Skin Imaging Using Ultrasound Imaging, Optical Coherence Tomography, Confocal Microscopy, and Two-Photon Microscopy in Cutaneous Oncology
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, South Korea
- 2Department of Mechanical Engineering, Pohang University of Science and Technology, Pohang-si, South Korea
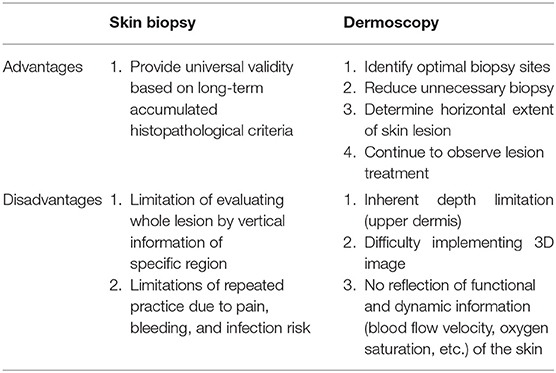
With the recognition of dermoscopy as a new medical technology and its available fee assessment in Korea comes an increased interest in imaging-based dermatological diagnosis. For the dermatologist, who treats benign tumors and malignant skin cancers, imaging-based evaluations can assist with determining the surgical method and future follow-up plans. The identification of the tumor's location and the existence of blood vessels can guide safe treatment and enable the use of minimal incisions. The recent development of high-resolution microscopy based on laser reflection has enabled observation of the skin at the cellular level. Despite the limitation of a shallow imaging depth, non-invasive light-based histopathologic examinations are being investigated as a rapid and pain-free process that would be appreciated by patients and feature reduced time from consultation to treatment. In the United States, the current procedural terminology billing code was established for reflectance confocal microscopy in 2016 and has been used for the skin cancer diagnosis ever since. In this review, we introduce the basic concepts and images of ultrasound imaging, optical coherence tomography, confocal microscopy, and two-photon microscopy and discuss how they can be utilized in the field of dermatological oncology.
Introduction
Efforts to diagnose skin cancer without skin biopsy are ongoing. The diagnoses of patients with suspected skin cancer are confirmed by punch biopsy followed by histopathological examination, which involve the collection of a small portion of the entire lesion to diagnose skin cancer (1). In this case, since only vertical information of a specific region is acquired, dermoscopy can supplement horizontal information of the entire lesion to identify the most suitable biopsy site. However, dermoscopy has an inherent depth limit confined to the upper dermis (Table 1).
