- 1Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, China-Japan Friendship Hospital, Beijing, China
- 3Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Objective: To evaluate neurological function and its influencing factors in patients with anti-γ -aminobutyric acid B receptor (GABABR) encephalitis.
Methods: This was a clinical cohort study of patients diagnosed with anti-GABABR encephalitis; long-term follow-up was performed by telephone. Clinical factors associated with prognosis were analyzed, including clinical manifestations, laboratory examinations, imaging features, tumor comorbidities and therapeutic responses.
Results: Twenty-two patients with anti-GABABR encephalitis were evaluated (median age: 55 years). Lung cancer was detected in eight patients. All were with serum tumor markers (mainly NSE), and three of them had additional onconeuronal antibodies. The patients with tumors were older than the patients without tumors and more likely to develop status epilepticus (62.5% vs. 14.3%; p = 0.052), central hypoventilation (50% vs. 7.1%; p = 0.039), and hyponatremia (87.5% vs. 14.3%; p = 0.001). The patients with tumors had higher mortality (87.5% vs. 0%; p < 0.05). Although 92.9% of the patients without tumors became functionally independent (mRS ≤2), sequelae of symptomatic seizures, neuropsychiatric symptoms, and cognitive impairment were still observed in 14.3%, 21.4%, and 21.4% of patients, respectively.
Conclusions: (1) Elderly patients with anti-GABABR antibodies, especially those with severe symptoms, serum tumor markers, and additional onconeuronal antibodies, should be screened for lung cancer. (2) Anti-GABABR encephalitis with tumors has a poor prognosis. (3) Most patients without tumors achieve self-care, but some still experience remaining neurological deficits.
Introduction
With the finding of autoantibodies, cases of encephalitis that were previously presumed to be viral or idiopathic in etiology have been determined to be caused by autoimmune mechanisms. Moreover, these autoantibodies can also be used to distinguish autoimmune encephalitis (AE) in various subgroups with distinct clinical phenotypes and different prognoses (1, 2). Anti-γ-aminobutyric acid B receptor (GABABR) encephalitis is one form of AE that is caused by autoantibodies targeting GABABRs and was first reported in 2010 by Lancaster et al. (3). It is a relatively rare disease, accounting for approximately 5% of all cases of autoimmune synaptic encephalitis (4). Most of the previously described patients had typical features of limbic encephalitis (LE) such as seizures, memory deficits or behavioral problems, which were mainly related to the inhibition of the high-density expression of GABABR in the hippocampus (5). A subset of patients also present with cerebellar ataxia (6) and opsoclonus-myoclonus (7). Additionally, GABABR autoimmunity likely progresses to severe symptoms of status epilepticus and consciousness disturbance, and severe complications such as respiratory failure; therefore, these patients require extensive critical care management (3). Previous studies showed that the fatality rates of this disorder range between 22% and 45% (3, 8–12).
It has been reported that lung cancer or tumors of neuroendocrine origin may be observed in patients with anti-GABABR encephalitis. However, there are limited data about the difference in functional outcomes between patients with paraneoplastic and idiopathic anti-GABABR encephalitis or the correlation between the clinical manifestations and neurological outcomes. To further identify the potential factors that affect these outcomes, we analyzed clinical features, laboratory evaluation results, imaging features, electroencephalogram (EEG) features, and treatment responses. These data may help promote recognition of the disorder, which is crucial for improving oncologic and neurologic outcomes.
Methods
Study Participants and Setting
Anti-GABABR encephalitis patients who were consecutively admitted to the Department of Neurology at Xuanwu Hospital Capital Medical University between December 2011 and January 2020 were enrolled in this cohort study. The inclusion criteria were as follows: (1) anti-GABABR encephalitis was diagnosed based on the following criteria (13): 1) positive results of GABABR antibodies in serum or cerebral spinal fluid (CSF); the presence of anti-GABABR antibodies was measured using fixed cell-based indirect immunofluorescence test (IIFT) kits (Euroimmun AG, Lübeck, Germany). Other antibodies were also examined by IIFT, including antibodies against neuronal cell surface antigens N-methyl-D-aspartate (NMDA) receptor, a-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid (AMPA) receptors 1 and 2, contactin associated protein 2 (CASPR2), leucine-rich gliomainactivated protein 1 (LGI1), as well as antibodies against intracellular neuronal antigens Hu, Ri, Yo, glutamic acid decarboxylase 65 (GAD65), amphiphysin, collapsin response mediator protein (CV2), sry-related box genes (SOX1) and paraneoplastic antigen Ma2 (PNMA2). 2) clinical data indicative of LE, which was defined as encephalitis with predominant clinical involvement of the limbic system (subacute onset of working memory deficits, seizures, or psychiatric symptoms) or magnetic resonance imaging (MRI) fluid-attenuated inversion recovery (FLAIR)/T2 abnormalities in the medial temporal lobes. (2) Other disorders were reasonably excluded. (3) Informed consent was obtained from the patient’s family. The exclusion criteria were as follows: (1) the patient was lost to follow-up; and (2) the patient had cognitive deficits or epilepsy prior to the onset of anti-GABABR encephalitis. This study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (2020-104).
