- 1Department of Gastroenterology, Xinqiao Hospital, Army Medical University, Chongqing, China
- 2Department of Medicinal Chemistry, College of Pharmacy, Army Medical University, Chongqing, China
- 3Department of Foreign Languages, Army Medical University, Chongqing, China
- 4Institute of Cardiovascular Diseases, Xinqiao Hospital, Army Medical University, Chongqing, China
Considered as the most popular pathogen worldwide, Helicobacter pylori is intensively associated with diverse gastric diseases, including gastric ulcers, chronic progressive gastritis, and gastric cancer. Aside from its pathogenic effect on gastric diseases, growing evidences reveal that H. pylori may be related to numerous extragastric diseases. In this article, we reviewed recent studies and systematically elucidated that H. pylori may interfere with many biological processes outside the stomach and influence the occurrence of various extragastric diseases. Many epidemiological studies have indicated that H. pylori plays a pathogenic role in COVID-19, atherosclerosis, hyperemesis gravidarum and several other extragastric diseases, while the effect of H. pylori is currently under investigation in gastroesophageal reflux disease, asthma, and inflammatory bowel disease. Moreover, we also summarized the possible pathogenic mechanisms of H. pylori that may be related to chronic systemic inflammation and molecular mimicker. Taken together, this review provides a new perspective on the role of H. pylori in extragastric diseases and explores the possible mechanisms, which may help guide clinical treatment.
Introduction
Helicobacter pylori is recognized as the most popular human pathogen, which infects nearly half of the population worldwide (approximately 4.4 billion people) (Hooi et al., 2017). Exposure to H. pylori may bring about lifelong chronic progressive gastritis, and 1–10% of infected individuals will have clinical complications, including gastric intestinal metaplasia, peptic ulcer disease, atrophy of gastric mucosa, gastric cancer (GC), and mucosa-associated lymphoid tissue (MALT) lymphoma (Yamaoka, 2018). The World Health Organization (WHO) has categorized H. pylori as one of the Class 1 carcinogens (No authors listed, 1994; Plummer et al., 2015). Previous studies have mostly focused on the role of H. pylori in inflammation and tumor development of the stomach. Several clinical trials have proven that the eradication of H. pylori reduces the incidence of GC (Lee et al., 2016) and atrophic gastritis (Choi et al., 2018). Management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline in 2019 (Pimentel-Nunes et al., 2019) recommended prevention aims for H. pylori due to its role in gastric carcinogenesis, precancerous and early cancer lesions. Almost all previous clinical studies on H. pylori have suggested H. pylori eradication for patients suffering from gastric and intestinal metaplasia or chronic atrophic gastritis.
However, growing evidences reveal that H. pylori infection may be related to numerous extragastric diseases of various systems throughout the human body in addition to the pathogenetic effects on gastric diseases. For example, H. pylori has been described to be related to some blood system diseases. A separate meta-analysis of 15 observational studies proved that iron deficiency anemia (IDA) was more common among H. pylori-positive individuals than H. pylori-negative controls (OR = 2.2; 95% CI = 1.5–3.2) (Qu et al., 2010). H. pylori infection was also found to be more prevalent in adolescents suffering from IDA (Xia et al., 2012). In the reproductive system, a more significant incidence of H. pylori in pregnant women suffering from hyperemesis gravidarum was observed in a meta-analysis (Li et al., 2015). Some endocrine and metabolic diseases are also closely related to H. pylori. As shown in a meta-analysis, H. pylori-positive subjects with type 1 diabetes had a higher level of glycosylated hemoglobin than uninfected patients (Dai et al., 2015). Apart from the diseases mentioned above, H. pylori infection may also cause disorders in many other human systems (Razuka-Ebela et al., 2018). Moreover, studies on pathogenic mechanisms have shown that H. pylori can stimulate macrophages, T cells, B cells and other inflammatory cells to accelerate chronic systemic inflammation, interfere with normal physiological processes and ultimately becomes a crucial risk factor for atherosclerosis, insulin resistance, etc. (Franceschi et al., 2014). Similar antigens between H. pylori and human tissues may also lead to vitamin B deficiency, pernicious anemia and atherosclerosis (Chmiela and Gonciarz, 2017). The latest American College of Gastroenterology (ACG) Clinical Guideline in 2017 proposed associations between numerous extragastric disorders and H. pylori infection, aiming at raising the concern amid clinical workers to attach great importance on H. pylori and confronting these diseases in clinical practice (Chey et al., 2017).
In this article, we aim to elucidate the correlation of H. pylori and many extragastric diseases, which is necessary to refine the understanding of the pathogenic processes of H. pylori and help improve clinical prognosis and guide management. We reviewed latest studies and found that H. pylori may be associated with several extragastric diseases of various systems throughout the human body. In addition, we also explored the promising pathogenic mechanisms of H. pylori infection. Ultimately, we sought to improve and refine clinical guidelines and benefit patients suffering from the mentioned extragastric diseases and H. pylori infection.
Respiratory disease
The relation of H. pylori infection with asthma has attracted extensive attention. For example, Zuo et al. (2021) found that H. pylori had a protective effect on allergic asthma by regulating Thl7/Tregs and the Th1/Th2 balance, reducing HSP70 and DCs, stimulating TLRs, and inhibiting gastroesophageal reflux. There are three well-known hypotheses related to the pathogenesis, including the gut-lung axis theory, the “disappearing microbiota” hypothesis and the hygiene hypothesis, all of them supporting the protective effect of H. pylori. In addition, therapeutic products made by H. pylori (such as H. pylori extract) have also been utilized to treat and prevent asthma. Perinatal H. pylori exposure reduced inflammation of the allergic airway in the offspring as well, providing a promising target for interventional therapy of asthma (Zuo et al., 2021). H. pylori can modulate anti-Th2 inflammation activity through neutrophil-activating protein (NAP) and contribute to allergic asthma, and purifying rNAP before sensitization can significantly reduce the accumulation of eosinophils in the lung tissue of asthmatic mice. It is worth noting that H. pylori treatment decreases the levels of IL-4, IL-13, and serological IgE, and increases the levels of IL-10 and IFN-γ (Zhou et al., 2017). This study suggests that eradication of H. pylori may have a preventive effect on the suppression of allergic asthma. However, it was not supported that H. pylori or its specific antigens provided protective antigens that reduced the occurrence of allergic asthma in a meta-analysis (Miftahussurur et al., 2017). Similarly, another cohort study published in 2017 showed that H. pylori was significantly associated with a 1.38-fold increased risk of asthma in adults. In addition, the risk of asthma in adults with H. pylori infection was still 1.85 times higher than that in H. pylori uninfected people (Wang et al., 2017). Thus, the protective effect of H. pylori on allergic asthma is controversial.
Helicobacter pylori may also promote the progression and evolution of chronic obstructive pulmonary disease (COPD). H. pylori-positive subjects showed a lower FEV1 (L) at baseline than H. pylori-negative patients, although no significant discrepancy in the decline rate between the two groups (p-value = 0.35) was shown (Sze et al., 2015). Socioeconomic status (SES) is a prognostic indicator for COPD. Interestingly, this study also found that years of education (on behalf of SES during childhood) were intensively associated with H. pylori status and might have effects on adult height. However, no significant difference was found in H. pylori seropositivity between individuals with GOLD 1 (global initiative for chronic obstructive lung disease) and GOLD 2 severity (Sze et al., 2015). A cohort study involving 3,619 subjects showed that neither H. pylori infection nor eradication treatment was related to COPD progression or lung dysfunction on a general population health screen. In summary, H. pylori may not be an intensively aggravated factor in lung function or COPD (Lee et al., 2020).
It is worth noting that H. pylori infection may also be associated with COVID-19. A large number of emerging results show that people infected with H. pylori may be more vulnerable to severe form of COVID-19 (Balamtekin et al., 2021). Besides, the inflammatory activation caused by H. pylori infection may enhance the respiratory inflammatory response of COVID-19, recruit inflammatory cells and promote sustained production of TNF-α, IL-8, and IL-1β, as well as endothelial dysfunction markers such as V-CAM and ICAM, leading to subsequent virus-mediated acute lung injury. H. pylori may also aggravate acute respiratory distress syndrome (ARDS), which is a serious complication threatening numerous COVID-19 patients (Gonzalez et al., 2022). However, there was no significant difference in loss of smell, dyspnea, fever, and dry cough between COVID-19 patients with or without H. pylori infection. At present, there is no evidence showing that H. pylori infection significantly increases the risk of chronic pulmonary fibrosis and COPD among patients with COVID-19 (Balamtekin et al., 2021). The possible reason may be that H. pylori infection only affects the acute progression of COVID-19, but not the chronic course.
Studies have found that H. pylori pathogen-derived proteins (such as VacA) are found in lung biopsy specimens and bronchoalveolar lavage fluid of lung cancer. These proteins can aggravate the progress of airway diseases, promote the H. pylori infection inflammatory status (anti-H. pylori IgG and IgM) and recruit B cells, and finally accelerate the occurrence of lung cancer. Besides, eradication of H. pylori was significantly correlated with the decrease of lung cancer marker CEA. This explained that H. pylori may be of benefit for the treatment of lung cancer (Xu et al., 2018a). Of concern, there is a currently ongoing clinical trial investigating the association between H. pylori strain specific blood biomarkers and lung cancer risk (PLCO2019-1026), which may help understand of H. pylori infection and lung cancer risk, identify markers for lung cancer risk, and provide new information for a feasible cancer prevention strategy.
Although recent studies suggested an association between H. pylori infection and respiratory diseases, further studies are necessary to confirm a causal relationship. Moreover, the roles of other risk factors, such as air pollution or smoking habits, as well as the latent molecular mechanisms should also be considered (GonzAlez et al., 2018; Supplementary Table S1).
