- Department of Dermatology, University of Occupational and Environmental Health, Kitakyushu, Japan
Humans are exposed to various external environmental factors. Food intake is one of the most influential factors impacting daily lifestyle. Among nutrients obtained from foods, omega-3 polyunsaturated fatty acids (PUFAs) have various beneficial effects on inflammatory diseases. Furthermore, omega-3 PUFA metabolites, including resolvins, are known to demonstrate strong anti-inflammatory effects during allergic and inflammatory diseases; however, little is known regarding the actual impact of these metabolites on skin diseases. In this review, we focused on metabolites that have strong anti-inflammatory actions in various inflammatory diseases, as well as those that present antitumor actions in malignancies, in addition to the actual effect of omega-3 PUFA metabolites on various cells.
Introduction
The environment is fundamental for humans to live on earth and influences various physiological and pathological functions in the human body (1, 2). Food intake is an essential task for animals to drive their body and allows us to reconstruct body structure from nutrients (3). Among nutrients derived from foods, fatty acids are a component of cells and are known to control various cellular functions (4, 5). Omega-3 polyunsaturated fatty acids (PUFAs) are composed of 18 or more carbon chains, with a double bond three atoms away from the terminal methyl group, and are mainly classified into three representative lipids: α-linoleic acid (ALA), docosahexaenoic acids (DHA), and eicosapentaenoic acid (EPA). ALA is enzymatically converted to EPA and subsequently converted into DHA (6). These conversions primarily occur in the liver and are extremely limited owing to the enzyme concentration in the human body (7–9). Therefore, it is reasonable to derive DHA and EPA directly from foods and/or dietary supplements enriched in fish oils.
Omega-3 PUFAs have been known to demonstrate anti-inflammatory actions in various inflammatory diseases, including psoriasis, inflammatory bowel disease, asthma, and rheumatoid arthritis (10–12). In recent studies, omega-3 PUFA metabolites, such as resolvins (Rvs) and maresins, have revealed potent anti-inflammatory actions. Protectins (PD) and D-series Rvs are converted from DHA by 15-lipoxygenase, whereas E-series Rvs are produced from EPA by the cytochrome P450 pathways or acetylated cyclooxygenase-2. These metabolites have strong anti-inflammatory actions in various inflammatory diseases, such as animal models of asthma (13) and colitis (14), as well as antitumor actions in malignancies; however, little is known regarding their role in skin diseases. This review focused on the therapeutic potential of omega-3 PUFA metabolites for inflammatory skin diseases, as well as antitumor actions against cutaneous tumors.
Anti-Inflammatory Action on Immune Cells and Epithelial Cells
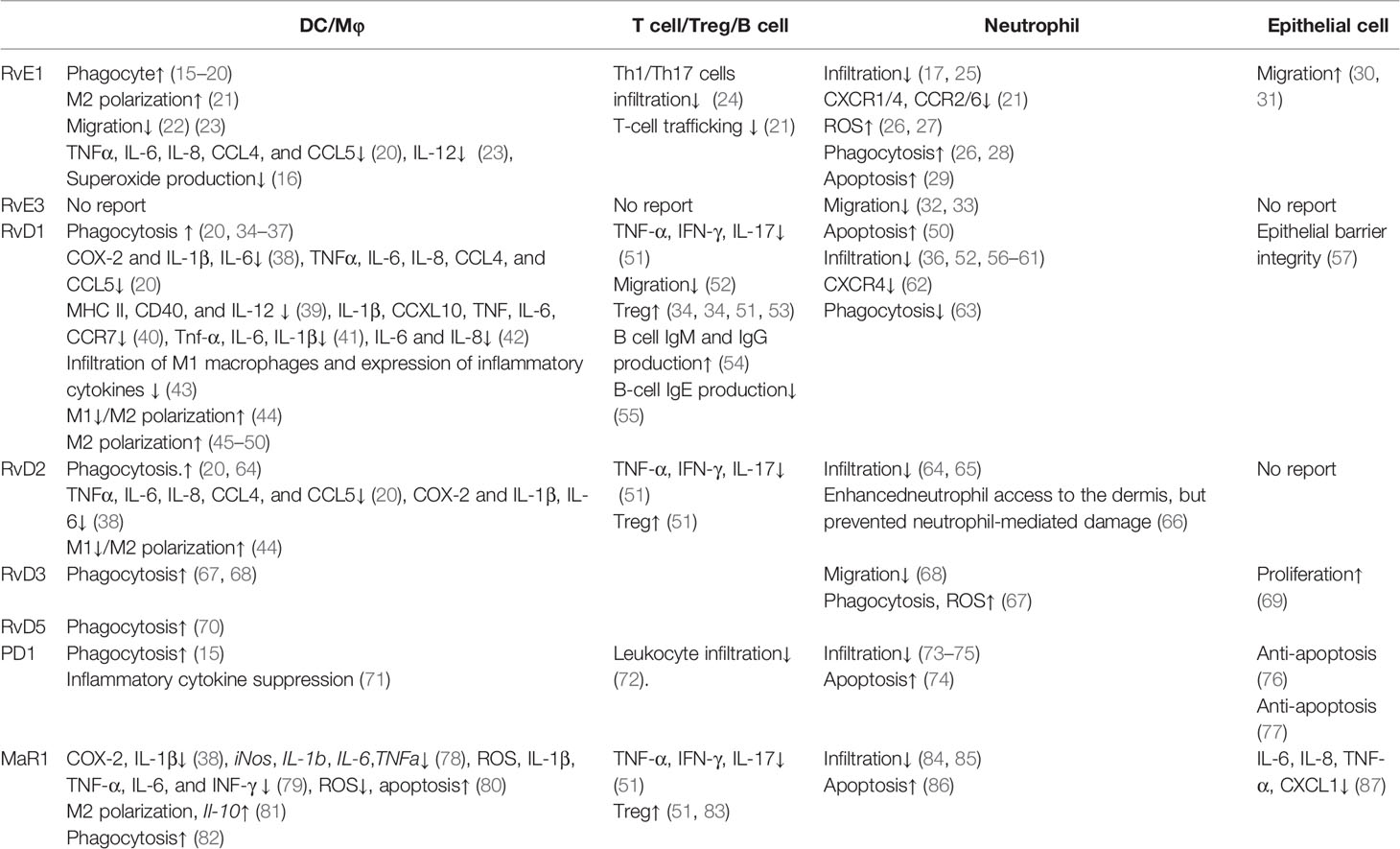
Reportedly, omega-3 PUFA metabolites have demonstrated various actions on immune and epithelial cells. In this section, we first reviewed the influence of omega-3 PUFA metabolites on each cell type, including dendritic cells (DCs)/macrophages, T cells/regulatory T cells (Tregs)/B cells, neutrophils, and epithelial cells, which are known to be present in the skin (Table 1).
