Semantic Memory and Lexical Availability in Parkinson’s Disease: A Statistical Learning Study
- 1Facultad de Psicología, Universidad del Valle, Santiago de Cali, Colombia
- 2Centro de Investigación en Neurociencia Clínica y Comportamental (CINCCO), Universidad del Valle, Santiago de Cali, Colombia
- 3Centro Médico de Atención Neurológica “Neurólogos de Occidente”, Santiago de Cali, Colombia
- 4Instituto Neurológico del Pacifico, Santiago de Cali, Colombia
- 5Departamento de Lenguas, Universidad Pedagógica Nacional, Bogotá, Colombia
- 6Centre for Change and Complexity in Learning, University of South Australia, Adelaide, SA, Australia
Parkinson’s disease (PD) is a neurodegenerative disorder that causes a progressive impairment in motor and cognitive functions. Although semantic fluency deficits have been described in PD, more specific semantic memory (SM) and lexical availability (LA) domains have not been previously addressed. Here, we aimed to characterize the cognitive performance of PD patients in a set of SM and LA measures and determine the smallest set of neuropsychological (lexical, semantic, or executive) variables that most accurately classify groups. Thirty early-stage non-demented PD patients (age 35–75, 10 females) and thirty healthy controls (age 36–76, 12 females) were assessed via general cognitive, SM [three subtests of the CaGi battery including living (i.e., elephant) and non-living things (i.e., fork)], and LA (eliciting words from 10 semantic categories related to everyday life) measures. Results showed that PD patients performed lower than controls in two SM global scores (picture naming and naming in response to an oral description). This impairment was particularly pronounced in the non-living things subscale. Also, the number of words in the LA measure was inferior in PD patients than controls, in both larger and smaller semantic fields, showing a more inadequate recall strategy. Notably, the classification algorithms indicated that the SM task had high classification accuracy. In particular, the denomination of non-living things had a classification accuracy of ∼80%. These results suggest that frontostriatal deterioration in PD leads to search strategy deficits in SF and the potential disruption in semantic categorization. These findings are consistent with the embodied view of cognition.
Introduction
How are concepts stored in our minds? Since the conceptual framework of Collins and Quillian (1969), theoretical approaches have emerged in the field of semantic memory (SM) (Tulving, 1972; Caramazza and Hillis, 1991; Ullman, 2001, 2004; Caramazza and Mahon, 2006; Gainotti, 2015; Kumar, 2021). Neuroimaging studies have highlighted the involvement of modality-specific (sensory, cognitive, and motor) and multimodal neural circuits distributed in the frontal, temporal, and parietal cortex (Simons and Spiers, 2003; Binder and Desai, 2011; Quiroga, 2012). These findings have made it possible to identify a widely distributed cortical network associated with declarative memory.
Semantic fluency (SF) (Bousfield and Sedgewick, 1944) has been a classic SM measure in clinical and experimental neuropsychology. SF is the ability to identify specific categories (i.e., concepts, items, names, and objects) through association in a long-term memory store (Capitani et al., 2003; Robinson et al., 2012). Lexical availability (LA) tasks, which are typically used to identify the potential lexicon that a speaker possesses (of a mother tongue or a foreign language), have essentially the same features of the semantic fluency task (Hernández-Muñoz et al., 2014) with the critical addition of having defined categories (semantic fields) that are relevant to the everyday life of a speaking community, making them especially useful for SF studies.
The critical role of frontal and temporal cortical areas in SF performance has been well-studied. Neuropsychological studies have made it possible to partially identify the neural substrates of the conceptual organization and SM impairments’ characteristics. Patients with frontal damage have shown monitoring deficits and poor strategies during the retrieval process (Warrington and Shallice, 1984; Baldo and Shimamura, 1998; Stuss et al., 1998; Troyer et al., 1998; Schwartz and Baldo, 2001; Fuster, 2008; Squire, 2009; Squire and Wixted, 2011; Robinson et al., 2012). These deficits have also been reported in the behavioral variant of frontotemporal dementia (bvFTD) (Burgess and Shallice, 1997; Mayr, 2002; Reverberi et al., 2006, 2014; Possin et al., 2013). Furthermore, temporal lobe damage has been associated with worse performance on semantic fluency tasks (Campanella et al., 2010). Similar findings have been reported in the semantic variant of primary progressive aphasia (sv-PPA) (Hodges et al., 1992; Catricalà et al., 2014; Reverberi et al., 2014; Migliaccio et al., 2016).
Semantic categorization (SC) is a fundamental ability to recognize and classify an object. Indeed, identifying whether a stimulus is a living or non-living object allows us to make inferences and predictions about its behavior and its relationship with the context (Binder and Desai, 2011). The dissociation between semantic categories has been previously addressed. In their seminal work, Damasio and Tranel (1993) reported the dissociated naming performance for objects and verbs in three patients with predominantly frontal or temporal lesions. Recently, the study of neurodegenerative motor disorders also supports the differential role of frontal (motor and premotor) areas in action-verb processing (De Renzi and Di Pellegrino, 1995; Bak et al., 2001, 2006). A relevant dissociation deficit found in PD patients is that of manipulated vs. non-manipulated object naming. These patients perform lower (i.e., accuracy of responses) than controls when naming manipulated objects, but their performance is similar when naming non-manipulated objects (Johari et al., 2019). Notably, response times in manipulated object naming tasks seem to improve in early PD patients receiving both pharmacological and subthalamic DBS treatment (but not pharmacological treatment alone), contrary to non-manipulated object naming. However, accuracy seems to improve for neither type of object (Phillips et al., 2012).
SM is not limited to cortical regions but also extends into the subcortical areas. Currently, it is recognized the role of the basal ganglia in SM (Copland, 2003; Crosson et al., 2003; Longworth et al., 2005; Cardona et al., 2013). Several studies have shown that SM is impaired in Parkinson’s disease (PD) patients (Henry and Crawford, 2004; Kudlicka et al., 2011; Angwin et al., 2017). However, the cortico-subcortical circuits’ role in PD in categorizing and storing information in the living vs. non-living categories is not clear.
The purpose of the present study was to characterize the cognitive performance of PD patients using a comprehensive set of LA and SM tasks that included living/non-living categories. Importantly, this study aimed to determine the smallest set of neuropsychological (executive, semantic, or lexical) variables that could better classify participants as being PD or control with high accuracy. To our knowledge, the current research is the first to study LA to explore semantic fluency in PD.
