- 1Anesthesia and Intensive Care, San Martino Policlinico Hospital, IRCCS for Oncology and Neuroscience, Genoa, Italy
- 2Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy
- 3Laboratório de Neurobiologia Comparada e do Desenvolvimento, Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 4Keenan and Li Ka Shing Knowledge Institute, University Health Toronto—St. Michael's Hospital, Toronto, ON, Canada
- 5Laboratory of Pulmonary Investigation, Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 6Rio de Janeiro Network on Neuroinflammation, Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ), Rio de Janeiro, Brazil
The microbiota–gut–brain axis is considered a central regulator of the immune system after acute ischemic stroke (AIS), with a potential role in determining outcome. Several pathways are involved in the evolution of gut microbiota dysbiosis after AIS. Brain–gut and gut–brain signaling pathways involve bidirectional communication between the hypothalamic–pituitary–adrenal axis, the autonomic nervous system, the enteric nervous system, and the immune cells of the gut. Alterations in gut microbiome can be a risk factor and may also lead to AIS. Both risk factors for AIS and gut-microbiome composition are influenced by similar factors, including diabetes, hypertension, hyperlipidemia, obesity, and vascular dysfunction. Furthermore, the systemic inflammatory response after AIS may yield liver, renal, respiratory, gastrointestinal, and cardiovascular impairment, including the multiple organ dysfunction syndrome. This review focus on biochemical, immunological, and neuroanatomical modulation of gut microbiota and its possible systemic harmful effects after AIS, as well as the role of ischemic stroke on microbiota composition. Finally, we highlight the role of gut microbiota as a potential novel therapeutic target in acute ischemic stroke.
Introduction
Acute ischemic stroke (AIS) is the second leading cause of death worldwide, accounting for up to 25% of global lifetime risk (1). Great effort has been invested into identifying risk factors, elucidating pathogenesis, and discovering implications for outcomes (2). Post-AIS infection has been identified as a key cause of death and prolonged hospitalization after stroke (3). Recent advances have demonstrated, for instance, that peripheral adaptive immunity is activated and recruited into the brain within the first few hours/days after AIS (4), and that its cells might regulate and be regulated by the gut microbiota (5). A microbiota is defined as an ecological unit composed of microorganisms within a specific (micro) environment, while the microbiome is the genetic material of these microorganisms (6). Dysbiosis is defined as a microbial imbalance in composition and function of the microbiota, occurring in several animal models of AIS which demonstrates that gut microbiota can regulate the neuroinflammatory response, influencing brain recovery (7). Several studies have focused on the relationship between the intestinal microbiome and AIS, confirming the existence of a bidirectional microbiota–gut–brain axis (8). In fact, alterations in gut microbiome can be a risk factor for AIS, and vice-versa; AIS may lead to changes in gut microbiome, impacting on peripheral organs and leading to severe liver, renal, respiratory, gastrointestinal and cardiovascular impairment, including the multiple organ dysfunction syndrome (MODS) (9). The aim of this review is to highlight the pathophysiology potentially involved in gut microbiota modulation after AIS, and its implication for therapy and outcome.
Pathophysiology
Brain–Gut–Microbiota Axis
Communication Pathways
Recent evidence confirms the existence of bidirectional communication pathways linking the brain and the gut. The pathways involved in brain-gut axis include sympathetic and parasympathetic activation, the hypothalamic–pituitary–adrenal axis, and the immune system at a central level (8). The hypothalamic–pituitary–adrenal axis is a key communication mechanism between the brain and gut, particularly in response to a variety of stressful and stimuli (10). The autonomic nervous system, after integrating neuronal and neuroendocrine signaling, modulates intestinal homeostasis, thus enhancing inflammation in gut tissue, reducing the number of goblet cells in the cecum, and impairing mucin production in the overall intestine (8). The autonomic system is further responsible for controlling gut motility, permeability, fluid maintenance, bile secretion, bicarbonate and mucus production, intestinal fluid handling, and the mucosal neuroimmune response (11). Peripheral connections from the gut to the brain consist of the enteric and autonomic nervous systems, alongside the autonomic nervous system and various neuroimmune and neuroendocrine pathways (Figure 1) (8). These include serotonin, gamma-amino butyric acid (GABA), catecholamines (12), cholecystokinin, glucagon-like peptide-1, and neuropeptide Y (7). Bottom-up signals triggered upon stimulation of hepatic and celiac branches of the vagus nerve by microbial compounds, metabolites, and hormones released from the gut are carried by afferent vagal branches to the brain (13).
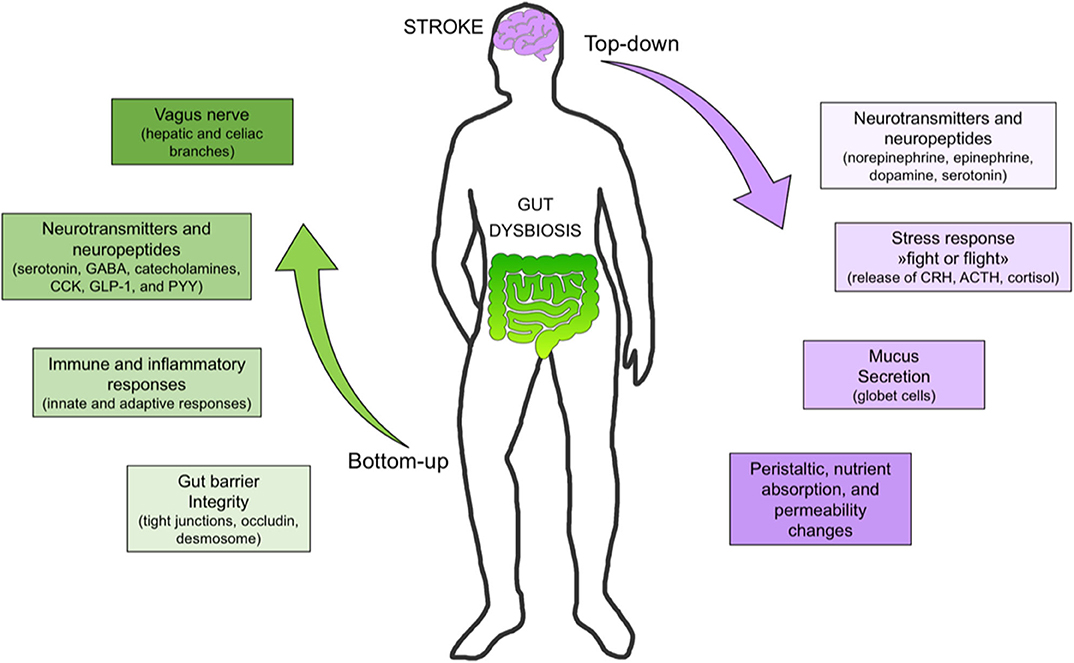
Figure 1. Bottom-up and top-down signaling in stroke. Bottom-up signaling from the gut to the brain includes barrier integrity maintenance, immune response (e.g., immunoglobulin A secretion), neurotransmitter and neuropeptide release, short-chain fatty acid (e.g., butyrate) release, and vagus nerve activation. Top-down signaling includes release of neurotransmitters (e.g., dopamine, serotonin), stress response (e.g., cortisol release), mucus secretion, and peristalsis control. [SCFA, short chain fatty acid; DA, dopamine; 5HT, serotonin; Ig, immunoglobulin, PYY, peptide YY; GLP-1, glucagon-like peptide-1; CKK, cholecystokinin; GABA, gamma amino butyric acid].