DCs/Macrophages
DCs and macrophages play central roles in the acquired immune system to determine the direction of immune responses. As antigen-presenting cells, they take up antigens via phagocytic mechanisms to present antigens to T cells, to determine the direction of the immune response.
Phagocytosis is promoted by resolvin E1 (RvE1) (15–20), resolvin D1 (RvD1) (20, 34–37), resolvin D2 (RvD2) (20, 64), resolvin D3 (RvD3) (67, 68), resolvin D5 (RvD5) (70), protectin D1 (PD1) (15), and maresin 1 (MaR1) (82). For apoptotic cells, the macrophage phagocytosis is enhanced by RvE1 (15), PD1 (15), and RvD1 (34). Phagocyte-dependent bacterial clearance is enhanced by maresin 1 (MaR1) (82), RvD2 (64), RvD3 (67), and RvD5 (70).
Inflammatory cytokine production is negatively regulated by RvE1 (16, 20, 23), RvD1 (20, 38, 40–42), RvD2 (20, 38), RvD3 (71), and MaR1 (38, 78–80). RvE1, RvD1, and RvD2 suppress chemokine (C-C motif) ligand 4 (CCL4), CCL5, interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α induced by human monocyte-derived macrophages co-cultured with tumor cell debris (20), IL-12 by DCs stimulated with pathogen extract (23), and superoxide production by macrophages stimulated with cigarette smoke (16). In macrophages stimulated with lipopolysaccharide (LPS), RvD1, RvD2, and MaR1 suppress IL-1β and IL-6 (38, 78, 79). In contrast, MaR1 promotes the production of the inhibitory cytokine IL-10 (81).
DCs/macrophages play central roles in antigen presentation, as well as migration into antigen-presenting sites for T cells to initiate their active forms. RvD1 suppresses antigen presentation by suppressing major histocompatibility complex (MHC) class II and CD40 expression (39). Additionally, DC migration and infiltration into inflamed tissues are negatively regulated by RvE1 (22, 23) and RvD1 (43). RvE1 suppresses the migration of DCs in the skin and into draining lymph nodes, attenuating the acquired immune response (22), while RvD1 attenuates the increased infiltration of M1 macrophages (43).
Owing to the characteristics of antigen-presenting cells as a control tower in the immune response, the polarization of macrophages determines the direction of inflammatory responses. M1 macrophages are induced by interferon (IFN)-γ, a microbial component. M1 macrophages produce inflammatory cytokines and induce effector cells in polarized Th1 responses. M1 polarization is reportedly suppressed by RvD1 (44) and RvD2 (44). M2 macrophages are generated during enhanced Th2 reactions, promoting parasite killing (88), tissue repair (89), and immunoregulatory functions (90). M2 polarization is positively regulated by RvD1 (44–50), RvD2 (44), and MaR1 (91).
T Cells/Tregs/B Cells
T cells can respond to pathogens by direct contact with the antigen derived from the pathogen and need to migrate to sites in the presence of the antigen. Reportedly, T cell migration is regulated by RvE1 (21, 24), RvD1 (52), and PD1 (72). RvE1 decreases the infiltration of Th1 and Th17 cells (24). RvE1 suppresses T cell infiltration by decreasing the production of RANTES in vascular smooth muscle cells (21).
After the initiation of immune responses by antigen-presenting cells, naïve T cells develop T cell responses as an appropriate direction of inflammatory immune responses. RvD1, RvD2, and PD1 suppress inflammatory cytokine production (51). RvD1, RvD2, and PD1 reduce Th1 and Th17 cytokine production (51).
Tregs are promising cells for the maintenance of immune homeostasis and tolerance (92). T cell‐mediated autoimmune diseases and allergies are closely related to their deficiency or dysfunction. RvD1, RvD2, and PD1 are known to contribute to the inhibition of immune reactions by increasing Tregs (51).
B cells play an important role in the adaptive immune system. The activation of B cells is induced following antigen recognition, differentiating to form plasma cells for antibody secretion. RvD1 influences immunoglobulin production by B cells and increases IgM and IgG production (54), as well as reduces IgE production (55).