Materials and Methods
Participants
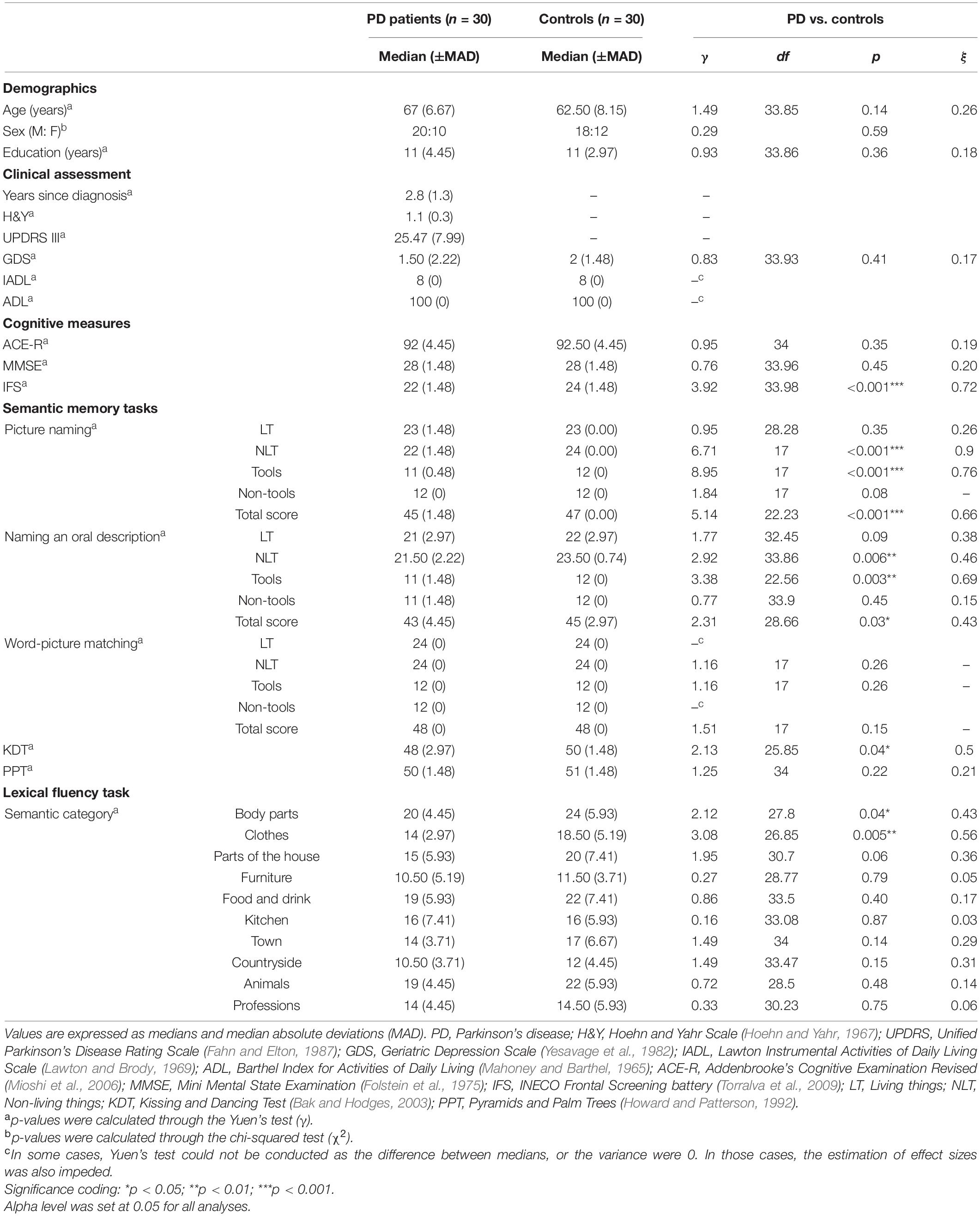
The study comprised thirty early-stage non-demented PD patients and thirty healthy controls (all right-handed). PD patients’ clinical diagnosis was established by an expert neurologist (J.D) following the United Kingdom PD Society Brain Bank Criteria (Hughes et al., 1992). Their motor symptoms and disease stage were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn and Elton, 1987) and the Hoehn and Yahr scale (H&Y) (Hoehn and Yahr, 1967), respectively. All patients were receiving antiparkinsonian therapy and evaluated during the “on” phase of their medication. Control subjects were matched for age, sex, and years of education (see Table 1).
No subject in any group presented a history of alcohol/drug abuse, physical or psychiatric conditions, or other neurological illnesses. Also, the groups were comparable in terms of their independent living skills and depressive symptoms, as measured with the Lawton Instrumental Activities of Daily Living Scale (IADL) (Lawton and Brody, 1969) and the Barthel Index for Activities of Daily Living (ADL) (Mahoney and Barthel, 1965), and the Geriatric Depression Scale (GDS) (Yesavage et al., 1982; Gomez-Angulo and Campo-Arias, 2011), respectively (see Table 1). All participants provided written informed consent in agreement with the Declaration of Helsinki. The Ethical Research Committee of Universidad del Valle (CIREH 203-015, CI 5278) approved all the study procedures.
Materials
General Cognitive State and Executive Functioning
The participant’s general cognitive state was assessed using the Addenbrooke’s Cognitive Examination Revised (ACE-R) (Sarasola et al., 2005; Mioshi et al., 2006; Reyes et al., 2009), which allows to simultaneously calculate the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) score. This instrument has been extensively used in neurodegenerative diseases (Mioshi et al., 2006; McColgan et al., 2012; Hsieh et al., 2013). The maximum total score in the ACE-R is 100 points (see Supplementary Section 1).
Furthermore, subjects’ executive functioning was examined through the INECO Frontal Screening (IFS) (Torralva et al., 2009), a validated test to measure executive dysfunction in neurodegeneration (Gleichgerrcht et al., 2011; Broche-Pérez et al., 2019; Moreira et al., 2019). This test comprises the following eight subtests: (1) motor programming (Luria series, “fist, edge, palm”); (2) conflicting instructions (hitting the table once when the administrator hits it twice, or hitting it twice when the administrator hits it only once); (3) motor inhibitory control; (4) numerical working memory (backward digit span); (5) verbal working memory (months backward); (6) spatial working memory (modified Corsi tapping test); (7) abstraction capacity (inferring the meaning of proverbs), and (8) verbal inhibitory control (modified Hayling test). The maximum total score in the IFS is 30 points.
Semantic Memory Tasks
CaGi Battery
The participants performed a previously Spanish adapted version (Moreno-Martínez and Rodríguez-Rojo, 2015; Navarro et al., 2020) of the CaGi battery (Catricalà et al., 2013), which has been widely used in neurodegenerative conditions (Catricalà et al., 2013, 2014, 2015; Della Rosa et al., 2014). This battery includes a set of 48 stimuli belonging to both living (12 animals and 12 vegetables) and non-living entities (12 tools and 12 non-tools).
Specifically, we used the following three subtests: (a) picture naming task, asking the participants to name colored pictures, (b) naming in response to an oral description requiring examinees to name each stimulus after listening to its verbal description (i.e., “It grows in clusters, has a round shape, is used to make wine.”), and (c) word-picture matching task, requiring subjects to select, from three pictures, the one corresponding to the spoken word. Correct and incorrect responses were assigned scores of 1 and 0, respectively. Thus, the maximum global score in each task is 48 points.
Pyramids and Palms Trees and Kissing and Dancing Tests
The subjects performed the picture version of two additional tasks assessing semantic memory for objects and actions: the Pyramids and Palms Trees test (PPT) (Howard and Patterson, 1992) and the Kissing and Dancing test (KDT) (Bak and Hodges, 2003). Both tests have been previously used in neurodegenerative diseases (Bak et al., 2001, 2006; Ibáñez et al., 2013). In the PPT, participants are shown 52 triplets of object drawings (1 target, 1 correct match semantically related, and 1 distractor non-semantically related) and asked to match the target picture with the one semantically related. The KDT task structure is analogous to the PPT, but stimuli consisted of pictures depicting actions instead of objects. In both tests, one point is earned for each correct answer, resulting in global scores out of 52.
Lexical Fluency Measures
LA was measured using 10 semantic categories (SC) of the Pan-Hispanic project (PPHDL available at www.dispolex.com), based on the indications for defining the fundamental lexicon of a language (Sánchez and Aguirre, 1992). SC represented an area related to everyday life, including (1) parts of the body, (2) clothes, (3) parts of the house, (4) furniture, (5) food and drinks, (6) kitchen, (7) town, (8) countryside, (9) animals, and (10) professions. In each SC, the participants were asked to orally generate words for 2 min, avoiding producing proper nouns or repeating words. The participants’ answers were recorded and analyzed offline. One point was assigned for each correct generated word.
Statistical Analysis
Between-Group Comparisons and Statistical Learning Analysis
Normality was evaluated using the Shapiro-Wilk test. Since the assumption of normality was not met, we tried several transformations but none of them normalized the data, so we retained the original scores and proceeded using Yuen (1974)’s test (γ) for between groups comparisons of demographic and behavioral data. Sex was analyzed using the chi-squared test (χ2). The statistical significance level was set at p < 0.05 for all analyses. Effect sizes were calculated through Wilcox and Tian’ξ (2011), implemented in the WRS2 package (Mair and Wilcox, 2020).
