- 1SAMRC Microbial Water Quality Monitoring Centre, University of Fort Hare, Alice, South Africa
- 2Applied and Environmental Microbiology Research Group, Department of Biochemistry and Microbiology, University of Fort Hare, Alice, South Africa
- 3Department of Microbiology, Obafemi Awolowo University, Ife, Nigeria
- 4Department of Microbiology, Adekunle Ajasin University, Akungba-Akoko, Nigeria
- 5Department of Environmental Health Sciences, University of Sharjah, Sharjah, United Arab Emirates
Water resources contaminated with pathogenic Vibrio species are usually a source of devastating infection outbreaks that have been a public health concern in both developed and developing countries over the decades. The present study assessed the prevalence of six medically significant Vibrio species in some water resources in Eastern Cape Province, South Africa for 12 months. We detected vibrios in all the 194 water samples analyzed using polymerase chain reaction (PCR). The prevalence of Vibrio cholerae, Vibrio mimicus, Vibrio fluvialis, Vibrio vulnificus, Vibrio alginolyticus, and Vibrio parahaemolyticus in freshwater samples was 34, 19, 9, 2, 3, and 2%, and that in brackish water samples was 44, 28, 10, 7, 46, and 51%, respectively. The population of the presumptive Vibrio spp. isolated from freshwater (628) and brackish water (342) samples that were confirmed by PCR was 79% (497/628) and 85% (291/342), respectively. Twenty-two percent of the PCR-confirmed Vibrio isolates from freshwater (n = 497) samples and 41% of the PCR-confirmed Vibrio isolates from brackish water samples (n = 291) fall among the Vibrio species of interest. The incidences of V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus amidst these Vibrio spp. of interest that were recovered from freshwater samples were 75, 14, 4, 6, 1, and 1%, whereas those from brackish water samples were 24, 7, 3, 3, 47, and 18%, respectively. Our observation during the study suggests pollution as the reason for the unusual isolation of medically important vibrios in winter. Correlation analysis revealed that temperature drives the frequency of isolation, whereas salinity drives the composition of the targeted Vibrio species at our sampling sites. The finding of the study is of public health importance going by the usefulness of the water resources investigated. Although controlling and preventing most of the factors that contribute to the prevalence of medically important bacteria, such as Vibrio species, at the sampling points might be difficult, regular monitoring for creating health risk awareness will go a long way to prevent possible Vibrio-related infection outbreaks at the sampling sites and their immediate environment.
Introduction
The Vibrio genus is made up of over one hundred species (Romalde et al., 2014; Fernández-delgado et al., 2015) of which about 12 have been associated with human infections. The three major human pathogens in the Vibrio genus are Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus (Guardiola-avila et al., 2015; Kokashvili et al., 2015; Jones, 2017). Pathogenic members of the Vibrio genus are very common in the aquatic environment and can cause water- and food-related infections (Ajin et al., 2016; Kirchberger et al., 2016; Machado and Bordalo, 2016; Shaheen et al., 2016). Infections caused by these human pathogenic Vibrio species include cholera and vibriosis, e.g., wound infections, septicemia, and gastroenteritis, which is often self-limiting (Baker-Austin et al., 2018). Human pathogenic Vibrio species include V. carchariae, V. mimicus, V. cincinnatiensis, V. fluvialis, V. cholerae, V. parahaemolyticus, V. vulnificus, V. alginolyticus (now Grimontia hollisae), V. furnissii, V. metschnikovii, V. hollisae, and V. damsela (Photobacterium hollisae; Finkelstein, 1996; Levinson and Jawetz, 1996; Turner, 1997; Morris, 2019). The most notable human Vibrio pathogens are V. cholerae, V. parahaemolyticus, V. vulnificus, and V. fluvialis (CDC, 1999; Finkelstein et al., 2002; Kothary et al., 2003; Chakraborty et al., 2006). On the other hand, V. mimicus, which diverged from a common ancestor as V. cholerae, and V. alginolyticus, which was formerly classified as V. parahaemolyticus, are emerging human pathogens (Mustapha et al., 2013; Guardiola-avila et al., 2015). V. cholerae causes cholera, whereas other human pathogenic members of the Vibrio genus cause infections generally refer to as vibriosis (Baker-Austin et al., 2018). The vehicles of transmission of the etiological agents of cholera and vibriosis to humans are water and food most especially seafood (Di et al., 2017). Water- and food-related diseases continue to be a huge problem for humanity (Tantillo et al., 2004). To circumvent the scourge of cholera and vibriosis, localized monitoring of the environment for etiological agents of the infections was recommended, and this should be done without prejudice to either Vibrio-related outbreak is ongoing or not (CDC, 2015). To better protect human health, monitoring the environment for agents of waterborne and foodborne infections, such as human pathogenic Vibrio spp., has been emphasized in the literature (European Commission, 2001; Baffone et al., 2006; Jones and Oliver, 2009). Members of the Vibrio genus are halophiles; thus, they are not expected to be found in freshwater resources, and this may explain why studies on the prevalence of human pathogenic Vibrio spp. in freshwater are relatively small. However, the ability of Vibrio spp. to adapt to varying ecological niches (Ceccarelli and Colwell, 2014; Payne et al., 2016; Osunla and Okoh, 2017) supported the need to investigate the role of freshwater resources in the spread of etiological agents of cholera and vibriosis.
Of the approximately 139 serogroups of V. cholerae, only O1 and O139 cause pandemic and epidemic cholera, but the non-O1/non-O139 serogroups also cause sporadic cholera-like infections (Glenn Morris, 1994; Rippey, 1994; Sharma et al., 1998; Deen et al., 2019). On the other hand, V. parahaemolyticus causes most of the seafood-related diarrhea infections in Florida (Klontz et al., 1993; Letchumanan et al., 2015), and it causes septicemia occasionally (Rippey, 1994; Letchumanan et al., 2015). V. vulnificus biotype 1 is the most deadly of all Vibrio species because of its high invasiveness and its fatality rate that is higher than that of any other bacteria (Oliver et al., 1991; Oliver and Bockian, 1995; Bisharat, 2002; Bisharat et al., 2005; Drake et al., 2007). V. fluvialis commonly cause food poisoning (Bhattacharjee et al., 2010; Kanungo et al., 2012; Chowdhury et al., 2013), and it is an emerging pathogen that possesses epidemic potentials (Vinothkumar et al., 2013). Cholera and vibriosis outbreaks had been reported from several parts of the globe. The isolation of these pathogens from various aquatic milieu around the globe (Sarkar et al., 1985; Venkateswaran et al., 1989; Barua and Greenough, 1991; Kazi et al., 2005; Binh et al., 2009; Safa et al., 2009; Grim et al., 2010; Reimer et al., 2011; Esteves et al., 2015; Okoh et al., 2015; Di et al., 2017; Abioye and Okoh, 2018) has been reported. The occurrence of cholera in the Eastern Cape Province (ECP) has been well documented over the years (Mudzanani et al., 2003; Mema, 2008). The previous studies carried out in our laboratory (Applied and Environmental Microbiology Research Group, Department of Biochemistry and Microbiology, University of Fort Hare, South Africa) confirmed the occurrence of medically important Vibrio species in some wastewater treatment plants (WWTPs) of the ECP and their receiving watershed, especially those that discharge poorly treated final effluent (Igbinosa et al., 2009, 2011a,b; Nongogo and Okoh, 2014; Okoh et al., 2015).
These previous findings support the need for spatial and temporal investigation of the presence and distribution of these bacterial species in freshwater resources in the province since there is interconnectivity between WWTPs receiving watershed and other freshwater resources most especially rivers. Going by the scarcity of freshwater resources in South Africa, this kind of investigation is needful since all available freshwater resources in the country are potential alternatives to regular sources of treated freshwater during freshwater scarcity. Some of the anthropogenic activities that contribute to the occurrence of pathogenic Vibrio spp. in the aquatic environment include indiscriminate dumping of refuses into water bodies, discharge of poorly treated wastewater from the treatment plant into receiving watershed, run-off from farms especially farmland treated with manure, and defecating and urinating into water bodies by humans and grazing animals (Nevondo and Cloete, 2001; USEPA, 2001; Rees et al., 2010; Abakpa et al., 2013). Contamination due to the aforementioned factors is evident around some of the freshwater resources in the ECP. This is not a surprise since most of the ECP waterbodies are not well protected (Christine et al., 2013). Diseases outbreak in Eastern Cape as a result of watershed contaminated with poorly treated effluent from WWTPs has occurred in the past (Cottle and Deedat, 2002). Unfortunately, recent studies showed that the problem persists in the ECP (Mema, 2008; Nongogo and Okoh, 2014; Okoh et al., 2015; Edokpayi et al., 2017). Poorly treated WWTP’s effluent has been identified as a significant contributor of pathogens to the water milieu of the ECP (Okeyo et al., 2018).
Vibrio spp. of medical importance including four of the six Vibrio spp. focused on in this study have been reported from various WWTPs of Eastern Cape and their receiving watershed. A significant amount of V. cholerae, V. parahaemolyticus, V. metschnikovii, V. fluvialis, and V. vulnificus were reportedly isolated from final effluents in some WWTPs in Nkonkobe rural community, Chris Hani, and Amathole district municipalities (Igbinosa et al., 2009, 2011a,b; Nongogo and Okoh, 2014). A more comprehensive study on final effluents of 14 WWTPs in Amathole and Chris Hani district municipalities reported 66.8% Vibrio spp. prevalence of the 1,000 randomly selected isolates recovered from the WWTPs. Of the 300 confirmed Vibrio spp., 68.2% belong to one of V. parahaemolyticus, V. fluvialis, and V. vulnificus (Okoh et al., 2015). The aforementioned is of potential health risk to individuals using watershed for recreational, agricultural, and domestic purposes most especially at the downstream of WWTP final effluents discharge points. In some studies from other provinces of South Africa, the isolation of Vibrio harveyi, V. parahaemolyticus, V. cholerae, V. mimicus, and V. vulnificus from tap, borehole, and dam in North West province (Maje et al., 2020) and V. cholerae from four WWTPs located in Gauteng Province (Dungeni et al., 2010) has also been reported. Health risks that an individual using these water resources could be exposed to include gastrointestinal infections caused by V. parahaemolyticus, V. mimicus, and V. fluvialis; wound infections caused by V. vulnificus and V. harveyi; cholera caused by V. cholerae; and cholera-like bloody diarrhea caused by V. fluvialis (Ramamurthy et al., 1994; Igbinosa and Okoh, 2010; Jones, 2017; Brehm et al., 2020).
The occurrences of dysfunctional WWTPs that discharge unacceptable final effluent (Herbig and Meissner, 2019) and the lack of adequate protection for surface water in the ECP suggest that pathogenic vibrios could have contaminated some water resources in the province. Although extensive work has been done on the contribution of WWTPs to the abundance of Vibrio spp. in the province, however, work on major rivers, their tributaries, and brackish water resources is still limited.
The findings of Momba et al. (2006), which confirmed the presence of toxigenic V. cholerae in the surface and groundwater of rural locality of Nkonkobe Local Municipality of Amathole District, Eastern Cape Province, South Africa, showed that surface water is of significant importance to the understanding of the abundance of Vibrio spp. in the province water milieu. However, the study by Momba et al. (2006) reported for toxigenic V. cholerae alone but not for non-cholera causing Vibrio pathogens of public health importance. In addition, the study was carried out more than a decade ago, and it does not cover all the important surface water resources in the province. The only one report found on the occurrence of V. vulnificus while developing this manuscript was from the KwaZulu-Natal beach, but the information was found in local news (Comins, 2012) rather than in research articles publishing outlets. The other few reports on occurrences of cholera and non-cholera causing Vibrio pathogens in rivers were carried out in KwaZulu-Natal, Mpumalanga, and Limpopo provinces, South Africa (Bessong et al., 2009; Madoroba and Momba, 2010; Ntema et al., 2014; Marie and Lin, 2018). Although studies on occurrences of cholera and non-cholera causing Vibrio pathogens have been carried out in the ECP in the past, they were limited to up, final effluents discharge points, and some meters downstream of the receiving watershed for the WWTPs (Igbinosa et al., 2009, 2011a,b; Nongogo and Okoh, 2014; Okoh et al., 2015). Therefore, our understanding of the abundance and distribution of Vibrio spp. beyond the targeted points along the receiving watershed of the WWTPs and rivers that do not serve as receiving watershed for WWTPs in the province is still unclear. Based on the aforementioned, information on the occurrence of the etiological agents of cholera and vibriosis in South Africa freshwater and brackish water resources at the moment is limited most especially in the ECP. Going by the advice of the CDC (2017) to monitor the environment for Vibrio pathogens as a means of preventing cholera and vibriosis outbreaks, this study assesses the microbial quality of some important freshwater and brackish water sources in the ECP based on the presence or absence of six medically important Vibrio pathogens. The six medically important Vibrio spp. targeted were V. cholerae, V. parahaemolyticus, V. vulnificus, V. fluvialis, V. mimicus, and V. alginolyticus. The last two have not been reported from this region before now. It is intended that the study will contribute to the information needed for the epidemiology of the etiological agents of cholera and vibriosis in the aquatic milieu of the province most especially rivers and brackish water resources. It was also anticipated that this study will encourage Vibrio pathogens monitoring programs in the brackish and freshwater milieu of the ECP most especially those that humans access regularly.