Neutrophils
Neutrophils have various functions, including phagocytosis and antimicrobial peptide production (93). Reportedly, neutrophil phagocytosis is positively regulated by RvE1 (26, 28), RvD1 (63), and RvD3 (67). In phagocytes, NADPH oxidase is essential for neutrophil microbicidal activity (94). Furthermore, reactive oxygen species (ROS) generation in neutrophils is promoted by RvE1 (26, 27) and RvD3 (67). In addition, neutrophil migration is suppressed by RvE1 (17, 25), RvE3 (32, 33), RvD1 (36, 52, 56–61), RvD2 (64, 65), RvD3 (68), PD1 (73–75), and MaR1 (84, 85). RvE1 decreases chemokine receptor expression, including C-C chemokine receptor type 2 (CCR2), CCR6, chemokine (C-X-C motif) receptor 1 (CXCR1), and CXCR4 (21), as well as chemotaxis (26). RvD1 decreases CXCR4 expression (62). Moreover, RvD2 allows neutrophils to enhance their access to the dermis; however, RvD2 demonstrates a protective role against neutrophil-mediated damage (66).
Apoptotic neutrophils progress toward secondary necrosis mediated by the release of caspase 3-processed danger signals (93). Neutrophil apoptosis is reportedly enhanced by RvE1 (29), RvD1 (50), PD1 (74), and MaR1 (86). These metabolites enhance apoptotic cell phagocytosis of macrophages, suppressing secondary necrosis-mediated inflammation.
Epithelial Cells
Epithelial cells act as the first line of defense against the external environment. As the outermost organ layer, epithelial cells induce inflammatory cytokine and chemokine production to amplify the response to external stimuli. Inflammatory cytokine production is negatively regulated by MaR1 (87). Furthermore, MaR1 suppresses the production of CXCL1, IL-6, IL-8, and TNF-α by bronchial epithelial cells (87).
As the outer organ layer, epithelial cells exhibit migration and proliferation to shield the defect of the first line of defense against external stimuli. RvE1 enhances epithelial cell migration (30, 31), RvD3 promotes lung epithelial cell proliferation (69), while RvD1 and PD1 exhibit protective effects toward epithelial cells. Furthermore, RvD1 promotes epithelial barrier integrity (57). PD1 is shown to afford protection against repetitive oxidative stress-induced apoptosis (76, 77).
Different Functions of PUFA Metabolites on Immune Cells and Epithelial Cells
There are some different effects of PUFA metabolites in immune cells and epithelial cells. RvE1 suppresses immune cell migration (22, 23) while it promotes epithelial cell migration (30, 31). In addition, PD1 promotes apoptosis of neutrophils (74); however, it enhances anti-apoptosis in epithelial cells (76, 77). Although the detailed mechanism remains unclear, a possible other point of action might exist in each different type of cell, because they are located in different body sites and surface layers, which are given the appropriate role in the body.
The Anti-Inflammatory Action of PUFA Metabolites on Skin Diseases
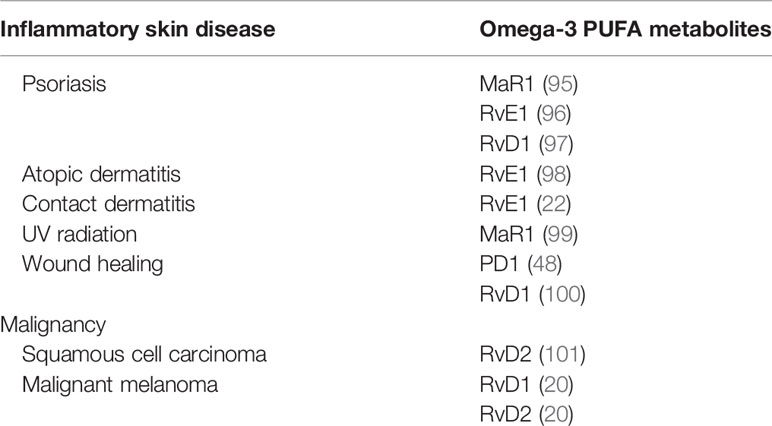
Several reports have highlighted the anti-inflammatory actions of omega-3 PUFA metabolites on inflammatory skin diseases, including psoriasis, atopic dermatitis, contact hypersensitivity, and ultraviolet (UV) radiation. Furthermore, the antitumor effects of PUFA metabolites on squamous cell carcinoma and melanoma have been reported (Table 2).
Psoriasis and PUFA Metabolites
Psoriasis is a representative inflammatory skin disease characterized by scaly erythematous plaques with epidermal hyperplasia (102). Although the underlying mechanism of psoriasis remains unclear, recent studies have revealed some predominant pathways underlying pathological conditions, as well as the contribution of the TNF/IL-23/IL-17 axis (103). Current biologics targeting IL-17, IL-23, and TNF-α play critical roles in the pathogenesis of psoriasis (104).
Reportedly, MaR1 impairs imiquimod-induced psoriasis-like skin inflammation and IL-23 subcutaneous injection-induced skin inflammation (95). MaR1 decreases lymphocyte and neutrophil infiltration, dermal edema, and epithelial hyperplasia. MaR1 inhibits the production of IL-17A by CD4+ and γδTCRmid+ cells. Consequently, MaR1 attenuates IL-23 receptor expression on CD4+ and γδTCRmid+ cells by inhibiting retinoic acid-related orphan receptor gamma t (RORγt) in clathrin-dependent IL-23 receptor internalization.