Data Collection
A uniformly designed form was used to record patient demographics, clinical characteristics, laboratory results, imaging findings, EEG data, tumor association, immunotherapy selection and therapeutic responses. Tumor screenings, such as CT thorax/abdomen, were conducted for all patients with anti-GABABR encephalitis. In 22 patients enrolled in this study, positron emission tomography/computed tomography (PET/CT) was performed in 17 (77%), and of 16 patients who were tumor negative on CT thorax/abdomen, 11 (69%) underwent PET/CT. Eight serum tumor markers, including alpha-fetoprotein (AFP), neuron specific enolase (NSE), cytokerantin-19-fragment (Cyfra21-1), carbohydrate antigen (CA)125, CA199, CA153, CA72-4, and carcinoembryonic antigen (CEA), were also tested in all. Based on tumor comorbidity, the patients were divided into two groups: a group with tumors and a group without tumors. Additionally, based on the severity of clinical symptoms [with coma (Glasgow Coma Scale ≤ 8), status epilepticus, or mechanical ventilation] or admission to the intensive care unit (ICU), the patients were divided into two groups: a nonsevere group and a severe group. Moreover, based on the antibody titers in the CSF, the patients were divided into two groups: the low-titer group and the high-titer group. The low-titer group included patients who were with titer of GABABR antibodies equal to or less than 1:32 in the CSF. The high-titer group included patients who were strongly positive [titer of 1:100 and above] for GABABR antibodies in the CSF. Immunotherapeutic methods and therapeutic responses were also recorded.
Treatment and Prognosis Evaluation
All patients received tumor screening, symptomatic treatment, and immunotherapy. Immunotherapy included first-line therapy [glucocorticoid therapy (5 d, IV methylprednisolone, followed by oral prednisolone), IV immunoglobulin (5 d, 0.4 g/kg), or plasma exchange], and second-line immunosuppressants (cyclophosphamide, rituximab, or mycophenolate mofetil) when necessary.
Since it was not easy to assess residual cognitive function in a large geographically dispersed population or in patients whose functional status prevented clinical visits, telephone interviews were conducted. For the enrolled patients, neurological outcomes, including motor disability (modified Rankin Scale, mRS), quality of daily life (activities of daily living, ADL), remote symptomatic epilepsy, neuropsychiatric symptoms (neuropsychiatric inventory, NPI), and cognitive ability (modified telephone interview for cognitive status, TICS-M), were evaluated. For the patients whose condition could not be clearly investigated by telephone follow-up, a clinical interview was also added. mRS scores included a motor disability scale ranging from 0 for no symptoms to 6 for death. The scale was divided into good (mRS ≤2) and poor (mRS ≥3) outcomes. The ADL instrument is a 14-item (4 points for each item) scale used to evaluate the activities of daily life that included six items for basic physical self-maintenance (PSM) and eight items for instrumental ADL (IADL). The Neuropsychiatric Inventory (NPI) describes 12 behavioral symptoms, including delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep behavior and appetite changes, and can be used to assess neuropsychiatric symptoms (NPSes). NPSes were considered to exist if the NPI scores were ≥ 1. The TICS-M (50 points) is a 13-item cognitive screening instrument. It includes items sensitive to amnestic mild cognitive impairment (delayed recall), which has been reported as a sensitive and specific indicator for dementia. Cognitive impairment was defined as a TICS-M score lower than the cutoff value of 34 for mild cognitive impairment.
Statistical Analysis
Statistical analyses were performed with the statistical software SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Patient characteristics were summarized by expressing categorical variables as counts (proportions) and continuous variables as medians (interquartile ranges, IQRs). Differences among groups were studied using Fisher’s exact tests and Mann–Whitney U tests with a significance level of 0.05.
Results
Clinical Features and Ancillary Examination Investigation
With the exception of four patients who were lost to follow-up and excluded from this study, thirteen males and nine females, with an average age of 55 years, were evaluated. A summary of the clinical information of the 22 patients with anti-GABABR encephalitis is presented in Table 1. In this study, the most characteristic presentation was seizures, which occurred at diagnosis in 100% of patients and presented as the initial symptom in 81.8% of patients. Other limbic symptoms characterized by memory deficits and psychiatric disorders were also common. Moreover, in 68.2% of the 22 patients, the clinical diagnosis of LE was confirmed by MRI findings of increased unilateral (5 patients) or bilateral (10 patients) FLAIR/T2 signal abnormalities in the medial temporal lobes. EEG data were available for 21 patients; the findings were unremarkable in 5 patients and showed generalized or focal slowing in the remaining 16 patients. Among these 16 patients, 56.3% (9/16) had foci of epileptic activity. All patients in this study underwent lumbar puncture. GABABR antibodies were found in 100% of CSF samples and in 86.4% of serum samples.
