- 1Division of Pharmacology, Faculty of Science, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, Netherlands
- 2Global Centre of Excellence Immunology, Danone Nutricia Research B.V., Utrecht, Netherlands
- 3Global Centre of Excellence Human Milk Research and Analytical Sciences, Danone Nutricia Research B.V., Utrecht, Netherlands
- 4Division of Chemical Biology and Drug Discovery, Faculty of Science, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, Netherlands
- 5Center for Translational Immunology, University Medical Center Utrecht, Utrecht, Netherlands
The prevalence and incidence of allergic diseases is rising and these diseases have become the most common chronic diseases during childhood in Westernized countries. Early life forms a critical window predisposing for health or disease. Therefore, this can also be a window of opportunity for allergy prevention. Postnatally the gut needs to mature, and the microbiome is built which further drives the training of infant's immune system. Immunomodulatory components in breastmilk protect the infant in this crucial period by; providing nutrients that contain substrates for the microbiome, supporting intestinal barrier function, protecting against pathogenic infections, enhancing immune development and facilitating immune tolerance. The presence of a diverse human milk oligosaccharide (HMOS) mixture, containing several types of functional groups, points to engagement in several mechanisms related to immune and microbiome maturation in the infant's gastrointestinal tract. In recent years, several pathways impacted by HMOS have been elucidated, including their capacity to; fortify the microbiome composition, enhance production of short chain fatty acids, bind directly to pathogens and interact directly with the intestinal epithelium and immune cells. The exact mechanisms underlying the immune protective effects have not been fully elucidated yet. We hypothesize that HMOS may be involved in and can be utilized to provide protection from developing allergic diseases at a young age. In this review, we highlight several pathways involved in the immunomodulatory effects of HMOS and the potential role in prevention of allergic diseases. Recent studies have proposed possible mechanisms through which HMOS may contribute, either directly or indirectly, via microbiome modification, to induce oral tolerance. Future research should focus on the identification of specific pathways by which individual HMOS structures exert protective actions and thereby contribute to the capacity of the authentic HMOS mixture in early life allergy prevention.
Introduction
Human milk is unique in its composition as it covers all nutritional and physiological infant requirements during the first months of life (1). Therefore, investigating the biological activity of components derived from human breast milk is an area of great interest, in order to identify specific components that support proper immune development in the infant when breastfeeding is not possible. The first indications of a link between breastfeeding and allergy outcome later in life has been published almost a century ago (2). Since then, numerous studies have been conducted to substantiate this suspected link (3–8). Breastmilk is the gold standard in early life nutrition, because of its large range of bio-active protective nutrients essential for healthy development of the microbiome and gastro-intestinal and immune maturation. However, it can also transfer allergens which may cause allergic reactions in atopic or allergic infants. Therefore, the conflicting data presented by these studies demonstrate the importance of studies further evaluating the biological activities of specific constituents found in human milk (9), such as human milk oligosaccharides (HMOS).
HMOS are the third most abundant component of human breast milk after lactose and lipids. The concentration of total HMOS in human breast milk ranges from 5 to 15 g/L, depending on the stage of lactation and genetic background of the mother (10, 11). More than two hundred structurally different forms of HMOS have been identified (12–14). Different structural and functional groups of HMOS have been related to various effects on several aspects of the immune system (15–19), highlighting the need for a diverse mixture of oligosaccharides in neonatal nutrition for optimal immune development.
Maturation of the immune system in the gastrointestinal tract is linked to proper systemic immunity and the establishment of effective oral tolerance for harmless food proteins and commensal bacteria of the host microbiome (20). As microbial colonization coincides with a rapidly maturing immune system in infants, microbial dysbiosis may therefore disturb development of the gastro-intestinal tract and immune system (21). Microbial dysbiosis and immature immune responses are thought to play a crucial role in e.g., necrotic enterocolitis (NEC), a disease characterized by inflammation and necrosis of the intestines affecting especially premature infants (22), whose immune system is not yet fully developed. Pathologies such as NEC and allergic diseases share common ground, as both have been linked to impaired microbial colonization and improper immune maturation.
One of the specific contributions of HMOS in human milk is its prebiotic capacity. Modulation of the infant's microbiome composition into a bifidogenic profile has been shown to have beneficial effects on infant health. Therefore, prebiotics, such as galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS), have shown several beneficial immune and microbiome developments in infants (23–25). The specific combination of 90% short-chain (sc)GOS with 10% long-chain (lc)FOS resemble the molecular size distribution of the neutral HMOS fraction found in human milk (26). Prebiotic supplementation with scGOS and lcFOS reduces the incidence of allergy development (26–31). Murine models for both food allergy and house dust mite induced allergic asthma demonstrated the preventive effects of non-digestible oligosaccharides (29, 30). Moreover, scGOS/lcFOS supplemented infant formula in neonates decreased the prevalence of atopic dermatitis and other allergic manifestations (26–28).
Currently, only a small number of in vivo studies have investigated immunomodulatory properties and immune development capacities of HMOS. Thus, there are a limited amount of studies that attribute immune development properties to HMOS and individual HMOS structures. Several studies describing immunomodulatory effects of scGOS and lcFOS have been included in this review as they may serve as a framework in which future research could focus on elucidating how immune related mechanisms may be affected by HMOS. In addition, almost no clinical trials have investigated the effects of HMOS supplementation, although the association between the presence of specific HMOS biologically available in human milk and the prevalence of infectious diseases (32–34) or allergic diseases (35–37) has been indicated. The possible biological functions of HMOS gain support from studies that show a potential protective effect of prebiotic administration in in vitro models, animal models and human studies against development of asthma or allergy (28, 35, 38, 39). Most of the HMOS are not digested in the upper part of the gastrointestinal tract, but are fermented by local microbiota (40). A large proportion of HMOS will reach the colon intact (40), where they can serve as prebiotics for the colonic microbiota of the infant. Although a large portion of HMOS is metabolized by gut microbiota, some cross the intestinal (sub)mucosa and enter systemic circulation (13, 41, 42), thereby potentially modulating systemic immune functions. This means that HMOS may influence immunity and potentially not only the intestinal microbiome but also the microbiome composition in the lungs, providing a possible explanation for the observation that breastfed infants are less likely to develop asthma during childhood (43). In addition, reduced occurrence (up to 50% reduction) of atopic dermatitis, asthma, recurrent wheeze and food allergy in infants supplemented with prebiotics in early life has been observed (27, 28, 44–46). Despite these observations, little is known regarding the systemic distribution of HMOS in the infant, and how it may influence processes outside the gastrointestinal tract.
The complexity and abundance of oligosaccharides in human milk is unique amongst mammals (47). HMOS play an essential role in the postnatal growth and development of the mucosal immune system. HMOS are made up of monosaccharide units such as glucose (Glc), galactose (Gal), fucose (Fuc), N-acetylglucosamine (GlcNAc), and sialic acid with N-acetylneuramic acid (Neu5Ac). HMOS synthesis follows a distinct pattern of formation. Each structure has a Gal-Glc unit at the reducing terminus, also known as a lactose unit, containing a β1–4 glycosidic linkage. Elongation of lactose can occur by addition of Gal-GlcNAc units via a β1–3 or β1–6 glycosidic bond to form the linear or branched core structures (see Figure 1). The HMOS core structure can be further modified through the addition of Fuc or Neu5Ac residues (48).
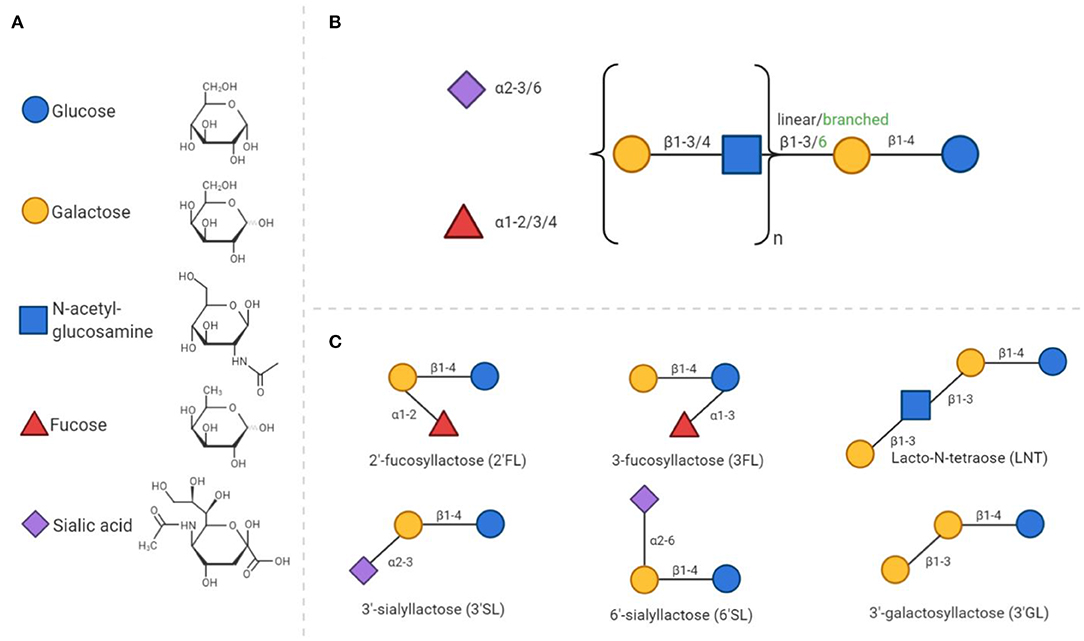
Figure 1. General composition of human milk oligosaccharides and synthetic analogs. (A) All HMOS consist of only 5 different monosaccharides. The chemical structures of these monosaccharides are presented in a D- configuration. (B) The composition of HMOS follows a distinct structure. Elongation of the core structure and decoration with fucose and/or sialic acid residues leads to the large number of different structures discovered to date. (C) As examples, six simple oligosaccharide structures are displayed.
The unique diversity of HMOS also includes galactosyllactoses, with structures based on the elongation of lactose and further galactose residues (49, 50). These types of linkages are indigestible, but fermentable by specific bacteria; leading to the large number of ~200 distinct structures identified to date. Decoration of the core structure with sialic acid, results in an acidic structure, whereas all other HMOS, including those containing fucose groups, are considered neutral. The composition of HMOS produced by a mother is determined by genetic polymorphisms in genes encoding fucosyltransferases FUT2 [Secretor (Se) gene] and FUT3 [Lewis (Le) gene]. Both genes are polymorphic, the individual expression of these genes are accountable for variable enzyme activity and corresponding variation in HMOS profiles in breast milk (11). Recent data has even indicated that these genetic polymorphisms in mothers, impact immunologic outcome of their children later in life. This effect was demonstrated in children, with a hereditary high risk of developing allergic diseases, who were fed breast milk of FUT2 expressing mothers which decreased the incidence of allergic manifestation of these children at 2 years of age (36). However, from this study it cannot be concluded that solely this genetic polymorphism is related to the allergic outcome of the infant, as many genetic, nutritional and environmental factors contribute to the immune development in neonates.
