Case report: Muscular tuberculosis with lower-extremity muscular masses as the initial presentation: Clinicopathological analysis of two cases and review of the literature
- 1Department of Neurology, Suzhou Hospital of Anhui Medical University, Suzou, China
- 2Department of Neurology, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Pathology, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Tuberculosis (TB) is a threat to public health that mostly affects people in developing countries. TB presenting as a soft tissue mass is rare and is usually seen in patients with muscular tuberculosis (MT).
Case presentation: In this study, we present the clinical, radiographic, and pathological features of two cases and retrospective evaluations of an additional 28 patients who were diagnosed with MT. More patients were men (60.9%) than women (39.1%), with a male-to-female ratio of 1.6:1. The average age among male and female patients was 38.9 and 30.1 years, respectively. MT usually presents with painful or painless muscular nodules on the lower limbs. Imaging findings, including ultrasound, CT, and MRI, can be used to identify lesions and sites for biopsy. The most typical histopathological feature of MT is granulomatous inflammation with caseous necrosis and epithelioid granulomata. Acid-fast bacilli stain and polymerase chain reaction (PCR) assays are helpful in identifying tubercle bacillus.
Conclusion: We describe two MT cases with lower-extremity muscular masses as the initial presentation. The results suggest that muscle biopsy and pathological analysis remain necessary for diagnosis. Most of the patients could be cured with standard antituberculosis therapy.
1. Introduction
Tuberculosis (TB) is a public health threat throughout the world and ~10 million people contract TB every year. The bacteria usually attack the lungs, butcan also attack other parts of the body, such as the kidney, spine, and brain. Extrapulmonary cases of TB are distributed among the lymph nodes (47%), pleural cavity (30%), abdomen (10%), bones and joints (8%), CNS (2%), and other sites (3%). The least frequent location of TB is intramuscular (1, 2). TB presenting as a soft tissue mass is rare and is usually seen in patients with muscular tuberculosis (MT). MT mainly presents in the form of muscle masses at a single site or multiple sites with swelling, weakness, pain, or painlessness (3, 4). The rarity of this condition often leads to failure to consider MT in the differential diagnosis, resulting in delayed therapy.
Here, we report two MT cases with muscular nodules on the lower limbs as the initial presentation. Furthermore, we present a retrospective evaluation of an additional 28 patients diagnosed with typical MT, drawn from 27 articles in the PubMed database published between 2000 and 2022, with the aim of determining the clinicopathological characteristics of MT and establishing definitive differential diagnosis criteria.
2. Case presentation
2.1. Case 1
A 66-year-old woman presented with a 1-month history of a painful isolated mass on the inside of the left calf. She also complained of bilateral numbness in her toes. A history of contact with patients with suspected TB could be traced: her father had had pulmonary TB 10 years previously, and her daughter had had tuberculous pleurisy 7 years previously. She was in good general condition and did not complain of systemic symptoms such as night sweats, weight loss, anorexia, fatigue, or intermittent fever. Physical examination revealed a tender mass with irregular indistinct borders in her left calf. Movement was severely restricted by knee pain. She had a slight decrease in sensation and swelling of the lower extremities. The local temperature was not elevated, and the skin over the mass was normal. No obvious mass was palpable on the right leg. Further clinical examinations, including hemogram, erythrocyte sedimentation rate (ESR), procalcitonin test (PCT), liver and renal function tests, antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies (ANCA), fungal G-test, and tumor marker levels, returned results within normal limits. Serological rheumatoid factor (RF) was elevated to 33.8 iu/ml (reference range: up to 15 iu/ml), serological high sensitivity C-reactive protein (CRP) waselevated to 7.48 mg/L (reference range: up to 3 mg/L), and antistreptolysin O (ASO) was positive. Tuberculin antibody IgG, IgM, γ-interferon release test, purified protein derivative (PPD) skin test, acid-fast bacilli smear, and tissue culture of sputum were negative. Electromyography (EMG) indicated reduced motor and sensory nerve conduction velocity in the lower limbs (MCV: 25.8–33.1 m/s, SCV: 0–34.3 m/s), accompanied by decreased compound motor action potential (CMAP: 1.6–12.1 mV) and sensory nerve action potential amplitude (SNAP: 0–2.1 uV). Distal motor latency and F wave were normal. The duration and amplitude of motor unit potential (MUP) were increased, without spontaneous activity, indicating extensive chronic neurogenic lesions.
