- Ruminant Diseases and Immunology Research Unit, National Animal Disease Center, Agricultural Research Service, USDA, Ames, Iowa
Although most commonly associated with the infection of domestic livestock, the replication of pestiviruses, in particular the two species of bovine viral diarrhea virus (BVDV), occurs in a wide range of free ranging cervids including white-tailed deer, mule deer, fallow deer, elk, red deer, roe deer, eland and mousedeer. While virus isolation and serologic analyses indicate that pestiviruses are circulating in these populations, little is known regarding their impact. The lack of regular surveillance programs, challenges in sampling wild populations, and scarcity of tests and vaccines compound the difficulties in detecting and controlling pestivirus infections in wild cervids. Improved detection rests upon the development and validation of tests specific for use with cervid samples and development and validation of tests that reliably detect emerging pestiviruses. Estimation of impact of pestivirus infections on herd health will require the integration of several disciplines including epidemiology, cervid natural history, veterinary medicine, pathology and microbiology.
Introduction
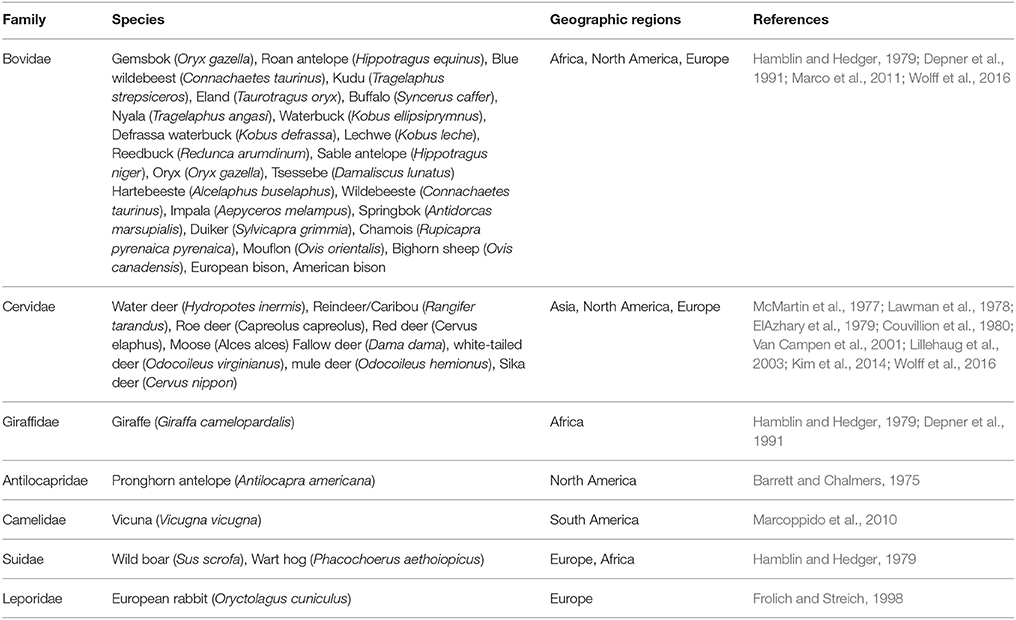
The recognized species of the Pestivirus genus include bovine viral diarrhea virus types 1 (BVDV1) and 2 (BVDV2), classical swine fever virus (CSFV), and border disease virus (BDV) (Simmonds et al., 2012). In addition to these four species, five putative species have been proposed; Bungowannah virus, giraffe virus, HoBi-like virus, pronghorn virus (PHV) and atypical porcine pestivirus. All four of the recognized species have been isolated from free ranging wildlife populations and two of the putative species, giraffe virus and PHV, have only been isolated from free ranging wildlife species (Table 1). Despite abundant evidence that pestiviruses currently circulate in wildlife populations, the full impact of exposure and prevalence of these infections are largely unknown. The limited information available regarding prevalence is mainly in the form of serological surveys (Table 2). Even though these studies have been limited and sporadic, they have demonstrated that a wide range of wildlife species havea wide range of wildlife species has been infected by pestiviruses. Further, controlled studies have shown that pestiviruses infect wild species and once infected they may transmit virus (Grondahl et al., 2003; Uttenthal et al., 2005, 2006; Duncan et al., 2008a; Nelson et al., 2008; Passler et al., 2010; USDA, 2010; Pruvot et al., 2014). While it is possible that positive serology results may be due to contact with domestic species, the high prevalence of seropositive samples within some isolated wild life populations without close contact with domestic species suggest that pestiviruses are being maintained independently within wildlife populations. This is illustrated by a study in which the geographic location of BVDV antigen-positive cattle and BVDV-seropositive white-tailed deer were analyzed using the dual kernel density estimation method. An exploratory cluster analysis revealed 1 significant cluster of BVDV antigen-positive herds and 2 significant clusters of BVDV-seropositive deer. There was no spatial overlap between the clusters suggesting that BVDV is maintained independently in domestic livestock herds and in the white-tailed deer population.(Kirchgessner et al., 2013).