Gut Dysbiosis and Neurological Disorders
Molecular biology has made great strides in characterizing which microorganisms co-inhabit each host and how this community may change over time. Each individual shows a unique microbiota profile with specific functions, such as immunomodulation, protection against pathogens, maintenance of gut mucosal barrier integrity, and control of nutrient metabolism (14). The largest population of commensal bacteria, comprising more than 1,000 species, is located in the digestive tract (15). Mucosal bacteria are more interactive with host cells when compared to luminal bacteria, influencing host gene expression and healing (16). This occurs, in part, due to the presence of the intestinal mucus layer, an essential component of the protective barrier between the intestinal epithelium and the gut lumen (17). The gut microbiota is composed of several bacterial species, of which 90% is represented by Firmicutes and Bacteroidetes. The Firmicutes phylum is predominantly composed of clostridial genera (14). Dysbiosis is usually caused by several mechanisms, such as infection, inflammation, diet, xenobiotics, and genetics, although the full list of lifestyle factors involved in microbiota modulation is still unknown (18). Inflammation compromises the microbiotic defense against pathogens causing dysbiosis, since the innate and adaptive immune systems are essential in regulation of microbiota homeostasis (19). The enteric nervous system is composed of the submucosal and the myenteric plexuses, which control intestinal motility and fluid movement (20). Their function is influenced by the activation of pattern recognition receptors (PRRs), especially toll-like receptors (TLRs) (21). Innate immunity primarily recognizes gut microbes by pathogen-associated molecular patterns (PAMPs), which are PRRs. The stimulation of PAMPs such as TLR-2 is responsible for hippocampal neurogenesis, while TLR-4 exhibits the opposite mechanisms (22). TLR-4 is involved in the development of learning and memory (23), and inhibits retinal neurogenesis and differentiation through pathways mediated by myeloid differentiation primary response protein (MYD)-88 and nuclear factor-κB (NF-κB) (24). The loss of MYD-88 in epithelial cells yields increased bacterial translocation and altered bacterial microbiota (25). TLR-5 controls intestinal microbial ecology, preventing dysbiosis (26). Nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors (NLRs) are also determinants of microbial dysbiosis. Among NOD receptor subtypes, NOD1 is implicated in binding peptidoglycan from Gram-negative bacteria (27). NOD2 is involved in increased burden of commensals and mucosa-associated bacteria (28), its absence predisposing to dysbiosis. In contrast, NOD6 is involved in the maintenance of a stable intestinal microbial community (29). NOD2, which is responsible for intestinal homeostasis, activates kinase receptor-interacting protein (RIPK)-2 and NF-kB, which are involved in the control of the production of antimicrobial peptides (AMPs) and mucin. AMP expression is also influenced by flagellin and lipoproteins, which interact with TLR-5 on dendritic and epithelial cells (29). Additionally, some NLR proteins create a multiprotein complex known as the inflammasome. The inflammasome activates caspase-1, which processes interleukin (IL)-1β and IL-18 precursors. Overall, inflammasome activation results in IL-1β and IL-18 secretion and changes in several immune cell populations (30). Regarding the adaptive immune response, compelling evidence suggests that B cells play an important role in microbiota homeostasis through the secretion of immunoglobulin (Ig)A (31). IgA selection is promoted by T helper follicular cells. This mechanism of IgA secretion is regulated by death protein-1, which manages the microbiome at the intestinal level (32). Invariant natural killer T-cells are also involved in microbiota regulation (33). Specifically, a symbiotic relationship between the immune system in the intestine and the microbiota exists. In fact, the intestinal immune system protects the organism against pathogens germs, while the microbiota maintains the intestinal immune system, inducing secretion of IgG from plasma cells, IL-17- and IL-22-from helper T cells (Th-17 cells), which are situated in the mucosal lamina propria, and represent the mediators of microbiota-immune system crosstalk. IgA directly promotes colonization by mutualistic microbes, competing with invasive pathogens. IL-17 and IL-22 maintain the mucosal barrier system homeostasis, which protects the organism stimulating the expression anti-microbial molecules in intestinal epithelial cells. Moreover, T regulator (Treg) cells contribute to immune homeostasis in non-lymphoid tissues including the gastrointestinal tract, recruited by T-cell antigen receptor (TCR) and cytokine signals expression, differentiating into suppressive effector Treg cells with IL-10 production. IL-10 holds anti-inflammatory effect, contributing to preserve gut homeostasis. Dysbiosis is correlated to an increased susceptibility to intestinal inflammation, because of reduced IgA, IL-22, and IL-10 production, and IL-17R impairment (34). The activation of innate and adaptive immune responses at mucosal surfaces during inflammation, autoimmunity, and infection explains why microbiota composition could play an essential role in modulating immune response in other organs, such as the brain (35). Evidence from germ-free mice suggests correlations among an immature microglial phenotype, altered immune response, and brain pathology. This can be modulated by short-chain fatty acids and microbiota-derived bacterial fermentation products (36). Microglia are essential for the maintenance of tissue homeostasis, synaptic remodeling, and scavenging of pathogens, molecules and death cells, and have been associated with neuropsychiatric and neurological disease in humans (36). Under germ-free conditions, microglial polarization (especially M1>M2) is reduced, suggesting that reduced complexity of microbiota impairs microglial function (36). Several neurological diseases correlate with blood–brain barrier impairment, in which microbiota dysregulation may play a role. These include Parkinson's disease, Alzheimer's disease, several mental disorders, and autism spectrum disorders (24). Experimental research has hypothesized that increased blood–brain barrier permeability might be induced by the loss of normal microbiota diversity (37). Besides, microbiota diversity is not only associated with blood–brain barrier permeability, but also plays a crucial role in hippocampal and microglial brain morphology (24). Additionally, several neurological disorders are associated with an enhanced local inflammatory response, which can contribute to systemic cytokine release and increased tissue permeability, thus altering microbiota homeostasis (24). In fact, dysbiosis of the intestinal microbiota in neurocritically ill patients has been correlated to mortality at 180 days (38).
The Brain–Gut–Microbiota Axis in AIS
Local and systemic inflammatory responses are enhanced after AIS (4). Monocytes (innate response) enter the brain within 24 h, reaching maximum numbers around 3–5 days after AIS (39). They differentiate into macrophages with a distinct molecular signature compared to microglia (40). Inflammatory monocytes have been shown to exert protective effects following AIS in mice (41), whereas the role of patrolling monocytes is less clear (42). Neutrophils play a controversial role in AIS. Although several mechanisms (43) (such as the production of reactive oxygen species and the release of metalloproteinases) have been attributed to neutrophils, the existence of neuroprotective N2 neutrophils has also been reported (44). A recent study in mice identified the triggering receptor expressed on myeloid cells (TREM)-1 as an interesting target for pharmacotherapies aimed at reducing the pro-inflammatory response of peripheral and intestinal myeloid cells after AIS (45).
TREM1 is a potent enhancer of innate immune responses, it acts by synergizing with classical PRRs, thus inducing the production of proinflammatory cytokines and chemokines, including IL-8, monocyte chemoattractant protein-1 (MCP-1), MCP-3 and macrophage inflammatory (MIP-1α), and inhibition of IL-10 (45). Furthermore, neurotoxic mechanisms activate the release of pro-inflammatory cytokines such as interleukin-21 from cluster of differentiation (CD)-4+ T cells within 24 h after AIS (46) and IL-17 from γδT-cells (47). IL-17 acts by recalling neutrophils through chemokine release, whereas IL-21 and perforin exert direct neurotoxic effects in the brain (48). Clinical trials have failed to provide evidence of beneficial effects of neutrophil blockade in AIS patients (49). γδT-cells express the chemokine receptor (CCR)-6, which is essential for their infiltration into the AIS lesion (47). In the first 2-3 days after AIS, lymphocytes arrive at the ischemic lesion (adaptive response) (4). In particular, T helper-1 and-17 subpopulations activate neuroinflammation, whereas regulatory T-cells have a neuroprotective action due to their anti-inflammatory properties (50). The consequences of this remain unclear, as detrimental effects to regulatory T cells after AIS have been reported (51). Release into the systemic circulation of cytokines and chemokines produced in the brain after AIS may exert a potential influence on microbiota composition and dysbiosis (52). T lymphocytes, especially regulatory and γδT-cells, play a pivotal role in how the microbiota can modify infarct size and neurological function after AIS (7, 53). T lymphocytes have been shown to migrate from the Peyer patches of the small intestine or from the intestinal lamina propria to the brain and/or the leptomeninges following AIS (7). Increased gut permeability after AIS can result in bacterial translocation and lung infection in mice (17). Local inflammatory responses at the intestinal level in AIS are depicted in Figure 2. Despite some degree of controversy arising from study heterogeneity, pneumonia—presumably secondary to immune dysregulation and bacterial translocation—is the most common acute complication after AIS (54). Immunogenic endotoxins from the microbiota, such as lipopolysaccharide (LPS), can promote neuroinflammation by a direct mechanism and/or by inducing migration of peripheral immune cells to the brain (55). An interesting experimental study showed that AIS in cynomolgus monkeys induced a long-term, persistent increase in levels of LPS and pro-inflammatory cytokines in plasma, which correlated with the relative abundance of the phylum Bacteroidetes in the gut microbiota. Additionally, intestinal dysbiosis and mucosal damage persisted for up to 12 months after AIS (52). Clinical evidences (56, 57) concluded that after AIS the commensal flora changes in favor of opportunistic pathogens, such as Enterobacter, Megasphaera, Oscillibacter, Desulfovibrio, Odoribacter, and Akkermansia (58), instead of commensals such as Bacteroides, Prevotella, and Faecalibacterium. Although several mechanisms involved in the microbiota-regulated immune response after AIS have been identified, our understanding about microbiota modulation following AIS is incompletely understood.