Chest computed tomography (CT) of case 1 revealed scattered bilateral inflammatory changes in the inferior lobes of both lungs and multiple nodular thickening on both sides of the pleura. Bilateral ultrasound of the calves suggested a 50 × 16-mm hypoechoic lesion in the left soleus muscle (Figure 1A) and a 43 × 6-mm hypoechoic lesion in the medial head of the gastrocnemius muscle. Additionally, 18 F-fluorodeoxyglucose positron emission tomography–computed tomography (18 F-FDG-PETCT) revealed multiple hypermetabolic lesions, which involved the left soleus muscle, the left musculus fibularis longus, the right soleus muscle, and the tibialis anterior muscle. The largest lesion was located on the middle of the left soleus muscle measuring 33.8 × 20 × 42 mm, and the maximum standardized uptake value (SUVmax) was 15.9 (Figure 1B). Magnetic resonance imaging (MRI) examination showed abnormal mass signals involving the inner front edge of the gastrocnemius muscle. The lesion was enhanced by gadolinium on the T1-weighted turbo spin-echo (T1W-TSE) image, and T2-weighted spectral presaturation attenuated inversion recovery (T2W-SPAIR) showed a hyperintense mass. There was a similar lesion in the gastrocnemius muscle of the right leg (Figures 1C, D). Pelvis and lumbar spine MRI showed mild lumbar disk herniation, with no other abnormal findings.
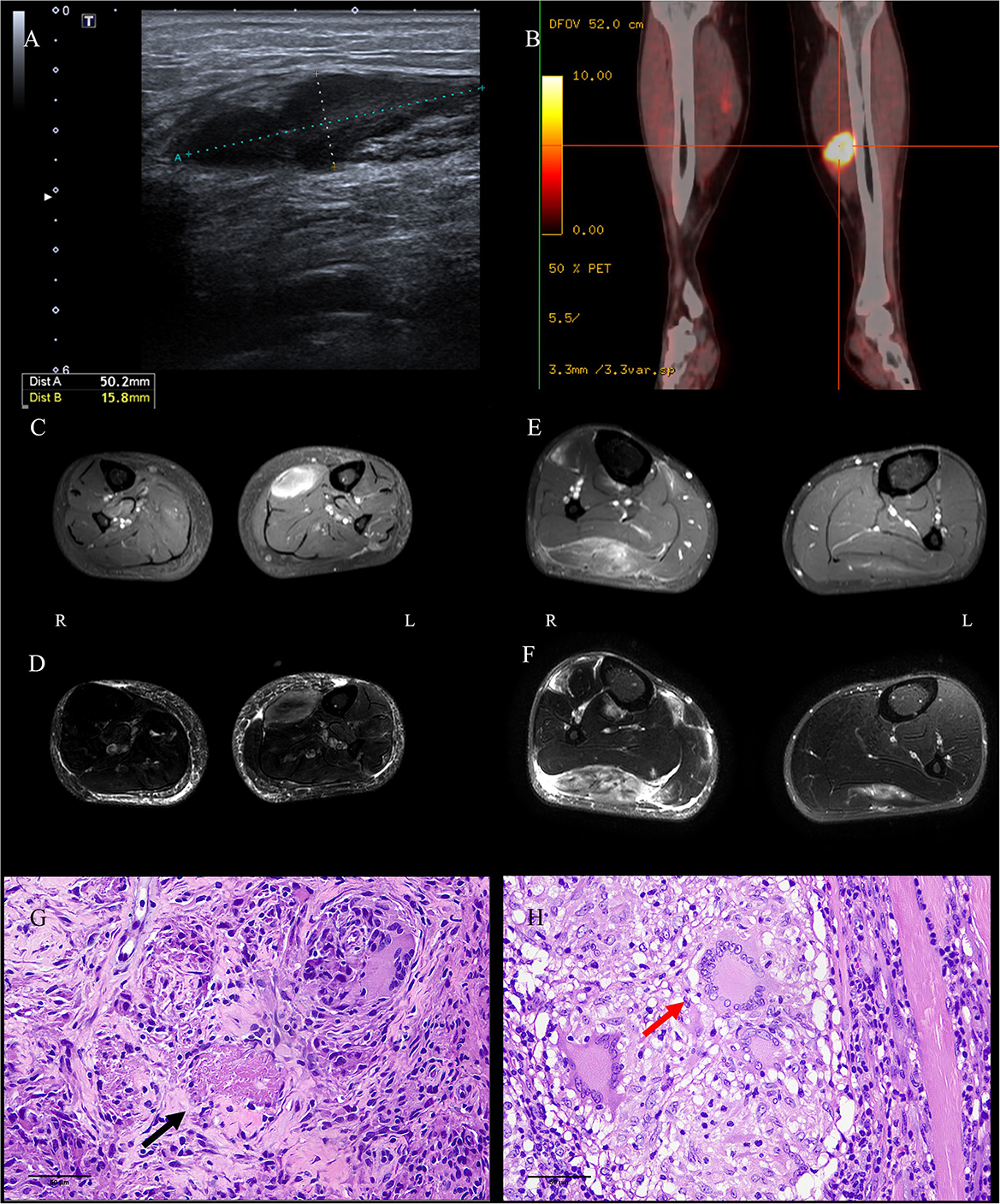
Figure 1. Images and pathological findings of the muscle masses. The lesion in the left soleus muscle of P1 was found to be hypoechoic on ultrasound (A), hypermetabolic on PETCT (B), enhanced on axial enhanced T1WI-TES (C), and light-hyperintense on T2W-SPAIR (D). Several well-formed epithelioid granulomas centered by caseous necrosis were observed [(G), HE, bar = 50 μm]. The lesion in the right gastrocnemius muscle of P2 was identified via enhancement on axial enhanced T1WI-TES (E) and hyperintensity on T2W-SPAIR (F). Three Langerhans cells in granulomas were noticed in the sections [(H), HE, bar = 50 μm]. P, patient; PETCT, positron emission tomography–computed tomography; T1W-TSE, T1-weighted turbo spin echo; T2W-SPAIR, T2-weighted spectral presaturation attenuated inversion recovery.
