A Possible Role of Allatostatin C in Inhibiting Ecdysone Biosynthesis Revealed in the Mud Crab Scylla paramamosain
- 1College of Fisheries, Jimei University, Xiamen, China
- 2College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
C-type allatostatins (C-type ASTs) are a family of structurally related neuropeptides found in a wide range of insects and crustaceans. To date, the C-type allatostatin receptor in crustaceans has not been deorphaned, and little is known about its physiological functions. In this study, we aimed to functionally define a C-type ASTs receptor in the mud crab, Scylla paramamosian. We showed that C-type ASTs receptor can be activated by ScypaAST-C peptide in a dose-independent manner and by ScypaAST-CCC peptide in a dose-dependent manner with an IC50 value of 6.683 nM. Subsequently, in vivo and in vitro experiments were performed to investigate the potential roles of ScypaAST-C and ScypaAST-CCC peptides in the regulation of ecdysone (20E) and methyl farnesoate (MF) biosynthesis. The results indicated that ScypaAST-C inhibited biosynthesis of 20E in the Y-organ, whereas ScypaAST-CCC had no effect on the production of 20E. In addition, qRT-PCR showed that both ScypaAST-C and ScypaAST-CCC significantly decreased the level of expression of the MF biosynthetic enzyme gene in the mandibular organ, suggesting that the two neuropeptides have a negative effect on the MF biosynthesis in mandibular organs. In conclusion, this study provided new insight into the physiological roles of AST-C in inhibiting ecdysone biosynthesis. Furthermore, it was revealed that AST-C family peptides might inhibit MF biosynthesis in crustaceans.
Introduction
Allatostatins (ASTs), a class of peptides found in insects, have a potent effect on the production of juvenile hormone (JH) in corpora allata (Stay and Tobe, 2007). Three distinct families of allatostatin peptides known as A-type, B-type, and C-type ASTs, have been identified in arthropods based on structural difference (Verlinden et al., 2015). The C-type AST has a conserved pentapeptide P–I–S–C–F in the C-terminus and a disulfide bridge between the cysteine residues located at positions 7 and 14, which was first isolated in the tobacco hornworm Manduca sexta (Kramer et al., 1991). Three members of C-type ASTs, including AST-C peptide, AST-CC peptide, and AST-CCC peptide, have been recorded in arthropods. AST-C and AST-CC are unblocked peptides whereas AST-CCC has an amidated C-terminus in most insect species, but AST-CCC peptides in Hemiptera are not amidated (Veenstra, 2009, 2016). The allatostatic role was initially assigned to allatostatin neuropeptides, but further research revealed pleiotropic roles in different species, such as inhibition of the spontaneous foregut contractions in the tomato moth (Lacanobia oleracea) (Matthews et al., 2007), stimulation of proteases release from the gut in the red flour beetle (Tribolium castaneum) (Audsley et al., 2013), and modulation of immunity and seasonal evening activity in the fruitfly (Drosophila melanogaster) (Bachtel et al., 2018; Diaz et al., 2019).
A large number of C-type AST family peptides have been identified using mass spectrometry and transcriptome sequencing in crustacean species (Stemmler et al., 2010; Bao et al., 2015; Christie, 2016, 2020; Veenstra, 2016), but there has been limited research into their biological functions. Besides regulating the cardiac and foregut activity of the American lobster, Homarus americanus and the crab, Cancer borealis (Dickinson et al., 2009, 2018; Ma et al., 2009; Wiwatpanit et al., 2012), allatostatin C has been showed to inhibit the ovarian development in our recently study of the mud crab, Scylla paramamosain (Liu et al., 2019). The blocked C-type AST peptides (AST-C and AST-CC) and unblocked peptide (AST-CCC) had different distribution patterns in the stomatogastric nervous system in H. americanus, indicating that they may play distinct roles in crustaceans (Christie et al., 2018).
The cognate receptors for C-type AST peptide, Drostar-1 and -2, which were structurally related to the mammalian somatostatin receptor, were first characterized in D. melanogaster by reverse pharmacology using Xenopus oocytes (Kreienkamp et al., 2002). Inhibition of Drostar-1 and -2 by RNA interference caused an increase in the methyl farnesoate (MF) and juvenile hormone III (JH III) (Wang et al., 2012). Subsequently, receptors for C-type ASTs were deorphaned in other insect species, including Aedes aegypti (Mayoral et al., 2010), T. castaneum (Audsley et al., 2013), Apis mellifera (Urlacher et al., 2016), Carausius morosus (Şahbaz and Lyison, 2019), and Bombyx mori (Čižmár et al., 2019), as well as the starfish Asterias rubens, which is an echinoderm (Zhang et al., 2020). In A. aegypti, for example, allatostatin C peptide inhibits the level of cAMP in cytoplasm after binding to its receptor expressed in human embryonic kidney 293T (HEK293T) cells (Mayoral et al., 2010). Interestingly, the C-type AST receptor gene is duplicated in Diptera insects, and different members of the C-type AST family can activate a same receptor (Verlinden et al., 2015; Urlacher et al., 2016). Although transcriptome sequencing showed a putative C-type AST receptor in the Y-organ of the green shore crab, Carcinus maenas, and the blackback land crab, Gecarcinus lateralis (Oliphant et al., 2018; Tran et al., 2019), C-type AST receptors have not been yet functionally deorphaned in any crustacean species. Therefore, the identification of a C-type ASTs receptor in crustaceans will have significant implications for future research into physiological functions of C-type ASTs.
In our previous study, we used molecular cloning to isolate a putative receptor for C-type ASTs from S. paramamosain, and revealed that it was expressed in the Y-organ and mandibular organ (Liu et al., 2019), indicating that C-type ASTs have a potential role in the regulation of ecdysone and MF biosynthesis. Ecdysone, a hormone in arthropods plays a crucial role in molting, growth, and reproduction, among other processes (Nagaraju, 2011; Gong et al., 2015; Hyde et al., 2019). The biosynthesis of ecdysone in crustacean is known to be negatively regulated by molt-inhibiting hormone (MIH), which is produced by the eyestalk ganglion (Nakatsuji and Sonobe, 2004). In silkworm, B. mori, ecdysone biosynthesis is inhibited by B-type ASTs, which is also termed as prothoracicostatic peptide (PTSP) (Yamanaka et al., 2010). The effects of C-type AST in ecdysone biosynthesis in crustaceans and insects has not been yet reported. Methyl farnesoate is a homologous hormone to insect JH with the same biosynthesis pathway as JH (Homola and Chang, 1997; Sin et al., 2015). Methyl farnesoate is involved in the regulation of protein metabolism, molting and metamorphosis, reproduction, and other processes in crustaceans (reviewed by Homola and Chang, 1997; Nagaraju, 2011). The inhibitory role of C-type AST is not conserved in insects, and it is not clear whether it regulates the biosynthesis of MF in crustaceans.
Mud crab (S. paramamosain) is a popular aquaculture species found in Indo-Pacific regions (Walton et al., 2006). It has great economic values due to its high nutritional benefits and fast growth rate. In recent years, S. paramamosain has drawn increasing attention in the fishing and aquaculture industries in China, as well as in Southeast Asian countries (VinceCruz-Abeledo et al., 2021). In this study, we used in vivo and in vitro experiments to functionally characterize the C-type ASTs receptor in S. paramamosain, as well as to examine the potential effects of ScypaAST-C and ScypaAST-CCC on the regulation of ecdysone and MF production. Recently, four cytochrome P450 (CYP) enzymes, namely CYP306A1 (phantom), CYP302A1 (disembodied), CYP315A1 (shadow), and CYP314A1 (shade), that are involved in the ecdysteroid biosynthesis pathway have been identified (Christiaens et al., 2010). Disembodied and shadow genes were identified from S. paramamosain transcriptome data, and these two genes expression in the Y-organ were studied as indicators of 20E biosynthesis. In addition, in order to investigate the effect of ScypaAST-C and ScypaAST-CCC peptides on MF biosynthesis, six MF biosynthetic enzyme genes, including acetoacetyl-CoA thiolase (thiol), 3-hydroxy-3-methylglutaryl-CoA synthase (hmgs), 3-hydroxy-3-methylglutaryl-CoA reductase (hmgr), mevalonate kinase (mk), diphosphomevalonate decarboxylase (ppmd), and farnesyl diphosphate synthase (fpps), expression in the mandibular organ were studied.