Synthetically manufactured HMOS or HMOS produced by genetically engineered bacteria, such as 2'-fucosyllactose (2'FL) (51), 3-fucosyllactose (3FL) (52, 53), lacto-N-neotetraose (LNnT) (54), 3'-sialyllactose (3'SL), 6'-sialyllactose (6'SL) (55), and 3'-galactosyllactose (3'GL) (56) have become commercial available just recently. This provides the opportunity to study specific pathways by which individual HMOS structures exert their protective immunologic effects in infants.
Allergic Sensitization and the Role of the Epithelial Barrier
The prevalence of allergic diseases is rising tremendously, particularly in Westernized regions (57). An allergic disease is an immunological result of complex interactions between genetic, environmental and lifestyle factors mainly triggered by harmless substances (58). Reduced microbial exposure and diversity is one of the many factors that may contribute to the rise in allergic disease prevalence. In allergic sensitization, a harmless, for example food-derived or airborne protein, crosses the mucosal lining and is presented by antigen presenting cells that drive T helper 2 (TH2) biased immunity contributing to IgE isotype switching of B-cells. Mucosal surfaces with epithelial barriers provide the body with protection from external factors, ensuring that only specific components and nutrients can pass through the epithelium and enter systemic circulation. Allergic sensitization has been linked to dysfunction of the epithelial barrier, both in the intestine and skin (59, 60). Epithelial barrier integrity depends, among other factors, on the mucus layer covering the single layer of epithelial cells. The mucus layer in the intestines prevents the majority of pathogens and intestinal contents from making direct contact with the epithelial cells (61). In humans, the most abundant protein present in the intestinal mucus layer is mucin 2, which is secreted by goblet cells (62). Several factors, including the microbiota, can influence the composition and therefore the protective effects of the mucus (63). Gut maturation takes place the first couple of weeks after birth rendering a leaky barrier in the first weeks of life (64). This can help to organize oral tolerance induction, but it also provides a risk for allergic sensitization.
Tight junctions strengthen apical connections between epithelial cells that cover the underlying connective tissue, thereby contributing to barrier function. Epithelial tight junction proteins tightly regulate paracellular compartments, preventing transport of large molecules, such as proteins and lipids or microbes and microbial products into the underlying tissue (65). These tight junctions are apically present and are crucial for epithelial barrier integrity. Upon epithelial injury, antigens can cross the epithelium more easily. Cytokines, such as interleukin-8 (IL-8), IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), are produced by the epithelial cells as a response to stress and damage (66). These epithelial cell secreted cytokines influence neighboring dendritic cells (DCs) (67). Generally, DCs in the gastrointestinal tract are hyporesponsive and favor tolerogenic response to prevent unnecessary inflammatory responses to antigens and microbes (68). IL-25, IL-33, and TSLP stimulate the uptake and processing of foreign antigens by DCs and drive these DCs to promote development of TH2 cells from naïve T cells (69, 70). Consequently, IL-4 and IL-13 produced by the TH2 cells induces the activation and class-switching of B cells to produce allergen-specific IgE (67). The secreted IgE will bind to the high-affinity Fc receptors on the surface of mast cells. Upon a consequent encounter, the allergen crosslinks the IgE bound to the mast cells, triggering the mast cell to degranulate and release inflammatory mediators, such as histamine, causing the symptoms of allergic disease (71).
Newborns may be particularly susceptible to developing allergic diseases since the immune system after birth is dominated by TH2 responsiveness (72). Immune maturation involves shifting toward a more T helper 1 (TH1) prone and regulatory type, which favors the development of adequate immune protection and balanced immune responses (73). The importance of the epithelial barrier and mucosal homeostasis in prevention of allergic sensitization has sparked interest. HMOS may help to support this function by stimulating proper epithelial maturation and microbial colonization (74–76).
HMOS Shape the Microbiota of Neonates
The first 1,000 days of life are critical for the development of a diverse, stable gut microbiome (76–78). The initial microbial composition of the gut is determined by host genetics and environmental factors, such as health status, mode of delivery and diet (79). The first bacteria to colonize neonate's intestines are Enterobacteriaceae and Staphylococcus (80), followed by bifidobacteria and lactic acid bacteria (81). Proper colonization is essential for optimal development and health, as the establishment of a rich and diverse microbiome is related to a decreased prevalence of allergic (82), metabolic and other immunologic diseases later in life (83, 84).
HMOS promote the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus species (85, 86). Therefore, HMOS are known for their prebiotic effects and as players in shaping the microbiota of infants as depicted in Figure 2. The microbiota supporting effects of HMOS were observed when the gut colonization in breast-fed and formula-fed infants was compared, while addition of scGOS/lcFOS to formula milk was found to bring the microbiome composition closer to that of breastfed infants (87, 88). The microbiota are capable of fermenting oligosaccharides, however the capacity to degrade HMOS is strain-specific and depends on the presence of several genes (89, 90). Several strains of Bifidobacterium are well-adapted to digest purified natural HMOS into metabolites such as short chain fatty acids (SCFA) (90–93). Glycosyl hydrolases (GH), expressed by bifidobacteria, cleave monosaccharides from the HMOS and making them available for utilization by the microbe (94). This enzymatic degradation can either occur by membrane-associated extracellular GHs (95) or, as is the case for Bifidobacterium infantis, intact HMOS are transported into the cell by Solute Binding Proteins (96) and broken down by GHs inside the cytoplasm (97). The available monosaccharides are assimilated in central metabolic pathways and consequently release large volumes of e.g., SCFAs (98).

Figure 2. Overview of the possible functions of HMOS related to the prevention of allergic diseases. The diversity in structures suggests engagement in several mechanisms related to maturation of the infant's gastrointestinal tract. (1) HMOS have shown to function as prebiotics and therefore stimulate growth of commensal bacteria. In addition, HMOS have shown to bind pathogens, thereby preventing binding of these pathogens to the intestinal epithelium itself and possible consequent infections. SCFAs produced during HMOS fermentation can enhance epithelial barrier integrity and locally and systemically modify immune responses. (2) HMOS can promote mucus production and epithelial tight junction integrity, thereby supporting the physical barrier between the intestinal epithelium and the gut content. (3) Several mechanisms by which HMOS directly affect the immune function have been described. Modulation of the response of DCs is one of those described mechanisms which may be relevant for the instruction of protective mucosal immune development. (4) Transportation of a small fraction of HMOS over the intestinal epithelium, results in systemic availability of these structures. This suggests an immunomodulatory role for HMOS, also beyond the gastrointestinal tract. All these HMOS related mechanisms can potentially enhance tolerance induction and therefore possibly prevent allergic diseases. Adjusted from Ayechu-Muruzabal et al. (48).
Both B. longum and B. bifidum, the major intestinal bacteria found in breastfed infants, are remarkably well-equipped to metabolize HMOS. In contrast, B. adolescentis is often associated with the adult intestinal microbiota, and is a less effective HMOS metabolizer (81, 91, 93). In contrast to Bifidobacterium spp., Bacteroides spp. are not specifically adapted to metabolize HMOS, but degradation of plant polysaccharides by Bacteroides spp. has been indicated (90). As plant-derived oligosaccharides are structurally comparable to human oligosaccharides, the capacity of multiple Bacteroides strains to metabolize HMOS is not unexpected (89). Providing a substrate for commensal gut bacteria results in a competitive growth advantage for these bacteria, enhancing proper colonization in the infants intestine and reducing growth conditions for and colonization by pathogenic bacteria (99, 100).
Unlike several species of commensal gut bacteria discussed previously, certain pathogenic species do not use HMOS as carbohydrate source for growth, including Clostridium difficile, Enterococcus faecalis and Escherichia coli (89). In addition, HMOS can actively bind to several pathogenic microbes and thereby possibly prevent adhesion as first step of infection (101). Infant formula can be supplemented with the prebiotics scGOS and lcFOS in order to promote the growth of various Bifidobacterium and Lactobacillus strains (102). However, these oligosaccharides do not contain terminal fucose or sialic acid residues, hence missing out biological function of HMOS related to these specific functional groups (103).
Proper colonization of the gut promotes intestinal barrier function and immune maturation (104). The establishment of a rich and diverse microbiome is related to a decreased prevalence of allergic diseases (82). Prebiotics like HMOS can support the growth and function of commensal bacteria and therefore possibly enhance gut microbial diversity. The association between microbial diversity and development of allergic diseases (83, 105) and the role of HMOS in this context, has yet to be elucidated.
Metabolites of HMOS Influence Intestinal Barrier Integrity and Immune Function
As described in previous section, HMOS are digested by intestinal bacteria, resulting in various metabolites, among which SCFA are well-known for immunomodulatory properties. The fermentation of major HMOS by bifidobacteria and lactobacilli into SCFA is very efficient (81), hence these bacteria are the dominant suppliers of SCFA in the infant's colon. Butyrate, propionate, and acetate are SCFA metabolites that have gained interest in recent years due to their proposed health benefits. Butyrate is mainly utilized by the epithelial cells, whereas acetate and propionate can be transported across the epithelial barrier to become systemically available in low levels via the bloodstream as depicted in Figure 2 (106).
Upon absorption by the colonic epithelial cells, SCFA promote several functions of the epithelial barrier. The mucus layer covering the epithelial cells is essential to maintain epithelial barrier integrity. SCFA enhance the mucus secretion by upregulating the expression of mucin 2 (107). Acetate, produced in high levels by Bifidobacterium and Bacteroides species, increases the expression of genes related to mucus and support goblet cell differentiation (108–110). In addition, SCFA are known to protect against inflammatory insults and fortify the tight junction barrier (111). Promoting and enhancing the epithelial integrity may be of relevance in preventing allergic diseases, as a disrupted intestinal epithelial layer could lead to a compromised local tolerance response in which food allergens are able to reach underlying immune cells intact (112).