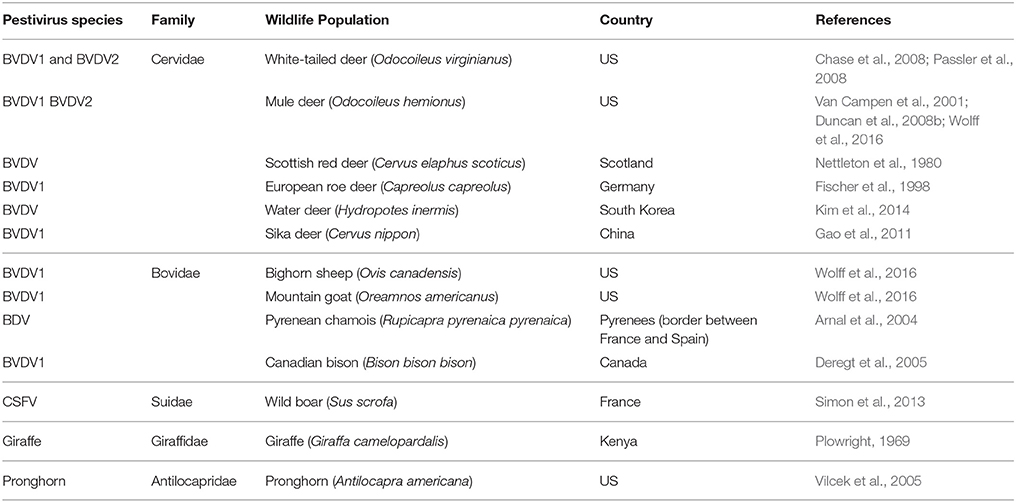
Table 1. Detection of pestivirus species in samples collected from free ranging wildlife populations.
The purpose of this article is to review reports regarding pestivirus infections in wild cervids and to summarize some of the challenges involved in determining the impact of pathogens infecting free ranging cervids.
Surveillance Based on Detection or Isolation of Pestiviruses
Pestiviruses, principally BVDV1 and BVDV2, have been detected in samples collected from free ranging cervid populations (Table 1). However, isolations or detection by PCR tend to be a rare event among the populations surveyed (for references see Table 1). Cattle may be acutely or persistently infected with BVDV (Evermann and Barrington, 2005). Similarly it has been demonstrated that, under experimental conditions, cervids may be acutely or persistently infected with pestiviruses such as BVDV1 or BVDV2 (Passler et al., 2007, 2009; Ridpath et al., 2007, 2008). Experimental infections with typical field strains of BVDV in immunocompetent cattle and white tailed deer tend to be mild or asymptomatic (Ridpath et al., 2007, 2013). The majority of the surveys conducted to date relied on serum or ear notch samples which, at least in cattle, are better for detecting persistent infections than acute infections (Ridpath et al., 2002; Liebler-Tenorio et al., 2004). Further, based on the pattern of viral antigen present in various tissues it appears that the pestivirus positive deer harvested from free ranging populations were probably persistently rather than acutely infected. In cattle, persistently infected animals make up less than one percent of the population at slaughter but have a significant impact on the health of cohorts (Hessman et al., 2009). The detection of persistently infected animals (PI) in any population, domestic or free ranging, is significant as PIs act as efficient vectors for keeping the virus in circulation. However, persistent infections in deer are only established if the fetus is infected in the first one third of pregnancy (Ridpath et al., 2008, 2012). Thus, infections of the fetus occurring during the final two thirds of pregnancy and all infections of animals post-birth result in acute infections rather than persistent infections. Failure to detect acutely infected animals will lead to underestimation of infection rate. Therefore, while detection of PIs yields significant information it cannot be used as a measure of prevalence of infection.
Serological Surveys
Antibodies against pestiviruses have been identified from serum collected from seven different families of free ranging wildlife; Antilocapridae, Bovidae, Giraffidae, Cervidae, Suidae, Camelidae, and Leporidae with the greatest number of wildlife host species in the Bovidae and Cervidae families (Table 2). In North America, the largest numbers of wild ruminants are found in the Cervidae family (Flather et al., 2009) with five species being represented: moose (Alces alces), elk/wapiti (Cervus elaphus), caribou/reindeer (Rangifer tarandus), mule deer (Odocoileus hemionus), and white-tailed deer (Odocoileus virginianus) (Conner et al., 2008). Pestivirus neutralizing antibodies have been detected in free ranging populations of all five species (Table 2). A limitation of serological surveys is that the level of antigenic cross reactivity between pestivirus species makes it difficult to absolutely identify the pestivirus species elicited the immune response (Dubovi, 2013).