A muscle biopsy of the left gastrocnemius was performed in case 1; the result showed multiple nodular epithelioid granulomas embedded among the muscle fibers. Caseous necrosis was found under the microscope (Figure 1G). Microscopic examination for acid-fast bacilli stain and cytokeratin (AE1/AE3) was completely negative. CD3, CD31, CD34, desmin, Kp1, and PGM1 staining were weakly positive. The Ki67 labeling index was low (10%). PCR for the mycobacterium tuberculosis complex in blood, urine, sputum, and paraffin-embedded samples from case 1 returned negative results; however, the result was positive in frozen sections of muscles. A diagnosis of MT was considered in case 1, despite the fact that all the tests used for diagnosis of TB were negative, with the exceptions of PCR and histopathology (5, 6).
2.2. Case 2
A 50-year-old woman presented with a mass in the right calf for 4 months. There were no symptoms of cough or weight loss, and no history of intramuscular injection at the site concerned or of any contact with TB. Physical examination revealed an indurated nodule with no tenderness or elevation of local temperature, about 8 × 5 cm in size. The borders of mass were not well-demarcated. There was no abnormality of distal vascularity or sensation, and no limitation of movement. Laboratory tests revealed a normal blood count, and liver function tests, ASO titers, and renal functions were not deranged. An extensive laboratory workup, including ANA, ANCA, CRP, tumor marker levels, thyroid function tests, and inflammatory markers, returned results within normal limits. The outcomes of several infection tests for syphilis, hepatitis B, hepatitis C, and HIV were negative.