Materials and Methods
Study Areas and Sample Collection
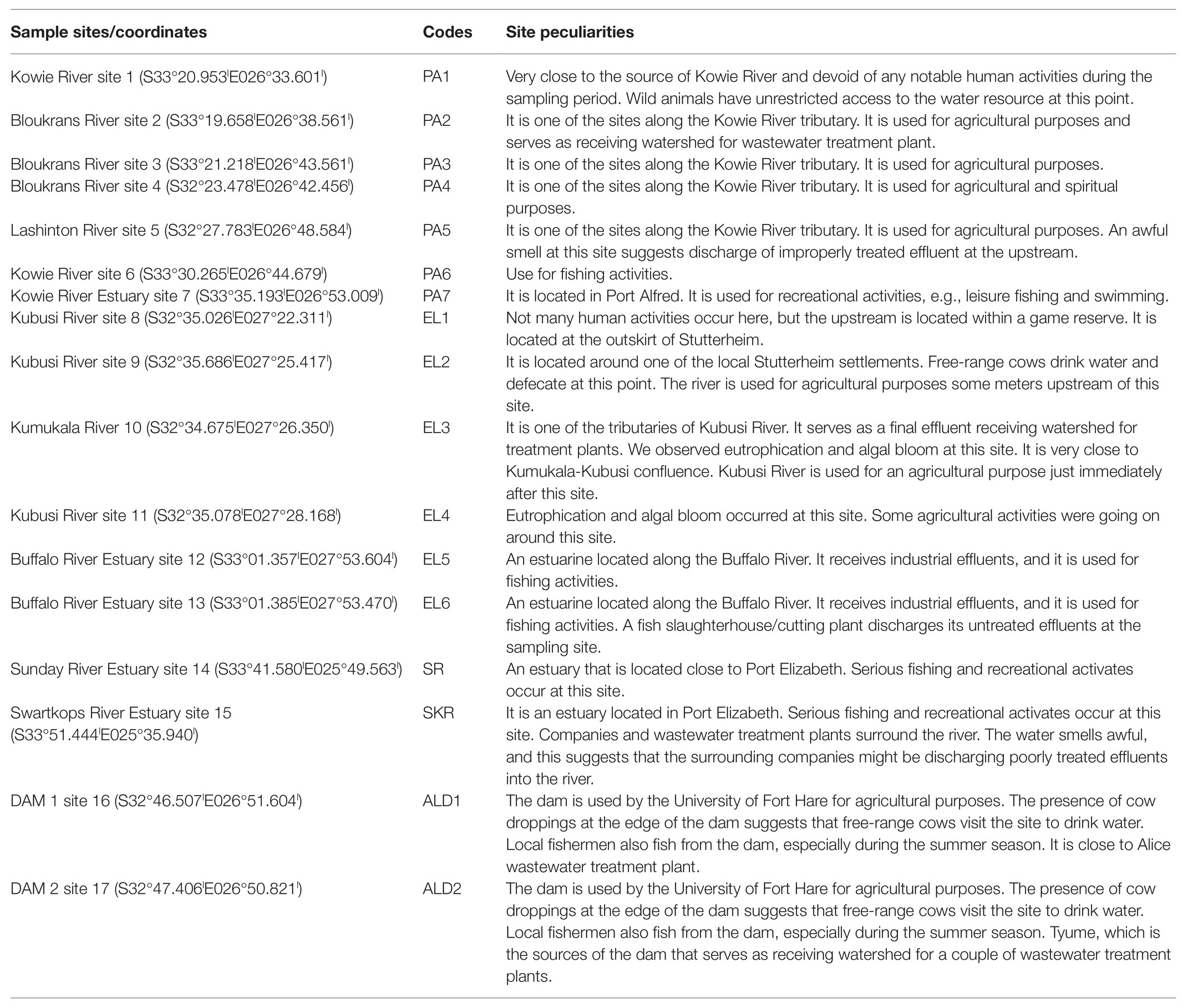
Water samples were collected from freshwater and brackish water resources located along Kowie and tributaries, Kubusi, Sunday, Swartkops, and Buffalo Rivers as shown in Figure 1. Two freshwater dams in Amathole District Municipality were also included in the study, and their coordinates are S32°46.507lE026°51.604l and S32°47.406lE026°50.821l. The dams are used for fishing by the local anglers. The water sampling method employed is as documented in the previous study carried out in our laboratory (Igbinosa et al., 2011b; Okoh et al., 2015). Briefly, water samples were collected at 3–5 different points at each sampling site into 1-L sterile sterilin bottles by midstream dipping of sample bottles at 25–30 cm down the water column. The sample bottle cap was replaced while the sample bottle was still beneath the water column, transferred into cooler boxes containing ice, and afterwards transferred to the Applied and Environmental Microbiology Research Group (AEMREG) laboratory, University of Fort Hare, Alice. All the samples collected were analyzed within 6 h of collection, and samples were collected once a month for 1 year. The characteristics of each of the sampling sites are given in Table 1.
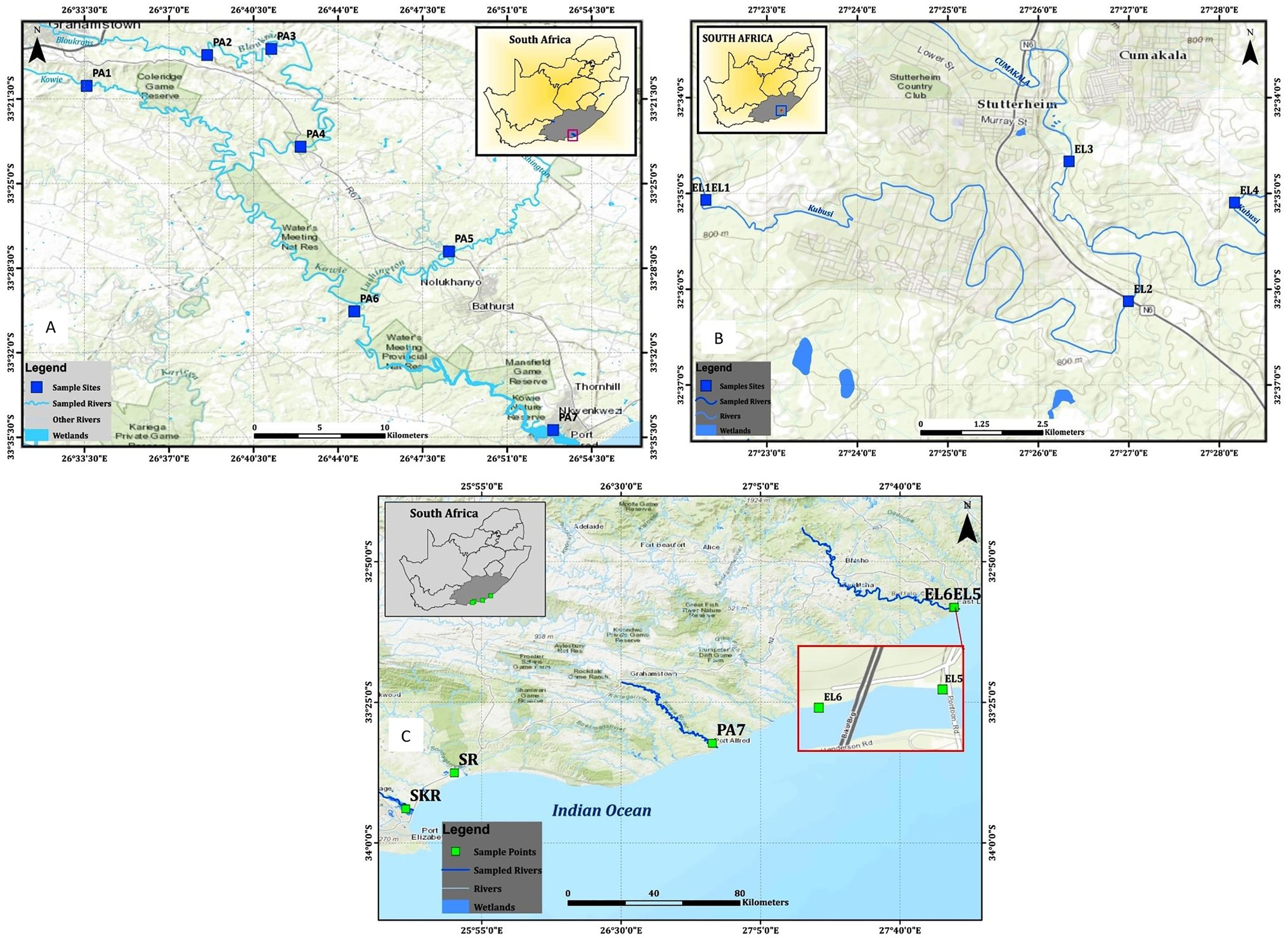
Figure 1. Maps showing locations of the sampling sites, (A) sampling sites along the Kowie River, (B) sampling sites along the Kubusi River, and (C) sampling sites at Buffalo, Sunday, and Swartkops Rivers.
Temperature, Salinity, and Presumptive Vibrio Density Determination
The salinity and temperature were determined using a multiparameter ion-specific meter (version HI98195; Hanna Instruments) following the manufacturer’s guidelines. The presumptive Vibrio density (PVD) was determined following the membrane filtration technique described in the literature (Nongogo and Okoh, 2014). A 100 ml of raw or diluted water samples, as the case may be, was filtered with the aid of a vacuum pump using a 0.45 μm membrane filter. Afterwards, the resulting membrane filters were placed on sterile thiosulfate citrate bile salts sucrose (TCBS) plates and incubated for 24–48 h at 37°C in triplicates. The mean of the yellow and green colonies on the TCBS triplicate plates per sample was recorded as PVD.
Total Vibrio spp. Density
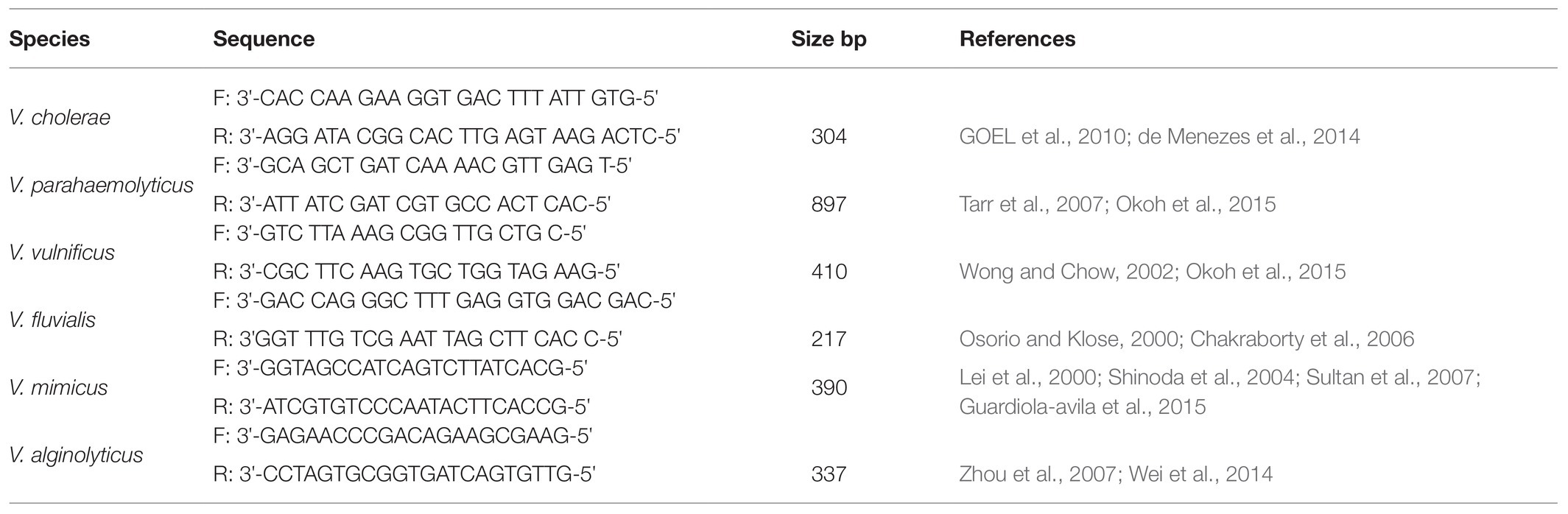
The most probable number coupled with polymerase chain reaction (MPN-PCR) method detailed in the literature was adapted for determining the total Vibrio spp. density (TVD) in water samples (Ahmad et al., 2011; Copin et al., 2012; Ramos et al., 2014; Abioye and Okoh, 2018). As a statistical-based method, MPN only estimates the viable numbers of bacteria in a sample by the principle of extinction dilution, i.e., inoculating liquid medium in 10-fold dilutions to a dilution factor at which no turbidity is observed after the incubation period. Unfortunately, the turbidity observed could be due to the presence of other bacteria other than the organism of interest. To overcome this shortcoming and make the methods more accurate and precise, MPN turbid tubes were subjected to PCR to ascertain the presence of the organism of interest. In this approach, MPN turbid tubes that tested negative for the organism of interest when subjected to PCR are counted as false positive and are not useful for calculating the density. Thus, this approach corrects for the shortcoming of using only MPN for determining the density of specific bacteria species. This is called the MPN-PCR method. Briefly, a 10-fold serial dilution of water sample was prepared up to the power of five, and afterwards, 1 ml of aliquots from raw and each of the diluted water sample was aseptically introduced into test tubes containing 10 ml of sterile freshly prepared alkaline peptone water (APW) in triplicates and incubated at 37°C for 24 h. Turbid tubes were separated, and total genomic DNA was extracted from each turbid tube by the boiling method (Maugeri et al., 2006). For the total genomic DNA extraction, 1 ml of aliquot from the turbid tubes was aseptically transferred into a sterile microcentrifuge tube and centrifuged for 2 min at a speed of 11,000 × g using a MiniSpin microcentrifuge. After centrifugation, the supernatant was discarded while 200 μl of sterile distilled water was added to the cell pellet at the bottom of the microcentrifuge tubes and vortexed to form a solution containing evenly distributed cells. The cells in the solution were afterwards lysed with the aid of an AccuBlock (Digital dry bath; Labnet) for 15 min at 100°C, and the solution of the lysed cells was centrifuged at a speed earlier mentioned. The supernatant that was the genomic extraction was subjected to 25 μl PCR reaction to confirm the presence of Vibrio spp. using a primer that targets a variable region of 16S rRNA gene that is specific for members of the Vibrio genus. Furthermore, to determine the absolute density of each of the targeted Vibrio spp., DNA templates from MPN tubes that were positive for Vibrio spp. were subjected to another round of PCR that targeted each of the six Vibrio spp. using species-specific primers used for delineation (Table 2). The composition of the PCR reaction was 5 μl of DNA template, 12.5 μl of one Taq 2X Master Mix Standard Buffer (BioLabs, UK), 1 μl each of 10 μM of forward and reverse primers, and 5.5 μl of nuclease-free water. The concentration of the DNA in templates ranges between 80 and 195 ng/μl through the experiment. The positive controls used for the PCR assay were V. parahaemolyticus (DSM 10027), V. vulnificus (DSM 10143), V. fluvialis (DSM 19283), V. mimicus (DSM 19130), and V. alginolyticus (DSM 2171) and one locally isolated V. cholera. The TVD was determined by extrapolating the equivalent MPN values for turbid tubes that were positive for members of the Vibrio genus and each of the targeted Vibrio spp. using bacteriological analytical manual (BAM) Excel spreadsheet (Blodgett, 2015). The detection limit for the MPN-PCR method is <3 MPN/ml (<0.477 log MPN/ml). The forward sequence for the Vibrio genus-specific primer used was 3'CGG TGA AAT GCG TAG AGA T5', whereas that for the reverse sequence was 3'TTA CTA GCG ATT CCG AGT TC5'. The thermal cycler condition for the PCR assay was 15 min at 93°C for initial denaturation, 35 cycles of 92°C for 40 s, 57°C for 1 min, and 72°C for 1.5 min for denaturation, annealing, and elongation, respectively, and 75°C for 7 min for the final extension. The resulting amplicons were electrophoresed on 1.5% agarose, and pictures of amplified DNA bands on agarose gel were viewed and taken using an ultraviolet (UV) transilluminator following the manufacturer’s guideline. The expected amplicon size was 663 bp (Kwok et al., 2002; Okoh et al., 2015).
Isolation of Presumptive Vibrio spp. From Water Samples
To maximize the isolation of the Vibrio species of interest, a water sample (100 ml) was filtered as articulated in the Temperature, Salinity, and Presumptive Vibrio Density Determination section, and the resulting membrane filter was introduced into a conical flask containing sterile 100 ml APW. The conical flask with its content was perturbed gently for about 1–2 min before incubating the set-up for 24 h at 37°C (Ntema et al., 2010). The set-up was observed on hourly bases, and as soon as there was the formation of the pellicle at the surface of the incubated APW, a loopful just below the pellicle was carefully streaked on sterile TCBS (Ceccarelli et al., 2015). The newly streaked TCBS plates were incubated for 24 h at 37°C. At the expiration of the incubation period, colonies (5–10 per plate) with typical Vibrio yellowish and greenish morphology were carefully picked, re-streaked on fresh sterile TCBS, and incubated for 24 h at 37°C. After the incubation period, a colony from TCBS plates with uniform colonies’ morphology were transferred to fresh 1% NaCl nutrient agar plates. Resulting colonies were Gram stained and observed under a microscope to ensure the purity of the isolated presumptive Vibrio species to be stocked. Twenty percent glycerol stock of pure isolates were prepared afterwards and stored at −80°C for PCR analysis.