To observe lesions deep to the upper dermis, the maximum depth that can be observed with dermoscopy, non-invasive techniques, such as confocal microscopy, multiphoton microscopy, optical coherence tomography, and ultrasound must be used. Although each operation principle is different, they all use the reflection characteristic as if it is mirrored, and the skin's depth and resolution differ among device types (Table 2). Here we briefly discuss each available device and its clinical use in the dermatology field.
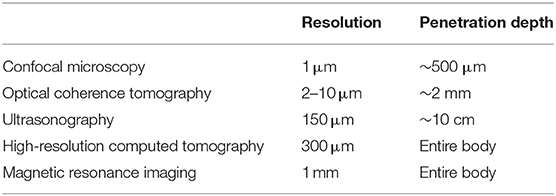
Table 2. Device resolution and imaging depths1.
Ultrasound Imaging
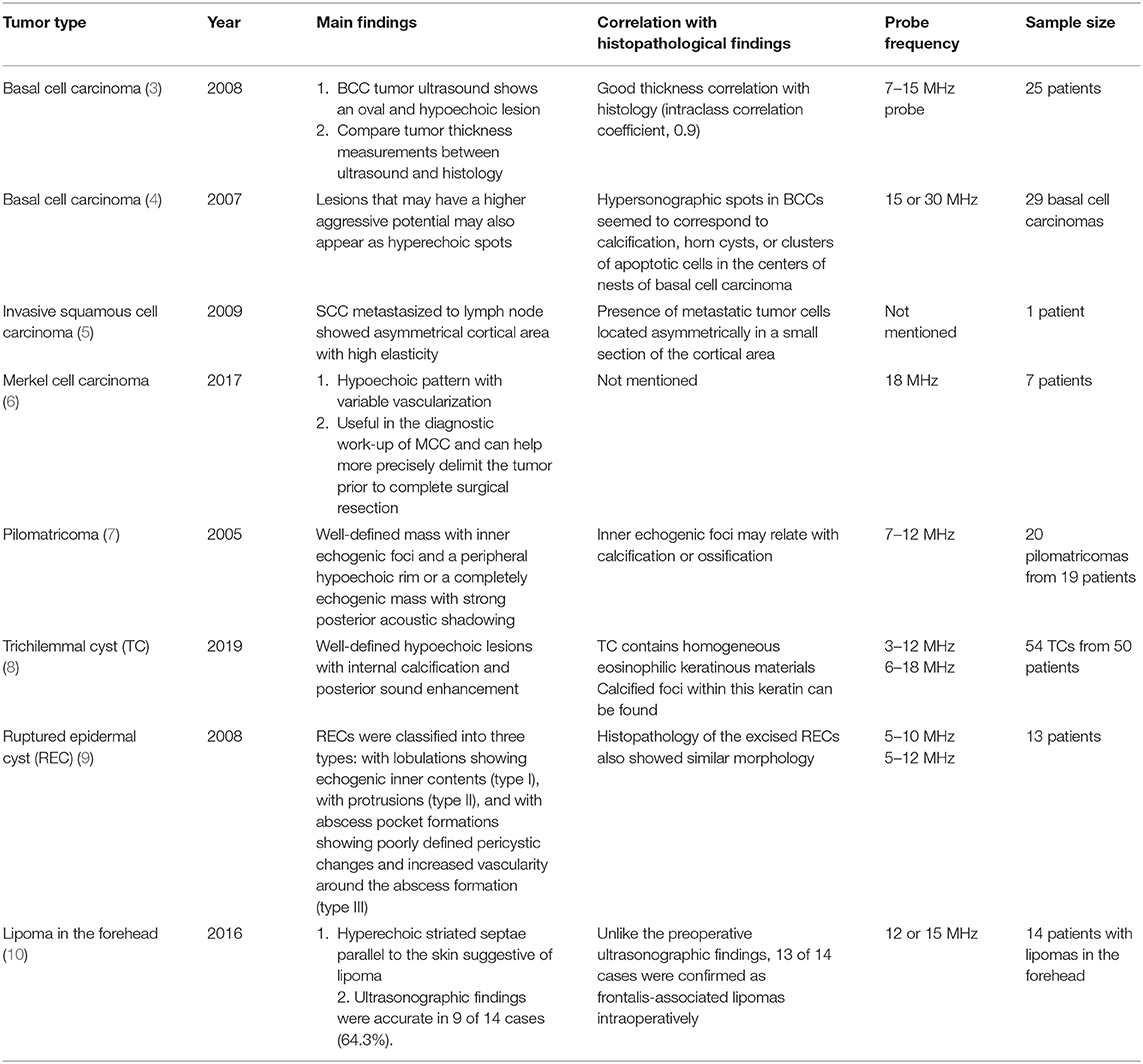
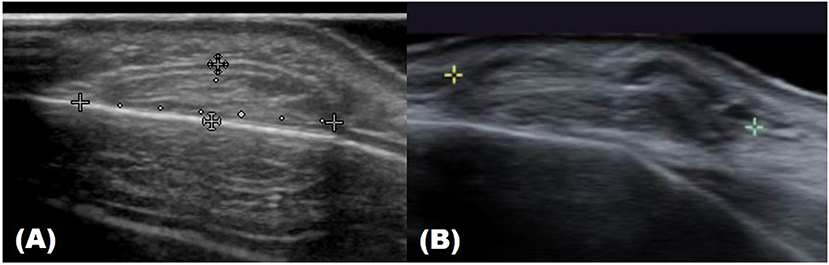
Ultrasound imaging uses high-frequency sound waves that cannot be heard by the human ear. When it is sent inside the human body, the degree of absorption and reflection is cut off depending on the constituents and the reflected sound waves are sensed and imaged (2). Therefore, the probe that sends and detects the sound waves forms the core equipment for ultrasound technology. Higher-frequency (MHz) sound waves enable high-resolution observation of the skin surface, but the observable depth decreases. In the field of dermatology, ultrasound is mainly used to identify benign tumor type and extent (Table 3). Before surgery, it can provide information about tumor type and size, locate the existence of surrounding vessels, identify the best location for the incision, and set the range while viewing the ultrasound screen in real time with the patient. It can also help the clinician evaluate whether the tumor was completely removed after surgery (Figure 1).
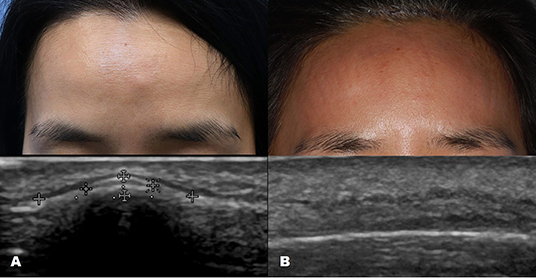
Figure 1. Ultrasound images of forehead osteoma. (A) Before excision. (B) After excision performed through a remote incision above the hairline.
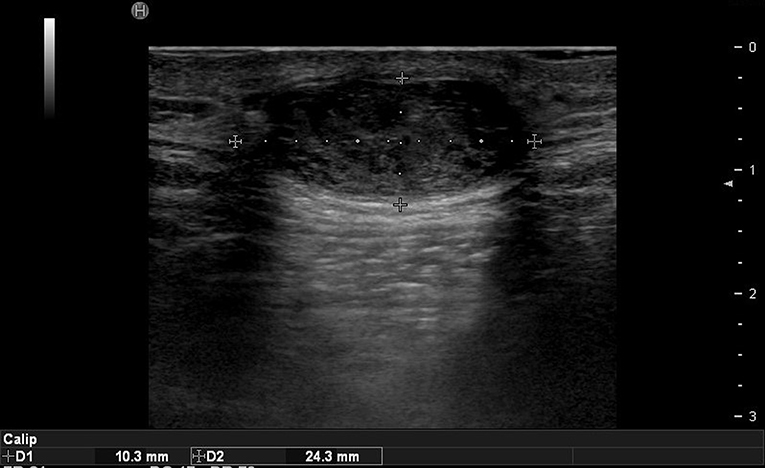
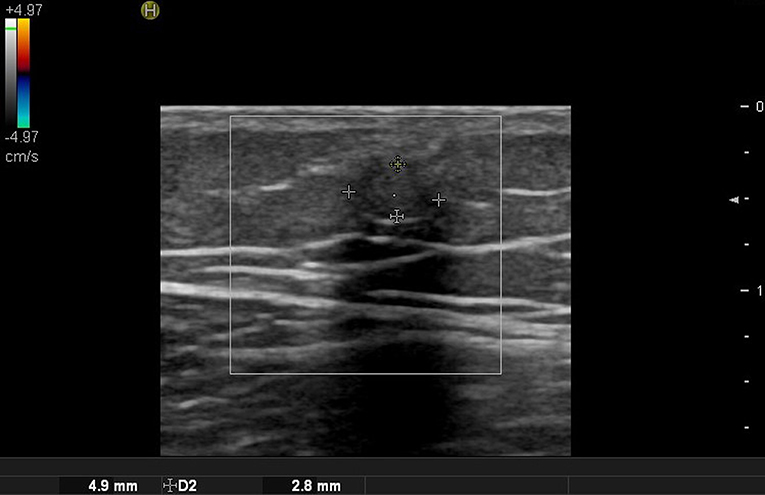
In the case of epidermoid cysts, one of the most common benign tumors, it is often seen as a well-defined ovoid-shaped heterogeneous hypoechoic lesion in the subcutaneous layer with strong posterior acoustic enhancement (Figure 2). Ultrasonographic findings corresponding to epidermal cyst rupture include pericystic changes, increased vascularity, deep abscess formation, and others (9). Trichilemmal cyst, a benign appendage lesion derived from the outer root sheath of the hair follicle, is often seen as a well-defined hypoechoic lesion with internal calcification and posterior sound enhancement (Figure 3) (8). Identifying these sites just prior to surgery and optimizing the incision site and approach can improve the success rate and reduce recurrence rates.