Ultrasound of the right calf was performed in case 2 and suggested a hypoechoic lesion. The patient in case 2 also underwent musculoskeletal MRI examination, and the result showed similar lesions to case 1 in the soleus muscle and medial posterior part of the right tibia and fibula (Figures 1E, F). According to radiological imaging findings, both patients had other painless muscular nodules at multiple sites throughout their entire bodies.
Resections and histopathologic observations of lesions were performed in case 2. Granulomatous inflammation, mainly composed of epithelioid histiocytes and Langerhans giant cells, was observed under the microscope (Figure 1H). Histopathologic examination showed the presence of non-necrotizing epithelioid granulomas compatible with TB. Immunohistochemistry against CD4/CD8, MHC-I, and MAC (C5b9) was negative. Moreover, the acid-fast bacilli stain was negative. PCR for TB was positive in case 2 in paraffin-embedded and frozen sections of muscles. A diagnosis of MT was considered in case 2, despite the lack of detectable focus of tubercular infection except in the PCR and histopathology results (5, 6).
Many differential diagnoses were considered in both cases, including deep vein thrombosis, a soft tissue tumor, or a pyogenic abscess. Based on the diagnosis of MT, the patients in cases 1 and 2 were both prescribed standard oral antituberculosis treatment with four drugs, namely, isoniazid, rifampicin, ethambutol, and pyrazinamide, for 2 months. After treatment, the patients' symptoms were relieved, and the nodules in the calves appeared to decrease in size. Further courses of isoniazid and rifampicin were administered. At the time of writing, the patients are undergoing follow-up.
3. Discussion
In recent years, as the incidence of pulmonary TB has rebounded, extrapulmonary TB has become a common disease (7). MT without bony involvement is still a rare presentation, as it has been since it was first reported in 1886. Since 2000, only 28 cases of MT have been reported in the published literature (3, 6, 8–32). Combining these with the two new cases reported here, we reviewed all 30 cases; the clinical profiles are summarized in Table 1.
MT occurred more often in men (60.9%) than in women (39.1%), with a male-to-female ratio of 1.6:1. The average age among male and female patients was 38.9 and 30.1 years, respectively (range: 9–83 years). All patients presented with chronic occult onset, ranging from 2 weeks to 2 years, and symptoms gradually became more aggravated. There were 11 cases of patients who presented with masses as the main clinical manifestation, and the other 19 patients presented with swelling. Among the 11 cases, seven were recorded as having nodular mass in the lower extremities (3, 8, 13, 19). Pain (20/30) was the most commonly presenting clinical feature. The thigh and calf (9/30, respectively) were the most common sites of involvement. This symptom and location were distinctive features for MT and should be included in the differential diagnostic criteria for other muscular nodular diseases. In five cases (including case 1 in the present report), the patient had suffered from pulmonary TB or had a history of contact with patients with suspected TB (3, 14, 21, 29). At present, it is generally agreed that hematogenous dissemination plays an important role in MT, although in some cases it was transmitted through an infected needle (3, 6, 15, 20, 21). Skeletal muscles were usually spared by TB because these are a poor host for mycobacterium tuberculosis (30). In consideration of MT occurring as a secondary infection, patients in four cases had been treated with steroids, and long-term chronic pulmonary inflammation or decreased immunity may therefore be a potential risk factor for MT (3, 9, 18, 27, 29). The clinical symptom in case 1 was painful isolated masses in the lower limbs, and EMG suggested potential generalized involvement of peripheral nerves with axonal damage. We speculate that MT may affect the peripheral nerves, which has not been reported previously. Unfortunately, further biopsy of the sural nerve was not performed to prevent secondary infection.
