- 1State Key Laboratory of Agricultural Microbiology, College of Life Science and Technology, Huazhong Agricultural University, Wuhan, China
- 2Key Laboratory of Agro-Microbial Resource and Development, Ministry of Agriculture, Wuhan, China
Poly-3-hydroxybutyrate (PHB) is a natural polymer synthesized by many bacteria as a carbon-energy storage material. It was accumulated maximally prior to the spore formation but was degraded during the process of sporulation in Bacillus thuringiensis. Intriguingly, B. thuringiensis also accumulates large amounts of insecticidal crystal proteins (ICPs) during sporulation, which requires considerable input of carbon and energy sources. How PHB accumulation affects sporulation and ICP formation remains unclear to date. Intuitively, one would imagine that accumulated PHB provides the energy required for ICP formation. Yet our current data indicate that this is not the case. First, growth curves of the deletion mutants of phaC (encoding the PHB synthase) and phaZ (encoding the PHB depolymerase) were found to be similar to the parent strain BMB171; no difference in growth rate could be observed. In addition we further constructed the cry1Ac10 ICP gene overexpression strains of BMB171 (BMB171-cry), as well as its phaC and phaZ deletion mutants ΔphaC-cry and ΔphaZ-cry to compare their spore and ICP production rates. Again, not much change of ICP production was observed among these strains either. In fact, PHB was still degraded in most ΔphaZ-cry cells as observed by transmission electron microscopy. Together these results indicated that there is no direct association between the PHB accumulation and the sporulation and ICP formation in B. thuringiensis. Some other enzymes for PHB degradation or other energy source may be responsible for the sporulation and/or ICP formation in B. thuringiensis.
Introduction
Bacillus thuringiensis is a ubiquitous Gram-positive, rod-shaped, and spore-forming bacterium that produces poly-3-hydroxybutyrate (PHB) and insecticidal crystal proteins (ICPs). PHB is a linear biopolymer consisting of (R)-3-hydroxybutyrate (3HB) monomers. It can be accumulated as insoluble cytoplasmic granules under over-nutrition state and/or in the absence of one or more essential nutritional elements in B. thuringiensis (Jendrossek, 2009). When energy supplies are exhausted, it can then be served as an alternate energy source (Anderson and Dawes, 1990; Jendrossek and Pfeiffer, 2014; Prieto et al., 2016).
The biochemical pathways for PHB synthesis and degradation have been studied in great details in bacteria such as Ralstonia eutropha H16, B. megaterium, Rhodospirillum rubrum, and so on (Jendrossek and Handrick, 2002). The PHB synthesis requires three enzymatic steps, starting from the condensation of two molecules of acetyl-coenzyme A to form one molecule of acetoacetyl-CoA (condensation reaction, catalyzed by the PhaA thiolase), followed by the reduction of acetoacetyl-CoA to 3-hydroxybutyryl-CoA (3HB-CoA) (reduction reaction, catalyzed by the PhaB reductase), the monomeric precursor for PHB. Finally, the 3HB-CoA monomers are polymerized to form PHB by the PHB synthase (PhaC) (Anderson and Dawes, 1990; Steinbuchel et al., 1992; Madison and Huisman, 1999; Chen, 2009). Likewise, three enzymes of PhaZ, BdhA and AacS are involved for its degradation, in which the most crucial step is the depolymerization of PHB into 3HB-CoA catalyzed by the PHB depolymerase (PhaZ). This enzyme is currently the only known PHB depolymerase observed to date (Tseng et al., 2006; Jendrossek and Pfeiffer, 2014).
The process of sporulation and ICP accumulation requires considerable input of carbon and energy sources (Wang et al., 2013a,b). As early paper (Slepecky and Law, 1961) reported that PHB fueled the sporulation process in B. megaterium. It was also reported that PHB was accumulated maximally prior to the spore formation and was degraded during the process of sporulation in B. cereus (Kominek and Halvorson, 1965; Valappil et al., 2007). However, how PHB affects sporulation and parasporal crystal formation is still controversial to date. It is generally believed that PHB degradation can provide energy and carbon sources required for the sporulation and parasporal crystal formation. For example, Navarro et al. (2006) found a linear relationship between the PHB accumulation and the parasporal crystal formation in B. thuringiensis. Recently, a phaC deletion mutant from B. thuringiensis BMB 171 was constructed, and found to result in a growth delay and sporulation-deficient phenotype (Chen et al., 2012). However, another group reported that spore formation is not impaired in a phaC deletion mutant in B. thuringiensis (Chen et al., 2010). In many cases, utilization of PHB does not seem to be imperative for sporulation, because many spore-forming genus Bacillus that cannot synthesize PHB still sporulate normally. Most Bacillus species such as B. subtilis exhibits natural PHB-negative phenotype, and bioimformatic analysis reveals no known pha genes or sequences in their genomes (Singh et al., 2009), indicating that PHB has no direct correlation with the bacterial sporulation. McCool and Cannon (2001) constructed a phaPQRBC operon deletion strain of B. megaterium. However, other than the PHB-negative phenotype, it showed no apparent phenotypic difference and exhibited similar growth rate as its progenitor. Based on these conflicting results, the relationship between PHB accumulation and sporulation and ICP formation still remains obscure. In this study, we have used the markerless gene deletion method, which is believed to cause the least perturbation to genome, to knock-out the phaC and phaZ genes respectively in order to carefully examine the influence of PHB on sporulation and ICP formation in B. thuringiensis BMB171.
Materials and Methods
Strains, Plasmids, Primers and Growth Conditions
The strains and plasmids, as well as the primers used in this study, were listed in Table 1 and Table S1, respectively. Escherichia coli were cultured at 37°C in lysogeny broth (LB) medium (g/L: tryptone, 10; yeast extract, 5; NaCl, 10). The medium was adjusted to pH 7.0 before autoclaving at 121°C for 15 min. Unless otherwise specified, B. thuringiensis strains were cultured at 28°C in the GYS medium (g/L: glucose, 1.00; yeast extract, 2.00; K2HPO4⋅3H2O, 0.66; (NH4)2SO4, 2.00; MgSO4⋅7H2O, 0.04; MnSO4⋅H2O, 0.04; CaCl2, 0.08). The medium was autoclaved at 115°C for 30 min after adjusting the pH to 7.8.
Construction of the Integrating Plasmids for Gene Deletion
To construct the plasmids for phaC (BMB171_RS06655, old locus lag BMB171_C1161) and phaZ (BMB171_RS16270, old locus lag BMB171_C2975) deletions, upstream homologous arms of UphaC and UphaD and downstream homologous arms of DphaC and DphaZ (homologous to the 5′ and 3′ uncoding regions of the target genes) of approximately 750 bp were amplified from the BMB171 genomic DNA by PCR, using primer pairs of DphaCU F/DphaCU R, DphaCD F/DphaCD R, DphaZU F/DphaZU R and DphaZD F/DphaZD R, respectively (Table S1). Then, UphaC (or UphaZ) was inserted into plasmid pRP1028 between the Mlu I and BamH I sites to construct the plasmid pRP1028-UphaC (or pRP1028-UphaZ). Next, DphaC (or DphaZ) was inserted into plasmid pRP1028-UphaC (or pRP1028-UphaZ) between the BamH I and Kpn I sites to construct the integrating plasmid pRP1028-UDphaC (pRP1161) [or pRP1028-UDphaZ (pRP2975)] (Figure S1). pRP1028 was a shuttle plasmid with a temperature-sensitive suicide B. thuringiensis replicon. The resulting integrating plasmids were further verified by sequencing.