Molecular Identification of Presumptive Vibrio Isolates
A total genomic DNA was extracted from the presumptive Vibrio isolates using the boiling method as described earlier in the Total Vibrio spp. Density section. The DNA was extracted from a colony of an 18-h-old pure culture of presumptive Vibrio spp. isolates. A solution of the colony prepared in a microcentrifuge containing 200 μl of sterile distilled water was boiled, and total genomic DNA was afterwards extracted from the cell solutions as earlier described in the Total Vibrio spp. Density section. This was followed by the confirmation of all the presumptive isolates as member of the Vibrio genus or otherwise following the PCR protocol detailed in the Total Vibrio spp. Density section. All PCR-confirmed Vibrio isolates were further delineated into the six Vibrio spp. targeted in this study.
Delineation of the PCR-Confirmed Vibrio Species
A set of species-specific primers were employed in PCR assay for delineating confirmed Vibrio spp. into the Vibrio species of interest. The respective primers employed for the identification of V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus targeted the conserved region on OmpW, vhm, ToxR, GroEl, GyrB, and fla E genes that are specific for the organisms of our interest. V. cholera and V. mimicus were simultaneously identified using a duplex PCR protocol, whereas V. vulnificus, V. fluvialis, and V. alginolyticus were also simultaneously identified by employing a triplex PCR protocol. V. parahaemolyticus isolates were identified using a simplex PCR protocol. The primer sequences, sources, and expected amplicon sizes are given in Table 2, and all the PCR protocols were as earlier articulated in our previous study (Abioye and Okoh, 2018). The resulting amplicons were electrophoresed, bands on gels were viewed, and their pictures were taken using a UV transilluminator following the manufacturer’s guideline.
Statistical Analysis
Results for freshwater and brackish water samples were distinctively treated on seasonal and annual bases. Density across sites was statistically compared using the Kruskal-Wallis test and the Games-Howell post hoc test at p ≤ 0.05. Spearman correlation analysis was carried out to understand the relationship between PVD, TVD, temperature, and salinity at our sampling sites since salinity and temperature have been reported to modulate the ecology of Vibrio spp. differently at different geographical locations. The frequency of isolation (relative density) and absolute density of each of the six targeted Vibrio spp. were also correlated with temperature and salinity to establish species-based relationship. Kruskal-Wallis test and Spearman correlation analysis were used because our variables (temperature, density, salinity, and frequency of isolation) were not normally distributed.
Excel version 2013 and SPSS version 2020 were used to organize and analyze our data.
Result and Discussion
Temperature, Salinity, and Vibrio spp. Density
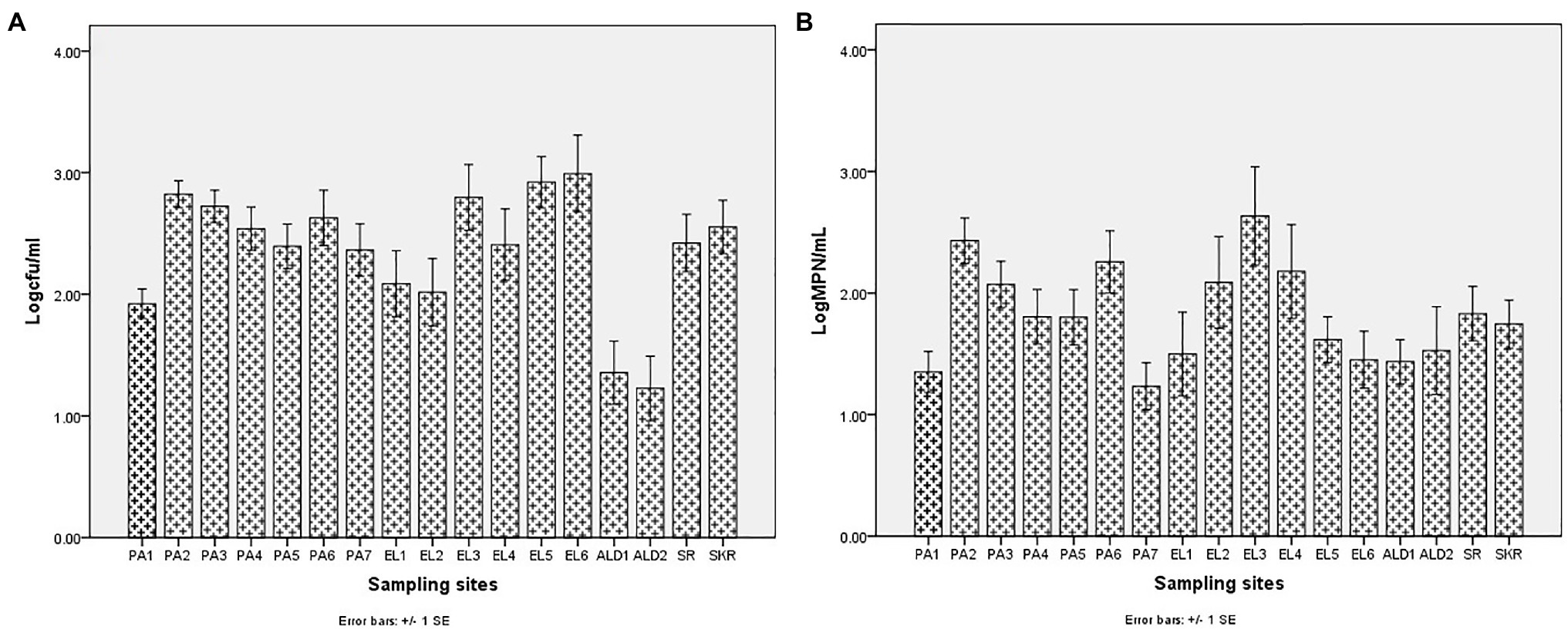
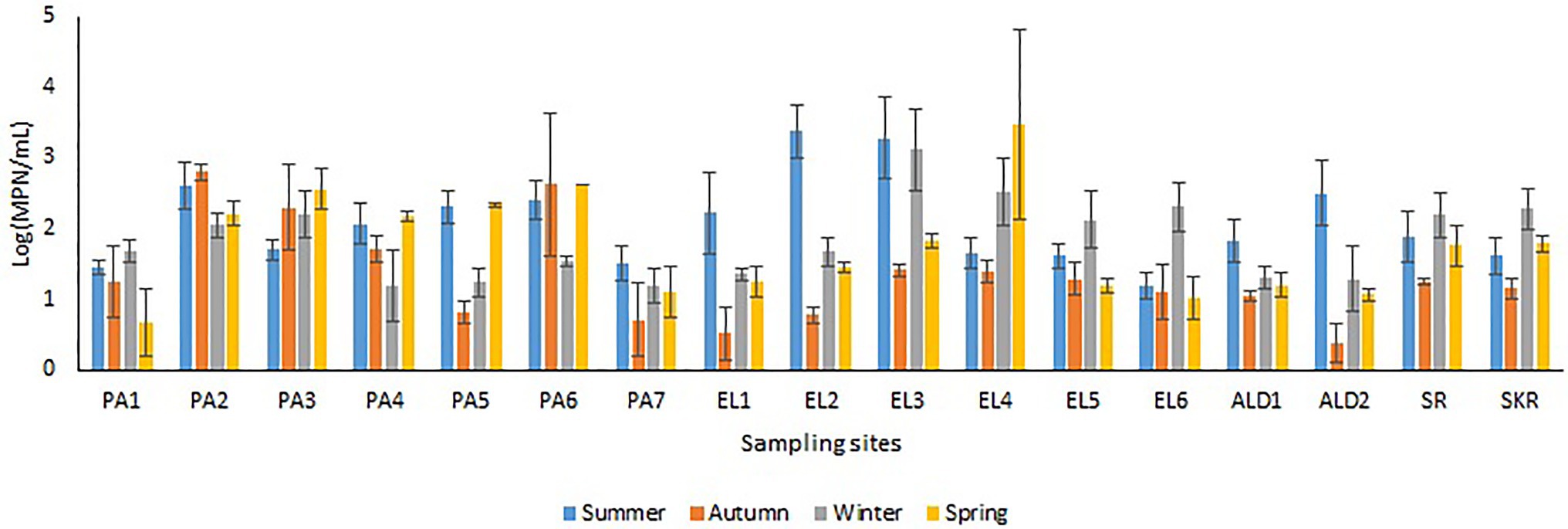
The average annual temperature and salinity of water samples at the sampling points were as given in Table 3. Salinity ranges between 0.06 ± 0.04 PSU at site EL1 and 2.03 ± 0.47 PSU at site PA5 for freshwater sampling sites, whereas it ranges between 16.14 ± 3.91 PSU at site SR and 31.65 ± 4.19 PSU at site PA7 for brackish water sampling sites. The temperature range was between 15.31 ± 4.25°C at site EL1 and 21.50 ± 3.67°C at site PA6 for freshwater sampling sites, and it was between 18.74 ± 2.12°C at site EL6 and 22.01 ± 4.47°C at site SR for brackish water sampling sites. During the study regime, 194 water samples were collected and analyzed for PVD and TVD. The mean annual PVD and TVD per site (Figure 2) differ across the sampling sites. The average annual PVD ranges between 1.23 ± 0.83 log CFU/ml at site ALD2 and 2.92 ± 0.93 log CFU/ml at site EL4 for freshwater samples, whereas that for brackish water samples ranges between 2.36 ± 0.72 log CFU/ml at site PA7 and 2.99 ± 1.0 log CFU/ml at site EL6. On the other hand, the range of annual average TVD was 1.35 ± 0.56 log MPN/ml at site PA1 to 2.63 ± 1.28 log MPN/ml at site EL3 for freshwater samples. The range for brackish water samples was 1.23 ± 0.64 log MPN/ml at site PA7 to 1.83 ± 0.71 log MPN/ml at site SR. The PVD at all sampling sites was more than that at site PA1 except for sites ALD1 and ALD2. This observation was significant at p ≤ 0.05 for all sampling sites except for sites PA7, EL1, EL2, EL4, ALD1, and SR. A similar scenario was observed for TVD except that TVD at site PA1 was greater than TVD at site PA7. The observation for TVD was only significant for PA2 > PA1. The densities of each of the targeted species are as given in Supplementary Table SS1B. A density greater than 0.477 log MPN/ml, which was the maximum detection limit of the three tubes by the five dilutions MPN-PCR density determination method employed, was recorded mainly in summer but scarcely recorded in winter months. Of the samples with >0.477 log MPN/ml V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus densities, 82, 72, 63, 100, 87, and 58%, respectively, were collected in summer months.
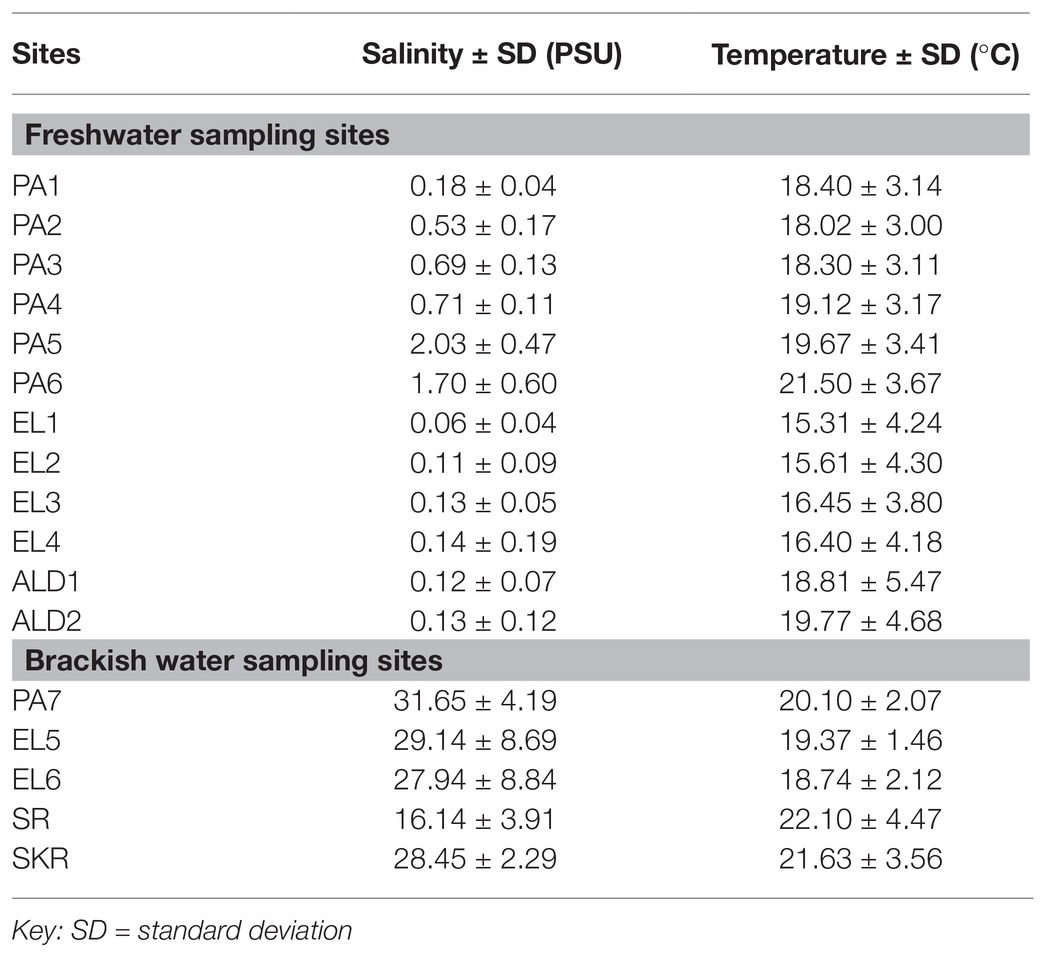
Table 3. Average annual salinity and temperature at the freshwater and brackish water sampling sites.
