- 1Department of Rehabilitation Medicine, University of Washington, Seattle, WA, United States
- 2Department of Mechanical Engineering, University of Washington, Seattle, WA, United States
Background: Stroke is one of the most common neurologic injuries worldwide. Over decades, evidence-based neurorehabilitation research and advancements in wireless, wearable sensor design have supported the deployment of technologies to facilitate recovery after stroke. Surface electromyography (sEMG) is one such technology, however, clinical application remains limited. To understand this translational practice gap and improve clinical uptake, it is essential to include stakeholder voices in an analysis of neurorehabilitation practice, the acceptability of current sEMG technologies, and facilitators and barriers to sEMG use in the clinic and the community. The purpose of this study was to foreground the perspectives of stroke survivors to gain a better understanding of their experiences in neurorehabilitation, the technologies they have used during their recovery, and their opinions of lab-designed and commercially-available sEMG systems.
Methods: A qualitative, phenomenological study was completed. In-depth, semi-structured interviews were conducted with eight stroke survivors (age range 49–78 years, 6 months to 12 years post-stroke) and two caregivers from a large metropolitan region. A demonstration of four sEMG systems was provided to gather perceptions of sensor design, features and function, and user interface. Interviews were audio-recorded, transcribed verbatim, and coded for analysis using constant comparison until data saturation was reached.
Results: Three themes emerged from the data: (1) “Surface EMG has potential….but…” highlights the recognition of sEMG as a valuable tool but reveals a lack of understanding and need for clear meaning from the data; (2) “Tracking incremental progress over days or years is important” highlights the persistence of hope and potential benefit of sEMG in detecting small changes that may inform neurorehabilitation practice and policy; and (3) “Neurorehabilitation technology is cumbersome” highlights the tension between optimizing therapy time and trying new technologies, managing cost, logistics and set-up, and desired technology features.
Conclusion: Further translation of sEMG technology for neurorehabilitation holds promise for stroke survivors, but sEMG system design and user interface needs refinement. The process of using sEMG technology and products must be simple and provide meaningful insight to recovery. Including stroke survivors directly in translational efforts is essential to improve uptake in clinical environments.
Introduction
Over the past decades, there has been a prolific amount of research and development of technology to enhance both the understanding of neurologic injuries and the application of evidence-based neurorehabilitation interventions. Surface electromyography (sEMG) is one such technology that has undergone rapid advancement in development, but has yet to reach its full translational potential to help drive neurorehabilitation and maximize recovery. Understanding this translational gap must consider multiple factors across a complex landscape of healthcare provision, especially given the public/private healthcare model in the United States. Successful deployment of sEMG in clinical environments relies on an interaction of system design, funding, translational research findings, clinician training, and user acceptance, among many other factors. While user acceptance of neurorehabilitation technology is just a small piece of a much larger puzzle, it is an essential one, and a more explicit understanding of the perceptions and experiences of individuals with neurologic injury, such as stroke, is warranted to better understand the barriers, facilitators, and untapped potential of sEMG technology in clinical neurorehabilitation,
Stroke is one of the most common neurologic injuries worldwide (1, 2). Recent global statistics estimate nearly 14 million new instances of stroke annually; stroke related healthcare costs in the US alone have topped $750 billion annually and are projected to increase as a result of the aging population (3, 4). Further, the psychosocial and functional impacts of stroke are also significant, leading to stress, isolation, and potential comorbid health conditions (5, 6). While neurorehabilitation is a central feature of recovery for individuals with stroke, outcomes can be disparate and long-term impairment is common, further influenced by the extent to which stroke survivors have the geographic, financial, healthcare, and socio-emotional resources to maximize recovery following their injury (1). It is because of this significant impact of stroke at both individual and institutional levels that the field of neurorehabilitation must engage in a deeper exploration of the translation of advanced healthcare technologies into clinical settings to enhance our knowledge and provision of care during recovery from neurologic injuries.
Surface EMG today is used in research and clinical environments across a wide variety of physiological and engineering applications relating to rehabilitation, sport performance, occupational performance, and beyond (7). More specific to neurorehabilitation, foundational literature in the mid-twentieth century described sEMG as a useful tool to characterize neuromuscular patterns, demonstrated the relative contribution of different muscles in functional movement, and in some cases, assisted in prognosis of recovery following neurologic injury (8, 9). Across many subsequent decades, researchers have used sEMG to examine factors in participants with and without neurologic impairments such as interlimb coordination, muscle activation and co-activation patterns, response to biofeedback, and most recently, as a tool to determine treatment appropriateness and costs in stroke survivors with gait impairments (7, 10–14). Despite these advances, a significant body of literature supporting the use of sEMG, and the establishment of expert guidelines for sEMG implementation through SENIAM (Surface EMG Non-Invasive Assessment of Muscles), a lack of clinical translation of sEMG technology has also been recognized by researchers (7, 15–18).
