Genetic Susceptibility to Antisynthetase Syndrome Associated With Single-Nucleotide Variants in the IL1B Gene That Lead Variation in IL-1β Serum Levels
- 1HLA Laboratory, Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas, Mexico City, Mexico
- 2Unidad de Medicina Experimental, Facultad de Medicina, Universidad Nacional Autónoma de México, Mexico City, Mexico
- 3Interstitial Lung Disease and Rheumatology Unit, Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas, Mexico City, Mexico
- 4Translational Research Laboratory on Aging and Pulmonary Fibrosis, Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas, Mexico City, Mexico
The antisynthetase syndrome (ASSD) is an autoimmune disorder characterized by myositis, arthritis, mechanic's hands, fever, Raynaud phenomenon, and interstitial lung disease (ILD). We aimed to evaluate single-nucleotide polymorphisms in the interleukin 1B (IL1B) gene and their association between ILD with antisynthetase autoantibodies, as well as IL-1β serum levels. The most frequent antisynthetase autoantibody was anti-Jo1. The most frequent tomographic pattern was non-specific interstitial pneumonia, whereas in the anti-Jo1 subjects, it was organized pneumonia. Anti-Jo1 patients tend to have more significant arthritis, and Raynaud phenomenon have higher levels of creatinine phosphokinase. In the IL1B gene, the GG genotype and G allele of rs1143634 [odds ratio (OR) = 2.21 and OR = 2.60, respectively, p < 0.05] are associated with an increased risk, as well as with the dominant and recessive models (p < 0.05). This finding is maintained after logistic regression analysis adjusting for potential confounding variables (p < 0.05). Subjects with the rs16944/AG heterozygous genotype had higher serum levels of IL-1β compared to homozygous (p < 0.05). In conclusion, rs1143634 is associated with a higher risk of ASSD. Also, the GA genotype is associated with higher levels of IL-1β in ASSD patients.
Introduction
The antisynthetase syndrome (ASSD) is an autoimmune disorder characterized by diverse clinical manifestations, including myositis, arthritis, mechanic's hands, fever, Raynaud phenomenon, and interstitial lung disease (ILD), as well as the presence of aminoacyl-transfer RNA synthase (ARS) autoantibodies (1, 2). The ASSD was firstly associated with idiopathic inflammatory myopathies (IIMs); however, previously, it has been described that many of these patients may have only slight myositis clinical manifestations and not fulfill with the Bohan and Peter criteria for an inflammatory myopathy (3–5).
The ARS autoantibodies include anti-Jo1 (anti-histidyl), anti-EJ (anti-glycyl), anti-OJ (anti-isoleucyl), anti-PL7 (anti-threonyl), anti-PL12 (anti-alanyl), anti-SC (anti-lysil), anti-KS (anti-asparaginyl), anti-JS (anti-glutaminyl), anti-Ha or anti-YRS (anti-threonyl), anti-tryptophanyl, and anti-Zo (anti-phenylalanyl), with anti-Jo1 being the most common (6). Differences have been described according to the different ARS autoantibodies. For example, PL7 and PL12 are associated with early and severe ILD (7, 8). Also, anti-Jo1 is associated with more muscle involvement and better prognosis (9, 10).
However, despite the clinical characteristics of ASSD have been widely described and are a topic of intensive research, little is known about this nosological entity's genetic background. Previous reports of single-nucleotide polymorphisms (SNPs) in proinflammatory genes associated with IIM, such as PTPN22, PLCL1, and TNF, have been described (11–13). However, despite that interleukin 1β (IL-1β) has been associated with other autoimmune disorders such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), the association between SNPs in the IL1B gene and IIM has been poorly described (14, 15). There is only one previous report of IL1RN VNTR polymorphism associated with dermatomyositis (DM) in a Bulgarian population (16). On the other hand, studies related to ILD with ARS autoantibodies are relatively scarce. We aimed to evaluate SNPs in IL1B, IL-1β serum levels, and their association between ILD with ARS autoantibodies.
Materials and Methods
Subjects Included
Case Group
One hundred fifty-four patients with ASSD diagnosis were included. All of them were evaluated and managed in the Interstitial Lung Disease and Rheumatology Unit (ILD&RU) at the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas (INER) in México City. Patients in the ILD&RU are evaluated by a multidisciplinary group (pulmonologists, radiologists, and rheumatologists). Included patients were ≥18 years old, born as Mexican mestizos (MMs), no biological relation among themselves or with the patients or controls, with the diagnosis of ILD confirmed by high-resolution computed tomography (HRCT) and be positive to at least one of the following autoantibodies: anti-Jo1, anti-PL7, anti-PL12, and anti-Ej (subjects positive only for anti-OJ antibody were excluded). According to the manufacturer's instructions, all autoantibodies were measured by the Myositis Profile 3 immunoblot 16 strips EUROLINE panel (EUROIMMUN AG, Lübeck, Germany). Also, we included baseline pulmonary function tests, such as single-breath carbon monoxide diffusing capacity (DLco) and spirometry. Furthermore, baseline serum creatinine phosphokinase (CPK) levels were recorded, as well as the history of Raynaud phenomenon, arthritis, mechanic's hands, fever, and smoking history. Patients were evaluated between January 2008 and January 2019. Besides, the case group was divided into anti-Jo1 and non–anti-Jo1 patients for further analyses.
Control Group
A group of five hundred six healthy volunteer subjects (HS) was also included. These subjects had the following characteristics: clinically healthy (with neither chronic nor acute diseases self-reported), ≥18 years old, men and women, born as MMs (no biological relation among themselves or with the patients or controls), and no history of family pulmonary or inflammatory/autoimmune diseases. All subjects had at least three generations born in Mexico (parents and grandparents) and were considered MMs. We have previously demonstrated that this criterion is a good proxy of Mexican ancestry evaluated by ancestry-informative markers (17). All participants underwent a background questionnaire of inherited pathologies, excluding subjects who reported suffering from some lung and chronic inflammatory disease.
Ethics Approval and Informed Consent
The Institutional Committees for Research, Ethics in Research, and Biosecurity of the INER approved this study (approval code numbers: C08-17, B11-19). All participants were previously invited to participate in the protocol; they signed a written informed consent document and provided with a privacy statement describing personal data protection.