Many serological surveillance studies in wildlife arise out of pestivirus control programs aimed at clearing a pestivirus species, such as BVDV1 and BVDV2, from domestic animal populations. The primary goal of many of these studies is to determine if wildlife species can serve as virus reservoirs for domestic species, not to determine the level of infection in wildlife populations. The significant problem with these serological surveillance studies is that the level of neutralizing antibodies is only determined against one of the four recognized pestivirus species and this may result in underestimation of infection with emerging pestivirus species. This was noted by the authors in one of the earliest large scale serology surveys of wildlife which used samples collected from free ranging ungulates residing in Africa (Hamblin and Hedger, 1979). This survey evaluated 3359 sera, collected from multiple species of wildlife in nine African countries, for neutralizing antibodies against BVDV. At that point in history, the BVDV2 species had not yet been identified. Thus, the laboratory reference strains used in this study only belonged to the BVDV1 species. Neutralizing antibodies were detected in sera from 17 different species. The authors noted that because pestiviruses are cross reactive it is possible that the serum neutralizing antibodies reported in their study, may be due to cross neutralization with “other viruses as yet unrecognized.” It is also highly possible that antibodies against pestiviruses with limited cross reactivity with BVDV1 could have been missed in this and other studies.
Aside from an interest in pestiviruses that impact domestic species there are other reasons for the use of classic pestivirus strains in assays. Firstly, cytopathic reference strains from each of these species are readily available. This is not true of all emerging pestivirus species. To date only noncytopathic strains of the Giraffe and Pronghorn species are available. When cytopathic strains are used in virus neutralization (VN) tests, end points may be determined by observation of the cell monolayer. End point determination using noncytopathic strains requires secondary detection methods such as immunofluorescence, immunohistochemistry staining or polymerase chain reaction. Use of such secondary detection methods is time and cost prohibitive for large-scale surveillance projects.
Another consideration is that frequently emerging viruses, such as pronghorn virus (Vilcek et al., 2005) or atypical porcine pestivirus (Hause et al., 2015), do not initially grow well in cell lines commonly used in the laboratory (Vilcek et al., 2005). Finally, the pestivirus that the wild population was infected with may not yet have been isolated and characterized.
While there are valid reasons why serological surveys, based on VN tests, use reference strains from the four recognized species, it is highly probable that when these assays are used in such surveys they miss titers resulting from exposure to emerging viruses that are genetically distant and antigenically distinct. The greater the genetic difference between pestiviruses, the lower the cross reactivity (Ridpath et al., 2010; Bauermann et al., 2012). For example, the emerging bovine pestivirus species known as HoBi-like virus, while distinct, is closer to the two BVDV species than to other emerging pestivirus such as pronghorn virus. In one study it was shown that a serum collected from a bovid infected by a HoBi-like viruses had a greater than 1/500 titer against a HoBi-like virus, averaged a greater than 1/300 titer against BVDV2 strains but did not neutralize the pronghorn virus (Bauermann et al., 2012).
While commercial ELISA kits are available for detecting antibodies against the classic pestiviruses, particularly BVDV, the limited cross reactivity that exists between emerging pestiviruses and classic pestiviruses make these tests unreliable for detecting antibodies resulting from infection by emerging pestiviruses (Bauermann et al., 2012). Further, these commercial tests are not designed to differentiate between antibodies raised against different pestivirus species.
While performing serology on a one time collection of samples from a population can give information on the occurrence and prevalence of exposure, it does not yield information on when the exposure occurred. To estimate time of exposure, multiple samples over time must be collected and archived.
Challenges in the Collection of Representative Samples
Ideally samples should be representative of the population under study including biological, spatial, and temporal variables (Stallknecht, 2007). Further, samples must be collected while virus is present in tissues and tissues must be tested using technologies that maximize the probability of detecting the agent (Thurmond, 2003). Issues of access, cost and feasibility frequently preclude the gathering of such ideal samples.
If the goal is to detect a pestivirus the sample must be collected while the animal is still viremic. This not a problem with persistently infected cervids but is a problem with acutely infected cervids where the window of detectable viremia may be less than 5 days (Ridpath et al., 2007).
