She Doesn’t Even Go Here: The Role of Inflammatory Astrocytes in CNS Disorders
- 1Department of Clinical Neurosciences, Hotchkiss Brain Institute and Snyder Institute for Chronic Diseases, University of Calgary, Calgary, AB, Canada
- 2Department of Cell Biology & Anatomy, Hotchkiss Brain Institute and Snyder Institute for Chronic Diseases, University of Calgary, Calgary, AB, Canada
Astrocyte heterogeneity is a rapidly evolving field driven by innovative techniques. Inflammatory astrocytes, one of the first described subtypes of reactive astrocytes, are present in a variety of neurodegenerative diseases and may play a role in their pathogenesis. Moreover, genetic and therapeutic targeting of these astrocytes ameliorates disease in several models, providing support for advancing the development of astrocyte-specific disease modifying therapies. This review aims to explore the methods and challenges of identifying inflammatory astrocytes, the role these astrocytes play in neurological disorders, and future directions in the field of astrocyte heterogeneity.
Introduction
Astrocytes are the most abundant glial (non-neuronal) cell type of the central nervous system (CNS). Among many functions, they play a critical role in maintaining blood-brain barrier function (Engelhardt, 2003), supporting neurons and other glia (Jessen, 2004), and reacting to changes in both the local (Henrik Heiland et al., 2019; Shigetomi et al., 2019) and external environment (Rothhammer et al., 2016; Wheeler et al., 2020b). Beyond these homeostatic functions, astrocytes can respond to several stimuli and subsequently display profound genetic, morphological, and functional changes in a process termed reactive astrogliosis (Sofroniew, 2009; Escartin et al., 2021; Figure 1). Reactive astrogliosis can be triggered by injury (Cotrina et al., 2015; Okada et al., 2018), inflammation (Hansson et al., 2016), or stress (Chen et al., 2020; Kogel et al., 2021), and can result in a feed-forward process, where an initial stimulus induces a reactive astrocyte response, which triggers the release of intracellular and soluble factors that further drive this response. Reactive astrogliosis, reactive astrocyte response, and (astro)gliosis are terms often used interchangeably in the field of astrocyte biology. While their exact definition might differ between studies, potentially due to the heterogeneity in reactive astrocyte phenotypes and functions (see below), they generally refer to astrocyte responses to stimuli beyond physiological functions, as described above.
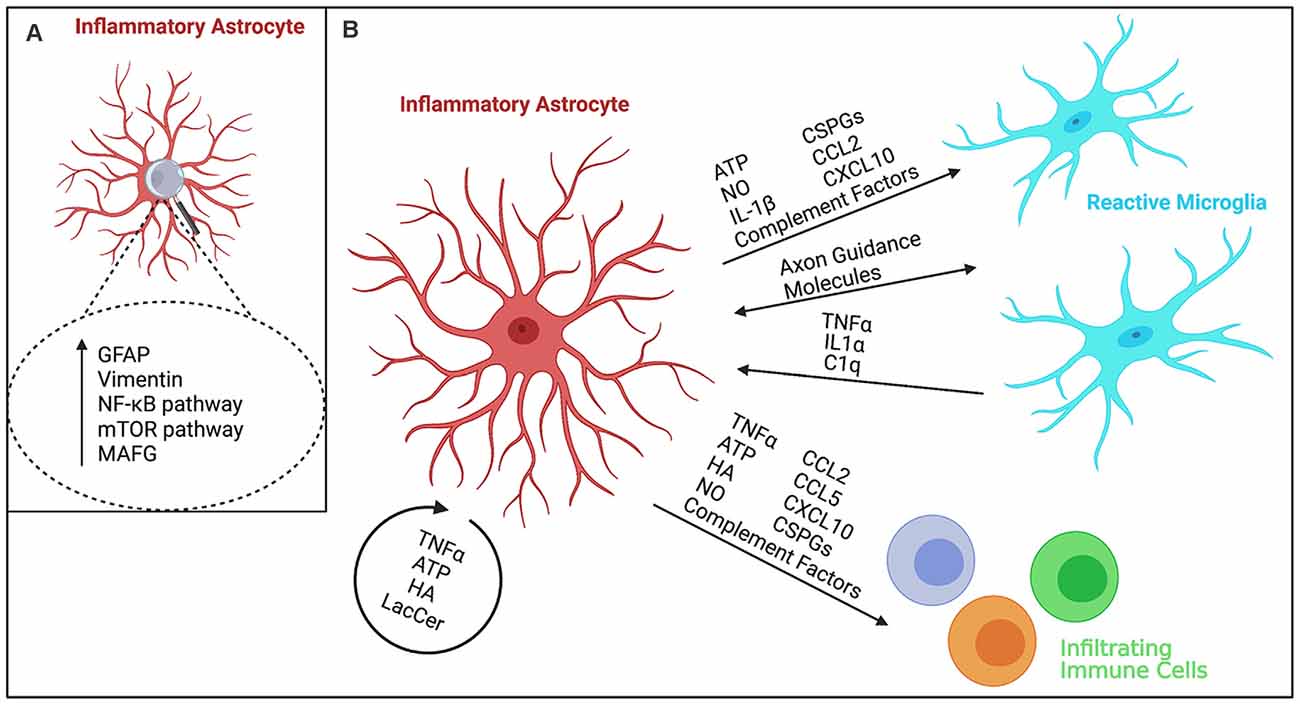
Figure 1. Schematic representation of inflammatory astrocytes and their interactions with other cells during neuroinflammation. (A) Markers upregulated in inflammatory astrocytes and pathways regulating their activation. While GFAP and Vimentin are commonly upregulated in reactive astrocytes (Sofroniew, 2009; Escartin et al., 2021), inflammatory astrocytes show increased expression of the NF-κB pathway (Zamanian et al., 2012; Lian et al., 2015) and activation of MAFG and mTOR signaling (Sofroniew, 2009; Zamanian et al., 2012; Li et al., 2015; Wheeler et al., 2020a). (B) Schematic overview of various known cytokine, chemokine, ionic, and protein interactions of inflammatory astrocytes with neighboring cells. Inflammatory astrocytes can affect microglia and infiltrating immune cells by secreting immune factors such as cytokines and chemokines (Sofroniew, 2009; Zamanian et al., 2012; Liddelow et al., 2017; Clark et al., 2019), complement proteins (Zamanian et al., 2012; Lian et al., 2016; Liddelow et al., 2017), as well as extracellular matrix molecules such as hyaluronan (HA; Kuipers et al., 2016; Nagy et al., 2019) and chondroitin sulfate proteoglycans (CSPGs; Keough et al., 2016; Stephenson et al., 2018), and cytotoxic factors, such as nitric oxide (NO), adenosine triphosphate (ATP; Orellana et al., 2011), and mitochondrial fragments (Joshi et al., 2019). These cells can, in turn, affect astrocyte reactivity as well (Colombo and Farina, 2016; Rothhammer et al., 2016; Liddelow et al., 2017; Williams et al., 2020; Clark et al., 2021). In particular, microglia have been shown to affect inflammatory astrocyte function (Liddelow et al., 2017; Yun et al., 2018; Joshi et al., 2019), while concurrently inflammatory astrocytes release many microglia-activating factors (Zamanian et al., 2012; Guedes et al., 2018) resulting in a feed-forward loop of activation. In addition, microglia-astrocyte crosstalk (Matejuk and Ransohoff, 2020) has been implicated in driving disease pathology, for example through the release of chemokines/cytokines (Itoh et al., 2017) and direct protein-protein interaction through axon guidance molecules, such as Sema4D/PlexinB2 and EphrinB3/EphB3 (Clark et al., 2021). Astrocytes can also activate themselves in an autocrine manner through the release of cytokines (Sofroniew, 2009; Zamanian et al., 2012; Escartin et al., 2021), ATP (Sofroniew, 2009; Zamanian et al., 2012), inflammatory HA (Kuipers et al., 2016; Nagy et al., 2019), and certain glycolipids such as lactosylceramide (LacCer; Mayo et al., 2014).
While the concept of (reactive) astrogliosis has been known since it was first observed early in the history of neuroscience (Rindfleisch, 1863; Müller, 1904), the heterogeneity of astrocyte phenotypes, function, and reactivity are becoming increasingly appreciated (Escartin et al., 2021). Due to this heterogeneity, the role of reactive astrogliosis in disease processes, such as neuroinflammation, is often multifaceted and remains an active area of research in the field. For example, reactive astrocytes can contribute to inflammation by promoting immune responses, but can also suppress these responses (Cordiglieri and Farina, 2010).
In parallel, at least two distinct types of reactive astrocytes were identified in initial studies examining the heterogeneity of astrocyte responses—inflammatory/neurotoxic and neuroprotective astrocytes, originally referred to as “A1” and “A2” astrocytes—analogous to proinflammatory M1 and anti-inflammatory M2 macrophages.