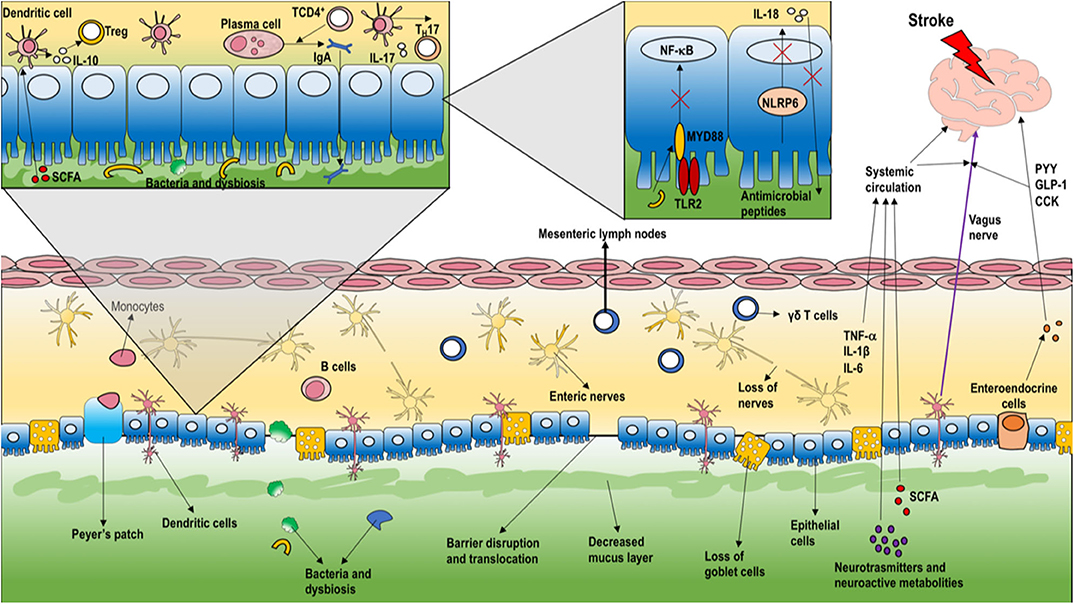
Figure 2. Local intestinal inflammatory response in stroke. As the first intestinal response to AIS, the enteric barrier is disrupted, thus compromising the ability of the microbiota to defend against intestinal pathogens. The microbiota stimulates release of metabolites, neurotransmitters, indoles, short-chain fatty acids, and bile acids that can reach the brain, thus modulating neurons, microglia, astrocytes, and neurovascular unit function. Vagal afference activate neuroendocrine system to release peptides [peptide YY (PYY), glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK)], as well as inflammatory response to enhance pro-inflammatory cytokines release (e.g., interleukin (IL)-21) from cluster of differentiation (CD)-4+ T-cells and IL-17 from γδT-cells. IL-17 acts to recall neutrophils by releasing chemokines. Moreover, T lymphocytes can migrate from the Peyer patches of the small intestine or from the intestinal lamina propria to the brain and/or the leptomeninges. As depicted in the inset, the loss of myeloid differentiation primary response protein (MYD)-88 in epithelial cells results in increased bacterial translocation and altered bacterial microbiota. Moreover, nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors-6 (NLRP6) are involved in the maintenance of a stable intestinal microbial community. Usually, NLR proteins create a multiprotein complex named the inflammasome, activating IL-1β and IL-18 secretion and changes in several immune cell populations. B-cells also play an important role in microbiotic homeostasis through secretion of immunoglobulin A, promoted by TCD4+ on plasma cells. Additionally, dendritic cells are stimulated by T regulatory cells to increase the secretion of IL-10. [PYY, peptide YY; GLP-1, glucagon-like peptide-1; CCK, cholecystokinin; IL, interleukin; CD, cluster of differentiation; MYD, myeloid differentiation primary response protein; NOD, nucleotide binding oligomerization domain-containing protein; NLRP, nucleotide-binding oligomerization domain-containing protein receptor; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone].
Clinical Implications
Alterations in the gut microbiota composition have been identified in various neurological diseases, including cognitive dysfunction, autism, neurodegenerative disorders, and cerebrovascular diseases (24). The gut microbiome is pivotal for brain function and behavior, as demonstrated by the fact that gut dysbiosis is associated with impairment of the blood–brain barrier, behavioral deficits, and alterations of synaptic plasticity (59). We will discuss how alterations in the gut microbiota during AIS affect neurologic outcomes, risk factors, and occurrence of AIS-associated pneumonia, as well as cardiovascular, gastrointestinal, hepatic, renal complications and development of the multiple organ dysfunction syndrome, thus potentially affecting clinical outcomes and functional recovery (Figure 3) (6).
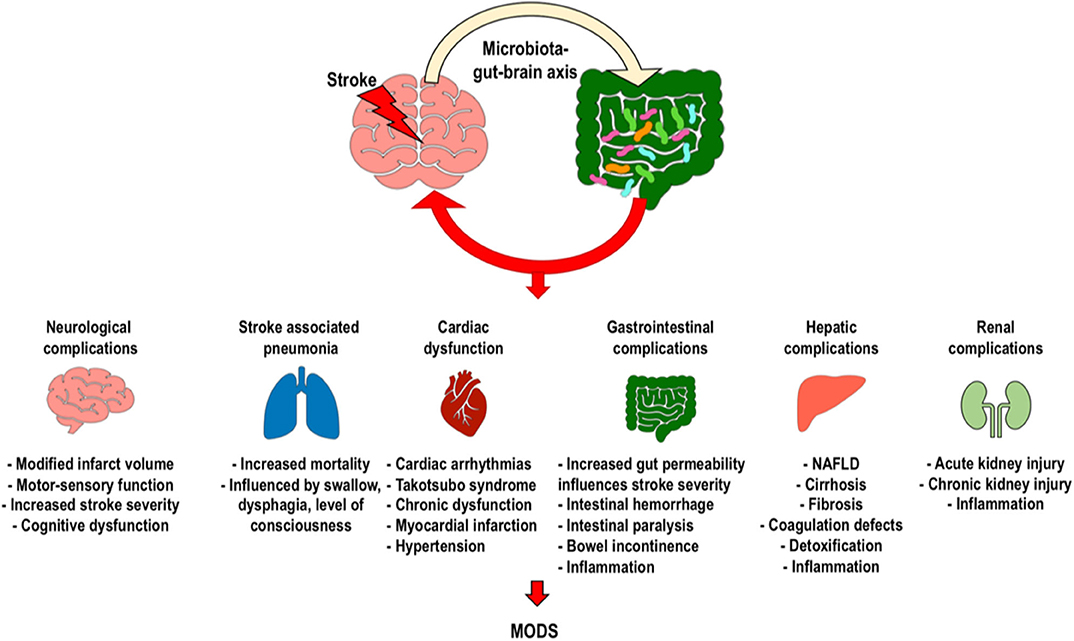
Figure 3. Clinical implications of intestinal dysbiosis after ischemic stroke. Changes caused by stroke on the gut microbiota can induce neurological complications, stroke-associated pneumonia, cardiac dysfunction, gastrointestinal complications, and renal dysfunction, with possible development of the systemic inflammatory response and multiple organ dysfunction syndromes. [MODS, multiple organ dysfunction syndrome].
Influence of Gut Dysbiosis on AIS Outcomes
Gut dysbiosis in AIS patients is associated with host metabolism and inflammation (60, 61). Increased levels of opportunistic pathogens in the gut have been associated with higher risk of developing AIS (62). A clinical study that compared asymptomatic controls to patients with AIS reported significant changes in the gut microbiota composition in terms of increased opportunistic pathogens, with this finding persisting for at least 3 weeks after AIS (57). Gut dysbiosis was measured in AIS patients by developing a score called the “stroke dysbiosis index (SDI),” which has shown good correlation in predicting severe stroke and unfavorable outcome (56).