Construction of phaC and phaZ Deletion Strains
The markerless gene deletion system was successfully developed for BMB171 based on an I-SceI mediated replacement method as established in B. anthracis by Janes and Stibitz (2006). The detailed procedures have been well described in a previous publication (Zheng et al., 2015), and will only be briefly accounted here taking deletion of phaC as the example. Firstly, BMB171 (recipient strain), E. coli DH5α containing the integrating plasmid pRP1028-UDphaC (donor strain) and E. coli DH5α containing the helper plasmid pSS1827 (helper strain) used for conjugational transfer (triparental mating) were grown in LB medium. The plasmid pRP1028-UDphaC was integrated into the BMB171 chromosome to form the plasmid-integrated strain by homologous single cross-over recombination. Plasmid-integrated strains were verified by PCR using primer pairs IphaC F/UniversalI R (Table S1). Secondly, the expression plasmid pSS4332 (in DH5α-pSS4332), which produced the restriction endonuclease I-SceI, was conjugationally transfered into the plasmid-integrated strain by a triparental mating system with the help of E. coli DH5α-pSS1827 as well. The I-Sce I endonuclease can recognize and cleave the highly specific 18 bp DNA target site within the integrated plasmid, resulting in chromosomal double-stranded break and thus stimulated the host genetic repair by homologous recombination between the flanking repeat sequences. As a result, there is approximately 50% possibility to delete the target gene from chromosome. Positive deletion strains of phaC were verified by PCR and sequenced (Figures S2 and S3). Finally, the plasmid pSS4332 in the phaC deletion strain was eliminated by continuous passage for ten times in the LB medium at 28°C. Those strains in which the pSS4332 plasmid was removed were sensitive against kanamycin. Deletion of phaZ was performed using the same method (Figures S2 and S4).
RNA Extraction, cDNA Synthesis and RT-qPCR
Twenty mililiter of a cultured sample at 48 h in LB medium was collected, and pelleted cells were grounded in liquid nitrogen. The procedures for RNA extraction and cDNA synthesis were carried out as previously described (Wang et al., 2013b; Zheng et al., 2015). Each experiment was repeated in triple.
Extraction and Determination of ICP Concentrations
Strains of BMB171-cry, ΔphaC-cry and ΔphaZ-cry were obtained by transformation of the cry1Ac10 promoter and cry gene-containing plasmid (pBMB43-304) (Qi et al., 2015) into the BMB171, ΔphaC and ΔphaZ strains, respectively. pBMB43-304 is a derivative of the low copy number shuttle plasmid pHT304 (Arantes and Lereclus, 1991), into which a cry gene was inserted between the two HindIII sites. The plasmid pBMB43-304 was maintained by growing the bacteria in the presence of 25 μg/mL erythromycin. For the extraction of the ICP (Cry1Ac10), strains were grown at 28°C and 200 rpm for 20 h in GYS medium supplemented with 25 μg/mL erythromycin. After optical density measurement, cells were diluted to a final OD600 of 1.0, and 20 mL of each culture was separately collected by centrifuge at 6000 × g for 15 min (AG Eppendorf, Hamburg, Germany). Procedure for the extraction of the ICP was carried out according to a previous study (Wang et al., 2013a). Finally, the ICP was visualized by SDS-PAGE and the ICP concentrations were measured by the Bradford method.
PHB Assays
The PHB contents of B. thuringiensis cells were determined by UV absorption spectroscopy at 245 nm (Law and Slepecky, 1961) using PHB extracted from BMB171 as standard.
Transmission Electron Microscopy
Sample preparation for transmission electron microscopy was carried out according to the method described in the literature (Tian et al., 2005) with slight modification. Strains were collected at 9 and 19 h, centrifuged at 6000 × g for 3 min, and washed three times with PBS buffer (pH 7.2). Pellets were then resuspended in 2.5% glutaraldehyde phosphate buffer, fixed at 4°C for 24 h. After washing three times with PBS buffer (pH 7.2), cells were dehydrated gradually using different concentrations of ethanol (20%, 50%, 70%, 80%, 90%, and 100%). Each process was carried out twice for 15 min each. Samples were stored in vacuum freeze drier overnight. Thin sections were examined on a HITACHI H-7000FA transmission electron microscope (Hitachi, Ibaraki, Japan).
Virulence Assays
The larvae of Heliothis armigera were reared at 28°C with a light/dark cycle of 12:12 h. Artificial diet comprised 4 g yeast extraction, 7 g bean flour, 0.5 g vitamin C, 1.5 g agar, 36% acetic acid and 1 g penicillin per 100 mL of water. The medium was transferred into 24-hole cell culture plates (Corning, USA), 1 mL per well. 200 mL of cells grown in GYS medium for 20 h were harvested by centrifugation (8,500 × g, 5 min, 4°C) and suspended in 20 mL distilled water. Cells were then diluted 10,000-fold, with 100 μl of diluted cells applied to each well, allowing it to dry automatically. One first-instar larvae was used for feeding assay per well, with mortality recorded at indicated dates. Meanwhile, body length and weight were measured at the 6th day. Three repeats per bioassay were performed using 24 larvae for each strain (Fiuza et al., 2013).
Spore Count
Besides BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains, BMB171 and BMB171-pHT (BMB171 harboring empty plasmid pHT304) strains were used as controls. OD600 of each strain grown in the LB medium at 28°C for 72 h were measured, and diluted to a final OD600 of 1.0. Cells were then heated to 65°C for 30 min, followed by gradient dilution (ten times), after that 100 μL of a series of diluents were spreaded onto LB plates. CFU (Colony-forming units) per mL were then counted.
Results
PHB Contents Changed upon phaC or phaZ Deletion
In previous reports, phaC and phaZ were identified as the genes encoding PHB synthetase and degradation enzyme in B. thuringiensis respectively (Tseng et al., 2006; Chen et al., 2010). To disrupt the PHB metabolism pathway, phaC and phaZ were deleted from the parent strain BMB171 by the markerless gene deletion method, with the corresponding strains named as ΔphaC and ΔphaZ, respectively. The growth curves of BMB171, ΔphaC andΔphaZ strains in GYS medium at 28°C were determined. No obvious difference was observed for all three strains, and they all entered the stationary phase at approximate 11 h (Figure 1).