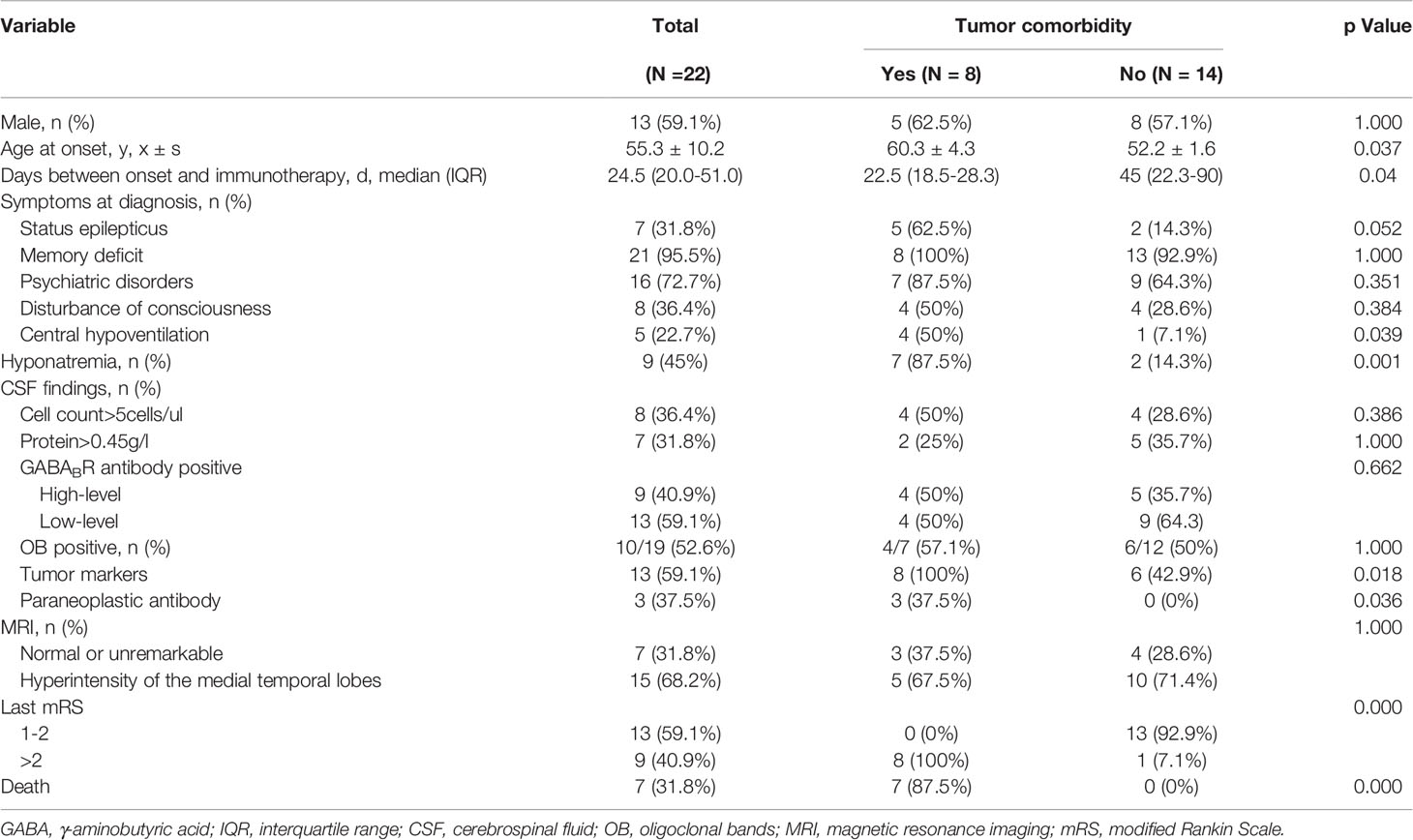
Table 1 Clinical features of 22 patients with anti- GABABR encephalitis and comparison between the group with tumors and without tumors.
Treatment and Outcomes
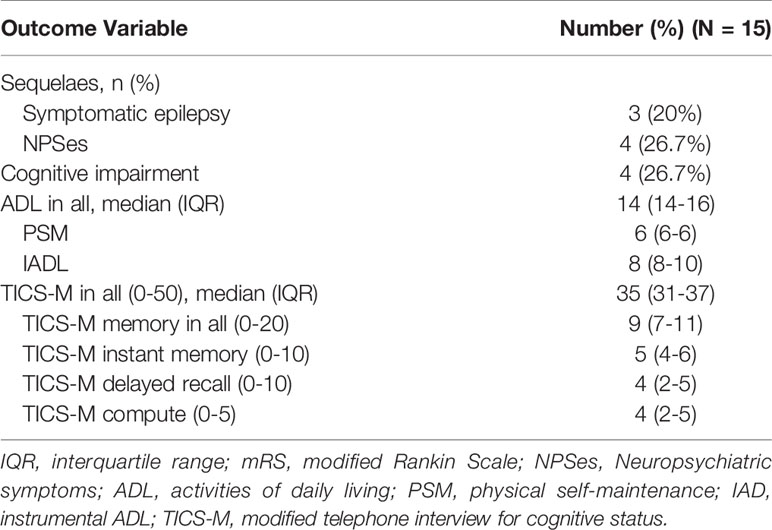
Overall, 31.8% (7/22) of patients exhibited resistance to the initial immunotherapy, including 50% (4/8) of patients with tumors and 21.4% (3/14) of patients without tumors (p =0.343). The median follow-up period with these 22 patients of anti-GABABR encephalitis was 13 months (range: 6–75). The mRS scores ranged from 2-5 (median 4) during acute illness, and the changes of mRS over time in patients with and without response to first-line immunotherapy were shown in Figure 1. Seven patients with lung cancer died during follow-up, and 13 (59.1%) had favorable outcomes during the recovery phase. The functional outcomes of the 15 survivors with anti-GABABR encephalitis are shown in Table 2. At the last follow up, the median ADL score of these patients was 14 points and the median TICS-M score was 35 points. The median follow-up period for them was 42 months (range: 8–75). Eleven patients had legacy symptoms, including symptomatic epilepsy (20%), neuropsychiatric symptoms (26.7%) and cognitive impairment (26.7%).