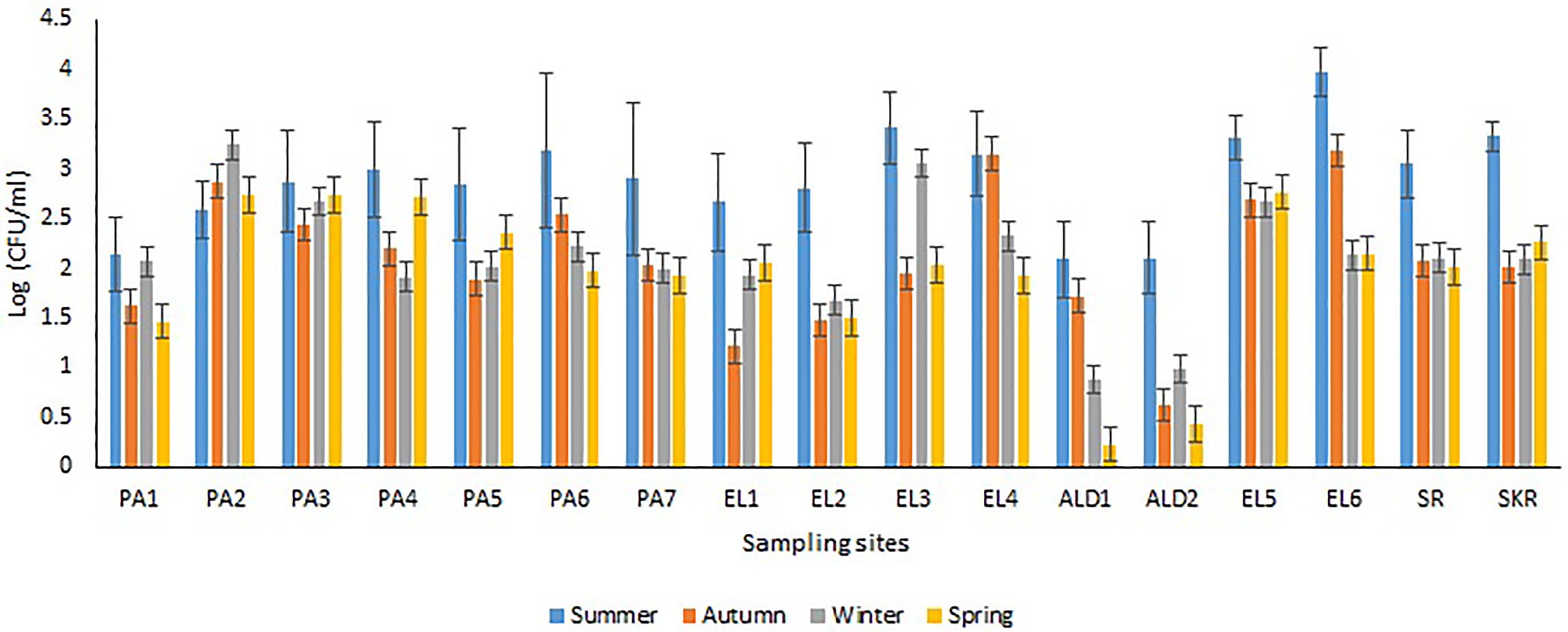
Sites PA1, EL1, ALD1, and ALD2 were anticipated to be pristine and have relatively low microbial load because PA1 and EL1 were around the sources of Kowie and Kubusi Rivers, respectively, whereas sites ALD1 and ALD2 were in a controlled environment. However, in some instance, PVD and TVD at these supposedly said pristine sampling sites were greater than PVD and TVD at other sampling sites, e.g., the mean annual Vibrio density at PA1 was greater than that at PA7, which are more exposed to anthropogenic activities. The unanticipated observation could be as a result of the easy access and activities of wild and domesticated animals around the water resources at PA1. South Africa water resources have been adjudged unprotected (Christine et al., 2013; Colvin et al., 2016) while domesticated and wild animals activities around surface water generally cause contamination of water resources with pathogens (Cabral, 2010; McAllister and Topp, 2012; Decol et al., 2017; Topalcengiz et al., 2017; Liu et al., 2018). Furthermore, domestic wastes from informal settlements along the riverbanks, effluents from overloaded and poorly maintained treatment plants, polluted runoff, and solid wastes that are potential contributors of pathogens to surface water (Fatoki et al., 2001; Chigor et al., 2012; Edokpayi et al., 2017; Adams et al., 2019) were evident at our sampling sites. In addition, droppings from free-range and grazing cows were observed at most of our freshwater sampling sites. Animals droppings are major contributors of pathogenic Vibrio spp., e.g., toxigenic V. cholerae into human environments (Dufour et al., 2013; Momba and Azab El-Liethy, 2017; Penakalapati et al., 2017; Delahoy et al., 2018; Munshi et al., 2019). As anticipated, sites SR, SKR, PA7, EL5, and EL6 located at estuaries where Vibrio spp. are commonly isolated and site EL6 that also serves as receiving watershed for fish slaughterhouse/cutting plant had relatively high Vibrio density than some of the freshwater sampling sites. Reports have shown that effluents from fish slaughterhouses contribute significantly to Vibrio spp. load in receiving watershed (Novotny et al., 2004; Skall and Olesen, 2011). Surprisingly, the densities of Vibrio at some of the freshwater sampling sites were also significantly more than those at some of the brackish water sampling sites (Figures 2A,B). This observation could be as a result of the type of industrial effluent and other pollutants that are usually discharged into the water environments around the brackish water sampling sites (Adeniji et al., 2017a,b). Industrial waste has been reported to modulate the microbial biodiversity of the aquatic ecosystem (Li et al., 2017; Jordaan et al., 2019), and this could be on a downward trend for microbial populations when such waste is toxic rather than nutrient-rich. Vibrio spp. density (PVD and TVD) also varies across seasons with relatively high density recorded in summer as earlier reported (Igbinosa et al., 2011a,b; Kokashvili et al., 2015); however, few exceptions were observed (Figures 3, 4). A relatively high PVD and TVD in winter recorded at sites PA2 and EL3 can be traced to pollution. The two sites serve as receiving watershed for dysfunctional WWTPs (Maclennan, 2019), and dysfunctional WWTPs usually contaminate the water system with pathogens (Edokpayi et al., 2017). The highest density for each of the targeted Vibrio spp. (Supplementary Table SS1B) was recorded at sites (PA2, PA5, EL3, SR, and SKR) where we observed a high level of pollution.
Statistically speaking, a significantly positive weak correlation (r = 0.201, p = 0.018) between PVD and salinity was observed for freshwater but a significantly negative weak correlation (r = −0.327, p = 0.014) for brackish water samples. The correlation between TVD and salinity was not significant for freshwater (r = 0.058, p = 0.503) and brackish water (r = −0.163, p = 0.203) samples. The correlation coefficients between PVD vs. temperature (r = 0.133, p = 0.121 for freshwater; r = 0.2, p = 0.14 for brackish water) and TVD vs. temperature (r = 0.018, p = 0.252 for freshwater; r = −0.068, p = 0.619 for brackish water) were not significant too. The lack of correlation between salinity, temperature, and Vibrio density had been reported in the literature (Randa et al., 2004; De Souza Costa Sobrinho et al., 2010; Paranjpye et al., 2015; Nilsson et al., 2019). The finding suggests that the contribution of salinity and temperature to the Vibrio genus density at our sampling sites is small, and that some other physicochemical and biological factors could be key to Vibrio density dynamics at our sampling sites. Interestingly, the literature has shown that parameters, such as dissolved oxygen and chemical and biological oxygen demands, have a strong relationship with Vibrio spp. density and the aforementioned parameters are good indexes for detecting water resources polluted with organic waste that may contain pathogens (Sharma and Chaturvedi, 2007; Prasanthan et al., 2011; Mezgebe et al., 2015; Di et al., 2017; Rabea et al., 2019). Unfortunately, organic waste pollution can occur at any time and cause unexpected offshoot in microbial density and diversity in an ecological niche. This, thus, explain the observation of the highest Vibrio density in seasons other than summer at some of our sampling sites because we observed that sources of organic waste pollutants are close to these sampling sites.
Effects of Salinity and Temperature on the Density, Frequency of Isolation, and Seasonality of the Targeted Vibrio Species
The frequency of isolation of each of the Vibrio species of interest in freshwater and brackish water samples per sampling month and the impact of temperature and salinity on the frequency of detection are given Figure 5. The seasonal variation in the frequency of isolation of the Vibrio spp. of interest is given in Figure 6. The results show that the organism of interest was frequently isolated in summer than other seasons. Surprisingly, while the isolation frequency of organisms of interest in freshwater samples followed a similar pattern as salinity and temperature, only temperature did for brackish water samples. It is an established fact that salinity and temperature are important environmental factors that influence Vibrio spp. density, but the literature has also shown that the correlation between these factors and Vibrio spp. density differs for different geographical locations and sample types (Kelly, 1982; Kaspar and Tamplin, 1993; Fukushima and Seki, 2004; Randa et al., 2004; Igbinosa et al., 2011a; Takemura et al., 2014; Kokashvili et al., 2015; Colwell, 2016; Urquhart et al., 2016). For example, Kokashvili et al. (2015) observed a linear correlation between the two factors and frequency of isolation of some medically important Vibrio species but a negative correlation between temperature and V. metschnikovii at the marine and freshwater sampling sites. In addition, Randa et al. (2004) observed a significant moderate negative correlation between salinity and abundance of V. vulnificus in estuarine water samples, whereas Colwell (2016) reported a non-significant negative correlation between temperature and the abundances of V. parahaemolyticus and V. vulnificus in estuary water samples. In this study, the frequency of isolation of at least one of the targeted species from freshwater samples showed a significant positive relationship with salinity and temperature (Table 4). However, only temperature had a significant positive relationship with the frequency of isolation of at least one of the targeted Vibrio spp. in brackish water samples. Interestingly, correlation coefficients and p values for the relationship between frequency of isolation of at least one of the Vibrio spp. and temperature were very similar for the two water types (Table 4). This shows that temperature modulates the chances of isolating at least one of the Vibrio spp. of interest from the two water types in a similar way. However, at species level, the correlation analysis (Table 4) showed that temperature is more effective as guide for the isolation of V. cholerae, V. mimicus, and V. fluvialis (generally called non-halophilic) from freshwater and V. alginolyticus, V. vulnificus, and V. parahaemolyticus (generally called halophilic) from brackish water. In their work, Liu et al. (2016) and Mahoney et al. (2010) reported that temperature is more relevant to the isolation of non-halophilic vibrios from freshwater and halophilic vibrios from brackish water than salinity. Consequently, we adjudged from our data (Figure 5) that a temperature range between 20 and 24°C is the optimum temperature for the isolation of V. cholerae, V. mimicus, and V. fluvialis from freshwater sampling sites and V. alginolyticus, V. vulnificus, and V. parahaemolyticus from brackish water sampling sites. It was further inferred that a salinity range between 5 and 9 PSU should be used in adjunct with a temperature range of 20 and 24°C for the isolation of V. cholerae at the freshwater sampling sites. Our submission is in concordance with earlier studies that showed that relatively high frequency of isolation of medically important Vibrio spp. is usually achieved in summer when the temperature is above 15°C and salinity is between 5 and 25 PSU (Jones and Summer-Brason, 1998; DePaola et al., 2003; Urquhart et al., 2016).
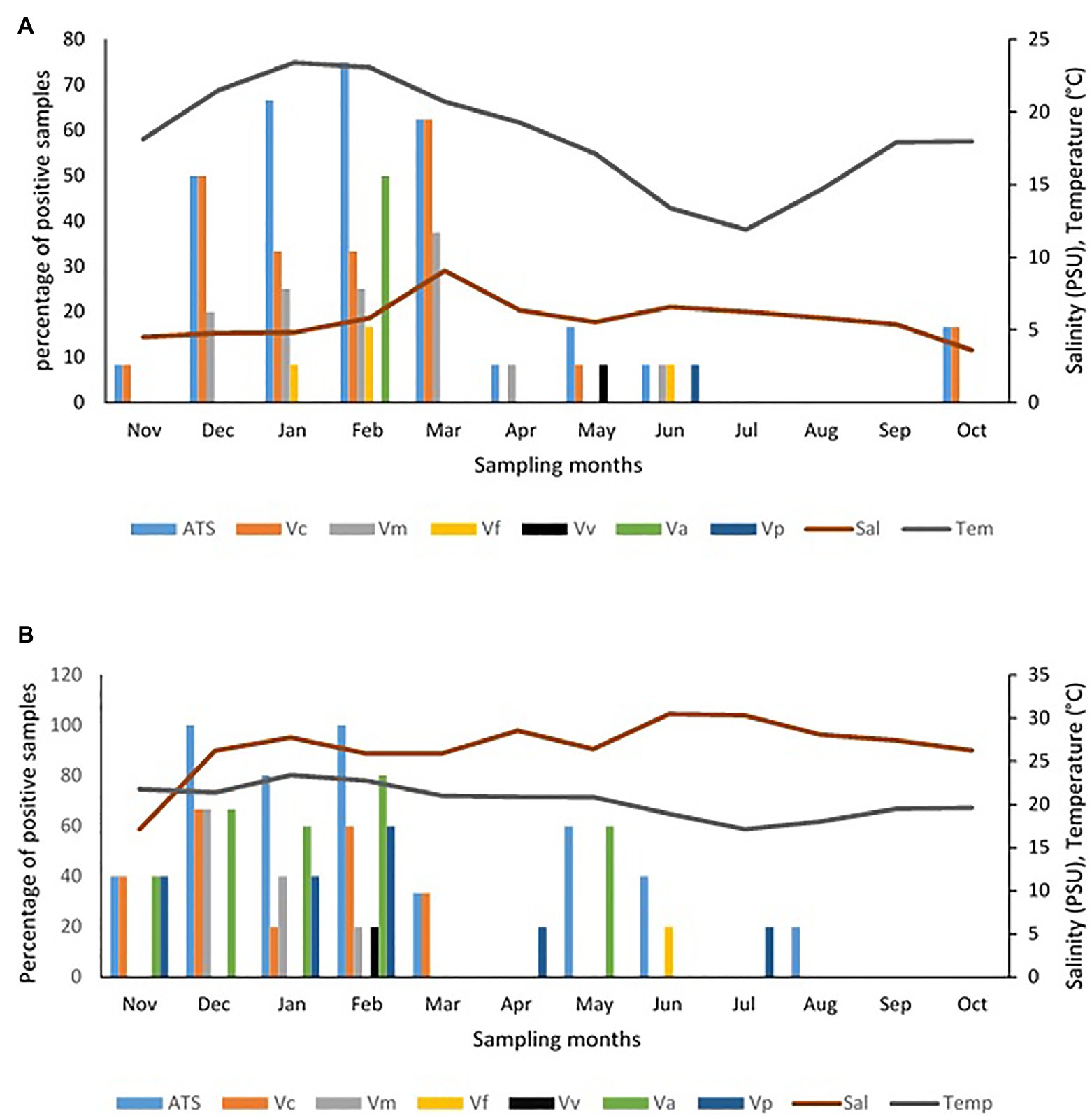
Figure 5. Influence of temperature and salinity on the detection of targeted Vibrio spp. in water samples. (A) Freshwater samples, (B) brackish water samples. Key: Va = V. alginolyticus, Vc = V. cholerae, Vf = V. fluvialis, Vm = V. mimicus, Vp = V. parahaemolyticus, Vv = V. vulnificus, ATS = all targeted Vibrio species.