One potential reason for the slow clinical uptake of sEMG and related neurorehabilitation technologies may be the paucity of perspectives in research from clinicians as providers of sEMG assessment or intervention, and individuals with neurologic conditions and their caregivers as recipients of sEMG assessment or intervention. Considering sEMG alongside other neurorehabilitation technologies more broadly, the literature is lacking a clear picture of how and how often these technologies are used in clinics across the US, and how technology users and their caregivers respond to the design, logistics of use, and output of the devices. However, user and caregiver perspectives are a key untapped resource in the design and implementation of rehabilitation technologies such as sEMG, and have the potential to richly contextualize the barriers and facilitators that affect technology acceptance and use. For example, within the broader realm of neurorehabilitation technology, Alt Murphy et al. (19) recently published a qualitative analysis of participant responses to a novel wearable sensor garment to monitor physiologic and movement parameters for individuals with stroke, Parkinson's Disease, or Epilepsy. The authors reported that responses to the upper body garment was acceptable, but participants noted challenges with fit and comfort and felt uncertain about consistent monitoring and privacy (19). Another study noted similar comfort issues with wearable sensors, but highlighted that despite the discomfort, participants preferred the automated data tracking features of the sensors compared to more time-intensive activities such as completing activity or symptom diaries (20).
Additional qualitative work with stroke survivors and clinicians has also explored perspectives and experiences of the rehabilitation process itself, as well as technologies such as virtual reality, gaming, robotic exoskeletons, or other wearable devices, but little work has focused specifically on sEMG (21–29). One study included gaming as part of a structured, enriched rehabilitation environment, which garnered positive responses from participants who noted increased motivation to move as well as friendly competition between other participants on the unit (29). Perceptions of virtual reality systems varied, with one study reporting low rates of side effects but high rates of perceived exertion by stroke survivors (21), and another describing how users felt enjoyment and motivation using a novel technology they would not otherwise have had access to, but felt that the experiences with virtual reality did not translate into improved functional carryover (23). Many studies have examined robotic applications for stroke rehabilitation, but very few have included survivor perspectives. Those that have describe user priorities of cost, better movement quality, endurance, practicality, and appropriate training and support, but also highlight technology acceptance issues as a potential barrier for clinical or home use (30–33). One set of studies investigated the preliminary use of sEMG as a control mechanism for a gaming system in chronic stroke survivors, finding significant pre and post intervention sEMG changes, and qualitative outcomes which indicated most participants would recommend neurogaming to others for enjoyment, despite a lack of reported functional carryover (26, 34). Our recent work has explored rehabilitation clinicians' perspectives of the use of sEMG in practice with individuals with neurologic conditions, who noted the potential benefits of objective recovery tracking, muscle training, and patient motivation, but also acknowledged barriers to sEMG use such as time, training, and access to funds and technical support for sEMG equipment (35).
The literature notes that the introduction of novel healthcare technologies into existing clinical practices can be challenging, as the process often disrupts engrained care routines (36). Resistance to new technology integration, as well as distinct ways of evaluating the utility of technology from professional and lay perspectives are common (37). This has consequences for both healthcare providers as well as patients. For example, healthcare providers have noted translational difficulties, including challenges with clearly communicating results to patients and using technology outputs to meaningfully guide treatment decisions. Patients have expressed uncertainty about the purpose of technology as a part of their care, and a failure to receive meaningful results from their providers (37). Applied to rehabilitation, it is reasonable to expect that there may be similar challenges when considering the implementation of sEMG technology, especially considering the introduction of a high-tech, objective, instrumented assessment tool juxtaposed with clinical standards that typically involve low-tech, subjective, scaled tools such as manual muscle testing or dynamometry. Experiences such as these underscore that clinician training, communication about technology intent, impact, and translational capacity to assist in healthcare decision-making are important factors to consider in improving uptake of technology in clinical settings.
The purpose of this early-stage study was to foreground the perspectives of stroke survivors and gain a better understanding of their experiences in neurorehabilitation, the technologies they have used during their recovery, and their introductory perceptions of one lab-designed prototype and three commercially available sEMG systems. Centering these perspectives is critical to understanding the barriers and untapped potential of sEMG and other neurorehabilitation technologies that may support the recovery of individuals with neurologic injuries. This qualitative work complements and builds upon past milestones in sEMG research across rehabilitation and engineering fields. It offers a preliminary look at baseline user perspectives to inform more robust research in the future, and provides a unique opportunity to leverage user-centered perspectives to support potential innovations in sEMG design, implementation, and outcomes.