Materials and Methods
Animals
Female crabs at the intermolt stages (carapace width 11.9 ± 0.56 cm, body weight 317.1 ± 25.5 g) were purchased from a local fish market in Xiamen city, Fujian Province, China, and then transported to the laboratory in Xiamen university. Crabs were housed individually in a rectangular tank filled with seawater for a week. The salinity of the water was set at 28 ± 0.5 ppt and the water temperature was maintained at 28 ± 2°C. During this period, crabs were fed with clam Ruditapes philippinarum to satiation once a day.
Peptides
According to the deduced amino acids of the preprohormone, mature allatostatin C (QIRYHQCYFNPISCF-OH, a disulfide bridge present between C7 and C14; GenBank accession number: MK314113) and allatostatin CCC (RSYWKQCAFNAVSCF-NH2, a disulfide bridge present between C7 and C14; GenBank accession number: ALQ28598.1) were synthesized by GL Biochem (Shanghai, China), with a purity of 98%, for the subsequent experiments.
Molecular Cloning and Plasmid Construction
Total RNA from the Y-organ of S. paramamosain was extracted using TRIzol® reagent (Invitrogen), according to the manufacturer’s instructions. The cDNA was reverse-transcribed with a RevertAid First Strand cDNA Synthesis Kit (Fermentas) utilizing random primers and 1 μg of total RNA according to the given directions. The open reading frame of ScypaAST-CR (GenBank accession number: MK314114) was amplified with AST-CR-F and AST-CR-R primers (Supplementary Table 1), and then inserted into pEGFP-N1 vector by restriction enzymes of EcoRI and BamHI. Finally, it was sequenced to validate the veracity and orientation.
Cell Culture and Receptor Assay
HEK293T cells were cultured in dulbecco’s modified eagle medium (DMEM, Gibco) supplemented with 10% inactivated fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma) at 37°C, 5% CO2. Cells grown in a monolayer to approximately 60% confluency in a 6-well plant were transiently transfected with 1 μg of pFGFP-N1-ScypaAST-CR plasmid, 0.5 μg of cAMP reporter gene pCRE-luc, and 0.2 μg of internal control gene pRL-TK using Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s recommendations. The mock cells were transfected with empty pEGFP-N1 instead.
To examine the expression and localization of ScypaAST-CR in HEK293T cells, transfected cells cultured for 24 h were fixed in 4% paraformaldehyde for 20 min. Subsequently, cells were stained with nuclear dye DAPI (Invitrogen) for 10 min and then with membrane probe DiI (Beyotime, China) for 15 min. The stained cells were imaged using a fluorescence confocal microscope (LSM 780, Zeiss) and the pictures were processed using Zeiss 20.0 software.
Transfected cells were detached from the culture flask 24 h after transfection and transferred to 96 well plates at a confluence of approximately 90%. After 12 h, the cells were stimulated with 0.1 μM forskolin for 15 min, thereafter, they were washed with HBSS buffer (Sigma). The cells were then challenged for 6 h with a dilution series of ScypaAST-C and ScypaAST-CCC peptides dissolved in same DMEM. Finally, luciferase activity was detected by GloMax® 20/20 Luminometer (Promega) using a Dual-Luciferase® Reporter Assay System Kit (Promega).
In vivo Effect of AST-C and AST-CCC on Y-Organ and Mandibular Organ
To explore the potential effect of C-type ASTs peptide on the biosynthesis of 20E and MF in S. paramamosain, an in vivo experiment with injection of ScypaAST-C or ScypaAST-CCC peptides was performed. Before the experiment, the crabs were housed for a week under the same condition for acclimation as described in section “Animals.” The animals were randomly divided into four groups, each with seven individuals (n = 7). Before injection, a group of animals was randomly sampled and set as a pre-injection control. Subsequently, crabs were given 100 μL of crustacean saline that containing ScypaAST-C or ScypaAST-CCC peptides (10 ng/g body weight). The saline control group was treated with 100 μL of crustacean salt instead. Injections were performed at every 4 days, with a microsyringe (Hamilton, Switzerland) into the arthrodial membrane located at the fifth swimming leg. The injections were continued for about half a month, and on day 16, almost 24 h after the fourth injection, the crabs were put on ice for anesthetization and 1 mL of the hemolymph was extracted for 20-hydroxyecdysone (20E) assay. Finally, the animals were euthanized, and Y-organ and mandibular organ samples were dissected for gene expression analyses.
In vitro Effect of AST-C and AST-CCC on Y-Organ and Mandibular Organ
We conducted an in vitro experiment that applied ScypaAST-C and ScypaAST-CCC peptide to the explants of Y-organ to confirm the role of C-type ASTs in regulating 20E biosynthesis in S. paramamosain. The crabs at the intermolt stage were anesthetized on ice for 10 min and sterilized in 75% ethanol for 10 min. Y-organ samples were immediately taken from the crabs and gently washed five times with crab saline containing antibiotics penicillin G (300 IU/mL) and streptomycin (300 mg/mL). Subsequently, the Y-organs were cultured in L15 medium that contained antibiotics penicillin G (300 IU/mL) and streptomycin (300 mg/mL). Each crab has two Y-organs, one was used as a control and the other was treated with ScypaAST-C or ScypaAST-CCC peptide at a final concentration of 10–6 M. Each treatment was repeated five times (n = 5). After incubation for 12 h, Y-organ samples were used to examine gene expression of the 20E biosynthetic enzyme, and the medium was used to perform a 20-hydroxyecdysone assay.
To explore the effects of C-type ASTs on MF biosynthesis in the mandibular organ of S. paramamosain, we performed an in vitro experiment in which we added ScypaAST-C or ScypaAST-CCC peptide to the explants of mandibular organ. Similarly, one mandibular organ was cultured for 12 h in L15 medium containing ScypaAST-C or ScypaAST-CCC peptide at a final concentration of 10–6 M, whereas the other one was cultured in the L15 medium without any peptides as the control (n = 5). Finally, the mandibular organ was collected for MF biosynthetic enzyme gene expression analysis.
RNA Extraction, cDNA Synthesis, and qPCR Analysis
Total RNA was extracted from homogenized samples of Y-organ and mandibular organ using TRIzol® reagent (Invitrogen), according to the manufacturer’s instructions. The quantity and quality of RNA was determined using a Nanodrop spectrophotometer (Thermo Scientific). After removing any potential genomic DNA contamination with DNase I (Invitrogen), 1 μg of total RNA was reverse-transcribed with a RevertAid First Strand cDNA Synthesis Kit (Fermentas) using random primers according to the manufacturer’s protocol. The generated cDNAs were diluted four folds for qPCR analysis. The reaction was 20 μL in a total volume that containing 10 μL 2X PCR Master Mix with SYBR Green, 2 μL dilute cDNA, 0.8 μL each primer (1 mM), and 6.4 μL deionized water. The qPCR reaction was run using 7500 Fast Real-time PCR machine under the following conditions: 94°C for 3 min, followed by 40 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 30 s, and 72°C for 10 min. The reactions were completed in triplicate and the results were normalized to the internal control gene β-actin. In this study, the 20E and MF biosynthetic enzyme genes were identified through transcriptome sequencing (see Supplementary Files 1, 2). The primers used for qPCR analysis were listed in Supplementary Table 1.
Determination the Concentration of 20-Hydroxyecdysone
The determination of 20-hydroxyecdysone concentrations was performed using a 20E enzyme immunoassay kit (Arbor assays, United States) (Mckinney et al., 2017). For sample preparation, 1 mL hemolymph or 0.5 mL medium was extracted by adding three volumes of chilled methanol followed by vortexing for 30 s. The mixture was centrifuged at 10,000 g for 10 min at 4°C, and the supernatant containing 20E was carefully collected. The supernatant was then dried completely in a centrifugal concentrator at 30°C for 2–3 h before being dissolved in 125 μL assay buffer for 20E analysis as directed by the manufacturer. Each set of standards and samples was run in duplicate. Finally, the data were calculated using Arbor assay, an online tool1.
Statistical Analysis
The qRT-PCR data were processed using the 2–ΔΔCt method, and then analyzed statistically. The Levene’ test was used for homoscedasticity, and statistical significance (P < 0.05) was evaluated using one-way analysis of variance (ANOVA) or Student’s t test in SPSS 20.0. The results were presented as mean ± SEM.
Results
ScypaAST-CR Is Expressed and Activated in HEK293T Cells
In this study, we assessed the functionality of ScypaAST-CR by assaying cAMP accumulation in response to ScypaAST-C or ScypaAST-CCC stimulation. The levels of intracellar cAMP were determined by luciferase activity. The fusion protein, ScypaAST-CR-EGFP was successfully expressed in HEK293T cells and localized in the cell membrane (Figure 1).