Pilomatricoma, a benign superficial tumor of the hair follicle, is often seen as a well-defined mass with inner echogenic foci and a peripheral hypoechoic rim or a completely echogenic mass with strong posterior acoustic shadowing in the subcutaneous layer on ultrasonography (Figure 4) (7). Pilomatricoma often shows angiographic findings and may be difficult to differentiate from hemangioma.
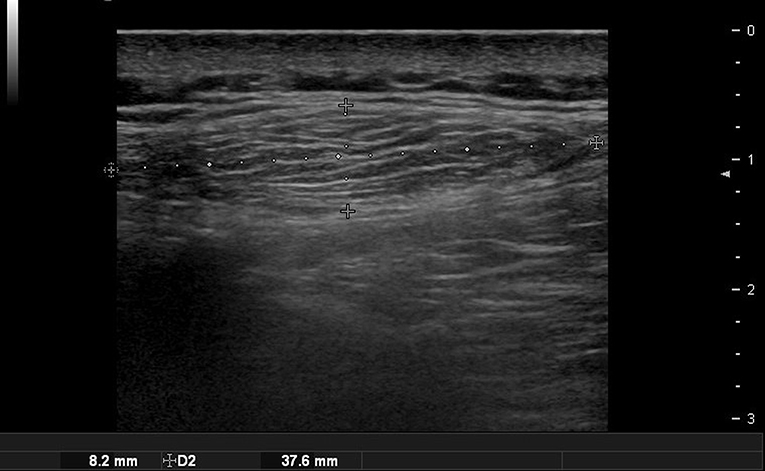
A lipoma appears as a well-defined hypoechoic mass with multiple echogenic strands on ultrasound (Figure 5). If the encapsulation is well-formed, it is easier to remove. Ultrasonography is especially useful for diagnosing and treating lipoma in the forehead. A lipoma occurring in the forehead is often located under the frontalis muscles, and it is important to confirm its precise position using preoperative ultrasonography. It typically has a semispherical shape when located under the muscles and an ovoid shape when it is located in the subcutaneous fat layer (Figure 6) (11). However, this is not always the case, so a comprehensive judgment should be made by checking whether it is close to the periosteum or using a special technique that uses the angulation of the probe to point out the lateral borders of the lesion (12).
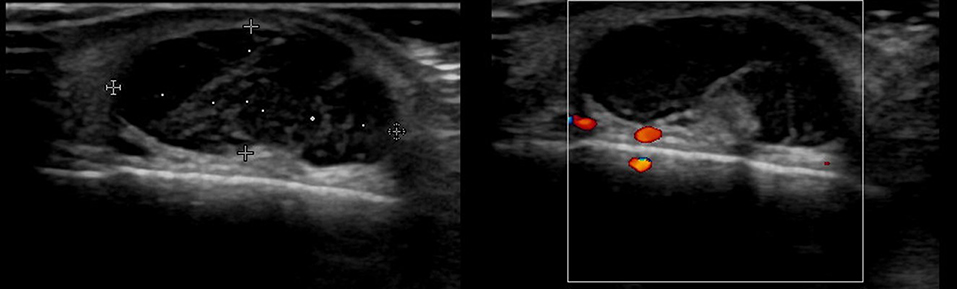
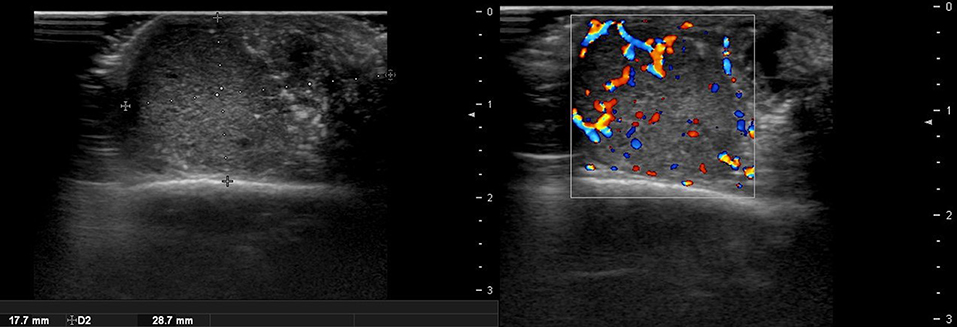
There are no obvious criteria that can diagnose malignant cutaneous tumors using ultrasound imaging. However, tumor size >5 cm, infiltrated margins, rapid clinical growth, moderate to severe intratumoral hypervascularity (Figure 7), and an absence of the typical features of benign tumors are highly suggestive of malignancy (13, 14). High-definition ultrasound with transducers up to 70 MHz, which can observe more detail, has been used to diagnose cutaneous angiosarcoma of the breast and is expected to be useful for the identification of malignant skin cancers (15).