Inflammatory “A1” astrocytes are a classification of reactive astrocytes that are characterized by their neurotoxic, proinflammatory phenotype (Liddelow et al., 2017). They were first defined, alongside their neuroprotective counterparts, “A2” astrocytes, through pioneering experiments conducted in the Barres lab (Zamanian et al., 2012). To assess whether reactive astrocyte responses differ based on the insult given, they analyzed differentially expressed genes in reactive astrocytes that were induced either by experimental ischemic stroke or by neuroinflammation [through systemic administration of lipopolysaccharide (LPS; Zamanian et al., 2012)] and subsequently defined the two distinct activation states described above. However, similar to the evolution of the concept of M1/M2 macrophages, which has been expanded into a more continuous and plastic activation model, recent advances in single cell RNA sequencing (Wheeler et al., 2020a), as well as further analysis of the regional (Itoh et al., 2017; Boisvert et al., 2018; Williams et al., 2020) and phenotypic (Wheeler et al., 2020a) diversity of astrocytes, have made it apparent that the heterogeneity of (reactive) astrocytes extends beyond these two distinct states. In fact, a recent consensus review clarifying various idiosyncrasies in the field of astrocyte biology highlights the need to abandon the limited categorization of A1/A2 astrocytes, as the understanding of distinct astrocyte states has evolved beyond a binary paradigm (Escartin et al., 2021). Instead, a spectrum of reactive astrocyte states, characterized by gene expression signatures, as well as functional features, more accurately reflects astrocyte responses in neuropathology. Nevertheless, it is clear that under certain pathological conditions, astrocytes can adopt distinct inflammatory features and markers for this inflammatory phenotype are becoming more refined. Therefore, in this review, we will focus on the current state of literature on the role of inflammatory astrocytes in various neurological disorders.
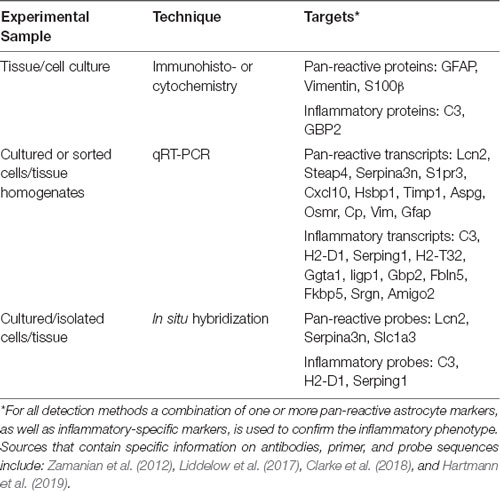
Genes that are differentially upregulated in inflammatory astrocytes (the originally coined “A1” astrocytes) have been identified as critical players in various proinflammatory pathways, including the antigen presentation pathway, the complement pathway, and the interferon response pathway (Zamanian et al., 2012). Activation of the complement pathway can result in detrimental neuroinflammation (Lian et al., 2015; Okrój and Potempa, 2019), and complement component 3 (C3) is markedly enriched in inflammatory astrocytes compared to resting and neuroprotective astrocytes. Therefore, it is now frequently used in histology to identify inflammatory astrocytes, along with the upregulation of general reactive astrocyte markers, such as glial fibrillary acidic protein (GFAP; Escartin et al., 2021; Table 1). In addition, a common method to identify inflammatory astrocytes in vitro or ex vivo is to assess the expression of a set of genes that were found to be uniquely upregulated in the originally defined “A1” astrocytes (Liddelow et al., 2017), by quantitative PCR (Table 1). Because there is not one specific marker for this subtype and inflammatory genes can be expressed by other cell types (or even other astrocyte subpopulations) as well, particularly under neuroinflammatory conditions, a combination of markers should be used to properly determine the inflammatory phenotype of reactive astrocytes and rule out contamination of other cell types. In addition, functional features should also be taken into account when defining whether a particular astrocyte subtype observed in a neuropathological condition has inflammatory capacities.
After their first genomic identification, subsequent studies have shown that in vitro, inflammatory astrocytes lose many of the homeostatic functions that astrocytes are known for, such as providing neurotrophic support, promoting synaptogenesis, and phagocytosis of synapses (Liddelow et al., 2017). It was shown that inflammatory astrocytes could be induced by soluble factors secreted by LPS-stimulated microglia. Of these factors, IL-1α, TNFα, and C1q, most potently in combination, were shown to be sufficient and necessary to polarize astrocytes to an inflammatory phenotype (Liddelow et al., 2017). Similarly, culturing naïve astrocytes with microglia conditioned media from Amyotrophic Lateral Sclerosis (ALS; Joshi et al., 2019) or Alzheimer’s Disease (AD; Xu et al., 2018) models resulted in these astrocytes taking on an inflammatory phenotype.
Inflammatory astrocytes have been the primary focus of neurological disease research, in part because techniques to identify neuroprotective astrocytes have remained elusive, whereas inflammatory astrocytes are more readily identified using the methods described above. As such, the potential role of inflammatory/neurotoxic astrocytes in neurodegenerative and neuroinflammatory diseases has recently been the subject of an increasing number of studies. Here, we discuss the roles that inflammatory astrocytes (may) play in these diseases, the efforts that are being made to pharmacologically target inflammatory astrocytes, and the limitations in studying this specific phenotype.
Alzheimer’s Disease
Alzheimer’s disease is a progressive, neurodegenerative disease characterized by the accumulation of amyloid-beta plaques and neurofibrillary tangles of the microtubule-associated protein tau (Dickson and Vickers, 2001). The exact role of astrocytes in the propagation (or “seeding”) of tau tangles is debated and is a growing, active area of research. Astrocytes have been observed to internalize tau. However, it is yet unclear whether (or when) this internalization leads to degradation or propagation of tau, and whether this contributes to the induction of an inflammatory phenotype in astrocytes (Kovacs, 2020; Reid et al., 2020; Fleeman and Proctor, 2021). In AD, the presence of reactive astrocytes often precedes the formation of the disease’s characteristic histopathologies (Heneka et al., 2005; Orre et al., 2014). Moreover, a recent single cell analysis of non-neuronal cell populations in the 5xFAD transgenic mouse model of AD revealed a transient astrocyte response as the disease progresses, from a GFAP-low state to a GFAP-high state, as well as an AD-specific population termed “disease-associated astrocytes” (Habib et al., 2020). As such, there is great interest in determining the role of these reactive astrocytes in the pathogenesis of AD, and advances in RNA sequencing technology drive increasingly refined analyses of their phenotypes and functions.
To quantify inflammatory astrocyte responses, the density of C3+ cells with astrocyte morphology was analyzed in post-mortem AD tissue. C3+ astrocyte-like cells were found to be enriched in the upper cerebral cortex of patients. Interestingly, control tissue also showed significant numbers of C3+ astrocyte-like cells, notably in the lower cerebral cortex and white matter (King et al., 2020). In another study, AD subjects had significantly more C3+ reactive astrocytes compared to matched controls in the entorhinal cortex, one of the first brain regions affected in AD, and the hippocampus (Balu et al., 2019). The majority of these C3+ astrocytes also co-expressed serine racemase (SR); however, the density of these astrocytes was concentrated primarily in superficial rather than deep layers. SR is an enzyme that is critical for the conversion of L-serine to D-serine, which can bind to NMDA receptors. These human results were confirmed with a murine model of AD using aged TgF344-AD rats and it was additionally found that these rats had increased activation of signaling pathways associated with extrasynaptic NMDAR activation in the hippocampus (Balu et al., 2019). As inflammatory astrocytes have been shown to lose normal astrocyte functions (Liddelow et al., 2017) and extrasynaptic NMDAR activation is linked to the deleterious effects of glutamate on plasticity and neuronal survival (Bading, 2017), these results implicate a potential involvement of inflammatory astrocytes in the progression of AD. Indeed, in a murine tauopathy model, astrocytes were shown to display an inflammatory expression profile in the early stages of neurodegeneration. In addition, C3 immunoreactivity was confined to reactive astrocytes and genetic deletion of C3 resulted in reduced neuronal loss, suggesting that these inflammatory astrocytes might contribute to tau-driven pathology (Wu et al., 2019). In another murine AD model, activation of melanocortin receptors by its agonist D-Tyrosine resulted in a significant decrease in GFAP+/C3+ astrocytes in the CA1 region of the hippocampus (Lau et al., 2021). This decrease in inflammatory astrocyte numbers correlated with a significant decrease in amyloid plaques deposition and critical levels of toxic amyloid-β isomers in the hippocampus (Lau et al., 2021). These findings suggest that targeting GFAP+/C3+ astrocytes might be a potential therapeutic avenue in the treatment of AD. In addition, another study showed that in vitro, inflammatory astrocyte induction can be blocked by exogenously applied milk fat globule epidermal growth factor 8 (MFG-E8), production of which is reduced in these inflammatory astrocytes (Xu et al., 2018). In a study highlighting the glial effects of amyloid-β exposure in an AD model, activation of the NF-κB pathway, known to be involved in inflammatory astrocyte induction, was detected in astrocytes and subsequent neuronal release of C3 resulted in synaptic dysfunction (Lian et al., 2015). These studies highlight potential pathways to modulate inflammatory astrocyte activation and improve AD pathology.