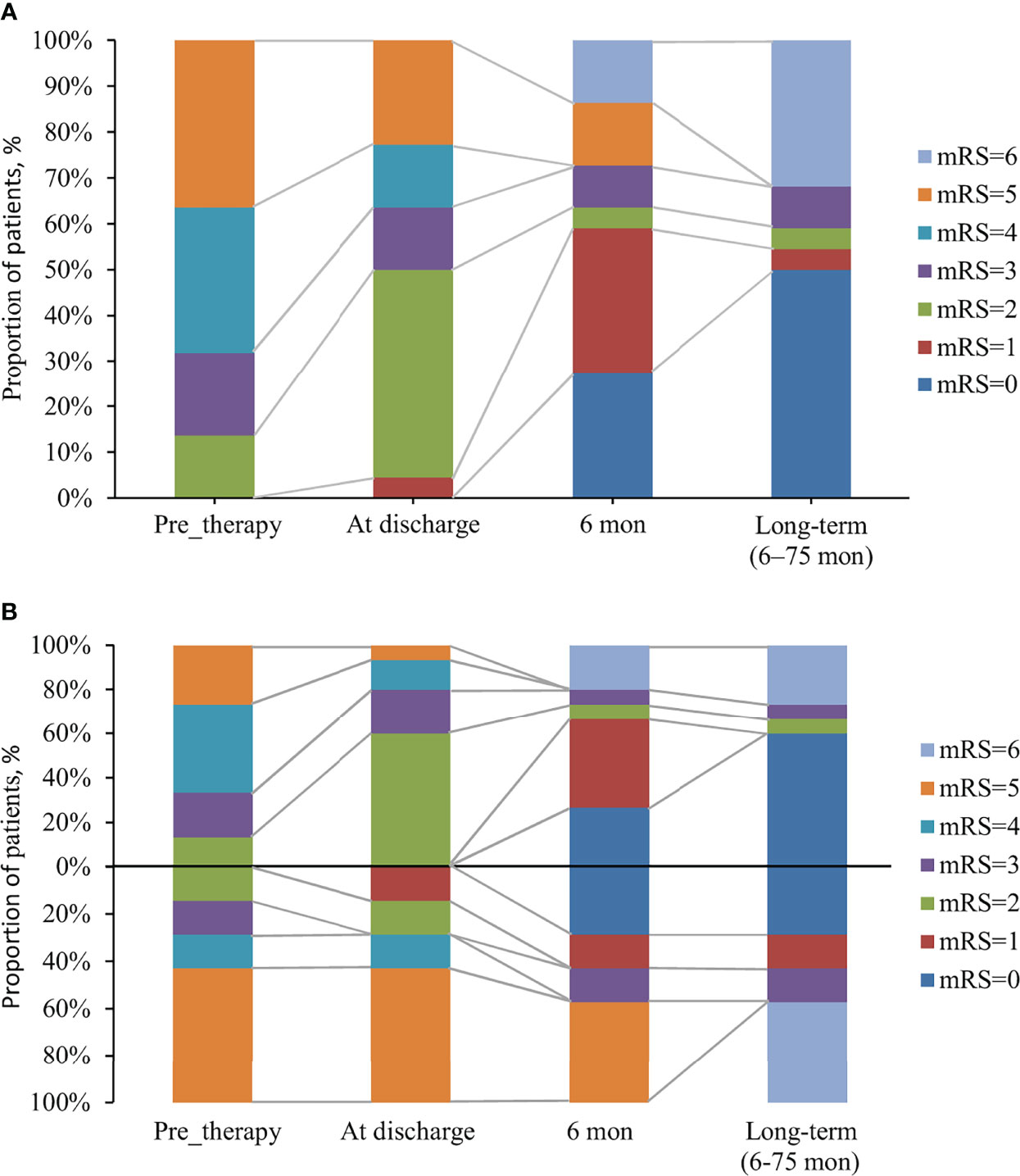
Figure 1 The changes of mRS over time in anti-GABABR encephalitis patients with and without response to first-line immunotherapy. (A) The changes of mRS in 22 patients with anti-GABABR encephalitis. (B) 22 patients were divided into two groups: a group with response to first-line immunotherapy (above the x-axis, n = 15) and a group with no response (below the x-axis, n = 7), based on the effects of first-line immunotherapy.