Optical Coherence Tomography
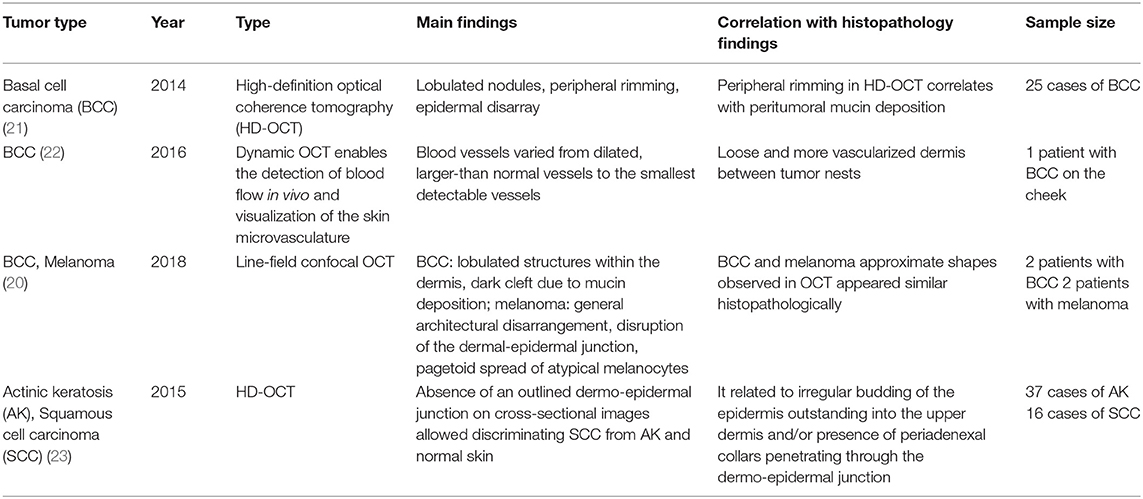
Optical coherence tomography (OCT), a three-dimensional (3D) imaging technique based on low coherence interferometry, creates an image by detecting the interference phenomena from light scattering or reflection as it passes through different layers of skin via the time domain or Fourier-domain method. OCT non-invasively provides skin images similar to the B mode of ultrasound to a depth of 1–2 mm and a resolution of 2–10 μm with high imaging speed. Functional OCT techniques that can provide additional information, such as polarization and vasculature were recently developed and applied for the detection of abnormal vasculature of a port-wine stain or skin cancer (16–18). Our research group developed a device that matches an OCT image with that obtained by dermoscopic imaging and provides more information than dermoscopy alone (19). Through this, we expect to be able to assess the extent of scar treatment (Figure 8). It is expected that a stage of nevus flammeus will be established, and treatment feasibility and degree will be evaluated (Figure 9). The limitations of OCT are limited depth of examination and lack of resolution to observe cancer cell morphology. Line-field confocal OCT, which can reveal comprehensive structural mapping of the skin at the cellular level with an isotropic spatial resolution of ~1 μm to a depth of ~500 μm, was recently reported to correlate with conventional histopathological images of skin tumors (20). Key articles comparing OCT and histopathology are summarized in Table 4.
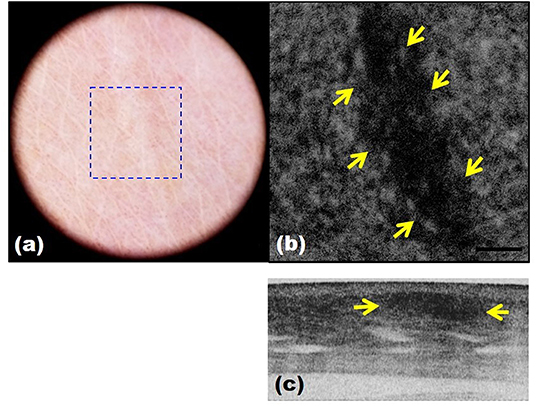
Figure 8. Scar images by dermoscopy-guided multifunctional optical coherence tomography (OCT). (a) Dermoscopic image. (b,c) Intensity OCT showing a dark area and frequent banding pattern due to stronger light scattering and birefringence.
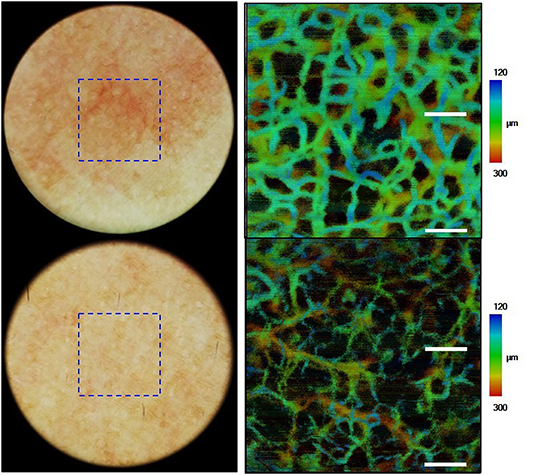
Figure 9. Images of nevus flammeus and normal skin acquired by dermoscopy-guided angiographic optical coherence tomography.
Confocal Microscopy
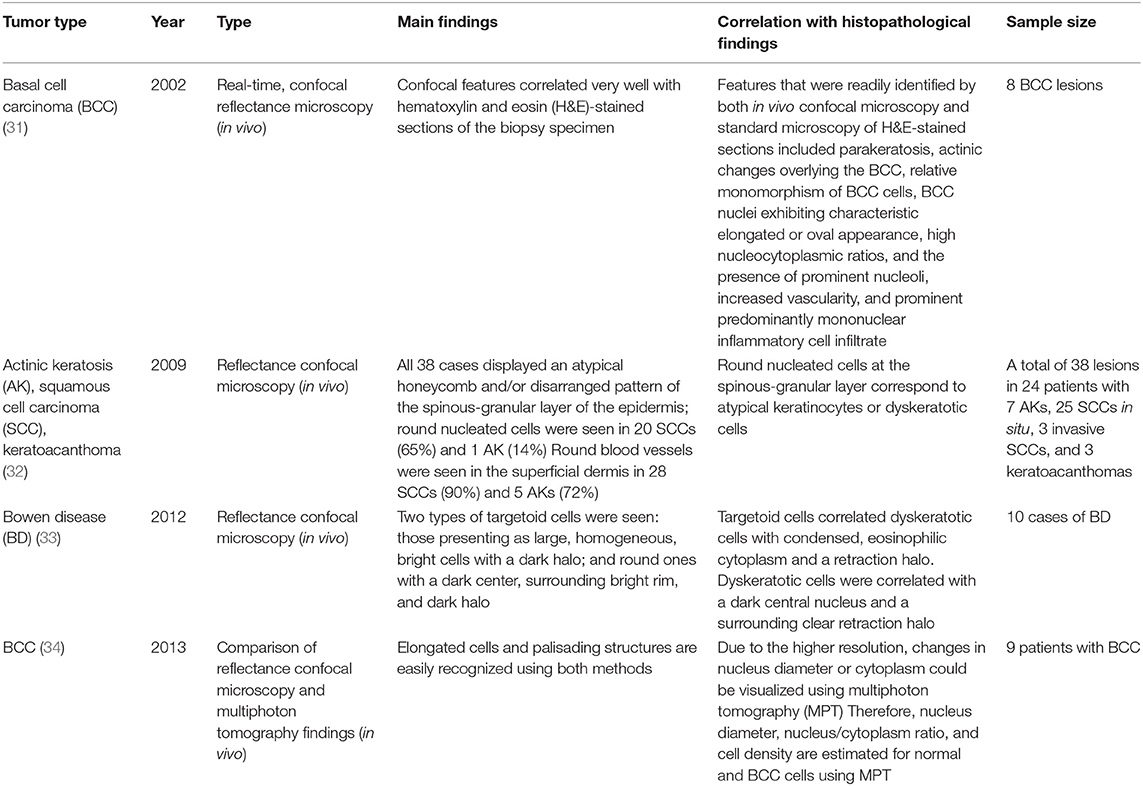
Confocal microscopy is based on the existence of one focal point when a laser, used as a light source, is reflected off a subject. The “out of focus” signal is blocked by a pinhole, and contrast is generated by reflections at the interfaces of tissue and cellular structures due to variations of the index of refraction. Since image acquisition is not possible with a single signal point, imaging occurs by scanning across several pinholes. Imaging up to a depth of 100–200 