- 1Faculty of Food Science and Engineering, Dunarea de Jos University of Galati, Galati, Romania
- 2Institute of Microbiology and Biotechnology, Ulm University, Ulm, Germany
Listeria monocytogenes is a human food-borne facultative intracellular pathogen that is resistant to a wide range of stress conditions. As a consequence, L. monocytogenes is extremely difficult to control along the entire food chain from production to storage and consumption. Frequent and recent outbreaks of L. monocytogenes infections illustrate that current measures of decontamination and preservation are suboptimal to control L. monocytogenes in food. In order to develop efficient measures to prevent contamination during processing and control growth during storage of food it is crucial to understand the mechanisms utilized by L. monocytogenes to tolerate the stress conditions in food matrices and food processing environments. Food-related stress conditions encountered by L. monocytogenes along the food chain are acidity, oxidative and osmotic stress, low or high temperatures, presence of bacteriocins and other preserving additives, and stresses as a consequence of applying alternative decontamination and preservation technologies such high hydrostatic pressure, pulsed and continuous UV light, pulsed electric fields (PEF). This review is aimed at providing a summary of the current knowledge on the response of L. monocytogenes toward these stresses and the mechanisms of stress resistance employed by this important food-borne bacterium. Circumstances when L. monocytogenes cells become more sensitive or more resistant are mentioned and existence of a cross-resistance when multiple stresses are present is pointed out.
Introduction
Along the food chain, bacteria are constantly exposed to a wide range of stress factors, which affect their activity and viability. These stresses are either intrinsic to the food matrix or extrinsic factors intentionally applied to preserve food or imposed onto the organisms upon consumption by the host (Ruiz et al., 2017). L. monocytogenes is an important food-borne pathogen (Schlech et al., 1983) that frequently causes food recalls1,2 and disease outbreaks with significant case numbers and a mortality rate of 20–30%3 worldwide (Buchanan et al., 2017). This organism is known for its ability to survive or even to replicate under a wide range of environmental stress conditions (Gandhi and Chikindas, 2007; Ferreira et al., 2014; Gahan and Hill, 2014). Resistance to stress supports colonization and persistence of L. monocytogenes in various niches along the food chain and thus formation of reservoirs for contamination (Berrang et al., 2010; Leong et al., 2014, 2015; Bolocan et al., 2016). Moreover, it ultimately contributes to the ability of this bacterium to infect humans (Sleator et al., 2009).
The stresses encountered by L. monocytogenes in foods include those that are a consequence of various methods of preservation, including traditional ones as acidic pH due to fermentation by e.g., lactic acid bacteria (LAB), and osmotic stress by increased salt concentrations and more contemporary ones as using of growth inhibitors including bacteriocins and other food preservatives (Leroy and de Vuyst, 2004; Albarracín et al., 2011; Johnson et al., 2017). It should be mentioned that bacteriocins, which are small antimicrobial peptides, may either be naturally produced by bacteria used for food fermentation or can be added exogenously as a preserving additive. On the other hand, there are measures of food preservation that are rather technical in nature and are designed either to kill pathogens and spoilage microorganism at the processing stage [thermal treatments and its alternatives high pressure, pulsed electric fields (PEF), radiation] or even to protect foods during their storage (low temperatures/refrigeration, low oxygen concentrations, presence of protective gases in the surrounding atmosphere) (Morris et al., 2007; Rascon Escajeda et al., 2018).
The same stress may occur on several occasions along the food chain. For example, L. monocytogenes may be exposed to pH and osmotic stress first in the food matrix as consequence of fermentation or food preservation and subsequently in the host gastrointestinal tract. In this respect, it is of importance that resistance to different stresses is interconnected. For example incubation of L. monocytogenes at low temperatures enhances its resistance to high salt concentrations (Schmid et al., 2009). Likewise, osmotic stress in L. monocytogenes can lead to cross-protection against other causes of injury, including heat, ethanol, acidity, alkalinity, and oxidative stress (Melo et al., 2015). This is, at least partially explained by the fact that the stress signal received by the two component systems liaRS, lisRK, cesRK, agrCA, and virRS, which have been demonstrated to play a role in the stress response (Kang et al., 2015; Pöntinen et al., 2015, 2017), converge on the level of SigB, which is the alternative sigma factor σB that controls the general stress response in L. monocytogenes and other Gram-positive bacteria (Kazmierczak et al., 2003; Chaturongakul and Boor, 2006; Abram et al., 2008). For L. monocytogenes, SigB has been shown to be involved in the resistance to acidity (Wemekamp-Kamphuis et al., 2004b), osmotic stress (Fraser et al., 2003), cold and freezing stress (Becker et al., 2000), oxidative stress (Chaturongakul and Boor, 2004), and high hydrostatic pressure (Wemekamp-Kamphuis et al., 2004b). Appropriate resistance mechanisms are triggered by activation of σB-dependent promoters (Van Schaik and Abee, 2005). The extremely high tolerance to stressful conditions makes L. monocytogenes a major concern in food processing and a suitable model organism to study resistance mechanisms to stress conditions encountered in food and food processing environments.
Some of the mechanisms (and consequences) of resistance of L. monocytogenes have been expertly reviewed previously (Doyle et al., 2001; Tasara and Stephan, 2006; Thévenot et al., 2006; Gandhi and Chikindas, 2007; Lungu et al., 2009; NicAogáin and O’Byrne, 2016). However, most of these reviews focus on osmotic, pH, and temperature stress. With the present review we aim at providing a summary of the current knowledge on resistance and associated mechanisms of L. monocytogenes with a clear focus on stressful conditions that arise from traditional or alternative methods of food processing, preservation and decontamination. While we will touch upon stresses reviewed elsewhere (acidic pH, osmolarity, high and low temperatures, oxidative stress), we will also discuss resistance of L. monocytogenes to other stress conditions that have not gained as much attention (bacteriocins, pulsed or continuous UV radiation or visible light, electrical fields, high pressure). A deeper understanding of the mechanisms used by L. monocytogenes to survive and proliferate in food products may help food specialists to design efficient preservation methods that will extend shelf lives and provide a better protection of consumers against this pathogen while at the same time maintain the sensory and nutritional properties of the food products.
Resistance of L. monocytogenes to Stress During Food Processing and Storage
Resistance to Thermal Stress
Thermal treatments and temperature control are strategies that have been applied in food production and preservation for centuries to prevent or limit contamination and outgrowth of food-borne pathogens. However, the efficacy of thermal treatments against L. monocytogenes is limited by the intrinsic ability of this pathogen to survive and actively replicate at temperatures between −0.4 and 45°C (Chaturongakul et al., 2008; Chan and Wiedmann, 2009).
Resistance to Thermal Treatments
Mild thermal treatments (<100°C) are largely applied in food processing in order to inactivate vegetative microbial cells of food-spoilage bacteria and food-borne pathogens. Such treatments ensure food safety and prolonged shelf life as long as food products are properly packed and adequately stored (Van Boekel et al., 2010). Despite these benefits, thermal treatments can have a negative impact on the quality of food affecting the nutritional value and sensory properties (Hardy et al., 1999). However, the main concern with thermal processing of foods remains the ability of sublethally injured pathogenic bacteria to recover and grow during post-processing storage. This is of particular relevance for L. monocytogenes with its ability to grow in a wide temperature range (Sörqvist, 1993; Mackey et al., 1994). Although L. monocytogenes does not manifest an extraordinary resistance to high temperatures, it was shown to be more heat tolerant than other non-spore-forming pathogens such as Salmonella and E. coli (Abdel Karem and Mattar, 2001; Huang, 2004; Sallami et al., 2006). Factors that influence the resistance of L. monocytogenes to heat vary among strains, bacterial cells’ age, test and growth conditions, previous environmental stresses, or food components (Doyle et al., 2001).
L. monocytogenes has been shown to survive the minimum high-temperature, short-time treatment imposed by U.S. Food and Drug Administration (71.7°C, 15 s) in the case of milk collected from deliberately contaminated cows. Early studies raised the possibility that polymorphonuclear leukocytes present in milk may have a protective effect on L. monocytogenes during heat treatments residing inside these cells (Fleming et al., 1985; Doyle et al., 1987). However, subsequent reports showed that, in naturally contaminated milk, L. monocytogenes was not able to resist temperatures greater than 67.5°C combined with a holding time of 16.2 s (Farber et al., 1988). Nevertheless, the conditions associated with dairy products seem to influence the resistance of L. monocytogenes to heat treatments. For instance, Casadei and colleagues showed that limited access to essential nutrients in butter and the physical structure of this food could induce a starvation state in L. monocytogenes cells correlated with cross-resistance to other types of stress. In this case L. monocytogenes Scott A grown within this food matrix was four times more resistant to a treatment at 60°C than the same strain grown in TSB broth (Casadei et al., 1998). Furthermore, L. monocytogenes was shown to survive relatively high temperatures in heat-treated meat (Farber et al., 1989; Gaze et al., 1989; Murphy et al., 2003), egg products (Bartlett and Hawke, 1995; Monfort et al., 2012) and vegetables such as mushrooms and peas (Mazzotta, 2001a).
Resistance of L. monocytogenes strains to heat can vary significantly among serotypes (Sörqvist, 1994). In one study, strains belonging to serotype 1/2a showed relatively low tolerance to heat (up to 2 log CFU/mL), while strains representing serotypes 1/2b and 4b exhibited an extensive variability (from undetectable to 4 log CFU/mL). The highest heat tolerance was recorded for a serotype 7 strain (5 log CFU/mL) (Shen et al., 2014).
L. monocytogenes cells exposed to sublethal stresses prior to thermal challenge can become considerably more heat resistant. Shen and colleagues found that exposure of L. monocytogenes to a temperature of 48°C for 30 min led to heat stress adaptation among bacterial cells. Moreover, subjection to this mild stress for a short period of time did not affect the capacity of growing (Shen et al., 2014). Salt was also shown to potentiate the ability of L. monocytogenes to withstand thermal treatments (Jørgensen et al., 1995). For instance, the D63°C value, which is the time required to kill 90% of bacteria when exposed to the temperature of 63°C, for Scott A strain inoculated in egg products with 10% NaCl increased approximately 6 times in comparison with that of the same strain processed in egg products without salt (Bartlett and Hawke, 1995). This may be due to the protective effect of decreased water activity in the growth medium (Shebuski et al., 2000). Acidity is another factor that can influence bacteria’s thermotolerance. Acid-adaptation in fruit juices was shown to substantially increase the resistance of L. monocytogenes to subsequent heat treatment (Mazzotta, 2001b). Also, L. monocytogenes displays an increased heat tolerance and a significantly increased D60°C value (2.2 min) in stationary compared to exponential growth phase (0.6 min) (Jørgensen et al., 1999).
At the molecular level, the response of L. monocytogenes to 48°C involves the expression of genes belonging to specific heat-shock regulons, namely class I and class III heat-shock genes, and genes of the SigB-dependent class II stress response. The upregulation of recA expression, an activator of SOS response implicated in DNA repair could be also observed (Van der Veen et al., 2007). Class I heat-shock genes (grpE, dnaK, dnaJ, groEL, and groES) encode for heat-shock proteins (HSPs) that act as intra-cellular chaperones whose expression is increased when denatured proteins accumulate in cytoplasm (Figure 1Ba). The role of HSPs is to stabilize and assemble partially unfolded proteins, preventing their aggregation under stress conditions. Under physiological growth conditions at ambient temperature, expression of class I heat-shock genes is controlled by the HrcA repressor (Figure 1Aa), which in turn is encoded by the first gene of the dnaK operon (Hendrick and Hartl, 1993; Hanawa et al., 2000; Hartl and Hayer-Hartl, 2002). Class III heat-shock genes encode for ATP-dependent proteases (ClpC, ClpP, and ClpE) required for degradation of misfolded proteins under stress conditions including high temperature (Figure 1Bb). These proteases are negatively regulated by the CtsR repressor (Figure 1Ab), which is the product of the first gene of the clpC operon (Nair et al., 2000). The ClpL protease was recently found to play a considerable role in the elevated temperature tolerance of L. monocytogenes AT3E. This finding was observed upon curing the strain from plasmid pLM58, which harbors clpL, resulting in a strain with reduced heat resistance. Moreover, insertion of clpL increased the resistance of a heat-sensitive L. monocytogenes strain (Pöntinen et al., 2017).