Methods
All procedures in this study were approved by the authors' institutional review board, and written consent was obtained by all participants prior to initiation of study procedures. Participant names are pseudonyms to protect privacy and confidentiality. This qualitative study was conducted using a phenomenological approach, which is ideal for understanding the lived experiences of a group of participants with similar characteristics (38). Ultimately, the goal of phenomenological research is to describe and interpret a given phenomenon through the lens of individuals with first-hand knowledge of the event or experience, in this case, the experience of having and living with stroke and experiencing neurorehabilitation (39, 40). While this lived experience may or may not have included technology use during recovery, it provided a shared foundation from which to obtain informed perceptions of sEMG as a potential part of this experience.
Research Team Background
This study was conducted by a multidisciplinary research team, including a physical therapist with qualitative research expertise, mechanical engineer with wearable sensor expertise, and two research scientists. All members of the team had extensive training and experience in the clinical application of sEMG systems to track muscle activity changes in stroke survivors. In addition to this qualitative work, the research team was concurrently collecting sEMG data from stroke survivors in acute care and community-based settings. Therefore, the researchers were well-positioned to engage with and provide baseline information to the participants about sEMG technology.
Study Procedures
Semi-structured interviews were conducted to obtain primary source thoughts, opinions, and interpretations from the participants, using an interview guide that was developed by the authors, edited for content until consensus was reached, and piloted with a volunteer to ensure clarity of question content and order. Figure 1 contains a list of sample questions from the interview guide.

Figure 1. Sample Semi-Structured Interview Questions: These questions were among those asked of each participant during the semi-structured interview. Responses were audio-recorded and transcribed verbatim.
During each interview, a brief demonstration of four, research lab-owned sEMG systems was conducted. This included one lab-designed sEMG sensor prototype as well as three commercially available sEMG systems, chosen to represent a broad range of system design, aesthetic, and capabilities: (A) MC10 BiostampRC® (Lexington, MA, USA); (B) Thalamic Labs Myo™ Armband (Kitchener, Ontario, CAN); (C) Delsys Trigno™ (Natick, MA, USA); and (D) Epidermal Sensor System (Austin, TX, USA, patent pending), a lab-designed prototype with sensor filament embedded in medical tape. See Figure 2 for images of each sEMG system. Participants were able to examine each system and were briefed on features including functionality and purpose, battery life, skin preparation needs, anatomical placement, user interface, and cost. Participants were also oriented to print versions of sample signal outputs from each system, since time constraints prohibited real-time system use. To minimize the potential for biased responses, the research team refrained from endorsing any given system and only provided pre-scripted, general purpose information about each system and the clinical applications of sEMG to assist the participants in offering informed perceptions. Participants were able to ask clarifying questions about the purpose and features of the systems, and self-assessed their understanding of the information presented prior to continuing the interview. Feedback about the features and perceived utility of each sEMG system was then solicited from each participant.

Figure 2. Sample Commercial and Lab-Based sEMG Sensors: A brief demonstration and feature discussion for these four sEMG systems was conducted during each participant interview. Systems were available for physical inspection, but not applied to the participants. (A) MC10 Biostamp® (B) Thalamic Labs Myo™ Armband; (C) Delsys Trigno™; and (D) Epidermal Sensor System (patent pending).
Interviews were conducted at a location of the participant's choosing, with half the interviews taking place at the participant's home, and half taking place in university settings such as an office or research lab. All interviews were audio-recorded, transcribed verbatim, and de-identified.