Diagnostic tests for TB disease had been performed on all 30 patients, including PPD skin test (9/13), acid-fast bacilli smear (9/22), tissue culture of sputum or blood (0/8), tissue culture of the lesion (17/23), PCR for MT (8/9), chest X-ray or CT (8/23), ultrasound (17/18), CT (13/13), MRI (21/21), and biopsy (25/26) of the lesion (Figure 2) (3, 6, 18–32). Pathological information was provided for 26 patients. The most common histological features of MT included granulomatous inflammation, caseous necrosis, and epithelioid granulomata. The TB test could be partially negative, which does not rule out TB infection (26). Among the available modalities, serological tests and PPD skin tests were not recommended in the diagnosis of MT or the initiation of antituberculosis treatment. Bacterial culture of the lesion helps to demonstrate the etiological organism, but culturing was not sensitive in sputum or blood. It should be highlighted that in previous studies, most of the patients were diagnosed as MT based on muscle biopsy, even though some of these patients (5/26) did not have a positive result associated with TB infection (12, 30). TB pathognomonic lesions could be found at any site of TB infection. This was a characteristic granulomatous inflammatory reaction against mycobacterium tuberculosis bacilli from the host's cell-mediated immunity (33). The diagnosis of MT mainly depended on biopsy and bacteriological culture traditionally. PCR was reported recently to be a useful method to determine TB (5). In our cases, TB was not detected by acid-fast bacilli stain, but PCR for TB proved positive in preserved frozen sections of muscles. However, PCR was negative in the blood, urine, and sputum in case 1, probably because the trace amounts of TB DNA were not detected. Sometimes, DNA-related tests using paraffin-embedded samples showed inconsistent results. This indicates that PCR is more sensitive than acid-fast bacilli stain, especially at the site of infection. This is consistent with earlier findings (3, 32).
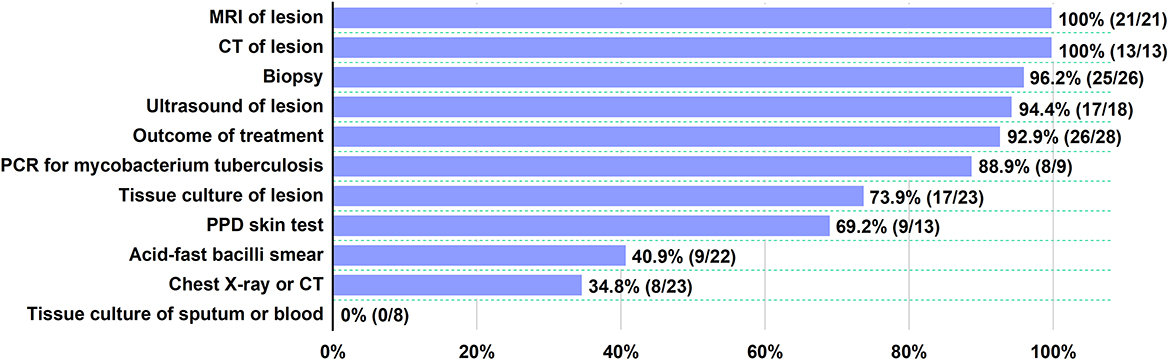
Figure 2. Diagnostic tests for TB disease. For each test, the proportion of positive patients is indicated.
Imaging findings, including ultrasound, CT, and MRI, could identify lesions and sites for biopsy (28, 34). The evaluation should begin with ultrasonography. The tissue masses of the soft parts had a hypoechogenic appearance in most cases. Sonography allowed for differentiation between solid and liquid lesions, and some soft tissue tumors, hematomas, and cystic diseases could be distinguished (35). However, ultrasound was a very sensitive but non-specific examination. In general, a soft tissue mass of unknown character in the lower extremity requires further workup via MRI, because this may confirm the size and nature of the mass more clearly than ultrasound, particularly in indicated tissue swelling and deeper tissue damage (36).