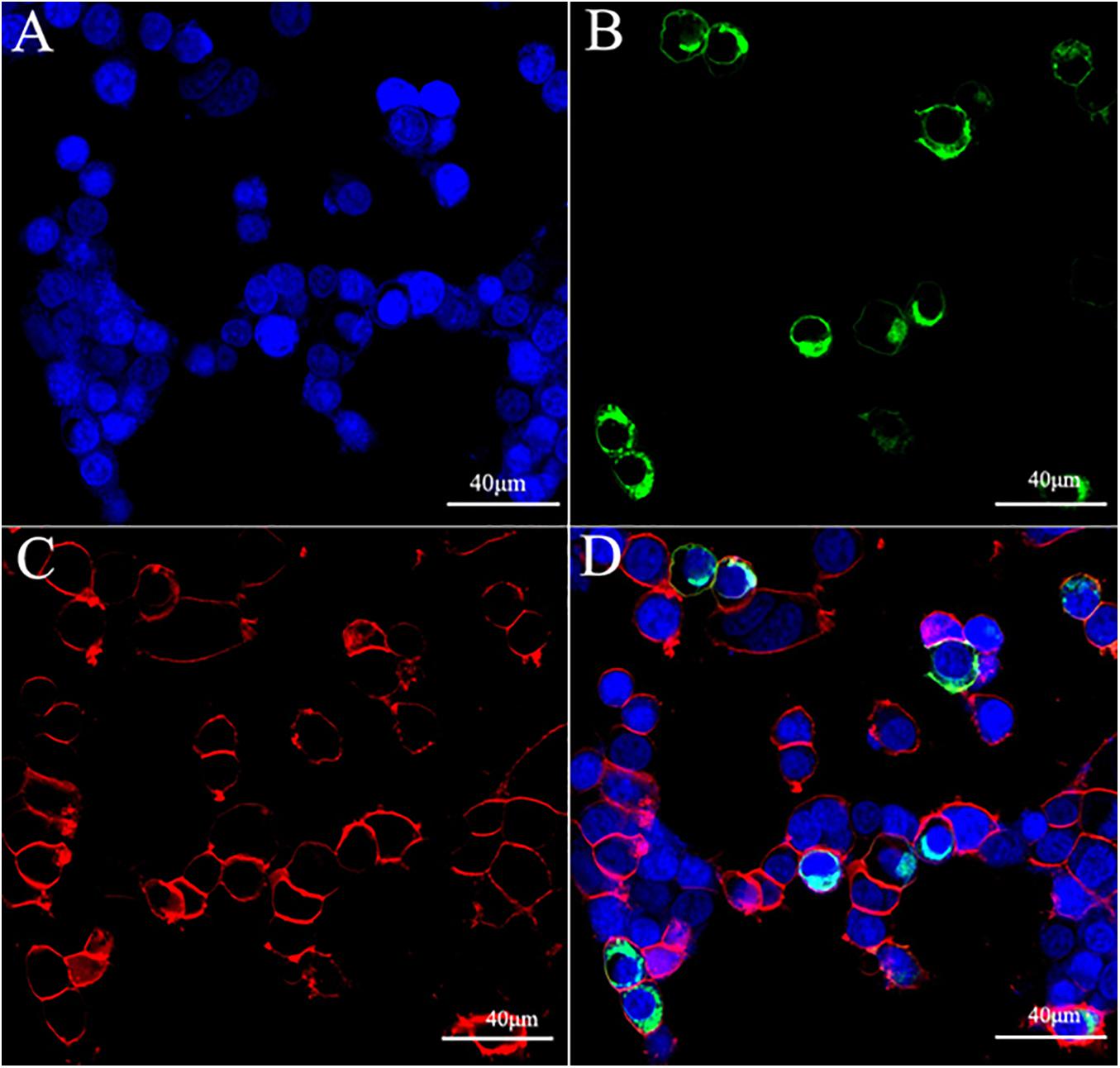
Figure 1. Expression and localization of ScypaAST-CR-EGFP in the HEK293T cell. (A) Nucleus labeled by DAPI (blue); (B) ScypaAST-C-EGFP; (C) cell membrane stained by Dil (red); (D) merge.
It was discovered that the cytoplasmic cAMP levels increased significantly in HEK293T cell after adding forskolin (Figure 2A). The ScypaAST-CR was activated by ScypaAST-C and ScypaAST-CCC at concentration of 10–6 M, resulting a significant inhibition of forskolin-stimulated luciferase activity (P < 0.0001). There was no significant change in luciferase activity in mock-transfected HEK 293T cells (Figure 2A). Surprisingly, the ScypaAST-C induced inhibition was dose-independent, but ScypaAST-CCC induced inhibition was dose-dependent with an IC50 value of 6.683 nM (Figure 2B).
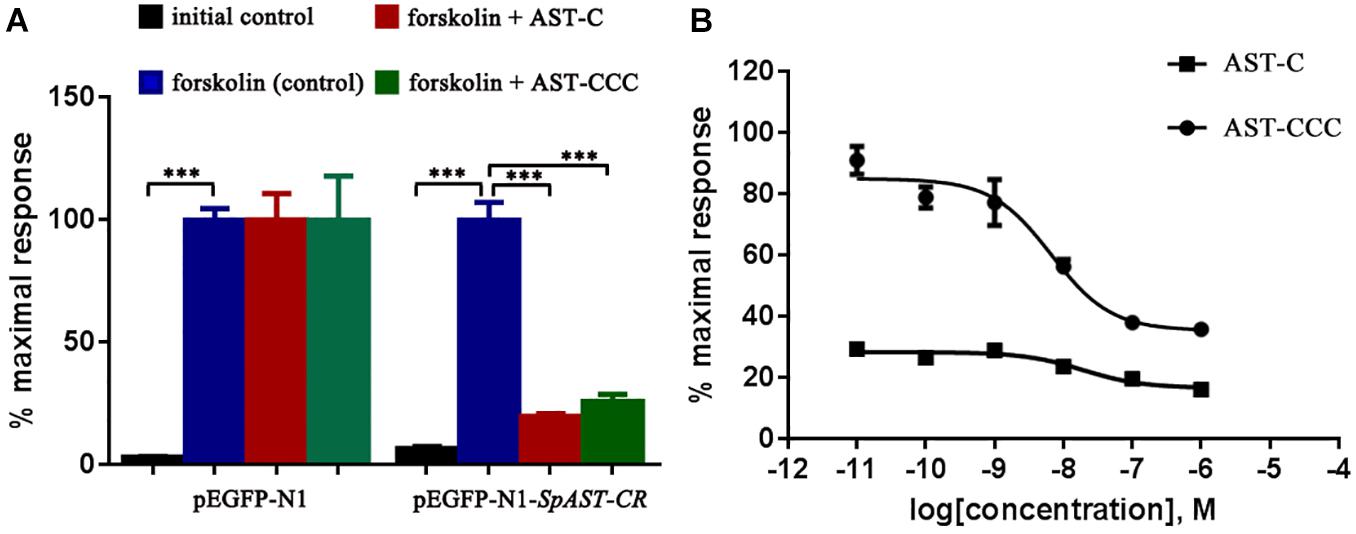
Figure 2. Functional characterization of ScypaAST-CR in HEK293T cells in vitro. (A) ScypaAST-CR was activated after stimulation with ScypaAST-C or ScypaAST-CCC at concentration of 10–6 M, resulting in a significant inhibition of forskolin-stimulated luciferase activity (***P < 0.0001). (B) Receptor activation following stimulation by different concentrations of ScypaAST-C and ScypaAST-CCC peptides was determined by monitoring intracellular cAMP levels. Curves were obtained by fitting the data using non-linear regression analysis in the GraphPad Prism software.
ScypaAST-C Inhibits the Biosynthesis of 20E in the Y-Organ of S. paramamosain
The functions of C-type ASTs in the regulation of 20E biosynthesis in the Y-organ of the mud crab S. paramamosain was investigated through in vivo and in vitro experiments. The results revealed that the concentration of 20E in the hemolymph of S. paramamosain ranged from 20.49 to 73.69 ng/mL (Figure 3). The levels of hemolymph 20E levels were not significantly affected by ScypaAST-C or ScypaAST-CCC peptides injection (P > 0.05). Additionally, the qPCR results suggested that the expression of the disembodied gene was significantly decreased by the ScypaAST-C peptide but not by the ScypaAST-CCC peptide (Figure 4A). The amount of shadow gene expression in the Y-organ was not significantly changed after the administration of ScypaAST-C or ScypaAST-CCC peptides (Figure 4B).