Both passive and active surveillance systems may be used to obtain cervid samples. Passive surveillance, which relies upon the observation and subsequent testing of an animal displaying clinical signs of disease or collection of samples from animals that have died of disease, is problematic for detecting infection with viruses, such as pestiviruses, which don't cause severe clinical disease. Passive surveillance tends to under estimate the impact of diseases that have significant mortality rates let alone those that result in subclinical disease. This is illustrated by an outbreak of hemorrhagic disease in white-tailed deer that occurred in Missouri. While it was estimated that the outbreak resulted in an 8% mortality rate, not one case of mortality or morbidity was reported by the public. The occurrence and extent of the outbreak were only noted because of surveillance conducted on 100 radio-monitored deer (Beringer et al., 2000). Some surveys for BVDV in deer have depended on getting samples from deer that were harvested by hunters (Duncan et al., 2008b; Passler et al., 2008). Hunting licenses usually require that the harvested animals are adults and most hunters desire to harvest healthy specimens. Thus, hunter harvested samples tend to represent healthy animals that have lived to sexual maturity, and based in studies in cattle, restricting surveys to healthy adults may result in underestimation of the incidence of persistent infection. In cattle it has been observed that animals persistently infected (PI) with BVDV are more frequently found among young stock than older stock because some (but not all) PI cattle succumb in the first year of life (Houe, 1992).
Even though hunter harvested samples may be skewed against including PI animals, BVDV PI animals have been detected in these samples (Van Campen et al., 2001; Chase et al., 2004; Duncan et al., 2008b; Passler et al., 2008) albeit at a low rate varying from 0.03 to 0.2%. The presence of PI deer indicates that BVDV circulates in these populations; however, their impact is difficult to assess.
The design of active surveillance systems requires an understanding of the social organization of the species to be studied. Unlike domestic livestock, wild deer do not confine their activities to large herd groups, cannot be rounded up without damaging ecosystems and social grouping, and are not amenable to handling. Populations are frequently divided into small breeding groups based on age and gender and contact between groups and make up within groups may change with the season. Neonates are frequently hidden rather than grazing with the herd.
The ideal surveillance program would include samples collected at multiple time points allowing retrospective analysis (Stallknecht, 2007). Archived samples are fundamental to estimating the introduction of a pathogen or detecting an increase in the incidence of infection.
Assessing the Impact of Pestivirus Infections
It is easier to assess the impact of infection with high virulence pestivirus strains that result in clinically severe acute disease such as classic swine fever in swine or hemorrhagic syndrome in cattle. However, the impact of lower virulence pestiviruses is harder to assess, even in domesticated species. Previous studies using captive deer have demonstrated white-tailed deer infected by pestiviruses such as BVDV1, BVDV2, and PHV display very mild clinical signs even though they are undergoing significant immune suppression (Van Campen et al., 1997; Vilcek et al., 2005; Ridpath et al., 2007, 2008, 2012). While the immune suppression may lead to reduction in herd health and numbers, the contribution of pestivirus infections to the problem may be difficult to establish. The prevalence of BVDV persistent infection in cattle, while low, has significant impact on production. Lonergan et al. determined that while PI cattle represent only 0.3% of the cattle population on arrival in feedlots, they accounted for 2.6% of chronically ill cattle and 2.5% of cattle that died during the observation period (Loneragan et al., 2005). Perhaps more importantly, exposure to PI animals has a significant impact on the health of cohorts. In the same study it was found that the risk of initial treatment for respiratory tract disease was 43% greater in cattle exposed to a PI animal, compared with those not exposed to a PI animal. Overall, 15.9% of initial respiratory tract disease events were attributable to exposure to a PI animal. In a subsequent study, Hessman et al. (2009) demonstrated that aside from overt disease, growth rates and feed conversion were negatively affected by the presence of PI cattle in feedlots. Comparing cattle lots with direct exposure to a PI with those without direct exposure revealed significant deficits in all performance outcomes associated with PI exposure. In the wild, where the rule is survival of the fittest, pestivirus infections which reduce efficiency in feed conversion and resistance to disease could be instrumental in a decline in animal numbers and population health.
Conclusions
The limited serologic surveillance that has been published focused on the levels of neutralizing antibodies against the recognized pestivirus species. Such studies may underestimate exposure to emerging pestiviruses. The value of serological studies is greatly enhanced if sequential testing of the same population over is conducted. Samples, collected from the same population, over time allows detection of changes in exposure patterns.