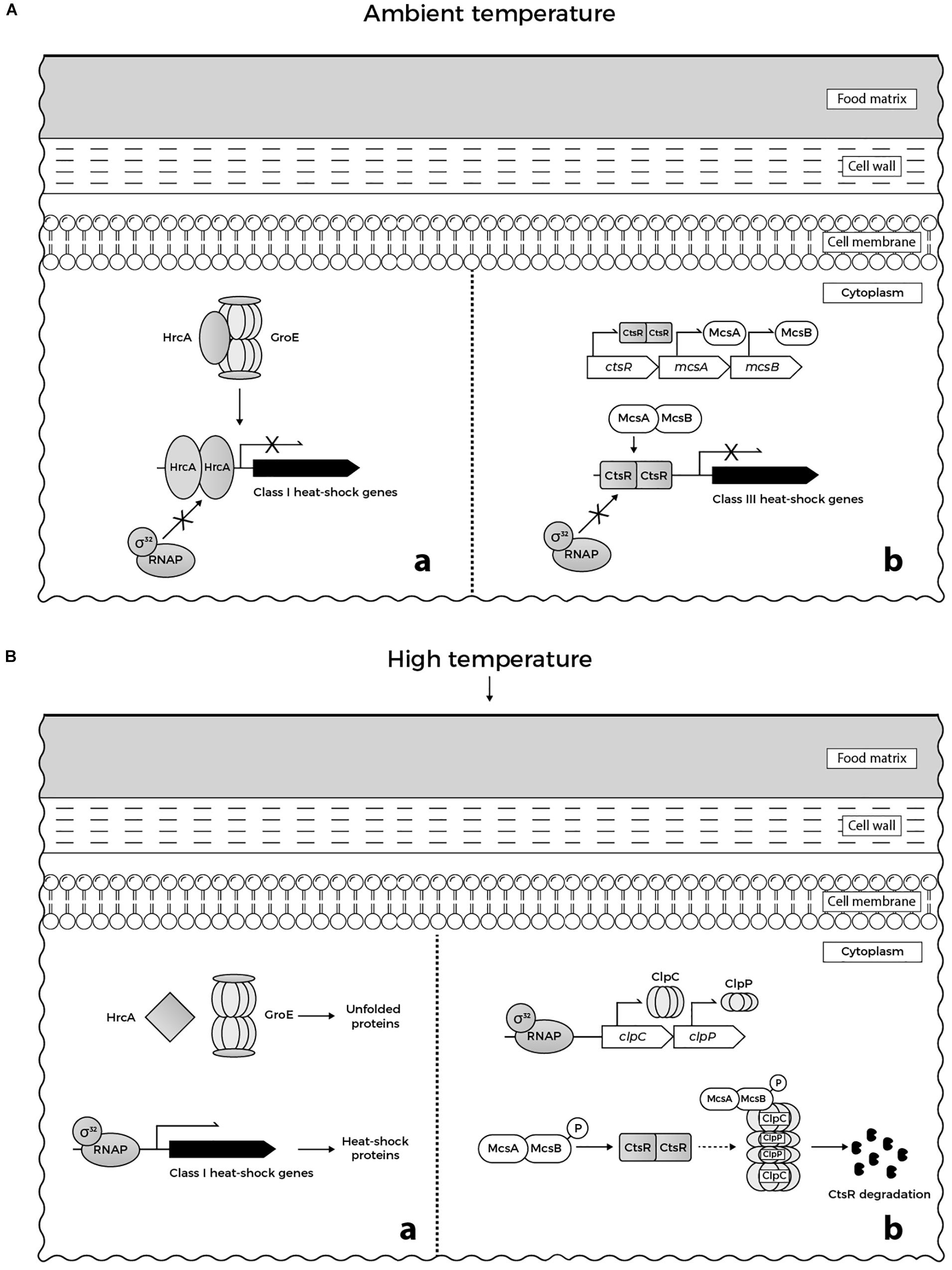
FIGURE 1. (A) Regulation of heat-shock genes in L. monocytogenes under ambient temperature in food matrices: (a) GroE chaperonine ensures the adequate folding of HrcA repressor. When folded correctly, the repressor binds to its target promoters, preventing expression of class I heat-shock genes. (b) McsB (tyrosine kinase) and its co-activator McsA (zinger finger protein) are involved in the regulation of CtsR repressor activity. CtsR, stabilized by McsA, binds to its target promoters, preventing expression of class III heat-shock genes. (B) Regulation of heat-shock genes in L. monocytogenes under heat stress in food matrices: (a) GroE is titrated by unfolded proteins that accumulate in cytoplasm and cannot interact with HrcA. When denatured upon heat stress, misfolded HrcA is unable to bind to the target DNA. Consequently, RNA polymerase-σ32 binds to the target promoters allowing the transcription of class I heat shock genes. (b) Similarly, CtsR undergoes heat-induced conformational changes that prevent its interaction with the target promoters. This allows binding of RNA polymerase-σ32 to the promoters of clp genes inducing the transcription of clpC and clpP. Following temperature-dependent autophosphorylation, McsB, assisted by McsA, targets CtsR to degradation by ClpCP protease (based on Krüger et al., 2001; Roncarati and Scarlato, 2017; Roncarati and Scarlato, 2018).
Resistance to Low Temperatures
L. monocytogenes is considered a psychrotolerant bacterium due to its ability to grow at temperatures as low as −0.4°C (Chan and Wiedmann, 2009). This tolerance to cold stress is responsible for the frequent detection of L. monocytogenes in refrigerated food products, especially meat, poultry, and seafood (Tasara and Stephan, 2006). Low temperatures result in decreased metabolic rates and changes in membrane composition, expression of cold shock proteins (Csps), and uptake of cryoprotective compounds (Phadtare et al., 1999; Neunlist et al., 2005; Cordero et al., 2016).
The alterations in the membrane in response to cold stress comprise a reduced chain length of fatty acids, an increase in the concentration of unsaturated fatty acids, and altered ratios of iso- and anteiso-branched fatty acids (Püttman et al., 1993; Russell et al., 1995; Neunlist et al., 2005). These changes maintain fluidity of the membrane at low temperatures and prevent formation of a gel-like state that may result in leakage of cytoplasmic content (Beales, 2004).
Csps are small proteins (65–70 amino acids long) with a highly conserved structure. They bind to single-stranded nucleic acid molecules via their ribonucleoprotein binding motifs RNP1 and RNP2 (Horn et al., 2007). This stabilizes the conformation of the nucleic acid and prevents degradation (Barria et al., 2013). Thus, Csps act as molecular chaperones that facilitate replication, transcription, and translation at low temperatures (Lee et al., 2012). CspA, CspB, and CspD contribute to resistance to low temperatures albeit with different importance (Schmid et al., 2009). Interestingly, they also seem to be involved in the resistance to osmotic stress (Schmid et al., 2009). The ferritin-like protein (Flp) was highly induced in response to cold shock suggesting it is involved in response to cold stress (Hébraud and Guzzo, 2000). Chan et al. (2007) determined the cold shock regulon by genome-wide expression analysis and could show that expresion of 105 and 170 genes was increased during growth on 4°C vs. 37°C in logarithmic- and stationary-phase with an overlap of 30 genes including cspL. Of these 30 genes, many are involved in membrane and cell wall function, lipid metabolism, transcription or translation.
Another mechanism of L. monocytogenes to counteract cold stress is the import of osmolytes such as glycine betaine, carnitine, γ-butyrobetaine, proline betaine, and 3-dimethylsulphoniopropionate as cryoprotectants. In the above mentioned genome-wide transcriptional analysis, the opuCABCD operon, which encodes a carnitine transporter, and gbuC encoding the substrate binding protein of a glycine betaine transporter showed increased expression in exponential growth phase at 4°C compared to 37°C (Chan et al., 2007). Similarly, expression of opuCA and betL were increased after exposure of L. monocytogenes S1 to cold and freezing stress as shown by quantitative RT-PCR (Miladi et al., 2016). This confirmed previous observations showing that Gbu-mediated betaine uptake improves growth under cold stress and uptake of betaine via BetL and OpuC transport betaine slightly improves cryotolerance (Angelidis and Smith, 2003). In the same study, OpuC was shown to be the main carnitine transporter, which provided markedly higher resistance to cold stress than betaine uptake.
Resistance to Acidity
Acidification is a method of food preservation widely applied to dairy, meat and vegetable products for centuries and is primarily achieved by fermentation by bacteria either present in the raw food or added as starter cultures (Hill et al., 2017). The preserving effect is achieved, on the one hand, by the metabolic end products, which are weak organic acids (e.g., acetate, lactate) that have anti-microbial activity, and, on the other hand, by inhibition of microbial growth at low pH (Caplice and Fitzgerald, 1999).
Both planktonic and surface attached cells of L. monocytogenes display adaptive acid tolerance response (ATR), i.e., bacteria pre-exposed to mild acid stress (pH 5.0) showed higher survival to subsequent challenge at a lower pH (3.0) compared to untreated bacteria (Davis et al., 1996; Chorianopoulos et al., 2011). The extent of ATR may be influenced by the structural properties of the food matrices. For example, L. monocytogenes grown on the surface of meat product slices formulated with potassium lactate and sodium diacetate exhibited higher resistance to a pH of 1.5 than the same bacteria exposed to the same pH in homogenates of the meat product (Skandamis et al., 2012). Similar observations were made for L. monocytogenes incubated on tomato, lettuce or in culture media for 5 days at 5°C. Bacteria incubated on vegetables were more tolerant to exposure to acidic conditions induced by lactic acid, acetic acid or hydrochloric acid than those kept in tryptic soy broth under the same conditions (Poimenidou et al., 2016).
L. monocytogenes has several mechanisms to maintain its internal pH (pHi) under acid stress (Table 1) including the F0F1-ATPase (Cotter et al., 2000), the glutamatic acid decarboxylase (GAD; Feehily et al., 2014), and the arginine and agmatine deiminases (ADI and AgDI; Lund et al., 2014). The F0F1-ATPase is involved in ATR initiation during mild pH stress (Mclaughlin and Rees, 2009). The GAD system confers resistance to more severe acidic conditions (pH < 4.5; Karatzas et al., 2012) and has also been shown to be activated as result of reduced oxygen availability associated with food atmosphere packaging (Francis et al., 2007; Sewell et al., 2015). It is comprised of two proteins, a cytoplasmic glutamate decarboxylase (GadA or GadB) and a glutamate/GABA antiporter (GadC) located in the cytoplasmic membrane (Cotter et al., 2005). The role of the GAD system is to increase pHi by converting extracellular glutamate to Γ-aminobutyrate (GABA) in an enzymatic reaction that reduces the intracellular proton concentration (Cotter et al., 2001). The ADI and AgDI systems are both involved in the response of L. monocytogenes to extreme acidity (Ryan et al., 2009; Soares and Knuckley, 2016). ADI imports arginine molecules from the extracellular environment, converting them to ornithine, CO2, ammonia (NH3), and ATP. NH3 is then protonated to ammonium (NH4), which increases pHi (Cotter and Hill, 2003). The same is true for AgDI, which converts agmatine into putrescine and NH3 (Chen et al., 2011).
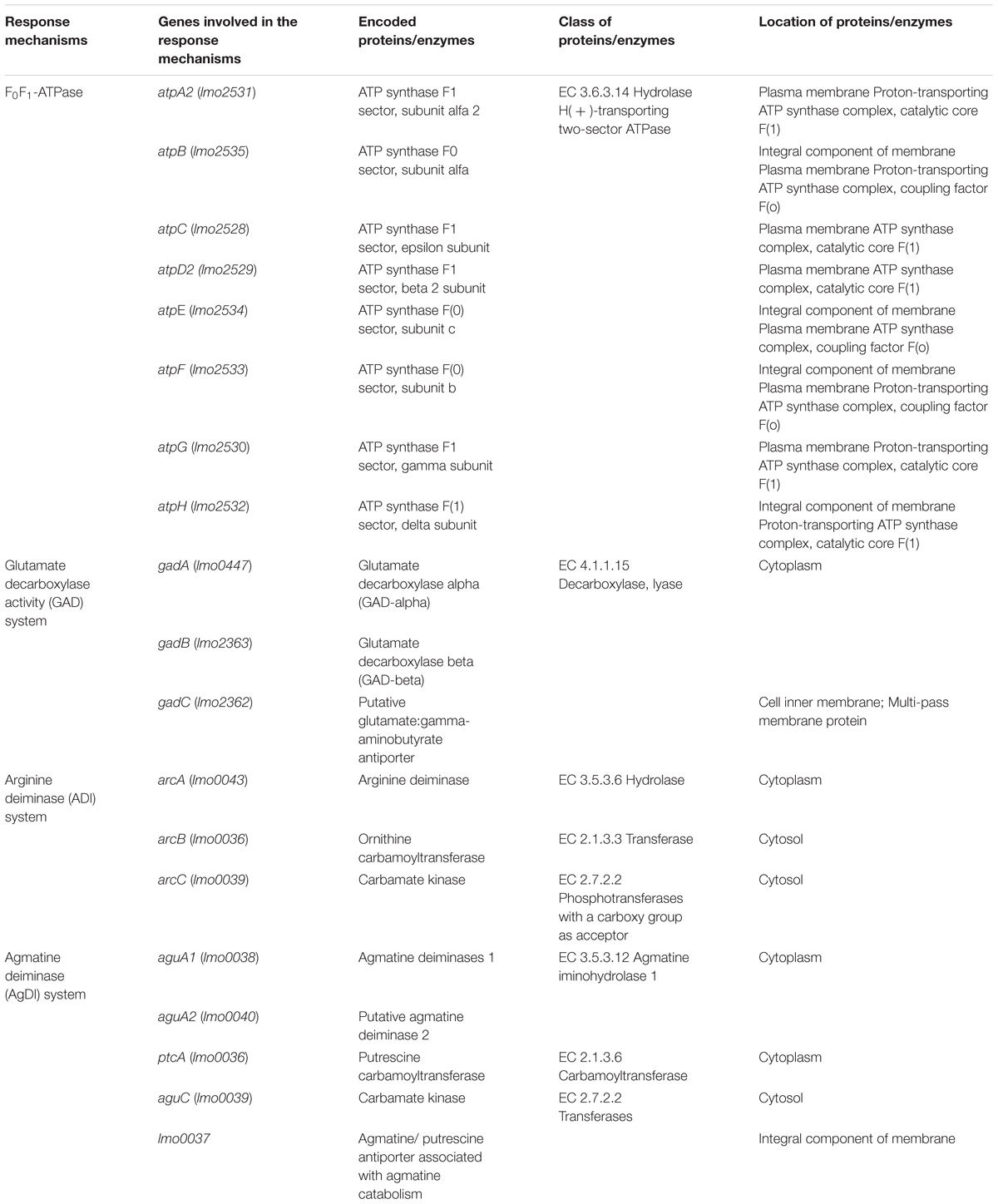
TABLE 1. Genes involved in the acidity resistance of L. monocytogenes (data retrieved from two databases, The Universal Protein Resource (UniProt) and The National Center of Biotechnology Information (NCBI), respectively).
Resistance to Osmotic Stress
Osmotic stress in food is mostly the result of increased concentrations of salts or sugars that are added to improve the sensory properties and as preserving agents to increase the shelf life of seafood, cheese, salami, pickles, jams, or syrups (Burgess et al., 2016). The presence and concentration of these additives determine water activity (Duché et al., 2002b) and affect bacterial cells by challenging the osmotic balance between cytoplasm and extracellular environment (Bae et al., 2012).