AIS Risk Factors and Gut Dysbiosis
AIS patients often show significant changes in microbial flora that may be independent of relevant comorbidities (hypertension, age, and type 2 diabetes) (56). However, alterations in the gut microbiota are known to elicit hypertension in rats, raising doubts as to the preceding evidence (63). Like hypertension, obesity and body composition in both sexes are associated with metabolic syndrome and higher cardiovascular and cerebrovascular risk, which are influenced by inflammation and gut permeability, thereby suggesting a role of gut microbiota in the prevention of these diseases (64).
Hypertension
Diabetes and hypertension have been identified as systemic activators of immune response and are two of the most important risk factors for AIS (65). Intensive management of hypertension in AIS patients is recommended to reduce the risk of chronic myocardial dysfunction and cardiac remodeling (66–68). Hypertensive and pre-hypertensive patients have a different gut microbiota composition as compared to non-hypertensive patients. The finding of altered microbiota composition in pre-hypertensive patients suggests that dysbiosis most commonly appears before clinical hypertension, rather than hypertension being the causative factor of dysbiosis (63).
Obesity and Diabetes Mellitus
These metabolic disorders are known to increase the risk of cerebrovascular diseases, such as AIS (6). The gut microbiota plays paramount roles in the regulation of satiety, thermogenesis, and energy balance, due to its ability to manage host immune cell activation and maturation and neuro-endocrine and hormonal functions. Furthermore, dysbiosis activates pro-inflammatory mechanisms that are implicated in diabetes and cardiovascular disease. In particular, trimethylamine N-oxide (TMAO) may be implicated in the pathogenesis of cardiovascular, metabolic and cerebrovascular diseases (69). In fact, in patients with type I diabetes, higher concentrations of plasma TMAO were associated with mortality, cardiovascular events, and micro and macrovascular complications (70). Elevated TMAO levels within the first hours after AIS are associated with poor stroke outcomes (71).
Vascular Dysfunction
Vascular dysfunction can influence mortality and outcome following AIS. Bacterial metabolites such as short-chain fatty acids, nitrites, flavanol, TMAO, indoles, and sulfidic acid have been identified as causative factors of vascular dysfunction. Short chain fatty acids and sulfidic acid are particularly capable of inducing vasodilatation, whereas indole and trimethylamine N-oxide increase the production of reactive oxygen species, thus reducing cerebral vasodilatation (63, 72).
Aging
According to recent investigations, the gut microbiota undergoes several modifications during the life course. Specifically, immune function, microbiota variability, and anti-inflammatory properties are reduced in older adults.
AIS-Associated Pneumonia
The mechanism underlying immune dysregulation and reduced antimicrobial defense after AIS is still unclear. Infection rates range between 5 and 65%; this variability is attributed to the heterogeneity of the current definition (73). Stanley et al. suggested a role for bacterial translocation in the pathogenesis of AIS-associated pneumonia (17). Pneumonia is a typical example of dysbiosis-related infection. Pneumonia correlates with an increased mortality rate (73). A recent meta-analysis including 137,817 AIS patients from 87 studies confirmed an overall infection incidence of 30%, with pneumonia accounting for up to 10% (74). Dysphagia occurs in 50–55% of AIS patients (75), and the incidence of AIS-associated aspiration pneumonia ranges from 4 to 57% (76). Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Enterobacter species are commonly identified, especially in the intensive care unit (77). Another important factor leading to pneumonia after AIS is immunosuppression (4). In post-AIS mice, aspiration of only 200 colony-forming units of Streptococcus pneumoniae was enough to trigger pneumonia and bacteremia compared to sham-operated mice, in which up to 20,0000 colony forming units were required to induce pneumonia (78).
Cardiovascular Complications
The incidence of cardiovascular events is increased after AIS (79), accounting for up to 39% of complications in AIS patients without previous cardiac events (80). The most common disturbances include cardiac arrhythmias, stress-induced cardiomyopathy (Takotsubo syndrome), autonomic dysfunction, myocardial infarction, and arterial hypertension (81). According to recent evidence, the occurrence of cardiovascular and cerebrovascular diseases correlates with dysbiosis (82). TMAO, a byproduct of the intestinal microbiota, is a potential novel biomarker of cardiovascular events, including AIS (83), and also associated with poor outcomes (84).
Gastrointestinal Complications
Water and nutrients are absorbed by the gut–blood barrier, which also prevents the passage of toxins and pathogens into the blood (85). Intestinal permeability is modified by AIS-induced inflammation, thus enhancing microbiota dysregulation (85). Moreover, increased intestinal permeability has been associated with greater AIS severity (85), as well as imbalance of the intestinal flora in favor of pathogens, thereby influencing outcome and post-AIS mortality (86). After AIS, up to 50% of patients develop gastrointestinal complications such as hemorrhage, intestinal paralysis, bowel incontinence, and dysphagia, which are considered partially responsible for poor neurological outcomes and increased mortality (53, 87). This deterioration of outcomes is mainly attributed to increased immune activation in the gut, with systemic migration of lymphocytes to the brain (53). It is interesting to note that patients with Crohn's disease have an increased susceptibility to AIS, further implying a bidirectional association between gut dysbiosis and AIS (88).
Hepatic Complications
The potential association between liver disease, AIS, and gut microbiota dysregulation is poorly understood (89). Since the systemic coagulation cascade is managed mostly by pro- and anticoagulant factors produced by the liver, hepatic dysfunction can cause coagulopathies with bleeding or thrombotic complications, and has consequently been identified as an important risk factor for AIS (90). Among hepatic disorders, non-alcoholic fatty acid disease (NAFLD), cirrhosis, and liver fibrosis are the most common factors associated with AIS (89, 91). The liver plays a major role in the biotransformation of drugs and toxins. Trimethylamine is converted by hepatic enzymes into TMAO, which is also implicated in platelet hyperreactivity, atherosclerosis (92), inflammation, and cholesterol metabolism (93). The gut microbiota is able to modulate cholesterol transport by activating of specific enzymes that suggests a role in modulation of risk factors involved in the development of AIS (94).
Renal Complications
Risk factors for kidney injury and AIS are very similar, and atherosclerosis plays a crucial role in both contexts (95). In a recent meta-analysis, the incidence of acute kidney injury in AIS patients reached up to 9.6%, and was associated with increased overall mortality rate (96), in-hospital mortality, and neurological deterioration (97). Uremic toxins are waste products of microbiota that can cross the intestinal blood–barrier because of increased gut permeability after AIS, and thus reach the systemic circulation (98). In support of a purported gut–brain–kidney axis, an experimental study demonstrated that rats affected by both acute kidney injury and AIS displayed neuronal loss; glial, macrophage, and microglial upsurge; and increased circulating IL-6 and IL-1β levels (99). Following a systemic inflammatory process, high level of microbiota waste products such as TMAO are found in patients with chronic kidney disease, suggesting that TMAO is inversely associated with glomerular filtration rate and should be considered as a new clinical marker of renal medullary damage, hypertension, and heart disease (100). TMAO plasma concentrations are elevated in patients with chronic kidney disease, and predict poor long-term survival; in animal models, TMAO is associated with tubulointerstitial fibrosis and renal dysfunction (101).
Multiple Organ Dysfunction Syndrome
The gut is the most colonized system of the body. It is home to a broad range of commensal and pathogenic microorganisms—up to trillions of different types of bacteria. The intestinal mucosal barrier activates a specialized local immune response due to the existence of organized gut-associated lymphoid tissue (GALT) (102). Leaky gut (i.e., increased intestinal permeability) can determine the release of intestinal lumen bacteria and toxins into the circulation, thus enhancing systemic inflammation and causing sepsis or a systemic inflammatory response (9). Indeed, the systemic inflammatory response syndrome has been detected as a complication of systemic inflammation after AIS (9). A study conducted on 1,500 AIS patients revealed that systemic inflammation at admission was associated with infarct volume, functional outcome, and clinical severity (103). Moreover, within the first days after AIS, white blood cell counts, body temperature, and C-reactive protein levels were higher; therapeutic thrombolysis attenuated this inflammatory response (103). Systemic inflammatory response can result in MODS in up to 12% of AIS patients, increasing the mortality rate up to 80%. (104) Among risk factors for MODS after AIS, a low Glasgow Coma Score, advanced age, hypoglycemia, hyperglycemia, leukocytosis, and history of chronic organ dysfunction have been identified (104). Unfortunately, clinical data on multiorgan failure and systemic inflammatory response in AIS patients is scant, and their correlation with microbiota manipulation poor.
