- 1Institute of Traditional Medicine, National Yang-Ming University, Taipei, Taiwan
- 2Division of Gastroenterology, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan
- 3Department of Medical Research, MacKay Memorial Hospital, New Taipei City, Taiwan
- 4Department of Medicine, MacKay Medical College, New Taipei City, Taiwan
- 5Mackay Junior College of Medicine, Nursing, and Management, New Taipei City, Taiwan
- 6MacKay Children’s Hospital, Taipei, Taiwan
- 7Institute of Biomedical Informatics, Center for Systems and Synthetic Biology, National Yang-Ming University, Taipei, Taiwan
- 8Department of Chemical Engineering, National United University, Miaoli, Taiwan
- 9Department of Radiation Oncology, MacKay Memorial Hospital, Taipei, Taiwan
Adjuvant 5-fluorouracil (5-FU)-based chemotherapy, including FOLFOX (5-FU, leucovorin, and oxaliplatin), is recommended for colorectal cancer. However, intestinal mucositis remains a common adverse effect for which no effective preventive strategies are available. To develop a convenient and novel way to alleviate mucositis, we investigated the effect of Lactobacillus casei variety rhamnosus (Lcr35) on FOLFOX-induced mucosal injury. BALB/c mice subcutaneously injected with syngeneic CT26 colorectal adenocarcinoma cells were orally administered Lcr35 daily before, during, and after 5-day injection of FOLFOX regimen, for 14 days. The following methods were used: diarrhea score for toxicity, ELISA for cytokine production, histopathology for intestinal injury, immunohistochemistry for apoptosis/proliferation and regulatory proteins, RT-PCR for cytokine mRNA expression, and DNA sequencing for fecal gut microbiota. FOLFOX administration to colorectal cancer-bearing mice significantly inhibited tumor growth and the accompanying marked diarrhea and intestinal injury histologically characterized by the shortening of villi and destruction of intestinal crypts. Preventive administration of Lcr35 dose-dependently reduced the severity of diarrhea and intestinal mucositis without affecting the anti-tumor effect of FOLFOX. The numbers of apoptotic, NF-κB-, and BAX-activated cells increased after FOLFOX, and these responses were mitigated by Lcr35. TNF-α and IL-6 upregulation by FOLFOX treatment was attenuated by Lcr35. The fecal gut microbiota composition of Firmicutes and Bacteroidetes disturbed by FOLFOX was significantly reversed by Lcr35 toward a preferential profile. In conclusion, the oral probiotic Lcr35 prevented FOLFOX-induced intestinal mucositis in colorectal cancer-bearing mice. The putative mechanism might involve modulation of gut microbiota and proinflammatory responses with suppression of intrinsic apoptosis in intestinal injury.
Introduction
Gastrointestinal toxicity due to chemotherapeutic drugs is a major cause of morbidity and mortality in cancer patients. Mucositis and diarrhea are the most significant enterotoxicities. Lesions associated with mucositis result in pain, decreased quality of life, increased length of hospitalization, higher risk of infection, and modification of anti-neoplastic treatment regimens (Sonis et al., 2004; Sharma et al., 2005; Lee et al., 2014). The pathophysiology of chemotherapy-induced mucositis remains unclear and involves a complex and dynamic array of biological events (Logan et al., 2007; Lee et al., 2014). Some studies have suggested a five-stage process, including an initiation phase, a message generation phase, a signaling and amplification phase, an ulceration phase, and a spontaneous healing phase (Sonis et al., 2004; Logan et al., 2007). The pathophysiology might include decreased villi length and disruption of crypt cell homeostasis, and several pathogenic elements are involved, including direct toxicity, a change in the balance of bowel microbial flora, oxidative stress, apoptosis, hypoproliferation, and abnormal inflammation. Recent studies have revealed that chemotherapeutics affect the intestinal microbial composition (Stringer et al., 2009a,b) and fecal microbiota (Touchefeu et al., 2014). However, no well-established or up-to-date therapeutic strategy is available to manage chemotherapy-induced intestinal mucositis (Sharma et al., 2005). Thus, the development of an effective intervention against chemotherapy-related mucositis is urgently needed for oncological supportive care.
Probiotics are live microorganisms that, similar to certain drugs or food supplements, help maintain a beneficial microbial balance in the digestive tract of humans and other hosts. Probiotics have been tested in multiple indications, including gastrointestinal disorders (for the prevention and treatment of infectious and antibiotic-induced diarrhea), treatment of liver insufficiency, lactose intolerance, inflammatory bowel disease, irritable bowel syndrome, and anti-tumorigenic activities (Liong, 2008; Jacouton et al., 2017). Certain strains of Lactobacillus have been recommended in the treatment and prevention of diarrhea and inflammatory bowel disease to improve the integrity of the intestinal tissue (Eizaguirre et al., 2002). Experimental and clinical evidence suggest that probiotics might have a beneficial effect on the toxicity of anticancer therapy (Mego et al., 2013). The most commonly used probiotic organisms are lactic acid bacteria, especially those belonging to the genus Lactobacillus. We previously demonstrated that Lactobacillus attenuates the barrier disruption of intestinal epithelial cells caused by Salmonella lipopolysaccharide administration and ameliorates chemotherapy-induced intestinal mucositis in a healthy mouse model (Yeung et al., 2013, 2015). Thus, probiotics, by manipulating gut microbiota, may ameliorate inflammation, and protect the epithelium by maintaining intestinal epithelial integrity and reduce the severity of mucositis.
Colorectal cancer is one of the most common types of cancer and a lethal disease worldwide (Brenner et al., 2014). For several decades, regimens based on 5-fluorouracil (5-FU), an anti-metabolite anticancer agent, have been the first-choice chemotherapy for colorectal cancer (Brenner et al., 2014). Among regimens based on 5-FU with the cytotoxic agent oxaliplatin, FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) has been widely used as standard chemotherapy for advanced and metastatic colorectal cancer (de Gramont et al., 2000; Giacchetti et al., 2000; Mohelnikova-Duchonova et al., 2014). While 5-FU alone has an objective response rate of ∼20%, the combination of oxaliplatin with 5-FU/folinic acid results in significantly increased response rates and improved survival (Mohelnikova-Duchonova et al., 2014). The 5-FU is a pyrimidine analog (Johnson and Rogers, 1964) that is transformed inside the cell into different cytotoxic metabolites, which are then incorporated into DNA and RNA, finally inducing cell cycle arrest and apoptosis. Oxaliplatin, a platinum derivative, functions by forming both inter- and intrastrand crosslinks in DNA to prevent DNA replication and transcription, resulting in apoptosis (Graham et al., 2004). Gastrointestinal toxicity is potentiated in the combination therapy of oxaliplatin with 5-FU in clinical studies; however, the underlying mechanism remains unclear (Kuebler et al., 2007; Bano et al., 2014; Lee et al., 2014).
Our previous researches have reported anti-inflammatory effects of the probiotic strain Lactobacillus casei variety rhamnosus (Lcr35) on lipopolysaccharide-induced inflammation and epithelial barrier dysfunction in a co-culture model using Caco-2/peripheral blood mononuclear cells (Fang et al., 2010). Furthermore, we recently demonstrated that this strain attenuates chemotherapy-induced intestinal mucositis in healthy mice (Yeung et al., 2015). The 5-FU-induced diarrhea and damage in jejunal villi was ameliorated following Lcr35 administration. Lcr35 suppressed the upregulation of pro-inflammatory cytokines expression in intestinal mucositis tissues following 5-FU treatment. No bacterial translocation was found in the safety study of Lcr35 (Yeung et al., 2015). However, the beneficial role of Lactobacillus casei variety rhamnosus in FOLFOX-induced intestinal mucositis of colorectal cancer model remains to be assessed. Subcutaneously injected colorectal carcinoma murine models have been widely used in translational research (Liu et al., 2005; Doi et al., 2010; Kim et al., 2012; Evans et al., 2016; Gordon et al., 2017). In the current study, we further investigated the protective effect of Lactobacillus casei variety rhamnosus on intestinal mucosal injury induced by 5-FU-based chemotherapy (FOLFOX) in subcutaneously injected colon cancer mice. Our results revealed that probiotic Lcr35 did not interfere anti-tumor effect of FOLFOX. Lcr35 ameliorated diarrhea and repaired intestinal mucosa damage following FOLFOX treatment. The possible mechanism(s) of Lcr35 was also elucidated.
Materials and Methods
Chemotherapy Regimen Administration
Chemotherapy regimen (FOLFOX) with 5-fluorouracil (5-FU, Sigma F6627), leucovorin (LV, Sigma F7878), and oxaliplatin (Sigma O9512) was injected intraperitoneally (i.p.) to cause mucositis and diarrhea. The drug-dosing schedule was based upon that used in previously published studies and our preliminary dose-finding experiments (El-Salhy et al., 2005; Wagner et al., 2009). The 5-FU and LV were injected i.p. at a single dose (30 and 10 mg/kg, respectively) for 5 consecutive days (days 0–4). On the first day (day 0), the experimental animals also received oxaliplatin (1 mg/kg, i.p.) 1 h after 5-FU/LV administration. Saline was injected i.p. in control groups.
Probiotic Preparation
Lactobacillus casei variety rhamnosus (Lcr35) (Antibiophilus®) was diluted in sterile saline and administered by oral gavage. The mice received 100 μL of saline or suspension containing 1 × 103-7 CFU of the probiotics cocktail daily during the experiment.
Cell Culture
The CT26 cells, N-nitroso-N-methyl urethane-induced mouse colon carcinoma cells of BALB/c origin (Brattain et al., 1980), were purchased from the American Type Culture Collection. Cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (Hyclone) at 37°C in a humidified incubator with 50 mL/L CO2. The cell cultures were passaged every 2–3 days with TEG solution (0.25% trypsin, 0.1% EDTA, and 0.05% glucose in Hanks’ balanced salt solution) and were maintained in exponential growth.
Animal Experiments
Male, 6- to 8-week-old BALB/c mice weighing 22–24 g were obtained from Taiwan’s National Laboratory Animal Center and were maintained under a 12-h light/dark cycle at a temperature of 22 ± 1°C and a humidity of 55 ± 10% (Yeung et al., 2015). Animal studies were performed in accordance with institutional ethical guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of MacKay Memorial Hospital (Taiwan) (MMH-A-S-104-15). All mice were given ad libitum access to autoclaved food (laboratory-autoclavable rodent diet 5010) and water. The mice were randomly divided into control and experimental groups. All mice were inoculated with CT26 cells (4 × 106 cells) by subcutaneous injection into the right gluteal region. Treatment was started when the tumors reached 0.5 cm in diameter (insert in Figure 1A); the mice were injected saline or FOLFOX i.p. daily for 5 days (Figure 1A).