Heart and circulatory disease
The association of H. pylori infection with coronary artery disease has also been investigated. One study showed that H. pylori infection significantly reduced endothelium-dependent flow-mediated vasodilation in a young group and strongly repressed acetylcholine-induced endothelium-dependent aortic relaxation without altering nitroglycerin-induced endothelium-dependent vascular relaxation in mice. In addition, H. pylori eradication in both human subjects and mice obviously improved endothelium-dependent vasodilation (Xia et al., 2020). Infection with serum CagA+ H. pylori can induce cardiovascular disease and coronary heart disease (Sharma and Aggarwal, 2015). Mechanisms by which CagA+ H. pylori causes atherosclerosis include increasing the production of COX-1/2 from the vascular endothelium, thereby stimulating the synthesis of thromboxane A2 (TXA2) and prostaglandin to induce platelet aggregation. In addition, H. pylori releases many cytokines, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), IL-1 and free radicals, causing atherosclerosis and oxidative stress. Furthermore, an aberrant immune reaction is considered to play a role in atherosclerotic plaque rupture and destabilization by the cross-reactivity between antibodies and CagA vascular wall antigens (de Boer et al., 2000; Byrne et al., 2003; Guo et al., 2007; Feletou et al., 2011). Therefore, as H. pylori infection can lead to endothelial dysfunction, dyslipidemia and hyperhomocysteinemia, H. pylori eradication therapy is recommended as a possible secondary cardiovascular prevention strategy (Zuin et al., 2016).
Myocardial infarction (MI) is the most dire and serious outcome for patients with CAD due to its fatal influence on survival quality. A meta-analysis including more than 20,000 subjects and 26 studies found that H. pylori infection is a risk factor for MI, even among young participants (Liu et al., 2015).
A cohort study that included 12,836 participants showed that H. pylori may also significantly increase the risk of carotid atherosclerosis in Chinese men under 50 years old (Zhang et al., 2019). Another study indicated that non-alcoholic fatty liver disease (NAFLD) caused by infection with H. pylori increases the formation of carotid artery plaques (Yu L. Y. et al., 2019).
After adjusting for potential cofactors, a trial that included 5,168 study participants revealed an association between high blood pressure and H. pylori. In this study, H. pylori was related to an increased risk of hypertension (95% CI = 1.04–1.46; OR = 1.23). Compared with individuals without H. pylori infection, infected subjects showed a 0.735 mmHg increase in diastolic blood pressure (95% CI = 0.101–1.369) and a 0.723 mmHg increase in mean arterial pressure (95% CI = 0.034–1.413) (Wan et al., 2018; Supplementary Table S1).
Digestive disease
Eosinophilic esophagitis (EoE) is a kind of disease mediated by the immune response. A meta-analysis by Doulberis et al. (2020a) revealed that H. pylori infection is one of the protective factors against EoE. However, in 2018, a prospective case–control study conducted in 23 centers reported that H. pylori was not negatively associated with EoE, neither in adults nor in children (Molina-Infante et al., 2018). Thus, the effect of H. pylori infection on EoE still needs further study.
In developing countries, esophageal squamous cell carcinoma is a prevalent esophageal disorder. Currently, there is no definite evidence showing that H. pylori infection contributes to the incidence of esophageal squamous cell carcinoma. A meta-analysis of 35 studies with 345,886 participants indicated that there was no crucial association between esophageal squamous cell carcinoma and H. pylori infection (Gao et al., 2019). However, a study that included 95 esophageal squamous cell carcinoma patients showed a statistically significant negative association between esophageal squamous cell carcinoma and H. pylori infection via testing gastric biopsy materials from the patients (Poyrazoglu et al., 2017).
Some studies have proposed a different relationship between H. pylori and gastroesophageal reflux disease (GERD). An analysis of GERD patients found a higher prevalence of H. pylori infection among patients with peptic ulcers (Jie et al., 2019). In contrast, a prospective clinical study of 124 patients with GERD, revealed that H. pylori infection reduced esophageal acid exposure, enhanced lower esophageal sphincter pressure, and improved esophageal peristalsis. Thus, H. pylori may be protective factors for GERD (Liu et al., 2018). However, interestingly, H. pylori eradication did not increase the incidence of GERD. In summary, more studies are needed to determine this pathogenesis.
Several clinical trials have found a relationship between hepatocellular carcinoma (HCC) and H. pylori, which was detected in liver samples from individuals with HCC, but this presence cannot support a definite causal relationship (Okushin et al., 2018).
Cholelithiasis and chronic cholecystitis are quite prevalent worldwide. A meta-analysis found that the chronic cholecystitis/cholelithiasis group was more prevalent in H. pylori infected gallbladder than the control group in 17 studies (Wang et al., 2021).
The supposed role of H. pylori infection in gallstones and gallbladder polyps is still debated. A retrospective study showed that H. pylori infection was related to gallstones and gallbladder polyps in a Chinese population (Xu et al., 2018b), whereas this relation was not supported in another case–control matched study of a Chinese population (Zhang et al., 2020). Thus, the role of H. pylori in cholecystic polyps and gallstones requires further research.
Non-alcoholic fatty liver disease is a kind of liver injury that is induced by metabolic stress. A meta-analysis of 21 studies indicated that H. pylori infection was one of the factors contributing to NAFLD progression in the Asian population (Liu et al., 2019), but H. pylori infection was not an independent risk factor for NAFLD revealed by a cross-sectional study in China (Fan et al., 2018). One hypothesis is that H. pylori infection may cause chronic low-level systemic inflammation, which increases the concentration of inflammatory cytokines, such as IL-6 and TNF-α, stimulating IKK/NF-κB signaling and leading to insulin resistance. H. pylori infection may also restrain leptin release from white adipose tissue, which in turn leads to liver stearoyl-CoA desaturase, thereby stimulating fat and VLDL-C deposition in liver tissue. Another hypothesis is that H. pylori infection may cause dysbiosis of gastrointestinal flora, increase serum lipopolysaccharide, accelerate the systemic inflammatory response and increase the expression of IL-6, TNF-α, and C-reactive protein, which results in reduced lipoprotein activity followed by dyslipidemia (Cheng et al., 2017). Notably, an ongoing clinical study may contribute to reveal the risk of NAFLD due to H. pylori infection by investigating the genome-wide association of H. pylori infection (PLCO-989).
Helicobacter pylori infection might play a protective role in inflammatory bowel disease (IBD) reported by a meta-analysis (Imawana et al., 2020). Besides, another meta-analysis of clinical studies including 1,748 individuals, also indicated an association between CagA seropositivity and lower odds of IBD (Tepler et al., 2019; Supplementary Table S2).
Viral hepatis has also been found to be related to H. pylori infection. Esmat et al. (2012) found the existence of CagA gene of H. pylori in liver samples of patients with hepatitis C virus (HCV)-related chronic hepatitis. A multivariate analysis further indicated that positive anti-H. pylori antibody was independently and significantly related to cirrhosis in individuals with HCV-related chronic hepatitis (Queiroz et al., 2006). Moreover, clinical reports also suggested an association between H. pylori and HBV-related liver diseases. A meta-analysis of a Chinese population demonstrated that the infection rate of H. pylori in patients with HBV-related liver diseases had a positive relation with the increase of disease severity. In addition, the rate of H. pylori positivity in chronic HBV patients was 2.44-fold higher than that in healthy controls (Wang et al., 2016). Therefore, the prevalence of H. pylori may promote the progression of HBV-related liver diseases. However, the relationship between H. pylori infection and HAV is usually overestimated by confounding factors such as socio-economic status and age, and eliminating interference of these factors would reduce this correlation (BinSaeed, 2010).
It has been found that H. pylori can interact with the gut microbiome and affect extragastric diseases progression. Heimesaat et al. (2014) found that with long-term H. pylori infection, gut microbiome showed a lower level of Lactobacillus spp. and a significant higher loads of E. coli, Bacteroides/Prevotella spp., and Enterococcus spp. than H. pylori-negative subjects. In addition, H. pylori permits more microorganisms to pass through the gastric acid barrier and colonize the distal gut, increasing gut microbiota diversity through hypergastrinemia and hypochlorhydria (Lopetuso et al., 2014). Subsequently, low level of beneficial gut bacteria (such as Lactobacillus spp.) may lead to the proliferation of some harmful bacteria and damage gut barrier function. This also causes the immune imbalance and mediates several chronic inflammatory diseases mentioned above (Sanders et al., 2019). Furthermore, H. pylori infection-related gut microbiome alternation may decrease insulin sensitivity and lead to diabetes, and may also lead to abnormal lipid metabolism, increasing the risk of NAFLD (He et al., 2016). Recovery of gut microbiome balance was observed after H. pylori eradication (Chen et al., 2021). Taken together, H. pylori can induce the gut microbiome alternation and lead to the progression of several extragastric diseases (Figure 1).