All experiments were performed following the relevant guidelines and regulations. The STREGA (STrengthening the REporting of Genetic Association) guidelines were considered to design this genetic association study (18).
DNA Extraction
The DNA was extracted from peripheral blood cells via venipuncture using EDTA tubes from all 668 subjects' using the commercial BDtract Genomic DNA isolation kit (Maxim Biotech, San Francisco, CA, USA). The DNA was quantified by UV absorption spectrophotometry at the 260-nm wavelength employing a NanoDrop 2000 device (Thermo Scientific, Wilmington, DE, USA). Contamination with organic compounds and proteins was determined by measuring the ratio absorbance at 280 and 260 nm. Samples were considered of good quality when the ratio was ~1.8.
SNP Selection
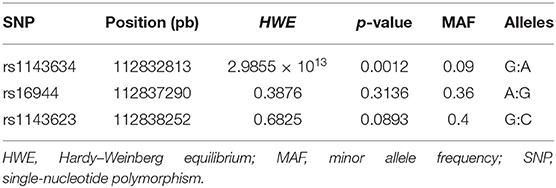
SNPs were selected based on a bibliographic search in PubMed (NCBI), identifying polymorphisms in IL1B previously associated with IIM and other autoimmune diseases, such as RA and SLE. Additionally, according to the HapMap project, we considered a minor allelic frequency (MAF) higher than 5% in the Mexican population in Los Angeles. Table 1 summarizes the principal characteristics of the evaluated SNPs.
SNP Genotyping
The SNPs' allele discrimination was performed using commercial TaqMan probes (Applied Biosystems, San Francisco, CA, USA) at a concentration of 20 × in total subjects included. SNPs evaluated were rs1143634, rs16944, and rs1143623 in the IL1B gene, using quantitative polymerase chain reaction (qPCR) in a 7300 Real-Time PCR System (Applied Biosystems/Thermo Fisher Scientific Inc., Singapore), and the analysis performed by sequence detection software version 1.4 software (Applied Biosystems, CA, USA). Also, three controls without template (contamination controls) included each genotyping plate, and 5% of the genotyped in duplicate as controls for allele assignment.
Besides, to determine the haplotype structure in the IL1B gene associated with ASSD susceptibility, we applied Haploview software version 4.2.
Measurement of IL-1β Serum Levels
Once performing the association analyses, we selected sera samples from 62 ASSD patients, carrying genotypes of the three SNPs evaluated. Sample selection was performed to identify representability for each genotype from all SNPs. For the rs1143634, we selected 58 with GG and 4 with GA genotypes. For the rs16944 group, we included 33 with AA, 25 with AG, and 4 with GG genotypes. For the rs1143623, we chose 5 with CC, 28 with CG, and 29 with GG genotypes. Differences in the patients' number, according to genotypes, are due to serum samples availability and the genotype frequencies. The serum levels were measured by the enzyme-linked immunosorbent assay (ELISA) technique; the detectable range of IL-1β was 14.06–900 pg/mL (human IL-1 beta ELISA Kit, ab214025, Abcam Plc, Cambridge, UK). A total of 50 μL of serum for each sample was centrifuged and prepared for analysis, following the manufacturer's protocol.
Statistical Analysis
The differences between groups were assessed by determining and comparing the allele and genotype frequencies. The statistical significance was assessed using SPSS v20.0 (SPSS Inc., Chicago, IL, USA) and Epi Info 7.1.4.0 statistical software (19). The allele and genotype frequencies between groups were analyzed using the χ2-test. The results were considered to be significant when p < 0.05; similarly, the odds ratios (ORs) with 95% confidence intervals (CIs) were estimated to determine the strength of the association. Comparisons made between ASSD and HS are shown. Also, the ASSD patients are divided into anti-Jo1+ and non–anti-Jo1.
A logistic regression analysis was performed to adjust for potential confounding variables [sex, age, and body mass index (BMI)] using Plink v. 1.07.
Results
Demographic Variables in Case and Control Groups
One hundred fifty-four patients with at least one ARS autoantibody and ILD diagnosed by HRCT were included, and 506 healthy subjects as the control group. The ASSD group was older and predominantly women compared with the HS group (p < 0.05). There were no significant differences in BMI.
Furthermore, 37% of the ASSD patients are smokers with 21 years of smoking, 5 cigarettes per day, and 6 pack-years' history. The most frequent clinical manifestation was arthritis (70.13%), followed by mechanic's hands, fever, and Raynaud phenomenon. Also, the most frequent ARS autoantibody was anti-Jo1 (43.51%), and the most frequent HRCT pattern was non-specific interstitial pneumonia (NSIP) (45.70%). Table 2 shows the complete results.