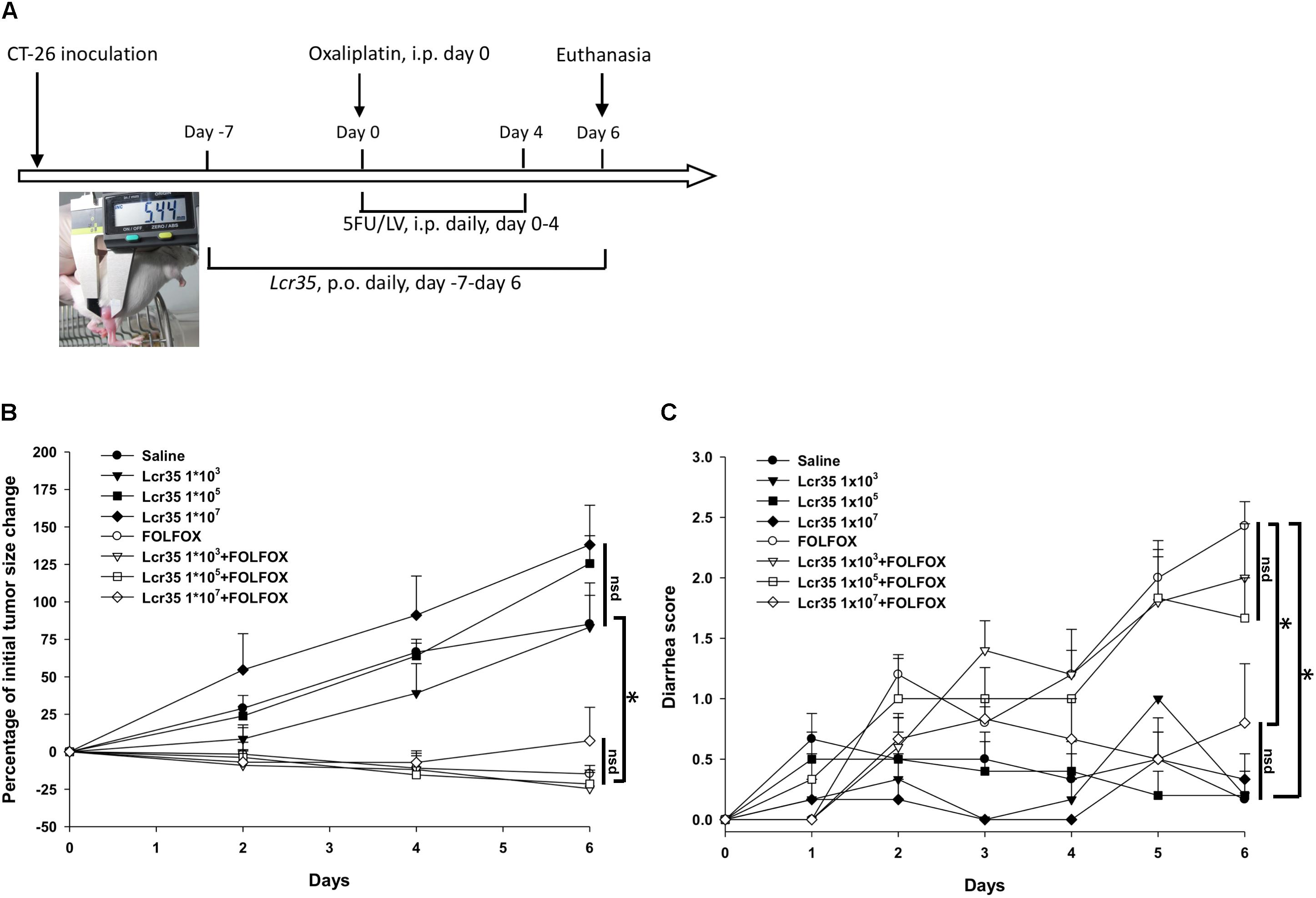
FIGURE 1. Effects of Lcr35 in subcutaneously injected colorectal cancer mice challenged with FOLFOX (n = 6 for each group). (A) Protocols. (B) Antitumor activity in percentage. Tumor sizes are expressed as a percentage of that at day 0. (C) Diarrhea severity. Mice in each group were inoculated with CT26 cells (4 × 106 cells) by subcutaneous injection into the right gluteal region. Treatment was started when the tumors reached 0.5 cm in diameter as measured with a caliper (insert in A); the mice were injected saline or FOLFOX i.p. daily for 5 days. Briefly, subcutaneously injected colorectal cancer mice in each control group and experimental group were orally administrated saline or probiotic suspension of Lcr35 (1 × 103-7 CFU/daily) 7 days before, during (days 0–4) and 2 days (days 5, 6) after FOLFOX administration i.p. for 14 days in total. Details of the experimental procedures are given in the Materials and Methods. ∗p < 0.05. nsd, no significant difference.
To evaluate the effect of probiotics, mice in the control and experimental groups were orally administered saline or a suspension of Lcr35, respectively, daily, 7 days before, during, and 2 days after FOLFOX administration, for a total of 14 days (Figure 1A, Table 1).
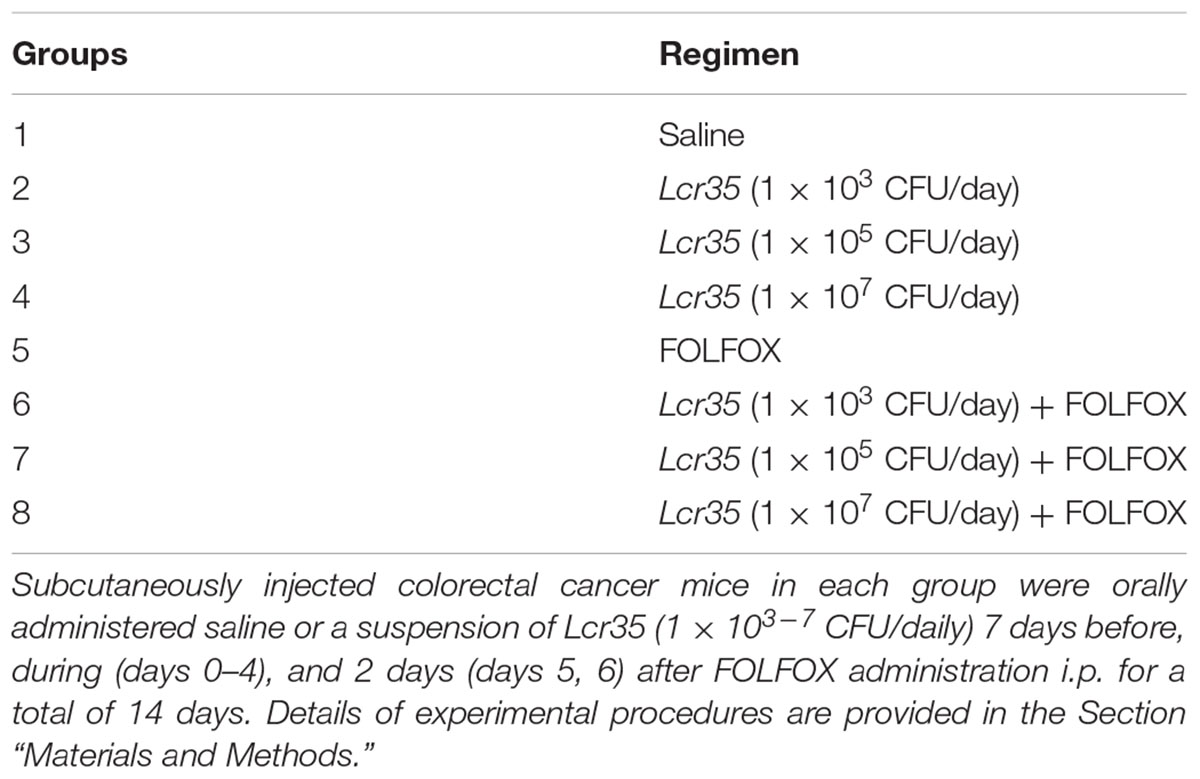
TABLE 1. Animal groups for evaluating the effects of Lcr35 on FOLFOX-induced intestinal mucositis in subcutaneously injected colorectal cancer mice (n = 6 in each group).
Disease severity, including body weight, size of injected tumors, and diarrhea severity, was determined daily by a single observer. A caliper was used to measure the largest (a) and smallest (b) diameter, and the tumor volume was estimated according to the formula 0.5ab2 (Liu et al., 2005). Diarrhea severity was assessed by using Bowen’s score system (Bowen et al., 2007) and was classified into four grades according to the stool consistency: 0, normal stool; 1, slightly wet and soft stool, indicating mild diarrhea; 2, wet and unformed stool, indicating moderate diarrhea; and 3, watery stool, indicating severe diarrhea. Mice were euthanized 2 days after complete FOLFOX administration, and tissue and blood samples were harvested for histological and biochemical analyses (Figure 1A).
Histological Analysis of Intestinal Injury
Intestinal tissues were removed and each harvested specimen was processed and fixed in 10% buffered neutral formalin for 2 h, dehydrated in an ascending series of ethanol concentrations, cleared in xylene, and embedded in paraffin wax. Sections of 4 μm thickness were cut and mounted on glass slides. Sections were routinely stained with hematoxylin and eosin (H&E) (Stringer et al., 2009b). Images were acquired using a 20× magnification objective. Specimens were viewed under a TissueFAXS automatic scanning system, captured by a digital camera, and analyzed with HistoQuest software (TissueGnostics) (Haisan et al., 2013). Villus height and crypt depth in the small intestine were measured for whole, well-oriented villi and crypts per small-intestinal tissue section per mouse, and the values were averaged.
Immunohistochemical Analysis of Intestinal Injury
Immunohistochemistry was used to detect protein expression in the intestinal tissues. The sections were dewaxed with xylene and gradually hydrated. Heat-induced antigen retrieval was achieved by using 10 mM sodium citrate (pH 6) or EDTA (pH 8) buffer at 98°C for 15 min. Endogenous peroxidase activity was quenched with hydrogen peroxide for 10 min. Then, the sections were incubated in protein block solution for 10 min and rinsed with TBST. The sections were incubated with the primary antibodies at the indicated dilution at room temperature for 1 h or at 4°C overnight. As a negative control, a set of slides was processed without primary antibody. The following antibodies were used: anti-Ki-67 (ab16667; Abcam, 1:200 dilution); anti-CD44 (ab157107; Abcam, 1:2,000 dilution); anti-NF-κB (ab28856; Abcam, 1:50 dilution); anti-BAX (#14796; Cell Signaling Technology, 1:100 dilution); anti-BCL-2 (ab32124; Abcam, 1:100 dilution); and anti-caspase 8 (ab4052; Abcam, 1:50 dilution). The Polink-2 plus Polymer HRP Detection System (GBI Labs, Mukilteo) was used as the detection system. The 3,3-Diaminobenzidine was used as the chromogen and sections were counter-stained with hematoxylin. Apoptosis was quantified by terminal deoxyribonucleotide transferase (TdT)-mediated nick-end labeling (TUNEL) assay for detecting DNA breaks in the cells of various intestinal segments [In Situ Cell Death Detection Kit, POD (Roche)]. Goblet cells were stained with periodic acid–Schiff (PAS)/Alcian Blue (AB) stain (Alcian Blue-PAS Stain Kit; ScyTek Laboratories). Specimens were viewed under a TissueFAXS automatic scanning system, and images were captured with a digital camera and analyzed using HistoQuest software (TissueGnostics). Multiple (seven) images of intestinal tissue stained with PAS/AB, Ki67, CD44, TUNEL, NF-κB, BAX, BCL-2, and caspase-8 were acquired on high-power fields (200×) of four groups by using software for image analysis.
Real-Time Quantitative (q)PCR
Total RNA from jejunum tissues was isolated using TRI Reagent® RNA isolation Reagent (Sigma) according to the manufacturer’s instructions for animal tissue. Template cDNA was synthesized from RNA using reverse transcription with oligo(dT) (Stringer et al., 2009b) primers (Fermentas). DNA detection and amplification by qPCR was carried out on an ABI 7500 Sequence Detection System with system software version 1.2.3 (Applied Biosystems). Cytokines, including TNF-α, IL-1β, IL-6, and IL-10 were detected using the Maxima SYBR Green/ROX Q-PCR Master Mix (Applied Biosystems), with 100 nM of each of the forward and reverse primers and 1 ng of DNA per reaction. PCR cycles were as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Pairs of oligonucleotide primers specific to TNF-α, IL-1β, IL-6, IL-10, and the 18S rRNA housekeeping gene were used. The qPCR data were analyzed following the 2-ΔΔCt method using the 18S rRNA gene as an internal control. The relative quantity of the target transcript was described as a fold increase relative to the reference sample and 18S rRNA. Duplicate samples were routinely used for the determination of DNA by qPCR, and mean values were calculated.