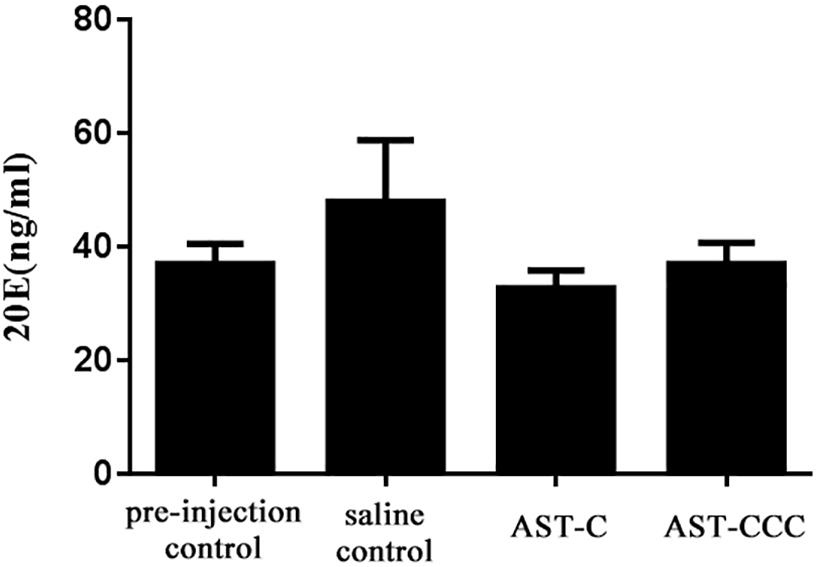
Figure 3. In vivo effect of synthetic ScypaAST-C and ScypaAST-CCC peptides on 20E titer in the hemolymph of S. paramamosain. Data were shown as means ± SEM (n = 7). No significant change in the hemolymph 20E titer was detected after administration of ScypaAST-C or ScypaAST-CCC peptide.
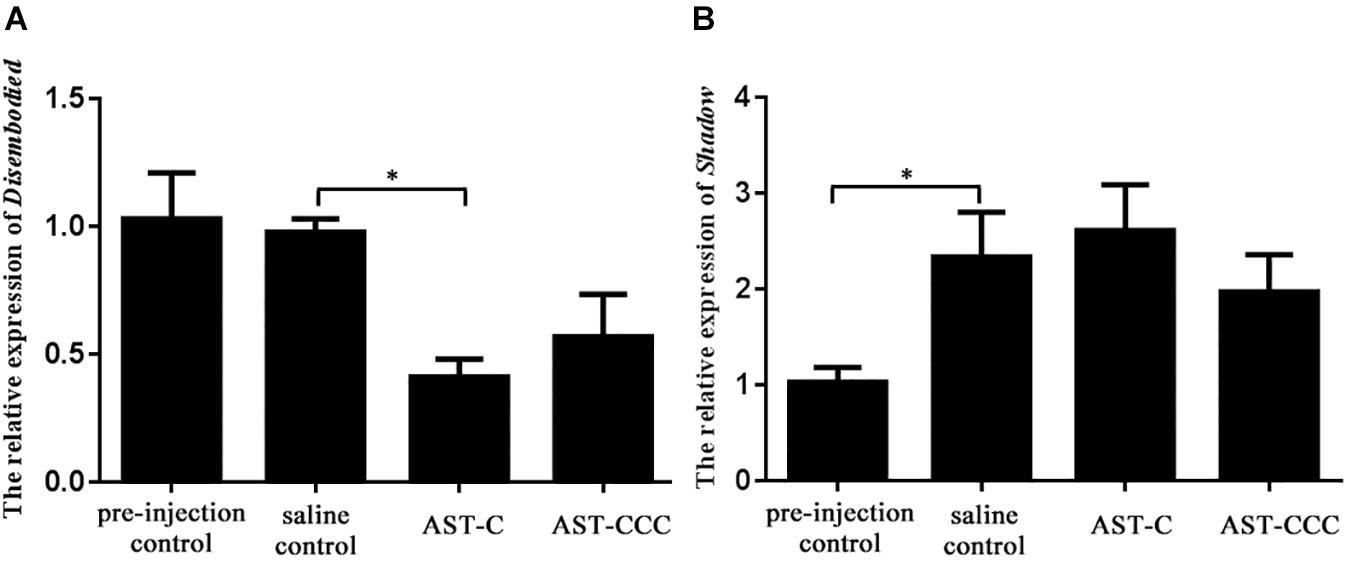
Figure 4. In vivo effect of synthetic ScypaAST-C and ScypaAST-CCC peptides on the abundance of 20E biosynthetic enzyme gene expression in Y-organ. (A) The abundance of disembodied gene expression in Y-organ; (B) the abundance of shadow gene expression in Y-organ. Result were shown as means ± SEM (n = 7). Asterisk “∗” on the error bars indicates the significant difference from the saline control (P < 0.05).
The in vitro experiment showed that the secretion of 20E by Y-organ was significantly inhibited by ScypaAST-C peptide. However, the ScypaAST-CCC peptide had no effect on the synthesis of 20E in Y-organ explant (Figure 5). There were no significant changes in the abundance of both disembodied and shadow genes in the Y-organ after addition of ScypaAST-C or ScypaAST-CCC peptide in vitro (Figure 6).
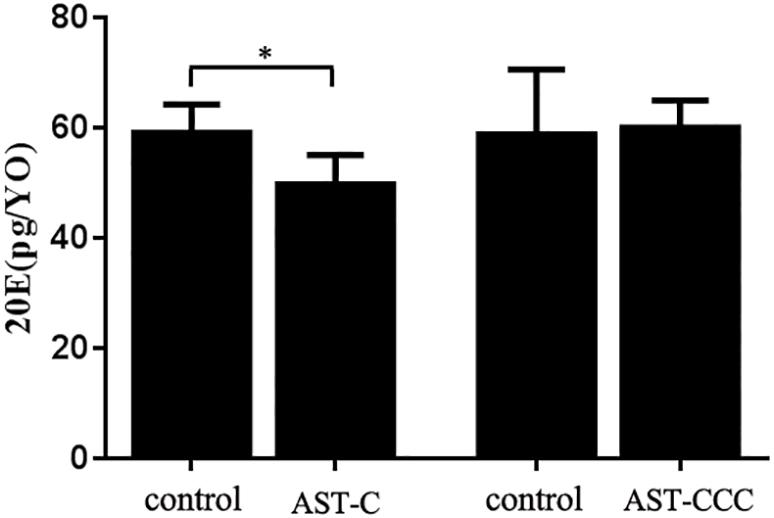
Figure 5. In vitro effects of ScypaAST-C and ScypaAST-CCC peptides on the secretion of 20E by Y-organ of S. paramamosain. The secretion of 20E by Y-organ was significantly inhibited by ScypaAST-C but not effected by ScypaAST-CCC. Data were shown as means ± SEM (n = 5). Asterisk “∗” on the error bars indicates the significantly difference from the control (P < 0.05).