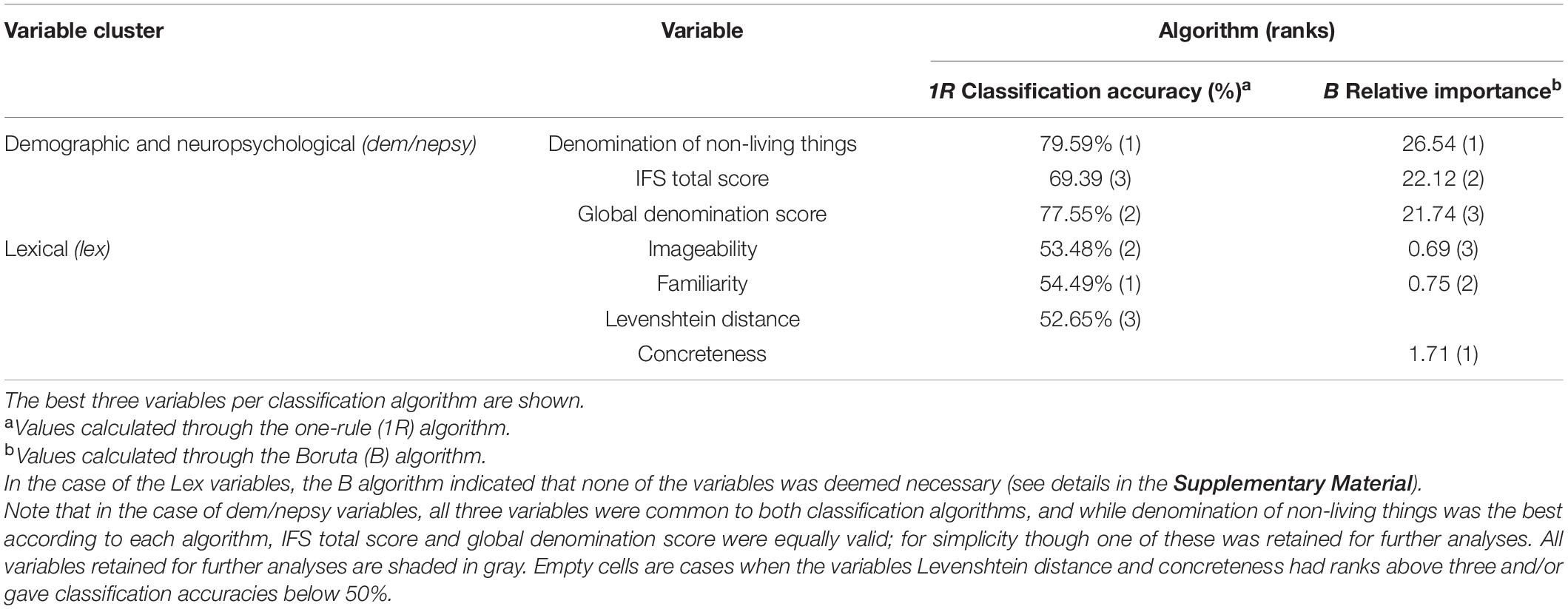
Additionally, statistical learning analyses were conducted to explore which measures best classify groups using the smallest possible set of variables. The predictors were categorized into demographic and neuropsychological (dem/nepsy) and lexical (lex) clusters. The Dem/nepsy cluster included age, years of education, sex, ACE-R, MMSE, IFS, working memory index, the CaGi battery total scores, and the living/non-living subscores, the KDT, and the PPT scores as predictors. The SC of the LA task was introduced as a covariate in this cluster. The lex cluster included log-frequency, number of letters, orthographic neighborhood, number of phonemes, number of syllables, familiarity, imageability, and concreteness as predictors.
Then, each cluster of variables was submitted to “one rule” (1R) (Holte, 1993) and Boruta (B) (Kursa and Rudnicki, 2010) classification algorithms, which rank the variables according to their classification accuracy (1R) and relative importance (B), respectively. The three strongest classifiers identified by each algorithm were kept.
Finally, four logistic regression models were conducted to ascertain which combination of variables had the highest predicting level (see Table 2). Each model included a combination of two of the strongest classifiers of the dem/nepsy and lex clusters as independent variables and group (PD patients and controls) as the dependent variable, following the structure group ∼ lex + dem/nepsy. The models were fitted using the standard GLM with a binomial distribution (logit link function). The best classification model was represented via classification trees and spinograms (Everitt and Hothorn, 2014). All analyses were conducted using R version 3.6 (R Core Team., 2020). The R codes and data sets are available at https://figshare.com/projects/memory_and_lexicality_in_Parkinson/99800.
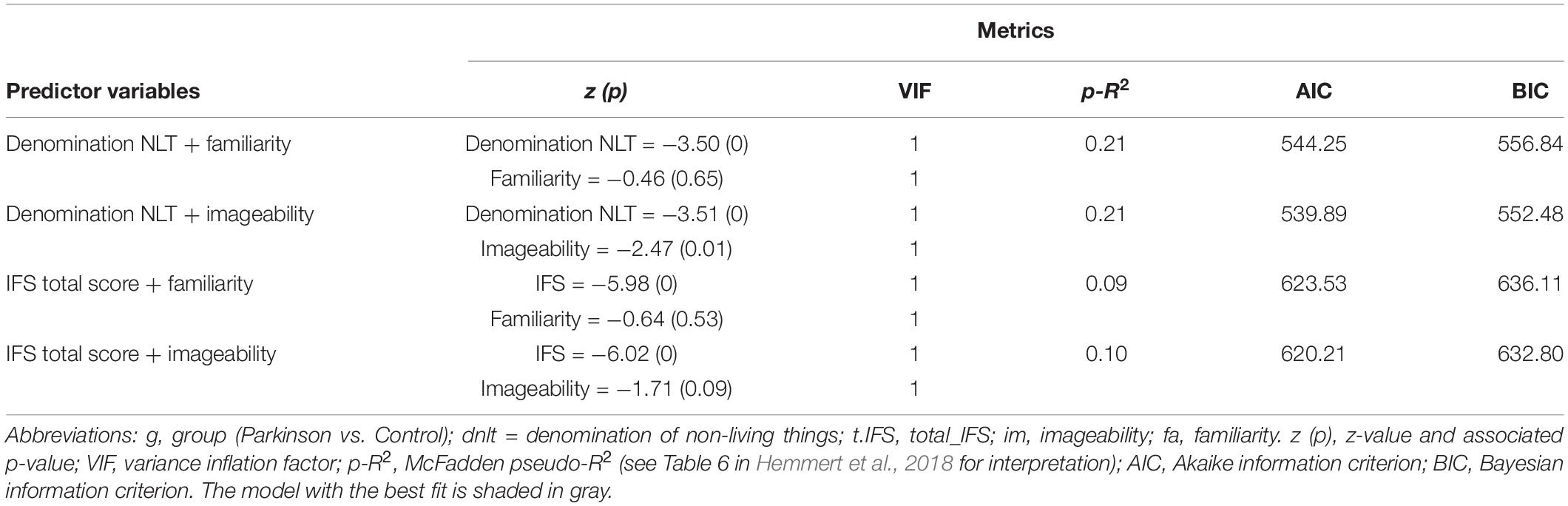
Table 2. Logistic regression models combining the four variables suggested by the classification algorithms.
Lexical Availability Analysis
First Step
All perseverative responses were excluded. We used the lexical statistical program Dispolex (available at http://www.dispolex.com) following previous studies (Samper-Padilla, 1998; Bartol-Hernández and Hernández-Muñoz, 2003; Hernández-Muñoz et al., 2006, 2014; Mateus and Santiago, 2006; López-Morales, 2014). This program provided us: (a) the total number of words’ occurrences (tokens), (b) each lexical unit (types) counts, (c) the average number of responses, and (d) the frequency and position of each word in each semantic category (LA index), and (e) the degree of coincidence in informants’ word response (lexical cohesion index) (Echeverría, 1991; Hernández-Muñoz, 2010).
Second Step
In each category, words with a frequency of appearance lower than 4.17% (frequency equal to 1) were excluded. Subsequently, a lexical properties analysis was conducted by identifying: (a) orthographic structure: word frequency and number of letters, (b) orthographic neighborhoods: Levenshtein distance (Levenshtein, 1966), (c) phonological structure: number of phonemes and number of syllables, and (d) word’s subjective ratings: familiarity, imageability, and concreteness.
These linguistic variables for Latin American Spanish were identified in the web interface to Spanish word frequency data and other word properties based on written and subtitle corpora (Duchon et al., 2013) (available at https://www.bcbl.eu/databases/espal/).
Results
General Cognitive State
No between-group differences were observed in the ACE-R [γ(34) = 0.95, p = 0.35, ξ = 0.19] and the MMSE [γ(33.96) = 0.76, p = 0.45, ξ = 0.20] total scores. However, PD patients performed lower than controls in the IFS total score [γ(33.98) = 3.92, p < 0.001, ξ = 0.72], the digits backward subtest [γ(28.66) = 2.65, p = 0.01, ξ = 0.44], the working memory index [γ(33.96) = 2.22, p = 0.03, ξ = 0.46], and marginally lower in the verbal inhibitory control subtest [γ(33.31) = 1.76, p = 0.09, ξ = 0.38] (see Table 1 and Supplementary Table 1).