In addition, SCFA interact with DC and T cells and therefore modulate inflammatory immune responses. Many of the protective effects of SCFA have been attributed to the interaction with G protein-coupled receptors (GPR) present on intestinal epithelial cells and immune cells (113). Moreover, GPR-independent regulation of the immune response via T cell modulation has been shown in a murine model (114). In this model, SCFA regulate cytokine production via mammalian target of rapamycin (mTOR) by inhibiting histone deacetylase (HDAC) in T cells. In a previous study, butyrate effectively inhibited several HDACs in various cells, among which those that promote the transcription of FoxP3 in T cells, leading to increased expression of this hallmark transcription factor of regulatory T (Treg) cells (115, 116). In addition, inhibition of maturation and differentiation of macrophages and DCs has been demonstrated (117). Suppression of inflammatory responses by butyrate was shown to involve inhibition of the NF-κB pathway in inflammatory cells such as macrophages in the lamina propria (118).
Interestingly, recently it was found that the microbiome of infants who develop allergic diseases during childhood have a reduced genetic potential for butyrate production from complex carbohydrates, supporting the importance of SCFA production in protecting the infant from developing allergic diseases (119). Therefore, supporting the microbial development may be of interest in infants more susceptible to developing allergic diseases (120, 121). All together, as bacterial metabolites of HMOS, SCFA may contribute to the immunomodulatory and protective effects against allergic disease development.
HMOS Strengthening the Intestinal Epithelial Integrity
Beyond their fermentation products, HMOS themselves may directly provide protection from intestinal epithelial barrier dysfunction (122), by promoting epithelial barrier maturation and mucus production (75) (illustrated in Figure 2). A mixture of human milk derived HMOS was shown to increase mucus production after 24 h of in vitro treatment in two different intestinal epithelial cell lines. The improved mucus production was linked to an upregulation of Muc2. In addition, apart from increased mucus production, HMOS could protect against pathogen induced barrier disruption as determined by means of transepithelial electrical resistance (TEER) (123). Furthermore, pollution induced loss of epithelial barrier integrity could be prevented by scGOS and 3'GL as measured in both TEER values and luciferase yellow flux across the intestinal epithelial monolayer in Caco-2 cells (124, 125). It was also demonstrated that supplementation with scGOS resulted in a significant increased rate of tight junction reassembly (124). Interestingly, the galactosyllactose with a β1–3 glycosidic linkage was effective in protecting the intestinal barrier function, whereas the galactosyllactose with an α1–3 glycosidic linkage did not prevent the deoxynivalenol (DON)-induced disrupted intestinal barrier (125). The protective effect of 3'GL on the intestinal epithelial barrier under challenge is structure-specific, which supports the notion that it is critical to understand the function and diversity of the structures within the total pool of HMOS, including the specific benefits of 3'GL within early life nutrition. These studies show that HMOS may directly promote proper development of the intestinal barrier, which strengthens the physical barrier between the intestinal epithelium and the gut content, contributing to lower antigenic load and mucosal homeostasis, which may help to decrease sensitization to food allergens.
In addition to this, the immunologic effects that are mediated through interaction between the intestinal epithelium and the underlying mucosal immune system should be addressed. Administration of synthetic HMOS 6'SL to antigen-antibody complex activated intestinal epithelial cells in vitro and resulted in a dose-dependent decrease of IL-8 and CCL20 secretion. Whereas, administration of 2'FL selectively reduced the secretion of CCL20 from the two cell lines used in this study (38). Similarly, a decrease of cytokine and chemokine production was observed upon TNFα stimulation of these cells after 6'SL exposure. Furthermore, comparable outcomes were observed for 3'GL, 4'GL, and 6'GL in an in vitro model for the infant intestinal epithelium (50). However, this decrease in cytokine production was not observed when two different intestinal cell lines were exposed to 2'FL (38). Additionally, it was observed that 3'SL, which is an isomer of 6'SL, downregulated the production of pro-inflammatory cytokines in Caco-2 intestinal cells by inhibition of the NF-κB pathway in a PPARγ dependent manner (126). These observations indicate that different functional groups and structures of HMOS exert the anti-inflammatory effects via different mechanisms. Silencing exaggerated or unwanted epithelial cell activation is essential for maintaining mucosal homeostasis.
Data indicated that mice, fed a diet supplemented with GOS for 2 weeks prior to exposure to DON, maintain their normal cellular distribution, as measured by villus height in the proximal small intestine (124). A study in suckling rats investigated the effects of 2'FL on mucosal immunomodulation (19). After treatment with 2'FL for 16 days an overall lower presence of inflammatory cytokines in the intestines compared to a reference group was observed, whereas the ratio of TH1/TH2 cytokines remained unchanged. In addition, the height and area under the villi present in the intestines was significantly increased upon supplementation with 2'FL, pointing to a positive effects of this prebiotic on intestinal growth (19). This is linked to the observation that 2'FL and scGOS/lcFOS in early life alter gut microbiome development while supporting vaccination responses (18, 127, 128).
In the light of NEC, especially sialylated oligosaccharides have shown promising outcomes in vivo in prevention and development of necrotic intestinal lesions (122). Several studies in neonatal rats have reported reduced pathology scores upon intervention with HMOS mixture (129), or single HMOS alone (130, 131). Although sialylated oligosaccharides have been identified as the protective agents (129), intervention with 2'FL has also resulted in a reduced pathology score in rats (130). Dietary supplementation of 2'FL in preterm pigs had no significant effects on intestinal structure, digestive function and the development of NEC (132). Nonetheless, pooled HMOS, rather than single HMOS, have consistently shown to be most effective in preventing development of NEC (122).
Moreover, it has been shown that HMOS provision early in life can protect against the development of autoimmune diabetes in NOD-mice (133). The number of in vivo studies looking into the immunomodulatory effects of single HMOS are rather limited, and currently restricted to only the simple short chain structures. In a murine model for hen's egg allergy, 2'FL or 6'SL were found to reduce allergy symptoms in association with the induction of IL-10+ Treg cells (39). Prebiotic mixtures, such as scGOS and lcFOS, have been studied more extensively for immunomodulatory effects in vivo, showing promising results with regards to preventing allergic diseases, such allergic asthma and food allergy and these effects also link to the induction of Treg responses (134–137). This implies a need for additional in vivo studies to gain insight in the properties of (single) HMOS to modulate gut maturation and the development of the mucosal immune system. Combining these studies, the direct effects of HMOS on the intestinal epithelial integrity and activation status and possibly the mucosal immune system are only started to be elucidated. The exact mechanisms and pathways involved are not yet fully understood. However, some of the receptors involved in HMOS signaling are identified and will be discussed in the following section.
HMOS Bind to and Act as Receptors
One potential role of HMOS to modulate the infant's immune system is through receptor binding properties. In fact, multiple classes of human receptors have been described to interact with specific structures of HMOS, as summarized in Table 1. These receptors are mainly expressed by innate, adaptive immune cells and epithelial cells, they may therefore play a key role in mucosal immunomodulatory effects of HMOS (145).
Glycan Receptors
Glycan-binding receptors, also known as lectins, are particularly effective in binding HMOS. Many of the receptors belonging to the lectin family are involved in modulation of immune pathways. Lectin receptors consist of several subcategories, such as: membrane bound C-type lectins, sialic acid binding immunoglobulin-like lectins (Siglecs) and soluble type galectins.
The C-type lectin receptor dendritic cell-specific ICAM-grabbing non-integrin (DC-SIGN) is present on the surface of DCs and macrophages. It is usually involved in phagocytosis of pathogens upon recognizing pathogen-related glycoproteins. DC-SIGN has an affinity for HMOS containing α-linked fucose residues (138). A high affinity for 2'FL and 3FL (2 major structures of HMOS) may be of distinct physiological relevance in modulating immune responses in infants. DC-SIGN is expressed by cells in the gastrointestinal tract (139) and this receptor can promote allergen uptake by DCs. This may lead to subsequent TH2 cell polarization as seen in patients with atopic dermatitis (146). Therefore, even though DC-SIGN can confer protective regulatory immunity in a pre-clinical model for auto-immune disease (147), DC-SIGN signaling may be involved in the sensitization phase of allergic diseases as allergens are capable of DC activation via DC-SIGN binding (148). An HMOS mixture derived from human milk was found to lower the expression of DC-SIGN on DC (140). This indicates that HMOS may be able to reduce DC-SIGN driven allergic sensitization through suppression of DC-SIGN expression on DC and via blocking the DC-SIGN receptor.
Siglecs are expressed by several immune cells that are involved in allergic effector responses, such as eosinophils and mast cells. Siglecs have been associated with binding of sialylated HMOS, although previous results show only affinity of siglec-1, -5, -7, -9, and -10 to 3'SL and 6'SL (141), and more recent data show a more limited binding affinity of Siglecs for HMOS (138). Siglec-9 provides low binding affinity for 3'SL and 6'SL, while siglec-5 has very low affinity for only 3'SL. This study found no other Siglecs to bind sialylated HMOS (138). Hence, the presence of sialic acid alone is not sufficient to ensure functional binding to a Siglec receptor (138). Siglec-7 and siglec-8 have been associated with allergy related immune mechanism (149, 150) making these potential targets for immune modulation by HMOS in relation to allergy prevention.
Galectins are another group of β-galactoside-binding receptors that bind carbohydrate moieties or glycan structures present on proteins. Moreover, galectins are expressed on and/or secreted by several immune cells and intestinal epithelial cells (151). These receptors can directly forward signals into the cell upon binding to a ligand, but galectins can also be secreted from cells (152). In the secreted form, galectins can act as ligands and bind to receptors, such as TIM-3 and CD44 on other mucosal immune cells (153). Galectins such as galectin-9 have shown to induce Treg cells (154–156). The binding of HMOS to galectins may directly modify galectin release and affect interactions of galectins with other cells, potentially resulting in immune modulation. Of the thirty-two different HMOS structures tested for binding to four galectins (galectin-1, -3, -7, and -9) (142), a total of 25 of these structures were recognized by all four galectins. Significant differences in affinity for each HMOS were observed, i.e., 2'FL, 3'SL, and LNnT were shown to bind galectins, whereas 3FL and 6'SL did not. 2'FL, the most common HMOS in human milk, binds with moderate-to-high affinity to all four galectins, while 3FL a structure very similar to 2'FL, not or weakly binds to any of the four galectins included in the study (142). Similar results were obtained in a different report, including galectin-1, -3, and -7 (143). These findings are supported by a previous study (144), suggesting that all included galectins showed affinity for LNnT, but had no affinity for 6'SL. This study also highlighted the evolutionary conserved binding affinity of galectins for glycans. Galectin-9 is a particularly promising target in allergy prevention strategies, as exposure of intestinal epithelial cells to scGOS/lcFOS together with bacterial CpG DNA or synthetic CpG ODN promoted the secretion of galectin-9 in vitro, which resulted in enhanced secretion of IFNγ and IL-10 production by underlying immune cells (154, 157). These cytokines are related to a regulatory type of TH1 polarization and suppress TH2 cell activation. Experiments with dietary interventions including scGOS/lcFOS enhanced local and/or systemic galectin-9 levels in murine and human allergy in association with symptom reduction (137). Furthermore, galectins can become systemically available and dampen allergic effector responses as shown in a murine model of food allergy (137).