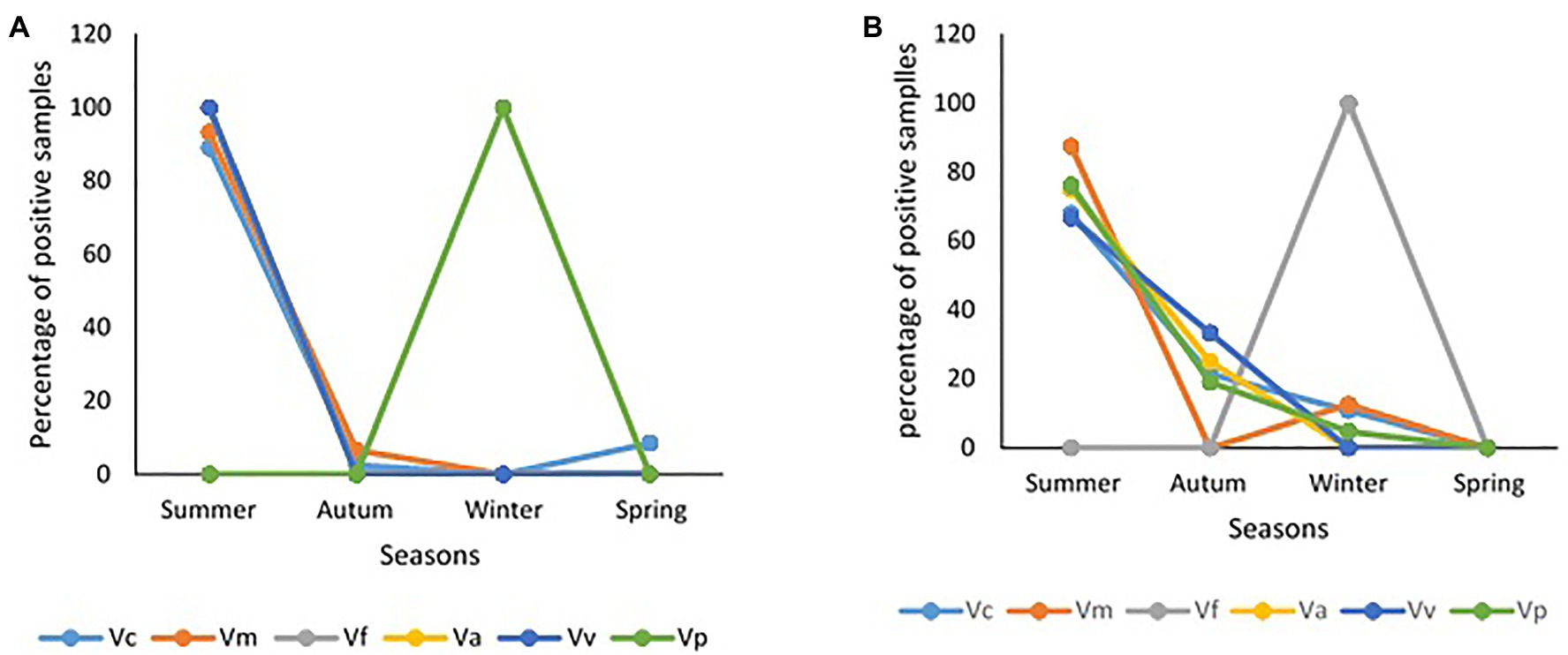
Figure 6. Seasonality of samples positive for targeted Vibrio spp. (A) freshwater samples, (B) brackish water samples.
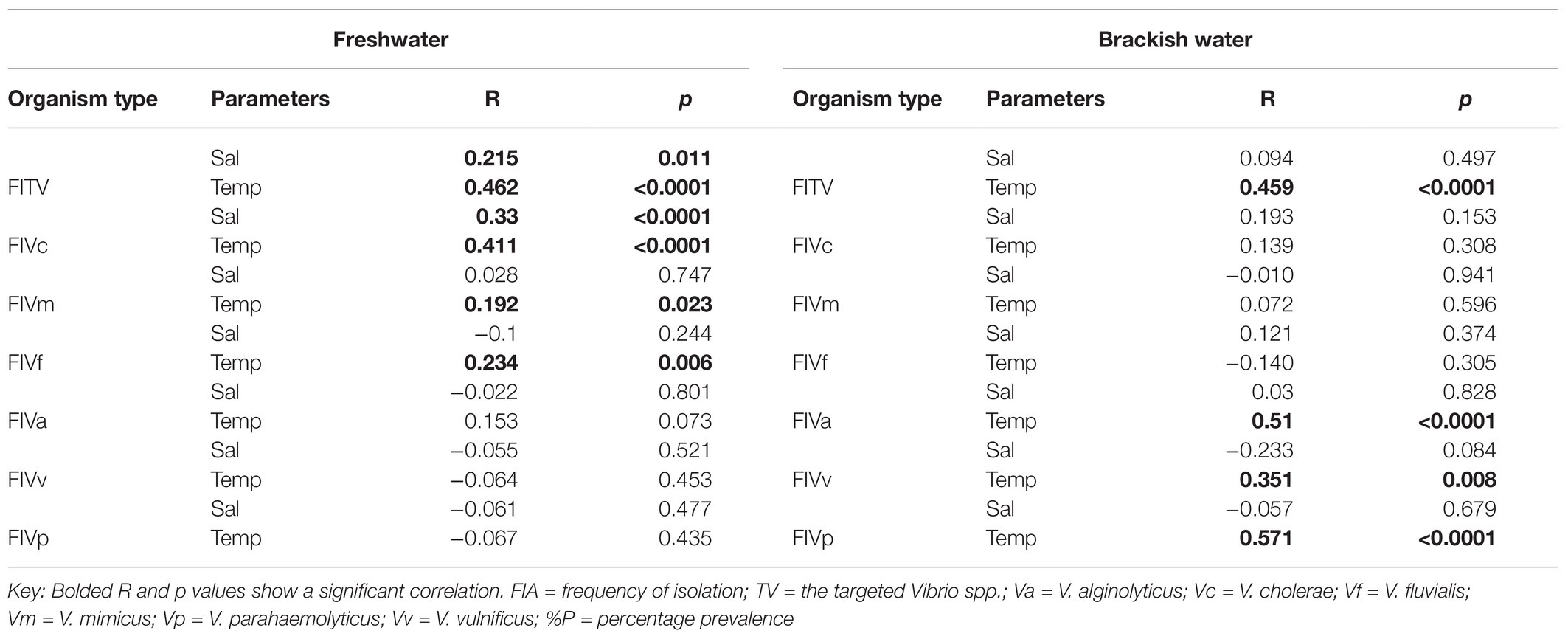
Table 4. Correlation analysis between the frequency of isolation of targeted Vibrio spp. from water samples, temperature, and salinity.
It is good to point out that targeted Vibrio spp. from few samples at sites ALD1, ALD2, EL5, and SKR were isolated in winter in this study. This could be attributed to the level of pollution at these sites as earlier discussed and affirm the possibility of isolating medically important Vibrio spp. in usual season because of pollution and contamination that is not season dependent. It has been earlier reported by Kopprio et al. (2020) and Watkins and Cabelli (1985) that warmer temperatures and sewage pollution are reasons for the abundance of pathogenic Vibrio spp. in the aquatic milieu. In addition, the studies carried out by Jaiani et al. (2013) and Janelidze et al. (2011) suggest that temperature affects Vibrio abundance, whereas salinity affects Vibrio species composition of any ecological niche. It is important to note here that a similar scenario discussed above was observed when absolute densities of targeted Vibrio spp. were statistically analyzed (Supplementary Tables SS1B,C; Supplementary Figures SS1A,B).
Prevalence of the Six Targeted Vibrio Species of Medical Importance
The Kruskal-Wallis test showed that the average annual absolute density of each of the targeted Vibrio spp. was significantly different across sites at p < 0.05. The Games-Howell post hoc test showed the site comparisons with significant differences (Supplementary Table SS1D). The comparisons of absolute density of targeted species between freshwater and brackish water sites that showed significant difference revealed that V. cholerae and V. mimicus are more abundant in freshwater than in brackish water, whereas V. vulnificus, V. alginolyticus, and V. parahaemolyticus are more abundant in brackish water than in freshwater. Furthermore, seasonal comparison of absolute density (Supplementary Table SS1E) showed that all the targeted species proliferate in summer significantly than in other seasons of the year. The relative abundance of each of the six targeted Vibrio spp. in isolates recovered at the sampling sites is shown in Figures 7A,B, whereas the incidence of each of the targeted species among the total Vibrio spp. isolates recovered from each of the water sample types is given in Figures 7C,D. The prevalence of each of the targeted Vibrio spp. per sampling site is given in Figures 7E,F. At least one of the targeted Vibrio species was detected and isolated from each of the sampling sites. The prevalence of V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus in freshwater samples was 34, 19, 9, 2, 3, and 2%, respectively. On the other hand, the prevalence of V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus in brackish water samples was 44, 28, 10, 7, 46, and 51%, respectively. A total of 628 and 342 presumptive isolates from freshwater and brackish water samples, respectively, were analyzed in this study. Of the freshwater presumptive isolates, 79% were confirmed as Vibrio spp., whereas 85% of presumptive isolates from brackish water samples were confirmed as Vibrio spp. Twenty-two and 41% of the PCR-confirmed isolates from freshwater samples and brackish water samples, respectively, fall among the targeted Vibrio species. The samples of gel electrophoresis pictures for the confirmation and speciation of Vibrio spp. into the six targeted species are given in Figure 8. The incidence of V. cholerae, V. mimicus, V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus of all the six targeted Vibrio species recovered from freshwater samples was 75, 14, 4, 6, 1, and 1%, whereas that for brackish water was 24, 7, 3, 47, 3, and 18%, respectively. The predominant species of the freshwater samples were V. cholerae and V. mimicus, whereas those of the brackish water samples were V. alginolyticus, V. cholerae, and V. parahaemolyticus. The prevalence of the remaining four (V. fluvialis, V. vulnificus, V. alginolyticus, and V. parahaemolyticus) of the six targeted Vibrio spp. among Vibrio isolates from freshwater samples and three (V. mimicus, V. fluvialis, V. vulnificus) of the six targeted Vibrio species in brackish water samples were, respectively, low. Our result showed population diversity of targeted Vibrio species in freshwater and brackish water samples. Halophilic vibrios were more abundant in brackish water, whereas non-halophilic vibrios were abundant in freshwater samples. The six targeted Vibrio spp. were more diverse in brackish water sample than in freshwater sample. These findings corroborate some earlier reports and suggest that salt concentration modulates the diversity of vibrios in water resources (Elhadi, 2013; Mookerjee et al., 2015; de Menezes et al., 2017; Fu et al., 2019). The result (Figure 7; Supplementary Table SS1A) of the present study showed that the possibility of contracting infections, such as cholera and cholera-like infections, caused by the non-halophilic vibrios is higher at the freshwater sampling sites most especially sites PA4, PA5, PA6, and ALD1 where most of the halophilic vibrios were recovered than at the brackish water sampling sites. Likewise, the chances of contracting gastroenteritis, wound infections, and other vibriosis peculiar to halophilic vibrios are higher at the brackish sampling sites most especially sites EL6, SR, and SKR. Furthermore, the coexistence of medically important non-halophilic and halophilic vibrios at the same ecological niche in most of our sampling sites is of public health concern. As the density and diversity of cholera and vibriosis causing Vibrio species increase in a single ecological niche, the chances of acquiring the infections they cause also increase. Furthermore, the possibility of exchanging genetic materials, such as virulence and antibiotics resistance determinants, will be high. The coexistence of medically important Vibrio species within the same ecological niche has been reported in an earlier study (Kokashvili et al., 2015). Although the present study focused on freshwater and brackish water that have not been investigated for members of the Vibrio genus before, Vibrio spp. of medical importance including four of the six Vibrio spp. focused on in this study have been reported from various WWTPs of Eastern Cape and their receiving watershed. A significant amount of V. cholerae, V. parahaemolyticus, V. metschnikovii, V. fluvialis, and V. vulnificus were reportedly isolated from final effluents in some WWTPs in Nkonkobe rural community, Chris Hani, and Amathole district municipalities (Igbinosa et al., 2009, 2011a,b; Nongogo and Okoh, 2014). A more comprehensive study on final effluents of 14 WWTPs in Amathole and Chris Hani district municipalities reported 66.8% Vibrio spp. prevalence of the 1,000 randomly selected isolates recovered from the WWTPs. Of the 300 confirmed Vibrio spp., 68.2% belong to one of V. parahaemolyticus, V. fluvialis, and V. vulnificus (Okoh et al., 2015). All these studies, including the present one, show that medically important Vibrio spp. are present in the aquatic milieu of the ECP. The aforementioned is of potential health risk to individuals using the water resources studied and watershed for recreational, agricultural, and domestic purposes most especially at the downstream of WWTP final effluents discharge points. In some studies from other provinces of South Africa, the isolation of V. harveyi, V. parahaemolyticus, V. cholerae, V. mimicus, and V. vulnificus from tap, borehole, and dam in North West province (Maje et al., 2020) and V. cholerae from four WWTPs located in Gauteng Province (Dungeni et al., 2010) has also been reported. Health risks that an individual using these water resources could be exposed to include gastrointestinal infections caused by V. parahaemolyticus, V. mimicus, and V. fluvialis; wound infections caused by V. vulnificus and V. harveyi; cholera caused by V. cholerae; and cholera-like bloody diarrhea caused by V. fluvialis (Ramamurthy et al., 1994; Igbinosa and Okoh, 2010; Jones, 2017; Brehm et al., 2020).