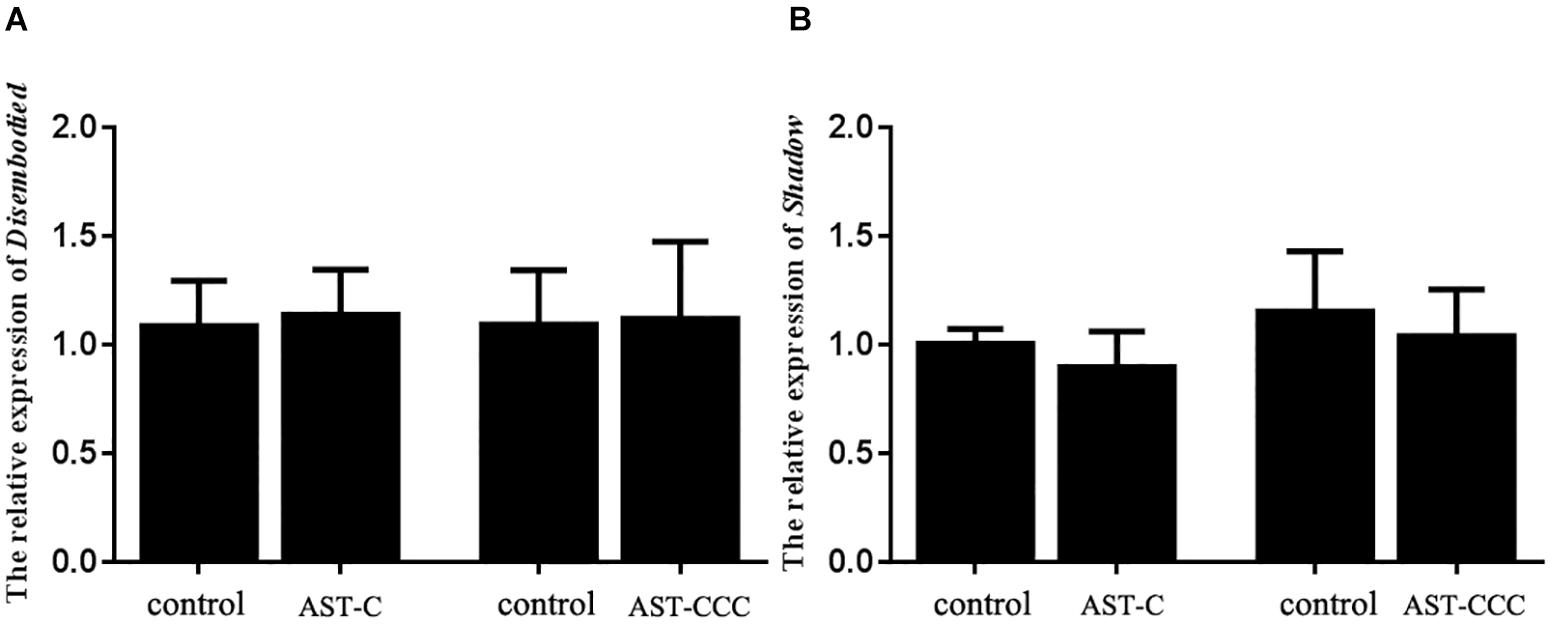
Figure 6. In vitro effects of ScypaAST-C and ScypaAST-CCC peptides on the abundance of 20E biosynthetic enzyme gene expression in the Y-organ. (A) The abundance of disembodied gene expression in Y-organ; (B) the abundance of shadow gene expression in Y-organ. Data were shown as means ± SEM (n = 5). No significant change in the abundance of disembodied and shadow expression in the Y-organ was detected after addition of ScypaAST-C or ScypaAST-CCC peptide.
C-Type ASTs Have Potential Effects on the MF Biosynthesis
The qPCR results revealed that ScypaAST-C peptide injection had no effect on the expression of thiol, hmgs, hmgr, mk, or fpps genes in the mandibular organ, but significantly induced the expression of ppmd gene (Figure 7). However, the quantity of hmgs, hmgr, mk, and fpps transcripts was decreased after the administration of ScypaAST-CCC peptide (Figure 7).
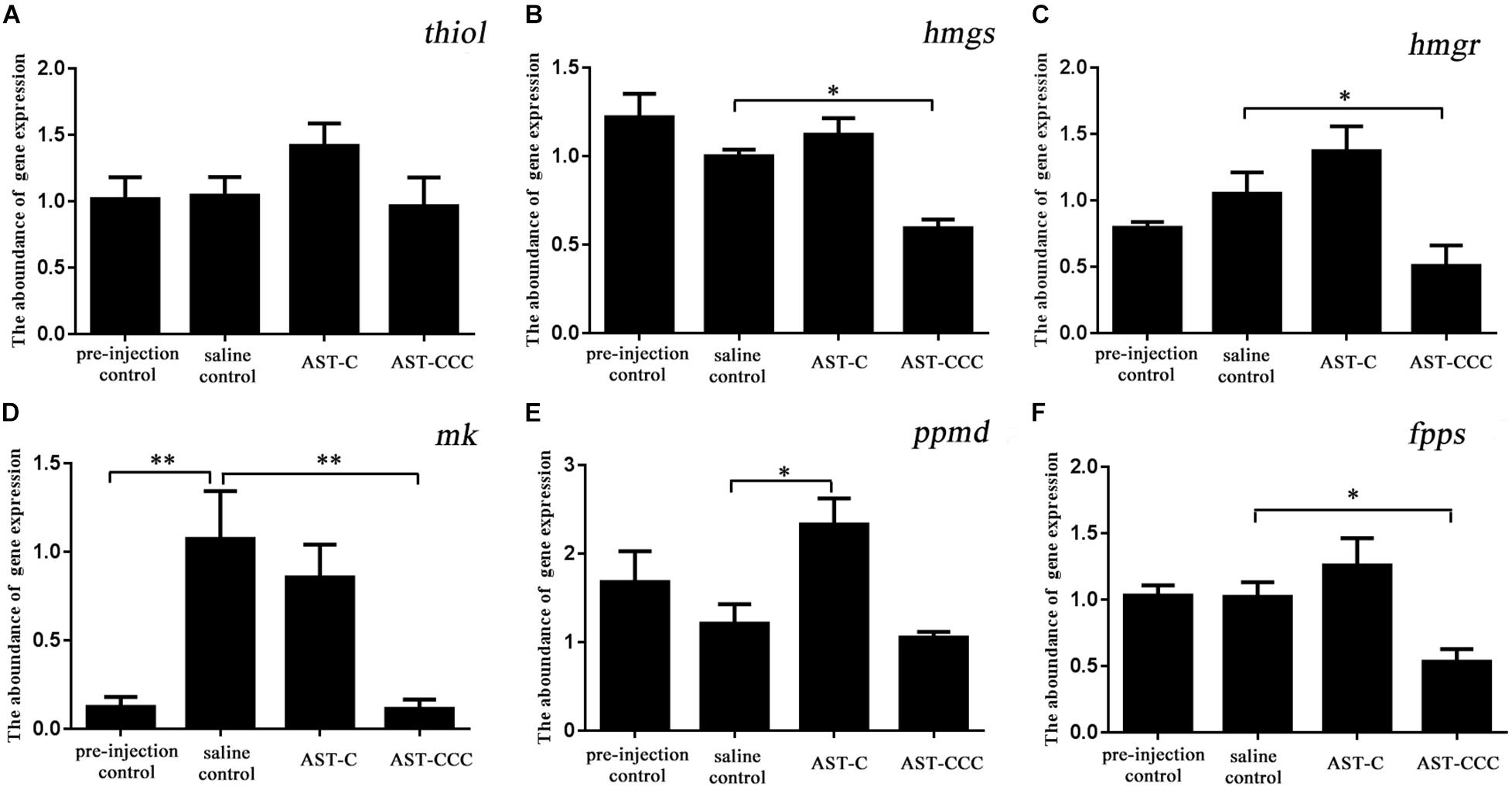
Figure 7. In vivo effects of ScypaAST-C and ScypaAST-CCC peptides on the abundance of MF biosynthetic enzyme genes in the mandibular organ. (A) thiol: acetoacetyl-CoA thiolase; (B) hmgs: 3-hydroxy-3-methylglutaryl-CoA synthase; (C) hmgr: 3-hydroxy-3-methylglutaryl-CoA reductase; (D) mk: mevalonate kinase; (E) ppmd: diphosphomevalonate decarboxylase; (F) fpps: farnesyl diphosphate synthase. Asterisks on the error bars indicates significant difference from the saline control (∗P < 0.05; ∗∗P < 0.01).
The results of qRT-PCR revealed that the ScypaAST-C peptide significantly reduced the amount of thiol and hmgs transcripts in the mandibular organ. However, there was no significant difference in the abundance of hmgs, mk, ppmd, and fpps transcripts between the control and ScypaAST-C treatment groups (Figure 8). In contrast, ScypaAST-CCC had no effect on the expression of thiol, hmgs, hmgr, and mk genes in the mandibular organ, but it significantly inhibited the expression of ppmd and fpps genes (Figure 8).