Outcome Comparisons Between Groups With and Without Tumors
Overall, our study found that 36.4% (8/22) of patients had tumors, and all of these patients were found to have lung cancers. Among these eight patients, seven of them had small cell lung cancer (SCLC) and the other one with bone metastasis did not have pathologic confirmation. As shown in Table 1, the median age of the group with tumors was older than that of the group without tumors [60 (range: 55-69) vs. 52 (range: 33-77), P=0.037]. Individuals with neoplastic anti-GABABR encephalitis were more likely to develop status epilepticus (p = 0.052), central hypoventilation (p = 0.039), and hyponatremia (p = 0.001). Moreover, significantly higher levels of serum tumor markers were found in this cohort (p = 0.018). Among them, NSE was the most common tumor markers, which was high in 50% (4/8) of the group with tumors and 14% (2/14) of the group without tumors, respectively. Three patients with SCLC had additional onconeuronal antibodies in serum: to Hu in two patients and to CV2 in one patient. Although patients harboring an underlying tumor had a shorter time to immunotherapy initiation, they had a tendency toward higher mortality (87.5% vs. 0%; p < 0.05). In eight patients with lung cancer, seven died within 1–12 months due to neoplastic complications, and the remaining patient survived but did not achieve self-care (mRS = 3), who had the sequelaes of symptomatic seizures, neuropsychiatric symptoms, cognitive impairment (TICS-M=13) and decreased quality of daily life (ADL=39). Inspiringly, none of the patients without tumors had died by the last follow-up, and 92.9% of these patients became functionally independent (mRS ≤ 2). However, sequelaes of symptomatic seizures, neuropsychiatric symptoms, and cognitive impairment persisted in 14.3%, 21.4%, and 21.4% of these patients, respectively.
Comparison of Outcomes Between Groups With Severe and Nonsevere Symptoms
The incidence of severe symptom status epilepticus, coma and central hypoventilation was 31.8%, 31.8% and 22.7%, respectively. Eleven patients required intensive care, and the patients were then divided into a nonsevere group (11) and a severe group (11). The clinical characteristics and prognosis of anti-GABABR encephalitis patients between the two groups are shown in Table 3. The number of CSF cells was significantly higher in the severe group than in the nonsevere group (p < 0.05). Although there was a tendency of longer hospital stays in the severe group (p = 0.051), no significant differences between the two groups were found in terms of demographics, main accessory examinations or functional outcomes.
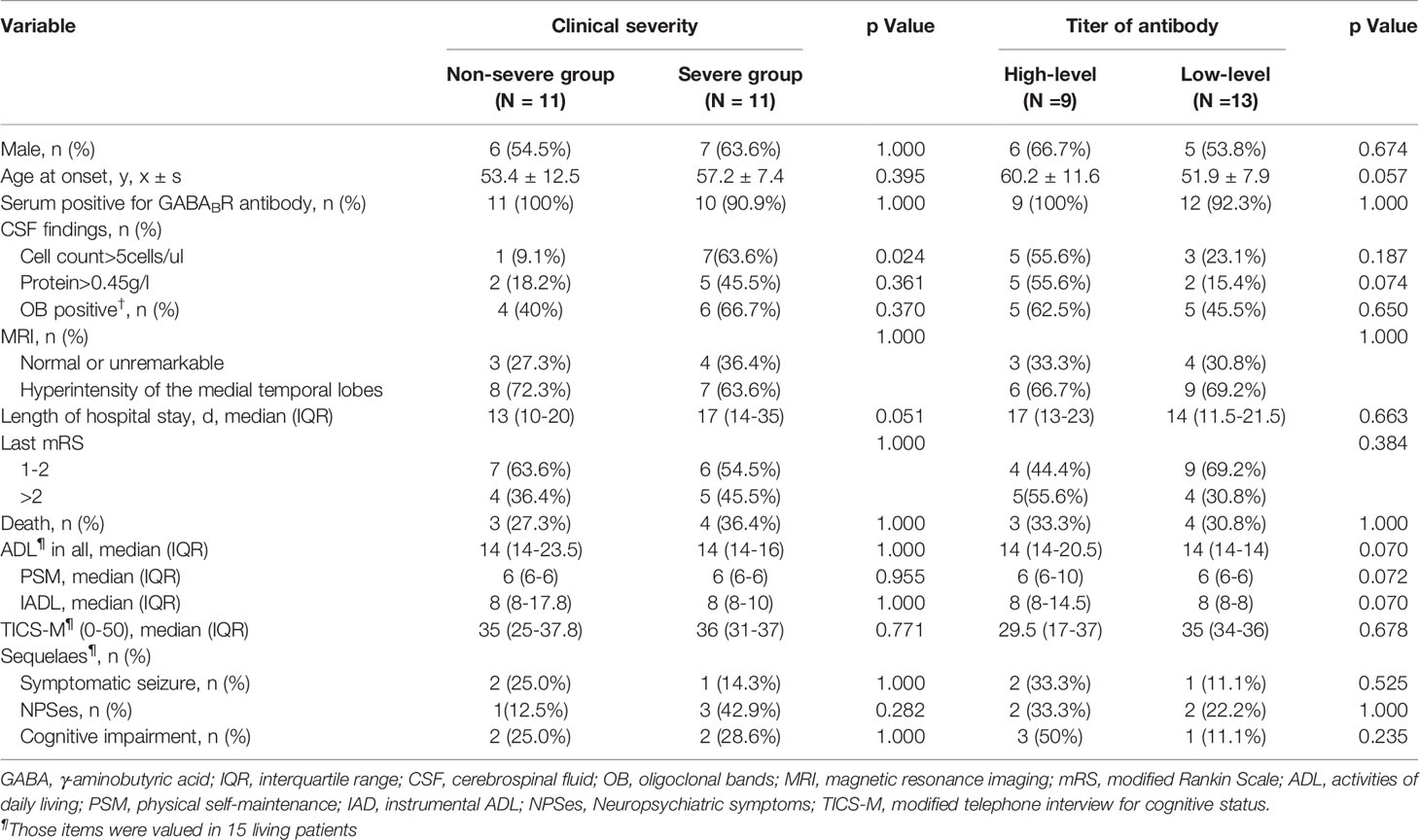
Table 3 Comparison between anti-GABABR encephalitis patients with two levels of severities of clinical symptoms, and with two titer levels of anti-GABABR antibodies.