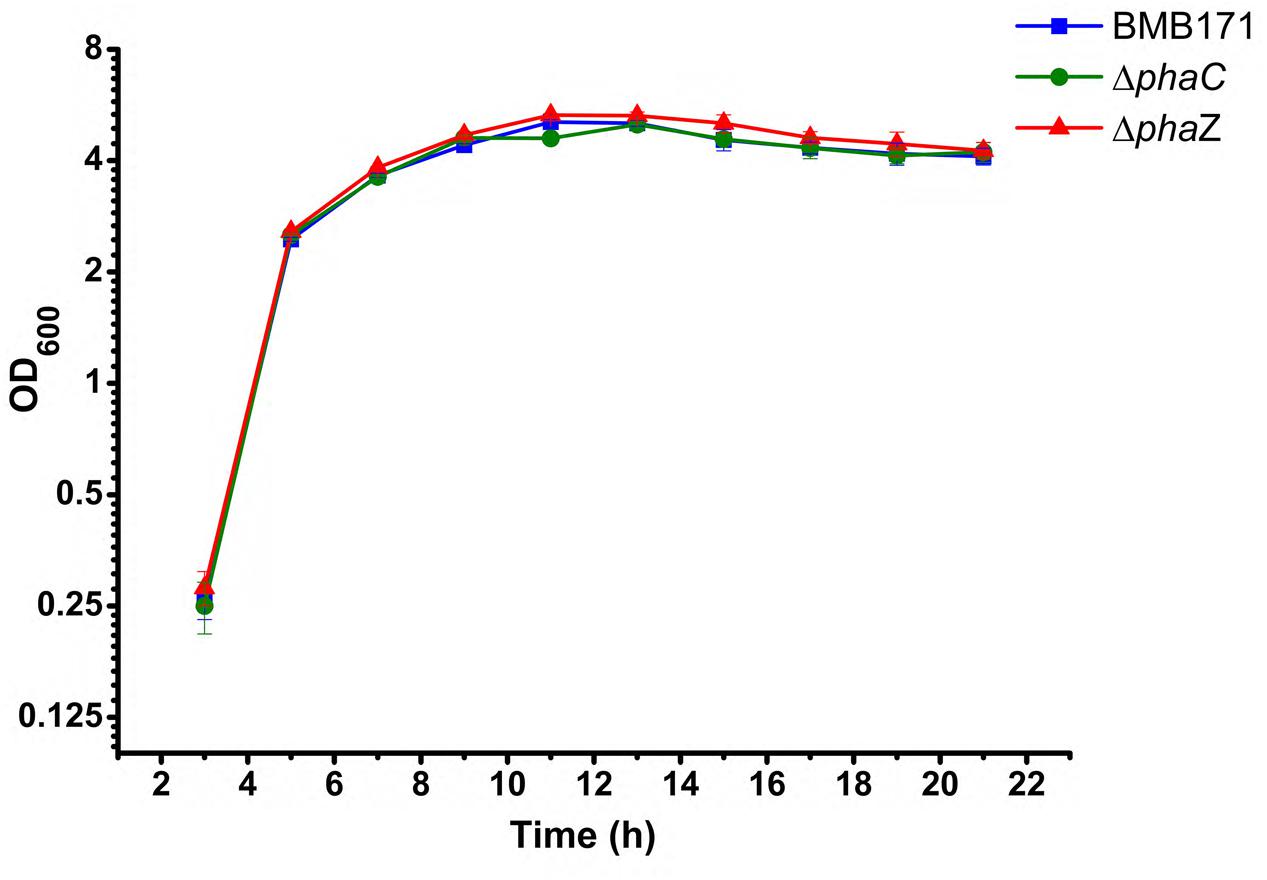
FIGURE 1. Growth curves of the BMB171, ΔphaC and ΔphaZ strains in the GYS medium at 28°C. A bar for each sampling time point represents standard error of the mean from three batches.
The amount of PHB produced was measured during the whole growth period. For the parent strain BMB171, it was found that its intracellular PHB level started to accumulate from 3 h and reached to a maximum level at 11 h, after which it descended rapidly to half amount at about 16 h and approximately to zero at 21 h (Figure 2). No PHB could be detected in the ΔphaC mutant over the whole growth cycle, indicating that PHB accumulation in the ΔphaC mutant was totally abolished. This data indicated that PhaC was absolutely required for the PHB synthesis (Figure 2). As for the ΔphaZ mutant, the accumulation stage was similar, but there was a delay in the degradation stage. It began to degrade at 16 h, with about half amount left at 21 h. Since PHB degradation wasn’t completely abolished in ΔphaZ, we speculated that there were some other PHB degradation enzymes or pathways existing in B. thuringiensis.
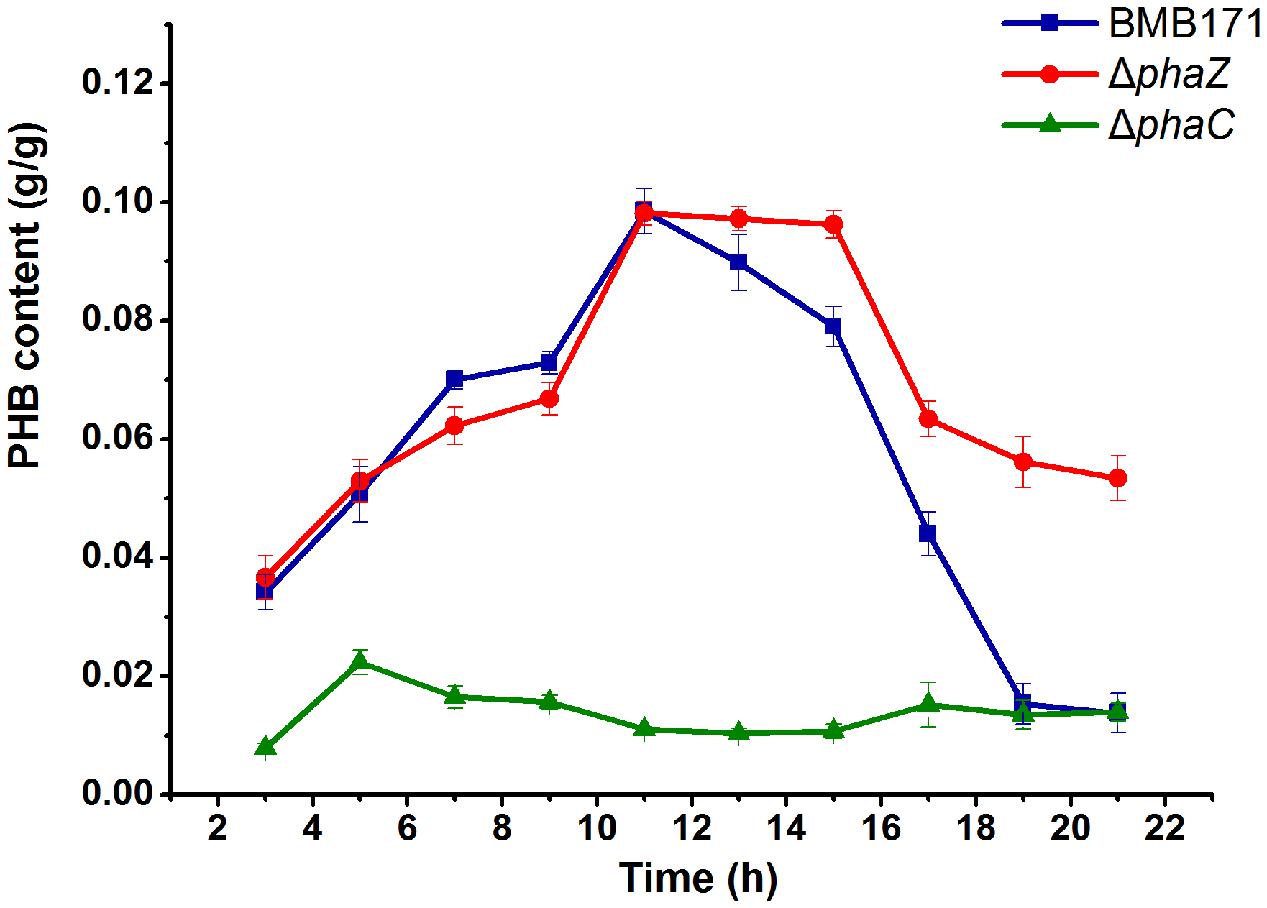
FIGURE 2. PHB contents of BMB171, ΔphaC and ΔphaZ cells. Cells of indicated strains were grown on the GYS medium at 28°C. Samples were taken at selected time points. Cell dry weight (g/L) and PHB concentration (g/L) were measured, respectively. PHB content (g/g) was calculated by dividing the PHB concentration by the cell dry weight. Each experiment was carried out in triplicate, mean values and standard deviations were calculated.
Cell Morphology Didn’t Change in the phaC and phaZ Deletion Strains
BMB171 is an acrystalliferous strain that doesn’t produce ICPs (Li et al., 2000; He et al., 2010). To investigate its morphological changes in the absence of phaC and phaZ, the ICP coding gene cry1Ac10-containing plasmid pBMB43-304 (Qi et al., 2015) needs to be introduced into this strain, and the ΔphaC and ΔphaZ mutants. The corresponding strains were named as BMB171-cry, ΔphaC-cry and ΔphaZ-cry, respectively. Cell morphologies during the exponential phase and sporulation phase were observed using transmission electron microscope. The cell size and shape of the mutants were found to be similar as the parent strain (Figure 3). The spore and parasporal crystal morphologies of ΔphaC-cry and ΔphaZ-cry also exhibited no major difference compared to those of BMB171-cry under the similar growth conditions.