Many studies rely on samples generated from deer harvested by hunters. However, such samples may yield skewed data as the majority of hunter-generated samples come from healthy, primarily male adults. Further, the tests available are designed for detection of recognized pestiviruses in domestic species. The reagents used may not be appropriate for wild cervids or emerging pestiviruses that are only distantly related to the recognized pestivirus species. In particular, cell cultures derived from domestic species may not work for the propagation of viruses that are adapted to cervid hosts. In summary, the full impact of pestiviruses on cervid populations may not be recognized at this time.
Improved detection rests upon the development and validation of tests specific for use with cervid samples and the development and validation of tests that reliably detect emerging pestiviruses. Estimation of the impact of pestivirus infections will require the integration of several disciplines including epidemiology, cervid sociology, veterinary medicine, pathology and microbiology.
Author Contributions
JR organized and drafted article. JN reviewed and amended article. Both authors agree to be accountable for this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arnal, M., Fernandez-de-Luco, D., Riba, L., Maley, M., Gilray, J., Willoughby, K., et al. (2004). A novel pestivirus associated with deaths in Pyrenean chamois (Rupicapra pyrenaica pyrenaica). J. Gen. Virol. 85, 3653–3657. doi: 10.1099/vir.0.80235-0
Barrett, M. W., and Chalmers, G. A. (1975). A serologic survey of pronghorns in Alberta and Saskatchewan, 1970-1972. J. Wildl. Dis. 11, 157–163. doi: 10.7589/0090-3558-11.2.157
Bauermann, F. V., Flores, E. F., and Ridpath, J. F. (2012). Antigenic relationships between Bovine viral diarrhea virus 1 and 2 and HoBi virus: possible impacts on diagnosis and control. J. Vet. Diagn. Invest. 24, 253–261. doi: 10.1177/1040638711435144
Beringer, J., Hansen, L. P., and Stallknecht, D. E. (2000). An epizootic of hemorrhagic disease in white-tailed deer in Missouri. J. Wildl. Dis. 36, 588–591. doi: 10.7589/0090-3558-36.3.588
Chase, C. C., Braun, L. J., Leslie-Steen, P., Graham, T., Miskimins, D., and Ridpath, J. F. (2008). Bovine viral diarrhea virus multiorgan infection in two white-tailed deer in southeastern South Dakota. J. Wildl. Dis. 44, 753–759. doi: 10.7589/0090-3558-44.3.753
Chase, C., Miskimmins, D., Graham, T., Braun, L., Steen, P., and Ridpath, J. (2004). “Evidence of bovine viral diarrhea virus persistent infection in two white-tail deer in southeastern South Dakota,” in 37th Annual Conference of American Association of Bovine Practitioners (Fort Worth, TX), 169.
Conner, M. M., Ebinger, M. R., Blanchong, J. A., and Cross, P. C. (2008). Infectious disease in cervids of North America: data, models, and management challenges. Ann. N.Y. Acad. Sci. 1134, 146–172. doi: 10.1196/annals.1439.005
Couvillion, C. E., Jenney, E. W., Pearson, J. E., and Coker, M. E. (1980). Survey for antibodies to viruses of bovine virus diarrhea, bluetongue, and epizootic hemorrhagic disease in hunter-killed mule deer in New Mexico. J. Am. Vet. Med. Assoc. 177, 790–791.
Depner, K., Hubschle, O. J., and Liess, B. (1991). Prevalence of ruminant pestivirus infections in Namibia. Onderstepoort J. Vet. Res. 58, 107–109.
Deregt, D., Tessaro, S. V., Baxi, M. K., Berezowski, J., Ellis, J. A., Wu, J. T., et al. (2005). Isolation of bovine viral diarrhoea viruses from bison. Vet. Rec. 157, 448–450. doi: 10.1136/vr.157.15.448
Dubovi, E. J. (2013). Laboratory diagnosis of bovine viral diarrhea virus. Biologicals 41, 8–13. doi: 10.1016/j.biologicals.2012.06.004
Duncan, C., Ridpath, J., Palmer, M. V., Driskell, E., and Spraker, T. (2008a). Histopathologic and immunohistochemical findings in two white-tailed deer fawns persistently infected with Bovine viral diarrhea virus. J. Vet. Diagn. Invest. 20, 289–296. doi: 10.1177/104063870802000305
Duncan, C., Van Campen, H., Soto, S., LeVan, I. K., Baeten, L. A., and Miller, M. W. (2008b). Persistent Bovine viral diarrhea virus infection in wild cervids of Colorado. J. Vet. Diagn. Invest. 20, 650–653. doi: 10.1177/104063870802000521
ElAzhary, M. A., Roy, R. S., and Frechette, J. L. (1979). Serological evidence of IBR and BVD infection in caribou (Rangifer tarandus). Vet. Rec. 105:336. doi: 10.1136/vr.105.14.336
Evermann, J. F., and Barrington, G. M. (2005). “Clinical features,” in Bovine Viral Diarrhea Virus: Diagnosis, Management and Control, eds S. M. Goyal and J. F. Ridpath (Ames, IA: Blackwell Publishing), 105–120.