Comparison of Outcomes Between Groups With High-Titer and Low-Titer Antibody Levels
Based on the antibody titers, the enrolled patients were divided into two groups: a high-titer group (9) and a low-titer group (13). The clinical characteristics and prognosis between the two groups are shown in Table 3. Overall, no significant differences were observed between the two groups in terms of demographics, clinical manifestations, main accessory examinations or functional outcomes.
Discussion
We provide a comprehensive investigation in a relatively large cohort (22 patients) of rare AE in China. In evaluating neurological function and its influencing factors in patients with anti-GABABR encephalitis, this study revealed several novel findings. (1) In patients with anti-GABABR encephalitis, tumors occur more often in older patients, especially those with serum tumor markers and additional onconeuronal antibodies; and paraneoplastic patients are more likely to develop status epileptics, central hypoventilation and hyponatremia. (2) Although patients with tumors have a shorter time to immunotherapy initiation, they have a poor prognosis and high mortality. (3) After immunotherapy, although a majority of the patients without tumors achieved self-care, some of them still experienced neurological deficits.
It has been reported as paraneoplastic or idiopathic in case series of anti-GABABR encephalitis, and LE patients with anti-GABABR antibodies are either with or without underlying tumors. Here, we showed a tumor rate of 36% with the vast majority of the neoplastic cases being SCLC, which is similar to the prevalence (33.3%-38.7%) of tumors in two studies in China (10, 12). However, as in other previous studies, approximately 50% of cases have an associated tumor (3, 8, 14, 15). We cannot exclude the possibility that tumors were detected after discharge in the four patients who were lost to follow-up. Moreover, inadequate follow-up or tumor screening may play a role, as not all patients in this cohort were followed up for more than four years or received PET/CT as a routine tumor screening method during the acute phase and the follow-up period.
In addition to anti-GABABR antibody, three patients with SCLC had additional onconeuronal antibodies (to Hu in two patients and to CV2 in one patient). Because anti-Hu antibody recognize a family of RNA-binding proteins expressed in the nuclei of neurons and SCLC cells, it is highly associated with SCLC. As reported previously, it is the most frequently detected antibody in paraneoplastic seizures or status epilepticus (16). Lung tumor can be found in 77% of anti-Hu antibody positive patients with paraneoplastic encephalomyelitis/sensory neuropathy (17). Similarly, the most commonly reported autoantibodies associated with GABABR autoimmune disease were anti-Hu (10.8%), anti-SOX1 (10.8%) and anti-GAD65 (8.5%), as summarized by a review of 94 cases with anti-GABABR encephalitis (18). The coexistence of all these onconeuronal antibodies suggested autoimmunity caused by tumors, ie. an immune response against onconeuronal antibody expressing tumor cells that also destroy onconeuronal antibody expressing neurons. Therefore, if the patient shows GABABR antibody along with other onconeuronal antibodies, SCLC should be considered until proven otherwise. In another study, level of serum pro-gastrin releasing peptide (ProGRP) was higher in anti-GABABR encephalitis patients than in normal population, and the level of serum ProGRP showed significant difference between SCLC and non-SCLC subgroup (15). It is a pity that proGRP was not a routine test in our study. However, NSE as another tumor marker, was found to be more frequently in the patients of anti-GABABR encephalitis with lung cancer. Although elevated NSE may be caused by severe brain injury and can be found in patients without tumor, it was detected more often in neoplastic cases, suggesting its potential as a tumor marker for SCLC in cases with anti-GABABR encephalitis. Therefore, we infer the tests of serum paraneoplastic antibodies associated with GABABR autoimmune disease and tumor markers associated with SCLC, which can initially suggest whether the patient has tumors (such as SCLC), in order to expect early detection of potential tumors and timely treatment.