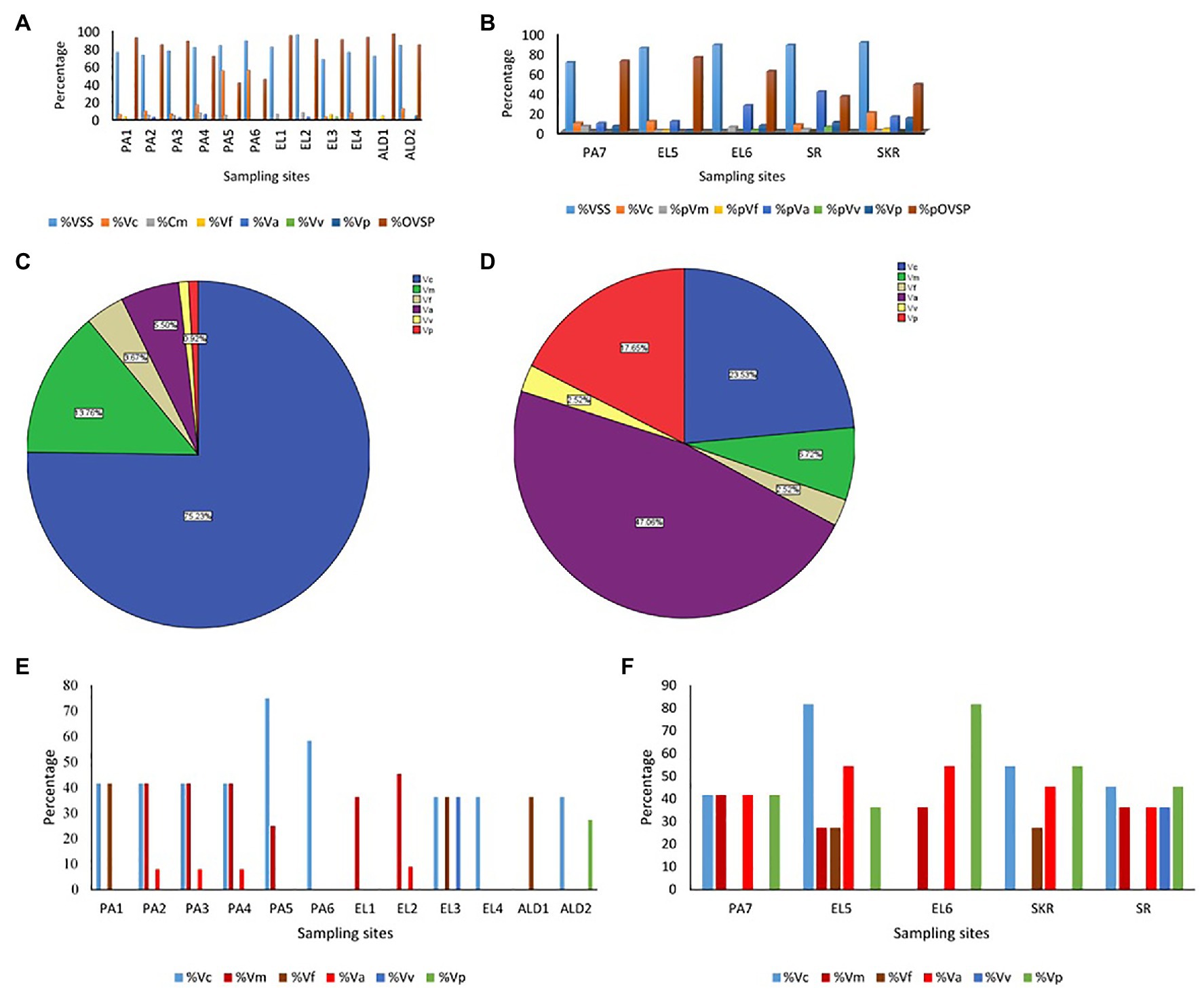
Figure 7. (A,B) Prevalence of targeted Vibrio spp. in isolates recovered per sampling sites. (C,D) Distribution of targeted Vibrio spp. among isolates recovered from samples. (E,F) Detection rate of targeted Vibrio spp. in samples per sampling site. (A,E) Freshwater sampling sites, (B,F) brackish water sampling sites, (C) isolates from freshwater samples, (D) isolates from brackish water samples, % = percentage, VSS = Vibrio spp. positive samples, Vc = Vibrio cholerae, Vm = Vibrio mimicus, Vf = Vibrio fluvialis, Va = Vibrio alginolyticus, Vp = V. parahaemolyticus, OVSP = other Vibrio spp.
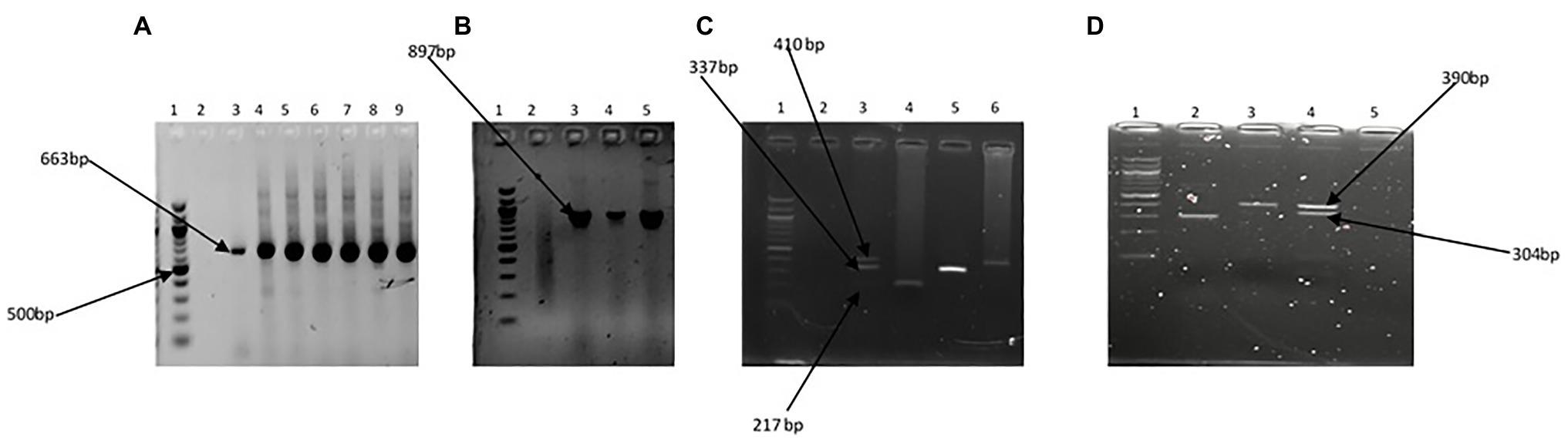
Figure 8. Gel pictures showing PCR amplification products of the specific regions of 16S rRNA gene for the Vibrio genus (A), fla E gene for V. parahaemolyticus (B), GroEl, ToxR, and GyrB genes in triplex PCR for V. vulnificus, V. fluvialis, and V. alginolyticus, respectively (C), and OmpW and vhm genes in duplex PCR for V. cholerae and V. mimicus, respectively. Lane 1 (A–D) 100 bp molecular marker, lane 2 (A–C) and lane 5 (D) negative control, lane 3 (A–C) and lane 4 (D) positive controls, and lanes 4–9 (A), lanes 4 and 5 (B), lanes 4–6 (C), and lanes 2 and 3 (D) positive isolates.
Summary and Conclusion
The present study confirmed the presence of Vibrio species of medical importance in both freshwaters (Kowie River, Bloukrans River, Lashinton River, Kubusi River, and two dams in Amathole District Municipality) and brackish water (Buffalo, Sunday, Kowie, and Swartkops estuaries) of the Eastern Cape, Province of South Africa. It is worth mentioning that humans have regular contact with more than 88% of our sampling sites and all the sampling sites had not been investigated for the occurrence of Vibrio spp. of medical importance before. The predominant species at the sampling sites showed that the chances of contracting cholera and cholera-like infection are high at the freshwater sampling sites than at the brackish water sampling sites. On the other hand, the chances of contracting other vibriosis are high at the brackish water sampling sites than at the freshwater sampling sites. The findings of the study also suggest pollution as the reason for the isolation of medically important vibrios in the unusual season at some of the sampling sites. It was also observed that temperature drives isolation frequency, whereas salinity drives the composition of the targeted Vibrio species at both freshwater and brackish water sampling sites. Although the virulence status of the isolated medically important Vibrio species was not elucidated in this study, the confirmation of their presence in the water resources investigated for the first time is a significant finding. This finding is an eye opener to the potential threat that the water resources investigated pose to public health in terms of cholera and vibriosis. Therefore, our findings are of public health importance going by the usefulness of the water resources investigated. Although controlling and preventing most of the identified factors that could be contributing to the prevalence of medically important Vibrio species at the sampling points might be difficult, regular monitoring will go a long way to prevent possible Vibrio-related infection outbreaks.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
OA and AIO initiated the research topic. AIO provided the materials for the study. OA structured the methods and carried out the statistical analysis and wrote the manuscript. OA and ACO carried out the experiment. ACO and AIO proofread and corrected the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Water Research Commission (grant number: K5 2432), South Africa and Medical Research Council, South Africa.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.617703/full#supplementary-material
References
Abakpa, G. O., Umoh, V. J., Ameh, J. B., and Al, E. T. (2013). Occurrence of Vibrio cholerae in some households engaged in livestock farming in some parts of Zaria, Nigeria. Adv. Microbiol. 3, 128–131. doi: 10.4236/aim.2013.31020
Abioye, O. E., and Okoh, A. I. (2018). Limpet (Scutellastra cochlear) recovered from some estuaries in the eastern Cape Province, South Africa act as reservoirs of pathogenic Vibrio species. Front. Public Health 6:237. doi: 10.3389/fpubh.2018.00237
Adams, J., Pretorius, L., and Snow, G. (2019). Deterioration in the water quality of an urbanised estuary with recommendations for improvement. Water SA 45, 86–96. doi: 10.4314/wsa.v45i1.10
Adeniji, A., Okoh, O., and Okoh, A. (2017a). Petroleum hydrocarbon fingerprints of water and sediment samples of Buffalo River estuary in the eastern Cape Province, South Africa. J. Anal. Methods Chem. 2017:2629365. doi: 10.1155/2017/2629365
Adeniji, A., Okoh, O., and Okoh, A. (2017b). Petroleum hydrocarbon profiles of water and sediment of algoa bay, Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 14:1263. doi: 10.3390/ijerph14101263
Ahmad, N., Ghazali, F. M., Cheah, Y. K., and Chilek, T. Z. T. (2011). Prevalence and quantification of Vibrio species and Vibrio parahaemolyticus in freshwater fish at hypermarket level. Int. Food Res. J. 18, 689–695.
Ajin, A., Silvester, R., Alexander, D., Nashad, M., and Abdulla, M. H. (2016). Characterization of blooming algae and bloom-associated changes in the water quality parameters of traditional pokkali cum prawn fields along the South West coast of India. Environ. Monit. Assess. 188, 1–10. doi: 10.1007/s10661-016-5133-6
Baffone, W., Tarsi, R., Pane, L., Campana, R., Repetto, B., Mariottini, G. L., et al. (2006). Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ. Microbiol. 8, 1299–1305. doi: 10.1111/j.1462-2920.2006.01011.x
Baker-Austin, C., Oliver, J. D., Alam, M., Ali, A., Waldor, M. K., Qadri, F., et al. (2018). Vibrio spp. infections. Nat. Rev. Dis. Prim. 4:8. doi: 10.1038/s41572-018-0005-8
Bessong, P. O., Odiyo, J. O., Musekene, J. N., and Tessema, A. (2009). Spatial distribution of diarrhoea and microbial quality of domestic water during an outbreak of diarrhoea in the Tshikuwi Community in Venda, South Africa. J. Health Popul. Nutr. 27, 652–659. doi: 10.3329/jhpn.v27i5.3642
Bhattacharjee, S., Bhattacharjee, S., Bal, B., Pal, R., Niyogi, S. K., and Sarkar, K. (2010). Is Vibrio fluvialis emerging as a pathogen with epidemic potential in coastal region of eastern India following cyclone Aila? J. Health Popul. Nutr. 28, 311–317. doi: 10.3329/jhpn.v28i4.6036
Binh, M. N., Je, H. L., Ngo, T. C., Seon, Y. C., Nguyen, T. H., Dang, D. A., et al. (2009). Cholera outbreaks caused by an altered Vibrio cholerae O1 El tor biotype strain producing classical cholera toxin B in Vietnam in 2007 to 2008. J. Clin. Microbiol. 47, 1568–1571. doi: 10.1128/JCM.02040-08
Bisharat, N., Cohen, D. I., Harding, R. M., Falush, D., Crook, D. W., Peto, T., et al. (2005). Hybrid Vibrio vulnificus. Emerg. Infect. Dis. 11, 30–35. doi: 10.3201/eid1101.040440
Blodgett, R., (2015). BAM Appendix 2: Most Probable Number from Serial Dilutions | FDA [WWW Document]. Available at: https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions (Accessed June 23, 2020).
Brehm, T. T., Berneking, L., Rohde, H., Chistner, M., Schlickewei, C., Sena Martins, M., et al. (2020). Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: a case report and review of literature. BMC Infect. Dis. 20:104. doi: 10.1186/s12879-020-4789-2
Cabral, J. P. S. (2010). Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 7, 3657–3703. doi: 10.3390/ijerph7103657
CDC (1999). Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from long Island sound--Connecticut, New Jersey, and New York, 1998. MMWR Morb. Mortal. Wkly Rep. 48, 48–51.
CDC (2015). Recommendations for the Use of Antibiotics for the Treatment of Cholera [WWW Document]. Available at: https://www.cdc.gov/cholera/treatment/antibiotic-treatment.html (Accessed December 16, 2019).
CDC (2017). Vibriosis | 2017 Case Definition. Available at: https://wwwn.cdc.gov/nndss/conditions/vibriosis/case-definition/2017/ (Accessed June 21, 2020).
Ceccarelli, D., Chen, A., Hasan, N. A., Rashed, S. M., Huq, A., and Colwell, R. R. (2015). Non-O1/non-O139 Vibrio cholerae carrying multiple virulence factors and V. cholerae O1 in the Chesapeake Bay, Maryland. Appl. Environ. Microbiol. 81, 1909–1918. doi: 10.1128/AEM.03540-14
Ceccarelli, D., and Colwell, R. R. (2014). Vibrio ecology, pathogenesis, and evolution. Front. Microbiol. 5:256. doi: 10.3389/fmicb.2014.00256
Chakraborty, R., Sinha, S., Mukhopadhyay, A. K., Asakura, M., Yamasaki, S., Bhattacharya, S. K., et al. (2006). Species-specific identification of Vibrio fluvialis by PCR targeted to the conserved transcriptional activation and variable membrane tether regions of the toxR gene. J. Med. Microbiol. 55, 805–808. doi: 10.1099/jmm.0.46395-0
Chigor, V. N., Sibanda, T., and Okoh, A. I. (2012). Studies on the bacteriological qualities of the Buffalo River and three source water dams along its course in the Eastern Cape Province of South Africa. Environ. Sci. Pollut. Res. Int. 20, 4125–4136. doi: 10.1007/s11356-012-1348-4
Chowdhury, G., Ghosh, S., Pazhani, G. P., Paul, B. K., Maji, D., Mukhopadhyay, A. K., et al. (2013). Isolation and characterization of pandemic and nonpandemic strains of Vibrio parahaemolyticus from an outbreak of diarrhea in north 24 Parganas, West Bengal, India. Foodborne Pathog. Dis. 10, 338–342. doi: 10.1089/fpd.2012.1340
Christine, C., Sindiswa, N., and Imelda, H. (2013). An Introduction to South Africa’s water sources areas [WWW Document]. Available at: www.journeyofwater.co.za (Accessed January 14, 2020).
Colvin, C., Muruven, D., Lindley, D., and Schachtschneider, K. (2016). Water: Facts and Futures Rethinking South Africa’s Water Future [WWW Document]. Available at: http://awsassets.wwf.org.za/downloads/wwf009_waterfactsandfutures_report_web__lowres_.pdf (Accessed January 14, 2020).
Colwell, J. J. (2016). The Effects of Temperature and Salinity on Vibrio Species in Breton Sound Estuary. LSU Master’s Theses.
Comins, L. (2012). Deadly bug hits beaches. Available at: https://www.iol.co.za/news/south-africa/kwazulu-natal/deadly-bug-hits-beaches-1227227 (Accessed June 01, 2020).