Pattern Recognition Receptors
Toll-like receptors (TLR) are a family of receptors known to sense common molecules of pathogenic or commensal microorganisms, such as TLR4 ligand lipopolysaccharide (LPS) or TLR9 ligand bacterial CpG DNA. Decreased formation of the three-component complex TLR4, CD14, and LPS, inhibits subsequent pro-inflammatory immune signaling (158). Xiao et al. showed an increase in LPS receptor TLR4 mRNA expression upon stimulation with pooled HMOS isolated from human milk in monocytic derived dendritic cells (moDC) in vitro, yet protein levels of this receptor were not increased (140). In addition to affecting TLR4 transcription, HMOS suppress the expression of cluster of differentiation (CD)14, a coreceptor of TLR which is necessary to recognize LPS. 2'FL significantly suppresses CD14 in intestinal epithelial cells (16). In contrast to suppression of inflammation via TLR4 by 2'FL, pro-inflammatory properties related to TLR4 modulation have been described for synthetic 3'SL. In a TLR4-dependent manner, 3'SL was shown to induce intestinal inflammation (15). This pro-inflammatory effect of 3'SL can be explained by mimicking possible structural aspects of pathogenic bacteria, thereby educating and preparing the immune system for possible pathogenic encounters later in life. However, the phenotypical changes of DCs by 3'SL may have been due to LPS contamination of the oligosaccharide during synthesis, since pre-exposure to LPS may contribute to TLR4 silencing (159). However, LPS-containing bacteria are normal components of a healthy intestinal microbiome (160). In this respect, the low level of endotoxins present in purified HMOS used in in vivo studies would be minimal compared to the vast amount of endotoxin triggers the infant receives directly after birth. The contradicting results regarding HMOS-induced modulation of TLR4 show that we are only beginning to elucidate the possible immunomodulatory effects of HMOS. In addition, as synthetic (s)HMOS are either derived from enzymatically-processed lactose or produced by E. coli. In the latter situation a second possible immune trigger from bacterial byproducts may add to the biological effects of sHMOS structure. The origin of HMOS may influence the immunomodulatory effect, therefore an overview of the source and main outcomes of the studies referred to in this review is provided in Table 2.
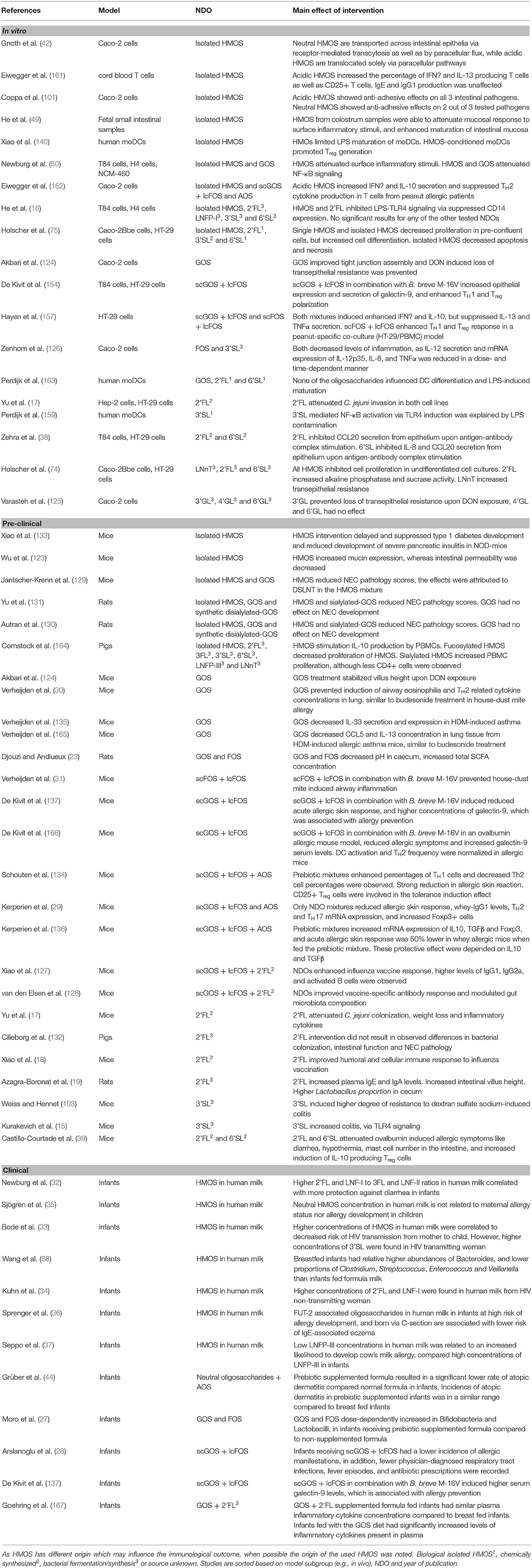
Table 2. Overview of studies included in this review, which describe effects of non-digestible oligosaccharides (NDO) on immune function.
Pathogen Binding
Besides binding to receptors on the cell membrane, HMOS can act as soluble receptors and bind to several pathogenic bacteria, thereby preventing binding to the intestinal epithelium and subsequent infection (101). Both in vitro and in vivo studies show that 2'FL attenuated Campylobacter jejuni infection (17, 168). However, Coppa et al. did not find inhibition of adhesion of Escherichia coli, Vibrio cholerae and Salmonella fyris in an in vitro intestinal epithelial setting with 2'FL (101). Nonetheless, inhibition of adhesion was observed with 3'SL, 6'SL and 3FL and combinations of these sHMOS. There was a diminished growth of Streptococcus agalactiae (group B Streptococcus) upon incubation with human pooled natural HMOS, that was attributed to the neutral fraction of the HMOS (169). This effect was supported by other studies, as pooled HMOS inhibited growth of group B Streptococcus (GBS) and prevented biofilm formation, although the effects of single HMOS were GBS strain specific (170–172). In this study, the effects of HMOS were compared to scGOS. scGOS did not diminish the growth of group B Streptococcus (169), showing that the structures in scGOS in this respect do not exert similar effects as the mentioned HMOS subtypes. These studies indicate that HMOS can also function as decoy receptors, thereby inhibiting growth and adhesion of pathogens in the gastrointestinal tract.
As antibiotic resistance is a growing problem, alternative antibacterial treatments are being investigated (173), including the use of HMOS to potentiate antibiotic functioning (174). It has been recently demonstrated that when exposed to HMOS, GBS becomes sensitive for trimethoprim, an antibiotic to which these bacteria are normally resistant. A significant decrease in metabolic pathways related to membrane construction was observed (175). Furthermore, HMOS were able to sensitize GBS to several antibiotics, such as erythromycin, gentamycin and clindamycin. In addition, an increased sensitivity to gentamycin, when combined with HMOS, in Staphylococcus aureus and Acinetobacter baumanii was also observed. However, these potentiating effects were obtained for β-lactams and glycopeptides (176). Next to the above reported antibacterial properties, similarly some viral inhibiting interactions have been described (177). These interactions include binding of 2'FL to conserved epitopes, which are involved in binding to host cells, on norovirus (178, 179). Next to 2'FL, also 3'SL and 6'SL showed to inhibit cell binding in a rotavirus in vitro model (180). Some promising results of HMOS intervention have even been observed for influenza and HIV infections (177).
HMOS Interact with Immune Cells
HMOS have been detected in the blood, feces and urine of breastfed term and preterm infants (181–184). In breastfed infants, HMOS concentrations in urine appear to be around 10 times higher than in serum (184), which can be explained by clearance of substances from a larger volume of blood and accumulation in a small volume of urine. Direct effects have been demonstrated in vitro in bone marrow-derived dendritic cells (BMDC) treated with 2'FL. There was an increase in the percentage of CD40+ and CD86+ BMDCs upon exposure to 2'FL (18). Direct modulation of human moDCs was not found for 2'FL, 6'SL and scGOS (163), but the idea of possible moDC modulation via other HMOS cannot be excluded. BMDC exposed to 2'FL and stimulated by influenza vaccination had a greater capacity to induce CD4+ T cell proliferation in fresh whole splenocytes (18). Low concentrations of a mixture of acidic HMOS, purified from human milk, can alter cytokine production in cord blood mononuclear cells (CBMC) (161). The production of IFNγ and IL-10 in CBMCs was increased upon exposure to acidic HMOS, while IL-13 production remained unaltered, pointing to skewing of the balance toward a regulatory type TH1 response. Similar effects were observed in a prior study exposing CBMC to acidic HMOS, which resulted in decreased IL-13 production in T cells (162). Mast cell function and direct effects of HMOS on mast cell degranulation were investigated in a murine food allergy model (39). In vitro exposure of bone marrow-derived mast cells to 6'SL resulted in significant inhibition of IgE-dependent mast cell degranulation, but only at a relatively high concentration of 1 mg/mL. However, in this same study, 2'FL did not significantly inhibit mast cell activation. Both 6'SL and 2'FL induce IL10+ Treg cells and thereby indirectly stabilize the degranulation of mast cells, in association with reduced food allergy symptoms (39). Hence, HMOS may have the capacity to modulate the immune response via various mechanisms, as indicated by the direct effects of HMOS on several immune cell types.
In the above described murine model for food allergy, 2'FL and 6'SL reduced food allergy symptoms via inducing Treg cells and modulating mast cells (39). After 2'FL and 6'SL treatment during challenge in ovalbumin sensitized mice enhanced the capacity of CD4+CD25+ Treg cells to inhibit mast cell degranulation ex vivo (39), indicating that specific sHMOS support Treg cell function. Similar results were found using scGOS and lcFOS in combination with acidic oligosaccharides or B. breve in prevention of food- (29, 166) or asthma-allergy in mice (31, 165). In piglets, either sow-reared or formula fed, peripheral blood mononuclear cells (PBMCs) were isolated (164). PBMCs from formula fed piglets showed more proliferation than sow-reared piglets upon LPS stimulation ex vivo, while ex vivo addition of sHMOS 2'FL normalized this increased proliferation. The percentage of T helper cells was higher in formula fed piglets compared to sow-reared piglets. Ex vivo added synthetic fucosylated and sialylated oligosaccharides downsized the expansion of the TH cell population in the formula fed piglets, while the cytotoxic T cell population remained unaffected by ex vivo sHMOS treatment (164). These results indicate that fucosylated and sialylated oligosaccharides may possess immune regulatory properties, potentially modulating an allergic inflammatory response.