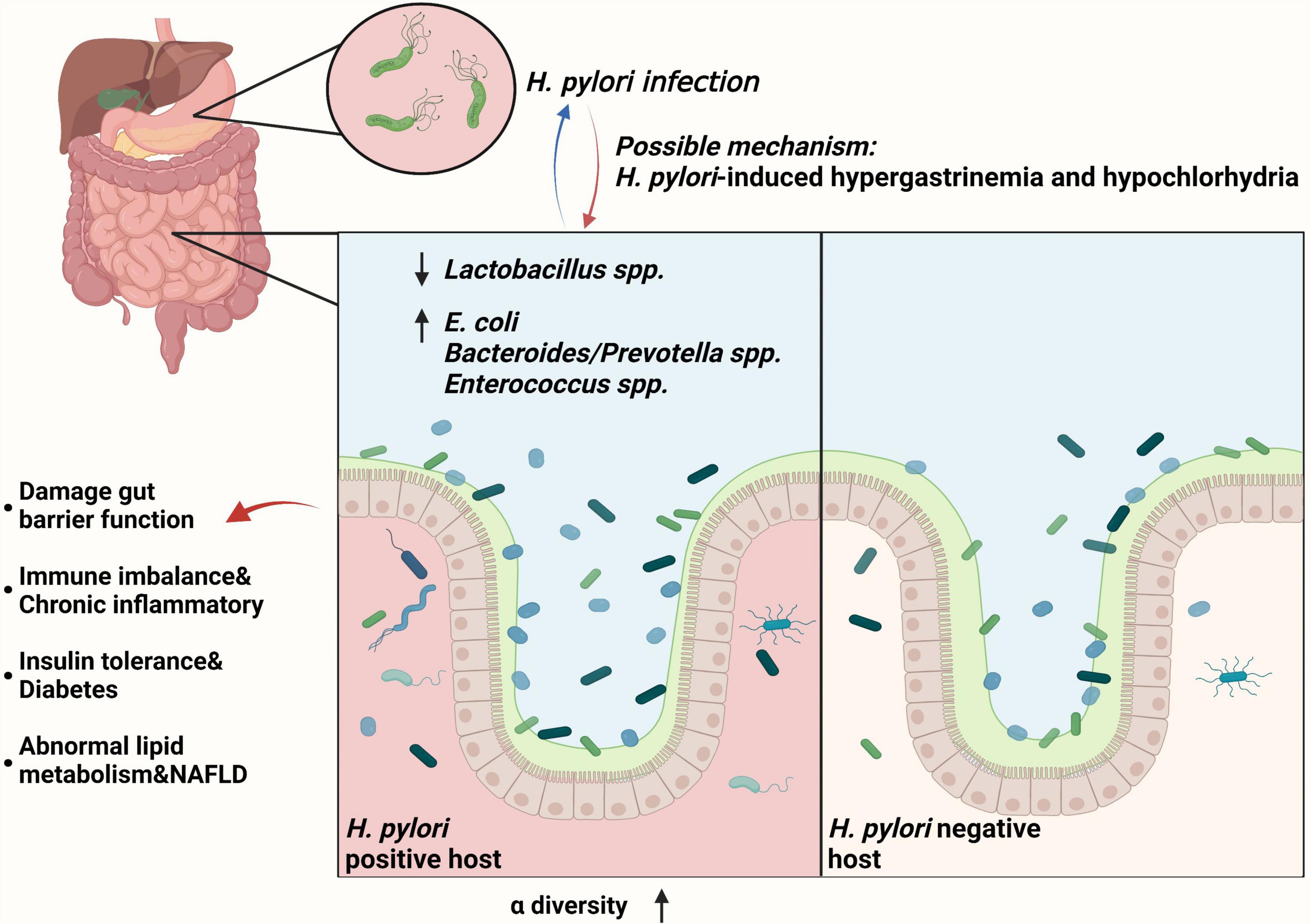
Figure 1. Helicobacter pylori infection can interact with the gut microbiome and affect extragastric diseases progression. With chronic H. pylori infection, gut microbiome showed a lower level of Lactobacillus spp. and a significant higher loads of E. coli, Bacteroides/Prevotella spp., and Enterococcus spp., increasing α-diversity of gut microbiota (Heimesaat et al., 2014). For the mechanism, gut microbiota changes may be triggered by H. pylori-induced gastric immune pathogenesis, including hypergastrinemia and hypochlorhydria (Lopetuso et al., 2014). Subsequently, low level of beneficial gut bacteria (such as Lactobacillus spp.) may lead to the proliferation of some harmful bacteria and damage gut barrier function (Sanders et al., 2019). This also leads to the immune imbalance and chronic inflammatory, insulin tolerance and diabetes, and abnormal lipid metabolism and NAFLD (He et al., 2016).
Blood system disease
It is found that H. pylori infection is closely related to MALT lymphoma. H. pylori-induced T cells can promote macrophages to secrete APRIL, which is an important cytokine that promotes the progression of MALT lymphoma (Planelles et al., 2008; Zhang et al., 2015). H. pylori may also directly drive CagA protein into B cells, leading to increased Bcl-2 expression, activating extracellular signal-regulated kinase and inhibiting apoptosis, which finally promote MALT lymphoma progression (Kuo et al., 2014). Besides, CagA+ H. pylori-infected MALT lymphoma patients significantly delayed the progression of MALT lymphoma after H. pylori eradication treatment (Kuo et al., 2014). Infected by H. pylori, normal B cells are driven into malignant clones by three kinds of chromosomal translocations, t (14;18) (q32; q21), t (1;14) (p22; q32), and t (11;18) (q21; q21), activating NF-κB signaling and regulating apoptosis, inflammation, and immunity (Bertoni and Zucca, 2006; Ruskone-Fourmestraux et al., 2011; Bautista-Quach et al., 2012; Zullo et al., 2014). Among them, t (Dai et al., 2015; Miftahussurur et al., 2017) (q21; q21) may be conducive to the occurrence of MALT lymphoma (Streubel et al., 2006).
Many studies have proven that H. pylori infection leads to IDA. The Maastricht III European guidelines for people with unknown sarcopenic anemia recommend an H. pylori infection test and germ eradicate therapy (Malfertheiner et al., 2007). Flores et al. (2017) found that CagA protein is significant in alteration of iron metabolism in gastric adenocarcinoma cells of H. pylori-infected humans, and this is mediated by transferrin endocytosis and increasing iron uptake.
It has been reported that the lack of vitamin B12 absorption contributes to pernicious anemia and H. pylori also plays a role in this process. H. pylori infection changes intragastric pH, leading to vitamin B12 malabsorption (Cohen et al., 2000). In addition, H. pylori may also evoke an antigen similar to antibodies against the H+K+-adenosine triphosphate protein to inhibit vitamin B12 absorption (Claeys et al., 1998). Besides, an ongoing clinical study may help reveal the risk of vitamin B12 deficiency due to H. pylori infection by investigating the genome-wide association of H. pylori infection (PLCO-989).
The role of H. pylori in Idiopathic or Immune Thrombocytopenic Purpura (ITP) has also been investigated. A meta-analysis of six studies involving 241 patients proved that H. pylori eradication is an effective treatment for ITP patients (Kim B. J. et al., 2018). Lei et al. (2021) reported that H. pylori can promote platelet destruction in mice, and the mechanisms may be related to activating NF-κB/IL-17 signaling.
Antiphospholipid syndrome is characterized by both venous and arterial thrombosis, and often leads to abortions, premature birth, and preeclampsia. Cicconi et al. (2001) reported that after the eradication of H. pylori, the antiphospholipid syndrome of a case disappeared (Supplementary Table S3).
Endocrine and metabolic disease
Diabetes is the most prevalent metabolic disorder worldwide, killing approximately four million people each year. A meta-analysis of 9,559 individuals found that the effects of H. pylori on type 1 and 2 diabetes and diabetes mellitus (both types) were 1.19 (95% CI = 0.98–1.45), 1.43 (95% CI = 1.11–1.85) and 1.17 (95% CI = 0.94–1.45), respectively, indicating that H. pylori-infected individuals would have a higher risk of diabetes. According to an analysis of geographical subpopulation regions, the infection risk of H. pylori in the Asian population was slightly higher than that in other populations (Mansori et al., 2020). In contrast, a cross-sectional study showed that there was no significant correlation between H. pylori and diabetes, though it has been estimated that H. pylori may be associated with an increased risk of diabetes in Chinese females (Man et al., 2020). Moreover, an ongoing clinical study may help reveal the association between diabetes and H. pylori infection by investigating the genome-wide association of H. pylori infection (PLCO-989).
Obesity has become a crucial public health problem. The impact of H. pylori on obesity or overweight is still unclear. A meta-analysis including 22 articles and 178,033 samples showed that obesity was associated with H. pylori, which may increase the risk of obesity (OR = 1.2) (Xu et al., 2019). However, from a retrospective study of 3,039 subjects, H. pylori was not related to obesity or overweight observed in a Chinese population (P = 0.321) (Xu et al., 2017). More investigation of the relationship between H. pylori infection and obesity are still needed.
The relation of H. pylori with autoimmune thyroid diseases (AITDs) also needs more research to clarify. A meta-analysis of 15 articles that included 3,046 cases showed that H. pylori was positively correlated with HT and GD (HT: 95% CI: 1.44–3.23, OR = 2.16; GD: 95% CI: 1.68–4.61, OR = 2.78), and CagA+ H. pylori was positively related to AITD (95% CI: 1.07–3.70, OR = 1.99) (Hou et al., 2017). Nevertheless, another study proposed that this pathogenesis might be caused by molecular mimics and an increased inflammatory state (Figura et al., 2019; Supplementary Table S3).
Nerve disease
Alzheimer’s disease (AD), as a kind of nerve disease characterized by neurodegeneration, has also been studied for a possible association with H. pylori infection. Beydoun et al. (2018) found a direct relationship between AD mortality and H. pylori seropositivity in their retrospective cohort study that included 16,970 participants. In addition, a systematic study also revealed that AD may be associated with gastrointestinal microbiota dominated by H. pylori (Katsinelos et al., 2019).
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world. Although the pathogenesis of PD remains unclear, H. pylori eradication was found to intensively improve the clinical symptoms of PD in a prospective cohort study. H. pylori eradication not only increased the normal motor function time (also known as ‘on’ time) of the day, but also improved gastrointestinal symptoms and reduced fatigue symptoms (Lolekha et al., 2021). Another case–control study found that the positive serum of H. pylori was related to the adverse reaction and higher dosage of levodopa, and H. pylori eradication improved the prognosis of patients (Mridula et al., 2017). A meta-analysis of 13 studies also found that H. pylori infection was significantly associated with adverse drug response, higher levodopa equivalent daily dose (LEDD) and severer motor symptoms in PD patients (Zhong et al., 2022).
A descriptive analytical cross-sectional study in Iran showed that H. pylori was related to the etiology of restless legs syndrome (RLS). Proinflammatory cytokines released by H. pylori infection, such as IL-6, have been shown to increase production of hepcidin, which affects iron transport in healthy human, resulting in an iron deficiency in the CNS and causing RLS (Rezvani et al., 2018).
The etiology of multiple sclerosis (MS) is the complex interaction of environmental and genetic factors. Bacterial exposure has been identified as one of the many pathogenic factors of MS (Cossu et al., 2018). As shown in a meta-analysis conducted in Western countries, the presence of bacteria was negatively correlated with MS (Jaruvongvanich et al., 2016). In Asian countries, H. pylori antigen antibodies were more common in patients with aquaporin 4 antibody-positive neuromyelitis optica spectrum disorders (NMOSDs) but negative in patients with MS (Yoshimura et al., 2013). The above results suggested that H. pylori may be a protective factor by manipulating pattern-recognition receptors (PRRs) (Efthymiou et al., 2017) and inhibiting Th1/Th17-cell responses (Salama et al., 2013). A recent seroprevalence study showed that antibodies against VacA were frequently detected in patients with secondary progressive MS (Efthymiou et al., 2017). Aside from the local role of H. pylori, the direct regulation was observed in the brain-intestinal axis (Kountouras et al., 2015).
Guillain–Barré syndrome (GBS) is a serious peripheral nerve autoimmune demyelinating disease that often occurs after bacterial infection. A meta-analysis revealed that there was an intensive relationship between GBS and H. pylori antibodies, especially in cerebrospinal fluid, suggesting that H. pylori is significant in GBS pathophysiology (Dardiotis et al., 2020; Supplementary Table S4).