Fischer, S., Weiland, E., and Frolich, K. (1998). Characterization of a bovine viral diarrhea virus isolated from roe deer in Germany. J. Wildl. Dis. 34, 47–55. doi: 10.7589/0090-3558-34.1.47
Flather, C. H., Knowles, M. S., and Brady, S. J. (2009). “Population and harvest trends of big game and small game species,” in A Technical Document in the Support of the USDA Forest Service Interim Update of the 2000 RPA Assessment (Fort Collins, CO), 34.
Frolich, K., and Streich, W. J. (1998). Serologic evidence of bovine viral diarrhea virus in free-ranging rabbits from Germany. J. Wildl. Dis. 34, 173–178. doi: 10.7589/0090-3558-34.1.173
Gao, Y., Wang, S., Du, R., Wang, Q., Sun, C., Wang, N., et al. (2011). Isolation and identification of a bovine viral diarrhea virus from sika deer in china. Virol. J. 8, 83. doi: 10.1186/1743-422X-8-83
Grondahl, C., Uttenthal, A., Houe, H., Rasmussen, T. B., Hoyer, M. J., and Larsen, L. E. (2003). Characterisation of a pestivirus isolated from persistently infected mousedeer (Tragulus javanicus). Arch. Virol. 148, 1455–1463. doi: 10.1007/s00705-003-0130-9
Hamblin, C., and Hedger, R. S. (1979). The prevalence of antibodies to bovine viral diarrhoea/mucosal disease virus in African wildlife. Comp. Immunol. Microbiol. Infect. Dis. 2, 295–303. doi: 10.1016/0147-9571(79)90017-1
Hause, B. M., Collin, E. A., Peddireddi, L., Yuan, F., Chen, Z., Hesse, R. A., et al. (2015). Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J. Gen. Virol. 96, 2994–2998. doi: 10.1099/jgv.0.000251
Hessman, B. E., Fulton, R. W., Sjeklocha, D. B., Murphy, T. A., Ridpath, J. F., and Payton, M. E. (2009). Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhea virus in a starter feedlot. Am. J. Vet. Res. 70, 73–85. doi: 10.2460/ajvr.70.1.73
Houe, H. (1992). Age distribution of animals persistently infected with bovine virus diarrhea virus in twenty-two Danish dairy herds. Can. J. Vet. Res. 56, 194–198.
Kim, S. H., Choi, H., Yoon, J., Woo, C., Chung, H. M., Kim, J. T., et al. (2014). Pathogens in water deer (Hydropotes inermis) in South Korea, 2010-12. J. Wildl. Dis. 50, 478–483. doi: 10.7589/2013-06-137
Kirchgessner, M. S., Dubovi, E. J., and Whipps, C. M. (2013). Spatial point pattern analyses of Bovine viral diarrhea virus infection in domestic livestock herds and concomitant seroprevalence in wild white-tailed deer (Odocoileus virginianus) in New York State, USA. J. Vet. Diagn. Invest. 25, 226–233. doi: 10.1177/1040638713479121
Lawman, M. J., Evans, D., Gibbs, E. P., McDiarmid, A., and Rowe, L. (1978). A preliminary survey of British deer for antibody to some virus diseases of farm animals. Br. Vet. J. 134, 85–91.