RvE1 impairs imiquimod-induced psoriatic dermatitis (96). Furthermore, IL-17-producing cells and neutrophils are reduced in the skin following RvE1 treatment. Reportedly, IL-23 and IL-17 are downregulated by RvE1. IL-23 production by DCs, as well as the migration of DCs and IL-17 producing cells, is suppressed by RvE1.
RvD1 reduces acanthosis and hyperkeratosis induced by imiquimod (97). Inflammatory cell infiltration into the dermis is reduced following treatment with RvD1. Consistently, IL-17, IL-23, and TNF-α are decreased by RvD1.
Atopic Dermatitis (AD) and PUFA Metabolites
AD is a representative Th2-mediated chronic inflammatory skin disease characterized by inflamed and irritative itchy skin inflammation (105). Several factors are involved in the various environmental factors that drive Th2 dominant skin inflammation, including skin barrier disruption (106, 107), pathogens (108), and prostanoids (109). In NC/Nga mice, RvE1 demonstrates anti-inflammatory actions in repeated hapten application-induced AD-like skin inflammation (98). RvE1 suppresses IL-4 and IFN-γ production by T cells, as well as serum IgE levels, and reduces the infiltration of eosinophils, mast cells, and T cells in skin lesions.
Contact Dermatitis and PUFA Metabolites
Contact dermatitis is a common cutaneous allergic reaction that depends on the acquired immune response (110, 111). RvE1 impairs the inflammatory response in contact hypersensitivity during the sensitization and elicitation phases (22). During the sensitization phase, RvE1-treated mice exhibit significantly reduced DC migration into draining lymph nodes, subsequently reducing the number of central and effector memory T cells. Consistently, antigen-specific T cell proliferation and IFN-γ production are reduced by RvE1. In the elicitation phase, RvE1 impairs DC cluster formation, which is essential for the development of elicitation phase inflammation, subsequently suppressing the number of IFN-γ–producing CD8+ T cells in the skin.
UV Radiation and MaR1
UV radiation is a representative environment-related skin inflammation that causes acute inflammation characterized by skin inflammation after sun exposure (112). MaR1 suppresses skin swelling as well as macrophage infiltration induced by UVB irradiation (99). Furthermore, MaR1 inhibits UVB irradiation-induced keratinocyte apoptosis, production of inflammatory cytokines, IL-1β and TNFα, and oxidative stress.
Wound Healing and PUFA Metabolites
Damage induced by external factors destroys the skin surface, resulting in skin defects that appear as a wound. As the skin acts as a barrier against the external environment, wounds allow outside pathogens and irritants to infiltrate the body and cause skin inflammation (113). PD1 and RvD1 promote skin wound healing (48, 100). In diabetic wounds, RvD1 enhances macrophage phagocytosis, promoting wound closure owing to the reduced number of apoptotic cells (48). Furthermore, PD1 promotes wound closure (100). Skin wounds promote the synthesis of PD1 in the skin; however, in diabetes, the skin suppresses PD1 production. Macrophages are one of the main sources of PD1 in skin wounds and are known to contribute to the development of inflammation and oxidative stress reactions during acute inflammation in diabetic wounds.
Antitumor Effects of PUFA Metabolites
Squamous cell carcinoma is a keratinocyte-derived malignancy, and the advanced clinical stage of this malignancy remains refractory to current systemic treatments (114). RvD2 suppresses squamous cell carcinoma development (101) and decreases inflammatory chemokines and cytokines, including CXCL10, IL-6, monocyte chemoattractant protein-1 (MCP-1), and TNF-α, by cancer cells. In squamous cell carcinoma, RvD2 decreases the infiltration of neutrophils and suppresses myeloperoxidase (MPO) activity. Additionally, RvD2 enhances the M2 macrophage population and their efferocytosis.
Malignant melanoma is a malignancy derived from melanocytes with severe life-threatening clinical behavior owing to a lack of radical treatment (115). RvD1 and RvD2 suppress melanoma development (20). Furthermore, RvD1 or RvD2 inhibit lung metastasis of melanoma cells and regulate the production of chemokines and cytokines, including CCL4, CCL5, IL-6, IL-8, and TNF-α, by human macrophages, providing antitumor immunity.
Clinical Trial of Omega3 PUFA for Cutaneous Skin Diseases
Finally, we reviewed the clinical trials of omega 3 PUFAs for cutaneous skin diseases. There are several trials for atopic dermatitis and psoriasis.
The intake of omega-3 supplement improves the Scoring in Atopic Dermatitis (SCORAD) score (116). A double-blind, randomized, placebo-controlled trial showed AD patients who received daily ω3 fatty acid supplementation (fish oil, 10%; 200 mL) show high serum EPA concentration and a decreased disease severity of AD (117).
Psoriasis patients with obesity received energy-restricted foods enriched of ω3 PUFAs (average 2.6 g/d), and these patients showed impaired Psoriasis Area Score Index (PASI) score and Dermatological Life Quality Index (118). A double-blind, randomized study in multicenter trials showed the group of intaking daily an ω3 fatty acid (Omegavenous; 200 ml/day with 4.2 gm of both EPA and DHA) decreased total PASI score without serious side effects (119).
Although there have been no clinical trials of cutaneous malignancies, head and neck squamous cell carcinoma has been reported. Supplementation of daily 2 g EPA intakes impairs the production of serum pro-inflammatory cytokines, reduction of body weight and lean body mass, and increases quality of life in patients with squamous cell carcinoma in head and neck (120).