Although clinical trials in this area of research are scarce, data from an initial study indicate that addition of 2'FL to infant formula lowers concentrations of pro-inflammatory cytokines in plasma compared to infants fed a control formula (167). In addition, the decrease of these cytokines in the 2'FL supplemented infants was comparable to the low level of inflammatory cytokines that was measured in plasma of breastfed infants (167). As such, it should be carefully considered whether the effects observed in any of the in vivo and clinical studies are caused by a direct effect of the HMOS or indirect immunomodulatory effects as a result of microbiome modulation.
A convincing body of evidence is missing to ascribe clear immune development properties to HMOS and individual HMOS structures, since only a small number of in vivo studies describe immunomodulatory properties and immune maturation. In addition, the exact properties of the different groups of HMOS to modulate the immune system are not clear. Therefore, several studies illustrating immunomodulatory effects of scGOS and lcFOS have been described here and summarized in Table 2, as they may propose a framework in which future research could focus to elucidate immune related mechanisms affected by HMOS. As synthetically produced HMOS have become available recently, studying these may contribute to acquiring knowledge of the exact properties of HMOS and their specific functional groups in more detail and promote research focussing on allergy prevention. Development of adequate in vitro models for allergic sensitization including intestinal epithelial cells and/or dendritic cells, may help understanding the direct immunomodulatory effects of HMOS and their possible role in allergy prevention.
Conclusion
The increasing prevalence of allergic diseases has sparked interest in the role of early life nutrition and allergy development. Dietary components drive early life microbiome development as well as gut and immune maturation. HMOS in breast milk exhibit various microbiome modulating as well as mucosal immune maturation properties, which are not yet fully understood. However, in recent years several pathways involved in the effects of HMOS have been elucidated, including their capacities to fortify the microbiome composition and the release of fermentation products including SCFAs, as well as direct binding to pathogens and interactions with the gastrointestinal epithelium and local and systemic immune cells (as illustrated in Figure 2). Specific structural groups of HMOS may target several aspects of the immune system and modify immune function, thereby highlighting the need for further research on this topic. In addition, a more diverse mixture of oligosaccharide structures in neonatal formula nutrition may more closely resemble the HMOS composition as available in human breast milk and provide extra benefit for the child. Future research should focus on uncovering the mechanisms and pathways by which HMOS and the specific functional groups present in these HMOS may exert immunomodulatory actions. Ultimately, it would be of utmost value to identify whether specific HMOS structures are capable of contributing to early life allergy prevention.
Author Contributions
MZ and NW have written the review. JG, GF, BL, and LW supervised the program. BL and LW have discussed and edited the manuscript. BS made specific contribution to the program with regard to functional oligosaccharides. All authors listed have approved for publication.
Funding
This study was financially supported by a Dutch government TKI-Health Holland public-private funding for the project with the acronym HMOS for ALL, project number LSHM18037.
Conflict of Interest
JG is head of the Division of Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of Science at Utrecht University and partly employed by Danone Nutricia Research B.V. BS and BL are employed by Danone Nutricia Research B.V. BS has an associated position at Utrecht Institute for Pharmaceutical Sciences, CBDD, Faculty of Science at Utrecht University. BL is affiliated at and leading a strategic alliance between Danone Nutricia Research B.V. and the University Medical Centre Utrecht/Wilhelmina Children's Hospital. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Garwolinska D, Namieśnik J, Kot-Wasik A, Hewelt-Belka W. Chemistry of human breast milk - a comprehensive review of the composition and role of milk metabolites in child development. J Agric Food Chem. (2018) 66:11881–96. doi: 10.1021/acs.jafc.8b04031
2. Grulee C, Sanford H. The influence of breast and artificial feeding on infantile eczema. J Pediatr. (1936) 9:223–5. doi: 10.1016/S0022-3476(36)80058-4
3. Nwaru BI, Craig LCA, Allan K, Prabhu N, Turner SW, Mcneill G, et al. Breastfeeding and introduction of complementary foods during infancy in relation to the risk of asthma and atopic diseases up to 10 years. Clin Exp Allergy. (2013) 43:1263–73. doi: 10.1111/cea.12180
4. Nwaru BI, Takkinen HM, Niemelä O, Kaila M, Erkkola M, Ahonen S, et al. Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol. (2013) 131:78–86. doi: 10.1016/j.jaci.2012.10.028
5. Lowe AJ, Thien FCK, Stoney RM, Bennett CM, Hosking CS, Hill DJ, et al. Associations between fatty acids in colostrum and breast milk and risk of allergic disease. Clin Exp Allergy. (2008) 38:1745–51. doi: 10.1111/j.1365-2222.2008.03073.x
6. Wijga AH, Van Houwelingen AC, Kerkhof M, Tabak C, De Jongste JC, Gerritsen J, et al. Breast milk fatty acids and allergic disease in preschool children: the prevention and incidence of asthma and mite allergy birth cohort study. J Allergy Clin Immunol. (2006) 117:440–7. doi: 10.1016/j.jaci.2005.10.022
7. Lee MT, Wu CC, Ou CY, Chang JC, Liu CA, Wang CL, et al. A prospective birth cohort study of different risk factors for development of allergic diseases in offspring of non-atopic parents. Oncotarget. (2017) 8:10858–70. doi: 10.18632/oncotarget.14565
8. Elbert NJ, van Meel ER, den Dekker HT, de Jong NW, Nijsten TEC, Jaddoe VWV, et al. Duration and exclusiveness of breastfeeding and risk of childhood atopic diseases. Allergy Eur J Allergy Clin Immunol. (2017) 72:1936–43. doi: 10.1111/all.13195
9. Munblit D, Peroni DG, Boix-Amorós A, Hsu PSB, Van't Land Gay MCL, et al. Human milk and allergic diseases: an unsolved puzzle. Nutrients. (2017) 9:894. doi: 10.3390/nu9080894
10. Thurl S, Munzert M, Henker J, Boehm G, Mller-Werner B, Jelinek J, et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. (2010) 104:1261–71. doi: 10.1017/S0007114510002072
11. Kunz C, Meyer C, Collado MC, Geiger L, García-Mantrana I, Bertua-Ríos B, et al. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr. (2017) 64:789–98. doi: 10.1097/MPG.0000000000001402
12. Thurl S, Munzert M, Boehm G, Matthews C, Stahl B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr Rev. (2017) 75:920–33. doi: 10.1093/nutrit/nux044
13. Moossavi S, Miliku K, Sepehri S, Khafipour E, Azad MB. The prebiotic and probiotic properties of human milk: implications for infant immune development and pediatric asthma. Front Pediatr. (2018) 6:1–7. doi: 10.3389/fped.2018.00197
14. Ramani S, Stewart CJ, Laucirica DR, Ajami NJ, Robertson B, Autran CA, et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat Commun. (2018) 9:1–12. doi: 10.1038/s41467-018-07476-4
15. Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci USA. (2013) 110:17444–9. doi: 10.1073/pnas.1306322110
16. He YY, Liu SB, Kling DE, Leone S, Lawlor NT, Huang Y, et al. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. (2016) 65:33–46. doi: 10.1136/gutjnl-2014-307544
17. Yu ZT, Nanthakumar NN, Newburg DS. The human milk oligosaccharide 2′-fucosyllactose quenches campylobacter jejuni–induced inflammation in human epithelial cells HEp-2 and HT-29 and in mouse intestinal mucosa. J Nutr. (2016) 146:1980–90. doi: 10.3945/jn.116.230706
18. Xiao L, Leusink-Muis T, Kettelarij N, van Ark I, Blijenberg B, Hesen NA, et al. Human milk oligosaccharide 2'-fucosyllactose improves innate and adaptive immunity in an influenza-specific murine vaccination model. Front Immunol. (2018) 9:452. doi: 10.3389/fimmu.2018.00452
19. Azagra-Boronat I, Massot-Cladera M, Mayneris-Perxachs J, Knipping K, van't Land B, Tims S, et al. Immunomodulatory and prebiotic effects of 2′-fucosyllactose in suckling rats. Front Immunol. (2019) 10:1773. doi: 10.3389/fimmu.2019.01773
20. Mowat AMI. To respond or not to respond - a personal perspective of intestinal tolerance. Nat Rev Immunol. (2018) 18:405–15. doi: 10.1038/s41577-018-0002-x
21. Houghteling PD, Walker WA. From birth to ‘immuno-health', allergies and enterocolitis. J Clin Gastroenterol. (2015) 49(Suppl. 1):S7–12. doi: 10.1097/MCG.0000000000000355
22. Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. (2001) 15:1398–403. doi: 10.1096/fj.00-0833hyp
23. Djouzi Z, Andlueux C. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br J Nutr. (1997) 78:313–24. doi: 10.1079/bjn19970149
24. Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr. (2002) 34:291–5. doi: 10.1097/00005176-200203000-00014
25. Fanaro S, Jelinek J, Stahl B, Boehm G, Kock R, Vigi V. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: effect on intestinal flora, stool characteristics, and pH. J Pediatr Gastroenterol Nutr. (2005) 41:186–90. doi: 10.1097/01.mpg.0000172747.64103.d7
26. Van Hoffen E, Ruiter B, Faber J, M'Rabet L, Knol E F, Stahl B, et al. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy Eur J Allergy Clin Immunol. (2009) 64:484–7. doi: 10.1111/j.1398-9995.2008.01765.x
27. Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. (2006) 91:814–9. doi: 10.1136/adc.2006.098251
28. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. (2008) 138:1091–5. doi: 10.1093/jn/138.6.1091