Gut Microbiota Analysis
DNA Extraction
Stool was sampled from mice (n = 4/group) and immediately stored at -80°C. DNA from fecal material was extracted with a QIAamp® DNA Stool Mini Kit (Qiagen) according to the manufacturer’s instructions. The concentration was determined by using a NanoDrop 2000 Spectrophotometer (Thermo Scientific).
Sequence Analysis
The hypervariable region V3–V4 of bacterial 16S rRNA genes was amplified by PCR using bar-coded universal primers chosen according to (Nossa et al., 2010). Library construction and amplicon sequencing were conducted with Genomics BioScience. A pair-end library (insert size of 490 bp for each sample) was constructed with TruSeq Nano DNA Library Prep kit (Illumina) and high-throughput sequencing was carried out on an Illumina MiSeq sequencer with a MiSeq Reagent Kit v3 (Illumina). The sequences of 2 × 300-bp pair-end reads were produced from the sequencer following the manufacturer’s instructions. The pair-reads were merged into amplicon sequences using PEAR (Zhang et al., 2014) and these amplicon sequences were checked for the existence of the primers, duplicates were removed, and short sequences and chimeric reads were filtered out to generate effective reads. Effective reads were analyzed to generate operational taxonomic units (OTUs). Further 16S rDNA analysis (OTU picking and taxonomic assignment) and data visualization were conducted using Quantitative Insights Into Microbial Ecology (QIIME) version 1.8.0 (Caporaso et al., 2010) with the Greengenes 16S rRNA Taxonomy Database (gg_13_8). Taxonomy (i.e., phyla and OTUs) was analyzed by one-way ANOVA.
Lactobacillus Growth Assay
Bacterial Strains, Growth Media, and Culture Conditions
Lactobacillus casei variety rhamnosus (Lcr35) (Antibiophilus®) was routinely activated and cultivated statically at 37°C for 24 h under aerobic conditions in MRS broth (BD 288130).
Determination of Minimum Inhibitory Concentrations (MICs)
Adjust Lcr35 concentration to obtain an approximate final concentration of 3 × 105 CFU/mL (Florez et al., 2016). The MICs of 5-fluorouracil (5-FU, Sigma F6627) and oxaliplatin (Sigma O9512) for Lcr35 were determined using a broth dilution test for antibacterial testing as recommended by Clinical and Laboratory Standards Institute (CLSI) protocol (Balouiri et al., 2016). Aliquots of 100 μL of the diluted cell suspensions were added to MRS Agar plates (BD 288310), with the concentration of the 5-FU and oxaliplatin would be two-fold dilutions from 0 to 512 μg/mL. Absorbance was measured according to the CLSI guidelines (Balouiri et al., 2016). MICs were established as the lowest concentration of 5-FU and oxaliplatin at which no growth was observed. The experiments were performed in triplicate.
Statistical Analysis
Results are presented as the mean ± standard error of the mean (SEM). Statistical significance (p < 0.05) was determined by one-way ANOVA. Data were analyzed with IBM SPSS software (version 21.0; SPSS Institute).
Results
Effects of Lcr35 on Subcutaneously Injected Colorectal Cancer Mice Challenged With FOLFOX
Subcutaneously injected colorectal cancer mice were divided into eight treatment groups (Table 1), and experimental animals received dosage of Lcr35 (1 × 105-7 CFU/daily) orally 7 days before, during (days 0–4), and 2 days (days 5, 6) after FOLFOX treatment (Figure 1A). After completion of the experiment, no animal exhibited signs of marked adverse effects, such as bloody stool passage or cachexia. No mortality was noted.
Size of subcutaneously injected tumors was recorded. Tumor growth was significantly prevented in colon cancer-bearing mice challenged with FOLFOX as compared to saline controls (Figure 1B). At the end of the study (day 6), tumor size was significantly decreased in the FOLFOX group as compared to the saline control group (-17.75 ± 5.63% vs. 85.15 ± 19.17%; p < 0.005). Lcr35 (1 × 103-7 CFU/daily) alone did not affect tumor growth as compared to the saline control group (day 6; Lcr35 1 × 103 vs. saline, p = 0.94; Lcr35 1 × 105 vs. saline, p = 0.15; Lcr35 1 × 107 vs. saline, p = 0.06) (Figure 1B). Furthermore, Lcr35 (1 × 103-7 CFU/daily) did not affect anti-tumorigenic activities of FOLFOX as compared to the FOLFOX group (day 6; Lcr35 1 × 103 + FOLFOX vs. FOLFOX, p = 0.75; Lcr35 1 × 105 + FOLFOX vs. FOLFOX, p = 0.81; Lcr35 1 × 107 + FOLFOX vs. FOLFOX, p = 0.42).
Diarrhea scores were recorded daily, and the results of all groups were compared. In the four saline-treated groups (with or without probiotics), no significant diarrhea was detected (day 6; Lcr35 1 × 103 vs. saline, p = 0.94; Lcr35 1 × 105 vs. saline, p = 0.94; Lcr35 1 × 107 vs. saline, p = 0.69), and their total scores remained < 1 throughout the experiment. However, after FOLFOX injection, the mice experienced the strongest diarrhea on day 6 (2 days after completion of FOLFOX treatment) as compared with saline group (2.7 ± 0.3 vs. 0.2 ± 0.2, p < 0.005; Figure 1C). On day 6, diarrhea severity was clearly attenuated in mice pretreated with the highest dose of Lcr35 (1 × 107 CFU/daily) in the FOLFOX group (0.8 ± 0.5 vs. 2.7 ± 0.3, p < 0.005; Figure 1C).
Effects of Lcr35 on FOLFOX-Induced Intestinal Mucosal Damage in Subcutaneously Injected Colorectal Carcinoma Mice
On day 6, FOLFOX caused substantial histological changes in the intestinal mucosal layer (Figure 2A), including flattened epithelial layer, shortened villi, and lamina propria with inflammatory cell infiltration in the intestine. The crypts were thickened.
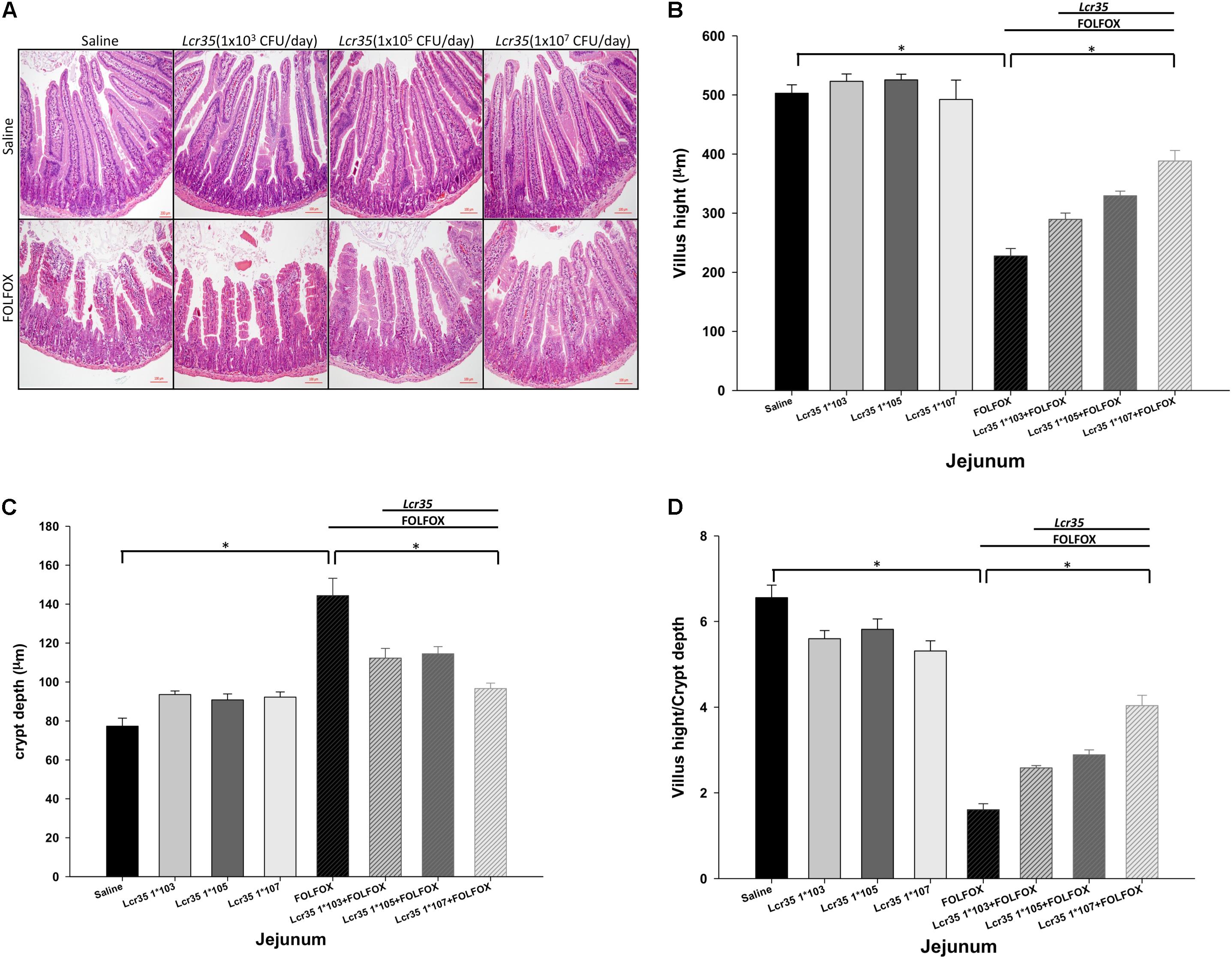
FIGURE 2. Effects of Lcr35 on histological changes in the jejunum of subcutaneously injected colorectal carcinoma mice presenting FOLFOX-induced intestinal mucositis (n = 6 for each group). Segments of the jejunum were harvested for (A) hematoxylin and eosin staining (scale bar = 100 μm) and measurement of (B) villus height, (C) crypt depth, and (D) villus height-to-crypt depth ratio per mouse. The mice received saline or Lcr35 (1 × 103-7 CFU/daily) daily during the experiment. Values are presented as the mean ± SEM. ∗p < 0.05.
FOLFOX significantly decreased villus height in the jejunum as compared to the control groups (Figure 2B). This effect was significantly and dose-dependently abrogated by Lcr35 (1 × 105-7 CFU/daily) in FOLFOX-injected mice, resulting in significantly lengthened villi as compared to the FOLFOX group (Figure 2B). Additionally, FOLFOX significantly increased intestinal crypt depth as compared to the control group (Figure 2C), while the crypt depth in the jejunum was significantly and dose-dependently restored by Lcr35 (1 × 105-7 CFU/daily; Figure 2C). Changes in villus height-to-crypt depth ratio were similar to those in villus height. FOLFOX markedly decreased the ratio in jejunal sections when compared to the control groups. These effects were also abrogated by Lcr35 (1 × 105-7 CFU/daily, p < 0.05; Figure 2D). Lcr35 treatment alone did not markedly affect intestinal histology (Figures 2A–D). Therefore, oral administration of the probiotics Lcr35 at the highest dose, 1 × 107 CFU/daily, was the most effective in ameliorating FOLFOX-induced intestinal mucosal damage in colorectal carcinoma-bearing mice, as characterized by villus height (388.21 ± 17.83 vs. 227.46 ± 12.84 μm, p < 0.05), crypt depth (96.68 ± 2.79 vs. 144.39 ± 8.88 μm, p < 0.05), and villus height-to-crypt depth ratio (4.04 ± 0.24 vs. 1.61 ± 0.14, p < 0.05).