Liebler-Tenorio, E. M., Ridpath, J. E., and Neill, J. D. (2004). Distribution of viral antigen and tissue lesions in persistent and acute infection with the homologous strain of noncytopathic bovine viral diarrhea virus. J. Vet. Diagn. Invest. 16, 388–396. doi: 10.1177/104063870401600504
Lillehaug, A., Vikoren, T., Larsen, I. L., Akerstedt, J., Tharaldsen, J., and Handeland, K. (2003). Antibodies to ruminant alpha-herpesviruses and pestiviruses in Norwegian cervids. J. Wildl. Dis. 39, 779–786. doi: 10.7589/0090-3558-39.4.779
Loneragan, G. H., Thomson, D. U., Montgomery, D. L., Mason, G. L., and Larson, R. L. (2005). Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J. Am. Vet. Med. Assoc. 226, 595–601. doi: 10.2460/javma.2005.226.595
Marco, I., Cabezon, O., Rosell, R., Fernandez-Sirera, L., Allepuz, A., and Lavin, S. (2011). Retrospective study of pestivirus infection in Pyrenean chamois (Rupicapra pyrenaica) and other ungulates in the Pyrenees (NE Spain). Vet. Microbiol. 149, 17–22. doi: 10.1016/j.vetmic.2010.09.032
Marcoppido, G., Parreno, V., and Vila, B. (2010). Antibodies to pathogenic livestock viruses in a wild vicuna (Vicugna vicugna) population in the Argentinean Andean altiplano. J. Wildl. Dis. 46, 608–614. doi: 10.7589/0090-3558-46.2.608
McMartin, D. A., Snodgrass, D. R., and Corrigall, W. (1977). Bovine virus diarrhoea antibody in a Scottish red deer. Vet. Rec. 100:187. doi: 10.1136/vr.100.9.187-b
Nelson, D. D., Dark, M. J., Bradway, D. S., Ridpath, J. F., Call, N., Haruna, J., et al. (2008). Evidence for persistent Bovine viral diarrhea virus infection in a captive mountain goat (Oreamnos americanus). J. Vet. Diagn. Invest. 20, 752–759. doi: 10.1177/104063870802000606
Nettleton, P. F., Herring, J. A., and Corrigall, W. (1980). Isolation of bovine virus diarrhoea virus from a Scottish red deer. Vet. Rec. 107, 425–426. doi: 10.1136/vr.107.18.425
Passler, T., Ditchkoff, S. S., Givens, M. D., Brock, K. V., DeYoung, R. W., and Walz, P. H. (2010). Transmission of bovine viral diarrhea virus among white-tailed deer (Odocoileus virginianus). Vet. Res. 41, 20. doi: 10.1051/vetres/2009068
Passler, T., Walz, P. H., Ditchkoff, S. S., Brock, K. V., Deyoung, R. W., Foley, A. M., et al. (2009). Cohabitation of pregnant white-tailed deer and cattle persistently infected with Bovine viral diarrhea virus results in persistently infected fawns. Vet. Microbiol. 134, 362–367. doi: 10.1016/j.vetmic.2008.08.012
Passler, T., Walz, P. H., Ditchkoff, S. S., Givens, M. D., Maxwell, H. S., and Brock, K. V. (2007). Experimental persistent infection with bovine viral diarrhea virus in white-tailed deer. Vet. Microbiol. 122, 350–356. doi: 10.1016/j.vetmic.2007.01.028
Passler, T., Walz, P. H., Ditchkoff, S. S., Walz, H. L., Givens, M. D., and Brock, K. V. (2008). Evaluation of hunter-harvested white-tailed deer for evidence of bovine viral diarrhea virus infection in Alabama. J. Vet. Diagn. Invest. 20, 79–82. doi: 10.1177/104063870802000116
Plowright, W. (1969). “Other virus diseases in relation to the JP15 programme,” in Joint Campaign Against Rinderpest, Proceedings of the First Technical Review Meeting, Phase IV (Mogadiscio), 19–23.
Pruvot, M., Kutz, S., van der Meer, F., Musiani, M., Barkema, H. W., and Orsel, K. (2014). Pathogens at the livestock-wildlife interface in Western Alberta: does transmission route matter? Vet. Res. 45:18. doi: 10.1186/1297-9716-45-18
Ridpath, J. F., Driskell, E. A., Chase, C. C., Neill, J. D., Palmer, M. V., and Brodersen, B. W. (2008). Reproductive tract disease associated with inoculation of pregnant white-tailed deer with bovine viral diarrhea virus. Am. J. Vet. Res. 69, 1630–1636. doi: 10.2460/ajvr.69.12.1630
Ridpath, J. F., Falkenberg, S. M., Bauermann, F. V., Vanderley, B. L., Do, Y., Flores, E. F., et al. (2013). Comparison of acute infection of calves exposed to a high-virulence or low-virulence bovine viral diarrhea virus or a HoBi-like virus. Am. J. Vet. Res. 74, 438–442. doi: 10.2460/ajvr.74.3.438
Ridpath, J. F., Fulton, R. W., Kirkland, P. D., and Neill, J. D. (2010). Prevalence and antigenic differences observed between Bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J. Vet. Diagn. Invest. 22, 184–191. doi: 10.1177/104063871002200203
Ridpath, J. F., Hietala, S. K., Sorden, S., and Neill, J. D. (2002). Evaluation of the reverse transcription-polymerase chain reaction/probe test of serum samples and immunohistochemistry of skin sections for detection of acute bovine viral diarrhea infections. J. Vet. Diagn. Invest. 14, 303–307. doi: 10.1177/104063870201400405
Ridpath, J. F., Mark, C. S., Chase, C. C., Ridpath, A. C., and Neill, J. D. (2007). Febrile response and decrease in circulating lymphocytes following acute infection of white-tailed deer fawns with either a BVDV1 or a BVDV2 strain. J. Wildl. Dis. 43, 653–659. doi: 10.7589/0090-3558-43.4.653
Ridpath, J. F., Neill, J. D., and Chase, C. C. (2012). Impact of BVDV infection of white-tailed deer during second and third trimesters of pregnancy. J. Wildl. Dis. 48, 758–762. doi: 10.7589/0090-3558-48.3.758
Simmonds, P., Becher, P., Collett, M. S., Gould, E. A., Heinz, F. X., Meyers, G., et al. (2012). Genus Pestivirus in Ninth Report of the International Committee on Taxonomy of Viruses. eds A. M. Q. King, M. J. Adams, E. B. Carstens, and E. J. Lefkowitz (New York, NY), 971–1014.