Conclusion
There are a limited number of research and clinical trials for investigations of the effects of ω3 PUFA in skin diseases. According to previous studies assessing immune cells, various beneficial effects are expected in skin diseases. Furthermore, there are few reports available on keratinocytes, which are a major component of cells present in the epidermis. The detailed action of keratinocytes in the skin needs to be clarified in future research. Because the incidences of inflammatory skin diseases and malignancies are currently increasing, future basic research and clinical trials for ω3 PUFAs in dermatology fields are expected give us a beneficial information for the daily clinical treatment for skin diseases.
Author Contributions
YS and MN wrote this manuscript, and NS-S conducted a critical review of this paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pamenter ME, Hall JE, Tanabe Y, Simonson TS. Cross-Species Insights Into Genomic Adaptations to Hypoxia. Front Genet (2020) 11:743. doi: 10.3389/fgene.2020.00743
2. Stellingwerff T, Peeling P, Garvican-Lewis LA, Hall R, Koivisto AE, Heikura IA, et al. Nutrition and Altitude: Strategies to Enhance Adaptation, Improve Performance and Maintain Health: A Narrative Review. Sports Med (2019) 49(Suppl 2):169–84. doi: 10.1007/s40279-019-01159-w
3. Pereira LJ, van der Bilt A. The influence of oral processing, food perception and social aspects on food consumption: a review. J Oral Rehabil (2016) 43(8):630–48. doi: 10.1111/joor.12395
4. Causeret C, Bentejac M, Bugaut M. Proteins and enzymes of the peroxisomal membrane in mammals. Biol Cell (1993) 77(1):89–104. doi: 10.1016/S0248-4900(05)80178-9
5. Chintalapati S, Kiran MD, Shivaji S. Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol (Noisy-le-grand) (2004) 50(5):631–42.
6. Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care (2004) 7(2):137–44. doi: 10.1097/00075197-200403000-00006
7. Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduct Nutr Dev (2005) 45(5):581–97. doi: 10.1051/rnd:2005047
8. Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br J Nutr (2002) 88(4):355–63. doi: 10.1079/BJN2002662
9. Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr (2002) 88(4):411–20. doi: 10.1079/BJN2002689
10. Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet (1978) 2(8081):117–9. doi: 10.1016/S0140-6736(78)91505-2
11. Horrobin DF. Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of eicosapentaenoic acid (EPA), a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med Hypotheses (1987) 22(4):421–8. doi: 10.1016/0306-9877(87)90037-5
12. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation (2017) 135(15):e867–84. doi: 10.1161/CIR.0000000000000482
13. Siddiquee A, Patel M, Rajalingam S, Narke D, Kurade M, Ponnoth DS. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol Immunotoxicol (2019) 41(2):250–7. doi: 10.1080/08923973.2019.1584903
14. Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, et al. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm bowel Dis (2010) 16(1):87–95. doi: 10.1002/ibd.21029
15. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature (2007) 447(7146):869–74. doi: 10.1038/nature05877
16. Takamiya R, Fukunaga K, Arita M, Miyata J, Seki H, Minematsu N, et al. Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress. FEBS Open Bio (2012) 2:328–33. doi: 10.1016/j.fob.2012.10.001
17. Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest (2011) 121(2):569–81. doi: 10.1172/JCI42545
18. Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem (2010) 285(5):3451–61. doi: 10.1074/jbc.M109.044131
19. Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol (2008) 180(5):3512–9. doi: 10.4049/jimmunol.180.5.3512
20. Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med (2018) 215(1):115–40. doi: 10.1084/jem.20170681
21. Liu G, Gong Y, Zhang R, Piao L, Li X, Liu Q, et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. FASEB J (2018) 32(10):5413–25. doi: 10.1096/fj.201800173R
22. Sawada Y, Honda T, Hanakawa S, Nakamizo S, Murata T, Ueharaguchi-Tanada Y, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med (2015) 212(11):1921–30. doi: 10.1084/jem.20150381
23. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med (2005) 201(5):713–22. doi: 10.1084/jem.20042031
24. Wang H, Zhao Q, Luo D, Yin Y, Li T, Zhao M. Resolvin E1 Inhibits Corneal Allograft Rejection in High-Risk Corneal Transplantation. Invest Ophthalmol Vis Sci (2018) 59(10):3911–9. doi: 10.1167/iovs.18-24562
25. Zhang J, Wang M, Ye J, Liu J, Xu Y, Wang Z, et al. The Anti-inflammatory Mediator Resolvin E1 Protects Mice Against Lipopolysaccharide-Induced Heart Injury. Front Pharmacol (2020) 11:203. doi: 10.3389/fphar.2020.00203
26. Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One (2007) 2(12):e1316. doi: 10.1371/journal.pone.0001316
27. Unno Y, Sato Y, Fukuda H, Ishimura K, Ikeda H, Watanabe M, et al. Resolvin E1, but not resolvins E2 and E3, promotes fMLF-induced ROS generation in human neutrophils. FEBS Lett (2018) 592(16):2706–15. doi: 10.1002/1873-3468.13215
28. Herrera BS, Hasturk H, Kantarci A, Freire MO, Nguyen O, Kansal S, et al. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect Immun (2015) 83(2):792–801. doi: 10.1128/IAI.02444-14