Effects of Lcr35 on Mucus Barrier Function of Villi and Proliferation, Regeneration, and Apoptosis of Crypt in Subcutaneously Injected Colorectal Cancer Mice After FOLFOX Treatment
Compared to the control group, PAS/AB staining showed a significant decrease in mucin-filled goblet cell number in the intestinal villus after FOLFOX injection (4.66 ± 0.64 vs. 25.63 ± 3.04 cells/crypt, p < 0.005). Lcr35 at the highest dose, 1 × 107 CFU/daily, did not significantly reduce goblet cell damage in the Lcr35/FOLFOX group, suggesting that Lcr35 prevented villus damage without significantly affecting goblet cell differentiation and mucus barrier function in the intestine (Figures 3A,B).
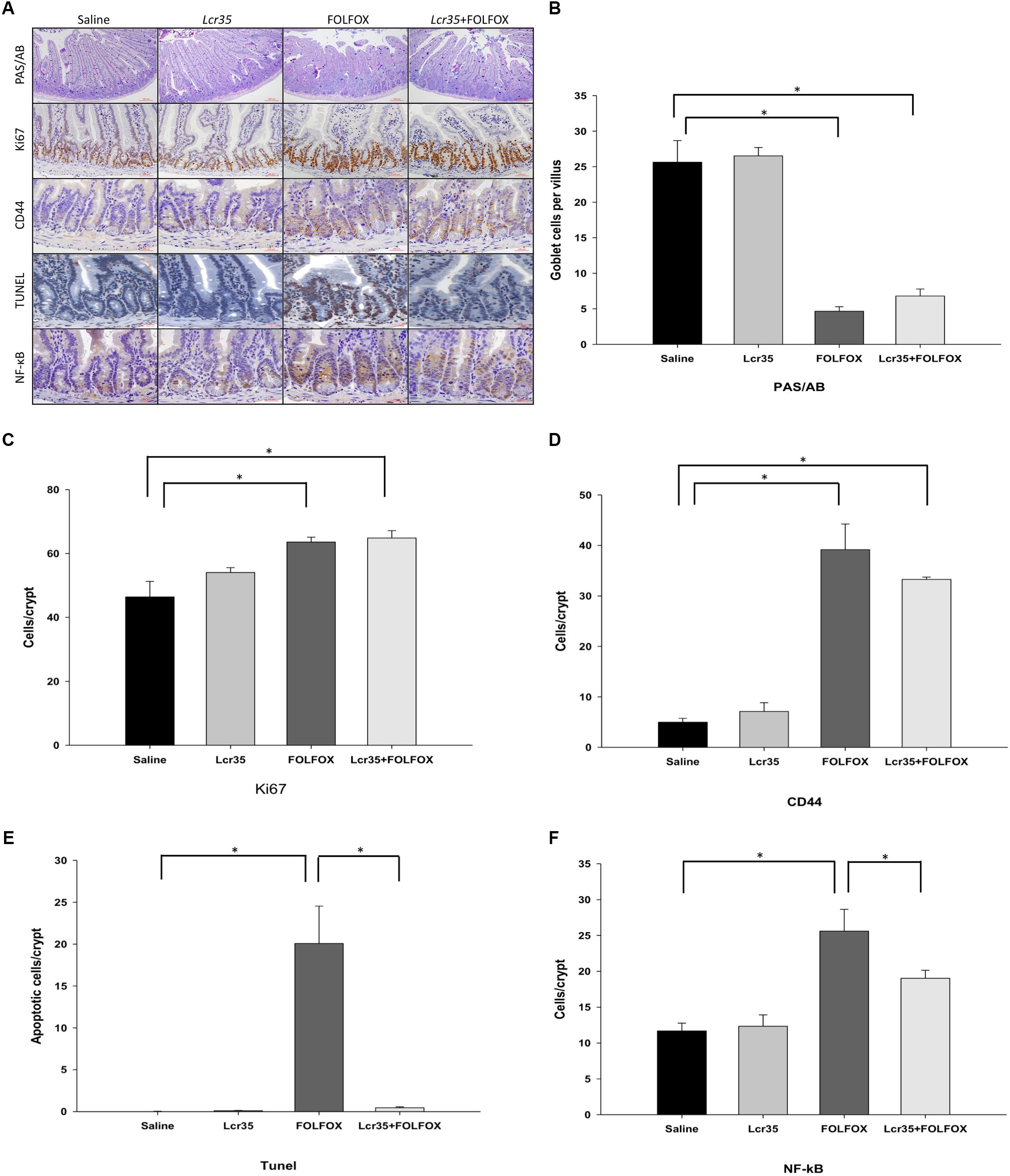
FIGURE 3. Effects of Lcr35 on goblet cells, proliferation, regeneration, apoptosis, and NF-κB activity in FOLFOX-induced intestinal damage in subcutaneously injected colorectal carcinoma mice. (A) Immunohistochemical staining of jejunum sections was used to determine Lcr35 effects on PAS (dark purple)/AB staining (goblet cells), Ki67 (brown) expression to detect proliferative activity, CD44 (brown) staining to detect regeneration activity, TUNEL (brown) to detect DNA breaks, and NF-κB activity (brown) in the intestine using antibodies. Quantification of the staining intensity of (B) PAS/AB in the intestinal villus, (C) Ki67, (D) CD44, (E) TUNEL, and (F) NF-κB in the intestinal crypt in (A). Scale bar = 100 μm. The mice received saline or Lcr35 (1 × 107 CFU/daily) daily during the experiment. Results are representative of four individual experiments and are presented as the mean ± SEM. ∗p < 0.05.
Proliferative activity in intestinal crypts was estimated by crypt Ki67 expression in jejunal segments (Figures 3A,C). A total of 2 days after FOLFOX injection, the number of Ki67-positive cells was significantly increased in the FOLFOX group as compared to the saline group (63.57 ± 1.54 vs. 46.43 ± 4.85 cells/crypt, p < 0.01). Lcr35 (1 × 107 CFU/daily) administration did not affect the proliferative activity after FOLFOX injection in the Lcr35/FOLFOX group (Figures 3A,C).
To establish whether crypts in jejunal segments were repopulated by intestinal progenitor cells, we stained the cells with an antibody against CD44. Compared to the saline group, Lcr35 (1 × 107 CFU/daily) alone had no effect on the level of CD44. The number of CD44-positive cells at the bottom of the crypts significantly increased after FOLFOX or Lcr35/FOLFOX treatment (39.18 ± 5.06 or 33.28 ± 0.47 vs. 4.98 ± 0.77 cells/crypt, p < 0.05; Figures 3A,D). However, the number of CD44-positive cells was not different between the Lcr35/FOLFOX and FOLFOX groups (Figures 3A,D).
The TUNEL staining was performed to detect apoptotic cells in the intestinal tissues. FOLFOX administration caused a marked increase in TUNEL-positive apoptotic cells in the intestinal crypts. The number of apoptotic cells in the FOLFOX-treated group was about 20-fold that of the saline group (Figures 3A,E). Lcr35 (1 × 107 CFU/daily) significantly reduced FOLFOX-increased apoptotic cells (20.07 ± 4.47 vs. 0.45 ± 0.12 cells/crypt, p < 0.0001). Lcr35 accelerated the disappearance of TUNEL-positive cells in the Lcr35/FOLFOX group across the crypts of jejunal segments (Figures 3A,E).
Immunohistochemical staining with an antibody against the NF-κB p65 subunit revealed that, compared to the saline group, there were many p65-reactive cells in the crypts of FOLFOX-treated intestinal tissues (Figures 3A,F). Lcr35 (1 × 107 CFU/daily) significantly reduced the FOLFOX-induced increase in the number of p65-reactive cells in the intestine (19.03 ± 1.12 vs. 25.60 ± 3.05 cells/crypt, p < 0.05). These findings indicated that FOLFOX-induced NF-κB activity, while Lcr35 inhibited FOLFOX-induced NF-κB activity across the crypts of jejunal segments (Figures 3A,F).
Effects of Lcr35 on BAX, BCL-2, and Caspase-8 Expression in the Intestine of Subcutaneously Injected Colorectal Cancer Mice After FOLFOX Treatment
To elucidate the role of apoptotic cascades, the expression of caspase-8, BAX, and BCL-2 proteins was measured. To assess whether Lcr35 had an effect on the expression and localization of mitochondria-associated apoptotic proteins, the numbers of BAX- and BCL-2-positive cells in the small intestines from mice in each group were quantified by immunohistochemical staining. Compared to the saline group, there were many BAX-positive cells in the crypts of FOLFOX-treated intestinal tissues (Figures 4A,B). Lcr35 (1 × 107 CFU/daily) significantly reduced the FOLFOX-induced increase in the number of BAX-positive cells in the intestinal crypts (23.83 ± 2.78 vs. 41.66 ± 3.69 cells/crypt, p < 0.05). These findings indicated that FOLFOX evoked BAX expression, while Lcr35 inhibited FOLFOX-induced BAX expression in the intestine. Compared to the saline group, there were many BCL-2-positive cells in the crypts of FOLFOX-treated intestine (Figures 4A,C). Lcr35 had no significant effect on the FOLFOX-induced increase in BCL-2 expression in the intestine. However, changes in the BAX/BCL-2 ratio were similar to that in BAX. FOLFOX markedly increased the ratio as compared to that observed in the saline control. These effects were reduced by Lcr35 (1 × 107 CFU/daily) administration in FOLFOX-injected mice (0.703 ± 0.06 vs. 1.03 ± 0.07 BAX/BCL-2 ratio, p < 0.05; Figure 4D).
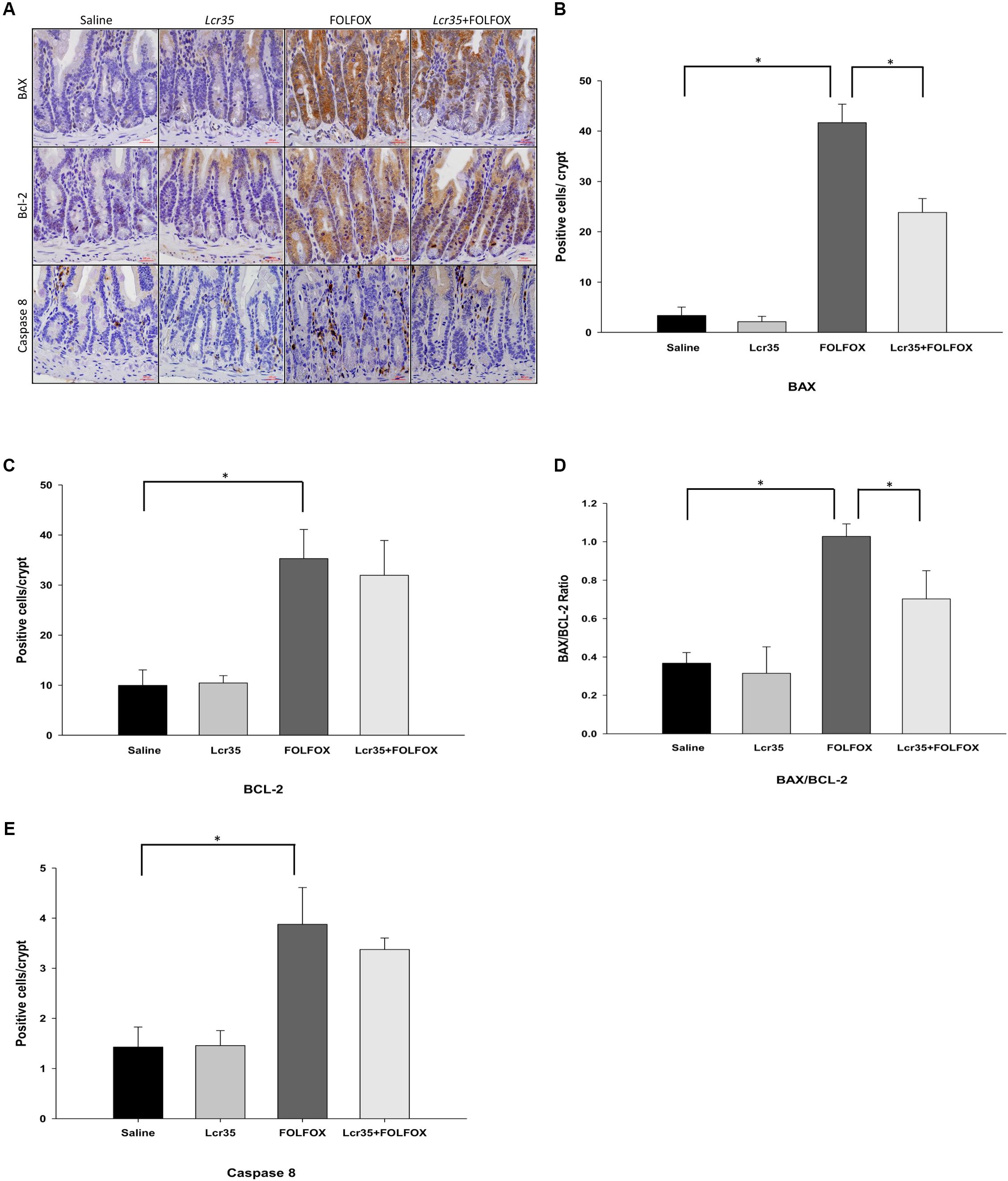
FIGURE 4. Effect of Lcr35 on BAX, BCL-2, and caspase 8 expression in FOLFOX-induced intestinal damage in subcutaneously injected colorectal carcinoma mice. Immunohistochemical staining of jejunum sections and quantification of the staining intensity in the crypts of the intestine were conducted to determine Lcr35 effects on (A,B) BAX, (A,C) BCL-2 activity (brown), (D) BAX/BCL-2 ratio, and (A,E) caspase 8 (brown) using antibodies. Scale bar = 100 μm. The mice received saline or Lcr35 (1 × 107 CFU/daily) daily during the experiment. Results are representative of four individual experiments and are presented as the mean ± SEM. ∗p < 0.05.