Ophthalmic disease
Glaucoma is a leading cause of blindness worldwide. A meta-analysis that included 15 studies and 2,664 participants found that H. pylori infection was associated with non-heterogeneous glaucoma (Doulberis et al., 2020b). Following H. pylori eradication therapy, a significant (p = 0.005) reduction in intraocular pressure (IOP) was found after 2 months of follow-up, showing that H. pylori eradication may be positive in glaucoma therapy (Ala et al., 2020).
A meta-analysis found a higher H. pylori prevalence among central serous chorioretinopathy (CSR) patients (Bagheri et al., 2017). In addition, some studies have indicated that CagA antigen antibodies might cross-react with vascular endothelial antigens to promote the occurrence of vascular wall injury and atherosclerosis (Franceschi et al., 2002). As atherosclerosis is one of the most significant risk factors for CSR, H. pylori may play a pathogenic role in CSR and injure the vascular endothelium through similar antigens and cross-reactivity (Supplementary Table S5).
Dermatological disease
Alopecia areata is an inflammatory alopecia mediated by immunity that appears in all age and ethnic groups. The results of a case–control study including 162 examples showed that H. pylori infection may have a pathogenic effect on alopecia areata (Behrangi et al., 2017). H. pylori can promote chronic immune responses and local inflammatory, leading to sustained release of inflammatory mediators including PAF, LTC4, IFN-γ, TNF-α, and IL-1. These mediators may contribute to the occurrence of alopecia areata.
Besides, a meta-analysis of 11 studies and 1,741 examples revealed that H. pylori was also associated with psoriasis and that H. pylori+ individuals had a higher score on the Psoriasis Area and Severity Index (PASI) (Yu M. et al., 2019). However, a population-based longitudinal cohort study found no correlation between H. pylori and psoriasis (Wu et al., 2020). Thus, more studies are necessary to determine the relationship between psoriasis and H. pylori.
Similarly, a meta-analysis of 27 studies confirmed that H. pylori was related to the rosacea process (Yang, 2018), and H. pylori-infected individuals had a higher risk of suffering from rosacea.
Urticaria, a prevalent dermatological disease has also been reported to have a relation with H. pylori. Some studies found that the level of H. pylori antigens in individuals with chronic urticaria was significantly higher than that in controls. The eradication of H. pylori alleviated the symptoms of these patients, which supported an impact of H. pylori on pathogenesis (Erdem et al., 2020; Supplementary Table S5).
Urinary disease
Helicobacter pylori is significantly related to immunoglobulin A (IgA) nephropathy, membranous nephropathy, Henoch–Schonlein purpura nephritis, diabetic nephropathy and other urinary diseases (Moriyama et al., 2007). H. pylori antigens were found in pathological tissues of these diseases (Li et al., 2013). A study indicated that H. pylori was probably a risk factor for kidney damage in patients with H. pylori+ peptic ulcers, and eradication of H. pylori may alleviate kidney damage and prevent chronic processes (Pan et al., 2019). Another study revealed that H. pylori infection may lead to a strong mucosal immune response and play a pathogenic role in IgA nephropathy based on renal tubular injury (Zhu et al., 2016; Supplementary Table S5).
Reproductive disease
Previous studies have revealed that in men with fertility problems, the prevalence of H. pylori was much higher. Some immunocytochemical studies emphasized that serum samples from infected men (as well as anti-H. pylori hyperimmune serum) reacted with the equatorial segment and the flagella (especially abundant in tubulin) of sperm (Figura et al., 2002). However, in 2020, a cross-sectional study found that there was no difference in anti-Müllerian hormone (AMH) levels and sperm parameters in Chinese patients based on H. pylori infection history (Feng et al., 2020).
A cohort study showed that there was no significant relationship between subsequent prostate cancer risk and H. pylori-infected peptic ulcers (Fang et al., 2020). To date, the relationship of prostate cancer (PCa), benign prostatic hyperplasia (BPH), and H. pylori needs to be further studied.
Hyperemesis gravidarum (HG) is characterized by excessive vomiting and severe nausea that begins before the end of 22 weeks of pregnancy (World Health Organization, 2016). A study showed that in the stomach of women with HG, H. pylori was more prevalent, and there was a significant positive correlation between H. pylori serum levels and HG symptoms (Bustos et al., 2017; Supplementary Table S5).
Other diseases
Laryngeal cancer is a serious disease threatening human health. A prospective controlled study found that in cases of H. pylori ureA gene-positive laryngeal cancer, 46.7–49.3% of 75 were also CagA positive. The CagA gene in laryngeal cancer greatly reduced the survival rate and increased the possibility of recurrence (Burduk, 2013).
Some studies revealed an association between oral diseases and H. pylori infection. Okuda et al. (2000) found the expression of H. pylori in the dental plaques in 12 of 54 H. pylori infected subjects. Moreover, a study reported that some oral samples expressed the H. pylori ureA gene, and the primary host of oral infection was identified as dental pulp (Iwai et al., 2019). The presence of H. pylori may be harmful to the oral environment. Recurrent aphthous stomatitis (RAS) is regarded as a recurrent painful ulcerative disease that regularly impacts mucosa in the oral cavity. Gao et al. (2021) reported a RAS case with a history of 24 years that was cured after treatment for H. pylori, indicating that eradication of H. pylori might relieve RAS symptoms and is a promising RAS therapy.
In addition to the standard drug regimen, the clinical practice of appending antidepressants to the treatment of H. pylori eradication is not quite explicit. A meta-analysis that included three RCTs, two review articles, one cohort study, four prospective studies, and eight cross-sectional studies found that individuals with functional dyspepsia who did not improve after H. pylori eradication (Al Quraan et al., 2019). Another study found that stress/anxiety/depression (SAD) and H. pylori infection were significantly prevalent in patients with functional dysplasia (FD) (Kabeer et al., 2017). A cohort study showed that in the general Chinese adult population, H. pylori infection was related to depressive symptoms in women but not men (Gu et al., 2019; Supplementary Table S5).
Discussion
Previously, H. pylori infection was mostly considered as a risk factor for gastric disorders. However, growing evidences show that H. pylori infection presents more complexity and tends to be associated with almost every system in the human body. From our perspective, H. pylori can produce many kinds of bacterial toxins and induce numerous extragastric diseases in the human body, such as asthma, COPD, ITP and psoriasis. We summarized these diseases in terms of the human system, listed them methodically in this article and showed a schematic diagram (Figure 2). Interestingly, recent studies mentioned in this review partially elucidate the potential pathogenesis of these extragastric diseases caused by H. pylori infection. We synthesized the results of these studies and proposed two promising hypotheses. (i) Since H. pylori can induce several inflammatory factors, such as IL-1/2/6/8/10, TNF-α and IFN-γ, these factors may lead to chronic low-level systemic inflammation in the human body and ultimately represent diseases. Typical disorders due to H. pylori-induced inflammatory factor turbulence include atherosclerosis, insulin resistance, blood-brain barrier damage, brain neurodegenerative disease and decreased sperm motility (Figure 3). (ii) H. pylori antigen, like the antigen components of host leads to molecular mimicker and cross-antigen reactions, which cause autoimmune attacks and relevant diseases. Typical diseases chiefly rely on this mechanism, including cross reaction between CagA antibody and vascular wall inducing atherosclerosis; H. pylori and gastric H+K+ATPase cross antigen contributes to vitamin B12 deficiency; arteriosclerosis of fundus for autoimmune reaction induces central serous choroidal retinopathy (CSR); and cross-antigen reactivity between spermatogenesis related proteins, sperm motility related proteins and H. pylori contributes to hypomotility of sperm (Figure 4). In a word, it is believed that the two hypotheses contribute to deciphering the reasons why H. pylori is associated with disorders in many systems of the human body (Franceschi et al., 2014; Chmiela and Gonciarz, 2017).

Figure 2. Summary schematic of the systematic effect of H. pylori infection. In red, the manifestations for which H. pylori infection represents a risk effect. In green, although some studies revealed that H. pylori showed a positive effect on these diseases, there are still many controversial aspects and further research is needed. In gray, we show the manifestations for which H. pylori infection shows an insignificant effect.