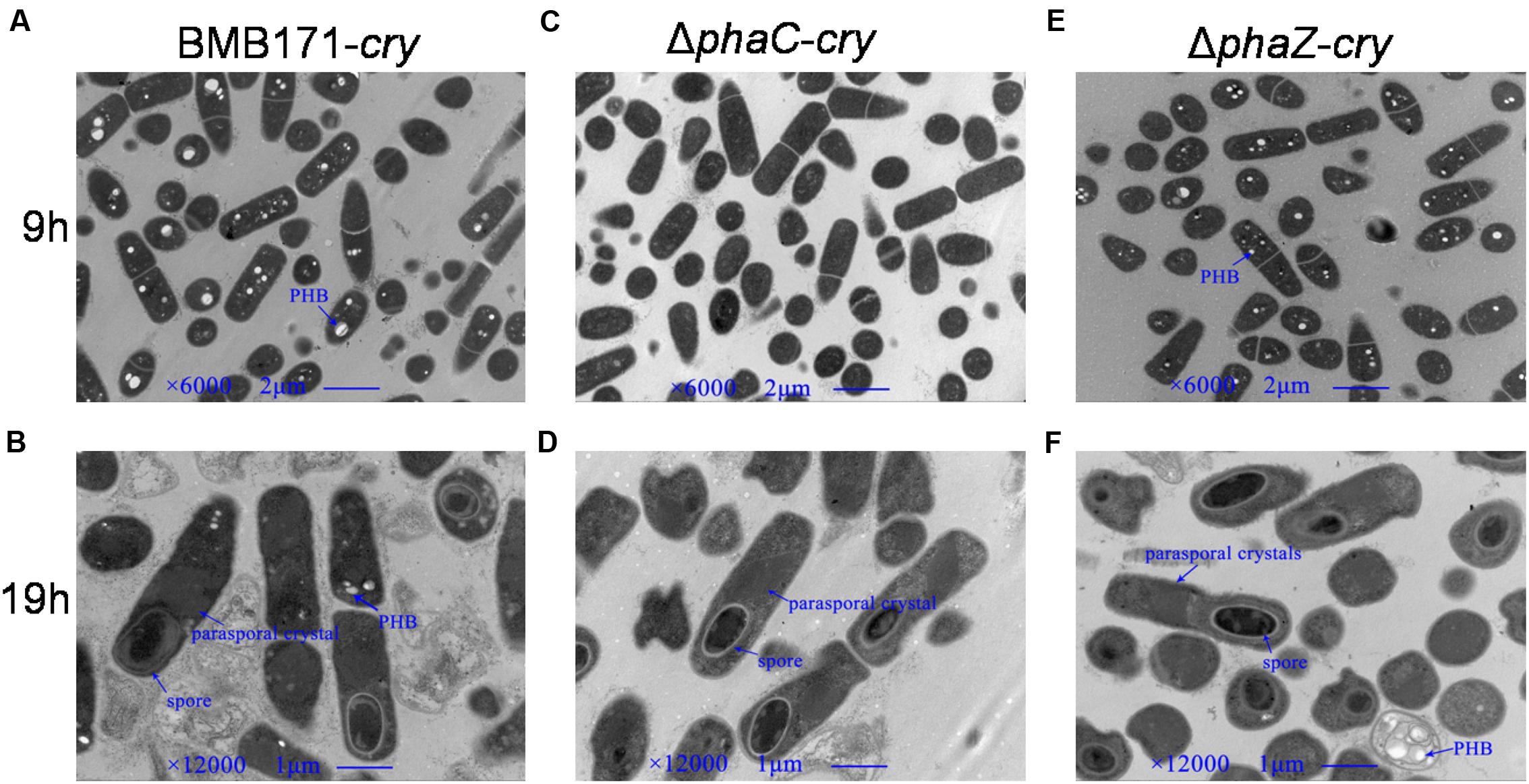
FIGURE 3. Transmission electron microscopic observations of PHB (white granule), spore (black ellipse) and parasporal crystal (dark diamond). (A,C), and (E), the electron micrographs of BMB171-cry, ΔphaC-cry and ΔphaZ-cry at 9 h; (B,D), and (F), the corresponding micrographs at 19 h.
ICP Formation and Toxicity Were Not Changed in the ΔphaC-cry and ΔphaZ-cry Strains
To be more quantitative, we have further measured the ICP amounts to investigate the influence of phaC or phaZ deletion on ICP formation. As shown in Figure 4, all the three strains produce the ICP without conspicuous difference in their amounts (“ns” indicates not significant, P > 0.05). It is well known that ICPs are virulent to the Helicoverpa armigera larvae. To test whether the toxicities differ in these strains, virulence assays were also performed and the survival rates of the H. armigera larvaes fed with these bacteria were measured (H2O and BMB171 were set as control groups). Table 2 shows that the survival rates of BMB171-cry, ΔphaC-cry and ΔphaZ-cry were almost similar even after six days (ns, P > 0.05). The body length and weight also didn’t differ much among these three strains (ns, P > 0.05). Together, these results indicate that the insecticidal activities of the ICP produced by the three strains were comparable.
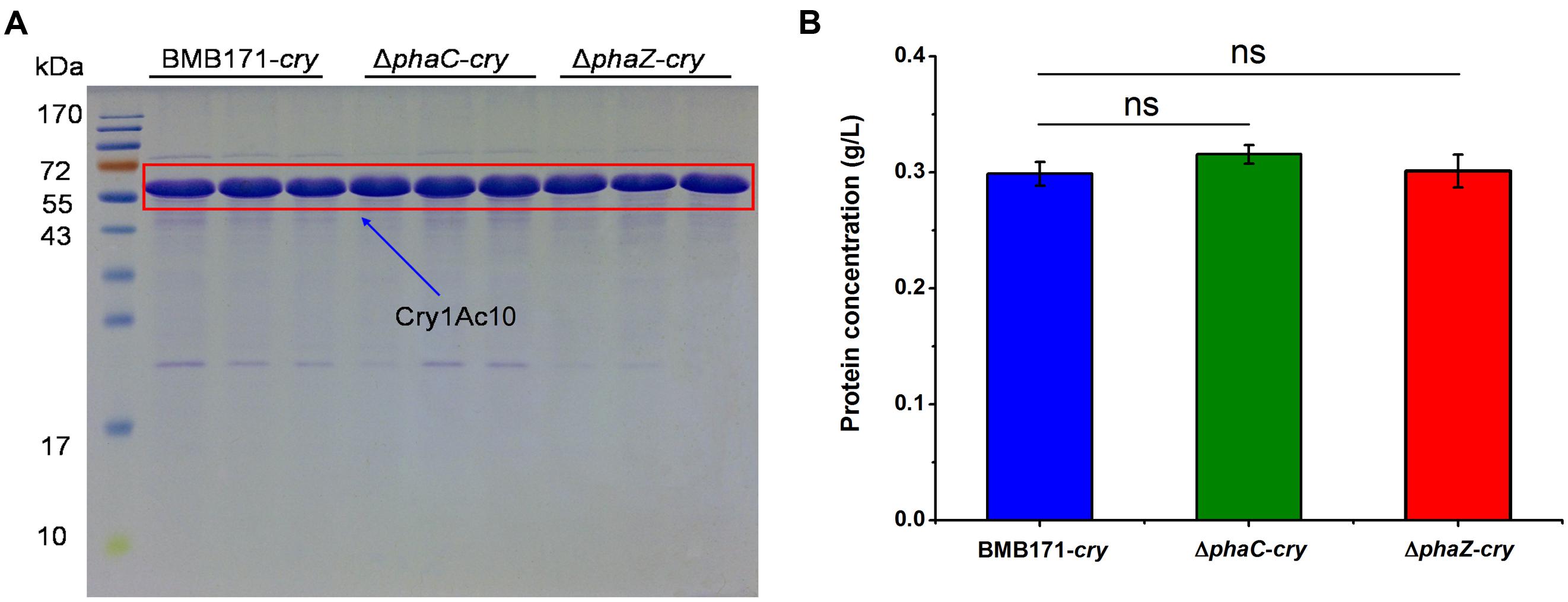
FIGURE 4. Effect of phaC or phaZ deletion on ICP formation. (A) ICP in BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains was separated by SDS-PAGE. ICP was boxed in red; (B) ICP concentrations measured in BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains. The values were means ± standard deviations for triplicate assays. Significances of differences by Student’s t-test are indicated (ns, P > 0.05).