In response to elevated concentrations of salt, L. monocytogenes accumulates osmolytes, known also as compatible solutes, such as carnitine and glycine betaine in the cytoplasm to reduce osmotic pressure and water loss (Duché et al., 2002a). Besides their property to keep turgor pressure under control, compatible solutes were also shown to stabilize enzymes’ structure and function during stress (Lippert and Galinski, 1992). This mechanism is mediated by an increased expression of genes encoding for proteins involved in the transport of the respective compatible solutes (Cacace et al., 2010; Bae et al., 2012). These are the main carnitine transport system encoded by opuCABCD operon, the glycine betaine porter II system, encoded by gbuABC, and the sodium-motive-force-dependent glycine betaine uptake system, encoded by betL (Chan et al., 2007; Figure 2). L-carnitine is present in raw meat in relevant quantities (Vermassen et al., 2016), while glycine betaine is found in vegetables (e.g., sugar beet, spinach, cereals) (Sleator et al., 1999). OpuCABCD couples ATP hydrolysis to osmolyte transport across the cytoplasmic membrane (Wemekamp-Kamphuis et al., 2004a). This system is formed of OpuCA that hydrolyses ATP providing the energy for transport of the substrate by a complex consisting of the two transmembrane proteins OpuCB and OpuCD and a solute-binding protein OpuCC (Fraser et al., 2000). While BetL is involved in the primary response of L. monocytogenes to salt, GbuABC seems to administer the capacity of this bacterium to tolerate such stress during a long-term exposure (Sleator et al., 2003b).
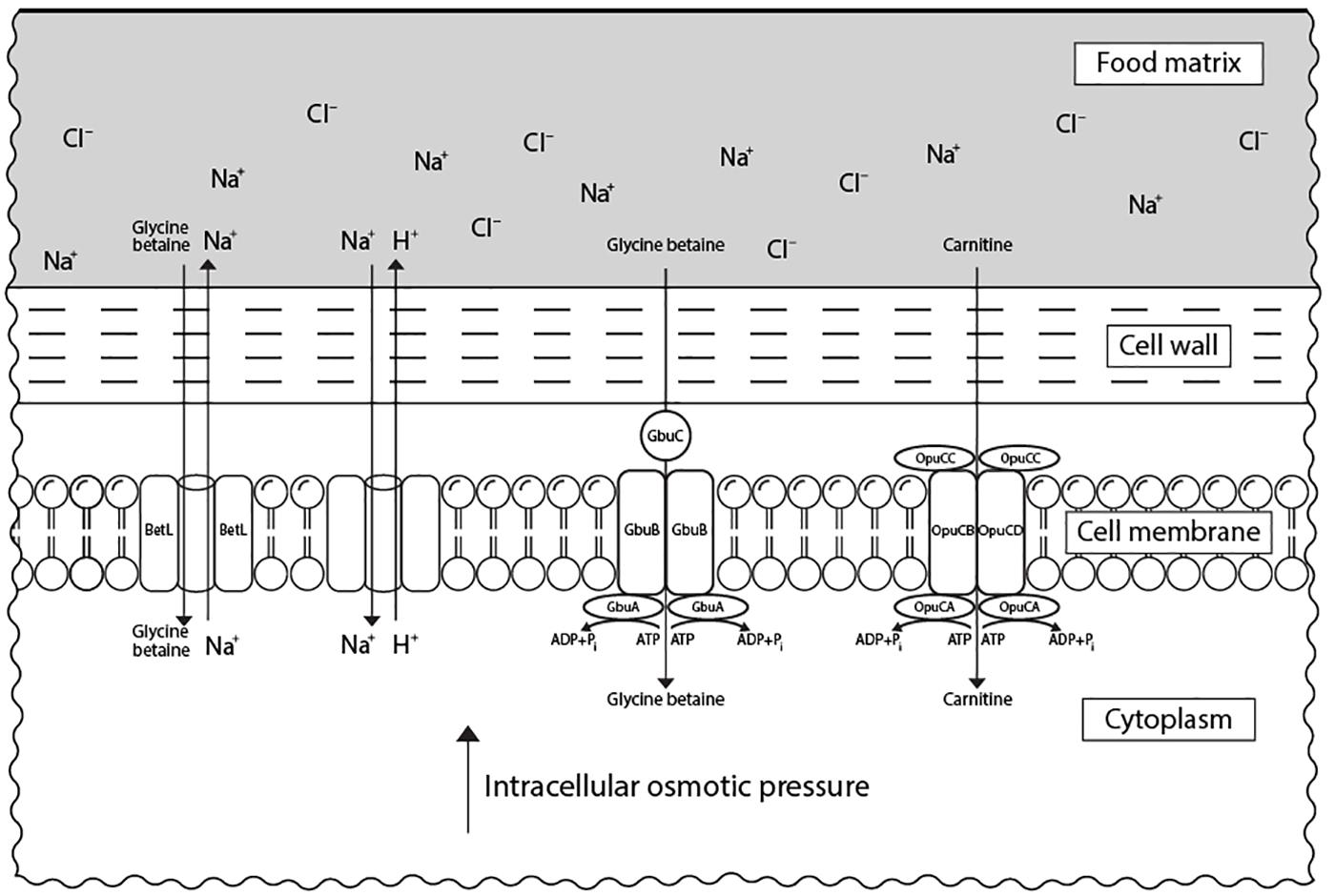
FIGURE 2. Transport systems of compatible solutes in L. monocytogenes associated with its resistance to osmotic stress in food matrices. Under salt stress, L. monocytogenes accumulates compatible solutes (carnitine and glycine betaine) via specific transport systems from the external medium (food matrix). Carnitine is transported via OpuCABCD system, while glycine betaine can be accumulated via both GbuABC and BetL systems. The presence of compatible solutes in cytoplasm leads to an increase in the intracellular osmotic pressure which restores cell turgor.
In response to osmotic stress, L. monocytogenes can also adjust expression levels of genes other than those associated with osmolytes accumulation. For example, growth of L. monocytogenes under salt stress resulted in increased expression of genes for Csps, especially cspA and cspD, promoting also adaptation to cold stress. The chaperone activity of these proteins is thought to facilitate the repair of DNA lesions, since NaCl has been shown to induce DNA breaks (Dmitrieva et al., 2004; Schmid et al., 2009). On the other hand, Bae and colleagues showed that presence of salt in the growth medium led to a decreased expression of genes associated with carbohydrate PTS systems in L. monocytogenes including those related to uptake of β-glucoside, galactitol, fructose, and cellobiose. This suggests a possible connection between a significantly lower growth rate and reduced uptake of carbohydrates under osmotic stress (Bae et al., 2012).
While accumulation of compatible solutes plays the main role in L. monocytogenes’ survival to hyper-osmotic shock, a potential response of this bacterium to hypo-osmotic conditions may be mediated by mechanosensitive channels. Bacterial mechanosensitive ion channels regulate turgor pressure by assisting efflux of osmolytes (Perozo and Rees, 2003). So far, genes for two putative mechanosensitive channels have been identified in L. monocytogenes. The lmo2064 gene shows significant homology to mscL from E. coli, which encodes for a large-conductance mechanosensitive channel (MscL). Additionally, lmo1013 is similar to mscS of Streptococcus pneumoniae encoding for a small-conductance mechanosensitive channel (MscS) (Sleator et al., 2003a; Renier et al., 2012). After subjection to osmotic downshock, L. monocytogenes cells have been shown to release almost instantaneously betaine and L-carnitine, which may be linked to the activity of these channels (Verheul et al., 1997a).
Resistance to Bacteriocins
Bacteriocins are antimicrobial peptides produced by a wide range of LAB and are mostly active against Gram-positive bacteria including L. monocytogenes (Cotter et al., 2013; Chikindas et al., 2018). Bacteriocins are natural and safe food additives for a wide range of food products including fruits, vegetables, dairy products, and meat, that are either produced in situ by LAB used for food fermentation or added exogenously (Silva et al., 2018). Most bacteriocins are highly specific for their target organisms and kill their targets by inhibiting growth, disruption of membrane homeostasis and pore formation (Zhang and Gallo, 2016).
The only bacteriocin approved as preserving additive in food is nisin, which belongs to the class I bacteriocins and has a broad activity against various Gram-positive bacteria (Cleveland et al., 2001). Nisin is widely used in dairy and meat products with the purpose to inhibit the growth of food-borne pathogens including L. monocytogenes and Clostridium botulinum (Gharsallaoui et al., 2016). The antimicrobial activity of this bacteriocin is mediateded by two mechanisms. Nisin inhibits the cell wall biosynthesis by binding and sequestering lipid II, which is an essential carrier molecule for peptidoglycan building blocks. Moreover, nisin-lipid II complexes form pores in the membrane leading to permeabilization (Wiedemann et al., 2001).
Resistance of L. monocytogenes to nisin has been associated with a series of changes in the cytoplasmic membrane composition aiming to prevent the peptide from crossing this barrier. The studies conducted on nisin-resistant (Nisr) cells noticed a reduction in the content of phospholipids with particular emphasis on phosphatidylglycerol and diphosphatidylglycerol, major components correlated with the interaction between nisin and membrane (Ming and Daeschel, 1995; Verheul et al., 1997b). In addition, it was indicated an increase in the proportion of straight-chain fatty acids to the detriment of branched-chain fatty acids, changes that result in a less fluid and, in the same time, more rigid cell membrane (Ming and Daeschel, 1993). The alterations in the cell wall of Nisr strains of L. monocytogenes have been also investigated. The resistance of Nisr cells to the degradation action of lysozyme and their sensitivity to benzylpenicillin and ampicillin suggested compositional changes that occurred at the level of this cellular component (Crandall and Montville, 1998). One example could be the D-alanine esterification of teichoic acids (Vadyvaloo et al., 2004). However, a recent transcriptomic analysis of L. monocytogenes survival cells following the exposure to a high nisin concentration reported the downregulation of dltA and dltB, implying that D-alanine residues are not involved in the elevated resistance to this bacteriocin. The study also emphasized the expression regulation of two cell-wall associated genes: downregulation of lmo2714 encoding for a peptidoglycan anchored protein and upregulation of lmo2522 encoding for a cell wall-binding protein with possible implication in nisin tolerance (Wu et al., 2018).
In L. monocytogenes, nisin resistance is directly mediated by VirR (Grubaugh et al., 2018), the response regulator of the VirRS two component system previously described to be involved in resistance to stress (Mandin et al., 2005). However, in the case of nisin an ABC-transporter encoded by virAB seems to be responsible in perception of the stressor instead of the VirS receptor histidine kinase (Grubaugh et al., 2018). VirR mediates the resistance to nisin and other stresses of the cell envelop by regulating the dltABCD operon (Kang et al., 2015) that is responsible for modification of lipoteichoic acids (Abachin et al., 2002). Other two component systems that were shown to be involved in resistance to nisin are LiaRS and LisRK (Cotter et al., 2002; Collins et al., 2012; Bergholz et al., 2013). Genes/operons and their products regulated by these TCS with a reported role in resistance to nisin are lmo2229 (Gravesen et al., 2004; Collins et al., 2012), telA (Collins et al., 2010b), mprF (Thedieck et al., 2006), anrAB (Collins et al., 2010a), and dltABCD (Abachin et al., 2002). With the exception of telA, all these genes have a known role in metabolism/biosynthesis of components of the membrane or cell wall. Similar to e.g., resistance to pH or salt stress, nisin resistance can also be induced by other stresses, e.g., increased salt concentrations (Bergholz et al., 2013).
Recently, a number of class II bacteriocins with activity against L. monocytogenes have been isolated and characterized including pediocins, sakacin P, leucocins, enterococin, mesentericin Y105, garvicin, linocin M18, and others (Eppert et al., 1997; Tosukhowong et al., 2012; Perez et al., 2014; Ovchinnikov et al., 2016; Ríos et al., 2018). L. monocytogenes and other bacteria are able to develop resistance to bacteriocins. Natural resistance is observed with a frequency of 1–8% depending on the bacteriocin and the L. monocytogenes strain tested (Macwana and Muriana, 2012). Consistent with the receptors and mechanisms of action of bacteriocins, resistant strains show altered expression or mutations in certain phosphotransferase systems (PTSs) (Vadyvaloo et al., 2002, 2004; Tymoszewska et al., 2017). For instance, a spontaneous leucocin-resistant mutant of L. monocytogenes B73 was lacking a putative IIAB subunit of a mannose PTS (Ramnath et al., 2000). Furthermore, pediocin PA-1-resistant L. monocytogenes 412 mutants overexpressed gene fragments associated with a β-glucoside-specific PTS (Gravesen et al., 2000). Similar results of other studies suggest that resistance of L. monocytogenes to class IIa bacteriocins is correlated with a general mechanism consisting of a lack in EII subunits of mannose PTS and a compensatory upregulation of the β-glucoside PTS genes (Dalet et al., 2001; Gravesen et al., 2002).
Resistance of L. monocytogenes to Stress During Processing and Decontamination Using Alternative Technologies
In recent years, a number of novel technologies are applied by the industry for production and preservation of minimally processed foods and diminish the impact of chemical substances on the environment. Consequently this results in new stress conditions encountered by L. monocytogenes.
Resistance to High Hydrostatic Pressure
High pressure processing (HPP) is a technology used in food preservation as an alternative to thermal treatments, aiming to destroy food spoilage microorganisms and food-borne pathogens (Huang et al., 2014). Depending on the food and spoilage organisms, pressures applied for sterilization are usually between 250 and 700 MPa. Bacterial cells subjected to HPP treatments display morphological and physiological changes that may be reversible depending on pressure and holding time. Primary effects of HPP are an increase in the permeability of the cell membrane, the disruption of the protein structure and function, and, as a consequence, inhibition of the metabolism, replication, and transcription (Huang et al., 2014).