29. Kerperien J, Jeurink PV, Wehkamp T, van der Veer A, van de Kant HJG, Hofman GA, et al. Non-digestible oligosaccharides modulate intestinal immune activation and suppress cow's milk allergic symptoms. Pediatr Allergy Immunol. (2014) 25:747–54. doi: 10.1111/pai.12311
30. Verheijden KAT, Willemsen LEM, Braber S, Leusink-Muis T, Delsing DJM, Garssen J, et al. Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model. Respir Res. (2015) 16:1–9. doi: 10.1186/s12931-015-0171-0
31. Verheijden KAT, Willemsen LEM, Braber S, Leusink-Muis T, Jeurink PV, Garssen J, et al. The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V. Eur J Nutr. (2016) 55:1141–51. doi: 10.1007/s00394-015-0928-8
32. Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, de Lourdes Guerrero M, et al. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. (2004) 14:253–63. doi: 10.1093/glycob/cwh020
33. Bode L, Kuhn L, Kim H Y, Hsiao L, Nissan C, Sinkala M, et al. Human milk oligosaccharides and postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. (2012) 96:831–9. doi: 10.3945/ajcn.112.039503.1
34. Kuhn L, Kim H.-Y, Hsiao L, Nissan C, Kankasa C, Mwiya M, et al. Oligosaccharide composition of breast milk influences survival of uninfected children born to HIV-infected mothers in lusaka, zambia. J Nutr. (2015) 145:66–72. doi: 10.3945/jn.114.199794
35. Sjögren YM, Duchén K, Lindh F, Björkstén B, Sverremark-Ekström E. Neutral oligosaccharides in colostrum in relation to maternal allergy and allergy development in children up to 18 months of age. Pediatr Allergy Immunol. (2007) 18:20–6. doi: 10.1111/j.1399-3038.2006.00486.x
36. Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr. (2017) 56:1293–301. doi: 10.1007/s00394-016-1180-6
37. Seppo A, Autran CA, Bode L, Jarvinen KM. Human milk oligosaccharides and development of cow's milk allergy in infants. J Allergy Clin Immunol. (2017) 139:708–11. doi: 10.1016/j.jaci.2016.08.031
38. Zehra S, Khambati I, Vierhout M, Mian MF, Buck R, Forsythe P. Human milk oligosaccharides attenuate antigen–antibody complex induced chemokine release from human intestinal epithelial cell lines. J. Food Sci. (2018) 83:499–508. doi: 10.1111/1750-3841.14039
39. Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy Eur J Allergy Clin Immunol. (2015) 70:1091–102. doi: 10.1111/all.12650
40. Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. (2000) 71:1589–96. doi: 10.1093/ajcn/71.6.1589
41. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. (2015) 91:619–22. doi: 10.1016/j.earlhumdev.2015.09.001
42. Gnoth MJ, Rudloff S, Kunz C, Kinne RKH. Investigations of the in vitro transport of human milk oligosaccharides by a caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J Biol Chem. (2001) 276:34363–70. doi: 10.1074/jbc.M104805200
43. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-Analysis. Am J Epidemiol. (2014) 179:1153–67. doi: 10.1093/aje/kwu072
44. Grüber C, Van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. (2010) 126:791–7. doi: 10.1016/j.jaci.2010.07.022
45. Cuello-Garcia CA, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. World allergy organization-mcmaster university guidelines for allergic disease prevention (GLAD-P): prebiotics. World Allergy Organ J. (2016) 9:1–10. doi: 10.1186/s40413-016-0102-7
46. Wopereis H, Sim K, Shaw A, Warner JO, Knol J, Kroll JS. Intestinal microbiota in infants at high risk for allergy: effects of prebiotics and role in eczema development. J Allergy Clin Immunol. (2018) 141:1334–42.e5. doi: 10.1016/j.jaci.2017.05.054
47. Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. (2007) 137:847–9S. doi: 10.1093/jn/137.3.847s
48. Ayechu-Muruzabal V, van Stigt AH, Mank M, Willemsen LEM, Stahl B, Garssen J, et al. Diversity of human milk oligosaccharides and effects on early life immune development. Front Pediatr. (2018) 6:239. doi: 10.3389/fped.2018.00239
49. He Y, Liu S, Leone S, Newburg DS. Human colostrum oligosaccharide modulate major immunologic pathways of immature human intestine. Physiol Behav. (2014) 7:1326–39. doi: 10.1038/mi.2014.20
50. Newburg DS, Ko JS, Leone S, Nanthakumar NN. Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3′-, 4-, and 6′-galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo. J Nutr. (2016) 146:358–67. doi: 10.3945/jn.115.220749
51. Liu JJ, Kwak S, Pathanibul P, Lee JW, Yu S, Yun EJ, et al. Biosynthesis of a functional human milk oligosaccharide, 2′-fucosyllactose, and l -fucose using engineered Saccharomyces Cerevisiae. Synth Biol. (2018) 7:2529–36. doi: 10.1021/acssynbio.8b00134
52. Yu J, Shin J, Park M, Seydametova E, Jung SM, Seo JH, et al. Engineering of α-1,3-fucosyltransferases for production of 3-fucosyllactose in Escherichia Coli. Metab Eng. (2018) 48:269–78. doi: 10.1016/j.ymben.2018.05.021
53. Jung SM, Park YC, Seo JH. Production of 3-fucosyllactose in engineered Escherichia Coli with α-1,3-fucosyltransferase from Helicobacter Pylori. Biotechnol J. (2019) 14:1–7. doi: 10.1002/biot.201800498
54. Chen C, Zhang Y, Xue M, Liu XW, Li Y, Chen X, et al. Sequential one-pot multienzyme (OPME) synthesis of lacto-N-neotetraose and its sialyl and fucosyl derivatives. Chem Commun. (2015) 51:7689–92. doi: 10.1039/c5cc01330e
55. Guo Y, Jers C, Meyer AS, Li H, Kirpekar F, Mikkelsen JD. Modulating the regioselectivity of a Pasteurella Multocida sialyltransferase for biocatalytic production of 3′- and 6′-sialyllactose. Enzyme Microb Technol. (2015) 78:54–62. doi: 10.1016/j.enzmictec.2015.06.012
56. Akiyama K, Takase M, Horikoshi K, Okonogi S. Production of galactooligosaccharides from lactose using a β-glucosidase from thermus sp. Z-1. Biosci Biotechnol Biochem. (2001) 65:438–41. doi: 10.1271/bbb.65.438
57. West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. (2015) 45:43–53. doi: 10.1111/cea.12332
58. Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. (2017) 140:1–12. doi: 10.1016/j.jaci.2017.05.010
59. Yu LCH. Intestinal epithelial barrier dysfunction in food hypersensitivity. Allergy J. (2012) 2012:596081. doi: 10.1155/2012/596081
60. Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. (2012) 122:440–7doi: 10.1172/JCI57416DS1
61. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. (2009) 9:799–809. doi: 10.1038/nri2653
62. Arike L, Holmén-Larsson J, Hansson GC. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology. (2017) 27:318–28. doi: 10.1093/glycob/cww134
63. Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. (2015) 16:164–77. doi: 10.15252/embr.201439263
64. Ma B, Mccomb E, Gajer P, Yang H, Humphrys M, Okogbule-Wonodi AC, et al. Microbial biomarkers of intestinal barrier maturation in preterm infants. Front Microbiol. (2018) 9:1–14, (2018). doi: 10.3389/fmicb.2018.02755
65. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. (2013) 70:631–59. doi: 10.1007/s00018-012-1070-x
66. Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC, Moog F, et al. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. (2016) 137:984–97. doi: 10.1016/j.jaci.2016.02.004
67. Gour N, Wills-Karp M. IL-4 and IL13 signalling in allergic airway disease. Cytokine. (2015) 75:68–78. doi: 10.1016/j.cyto.2015.05.014
68. Mann ER, Li X. Intestinal antigen-presenting cells in mucosal immune homeostasis: crosstalk between dendritic cells, macrophages and B-cells. World J Gastroenterol. (2014) 20:9653–64. doi: 10.3748/wjg.v20.i29.9653
69. Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. (2015) 136:59–68.e14. doi: 10.1016/j.jaci.2014.11.037
70. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. (2012) 129:104–11. doi: 10.1016/j.jaci.2011.08.031
71. Valitutti S, Joulia R, Espinso E. The mast cell antibody-dependent degranulatory synapse. Immune Synapse. (2017) 1584:487–95. doi: 10.1007/978-1-4939-6881-7_30
72. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. (2003) 8:223–46. doi: 10.1111/pim.12500
73. Simon AK, Hollander GA, Mcmichael A, Mcmichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
74. Holscher HD, Davis S R, Tappenden KA. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J Nutr. (2014) 144:586–91. doi: 10.3945/jn.113.189704
75. Holscher HD, Bode L, Tappenden KA. Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro. J Pediatr Gastroenterol Nutr. (2017) 64:296–301. doi: 10.1097/MPG.0000000000001274
76. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/j.chom.2015.04.004
77. Selma-Royo M, Tarrazó M, García-Mantrana I, Gómez-Gallego C, Salminen S, Collado M C. Shaping microbiota during the first (1000) days of life. In: Guandalini S, Indrio F, editors. Probiotics and Child Gastrointestinal Health: Advances in Microbiology, Infectious Diseases and Public Health Volume. Springer International Publishing (2019). p. 3–24
78. Dzidic M, Boix-Amorós A, Selma-Royo M, Mira A, Collado M. Gut microbiota and mucosal immunity in the neonate. Med Sci. (2018) 6:56. doi: 10.3390/medsci6030056
79. Wang M, Monaco M H, Donovan S M. Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonatal Med. (2016) 21:380–7. doi: 10.1016/j.siny.2016.04.004
80. Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun. (2016) 7:1–12. doi: 10.1038/ncomms11939
81. Yu ZT, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. (2013) 23:1281–92. doi: 10.1093/glycob/cwt065
82. Cukrowska B. Microbial and nutritional programming—the importance of the microbiome and early exposure to potential food allergens in the development of allergies. Nutrients. (2018) 10:1541. doi: 10.3390/nu10101541
83. Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. (2015) 7:307. doi: 10.1126/scitranslmed.aab2271
84. Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, et al. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. (2015) 6:1–9. doi: 10.1128/mBio.02419-14
85. Underwood MA, Davis JCC, Kalanetra KM, Gehlot S, Patole S, Tancredi DJ, et al. Digestion of human milk oligosaccharides by bifidobacterium breve in the premature infant. J Pediatr Gastroenterol Nutr. (2017) 65:449–55. doi: 10.1097/MPG.0000000000001590