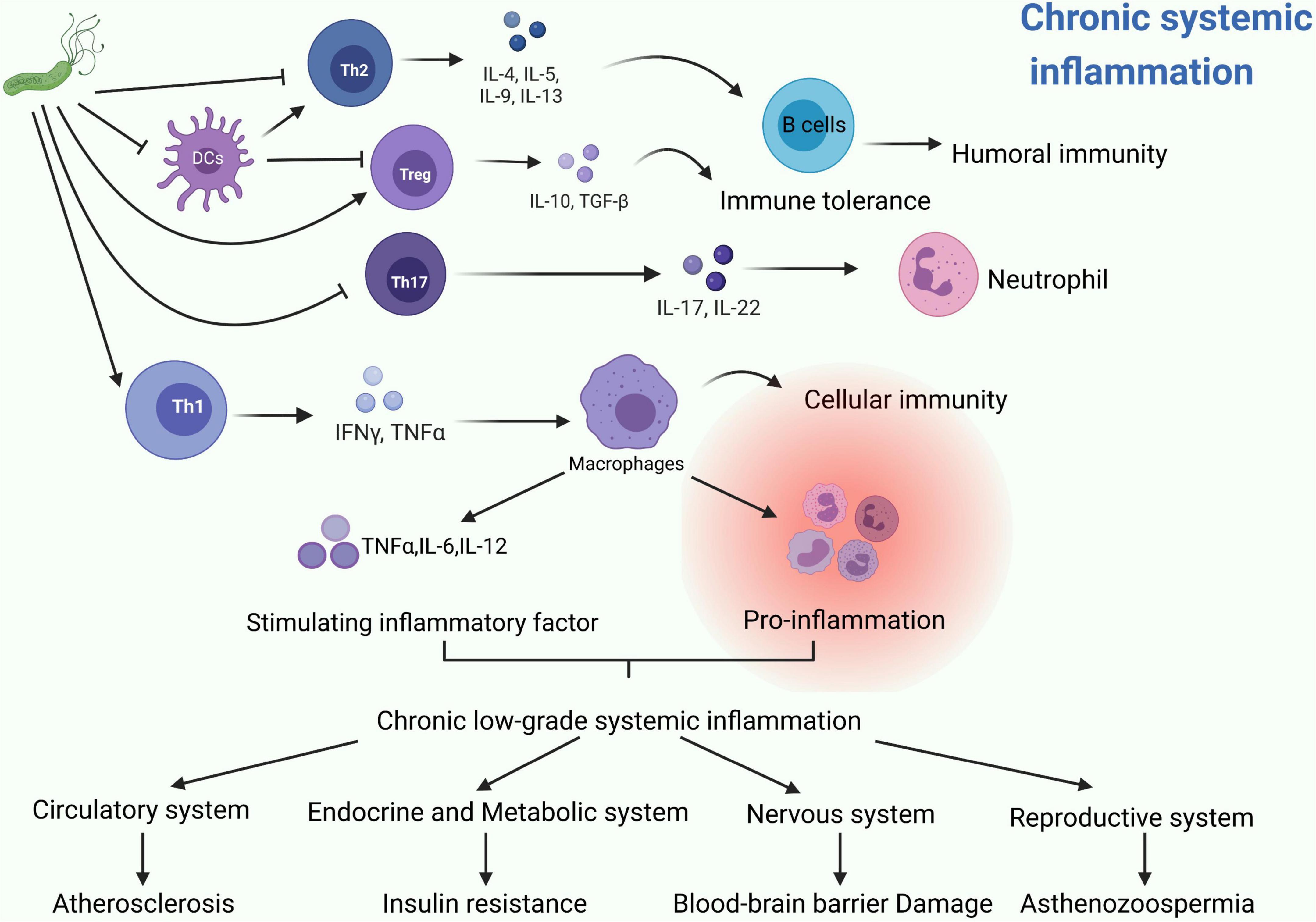
Figure 3. The possible common mechanisms by which H. pylori induces these systemic diseases can be summarized into two promising hypotheses: Hypotheses 1. Since Helicobacter pylori can induce several inflammatory factors, such as IL-1/2/6/8/10, TNF-α and IFN-γ, these factors may lead to chronic low-level systemic inflammation in the human body and ultimately represent diseases (Franceschi et al., 2014). Typical disorders due to H. pylori-induced inflammatory factors turbulence include: atherosclerosis (de Boer et al., 2000), insulin resistance (Cheng et al., 2017), blood-brain barrier damage, brain neurodegenerative disease (Efthymiou et al., 2017), and decreased sperm motility (Figura et al., 2002).
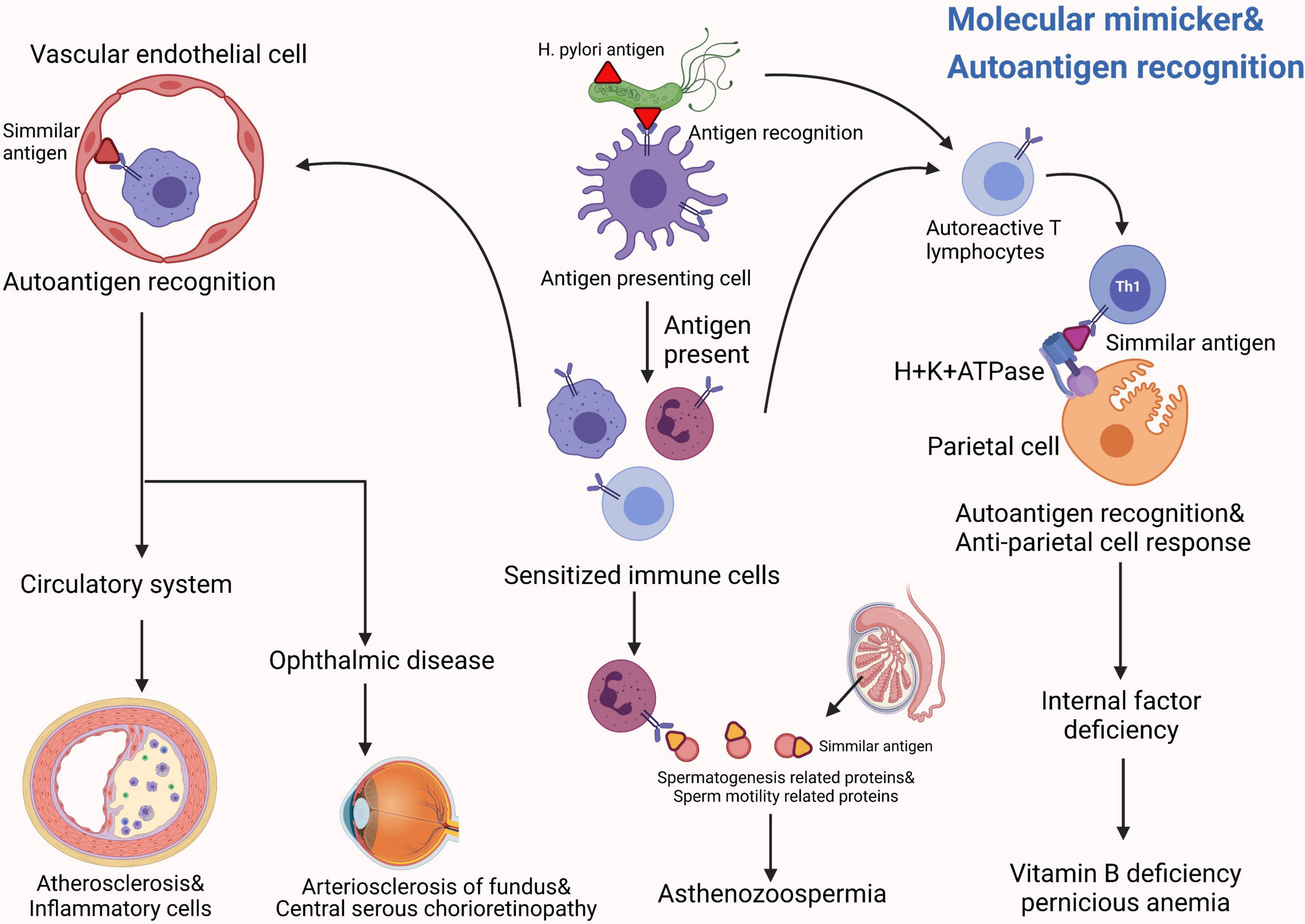
Figure 4. The possible common mechanisms by which H. pylori induces these systemic diseases can be summarized into two promising hypotheses: Hypotheses 2. H. pylori antigen, like the antigen components of host leads to molecular mimicker and cross-antigen reactions, which cause autoimmune attacks and relevant diseases (Chmiela and Gonciarz, 2017). Typical diseases that chiefly rely on this mechanism include: a cross reaction between the CagA antibody and the vascular wall induces atherosclerosis (Guo et al., 2007); H. pylori and gastric H+K+ATPase cross antigen contributes to vitamin B12 deficiency (Claeys et al., 1998); arteriosclerosis of fundus for autoimmune reaction induces central serous choroidal retinopathy (CSR) (Franceschi et al., 2002); cross-antigen reactivity between spermatogenesis-related proteins, sperm motility related proteins and H. pylori contributes to hypomotility of sperm (Figura et al., 2002).
There are still some limitations of current studies that need to be improved. First, at present, the sample size of H. pylori-related extragastric diseases in most studies is generally insufficient. Larger sample sizes and broader clinical trials are beneficial to decipher the correlation between various clinical diseases and H. pylori, and the control of confounding factors is necessary. In addition, the pathogenic effect of H. pylori in some extragastric diseases, such as gastroesophageal reflux disease, asthma, and IBD, are still controversial (Figure 2). Some studies even proposed that H. pylori may have a certain protective effect on some diseases (such as GERD) (Scida et al., 2018). And most studies are only correlation studies without explanation of causality. The proof of Evidence-based medicine is not strong enough. It also needs to clarify causality with the help of animal model research of disease and in-depth molecular mechanism research. What’s more, the hypothesis (i) about systemic inflammation is limited for the heterogeneity of participants and the control of confounding factors is often incomplete (Kim T. J. et al., 2018). Therefore, the establishment of H. pylori infection model based on specific inflammatory markers (such as CRP and PLR) and the study of inflammatory-activated pathways are of great significance to reveal the systemic effects of H. pylori. Furthermore, there is high heterogeneity in the research on the relationship between H. pylori and the development of autoimmune diseases, and the differences of their distribution patterns make the research results controversial. At present, it is found that H. pylori infection may increase susceptibility to autoimmune diseases by stimulating cell damage, chronic inflammatory, and polyclonal lymphocyte activation (Youssefi et al., 2021). Aside from that, several intervention variables, including antibiotic treatment, microbiota, and host genome polymorphism may also be involved in the self-recognition of anti-H. pylori antibodies. The pathogenesis of gastric immunity induced by H. pylori, including hypergastrinemia and hypochlorhydria, may lead to changes in gastrointestinal microbiome. Nevertheless, the exact potential mechanism needs to be further clarified to confirm the systematic effects of H. pylori infection. In addition, in current clinical practice, the first-line treatment for most H. pylori-related extragastric diseases remains H. pylori eradication. However, H. pylori treatment to prevent allergic asthma and coronary artery disease has showed promising clinical outcomes (Zuin et al., 2016; Zhou et al., 2017). Thus, it is worth exploring that H. pylori preventive control strategies may be valuable for the contribution of other extragastric diseases. In general, most of previous articles on the extragastric diseases caused by H. pylori infection have limitations on the finite sample size, unclear pathogenic mechanism, and the limitation of H. pylori detection means (Supplementary Table S6).
Helicobacter pylori infection can induce several extragastric diseases through many pathways, and different types of H. pylori may contribute to different kinds of diseases because of their specific bacterial toxins and pathogenies. It is generally accepted that the systemic effects of H. pylori infection should not be neglected. Although H. pylori has been discovered over more than 100 years ago, many aspects of H. pylori still need further studies. For clinical practitioners, the impact of H. pylori infection on extragastric diseases should be taken into more consideration.