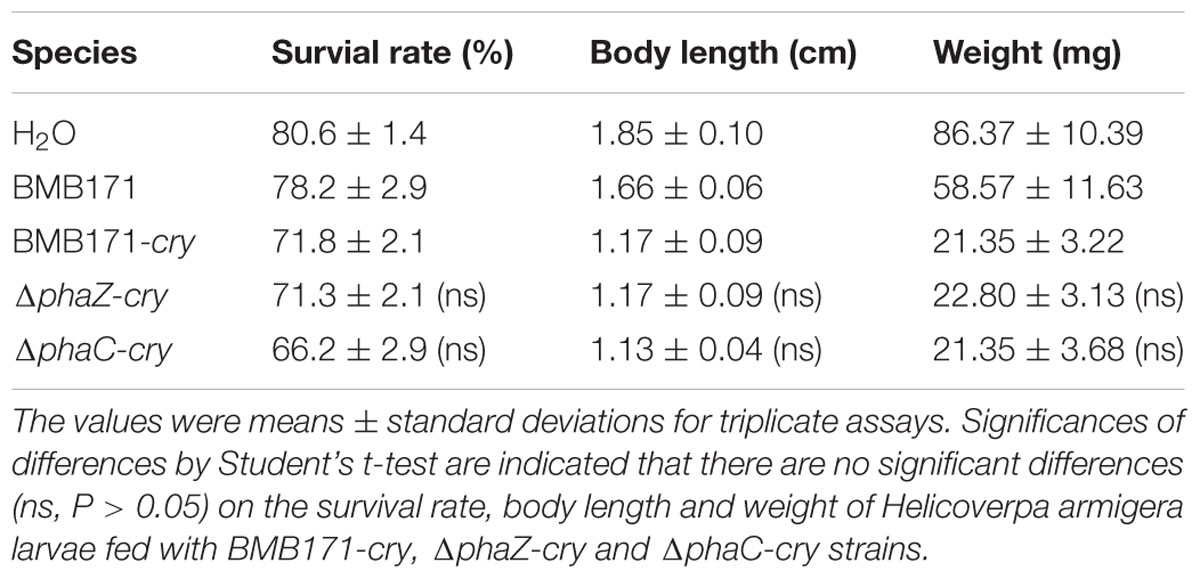
TABLE 2. Survival rates of Helicoverpa armigera larvae fed with BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains.
PhaC or PhaZ Is Not Required for Sporulation
As observed by the transmission electron microscopy in the Figure 3, both the ΔphaC-cry and ΔphaZ-cry strains are able to sporulate similar to the BMB171-cry strain, which is different to the previous result that sporulation was abolished when phaC was deleted from BMB171 (Chen et al., 2012). It is thus important to learn what caused the difference. To make the comparison more objective, three B. thuringiensis strains were cultivated in the same condition as Chen’s experiment, and their spore numbers were counted. No significant difference for the BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains (Figure 5) seems to be present (ns, P > 0.05). In addition, several sporulation-related genes were selected to measure their relative expression levels by RT-qPCR experiments using 16S rRNA gene as an internal control. The sporulation-related genes include those expressed in the pre-divisional cells (spoIIAB and spoIIGA), early mother cells (spoIID) and late mother cells (cotH and gerE) (de Hoon et al., 2010). If the sporulation pathway was impaired in those strains, gene expression level will change. Our data showed that the transcription levels of most sporulation-related genes such as spoIIAB, spoIIGA, spoIID, cotH and gerE didn’t change significantly (Figure 6, ns, P > 0.05). Taken together, these results indicate that the spore-forming ability and transcription of sporulation-related genes were not much impaired in the three strains demonstrating that there is no relationship between the PHB accumulation and sporulation in B. thuringiensis.
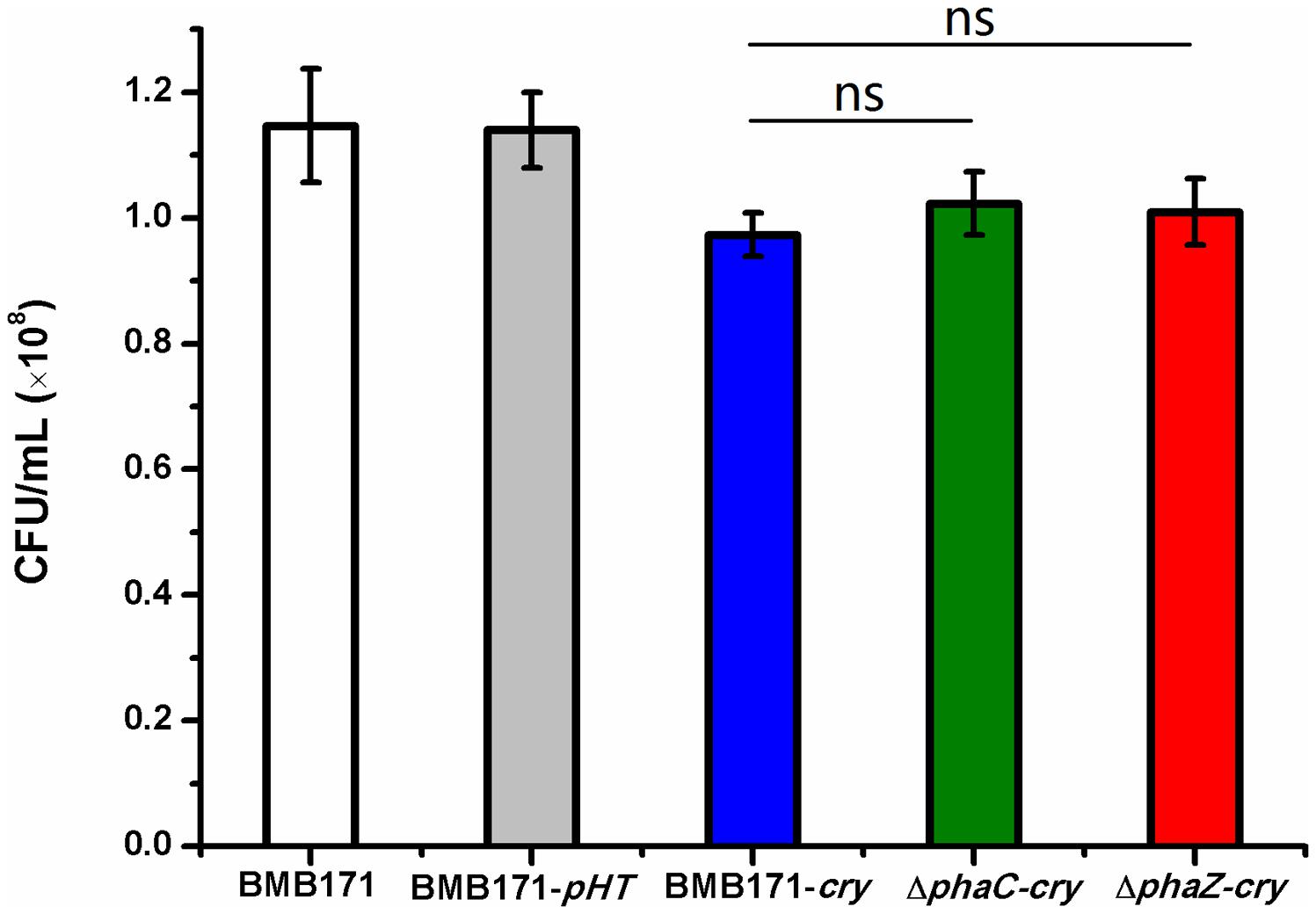
FIGURE 5. Spore formation in BMB171, BMB171-pHT, BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains in LB at 72 h. Spore numbers per mL were indicated by CFU/mL. Each experiment was carried out in triplicate, with mean values and standard deviations calculated. Significances of differences among BMB171-cry, ΔphaC-cry and ΔphaZ-cry by Student’s t-test are indicated (ns, P > 0.05).