Participants
Participants were recruited via convenience sampling through stroke clinics and rehabilitation professional contacts across a large metropolitan area. To be included in the study, participants must have been over the age of 18 years, have the cognitive capability to consent for themselves, have had any type of stroke in the past or be the spouse or caregiver of the stroke survivor, and demonstrate proficiency communicating in English. Potential participants were excluded from the study if they were under the age of 18 years, not able to cognitively consent for themselves, or communicate proficiently in English. Stroke survivors with aphasia or limited communication capabilities were included in the study along with their spouses/caregivers. Ten participants across the Seattle metropolitan region completed the study, including eight stroke survivors and two spouses/caregivers. Of the stroke survivors, four were male and four were female, ranging in age from 49 to 78 years old (mean = 65 years), and ranging from 6 months to 12 years post-stroke (mean = 4.75 years). One male and one female spouse/caregiver participated in the interviews with their respective partners. Two participants had mild to moderate expressive aphasia, one participated in the interviews independently, and another participated to the greatest extent possible with the assistance of his spouse. Most participants (n = 8) had post-secondary vocational training or college degrees, with employment backgrounds including business and finance administration, teaching, musician, and aerospace engineering. One participant had previously worked in a research and development capacity with activity monitoring technology for nearly 20 years, and another had participated in a prior research study using sEMG for serious gaming. The remaining participants did not have prior experience with sEMG, though many had used or trialed related neuromuscular rehabilitation technologies such as electrical stimulation or biofeedback, as well as ubiquitous lay technologies such as commercial fitness or activity trackers, following their stroke.
Data Analysis
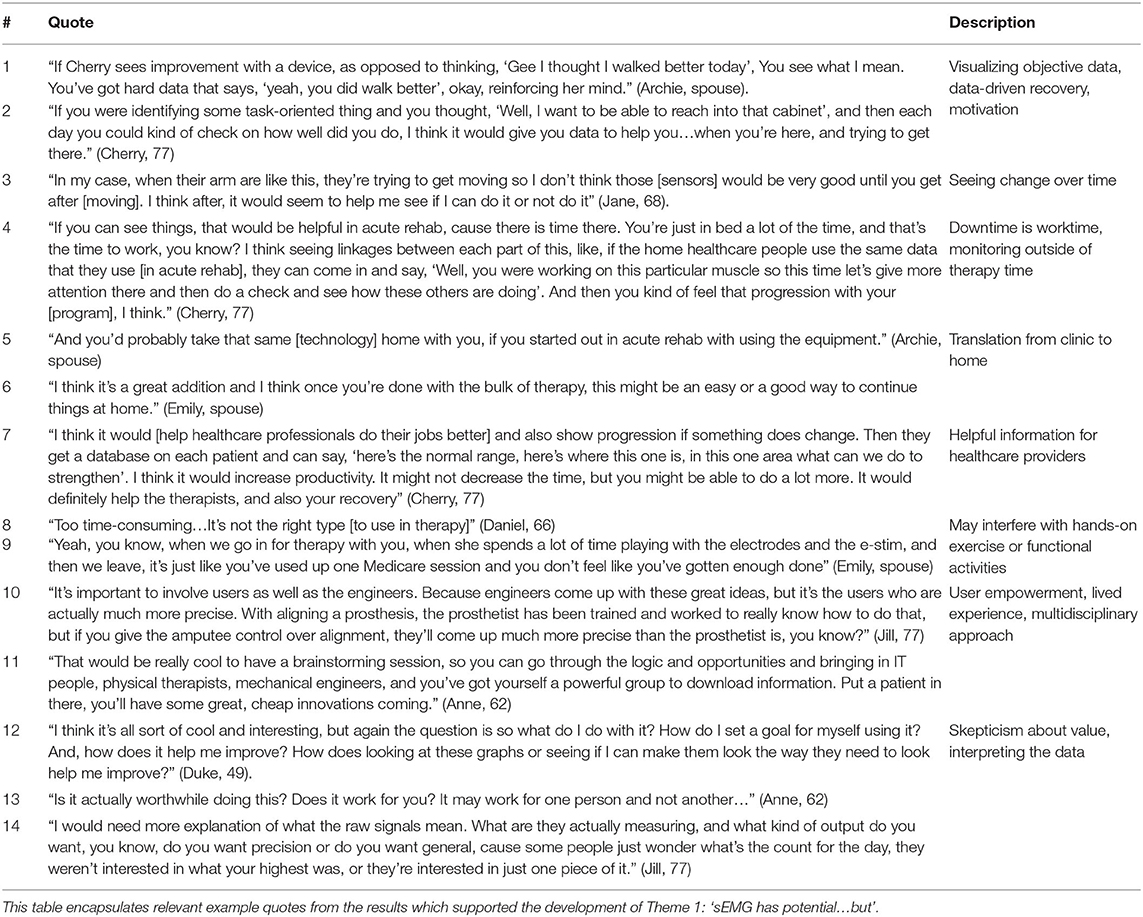
De-identified transcripts were analyzed inductively for their responsiveness to the research purpose, and coded using constant comparison until data saturation was reached and themes grounded in the participants' perceptions and experiences emerged (38, 39). The authors engaged first in open coding, followed by further, independent content analysis and focused coding and discussion among the team to consolidate focused codes into themes. All themes were created with >95% agreement, and any differences in interpretation were resolved by discussion until consensus was achieved. Interview participants engaged in member checking, by which they were provided an opportunity to review a summary of the findings and ask questions so the research team could confirm accuracy and avoid misinterpretation of the results (38). A “thick description” of participant perceptions and experiences, supported directly by verbatim quotations, is presented to allow reader-driven determination of data credibility (39). Table 1 shows a detailed example of the structured coding process.