FOLFOX administration caused a marked increase in the number of caspase 8-positive cells in the intestinal crypts as compared to the saline group. The Lcr35 (1 × 107 CFU/daily) administration had no effect on the FOLFOX-induced increase in caspase-8 protein expression across jejunal segments (Figures 4A,E).
Effects of Lcr35 on the Regulation of IL-1β, IL-6, TNF-α, and IL-10 mRNA Expression in the Jejunum of Subcutaneously Injected Colorectal Cancer Mice Challenged by FOLFOX
After euthanasia, the effects of Lcr35 (1 × 107 CFU/daily) treatment on IL-1β, IL-6, TNF-α, and IL-10 mRNA expression in the jejunum of mice treated with FOLFOX were determined. IL-1β, IL-6, TNF-α, and IL-10 mRNA expression in the jejunum tissues was markedly upregulated in the FOLFOX-challenged group (Figures 5A–D). Lcr35 (1 × 107 CFU/daily) treatment significantly suppressed FOLFOX-induced IL-6 and TNF-α upregulation in jejunum tissues (1.76 ± 0.45 vs. 3.36 ± 0.35 and 1.12 ± 0.22 vs. 1.77 ± 0.15, respectively, p < 0.0.5; Figures 5B,C).
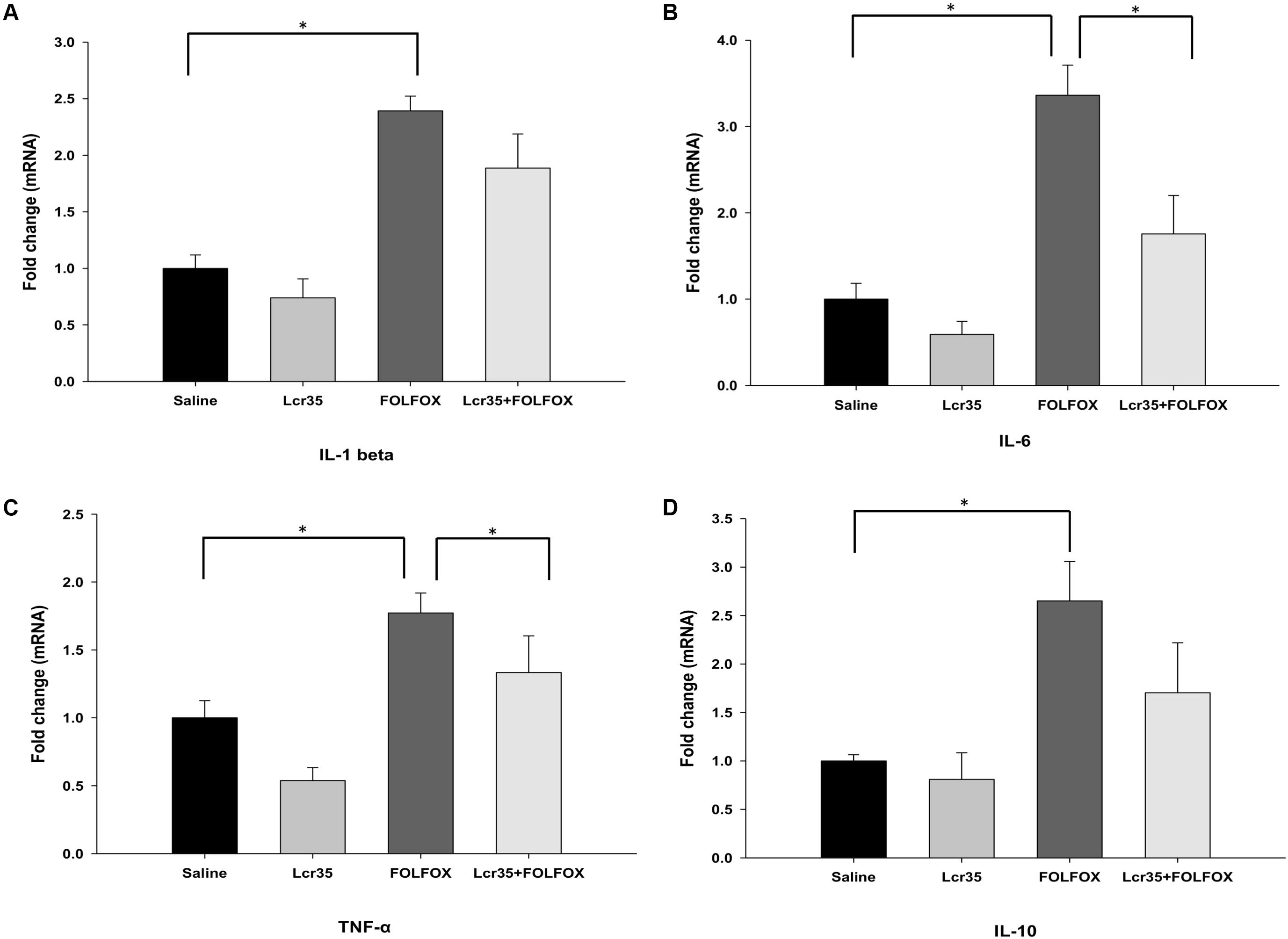
FIGURE 5. Effects of Lcr35 on Il-1β, Il-6, Tnf-α, and Il-10 mRNA expression in the jejunum of subcutaneously injected colorectal cancer mice challenged with FOLFOX. Gene expression of (A) IL-1β, (B) IL-6, (C) TNF-α, and (D) IL-10 was determined by Q-PCR in jejunum tissues. The mice received saline or Lcr35 (1 × 107 CFU/daily) daily during the experiment. Values are presented as the mean ± SEM. ∗p < 0.05.
Effects of Lcr35 on Gut Microbiota Composition in Stool From Subcutaneously Injected Colorectal Cancer Mice Challenged With FOLFOX
Fecal gut microbiota composition was determined by next-generation sequencing. Taxonomic analysis at the phylum level indicated that FOLFOX changed the gut microbiota composition. Lcr35 altered this composition in the FOLFOX-challenged group as compared to the saline group (Figure 6A). Bacteroidetes and Firmicutes were the two major phyla in all groups. FOLFOX significantly increased the abundance of Firmicutes (F) as compared to the saline control (53.92 ± 8.11 vs. 24.23 ± 7.28 relative abundance (%), p < 0.05; Figure 6B). Moreover, FOLFOX significantly decreased the abundance of Bacteroides (B) as compared to the saline control [40.27 ± 6.94 vs. 73.64 ± 7.56 relative abundance (%), p < 0.05; Figure 6C]. Changes in the F/B ratio were similar to those in Firmicutes abundance. FOLFOX markedly increased the ratio as compared to the saline control. These effects were abrogated by Lcr35 (1 × 107 CFU/daily) administration in FOLFOX-injected mice (0.67 ± 0.03 vs. 1.50 ± 0.48 F/B ratio, p < 0.05; Figure 6D). Lcr35-treatment alone did not affect fecal gut microbiota composition. Thus, oral administration of probiotics, Lcr35, specifically altered FOLFOX-induced gut microbiota change in subcutaneously injected colorectal carcinoma mice.
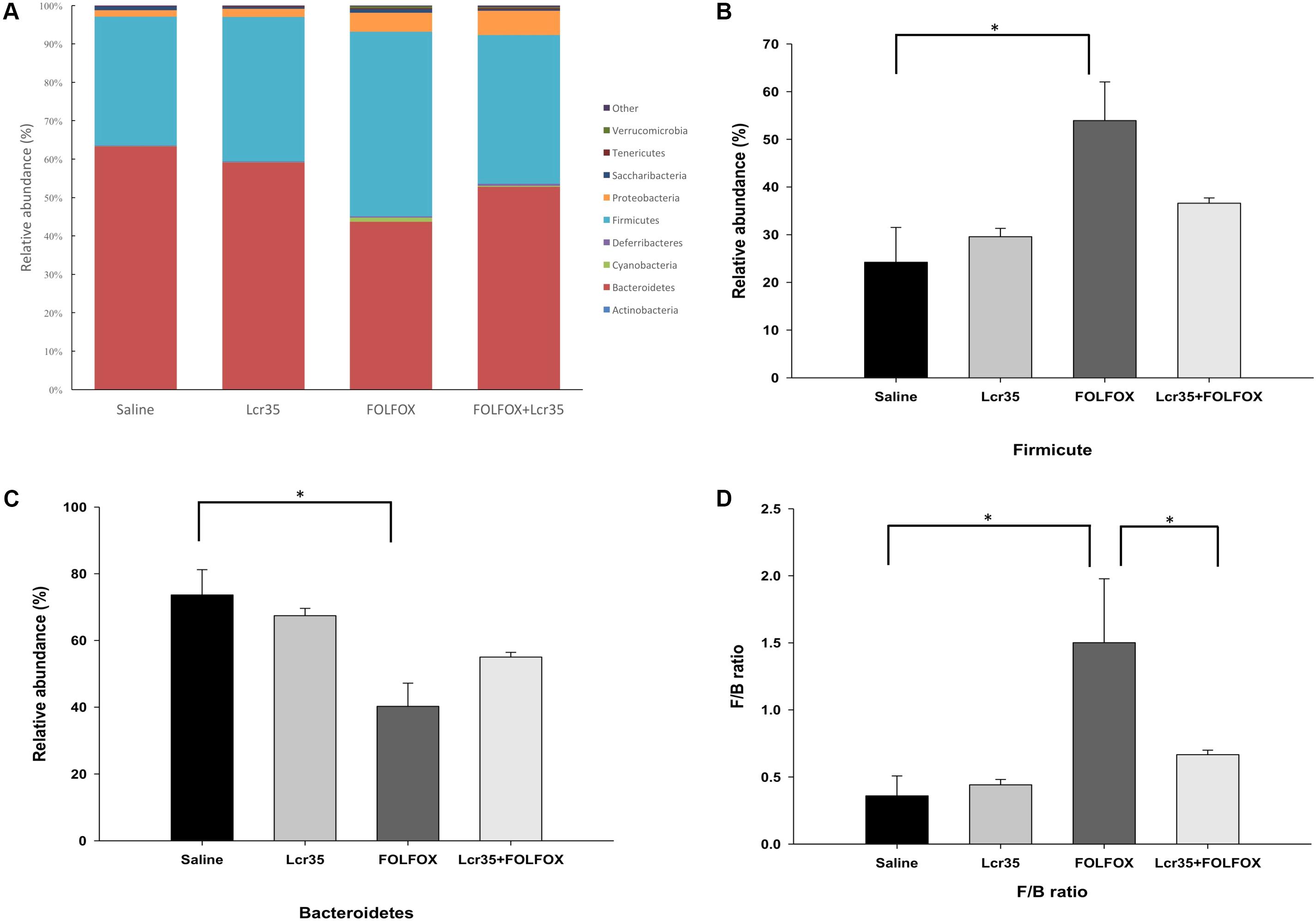
FIGURE 6. Effects of Lcr35 on changes in the gut microbiota from stool of subcutaneously injected colorectal cancer mice challenged with FOLFOX. Gut microbiota composition was determined by (A) taxonomy at phylum level, (B) Firmicutes, (C) Bacteroidetes, and (D) Firmicutes-to-Bacteroidetes (F/B) ratio. The mice received saline or Lcr35 (1 × 107 CFU/daily) daily during the experiment. Values are presented as the mean ± SEM. ∗p < 0.05.