The differential diagnosis list for MT is broad, especially in the case of some patients presenting clinically with muscle nodules. The most common differentials include schwannomas, lipoma, liposarcoma, ganglionic cyst, focal myositis, and hydatid cyst of the muscle. Because of a suspicion of malignancy, exploration was undertaken and an open biopsy was conducted on the nodule in two patients. Focal myositis could also present with similar clinical and imaging presentation, but the pathological features such as inflammatory infiltration and muscle fiber atrophy, necrosis, and regeneration were not obvious in the muscle pathology of these two patients. In fact, during diagnosis, oncological vigilance should be maintained, because MT presentation is similar to that of some patients with tumors.
The main treatments were standard antituberculosis therapies and lesion removal. Observations of therapeutic effects and prognostic data were available in 28 cases (including ours). Almost all diagnosed patients were treated with standardized application of TB drugs, and most had good prognoses (26/28) (3, 6, 9–13, 24–32). Only two patients died of another fatal disease during therapy (cerebrovascular hemorrhage; profound shock and multiorgan failure) (9, 18).
In conclusion, we have described two patients who presented with muscular nodules on the lower limbs as the initial manifestation of tuberculosis and presented the corresponding clinical, imaging, and pathological features. Although MT is a rare entity, it should be considered in a differential diagnosis when a patient presents with single or multiple intramuscular masses, especially in tubercular endemic areas. PCR is a sensitive and useful method to confirm MT.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No: 2021-219). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
X-wZ: data acquisition, data analysis, interpretation of data, and drafting of the manuscript for intellectual content. X-hL: data acquisition, analysis of data, and revision of the manuscript for intellectual content. K-lJ, CZ, S-hL, and LC: data acquisition. PZ and Z-yL: funding, study design, conceptualization, data analysis, interpretation of data, manuscript revision, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This project is supported by the National Natural Science Foundation of China (Nos. 81870889 and 81571086, project manager: LC and No. 81972500, project manager: Z-yL).
Acknowledgments
We would like to thank the patients and their family members for their support and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Natarajan A, Beena PM, Devnikar AV, Mali S. A systemic review on tuberculosis. Indian J Tuberc. (2020) 67:295–311. doi: 10.1016/j.ijtb.2020.02.005
2. Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. Dtsch Arztebl Int. (2019) 116:729–35. doi: 10.3238/arztebl.2019.0729
3. Zeng Y, Liu Y, Xie Y, Liang J, Kuang J, Lu Z, et al. Muscular tuberculosis: a new case and a review of the literature. Front Neurol. (2019) 10:1031. doi: 10.3389/fneur.2019.01031
4. Gou L-j, Su J-m, Zhao Y, Zhang F-C. Clinical analysis of 20 cases of muscular tuberculosis. Zhonghua Yi Xue Za Zhi. (2012) 92:206–8. doi: 10.3760/cma.j.issn.0376-2491.2012.03.016
5. Lira LA, Santos FC, Carvalho MS, Montenegro RA, Lima JF, Schindler HC, et al. Evaluation of a IS6110-taqman real-time PCR assay to detect mycobacterium tuberculosis in sputum samples of patients with pulmonary TB. J Appl Microbiol. (2013) 114:1103–8. doi: 10.1111/jam.12119