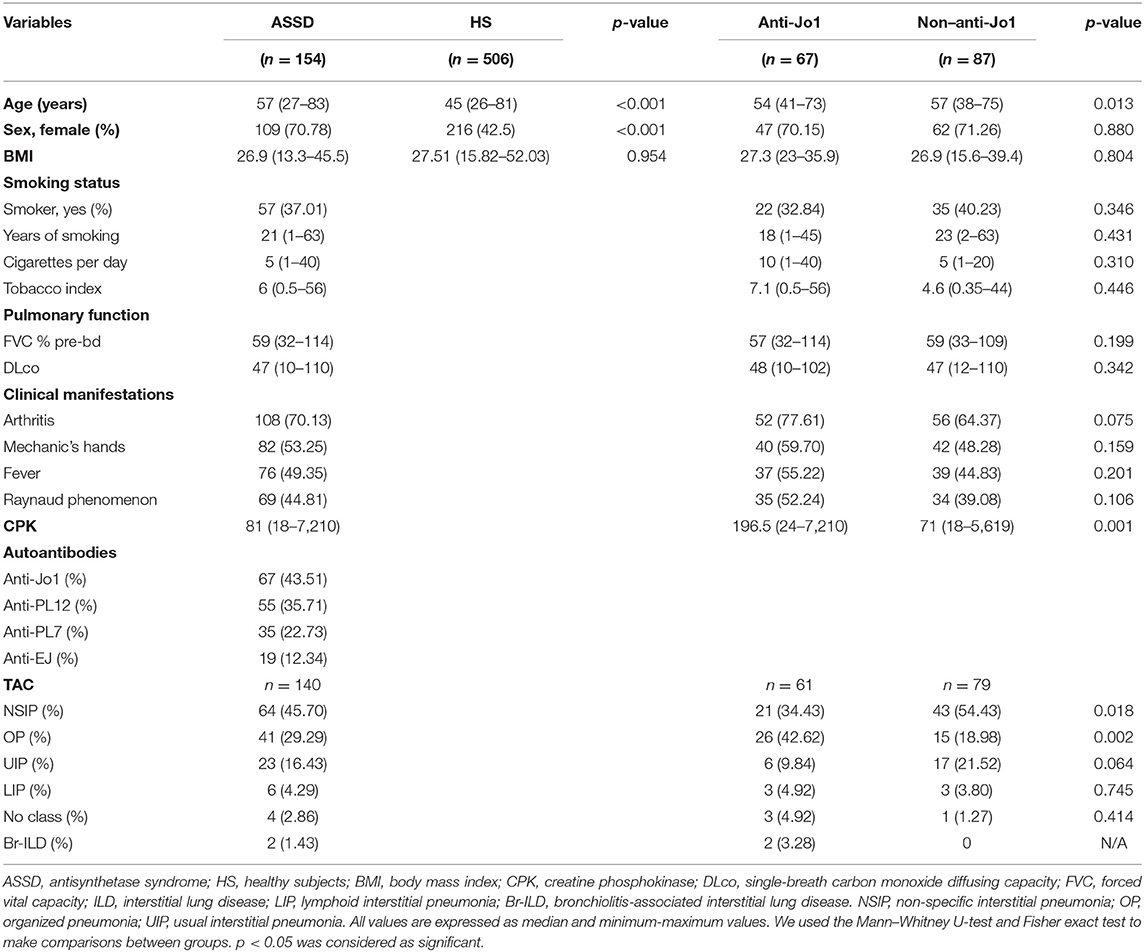
Table 2. Demographic and clinical variables from ASSD and HS groups and among ASSD anti-Jo1 and non–anti-Jo1.
Demographic Variables in Anti-Jo1 and Non–Anti-Jo1 Groups
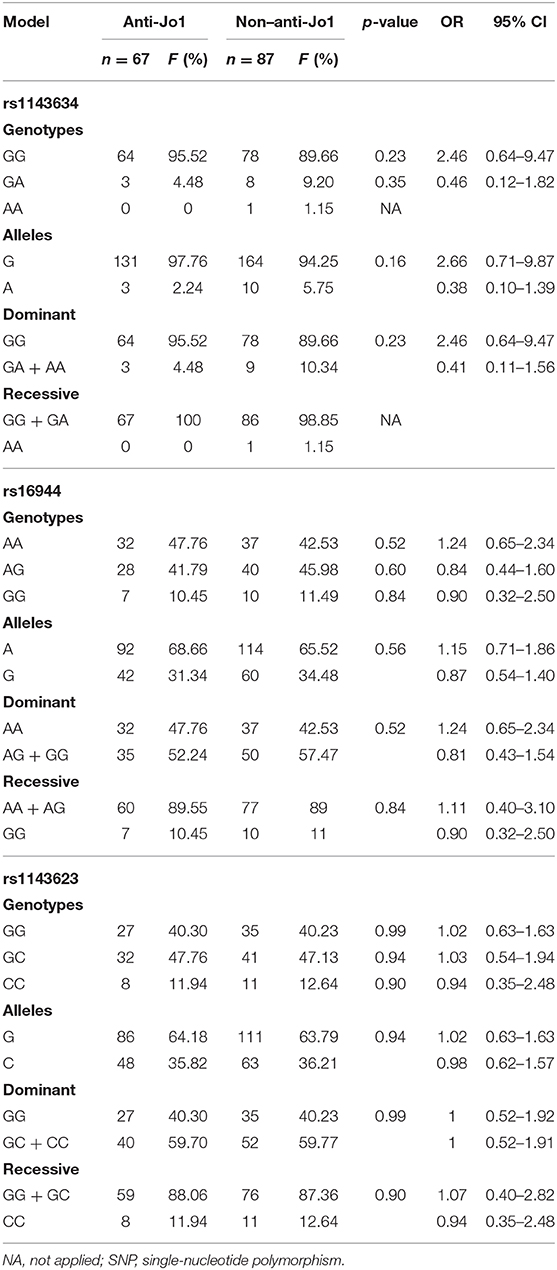
Besides, we divided the case group into anti-Jo1 and non–anti-Jo1, comparing them to each other. Sixty-seven patients were included in the anti-Jo1 group, whereas 87 were included in the non–anti-Jo1 group. Patients included in the non–anti-Jo1 group were older than the anti-Jo1 subjects (p < 0.05). We did not find statistically significant differences between groups comparing sex, BMI, smoking status, pulmonary function (FVC-Pb and DLco), and clinical manifestations, such as mechanic's hands and fever and Raynaud phenomenon (p > 0.05). However, those subjects in the anti-Jo1 group tended to present more arthritis (p = 0.075). Also, anti-Jo1 patients present the most significant muscle involvement, represented by higher levels of CPK (p = 0.001). Interestingly, the most frequent HRCT pattern in the anti-Jo1 group was organized pneumonia (OP) (42.62 vs. 18.98%, p = 0.002), whereas in the non–anti-Jo1 group, it was NSIP (54.43 vs. 34.43%, p = 0.018). Complete results are shown in Table 2.
Allele and Genotype Frequencies
We evaluated three SNPs (rs1143623, 1143634, and rs16944) in the IL1B. Table 3 shows the full genetic association results.