In this study, the median age (60) of the paraneoplastic group was higher than that (52) of the group without tumors, suggesting that tumors occur more often in older patients. Interestingly, it was reported that when anti-GABABR encephalitis occurs before the age of 45, there seems to be no association with cancer (3, 8, 19). Furthermore, paraneoplastic patients were more likely to develop status epileptics, central hypoventilation and hyponatremia in this study. Therefore, in elderly patients with probable AE with unknown etiology, especially who are accompanied by critically ill situations, testing for anti-GABABR antibodies and suspicion of occult neoplasm is important.
Nonparaneoplastic anti-GABABR encephalitis seems to better respond to immunomodulatory treatment and have a better prognosis. From this study, 31.8% (7/22) of patients had resistance to first-line immunotherapy, including 50% (4/8) of patients with tumors and 21.4% (3/14) of patients without tumors. The mortality rate of patients with tumor comorbidity was as high as 87.5%, suggesting that the prognosis of anti-GABABR encephalitis with lung cancer was very poor. In a previous study, GABAB receptor was also found to be expressed in lung cancer tissues of the patients diagnosed with anti-GABABR encephalitis by immunohistochemistry (14). It hypotheses that ectopic expression of neuronal proteins by the tumor may lead to the reduction of immune tolerance for these proteins, which thus contributes to cancer-induced immune responses directed toward neuronal proteins and then the development of the autoimmune encephalitis. Early tumor resection and more aggressive upfront immunotherapy may improve the prognosis. In this cohort, deaths did not occur in the group without tumor comorbidity, which is inspiring. The results of the last follow-up of the 15 patients who survived showed that the patients could basically take care of themselves in their daily lives. However, instrumental activities of daily living were decreased, and the TICS-M suggested severe memory deficits in those patients. In previous report, Maureille et al. (11). retrospectively reported that two years after onset, massive anterograde amnesia affected all surviving patients with anti-GABABR encephalitis in France. The lack of improvement in anterograde amnesia with treatment suggests neuronal death rather than functional impairment of synaptic transmission. One possible cause could be excitotoxicity due to extended SE (11), but the hypothesis of T cell mediated cytotoxicity could also be considered (11).
Previously, antibody titers against GABABR in the serum ranged from 1:40 to 1:10,240 but did not correlate with disease severity (3). Consistent with that, no correlation between the antibody titers in CSF and disease severity was observed in this study. In addition, no correlation between the antibody titers in CSF and functional outcomes was found. Although there was a tendency for longer stays in the severe group in the hospital stay, there were no significant differences in functional outcomes between the nonsevere group and the severe group. Possible explanations are that bad functional outcomes were mainly caused by tumors or their complications.
The main limitation of this study was the small number of patients with anti-GABABR encephalitis, and more data are needed to get more accurate statistics due to the lower incidence of anti-GABABR encephalitis and fewer patients. The study was also subject to referral bias because the data were collected from a highly reputed national referral hospital known for neurology. In addition, the correlation between the immunotherapy and neurological outcomes is still uncertain, and the optimization of therapy needs to be further investigated. However, the findings from this study have several practical implications. The identification of elderly patients with anti-GABABR antibodies, especially those with severe symptoms, serum tumor markers or additional onconeuronal antibodies, should prompt tumor screening with CT of the thorax/abdomen and PET/CT. Although patients with tumors had a shorter time to immunotherapy initiation, they had a higher mortality. After immunotherapy, although a majority of the patients without tumors achieved self-care, some of them were still experienced neurological deficits.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (2020-104). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WC designed and administrated the study, analyzed data and drafted paper. YW and XG participated within the investigation, data curation and analysis. LG, ZH, YL, QX, and GL took part in investigation. YZ and YS provided the resources, supervised the study and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key Research and Development Program of China (No. 2020YFC2005403), Beijing Municipal Science & Technology Commission (No. Z211100002921030) and Beijing Municipal Administration of Hospitals Incubating Program (No. PX2020035).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgements
The authors appreciate the supports of Beijing Key Laboratory of Neuromodulation and Beijing Institute for Brain Disorders.