86. Thongaram T, Hoeflinger JL, Chow JM, Miller MJ. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and Lactobacilli. J Dairy Sci. (2017) 100:7825–33. doi: 10.3168/jds.2017-12753
87. Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. (2005) 71:2318–24. doi: 10.1128/AEM.71.5.2318
88. Wang M, Li M, Wu S, Lebrilla CB, Chapkin RS, Ivanov I, et al. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. (2015) 60:825–33. doi: 10.1097/MPG.0000000000000752
89. Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. (2010) 58:5334–40. doi: 10.1021/jf9044205
90. Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect. (2012) 18(Suppl. 4):12–5. doi: 10.1111/j.1469-0691.2012.03863.x
91. Ward RE, Niñonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium Infantis and Lactobacillus Gasseri. Appl Environ Microbiol. (2006) 72:4497–9. doi: 10.1128/AEM.02515-05
92. Ward RE, Niñonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. (2007) 51:1398–405. doi: 10.1002/mnfr.200700150
93. LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. (2007) 55:8914–9. doi: 10.1021/jf0710480
94. Sela DA, Garrido D, Lerno L, Wu S, Tan K, Eom HJ, et al. Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl Environ Microbiol. (2012) 78:795–803. doi: 10.1128/AEM.06762-11
95. Kitoaka M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv Nutr An Int Rev J. (2012) 3:422–9S. doi: 10.3945/an.111.001420
96. Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE. (2011) 6:e17315. doi: 10.1371/journal.pone.0017315
97. Garrido D, Ruiz-Moyano S, Lemay DG, Sela DA, German JB, Mills DA. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci Rep. (2015) 5:1–18. doi: 10.1038/srep13517
98. Kim JH, An HJ, Garrido D, German JB, Lebrilla CB, Mills DA. Proteomic analysis of bifidobacterium longum subsp. infantis reveals the metabolic insight on consumption of prebiotics and host glycans. PLoS ONE. (2013) 8:e57535. doi: 10.1371/journal.pone.0057535
99. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. (2016) 167L:1339–53.e21. doi: 10.1016/j.cell.2016.10.043
100. Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. (2018) 23:705–15. doi: 10.1016/j.chom.2018.05.012
101. Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, et al. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia Coli, Vibrio cholerae, and Salmonella fyris. Pediatr Res. (2006) 59:377–82. doi: 10.1203/01.pdr.0000200805.45593.17
102. Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. (2005) 71:2318–24. doi: 10.1128/AEM.71.5.2318-2324.2005
103. Weiss GA, Hennet T. The role of milk sialyllactose in intestinal bacterial colonization. Adv Nutr An Int Rev J. (2012) 3:483–8. doi: 10.3945/an.111.001651
104. Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. (2017) 5:e1373208. doi: 10.1080/21688370.2017.1373208
105. Haahtela T, Laatikainen T, Alenius H, Auvinen P, Fyhrquist N, Hanski I, et al. Hunt for the origin of allergy - comparing the finnish and russian karelia. Clin Exp Allergy. (2015) 45:891–901. doi: 10.1111/cea.12527
106. Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, Macfarlane T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. (1987) 28:1221–27. doi: 10.1136/gut.28.10.1221
107. Willemsen LEM, Koetsier MA, Van Deventer SJH, Van Tol EAF. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut. (2003) 52:1442–7. doi: 10.1136/gut.52.10.1442
108. Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. (2009) 9:197. doi: 10.1186/1471-2180-9-197
109. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. (2011) 469:543–9. doi: 10.1038/nature09646
110. Wrzosek L, Miquel S, Noordine ML, Bouet S, Chevalier-Curt MJ, Robert V, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. (2013) 11:61. doi: 10.1186/1741-7007-11-61
111. Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of nlrp3 inflammasome and autophagy. Cell Physiol Biochem. (2018) 49:190–205. doi: 10.1159/000492853
112. König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. (2016) 7:e196. doi: 10.1038/ctg.2016.54
113. Thorburn AN, McKenzie CI, Shen S, Stanley D, MacIa L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. (2015) 6:7320. doi: 10.1038/ncomms8320
114. Park J, Kim M, Kan SG, Hopf Jannasch A, Cooper B, Patterson J, et al. Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. (2015) 8:80–93. doi: 10.1038/mi.2014.44
115. Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. (2007) 109:1123–30. doi: 10.1182/blood-2006-04-019711
116. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. (2014) 111:2247–52. doi: 10.1073/pnas.1322269111
117. Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. (2002) 130:245–55. doi: 10.1046/j.0009-9104.2002.01977.x
118. Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberger F, et al. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. (2002) 37:458–66. doi: 10.1080/003655202317316105
119. Cait A, Cardenas E, Dimitriu P, Amenyogbe N, Dai D, Cait J, et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J Allergy Clin Immunol. (2019)144:1638–47. doi: 10.1016/j.jaci.2019.06.029
120. Forsberg A, West CE, Prescott SL, Jenmalm MC. Pre- and probiotics for allergy prevention: time to revisit recommendations?. Clin Exp Allergy. (2016) 46:1506–21. doi: 10.1111/cea.12838
121. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20:159–66. doi: 10.1038/nm.3444
122. Bering SB. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients. (2018) 10:1461–76. doi: 10.3390/nu10101461
123. Wu RY, Li B, Koike Y, Määttänen P, Miyake H, Cadete M, et al. Human milk oligosaccharides increase mucin expression in experimental necrotizing enterocolitis. Mol Nutr Food Res. (2019) 63:1–11. doi: 10.1002/mnfr.201800658
124. Akbari P, Braber S, Alizadeh A, Verheijden KAT, Schoterman MHC, Kraneveld AD, et al. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell. J Nutr. (2015) 145:1604–13. doi: 10.3945/jn.114.209486
125. Varasteh S, van't Land B, Giziakis L, Mank M, Stahl B, Wierstema S, et al. Human milk oligosaccharide 3′-galactosyllactose can protect the intestinal barrier to challenges. In: Proceedings of the 5th Anual Meeting of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. Glasgow (2019). p. 5–8.
126. Zenhom M, Hyder A, de Vrese M, Heller K J, Roeder T, Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal caco-2 cells via activation of pparγ and peptidoglycan recognition protein 3. J. Nutr. (2011) 141:971–7. doi: 10.3945/jn.110.136176
127. Xiao L, Engen PA, Leusink-Muis T, Van Ark I, Stahl B, Overbeek SA, et al. The combination of 2-fucosyllactose with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides that enhance influenza vaccine responses is associated with mucosal immune regulation in mice. J Nutr. (2019) 149:856–69. doi: 10.1093/jn/nxz006
128. van den Elsen LWJ, Tims S, Jones AM, Stewart A, Stahl B, Garssen J, et al. Prebiotic oligosaccharides in early life alter gut microbiome development in male mice while supporting influenza vaccination responses. Benef Microbes. (2019) 10:279–91. doi: 10.3920/BM2018.0098
129. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. (2012) 61:1417–25. doi: 10.1136/gutjnl-2011-301404
130. Autran CA, Schoterman MHC, Jantscher-krenn E, Kamerling JP, Bode L. Sialylated galacto-oligosaccharides and 2′ -fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr. (2016) 116:294–99. doi: 10.1017/S0007114516002038
131. Yu H, Lau K, Thon V, Autran C A, Jantscher-krenn E, Xue M, et al. Synthetic disialyl hexasaccharides protect neonatal rats from necrotizing enterocolitis. Angew Chemie Int Ed. (2014) 53:6687–91. doi: 10.1002/anie.201403588
132. Cilieborg MS, Bering SB, Østergaard VM, Jensen ML, Krych Ł, Newburg DS, et al. Minimal short-term effect of dietary 2'-fucosyllactose on bacterial colonisation, intestinal function and necrotising enterocolitis in preterm pigs. Br J Nutr. (2016) 116:834–41. doi: 10.1017/S0007114516002646
133. Xiao L, Van't Land B, Engen PA, Naqib A, Green SJ, Nato A, et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci Rep. (2018) 8:1–15. doi: 10.1038/s41598-018-22052-y
134. Schouten B, Van Esch BCAM, Hofman GA, De Kivit S, Boon L, Knippels LMJ, et al. A potential role for CD25+regulatory T-cells in the protection against casein allergy by dietary non-digestible carbohydrates. Br J Nutr. (2012) 107:96–105. doi: 10.1017/S0007114511002637
135. Verheijden KAT, Akbari P, Willemsen LEM, Kraneveld AD, Folkerts G, Garssen J, et al. Inflammation-induced expression of the alarmin interleukin 33 can be suppressed by galacto-oligosaccharides. Int Arch Allergy Immunol. (2015) 167:127–36. doi: 10.1159/000437327
136. Kerperien J, Veening-Griffioen D, Wehkamp T, Van Esch BCAM, Hofman GA, Cornelissen P, et al. IL-10 receptor or TGF-β neutralization abrogates the protective effect of a specific nondigestible oligosaccharide mixture in cow-milk-allergic mice. J Nutr. (2018) 148:1372–9. doi: 10.1093/jn/nxy104
137. De Kivit S, Saeland E, Kraneveld AD, Van De Kant HJG, Schouten B, Van Esch BCAM, et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy Eur J Allergy Clin Immunol. (2012) 67:343–52. doi: 10.1111/j.1398-9995.2011.02771.x
138. Noll AJ, Yu Y, Lasanajak Y, Duska-McEwen G, Buck RH, Smith DF, et al. Human DC-SIGN binds specific human milk glycans. Biochem J. (2016) 473:1343–53. doi: 10.1042/BCJ20160046
139. Koning N, Kessen SFM, Van Der Voorn JP, Appelmelk BJ, Jeurink PV, Knippels LMJ, et al. Human milk blocks DC-SIGN-pathogen interaction via MUC1. Front Immunol. (2015) 6:1–9. doi: 10.3389/fimmu.2015.00112
140. Xiao L, van De Worp WRPH, Stassen R, van Maastrigt C, Kettelarij N, Stahl B, et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur J Immunol. (2019) 49:1001–14. doi: 10.1002/eji.201847971
141. Zou Z, Chastain A, Moir S, Ford J, Trandem K, Martinelli E, et al. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS ONE. (2011) 6:e24559. doi: 10.1371/journal.pone.0024559