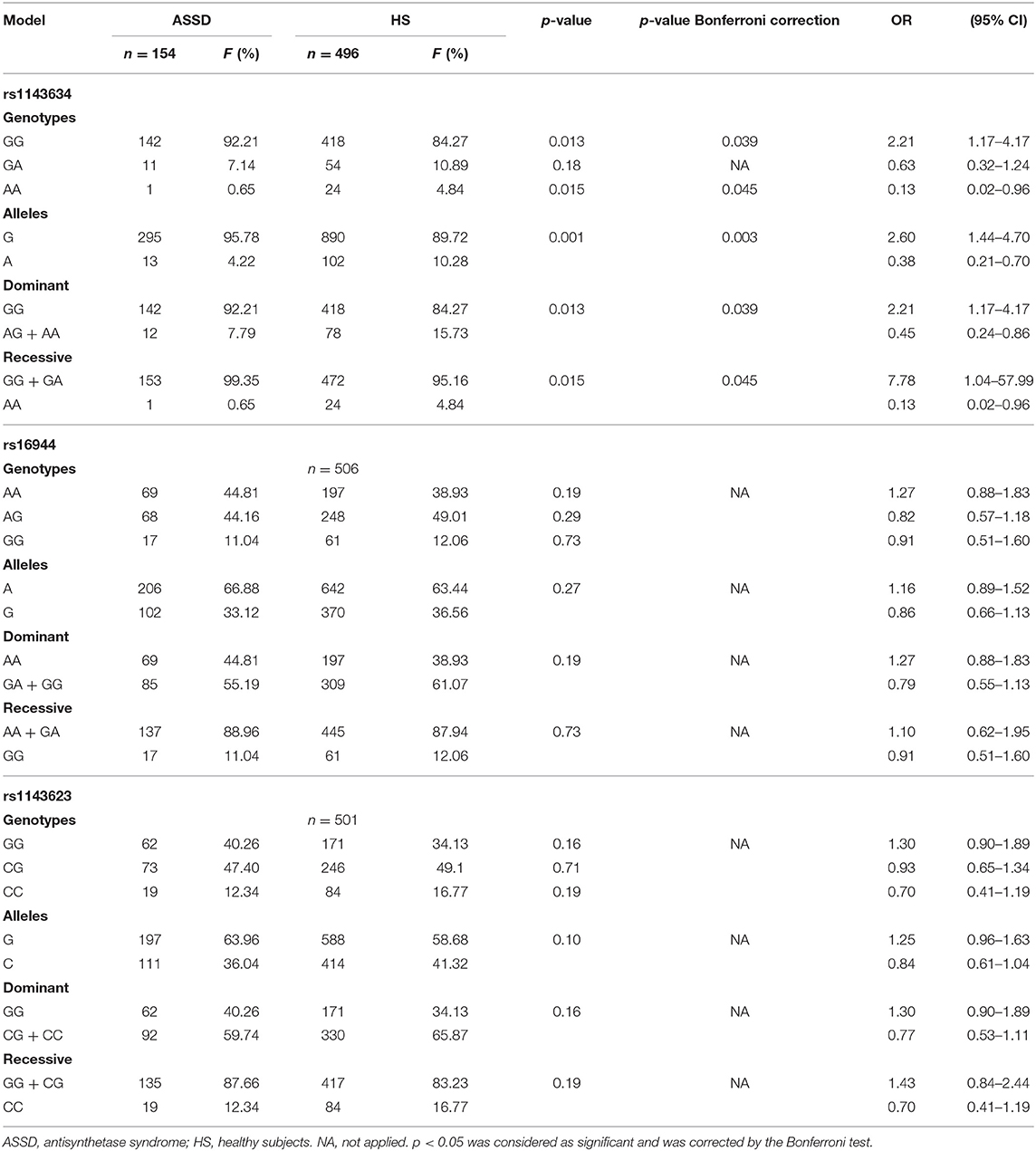
Table 3. Allele and genotype frequencies and genetic models of IL1B SNPs in case and control groups.
Case and Control Groups
In the HS group, we genotyped 496 HS for rs1143634, 506 for rs16944, and 501 for rs1143623. We did not find statistically significant differences with allele and genotype frequencies between case and control group comparison for the rs1143623 and rs16944, and neither with dominant nor recessive models (p > 0.05). However, for the rs1143634, we found significant association with an increased risk of ASSD with G allele (p = 0.001, OR = 2.60, 95% CI = 1.44–4.70), which are maintained after Bonferroni correction (p = 0.003); in contrast, the A allele offered a reduced risk (p = 0.001, OR = 0.38, 95% CI = 0.21–0.70) for ASSD.
Regarding genotype frequencies, we found a statistically significant association (p = 0.015) with a reduced risk of ASSD with AA genotype (OR = 0.13, 95% CI = 0.02–0.96). Conversely, we found a significant association (p = 0.013) with a higher risk of ASSD with GG genotype (OR = 2.21, 95% CI = 1.17–4.17). In addition, in the dominant model, we found significant association (p = 0.013) with a reduced risk with AG + AA genotypes (OR = 0.45, 95% CI 0.24–0.70) and higher risk with GG genotype (OR = 2.21, 95% CI = 1.17–4.17). Additionally, in the recessive model, we also found a significant association (p = 0.015) with reduced risk with AA genotype (OR = 0.13, 95% CI = 0.02–0.96) and higher risk with GG + GA genotypes (OR = 7.78, 95% CI = 1.04–57.99), these associations are maintained after Bonferroni correction (p = 0.045). The complete results' list can be consulted in detail in Table 3.
Logistic Regression Analysis
Logistic regression was performed to adjust for possible confounding covariables. For rs1143634/A allele, we found statistically significant differences comparing ASSD vs. HS in additive model, suggesting a reduced risk of ASSD (p = 0.027, OR = 0.46, 95% CI = 0.23–0.92) and adjusting for sex (p = 1.21E-07) and age (p = 1.79E-14). Additionally, we found significant differences in adjusting for sex and age for the same comparison with rs16944/G and rs1143623/C (p < 0.05). The results are shown in Supplementary Table 1.
Anti-Jo1+ and Non–Anti-Jo1 Groups
Regarding allele frequencies, we did not find statistically significant differences between the three SNPs evaluated. Besides, we did not find significant association when we compared genotype frequencies and neither with dominant and recessive genetic association models (p > 0.05). Complete results are shown in Table 4.