29. El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A (2012) 109(37):14983–8. doi: 10.1073/pnas.1206641109
30. Zhang F, Yang H, Pan Z, Wang Z, Wolosin JM, Gjorstrup P, et al. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Invest Ophthalmol Vis Sci (2010) 51(11):5601–9. doi: 10.1167/iovs.09-4468
31. Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, García AJ, et al. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci U S A (2020) 117(17):9477–82. doi: 10.1073/pnas.1921335117
32. Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K, et al. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J Biochem (2013) 153(4):355–60. doi: 10.1093/jb/mvs151
33. Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem (2012) 287(13):10525–34. doi: 10.1074/jbc.M112.340612
34. Luo B, Han F, Xu K, Wang J, Liu Z, Shen Z, et al. Resolvin D1 Programs Inflammation Resolution by Increasing TGF-β Expression Induced by Dying Cell Clearance in Experimental Autoimmune Neuritis. J Neurosci (2016) 36(37):9590–603. doi: 10.1523/JNEUROSCI.0020-16.2016
35. Markworth JF, Brown LA, Lim E, Floyd C, Larouche J, Castor-Macias JA, et al. Resolvin D1 supports skeletal myofiber regeneration via actions on myeloid and muscle stem cells. JCI Insight (2020) 5(18):e137713. doi: 10.1172/jci.insight.137713
36. Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, et al. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol (2016) 9(5):1278–87. doi: 10.1038/mi.2015.129
37. Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep (2013) 3:1940. doi: 10.1038/srep01940
38. Caron JP, Gandy JC, Brown JL, Sordillo LM. Docosahexaenoic acid-derived oxidized lipid metabolites modulate the inflammatory response of lipolysaccharide-stimulated macrophages. Prostagland Other Lipid Mediat (2018) 136:76–83. doi: 10.1016/j.prostaglandins.2018.05.006
39. Hua J, Jin Y, Chen Y, Inomata T, Lee H, Chauhan SK, et al. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Invest Ophthalmol Vis Sci (2014) 55(9):5944–51. doi: 10.1167/iovs.14-14356
40. Sun AR, Wu X, Liu B, Chen Y, Armitage CW, Kollipara A, et al. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci Rep (2019) 9(1):426. doi: 10.1038/s41598-018-36909-9
41. Kain V, Halade GV. Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J Cell Physiol (2019) 234(4):3910–20. doi: 10.1002/jcp.27165
42. Cox R Jr., Phillips O, Fukumoto J, Fukumoto I, Tamarapu Parthasarathy P, Mandry M, et al. Resolvins Decrease Oxidative Stress Mediated Macrophage and Epithelial Cell Interaction through Decreased Cytokine Secretion. PLoS One (2015) 10(8):e0136755. doi: 10.1371/journal.pone.0136755
43. Wang M, Liu M, Zhang J, Liu J, Ye J, Xu Y, et al. Resolvin D1 protects against sepsis-induced cardiac injury in mice. Biofactors (2020) 46(5):766–76. doi: 10.1002/biof.1668
44. Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med (2020) 24(14):8045–56. doi: 10.1111/jcmm.15436
45. Wang CS, Maruyama CL, Easley JT, Trump BG, Baker OJ. AT-RvD1 Promotes Resolution of Inflammation in NOD/ShiLtJ mice. Sci Rep (2017) 7:45525. doi: 10.1038/srep45525
46. Kang JW, Lee SM. Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing M2 macrophage polarization and efferocytosis. Biochim Biophys Acta (2016) 1861(9 Pt A):1025–35. doi: 10.1016/j.bbalip.2016.06.002
47. Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One (2013) 8(3):e58258. doi: 10.1371/journal.pone.0058258
48. Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes (2013) 62(2):618–27. doi: 10.2337/db12-0684
49. Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, et al. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol (2012) 189(4):1983–91. doi: 10.4049/jimmunol.1101665
50. Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A (2010) 107(4):1660–5. doi: 10.1073/pnas.0907342107
51. Chiurchiù V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med (2016) 8(353):353ra111. doi: 10.1126/scitranslmed.aaf7483
52. Shi J, Zhang X, Jiang L, Zhang L, Dong Y, Midgley AC, et al. Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration. Acta Biomater (2019) 97:360–73. doi: 10.1016/j.actbio.2019.07.037
53. Luan H, Wang C, Sun J, Zhao L, Li L, Zhou B, et al. Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway. Front Physiol (2020) 11:285. doi: 10.3389/fphys.2020.00285
54. Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol (2012) 189(2):1036–42. doi: 10.4049/jimmunol.1103483
55. Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, Phipps RP. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol (2016) 46(1):81–91. doi: 10.1002/eji.201545673
56. Murakami T, Suzuki K, Tamura H, Nagaoka I. Suppressive action of resolvin D1 on the production and release of septic mediators in D-galactosamine-sensitized endotoxin shock mice. Exp Ther Med (2011) 2(1):57–61. doi: 10.3892/etm.2010.170
57. Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol (2012) 180(5):2018–27. doi: 10.1016/j.ajpath.2012.01.028
58. Chen J, Shetty S, Zhang P, Gao R, Hu Y, Wang S, et al. Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Toxicol Appl Pharmacol (2014) 277(2):118–23. doi: 10.1016/j.taap.2014.03.017
59. Kim KH, Park TS, Kim YS, Lee JS, Oh YM, Lee SD, et al. Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration. Int J Chron Obstruct Pulmon Dis (2016) 11:1119–28. doi: 10.2147/COPD.S100198