Therapeutic Strategies
Diet
Diet is a major determinant of gut microbiota composition. Indeed, dietary diversity has been associated with regulation of insulin resistance, susceptibility to infections, and TMAO concentration, among other changes (105). The production of TMAO by gut microbiota can be increased by high intake of L-carnitine and phosphatidylcholine, which is an excellent dietary source of choline commonly found in red meat and eggs (106). Moreover, antibiotic treatment has been found to reduce TMAO generation, whereas, discontinuation of antibiotics caused an increase in TMAO levels (84). High-fat diets such as the ketogenic diet [commonly used for its antiseizure properties by increasing serum ketones and reducing neuronal apoptosis (107)] have also been found to increase TMAO concentration in humans (108), whereas the Mediterranean diet and vegetarian diets reduced TMAO production (94). The Mediterranean diet has been widely investigated in recent decades for its potential health benefits. Indeed, the consumption of cereals, nuts, vegetables, legumes, fruit, and fish can mitigate the incidence of neurodegenerative disorders, psychiatric diseases (109), and cardiovascular dysfunction (110). A study reported that patients with higher Mediterranean Diet Score Stratification had a lower Firmicutes/Bacteroidetes ratio, thus suggesting anti-inflammatory effects of the Mediterranean diet and its modulation of gut microbiota (111). Protein-rich diets have been associated with conflicting results. Although amino acids are considered essential for synthesis of neurotransmitters (112), long-term adherence to a high-protein diet is associated with harmful effects on gut microbiota composition (113). There has been growing interest in the role of dietary fiber in gut microbiota composition. Fiber is mainly fermented by Firmicutes and other bacterial species, thus increasing the production of short chain fatty acids (SCFAs) such as acetate, propionate, and butyrate (114). SCFAs have also been suggested to improve post-stroke recovery via immune mechanisms (115). There is little evidence on the impact of fiber-rich diets on AIS outcome, but, as demonstrated in experimental settings, the immunomodulatory effect of fibers seems to be anti-inflammatory.
Probiotics and Prebiotics
Probiotics are defined as “live micro-organisms which induce benefits in the host,” and although they are largely assumed to be safe and useful, four broad categories of adverse effects have been identified: (1) systemic infections, (2) deleterious metabolic activities, (3) excessive immune stimulation, and (4) undesired gene transfer (116). Importantly, in immunocompromised patients, probiotic administration has been associated with the development of sepsis (116). On the other hand, probiotics act through acid lactic fermentation in the gut, thus improving the balance between pathogens and commensals, enhancing immune regulation of the intestinal system, and inhibiting bacterial toxin production (117). Prebiotics are not digested in the small bowel, but are active in the colon, where they are fermented by bacteria. Carbohydrates are an example of prebiotics (117). Prebiotics are capable of influencing the production of SCFAs and regulating mucin production and local inflammatory response into the GALT, thus stimulating phagocytosis by macrophages (117). In a meta-analysis of thirteen clinical trials, treatment with prebiotics reduced neither intensive care unit mortality nor in-hospital mortality. However, administration of prebiotics resulted in declines in both incidence of pneumonia associated with intensive care unit stay and in-hospital length-of-stay (118). Therefore, based on this limited clinical evidence, prebiotics might be hypothesized to reduce the risk of pneumonia, which is particularly high in AIS patients (73).
Antibiotics
To date, no clear evidence is available in support of prophylactic antibiotic treatment within the first hours after AIS to control dysbiosis (77). A recent 1,224-patient study demonstrated that prophylactic antibiotics administered immediately following AIS, and primarily in those patients affected by post-AIS dysphagia, did not reduce the incidence of pneumonia (119). A second prospective randomized study confirmed a reduction in overall infections after prophylactic antibiotic therapy with 2 g of intravenous ceftriaxone every 24 h for 4 days relative to standard care, but this did not affect the incidence of post-AIS pneumonia or functional outcome scores at 2 months (120). In 2017, a randomized controlled trial of 227 patients compared standard care plus ultrasensitive procalcitonin-guided antibiotic treatment to standard care alone. When procalcitonin reached a value greater than 0.05 ng/ml, prophylactic antibiotics were administered following local guidelines. There were no beneficial effects on functional outcome at 90 days, nor on mortality rate (77).
Fecal Transplantation, Intra-Gastric Treatments, and Microbial Metabolites
Fecal Transplantation
Gut microbiota dysbiosis can influence the severity of brain injury (7). The effect of intestinal bacteria on neuronal function is defined as psychobiotics. Fecal microbiota transplantation is the transfer of donor fecal microbiota from healthy people to sick patients (121), and it has recently been identified as a possible strategy to correct dysbiosis in patients with neuropsychiatric disorders and those affected by AIS (122). Antibiotic administration reduced neurological impairment, blood lipid levels, and infarct volume, while transplanting stool rich in SCFAs (especially butyric acid) led to microbiota remodeling, increasing Lactobacillus species and improving intestinal microbiota, thus positively modulating the brain ischemic response (123). Fecal microbiota transplantation has proven effective for intestinal dysbiosis in clinical settings, although its use was associated with secondary bacteremia from E. coli infection in two patients with recurrent Clostridioides difficile infection, transmitted by the donor fecal microbiota (124). Nevertheless, this treatment is recognized as a novel alternative to antibiotic therapies against primary C. difficile infection in small cohorts of patients (124). Taken together, these studies suggest that fecal transplantation can broadly affect intestinal microbiota in recipients.
Intragastric Treatments and Microbial Metabolites
Intragastric treatment with Clostridium butyricum after bilateral common carotid artery occlusion resulted in reduced neuronal damage and cognitive impairment in rodents (125). These beneficial effects might be at least partially related to production of the microbial metabolite butyrate, which has been shown to be neuroprotective in models of AIS (126). In another study in rats, sodium butyrate mitigated blood–brain barrier permeability following AIS by reducing the activity of matrix metalloproteinase-9 (127). Among metabolites, GABA plays an important role in tryptophan–tryptamine–serotonin metabolism. Tryptophan is a precursor of both host and microbial metabolites (such as kynurenic acid (128) and quinolinic acid, which is associated with neurodegenerative diseases by neurotoxic modulation) with anti-inflammatory and neuroprotective properties (129). Further studies are underway and future research will be needed to reveal the impact of fecal microbiota manipulation and metabolite activity on neuronal function.
Environment
The environment has a great impact on daily life and health. The “halogenome theory of evolution” suggests that both the host species and the symbiotic microbiota, which together form a unit known as the halobiont, exert genetic selection in response to environmental demand (e.g., stress, temperature, diet, industry, etc.) (6). An example of harmful environment is contact with heavy metals like cadmium, mercury, and arsenic, which can modify the immune system towards nuclear changes and disturb gut microbiota composition (6). Likewise, bisphenol A, a compound widely used by the plastic industry, can disrupt intestinal microbiota with adverse effects (6). Antibiotic abuse can increase their levels in the environment, contaminating the soil, bodies of water, and waste, thus causing huge consequences at the global level (6).
In summary, various factors such as diet, probiotics, prebiotics, antibiotics, and the environment seem to be associated with meaningful changes in the gut microbiota. Alterations in both function and composition of this microbiota seems to profoundly affect risks and outcomes in AIS patients. As we move forward, the challenge will be to determine causal relationships and develop strategies to optimize microbiota composition in order to reduce risk and modulate outcomes and recovery (Figure 4).