Cerebral amyloid angiopathy (CAA) is a typical condition of AD pathology and is characterized by cerebrovascular deposition of amyloid protein. While the function of the amyloid protein remains elusive, its accumulation is toxic and known to induce apoptosis and drive neurodegeneration (Chow et al., 2010; Chen et al., 2017). In a murine model of early CAA, immune and glial responses were analyzed, and histology revealed perivascular reactive astrogliosis, identified by GFAP (reactive astrocytes), Thioflavin-S (vascular amyloid), and α-smooth muscle actin (vascular smooth muscle cells) immunoreactivity, in 9-month-old mice. Of note, this phenomenon was absent in 3-month-old mice, suggesting a temporal, progressive astrocyte response. Further characterization of this response revealed a robust inflammatory astrocyte presence, as defined by colocalization of C3 and GFAP, in the hippocampus and cerebellum (Taylor et al., 2020).
As the accumulation of plaques and tangles occurs before the onset of symptoms, and there is a significant benefit of early intervention to patients, there is a strong drive to establish biomarkers for early AD pathology, as well as to develop non-invasive treatments. In this regard, the retina has become a popular area of study given its common embryological origin with the brain (Paquet et al., 2007). In the pursuit of an early biomarker for AD, upregulation of IL-1β in microglia and the additional presence of inflammatory astrocytes, as determined by GFAP and C3 colocalization, was found in retinal tissue from AD patients, indicating that the inflammatory activation of astrocytes is a feature of early AD pathology (Grimaldi et al., 2019). In addition, in a study comparing astrocyte-derived exosomes (ADE) in the plasma of AD patients to those of matched controls, levels of complement proteins and cytokines were analyzed (Goetzl et al., 2019). Complement factors, including C3d and C1q, one of the factors able to induce inflammatory astrocytes in vitro, were significantly higher in ADE from AD patients. With respect to cytokine profiles, while there was greater overlap between the two groups, AD ADE contained higher levels of IL-6, TNF-α and IL-1β (Goetzl et al., 2019), cytokines known to be involved in reactive astrogliosis (Choi et al., 2014).
Moreover, exercise is thought to be of benefit as a treatment for AD due to its capacity to stimulate the release of neurotrophic factors (Prado Lima et al., 2018), decreasing deposition of amyloid-β plaques (Prado Lima et al., 2018), and improving tau pathology (Belarbi et al., 2011; Fleeman and Proctor, 2021). In a study using exercise to treat a murine model of AD, rotarod exercise therapy resulted in a decrease of inflammatory astrocytes, along with reduced amyloid-β deposition, neuronal loss, and cognitive decline, showing that astrocyte reactivity correlates with treatment effects as well (Nakanishia et al., 2021).
Huntington’s Disease
Huntington’s disease (HD) is a neurodegenerative disease, primarily affecting the basal ganglia, that is caused by a dominantly inherited CAG trinucleotide repeat expansion in the huntingtin gene on chromosome 4 (McColgan and Tabrizi, 2018). In humans, reactive (fibrillary) astrogliosis within the corpus striatum is used to classify progressive stages of HD (Rüb et al., 2016). It has been shown that astrocytes from HD patients become physiologically and morphologically activated when exposed to mutant huntingtin, as determined by increased GFAP staining and morphological changes—specifically, thicker processes and a larger somata (Faideau et al., 2010). Additionally, these reactive astrocytes have significantly decreased expression of the glutamate transporters GLAST and GLT-1, which leads to a subsequent decrease in a critical astrocyte function—glutamate uptake (Rose et al., 2018). This is of interest, as loss of physiological astrocyte functions is characteristic of in vitro generated inflammatory astrocytes (Liddelow et al., 2017).
Single-nucleus RNA sequencing of astrocytes derived from the postmortem anterior cingulate cortex of HD and control human tissue went beyond the “A1/A2” classification and identified several distinct astrocyte “states” as determined by differential gene pattern expression (Al-Dalahmah et al., 2020). Additionally, this study confirmed that astrocytes in the caudate nucleus of HD grades III and IV express markers of an inflammatory state, showing C3 staining alone and double immunostaining for C3 and GFAP (Al-Dalahmah et al., 2020). These results suggest that inflammatory astrocytes in the anterior cingulate cortex are associated with progressive stages of HD.
Genomic and proteomic analysis of striatal astrocytes shows only the inflammatory astrocyte-associated gene Serping1 to be consistently upregulated across human samples and murine models (Diaz-Castro et al., 2019). However, akin to what has been reported in previous inflammatory astrocyte literature (Liddelow and Barres, 2017), astrocytes from HD striatum undergo significant morphological and transcriptional changes. Moreover, these changes are largely reversed by lowering mutant Huntington protein specifically in astrocytes (Diaz-Castro et al., 2019) showing a direct effect of mutant protein on reactive astrogliosis and highlighting the potential for therapeutics targeting reactive astrocytes in HD.
Multiple Sclerosis
Multiple Sclerosis (MS) is a progressive autoimmune demyelinating disease, characterized by infiltration of peripheral immune cells that target myelin within the CNS, and resulting in focal neuroinflammatory lesions, demyelination, and neurodegeneration. Astrocytes are thought to be involved in MS pathogenesis due to their capacity to promote entry of peripheral immune cells to the CNS, as well as to directly affect inflammatory processes in lesion formation (Ponath et al., 2018). One of the most widely used animal models used in MS research is experimental autoimmune encephalomyelitis (EAE), which involves inducing a T cell-driven immune response against myelin that leads to infiltration of these cells into the CNS, activation of resident cells, including astrocytes, and subsequent destruction of myelin, and damage to axons and neurons (Rangachari and Kuchroo, 2013; Lassmann and Bradl, 2017). As the model is driven by an immune response, EAE is often used to study the role of infiltrating and resident inflammatory cells in demyelination, because it recapitulates the inflammatory milieu found in actively demyelinating MS lesions (Lassmann and Bradl, 2017). In addition, MS is often studied in conjunction with optic neuritis, which is also pathologically characterized by peripheral immune cell infiltration (Bettelli et al., 2003; Lassmann and Bradl, 2017). Reactive astrocytes can be found at various stages of MS lesions. In addition to their abundance in chronic lesions, reactive astrocytes are present in the center and the active edge of acutely demyelinating lesions, as well as in bordering white matter (Kuhlmann et al., 2017; Ponath et al., 2017, 2018). In parallel, astrocytes become reactive, as determined by enhanced expression of GFAP, early and throughout EAE pathogenesis (Wang et al., 2005; Luo et al., 2008; Pham et al., 2009). C3 containing astrocytes are abundantly present in the center, as well as the expanding edge of actively demyelinating MS lesions, and can also be found in chronic lesion stages. Interestingly, these C3+ astrocytes are often located in close proximity to activated microglia/macrophages (Ingram et al., 2014; Liddelow et al., 2017).
In EAE, inflammatory astrocytes, as defined by C3 staining and inflammatory-specific transcript analysis, are prevalent in the retina and optic nerve tissue and are associated with retinal ganglion cell loss (Jin et al., 2019). Additionally, the complement cascade was found to be one of the most significantly upregulated pathways in the optic nerve of EAE mice (Tassoni et al., 2019). These results suggest that inflammatory astrocytes could be a potential target against some common visual symptoms of MS resulting from optic nerve degeneration. Additionally, this study observed significantly more C3 expressing astrocytes within the optic nerve of female mice as compared to males (Tassoni et al., 2019). This is of note, as the prevalence of MS is significantly higher in women than in men.
Recently, a pro-inflammatory and neurotoxic signature was also found in an astrocyte subset that is greatly expanded during EAE, identified by single cell RNA sequencing analysis. This subset is characterized by activation of the NF-κB and inducible nitric oxide synthase (iNOS) pathways, reduction of the NRF2 pathway, which limits oxidative stress and inflammation, and increased expression of the master transcriptional regulator MAFG (Wheeler et al., 2020a). Moreover, an astrocyte subset with similar features can be found in a combined scRNAseq dataset containing data from MS and control tissue samples. This inflammatory subset is detected in the majority of patient samples (12 out of 20) and greatly expanded in samples from MS patients compared to control samples (25-fold; Wheeler et al., 2020a).