Semantic Memory Tasks
CaGi Battery
Picture Naming Task
PD patients globally scored lower than controls [γ(22.23) = 5.14, p < 0.001, ξ = 0.66]. Specifically, patients performed lower than controls in naming non-living things [γ(17) = 6.71, p < 0.001, ξ = 0.9] and tools [γ(17) = 8.95, p < 0.001, ξ = 0.76]. No significant between-group differences were observed in the denomination of living things [γ(28.28) = 0.95, p = 0.35, ξ = 0.26] and non-tools [γ(17) = 1.84, p = 0.08] (see Table 1).
Naming in Response to an Oral Description
PD patients globally performed lower than controls [γ(28.66) = 2.31, p = 0.03, ξ = 0.43]. Particularly, patients exhibited lower scores in naming non-living things [γ(33.86) = 2.92, p = 0.006, ξ = 0.46] and tools [γ(22.56) = 3.38, p = 0.003, ξ = 0.69]. The groups’ performance did not differ in naming living things [γ(32.45) = 1.77, p = 0.09, ξ = 0.38] and non-tools [γ(33.9) = 0.77, p = 0.45, ξ = 0.15] (see Table 1).
Word-Picture Matching
No significant differences between groups were observed in the global performance [γ(17) = 1.51, p = 0.15], and the denomination of living things (equal medians), non-living [γ(17) = 1.16; p = 0.26], tools [γ(17) = 1.16, p = 0.26] and non-tools categories (equal medians) (see Table 1).
Pyramids and Palms Trees and Kissing and Dancing Tests
KDT total score was lower in PD patients than in controls [γ(25.85) = 2.13, p = 0.04, ξ = 0.5], there being no significant between-group differences in the PPT scores [γ(34) = 1.25, p = 0.22, ξ = 0.21] (see Table 1).
Lexical Fluency Performance
Qualitatively, PD patients exhibited a lower total number of words (tokens) in large (i.e., countryside) and small (i.e., parts of the body) semantic categories (see Supplementary Section 2.1 and Supplementary Table 3).
Lexical Units Index
In PD patients, the two SC with the most different lexical units corresponded to animals (79 lexical units) and food and drinks (74 lexical units). In contrast, the least productive SC were countryside (33 lexical units) and furniture (38 lexical units). In Supplementary Table 3, there was no direct relationship between general lexical productivity and word types (a measure of lexical richness).
In controls, the most productive SC with the highest number of word types were food and drinks (83 lexical units) and body parts (74 lexical units). Like the PD group, the least productive SC were countryside (42 tokens) and furniture (43 lexical units).
Lexical Availability Index and Lexical Cohesion Index
Results are summarized in Supplementary Section 2.2, 2.3 and Supplementary Tables 1, 2.
Statistical Learning Analysis
In the dem/nepsy cluster, the denomination of non-living things, the global denomination score, and the total IFS score were the strongest variables for distinguishing between groups, correctly classifying 79.6% (58.3% of PD and 100% of controls), 77.5% (54.2% of PD patients and 100% of controls), and 69.4% (75% of PD patients and 64% of controls) of the overall cases, respectively. These variables also obtained the highest relative importance, only slightly varying in their order: denomination of non-living things (B = 26.54), total IFS score (B = 22.12), and global denomination score (B = 21.74) (see Table 3).
In the lex cluster, familiarity, imageability, and Levenshtein distance were the strongest predictors of group membership, successfully classifying 55% (58.8% of PD patients and 50% of controls), 53.5% (60% of PD patients and 48% of controls), and 52.7% (12.1% of PD patients and 91.6% of controls of the total cases, respectively. Besides, concreteness reached the highest relative importance (B = 1.71), followed by familiarity (B = 0.75) and imageability (B = 0.69) (see Table 3). Nevertheless, both classification algorithms indicated that these and other lex variables had classification accuracies near chance (1R) and low importance (B) (see Table 3).
Logistic Models
The model combining the denomination of non-living things (z = −3.51, p < 0.01) and imageability (z = −2.47, p = 0.01) reached the best fit (p-R2 = 0.21, AIC = 539.89, BIC = 552.48) (see Table 3). However, this model was not pursued given the results of the classification algorithms regarding the lex variables; as shown in Table 1, all lexical variables had classification accuracies near chance (1R algorithm) and very low importance (B algorithm). Thus, the model group ∼ dnlt was examined via a classification tree and a spinogram.
The classification tree results suggested that when a person produces less than 24 denominations of non-living things, there is about an 85% chance of being classified as a PD patient. If the person produces about 24 or more denominations, the chances of the person being classified as a PD patient are about 9% (Figure 1B). The spinogram further corroborates these approximate likelihoods and provides the observed counts for different bins (Figure 1A). It is important to stress that the cut-offs are merely approximations and need to be revised within the task context.
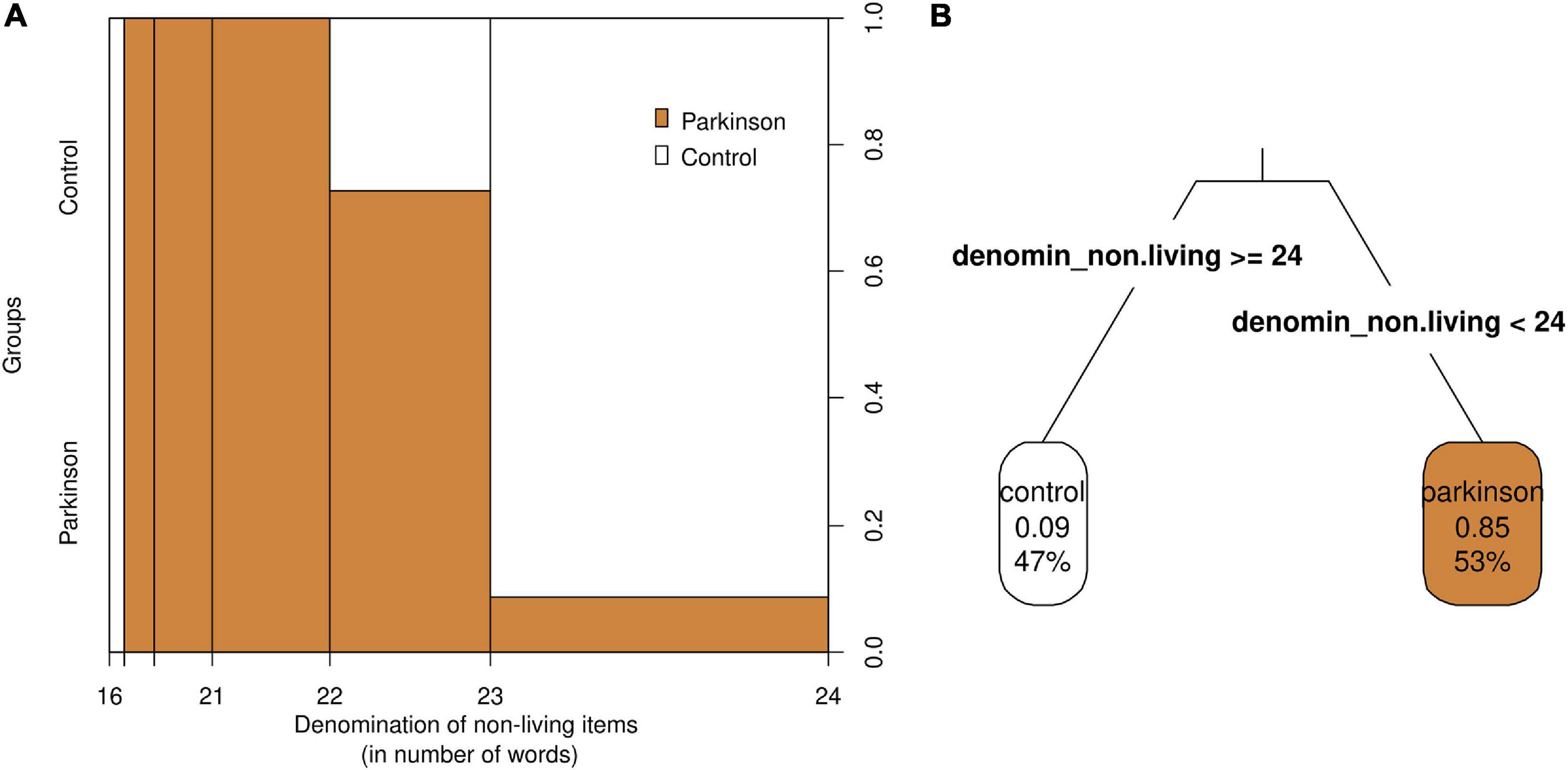
Figure 1. Spinogram (A) and classification tree (B) of the model group ∼ dnlt. (A) The widths of the bins in the x-axis in the spinogram represent the frequencies (number of participants that obtained a score) within each bin. For example, there were more observations between 23 and 24 denominations than between 16 and 21 denominations. Colors represent groups, white being for controls (always on top) and orange being for PD patients (always below). The right y-axis represents the proportion of subjects that belonged to each group in each of the bins. (B) The classification tree shows the likelihood of being classified as control or PD depending on a cut-off score of 24 in the denomination of non-living things subtask.