μm at a 1-μm resolution is possible. Confocal microscopy is capable of providing rapid bedside pathological analysis by producing images with subcellular resolution without skin biopsy and physical sectioning (24–26). There are two ways to use this approach for Mohs surgery. One is used in vivo and can help the identification of the surgical margins in a perioperative setting (27). It is also possible to check the remaining lesion using intraoperative images in vivo after removing the main skin cancer mass (28). The other is for ex vivo use, in which the surgical margins are removed and confocal microscopy is used to confirm whether the tumor remains within it (29). However, when used for detection in Mohs surgery, the grayscale confocal image was difficult to interpret by the surgeons. To improve this, each frozen specimen was stained with acridine orange (pH 6.0) and eosin (pH 6.0) and then scanned with confocal mosaicking microscopy to imitate hematoxylin and eosin-stained Mohs frozen sections. This approach and physician training can improve the accuracy of the non-melanoma skin cancer diagnosis (30). Key articles comparing confocal microscopy and histopathology are summarized in Table 5.
Confocal microscopy has also been applied to diagnose mammary and extramammary Paget's disease (EMPD) (37), frequently showing Paget cells predominantly within the epidermis (38). However, due to the limited depth of imaging (100–200 μm) when applied non-invasively, the invasion site is difficult to determine. A major limitation of this technique is that it can only provide morphological information and does not reflect the tissue's internal structure or functional state.
Two-photon Microscopy
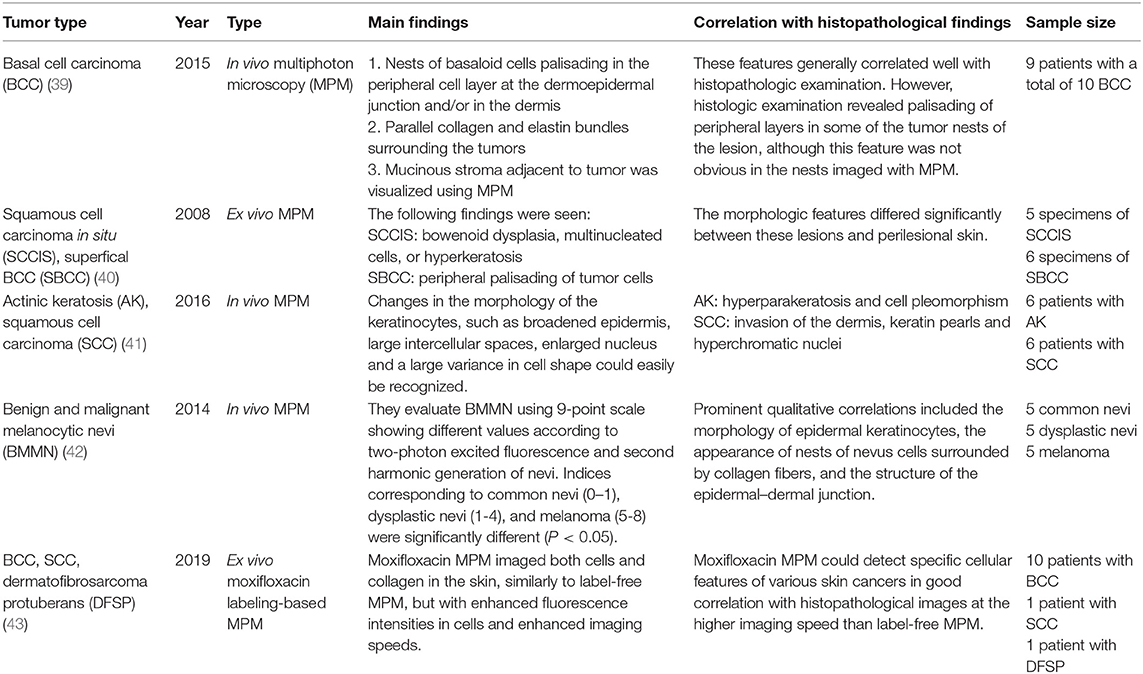
Two-photon microscopy (TPM) is a technique that uses the fluorescence released after excitation from simultaneously absorbing two photons with long wavelengths and low energy. TPM allows observation of vital phenomena in cells and in vivo at the molecular level. In particular, it has the advantage of being able to identify the distribution of collagen within the dermis using the second harmonic generation (SHG) produced when two photons simultaneously interfere. Non-invasive in vivo multi-photon microscopy (MPM) imaging also reportedly provides label-free contrast and reveals several characteristic features of basal cell carcinoma lesions (39). This feature correlates well with histopathological examination, findings, and SHG in particular shows collagen and elastin bundles around the tumor (Figure 10) (Table 6).