60. Xia H, Wang J, Sun S, Wang F, Yang Y, Chen L, et al. Resolvin D1 Alleviates Ventilator-Induced Lung Injury in Mice by Activating PPARγ/NF-κB Signaling Pathway. BioMed Res Int (2019) 2019:6254587. doi: 10.1155/2019/6254587
61. Zhang HW, Wang Q, Mei HX, Zheng SX, Ali AM, Wu QX, et al. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int Immunopharmacol (2019) 76:105877. doi: 10.1016/j.intimp.2019.105877
62. Yaxin W, Shanglong Y, Huaqing S, Hong L, Shiying Y, Xiangdong C, et al. Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J Surg Res (2014) 188(1):213–21. doi: 10.1016/j.jss.2013.11.1107
63. Sekheri M, El Kebir D, Edner N, Filep JG. 15-Epi-LXA(4) and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A (2020) 117(14):7971–80. doi: 10.1073/pnas.1920193117
64. Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature (2009) 461(7268):1287–91. doi: 10.1038/nature08541
65. Mizraji G, Heyman O, Van Dyke TE, Wilensky A. Resolvin D2 Restrains Th1 Immunity and Prevents Alveolar Bone Loss in Murine Periodontitis. Front Immunol (2018) 9:785. doi: 10.3389/fimmu.2018.00785
66. Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen (2013) 21(1):35–43. doi: 10.1111/j.1524-475X.2012.00853.x
67. Norris PC, Arnardottir H, Sanger JM, Fichtner D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostagland Leukot Essent Fatty Acids (2018) 138:81–9. doi: 10.1016/j.plefa.2016.01.001
68. Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol (2013) 20(2):188–201. doi: 10.1016/j.chembiol.2012.11.010
69. Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW, et al. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. Am J Pathol (2016) 186(7):1801–13. doi: 10.1016/j.ajpath.2016.03.011
70. Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun (2018) 9(1):59. doi: 10.1038/s41467-017-02538-5
71. Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J (2008) 22(10):3595–606. doi: 10.1096/fj.08-112201
72. Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem (2005) 280(52):43079–86. doi: 10.1074/jbc.M509796200
73. Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut (2015) 64(8):1236–47. doi: 10.1136/gutjnl-2014-306998
74. Li X, Li C, Liang W, Bi Y, Chen M, Dong S. Protectin D1 promotes resolution of inflammation in a murine model of lipopolysaccharide-induced acute lung injury via enhancing neutrophil apoptosis. Chin Med J (Engl) (2014) 127(5):810–4.
75. Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol (2006) 176(3):1848–59. doi: 10.4049/jimmunol.176.3.1848
76. Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res (2010) 90(6):718–25. doi: 10.1016/j.exer.2010.03.002
77. Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A (2007) 104(32):13152–7. doi: 10.1073/pnas.0705949104
78. Huang R, Vi L, Zong X, Baht GS. Maresin 1 resolves aged-associated macrophage inflammation to improve bone regeneration. FASEB J (2020) 34(10):13521–32. doi: 10.1096/fj.202001145R
79. Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol (2013) 191(8):4288–98. doi: 10.4049/jimmunol.1202743
80. Zhang P, Yin Y, Wang T, Li W, Li C, Zeng X, et al. Maresin 1 mitigates concanavalin A-induced acute liver injury in mice by inhibiting ROS-mediated activation of NF-κB signaling. Free Radic Biol Med (2020) 147:23–36. doi: 10.1016/j.freeradbiomed.2019.11.033
81. Martínez-Fernández L, González-Muniesa P, Laiglesia LM, Sáinz N, Prieto-Hontoria PL, Escoté X, et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J (2017) 31(5):2135–45. doi: 10.1096/fj.201600859R
82. Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest (2019) 129(12):5294–311. doi: 10.1172/JCI129448
83. Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J, et al. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann Rheum Dis (2018) 77(11):1644–52. doi: 10.1136/annrheumdis-2018-213511
84. Gong J, Wu ZY, Qi H, Chen L, Li HB, Li B, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol (2014) 171(14):3539–50. doi: 10.1111/bph.12714
85. Tang Q, Che C, Lin J, He H, Zhao W, Lv L, et al. Maresin1 regulates neutrophil recruitment and IL-10 expression in Aspergillus fumigatus keratitis. Int Immunopharmacol (2019) 69:103–8. doi: 10.1016/j.intimp.2019.01.032
86. Gong J, Liu H, Wu J, Qi H, Wu ZY, Shu HQ, et al. MARESIN 1 PREVENTS LIPOPOLYSACCHARIDE-INDUCED NEUTROPHIL SURVIVAL AND ACCELERATES RESOLUTION OF ACUTE LUNG INJURY. Shock (2015) 44(4):371–80. doi: 10.1097/SHK.0000000000000434
87. Nordgren TM, Heires AJ, Wyatt TA, Poole JA, LeVan TD, Cerutis DR, et al. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res (2013) 14(1):51. doi: 10.1186/1465-9921-14-51
88. Noël W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol (2004) 20(3):126–33. doi: 10.1016/j.pt.2004.01.004
89. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol (2004) 4(8):583–94. doi: 10.1038/nri1412
90. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol (2004) 25(12):677–86. doi: 10.1016/j.it.2004.09.015
91. Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J (2015) 29(6):2504–13. doi: 10.1096/fj.14-265363
92. Wing JB, Sakaguchi S. Foxp3+ T(reg) cells in humoral immunity. Int Immunol (2014) 26(2):61–9. doi: 10.1093/intimm/dxt060
93. Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol (2014) 15(7):602–11. doi: 10.1038/ni.2921
94. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) (2005) 53(3):199–206.