Effects of 5-FU and Oxaliplatin on Lactobacillus Growth
The MICs obtained for the Lactobacillus casei variety rhamnosus after 48 h of incubation. Lactobacillus casei variety rhamnosus grew at the highest concentrations of 5-FU and oxaliplatin (MICs > 512 μg/mL). Lactobacillus casei variety rhamnosus is resistant at the highest concentrations assayed [MICs > 512 μg/mL] to 5-FU and oxaliplatin in vitro. The 5-FU and oxaliplatin could not perturb the Lactobacillus casei variety rhamnosus.
Discussion
To our knowledge, this is the first report demonstrating the potential of probiotics to suppress FOLFOX-induced mucositis in a colorectal cancer mouse model in vivo. Continuous FOLFOX administration to subcutaneously injected colon cancer mice significantly prevented tumor growth. The probiotic Lcr35 dose-dependently ameliorated FOLFOX-induced severe diarrhea and intestinal mucosal injury in CT26 colorectal cancer-bearing mice. Furthermore, Lcr35 reduced FOLFOX-induced intestinal mucosal inflammation characterized by immunohistological change and pro-inflammatory cytokine expression. FOLFOX-induced intestinal mucosal apoptosis and fecal gut microbiota changes were also mitigated by Lcr35. Together, these results indicated that Lcr35 is clinically promising and relevant for the prevention or management of chemotherapy-induced mucositis.
Few studies have investigated gastrointestinal mucositis resulting from combined 5-FU and oxaliplatin chemotherapy in animal models and little is known regarding the pathophysiology of mucositis with this combination (Nukatsuka et al., 2012; Wang X. et al., 2015). Our study showed that marked diarrhea developed in the FOLFOX groups. Oral Lcr35 administration significantly attenuated diarrhea and improved diarrhea scores. Furthermore, histological analysis of intestinal mucosal injury indicated that FOLFOX caused significant villus shortening in the mouse model. This damage in jejunal villi was dose-dependently prevented by Lcr35 administration. Additionally, FOLFOX significantly lengthened the intestinal crypts, while Lcr35 administration restored the crypt depth in FOLFOX-treated mice. Besides villus shortening, FOLFOX caused significant decreases in the villus height-to-crypt depth ratio in the tumor-bearing mouse model, which was alleviated dose-dependently by Lcr35 administration in FOLFOX-injected mice, although the levels did not reach those observed in the normal saline group. Our results showing that FOLFOX-induced intestinal mucositis was consistent with that previously reported in a chemotherapy-induced intestinal mucositis mouse model (Justino et al., 2015; Wang X. et al., 2015; Yeung et al., 2015). However, the reported effects of probiotics on chemotherapy-induced mucositis on villus height and crypt depth are inconsistent (Prisciandaro et al., 2011b; Justino et al., 2015; Yeung et al., 2015; Tang et al., 2017), which might be owing to differences in probiotic strains, regimens, experimental protocols, or animal models used. Furthermore, these studies were mainly performed in healthy animal models. In contrast, we investigated chemotherapy-induced intestinal mucositis in tumor-bearing mice to mimic advanced colon cancer in humans.
The NF-κB is a transcription factor that regulates the expression of numerous genes that are critical for survival. NF-κB controls inflammation, cell growth, apoptosis, and cell cycle (Logan et al., 2007). It plays an important role in the pathobiology of mucositis. NF-κB activation induced by anti-neoplastic agents, such as 5-FU, is therefore thought to elicit inflammatory and apoptotic responses in the intestine (Logan et al., 2007; Chang et al., 2012). NF-κB activation results in the upregulation of the pro-inflammatory cytokines TNF, IL-1β, and IL-6 (Logan et al., 2007), leading to mucosal injury. Accordingly, inhibition of NF-κB has been suggested as an attractive strategy for the prevention of mucositis. In this study, immunohistological analysis indicated that NF-κB, induced by FOLFOX in the intestine, was the critical molecule regulating FOLFOX-induced intestinal mucositis. Lcr35 administration decreased FOLFOX-induced NF-κB activity in the intestine and improved mucositis, as evidenced by changes in the histological characteristics. These findings suggest that inhibition of NF-κB activity by Lcr35 might result in the suppression of inflammation and the sequential amelioration of mucositis in the intestine. Furthermore, different cytokines are responsible for the various stages of mucositis. Upregulation of pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-1β, and IL-6, causes mucosal injury, eliciting further tissue damage (Sonis, 2004; Logan et al., 2007). We showed that TNF-α, IL-1β, and IL-6 expression was significantly upregulated in the jejunum of mice in the FOLFOX group, while probiotic administration abrogated this effect of TNF-α and IL-6. Thus, Lcr35 attenuates the severity of intestinal mucositis induced by FOLFOX treatment in subcutaneously injected colorectal cancer mice through inhibition of the expression of pro-inflammatory cytokines involved in the pathogenesis of mucositis.
Apoptosis is a particularly critical event in intestinal mucositis induced by chemotherapeutic agents (Keefe et al., 2000; Bowen et al., 2006; Logan et al., 2007). Caspases and proteins from the BCL-2 family play an important role in apoptosis (Bowen et al., 2006). The activation of caspases 3, 8, and 9 plays a central role in early apoptosis, regulated by various factors, including the BCL-2 protein family (Bowen et al., 2006; Kumar, 2007). Activation of caspases in response to cancer chemotherapy can be initiated via two signaling pathways: the intrinsic and the extrinsic pathway (Fulda and Debatin, 2006). The intrinsic pathway is generally activated by DNA damage and when pro-apoptotic proteins such as BAX are released from mitochondria. This mitochondria-mediated apoptotic pathway, regulated by members of the BCL-2 family, depends on the balance of the anti-apoptotic protein, BCL-2, and the pro-apoptotic protein, BAX (Breckenridge and Xue, 2004). Several studies have demonstrated that 5-FU-induced apoptosis is accompanied by an increase in pro-apoptotic BAX expression and a decrease in anti-apoptotic BCL-2 expression through the intrinsic apoptotic pathway (Inomata et al., 2002; Wu et al., 2011). In contrast, the extrinsic pathway is initiated extracellularly via activation of death receptor signaling by factors such as TNF-α. Caspase-8 is an essential component of the extrinsic cell death pathway initiated by the TNF family members (Kumar, 2007). Caspase-3, the main downstream effector caspase, is activated following cleavage by caspase-8 or -9 (Kumar, 2007). In the present study, the number of TUNEL-positive apoptotic cells markedly increased in the intestinal crypts after FOLFOX administration. Lcr35 significantly reduced FOLFOX-induced apoptosis of the intestinal crypt cells, suggesting that Lcr35 ameliorates intestinal mucositis by suppressing apoptosis induced by FOLFOX. Furthermore, a remarkable increase in the BAX/BCL-2 ratio in the intestinal crypt cells of the FOLFOX group and a shift of the equilibrium of BCL-2 family members toward apoptosis were observed (Bowen et al., 2006). Lcr35 reduced the FOLFOX-induced increase in the BAX/BCL-2 ratio and induced a shift toward anti-apoptosis. Additionally, caspase-8 was activated in the intestinal crypt cells of the FOLFOX group; however, Lcr35 administration did not significantly reduce this activity. Thus, in intestinal mucositis, apoptosis in response to FOLFOX can be induced via both the extrinsic and intrinsic pathways, and Lcr35 predominantly reduced FOLFOX-induced apoptosis via the intrinsic pathway.
Several local and systemic inflammatory diseases are linked to gut microbiota, including inflammatory bowel disease, radiotherapy-induced diarrhea, obesity, and diabetes (van Vliet et al., 2010; Boulange et al., 2016). Chemotherapy is associated with a change in microbial diversity (Stringer et al., 2009a,b; van Vliet et al., 2010; Touchefeu et al., 2014). This change in microbial diversity coincides with the development of severe chemotherapy-induced mucositis (van Vliet et al., 2010). Commensal intestinal bacteria could be incorporated as a meaningful factor in the Sonic’s five-phase model of the pathogenesis of mucositis (van Vliet et al., 2010). Accordingly, manipulating gut microbiota might have therapeutic potential against chemotherapy-induced mucositis. In the current study, next-generation sequencing revealed that FOLFOX changed the gut microbiota composition, and oral administration of Lcr35 altered this compositional change. Further taxonomic analysis at the phylum level indicated that FOLFOX significantly increased the abundance of Firmicutes, decreased the abundance of Bacteroidetes, and increased the F/B ratio. These changes were restored by Lcr35 administration. Bacteroidetes and Firmicutes are the predominant phyla in humans and mice (Eckburg et al., 2005; Touchefeu et al., 2014). The balance between these two phyla (F/B ratio) appears to be critical for the regulation of radiotherapy or chemotherapy-related mucositis (Touchefeu et al., 2014; Wang A. et al., 2015). Additionally, gut microbiota can interact with the extracellularly located parts of Toll-like receptors (TLRs) and activate the NF-κB signaling pathway, which triggers the production of proinflammatory cytokines, resulting in the development of an inflammatory response (van Vliet et al., 2010; Touchefeu et al., 2014). Our data suggest that Lcr35 altered FOLFOX-induced gut microbiota change and influenced the pathogenesis of mucositis via the gut microbiota-TLRs-NF-κB signaling pathway in subcutaneously injected colorectal carcinoma mice.
The exact mechanisms by which probiotics exert their beneficial effects remain unknown. Potential mechanisms may include the inhibition of inflammatory pathways, maintenance of intestinal permeability and mucin secretion, prevention of cell apoptosis and oxidative damage, and prevention of pathogenic colonization by re-establishing the intestinal microflora (Prisciandaro et al., 2011a). Different probiotics demonstrate various beneficial effects. Moreover, not all studies have demonstrated beneficial effects of probiotics on chemotherapy-induced mucositis. This could be explained by the use of different probiotic strains and anti-neoplastic agents, and single or combination of probiotic strains in different mucositis animal models (Prisciandaro et al., 2011a). Prisciandaro et al. (2011a) have proposed that a combination of several probiotic strains may be more reliable and efficacious. In this study, we used a single strain, which mitigated chemotherapy-induced mucositis dose-dependently. NF-κB activated by FOLFOX may result in apoptotic signals and pro-inflammatory cytokine production in normal mucosal tissue and subsequently contribute to gastrointestinal injury. Probiotics (such as Lcr35) could modulate the gut flora composition, and inhibit inflammation and apoptosis.