There are various other models of MS that represent additional facets of its pathogenesis, such as the cuprizone model of demyelination and toxin-induced demyelination and remyelination (Lassmann and Bradl, 2017). However, studies exploring the presence and role of inflammatory astrocytes in these models are very limited.
One of the only known factors to correlate with MS progression is age, the majority of MS patients developing a progressive stage of the disease when they are between 40–50 years old (Tremlett and Zhao, 2017). This is significant, as both immune function and astrocyte functions (Palmer and Ousman, 2018), such as morphological changes (Jyothi et al., 2015), increased GFAP expression (Wu et al., 2005; Clarke et al., 2018), and activation of complement factors (Clarke et al., 2018), are known to change over time. In particular, aging astrocytes take on a more inflammatory phenotype (Clarke et al., 2018). In a study of over 1,000 proteins derived from the cerebrospinal fluid of 431 patients, a cluster of inflammatory astrocyte-derived proteins was found to be significantly upregulated in MS patients and had a significant, reproducible correlation with MS severity (Masvekar et al., 2019). Together, these findings suggest that inflammatory astrocytes may play an active role in various stages of MS pathogenesis and could provide a target for addressing damage to the optic nerve, as well as the CNS parenchyma of MS patients. Moreover, proteins derived from inflammatory astrocytes could prove to be a valuable biomarker to predict the progression of disease in MS.
Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by loss or degeneration of dopaminergic neurons in the substantia nigra in the midbrain and the development of Lewy bodies, protein aggregates that primarily contain the protein α-synuclein (Forno et al., 1986; Braak et al., 2003). It is a disease of unknown etiology commonly associated with aging and family history (Kalia and Lang, 2015). As a result of neurodegeneration, inflammation also plays a key role in PD. When neurons die, they release proinflammatory and cytotoxic molecules (Glass et al., 2010) that promote gliosis and immune responses. These responses lead to a feed-forward cycle wherein activated immune cells further respond by releasing additional proinflammatory factors (Lee et al., 2019), thereby perpetuating inflammation and neuronal damage.
The key contributors to PD pathogenesis with a proinflammatory relationship are astrocytes and microglia. A widely used model for PD is the MPTP model, based on the toxic properties of peripherally administered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which results in dopaminergic neurodegeneration in the striatum and substantia nigra, a pattern similar to the human disease (Meredith and Rademacher, 2011). In this model, systemic administration of LPS exacerbates microglial activation and induces the conversion of astrocytes to an inflammatory (C3+) phenotype (García-Domínguez et al., 2018). This shows that peripheral inflammation can trigger inflammatory astrocyte conversion under the conditions of dopaminergic neurodegeneration (García-Domínguez et al., 2018). In another murine model of PD, in which LPS is injected into the midbrain, genes associated with inflammatory astrocytes, as well as the potassium channel subunit Kir6.2, were upregulated in the substantia nigra (Song et al., 2021). Kir6.2 is induced by chronic metabolic stress, associated with the degeneration of dopaminergic neurons and can act as an inflammatory mediator (Liss et al., 2005; Du et al., 2014). Kir6.2 was shown to be expressed by astrocytes and its genetic deletion mitigated inflammatory astrocyte expression patterns and prevented dopaminergic neurodegeneration and behavioral deficits (Song et al., 2021). Additionally, it has been shown in vivo that NLY01, a glucagon-peptide-1 receptor agonist, is capable of blocking the astrocytic conversion to an inflammatory phenotype by preventing the microglial release of IL-1α, TNFα, and C1q (Yun et al., 2018). NLY01 was shown to be protective in two models of PD: the α-synuclein preformed fibrils (PFF) model of sporadic PD, and the progressive, lethal, constitutive α-synucleinopathy model (Yun et al., 2018). Furthermore, β-sitosterol-β-D-glucoside (BSSG) is a neurotoxin found in cyad seeds and its chronic consumption induces a progressive PD-like disease in humans and rats (Van Kampen and Robertson, 2017). In a study assessing the neuroinflammatory reaction to this neurotoxin, a significant induction of inflammatory astrocytes, verified by co-staining of C3 with GFAP or S100β, was observed with a single BSSG injection to the substantia nigra, correlating with loss of dopaminergic neurons (Luna-Herrera et al., 2020). Together, these studies illustrate the involvement of inflammatory astrocytes in the pathogenesis of PD and provide potential targets to regulate their induction.
Prion Diseases
Prion diseases are a group of neurodegenerative diseases caused by the conversion of prion protein (PrP) to an abnormal, misfolded form of the protein (PrPSc). This conversion is characterized by a shift of the normal prion protein α-helical structure to a β-pleated sheet structure, which forms amyloid deposits. The shift of PrP into PrPSc in prion diseases has a cascading effect, where the misfolded PrPSc protein acts as a seed and propagates the misfolding of additional proteins. However, the mechanism for this cascade is unknown. Among neurodegenerative disorders, prion diseases are unique because they can occur either spontaneously, genetically, or by transmission. The most common prion diseases are Creutzfeldt-Jakob Disease (CJD), its bovine equivalent Bovine Spongiform Encephalopathy (BSE, “mad cow disease”), and scrapie in sheep and goats (Belay, 1999; Geschwind, 2015).
Reactive astrogliosis is a hallmark of all prion diseases. Astrocytes play roles in prion diseases both in their capacity as proponents of neuroinflammatory response and as promotors of PrPSc spread and aggregation (Carroll and Chesebro, 2019). In a murine model of prion disease, induced via intracerebral injection of scrapie brain homogenate, reactive astrogliosis occurs early and throughout the clinical course and coincides with PrPSc deposition (Tribouillard-Tanvier et al., 2012). While prion diseases were originally thought to involve a limited neuroimmune response (Belay, 1999; Geschwind, 2015), analysis of cytokines and chemokines in the scrapie inoculation-induced mouse model showed that protein levels of, among many others, IL-1Ra, CXCL10 (IP-10), and CCL5 (RANTES) were significantly increased as the disease progressed (Carroll et al., 2015). These factors are also among many produced by in vitro activated human astrocytes (Choi et al., 2014). Additionally, the majority of the inflammatory genes upregulated in scrapie-inoculated mice can be induced by the NF-κB pathway, which is activated in these mice (Tribouillard-Tanvier et al., 2012; Carroll et al., 2015). This is of note as NF-κB pathway activation has been associated with C3 production by astrocytes as well (Lian et al., 2015).
Numerous inflammatory (C3+ or GBP2+) astrocytes can be found in tissues of both murine prion disease and human CJD cases (Hartmann et al., 2019), and expression of C3 and GBP2 is significantly upregulated in CJD brain tissue and is associated with disease duration and risk genotype (Ugalde et al., 2020). Blocking the induction of inflammatory astrocytes in prion-infected triple KO mouse (TKO-mice), lacking TNFα, IL-1α, and C1q expression, however, does not affect PrPSc protein titers or deposition. Moreover, the disease course is significantly accelerated in these mice, indicating that the inflammatory response of astrocytes might constitute a protective mechanism limiting the damaging effects of PrPSc accumulation (Hartmann et al., 2019). While their presence and involvement in the pathogenesis of prion diseases is apparent, the overall effect of their involvement, whether helpful or harmful, remains to be seen.
Discussion
Inflammatory astrocytes have been shown to play roles in inflammatory and neurodegenerative mechanisms of neurological disease. Of note, in the healthy aging brain, astrocytes have also been shown, in vivo, to take on a genetic profile similar to that seen in neuroinflammation-induced astrocytes (Clarke et al., 2018). Aging is a significant risk factor for many CNS pathologies (Palmer and Ousman, 2018; Hou et al., 2019); for example, it correlates with disease progression in MS (Confavreux and Vukusic, 2006; Koch et al., 2009, 2010; Hou et al., 2019). It is therefore critical to explore the role of these aging and potentially inflammatory astrocytes in the exacerbation of disease and injury. It is likely that aging-induced inflammatory astrocytes contribute to neuroinflammation and neurodegenerative processes in general, given the demonstrated neurotoxic functions of inflammatory astrocytes (Liddelow et al., 2017), their prevalence with age (Clarke et al., 2018), and their observed presence in many neurodegenerative diseases (Liddelow and Barres, 2017; Clarke et al., 2018; Goetzl et al., 2019; Hartmann et al., 2019; Tassoni et al., 2019; Song et al., 2021).