The effect of HPP on survival of L. monocytogenes was tested under various settings in different food products including cheese (Tomasula et al., 2014), fruit juice (Alpas and Bozoglu, 2003), jams (Préstamo et al., 1999), whole milk (Hayman et al., 2007), and RTE cooked meat products (Hereu et al., 2012). Overall, the results of these studies indicate that resistance of L. monocytogenes to HPP varies depending on the strain. For instance, when pressured with 350 MPa at 20°C, L. monocytogenes EGD-e displayed only 1.0 log CFU/mL reduction and was more resistant to this HPP treatment than LO28 strain (1.8 log CFU/mL reduction) and ScottA strain (3.2 log CFU/mL reduction) (Van Boeijen et al., 2008). In addition, the type, composition and matrix of food products have an impact on the resistance of bacteria to HPP. Vitamins, amino acids, and cations (Ca2+, Mg2+) may have protective effects. For example, Mg2+ is known to stabilize ribosome structure and Ca2+ strengthens the outer membrane (Niven et al., 1999). Also, elevated salt concentrations in a food product may induce uptake of compatible solutes, which in turn stabilize cells during HPP (Abe, 2007). In line with this, a mutant deficient in synthesis of the compatible solute proline showed increased sensitivity to HPP (Considine et al., 2011)
The effect of HPP on L. monocytogenes was investigated on the global transcriptomic level by microarray analysis with subsequent RT-PCR on some target genes (Bowman et al., 2008). This indicated that mRNA levels were reduced globally with increasing intensity and duration of the treatment. Nevertheless, HPP induced expression of genes associated with DNA repair, transcription, translation, cell division, protein secretion, motility, chemotaxis, and membrane and cell wall biosynthesis. On the other hand, reduced expression was observed for genes involved in carbohydrates’ uptake, energy metabolism and virulence. Surprisingly, HPP seemed to reduce expression of the general stress sigma factor SigB and part of the SigB regulon. One of the genes showing highest induction by HPP was cspL encoding a cold-shock protein. This suggests that HPP also induces cross-resistance to other stresses. For example, HPP resistance in semi-skimmed milk was higher than in buffer and the resistant isolate was also more resistant to heat, acid, and oxidative stress (Karatzas and Bennik, 2002).
Mutations in CtsR, a class III stress genes repressor (Nair et al., 2000), have been linked to spontaneous resistance of L. monocytogenes cells to HPP. Mutants with a stable resistance showed point mutations, insertions or deletions in the ctsR gene that negatively affected its activity. This loss in ctsR function in HPP resistant variants of L. monocytogenes was accompanied by increased expression of clpB, clpC, clpE, and clpP (Karatzas et al., 2003; Van Boeijen et al., 2010). Clp proteases have a clear role in degradation of misfolded or damaged proteins preventing their potentially harmful accumulation in bacterial cells (Krüger et al., 2000; Tomoyasu et al., 2001). Since protein denaturation is one of the consequences of HPP treatment (Moreirinha et al., 2016) increased Clp protease activity is in line with increased HPP tolerance in L. monocytogenes. However, isolation of resistant mutants that do not display these changes indicates that there may be other unknown mechanisms conferring resistance to HPP (Karatzas et al., 2005). Moreover, Chen et al. (2009) reported that different levels of HPP resistance among L. monocytogenes strains are not based on ctsR gene mutations.
L. mononcytogenes ScottA and a spontaneous HPP resistant isolate of this strain were shown to be more resistant to HPP in stationary compared to exponential growth phase (Karatzas and Bennik, 2002). Moreover, it seems that cells in stationary phase of growth do not exhibit the highest resistance to HPP treatment. L. monocytogenes cells found in long-term-survival phase showed even higher HPP tolerance, as transition back to log and stationary phases resulted in less survivors after pressurization. This phenomenon has been attributed to a change in cell morphology from rods to cocci that results in cytoplasmic condensation and, implicitly, reduction of intracellular water activity (Wen et al., 2009).
Resistance to UV-Light
Another more recent method of food decontamination, included under the umbrella of alternative technologies, is pulsed or continuous UV-light, which kills microorganisms found on the surface of food products as result of cross-contamination occurring during processing procedures such as cutting, slicing or packing (Gómez-López et al., 2007). Although approved by the United States and Food and Drug Administration (USFDA) for food application in 1996, the safety of this technology regarding the potential of permanent microbial inactivation still remains under question.
The bactericidal effect of UV light is caused by DNA damage as a consequence of the formation of photoproducts including cyclobutane-pyrimidine dimers (CPDs), pyrimidine 6-4 pyrimidone photoproducts (6-4PPs), and their Dewar isomers (Rastogi et al., 2010). Other mechanisms of bacteria inactivation caused by UV light are the photophysical and photothermal effects resulting in leakage of cellular content following the absorption of the high energy light pulses (Gómez-López et al., 2007). The efficacy of UV treatment in decontamination of food surfaces depends on a number of factors including the food product, distance and position of the product to the light source, energy level given by number and frequency of the light pulses, level of contamination and others (Gómez-López et al., 2007). The potential of UV-C light in L. monocytogenes inactivation was shown to be lower on fruits with smooth surface (apples and pears) compared to fruits with a rougher surface (cantaloupe, strawberry or raspberry) (Adhikari et al., 2015). UV light is also used as disinfection procedure to improve hygiene in food processing environments (Bintsis et al., 2000). The presence of organic materials such as food debris on stainless steel surfaces appeared to protect L. monocytogenes cells against UV-C radiation (Bernbom et al., 2011).
Several studies have been conducted in order to investigate the efficacy of L. monocytogenes inactivation by pulsed UV-light on/within various food matrices. A maximum of inactivation of L. monocytogenes ScottA on the skin side of salmon filets was achieved with 180 pulses of UV light of 5.6 J/cm2 at a distance of 8 cm for 60 s and efficacy was markedly lower on the muscle side (Ozer and Demirci, 2006). Similarly, the best inactivation rates of the same strain on chicken frankfurters was obtained with 180 pulses in 60 s at UV energy of 1.27/cm2 (Keklik et al., 2009).
L. monocytogenes has been reported to be more resistant to UV-light than other pathogens, such as E. coli (Beauchamp and Lacroix, 2012). However, very little is known regarding specific mechanisms of UV resistance in L. monocytogenes. Sublethal challenge with other stresses does not induce cross-resistance to UV light and UV resistance does not seem to depend on SigB, the general stress sigma factor of L. monocytogenes (Gayán et al., 2015). Global gene expression analysis of the response to both pulsed light (PL) and continuous ultraviolet treatment was conducted in L. monocytogenes 10403S (Uesugi et al., 2016). Although the overall amplitude of the changes in gene expression was low, a number of genes encoding for stress proteins, motility and transcriptional regulators were induced by UV exposure. However, no increased expression was observed for lmo0588. This gene encodes for a (putative) photolyase. This protein plays an important role in photoreactivation, which is the recovery of bacteria sublethally injured by UV light due to subsequent exposure of visible light (Gómez-López et al., 2007). During photoreactivation, photolyase binds and repairs the pyrimidine DNA lesions using light energy absorbed by its chromophores (Sinha and Häder, 2002). In fact, an increase in viability was observed for a UV-treated L. monocytogenes serotype 1/2b after incubation in daylight for only 90 min followed by storage under dark (Lasagabaster and Martínez de Marañón, 2017).
Resistance to Pulsed Electric Fields
Pulsed electric fields (PEF) processing is another non-thermal alternative technology for decontamination mainly used in liquid foods processing and thus is not limited to inactivation on the surface of a product. The treatment consists of short, highly intense pulses of electric fields applied to the products in order to achieve the inactivation of unwanted microorganisms (Góngora-Nieto et al., 2002). The inactivating effects of PEF are destabilization and, depending on the strength of the PEF, irreversible damage of the cytoplasmic membrane with formation of micropores and leakage of cytoplasmic content (Góngora-Nieto et al., 2002). Similar to HPP and UV light but unlike conventional thermal food processing technologies, such as pasteurization, this method is less detrimental to food matrices and better in preserving the sensory and nutritional characteristics of the product (Toepfl et al., 2007). The efficacy of inactivation by PEF is determined by a number of factors related to the process (strength, duration, frequency of the pulses, temperature, etc.), the food product (composition, conductivity, pH, etc.) and the microorganisms to be inactivated (species, growth phase, etc.) (Wouters et al., 2001).
In general, Gram-positive organisms are believed to be more resistant to PEF than Gram-negative bacteria, presumably due to the thicker cell wall and stiffening (lipo)teichoic acids (Lado and Yousef, 2002). For example, L. monocytogenes proved to be more PEF tolerant than Salmonella enteritidis and E. coli when treated in melon and watermelon juices (Mosqueda-Melar et al., 2007). Thus, PEF alone is probably not the method of choice for inactivation of L. monocytogenes. It has been recommended to combine PEF with other methods such as ozone (Unal et al., 2001), mild heat (Fleischman et al., 2004) or plants infusions with antimicrobial properties (Rivas et al., 2016) to decontaminate food products at risk for contamination with L. monocytogenes. Low inactivation rates were observed for L. monocytogenes in a Spanish vegetable-based beverage and this was attributed to the neutral pH of the product (Selma et al., 2006). In fact, in buffer inactivation rates of L. monocytogenes by PFE were higher at acidic pH (Álvarez et al., 2002; Gómez et al., 2005; Saldaña et al., 2009). Further data in buffered systems or culture media indicated that resistance to PEF was increased in stationary growth phase and in media with reduced water activities (Álvarez et al., 2002; Lado and Yousef, 2003) suggesting a cross-resistance with other stresses.
Besides membrane disruption, PEF was suggested to affect bacterial cells by denaturation of the membrane-bound proteins as result of localized overheating caused by the capacity of the formed pores to conduct electricity (Simpson et al., 1999). This might imply an involvement of chaperones in the response of L. monocytogenes to PEF. One study compared the expression levels of three major molecular chaperones, namely GroEL, GroES, and DnaJ, in a resistant and a sensitive L. monocytogenes strain treated with a sublethal PFE challenge and found a transient reduction in expression of these chaperones in the sensitive strain (Lado et al., 2004).
Somolinos and colleagues have shown no difference in the resistance to PEF processing between L. monocytogenes EGD-e and its isogenic ΔsigB mutant suggesting that SigB is not involved in the repair mechanism of injured cells as shown for thermal treatment of the same strains. Also, unlike heat challenge, mild acid shock applied to L. monocytogens cells did not increase the resistance to subsequent PEF treatment (Somolinos et al., 2010).
Resistance to Oxidative Stress
Under oxidative stress (bacterial) cells encounter high concentrations of oxygen radicals (Suo et al., 2014). This disturbs the normal redox state of cells leading to cell death due to the oxidative damage of proteins, lipids and nucleic acids. Bacteria use reduction pathways that repair damage of susceptible amino acids (cysteine and methionine) induced by reactive oxygen (ROS) or reactive chlorine species (RCS). ROS are a group of compounds containing oxygen on different redox states such as hydrogen peroxide, hydroxyl radical or peroxyl radical. In bacteria, these compounds activate enzymes such as superoxide dismutases (SOD), catalases, peroxidases and efflux pumps to counteract oxidative stress (Dröge, 2003; Archambaud et al., 2006).
Recently, Harter and colleagues revealed the presence of a novel stress survival islet (SSI-2) in L. monocytogenes ST121 and other strains isolated from food and food processing environments. The SSI-2 consists of two genes lin0464 and lin0465 (PfpI protease), that are upregulated after 10 min of exposure to oxidative stress. Lin0464 seems to be a positive gene regulator of lin0465, because the time frame of increased transcription of lin0465 is longer compared to that of lin0464 and because the constitutive expression of lin0464 has no effect on the survival rate in Δlin0465 mutant. Under alkaline or oxidative stress encountered in food processing environments, the expression of both genes offers L. monocytogenes ST121 the possibility to adapt and survive, an independent response mechanism from the alternative sigma factor (Harter et al., 2017).
Even if SigB is the main regulator of stress genes, its role in the oxidative stress resistance is controversial. A number of authors (Ferreira et al., 2001; Oliver et al., 2010) provided experimental data suggesting that, in L. monocytogenes, oxidative stress protection is conferred by σB since ΔsigB mutant cells are sensitive to this stress. Other studies suggested that sigB expression is harmful for stationary-phase L. monocytogenes (EGD-e and 10403S) cells grown aerobically, under oxidative stress conditions mediated by hydrogen peroxide. Furthermore, ΔsigB mutant proved, besides oxidative stress resistance, a stronger catalase activity upon addition of 30% H2O2, compared to the wild type. Interestingly, no difference was observed in the transcription of the catalase gene between the ΔsigB mutant and the wild type (Boura et al., 2016). All these discrepancies within the role of sigB in oxidative stress response may be explained by variation between strains (Moorhead and Dykes, 2003), oxidative agents tested, differences in growth phase, and oxygen tension of the culture (Boura et al., 2016).
In L. monocytogenes, the resistance to oxidative stress was also correlated with biofilm formation (Suo et al., 2012). Four genes related to oxidative stress, kat, perR (peroxide operon regulator), sigB and recA (recombinase A) were upregulated in a Δsod mutant, which produced more ROS than the wild-type L. monocytogenes 4b G (Suo et al., 2014). Also, a ΔperR L. monocytogenes mutant showed increased sensitivity to hydrogen peroxide stress. Moreover, catalase activity in these cells increased to a toxic level resulting in smaller colonies and changes in cell morphology compared to the wild type (Rea et al., 2005).
The anti-oxidative kat gene acts synergistically with sod gene (superoxide dismutase), both being involved in the protection against toxic effects of hydrogen peroxide and superoxide anion radicals (Suo et al., 2012). The sodA gene encodes for MnSOD, a cytosolic SOD enzyme which uses manganese in the catalytic reactions. Archambaud and colleagues reported that, during stationary phase, L. monocytogenes MnSOD activity is downregulated by phosphorylation at serine/threonine residues. MnSOD activity increases only when dephosphorylation is performed, condition that facilitates its secretion in the bacterial culture media via SecA2 pathway (Archambaud et al., 2006).
Other genes involved in the response to oxidative stress are fri, gltB, and gltC. Based on its iron-binding activity, fri-encoded ferritin detoxifies oxidative agents (Dussurget et al., 2005; Olsen et al., 2005). Huang and colleagues introduced a role of gltB and gltC gene products in oxidative stress and L. monocytogenes biofilm formation. GltC is a member of LysR-type transcriptional regulator and gltB encodes for a glutamate synthase regulated by GltC. Experiments with gltB and gltC mutants revealed a reduced ability to form biofilm and an increased sensitivity to oxidative stress (Huang et al., 2013).
Conclusion
L. monocytogenes is able to use diverse mechanisms to survive various stress conditions encountered in food matrices. This explains the efforts made by scientists to understand these mechanisms in order to develop more efficient methods to reduce L. monocytogenes occurrence in food and food related environments. With the present review, we aim at providing an overview of the current knowledge on food-related stress and stress resistance of L. monocytogenes. As observed for many other organisms, L. monocytogenes employs different survival mechanisms for the same stress or use the same mechanism for different stresses (heat-shock genes are expressed when L. monocytogenes is subjected to heat stress, HPP or PEF; cold-shock genes are expressed and osmolytes transport systems are activated when L. monocytogenes encounters cold or osmotic stress). However, compared to other organisms, the large number of mechanisms also increases the possibilities of this organism for cross-resistance.