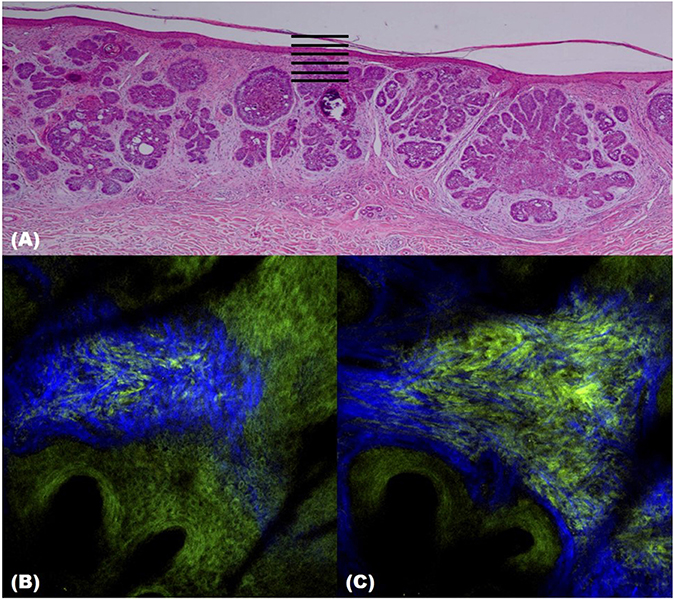
Figure 10. Two-photon microscopy (TPM) images of basal cell carcinoma (BCC). (A) Histopathological finding. (B,C) TPM images showing parallel collagen fibers (blue) surrounding a BCC tumor nest.
However, since TPM and MPM utilize weak endogenous fluorescence in tissue, there is a need for high excitation laser power and extension of pixel duration (44, 45). To overcome this limitation and reduce photodamage, moxifloxacin, an FDA-approved antibiotic, has been reported as a cell-labeling agent for MPM (46). Moxifloxacin has bright intrinsic multi-photon fluorescence, good tissue penetration, and high intracellular concentration. In addition, moxifloxacin-based MPM imaging is 10 times faster than imaging based on endogenous fluorescence (Figure 11) (46).
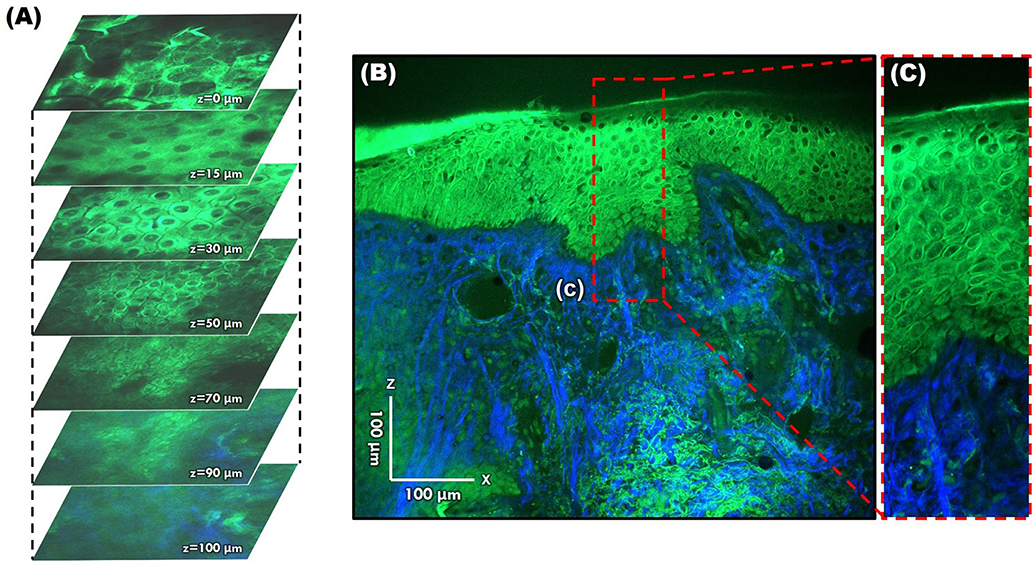
Figure 11. Moxifloxacin-based multi-photon microscopy images of normal skin. (A) En face images at different depths. (B,C) Cross-sectional view of the epidermis and dermis.
Although imaging depth remains a limitation, various methods to achieve a clear and high-resolution image are being developed. It is also expected that the diagnosis rate can be increased by tumor marker labeling. A recent report stated that in patients with EMPD, a subclinical extension can be assessed by MPM using whole-mount immunostaining with anti-cytokeratin 7 antibody to label Paget cells (35). These trials will be used in the ex vivo skin tissue to find the tumor's margins, and it is anticipated that it may replace frozen sections in the future. For more generalized clinical applications, the cost of the equipment is the greatest hinderance. MPM equipment is expensive because it uses a femtosecond laser (36).
Conclusion
In addition to ultrasonic devices that can closely observe the skin and deep structures, the development of dermatological equipment that unites laser and optical technology has shown visible progress. The principle of these devices is to analyze signals reflected or scattered from the skin, and there is a fundamental limitation that it is evaluated by looking into the mirror. These limitations are expected to improve in the near future by the development of fluorescent probes targeting tumors or diseases and will be used more actively for the diagnosis and treatment of skin lesions.
For dermatologists, this is a good opportunity to strengthen the specialty of dermatology. We are already familiar with laser equipment and have demonstrated a correlation between clinical and histopathological findings. When we use imaging equipment to further investigate a patient's skin and present objectively explainable data by linking “clinical imaging–histopathological findings,” a more robust doctor–patient relationship can be established.
Author Contributions
BO conceived the concept and wrote the manuscript. KK co-conceived the concept and drafted the figures and tables. KC co-conceived the concept and edited and improved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
OCT, optical coherence tomography; TPM, two-photon microscopy; EMPD, extramammary Paget's disease; SHG, second harmonic generation; MPM, multi-photon microscopy; BCC, basal cell carcinoma; SCC, squamous cell carcinoma; AK, actinic keratosis.