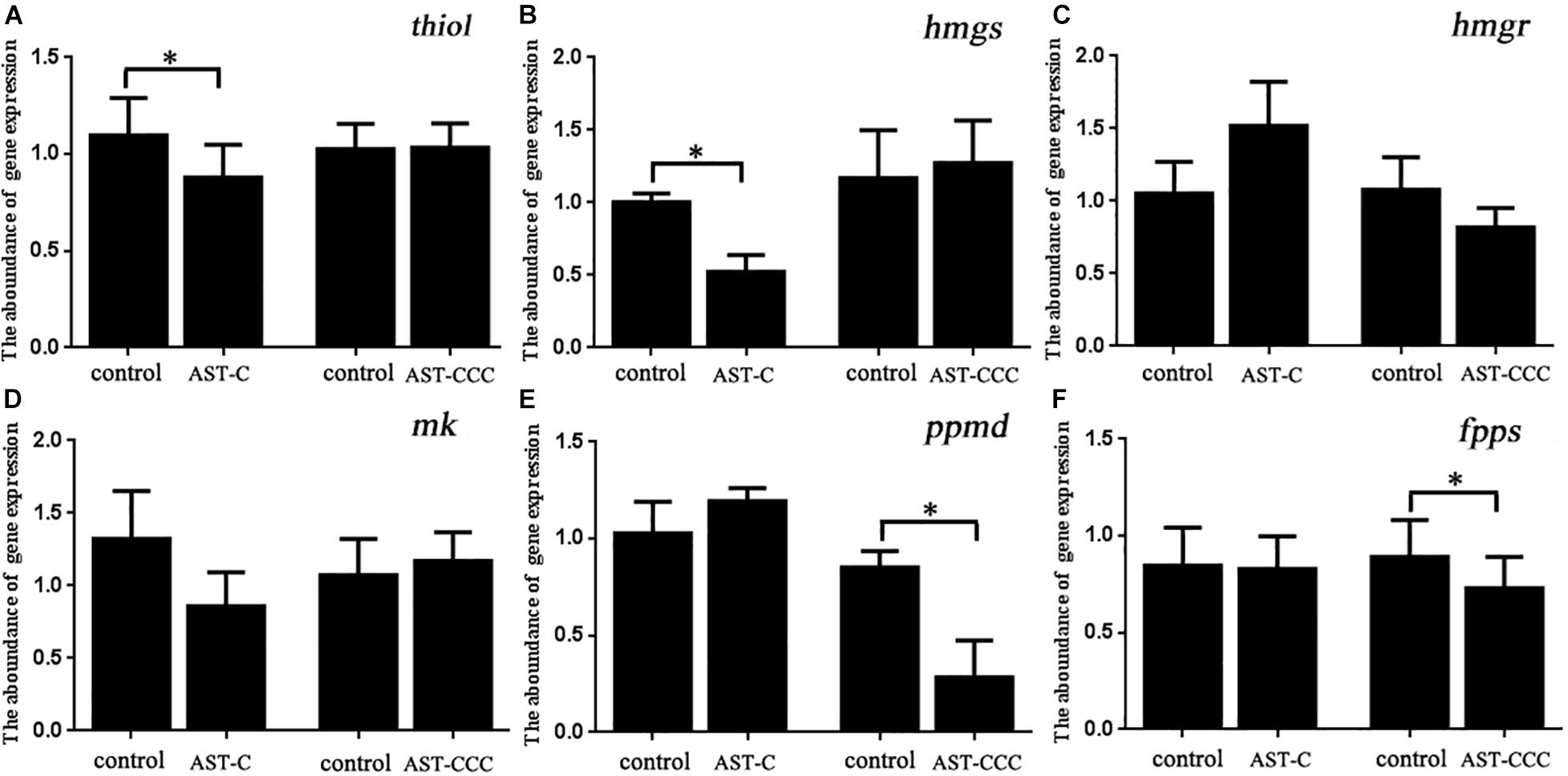
Figure 8. In vitro effects of ScypaAST-C and ScypaAST-CCC on the abundance of MF biosynthetic enzyme genes in the mandibular organ. (A) thiol: acetoacetyl-CoA thiolase; (B) hmgs: 3-hydroxy-3-methylglutaryl-CoA synthase; (C) hmgr: 3-hydroxy-3-methylglutaryl-CoA reductase; (D) mk: mevalonate kinase; (E) ppmd: diphosphomevalonate decarboxylase; (F) fpps: farnesyl diphosphate synthase. Asterisks “∗” on the error bars indicates significant difference from the control (P < 0.05).
Discussion
Recently, we used transcriptome sequencing and RACE cloning to isolate ScypaAST-CR, a putative receptor which had a strong structural resemblance to the somatostatin receptor from the mud crab S. paramamosain (Liu et al., 2019). C-type ASTs receptors with structural homology to the somatostatin receptor have been functionally characterized in several insects (Kreienkamp et al., 2002; Mayoral et al., 2010; Audsley et al., 2013; Urlacher et al., 2016; Čižmár et al., 2019; Şahbaz and Lyison, 2019) and echinoderm A. rubens (Zhang et al., 2020). However, the C-type ASTs receptor has been not yet deorphaned in crustaceans. Forskolin, a potent eukaryotic adenylate cyclase (AC) activator, can rapidly increase intracellular cAMP levels without receptors, so it was commonly used in receptor assay (Alasbahi and Melzig, 2012). In this study, the ligand-receptor activation of signaling pathway was demonstrated by using forskolin upon peptides stimulation. The synthetic peptides ScypaAST-C activated the putative receptor ScypaAST-CR in a dose-independent manner. In contrast, ScypaAST-CCC activated the putative receptor in a dose-dependent manner with an IC50 value of 6.683 nM, inhibiting of forskolin-stimulated cAMP accumulation (Figure 2). Therefore, we designated this candidate receptor as the S. paramamosain C-type ASTs receptor. Similar results were reported in T. castaneum and A. mellifera, where AST-C and AST-CC activated the same receptor (Audsley et al., 2013; Urlacher et al., 2016). The reduction in cytoplasmic cAMP suggested that ScypaAST-CR was coupled with Giα protein to inhibit the activity of AC. Surprisingly, ScypaAST-C induced inhibition was in a dose-independent manner, which contradicted the results recorded in insects (Mayoral et al., 2010; Audsley et al., 2013; Urlacher et al., 2016), and the reason for this was unknown.
To date, the physiological roles of C-type ASTs, and hence their receptors, in crustaceans have received little attention. In our previous study, we demonstrated that ScypaAST-CR was extensively expressed in the female S. paramamosian and was strongly expressed in the Y-organ (Liu et al., 2019). Transcripts of C-type AST receptor were also found in the Y-organ of the green shore crab, C. maenas and the blackback land crab, G. lateralis (Oliphant et al., 2018; Tran et al., 2019). These findings indicated that C-type ASTs may regulate the ecdysone biosynthesis in crustaceans. In this study, we performed both in vivo and in vitro experiments to examine the potential effects of ScypaAST-C and ScypaAST-CCC on ecdysone biosynthesis. The results revealed that ScypaAST-C significantly reduced the production of 20E in vitro, but that ScypaAST-CCC had no effect (Figure 5). In addition, injection of ScypaAST-C decreased the amount of disembodied expression in the Y-organ of S. paramamosian, however, no significant effect was observed after the administration of ScypaAST-CCC (Figure 3). Our result suggested that ScypaAST-C inhibited ecdysone biosynthesis in S. paramamosain, whereas ScypaAST-CCC had no effect on the biosynthesis of ecdysone. To our knowledge, this study is the first study to show that AST-C inhibits ecdysone biosynthesis in crustaceans.
Previously, it was reported that ecdysone biosynthesis was negatively regulated by MIH in crustaceans (Soumoff and O’Connor, 1982; Nakatsuji and Sonobe, 2004), and by PTSP in B. mori (Yamanaka et al., 2010). On Y-organ, AST-C displayed a physiological effect comparable to MIH in crustacean and PTSP in B. mori, implying that it was involved in the regulating molting and reproduction through inhibiting ecdysone biosynthesis (Gong et al., 2015; Hyde et al., 2019). Investigation in A. aegypti revealed that AST-C affected the production of acetyl coenzyme A (acetyl-CoA) (Nouzova et al., 2015). Cholesterol, the first step in the ecdysone biosynthesis pathway, was discovered to be biosynthesized from acetyl-CoA (Warren and Hetru, 1990; Gilbert et al., 2002; Do et al., 2009). Thus, we hypothesized that AST-C inhibited ecdysone biosynthesis in the Y-organ via obstructing cytoplasmic acetyl-CoA production.