6. Dhakal AK, Shah SC, Shrestha D, Banepali N, Geetika KC. Tuberculosis presenting as multiple intramuscular nodules in a child: a case report. J Med Case Rep. (2015) 9:72. doi: 10.1186/s13256-015-0543-6
7. Pang Y, An J, Shu W, Huo F, Chu N, Gao M, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008-2017. Emerg Infect Dis. (2019) 25:457–64. doi: 10.3201/eid2503.180572
8. Haq I, Moss K, Morris VH. Myalgia with lymphadenopathy. J R Soc Med. (2001) 94:521–2. doi: 10.1177/014107680109401008
9. Ergin F, Arslan H, Bilezikçi B, Agildere AM, Ozdemir N. Primary tuberculosis in the gluteal muscle of a patient with chronic renal failure. A rare presentation. Nephron. (2001) 89:463–6. doi: 10.1159/000046122
10. Franco-Paredes C, Blumberg HM. Psoas muscle abscess caused by Mycobacterium tuberculosis and Staphylococcus aureus: case report and review. Am J Med Sci. (2001) 321:415–7. doi: 10.1097/00000441-200106000-00008
11. Morris BS, Varma R, Garg A, Awasthi M, Maheshwari M. Multifocal musculoskeletal tuberculosis in children: appearances on computed tomography. Skeletal Radiol. (2002) 31:1–8. doi: 10.1007/s00256-001-0439-y
12. Trikha V, Gupta V. Isolated tuberculous abscess in biceps brachii muscle of a young male. J Infect. (2002) 44:265–6. doi: 10.1053/jinf.2002.0986
13. Bakshi G, Satish R, Shetty SV, Anjana J. Primary skeletal muscle tuberculosis. Orthopedics. (2003) 26:327–8. doi: 10.3928/0147-7447-20030301-15
14. Winzer K-J, Menenakos C, Braumann C, Mueller JM, Guski H. Breast mass due to pectoral muscle tuberculosis mimicking breast cancer in a male patient. Int J Infect Dis. (2005) 9:176–7. doi: 10.1016/j.ijid.2004.07.007
15. Trikha V, Varshney MK, Rastogi S. Isolated tuberculosis of the vastus lateralis muscle: a case report. Scand J Infect Dis. (2006) 38:304–6. doi: 10.1080/00365540500353267
16. Mascarenhas S, Tuffin JR, Hassan I. Tuberculous submasseteric abscess: case report. Br J Oral Maxillofac Surg. (2009) 47:566–8. doi: 10.1016/j.bjoms.2008.10.002
17. Narang S. Tuberculous pyomyositis of forearm muscles. Hand. (2009) 4:88–91. doi: 10.1007/s11552-008-9127-x
18. Huang C-C, Liu M-F, Lee N-Y, Chang C-M, Lee H-C, Wu C-J, et al. Fatal tuberculous myositis in an immunocompromised adult with primary Sjogren's syndrome. J Formos Med Assoc. (2010) 109:680–3. doi: 10.1016/S0929-6646(10)60110-6
19. Perez-Alonso AJ, Husein-Elahmed H, Duran CP, Caballero-Marcos L, Ramon JA. Isolated muscle tuberculosis. Med Mal Infect. (2011) 41:559–60. doi: 10.1016/j.medmal.2011.05.002
20. Arora S, Sabat D, Sural S, Dhal A. Isolated tuberculous pyomyositis of semimembranosus and adductor magnus: a case report. Orthop Surg. (2012) 4:266–8. doi: 10.1111/os.12011
21. Elshafie KT, Al-Hinai MM, Al-Habsi HA, Al-Hattali MS, Hassan O, Al-Sukaiti R, et al. massive tuberculosis abscess at the erector spinae muscles and subcutaneous tissues in a young man. Sultan Qaboos Univ Med J. (2013) 13:601–5. doi: 10.12816/0003325
22. Neogi DS, Bandekar SM, Chawla L. Skeletal muscle tuberculosis simultaneously involving multiple sites. J Pediatr Orthop B. (2013) 22:167–9. doi: 10.1097/BPB.0b013e328354b04d
23. Sökücü S, Sökücü SN, Kabukçuoglu Y, Kabukçuoglu F. Primary skeletal muscle tuberculosis at an unusual site. J Pak Med Assoc. (2013) 63:126–8.