Copin, S., Robert-Pillot, A., Malle, P., Quilici, M. L., and Gay, M. (2012). Evaluation of most-probable-number-PCR method with internal amplification control for the counting of total and pathogenic Vibrio parahaemolyticus in frozen shrimps. J. Food Prot. 75, 150–153. doi: 10.4315/0362-028X.JFP-11-165
Cottle, E., and Deedat, H. (2002). The cholera outbreak: a 2000–2002 case study of the source of the outbreak in the Madlebe Tribal Authority areas, uThungulu Region, KwaZulu-Natal. Durban: Health Systems Trust.
de Menezes, F. G. R., da Neves, S. S., Sousa, O. V., Vila-Nova, C. M. V. M., Maggioni, R., Theophilo, G. N. D., et al. (2014). Detecção de genes de virulência em estirpes de Vibrio cholerae isolados de estuários no Nordeste do Brasil. Rev. Inst. Med. Trop. Sao Paulo 56, 427–432. doi: 10.1590/S0036-46652014000500010
de Menezes, F. G. R., Rodriguez, M. T. T., de Carvalho, F. C. T., Rebouças, R. H., Costa, R. A., de Sousa, O. V., et al. (2017). Pathogenic Vibrio species isolated from estuarine environments (Ceará, Brazil) - antimicrobial resistance and virulence potential profiles. An. Acad. Bras. Cienc. 89, 1175–1188. doi: 10.1590/0001-3765201720160191
De Souza Costa Sobrinho, P., Destro, M. T., Franco, B. D. G. M., and Landgraf, M. (2010). Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo state, Brazil. Appl. Environ. Microbiol. 76, 1290–1293. doi: 10.1128/AEM.00861-09
Decol, L. T., Casarin, L. S., Hessel, C. T., Batista, A. C. F., Allende, A., and Tondo, E. C. (2017). Microbial quality of irrigation water used in leafy green production in Southern Brazil and its relationship with produce safety. Food Microbiol. 65, 105–113. doi: 10.1016/j.fm.2017.02.003
Deen, J., Mengel, M. A., and Clemens, J. D. (2019). Epidemiology of cholera. Vaccine 38, A31–A40. doi: 10.1016/j.vaccine.2019.07.078
Delahoy, M. J., Wodnik, B., McAliley, L., Penakalapati, G., Swarthout, J., Freeman, M. C., et al. (2018). Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health 221, 661–676. doi: 10.1016/j.ijheh.2018.03.005
DePaola, A., Nordstrom, J. L., Bowers, J. C., Wells, J. G., and Cook, D. W. (2003). Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69, 1521–1526. doi: 10.1128/AEM.69.3.1521-1526.2003
Di, D. Y. W., Lee, A., Jang, J., Han, D., and Hur, H. G. (2017). Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl. Environ. Microbiol. 83, e02680–e02616. doi: 10.1128/AEM.02680-16
Drake, S. L., Depaola, A., and Jaykus, L. (2007). An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Compr. Rev. Food Sci. Food Saf. 6, 120–144. doi: 10.1111/j.1541-4337.2007.00022.x
Dufour, A., Bartram, J., Bos, R., and Gannon, V. (2013). Animal waste, water quality and human health. Water Intell. Online 12, 1–10. doi: 10.2166/9781780401249
Dungeni, M., van Der Merwe, R. R., and Momba, M. N. B. (2010). Abundance of pathogenic bacteria and viral indicators in chlorinated effluents produced by four wastewater treatment plants in the Gauteng Province, South Africa. Water SA 36, 607–614. doi: 10.4314/wsa.v36i5.61994
Edokpayi, J. N., Odiyo, J. O., and Durowoju, O. S. (2017). “Impact of wastewater on surface water quality in developing countries: a case study of South Africa” in Water quality. ed. H. Tutu (Rijeka, Croatia: InTech), 401–416.
Elhadi, N. (2013). Occurrence of potentially human pathogenic Vibrio species in the coastal water of the Eastern Province of Saudi Arabia. Res. J. Microbiol. 8, 1–12. doi: 10.3923/jm.2013.1.12
Esteves, K., Hervio-Heath, D., Mosser, T., Rodier, C., Tournoud, M.-G., Jumas-Bilak, E., et al. (2015). Rapid proliferation of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae during freshwater flash floods in French Mediterranean Coastal Lagoons. Appl. Environ. Microbiol. 81, 7600–7609. doi: 10.1128/AEM.01848-15
European Commission (2001). Opinion of the scientific committee on veterinary measures relating to public health on Vibrio vulnificus and Vibrio parahaemolyticus (in raw and undercooked seafood).
Fatoki, O. S., Muyima, N. Y. O., and Lujiza, N. (2001). Situation analysis of water quality in the Umtata River catchment. Water SA 27, 467–473. doi: 10.4314/wsa.v27i4.4959
Fernández-delgado, A. M., Sanz, V., Giner, S., Contreras, M., and Michelangeli, F. (2015). Prevalence and distribution of Vibrio spp. in wild aquatic birds of the Southern Caribbean Sea, Venezuela, 2011–12. J. Wildl. Dis. 52, 621–626. doi: 10.7589/2015-06-154
Finkelstein, R., Edelstein, S., and Mahamid, G. (2002). Fulminant wound infections due to Vibrio vulnificus. Isr. Med. Assoc. J. 4, 654–655.
Finkelstein, R. A. (1996). Cholera, Vibrio cholerae O1 and O139, and Other Pathogenic Vibrios, Medical Microbiology. University of Texas Medical Branch at Galveston.
Fu, S., Hao, J., Yang, Q., Lan, R., Wang, Y., Ye, S., et al. (2019). Long-distance transmission of pathogenic Vibrio species by migratory waterbirds: a potential threat to the public health. Sci. Rep. 9:16303. doi: 10.1038/s41598-019-52791-5
Fukushima, H., and Seki, R. (2004). Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol. Ecol. 48, 221–229. doi: 10.1016/j.femsec.2004.01.009
Glenn Morris, J. Jr. (1994). “Non-O group 1 Vibrio cholerae strains not associated with epidemic disease,” in Vibrio Cholerae and Cholera. eds. I. K. Wachsmuth, P. A. Blake, and O. Olsvik (Wiley: American Society of Microbiology), 103–115.
Goel, A. K., Jain, M., Kumar, P., Kamboj, D. V., and Singh, L. (2010). Virulence profile and clonal relationship among the Vibrio cholerae isolates from ground and surface water in a cholera endemic area during rainy season. Folia Microbiol. 55, 69–74. doi: 10.1007/s12223-010-0011-z
Grim, C. J., Hasan, N. A., Taviani, E., Haley, B., Chun, J., Brettin, T. S., et al. (2010). Genome sequence of hybrid Vibrio cholerae O1 MJ-1236, B-33, and CIRS101 and comparative genomics with V. cholerae. J. Bacteriol. 192, 3524–3533. doi: 10.1128/JB.00040-10
Guardiola-avila, I., Noriega-orozco, L., and Acedo-félix, E. (2015). Presence of the Hemolysin gene of Vibrio mimicus in fish and seafood products in Sonora, México. J. Food Res. 4, 66–76. doi: 10.5539/jfr.v4n1p66
Herbig, F. J. W., and Meissner, R. (2019). Talking dirty-effluent and sewage irreverence in South Africa: a conservation crime perspective. Cogent Soc. Sci. 5:1701359. doi: 10.1080/23311886.2019.1701359
Igbinosa, E. O., Obi, L. C., and Okoh, A. I. (2009). Occurrence of potentially pathogenic vibrios in final effluents of a wastewater treatment facility in a rural community of the Eastern Cape Province of South Africa. Res. Microbiol. 160, 531–537. doi: 10.1016/j.resmic.2009.08.007
Igbinosa, E. O., and Okoh, A. I. (2010). Vibrio fluvialis: an unusual enteric pathogen of increasing public health concern. Int. J. Environ. Res. Public Health 7, 3628–3643. doi: 10.3390/ijerph7103628
Igbinosa, E. O., Obi, L. C., and Okoh, A. I. (2011a). Seasonal abundance and distribution of Vibrio species in the treated effluent of wastewater treatment facilities in suburban and urban communities of Eastern Cape Province, South Africa. J. Microbiol. 49, 224–232. doi: 10.1007/s12275-011-0227-x
Igbinosa, E. O., Obi, L. C., Tom, M., and Okoh, A. I. (2011b). Detection of potential risk of wastewater effluents for transmission of antibiotic resistance from Vibrio species as a reservoir in a peri-urban community in South Africa. Int. J. Environ. Health Res. 21, 402–414. doi: 10.1080/09603123.2011.572278
Jaiani, E., Kokashvili, T., Mitaishvili, N., Elbakidze, T., Janelidze, N., Lashkhi, N., et al. (2013). Microbial water quality of recreational lakes near Tbilisi, Georgia. J. Water Health 11, 333–345. doi: 10.2166/wh.2013.057
Janelidze, N., Jaiani, E., Lashkhi, N., Tskhvediani, A., Kokashvili, T., Gvarishvili, T., et al. (2011). Microbial water quality of the Georgian coastal zone of the Black Sea. Mar. Pollut. Bull. 62, 573–580. doi: 10.1016/j.marpolbul.2010.11.027
Jones, J. L. (2017). “Chapter 11 - Vibrio” in Foodborne Diseases. 3rd Edn. eds. C. E. R. Dodd, T. Aldsworth, R. A. Stein, D. O. Cliver, and H. P. Riemann (Academic Press), 243–252.
Jones, M. K., and Oliver, J. D. (2009). Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77, 1723–1733. doi: 10.1128/IAI.01046-08
Jones, S., and Summer-Brason, B. (1998). Incidence and detection of pathogenic “Vibrio” sp. in a northern New England estuary, USA. J. Shellfish Res. 17, 1665–1669.
Jordaan, K., Comeau, A. M., Khasa, D. P., and Bezuidenhout, C. C. (2019). An integrated insight into the response of bacterial communities to anthropogenic contaminants in a river: a case study of the Wonderfonteinspruit catchment area, South Africa. PLoS One 14:e0216758. doi: 10.1371/journal.pone.0216758
Kanungo, S., Sur, D., Ali, M., You, Y. A., Pal, D., Manna, B., et al. (2012). Clinical, epidemiological, and spatial characteristics of Vibrio parahaemolyticus diarrhea and cholera in the urban slums of Kolkata, India. BMC Public Health 12:830. doi: 10.1186/1471-2458-12-830
Kaspar, C. W., and Tamplin, M. L. (1993). Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59, 2425–2429. doi: 10.1128/AEM.59.8.2425-2429.1993
Kazi, A., Mumtaz, A., Nazir, R., Ismail, T., and Akbar, S. (2005). Determination of clobazam by visible spectrophotometry in pure and pharmaceutical preparations. Proc. Pak. Acad. Sci. 42:133.
Kelly, M. T. (1982). Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 44:820. doi: 10.1128/AEM.44.4.820-824.1982
Kirchberger, P. C., Orata, F. D., Barlow, E. J., Kauffman, K. M., Case, R. J., Polz, M. F., et al. (2016). A small number of phylogenetically distinct clonal complexes dominate a coastal Vibrio cholerae population. Appl. Environ. Microbiol. 82, 5576–5586. doi: 10.1128/AEM.01177-16
Klontz, K. C., Williams, L., Baldy, L. M., and Campos, M. (1993). Raw oyster-associated Vibrio infections: linking epidemiologic data with laboratory testing of oysters obtained from a retail outlet. J. Food Prot. 56, 977–979. doi: 10.4315/0362-028X-56.11.977
Kokashvili, T., Whitehouse, C. A., Tskhvediani, A., Grim, C. J., Elbakidze, T., Mitaishvili, N., et al. (2015). Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front. Public Health 3:232. doi: 10.3389/fpubh.2015.00232
Kopprio, G. A., Neogi, S. B., Rashid, H., Alonso, C., Yamasaki, S., Koch, B. P., et al. (2020). Vibrio and bacterial communities across a pollution gradient in the bay of Bengal: unraveling their biogeochemical drivers. Front. Microbiol. 11:594. doi: 10.3389/fmicb.2020.00594
Kothary, M. H., Lowman, H., McCardell, B. A., and Tall, B. D. (2003). Purification and characterization of enterotoxigenic E1 Tor-like hemolysin produced by Vibrio fluvialis. Infect. Immun. 71, 3213–3220. doi: 10.1128/IAI.71.6.3213-3220.2003
Kwok, A. Y. C., Wilson, J. T., Coulthart, M., Ng, L. K., Mutharia, L., and Chow, A. W. (2002). Phylogenetic study and identification of human pathogenic Vibrio species based on partial hsp60 gone sequences. Can. J. Microbiol. 48, 903–910. doi: 10.1139/w02-089
Lei, S., Miyoshi, S., Kewei, B., Nakamura, M., Hiura, M., Tomochika, K., et al. (2000). Presence of Hemolysin genes (vmh, tdh and hlx) in isolates of Vibrio mimicus determined by polymerase chain reaction. J. Health Sci. 46, 63–65. doi: 10.1248/jhs.46.63
Letchumanan, V., Yin, W., Lee, L., and Chan, K. (2015). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticu s isolated from retail shrimps in Malaysia. Front. Microbiol. 6:33. doi: 10.3389/fmicb.2015.00033
Levinson, W., and Jawetz, E. (1996). Medical Microbiology and Immunology 4th Edition by Levinson, Warren E., Jawetz, Ernest (1996) Paperback: Amazon.com: Books [WWW Document]. Availabe at: https://www.amazon.com/Medical-Microbiology-Immunology-Levinson-Paperback/dp/B011DBKQS0 (Accessed June 1, 2020).
Li, D., Jiang, X., Wang, J., Wang, K., and Zheng, B. (2017). Effect of sewage and industrial effluents on bacterial and archaeal communities of creek sediments in the Taihu Basin. Water 9:373. doi: 10.3390/w9060373
Liu, B., Liu, H., Pan, Y., Xie, J., and Zhao, Y. (2016). Comparison of the effects of environmental parameters on the growth variability of Vibrio parahaemolyticus coupled with strain sources and genotypes analyses. Front. Microbiol. 7:994. doi: 10.3389/fmicb.2016.00994
Liu, H., Whitehouse, C. A., and Li, B. (2018). Presence and persistence of Salmonella in water: the impact on microbial quality of water and food safety. Front. Public Health 6:159. doi: 10.3389/fpubh.2018.00159
Machado, A., and Bordalo, A. A. (2016). Detection and quantification of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in coastal waters of Guinea-Bissau (West Africa). EcoHealth 13, 339–349. doi: 10.1007/s10393-016-1104-1
Maclennan, S. (2019). Fish River sewage and Makhanda: DWS answers questions [WWW Document]. Avilable at: https://www.grocotts.co.za/2019/06/25/dws-takes-muni-to-court-over-sewage/ (Accessed May 22, 2020).
Madoroba, E., and Momba, M. N. (2010). Prevalence of vibrio cholerae in rivers of Mpumalanga province, South Africa as revealed by polyphasic characterization. Afr. J. Biotechnol. 9, 7295–7301.
Mahoney, J. C., Gerding, M. J., Jones, S. H., and Whistler, C. A. (2010). Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Appl. Environ. Microbiol. 76, 7459–7465. doi: 10.1128/AEM.01450-10
Maje, M. D., Kaptchouang Tchatchouang, C. D., Manganyi, M. C., Fri, J., and Ateba, C. N. (2020). Characterisation of Vibrio species from surface and drinking water sources and assessment of biocontrol potentials of their bacteriophages. Int. J. Microbiol. 2020:8863370. doi: 10.1155/2020/8863370
Marie, V., and Lin, J. (2018). Microbial indicators and environmental relationships in the Umhlangane River, Durban, South Africa. Open Life Sci. 13, 385–395. doi: 10.1515/biol-2018-0047
Maugeri, T. L., Carbone, M., Fera, M. T., and Gugliandolo, C. (2006). Detection and differentiation of Vibrio vulnificus in seawater and plankton of a coastal zone of the Mediterranean Sea. Res. Microbiol. 157, 194–200. doi: 10.1016/j.resmic.2005.06.007
McAllister, T. A., and Topp, E. (2012). Role of livestock in microbiological contamination of water: commonly the blame, but not always the source. Anim. Front. 2, 17–27. doi: 10.2527/af.2012-0039
Mema, V. (2008). Impact of Poorly Maintained Wastewater and Sewage Treatment Plants: Lessons From South Africa.