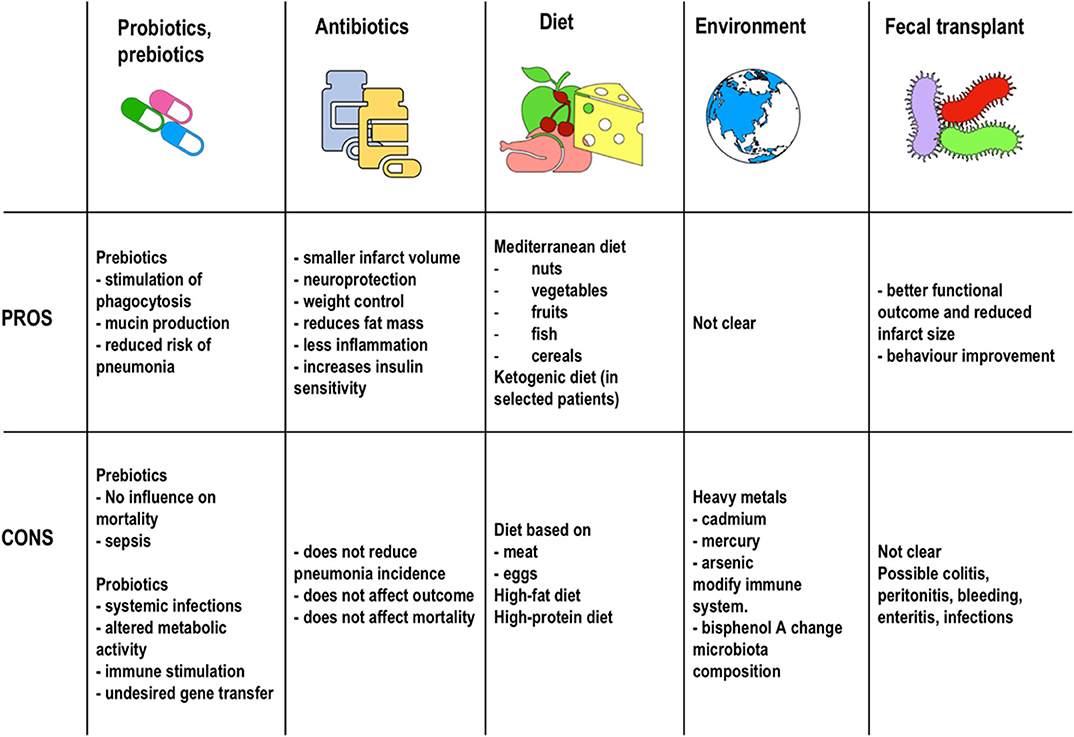
Figure 4. Therapeutic strategies. Pros and cons of the therapeutic strategies described in the literature to date for microbiota manipulation in stroke patients.
Conclusions
Translational microbiome research against the enhanced systemic inflammatory immune and neuroendocrine responses and on the impact of modulation of the environment, diet, and drugs on the so-called halobiont in AIS patients are limited. Since only few of these studies have demonstrated that antibiotic treatment, probiotics, exercise, or environmental changes could be essential for microbiota and outcome modulation, microbiota dysregulation after AIS remains a challenging target for new therapies.
Author Contributions
DB wrote the manuscript. DB, PR, PP-C, and FC designed the review. PP-C, FC, CS, PP, and PR revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Brazilian Council for Scientific and Technological Development (CNPq) and Rio de Janeiro State Research Foundation (FAPERJ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mr. Filippe Vasconcellos (São Paulo, Brazil) for his assistance in editing the manuscript.
Abbreviations
AMPs, antimicrobial peptides; AIS, acute ischemic stroke; CCR, chemokine receptor; CD, cluster of differentiation; GABA, gamma-aminobutyric acid; GALT, gut-associated lymphoid tissue; Ig, immunoglobulin; IL, interleukins; LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; MODS, multiple organ dysfunction syndrome; MYD, myeloid differentiation primary response protein; NAFLD, non-alcoholic fatty acid disease; NF-kB, nuclear factor-kappa B; NLRs, nucleotide-binding oligomerization domain-containing protein receptors; NOD, nucleotide-binding oligomerization domain-containing protein; PAMPs, pathogen associated molecular pattern; PRRs, pattern recognition receptor; RIPK, receptor-interacting protein kinase; SDI, stroke dysbiosis index; SCFA, short-chain fatty acid; TCR, T cell antigen receptor; TLRs, toll-like receptors; TMAO, trimethylamine N-oxide; Treg, T regulator lymphocytes; TREM, triggering receptor expressed on myeloid cells.
References
1. Roth GA, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
2. Poorthuis MHF, Algra AM, Algra A, Kappelle LJ, Klijn CJM. Female-and male-specific risk factors for stroke a systematic review and meta-analysis. JAMA Neurol. (2017) 74:75–81. doi: 10.1001/jamaneurol.2016.3482
3. Learoyd AE, Woodhouse L, Shaw L, Sprigg N, Bereczki D, Berge E, et al. Infections up to 76 days after stroke increase disability and death. Transl Stroke Res. (2017) 8:541–8. doi: 10.1007/s12975-017-0553-3
4. Samary CS, Pelosi P, Leme Silva P, Rocco PRM. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care. (2016) 20:391. doi: 10.1186/s13054-016-1573-1
5. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, DeRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
6. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu K V., Bastiaanssen TFS, Boehme M, et al. The microbiota-Gut-Brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
7. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ t cells. Nat Med. (2016) 22:516–23. doi: 10.1038/nm.4068
8. Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. (2016) 57:10–20. doi: 10.1016/j.bbi.2016.04.003
9. de Jong PR, González-Navajas JM, Jansen NJG. The digestive tract as the origin of systemic inflammation. Crit Care. (2016) 20:279. doi: 10.1186/s13054-016-1458-3
10. Barugh AJ, Gray P, Shenkin SD, MacLullich AMJ, Mead GE. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J Neurol. (2014) 261:533–545. doi: 10.1007/s00415-013-7231-5
11. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. (2016) 6:1239–78. doi: 10.1002/cphy.c150037
12. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
13. Wang FB, Powley TL. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. (2007) 329:221–30. doi: 10.1007/s00441-007-0413-7
14. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
15. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
16. Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. (2018) 8:568. doi: 10.1038/s41598-017-18904-8
17. Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. (2016) 22:1277–84. doi: 10.1038/nm.4194
18. David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. (2014) 15:R89. doi: 10.1186/gb-2014-15-7-r89
19. Urra X, Laredo C, Zhao Y, Amaro S, Rudilosso S, Renú A, et al. Neuroanatomical correlates of stroke-associated infection and stroke-induced immunodepression. Brain Behav Immun. (2017) 60:142–50. doi: 10.1016/j.bbi.2016.10.004
20. Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. (2014) 817:39–71. doi: 10.1007/978-1-4939-0897-4_3
21. Hyland NP, Cryan JF. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. (2016) 417:182–7. doi: 10.1016/j.ydbio.2016.06.027
22. Frederiksen HR, Haukedal H, Freude K. Cell type specific expression of toll-Like receptors in human brains and implications in alzheimer's disease. Biomed Res Int. (2019) 2019:7420189. doi: 10.1155/2019/7420189
23. Wang S, Zhang X, Zhai L, Sheng X, Zheng W, Chu H, et al. Atorvastatin attenuates cognitive deficits and neuroinflammation induced by aβ1-42 involving modulation of tLR4/TRAF6/NF-κB pathway. J Mol Neurosci. (2018) 64:363–73. doi: 10.1007/s12031-018-1032-3
24. Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. (2020) 17:1–20. doi: 10.1186/s12974-020-1705-z
25. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. (2008) 455:1109–13. doi: 10.1038/nature07336
26. Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of tLR-deficient mice. J Exp Med. (2012) 209:1445–56. doi: 10.1084/jem.20120504
27. Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through nOD1 regulates intestinal homeostasis. Nature. (2008) 456:507–10. doi: 10.1038/nature07450
28. Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. (2013) 123:700–11. doi: 10.1172/JCI62236
29. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. (2017) 17:219–32. doi: 10.1038/nri.2017.7
30. Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. Inflammasomes and metabolic disease. Annu Rev Physiol. (2014) 76:57–78. doi: 10.1146/annurev-physiol-021113-170324
31. Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. Microbial exposure during early life has persistent effects on natural killer t Cell function. Science. (2012) 336:485–9. doi: 10.1126/science.1217718
32. Lampron A, Pimentel-Coelho PM, Rivest S. Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration. J Comp Neurol. (2013) 521:3863–76. doi: 10.1002/cne.23363
33. Shen S, Kumar KP, Stanley D, Moore RJ, Hao Van TT, Wen SW, et al. Invariant natural killer t cells shape the gut microbiota and regulate neutrophil recruitment and function during intestinal inflammation. Front Immunol. (2018) 9:999. doi: 10.3389/fimmu.2018.00999
34. Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, et al. c-Maf-dependent treg cell control of intestinal tH17 cells and igA establishes host-microbiota homeostasis. Nat Immunol. (2019) 20:471–81. doi: 10.1038/s41590-019-0316-2
35. Berer K, Mues M, Koutrolos M, AlRasbi Z, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. (2011) 479:538–41. doi: 10.1038/nature10554
36. Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the cNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
37. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
38. Xu R, Tan C, Zhu J, Zeng X, Gao X, Wu Q, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care. (2019) 23:4. doi: 10.1186/s13054-019-2488-4
39. Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A, et al. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab. (2014) 34:450–9. doi: 10.1038/jcbfm.2013.217
40. Kronenberg G, Uhlemann R, Richter N, Klempin F, Wegner S, Staerck L, et al. Distinguishing features of microglia- and monocyte-derived macrophages after stroke. Acta Neuropathol. (2018) 135:551–68. doi: 10.1007/s00401-017-1795-6
41. Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, et al. Monocyte-derived macrophages contribute to spontaneous long-Term functional recovery after stroke in mice. J Neurosci. (2016) 36:4182–95. doi: 10.1523/JNEUROSCI.4317-15.2016
42. Michaud JP, Pimentel-Coelho PM, Tremblay Y, Rivest S. The impact of ly6C low monocytes after cerebral hypoxia-ischemia in adult mice. J Cereb Blood Flow Metab. (2014) 34:80. doi: 10.1038/jcbfm.2014.80
43. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
44. Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the pPARγ agonist rosiglitazone. Stroke. (2013) 44:3498–508. doi: 10.1161/STROKEAHA.113.002470
45. Liu Q, Johnson EM, Lam RK, Wang Q, Bo Ye H, Wilson EN, et al. Peripheral tREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. (2019) 20:1023–34. doi: 10.1038/s41590-019-0421-2
46. Clarkson BDS, Ling C, Shi Y, Harris MG, Rayasam A, Sun D, et al. T cell-derived interleukin (IL)-21 promotes brain injury following stroke in mice. J Exp Med. (2014) 211:595–604. doi: 10.1084/jem.20131377
47. Gelderblom M, Arunachalam P, Magnus T. γδ T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Front Cell Neurosci. (2014) 8:368. doi: 10.3389/fncel.2014.00368
48. Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, et al. Neutralization of the iL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. (2012) 120:3793–802. doi: 10.1182/blood-2012-02-412726
49. Veltkamp R, Gill D. Clinical trials of immunomodulation in ischemic stroke. Neurotherapeutics. (2016) 13:791–800. doi: 10.1007/s13311-016-0458-y
50. Singh V, Sadler R, Heindl S, Llovera G, Roth S, Benakis C, et al. The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab. (2018) 38:1293–8. doi: 10.1177/0271678X18780130
51. Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK, et al. Regulatory t cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. (2013) 121:679–91. doi: 10.1182/blood-2012-04-426734
52. Chen Y, Liang J, Ouyang F, Chen X, Lu T, Jiang Z, et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol. (2019) 10:661. doi: 10.3389/fneur.2019.00661
53. Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. (2016) 36:7428–40. doi: 10.1523/JNEUROSCI.1114-16.2016
54. Vermeij FH, Scholte OP, Reimer WJM, De Man P, Van Oostenbrugge RJ, Franke CL, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. (2009) 27:465–71. doi: 10.1159/000210093
55. Lukiw WJ, Cong L, Jaber V, Zhao Y. Microbiome-Derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-Glial (HNG) cells in primary culture. Front Neurosci. (2018) 12:896. doi: 10.3389/fnins.2018.00896
56. Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. (2019) 10:397. doi: 10.3389/fneur.2019.00397
57. Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of gut microbiota with reduced trimethylamine-n-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Hear Assoc. (2015) 4:e002699. doi: 10.1161/JAHA.115.002699
58. Li N, Weng X, Sun C, Wu X, Lu M, Si Y, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. (2019) 19:191. doi: 10.1186/s12866-019-1552-1
59. Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, et al. The microbiota regulate neuronal function and fear extinction learning. Nature. (2019) 574:543–8. doi: 10.1038/s41586-019-1644-y
60. Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE. (2017) 12:e0171521. doi: 10.1371/journal.pone.0171521
61. Bonsack B, Jiang RHY, Borlongan C V. A gut feeling about stroke reveals gut-brain axis' active role in homeostasis and dysbiosis. J Cereb Blood Flow Metab. (2020) 40:1132–4. doi: 10.1177/0271678X19900037
62. Zeng X, Gao X, Peng Y, Wu Q, Zhu J, Tan C, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-Producing bacteria in the gut. Front Cell Infect Microbiol. (2019) 9:4. doi: 10.3389/fcimb.2019.00004
63. Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. (2017) 49:96–104. doi: 10.1152/physiolgenomics.00081.2016
64. Avolio E, Gualtieri P, Romano L, Pecorella C, Ferraro S, Palma G, et al. Obesity and body composition in man and woman: associated diseases and the new role of gut microbiota. Curr Med Chem. (2019) 27:216–29. doi: 10.2174/0929867326666190326113607
65. Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. (2001) 104:2039–44. doi: 10.1161/hc4201.097944
66. Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care. (2016) 4:3. doi: 10.1186/s40560-016-0138-3
67. Ko SB, Yoon BW. Blood pressure management for acute ischemic and hemorrhagic stroke: the evidence. Semin Respir Crit Care Med. (2017) 38:718–25. doi: 10.1055/s-0037-1608777
68. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990-2015. JAMA. (2017) 317:165–82. doi: 10.1001/jama.2016.19043
69. Leustean AM, Ciocoiu M, Sava A, Costea CF, Floria M, Tarniceriu CC, et al. Implications of the intestinal microbiota in diagnosing the progression of diabetes and the presence of cardiovascular complications. J Diabetes Res. (2018) 2018:5205126. doi: 10.1155/2018/5205126
70. Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, et al. Utility of plasma concentration of trimethylamine n-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. (2019) 42:1512–20. doi: 10.2337/dc19-0048
71. Tan C, Wang H, Gao X, Xu R, Zeng X, Cui Z, et al. Dynamic changes and prognostic value of gut microbiota-Dependent trimethylamine-N-Oxide in acute ischemic stroke. Front Neurol. (2020) 11:29. doi: 10.3389/fneur.2020.00029
72. Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension novelty and significance. Hypertension. (2015) 65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315
73. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
74. Vermeij JD, Westendorp WF, van de Beek D, Nederkoorn PJ. Post-stroke infections and preventive antibiotics in stroke: update of clinical evidence. Int J Stroke. (2018) 13:913–20. doi: 10.1177/1747493018798557
75. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
76. Hannawi Y, Hannawi B, Rao CPV, Suarez JI, Bershad EM. Stroke-Associated pneumonia: major advances and obstacles stroke-Associated pneumonia. Cerebrovasc Dis. (2013) 35:430–43. doi: 10.1159/000350199
77. Ulm L, Hoffmann S, Nabavi D, Hermans M, Mackert BM, Hamilton F, et al. The randomized controlled sTRAWINSKI trial: procalcitonin-Guided antibiotic therapy after stroke. Front Neurol. (2017) 8:153. doi: 10.3389/fneur.2017.00153
78. Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. (2006) 37:2607–2. doi: 10.1161/01.STR.0000240409.68739.2b
79. Battaglini D, Robba C, Lopes da Silva A, dos Santos Samary C, Leme Silva P, Dal Pizzol F, et al. Brain-heart interaction after acute ischemic stroke. Crit Care. (2020) 24:163. doi: 10.1186/s13054-020-02885-8
80. Lavy S, Yaar I, Melamed E, Stern S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke. (1974) 5:775–80. doi: 10.1161/01.STR.5.6.775
81. Simula S, Muuronen AT, Taina M, Jäkälä P, Sipola P, Vanninen R, et al. Effect of middle cerebral artery territory ischemic stroke on qT interval. J Stroke Cerebrovasc Dis. (2014) 23:717–23. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.032
82. Battson ML, Lee DM, Weir TL, Gentile CL. The gut microbiota as a novel regulator of cardiovascular function and disease. J Nutr Biochem. (2018) 56:1–15. doi: 10.1016/j.jnutbio.2017.12.010
83. Wu C, Li C, Zhao W, Xie N, Yan F, Lian Y, et al. Elevated trimethylamine n-oxide related to ischemic brain lesions after carotid artery stenting. Neurology. (2018) 90:e1283–e90. doi: 10.1212/WNL.0000000000005298
84. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
85. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
86. Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of cultivatable gut microbiota by broad-Spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. (2016) 47:1354–63. doi: 10.1161/STROKEAHA.115.011800
87. Durgan DJ, Lee J, McCullough LD, Bryan RM. Examining the role of the microbiota-Gut-Brain axis in stroke. Stroke. (2019) 50:2270–77. doi: 10.1161/STROKEAHA.119.025140