Discussion
This study aimed to characterize the cognitive performance of PD patients using a comprehensive set of lexical fluency and SM tasks and determine the smallest set of measures that best classify the groups. The classification algorithms indicated that some of the SM tasks had the highest classification accuracies while none of the executive or lexical variables had reliably classified groups. In particular, the “denomination of non-living things” had the highest classification accuracy of ∼80%.
Semantic Memory in PD
PD patients showed an inferior performance in two naming tasks of CaGi measures. In line with previous studies, significant differences were observed in the visual and auditory input tasks (Portin et al., 2000; Rosenthal et al., 2017; Salmazo-Silva et al., 2017). Importantly, this inferior performance was most notable in the SM category of non-living things.
From an embodied perspective (Tirado et al., 2018; Khatin-Zadeh et al., 2021), these results could be attributed to PD patients’ difficulty to access manipulable objects’ semantic representation. Previous studies suggest that PD is associated with deficits in the semantic representation of actions/verbs that imply movement (Cardona et al., 2014; Bocanegra et al., 2015; Melloni et al., 2015; Suárez-García et al., 2021) or functional manipulability (Péran et al., 2009; Herrera et al., 2012; Bocanegra et al., 2017). This poor PD performance is associated with the disrupting basal ganglia-frontal circuit activated during action processing and object manipulation tasks. It has been shown that this circuit participates in the crucial coupling between motor and linguistic information (Pulvermüller, 2005; Pulvermüller et al., 2005; Melloni et al., 2015) and that its disruption hinders such coupling (Ibáñez et al., 2013). However, as this study did not include neurophysiological/neuroimaging measures, further evidence is needed to support this view. As the semantics of manipulable objects entails body movement, deterioration of the mentioned circuit might explain why PD patients have a challenging time accessing these semantic representations. This is further confirmed by the findings in the tools’ subcategory of the picture naming and naming on oral description tasks, in contrast to the non-tools subcategory (although there was a trend in the first task). These results converge with a growing corpus of research showing impairments in action semantics in PD and hint that the possibility of impairments in the semantic processing of non-living things is likely to be driven by the presence of motor representations (manipulability) in the semantic store of these objects.
As previous research has shown, manipulable objects naming is particularly impaired in PD (Johari et al., 2019). However, it might be possible to account for these deficits with techniques such as subthalamic DBS even in early PD (Phillips et al., 2012). The present findings also suggest that the comprehension of manipulable objects might deteriorate, so its treatment should also be explored through adjuvant electrical stimulation techniques.
Although PD patients did not present mild cognitive impairment, EF deficits were observed, especially in working memory and partially in verbal inhibitory control, as measured in the IFS scale by the digits backward task, and a shortened version of the Hayling test, respectively. These results agree with previous studies highlighting executive dysfunction as a frequent trait in PD’s initial stage (Barone et al., 2011; Khoo et al., 2013; Liu et al., 2017). Furthermore, while the IFS global score reached a high classification accuracy, it was not superior to that of denomination of non-living things, hinting that these semantic deficits might be more characteristic to PD than executive deficits.
Lexical Availability in PD
Meta-analysis has shown that non-demented PD patients have semantic fluency impairments (Henry and Crawford, 2004; Kudlicka et al., 2011). Some authors suggest a selective lexical retrieval impairment in PD and frontal patients (Rogers et al., 1998; Silveri et al., 2017; Johari et al., 2019). Tagini et al. (2018) speculate that this deterioration may be due to a low activation level (difficulty in initiation, bradyphrenia) that slows down the production rate throughout the task or a damaged semantic store.
No previous research has explored the lexical availability in PD. Our study’s total number of words per semantic field was inferior in the PD group in both large and small semantic categories. These results indicate that PD patients present an overall more deficient search strategy in the semantic store and deficits in switching from one subcategory to another than controls. The inferior performance shown in these semantic categories is expectable given the delay of speech initiation, bradyphrenia, and the fact that PD patients perform worse than healthy controls in all categories, although not all of them reached statistical significance.
Semantic fluency tasks are less automatic than naming or matching tasks (Fernandino et al., 2013; Salmazo-Silva et al., 2017). Several cognitive domains contribute to performance on fluency tasks (Rosen and Engle, 1997; Reverberi et al., 2006, 2014; Unsworth et al., 2011; Robinson et al., 2012; Tagini et al., 2018). In this way, generating search strategies and concepts’ internal organization is critical for satisfactory performance.
Limitations
This work has significant limitations. First, we did not use the complete CaGi battery, including the picture sorting, free generation of features, and sentence verification subtests due to the participants’ fatigue and/or disinterest. Another limitation is the absence of the switching and clustering index. Without these analyses, semantic proximity is unknown, and therefore, it cannot be inferred whether the observed deficits are associated with alterations in strategic retrieval processing or monitoring deficits. These limitations prevent a broader interpretation of the results. Finally, we acknowledge that the levodopa equivalent dose is a highly relevant variable missing in this study since previous studies have shown an effect of dopaminergic medication in semantic processing related to action (Boulenger et al., 2008; De Letter et al., 2012, 2020).
Conclusion
To summarize, our results suggest that semantic memory is affected in early-stage non-demented PD patients. More importantly, a potential dissociation between living and non-living things categories was found, consistent with previous findings in the study of cognition in PD and the embodied perspective of cognition. Future studies involving neuroimaging techniques can provide fine-grained spatial and functional brain information.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Research Committee of Universidad del Valle (CIREH 203-015, CI 5278). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JFC, GM-F, and FM-R developed the study concept and the study design. JG-C, CT-L, LT, JC, JD, and TJ performed the testing and data collection. JFC, FM-R, JG-C, CT-L, and GM-F performed the data analysis and interpretation. JFC, JG-C, CT-L, HU, SC, AT, LG, and JC drafted the manuscript. NO-C, FM-R, and GM-F provided the critical revisions. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by MINCIENCIAS (1106-744-55314), Sistema General de Regalías (BPIN2018000100059), and Universidad del Valle (Research Grant Scheme: CI5318).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.697065/full#supplementary-material
References
Angwin, A. J., Dissanayaka, N. N. W., Moorcroft, A., McMahon, K. L., Silburn, P. A., and Copland, D. A. (2017). A neurophysiological study of semantic processing in Parkinson’s disease. J. Int. Neuropsychol. Soc. : JINS 23, 78–89. doi: 10.1017/s1355617716000953
Bak, T. H., and Hodges, J. R. (2003). Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. J. Neurolinguistics 16, 169–181. doi: 10.1016/s0911-6044(02)00011-8
Bak, T. H., O’Donovan, D. G., Xuereb, J. H., Boniface, S., and Hodges, J. R. (2001). Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neuron disease–dementia–aphasia syndrome. Brain 124, 103–120. doi: 10.1093/brain/124.1.103
Bak, T. H., Yancopoulou, D., Nestor, P. J., Xuereb, J. H., Spillantini, M. G., Pulvermuller, F., et al. (2006). Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain 129, 321–332. doi: 10.1093/brain/awh701
Baldo, J. V., and Shimamura, A. P. (1998). Letter and category fluency in patients with frontal lobe lesions. Neuropsychology 12, 259–267. doi: 10.1037/0894-4105.12.2.259
Barone, P., Aarsland, D., Burn, D., Emre, M., Kulisevsky, J., and Weintraub, D. (2011). Cognitive impairment in non-demented Parkinson’s disease. Mov. Disord. 26, 2483–2495.
Bartol-Hernández, J. A., and Hernández-Muñoz, N. (2003). Dispolex: Base de Datos de la Disponibilidad Léxica. Salamanca: Universidad de Salamanca.