Author contributions
PL and CH contributed to the conception of the study. JH and YL were responsible for searching the literature, creating graphical illustrations, and writing the manuscript. QO, WC, WH, and LH contributed to manuscript review and read the submitted version. All authors contributed to the review and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2018YFA0507900), the National Natural Science Foundation of China (Grant No. 81902516), and the Frontiers in Medicine Project of Xinqiao Hospital, Army Medical University (2018YQYLY010). All figures were created with biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.972777/full#supplementary-material
References
Al Quraan, A. M., Beriwal, N., Sangay, P., and Namgyal, T. (2019). The Psychotic Impact of Helicobacter pylori Gastritis and Functional Dyspepsia on Depression: A Systematic Review. Cureus. 11:e5956. doi: 10.7759/cureus.5956
Ala, S., Maleki, I., Sanjari Araghi, A., Sahebnasagh, A., and Shahraki, A. (2020). Helicobacter pylori eradication in the management of glaucoma. Caspian J. Intern. Med. 11, 143–149.
Bagheri, M., Rashe, Z., Ahoor, M. H., and Somi, M. H. (2017). Prevalence of Helicobacter pylori Infection in Patients with Central Serous Chorioretinopathy: A Review. Med. Hypothesis Discov. Innov. Ophthalmol. 6, 118–124.
Balamtekin, N., Artuk, C., Arslan, M., and Gulsen, M. (2021). The Effect of Helicobacter pylori on the Presentation and Clinical Course of Coronavirus Disease 2019 Infection. J. Pediatr. Gastroenterol. Nutr. 72, 511–513.
Bautista-Quach, M. A., Ake, C. D., Chen, M., and Wang, J. (2012). Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J. Gastrointest. Oncol. 3, 209–225.
Behrangi, E., Mansouri, P., Agah, S., Ebrahimi Daryani, N., Mokhtare, M., Azizi, Z., et al. (2017). Association between Helicobacter Pylori Infection and Alopecia Areata: A Study in Iranian Population. Middle East J. Dig. Dis. 9, 107–110.
Bertoni, F., and Zucca, E. (2006). Delving deeper into MALT lymphoma biology. J. Clin. Invest. 116, 22–26. doi: 10.1172/JCI27476
Beydoun, M. A., Beydoun, H. A., Elbejjani, M., Dore, G. A., and Zonderman, A. B. (2018). Helicobacter pylori seropositivity and its association with incident all-cause and Alzheimer’s disease dementia in large national surveys. Alzheimers Dement. 14, 1148–1158. doi: 10.1016/j.jalz.2018.04.009
BinSaeed, A. A. (2010). Is there a link between seropositivity to Helicobacter pylori and hepatitis A virus? A systematic review. Int. J. Infect Dis. 14:e567–e571. doi: 10.1016/j.ijid.2009.09.003
Burduk, P. K. (2013). Association between infection of virulence cagA gene Helicobacter pylori and laryngeal squamous cell carcinoma. Med. Sci. Monit. 19, 584–591. doi: 10.12659/MSM.889011
Bustos, M., Venkataramanan, R., and Caritis, S. (2017). Nausea and vomiting of pregnancy - What’s new? Auton. Neurosci. 202, 62–72. doi: 10.1016/j.autneu.2016.05.002
Byrne, M. F., Murphy, J. F., Corcoran, P. A., Atherton, J. C., Sheehan, K. M., Cox, D., et al. (2003). Helicobacter pylori induces cyclooxygenase-1 and cyclooxygenase-2 expression in vascular endothelial cells. Scand. J. Gastroenterol. 38, 1023–1030. doi: 10.1080/00365520310005622
Chen, C. C., Liou, J. M., Lee, Y. C., Hong, T. C., El-Omar, E. M., and Wu, M. S. (2021). The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1–22.
Cheng, D. D., He, C., Ai, H. H., Huang, Y., and Lu, N. H. (2017). The Possible Role of Helicobacter pylori Infection in Non-alcoholic Fatty Liver Disease. Front. Microbiol. 8:743. doi: 10.3389/fmicb.2017.00743
Chey, W. D., Leontiadis, G. I., Howden, C. W., and Moss, S. F. A. C. G. (2017). Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 112, 212–239.
Chmiela, M., and Gonciarz, W. (2017). Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 23, 3964–3977.
Choi, I. J., Kook, M. C., Kim, Y. I., Cho, S. J., Lee, J. Y., Kim, C. G., et al. (2018). Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 378, 1085–1095. doi: 10.1056/NEJMoa1708423
Cicconi, V., Carloni, E., Franceschi, F., Nocente, R., Silveri, N. G., Manna, R., et al. (2001). Disappearance of antiphospholipid antibodies syndrome after Helicobacter pylori eradication. Am. J. Med. 111, 163–164. doi: 10.1016/s0002-9343(01)00738-0
Claeys, D., Faller, G., Appelmelk, B. J., Negrini, R., and Kirchner, T. (1998). The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 115, 340–347. doi: 10.1016/s0016-5085(98)70200-8
Cohen, H., Weinstein, W. M., and Carmel, R. (2000). Heterogeneity of gastric histology and function in food cobalamin malabsorption: absence of atrophic gastritis and achlorhydria in some patients with severe malabsorption. Gut 47, 638–645. doi: 10.1136/gut.47.5.638
Cossu, D., Yokoyama, K., and Hattori, N. (2018). Bacteria-Host Interactions in Multiple Sclerosis. Front. Microbiol. 9:2966. doi: 10.3389/fmicb.2018.02966
Dai, Y. N., Yu, W. L., Zhu, H. T., Ding, J. X., Yu, C. H., and Li, Y. M. (2015). Is Helicobacter pylori infection associated with glycemic control in diabetics? World J. Gastroenterol. 21, 5407–5416. doi: 10.3748/wjg.v21.i17.5407
Dardiotis, E., Sokratous, M., Tsouris, Z., Siokas, V., Mentis, A. A., Aloizou, A. M., et al. (2020). Association between Helicobacter pylori infection and Guillain-Barre Syndrome: A meta-analysis. Eur. J. Clin. Invest. 50:e13218.
de Boer, O. J., van der Wal, A. C., and Becker, A. E. (2000). Atherosclerosis, inflammation, and infection. J. Pathol. 190, 237–243.
Doulberis, M., Kountouras, J., and Rogler, G. (2020a). Reconsidering the “protective” hypothesis of Helicobacter pylori infection in eosinophilic esophagitis. Ann. N.Y. Acad. Sci. 1481, 59–71. doi: 10.1111/nyas.14449
Doulberis, M., Papaefthymiou, A., Polyzos, S. A., Bargiotas, P., Liatsos, C., Srivastava, D. S., et al. (2020b). Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms 8:894. doi: 10.3390/microorganisms8060894
Efthymiou, G., Dardiotis, E., Liaskos, C., Marou, E., Tsimourtou, V., Rigopoulou, E. I., et al. (2017). Immune responses against Helicobacter pylori-specific antigens differentiate relapsing remitting from secondary progressive multiple sclerosis. Sci. Rep. 7:7929. doi: 10.1038/s41598-017-07801-9
Erdem, Y., Altunay, I., Ozkur, E., and Sivaz, O. (2020). The Etiological Evaluation of Patients with Chronic Urticaria. Sisli Etfal Hastan Tip Bul. 54, 424–427.
Esmat, G., El-Bendary, M., Zakarya, S., Ela, M. A., and Zalata, K. (2012). Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: possible association with disease progression. J. Viral Hepat. 19, 473–479.
Fan, N., Peng, L., Xia, Z., Zhang, L., Wang, Y., and Peng, Y. (2018). Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Front. Microbiol. 9:73. doi: 10.3389/fmicb.2018.00073
Fang, C. W., Chen, C. H., Muo, C. H., and Wu, S. C. (2020). Risk of subsequent prostate cancer in peptic ulcer patients who received helicobacter pylori eradication therapy: an Asian population-based cohort study. BMC Urol. 20:135. doi: 10.1186/s12894-020-00706-2
Feletou, M., Huang, Y., and Vanhoutte, P. M. (2011). Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 164, 894–912. doi: 10.1111/j.1476-5381.2011.01276.x
Feng, C., Lv, P. P., Huang, C. C., Yang, S. Q., Yao, Q. P., Shen, J. M., et al. (2020). Sperm parameters and anti-Mullerian hormone remain stable with Helicobacter pylori infection: a cross-sectional study. BMC Urol. 20:188. doi: 10.1186/s12894-020-00725-z
Figura, N., Di Cairano, G., Moretti, E., Iacoponi, F., Santucci, A., Bernardini, G., et al. (2019). Helicobacter pylori Infection and Autoimmune Thyroid Diseases: The Role of Virulent Strains. Antibiotics 9:12 doi: 10.3390/antibiotics9010012
Figura, N., Piomboni, P., Ponzetto, A., Gambera, L., Lenzi, C., Vaira, D., et al. (2002). Helicobacter pylori infection and infertility. Eur. J. Gastroenterol. Hepatol. 14, 663–669.
Flores, S. E., Aitchison, A., Day, A. S., and Keenan, J. I. (2017). Helicobacter pylori infection perturbs iron homeostasis in gastric epithelial cells. PLoS One 12:e0184026. doi: 10.1371/journal.pone.0184026
Franceschi, F., Sepulveda, A. R., Gasbarrini, A., Pola, P., Silveri, N. G., Gasbarrini, G., et al. (2002). Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation 106, 430–434. doi: 10.1161/01.cir.0000024100.90140.19
Franceschi, F., Zuccala, G., Roccarina, D., and Gasbarrini, A. (2014). Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol. 11, 234–242.
Gao, H., Li, L., Zhang, C., Tu, J., Geng, X., Wang, J., et al. (2019). Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol. Res. Pract. 2019:1953497.
Gao, Y., Gupta, N., and Abdalla, M. (2021). Recurrent Aphthous Stomatitis Improved after Eradication Therapy for Helicobacter pylori. Case Rep. Gastrointest. Med. 2021:5543838. doi: 10.1155/2021/5543838
GonzAlez, I., Araya, P., and Rojas, A. (2018). Helicobacter Pylori Infection and Lung Cancer: New Insights and Future Challenges. Zhongguo Fei Ai Za Zhi 21, 658–662. doi: 10.3779/j.issn.1009-3419.2018.09.03
Gonzalez, I., Lindner, C., Schneider, I., Morales, M. A., and Rojas, A. (2022). Inflammation at the crossroads of Helicobacter pylori and COVID-19. Future Microbiol. 17, 77–80.