24. Meena M, Dixit R, Samaria JK, Kumaresan SHV. Tuberculosis of the triceps muscle. BMJ Case Rep. (2015) 2015:bcr-2014-207032. doi: 10.1136/bcr-2014-207032
25. Dabó H, Mineiro A, Carmelino J, Carvalho A, Gomes C. Skeletal muscle tuberculosis in an immunocompetent patient. Arch Bronconeumol. (2016) 52:340–1. doi: 10.1016/j.arbr.2015.10.013
26. Grigorakos L, Sgountzos V, Lazarescu D, Simopoulou S, Gkouni M, Markou N, et al. Primary thoracic muscle tuberculosis: two case reports. J Med Case Rep. (2016) 10:229. doi: 10.1186/s13256-016-0996-2
27. Kotecha D, Sardar M, Latimer MD. Tuberculosis presenting as a 'swollen calf'. BMJ Case Rep. (2016) 2016:bcr2016216340. doi: 10.1136/bcr-2016-216340
28. Alaya Z, Osman W. Isolated muscular tuberculosis: unusual location of the Koch bacillus. Pan Afr Med J. (2017) 26:158. doi: 10.11604/pamj.2017.26.158.11795
29. Al-Khazraji A, Takher J, Alkhawam H, Fabbri M. Primary tuberculous pyomyositis of the calf muscles. Am J Med Sci. (2017) 353:187–8. doi: 10.1016/j.amjms.2016.05.010
30. Fahad S, Baloch N, Din NU. Tuberculosis of the flexor carpi radialis muscle - a case report. J Pak Med Assoc. (2020) 70:1645–7. doi: 10.5455/JPMA.40799
31. Mohandes AF, Karam B, Alrstom A, Alasadi L, Bek MWR, Daher N, et al. Primary psoas tuberculosis abscess with an iliac bone lytic lesion: a case report. J Med Case Rep. (2022) 16:209. doi: 10.1186/s13256-022-03417-4
32. Zhang D, Qiao K, Zhou Z, Jiang J. Diagnostic difficulties in muscular tuberculosis coexistent with nasopharyngeal carcinoma. Jpn J Clin Oncol. (2022). doi: 10.1093/jjco/hyac084
33. Guler R, Ozturk M, Sabeel S, Motaung B, Parihar SP, Thienemann F, et al. Targeting molecular inflammatory pathways in granuloma as host-directed therapies for tuberculosis. Front Immunol. (2021) 12:733853. doi: 10.3389/fimmu.2021.733853
34. Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician. (2007) 75:695–700.
35. Gruber L, Gruber H, Luger AK, Glodny B, Henninger B, Loizides A. Diagnostic hierarchy of radiological features in soft tissue tumours and proposition of a simple diagnostic algorithm to estimate malignant potential of an unknown mass. Eur J Radiol. (2017) 95:102–10. doi: 10.1016/j.ejrad.2017.07.020
Keywords: tuberculosis, muscular tuberculosis, muscular mass, acid-fast bacilli stain, polymerase chain reaction
Citation: Zhu X-w, Luan X-h, Jiang K-l, Zhang C, Liu S-h, Cao L, Zhong P and Liu Z-y (2023) Case report: Muscular tuberculosis with lower-extremity muscular masses as the initial presentation: Clinicopathological analysis of two cases and review of the literature. Front. Med. 10:1106412. doi: 10.3389/fmed.2023.1106412
Received: 23 November 2022; Accepted: 13 February 2023;
Published: 14 March 2023.
Edited by:
Weiren Luo, The Second Affiliated Hospital of Southern University of Science and Technology, ChinaReviewed by:
Itu Singh, The Leprosy Mission Trust India, IndiaMaria Korzeniewska-Kosela, National Institute of Tuberculosis and Lung Diseases, Poland
Copyright © 2023 Zhu, Luan, Jiang, Zhang, Liu, Cao, Zhong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zhong, drzp1966@163.com; Zhi-yan Liu, zhiyanliu@shsmu.edu.cn
†These authors have contributed equally to this work
 Xiao-wei Zhu1†
Xiao-wei Zhu1†  Li Cao
Li Cao Ping Zhong
Ping Zhong Zhi-yan Liu
Zhi-yan Liu