Binder, J. R., and Desai, R. H. (2011). The neurobiology of semantic memory. Trends Cogn. Sci. 15, 527–536. doi: 10.1016/j.tics.2011.10.001
Bocanegra, Y., García, A. M., Lopera, F., Pineda, D., Baena, A., Ospina, P., et al. (2017). Unspeakable motion: selective action-verb impairments in Parkinson’s disease patients without mild cognitive impairment. Brain Lang. 168, 37–46. doi: 10.1016/j.bandl.2017.01.005
Bocanegra, Y., García, A. M., Pineda, D., Buriticá, O., Villegas, A., Lopera, F., et al. (2015). Syntax, action verbs, action semantics and object semantics in Parkinson’s disease: dissociability progression, and executive functions. Cortex 69, 237–254. doi: 10.1016/j.cortex.2015.05.022
Boulenger, V., Mechtouff, L., Thobois, S., Broussolle, E., Jeannerod, M., and Nazir, T. A. (2008). Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia 46, 743–756. doi: 10.1016/j.neuropsychologia.2007.10.007
Bousfield, W. A., and Sedgewick, C. H. W. (1944). An analysis of restricted associative responses. J. Gen. Psychol. 30, 149–165. doi: 10.1080/00221309.1944.10544467
Broche-Pérez, Y., Bartuste-Marrer, D., Batule-Domínguez, M., and Toledano-Toledano, F. (2019). Clinical utility of the INECO frontal screening for detecting mild cognitive impairment in Parkinson’s disease. Dementia Neuropsychol. 13, 394–402. doi: 10.1590/1980-57642018dn13-040005
Burgess, P., and Shallice, T. (1997). The Hayling and Brixton Tests: Test Manual. Bury St Edmunds, UK: Thames Valley Test Company.
Campanella, F., D’Agostini, S., Skrap, M., and Shallice, T. (2010). Naming manipulable objects: anatomy of a category specific effect in left temporal tumours. Neuropsychologia 48, 1583–1597. doi: 10.1016/j.neuropsychologia.2010.02.002
Capitani, E., Laiacona, M., Mahon, B., and Caramazza, A. (2003). What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cogn. Neuropsychol. 20, 213–261. doi: 10.1080/02643290244000266
Caramazza, A., and Hillis, A. E. (1991). Lexical organization of nouns and verbs in the brain. Nature 349, 788–790. doi: 10.1038/349788a0
Caramazza, A., and Mahon, B. Z. (2006). The organization of conceptual knowledge in the brain: the future’s past and some future directions. Cogn. Neuropsychol. 23, 13–38. doi: 10.1080/02643290542000021
Cardona, J. F., Gershanik, O., Gelormini-Lezama, C., Houck, A. L., Cardona, S., Kargieman, L., et al. (2013). Action-verb processing in Parkinson’s disease: new pathways for motor–language coupling. Brain Struct. Funct. 218, 1355–1373. doi: 10.1007/s00429-013-0510-1
Cardona, J. F., Kargieman, L., Sinay, V., Gershanik, O., Gelormini, C., Amoruso, L., et al. (2014). How embodied is action language? Neurological evidence from motor diseases. Cognition 131, 311–322. doi: 10.1016/j.cognition.2014.02.001
Catricalà, E., Della Rosa, P. A., Ginex, V., Mussetti, Z., Plebani, V., and Cappa, S. F. (2013). An Italian battery for the assessment of semantic memory disorders. Neurol. Sci. 34, 985–993. doi: 10.1007/s10072-012-1181-z
Catricalà, E., Della Rosa, P. A., Parisi, L., Zippo, A. G., Borsa, V. M., Iadanza, A., et al. (2015). Functional correlates of preserved naming performance in amnestic Mild Cognitive Impairment. Neuropsychologia 76, 136–152. doi: 10.1016/j.neuropsychologia.2015.01.009
Catricalà, E., Della Rosa, P. A., Plebani, V., Vigliocco, G., and Cappa, S. F. (2014). Abstract and concrete categories? Evidences from neurodegenerative diseases. Neuropsychologia 64, 271–281. doi: 10.1016/j.neuropsychologia.2014.09.041
Collins, A. M., and Quillian, M. R. (1969). Retrieval time from semantic memory. J. Verb. Learn. Verb. Behav. 8, 240–247. doi: 10.1016/s0022-5371(69)80069-1
Copland, D. (2003). The basal ganglia and semantic engagement: potential insights from semantic priming in individuals with subcortical vascular lesions, Parkinson’s disease, and cortical lesions. J. Int. Neuropsychol. Soc.: JINS 9:1041. doi: 10.1017/s1355617703970081
Crosson, B., Benefield, H., Cato, M. A., Sadek, J. R., Moore, A. B., Wierenga, C. E., et al. (2003). Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. J. Int. Neuropsychol. Soc.: JINS 9:1061. doi: 10.1017/s135561770397010x
Damasio, A. R., and Tranel, D. (1993). Nouns and verbs are retrieved with differently distributed neural systems. Proc. Natl. Acad. Sci. 90, 4957–4960. doi: 10.1073/pnas.90.11.4957
De Letter, M., Bruggeman, A., De Keyser, K., Van Mierlo, P., Buysse, H., Van Roost, D., et al. (2020). Subthalamic nucleus activity in the processing of body and mental action verbs in people with Parkinson’s disease. Brain Lang. 202:104738. doi: 10.1016/j.bandl.2019.104738
De Letter, M., Van Borsel, J., and Santens, P. (2012). An electrophysiological investigation of the effects of levodopa on semantic comprehension of action words in Parkinson’s Disease. J. Neurolinguist. 25, 95–103. doi: 10.1016/j.jneuroling.2011.09.001
De Renzi, E., and Di Pellegrino, G. (1995). Sparing of verbs and preserved, but ineffectual reading in a patient with impaired word production. Cortex 31, 619–636. doi: 10.1016/s0010-9452(13)80016-0
Della Rosa, P. A., Catricalà, E., De Battisti, S., Vinson, D., Vigliocco, G., and Cappa, S. F. (2014). How to assess abstract conceptual knowledge: construction, standardization and validation of a new battery of semantic memory tests. Funct. Neurol. 29:47.
Duchon, A., Perea, M., Sebastián-Gallés, N., Martí, A., and Carreiras, M. (2013). EsPal: one-stop shopping for spanish word properties. Behav. Res. Methods 45, 1246–1258. doi: 10.3758/s13428-013-0326-1
Echeverría, M. S. (1991). “Crecimiento de la disponibilidad léxica en estudiantes chilenos de nivel básico y medio,” in La Enseñanza del Español Como Lengua Materna, Actas del II Seminario Internacional Sobre ‘Aportes de La Lingüística a La Enseñanza del Español Como Lengua Materna, ed. H. L. Morales (Río Piedras: Universidad de Puerto Rico), 61–78.
Everitt, B., and Hothorn, T. (2014). A Handbook of Statistical Analyses Using R. Boca Raton: CRC Press.
Fahn, S., and Elton, R. L. (1987). “Unified Parkinson’s disease rating scale,” in Recent Developments in Parkinson’s Disease II, eds S. Fahn, C. D. Marsden, and M. Goldstein (New York, NY: Macmillan), 153–163.
Fernandino, L., Conant, L. L., Binder, J. R., Blindauer, K., Hiner, B., Spangler, K., et al. (2013). Parkinson’s disease disrupts both automatic and controlled processing of action verbs. Brain Lang. 127, 65–74. doi: 10.1016/j.bandl.2012.07.008
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatric Res. 12, 189–198.
Gainotti, G. (2015). Inborn and experience-dependent models of categorical brain organization. A position paper. Front. Hum. Neurosci. 9:2. doi: 10.3389/fnhum.2015.00002
Gleichgerrcht, E., Roca, M., Manes, F., and Torralva, T. (2011). Comparing the clinical usefulness of the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS) and the Frontal Assessment Battery (FAB) in frontotemporal dementia. J. Clin. Exp. Neuropsychol. 33, 997–1004. doi: 10.1080/13803395.2011.589375
Gomez-Angulo, C., and Campo-Arias, A. (2011). Geriatric depression scale (GDS-15 and GDS-5): a study of the internal consistency and factor structure. Universitas Psychol. 10, 735–743.
Hemmert, G., Schons, L., Wieseke, J., and Schimmelpfennig, H. (2018). Log-likelihood-based Pseudo-R2 in logistic regression: deriving sample-sensitive benchmarks. Sociol. Methods Res. 47, 507–531. doi: 10.1177/0049124116638107
Henry, J. D., and Crawford, J. R. (2004). Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J. Int. Neuropsychol. Soc.: JINS 10:608. doi: 10.1017/s1355617704104141
Hernández-Muñoz, N. (2010). Social aspects of oral and written lexical production in Spanish. SKY J. Linguist. 23, 101–123.