A confounding factor in all studies examining the role of astrocytes in disease pathogenesis is the fact that astrocyte and microglial activation commonly happens in concert. Due to obvious experimental challenges, many of the studies discussed in this review do not directly address the potential inflammatory feed forward cycle of inflammatory or generally reactive astrocytes and activated microglia that might contribute to the progression of disease (Figure 1). Inflammatory activation of astrocytes can result in the release of proinflammatory cytokines that activate microglia and mediate neurotoxic inflammation. In turn, the proinflammatory factors released by activated microglia can further activate inflammatory astrocytes (Liddelow et al., 2017), thereby creating a detrimental, inflammatory feed forward cycle that exacerbates disease severity. Therefore, disease-modifying treatments targeting inflammatory astrocytes are of great interest, as eliminating a main component of this inflammatory cycle can mitigate its damage.
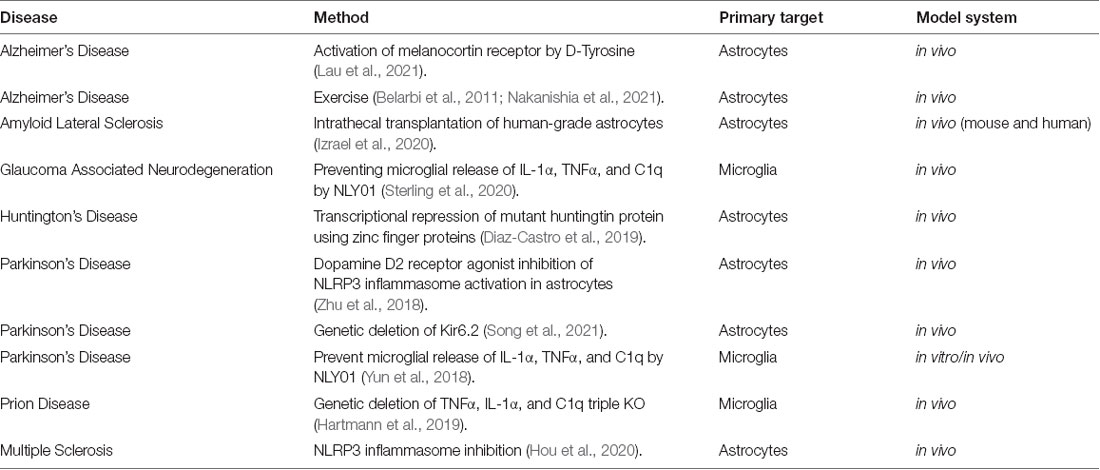
While still an area to be further explored, there have been some advances in therapeutically targeting inflammatory astrocytes, specifically, during disease (Table 2). NLY01, used to block the induction of inflammatory astrocytes by inhibiting the release of IL-1α, TNF-α and C1q from microglia, was successfully used in studies of PD (Yun et al., 2018) and glaucoma-associated neurodegeneration (Sterling et al., 2020) to ameliorate disease severity. With respect to ALS, studies regarding the transplantation of glial precursor cells demonstrated glial transplantation as a method to delay disease onset and ameliorate clinical symptoms (Kondo et al., 2014; Izrael et al., 2020). This has led to a current clinical study (National Library of Medicine, NCT03482050, 2018) of intrathecal transplantation of human-grade astrocytes in the hopes of reducing the large population of inflammatory astrocytes causing damage in patients with ALS (Izrael et al., 2020). These treatments show the potential of this avenue in disease management.
There are significant limitations in studying inflammatory astrocytes, as the mechanism underlying their induction has only been shown in vitro (Liddelow et al., 2017) and upregulation of genes associated with inflammatory astrocytes is not ubiquitous in all neurodegenerative diseases and their models. By extension, identifying the inflammatory nature of reactive astrocytes in particular settings is challenging. However, the previously mentioned recent consensus article provides clarity regarding markers and terminology to be used when describing reactive astrocytes (Escartin et al., 2021). Thus far, the methods used to identify inflammatory astrocytes are generally two-fold: co-expression of GFAP and C3 along with a transcription of a commonly defined subset of inflammatory astrocyte-specific genes. However, C3 upregulation is not unique to inflammatory astrocytes, as the complement cascade is activated in numerous inflammatory conditions (Markiewski and Lambris, 2007). Therefore, co-expression must be clearly shown and verified—preferably through quantitative techniques. Often these methods of identification are used individually or in conjunction to identify the presence of inflammatory astrocytes; however, studies that go beyond this correlation and delve into the mechanisms of inflammatory astrocyte induction and its consequences are still limited.
An additional challenge in determining the contribution of inflammatory astrocytes to neurological disorders is the innate limitations of using animal models. While rodent models provide valuable tools to dissect biological processes, there are various physiological differences between rodents and humans that need to be taken into account when extrapolating findings (Perlman, 2016). With regard to astrocyte responses, while general (reactive) gene expression profiles are similar between human and mouse astrocytes, differences in the molecular pathways induced by some stimuli do exist and it cannot be ruled out that the distinct expression profiles and functions of human astrocytes differ from those in the mouse models used to determine their role in neuropathology (Li et al., 2021). In addition to animal models, the postmortem tissue of patients is a valuable source to determine disease-specific mechanisms. However, many factors that are difficult (or impossible) to control can introduce variation in data and confound findings, such as the cause of death, stage of disease at the time of death, and postmortem interval (time from death to autopsy; Di Lullo and Kriegstein, 2017). For example, available tissue from postmortem sources is generally skewed towards the end or advanced stage of the disease, whereas biopsy material is often from cases that display an abnormal disease pattern. Moreover, obtaining control tissue (either from healthy individuals or non-related neurological conditions) that is properly matched for sex, gender, age, and lifestyle factors is challenging. Therefore, using a combination of techniques, models, and tissue sources is best suited to dissect the intricate interplay of the cellular and molecular mechanisms driving pathology. Moreover, the exact function of specific reactive astrocyte states or subtypes likely depends on the pathological context and stage of disease, due to the suggested transient and/or plastic nature of reactive astrocyte states/subtypes (Mayo et al., 2014; Habib et al., 2020). Only once the exact contribution of inflammatory astrocytes to the various stages of the disease has been mapped, targeting this population specifically at the appropriate stage could provide an effective treatment strategy.
Developments in the field of single cell RNA sequencing have advanced studies of reactive astrocyte responses beyond the initial binary classification of inflammatory/neurotoxic and neuroprotective astrocytes. For example, several unique clusters of reactive astrocytes were identified in EAE and MS tissue (Wheeler et al., 2020a), and differential effects of ablation of reactive astrocytes at different stages of EAE suggest that this astrocyte response might be transient and/or plastic (Mayo et al., 2014). In addition, transient and disease-specific reactive astrocyte populations were observed in the 5xFAD model of AD (Habib et al., 2020). Therefore, as discussed before, a refined view of astrocyte heterogeneity and plasticity allows for a more comprehensive classification of reactive astrocyte populations/states and potentially a greater understanding of their role in disease pathology.
Recent additional advances have also allowed for exploration in the field of cell-cell crosstalk. In a recent study using an mRNA barcoding technique that takes advantage of the pseudorabies virus’s capacity to spread between interacting cells (coined RABID-seq), labeling cells interacting with astrocytes showed that pathogenic astrocytes connected to microglia display an inflammatory signature, and that their crosstalk is mediated, amongst others, by axon guidance molecules (Clark et al., 2021). Advances such as these are critical as they allow for an understanding of the complex cellular interactions that perpetuate inflammation. However, it also forebodes that, as the narrative of the involvement of astrocytes in disease continues to develop, it may be that the classification of astrocyte subsets will be defined more by their function in relation to a specific disease state, rather than a specific binary phenotype based on gene expression signatures.