The physiological role of C-type AST in regulating JH biosynthesis is not conserved in insects and it was confined in Lepidoptera and mosquitoes (Verlinden et al., 2015), possibly due to the presence of the C-type AST receptor in the corpora allata. For instance, in D. melanogaster, A. aegypti, and T. castaneum, the C-type AST receptor was highly expressed in the corpora allata, supporting the aforementioned role (Mayoral et al., 2010; Wang et al., 2012). The sensitivity of corpora allata to C-type AST has been linked to the levels of expression of the C-type AST receptor gene in the corpora allata. Reducing the expression of C-type AST receptor gene in the corpora allata of D. melanogaster, resulted in an increase in MF and JH biosynthesis (Wang et al., 2012). The mandibular organ in crustaceans is homologous to corpora allata in insects and can produce MF (Homola and Chang, 1997). MF is the precursor of JH, and its physiological effect in crustaceans is similar to JH in insects (Homola and Chang, 1997; Sin et al., 2015). However, the potential function of C-type AST in the regulation of MF biosynthesis has not been yet studied in any crustacean species. In S. paramamosian, it was found that ScypaAST-CR was expressed in the mandibular organ, indicating that C-type ASTs might inhibit the biosynthesis of MF in mandibular organs (Liu et al., 2019). This was supported by our in vitro results, which indicated that ScypaAST-C and ScypaAST-CCC significantly reduced the abundance of MF biosynthetic enzyme genes in the mandibular organs (Figure 8). It was also discovered that ScypaAST-C injection had no effect on the expression of MF biosynthetic enzyme genes in mandibular organs, which is similar to the result observed in A. aegypti (Nouzova et al., 2015). Thus, the mechanism of ScypaAST-C action on MF biosynthesis in S. paramamosain might be similar to that of AST-C in A. aegypti. It was revealed that the target of AST-C is located before the entry point of acetyl-CoA in the JH biosynthesis pathway, and that AST-C inhibits JH biosynthesis by obstructing the production of cytoplasmic acetyl-CoA (Nouzova et al., 2015). In the in vitro experiment, ScypaAST-C decreased both the levels of thiol and hmgs gene expression, which could be due to a low level of cytoplasmic acetyl-CoA, which is the substrate of thiol and subsequently catalyzed by hmgs (Nagaraju, 2011). Limited studies have explored the functions of AST-CCC, and little is known regarding its physiological roles. Our results showed that injection of ScypaAST-CCC significantly reduced the abundance of hmgs, hmgr, mk, and fpps in mandibular organs, while application of ScypaAST-CCC to the explants of mandibular organ reduced the abundance of ppmd and fpps, indicating that ScypaAST-CCC might inhibit MF biosynthesis in a distinct mode from ScypaAST-C. Unfortunately, due to limited research conditions, in this study, the concentration of MF was not detected. Therefore, we could not define the inhibitory effect of ScypaAST-C and ScypaAST-CCC on MF at the hormone level. Consequently, we hypothesized that ScypaAST-C and ScypaAST-CCC served an inhibitory role in the biosynthesis of MF in S. paramamosain.
In this study, we characterized a cognate C-type AST receptor (ScypaAST-CR) in the mud crab, S. paramamosain, and showed that it can be activated by ScypaAST-C and ScypaAST-CCC. To our knowledge, this is the first time a C-type ASTs receptor has been functionally defined in crustacean species. In addition, we explored a novel role for AST-C in inhibiting ecdysone synthesis in a crustacean and suggested that ScypaAST-C and ScypaAST-CCC play an inhibitory role in regulating MF biosynthesis. In summary, this study provides a new insight into the physiological functions of C-type ASTs in crustaceans. C-type ASTs are short neuropeptides that can be easily synthesized and can thus be applied in the future development of mud crab aquaculture techniques.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
AL contributed to conceptualization, methodology, software, validation, formal analysis, investigation, data curation, visualization, and writing. HY contributed to conceptualization, methodology, validation, data curation, review and editing, supervision, and funding acquisition. WS and DL contributed to investigation. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number: 31972765).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.740251/full#supplementary-material
Footnotes
References
Alasbahi, R. H., and Melzig, M. F. (2012). Forskolin and derivatives as tools for studying the role of cAMP. Pharmazie 67, 5–13. doi: 10.1691/ph.2012.1642
Audsley, N., Vandersmissen, H. P., Weaver, R., Dani, P., Matthews, J., Down, R., et al. (2013). Characterisation and tissue distribution of the PISCF allatostatin receptor in the red flour beetle, Tribolium castaneum. Insect. Biochem. Mol. Biol. 43, 65–74. doi: 10.1016/j.ibmb.2012.09.007
Bachtel, N. D., Hovsepian, G. A., Nixon, D. F., and Eleftherianos, I. (2018). Allatostatin C modulates nociception and immunity in Drosophila. Sci. Rep. 8:7501. doi: 10.1038/s41598-018-25855-1
Bao, C. C., Yang, Y. N., Huang, H. Y., and Ye, H. H. (2015). Neuropeptides in the cerebral ganglia of the mud crab, Scylla paramamosain: transcriptomic analysis and expression profiles during vitellogenesis. Sci. Rep. 5:17055. doi: 10.1038/srep17055
Christiaens, O., Iga, M., Velarde, R. A., Rougé, P., and Smagghe, G. (2010). Halloween genes and nuclear receptors in ecdysteroid biosynthesis and signalling in the pea aphid. Insect. Mol. Biol. 19, 187–200. doi: 10.1111/j.1365-2583.2009.00957.x
Christie, A. E. (2016). Prediction of Scylla olivacea (Crustacea; Brachyura) peptide hormones using publicly accessible transcriptome shotgun assembly (TSA) sequences. Gen. Comp. Endocrinol. 230, 1–16. doi: 10.1016/j.ygcen.2016.03.008
Christie, A. E. (2020). Identification of putative neuropeptidergic signaling systems in the spiny lobster, Panulirus argus. Invertebr. Neurosci. 20:2. doi: 10.1007/s10158-020-0235-9
Christie, A. E., Miller, A., Fernandez, R., Dickinson, E. S., Jordan, A., Kohn, J., et al. (2018). Non-amidated and amidated members of the C-type allatostatin (AST-C) family are differentially distributed in the stomatogastric nervous system of the American lobster, Homarus americanus. Invertebr. Neurosci. 18:2. doi: 10.1007/s10158-018-0206-6
Čižmár, D., Roller, L., Pillerová, M., Sláma, K., and Žitňan, D. (2019). Multiple neuropeptides produced by sex-specific neurons control activity of the male accessory glands and gonoducts in the silkworm Bombyx mori. Sci. Rep. 9, 1–13. doi: 10.1038/s41598-019-38761-x
Diaz, M. M., Schlichting, M., Abruzzi, K. C., Long, X., and Rosbash, M. (2019). Allatostatin-C/AstC-R2 is a novel pathway to modulate the circadian activity pattern in Drosophila. Curr. Biol. 29, 13–22. doi: 10.1016/j.cub.2018.11.005
Dickinson, P. S., Armstrong, M. K., Dickinson, E. S., Fernandez, R., Miller, A., Pong, S., et al. (2018). Three members of a peptide family are differentially distributed and elicit differential state-dependent responses in a pattern generator-effector system. J. Neurophysiol. 119, 1767–1781. doi: 10.1152/jn.00850.2017
Dickinson, P. S., Wiwatpanit, T., Gabranski, E. R., Ackerman, R. J., Stevens, J. S., Cashman, C. R., et al. (2009). Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 212, 1140–1152. doi: 10.1242/jeb.028621
Do, R., Kiss, R. S., Gaudet, D., and Engert, J. C. (2009). Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway. Clin. Genet. 75, 19–29. doi: 10.1111/j.1399-0004.2008.01099.x
Gilbert, L. I., Rybczynski, R., and Warren, J. T. (2002). Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47, 883–916. doi: 10.1146/annurev.ento.47.091201.145302
Gong, J., Ye, H. H., Xie, Y. J., Yang, Y. N., Huang, H. Y., Li, S. J., et al. (2015). Ecdysone receptor in the mud crab: a possible role in promoting ovarian development. J. Endocrinol. 224, 273–287. doi: 10.1530/JOE-14-0526
Homola, E., and Chang, E. S. (1997). Methyl farnesoate: crustacean juvenile hormone in search of functions. Comp. Biochem. Physiol. B Biochem. Mol. Biol 117, 347–356.
Hyde, C. J., Elizur, A., and Ventura, T. (2019). The crustacean ecdysone cassette: a gatekeeper for molt and metamorphosis. J. Steroid Biochem. Mol. Biol. 185, 172–183.