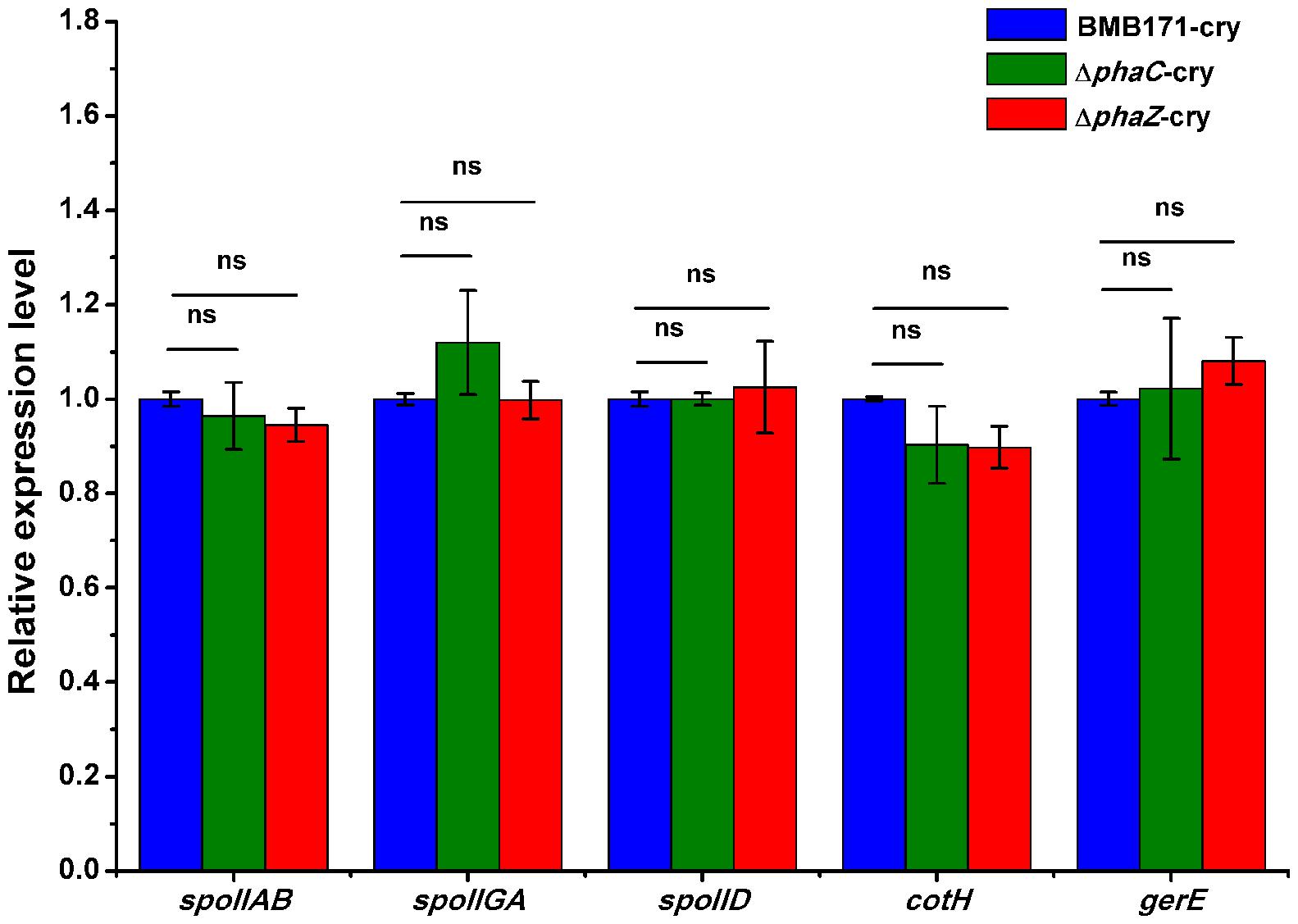
FIGURE 6. The relative expression levels of sporulation-related genes in BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains quantitated by RT-qPCR. The values were means ± standard deviations for triplicate assays. Significances of differences by Student’s t-test are indicated (ns, P > 0.05).
Bioinformatics Analysis Revealed That There Is No Relationship between the PHB Metabolism and the Sporulation in the Genus Bacillus
To further explore the relationship between PHB metabolism and sporulation, we expanded our investigation further through bioinformatics analysis. Since almost all species in the genus Bacillus except B. infantiscan sporulate, we wondered whether those species can also produce PHB. Distribution of phaC and phaZ genes in the 79 strains of the genus Bacillus with complete genomes from NCBI were investigated (Table S2). Screening of metabolic (KEGG) and genomic (NCBI) database for phaC and phaZ genes revealed that they were absent in the B. subtilis group and some other strains in genus Bacillus (Table S3). Since PhaC is required for PHB synthesis, we speculate that there is no PHB production in those strains lacking the phaC gene. Therefore, no relationship between the PHB accumulation and sporulation could be revealed from this bioinformatics study.
Discussion
In this study, cell morphologies of BMB171-cry, ΔphaC-cry and ΔphaZ-cry strains during the exponential and sporulation phases were observed using transmission electron microscope and phase contrast microscope. Interestingly, not much difference in their morphologies were observed. Other features, such as spore production rate and ICP concentration, were also carefully checked. Again, no significant change about these two characteristics was observed among the three strains. These observations were consistent with the data reported by McCool and Cannon (2001) and Chen et al. (2010), in which depletion of PHB metabolism pathway did not affect the sporulation and ICP formation. However, Chen et al. (2012) reported a conflict result even in the same B. thuringiensis strain. Comparison of methodologies used in these two experiments revealed that the only difference was the gene knockout method. In our study, we employed a markless gene deletion method to construct the ΔphaC and ΔphaZ mutant strains, while in Chen’s publication, a traditional knockout method was used, which required a higher screening temperature (42°C) that is close to the lethal temperature (around 45°C) of B. thuringiensis (Wu et al., 2011). This temperature difference could lead to a devastating effect on the physiological and genetic properties of this bacterium. Moreover, a resistance gene that was introduced into the chromosome to replace the target gene also increased the possibility of phenotypic change. Thus, we speculated that mutations in other genes other than phaC may lead to the lack of sporulation and ICP formation. As a matter of fact, we occasionally observed strains with abnormal phenotype when traditional knockout method was applied. If these unsure knockout strains were chosen for genetic study, inconsistent conclusions could be drawn. Based on our practice in the deletion of hundreds of genes in BMB171, we concluded that this system is easy to operate and allows for clean deletions of one or more genes within an operon (Zheng et al., 2015; Zhang et al., 2015, 2016). Because there is no need for a higher screening temperature, undesirable mutations could decrease, resulting with much more consistent results. Therefore, we strongly suggest using the I-secI endonuclease markless gene knockout method for gene knockout studies in B. thuringiensis and even in B. cereus group strains.