Hernández-Muñoz, N., Izura, C., and Ellis, A. W. (2006). Cognitive aspects of lexical availability. Eur. J. Cogn. Psychol. 18, 734–755.
Hernández-Muñoz, N., Izura, C., and Tomé, C. (2014). “Cognitive factors of lexical availability in a second language,” in Lexical Availability in English and Spanish as a Second Language, ed. R. M. Jiménez Catalán (Dordrecht: Springer Netherlands).
Herrera, E., Rodríguez-Ferreiro, J., and Cuetos, F. (2012). The effect of motion content in action naming by Parkinson’s disease patients. Cortex 48, 900–904. doi: 10.1016/j.cortex.2010.12.007
Hodges, J. R., Patterson, K., Oxbury, S., and Funnell, E. (1992). Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain 115, 1783–1806. doi: 10.1093/brain/115.6.1783
Hoehn, M. M., and Yahr, M. D. (1967). Parkinsonism: onset, progression and mortality. Neurology 17, 427–442. doi: 10.1212/wnl.17.5.427
Holte, R. C. (1993). Very simple classification rules perform well on most commonly used datasets. Machine Learn. 11, 63–91.
Howard, D., and Patterson, K. (1992). The Pyramids and Palm Trees Test. A Test of Semantic Access from Words and Pictures. Bury St. Edmunds, UK: Thames Valley Company.
Hsieh, S., Schubert, S., Hoon, C., Mioshi, E., and Hodges, J. R. (2013). Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dementia Geriatric Cogn. Disord. 36, 242–250. doi: 10.1159/000351671
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Ibáñez, A., Cardona, J. F., Dos Santos, Y. V., Blenkmann, A., Aravena, P., Roca, M., et al. (2013). Motor-language coupling: direct evidence from early Parkinson’s disease and intracranial cortical recordings. Cortex 49, 968–984. doi: 10.1016/j.cortex.2012.02.014
Johari, K., Walenski, M., Reifegerste, J., Ashrafi, F., Behroozmand, R., Daemi, M., et al. (2019). A dissociation between syntactic and lexical processing in Parkinson’s disease. J. Neurolinguistics 51, 221–235. doi: 10.1016/j.jneuroling.2019.03.004
Khatin-Zadeh, O., Eskandari, Z., Cervera-Torres, S., Ruiz-Fernández, S., Farzi, R., and Marmolejo-Ramos, F. (2021). The strong versions of embodied cognition: three challenges faced. Psychol. Neurosci. 14, 16–33. doi: 10.1037/pne0000252
Khoo, T. K., Yarnall, A. J., Duncan, G. W., Coleman, S., O’Brien, J. T., Brooks, D. J., et al. (2013). The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 80, 276–281.
Kudlicka, A., Clare, L., and Hindle, J. V. (2011). Executive functions in Parkinson’s disease: systematic review and meta-analysis. Mov. Disord. 26, 2305–2315. doi: 10.1002/mds.23868
Kumar, A. A. (2021). Semantic memory: a review of methods, models, and current challenges. Psychonomic Bull. Rev. 28, 40–80. doi: 10.3758/s13423-020-01792-x
Kursa, M., and Rudnicki, W. (2010). Feature selection with the Boruta algorithm∗. J. Statistical Softw. 36, 1–13. doi: 10.18637/jss.v036.i11
Lawton, M. P., and Brody, E. M. (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. doi: 10.1093/geront/9.3_part_1.179
Levenshtein, V. I. (1966). Binary codes capable of correcting deletions, insertions, and reversals. Soviet Physics - Doklady 10, 707–710.
Liu, G., Locascio, J. J., Corvol, J. C., Boot, B., Liao, Z., Page, K., et al. (2017). Prediction of cognition in Parkinson’s disease with a clinical–genetic score: a longitudinal analysis of nine cohorts. Lancet Neurol. 16, 620–629.
Longworth, C. E., Keenan, S. E., Barker, R. A., Marslen-Wilson, W. D., and Tyler, L. K. (2005). The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain 128, 584–596. doi: 10.1093/brain/awh387
López-Morales, H. (2014). “Lexical availability studies,” in Lexical Availability in English and Spanish as a Second Language, ed. R. M. Jiménez Catalán (Dordrecht: Springer Netherlands).
Mahoney, F. I., and Barthel, D. W. (1965). Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Maryland State Med. J. 14, 61–65.
Mair, P., and Wilcox, R. (2020). Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 52, 464–488. doi: 10.3758/s13428-019-01246-w
Mateus, G. E., and Santiago, A. W. (2006). Disponibilidad léxica en estudiantes bogotanos. Folios 24, 3–26.
Mayr, U. (2002). On the dissociation between clustering and switching in verbal fluency: comment on Troyer, Moscovitch, Winocur, Alexander and Stuss. Neuropsychologia 40, 562–566. doi: 10.1016/s0028-3932(01)00132-4
McColgan, P., Evans, J. R., Breen, D. P., Mason, S. L., Barker, R. A., and Williams-Gray, C. H. (2012). Addenbrooke’s Cognitive Examination-Revised for mild cognitive impairment in Parkinson’s disease. Mov. Disord. 27, 1173–1177.
Melloni, M., Sedeño, L., Hesse, E., García-Cordero, I., Mikulan, E., Plastino, A., et al. (2015). Cortical dynamics and subcortical signatures of motor-language coupling in Parkinson’s disease. Sci. Rep. 5:11899.
Migliaccio, R., Boutet, C., Valabregue, R., Ferrieux, S., Nogues, M., Lehéricy, S., et al. (2016). The brain network of naming: a lesson from primary progressive aphasia. PLoS One 11:e0148707. doi: 10.1371/journal.pone.0148707
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R., and Hodges, J. R. (2006). The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatric Psychiatry: J. Psychiatry Late Life Allied Sci. 21, 1078–1108. doi: 10.1002/gps.1610
Moreira, H. S., Costa, A. S., Machado, A., Castro, S. L., Lima, C. F., and Vicente, S. G. (2019). Distinguishing mild cognitive impairment from healthy aging and Alzheimer’s Disease: the contribution of the INECO Frontal Screening (IFS). PLoS One 14:e0221873. doi: 10.1371/journal.pone.0221873
Moreno-Martínez, F. J., and Rodríguez-Rojo, I. C. (2015). The Nombela 2.0 semantic battery: an updated Spanish instrument for the study of semantic processing. Neurocase 21, 773–785. doi: 10.1080/13554794.2015.1006644
Navarro, M. C., Marmolejo-Ramos, F., Vásquez, V., Carrea, B., Vélez, J. I., and Mebarak Chams, M. (2020). An exploratory study for assessment of multimodal semantic memory in Colombian Children. Int. J. Psychol. Res. 13, 49–58. doi: 10.21500/20112084.4847
Péran, P., Cardebat, D., Cherubini, A., Piras, F., Luccichenti, G., Peppe, A., et al. (2009). Object naming and action-verb generation in Parkinson’s disease: a fMRI study. Cortex 45, 960–971. doi: 10.1016/j.cortex.2009.02.019
Phillips, L., Litcofsky, K. A., Pelster, M., Gelfand, M., Ullman, M. T., and Charles, P. D. (2012). Subthalamic nucleus deep brain stimulation impacts language in early Parkinson’s disease. PLoS One 7:e42829. doi: 10.1371/journal.pone.0042829
Portin, R., Laatu, S., Revonsuo, A., and Rinne, U. K. (2000). Impairment of semantic knowledge in Parkinson disease. Arch. Neurol. 57, 1338–1343.