The ongoing trend toward healthier, minimally processed food products with unaltered sensory and nutritional properties demands new strategies for food preservation, while no compromises are accepted for food safety. Alternative treatments (e.g., high pressure, pulsed electrical field, UV light), have yielded promising results, but their application often allows to L. monocytogenes recovery. However, current data also suggest that combinations of these techniques with e.g., natural preserving additives such as bacteriocins may be feasible solutions. Nevertheless, the effects on and resistance of L. monocytogenes to such combinations of stresses need to be investigated.
Author Contributions
FB and LG-G drafted the manuscript and all authors revised and contributed to its final version.
Funding
This study was funded within the ERA-IB2 consortium “SafeFood” (ID: ERA-IB-16- 247 014) by grants of the Executive Agency for Higher Education, Research, Development and Innovation Funding in Romania to AN (International and European Cooperation – 250 Subprogramme 3.2 – Horizon 2020 – Contract No. 15/2017) and by the German Ministry for Education and Research to CR (Grant No. 031B0268). The activity of FB was partially funded by the Doctoral School of Mechanical and Industrial Engineering belonging to “Dunarea de Jos” University of Galati.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thankfully acknowledge Cornel Leuştean for providing professional technical support for graphical representation of the molecular mechanisms.
Footnotes
- ^ https://www.theguardian.com/business/2018/jul/06/uk-supermarkets-recall-frozen-vegetables-over-bacteria-fears (Accessed 31.07.2018)
- ^ https://www.fda.gov/AJAX/All/ (Accessed 31.07.2018)
- ^ http://www.who.int/csr/don/02-may-2018-listeriosis-south-africa/en/ (Accessed 10.10.2018)
References
Abachin, E., Poyart, C., Pellegrini, E., Milohanic, E., Fiedler, F., Berche, P., et al. (2002). Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43, 1–14. doi: 10.1046/j.1365-2958.2002.02723.x
Abdel Karem, H., and Mattar, Z. (2001). Heat resistance and growth of Salmonella enteritidis, Listeria monocytogenes and Aeromonas hydrophila in whole liquid egg. Acta Microbiol. Pol. 50, 27–35.
Abe, F. (2007). Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: perspectives from piezophysiology. Biosci. Biotechnol. Biochem. 71, 2347–2357. doi: 10.1271/bbb.70015
Abram, F., Starr, E., Karatzas, K. A. G., Matlawska-Wasowska, K., Boyd, A., Wiedmann, M., et al. (2008). Identification of components of the sigma B regulon in Listeria monocytogenes that contribute to acid and salt tolerance. Appl. Environ. Microbiol. 74, 6848–6858. doi: 10.1128/AEM.00442-08
Adhikari, A., Syamaladevi, R. M., Killinger, K., and Sablani, S. S. (2015). Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocytogenes on organic fruit surfaces. Int. J. Food Microbiol. 210, 136–142. doi: 10.1016/j.ijfoodmicro.2015.06.018
Albarracín, W., Sánchez, I. C., Grau, R., and Barat, J. M. (2011). Salt in food processing; usage and reduction: a review. Int. J. Food Sci. Technol. 46, 1329–1336. doi: 10.1111/j.1365-2621.2010.02492.x
Alpas, H., and Bozoglu, F. (2003). Efficiency of high pressure treatment for destruction of Listeria monocytogenes in fruit juices. FEMS Immunol. Med. Microbiol. 35, 269–273. doi: 10.1016/S0928-8244(02)00446-7
Álvarez, I., Pagán, R., Raso, J., and Condón, S. (2002). Environmental factors influencing the inactivation of Listeria monocytogenes by pulsed electric fields. Lett. Appl. Microbiol. 35, 489–493. doi: 10.1046/j.1472-765X.2002.01221.x
Angelidis, A. S., and Smith, G. M. (2003). Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl. Environ. Microbiol. 69, 7492–7498. doi: 10.1128/AEM.69.12.7492-7498.2003
Archambaud, C., Nahori, M.-A., Pizarro-Cerda, J., Cossart, P., and Dussurget, O. (2006). Control of Listeria superoxide dismutase by phosphorylation. J. Biol. Chem. 281, 31812–31822. doi: 10.1074/jbc.M606249200
Bae, D., Liu, C., Zhang, T., Jones, M., Peterson, S. N., and Wang, C. (2012). Global gene expression of Listeria monocytogenes to salt stress. J. Food Prot. 75, 906–912. doi: 10.4315/0362-028X.JFP-11-282
Barria, C., Malecki, M., and Arraiano, C. M. (2013). Bacterial adaptation to cold. Microbiology 159(Pt 12), 2437–2443. doi: 10.1099/mic.0.052209-0
Bartlett, F. M., and Hawke, A. E. (1995). Heat resistance of Listeria monocytogenes Scott A and HAL 957E1 in various liquid egg products. J. Food Prot. 58, 1211–1214. doi: 10.4315/0362-028X-58.11.1211
Beales, N. (2004). Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: a review. Compr. Rev. Food Sci. Food Saf. 3, 1–20. doi: 10.1111/j.1541-4337.2004.tb00057.x
Beauchamp, S., and Lacroix, M. (2012). Resistance of the genome of Escherichia coli and Listeria monocytogenes to irradiation evaluated by the induction of cyclobutane pyrimidine dimers and 6-4 photoproducts using gamma and UV-C radiations. Radiat. Phys. Chem. 81, 1193–1197. doi: 10.1016/j.radphyschem.2011.11.007
Becker, L. A., Evans, S. N., Hutkins, R. W., and Benson, A. K. (2000). Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 182, 7083–7087. doi: 10.1128/JB.182.24.7083-7087.2000
Bergholz, T. M., Tang, S., Wiedmann, M., and Boor, K. J. (2013). Nisin resistance of Listeria monocytogenes is increased by exposure to salt stress and is mediated via LiaR. Appl. Environ. Microbiol. 79, 5682–5688. doi: 10.1128/AEM.01797-13
Bernbom, N., Vogel, B. F., and Gram, L. (2011). Listeria monocytogenes survival of UV-C radiation is enhanced by presence of sodium chloride, organic food material and by biofilm formation. Int. J. Food Microbiol. 147, 69–73. doi: 10.1016/j.ijfoodmicro.2011.03.009
Berrang, M. E., Meinersmann, R. J., Frank, J. F., and Ladely, S. R. (2010). Colonization of a newly constructed commercial chicken further processing plant with Listeria monocytogenes. J. Food Prot. 73, 286–291. doi: 10.4315/0362-028X-73.2.286
Bintsis, T., Litopoulou-Tzanetaki, E., and Robinson, R. K. (2000). Existing and potential applications of ultraviolet light in the food industry – a critical review. J. Sci. Food Agric. 80, 637–645. doi: 10.1002/(SICI)1097-0010(20000501)80:6
Bolocan, A. S., Nicolau, A. I., Álvarez-Ordóñez, A., Borda, D., Oniciuc, E. A., Stessl, B., et al. (2016). Dynamics of Listeria monocytogenes colonisation in a newly-opened meat processing facility. Meat Sci. 113, 26–34. doi: 10.1016/j.meatsci.2015.10.016
Boura, M., Keating, C., Royet, K., Paudyal, R., O’Donoghue, B., O’Byrne, C. P., et al. (2016). Loss of SigB in Listeria monocytogenes strains EGD-e and 10403S confers hyperresistance to hydrogen peroxide in stationary phase under aerobic conditions. Appl. Environ. Microbiol. 82, 4584–4591. doi: 10.1128/AEM.00709-16
Bowman, J. P., Bittencourt, C. R., and Ross, T. (2008). Differential gene expression of Listeria monocytogenes during high hydrostatic pressure processing. Microbiology 154(Pt 2), 462–475. doi: 10.1099/mic.0.2007/010314-0
Buchanan, R. L., Gorris, L. G. M., Hayman, M. M., Jackson, T. C., and Whiting, R. C. (2017). A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 75, 1–13. doi: 10.1016/j.foodcont.2016.12.016
Burgess, C. M., Gianotti, A., Gruzdev, N., Holah, J., Knøchel, S., Lehner, A., et al. (2016). The response of foodborne pathogens to osmotic and desiccation stresses in the food chain. Int. J. Food Microbiol. 221, 37–53. doi: 10.1016/j.ijfoodmicro.2015.12.014
Cacace, G., Mazzeo, M. F., Sorrentino, A., Spada, V., Malorni, A., and Siciliano, R. A. (2010). Proteomics for the elucidation of cold adaptation mechanisms in Listeria monocytogenes. J. Proteomics 73, 2021–2030. doi: 10.1016/j.jprot.2010.06.011
Caplice, E., and Fitzgerald, G. F. (1999). Food fermentations: role of microorganisms in food production and preservation. Int. J. Food Microbiol. 50, 131–149. doi: 10.1016/S0168-1605(99)00082-3
Casadei, M. A., de Matos, R. E., Harrison, S. T., and Gaze, J. E. (1998). Heat resistance of Listeria monocytogenes in dairy products as affected by the growth medium. J. Appl. Microbiol. 84, 234–239. doi: 10.1046/j.1365-2672.1998.00334.x
Chan, Y. C., Raengpradub, S., Boor, K. J., and Wiedmann, M. (2007). Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 73, 6484–6498. doi: 10.1128/AEM.00897-07
Chan, Y. C., and Wiedmann, M. (2009). Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit. Rev. Food Sci. Nutr. 49, 237–253. doi: 10.1080/10408390701856272
Chaturongakul, S., and Boor, K. J. (2004). RsbT and RsbV contribute to σB-dependent survival under environmental, energy, and intracellular stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 70, 5349–5356. doi: 10.1128/AEM.70.9.5349-5356.2004
Chaturongakul, S., and Boor, K. J. (2006). σB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 72, 5197–5203. doi: 10.1128/AEM.03058-05
Chaturongakul, S., Raengpradup, S., Wiedmann, M., and Boor, K. J. (2008). Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 16, 388–396. doi: 10.1016/j.tim.2008.05.006
Chen, H., Neetoo, H., Ye, M., and Joerger, R. D. (2009). Differences in pressure tolerance of Listeria monocytogenes strains are not correlated with other stress tolerances and are not based on differences in CtsR. Food Microbiol. 26, 404–408. doi: 10.1016/j.fm.2009.01.007
Chen, J., Cheng, C., Xia, Y., Zhao, H., Fang, C., Shan, Y., et al. (2011). Lmo0036, an ornithine and putrescine carbamoyltransferase in Listeria monocytogenes, participates in arginine deiminase and agmatine deiminase pathways and mediates acid tolerance. Microbiology 157(Pt 11), 3150–3161. doi: 10.1099/mic.0.049619-0
Chikindas, M. L., Weeks, R., Drider, D., Chistyakov, V. A., and Dicks, L. M. T. (2018). Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 49, 23–28. doi: 10.1016/j.copbio.2017.07.011
Chorianopoulos, N., Giaouris, E., Grigoraki, I., Skandamis, P., and Nychas, G. (2011). Effect of acid tolerance response (ATR) on attachment of Listeria monocytogenes Scott A to stainless steel under extended exposure to acid or/ and salt stress and resistance of sessile cells to subsequent strong acid challenge. Int. J. Food Microbiol. 145, 400–406. doi: 10.1016/j.ijfoodmicro.2011.01.001
Cleveland, J., Montville, T. J., Nes, I. F., and Chikindas, M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Sci. Technol. 71, 1–20. doi: 10.1016/S0168-1605(01)00560-8
Collins, B., Guinane, C. M., Cotter, P. D., Hill, C., and Ross, P. R. (2012). Assessing the contributions of the lias histidine kinase to the innate resistance of Listeria monocytogenes to nisin, cephalosporins, and disinfectants. Appl. Environ. Microbiol. 78, 2923–2929. doi: 10.1128/AEM.07402-11
Collins, B., Curtis, N., Cotter, P. D., Hill, C., and Ross, R. P. (2010a). The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various β-lactam antibiotics. Antimicrob. Agents Chemother. 54, 4416–4423. doi: 10.1128/AAC.00503-10
Collins, B., Joyce, S., Hill, C., Cotter, P. D., and Ross, R. P. (2010b). TelA contributes to the innate resistance of Listeria monocytogenes to nisin and other cell wall-acting antibiotics. Antimicrob. Agents Chemother. 54, 4658–4663. doi: 10.1128/AAC.00290-10
Considine, K. M., Sleator, R. D., Kelly, A. L., Fitzgerald, G. F., and Hill, C. (2011). A role for proline synthesis and transport in Listeria monocytogenes barotolerance. J. Appl. Microbiol. 110, 1187–1194. doi: 10.1111/j.1365-2672.2011.04982.x
Cordero, N., Maza, F., Navea-Perez, H., Aravena, A., Marquez-Fontt, B., Navarrete, P., et al. (2016). Different transcriptional responses from slow and fast growth rate strains of Listeria monocytogenes adapted to low temperature. Front. Microbiol. 7:229. doi: 10.3389/fmicb.2016.00229
Cotter, P. D., Gahan, C. G. M., and Hill, C. (2000). Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. Int. J. Food Microbiol. 60, 137–146. doi: 10.1016/S0168-1605(00)00305-6
Cotter, P. D., Gahan, C. G. M., and Hill, C. (2001). A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40, 465–475. doi: 10.1046/j.1365-2958.2001.02398.x
Cotter, P. D., Guinane, C. M., and Hill, C. (2002). The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 46, 2784–2790. doi: 10.1128/AAC.46.9.2784-2790.2002
Cotter, P. D., and Hill, C. (2003). Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67, 429–453. doi: 10.1128/MMBR.67.3.429
Cotter, P. D., Ross, R. P., and Hill, C. (2013). Bacteriocins - a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. doi: 10.1038/nrmicro2937
Cotter, P. D., Ryan, S., Gahan, C. G. M., and Hill, C. (2005). Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl. Environ. Microbiol. 71, 2832–2839. doi: 10.1128/AEM.71.6.2832
Crandall, A. D., and Montville, T. J. (1998). Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64, 231–237.