Footnotes
1. ^Available online at: http://obel.ee.uwa.edu.au/research/fundamentals/introduction-oct/
References
1. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. (2016) 74:1–16; quiz 7–8. doi: 10.1016/j.jaad.2015.06.033
2. Kleinerman R, Whang TB, Bard RL, Marmur ES. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. (2012) 67:478–87. doi: 10.1016/j.jaad.2011.12.016
3. Bobadilla F, Wortsman X, Munoz C, Segovia L, Espinoza M, Jemec GB. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging. (2008) 8:163–72. doi: 10.1102/1470-7330.2008.0026
4. Uhara H, Hayashi K, Koga H, Saida T. Multiple hypersonographic spots in basal cell carcinoma. Dermatol Surg. (2007) 33:1215–9. doi: 10.1097/00042728-200710000-00009
5. Aoyagi S, Izumi K, Hata H, Kawasaki H, Shimizu H. Usefulness of real-time tissue elastography for detecting lymph-node metastases in squamous cell carcinoma. Clin Exp Dermatol. (2009) 34:e744–7. doi: 10.1111/j.1365-2230.2009.03468.x
6. Hernandez-Aragues I, Vazquez-Osorio I, Alfageme F, Ciudad-Blanco C, Casas-Fernandez L, Rodriguez-Blanco MI, et al. Skin ultrasound features of Merkel cell carcinoma. J Eur Acad Dermatol Venereol. (2017) 31:e315–8. doi: 10.1111/jdv.14102
7. Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med. (2005) 24:1397–402. doi: 10.7863/jum.2005.24.10.1397
8. He P, Cui LG, Wang JR, Zhao B, Chen W, Xu Y. Trichilemmal Cyst: clinical and sonographic features. J Ultrasound Med. (2019) 38:91–6. doi: 10.1002/jum.14666
9. Jin W, Ryu KN, Kim GY, Kim HC, Lee JH, Park JS. Sonographic findings of ruptured epidermal inclusion cysts in superficial soft tissue: emphasis on shapes, pericystic changes, and pericystic vascularity. J Ultrasound Med. (2008) 27:171–6; quiz 7–8. doi: 10.7863/jum.2008.27.2.171
10. Huh JW, Kim MS, Choi KH, Park HJ, Jue MS. The accuracy of ultrasonography on the location of lipomas in the forehead. Dermatol Surg. (2016) 42:191–4. doi: 10.1097/DSS.0000000000000598
11. Oh BH, Seo J, Chung KY. Surgical treatment of 846 patients with benign skin tumors: experience of a dermatologic surgeon in Korea. Korean J Dermatol. (2015) 53:202–8. Available online at: https://www.koreamed.org/article/0048KJD/2015.53.3.202
12. Wortsman X. The accuracy of ultrasonography on location of lipomas in forehead. Dermatol Surg. (2017) 43:158–9. doi: 10.1097/DSS.0000000000000835
13. Chiou HJ, Chou YH, Chiu SY, Wang HK, Chen WM, Chen TH, et al. Differentiation of benign and malignant superficial soft-tissue masses using grayscale and color doppler ultrasonography. J Chin Med Assoc. (2009) 72:307–15. doi: 10.1016/S1726-4901(09)70377-6
14. Hung EH, Griffith JF, Ng AW, Lee RK, Lau DT, Leung JC. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR Am J Roentgenol. (2014) 202:W532–40. doi: 10.2214/AJR.13.11457
15. Perrot JL, Habougit C, Biron Schneider AC, Couzan C, Tognetti L, Rubegni P, et al. Role of reflectance confocal microscopy and HD ultrasound in the diagnosis of cutaneous angiosarcoma of the breast. Ann Dermatol Venereol. (2019) 146:410–3. doi: 10.1016/j.annder.2018.12.008
16. Zhao S, Gu Y, Xue P, Guo J, Shen T, Wang T, et al. Imaging port wine stains by fiber optical coherence tomography. J Biomed Opt. (2010) 15:036020. doi: 10.1117/1.3445712
17. Mogensen M, Joergensen TM, Nurnberg BM, Morsy HA, Thomsen JB, Thrane L, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists and pathologists. Dermatol Surg. (2009) 35:965–72. doi: 10.1111/j.1524-4725.2009.01164.x
18. Gambichler T, Plura I, Schmid-Wendtner M, Valavanis K, Kulichova D, Stucker M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. (2015) 8:681–6. doi: 10.1002/jbio.201400085
19. Kwon S, Yoon Y, Kim B, Jang WH, Oh B, Chung KY, et al. Dermoscopy guided dark-field multi-functional optical coherence tomography. Biomed Opt Express. (2017) 8:1372–81. doi: 10.1364/BOE.8.001372
20. Dubois A, Levecq O, Azimani H, Siret D, Barut A, Suppa M, et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J Biomed Opt. (2018) 23:1–9. doi: 10.1117/1.JBO.23.10.106007
21. Gambichler T, Plura I, Kampilafkos P, Valavanis K, Sand M, Bechara FG, et al. Histopathological correlates of basal cell carcinoma in the slice and en face imaging modes of high-definition optical coherence tomography. Br J Dermatol. (2014) 170:1358–61. doi: 10.1111/bjd.12797
22. Ulrich M, Themstrup L, de Carvalho N, Manfredi M, Grana C, Ciardo S, et al. Dynamic optical coherence tomography in dermatology. Dermatology. (2016) 232:298–311. doi: 10.1159/000444706
23. Boone MA, Marneffe A, Suppa M, Miyamoto M, Alarcon I, Hofmann-Wellenhof R, et al. High-definition optical coherence tomography algorithm for the discrimination of actinic keratosis from normal skin and from squamous cell carcinoma. J Eur Acad Dermatol Venereol. (2015) 29:1606–15. doi: 10.1111/jdv.12954
24. Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. (1995) 104:946–52. doi: 10.1111/1523-1747.ep12606215
25. Ulrich M, Lange-Asschenfeldt S. In vivo confocal microscopy in dermatology: from research to clinical application. J Biomed Opt. (2013) 18:061212. doi: 10.1117/1.JBO.18.6.061212
26. New K, Petroll WM, Boyde A, Martin L, Corcuff P, Leveque J, et al. In vivo imaging of human teeth and skin using real-time confocal microscopy. J Scan Microsc. (1991) 13:369–72. doi: 10.1002/sca.4950130507