Possin, K. L., Feigenbaum, D., Rankin, K. P., Smith, G. E., Boxer, A. L., Wood, K., et al. (2013). Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology 80, 2180–2185. doi: 10.1212/wnl.0b013e318296e940
Pulvermüller, F. (2005). Brain mechanisms linking language and action. Nat. Rev. Neurosci. 6, 576–582. doi: 10.1038/nrn1706
Pulvermüller, F., Hauk, O., Nikulin, V. V., and Ilmoniemi, R. J. (2005). Functional links between motor and language systems. Eur. J. Neurosci. 21, 793–797. doi: 10.1111/j.1460-9568.2005.03900.x
Quiroga, R. Q. (2012). Concept cells: the building blocks of declarative memory functions. Nat. Rev. Neurosci. 13, 587–597. doi: 10.1038/nrn3251
R Core Team. (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reverberi, C., Cherubini, P., Baldinelli, S., and Luzzi, S. (2014). Semantic fluency: cognitive basis and diagnostic performance in focal dementias and Alzheimer’s disease. Cortex 54, 150–164. doi: 10.1016/j.cortex.2014.02.006
Reverberi, C., Laiacona, M., and Capitani, E. (2006). Qualitative features of semantic fluency performance in mesial and lateral frontal patients. Neuropsychologia 44, 469–478. doi: 10.1016/j.neuropsychologia.2005.05.011
Reyes, M. A., Lloret, S. P., Gerscovich, E. R., Martin, M. E., Leiguarda, R., and Merello, M. (2009). Addenbrooke’s cognitive examination validation in Parkinson’s disease. Eur. J. Neurol. 16, 142–147.
Robinson, G., Shallice, T., Bozzali, M., and Cipolotti, L. (2012). The differing roles of the frontal cortex in fluency tests. Brain 135, 2202–2214. doi: 10.1093/brain/aws142
Rogers, R. D., Sahakian, B. J., Hodges, J. R., Polkey, C. E., Kennard, C., and Robbins, T. W. (1998). Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain 121, 815–842. doi: 10.1093/brain/121.5.815
Rosen, V. M., and Engle, R. W. (1997). The role of working memory capacity in retrieval. J. Exp. Psychol. Gen. 126, 211–227.
Rosenthal, L. S., Salnikova, Y. A., Pontone, G. M., Pantelyat, A., Mills, K. A., Dorsey, E. R., et al. (2017). Changes in verbal fluency in Parkinson’s disease. Mov. Disord. Clin. Practice 4, 84–89.
Salmazo-Silva, H., Parente, M. A., de, M. P., Rocha, M. S., Baradel, R. R., Cravo, A. M., et al. (2017). Lexical-retrieval and semantic memory in Parkinson’s disease: the question of noun and verb dissociation. Brain Lang. 165, 10–20. doi: 10.1016/j.bandl.2016.10.006
Sánchez, L. J., and Aguirre, B. (1992). Léxico Fundamental del Español: Situaciones, Temas y Nociones. Madrid: SGEL. S.A. Sociedad General Española de Librería.
Sarasola, D., De Luján-Calcagno, M., Sabe, L., Crivelli, L., Torralba, T., Roca, M., et al. (2005). El Addenbrooke’s Cognitive Examination en español para el diagnóstico de demencia y para la diferenciación entre enfermedad de Alzheimer y demencia frontotemporal. Revista Neurol. 41, 717–721.
Schwartz, S., and Baldo, J. (2001). Distinct patterns of word retrieval in right and left frontal lobe patients: a multidimensional perspective. Neuropsychologia 39, 1209–1217. doi: 10.1016/s0028-3932(01)00053-7
Silveri, M. C., Traficante, D., Lo Monaco, M. R., Iori, L., Sarchioni, F., and Burani, C. (2017). Word selection processing in Parkinson’s disease: when nouns are more difficult than verbs. Cortex 100, 8–20. doi: 10.1016/j.cortex.2017.05.023
Simons, J. S., and Spiers, H. J. (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 4, 637–648. doi: 10.1038/nrn1178
Squire, L. R. (2009). The legacy of patient HM for neuroscience. Neuron 61, 6–9. doi: 10.1016/j.neuron.2008.12.023
Squire, L. R., and Wixted, J. T. (2011). The cognitive neuroscience of human memory since HM. Annu. Rev. Neurosci. 34, 259–288. doi: 10.1146/annurev-neuro-061010-113720
Stuss, D. T., Alexander, M. P., Hamer, L., Palumbo, C., Dempster, R., Binns, M., et al. (1998). The effects of focal anterior and posterior brain lesions on verbal fluency. J. Int. Neuropsychol. Soc. 4, 265–278. doi: 10.1017/s1355617798002653
Suárez-García, D. M. A., Birba, A., Zimerman, M., Diazgranados, J. A., Lopes da Cunha, P., Ibáñez, A., et al. (2021). Rekindling action language: a neuromodulatory study on Parkinson’s disease patients. Brain Sci. 11:887. doi: 10.3390/brainsci11070887
Tagini, S., Seyed-Allaei, S., Scarpina, F., Toraldo, A., Mauro, A., Cherubini, P., et al. (2018). When fruits lose to animals: disorganized search of semantic memory in Parkinson’s disease. Neuropsychology [online ahead of print] doi: 10.1037/neu0000429
Tirado, C., Khatin-Zadeh, O., Gastelum, M., Jones, N. L., and Marmolejo-Ramos, F. (2018). The strength of weak embodiment. Int. J. Psychol. Res. 11, 77–85. doi: 10.21500/20112084.3420
Torralva, T., Roca, M., Gleichgerrcht, E., Lopez, P., and Manes, F. (2009). INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia–Corrected Version. J. Int. Neuropsychol. Soc. 15, 777–786. doi: 10.1017/s1355617709990415
Troyer, A. K., Moscovitch, M., Winocur, G., Alexander, M. P., and Stuss, D. (1998). Clustering and switching on verbal fluency: the effects of focal frontal and temporal-lobe lesions. Neuropsychologia 36, 499–504. doi: 10.1016/s0028-3932(97)00152-8
Tulving, E. (1972). Episodic and Semantic Memory. En Organization of Memory. (pp. XIII, 423–XIII, 423). Oxford: Academic Press.
Ullman, M. T. (2001). A neurocognitive perspective on language: the declarative/procedural model. Nat. Rev. Neurosci. 2, 717–726. doi: 10.1038/35094573
Ullman, M. T. (2004). Contributions of memory circuits to language: the declarative/procedural model. Cognition 92, 231–270. doi: 10.1016/j.cognition.2003.10.008
Unsworth, N., Spillers, G. J., and Brewer, G. A. (2011). Variation in verbal fluency: a latent variable analysis of clustering, switching, and overall performance. Q. J. Exp. Psychol. 64, 447–466. doi: 10.1080/17470218.2010.505292
Warrington, E. K., and Shallice, T. (1984). Category specific semantic impairments. Brain 107, 829–853. doi: 10.1093/brain/107.3.829
Wilcox, R. R., and Tian, T. S. (2011). Measuring effect size: a robust heteroscedastic approach for two or more groups. J. Appl. Stat. 38, 1359–1368. doi: 10.1080/02664763.2010.498507
Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., et al. (1982). Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatric Res. 17, 37–49. doi: 10.1016/0022-3956(82)90033-4
Keywords: Parkinson’s disease, semantic memory, verbal fluency, lexical availability, embodied cognition
Citation: Cardona JF, Grisales-Cardenas JS, Trujillo-Llano C, Diazgranados JA, Urquina HF, Cardona S, Torres A, Torres LA, Gonzalez LM, Jaramillo T, Cediel J, Oñate-Cadena N, Mateus-Ferro G and Marmolejo-Ramos F (2021) Semantic Memory and Lexical Availability in Parkinson’s Disease: A Statistical Learning Study. Front. Aging Neurosci. 13:697065. doi: 10.3389/fnagi.2021.697065
Received: 18 April 2021; Accepted: 07 July 2021;
Published: 30 July 2021.
Edited by:
Adolfo M. García, Universidad de San Andrés, ArgentinaReviewed by:
Manuel De Vega, University of La Laguna, SpainKarim Johari, University of Iowa Hospitals and Clinics, United States
Copyright © 2021 Cardona, Grisales-Cardenas, Trujillo-Llano, Diazgranados, Urquina, Cardona, Torres, Torres, Gonzalez, Jaramillo, Cediel, Oñate-Cadena, Mateus-Ferro and Marmolejo-Ramos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan F. Cardona, felipe.cardona@correounivalle.edu.co
†These authors have contributed equally to this work
 Juan F. Cardona
Juan F. Cardona Johan S. Grisales-Cardenas
Johan S. Grisales-Cardenas Catalina Trujillo-Llano
Catalina Trujillo-Llano Jesús A. Diazgranados3
Jesús A. Diazgranados3  Hugo F. Urquina
Hugo F. Urquina Sebastián Cardona
Sebastián Cardona Alejandra Torres
Alejandra Torres Lina M. Gonzalez
Lina M. Gonzalez Judith Cediel
Judith Cediel Nelcy Oñate-Cadena
Nelcy Oñate-Cadena Fernando Marmolejo-Ramos
Fernando Marmolejo-Ramos