Dalet, K., Cenatiempo, Y., Cossart, P., and Héchard, Y. (2001). A σ54-dependent PTS permease of the mannose family is responsible for sensitivity Listeria monocytogenes to mesentericin Y105. Microbiology 147, 3263–3269. doi: 10.1099/00221287-147-12-3263
Davis, M. J., Coote, P. J., and O’Byrne, C. P. (1996). Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology 142, 2975–2982. doi: 10.1099/13500872-142-10-2975
Dmitrieva, N. I., Cai, Q., and Burg, M. B. (2004). Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc. Natl. Acad. Sci. U.S.A. 101, 2317–2322. doi: 10.1073/pnas.0308463100
Doyle, M. E., Mazzotta, A. S., Wang, T., Wiseman, D. W., and Scott, V. N. (2001). Heat resistance of Listeria monocytogenes. J. Food Prot. 64, 410–429. doi: 10.4315/0362-028X-64.3.410
Doyle, M. P., Glass, K. A., Beery, J. T., Garcia, G. A., Pollard, D. J., and Schultz, R. D. (1987). Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl. Environ. Microbiol. 53, 1433–1438.
Dröge, W. (2003). ““Oxidative stress and aging”,” in Hypoxia. Advances in Experimental Medicine and Biology, Vol. 543, eds R. C. Roach, P. D. Wagner, and P. H. Hackett (Boston, MA: Springer), 191–200. doi: 10.1007/978-1-4419-8997-0_14
Duché, O., Trémoulet, F., Glaser, P., and Labadie, J. (2002a). Salt stress proteins induced in Listeria monocytogenes. Appl. Environ. Microbiol. 68, 1491–1498. doi: 10.1128/AEM.68.4.1491
Duché, O., Trémoulet, F., Namane, A., and Labadie, J. (2002b). A proteomic analysis of the salt stress response of Listeria monocytogenes. FEMS Microbiol. Lett. 215, 183–188.
Dussurget, O., Dumas, E., Archambaud, C., Chafsey, I., Chambon, C., Hebraud, M., et al. (2005). Listeria monocytogenes ferritin protects against multiple stresses and is required for virulence. FEMS Microbiol. Lett. 250, 253–261. doi: 10.1016/j.femsle.2005.07.015
Eppert, I., Valdés-Stauber, N., Götz, H., Busse, M., and Scherer, S. (1997). Growth reduction of Listeria spp. caused by undefined industrial red smear cheese cultures and bacteriocin-producing Brevibacterium lines as evaluated in situ on soft cheese. Appl. Environ. Microbiol. 63, 4812–4817.
Farber, J. M., Hughes, A., Holley, R., and Brown, B. (1989). Thermal resistance of Listeria monocytogenes in sausage meat. Acta Microbiol. Hung. 36, 273–275.
Farber, J. M., Sanders, G. W., Speirs, J. I., D’Aoust, J.-Y., Emmons, D. B., and McKellar, R. (1988). Thermal resistance of Listeria monocytogenes in inoculated and naturally contaminated raw milk. Int. J. Food Microbiol. 7, 277–286. doi: 10.1016/0168-1605(88)90054-2
Feehily, C., Finnerty, A., Casey, P. G., Hill, C., Gahan, C. G. M., O’Byrne, C. P., et al. (2014). Divergent evolution of the activity and regulation of the glutamate decarboxylase systems in Listeria monocytogenes EGD-e and 10403S: roles in virulence and acid tolerance. PLoS One 9:e112649. doi: 10.1371/journal.pone.0112649
Ferreira, A., O’Byrne, C. P., and Boor, K. J. (2001). Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67, 4454–4457. doi: 10.1128/AEM.67.10.4454-4457.2001
Ferreira, V., Wiedmann, M., Teixeira, P., and Stasiewicz, M. J. (2014). Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J. Food Prot. 77, 150–170. doi: 10.4315/0362-028X.JFP-13-150
Fleischman, G. J., Ravishankar, S., and Balasubramaniam, V. M. (2004). The inactivation of Listeria monocytogenes by pulsed electric field (PEF) treatment in a static chamber. Food Microbiol. 21, 91–95. doi: 10.1016/s0740-0020(03)00015-7
Fleming, D. W., Cochi, S. L., MacDonald, K. L., Brondum, J., Hayes, P. S., Plikaytis, B. D., et al. (1985). Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312, 404–407. doi: 10.1056/NEJM198502143120704
Francis, G. A., Scollard, J., Meally, A., Bolton, D. J., Gahan, C. G. M., Cotter, P. D., et al. (2007). The glutamate decarboxylase acid resistance mechanism affects survival of Listeria monocytogenes LO28 in modified atmosphere-packaged foods. J. Appl. Microbiol. 103, 2316–2324. doi: 10.1111/j.1365-2672.2007.03466.x
Fraser, K. R., Harvie, D., Coote, P. J., and O’Byrne, C. P. (2000). Identification and characterization of an ATP binding cassette L-Carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66, 4696–4704. doi: 10.1128/AEM.66.11.4696-4704.2000
Fraser, K. R., Sue, D., Wiedmann, M., Boor, K., and O’Byrne, C. P. (2003). Role of σB in regulating the compatible solute uptake systems of Listeria monocytogenes: osmotic induction of opuC is σB dependent. Appl. Environ. Microbiol. 69, 2015–2022. doi: 10.1128/AEM.69.4.2015-2022.2003
Gahan, C. G., and Hill, C. (2014). Listeria monocytogenes: survival and adaptation in the gastrointestinal tract. Front. Cell. Infect. Microbiol. 4:9. doi: 10.3389/fcimb.2014.00009
Gandhi, M., and Chikindas, M. L. (2007). Listeria: a foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 113, 1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008
Gayán, E., Serrano, M. J., Pagán, R., Álvarez, I., and Condón, S. (2015). Environmental and biological factors influencing the UV-C resistance of Listeria monocytogenes. Food Microbiol. 46, 246–253. doi: 10.1016/j.fm.2014.08.011
Gaze, J. E., Brown, G. D., Gaskell, D. E., and Banks, J. G. (1989). Heat resistance of Listeria monocytogenes in homogenates of chicken, beef steak and carrot. Food Microbiol. 6, 251–259. doi: 10.1016/S0740-0020(89)80006-1
Gharsallaoui, A., Oulahal, N., Joly, C., and Degraeve, P. (2016). Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 56, 1262–1274. doi: 10.1080/10408398.2013.763765
Gómez, N., García, D., Álvarez, I., Condón, S., and Raso, J. (2005). Modelling inactivation of Listeria monocytogenes by pulsed electric fields in media of different pH. Int. J. Food Microbiol. 103, 199–206. doi: 10.1016/j.ijfoodmicro.2004.11.033
Gómez-López, V. M., Ragaert, P., Debevere, J., and Devlieghere, F. (2007). Pulsed light for food decontamination: a review. Trends Food Sci. Technol. 18, 464–473. doi: 10.1016/j.tifs.2007.03.010
Góngora-Nieto, M. M., Sepúlveda, D. R., Pedrow, P., Barbosa-Cánovas, G. V., and Swanson, B. G. (2002). Food processing by pulsed electric fields: treatment delivery, inactivation level, and regulatory aspects. Food Sci. Technol. 35, 375–388. doi: 10.1006/fstl.2001.0880
Gravesen, A., Kallipolitis, B., Holmstrøm, K., Høiby, P. E., Ramnath, M., and Knøchel, S. (2004). pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70, 1669–1679. doi: 10.1128/AEM.70.3.1669
Gravesen, A., Ramnath, M., Rechinger, K. B., Andersen, N., Jänsch, L., Héchard, Y., et al. (2002). High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148, 2361–2369. doi: 10.1099/00221287-148-8-2361
Gravesen, A., Warthoe, P., Knøchel, S., and Thirstrup, K. (2000). Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative β-glucoside-specific PTS system. Microbiology 146, 1381–1389. doi: 10.1099/00221287-146-6-1381
Grubaugh, D., Regeimbal, J. M., Ghosh, P., Zhou, Y., Lauer, P., Dubensky, T. W., et al. (2018). The VirAB ABC transporter is required for VirR regulation of Listeria monocytogenes virulence and resistance to nisin. Infect. Immun. 86, e901–e917. doi: 10.1128/IAI.00901-17
Hanawa, T., Kai, M., Kamiya, S., and Yamamoto, T. (2000). Cloning, sequencing, and transcriptional analysis of the dnak heat shock operon of Listeria monocytogenes. Cell Stress Chaperones 5, 21–29. doi: 10.1379/1466-1268(2000)005<0021:CSATAO>2.0.CO;2
Hardy, J., Parmentier, M., and Fanni, J. (1999). Functionality of nutrients and thermal treatments of food. Proc. Nutr. Soc. 58, 579–585. doi: 10.1017/s0029665199000762
Harter, E., Wagner, E. M., Zaiser, A., Halecker, S., Wagner, M., and Rychli, K. (2017). Stress survival Islet 2, predominantly present in Listeria monocytogenes strains of sequence type 121, is involved in the alkaline and oxidative stress responses. Appl. Environ. Microbiol. 83:e00827-17. doi: 10.1128/AEM.00827-17
Hartl, F. U., and Hayer-Hartl, M. (2002). Molecular chaperone in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858. doi: 10.1126/science.1068408
Hayman, M. M., Anantheswaran, R. C., and Knabel, S. J. (2007). The effects of growth temperature and growth phase on the inactivation of Listeria monocytogenes in whole milk subject to high pressure processing. Int. J. Food Microbiol. 115, 220–226. doi: 10.1016/j.ijfoodmicro.2006.10.019
Hébraud, M., and Guzzo, J. (2000). The main cold shock protein of Listeria monocytogenes belongs to the family of ferritin-like proteins. FEMS Microbiol. Lett. 190, 29–34. doi: 10.1016/S0378-1097(00)00310-4
Hendrick, J. P., and Hartl, F. U. (1993). Molecular chaperone functions of heat shock proteins. Annu. Rev. Biochem. 62, 349–384. doi: 10.1146/annurev.bi.62.070193.002025
Hereu, A., Dalgaard, P., Garriga, M., Aymerich, T., and Bover-Cid, S. (2012). Modeling the high pressure inactivation kinetics of Listeria monocytogenes on RTE cooked meat products. Innov. Food Sci. Emerg. Technol. 16, 305–315. doi: 10.1016/j.ifset.2012.07.005
Hill, D., Sugrue, I., Arendt, E., Hill, C., and Stanton, C. (2017). Recent advances in microbial fermentation for dairy and health [version 1; referees:3 approved]. F1000Res. 6:751. doi: 10.12688/f1000research.10896.1
Horn, G., Hofweber, R., Kremer, W., and Kalbitzer, H. R. (2007). Structure and function of bacterial cold shock proteins. Cell. Mol. Life Sci. 64, 1457–1470. doi: 10.1007/s00018-007-6388-4
Huang, H. W., Lung, H. M., Yang, B. B., and Wang, C. Y. (2014). Responses of microorganisms to high hydrostatic pressure processing. Food Control 40, 250–259. doi: 10.1016/j.foodcont.2013.12.007
Huang, L. (2004). Thermal resistance of Listeria monocytogenes, Salmonella heidelberg, and Escherichia coli O157:H7 at elevated temperatures. J. Food Prot. 67, 1666–1670. doi: 10.4315/0362-028x-67.8.1666
Huang, Y., Suo, Y., Shi, C., Szlavik, J., Shi, X. M., and Knøchel, S. (2013). Mutations in gltB and gltC reduce oxidative stress tolerance and biofilm formation in Listeria monocytogenes 4b G. Int. J. Food Microbiol. 163, 223–230. doi: 10.1016/j.ijfoodmicro.2013.02.023
Johnson, E. M., Jung, D. Y., Jin, D. Y., Jayabalan, D. R., Yang, D. S. H., and Suh, J. V. (2017). Bacteriocins as food preservatives: challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 7, 1–25. doi: 10.1080/10408398.2017.1340870
Jørgensen, F., Hansen, T. B., and Knøchel, S. (1999). Heat shock-induced thermotolerance in Listeria monocytogenes 13-249 is dependent on growth phase, pH and lactic acid. Food Microbiol. 16, 185–194. doi: 10.1006/fmic.1998.0222
Jørgensen, F., Stephens, P. J., and Knøchel, S. (1995). The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J. Appl. Bacteriol. 79, 274–281. doi: 10.1111/j.1365-2672.1995.tb03137.x
Kang, J., Wiedmann, M., Boor, K. J., and Bergholz, T. M. (2015). VirR-mediated resistance of Listeria monocytogenes against food antimicrobials and cross-protection induced by exposure to organic acid salts. Appl. Environ. Microbiol. 81, 4553–4562. doi: 10.1128/AEM.00648-15
Karatzas, K. A. G., and Bennik, M. H. J. (2002). Characterization of a Listeria monocytogenes Scott A isolate with high tolerance towards high hydrostatic pressure. Appl. Environ. Microbiol. 68, 3183–3189. doi: 10.1128/AEM.68.7.3183
Karatzas, K. A. G., Suur, L., and O’Byrne, C. P. (2012). Characterization of the intracellular glutamate decarboxylase system: analysis of its function, transcription, and role in the acid resistance of various strains of Listeria monocytogenes. Appl. Environ. Microbiol. 78, 3571–3579. doi: 10.1128/AEM.00227-12
Karatzas, K. A. G., Valdramidis, V. P., and Wells-Bennik, M. H. J. (2005). Contingency locus in ctsR of Listeria monocytogenes Scott A: a strategy for occurrence of abundant piezotolerant isolates within clonal populations. Appl. Environ. Microbiol. 71, 8390–8396. doi: 10.1128/AEM.71.12.8390-8396.2005
Karatzas, K. A. G., Wouters, J. A., Gahan, C. G. M., Hill, C., Abee, T., and Bennik, M. H. J. (2003). The CtsR regulator of Listeria monocytogenes contains a variant glycine repeat region that affects piezotolerance, stress resistance, motility and virulence. Mol. Microbiol. 49, 1227–1238. doi: 10.1046/j.1365-2958.2003.03636.x
Kazmierczak, M. J., Mithoe, S. C., Boor, K. J., and Wiedmann, M. (2003). Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185, 5722–5734. doi: 10.1128/JB.185.19.5722
Keklik, N. M., Demirci, A., and Puri, V. M. (2009). Inactivation of Listeria monocytogenes on unpackaged and vacuum-packaged chicken frankfurters using pulsed UV-light. J. Food Sci. 74, 431–439. doi: 10.1111/j.1750-3841.2009.01319.x
Krüger, E., Witt, E., Ohlmeier, S., Hanschke, R., and Hecker, M. (2000). The Clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins. J. Bacteriol. 182, 3259–3265. doi: 10.1128/jb.182.11.3259-3265.2000
Krüger, E., Zühlke, D., Witt, E., Ludwig, H., and Hecker, M. (2001). Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 20, 852–863. doi: 10.1093/emboj/20.4.852
Lado, B. H., Bomser, J. A., Dunne, C. P., and Yousef, A. E. (2004). Pulsed electric field alters molecular chaperone expression and sensitizes Listeria monocytogenes to heat. Appl. Environ. Microbiol. 70, 2289–2295. doi: 10.1128/AEM.70.4.2289
Lado, B. H., and Yousef, A. E. (2002). Alternative food-preservation technologies: efficacy and mechanisms. Microbes Infect. 4, 433–440. doi: 10.1016/S1286-4579(02)01557-5
Lado, B. H., and Yousef, A. E. (2003). Selection and identification of a Listeria monocytogenes target strain for pulsed electric field process optimization. Appl. Environ. Microbiol. 69, 2223–2229. doi: 10.1128/AEM.69.4.2223
Lasagabaster, A., and Martínez de Marañón, I. (2017). Comparative study on the inactivation and photoreactivation response of Listeria monocytogenes seafood isolates and a Listeria innocua surrogate after pulsed light treatment. Food Bioproc. Tech. 10, 1931–1935. doi: 10.1007/s11947-017-1972-6
Lee, J. H., Jeong, K. W., and Kim, Y. M. (2012). Purification and structural characterization of cold shock protein from Listeria monocytogenes. Bull. Korean Chem. Soc. 33, 2508–2512. doi: 10.5012/bkcs.2012.33.8.2508
Leong, D., Alvarez-Ordóñez, A., and Jordan, K. (2014). Monitoring occurrence and persistence of Listeria monocytogenes in foods and food processing environments in the Republic of Ireland. Front. Microbiol. 5:436. doi: 10.3389/fmicb.2014.00436