27. Couty E, Tognetti L, Labeille B, Douchet C, Habougit C, Couzan C, et al. In vivo reflectance confocal microscopy combined with the ‘spaghetti technique' for the identification of surgical margins of lentigo maligna: experience in 70 patients. J Eur Acad Dermatol Venereol. (2018) 32:e366–8. doi: 10.1111/jdv.14947
28. Flores ES, Cordova M, Kose K, Phillips W, Rossi A, Nehal K, et al. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: initial clinical experience. J Biomed Opt. (2015) 20:61103. doi: 10.1117/1.JBO.20.6.061103
29. Cinotti E, Perrot JL, Labeille B, Cambazard F, Rubegni P. Ex vivo confocal microscopy: an emerging technique in dermatology. Dermatol Pract Concept. (2018) 8:109–19. doi: 10.5826/dpc.0802a08
30. Mu EW, Lewin JM, Stevenson ML, Meehan SA, Carucci JA, Gareau DS. Use of digitally stained multimodal confocal mosaic images to screen for nonmelanoma skin cancer. JAMA Dermatol. (2016) 152:1335–41. doi: 10.1001/jamadermatol.2016.2997
31. Gonzalez S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. (2002) 47:869–74. doi: 10.1067/mjd.2002.124690
32. Rishpon A, Kim N, Scope A, Porges L, Oliviero MC, Braun RP, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. (2009) 145:766–72. doi: 10.1001/archdermatol.2009.134
33. Ulrich M, Kanitakis J, Gonzalez S, Lange-Asschenfeldt S, Stockfleth E, Roewert-Huber J. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. (2012) 166:451–3. doi: 10.1111/j.1365-2133.2011.10563.x
34. Ulrich M, Klemp M, Darvin ME, Konig K, Lademann J, Meinke MC. In vivo detection of basal cell carcinoma: comparison of a reflectance confocal microscope and a multiphoton tomograph. J Biomed Opt. (2013) 18:61229. doi: 10.1117/1.JBO.18.6.061229
35. Murata T, Honda T, Egawa G, Kitoh A, Dainichi T, Otsuka A, et al. Three-dimensional evaluation of subclinical extension of extramammary Paget disease: visualization of the histological border and its comparison to the clinical border. Br J Dermatol. (2017) 177:229–37. doi: 10.1111/bjd.15282
36. Tkaczyk E. Innovations and developments in dermatologic non-invasive optical imaging and potential clinical applications. Acta Derm Venereol. (2017) 218:5–13. doi: 10.2340/00015555-2717
37. Cinotti E, Galluccio D, Tognetti L, Habougit C, Manganoni AM, Venturini M, et al. Nipple and areola lesions: review of dermoscopy and reflectance confocal microscopy features. J Eur Acad Dermatol Venereol. (2019) 33:1837–46. doi: 10.1111/jdv.15727
38. Gonzalez S, Sanchez V, Gonzalez-Rodriguez A, Parrado C, Ullrich M. Confocal microscopy patterns in nonmelanoma skin cancer and clinical applications. Actas Dermosifiliogr. (2014) 105:446–58. doi: 10.1016/j.adengl.2014.04.007
39. Balu M, Zachary CB, Harris RM, Krasieva TB, Konig K, Tromberg BJ, et al. In vivo multiphoton microscopy of basal cell carcinoma. JAMA Dermatol. (2015) 151:1068–74. doi: 10.1001/jamadermatol.2015.0453
40. Paoli J, Smedh M, Wennberg AM, Ericson MB. Multiphoton laser scanning microscopy on non-melanoma skin cancer: morphologic features for future non-invasive diagnostics. J Invest Dermatol. (2008) 128:1248–55. doi: 10.1038/sj.jid.5701139
41. Klemp M, Meinke MC, Weinigel M, Rowert-Huber HJ, Konig K, Ulrich M, et al. Comparison of morphologic criteria for actinic keratosis and squamous cell carcinoma using in vivo multiphoton tomography. Exp Dermatol. (2016) 25:218–22. doi: 10.1111/exd.12912
42. Balu M, Kelly KM, Zachary CB, Harris RM, Krasieva TB, Konig K, et al. Distinguishing between benign and malignant melanocytic nevi by in vivo multiphoton microscopy. Cancer Res. (2014) 74:2688–97. doi: 10.1158/0008-5472.CAN-13-2582
43. Chang H, Jang WH, Lee S, Kim B, Kim MJ, Kim WO, et al. Moxifloxacin labeling-based multiphoton microscopy of skin cancers in Asians. Lasers Surg Med. (2019). doi: 10.1002/lsm.23138. [Epub ahead of print].
44. Thomas G, van Voskuilen J, Gerritsen HC, Sterenborg HJ. Advances and challenges in label-free nonlinear optical imaging using two-photon excitation fluorescence and second harmonic generation for cancer research. J Photochem Photobiol B. (2014) 141:128–38. doi: 10.1016/j.jphotobiol.2014.08.025
45. Dela Cruz JM, McMullen JD, Williams RM, Zipfel WR. Feasibility of using multiphoton excited tissue autofluorescence for in vivo human histopathology. Biomed Opt Express. (2010) 1:1320–30. doi: 10.1364/BOE.1.001320
Keywords: skin imaging, skin cancer, benign skin tumor, ultrasound, optical coherence tomography, confocal microscopy, two-photon microscopy
Citation: Oh BH, Kim KH and Chung KY (2019) Skin Imaging Using Ultrasound Imaging, Optical Coherence Tomography, Confocal Microscopy, and Two-Photon Microscopy in Cutaneous Oncology. Front. Med. 6:274. doi: 10.3389/fmed.2019.00274
Received: 08 July 2019; Accepted: 11 November 2019;
Published: 22 November 2019.
Edited by:
Yasuhiro Fujisawa, University of Tsukuba, JapanReviewed by:
Hassanin Al-Aasam, Luebeck University of Applied Sciences, GermanyRyota Tanaka, University of Tsukuba, Japan
Copyright © 2019 Oh, Kim and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kee Yang Chung, kychung@yuhs.ac
 Byung Ho Oh
Byung Ho Oh Ki Hean Kim
Ki Hean Kim Kee Yang Chung1*
Kee Yang Chung1*