Interestingly, our findings also indicate spontaneous regulation toward mucosa healing 2 days after FOLFOX cessation in this tumor mouse model (Logan et al., 2007). This negative feedback of mucositis included mucosal crypt proliferation, regeneration, and anti-apoptosis, assessed by Ki67, CD44, and BAX IHC staining, respectively. Additionally, the anti-inflammatory cytokine, IL-10, was also expressed (Sultani et al., 2012). Lcr35 administration had no significant effect on this negative feedback. Autophagy is essential for cell survival and differentiation. Recent evidence indicates that the intestinal epithelium and autophagy act in concert to maintain gut homeostasis and the intestinal epithelium can induce a protective autophagy (Baxt and Xavier, 2015). In our study, autophagy was not detected in mice after FOLFOX or Lcr35 administration (data not shown).
A number of studies have reported the benefits of probiotics against colorectal cancer (CRC) onset, mainly through participating in the modulation of the immune response and induction of cell apoptosis, antioxidant activity, and improving the community of gut microbiota (Ambalam et al., 2016; Meng et al., 2018). In vivo studies with chemical-induced animal models (1,2-dimethylhydrazine, DMH; 2,4,6-trinitrobenzenesulfonic acid, TNBS; azoxymethane, AOM, dextran sodium sulfate, DSS; MNNG) and knockout models have provided evidence that the administration of probiotics strains significantly protects against CRC (Ambalam et al., 2016; Meng et al., 2018). As a specific example, the probiotic strain L. casei BL23 displayed anti-tumor properties in a mouse allograft model of human papilloma virus-induced cancer and in a DMH-induced CRC model (Lenoir et al., 2016). Further, this strain prevented colitis-associated CRC development induced by AOM-DSS (Jacouton et al., 2017). Lactobacillus strains, L. plantarum and L. acidophilus, but not L. rhamnosus suppressed tumor growth in CT26 tumor-bearing mice (Chen et al., 2012; Hu et al., 2015). Our study revealed that Lcr35 alone showed no benefits in terms of suppression of CRC and did not interfere the anti-tumor effect of FOLFOX in CT26 subcutaneous injection mice. These different anti-tumor activities of probiotics might be owing to differences in probiotic strains, regimens, experimental protocols, or animal models used. Furthermore, the previous studies were mainly performed in spontaneous and chemically induced CRC in rodents and had the advantage of investigating the effects of the probiotics on the suppression of CRC development in the colorectal region (Evans et al., 2016). In contrast, our study was mainly to investigate the protective effect of probiotics on chemotherapy-induced gastrointestinal toxicity (side effect), including diarrhea and intestinal mucositis. The animal model used in our study was established to mimic patients with colon cancer suffering from chemotherapy-associated gastrointestinal toxicity and diarrhea.
To clarify the effect of 5-FU and oxaliplatin on the growth of Lcr35, we have determined minimum inhibitory concentrations (MICs) to evaluate the inhibitory role of 5-FU and oxaliplatin in vitro. In our study, Lactobacillus casei variety rhamnosus grew at the highest concentrations of 5-FU and oxaliplatin far higher than those reached in plasma during anticancer treatment (Bocci et al., 2000; Graham et al., 2000; Florez et al., 2016). Florez et al. (2016) also demonstrated that most species of Lactobacilli, including Lactobacillus casei and Lactobacillus rhamnosus, were resistant to the highest concentrations of 5-FU assayed (MICs > 128 μg/mL) in vitro (Florez et al., 2016). Accordingly, 5-fluorouracil and oxaliplatin do not inhibit Lactobacillus growth in vitro. To get a complete picture of the impact of 5-FU and oxaliplatin on the Lactobacillus growth, further research in vivo is needed.
This study has several limitations. First, analysis of the time-course and dose-dependent effects of probiotics will provide more information regarding their role in the pathogenesis of chemotherapy-induced mucositis. Second, further in vitro studies for elucidating anti-mucositis mechanism are required. Third, our analyses did not identify specific microorganisms that are related to chemotherapeutic injuries. We only demonstrated that quantitative alterations in the proportions of several phyla, such as Firmicutes and Bacteroidetes, might be useful biomarkers of chemotherapy exposure in the gut. Future studies should focus on the functional roles and taxonomic analysis of chemotherapy-related microorganisms in the gastrointestinal tract. Concerning the impact of tumor microenvironment, the orthotopic or carcinogen-induced CRC models is worthy to be applied for further validation (Terracina et al., 2015; Evans et al., 2016). Clinical studies are also required to demonstrate the beneficial effects of probiotics and to elucidate the safety and correct regimens for the management of chemotherapy-induced mucositis.
Conclusion
Our colorectal cancer murine model with intestinal mucositis induced by FOLFOX may be an effective model to investigate the mechanisms underlying intestinal injury and its possible interaction with drugs. The development of FOLFOX-induced mucositis involves changes in gut microbiota and might be “driven” by the activation of NF-κB. Activated NF-κB results in apoptotic signals and pro-inflammatory cytokine production, sequentially contributing to gastrointestinal injury. By modulation of the gut microbiota and proinflammatory responses with suppression of intrinsic apoptosis, Lcr35 mitigated FOLFOX-induced mucositis and may serve as an alternative therapeutic strategy for the prevention or management of chemotherapy-induced mucositis in future.
Author Contributions
C-WC, C-YL, T-HT, and Y-JC performed the study concept and design. C-WC, L-HL, and J-SCC performed the acquisition of data. C-WC, C-YL, L-HL, Y-HH, T-HT, and Y-JC performed the analysis and interpretation of data. H-CL, T-EW, C-HC, S-CS, T-HT, and Y-JC performed the critical revision of the manuscript for important intellectual content. C-WC, T-HT, and Y-JC drafted of the manuscript.
Funding
This research was supported in part by research grants from the Taipei MacKay Memorial Hospital (MMH-104-80, MMH-MM-10502, MMH-105-60, MMH-MM-10611, MMH-E-107-13, and MMH-107-69), Hsinchu MacKay Memorial Hospital (MMH-103-03 and MMH-104-03), and Ministry of Science Technology (MOST 106-2314-B-195-002-MY3) of Taiwan.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Taiwan Mouse Clinic for technical support in the animal experiments, the GenoInfo Core Facility(C1) funded by NCFPB of the Ministry of Science and Technology, Taiwan (MOST106-2319-B-010-001), for technical support with the 16S rRNA gene-based gut microbiota data analysis. They would like to thank National Core Facility for Biopharmaceuticals (NCFB, MOST 106-2319-B-492-002) and National Center for High-performance Computing (NCHC) of National Applied Research Laboratories (NARLabs) of Taiwan for providing computational resources and storage resources. In addition, they would like to thank Dr. Wen-Pin Chen (Department and Graduate Institute of Pharmacology, College of Medicine, National Taiwan University), Dr. Mei-Lien Cheng and Ms. Shwu-Fang Liaw (Department of Medical Research, MacKay Memorial Hospital, Taipei, Taiwan) for providing technical support. The authors acknowledge Editage for providing writing assistance.
References
Ambalam, P., Raman, M., Purama, R. K., and Doble, M. (2016). Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 30, 119–131. doi: 10.1016/j.bpg.2016.02.009
Balouiri, M., Sadiki, M., and Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 6, 71–79. doi: 10.1016/j.jpha.2015.11.005
Bano, N., Najam, R., Qazi, F., and Mateen, A. (2014). Gastrointestinal adverse effects in advanced colorectal carcinoma patients treated with different schedules of FOLFOX. Asian Pac. J. Cancer Prev. 15, 8089–8093. doi: 10.7314/APJCP.2014.15.19.8089
Baxt, L. A., and Xavier, R. J. (2015). Role of autophagy in the laintenance of intestinal homeostasis. Gastroenterology 149, 553–562. doi: 10.1053/j.gastro.2015.06.046
Bocci, G., Danesi, R., Di Paolo, A. D., Innocenti, F., Allegrini, G., Falcone, A., et al. (2000). Comparative pharmacokinetic analysis of 5-fluorouracil and its major metabolite 5-fluoro-5,6-dihydrouracil after conventional and reduced test dose in cancer patients. Clin. Cancer Res. 6, 3032–3037.
Boulange, C. L., Neves, A. L., Chilloux, J., Nicholson, J. K., and Dumas, M. E. (2016). Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 8:42. doi: 10.1186/s13073-016-0303-2
Bowen, J. M., Gibson, R. J., Cummins, A. G., and Keefe, D. M. (2006). Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support. Care Cancer 14, 713–731. doi: 10.1007/s00520-005-0004-7
Bowen, J. M., Stringer, A. M., Gibson, R. J., Yeoh, A. S., Hannam, S., and Keefe, D. M. (2007). VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol. Ther. 6, 1449–1454. doi: 10.4161/cbt.6.9.4622
Brattain, M. G., Strobel-Stevens, J., Fine, D., Webb, M., and Sarrif, A. M. (1980). Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 40, 2142–2146.
Breckenridge, D. G., and Xue, D. (2004). Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr. Opin. Cell Biol. 16, 647–652. doi: 10.1016/j.ceb.2004.09.009
Brenner, H., Kloor, M., and Pox, C. P. (2014). Colorectal cancer. Lancet 383, 1490–1502. doi: 10.1016/S0140-6736(13)61649-9
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chang, C. T., Ho, T. Y., Lin, H., Liang, J. A., Huang, H. C., Li, C. C., et al. (2012). 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappaB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One 7:e31808. doi: 10.1371/journal.pone.0031808
Chen, C. C., Lin, W. C., Kong, M. S., Shi, H. N., Walker, W. A., Lin, C. Y., et al. (2012). Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 107, 1623–1634. doi: 10.1017/S0007114511004934
de Gramont, A., Figer, A., Seymour, M., Homerin, M., Hmissi, A., Cassidy, J., et al. (2000). Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 18, 2938–2947. doi: 10.1200/JCO.2000.18.16.2938
Doi, Y., Okada, T., Matsumoto, H., Ichihara, M., Ishida, T., and Kiwada, H. (2010). Combination therapy of metronomic S-1 dosing with oxaliplatin-containing polyethylene glycol-coated liposome improves antitumor activity in a murine colorectal tumor model. Cancer Sci. 101, 2470–2475. doi: 10.1111/j.1349-7006.2010.01678.x
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591
Eizaguirre, I., Urkia, N. G., Asensio, A. B., Zubillaga, I., Zubillaga, P., Vidales, C., et al. (2002). Probiotic supplementation reduces the risk of bacterial translocation in experimental short bowel syndrome. J. Pediatr. Surg. 37, 699–702. doi: 10.1053/jpsu.2002.32256
El-Salhy, M., Hilding, L., Royson, H., and Tjomsland, V. (2005). Comparison between triple therapy with octreotide, galanin and serotonin vs. irinotecan or oxaliplatin in combination with 5-fluorouracil/leukovorin in human colon cancer. Int. J. Oncol. 27, 687–691.