Gu, Y., Zheng, L., Kumari, S., Zhang, Q., Liu, L., Meng, G., et al. (2019). The relationship between Helicobacter pylori infection and depressive symptoms in the general population in China: The TCLSIH cohort study. Helicobacter 24:e12632. doi: 10.1111/hel.12632
Guo, F. H., Yan, X. M., Fan, C. X., Zhao, F., Hu, Y., Xiao, D., et al. (2007). Cross-reactivity of anti-H pylori antibodies with membrane antigens of human erythrocytes. World J. Gastroenterol. 13, 3742–3746. doi: 10.3748/wjg.v13.i27.3742
He, C., Yang, Z., Cheng, D., Xie, C., Zhu, Y., Ge, Z., et al. (2016). Helicobacter pylori Infection Aggravates Diet-induced Insulin Resistance in Association With Gut Microbiota of Mice. EBioMedicine 12, 247–254. doi: 10.1016/j.ebiom.2016.09.010
Heimesaat, M. M., Fischer, A., Plickert, R., Wiedemann, T., Loddenkemper, C., Gobel, U. B., et al. (2014). Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS One 9:e100362. doi: 10.1371/journal.pone.0100362
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153, 420–429.
Hou, Y., Sun, W., Zhang, C., Wang, T., Guo, X., Wu, L., et al. (2017). Meta-analysis of the correlation between Helicobacter pylori infection and autoimmune thyroid diseases. Oncotarget 8, 115691–115700.
Imawana, R. A., Smith, D. R., and Goodson, M. L. (2020). The relationship between inflammatory bowel disease and Helicobacter pylori across East Asian, European and Mediterranean countries: a meta-analysis. Ann. Gastroenterol. 33, 485–494. doi: 10.20524/aog.2020.0507
Iwai, K., Watanabe, I., Yamamoto, T., Kuriyama, N., Matsui, D., Nomura, R., et al. (2019). Association between Helicobacter pylori infection and dental pulp reservoirs in Japanese adults. BMC Oral Health 19:267. doi: 10.1186/s12903-019-0967-2
Jaruvongvanich, V., Sanguankeo, A., Jaruvongvanich, S., and Upala, S. (2016). Association between Helicobacter pylori infection and multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 7, 92–97.
Jie, W., Qinghong, X., and Zhitao, C. (2019). Association of Helicobacter pylori infection with gastroesophageal reflux disease. J. Int. Med. Res. 47, 748–753.
Kabeer, K. K., Ananthakrishnan, N., Anand, C., and Balasundaram, S. (2017). Prevalence of Helicobacter Pylori Infection and Stress, Anxiety or Depression in Functional Dyspepsia and Outcome after Appropriate Intervention. J. Clin. Diagn. Res. 11, VC11–VC15.
Katsinelos, T., Doulberis, M., Polyzos, S. A., Papaefthymiou, A., Katsinelos, P., and Kountouras, J. (2019). Molecular Links Between Alzheimer’s Disease and Gastrointestinal Microbiota: Emphasis on Helicobacter pylori Infection Involvement. Curr. Mol. Med. 20, 3–12. doi: 10.2174/1566524019666190917125917
Kim, B.J., Kim, H. S., Jang, H. J., and Kim, J. H. (2018). Helicobacter pylori Eradication in Idiopathic Thrombocytopenic Purpura: A Meta-Analysis of Randomized Trials. Gastroenterol. Res. Pract. 2018:6090878. doi: 10.1155/2018/6090878
Kim, T. J., Pyo, J. H., Lee, H., Baek, S. Y., Ahn, S. H., Min, Y. W., et al. (2018). Lack of Association between Helicobacter pylori Infection and Various Markers of Systemic Inflammation in Asymptomatic Adults. Korean J. Gastroenterol. 72, 21–27.
Kountouras, J., Zavos, C., Polyzos, S. A., and Deretzi, G. (2015). The gut-brain axis: interactions between Helicobacter pylori and enteric and central nervous systems. Ann. Gastroenterol. 28:506.
Kuo, S. H., Yeh, K. H., Chen, L. T., Lin, C. W., Hsu, P. N., Hsu, C., et al. (2014). Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 4:e220. doi: 10.1038/bcj.2014.40
Lee, H. Y., Kang, H. R., Lee, J. K., Heo, E. Y., Choi, S. H., and Kim, D. K. (2020). The effect of Helicobacter pylori infection on the decline of lung function in a health screening population. Ann. Palliat. Med. 9, 3115–3122. doi: 10.21037/apm-20-850
Lee, Y. C., Chiang, T. H., Chou, C. K., Tu, Y. K., Liao, W. C., Wu, M. S., et al. (2016). Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 150, 1113–1124.e5.
Lei, H., Ma, Y., Tan, J., and Liu, Q. (2021). Helicobacter pylori Regulates the Apoptosis of Human Megakaryocyte Cells via NF-kappaB/IL-17 Signaling. Onco Targets Ther. 14, 2065–2074. doi: 10.2147/OTT.S268056
Li, L., Li, L., Zhou, X., Xiao, S., Gu, H., and Zhang, G. (2015). Helicobacter pylori Infection Is Associated with an Increased Risk of Hyperemesis Gravidarum: A Meta-Analysis. Gastroenterol. Res. Pract. 2015:278905. doi: 10.1155/2015/278905
Li, Q., Lin, X., Wu, Z., He, L., Wang, W., Cao, Q., et al. (2013). Immuno-histochemistry analysis of Helicobacter pylori antigen in renal biopsy specimens from patients with glomerulonephritis. Saudi J. Kidney Dis. Transpl. 24, 751–758. doi: 10.4103/1319-2442.113871
Liu, J., Wang, F., and Shi, S. (2015). Helicobacter pylori Infection Increase the Risk of Myocardial Infarction: A Meta-Analysis of 26 Studies Involving more than 20,000 Participants. Helicobacter 176–183. doi: 10.1111/hel.12188
Liu, L., Gao, H., Wang, H., Yu, W., Zhu, K., Zhang, Y., et al. (2018). Comparison of Esophageal Function Tests to Investigate the Effect of Helicobacter Pylori Infection on Gastroesophageal Reflux Disease (GERD). Med. Sci. Monit. 24, 4791–4797. doi: 10.12659/MSM.908051
Liu, R., Liu, Q., He, Y., Shi, W., Xu, Q., Yuan, Q., et al. (2019). Association between Helicobacter pylori infection and nonalcoholic fatty liver: A meta-analysis. Medicine 98:e17781.
Lolekha, P., Sriphanom, T., and Vilaichone, R. K. (2021). Helicobacter pylori eradication improves motor fluctuations in advanced Parkinson’s disease patients: A prospective cohort study (HP-PD trial). PLoS One 16:e0251042. doi: 10.1371/journal.pone.0251042
Lopetuso, L. R., Scaldaferri, F., Franceschi, F., and Gasbarrini, A. (2014). The gastrointestinal microbiome - functional interference between stomach and intestine. Best Pract. Res. Clin. Gastroenterol. 28, 995–1002.
Malfertheiner, P., Megraud, F., O’Morain, C., Bazzoli, F., El-Omar, E., Graham, D., et al. (2007). Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56, 772–781.
Man, S., Ma, Y., Jin, C., Lv, J., Tong, M., Wang, B., et al. (2020). Association between Helicobacter pylori Infection and Diabetes: A Cross-Sectional Study in China. J. Diabetes Res. 2020:7201379.
Mansori, K., Moradi, Y., Naderpour, S., Rashti, R., Moghaddam, A. B., Saed, L., et al. (2020). Helicobacter pylori infection as a risk factor for diabetes: a meta-analysis of case-control studies. BMC Gastroenterol. 20:77. doi: 10.1186/s12876-020-01223-0
Miftahussurur, M., Nusi, I. A., Graham, D. Y., and Yamaoka, Y. (2017). Helicobacter, Hygiene, Atopy, and Asthma. Front. Microbiol. 8:1034. doi: 10.3389/fmicb.2017.01034
Molina-Infante, J., Gutierrez-Junquera, C., Savarino, E., Penagini, R., Modolell, I., Bartolo, O., et al. (2018). Helicobacter pylori infection does not protect against eosinophilic esophagitis: results from a large multicenter case-control study. Am. J. Gastroenterol. 113, 972–979. doi: 10.1038/s41395-018-0035-6
Moriyama, T., Kaneko, T., Fujii, M., Tsubakihara, Y., Kawano, S., and Imai, E. (2007). High prevalence of Helicobacter pylori infection in Japanese patients with membranous nephropathy. Aliment. Pharmacol. Therapeutics 24, 189–193.
Mridula, K. R., Borgohain, R., Chandrasekhar Reddy, V., Bandaru, V., and Suryaprabha, T. (2017). Association of Helicobacter pylori with Parkinson’s Disease. J. Clin. Neurol. 13, 181–186.
No authors listed (1994). Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241.
Okuda, K., Ishihara, K., Miura, T., Katakura, A., Noma, H., and Ebihara, Y. (2000). Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol. Immunol. 44, 385–388. doi: 10.1111/j.1348-0421.2000.tb02510.x
Okushin, K., Tsutsumi, T., Ikeuchi, K., Kado, A., Enooku, K., Fujinaga, H., et al. (2018). Helicobacter pylori infection and liver diseases: Epidemiology and insights into pathogenesis. World J. Gastroenterol. 24, 3617–3625. doi: 10.3748/wjg.v24.i32.3617
Pan, W., Zhang, H., Wang, L., Zhu, T., Chen, B., and Fan, J. (2019). Association between Helicobacter pylori infection and kidney damage in patients with peptic ulcer. Ren. Fail 41, 1028–1034.
Pimentel-Nunes, P., Libanio, D., Marcos-Pinto, R., Areia, M., Leja, M., Esposito, G., et al. (2019). Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 51, 365–388. doi: 10.1055/a-0859-1883
Planelles, L., Medema, J. P., Hahne, M., and Hardenberg, G. (2008). The expanding role of APRIL in cancer and immunity. Curr. Mol. Med. 8, 829–844.
Plummer, M., Franceschi, S., Vignat, J., Forman, D., and de Martel, C. (2015). Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 136, 487–490. doi: 10.1002/ijc.28999
Poyrazoglu, O. B., Dulger, A. C., and Gultepe, B. S. (2017). Helicobacter Pylory infection in patients with esophageal squamous cell carcinoma. Clinics 72, 150–153. doi: 10.6061/clinics/2017(03)04
Qu, X. H., Huang, X. L., Xiong, P., Zhu, C. Y., Huang, Y. L., Lu, L. G., et al. (2010). Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J. Gastroenterol. 16, 886–896. doi: 10.3748/wjg.v16.i7.886
Queiroz, D. M., Rocha, A. M., Rocha, G. A., Cinque, S. M., Oliveira, A. G., Godoy, A., et al. (2006). Association between Helicobacter pylori infection and cirrhosis in patients with chronic hepatitis C virus. Dig Dis. Sci. 51, 370–373.