Author Contributions
JR performed literature searches, and structured and wrote the manuscript. HK gave structural and contextual input, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by an operating grant from the Multiple Sclerosis Society of Canada (MSSOC, grant # 3585 to HK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Dalahmah, O., Sosunov, A. A., Shaik, A., Ofori, K., Liu, Y., Vonsattel, J. P., et al. (2020). Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol. Commun. 8:19. doi: 10.1186/s40478-020-0880-6
Bading, H. (2017). Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med. 214, 569–578. doi: 10.1084/jem.20161673
Balu, D. T., Pantazopoulos, H., Huang, C. C. Y., Muszynski, K., Harvey, T. L., Uno, Y., et al. (2019). Neurotoxic astrocytes express the D-serine synthesizing enzyme, serine racemase, in Alzheimer’s disease. Neurobiol. Dis. 130:104511. doi: 10.1016/j.nbd.2019.104511
Belarbi, K., Burnouf, S., Fernandez-Gomez, F. J., Laurent, C., Lestavel, S., Figeac, M., et al. (2011). Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 43, 486–494. doi: 10.1016/j.nbd.2011.04.022
Belay, E. D. (1999). Transmissible spongiform encephalopathies in humans. Annu. Rev. Microbiol. 53, 283–314. doi: 10.1146/annurev.micro.53.1.283
Bettelli, E., Pagany, M., Weiner, H. L., Linington, C., Sobel, R. A., and Kuchroo, V. K. (2003). Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081. doi: 10.1084/jem.20021603
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N., and Allen, N. J. (2018). The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285. doi: 10.1016/j.celrep.2017.12.039
Braak, H., Del Tredici, K., Rüb, U., De Vos, R. A. I., Jansen Steur, E. N. H., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/s0197-4580(02)00065-9
Carroll, J. A., and Chesebro, B. (2019). Neuroinflammation, microglia and cell-association during prion disease. Viruses 11:65. doi: 10.3390/v11010065
Carroll, J. A., Striebel, J. F., Race, B., Phillips, K., and Chesebro, B. (2015). Prion infection of mouse brain reveals multiple new upregulated genes involved in neuroinflammation or signal transduction. J. Virol. 89, 2388–2404. doi: 10.1128/JVI.02952-14
Chen, G. F., Xu, T. H., Yan, Y., Zhou, Y. R., Jiang, Y., Melcher, K., et al. (2017). Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 38, 1205–1235. doi: 10.1038/aps.2017.28
Chen, Y., Qin, C., Huang, J., Tang, X., Liu, C., Huang, K., et al. (2020). The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif. 53:e12781. doi: 10.1111/cpr.12781
Choi, S. S., Lee, H. J., Lim, I., Satoh, J. I., and Kim, S. U. (2014). Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9:e92325. doi: 10.1371/journal.pone.0092325
Chow, V. W., Mattson, M. P., Wong, P. C., and Gleichmann, M. (2010). An overview of APP processing enzymes and products. Neuromol. Med. 12, 1–12. doi: 10.1007/s12017-009-8104-z
Clark, D. P. Q., Perreau, V. M., Shultz, S. R., Brady, R. D., Lei, E., Dixit, S., et al. (2019). Inflammation in traumatic brain injury: roles for toxic A1 astrocytes and microglial-astrocytic crosstalk. Neurochem. Res. 44, 1410–1424. doi: 10.1007/s11064-019-02721-8
Clark, I. C., Gutiérrez-Vázquez, C., Wheeler, M. A., Li, Z., Rothhammer, V., Linnerbauer, M., et al. (2021). Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372:eabf1230. doi: 10.1126/science.abf1230
Clarke, L. E., Liddelow, S. A., Chakraborty, C., Münch, A. E., Heiman, M., and Barres, B. A. (2018). Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U S A 115, E1896–E1905. doi: 10.1073/pnas.1800165115
Colombo, E., and Farina, C. (2016). Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 37, 608–620. doi: 10.1016/j.it.2016.06.006
Confavreux, C., and Vukusic, S. (2006). Natural history of multiple sclerosis: a unifying concept. Brain 129, 606–616. doi: 10.1093/brain/awl007
Cordiglieri, C., and Farina, C. (2010). Astrocytes exert and control immune responses in the brain. Curr. Immunol. Rev. 6, 150–159. doi: 10.2174/157339510791823655
Cotrina, M. L., Chen, M., Han, X., Iliff, J., Ren, Z., Sun, W., et al. (2015). Effects of traumatic brain injury on reactive astrogliosis and seizures in mouse models of Alexander disease. Brain Res. 1582, 211–219. doi: 10.1016/j.brainres.2014.07.029
Di Lullo, E., and Kriegstein, A. R. (2017). The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584. doi: 10.1038/nrn.2017.107
Diaz-Castro, B., Gangwani, M. R., Yu, X., Coppola, G., and Khakh, B. S. (2019). Astrocyte molecular signatures in huntington’s disease. Sci. Transl. Med. 11:eaaw8546. doi: 10.1126/scitranslmed.aaw8546
Dickson, T. C., and Vickers, J. C. (2001). The morphological phenotype of β-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience 105, 99–107. doi: 10.1016/s0306-4522(01)00169-5
Du, R. H., Tan, J., Yan, N., Wang, L., Qiao, C., Ding, J. H., et al. (2014). Kir6.2 knockout aggravates lipopolysaccharide-induced mouse liver injury via enhancing NLRP3 inflammasome activation. J. Gastroenterol. 49, 727–736. doi: 10.1007/s00535-013-0823-0
Engelhardt, B. (2003). Development of the blood-brain barrier. Cell Tissue Res. 314, 119–129. doi: 10.1007/s00441-003-0751-z
Escartin, C., Galea, E., Lakatos, A., O’Callaghan, J. P., Petzold, G. C., Serrano-Pozo, A., et al. (2021). Reactive astrocyte nomenclature, definitions and future directions. Nat. Neurosci. 24, 312–325. doi: 10.1038/s41593-020-00783-4
Faideau, M., Kim, J., Cormier, K., Gilmore, R., Welch, M., Auregan, G., et al. (2010). In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum. Mol. Genet. 19, 3053–3067. doi: 10.1093/hmg/ddq212
Fleeman, R. M., and Proctor, E. A. (2021). Astrocytic propagation of tau in the context of Alzheimer’s disease. Front. Cell. Neurosci. 15:645233. doi: 10.3389/fncel.2021.645233
Forno, L. S., Rasool, C. G., and Selkoe, D. J. (1986). Lewy bodies. N. Engl. J. Med. 314:122. doi: 10.1056/NEJM198601093140218
García-Domínguez, I., Veselá, K., García-Revilla, J., Carrillo-Jiménez, A., Roca-Ceballos, M. A., Santiago, M., et al. (2018). Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo mptp model of Parkinson’s disease. Front. Cell. Neurosci. 12:398. doi: 10.3389/fncel.2018.00398
Geschwind, M. D. (2015). Prion Diseases. Continuum (Minneap Minn) 21, 1612–1638. doi: 10.1212/CON.0000000000000251
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., and Gage, F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. doi: 10.1016/j.cell.2010.02.016
Goetzl, E. J., Schwartz, J. B., Abner, E. L., Jicha, G. A., Kapogiannis, D., Francisco, S., et al. (2019). High complement levels in astrocyte-derived exosomes of Alzheimer’s disease. Ann. Neurol. 83, 544–552. doi: 10.1002/ana.25172
Grimaldi, A., Pediconi, N., Oieni, F., Pizzarelli, R., Rosito, M., Giubettini, M., et al. (2019). Neuroinflammatory processes, A1 astrocyte activation and protein aggregation in the retina of Alzheimer’s disease patients, possible biomarkers for early diagnosis. Front. Neurosci. 13:925. doi: 10.3389/fnins.2019.00925
Guedes, J. R., Lao, T., Cardoso, A. L., and El Khoury, J. (2018). Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer’s disease-associated amyloid-β and tau pathologies. Front. Neurol. 9:549. doi: 10.3389/fneur.2018.00549
Habib, N., McCabe, C., Medina, S., Varshavsky, M., Kitsberg, D., Dvir-Szternfeld, R., et al. (2020). Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706. doi: 10.1038/s41593-020-0624-8
Hansson, E., Werner, T., Björklund, U., and Skiöldebrand, E. (2016). Therapeutic innovation: inflammatory-reactive astrocytes as targets of inflammation. IBRO Rep. 1, 1–9. doi: 10.1016/j.ibror.2016.06.001
Hartmann, K., Sepulveda-Falla, D., Rose, I. V. L., Madore, C., Muth, C., Matschke, J., et al. (2019). Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol. Commun. 7:83. doi: 10.1186/s40478-019-0735-1
Heneka, M. T., Sastre, M., Dumitrescu-Ozimek, L., Dewachter, I., Walter, J., Klockgether, T., et al. (2005). Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation 2:22. doi: 10.1186/1742-2094-2-22
Henrik Heiland, D., Ravi, V. M., Behringer, S. P., Frenking, J. H., Wurm, J., Joseph, K., et al. (2019). Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 10:2541. doi: 10.1038/s41467-019-10493-6
Hou, B., Zhang, Y., Liang, P., He, Y., Peng, B., Liu, W., et al. (2020). Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 11. doi: 10.1038/s41419-020-2565-2
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7
Ingram, G., Loveless, S., Howell, O. W., Hakobyan, S., Dancey, B., Harris, C. L., et al. (2014). Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol. Commun. 2:53. doi: 10.1186/2051-5960-2-53
Itoh, N., Itoh, Y., Tassoni, A., Ren, E., Kaito, M., Ohno, A., et al. (2017). Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl. Acad. Sci. U S A 115, E302–E309. doi: 10.1073/pnas.1716032115
Izrael, M., Slutsky, S. G., and Revel, M. (2020). Rising stars: astrocytes as a therapeutic target for ALS disease. Front. Neurosci. 14:824. doi: 10.3389/fnins.2020.00824
Jessen, K. R. (2004). Cells in focus: glial cells. Int. J. Biochem. Cell Biol. 36, 1861–1867. doi: 10.1016/j.biocel.2004.02.023
Jin, J., Smith, M. D., Kersbergen, C. J., Kam, T. I., Viswanathan, M., Martin, K., et al. (2019). Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 7:125. doi: 10.1186/s40478-019-0767-6
Joshi, A. U., Minhas, P. S., Liddelow, S. A., Haileselassie, B., Andreasson, K. I., Dorn, G. W., et al. (2019). Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 22, 1635–1648. doi: 10.1038/s41593-019-0486-0
Jyothi, H. J., Vidyadhara, D. J., Mahadevan, A., Philip, M., Parmar, S. K., Manohari, S. G., et al. (2015). Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol. Aging 36, 3321–3333. doi: 10.1016/j.neurobiolaging.2015.08.024
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Keough, M. B., Rogers, J. A., Zhang, P., Jensen, S. K., Stephenson, E. L., Chen, T., et al. (2016). An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 7:11312. doi: 10.1038/ncomms11312
King, A., Szekely, B., Calapkulu, E., Ali, H., Rios, F., Jones, S., et al. (2020). The increased densities, but different distributions, of both C3 and S100A10 immunopositive astrocyte-like cells in alzheimer’s disease brains suggest possible roles for both A1 and A2 astrocytes in the disease pathogenesis. Brain Sci. 10:503. doi: 10.3390/brainsci10080503
Koch, M., Kingwell, E., Rieckmann, P., Tremlett, H., Adams, D., Craig, D., et al. (2010). The natural history of secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 1039–1043. doi: 10.1136/jnnp.2010.208173
Kogel, V., Trinh, S., Gasterich, N., Beyer, C., and Seitz, J. (2021). Long-term glucose starvation induces inflammatory responses and phenotype switch in primary cortical rat astrocytes. J. Mol. Neurosci. [Online ahead of print]. doi: 10.1007/s12031-021-01800-2
Kondo, T., Funayama, M., Tsukita, K., Hotta, A., Yasuda, A., Nori, S., et al. (2014). Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice. Stem Cell Rep. 3, 242–249. doi: 10.1016/j.stemcr.2014.05.017
Kovacs, G. G. (2020). Astroglia and tau: new perspectives. Front. Aging Neurosci. 12:96. doi: 10.3389/fnagi.2020.00096.