References
1. Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-Surface Central Nervous System Autoantibodies: Clinical Relevance and Emerging Paradigms. Ann Neurol (2014) 76:168–84. doi: 10.1002/ana.24200
2. Tanaka K. Autoimmune Encephalitis-Update: Roles of Autoantibodies in the Pathogenesis. Rinsho Shinkeigaku (2014) 54:1107–9. doi: 10.5692/clinicalneurol.54.1107
3. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) Receptor in Limbic Encephalitis With Seizures: Case Series and Characterisation of the Antigen. Lancet Neurol (2010) 9:67–76. doi: 10.1016/S1474-4422(09)70324-2
4. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and Antibodies to Synaptic and Neuronal Cell Surface Proteins. Neurology (2011) 77:179–89. doi: 10.1212/WNL.0b013e318224afde
5. Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune Encephalitis: Recent Updates and Emerging Challenges. J Clin Neurosci (2014) 21:722–30. doi: 10.1016/j.jocn.2013.07.017
6. Jarius S, Steinmeyer F, Knobel A, Streitberger K, Hotter B, Horn S, et al. GABAB Receptor Antibodies in Paraneoplastic Cerebellar Ataxia. J Neuroimmunol (2013) 256:94–6. doi: 10.1016/j.jneuroim.2012.12.006
7. Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NK, Dalmau J. Autoantigen Diversity in the Opsoclonus-Myoclonus Syndrome. Ann Neurol (2003) 53:347–53. doi: 10.1002/ana.10462
8. Hoftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, et al. Encephalitis and GABAB Receptor Antibodies: Novel Findings in a New Case Series of 20 Patients. Neurology (2013) 81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f
9. Dogan Onugoren M, Deuretzbacher D, Haensch CA, Hagedorn HJ, Halve S, Isenmann S, et al. Limbic Encephalitis Due to GABAB and AMPA Receptor Antibodies: A Case Series. J Neurol Neurosurg Psychiatry (2015) 86:965–72. doi: 10.1136/jnnp-2014-308814
10. Guan HZ, Ren HT, Yang XZ, Lu Q, Peng B, Zhu YC, et al. Limbic Encephalitis Associated With Anti-Gamma-Aminobutyric Acid B Receptor Antibodies: A Case Series From China. Chin Med J (Engl) (2015) 128:3023–8. doi: 10.4103/0366-6999.168989
11. Maureille A, Fenouil T, Joubert B, Picard G, Rogemond V, Pinto AL, et al. Isolated Seizures Are a Common Early Feature of Paraneoplastic Anti-GABAB Receptor Encephalitis. J Neurol (2019) 266:195–206. doi: 10.1007/s00415-018-9132-0
12. Lin J, Li C, Li A, Liu X, Chen C, Gong X, et al. Long-Term Cognitive and Neuropsychiatric Outcomes of Anti-GABABR Encephalitis Patients: A Prospective Study. J Neuroimmunol (2021) 351:577471. doi: 10.1016/j.jneuroim.2020.577471
13. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A Clinical Approach to Diagnosis of Autoimmune Encephalitis. Lancet Neurol (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
14. Zhao XH, Yang X, Liu XW, Wang SJ. Clinical Features and Outcomes of Chinese Patients With Anti-Gamma-Aminobutyric Acid B Receptor Encephalitis. Exp Ther Med (2020) 20:617–22. doi: 10.3892/etm.2020.8684
15. Wu H, Wang Y, Wei K, Qiao S, Liu L, Zhang R, et al. Clinical Characteristics and Elevated ProGRP and Positive Oligoclonal Bands of 13 Chinese Cases With Anti-GABABR Encephalitis. Int J Dev Neurosci (2021) 81:492–501. doi: 10.1002/jdn.10121
16. Shavit YB, Graus F, Probst A, Rene R, Steck AJ. Epilepsia Partialis Continua: A New Manifestation of Anti-Hu-Associated Paraneoplastic Encephalomyelitis. Ann Neurol (1999) 45:255–8. doi: 10.1002/1531-8249(199902)45:2<255::AID-ANA18>3.0.CO;2-N
17. Sillevis Smitt P, Grefkens J, De Leeuw B, Van Den Bent M, Van Putten W, Hooijkaas H, et al. Survival and Outcome in 73 Anti-Hu Positive Patients With Paraneoplastic Encephalomyelitis/Sensory Neuronopathy. J Neurol (2002) 249:745–53. doi: 10.1007/s00415-002-0706-4
18. Mckay JH, Dimberg EL, Lopez Chiriboga AS. A Systematic Review of Gamma-Aminobutyric Acid Receptor Type B Autoimmunity. Neurol Neurochir Pol (2019) 53:1–7. doi: 10.5603/PJNNS.a2018.0005
Keywords: autoimmune encephalitis (AE), GABABR, Encephalitis (MeSH), prognosis, tumor
Citation: Chen W, Wang Y, Guo X, Gao L, Huang Z, Lin Y, Xue Q, Liu G, Zhang Y and Su Y (2022) A Prognostic Analysis of the Outcomes in Patients With Anti-γ-Aminobutyric Acid B Receptor Encephalitis. Front. Immunol. 13:847494. doi: 10.3389/fimmu.2022.847494
Received: 02 January 2022; Accepted: 24 March 2022;
Published: 19 April 2022.
Edited by:
Grant Schulert, Cincinnati Children’s Hospital Medical Center, United StatesReviewed by:
Alina Gonzalez-Quevedo, Instituto de Neurología y Neurocirugía, La Habana, CubaTerrence Thomas, KK Women’s and Children’s Hospital, Singapore
Copyright © 2022 Chen, Wang, Guo, Gao, Huang, Lin, Xue, Liu, Zhang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, zhangylq@sina.com; Yingying Su, suyingyingxwyy@163.com
 Weibi Chen
Weibi Chen Yunyun Wang
Yunyun Wang Xiaoyuan Guo1,3
Xiaoyuan Guo1,3 Yicong Lin
Yicong Lin Gang Liu
Gang Liu Yan Zhang
Yan Zhang