88. Keller JJ, Wang J, Hwang YL, Chou CC, Wang LH, Hsu JL, et al. Increased risk of stroke among patients with crohn's disease: a population-based matched cohort study. Int J Colorectal Dis. (2015) 30:645–53. doi: 10.1007/s00384-015-2132-y
89. Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: the reasons for geographic and racial differences in stroke cohort. PLoS ONE. (2018) 13:e0194153. doi: 10.1371/journal.pone.0194153
90. Tripodi A, Mannucci PM. Liver disease coagulopathy. N Engl J Med. (2011) 365:147–56. doi: 10.1056/NEJMra1011170
91. Parikh NS, Navi BB, Schneider Y, Jesudian A, Kamel H. Association between cirrhosis and stroke in a nationally representative cohort. JAMA Neurol. (2017) 74:927–32. doi: 10.1001/jamaneurol.2017.0923
92. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite tMAO enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165:111–24. doi: 10.1016/j.cell.2016.02.011
93. Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine n-oxide: the good, the bad and the unknown. Toxins. (2016) 8:26. doi: 10.3390/toxins8110326
94. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
95. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. (2014) 13:823–33. doi: 10.1016/S1474-4422(14)70026-2
96. Arnold J, Ng KP, Sims D, Gill P, Cockwell P, Ferro C. Incidence and impact on outcomes of acute kidney injury after a stroke: a systematic review and meta-analysis. BMC Nephrol. (2018) 19:283. doi: 10.1186/s12882-018-1085-0
97. Kumai Y, Kamouchi M, Hata J, Ago T, Kitayama J, Nakane H, et al. Proteinuria and clinical outcomes after ischemic stroke. Neurology. (2012) 78:1909–15. doi: 10.1212/WNL.0b013e318259e110
98. Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res. (2019) 42:123–40. doi: 10.1038/s41440-018-0144-z
99. Hénaut L, Grissi M, Brazier F, Assem M, Poirot-Leclercq S, Lenglet G, et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci Rep. (2019) 9:6432. doi: 10.1038/s41598-019-42933-0
100. Cho CE, Caudill MA. Trimethylamine-N-Oxide: friend, foe, or simply caught in the cross-Fire? Trends Endocrinol Metab. (2017) 28:121–30. doi: 10.1016/j.tem.2016.10.005
101. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine n-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. (2015) 116:448–55. doi: 10.1161/CIRCRESAHA.116.305360
102. Ahluwalia B, Magnusson MK, Öhman L. Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scand J Gastroenterol. (2017) 52:1185–93. doi: 10.1080/00365521.2017.1349173
103. Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. (2004) 35:2128–33. doi: 10.1161/01.STR.0000137607.61697.77
104. Liu HB, Tian J, Zhao JX, Song DB, Tian JK. [Study on the clinical epidemiological features of acute cerebral stroke inducing systemic inflammatory response syndrome and multiple organ dysfunction syndrome]. Zhonghua Liu Xing Bing Xue Za Zhi. (2008) 29:294–6.
105. Sanchez KK, Chen GY, Schieber AMP, Redford SE, Shokhirev MN, Leblanc M, et al. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell. (2018) 175:146–58.e15. doi: 10.1016/j.cell.2018.07.016
106. Ferguson JF, Allayee H, Gerszten RE, Ideraabdullah F, Kris-Etherton PM, Ordovás JM, et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a Scientific statement from the american heart association. Circ Cardiovasc Genet. (2016) 9:291–313. doi: 10.1161/HCG.0000000000000030
107. Cavaleri F, Bashar E. Potential synergies of β -Hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab. (2018) 2018:1076. doi: 10.1155/2018/7195760
108. Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, et al. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res. (2015) 35:858–64. doi: 10.1016/j.nutres.2015.07.002
109. Aridi YS, Walker JL, Wright ORL. The association between the mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. (2017) 9:674. doi: 10.3390/nu9070674
110. Tsivgoulis G, Psaltopoulou T, Wadley VG, Alexandrov AV, Howard G, Unverzagt FW, et al. Adherence to a mediterranean diet and prediction of incident stroke. Stroke. (2015) 46:780–5. doi: 10.1161/STROKEAHA.114.007894
111. Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. (2018) 9:890. doi: 10.3389/fmicb.2018.00890
112. Madsen L, Myrmel LS, Fjære E, Liaset B, Kristiansen K. Links between dietary protein sources, the gut microbiota, and obesity. Front Physiol. (2017) 8:1047. doi: 10.3389/fphys.2017.01047
113. Moreno-Pérez D, Bressa C, Bailén M, Hamed-Bousdar S, Naclerio F, Carmona M, et al. Effect of a protein supplement on the gut microbiota of endurance athletes: a Randomized, controlled, double-blind pilot study. Nutrients. (2018) 10:337. doi: 10.3390/nu10030337
114. Venegas DP, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:1486. doi: 10.3389/fimmu.2019.01486
115. Sadler R, Cramer J V., Heindl S, Kostidis S, Betz D, Zuurbier KR, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. (2020) 40:1162–73. doi: 10.1523/JNEUROSCI.1359-19.2019
116. Kothari D, Patel S, Kim SK. Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother. (2019) 111:537–47. doi: 10.1016/j.biopha.2018.12.104
117. Markowiak P, Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. (2017) 9:1021. doi: 10.3390/nu9091021
118. Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest. (2013) 143:646–55. doi: 10.1378/chest.12-1745
119. Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. (2015) 386:1835–44. doi: 10.1016/S0140-6736(15)00126-9
120. Westendorp WF, Vermeij J-D, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJLW, et al. The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. (2015) 385:1519–26. doi: 10.1016/S0140-6736(14)62456-9
121. Kim KO, Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clin Endosc. (2019) 52:137–43. doi: 10.5946/ce.2019.009
122. Evrensel A, Ceylan ME. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci. (2016) 14:231–7. doi: 10.9758/cpn.2016.14.3.231
123. Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. (2019) 148:104403. doi: 10.1016/j.phrs.2019.104403
124. Juul FE, Garborg K, Bretthauer M, Skudal H, Oines MN, Wiig H, et al. Fecal microbiota transplantation for primary clostridium difficile infection. NEJM. (2018) 378:2535–7. doi: 10.1056/NEJMc1803103
125. Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. (2016) 1642:180–8. doi: 10.1016/j.brainres.2016.03.042
126. Park MJ, Sohrabji F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. J Neuroinflammation. (2016) 13:300. doi: 10.1186/s12974-016-0765-6
127. Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of hDAC and mMP-9 inhibition. J Cereb Blood Flow Metab. (2011) 31:52–7. doi: 10.1038/jcbfm.2010.195
128. Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. (2013) 169:1211–27. doi: 10.1111/bph.12230
Keywords: microbiome, acute ischemic stroke, inflammation, dysbiosis, microbiota
Citation: Battaglini D, Pimentel-Coelho PM, Robba C, dos Santos CC, Cruz FF, Pelosi P and Rocco PRM (2020) Gut Microbiota in Acute Ischemic Stroke: From Pathophysiology to Therapeutic Implications. Front. Neurol. 11:598. doi: 10.3389/fneur.2020.00598
Received: 26 March 2020; Accepted: 22 May 2020;
Published: 25 June 2020.
Edited by:
Johannes Boltze, University of Warwick, United KingdomReviewed by:
Javier Ochoa-Reparaz, Eastern Washington University, United StatesYan He, Southern Medical University, China
Jia Yin, Southern Medical University, China
Copyright © 2020 Battaglini, Pimentel-Coelho, Robba, dos Santos, Cruz, Pelosi and Rocco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Rieken Macedo Rocco, prmrocco@biof.ufrj.br
 Denise Battaglini
Denise Battaglini Pedro Moreno Pimentel-Coelho
Pedro Moreno Pimentel-Coelho Chiara Robba
Chiara Robba Claudia C. dos Santos4
Claudia C. dos Santos4 Fernanda Ferreira Cruz
Fernanda Ferreira Cruz Paolo Pelosi
Paolo Pelosi Patricia Rieken Macedo Rocco
Patricia Rieken Macedo Rocco