PHB was generally believed to serve as a sink of carbon and reducing equivalents. However, this doesn’t rule out the possibility that bacteria may use other metabolic pathway to provide the energy required for sporulation and ICP formation. PHB oxidation involves a specific NADH dependent dehydrogenase (PhaB) (Madison and Huisman, 1999), which competes for tricarboxylicacid (TCA) cycle intermediates (for example, NADH, NADPH, ATP, and acetyl-CoA) in the electron transport system. When PHB accumulation is disrupted, more resources are accessible to TCA cycle. Transcriptome analyses of the wild type and mutated Pseudomonas putida showed that lack of PHA accumulation in the mutant altered transcription of many genes coding for enzymes involved in the central metabolic pathways, including carbohydrate, fatty acid, amino acid, nucleotide, cofactor and prosthetic group synthesis pathways (Escapa et al., 2012). Meanwhile, when the PHB-negative mutant R. eutropha PHB-4 and its wild type were subjected to proteome analyses, higher amounts of acetyl-CoA and pyruvate in the PHB-negative mutant were observed (Raberg et al., 2014). From these results we speculate that when PHB biosynthetic pathway is disrupted, other metabolic pathway could take place to provide the energy required for sporulation and ICP formation (Senior and Dawes, 1973; Jackson and Dawes, 1976; Page and Knosp, 1989; Aldor and Keasling, 2003).
Due to the absence of PHB depolymerase, ΔphaZ-cry may lost the ability to degrade PHB during stationary phase. However, when phaZ was deleted, most bacterial cells can still degrade PHB through an unknown mechanism. Thus deletion of phaZ didn’t seem to completely abolish PHB degradation. We speculate that some other pathways may be present to degrade PHB. In fact, many have tried to identify and isolate the PHB depolymerse. PHB is present in two different physical forms, an intracellular native PHB granule form (nPHB) and an extracellular denatured PHB granule form (ePHB). The nPHB exists in an amorphous state and is always coated with various proteins and phospholipase, while the ePHB is a partially crystalline polymer (Jendrossek and Handrick, 2002). The degradation enzymes for nPHB and ePHB displayed a low sequence similarity with rather different biochemical properties (Saegusa et al., 2001; York et al., 2003). Although the ePHB degradation enzyme has been extensively investigated in P. lemoignei (Kumar et al., 2000; Schober et al., 2000), not much is known about the nPHB degradation yet. Most methods to identify new nPHB depolymerase is based on sequence comparison with already known depolymerase, either by comparing their phaZ genes in the other species, or phaC genes (Tseng et al., 2006; Chen et al., 2009; Sznajder and Jendrossek, 2014), or through the blast search of the consensus motif. To date, only one PHB degradation enzyme (PhaZ) was well characterized in B. thuringiensis (Tseng et al., 2006; Wang et al., 2014). Obtained results indicated that these methods all suffer from some limitations. Further investigations are required to identify the novel potential nPHB depolymerase. From the results demonstrated in our work, however, we strongly believe that there must have other PHB depolymerases existent in B. thuringiensis, and characterization of these potential PHB depolymerases is currently undergoing.
Taken together, we have verified the relationship between PHB accumulation and sporulation and ICP formation in B. thuringiensis. This investigation povides a new perspective about the relationship between the important metabolic processes in B. thuringiensis, and supply information for designing novel metabolic engineering strategies for maximizing ICPs production or controlling sporulation process.
Author Contributions
XW performed molecular biology work, participated in study design and drafted the manuscript. ZL performed the phenotype assays and study design. XL (third author) performed the transmission electron microscopy observations, extraction and determination of ICP concentrations, and drafted the manuscript. HQ carried out gene deletions and the virulence assays. XC performed PHB assay and spore counting. XL (sixth author) carried out data analysis. JH conceived the study, participated in the study design, coordinated the research, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant 31270105), the National High-tech R&D Program of China (grant 2011AA10A205), and the Fundamental Research Funds for the Central universities (grant 2662015PY175).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00836
References
Aldor, I. S., and Keasling, J. D. (2003). Process design for microbial plastic factories: metabolic engineering of polyhydroxyalkanoates. Curr. Opin. Biotechnol. 14, 475–483. doi: 10.1016/j.copbio.2003.09.002
Anderson, A. J., and Dawes, E. A. (1990). Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54, 450–472.
Arantes, O., and Lereclus, D. (1991). Construction of cloning vectors for Bacillus thuringiensis. Gene 108, 115–119. doi: 10.1016/0378-1119(91)90495-w
Chen, D., Xu, D., Li, M., He, J., Gong, Y., Wu, D., et al. (2012). Proteomic analysis of Bacillus thuringiensis delta phaC mutant BMB171/PHB(-1) reveals that the PHB synthetic pathway warrants normal carbon metabolism. J. Proteomics 75, 5176–5188. doi: 10.1016/j.jprot.2012.06.002
Chen, G. Q. (2009). A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 38, 2434–2446. doi: 10.1039/b812677c
Chen, H. J., Pan, S. C., and Shaw, G. C. (2009). Identification and characterization of a novel intracellular poly(3-hydroxybutyrate) depolymerase from Bacillus megaterium. Appl. Environ. Microbiol. 75, 5290–5299. doi: 10.1128/AEM.00621-09
Chen, H. J., Tsai, T. K., Pan, S. C., Lin, J. S., Tseng, C. L., and Shaw, G. C. (2010). The master transcription factor Spo0A is required for poly(3-hydroxybutyrate) (PHB) accumulation and expression of genes involved in PHB biosynthesis in Bacillus thuringiensis. FEMS Microbiol. Lett. 304, 74–81. doi: 10.1111/j.1574-6968.2010.01888.x
de Hoon, M. J. L., Eichenberger, P., and Vitkup, D. (2010). Hierarchical evolution of the bacterial sporulation network. Curr. Biol. 20, R735–R745. doi: 10.1016/j.cub.2010.06.031
Escapa, I. F., Garcia, J. L., Buhler, B., Blank, L. M., and Prieto, M. A. (2012). The polyhydroxyalkanoate metabolism controls carbon and energy spillage in Pseudomonas putida. Environ. Microbiol. 14, 1049–1063. doi: 10.1111/j.1462-2920.2011.02684.x
Fiuza, L. M., Knaak, N., da Silva, R. F., and Henriques, J. A. (2013). Receptors and lethal effect of Bacillus thuringiensis insecticidal crystal proteins to the Anticarsia gemmatalis (Lepidoptera, Noctuidae). ISRN Microbiol. 2013:940284. doi: 10.1155/2013/940284
He, J., Shao, X. H., Zheng, H. J., Li, M. S., Wang, J. P., and Zhang, Q. Y. (2010). Complete genome sequence of Bacillus thuringiensis mutant strain BMB171. J. Bacteriol. 192, 4074–4075. doi: 10.1128/JB.00562-10
Jackson, F. A., and Dawes, E. A. (1976). Regulation of the tricarboxylic acid cycle and poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. J. Gen. Microbiol. 97, 303–312. doi: 10.1099/00221287-97-2-303
Janes, B. K., and Stibitz, S. (2006). Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 74, 1949–1953. doi: 10.1128/IAI.74.3.1949-1953.2006
Jendrossek, D. (2009). Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J. Bacteriol. 191, 3195–3202. doi: 10.1128/JB.01723-08
Jendrossek, D., and Handrick, R. (2002). Microbial degradation of polyhydroxyalkanoates. Ann. Rev. Microbiol. 56, 403–432. doi: 10.1146/annurev.micro.56.012302.160838
Jendrossek, D., and Pfeiffer, D. (2014). New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 16, 2357–2373. doi: 10.1111/1462-2920.12356
Kominek, L. A., and Halvorson, H. O. (1965). Metabolism of poly-beta-hydroxybutyrate and acetoin in Bacillus cereus. J. Bacteriol. 90, 1251–1259.
Kumar, A., Gross, R. A., and Jendrossek, D. (2000). Poly(3-hydroxybutyrate)-depolymerase from Pseudomonas lemoignei: catalysis of esterifications in organic media. Org. Chem. 65, 7800–7806. doi: 10.1021/jo000814y
Law, J. H., and Slepecky, R. A. (1961). Assay of poly-beta-hydroxybutyric acid. J. Bacteriol. 82, 33–36.