Leong, D., Alvarez-Ordóñez, A., Zaouali, S., and Jordan, K. (2015). Examination of Listeria monocytogenes in seafood processing facilities and smoked salmon in the Republic of Ireland. J. Food Prot. 78, 2184–2190. doi: 10.4315/0362-028X.JFP-15-233
Leroy, F., and de Vuyst, L. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 15, 67–78. doi: 10.1016/j.tifs.2003.09.004
Lippert, K., and Galinski, E. A. (1992). Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37, 61–65. doi: 10.1007/BF00174204
Lund, P., Tramonti, A., and De Biase, D. (2014). Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS 38, 1091–1125. doi: 10.1111/1574-6976.12076
Lungu, B., Ricke, S. C., and Johnson, M. G. (2009). Growth, survival, proliferation and pathogenesis of Listeria monocytogenes under low oxygen or anaerobic conditions: a review. Anaerobe 15, 7–17. doi: 10.1016/j.anaerobe.2008.08.001
Mackey, B. M., Boogard, E., Hayes, C. M., and Baranyi, J. (1994). Recovery of heat-injured Listeria monocytogenes. Int. J. Food Microbiol. 22, 227–237. doi: 10.1016/0168-1605(94)90174-0
Macwana, S., and Muriana, P. M. (2012). Spontaneous bacteriocin resistance in Listeria monocytogenes as a susceptibility screen for identifying different mechanisms of resistance and modes of action by bacteriocins of lactic acid bacteria. J. Microbiol. Methods 88, 7–13. doi: 10.1016/j.mimet.2011.09.009
Mandin, P., Fsihi, H., Dussurget, O., Vergassola, M., Milohanic, E., Toledo-Arana, A., et al. (2005). VirR, a response regulator critical for Listeria monocytogenes virulence. Mol. Microbiol. 57, 1367–1380. doi: 10.1111/j.1365-2958.2005.04776.x
Mazzotta, A. S. (2001a). Heat resistance of Listeria monocytogenes in vegetables: evaluation of blanching processes. J. Food Prot. 64, 385–387. doi: 10.4315/0362-028x-64.3.385
Mazzotta, A. S. (2001b). Thermal inactivation of stationary-phase and acid-adapted Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in fruit juices. J. Food Prot. 64, 315–320. doi: 10.4315/0362-028X-64.3.315
Mclaughlin, J., and Rees, C. E. D. (2009). “Genus I. Listeria,” in Bergey’s Manual of Systematic Bacteriology, 2nd Edn, eds P. De Vos, G. M. Garrity, D. Jones, N. R. Krieg, W. Ludwig, F. A. Rainey, et al. (New York, NY: Springer), 244–257.
Melo, J., Andrew, P. W., and Faleiro, M. L. (2015). Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: the role of stress responses. Food Res. Int. 67, 75–90. doi: 10.1016/j.foodres.2014.10.031
Miladi, H., Elabed, H., Ben Slama, R., Rhim, A., and Bakhrouf, A. (2016). Molecular analysis of the role of osmolyte transporters opuCA and betL in Listeria monocytogenes after cold and freezing stress. Arch. Microbiol. 199, 259–265. doi: 10.1007/s00203-016-1300-y
Ming, X., and Daeschel, M. A. (1993). Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Prot. 56, 944–948. doi: 10.4315/0362-028X-56.11.944
Ming, X., and Daeschel, M. A. (1995). Correlation of cellular phospholipid content with nisin resistance of Listeria monocytogenes Scott A. J. Food Prot. 58, 416–420. doi: 10.4315/0362-028X-58.4.416
Monfort, S., Sagarzazu, N., Gayán, E., Raso, J., and Álvarez, I. (2012). Heat resistance of Listeria species to liquid whole egg ultrapasteurization treatment. J. Food Eng. 111, 478–481. doi: 10.1016/j.jfoodeng.2012.02.014
Moorhead, S. M., and Dykes, G. A. (2003). The role of the sigB gene in the general stress response of Listeria monocytogenes varies between a strain of serotype 1/2a and a strain of serotype 4c. Curr. Microbiol. 46, 461–466. doi: 10.1007/s00284-002-3867-6
Moreirinha, C., Almeida, A., Saraiva, J. A., and Delgadillo, I. (2016). High-pressure processing effects on foodborne bacteria by mid-infrared spectroscopy analysis. LWT Food Sci. Technol. 73, 212–218. doi: 10.106/j.lwt.2016.05.041
Morris, C., Brody, A. L., and Wicker, L. (2007). Non-thermal food processing/ preservation technologies: a review with packaging implications. Packag. Technol. Sci. 20, 275–286. doi: 10.1002/pts.789
Mosqueda-Melar, J., Raybaudi-Massilia, R. M., and Martín-Belloso, O. (2007). Influence of treatment time and pulse frequency on Salmonella enteritidis, Escherichia coli and Listeria monocytogenes populations inoculated in melon and watermelon juices treated by pulsed electric fields. Int. J. Food Microbiol. 117, 192–200. doi: 10.1016/j.ijfoodmicro.2007.04.009
Murphy, R. Y., Duncan, L. K., Driscoll, K. H., Marcy, J. A., and Beard, B. L. (2003). Thermal inactivation of Listeria monocytogenes on ready-to-eat Turkey breast meat products during postcook in-package pasteurization with hot water. J. Food Prot. 66, 1618–1622. doi: 10.4315/0362-028X-66.9.1618
Nair, S., Derré, I., Msadek, T., Gaillot, O., and Berche, P. (2000). CtsR controls class III heat shock gene expression in the human pathogen Listeria monocytogenes. Mol. Microbiol. 35, 800–811. doi: 10.1046/j.1365-2958.2000.01752.x
Neunlist, M. R., Federighi, M., Laroche, M., Sohier, D., Delattre, G., Jacquet, C., et al. (2005). Cellular lipid fatty acid pattern heterogeneity between reference and recent food isolates of Listeria monocytogenes as a response to cold stress. Antonie van Leeuwenhoek J. Microbiol. 88, 199–206. doi: 10.1007/s10482-005-5412-7
NicAogáin, K., and O’Byrne, C. P. (2016). The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front. Microbiol. 7:1856. doi: 10.3389/fmicb.2016.01865
Niven, G. W., Miles, C. A., and Mackey, B. M. (1999). The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology 145, 419–425. doi: 10.1099/13500872-145-2-419
Oliver, H. F., Orsi, R. H., Wiedmann, M., and Boor, K. J. (2010). Listeria monocytogenes σB has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl. Environ. Microbiol. 76, 4216–4232. doi: 10.1128/AEM.00031-10
Olsen, K. N., Larsen, M. H., Gahan, C. G. M., Kallipolitis, B., Wolf, X. A., Rea, R., et al. (2005). The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology 151(Pt 3), 925–933. doi: 10.1099/mic.0.27552-0
Ovchinnikov, K. V., Chi, H., Mehmeti, I., Holo, H., Nes, I. F., and Diep, D. B. (2016). Novel group of leaderless multipeptide bacteriocins from Gram-positive bacteria. Appl. Environ. Microbiol. 82, 5216–5224. doi: 10.1128/AEM.01094-16
Ozer, N. P., and Demirci, A. (2006). Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV-light treatment. Int. J. Food Sci. Technol. 41, 354–360. doi: 10.1111/j.1365-2621.2005.01071.x
Perez, R. H., Zendo, T., and Sonomoto, K. (2014). Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb. Cell Fact. 13(Suppl. 1):S3. doi: 10.1186/1475-2859-13-S1-S3
Perozo, E., and Rees, D. C. (2003). Structure and mechanism in prokaryotic mechanosensitive channels. Curr. Opin. Struct. Biol. 13, 432–442. doi: 10.1016/s0959-440x(03)00106-4
Phadtare, S., Alsina, J., and Inouye, M. (1999). Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 2, 175–180. doi: 10.1016/S1369-5274(99)80031-9
Poimenidou, S. V., Chatzithoma, D., Nychas, G., and Skandamis, N. (2016). Adaptive response of Listeria monocytogenes to heat, salinity and low pH, after habituation on cherry tomatoes and lettuce leaves. PLoS One 11:e0165746. doi: 10.1371/journal.pone.0165746
Pöntinen, A., Lindström, M., Skurnik, M., and Korkeala, H. (2017). Screening of the two-component-system histidine kinases of Listeria monocytogenes EGD-e. LiaS is needed for growth under heat, acid, alkali, osmotic, ethanol and oxidative stresses. Food Microbiol. 65, 36–43. doi: 10.1016/j.fm.2017.01.018
Pöntinen, A., Markkula, A., Lindström, M., and Korkeala, H. (2015). Two-component-system histidine kinases involved in growth of Listeria monocytogenes EGD-e at low temperatures. Appl. Environ. Microbiol. 81, 3994–4004. doi: 10.1128/AEM.00626-15
Préstamo, G., Sanz, P. D., Fonberg-Broczek, M., and Arroyo, G. (1999). High pressure response of fruit jams contaminated with Listeria monocytogenes. Lett. Appl. Microbiol. 28, 313–316. doi: 10.1046/j.1365-2672.1999.00531.x
Püttman, M., Ade, N., and Hof, H. (1993). Dependence of fatty acid composition of Listeria spp on growth temperature. Res. Microbiol. 144, 279–283. doi: 10.1016/0923-2508(93)90012-Q
Ramnath, M., Beukes, M., Tamura, K., and Hastings, J. (2000). Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66, 3098–3101. doi: 10.1128/AEM.66.7.3098-3101.2000
Rascon Escajeda, L. F., Cruz Hernandez, M., Rodriguez Jasso, R. M., Charles Rodriguez, A. V., Robledo Olivo, A., Contreras Esquivel, J. C., et al. (2018). Discussion between alternative processing and preservation technologies and their application in beverages: a review. J. Food Process. Preserv. 42, 1–15. doi: 10.1111/jfpp.13322
Rastogi, R. P., Richa, K. A., Tyagi, M. B., and Sinha, R. P. (2010). Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010:592980. doi: 10.4061/2010/592980
Rea, R., Hill, C., and Gahan, C. G. M. (2005). Listeria monocytogenes perR mutants display a small-colony phenotype, increased sensitivity to hydrogen peroxide, and significantly reduced murine virulence. Appl. Environ. Microbiol. 71, 8314–8322. doi: 10.1128/AEM.71.12.8314-8322.2005
Renier, S., Micheau, P., Talon, R., Hébraud, M., and Desvaux, M. (2012). Subcellular localization of extracytoplasmic proteins in monoderm bacteria: rational secretomics-based strategy for genomic and proteomic analyses. PLoS One 7:e42982. doi: 10.1371/journal.pone.0042982
Ríos, C. N. S., Chalón, M. C., Navarro, S. A., and Bellomio, A. (2018). Pediocin-like bacteriocins: new perspectives on mechanism of action and immunity. Curr. Genet. 64, 345–351. doi: 10.1007/s00294-017-0757-9
Rivas, A., Sansano, S., Pérez, M. C. P., Martinez, A., and Rodrigo, D. (2016). “Antimicrobial effect of Stevia rebaudiana bertoni against Listeria monocytogenes in a beverage processed by pulsed electric fields (PEFs): combined effectiveness,” in 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Vol. 53, eds T. Jarm and P. Kramar (Singapore: Springer), 43–46. doi: 10.1007/978-981-287-817-5_10
Roncarati, D., and Scarlato, V. (2017). Regulation of heat-shock genes in bacteria: from signal sensing to gene expression output. FEMS Microbiol. Rev. 41, 549–574. doi: 10.1093/femsre/fux015
Roncarati, D., and Scarlato, V. (2018). The interplay between two transcriptional repressors and chaperones orchestrates Helicobacter pylori heat-shock response. Int. J. Mol. Sci. 19:E1702. doi: 10.3390/ijms19061702
Ruiz, L., Aertsen, A., Nguyen-The, C., Gänzle, M. G., and Alvarez-Ordóñez, A. (2017). Editorial: industrial and host associated stress responses in food microbes. Implications for food technology and food safety. Front. Microbiol. 8:1522. doi: 10.3389/fmicb.2017.01522
Russell, N. J., Evans, R. I., ter Steeg, P. F., Hellemons, J., Verheul, A., and Abee, T. (1995). Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28, 255–261. doi: 10.1016/0168-1605(95)00061-5
Ryan, S., Begley, M., Gahan, C. G. M., and Hill, C. (2009). Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ. Microbiol. 11, 432–445. doi: 10.1111/j.1462-2920.2008.01782.x
Saldaña, G., Puértolas, E., López, N., García, D., Álvarez, I., and Raso, J. (2009). Comparing the PEF resistance and occurrence of sublethal injury on different strains of Escherichia coli, Salmonella typhimurium, Listeria monocytogenes and Staphylococcus aureus in media of pH 4 and 7. Innov. Food Sci. Emerg. Technol. 10, 160–165. doi: 10.1016/j.ifset.2008.11.003
Sallami, L., Marcotte, M., Naim, F., Quattara, B., Leblanc, C., and Saucier, L. (2006). Heat inactivation of Listeria monocytogenes and Salmonella enterica serovar Typhi in a typical bologna matrix during an industrial cooking-cooling cycle. J. Food Prot. 69, 3025–3030. doi: 10.4315/0362-028x-69.12.3025
Schlech, W. F. III, Lavigne, P. M., Bortolussi, R. A., Allen, A. C., Haldane, E. V., Wort, A. J., et al. (1983). Epidemic listeriosis-Evidence for transmission by food. N. Engl. J. Med. 308, 203–206. doi: 10.1056/NEJM198301273080407
Schmid, B., Klumpp, J., Raimann, E., Loessner, M. J., Stephan, R., and Tasara, T. (2009). Role of cold shock proteins in growth of Listeria monocytogenes under cold and osmotic stress conditions. Appl. Environ. Microbiol. 75, 1621–1627. doi: 10.1128/AEM.02154-08
Selma, M. V., Salmerón, M. C., Valero, M., and Fernández, P. S. (2006). Efficacy of pulsed electric fields for Listeria monocytogenes inactivation and control in horchata. J. Food Saf. 26, 137–149. doi: 10.1111/j.1745-4565.2006.00038.x
Sewell, D., Allen, S. C. H., and Phillips, C. A. (2015). Oxygen limitation induces acid tolerance and impacts simulated gastro-intestinal transit in Listeria monocytogenes J0161. Gut Pathog. 7, 1–5. doi: 10.1186/s13099-015-0058-0
Shebuski, J. R., Vilhelmsson, O., and Miller, K. J. (2000). Effects of low water activity on the thermal tolerance of Staphylococcus aureus. J. Food Prot. 63, 1277–1281. doi: 10.4315/0362-028X-63.9.1277
Shen, Q., Jangam, P. M., Soni, K. A., Nannapaneni, R., Schilling, W., and Silva, J. L. (2014). Low, medium, and high heat tolerant strains of Listeria monocytogenes and increased heat stress resistance after exposure to sublethal heat. J. Food Prot. 77, 1298–1307. doi: 10.4315/0362-028x.jfp-13-423
Silva, C. C. G., Silva, S. P. M., and Ribeiro, S. C. (2018). Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 9:594. doi: 10.3389/fmicb.2018.00594
Simpson, R. K., Whittington, R., Earnshaw, R. G., and Russell, N. J. (1999). Pulsed high electric field causes ‘all or nothing’ membrane damage in Listeria monocytogenes and Salmonella typhimurium, but membrane H+-ATPase is not a primary target. Int. J. Food Microbiol. 48, 1–10. doi: 10.1016/S0168-1605(99)00022-7
Sinha, R. P., and Häder, D. P. (2002). UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1, 225–236. doi: 10.1039/b201230h
Skandamis, P. N., Gounadaki, A. S., Geornaras, I., and Sofos, J. N. (2012). Adaptive acid tolerance response of Listeria monocytogenes strains under planktonic and immobilized growth conditions. Int. J. Food Microbiol. 159, 160–166. doi: 10.1016/j.ijfoodmicro.2012.07.027
Sleator, R. D., Gahan, C. G., Abee, T., and Hill, C. (1999). Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65, 2078–2083.