Kuhlmann, T., Ludwin, S., Prat, A., Antel, J., Brück, W., and Lassmann, H. (2017). An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24. doi: 10.1007/s00401-016-1653-y
Kuipers, H. F., Rieck, M., Gurevich, I., Nagy, N., Butte, M. J., Negrin, R. S., et al. (2016). Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation and Th1 polarization. Proc. Natl. Acad. Sci. U. S. A. 113, 1339–1344. doi: 10.1073/pnas.1525086113
Lassmann, H., and Bradl, M. (2017). Multiple sclerosis: experimental models and reality. Acta Neuropathol. 133, 223–244. doi: 10.1007/s00401-016-1631-4
Lau, J. K. Y., Tian, M., Shen, Y., Lau, S. F., Fu, W. Y., Fu, A. K. Y., et al. (2021). Melanocortin receptor activation alleviates amyloid pathology and glial reactivity in an Alzheimer’s disease transgenic mouse model. Sci. Rep. 11:4359. doi: 10.1038/s41598-021-83932-4
Lee, Y., Lee, S., Chang, S. C., and Lee, J. (2019). Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch. Pharm. Res. 42, 416–425. doi: 10.1007/s12272-019-01133-0
Li, C. Y., Li, X., Liu, S. F., Qu, W. S., Wang, W., and Tian, D. S. (2015). Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem. Int. 83–84, 9–18. doi: 10.1016/j.neuint.2015.03.001
Li, J., Pan, L., Pembroke, W. G., Rexach, J. E., Godoy, M. I., Condro, M. C., et al. (2021). Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 12:3958. doi: 10.1038/s41467-021-24232-3
Lian, H., Litvinchuk, A., Chiang, A. C. A., Aithmitti, N., Jankowsky, J. L., and Zheng, H. (2016). Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of alzheimer’s disease. J. Neurosci. 36, 577–589. doi: 10.1523/JNEUROSCI.2117-15.2016
Lian, H., Yang, L., Cole, A., Sun, L., Chiang, A. C., Fowler, S. W., et al. (2015). NF-kB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85, 101–115. doi: 10.1016/j.neuron.2014.11.018
Liddelow, S. A., and Barres, B. A. (2017). Reactive astrocytes: production, function and therapeutic potential. Immunity 46, 957–967. doi: 10.1016/j.immuni.2017.06.006
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Liss, B., Haeckel, O., Wildmann, J., Miki, T., Seino, S., and Roeper, J. (2005). K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat. Neurosci. 8, 1742–1751. doi: 10.1038/nn1570
Luna-Herrera, C., Martínez-Dávila, I. A., Soto-Rojas, L. O., Flores-Martinez, Y. M., Fernandez-Parrilla, M. A., Ayala-Davila, J., et al. (2020). Intranigral administration of β -sitosterol- β -D-glucoside elicits neurotoxic A1 astrocyte reactivity and chronic neuroinflammation in the rat substantia nigra. J. Immunol. Res. 2020:5907591. doi: 10.1155/2020/5907591
Luo, J., Ho, P., Steinman, L., and Wyss-Coray, T. (2008). Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J. Neuroinflammation 5:6. doi: 10.1186/1742-2094-5-6
Koch, M., Kingwell, E., Rieckmann, P., and Tremlett, H. (2009). The natural history of primary progressive multiple sclerosis. Neurology 73, 1996–2002. doi: 10.1212/WNL.0b013e3181c5b47f
Markiewski, M. M., and Lambris, J. D. (2007). The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 171, 715–727. doi: 10.2353/ajpath.2007.070166
Masvekar, R., Wu, T., Kosa, P., Barbour, C., Fossati, V., and Bielekova, B. (2019). Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity. Mult. Scler. Relat. Disord. 28, 34–43. doi: 10.1016/j.msard.2018.11.032
Matejuk, A., and Ransohoff, R. M. (2020). Crosstalk between astrocytes and microglia: an overview. Front. Immunol. 11:1416. doi: 10.3389/fimmu.2020.01416
Mayo, L., Trauger, S. A., Blain, M., Nadeau, M., Patel, B., Alvarez, J. I., et al. (2014). Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156. doi: 10.1038/nm.3681
McColgan, P., and Tabrizi, S. J. (2018). Huntington’s disease: a clinical review. Eur. J. Neurol. 25, 24–34. doi: 10.1111/ene.13413
Meredith, G. E., and Rademacher, D. J. (2011). MPTP mouse models of Parkinson’s disease: an update. J. Parkinsons Dis. 1, 19–33. doi: 10.3233/JPD-2011-11023
Müller, E. (1904). Die multiple sklerose des Gehirns und Rückenmarks: ihre pathologie und behandlung; mit 5 tafeln. Pathol. Anat. Pathog. Fish 300–344.
Nagy, N., Kuipers, H. F., Marshall, P. L., Wang, E., Kaber, G., and Bollyky, P. L. (2019). Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 78–79, 292–313. doi: 10.1016/j.matbio.2018.03.022.
Nakanishia, K., Sakakima, H., Norimatsu, K., Otsuka, S., Takada, S., Tani, A., et al. (2021). Effect of low-intensity motor balance and coordination exercise on cognitive functions, hippocampal Aβ deposition, neuronal loss, neuroinflammation and oxidative stress in a mouse model of Alzheimer’s disease. Exp. Neurol. 337:113590. doi: 10.1016/j.expneurol.2020.113590
National Library of Medicine (U.S.). (2018, April 12–2020, June 22). A Study to Evaluate Transplantation of Astrocytes Derived From Human Embryonic Stem Cells, in Patients With Amyotrophic Lateral Sclerosis (ALS). Identifier NCT03482050. Available online at: https://clinicaltrials.gov/ct2/show/NCT03482050.