95. Saito-Sasaki N, Sawada Y, Mashima E, Yamaguchi T, Ohmori S, Yoshioka H, et al. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci Rep (2018) 8(1):5522. doi: 10.1038/s41598-018-23623-9
96. Sawada Y, Honda T, Nakamizo S, Otsuka A, Ogawa N, Kobayashi Y, et al. Resolvin E1 attenuates murine psoriatic dermatitis. Sci Rep (2018) 8(1):11873. doi: 10.1038/s41598-018-30373-1
97. Xu J, Duan X, Hu F, Poorun D, Liu X, Wang X, et al. Resolvin D1 attenuates imiquimod-induced mice psoriasiform dermatitis through MAPKs and NF-κB pathways. J Dermatol Sci (2018) 89(2):127–35. doi: 10.1016/j.jdermsci.2017.10.016
98. Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol (2012) 14(4):384–91. doi: 10.1016/j.intimp.2012.08.005
99. Cezar TLC, Martinez RM, Rocha CD, Melo CPB, Vale DL, Borghi SM, et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci Rep (2019) 9(1):3062. doi: 10.1038/s41598-019-39584-6
100. Hong S, Tian H, Lu Y, Laborde JM, Muhale FA, Wang Q, et al. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am J Physiol Cell Physiol (2014) 307(11):C1058–67. doi: 10.1152/ajpcell.00270.2014
101. Ye Y, Scheff NN, Bernabé D, Salvo E, Ono K, Liu C, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology (2018) 139:182–93. doi: 10.1016/j.neuropharm.2018.07.016
102. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol (2018) 201(6):1605–13. doi: 10.4049/jimmunol.1800013
103. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol (2017) 140(3):645–53. doi: 10.1016/j.jaci.2017.07.004
104. Tokuyama M, Mabuchi T. New Treatment Addressing the Pathogenesis of Psoriasis. Int J Mol Sci (2020) 21(20):7488. doi: 10.3390/ijms21207488
105. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers (2018) 4(1):1. doi: 10.1038/s41572-018-0001-z
106. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet (2006) 38(4):441–6. doi: 10.1038/ng1767
107. Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol (2012) 129(6):1538–46.e6. doi: 10.1016/j.jaci.2012.01.068
108. Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol (2016) 136(11):2192–200. doi: 10.1016/j.jid.2016.05.127
109. Sawada Y, Honda T, Nakamizo S, Nakajima S, Nonomura Y, Otsuka A, et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J Allergy Clin Immunol (2019) 144(5):1265–73.e9. doi: 10.1016/j.jaci.2019.06.036
110. Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol (2013) 133(2):303–15. doi: 10.1038/jid.2012.284
111. Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol (2014) 15(11):1064–9. doi: 10.1038/ni.2992
112. Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol (2019) 19(11):688–701. doi: 10.1038/s41577-019-0185-9
113. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol (2015) 173(2):370–8. doi: 10.1111/bjd.13954
114. Burton KA, Ashack KA, Khachemoune A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am J Clin Dermatol (2016) 17(5):491–508. doi: 10.1007/s40257-016-0207-3
115. Jenkins RW, Fisher DE. Treatment of Advanced Melanoma in 2020 and Beyond. J Invest Dermatol (2020) 141(1):23–31. doi: 10.1016/j.jid.2020.03.943
116. Eriksen BB, Kåre DL. Open trial of supplements of omega 3 and 6 fatty acids, vitamins and minerals in atopic dermatitis. J Dermatol Treat (2006) 17(2):82–5. doi: 10.1080/09546630600621946
117. Mayser P, Mayer K, Mahloudjian M, Benzing S, Krämer HJ, Schill WB, et al. A double-blind, randomized, placebo-controlled trial of n-3 versus n-6 fatty acid-based lipid infusion in atopic dermatitis. JPEN J Parenteral Enteral Nutr (2002) 26(3):151–8. doi: 10.1177/0148607102026003151
118. Guida B, Napoleone A, Trio R, Nastasi A, Balato N, Laccetti R, et al. Energy-restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: a randomized control clinical trial. Clin Nutr (Edinburgh Scotland) (2014) 33(3):399–405. doi: 10.1016/j.clnu.2013.09.010
119. Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J Off Publ Fed Am Societies Exp Biol (2016) 30(8):2792–801. doi: 10.1096/fj.201500155R
120. Solís-Martínez O, Plasa-Carvalho V, Phillips-Sixtos G, Trujillo-Cabrera Y, Hernández-Cuellar A, Queipo-García GE, et al. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr Cancer (2018) 70(4):663–70. doi: 10.1080/01635581.2018.1460678
Keywords: ω3 polyunsaturated fatty acids, resolvin, protectin, maresin, metabolites
Citation: Sawada Y, Saito-Sasaki N and Nakamura M (2021) Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 11:623052. doi: 10.3389/fimmu.2020.623052
Received: 29 October 2020; Accepted: 22 December 2020;
Published: 05 February 2021.
Edited by:
Laurence Macia, The University of Sydney, AustraliaReviewed by:
Hu Wang, Johns Hopkins University, United StatesIvan Milos Stankovic, University of Belgrade, Serbia
Copyright © 2021 Sawada, Saito-Sasaki and Nakamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Sawada, long-ago@med.uoeh-u.ac.jp
 Yu Sawada
Yu Sawada Natsuko Saito-Sasaki
Natsuko Saito-Sasaki Motonobu Nakamura
Motonobu Nakamura