Li, L., Yang, C., Liu, Z., Li, F., and Yu, Z. (2000). Screening of acrystalliferous mutants from Bacillus thuringiensis and their transformation properties. Wei Sheng Wu Xue Bao 40, 85–90. doi: 10.13343/j.cnki.wsxb.2000.01.015
Madison, L. L., and Huisman, G. W. (1999). Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63, 21–53.
McCool, G. J., and Cannon, M. C. (2001). PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J. Bacteriol. 183, 4235–4243. doi: 10.1128/JB.183.14.4235-4243.2001
Navarro, A. K., Farrera, R. R., Lopez, R., and Perez-Guevara, F. (2006). Relationship between poly-beta-hydroxybutyrate production and delta-endotoxin for Bacillus thuringiensis var. kurstaki. Biotechnol. Lett. 28, 641–644. doi: 10.1007/s10529-006-0029-0
Page, W. J., and Knosp, O. (1989). Hyperproduction of poly-β-hydroxybutyrate during exponential growth of Azotobacter vinelandii UWD. Appl. Environ. Microbiol. 55, 1334–1339.
Prieto, A., Escapa, I. F., Martínez, V., Dinjaski, N., Herencias, C., de la Peña, F., et al. (2016). A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ. Microbiol. 18, 341–357. doi: 10.1111/1462-2920.12760
Qi, M. X., Mei, F., Wang, H., Sun, M., Wang, G. J., Yu, Z. N., et al. (2015). Function of global regulator CodY in Bacillus thuringiensis BMB171 by comparative proteomic analysis. Microbiol. Biotechnol. 25, 152–161. doi: 10.4014/jmb.1406.06036
Raberg, M., Voigt, B., Hecker, M., and Steinbuchel, A. (2014). A closer look on the polyhydroxybutyrate- (PHB-) negative phenotype of Ralstonia eutropha PHB-4. PLoS ONE 9:e95907. doi: 10.1371/journal.pone.0095907
Saegusa, H., Shiraki, M., Kanai, C., and Saito, T. (2001). Cloning of an intracellular poly[D(-)-3-hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J. Bacteriol. 183, 94–100. doi: 10.1128/JB.183.1.94-100.2001
Schober, U., Thiel, C., and Jendrossek, D. (2000). Poly(3-hydroxyvalerate) depolymerase of Pseudomonas lemoignei. Appl. Environ. Microbiol. 66, 1385–1392. doi: 10.1128/AEM.66.4.1385-1392.2000
Senior, P. J., and Dawes, E. A. (1973). The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 134, 225–238. doi: 10.1042/bj1340225
Singh, M., Patel, S. K. S., and Kalia, V. C. (2009). Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Fact. 8:38. doi: 10.1186/1475-2859-8-38
Slepecky, R. A., and Law, J. H. (1961). Synthesis and degradation of poly-beta-hydroxybutyric acid in connection with sporulation of Bacillus megaterium. J. Bacteriol. 82, 37–42.
Steinbuchel, A., Hustede, E., Liebergesell, M., Pieper, U., Timm, A., and Valentin, H. (1992). Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol. Rev. 9, 217–230. doi: 10.1016/0378-1097(92)90313-D
Sznajder, A., and Jendrossek, D. (2014). To be or not to be a poly(3-hydroxybutyrate) (PHB) depolymerase: PhaZd1 (PhaZ6) and PhaZd2 (PhaZ7) of Ralstonia eutropha, highly active PHB depolymerases with no detectable role in mobilization of accumulated PHB. Appl. Environ. Microbiol. 80, 4936–4946. doi: 10.1128/AEM.01056-14
Tian, J., Sinskey, A. J., and Stubbe, J. (2005). Kinetic studies of polyhydroxybutyrate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J. Bacteriol. 187, 3814–3824. doi: 10.1128/JB.187.11.3814-3824.2005
Tseng, C. L., Chen, H. J., and Shaw, G. C. (2006). Identification and characterization of the Bacillus thuringiensis phaZ gene, encoding new intracellular poly-3-hydroxybutyrate depolymerase. J. Bacteriol. 188, 7592–7599. doi: 10.1128/JB.00729-06
Valappil, S. P., Boccaccini, A. R., Bucke, C., and Roy, I. (2007). Polyhydroxyalkanoates in gram-positive bacteria: insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek 91, 1–17. doi: 10.1007/s10482-006-9095-5
Wang, J., Mei, H., Qian, H., Tang, Q., Liu, X., Yu, Z., et al. (2013a). Expression profile and regulation of spore and parasporal crystal formation-associated genes in Bacillus thuringiensis. J. Proteome Res. 12, 5487–5501. doi: 10.1021/pr4003728
Wang, J., Mei, H., Zheng, C., Qian, H., Cui, C., Fu, Y., et al. (2013b). The metabolic regulation of sporulation and parasporal crystal formation in Bacillus thuringiensis revealed by transcriptomics and proteomics. Mol. Cell. Proteomics 12, 1363–1376. doi: 10.1074/mcp.M112.023986
Wang, Y. L., Lin, Y. T., Chen, C. L., Shaw, G. C., and Liaw, S. H. (2014). Crystallization and preliminary crystallographic analysis of poly(3-hydroxybutyrate) depolymerase from Bacillus thuringiensis. Acta Crystallogr. F Struct. Biol. Commun. 70, 1421–1423. doi: 10.1107/S2053230X14019347
Wu, D., He, J., Gong, Y., Chen, D., Zhu, X., Qiu, N., et al. (2011). Proteomic analysis reveals the strategies of Bacillus thuringiensis YBT-1520 for survival under long-term heat stress. Proteomics 11, 2580–2591. doi: 10.1002/pmic.201000392
York, G. M., Lupberger, J., Tian, J. M., Lawrence, A. G., Stubbe, J., and Sinskey, A. J. (2003). Ralstonia eutropha H16 encodes two and possibly three intracellular poly[D-(-)-3-hydroxybutyrate] depolymerase genes. J. Bacteriol. 185, 3788–3794. doi: 10.1128/JB.185.13.3788-3794.2003
Zhang, S., Hu, Y., Fan, Q., Wang, X., and He, J. (2015). Two-component system YvqEC-dependent bacterial resistance against vancomycin in Bacillus thuringiensis. Antonie van Leeuwenhoek 108, 365–376. doi: 10.1007/s10482-015-0489-0
Zhang, S., Li, X., Wang, X., Li, Z., and He, J. (2016). The two-component signal transduction system YvcPQ regulates the bacterial resistance to bacitracin in Bacillus thuringiensis. Arch. Microbiol. doi: 10.1007/s00203-016-1239-z [Epub ahead of print].
Keywords: poly-3-hydroxybutyrate, Bacillus thuringiensis, sporulation, insecticidal crystal proteins (ICPs), PHB synthase (PhaC), depolymerase (PhaZ)
Citation: Wang X, Li Z, Li X, Qian H, Cai X, Li X and He J (2016) Poly-β-hydroxybutyrate Metabolism Is Unrelated to the Sporulation and Parasporal Crystal Protein Formation in Bacillus thuringiensis. Front. Microbiol. 7:836. doi: 10.3389/fmicb.2016.00836
Received: 13 January 2016; Accepted: 18 May 2016;
Published: 15 June 2016.
Edited by:
Weiwen Zhang, Tianjin University, ChinaReviewed by:
Jingwen Zhou, Jiangnan University, ChinaLichuan Gu, Shandong University, China
Young Ha Rhee, Chungnam National University, South Korea
Copyright © 2016 Wang, Li, Li, Qian, Cai, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin He, hejin@mail.hzau.edu.cn
 Xun Wang
Xun Wang Zhou Li1
Zhou Li1 Jin He
Jin He