Haplotype Analysis
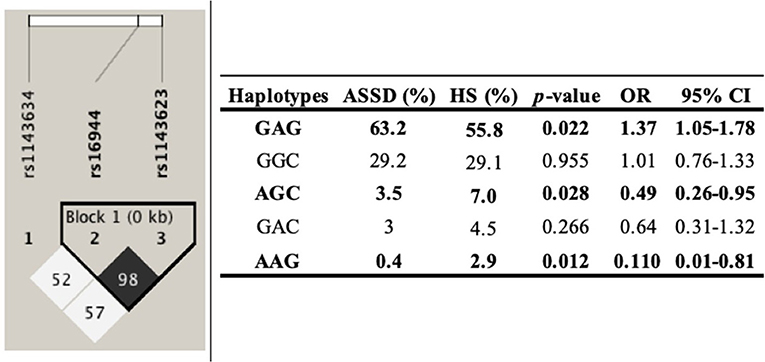
The haplotype analysis was carried out to determine its association with ASSD susceptibility. The analysis included the three SNPs in the IL1B, comparing ASSD vs. subjects in the control group. One of these polymorphisms evaluated (rs1143634) did not meet the Hardy–Weinberg equilibrium (p < 0.05). Haplotypes and their frequencies are summarized in Figure 1. The block shows that the haplotype shaped by rs16944 and rs1143623 is in high linkage disequilibrium (LD, r2 = 98). Moreover, according to the frequencies, GAG (conformed by all common alleles) haplotype is associated with a higher risk of ASSD (p = 0.022, OR = 1.37, 95% CI = 1.05–1.75). On the other hand, AGC (conformed by all minor alleles) and AAG (conformed by one minor allele and two common alleles) haplotypes are associated with reduced risk of ASSD (p = 0.028, OR = 0.49, 95% CI = 0.26–0.95; p = 0.012, OR = 0.110, 95% CI = 0.01–0.81).
IL-1β Serum Levels
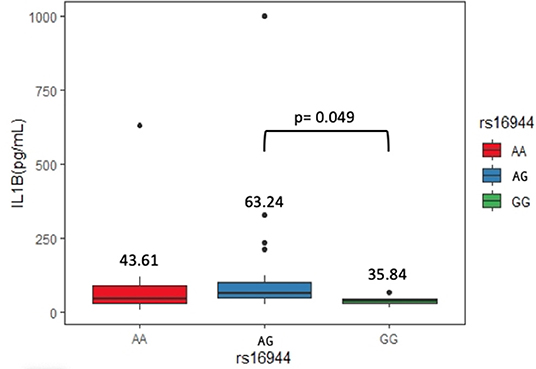
Sixty-two serum samples from the ASSD group were selected, carrying genotypes of the three SNPs evaluated. We did not find significant differences between serum levels of IL-1β in rs1143623 and rs1143634. On the other hand, AG genotype (63.24 pg/mL) from rs16944 showed higher levels of IL-1β than homozygous genotypes [AA (43.61 pg/mL) and GG (35.84 pg/mL)], being statistically significant (p = 0.049). Figure 2 shows these results.
Discussion
The ASSD is a rare systemic connective tissue disease, which usually affects joints, skin, muscles, and lungs. The clinical characteristics of the ASSD group showed that the median age was 57 years, and 70.6% were female. Also, arthritis was the most frequent clinical manifestation, whereas the most frequent ARS autoantibody was anti-Jo1 (41.36%). Also, NSIP was the most frequent tomographic pattern. These findings agree with two previous reports of our research group (1, 5).
Clinical manifestations can differ according to the different ARS autoantibodies. Pinal-Fernández et al. (10) showed that those anti-Jo1+ patients have more arthritis, muscle weakness, and Raynaud phenomenon. Rojas-Serrano et al. (6) described that anti-Jo1 patients had more arthritis, proximal muscle weakness, and higher levels of CPK than those non–anti-Jo1. These findings are similar to our current report and supported by the results shown in the largest ASSD patients cohort confirmed by the American and European NEtwork of Antisynthetase Syndrome collaborative group (20). They included 828 patients from 10 countries and 63 hospitals and found that anti-Jo1 was the most frequent ARS autoantibody, and these subgroups of patients had more muscle and articular involvement. These findings suggest that anti-Jo1 ARS could be associated with multiorgan involvement, whereas non–anti-Jo1 ARSs are mainly lung limited.
Interestingly, the most frequent tomographic pattern in anti-Jo1 patients was OP, whereas in non–anti-Jo1 patients, it was NSIP. These two tomographic patterns have been widely described as the most prevalent in the ASSD (6, 21), with inconsistent frequencies among studied populations. For example, in a previous study of our research group, we reported that in our ASSD cohort, the most frequent HRCT pattern was OP, followed by NSIP (1). Conversely, other studies have shown that NSIP is the most frequent tomographic pattern (7, 9, 22). This finding could be due to differences in the sample size, different inclusion criteria, and distinct population characteristics.
For most of the rheumatic diseases, a genetic susceptibility component has been established. SNPs located in proinflammatory gene cytokines have been associated with an important number of these autoimmune diseases. One of the most studied genes is IL1B, which has been associated in previous studies with RA and SLE (23–25). However, only a few studies are trying to find genetic associations between single-nucleotide variations and ASSD, with controversial results. Our analysis found some associations with rs1143634, being the first time that these associations in ASSD patients in an MM population are described. However, in a previous study by Beretta et al. (26) described that rs1143634 is associated with a more restrictive ILD pattern in patients with systemic sclerosis (SSc) in an Italian population. Although we found an association with a reduced risk of ASSD with the minor allele of rs1143634, this finding could suggest an indirect association with genetic variants that were not considered in this study, as Balding says in his report for candidate polymorphisms (27).
In contrast, Sugiura et al. (28), in a Japanese population, described that STAT4 rs7574865 was associated with a higher risk of DM/polymyositis (PM) but not with the presence of ILD in this group of patients. This finding is shared with SSc, whereas STAT4 rs7574865 is associated with a higher risk of SSc and is also associated with reduced risk of SSc-related ILD in a Caucasian population (29).
Also, in a Chinese Han population, Chen et al. (30, 31) found two SNPs in ETS1 (rs7117932 and rs6590330) and one SNP in CCL21 (rs951005) associated with a higher risk of DM/PM and with ILD related to IIM. Conversely, the same research group found a decreased frequency of minor allele rs7731626-A (ANKRD55) in DM-ILD and DM/PM-ILD patients, suggesting that the A variant may be protective against DM/PM-ILD (32). Even though we did not evaluate the same SNP, this finding agrees with our results because we also found that rs1143634 minor allele could play a protective role in ASSD.