Evans, J. P., Sutton, P. A., Winiarski, B. K., Fenwick, S. W., Malik, H. Z., Vimalachandran, D., et al. (2016). From mice to men: murine models of colorectal cancer for use in translational research. Crit. Rev. Oncol. Hematol. 98, 94–105. doi: 10.1016/j.critrevonc.2015.10.009
Fang, H. W., Fang, S. B., Chiang Chiau, J. S., Yeung, C. Y., Chan, W. T., Jiang, C. B., et al. (2010). Inhibitory effects of Lactobacillus casei subsp. rhamnosus on Salmonella lipopolysaccharide-induced inflammation and epithelial barrier dysfunction in a co-culture model using Caco-2/peripheral blood mononuclear cells. J. Med. Microbiol. 59, 573–579. doi: 10.1099/jmm.0.009662-0
Florez, A. B., Sierra, M., Ruas-Madiedo, P., and Mayo, B. (2016). Susceptibility of lactic acid bacteria, bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents. Int. J. Antimicrob. Agents 48, 547–550. doi: 10.1016/j.ijantimicag.2016.07.011
Fulda, S., and Debatin, K. M. (2006). Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25, 4798–4811. doi: 10.1038/sj.onc.1209608
Giacchetti, S., Perpoint, B., Zidani, R., Le Bail, N., Faggiuolo, R., Focan, C., et al. (2000). Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 18, 136–147. doi: 10.1200/JCO.2000.18.1.136
Gordon, S. R., Maute, R. L., Dulken, B. W., Hutter, G., George, B. M., McCracken, M. N., et al. (2017). PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499. doi: 10.1038/nature22396
Graham, J., Mushin, M., and Kirkpatrick, P. (2004). Oxaliplatin. Nat. Rev. Drug Discov. 3, 11–12. doi: 10.1038/nrd1287
Graham, M. A., Lockwood, G. F., Greenslade, D., Brienza, S., Bayssas, M., and Gamelin, E. (2000). Clinical pharmacokinetics of oxaliplatin: a critical review. Clin. Cancer Res. 6, 1205–1218.
Haisan, A., Rogojanu, R., Croitoru, C., Jitaru, D., Tarniceriu, C., Danciu, M., et al. (2013). Digital microscopy assessment of angiogenesis in different breast cancer compartments. Biomed. Res. Int. 2013:286902. doi: 10.1155/2013/286902
Hu, J., Wang, C., Ye, L., Yang, W., Huang, H., Meng, F., et al. (2015). Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J. Biosci. 40, 269–279. doi: 10.1007/s12038-015-9518-4
Inomata, A., Horii, I., and Suzuki, K. (2002). 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia? Toxicol. Lett. 133, 231–240. doi: 10.1016/S0378-4274(02)00204-7
Jacouton, E., Chain, F., Sokol, H., Langella, P., and Bermudez-Humaran, L. G. (2017). Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front. Immunol. 8:1553. doi: 10.3389/fimmu.2017.01553
Johnson, R. C., and Rogers, P. (1964). 5-Fluorouracil as a selective agent for growth of Leptospirae. J. Bacteriol. 87, 422–426.
Justino, P. F., Melo, L. F., Nogueira, A. F., Morais, C. M., Mendes, W. O., Franco, A. X., et al. (2015). Regulatory role of Lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother. Pharmacol. 75, 559–567. doi: 10.1007/s00280-014-2663-x
Keefe, D. M., Brealey, J., Goland, G. J., and Cummins, A. G. (2000). Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47, 632–637. doi: 10.1136/gut.47.5.632
Kim, S. D., Lee, H. Y., Shim, J. W., Kim, H. J., Baek, S. H., Zabel, B. A., et al. (2012). A WKYMVm-containing combination elicits potent anti-tumor activity in heterotopic cancer animal model. PLoS One 7:e30522. doi: 10.1371/journal.pone.0030522
Kuebler, J. P., Colangelo, L., O’Connell, M. J., Smith, R. E., Yothers, G., Begovic, M., et al. (2007). Severe enteropathy among patients with stage II/III colon cancer treated on a randomized trial of bolus 5-fluorouracil/leucovorin plus or minus oxaliplatin: a prospective analysis. Cancer 110, 1945–1950. doi: 10.1002/cncr.23013
Kumar, S. (2007). Caspase function in programmed cell death. Cell Death Differ. 14, 32–43. doi: 10.1038/sj.cdd.4402060
Lee, C. S., Ryan, E. J., and Doherty, G. A. (2014). Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J. Gastroenterol. 20, 3751–3761. doi: 10.3748/wjg.v20.i14.3751
Lenoir, M., Del Carmen, S., Cortes-Perez, N. G., Lozano-Ojalvo, D., Munoz-Provencio, D., Chain, F., et al. (2016). Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J. Gastroenterol. 51, 862–873. doi: 10.1007/s00535-015-1158-9
Liong, M. T. (2008). Safety of probiotics: translocation and infection. Nutr. Rev. 66, 192–202. doi: 10.1111/j.1753-4887.2008.00024.x
Liu, C. Y., Liao, H. F., Wang, T. E., Lin, S. C., Shih, S. C., Chang, W. H., et al. (2005). Etoposide sensitizes CT26 colorectal adenocarcinoma to radiation therapy in BALB/c mice. World J. Gastroenterol. 11, 4895–4898. doi: 10.3748/wjg.v11.i31.4895
Logan, R. M., Stringer, A. M., Bowen, J. M., Yeoh, A. S., Gibson, R. J., Sonis, S. T., et al. (2007). The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 33, 448–460. doi: 10.1016/j.ctrv.2007.03.001
Mego, M., Holec, V., Drgona, L., Hainova, K., Ciernikova, S., and Zajac, V. (2013). Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement. Ther. Med. 21, 712–723. doi: 10.1016/j.ctim.2013.08.018
Meng, C., Bai, C., Brown, T. D., Hood, L. E., and Tian, Q. (2018). Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics 16, 33–49. doi: 10.1016/j.gpb.2017.06.002
Mohelnikova-Duchonova, B., Melichar, B., and Soucek, P. (2014). FOLFOX/FOLFIRI pharmacogenetics: the call for a personalized approach in colorectal cancer therapy. World J. Gastroenterol. 20, 10316–10330. doi: 10.3748/wjg.v20.i30.10316
Nossa, C. W., Oberdorf, W. E., Yang, L., Aas, J. A., Paster, B. J., Desantis, T. Z., et al. (2010). Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 16, 4135–4144. doi: 10.3748/wjg.v16.i33.4135
Nukatsuka, M., Saito, H., Sakamoto, K., Nakagawa, F., Uchida, J., Kobunai, T., et al. (2012). Efficacy of combination chemotherapy using oral fluoropyrimidine S-1 with oxaliplatin (SOX) against colorectal cancer in vivo. Anticancer Res. 32, 2807–2812.
Prisciandaro, L. D., Geier, M. S., Butler, R. N., Cummins, A. G., and Howarth, G. S. (2011a). Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy-induced intestinal mucositis. Crit. Rev. Food Sci. Nutr. 51, 239–247. doi: 10.1080/10408390903551747
Prisciandaro, L. D., Geier, M. S., Butler, R. N., Cummins, A. G., and Howarth, G. S. (2011b). Probiotic factors partially improve parameters of 5-fluorouracil-induced intestinal mucositis in rats. Cancer Biol. Ther. 11, 671–677.
Sharma, R., Tobin, P., and Clarke, S. J. (2005). Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 6, 93–102. doi: 10.1016/S1470-2045(05)01735-3
Sonis, S. T. (2004). The pathobiology of mucositis. Nat. Rev. Cancer 4, 277–284. doi: 10.1038/nrc1318
Sonis, S. T., Elting, L. S., Keefe, D., Peterson, D. E., Schubert, M., Hauer-Jensen, M., et al. (2004). Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100, 1995–2025. doi: 10.1002/cncr.20162
Stringer, A. M., Gibson, R. J., Bowen, J. M., and Keefe, D. M. (2009a). Chemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of change. Curr. Drug Metab. 10, 79–83.
Stringer, A. M., Gibson, R. J., Logan, R. M., Bowen, J. M., Yeoh, A. S., Hamilton, J., et al. (2009b). Gastrointestinal microflora and mucins may play a critical role in the development of 5-fluorouracil-induced gastrointestinal mucositis. Exp. Biol. Med. 234, 430–441. doi: 10.3181/0810-RM-301
Sultani, M., Stringer, A. M., Bowen, J. M., and Gibson, R. J. (2012). Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemother. Res. Pract. 2012:490804. doi: 10.1155/2012/490804
Tang, Y., Wu, Y., Huang, Z., Dong, W., Deng, Y., Wang, F., et al. (2017). Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil-induced intestinal mucositis and dysbiosis in rats. Nutrition 33, 96–104. doi: 10.1016/j.nut.2016.05.003
Terracina, K. P., Aoyagi, T., Huang, W. C., Nagahashi, M., Yamada, A., Aoki, K., et al. (2015). Development of a metastatic murine colon cancer model. J. Surg. Res. 199, 106–114. doi: 10.1016/j.jss.2015.04.030
Touchefeu, Y., Montassier, E., Nieman, K., Gastinne, T., Potel, G., Bruley des Varannes, S., et al. (2014). Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 40, 409–421. doi: 10.1111/apt.12878
van Vliet, M. J., Harmsen, H. J., de Bont, E. S., and Tissing, W. J. (2010). The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 6:e1000879. doi: 10.1371/journal.ppat.1000879
Wagner, M., Roh, V., Strehlen, M., Laemmle, A., Stroka, D., Egger, B., et al. (2009). Effective treatment of advanced colorectal cancer by rapamycin and 5-FU/oxaliplatin monitored by TIMP-1. J. Gastrointest. Surg. 13, 1781–1790. doi: 10.1007/s11605-009-0948-x
Wang, A., Ling, Z., Yang, Z., Kiela, P. R., Wang, T., Wang, C., et al. (2015). Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One 10:e0126312. doi: 10.1371/journal.pone.0126312
Wang, X., Gao, J., Qian, L., Gao, J., Zhu, S., Wu, M., et al. (2015). Exogenous IL-1Ra attenuates intestinal mucositis induced by oxaliplatin and 5-fluorouracil through suppression of p53-dependent apoptosis. Anticancer Drugs 26, 35–45. doi: 10.1097/CAD.0000000000000142
Wu, Z. Q., Han, X. D., Wang, Y., Yuan, K. L., Jin, Z. M., Di, J. Z., et al. (2011). Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother. Pharmacol. 68, 87–96. doi: 10.1007/s00280-010-1451-5
Yeung, C. Y., Chan, W. T., Jiang, C. B., Cheng, M. L., Liu, C. Y., Chang, S. W., et al. (2015). Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One 10:e0138746. doi: 10.1371/journal.pone.0138746
Yeung, C. Y., Chiang Chiau, J. S., Chan, W. T., Jiang, C. B., Cheng, M. L., Liu, H. L., et al. (2013). In vitro prevention of Salmonella lipopolysaccharide-induced damages in epithelial barrier function by various Lactobacillus strains. Gastroenterol. Res. Pract. 2013:973209. doi: 10.1155/2013/973209
Keywords: FOLFOX, Lactobacillus, intestinal mucositis, apoptosis, gut microbiota
Citation: Chang C-W, Liu C-Y, Lee H-C, Huang Y-H, Li L-H, Chiang Chiau J-S, Wang T-E, Chu C-H, Shih S-C, Tsai T-H and Chen Y-J (2018) Lactobacillus casei Variety rhamnosus Probiotic Preventively Attenuates 5-Fluorouracil/Oxaliplatin-Induced Intestinal Injury in a Syngeneic Colorectal Cancer Model. Front. Microbiol. 9:983. doi: 10.3389/fmicb.2018.00983
Received: 15 January 2018; Accepted: 26 April 2018;
Published: 15 May 2018.
Edited by:
Mirian A. F. Hayashi, Federal University of São Paulo, BrazilReviewed by:
Nagendran Tharmalingam, Alpert Medical School, United StatesCésar López-Camarillo, Universidad Autónoma de la Ciudad de México, Mexico
Copyright © 2018 Chang, Liu, Lee, Huang, Li, Chiang Chiau, Wang, Chu, Shih, Tsai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tung-Hu Tsai, thtsai@ym.edu.tw Yu-Jen Chen, chenmdphd@gmail.com
†These authors have contributed equally to this work.
 Ching-Wei Chang
Ching-Wei Chang Chia-Yuan Liu
Chia-Yuan Liu Hung-Chang Lee
Hung-Chang Lee Yen-Hua Huang
Yen-Hua Huang Li-Hui Li
Li-Hui Li Jen-Shiu Chiang Chiau
Jen-Shiu Chiang Chiau Tsang-En Wang2,4,5
Tsang-En Wang2,4,5 Yu-Jen Chen
Yu-Jen Chen