Okada, S., Hara, M., Kobayakawa, K., Matsumoto, Y., and Nakashima, Y. (2018). Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 126, 39–43. doi: 10.1016/j.neures.2017.10.004
Okrój, M., and Potempa, J. (2019). Complement activation as a helping hand for inflammophilic pathogens and cancer. Front. Immunol. 9:3125. doi: 10.3389/fimmu.2018.03125
Orellana, J. A., Froger, N., Ezan, P., Jiang, J. X., Bennett, M. V. L., Naus, C. C., et al. (2011). ATP and glutamate released via astroglial connexin43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840. doi: 10.1111/j.1471-4159.2011.07210.x
Orre, M., Kamphuis, W., Osborn, L. M., Jansen, A. H. P., Kooijman, L., Bossers, K., et al. (2014). Isolation of glia from Alzheimer ’ s mice reveals in fl ammation and dysfunction. Neurobiol. Aging 35, 2746–2760. doi: 10.1016/j.neurobiolaging.2014.06.004
Palmer, A. L., and Ousman, S. S. (2018). Astrocytes and aging. Front. Aging Neurosci. 10:337. doi: 10.3389/fnagi.2018.00337
Paquet, C., Boissonnot, M., Roger, F., Dighiero, P., Gil, R., and Hugon, J. (2007). Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 420, 97–99. doi: 10.1016/j.neulet.2007.02.090
Perlman, R. L. (2016). Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health 2016, 170–176. doi: 10.1093/emph/eow014
Pham, H., Ramp, A., Klonis, N., Ng, S., Klopstein, A., Ayers, M., et al. (2009). The astrocytic response in early experimental autoimmune encephalomyelitis occurs across both the grey and white matter compartments. J. Neuroimmunol. 208, 30–39. doi: 10.1016/j.jneuroim.2008.12.010
Ponath, G., Park, C., and Pitt, D. (2018). The role of astrocytes in multiple sclerosis. Front. Immunol. 9:217. doi: 10.3389/fimmu.2018.00217
Ponath, G., Ramanan, S., Mubarak, M., Housley, W., Lee, S., Sahinkaya, F. R., et al. (2017). Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140, 399–413. doi: 10.1093/brain/aww298
Prado Lima, M. G., Schimidt, H. L., Garcia, A., Daré, L. R., Carpes, F. P., Izquierdo, I., et al. (2018). Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 115, E2403–E2409. doi: 10.1073/pnas.1718435115
Rangachari, M., and Kuchroo, V. K. (2013). Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 45, 31–39. doi: 10.1016/j.jaut.2013.06.008
Reid, M. J., Beltran-Lobo, P., Johnson, L., Perez-Nievas, B. G., and Noble, W. (2020). Astrocytes in Tauopathies. Front. Neurol. 11:572850. doi: 10.3389/fneur.2020.572850
Rindfleisch, E. (1863). Histologisches Detail zu der grauen Degeneration von Gehirn und Rückenmark. (Zugleich ein Beitrag zu der Lehre von der Entstehung und Verwandlung der Zelle). Arch. für Pathol. Anat. und Physiol. und für Klin. Med. 26, 474–483. doi: 10.1007/bf01878008
Rose, C. R., Felix, L., Zeug, A., Dietrich, D., Reiner, A., and Henneberger, C. (2018). Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 10:451. doi: 10.3389/fnmol.2017.00451
Rothhammer, V., Mascanfroni, I. D., Bunse, L., Takenaka, M. C., Jessica, E., Mayo, L., et al. (2016). Type 1 interferons and microbial metabolites of tryptophan modulate astrocyte activity and CNS inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597. doi: 10.1038/nm.4106
Rüb, U., Seidel, K., Heinsen, H., Vonsattel, J. P., den Dunnen, W. F., and Korf, H. W. (2016). Huntington’s disease (HD): the neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol. 26, 726–740. doi: 10.1111/bpa.12426
Shigetomi, E., Saito, K., Sano, F., and Koizumi, S. C. (2019). Aberrant calcium signals in reactive astrocytes: a key process in neurological disorders. Int. J. Mol. Sci. 20:996. doi: 10.3390/ijms20040996
Sofroniew, M. V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647. doi: 10.1016/j.tins.2009.08.002
Song, N., Zhu, H., Xu, R., Liu, J., Fang, Y., Zhang, J., et al. (2021). Induced expression of kir6.2 in A1 astrocytes propagates inflammatory neurodegeneration via Drp1-dependent mitochondrial fission. Front. Pharmacol. 11:618992. doi: 10.3389/fphar.2020.618992
Stephenson, E. L., Mishra, M. K., Moussienko, D., Laflamme, N., Rivest, S., Ling, C. C., et al. (2018). Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain 141, 1094–1110. doi: 10.1093/brain/awy033
Sterling, J. K., Adetunji, M. O., Guttha, S., Bargoud, A. R., Uyhazi, K. E., Ross, A. G., et al. (2020). GLP-1 receptor agonist nly01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 33:108271. doi: 10.1016/j.celrep.2020.108271
Tassoni, A., Farkhondeh, V., Itoh, Y., Itoh, N., Sofroniew, M. V., and Voskuhl, R. R. (2019). The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression. Sci. Rep. 9:10010. doi: 10.1038/s41598-019-46232-6
Taylor, X., Cisternas, P., You, Y., You, Y., Xiang, S., Marambio, Y., et al. (2020). A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy. J. Neuroinflammation 17:223. doi: 10.1186/s12974-020-01900-7
Tremlett, H., and Zhao, Y. (2017). Primary and secondary progressive MS have a similar age at onset of progression - NO. Mult. Scler. 23, 640–642. doi: 10.1177/1352458516684559
Tribouillard-Tanvier, D., Race, B., Striebel, J. F., Carroll, J. A., Phillips, K., and Chesebro, B. (2012). Early cytokine elevation, prpres deposition and gliosis in mouse scrapie: no effect on disease by deletion of cytokine genes IL-12p40 and IL-12p35. J. Virol. 86, 10377–10383. doi: 10.1128/JVI.01340-12
Ugalde, C. L., Lewis, V., Stehmann, C., McLean, C. A., Lawson, V. A., Collins, S. J., et al. (2020). Markers of A1 astrocytes stratify to molecular sub-types in sporadic Creutzfeldt-Jakob disease brain. Brain Commun. 2:fcaa029. doi: 10.1093/braincomms/fcaa029
Van Kampen, J. M., and Robertson, H. A. (2017). The BSSG rat model of Parkinson’s disease: progressing towards a valid, predictive model of disease. EPMA J. 8, 261–271. doi: 10.1007/s13167-017-0114-6
Wang, D., Ayers, M., Catmull, D., Hazlewood, L., Bernard, C., and Orian, J. (2005). Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia 51, 235–240. doi: 10.1002/glia.20199
Wheeler, M. A., Clark, I. C., Tjon, E. C., Li, Z., Zandee, S. E. J., Couturier, C. P., et al. (2020a). MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599. doi: 10.1038/s41586-020-1999-0
Wheeler, M. A., Jaronen, M., Covacu, R., Zandee, S. E. J., Rothhammer, V., Tjon, E. C., et al. (2020b). Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581.e18–596.e18. doi: 10.1016/j.cell.2018.12.012
Williams, J. L., Manivasagam, S., Smith, B. C., Sim, J., Vollmer, L. L., Daniels, B. P., et al. (2020). Astrocyte-T cell crosstalk regulates region-specific neuroinflammation. Glia 68, 1361–1374. doi: 10.1002/glia.23783
Wu, T., Dejanovic, B., Gandham, V. D., Gogineni, A., Edmonds, R., Schauer, S., et al. (2019). Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 28, 2111.e6–2123.e6. doi: 10.1016/j.celrep.2019.07.060
Wu, Y., Zhang, A. Q., and Yew, D. T. (2005). Age related changes of various markers of astrocytes in senescence-accelerated mice hippocampus. Neurochem. Int. 46, 565–574. doi: 10.1016/j.neuint.2005.01.002
Xu, X., Zhang, A., Zhu, Y., He, W., Di, W., and Fang, Y. S. X. (2018). MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J. Cell. Physiol. 234, 904–914. doi: 10.1002/jcp.26918
Yun, S. P., Kam, T. I., Panicker, N., Kim, S., Oh, Y., Park, J. S., et al. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 24, 931–938. doi: 10.1038/s41591-018-0051-5
Zamanian, J. L., Xu, L., Foo, L. C., Nouri, N., Zhou, L., Giffard, R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012
Keywords: astrocytes, reactive astrogliosis, heterogeneity, neuroinflammation, immune mediators, CNS disorders
Citation: Reid JK and Kuipers HF (2021) She Doesn’t Even Go Here: The Role of Inflammatory Astrocytes in CNS Disorders. Front. Cell. Neurosci. 15:704884. doi: 10.3389/fncel.2021.704884
Received: 04 May 2021; Accepted: 29 July 2021;
Published: 03 September 2021.
Edited by:
Jason R. Plemel, University of Alberta, CanadaReviewed by:
Steven Sloan, Emory University, United StatesKelly Ceyzériat, Université de Genève, Switzerland
Copyright © 2021 Reid and Kuipers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hedwich Fardau Kuipers, hedwich.kuipers@ucalgary.ca
 Jacqueline Kelsey Reid
Jacqueline Kelsey Reid Hedwich Fardau Kuipers
Hedwich Fardau Kuipers