Mezgebe, K., Gebrekidan, A., Hadera, A., and Weldegebriel, Y. (2015). Assessment of physico-chemical parameters of Tsaeda Agam River in Mekelle City, Tigray, Ethiopia. Bull. Chem. Soc. Ethiop. 29, 377–385. doi: 10.4314/bcse.v29i3.5
Momba, M., and Azab El-Liethy, M. (2017). “Vibrio Cholerae And Cholera Biotypes,” in Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project; Part 3: Specific Excreted Pathogens: Environmental and Epidemiology Aspects - Section 2: Bacteria. eds. A. Pruden, N. Ashbolt and J. Miller). eds. J. B. Rose and B. Jiménez-Cisneros. MI, UN: Michigan State University E. Lansing. Available at: http//www.waterpathogens.org/book/Vibrio (Accessed May 22, 2020).
Momba, M. N. B., Malakate, V. K., and Theron, J. (2006). Abundance of pathogenic Escherichia coli, Salmonella typhimurium and Vibrio cholerae in Nkonkobe drinking water sources. J. Water Health 4, 289–296. doi: 10.2166/wh.2006.011
Mookerjee, S., Batabyal, P., Sarkar, M. H., and Palit, A. (2015). Seasonal prevalence of Enteropathogenic Vibrio and their phages in the riverine estuarine ecosystem of South Bengal. PLoS One 10:e0137338. doi: 10.1371/journal.pone.0137338
Morris, J. (2019). Minor Vibrio and Vibrio-like species associated with human disease - UpToDate [WWW Document]. Available at: https://www.uptodate.com/contents/minor-vibrio-and-vibrio-like-species-associated-with-human-disease?search=minor-Vibrio-and-Vibrio-like-species-associated-with-human-disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (Accessed June 1, 2020).
Mudzanani, L., Ratsaka-Mathokoa, M., Mahlasela, L., Netshidzivhanii, P., and Mugero, C. (2003). Cholera. South African Health Review.
Munshi, S. K., Roy, J., and Noor, R. (2019). Microbiological investigation and determination of the antimicrobial potential of cow dung samples. S. J. Microbiol. 8, 34–37. doi: 10.3329/sjm.v8i1.42437
Mustapha, S., Mustapha, E. M., and Nozha, C. (2013). Vibrio alginolyticus: an emerging pathogen of foodborne diseases. Maejo Int. J. Sci. Technol. 2, 302–309.
Nevondo, T., and Cloete, T. (2001). The global cholera pandemic. Science in Africa [WWW Document]. Available at: http://www.scienceinafrica.com/global-cholera-pandemic (Accessed June 1, 2020).
Nilsson, W. B., Paranjpye, R. N., Hamel, O. S., Hard, C., and Strom, M. S. (2019). Vibrio parahaemolyticus risk assessment in the Pacific Northwest: It’s not what’s in the water. FEMS Microbiol. Ecol. 95:fiz027. doi: 10.1093/femsec/fiz027
Nongogo, V., and Okoh, A. I. (2014). Occurrence of vibrio pathotypes in the final effluents of five wastewater treatment plants in Amathole and Chris Hani District municipalities in South Africa. Int. J. Environ. Res. Public Health 11, 7755–7766. doi: 10.3390/ijerph110807755
Novotny, L., Dvorska, L., Lorencova, A., Beran, V., and Pavlik, V. (2004). Fish: a potential source of bacterial pathogens for human beings. Vet. Med. 49, 343–358. doi: 10.17221/5715-VETMED
Ntema, V. M., Potgieter, N., and Barnard, T. G. (2010). Detection of Vibrio cholerae and Vibrio parahaemolyticus by molecular and culture based methods from source water to household container-stored water at the point-of-use in South African rural communities. Water Sci. Technol. 61, 3091–3102. doi: 10.2166/wst.2010.222
Ntema, V. M., Potgieter, N., Van Blerk, G. N., and Barnard, T. G. (2014). Investigating the occurrence and survival of Vibrio cholerae in selected surface water sources in the KwaZulu-Natal province of South Africa Report to the Water Research Commission.
Okeyo, A. N., Nontongana, N., Fadare, T. O., and Okoh, A. I. (2018). Vibrio species in wastewater final effluents and receiving watershed in South Africa: implications for public health. Int. J. Environ. Res. Public Health. 1266:15. doi: 10.3390/ijerph15061266
Okoh, A. I., Sibanda, T., Nongogo, V., Adefisoye, M., Olayemi, O. O., and Nontongana, N. (2015). Prevalence and characterisation of non-cholerae Vibrio spp. in final effluents of wastewater treatment facilities in two districts of the Eastern Cape Province of South Africa: implications for public health. Environ. Sci. Pollut. Res. 22, 2008–2017. doi: 10.1007/s11356-014-3461-z
Oliver, J. D., and Bockian, R. (1995). In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol. 61, 2620–2623. doi: 10.1128/AEM.61.7.2620-2623.1995
Oliver, J. D., Nilsson, L., and Kjelleberg, S. (1991). Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl. Environ. Microbiol. 57:2640. doi: 10.1128/AEM.57.9.2640-2644.1991
Osorio, C. R., and Klose, K. E. (2000). A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among vibrio species. J. Bacteriol. 182, 526–528. doi: 10.1128/JB.182.2.526-528.2000
Osunla, C. A., and Okoh, A. I. (2017). Vibrio pathogens: a public health concern in rural water resources in sub-Saharan Africa. Int. J. Environ. Res. Public Health 14, 1–27. doi: 10.3390/ijerph14101188
Paranjpye, R. N., Nilsson, W. B., Liermann, M., Hilborn, E. D., George, B. J., Li, Q., et al. (2015). Environmental influences on the seasonal distribution of Vibrio parahaemolyticus in the Pacific Northwest of the USA. FEMS Microbiol. Ecol. 91:fiv121. doi: 10.1093/femsec/fiv121
Payne, S. M., Mey, A. R., and Wyckoff, E. E. (2016). Vibrio iron transport: evolutionary adaptation to life in multiple environments. Microbiol. Mol. Biol. Rev. 80, 69–90. doi: 10.1128/MMBR.00046-15
Penakalapati, G., Swarthout, J., Delahoy, M. J., Mcaliley, L., Wodnik, B., Levy, K., et al. (2017). Exposure to animal feces and human health: a systematic review and proposed research priorities. Environ. Sci. Technol. 51, 11537–11552. doi: 10.1021/acs.est.7b02811
Prasanthan, V., Udayakumar, P., and Ouseph, P. P. (2011). Influence of abiotic environmental factors on the abundance and distribution of Vibrio species in coastal waters of Kerala, India. Indian J. Geo-Mar. Sci. 40, 587–592.
Rabea, E., Bahia, B., Youssef, M., Ahlam, F., Ghita El, M., Bouchra, O., et al. (2019). Microbiological and physicochemical characterization of hospital effluents before and after treatment with two types of sawdust. J. Chem. 2019, 1–10. doi: 10.1155/2019/3275101
Ramamurthy, T., Albert, M. J., Huq, A., Colwell, R. R., Takeda, Y., Takeda, T., et al. (1994). Vibrio mimicus with multiple toxin types isolated from human and environmental sources. J. Med. Microbiol. 40, 194–196. doi: 10.1099/00222615-40-3-194
Ramos, R. J., Miotto, L. A., Miotto, M., Silveira Junior, N., Cirolini, A., da Silva, H. S., et al. (2014). Occurrence of potentially pathogenic Vibrio in oysters (Crassostrea gigas) and waters from bivalve mollusk cultivations in the South Bay of Santa Catarina. Rev. Soc. Bras. Med. Trop. 47, 327–333. doi: 10.1590/0037-8682-0069-2014
Randa, M. A., Polz, M. F., and Lim, E. (2004). Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 70, 5469–5476. doi: 10.1128/AEM.70.9.5469-5476.2004
Rees, G., Pond, K., and Kay, D. (2010). Safe management of shellfish and harvest waters. Water Intell. Online 12, 1–10. doi: 10.2166/9781780405797
Reimer, A. R., Domselaar, G. Van, Stroika, S., Walker, M., Kent, H., Tarr, C., et al. (2011). Comparative genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg. Infect. Dis. 17, 2113–2121. doi: 10.3201/eid1711.110794
Rippey, S. R. (1994). Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 7, 419–425. doi: 10.1128/CMR.7.4.419
Romalde, J. L., Diéguez, A. L., Lasa, A., and Balboa, S. (2014). New Vibrio species associated to molluscan microbiota: a review. Front. Microbiol. 4:413. doi: 10.3389/fmicb.2013.00413
Safa, A., Nair, G. B., and Kong, R. Y. C. (2009). Evolution of new variants of Vibrio cholerae O1. Trends Microbiol. 18, 46–54. doi: 10.1016/j.tim.2009.10.003
Sarkar, B. L., Nair, G. B., Banerjee, A. K., and Pal, S. C. (1985). Seasonal distribution of Vibrio parahaemolyticus in freshwater environs and in association with freshwater fishes in Calcutta. Appl. Environ. Microbiol. 49, 132–136. doi: 10.1128/AEM.49.1.132-136.1985
Shaheen, A., Saeed Baig, H., Shahana, A., and Kazmi, U. (2016). Microbial flora isolated from polluted and non-polluted coastal waters of Karachi. Pak. J. Bot. 48, 1703–1708.
Sharma, A., and Chaturvedi, A. N. (2007). Population dynamics of Vibrio species in the river Narmada at Jabalpur. J. Environ. Biol. 28, 747–751.
Sharma, C., Thungapathra, M., Ghosh, A., Mukhopadhyay, A. K., Basu, A., Mitra, R., et al. (1998). Molecular analysis of non-O1, non-O139 Vibrio cholerae associated with an unusual upsurge in the incidence of cholera-like disease in Calcutta, India. J. Clin. Microbiol. 36, 756–763.
Shinoda, S., Nakagawa, T., Shi, L., Bi, K., Kanoh, Y., Tomochika, K., et al. (2004). Distribution of virulence-associated genes in Vibrio mimicus isolates from clinical and environmental origins. Microbiol. Immunol. 48, 547–551. doi: 10.1111/j.1348-0421.2004.tb03551.x
Skall, H. F., and Olesen, N. J. (2011). Treatment of wastewater from fish slaugtherhouses. Evaluation and recommendations for hyginisation methods, National Veterinary Institute, Technical University of Denmark.
Sultan, Z., Mizuno, T., Sakurai, A., Takata, N., Okamoto, K., and Miyoshi, S.-I. (2007). Growth phase dependant activation of the precursor of Vibrio mimicus Hemolysin (Pro-VMH). J. Health Sci. 53, 430–434. doi: 10.1248/jhs.53.430
Takemura, A. F., Chien, D. M., and Polz, M. F. (2014). Associations and dynamics of vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 5:38. doi: 10.3389/fmicb.2014.00038
Tantillo, G. M., Fontanarosa, M., Pinto, A. Di, and Musti, M. (2004). Updated perspectives on emerging vibrios associated with human infections. Lett. Appl. Microbiol. 39, 117–126. doi: 10.1111/j.1472-765X.2004.01568.x
Tarr, C. L., Patel, J. S., Puhr, N. D., Sowers, E. G., Bopp, C. A., and Strockbine, N. A. (2007). Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J. Clin. Microbiol. 45, 134–140. doi: 10.1128/JCM.01544-06
Topalcengiz, Z., Strawn, L. K., and Danyluk, M. D. (2017). Microbial quality of agricultural water in Central Florida. PLoS One 12:e0174889. doi: 10.1371/journal.pone.0174889
Turner, J. W., (1997). Environmental factors and reservoir shifts contribute to the seasonality of pathogenic.
Urquhart, E. A., Jones, S. H., Yu, J. W., Schuster, B. M., Marcinkiewicz, A. L., Whistler, C. A., et al. (2016). Environmental conditions associated with elevated Vibrio parahaemolyticus concentrations in Great Bay Estuary, New Hampshire. PLoS One 11:e0155018. doi: 10.1371/journal.pone.0155018
USEPA (2001). Source Water Protection Practices Bulletin Managing Septic Systems to Prevent Contamination of Drinking Water. United States Environ. Prot. Agency i, July.
Venkateswaran, K., Kiiyukia, C., Takaki, M., Nakano, H., Matsuda, H., Kawakami, H., et al. (1989). Characterization of toxigenic vibrios isolated from the freshwater environment of Hiroshima, Japan. Appl. Environ. Microbiol. 55, 2613–2618. doi: 10.1128/AEM.55.10.2613-2618.1989
Vinothkumar, K., Kushwaha, A., and Ramamurthy, T. (2013). Triplex PCR assay for the rapid identi fi cation of 3 major Vibrio species, Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio fluvialis. Diagn. Microbiol. Infect. Dis. 76, 526–528. doi: 10.1016/j.diagmicrobio.2013.04.005
Watkins, W. D., and Cabelli, V. J. (1985). Effect of Fecal pollution on Vibrio parahaemolyticus densities in an estuarine environment. Appl. Environ. Microbiol. 49, 1307–1313. doi: 10.1128/AEM.49.5.1307-1313.1985
Wei, S., Zhao, H., Xian, Y., Hussain, M. A., and Wu, X. (2014). Multiplex PCR assays for the detection of Vibrio alginolyticus, Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae with an internal amplification control. Diagn. Microbiol. Infect. Dis. 79, 115–118. doi: 10.1016/j.diagmicrobio.2014.03.012
Wong, R. S. Y., and Chow, A. W. (2002). Identification of enteric pathogens by heat shock protein 60 kDa (HSP60) gene sequences. FEMS Microbiol. Lett. 206, 107–113. doi: 10.1111/j.1574-6968.2002.tb10994.x
Keywords: pathogenic Vibrio spp., temperature, salinity, public-health, brackish-water, freshwater
Citation: Abioye OE, Osunla AC and Okoh AI (2021) Molecular Detection and Distribution of Six Medically Important Vibrio spp. in Selected Freshwater and Brackish Water Resources in Eastern Cape Province, South Africa. Front. Microbiol. 12:617703. doi: 10.3389/fmicb.2021.617703
Edited by:
Rodrigo Gouvea Taketani, Rothamsted Research, United KingdomReviewed by:
Eiji Arakawa, National Institute of Infectious Diseases (NIID), JapanHelena D. M. Villela, Federal University of Rio de Janeiro, Brazil
Copyright © 2021 Abioye, Osunla and Okoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oluwatayo E. Abioye, abioyethayor@gmail.com
 Oluwatayo E. Abioye
Oluwatayo E. Abioye Ayodeji Charles Osunla
Ayodeji Charles Osunla Anthony I. Okoh
Anthony I. Okoh