142. Shams-Ud-Doha K, Kitova EN, Kitov PI, St-Pierre Y, Klassen JS. Human milk oligosaccharide specificities of human galectins. comparison of electrospray ionization mass spectrometry and glycan microarray screening results. Anal Chem. (2017) 89:4914–21. doi: 10.1021/acs.analchem.6b05169
143. El-Hawiet A, Chen Y, Shams-Ud-Doha K, Kitova EN, St-Pierre Y, Klassen JS. High-throughput label- and immobilization-free screening of human milk oligosaccharides against lectins. Anal Chem. (2017) 89:8713–22. doi: 10.1021/acs.analchem.7b00542
144. Hirabayashi J, Hashidate T, Arata Y, Nishi N. Oligosaccharide specificity for galectins: a search by frontal affinity chromotography. Biochim Biophys Acta. (2002) 1572:232–54. doi: 10.1016/s0304-4165(02)00311-2
145. Triantis V, Bode L, van Neerven RJJ. Immunological effects of human milk oligosaccharides. Front Pediatr. (2018) 6:190. doi: 10.3389/fped.2018.00190
146. Zhang Y, Luo Y, Li W, Liu J, Chen M, Gu H, et al. DC-SIGN promotes allergen uptake and activation of dendritic cells in patients with atopic dermatitis. J Dermatol Sci. (2016) 84:128–36. doi: 10.1016/j.jdermsci.2016.08.008
147. García-Vallejo JJ, Ilarregui JM, Kalay H, Chamorro S, Koning N, Unger WW, et al. CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG. J Exp Med. (2014) 211:1465–83. doi: 10.1084/jem.20122192
148. Kamalakannan M, Chang LM, Grishina G, Sampson HA, Masilamani M. Identification and characterization of DC-SIGN-binding glycoproteins in allergenic foods. Allergy Eur JAllergy Clin Immunol. (2016) 71:1145–55. doi: 10.1111/all.12873
149. Arakawa S, Suzukawa M, Ohshima N, Tashimo H, Asari I, Matsui H, et al. Expression of Siglec-8 is regulated by interleukin-5, and serum levels of soluble Siglec-8 may predict responsiveness of severe eosinophilic asthma to mepolizumab. Allergol Int. (2018) 67:S41–4. doi: 10.1016/j.alit.2018.03.006
150. Legrand F, Landolina N, Zaffran I, Emeh RO, Chen E, Klion AD, et al. Siglec-7 on peripheral blood eosinophils: surface expression and function. Allergy Eur J Allergy Clin Immunol. (2019) 74:1257–65. doi: 10.1111/all.13730
151. Nio-Kobayashi J. Tissue- and cell-specific localization of galectins, β-galactose-binding animal lectins, and their potential functions in health and disease. Anat Sci Int. (2017) 92:25–36. doi: 10.1007/s12565-016-0366-6
152. Hönig E, Schneider K, Jacob R. Recycling of galectin-3 in epithelial cells. Eur J Cell Biol. (2015) 94:309–15. doi: 10.1016/j.ejcb.2015.05.004
153. De Kivit S, Kraneveld AD, Garssen J, Willemsen LEM. Glycan recognition at the interface of the intestinal immune system: target for immune modulation via dietary components. Eur J Pharmacol. (2011) 668(Suppl. 1):S124–32. doi: 10.1016/j.ejphar.2011.05.086
154. De Kivit S, Kraneveld AD, Knippels LMJ, Van Kooyk Y, Garssen J, Willemsen LEM. Intestinal epithelium-derived galectin-9 is involved in the immunomodulating effects of nondigestible oligosaccharides. J Innate Immun. (2013):625–38. doi: 10.1159/000350515
155. Cummings RD. T cells are Smad'ly in Love with galectin-9. Immunity. (2014) 41:171–3. doi: 10.1016/j.immuni.2014.08.001
156. Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotaa C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. (2014) 41:270–82. doi: 10.1016/j.immuni.2014.06.011
157. Hayen SM, Otten HG, Overbeek SA, Knulst AC, Garssen J, Willemsen LEM. Exposure of intestinal epithelial cells to short- and long-chain fructo-oligosaccharides and CpG oligodeoxynucleotides enhances peanut-specific T Helper 1 polarization. Front Immunol. (2018) 9:923. doi: 10.3389/fimmu.2018.00923
158. Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, et al. CD14 controls LPS-induced endocytosis of Toll-loke receptor 4. Cell. (2011) 147:868–80. doi: 10.1016/j.cell.2011.09.051
159. Perdijk O, Joost Van Neerven RJ, Meijer B, Savelkoul HFJ, Brugman S. Induction of human tolerogenic dendritic cells by 3′-sialyllactose via TLR4 is explained by LPS contamination. Glycobiology. (2018) 28:126–30. doi: 10.1093/glycob/cwx106
160. Wassenaar TM, Zimmermann K. Lipopolysaccharides in food, food supplements, and probiotics: should we be worried?. Eur J Microbiol Immunol. (2018) 8:63–9. doi: 10.1556/1886.2018.00017
161. Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, et al. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res. (2004) 56:536–40. doi: 10.1203/01.PDR.0000139411.35619.B4
162. Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, et al. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. (2010) 21:1179–88. doi: 10.1111/j.1399-3038.2010.01062.x
163. Perdijk O, Joost van Neerven RJ, Van den Brink E, Savelkoul HFJ, Brugman S. The oligosaccharides 6'-sialyllactose, 2'-fucosyllactose or galactooligosaccharides do not directly modulate human dendritic cell differentiation or maturation. PLoS ONE. (2018) 13:e0200356. doi: 10.1371/journal.pone.0200356
164. Comstock SS, Wang M, Hester SN, Li M, Donovan SM. Select human milk oligosaccharides directly modulate peripheral blood mononuclear cells isolated from 10-d-old pigs. Br J Nutr. (2014) 111:819–28. doi: 10.1017/S0007114513003267
165. Verheijden KAT, Braber S, Leusink-Muis T, Thijssen S, Boon L, Kraneveld AD, et al. Regulatory T cell depletion abolishes the protective effect of dietary galacto-oligosaccharides on eosinophilic airway inflammation in house dust mite-induced asthma in mice. J Nutr. (2015) 146:831–7. doi: 10.3945/jn.115.224402
166. De Kivit S, Kostadinova AI, Kerperien J, Morgan ME, Ayechu-Muruzabal V, Hofman GA, et al. Dietary, nondigestible oligosaccharides and Bifidobacterium breve M-16V suppress allergic inflammation in intestine via targeting dendritic cell maturation. J Leukoc Biol. (2017) 102:105–15. doi: 10.1189/jib.3A0516-236R
167. Goehring KC, Marriage BJ, Oliver JS, Wilder JA, Barrett EG, Buck RH. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr. (2016) 146:2559–66. doi: 10.3945/jn.116.236919
168. Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. (2003) 278:14112–20. doi: 10.1074/jbc.M207744200
169. Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. (2017) 292:11243–9. doi: 10.1074/jbc.M117.789974
170. Ackerman DL, Doster RS, Weitkamp J.-H, Aronoff DM, Gaddy JA, and Townsend SD. Human milk oligosaccharides exhibit antimicrobial and anti-biofilm properties against group B Streptococcus. ACS Infect Dis. (2017) 3:595–605. doi: 10.1021/acsinfecdis.7b00064
171. Craft KM, Thomas HC, Townsend SD. Interrogation of human milk oligosaccharide fucosylation patterons for antimicrobial and antibiofilm trends in group B Streptococcus. ACS Infect Dis. (2018) 4:1755–65. doi: 10.1021/acsinfecdis.8b00234
172. Craft KM, Thomas HC, Townsend SD. Sialylated variants of lacto-N-tretraose axhibit antimicriobal activity against group B Streptococcus. Org Biomol Chem. (2019) 17:1893–900. doi: 10.1039/c8ob02080a
173. Lewis K. The science of antibiotic discovery. Cell. (2020) 181:29–45. doi: 10.1016/j.cell.2020.02.056
174. Craft KM, Townsend SD. The human milk glycome as a defense against infectious diseases: rationale, challenges and opportunities. ACS Infect Dis. (2018) 4:77–83. doi: 10.1021/acsinfecdis.7b00209
175. Chamber SA, Moore RE, Craft KM, Thmoas HC, Das R, Manning SD, et al. A Solution to antifolate resistance in group b streptococcus : untargeted metabolomics identifies human milk oligosaccharide-induced perturbations that result in potentiation of trimethoprim. MBio. (2020) 11:1–12. doi: 10.1128/mBio.00076-20
176. Craft KM, Gaddy JA, Townsend SD. Human milk oligosaccharides (HMOs) sensitize group B Streptococcus to clindamycin, erythromycin, gentamycin and minocycline on a strain specific basis. ACS Chem Biol. (2018) 13:2020–6. doi: 10.1021/acschembio.8b00661
177. Morozov V, Hansman G, Hanisch F, Schroten H, Kunz C. Human milk oligosaccharides as promising antivirals. Mol Nutr Food Res. (2018) 62:1–14. doi: 10.1002/mnfr.201700679
178. Weichert S, Koromyslova A, Singh BK, Hansman S, Jennewein S, Schroten H, et al. Structural basis for norovirus inhibition by human milk. J Virol. (2016) 90:4843–8. doi: 10.1128/JVI.03223-15
179. Koromyslova A, Tripathi S, Morozov V, Schroten H. Human norovirus inhibition by a human milk oligosaccharide. Virology. (2017) 508:81–9. doi: 10.1016/j.virol.2017.04.032
180. Laucirica DR, Triantis V, Schoemaker R, Estes MK, Ramani S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J Nutr. (2017) 147:1709–14. doi: 10.3945/jn.116.246090
181. Ruhaak LR, Stroble C, Underwood MA, Lebrilla CB. Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem. (2014) 406:5775–84. doi: 10.1007/s00216-014-8025-z
182. Albrecht S, Schols HA, Van Den Heuvel EGHM, Voragen AGJ, Gruppen H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. (2011) 346:2540–50. doi: 10.1016/j.carres.2011.08.009
183. Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr Int J Paediatr. (1996) 85:598–603. doi: 10.1111/j.1651-2227.1996.tb14095.x
Keywords: human milk oligosaccharides, mucosal immunity, allergic diseases, early life nutrition, sialyllactose, fucosyllactose, non-digestible oligosaccharides
Citation: Zuurveld M, van Witzenburg NP, Garssen J, Folkerts G, Stahl B, van't Land B and Willemsen LEM (2020) Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 11:801. doi: 10.3389/fimmu.2020.00801
Received: 29 November 2019; Accepted: 07 April 2020;
Published: 07 May 2020.
Edited by:
Maria Carmen Collado, Institute of Agrochemistry and Food Technology (IATA), SpainReviewed by:
Yaqing Qie, University of Texas MD Anderson Cancer Center, United StatesSteven Townsend, Vanderbilt University, United States
Copyright © 2020 Zuurveld, van Witzenburg, Garssen, Folkerts, Stahl, van't Land and Willemsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marit Zuurveld, m.zuurveld@uu.nl
 Marit Zuurveld
Marit Zuurveld Nikita P. van Witzenburg1
Nikita P. van Witzenburg1 Johan Garssen
Johan Garssen Gert Folkerts
Gert Folkerts Bernd Stahl
Bernd Stahl Belinda van't Land
Belinda van't Land Linette E. M. Willemsen
Linette E. M. Willemsen