebbapragada A, Shin LY, Dobson-Belaire W, Kimani J, Ngugi E, et al. Mucosal Neisseria gonorrhoeae coinfection during HIV acquisition is associated with enhanced systemic HIV-specific CD8 T-cell responses. AIDS (2008) 22:1729–37. doi: 10.1097/QAD.0b013e32830baf5e
50. Vieira VA, Avelino-Silva VI, Cerqueira NB, Costa DA, Costa PR, Vasconcelos RP, et al. Asymptomatic anorectal Chlamydia trachomatis and Neisseria gonorrhoeae infections are associated with systemic CD8+ T-cell activation. AIDS (2017) 31:2069–76. doi: 10.1097/QAD.0000000000001580
51. Liu Y, Islam EA, Jarvis GA, Gray-Owen SD, Russell MW. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-beta-dependent mechanisms. Mucosal Immunol. (2012) 5:320–31. doi: 10.1038/mi.2012.12
52. Witt K, Veale DR, Smith H. Resistance of Neisseria gonorrhoeae to ingestion and digestion by phagocytes of human buffy coat. J Med Microbiol. (1976) 9:1–12. doi: 10.1099/00222615-9-1-1
53. Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. (2009) 11:1074–87. doi: 10.1111/j.1462-5822.2009.01308.x
54. Liu Y, Liu W, Russell MW. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. (2014) 7:165–76. doi: 10.1038/mi.2013.36
55. Liu Y, Egilmez NK, Russell MW. Enhancement of adaptive immunity to Neisseria gonorrhoeae by local intravaginal administration of microencapsulated interleukin 12. J Infect Dis. (2013) 208:1821–9. doi: 10.1093/infdis/jit354
56. Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. (2005) 41 (Suppl 4):S263–8. doi: 10.1086/430787
57. Boslego JW, Tramont EC, Chung RC, Mcchesney DG, Ciak J, Sadoff JC, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine (1991) 9:154–62. doi: 10.1016/0264-410X(91)90147-X
58. Naess LM, Aarvak T, Aase A, Oftung F, Hoiby EA, Sandin R, et al. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine (1999) 17:754–64. doi: 10.1016/S0264-410X(98)00259-X
59. De Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, Sacchi CT, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet (1992) 340:1074–8. doi: 10.1016/0140-6736(92)93086-3
60. Whelan J, Klovstad H, Haugen IL, Holle MR, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection, Norway. Emerg Infect Dis. (2016) 22:1137–9. doi: 10.3201/eid2206.151093
Keywords: Neisseria gonorrhoeae, immune response, natural history, human infection, immunoglobulin, lymphocyte
Citation: Lovett A and Duncan JA (2019) Human Immune Responses and the Natural History of Neisseria gonorrhoeae Infection. Front. Immunol. 9:3187. doi: 10.3389/fimmu.2018.03187
Received: 06 November 2018; Accepted: 31 December 2018;
Published: 19 February 2019.
Edited by:
Scott D. Gray-Owen, University of Toronto, CanadaReviewed by:
Lee Mark Wetzler, Boston University, United StatesWilliam William Shafer, Emory University School of Medicine, United States
Copyright © 2019 Lovett and Duncan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph A. Duncan, jaduncan@med.unc.edu
 Angela Lovett
Angela Lovett Joseph A. Duncan
Joseph A. Duncan