IL1B polymorphisms have also been evaluated in other ILDs because of its profibrotic activity inducing fibroblast proliferation via platelet-derived growth factor (33). Volobaev et al. (34) showed in their study that an SNP in IL1B (rs16944) was associated with a higher risk of anthracosilicosis in coal miners in Russia. Also, it has been described that WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in a murine model of pulmonary fibrosis and an up-regulation of IL-1β in bronchoalveolar lavage fluid (BALF) in bleomycin-induced lung fibrosis in vivo (35). These findings suggest that IL-1β could play an essential role in several ILDs because of its proinflammatory and profibrotic properties.
In our knowledge, this is the first time that ASSD patients are divided into two subgroups (anti-Jo1 and non–anti-Jo1) and compared, taking into consideration the different autoantibodies to make allele and genotype associations from SNPs. These analyses were conducted because of the clinical and prognostic differences between the autoantibodies spectrum reported by different cohorts (6, 8, 20).
To the best of our knowledge, we described for the first time three haplotypes associated with higher and reduced risk of ASSD, even in IIM. However, haplotypes in the IL1B gene have previously been described in other inflammatory diseases such as osteoarthritis, RA, and SLE (15, 36, 37). Although one of the three SNPs does not meet the Hardy–Weinberg equilibrium, it has been previously described that for mestizo populations, this criterion is not always necessary to establish genetic associations because of the recombination events. The MM population has been previously described as a rich genetic variability product of many years of genetic recombinations between ancestral populations (Amerindian), Caucasian, and African descendants (38, 39).
Our study found that the AG genotype of rs16944 is associated with higher levels of IL-1β in ASSD patients. Several studies have identified higher levels of IL-1β in patients with diverse rheumatic and ILDs. For example, serum levels of IL-1β in idiopathic pulmonary fibrosis patients were significantly increased compared to healthy controls, as well as in BALF (40). Also, a higher expression of IL-1α, IL-1β, and transforming growth factor β in muscle tissue has been previously reported in patients with IIM (41). Previous reports have established that genetic variants in the IL1B gene may contribute to differences in the expression, translation, and secretion of IL-1β. Hall et al. (42) demonstrated that rs16944 promoter polymorphism could induce a greater affinity of the transcriptional factors, promoting a higher expression of IL-1β, which concurs with our results. Iglesias-Molli et al. (43) showed that the rs16944 heterozygous genotype is associated with changes in mRNA expression of IL-1β in patients with type 2 diabetes, and this is in agreement with previous publications where an IL-1β haplotype in its promoter region was associated with increased IL-1β mRNA (44).
This study is not free of limitations. First, we evaluated only three SNPs in the IL1B gene, and one of them did not meet the Hardy–Weinberg equilibrium. Second, we did not include a control group of subjects with IIM without ILD because our center attends only to those with pulmonary involvement. Additionally, we only were able to measure the IL-1β in only a single part of our cohort. However, we have one of the largest sample sizes for genetic association in ASSD. Also, we made an intracase analysis to evaluate the relationship between the SNPs and the different ARS autoantibodies.
In conclusion, rs1143634/G is associated with a higher risk of ASSD. Also, rs61944/GG genotype has a higher frequency in those patients positive for the anti-Jo1 autoantibody, and the GA genotype is associated with higher levels of IL-1β in ASSD patients. Three haplotypes are associated with ASSD; two of them (AGC and AAG) are associated with reduced risk of ASSD, whereas one is associated with higher risk. More studies are required to elucidate the role of proinflammatory cytokines in the disease and to offer new therapeutic targets for these patients.
Data Availability Statement
The datasets generated for this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: ClinVar system, VCV000869137, VCV000869138, and VCV000869139; https://www.ncbi.nlm.nih.gov/clinvar/?term=%22HLA%20Laboratory%2C%20Instituto%20Nacional%20de%20Enfermedades%20Respiratorias%20Ismael%20Cosio%20Villegas%22[submitter]+AND+%22IL1B%22[gene].
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Committees for Research, Ethics in Research, and Biosecurity of the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas (INER) approved this study (approval code numbers: C08-17, B11-19). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ER-M, JR-S, and RF-V: conceptualization. KN-Q and MG-P: data curation. MP-G and AG-C: formal analysis. IB-R: funding acquisition. ER-M, AG-C, KN-Q, EA-O, and MG-P: investigation. ER-M, AG-C, KN-Q, and EA-O: methodology. JR-S: project administration. MM, MG-P, IB-R, and JR-S: resources. MM, EA-O, and IB-R: software. MM, GP-R, IB-R, JR-S, and RF-V: supervision. MM and GP-R: validation. MM, GP-R, and JR-S: visualization. MP-G and RF-V: writing—original draft and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the allocated budget to research (RFV-HLA Laboratory) from the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas (INER).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas for the facilities to carry out this research. The authors acknowledge the support received from physicians and technicians from the ILD&RU at INER for confirmation of diagnosis, acquisition of data on lung function, HRCT and autoantibodies, and clinical care of the study participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.547186/full#supplementary-material
References
1. González-Pérez MI, Mejía-Hurtado JG, Pérez-Román DI, Buendía-Roldán I, Mejía M, Falfán-Valencia R, et al. Evolution of pulmonary function in a cohort of interstitial lung disease patients positive to antisynthetase antibodies. J Rheumatol. (2019) 47:415–23. doi: 10.3899/jrheum.181141
2. Witt LJ, Curran JJ, Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med. (2016) 23:218–26. doi: 10.1097/CPM.0000000000000171
3. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. (1975) 292:344–7. doi: 10.1056/NEJM197502132920706
4. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. (1975) 292:403–7. doi: 10.1056/NEJM197502202920807
5. Mejía M, Herrera-Bringas D, Pérez-Román DI, Rivero H, Mateos-Toledo H, Castorena-García P, et al. Interstitial lung disease and myositis-specific and associated autoantibodies: clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF). Respir Med. (2017) 123:79–86. doi: 10.1016/j.rmed.2016.12.014
6. Rojas-Serrano J, Herrera-Bringas D, Mejía M, Rivero H, Mateos-Toledo H, Figueroa JE. Prognostic factors in a cohort of antisynthetase syndrome (ASS): serologic profile is associated with mortality in patients with interstitial lung disease (ILD). Clin Rheumatol. (2015) 34:1563–9. doi: 10.1007/s10067-015-3023-x
7. Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev. (2012) 12:210–7. doi: 10.1016/j.autrev.2012.06.006
8. Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev. (2012) 11:739–45. doi: 10.1016/j.autrev.2012.01.006
9. Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. (2014) 73:227–32. doi: 10.1136/annrheumdis-2012-201800
10. Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, Albayda J, Paik JJ, Johnson C, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatol. (2017) 56:999–1007. doi: 10.1093/rheumatology/kex021
11. Chen S, Wang Q, Wu Z, Wu Q, Li P, Li Y, et al. Associations between TNF-α-308A/G polymorphism and susceptibility with dermatomyositis: a meta-analysis. PLoS ONE. (2014) 9:e102841. doi: 10.1371/journal.pone.0102841
12. Maundrell A, Lester S, Rischmueller M, Hill C, Cleland LG, Blumbergs P, et al. The PTPN22 gene is associated with idiopathic inflammatory myopathy. Muscle Nerve. (2017) 55:270–3. doi: 10.1002/mus.25222
13. Wang Q, Chen S, Li Y, Li P, Wu C, Wu Z, et al. Positive association of genetic variations in the phospholipase C-like 1 gene with dermatomyositis in Chinese Han. Immunol Res. (2016) 64:204–12. doi: 10.1007/s12026-015-8738-x
14. Mohammadoo-Khorasani M, Salimi S, Tabatabai E, Sandoughi M, Zakeri Z, Farajian-Mashhadi F. Interleukin-1β (IL-1β) and IL-4 gene polymorphisms in patients with systemic lupus erythematosus (SLE) and their association with susceptibility to SLE. Indian J Med Res. (2016) 143:591–6. doi: 10.4103/0971-5916.187107
15. Tolusso B, Pietrapertosa D, Morelli A, De Santis M, Gremese E, Farina G, et al. IL-1B and IL-1RN gene polymorphisms in rheumatoid arthritis: relationship with protein plasma levels and response to therapy. Pharmacogenomics. (2006) 7:683–95. doi: 10.2217/14622416.7.5.683
16. Kamenarska Z, Dzhebir G, Hristova M, Savov A, Vinkov A, Kaneva R, et al. IL-1RN VNTR polymorphism in adult dermatomyositis and systemic lupus erythematosus. Dermatol Res Pract. (2014) 2014:953597. doi: 10.1155/2014/953597
17. Pérez-Rubio G, Silva-Zolezzi I, Fernandez-Lopez JC, Camarena A, Velazquez-Uncal M, Morales-Mandujano F, et al. Genetic variants in IL6R and ADAM19 are associated with COPD severity in a Mexican Mestizo population. COPD. (2016) 13:610–5. doi: 10.3109/15412555.2016.1161017
18. Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E, et al. STrengthening the REporting of genetic association studies (STREGA)–an extension of the STROBE statement. Eur J Clin Invest. (2009) 39:247–66. doi: 10.1111/J.1365-2362.2009.02125.X
19. Dean AG, Arner TG, Sunki GG, Friedman R, Lantinga M, Sangam S, et al. Epi Info, a Database and Statistics Program for Public Health Professionals. (2004). Available online at: https://www.cdc.gov/epiinfo/index.html (accessed October 4, 2020).
20. Cavagna L, Trallero-Araguás E, Meloni F, Cavazzana I, Rojas-Serrano J, Feist E, et al. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J Clin Med. (2019) 8:2013. doi: 10.3390/jcm8112013
21. Waseda Y, Johkoh T, Egashira R, Sumikawa H, Saeki K, Watanabe S, et al. Antisynthetase syndrome: pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur J Radiol. (2016) 85:1421–6. doi: 10.1016/j.ejrad.2016.05.012
22. Jensen ML, Løkke A, Hilberg O, Hyldgaard C, Tran D, Bendstrup E, et al. European clinical respiratory journal clinical characteristics and outcome in patients with antisynthetase syndrome associated interstitial lung disease: a retrospective cohort study clinical characteristics and outcome in patients with antisynthetase syn. Eur Clin Respir J. (2019) 6:1583516. doi: 10.1080/20018525.2019.1583516
23. Jahid M, Rehan-Ul-Haq CD, Avasthi R, Ahmed RS. Association of polymorphic variants in IL1B gene with secretion of IL-1β protein and inflammatory markers in north Indian rheumatoid arthritis patients. Gene. (2018) 641:63–7. doi: 10.1016/j.gene.2017.10.051
24. Rong H, He X, Wang L, Bai M, Jin T, Wang Y, et al. Association between IL1B polymorphisms and the risk of rheumatoid arthritis. Int Immunopharmacol. (2020) 83:106401. doi: 10.1016/j.intimp.2020.106401
25. Umare V, Pradhan V, Rajadhyaksha A, Ghosh K, Nadkarni A. Predisposition of IL-1β (-511 C/T) polymorphism to renal and hematologic disorders in Indian SLE patients. Gene. (2018) 641:41–5. doi: 10.1016/j.gene.2017.10.039
26. Beretta L, Bertolotti F, Cappiello F, Barili M, Masciocchi M, Toussoun K, et al. Interleukin-1 gene complex polymorphisms in systemic sclerosis patients with severe restrictive lung physiology. Hum Immunol. (2007) 68:603–9. doi: 10.1016/j.humimm.2007.03.005
27. Balding DJ. A tutorial on statistical methods for population association studies. Nat Rev Genet. (2006) 7:781–91. doi: 10.1038/nrg1916
28. Sugiura T, Kawaguchi Y, Goto K, Hayashi Y, Tsuburaya R, Furuya T, et al. Positive association between STAT4 polymorphisms, and polymyositis/ dermatomyositis in a Japanese population. Ann Rheum Dis. (2012) 71:1646–50. doi: 10.1136/annrheumdis-2011-200839
29. Stock CJW, De Lauretis A, Visca D, Daccord C, Kokosi M, Kouranos V, et al. Defining genetic risk factors for scleroderma-associated interstitial lung disease. Clin Rheumatol. (2020) 39:1173–9. doi: 10.1007/s10067-019-04922-6
30. Chen S, Wang Q, Wu C, Wu Q, Li Y, Wu Z, et al. A single-nucleotide polymorphism of CCL21 rs951005 T>C is associated with susceptibility of polymyositis and such patients with interstitial lung disease in a Chinese han population. Clin Exp Rheumatol. (2015) 33:639–46.