Razuka-Ebela, D., Giupponi, B., and Franceschi, F. (2018). Helicobacter pylori and extragastric diseases. Helicobacter 23:e12520.
Rezvani, F., Sayadnasiri, M., and Rezaei, O. (2018). Restless legs syndrome in patients infected with Helicobacter pylori. Neurol. Res. 40, 581–585.
Ruskone-Fourmestraux, A., Fischbach, W., Aleman, B. M., Boot, H., Du, M. Q., Megraud, F., et al. (2011). EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 60, 747–758. doi: 10.1136/gut.2010.224949
Salama, N. R., Hartung, M. L., and Muller, A. (2013). Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 11, 385–399. doi: 10.1038/nrmicro3016
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., and Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616.
Scida, S., Russo, M., Miraglia, C., Leandro, G., Franzoni, L., Meschi, T., et al. (2018). Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 89, 40–43.
Sharma, V., and Aggarwal, A. (2015). Helicobacter pylori: Does it add to risk of coronary artery disease. World J. Cardiol. 7, 19–25. doi: 10.4330/wjc.v7.i1.19
Streubel, B., Seitz, G., Stolte, M., Birner, P., Chott, A., and Raderer, M. M. A. L. T. (2006). lymphoma associated genetic aberrations occur at different frequencies in primary and secondary intestinal MALT lymphomas. Gut 55, 1581–1585. doi: 10.1136/gut.2005.090076
Sze, M. A., Chen, Y. W., Tam, S., Tashkin, D., Wise, R. A., Connett, J. E., et al. (2015). The relationship between Helicobacter pylori seropositivity and COPD. Thorax 70, 923–929.
Tepler, A., Narula, N., Peek, R. M. Jr., Patel, A., Edelson, C., Colombel, J. F., et al. (2019). Systematic review with meta-analysis: association between Helicobacter pylori CagA seropositivity and odds of inflammatory bowel disease. Aliment Pharmacol. Ther. 50, 121–131. doi: 10.1111/apt.15306
Wan, Z., Hu, L., Hu, M., Lei, X., Huang, Y., and Lv, Y. (2018). Helicobacter pylori infection and prevalence of high blood pressure among Chinese adults. J. Hum. Hypertens. 32, 158–164. doi: 10.1038/s41371-017-0028-8
Wang, J., Chen, R. C., Zheng, Y. X., Zhao, S. S., Li, N., Zhou, R. R., et al. (2016). Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int. J. Infect. Dis. 50, 30–37. doi: 10.1016/j.ijid.2016.07.014
Wang, L., Chen, J., Jiang, W., Cen, L., Pan, J., Yu, C., et al. (2021). The Relationship between Helicobacter pylori Infection of the Gallbladder and Chronic Cholecystitis and Cholelithiasis: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021:8886085.
Wang, Y. C., Lin, T. Y., Shang, S. T., Chen, H. J., Kao, C. H., Wu, C. C., et al. (2017). Helicobacter pylori infection increases the risk of adult-onset asthma: a nationwide cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1587–1594. doi: 10.1007/s10096-017-2972-1
World Health Organization (2016). WHO recommendations on antenatal care for a positive pregnancy experience. WHO guidelines approved by the guidelines review committee. Geneva: World Health Organization.
Wu, M. C., Ma, K. S., Chen, H. H., Huang, J. Y., and Wei, J. C. (2020). Relationship between Helicobacter pylori infection and psoriasis: a nationwide population-based longitudinal cohort study. Medicine 99:e20632. doi: 10.1097/MD.0000000000020632
Xia, W., Zhang, X., Wang, J., Sun, C., and Wu, L. (2012). Survey of anaemia and Helicobacter pylori infection in adolescent girls in Suihua, China and enhancement of iron intervention effects by H. pylori eradication. Br. J. Nutr. 108, 357–362. doi: 10.1017/S0007114511005666
Xia, X., Zhang, L., Chi, J., Li, H., Liu, X., Hu, T., et al. (2020). Helicobacter pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism. J. Am. Heart Assoc. 9:e014120. doi: 10.1161/JAHA.119.014120
Xu, M. Y., Cao, B., Chen, Y., Musial, N., Wang, S., Yin, J., et al. (2018a). Association between Helicobacter pylori infection and tumor markers: an observational retrospective study. BMJ Open 8:e022374. doi: 10.1136/bmjopen-2018-022374
Xu, M. Y., Liu, L., Yuan, B. S., Yin, J., and Lu, Q. B. (2017). Association of obesity with Helicobacter pylori infection: A retrospective study. World J. Gastroenterol. 23, 2750–2756.
Xu, M. Y., Ma, J. H., Yuan, B. S., Yin, J., Liu, L., and Lu, Q. B. (2018b). Association between Helicobacter pylori infection and gallbladder diseases: A retrospective study. J. Gastroenterol. Hepatol. 33, 1207–1212.
Xu, X., Li, W., Qin, L., Yang, W., Yu, G., and Wei, Q. (2019). Relationship between Helicobacter pylori infection and obesity in Chinese adults: A systematic review with meta-analysis. PLoS One 14:e0221076. doi: 10.1371/journal.pone.0221076
Yamaoka, Y. (2018). How to eliminate gastric cancer-related death worldwide? Nat. Rev. Clin. Oncol. 15, 407–408.
Yang, X. (2018). Relationship between Helicobacter pylori and Rosacea: review and discussion. BMC Infect Dis. 18:318. doi: 10.1186/s12879-018-3232-4
Yoshimura, S., Isobe, N., Matsushita, T., Yonekawa, T., Masaki, K., Sato, S., et al. (2013). Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J. Neurol. Neurosurg. Psychiatry 84, 29–34. doi: 10.1136/jnnp-2012-302925
Youssefi, M., Tafaghodi, M., Farsiani, H., Ghazvini, K., and Keikha, M. (2021). Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J. Microbiol. Immunol. Infect. 54, 359–369. doi: 10.1016/j.jmii.2020.08.011
Yu, L. Y., Hu, K. C., Liu, C. J., Hung, C. L., Bair, M. J., Chen, M. J., et al. (2019). Helicobacter pylori infection combined with non-alcoholic fatty liver disease increase the risk of atherosclerosis: Focus in carotid artery plaque. Medicine 98, e14672. doi: 10.1097/MD.0000000000014672
Yu, M., Zhang, R., Ni, P., Chen, S., and Duan, G. (2019). Helicobacter pylori Infection and Psoriasis: A Systematic Review and Meta-Analysis. Medicina 55:645
Zhang, J., Zhang, Y., Chen, Y., Chen, W., Xu, H., and Sun, W. (2020). Helicobacter pylori is not a contributing factor in gallbladder polyps or gallstones: a case-control matching study of Chinese individuals. J. Int. Med. Res. 48:300060520959220.
Zhang, L., Chen, Z., Xia, X., Chi, J., Li, H., Liu, X., et al. (2019). Helicobacter pylori infection selectively increases the risk for carotid atherosclerosis in young males. Atherosclerosis 291, 71–77. doi: 10.1016/j.atherosclerosis.2019.10.005
Zhang, Y., Wei, Z., Li, J., and Liu, P. (2015). Molecular pathogenesis of lymphomas of mucosa-associated lymphoid tissue–from (auto)antigen driven selection to the activation of NF-kappaB signaling. Sci. China Life Sci. 58, 1246–1255. doi: 10.1007/s11427-015-4977-2
Zhong, R., Chen, Q., Zhang, X., Li, M., and Lin, W. (2022). Helicobacter pylori infection is associated with a poor response to levodopa in patients with Parkinson’s disease: a systematic review and meta-analysis. J. Neurol. 269, 703–711. doi: 10.1007/s00415-021-10473-1
Zhou, S., Huang, Y., Liang, B., Dong, H., Yao, S., Chen, Y., et al. (2017). Systemic and mucosal pre-administration of recombinant Helicobacter pylori neutrophil-activating protein prevents ovalbumin-induced allergic asthma in mice. FEMS Microbiol. Lett. 364. doi: 10.1093/femsle/fnw288
Zhu, T. T., Wang, L., Wang, H. L., He, Y., Ma, X., and Fan, J. M. (2016). Helicobacter pylori participates in the pathogenesis of IgA nephropathy. Ren. Fail. 38, 1398–1404. doi: 10.1080/0886022X.2016.1216713
Zuin, M., Rigatelli, G., Del Favero, G., Picariello, C., Meggiato, T., Conte, L., et al. (2016). Coronary artery disease and Helicobacter pylori infection: Should we consider eradication therapy as cardiovascular prevention strategy? Int. J. Cardiol. 223, 711–712. doi: 10.1016/j.ijcard.2016.08.320
Zullo, A., Hassan, C., Ridola, L., Repici, A., Manta, R., and Andriani, A. (2014). Gastric MALT lymphoma: old and new insights. Ann. Gastroenterol. 27, 27–33.
Keywords: Helicobacter pylori, extragastric diseases, pathological mechanism, systemic inflammation, molecular mimicry
Citation: He J, Liu Y, Ouyang Q, Li R, Li J, Chen W, Hu W, He L, Bao Q, Li P and Hu C (2022) Helicobacter pylori and unignorable extragastric diseases: Mechanism and implications. Front. Microbiol. 13:972777. doi: 10.3389/fmicb.2022.972777
Received: 19 June 2022; Accepted: 11 July 2022;
Published: 04 August 2022.
Edited by:
Muhammad Shahid Riaz Rajoka, Tohoku University, JapanReviewed by:
A. K. M. Humayun Kober, Chittagong Veterinary and Animal Sciences University, BangladeshAhmad Ud Din, Sichuan University, China
Copyright © 2022 He, Liu, Ouyang, Li, Li, Chen, Hu, He, Bao, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, lp25920150@126.com; Changjiang Hu, hcj888@tmmu.edu.cn
†These authors have contributed equally to this work
 Junjian He
Junjian He Yunyi Liu1†
Yunyi Liu1† Qin Ouyang
Qin Ouyang