Sleator, R. D., Watson, D., Hill, C., and Gahan, C. G. M. (2009). The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology 155, 2463–2475. doi: 10.1099/mic.0.030205-0
Sleator, R. D., Gahan, C. G. M., and Hill, C. (2003a). A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 69, 1–9. doi: 10.1128/AEM.69.1.1-9.2003
Sleator, R. D., Wood, J. M., and Hill, C. (2003b). Transcriptional regulation and posttranslational activity of the betaine transporter BetL in Listeria monocytogenes are controlled by environmental salinity. J. Bacteriol. 185, 7140–7144. doi: 10.1128/JB.185.24.7140-7144.2003
Soares, C. A., and Knuckley, B. (2016). Mechanistic studies of the agmatine deiminase from Listeria monocytogenes. Biochem. J. 473, 1553–1561. doi: 10.1042/BCJ20160221
Somolinos, M., Espina, L., Pagán, R., and Garcia, D. (2010). sigB absence decreased Listeria monocytogenes EGD-e heat resistance but not its pulsed electric fields resistance. J. Food Microbiol. 141, 32–38. doi: 10.1016/j.ijfoodmicro.2010.04.023
Sörqvist, S. (1993). Heat resistance of Listeria monocytogenes by two recovery media used with and without cold preincubation. J. Appl. Bacteriol. 74, 428–432. doi: 10.1111/j.1365-2672.1993.tb05150.x
Sörqvist, S. (1994). Heat resistance of different serovars of Listeria monocytogenes. J. Appl. Bacteriol. 76, 383–388. doi: 10.1111/j.1365-2672.1994.tb01644.x
Suo, Y., Huang, Y., Liu, Y., Shi, C., and Shi, X. (2012). The expression of superoxide dismutase (SOD) and a putative ABC transporter permease is inversely correlated during biofilm formation in Listeria monocytogenes 4b G. PLoS One 7:e48467. doi: 10.1371/journal.pone.0048467
Suo, Y., Liu, Y., Zhou, X., Huang, Y., Shi, C., Matthews, K., et al. (2014). Impact of sod on the expression of stress-related genes in Listeria monocytogenes 4b G with/without paraquat treatment. J. Food Sci. 79, M1745–M1749. doi: 10.1111/1750-3841.12545
Tasara, T., and Stephan, R. (2006). Cold stress tolerance of Listeria monocytogenes: a review of molecular adaptive mechanisms and food safety implications. J. Food Prot. 69, 1473–1484. doi: 10.4315/0362-028X-69.6.1437
Thedieck, K., Hain, T., Mohamed, W., Tindall, B. J., Nimtz, M., Chakraborty, T., et al. (2006). The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 62, 1325–1339. doi: 10.1111/j.1365-2958.2006.05452.x
Thévenot, D., Dernburg, A., and Vernozy-Rozand, C. (2006). An updated review of Listeria monocytogenes in the pork meat industry and its products. J. Appl. Microbiol. 101, 7–17. doi: 10.1111/j.1365-2672.2006.02962.x
Toepfl, S., Heinz, V., and Knorr, D. (2007). High intensity pulsed electric fields applied for food preservation. Chem. Eng. Prog. 46, 537–546. doi: 10.1016/j.cep.2006.07.011
Tomasula, P. M., Renye, J. A., Van Hekken, D. L., Tunick, M. H., Kwoczak, R., Toht, M., et al. (2014). Effect of high-pressure processing on reduction of Listeria monocytogenes in packaged Queso Fresco. J. Dairy Sci. 97, 1281–1295. doi: 10.3168/jds.2013-7538
Tomoyasu, T., Mogk, A., Langen, H., Goloubinoff, P., and Bukau, B. (2001). Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 40, 397–413. doi: 10.1046/j.1365-2958.2001.02383.x
Tosukhowong, A., Zendo, T., Visessanguan, W., Roytrakul, S., Pumpuang, L., Jaresitthikunchai, J., et al. (2012). Garvieacin Q, a novel class II bacteriocin from Lactococcus garvieae BCC 43578. Appl. Environ. Microbiol. 78, 1619–1623. doi: 10.1128/AEM.06891-11
Tymoszewska, A., Diep, D. B., Wirtek, P., and Aleksandrzak-Piekarczyk, T. (2017). The non-lantibiotic bacteriocin garvicin Q targets Man-PTS in a broad spectrum of sensitive bacterial genera. Sci. Rep. 7:8359. doi: 10.1038/s41598-017-09102-7
Uesugi, A. R., Hsu, L. C., Worobo, R. W., and Moraru, C. I. (2016). Gene expression analysis for Listeria monocytogenes following exposure to pulsed light and continuous ultraviolet light treatments. Food Sci. Technol. 68, 579–588. doi: 10.1016/j.lwt.2016.01.007
Unal, R., Kim, J.-G., and Yousef, A. E. (2001). Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Lactobacillus leichmannii by combinations of ozone and pulsed electric field. J. Food Prot. 64, 777–782. doi: 10.4315/0362-028x-64.6.777
Vadyvaloo, V., Arous, S., Gravesen, A., Héchard, Y., Chauhan-Haubrock, R., Hastings, J. W., et al. (2004). Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150, 3025–3033. doi: 10.1099/mic.0.27059-0
Vadyvaloo, V., Hastings, J. W., van der Merwe, M. J., and Rautenbach, M. (2002). Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68, 5223–5230. doi: 10.1128/AEM.68.11.5223
Van Boeijen, I. K. H., Chavaroche, A. A. E., Valderrama, W. B., Moezelaar, R., Zwietering, M. H., and Abee, T. (2010). Population diversity of Listeria monocytogenes LO28: phenotypic and genotypic characterization of variants resistant to high hydrostatic pressure. Appl. Environ. Microbiol. 76, 2225–2233. doi: 10.1128/AEM.02434-09
Van Boeijen, I. K. H., Moezelaar, R., Abee, T., and Zwietering, M. H. (2008). Inactivation kinetics of three Listeria monocytogenes strains under high hydrostatic pressure. J. Food Prot. 71, 2007–2013. doi: 10.4315/0362-028X-71.10.2007
Van Boekel, M., Fogliano, V., Pellegrini, N., Stanton, C., Scholz, G., Lalljie, S., et al. (2010). A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 54, 1215–1247. doi: 10.1002/mnfr.200900608
Van der Veen, S., Hain, T., Wouters, J. A., Hossain, H., de Vos, W. M., Abee, T., et al. (2007). The heat-shock response of Listeria monocytogenes comprises genes involved in heat shock, cell division, cell wall synthesis, and the SOS response. Microbiology 153, 3593–3607. doi: 10.1099/mic.0.2007/006361-0
Van Schaik, W., and Abee, T. (2005). The role of σB in the stress response of Gram-positive bacteria-targets for food preservation and safety. Curr. Opin. Biotechnol. 16, 218–224. doi: 10.1016/j.copbio.2005.01.008
Verheul, A., Glaasker, E., Poolman, B., and Abee, T. (1997a). Betaine and L-carnitine transport by Listeria monocytogenes ScottA in response to osmotic signals. J. Bacteriol. 179, 6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997
Verheul, A., Russell, N. J., van’T Hof, R., Rombouts, F. M., and Abee, T. (1997b). Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl. Environ. Microbiol. 63, 3451–3457.
Vermassen, A., Dordet-Frisoni, E., de la Foye, A., Micheau, P., Laroute, V., Leroy, S., et al. (2016). Adaptation of Staphylococcus xylosus to nutrients and osmotic stress in a salted meat model. Front. Microbiol. 7:87. doi: 10.3389/fmicb.2016.00087
Wemekamp-Kamphuis, H. H., Sleator, R. D., Wouters, J. A., Hill, C., and Abee, T. (2004a). Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70, 2912–2918. doi: 10.1128/AEM.70.5.2912
Wemekamp-Kamphuis, H. H., Wouters, J. A., de Leeuw, P. P. L. A., Hain, T., Chakraborty, T., and Abee, T. (2004b). Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 70, 3457–3466. doi: 10.1128/AEM.70.6.3457-3466.2004
Wen, J., Anantheswaran, R. C., and Knabel, S. J. (2009). Changes in barotolerance, thermotolerance, and cellular morphology throughout the life cycle of Listeria monocytogenes. Appl. Environ. Microbiol. 75, 1581–1588. doi: 10.1128/AEM.01942-08
Wiedemann, I., Breukink, E., van Kraaij, C., Kuipers, O. P., Bierbaum, G., de Kruijff, B., et al. (2001). Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276, 1772–1779. doi: 10.1074/jbc.M006770200
Wouters, P. C., Alvarez, I., and Raso, J. (2001). Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Technol. 12, 112–121. doi: 10.1016/S0924-2244(01)00067-X
Wu, S., Yu, P.-L., Wheeler, D., and Flint, S. (2018). Transcriptomic study on persistence and survival of Listeria monocytogenes following lethal treatment with nisin. J. Glob. Antimicrob. Resist. 15, 25–31. doi: 10.1016/j.jgar.2018.06.003
Zhang, L.-J., and Gallo, R. L. (2016). Antimicrobial peptides. Curr. Biol. 26, R14–R19. doi: 10.1016/j.cub.2015.11.017
Keywords: acidity, temperature, oxidative stress, osmolarity, high pressure, UV light, pulsed electric fields, bacteriocins
Citation: Bucur FI, Grigore-Gurgu L, Crauwels P, Riedel CU and Nicolau AI (2018) Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front. Microbiol. 9:2700. doi: 10.3389/fmicb.2018.02700
Received: 31 July 2018; Accepted: 23 October 2018;
Published: 13 November 2018.
Edited by:
Conor P. O’Byrne, National University of Ireland Galway, IrelandReviewed by:
Stephan Schmitz-Esser, Iowa State University, United StatesFrancisco Diez-Gonzalez, University of Georgia, United States
Copyright © 2018 Bucur, Grigore-Gurgu, Crauwels, Riedel and Nicolau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anca Ioana Nicolau, anca.nicolau@ugal.ro
 Florentina Ionela Bucur
Florentina Ionela Bucur Leontina Grigore-Gurgu
Leontina Grigore-Gurgu Peter Crauwels
Peter Crauwels Christian U. Riedel
Christian U. Riedel Anca Ioana Nicolau
Anca Ioana Nicolau