31. Chen S, Wen X, Li L, Li J, Li Y, Wang Q, et al. Single nucleotide polymorphisms in the ETS1 gene are associated with idiopathic inflammatory myopathies in a northern Chinese Han population. Sci Rep. (2017) 7:13128. doi: 10.1038/s41598-017-13385-1
32. Li L, Chen S, Wen X, Wang Q, Lv G, Li J, et al. Positive association between ankrd55 polymorphism 7731626 and dermatomyositis/polymyositis with interstitial lung disease in Chinese han population. Biomed Res Int. (2017) 2017:2905987. doi: 10.1155/2017/2905987
33. Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. (1989) 243:393–6. doi: 10.1126/science.2783498
34. Volobaev VP, Larionov AV, Kalyuzhnaya EE, Serdyukova ES, Yakovleva S, Druzhinin VG, et al. Associations of polymorphisms in the cytokine genes IL1β (rs16944), IL6 (rs1800795), IL12b (rs3212227) and growth factor VEGFA (rs2010963) with anthracosilicosis in coal miners in Russia and related genotoxic effects. Mutagenesis. (2018) 33:129–35. doi: 10.1093/mutage/gex047
35. Aumiller V, Balsara N, Wilhelm J, Günther A, Königshoff M. WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol. (2013) 49:96–104. doi: 10.1165/rcmb.2012-0524OC
36. Camargo JF, Correa PA, Castiblanco J, Anaya J-M. Interleukin-1b polymorphisms in Colombian patients with autoimmune rheumatic diseases. Genes Immun. (2004) 5:609–14. doi: 10.1038/sj.gene.6364133
37. Moxley G, Han J, Stern AG, Riley BP. Potential influence of IL1B haplotype and IL1A-IL1B-IL1RN extended haplotype on hand osteoarthritis risk. Osteoarthr Cartil. (2007) 15:1106–12. doi: 10.1016/j.joca.2007.03.022
38. Ambrocio-Ortiz E, Pérez-Rubio G, Ramírez-Venegas A, Hernández-Zenteno R, Del Angel-Pablo AD, Pérez-Rodríguez ME, et al. Effect of SNPs in HSP family genes, variation in the mRNA and intracellular Hsp levels in COPD secondary to tobacco smoking and biomass-burning smoke. Front Genet. (2020) 10:1307. doi: 10.3389/fgene.2019.01307
39. Silva-Zolezzi I, Hidalgo-Miranda A, Estrada-Gil J, Fernandez-Lopez JC, Uribe-Figueroa L, Contreras A, et al. Analysis of genomic diversity in Mexican mestizo populations to develop genomic medicine in Mexico. Proc Natl Acad Sci USA. (2009) 106:8611–6. doi: 10.1073/pnas.0903045106
40. Barlo NP, van Moorsel CHM, Korthagen NM, Heron M, Rijkers GT, Ruven HJT, et al. Genetic variability in the IL1RN gene and the balance between interleukin (IL)-1 receptor agonist and IL-1β in idiopathic pulmonary fibrosis. Clin Exp Immunol. (2011) 166:346–51. doi: 10.1111/j.1365-2249.2011.04468.x
41. Lundberg I, Ulfgren AK, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. (1997) 40:865–74. doi: 10.1002/art.1780400514
42. Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TWF, et al. Correlation of polymorphic variation in the promoter region of the interleukin-1? gene with secretion of interleukin-1? protein. Arthritis Rheum. (2004) 50:1976–83. doi: 10.1002/art.20310
43. Iglesias Molli AE, Bergonzi MF, Spalvieri MP, Linari MA, Frechtel GD, Cerrone GE. Relationship between the IL-1β serum concentration, mRNA levels and rs16944 genotype in the hyperglycemic normalization of T2D patients. Sci Rep. (2020) 10:9985. doi: 10.1038/s41598-020-66751-x
Keywords: antisynthetase syndrome, IL1B gene, IL1-beta, SNP, anti-Jo1, genetic association
Citation: Ponce-Gallegos MA, Ramos-Martínez E, García-Carmona A, Mejía M, Nava-Quiroz KJ, Pérez-Rubio G, Ambrocio-Ortiz E, González-Pérez MI, Buendía-Roldán I, Rojas-Serrano J and Falfán-Valencia R (2020) Genetic Susceptibility to Antisynthetase Syndrome Associated With Single-Nucleotide Variants in the IL1B Gene That Lead Variation in IL-1β Serum Levels. Front. Med. 7:547186. doi: 10.3389/fmed.2020.547186
Received: 26 May 2020; Accepted: 12 October 2020;
Published: 24 November 2020.
Edited by:
Savino Sciascia, University of Turin, ItalyReviewed by:
Baptiste Hervier, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceFederica Furini, University of Ferrara, Italy
Copyright © 2020 Ponce-Gallegos, Ramos-Martínez, García-Carmona, Mejía, Nava-Quiroz, Pérez-Rubio, Ambrocio-Ortiz, González-Pérez, Buendía-Roldán, Rojas-Serrano and Falfán-Valencia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramcés Falfán-Valencia, rfalfanv@iner.gob.mx; Jorge Rojas-Serrano, jorroser@gmail.com
 Marco Antonio Ponce-Gallegos
Marco Antonio Ponce-Gallegos Espiridión Ramos-Martínez
Espiridión Ramos-Martínez Adriana García-Carmona
Adriana García-Carmona Mayra Mejía
Mayra Mejía Karol J. Nava-Quiroz
Karol J. Nava-Quiroz Gloria Pérez-Rubio
Gloria Pérez-Rubio Enrique Ambrocio-Ortiz
Enrique Ambrocio-Ortiz Montserrat I. González-Pérez
Montserrat I. González-Pérez Ivette Buendía-Roldán
Ivette Buendía-Roldán Jorge Rojas-Serrano
Jorge Rojas-Serrano Ramcés Falfán-Valencia
Ramcés Falfán-Valencia