Simon, G., Le Dimna, M., Le Potier, M. F., and Pol, F. (2013). Molecular tracing of classical swine fever viruses isolated from wild boars and pigs in France from 2002 to 2011. Vet. Microbiol. 166, 631–638. doi: 10.1016/j.vetmic.2013.06.032
Stallknecht, D. E. (2007). Impediments to wildlife disease surveillance, research, and diagnostics. Curr. Top. Microbiol. Immunol. 315, 445–461. doi: 10.1007/978-3-540-70962-6_17
Thurmond, M. C. (2003). Conceptual foundations for infectious disease surveillance. J. Vet. Diagn. Invest. 15, 501–514. doi: 10.1177/104063870301500601
USDA (2010). Beef 2007–08, Prevalence and Control of Bovine Viral Diarrhea Virus on U.S. Cowcalf Operations, 2007–08. USDA: APHIS:VS, CEAH: FortCollins, CO.
Uttenthal, A., Grondahl, C., Hoyer, M. J., Houe, H., van Maanen, C., Rasmussen, T. B., et al. (2005). Persistent BVDV infection in mousedeer infects calves. Do we know the reservoirs for BVDV? Prev. Vet. Med. 72, 87–91. discussion: 215–219. doi: 10.1016/j.prevetmed.2005.08.006
Uttenthal, A., Hoyer, M. J., Grondahl, C., Houe, H., van Maanen, C., Rasmussen, T. B., et al. (2006). Vertical transmission of bovine viral diarrhoea virus (BVDV) in mousedeer (Tragulus javanicus) and spread to domestic cattle. Arch. Virol. 151, 2377–2387. doi: 10.1007/s00705-006-0818-8
Van Campen, H., Ridpath, J., Williams, E., Cavender, J., Edwards, J., Smith, S., et al. (2001). Isolation of bovine viral diarrhea virus from a free-ranging mule deer in Wyoming. J. Wildl. Dis. 37, 306–311. doi: 10.7589/0090-3558-37.2.306
Van Campen, H., Williams, E. S., Edwards, J., Cook, W., and Stout, G. (1997). Experimental infection of deer with bovine viral diarrhea virus. J. Wildl. Dis. 33, 567–573. doi: 10.7589/0090-3558-33.3.567
Vilcek, S., Ridpath, J. F., Van Campen, H., Cavender, J. L., and Warg, J. (2005). Characterization of a novel pestivirus originating from a pronghorn antelope. Virus Res. 108, 187–193. doi: 10.1016/j.virusres.2004.09.010
Wolff, P. L., Schroeder, C., McAdoo, C., Cox, M., Neldon, D. D., Evermann, J. F., et al. (2016). Evidence of bovine viral diarrhea virus infeections in three species of sympatric wild ungulates in Nevada: life history strategies may maintain endemic infection in wild populations. Front. Microbiol. 7:292. doi: 10.3389/fmicb.2016.00292
Keywords: pestivirus, cervids, wildlife diseases, surveillance, sampling
Citation: Ridpath JF and Neill JD (2016) Challenges in Identifying and Determining the Impacts of Infection with Pestiviruses on the Herd Health of Free Ranging Cervid Populations. Front. Microbiol. 7:921. doi: 10.3389/fmicb.2016.00921
Received: 15 January 2016; Accepted: 30 May 2016;
Published: 17 June 2016.
Edited by:
Slobodan Paessler, University of Texas Medical Branch, USAReviewed by:
Matthias Schweizer, Vetsuisse Faculty - University of Bern, SwitzerlandJames Frederick Evermann, Washington State University, USA
Copyright © 2016 Ridpath and Neill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia F. Ridpath, julia.ridpath@ars.usda.gov
 Julia F. Ridpath
Julia F. Ridpath John D. Neill
John D. Neill