Kramer, S. J., Toschi, A., Miller, C. A., Kataoka, H., Quistad, G. B., Li, J. P., et al. (1991). Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc. Natl. Acad. Sci. U.S.A. 88, 9458–9462. doi: 10.1073/pnas.88.21.9458
Kreienkamp, H. J., Larusson, H. J., Witte, I., Roeder, T., Birgül, N., Hönck, H. H., et al. (2002). Functional annotation of two orphan G-protein-coupled receptors. Drostar1 and-2, from Drosophila melanogaster and their ligands by reverse pharmacology. J. Biol. Chem. 277, 39937–39943. doi: 10.1074/jbc.M206931200
Liu, A., Liu, F., Shi, W. Y., Huang, H. Y., Wang, G. Z., and Ye, H. H. (2019). C-type allatostatin and its putative receptor from the mud crab serve an inhibitory role in ovarian development. J. Exp. Biol. 222:jeb207985. doi: 10.1242/jeb.207985
Ma, M., Szabo, T. M., Jia, C., Marder, E., and Li, L. (2009). Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides 30, 1660–1668. doi: 10.1016/j.peptides.2009.05.023
Matthews, H. J., Audsley, N., and Weaver, R. J. (2007). Interactions between allatostatins and allatotropin on spontaneous contractions of the foregut of larval Lacanobia oleracea. J. Insect. Physiol. 53, 75–83. doi: 10.1016/j.jinsphys.2006.10.007
Mayoral, J. G., Nouzova, M., Brockhoff, A., Goodwin, M., Hernandez-Martinez, S., Richter, D., et al. (2010). Allatostatin-C receptors in mosquitoes. Peptides 31, 442–450. doi: 10.1016/j.peptides.2009.04.013
Mckinney, D. A., Strand, M. R., and Brown, M. R. (2017). Evaluation of ecdysteroid antisera for a competitive enzyme immunoassay and extraction procedures for the measurement of mosquito ecdysteroids. Gen. Comp. Endocrinol. 253, 60–69. doi: 10.1016/j.ygcen.2017.08.028
Nagaraju, G. P. C. (2011). Reproductive regulators in decapod crustaceans: an overview. J. Exp. Biol. 214, 3–16. doi: 10.1242/jeb.047183
Nakatsuji, T., and Sonobe, H. (2004). Regulation of ecdysteroid secretion from the Y-organ by molt-inhibiting hormone in the American crayfish, Procambarus clarkii. Gen. Comp. Endocrinol. 135, 358–364. doi: 10.1016/j.ygcen.2003.11.001
Nouzova, M., Rivera-Perez, C., and Noriega, F. G. (2015). Allatostatin-C reversibly blocks the transport of citrate out of the mitochondria and inhibits juvenile hormone synthesis in mosquitoes. Insect. Biochem. Mol. Biol. 57, 20–26. doi: 10.1016/j.ibmb.2014.12.003
Oliphant, A., Alexander, J. L., Swain, M. T., Webster, S. G., and Wilcockson, D. C. (2018). Transcriptomic analysis of crustacean neuropeptide signaling during the moult cycle in the green shore crab, Carcinus maenas. BMC Genomics 19, 1–26. doi: 10.1186/s12864-018-5057-3
Şahbaz, B. D., and Lyison, N. B. (2019). Prediction and expression analysis of G protein-coupled receptors in the laboratory stick insect, Carausius morosus. Turk. J. Biol. 43, 77–88. doi: 10.3906/biy-1809-27
Sin, Y. W., Kenny, N. J., Qu, Z., Chan, K. W., Chan, K. W., Cheong, S. P., et al. (2015). Identification of putative ecdysteroid and juvenile hormone pathway genes in the shrimp Neocaridina denticulata. Gen. Comp. Endocrinol. 214, 167–176. doi: 10.1016/j.ygcen.2014.07.018
Soumoff, C., and O’Connor, J. D. (1982). Repression of Y-organ secretory activity by molt inhibiting hormone in the crab Pachygrapsus crassipes. Gen. Comp. Endocrinol. 48, 432–439. doi: 10.1016/0016-6480(82)90178-2
Stay, B., and Tobe, S. S. (2007). The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 52, 277–299. doi: 10.1146/annurev.ento.51.110104.151050
Stemmler, E. A., Bruns, E. A., Cashman, C. R., Dickinson, P. S., and Christie, A. E. (2010). Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta-or C-type allatostatin) from a non-insect. Gen. Comp. Endocrinol. 165, 1–10. doi: 10.1016/j.ygcen.2009.05.010
Tran, N. M., Mykles, D. L., Elizur, A., and Ventura, T. (2019). Characterization of G-protein coupled receptors from the blackback land crab Gecarcinus lateralis Y organ transcriptome over the molt cycle. BMC Genomics 20:74. doi: 10.1186/s12864-018-5363-9
Urlacher, E., Soustelle, L., Parmentier, M. L., Verlinden, H., Gherardi, M. J., Fourmy, D., et al. (2016). Honey bee allatostatins target galanin/somatostatin-like receptors and modulate learning: a conserved function? PLoS One 11:e0146248. doi: 10.1371/journal.pone.0146248
Veenstra, J. A. (2009). Allatostatin C and its paralog allatostatin double C: the arthropod somatostatins. Insect. Biochem. Mol. Biol. 39, 161–170. doi: 10.1016/j.ibmb.2008.10.014
Veenstra, J. A. (2016). Allatostatins C, double C and triple C, the result of a local gene triplication in an ancestral arthropod. Gen. Comp. Endocrinol. 230, 153–157. doi: 10.1016/j.ygcen.2016.04.013
Verlinden, H., Gijbels, M., Lismont, E., Lenaerts, C., Broeck, J. V., and Marchal, E. (2015). The pleiotropic allatoregulatory neuropeptides and their receptors: a mini-review. J. Insect. Physiol. 80, 2–14. doi: 10.1016/j.jinsphys.2015.04.004
VinceCruz-Abeledo, C. C., Solis, K. J., Angeles, A. D., Valdez, J. E. C., Ngo, C. A., and Ablan-Lagman, M. C. (2021). Comparison of morphometric identification of species in juvenile mangrove crabs (Genus Scylla) by automated and local approaches. Aquaculture 531:735917. doi: 10.1016/j.aquaculture.2020.735917
Walton, M. E., Le Vay, L., Lebata, J. H., Binas, J., and Primavera, J. H. (2006). Seasonal abundance, distribution and recruitment of mud crabs (Scylla spp.) in replanted mangroves. Estuar. Coast. Shelf Sci. 66, 493–500. doi: 10.1016/j.ecss.2005.09.015
Wang, C., Zhang, J., Tobe, S. S., and Bendena, W. G. (2012). Defining the contribution of select neuropeptides and their receptors in regulating sesquiterpenoid biosynthesis by Drosophila melanogaster ring gland/corpus allatum through RNAi analysis. Gen. Comp. Endocrinol. 176, 347–353. doi: 10.1016/j.ygcen.2011.12.039
Warren, J. T., and Hetru, C. (1990). Ecdysone biosynthesis: pathways, enzymes, and the early steps problem. Invertebr. Reprod. Dev. 18, 91–99. doi: 10.1080/07924259.1990.9672131
Wiwatpanit, T., Powers, B., and Dickinson, P. S. (2012). Inter-animal variability in the effects of C-type allatostatin on the cardiac neuromuscular system in the lobster Homarus americanus. J. Exp. Biol. 215, 2308–2318. doi: 10.1242/jeb.069989
Yamanaka, N., Hua, Y. J., Roller, L., Spalovská-Valachová, I., Mizoguchi, A., Kataoka, H., et al. (2010). Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proc. Natl. Acad. Sci.U.S.A. 107, 2060–2065. doi: 10.1073/pnas.0907471107
Keywords: mud crab, C-type allatostatin, receptor, ecdysone, methyl farnesoate
Citation: Liu A, Shi W, Lin D and Ye H (2021) A Possible Role of Allatostatin C in Inhibiting Ecdysone Biosynthesis Revealed in the Mud Crab Scylla paramamosain. Front. Mar. Sci. 8:740251. doi: 10.3389/fmars.2021.740251
Received: 12 July 2021; Accepted: 20 August 2021;
Published: 16 September 2021.
Edited by:
Carlo C. Lazado, Norwegian Institute of Food, Fisheries and Aquaculture Research (Nofima), NorwayReviewed by:
Klaus H. Hoffmann, University of Bayreuth, GermanySheila Ons, National University of La Plata, Argentina
Sirinart Techa, National Science and Technology Development Agency (NSTDA), Thailand
Copyright © 2021 Liu, Shi, Lin and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haihui Ye, hhye@jmu.edu.cn
 An Liu1
An Liu1  Haihui Ye
Haihui Ye