Results
Three major themes emerged from the data: (1) “Surface EMG has potential.but…”; (2) “Tracking incremental progress over days or years is important”; and (3) “Neurorehabilitation technology is cumbersome”. These themes inform an overarching construct that sEMG could be valuable for stroke survivors, but the process and products must be simple and meaningful to their recovery in order to achieve greater uptake in both clinical and community settings during rehabilitation. Within these themes, the participants identified several key features of existing sEMG systems that were appealing, as well as features that presented barriers to use. Participants also offered innovative ideas and solutions for future iterations of sEMG technology and key insights for improved technology translation in healthcare.
Theme 1: “Surface EMG Has Potential…but…”
The first theme highlights perspectives that sEMG could offer motivation, reinforcement, and provide more precise data during recovery, but this data is only valuable if its output is both meaningful for stroke survivors and clinically useful for rehabilitation professionals. Participants also identified that timing of sEMG application is a key consideration. In general, participants felt that having sEMG data would help clinicians in care planning and decision-making, however, there was some disagreement among participants as to if this was a valuable use of clinicians' time. Ultimately, participants felt excited about the potential of sEMG technology and an integrated approach to rehabilitation that included clinicians, engineers, and patients. However, participants also expressed a simultaneous need for further clarity about the impact of sEMG data on their recovery.
For example, a majority (n = 8) participants felt that sEMG could be a useful way to deliver more objective data to support their self-perceived assessment of functional recovery (Table 2, Quotes 1-2). In regard to optimal timing, there were differing opinions as to whether sEMG would be valued in acute rehabilitation prior to return of visible movement. One stroke survivor thought sEMG would be more useful after voluntary movement returned, however, this was an outlying view (Table 2, Quote 3). The remaining participants felt sEMG data could be applied early, to facilitate a more integrated approach to monitoring recovery, and over half of the participants noted that sEMG technology would translate well between clinical and home settings during the recovery process (Table 2, Quotes 4-6).
A majority of participants (n = 8) also highlighted the potential importance of sEMG in providing objective information to their clinical teams, noting that it could fill a gap for medical providers in challenging or ambiguous situations, and assist in making accurate prognostic decisions, providing concrete feedback to survivors and caregivers, or improve rehabilitation productivity (Table 2, Quote 7). One couple, in drawing from their previous experiences with electrical stimulation, had an alternative view, feeling like time spent with set up or monitoring of sEMG could interfere with other therapy activities and reduce time for hands-on functional activities or exercises with therapists (Table 2, Quotes 8-9).
Among over half the participants (n = 6), there was recognition of sEMG as a means to invite a multidisciplinary, user-centered technology experience into rehabilitation and recovery. Participants were excited about the prospect of brainstorming sessions to leverage existing technologies and innovate with new ideas, and highlighted the importance of the technology user as a primary stakeholder with lived expertise in regard to these healthcare advances (Table 2, Quotes 10-11). Despite these perceived benefits, reservations among participants remained regarding the interpretation of sEMG outputs and appraising the value of a potential investment in sEMG during recovery (Table 2, Quotes 12-14).
Theme 2: “Tracking Incremental Progress Over Days or Years Is Important”
The second theme reflects participants' experiences of small changes or progress months and years after stroke, the potential role of technology like sEMG in detecting such change during acute and long-term recovery, and the need for more data to document objective changes that may inform neurorehabilitation policy and practice.
For example, all participants discussed their recovery journey, highlighting processes of both self-discovery as well as harsh realities, noting that they surprised themselves and their medical team with changes long after their stroke. Depending on how recently the stroke occurred, these changes were still emerging (Table 3, Quotes 1-4). Progress was slower than expected, and even with positive experiences in recovery, long adjustment periods and fear were common threads (Table 3, Quote 5). As a part of this incremental recovery, a majority of participants (n = 7) noted they would appreciate the precision offered by sEMG and the potential to identify small changes during rehabilitation (Table 3, Quotes 6-7). The participants felt that the capability to monitor muscle activity and see these small changes was a benefit that outweighed the potential for discouragement if minimal or no progress was observed in a particular muscle group (Table 3, Quote 8).
All participants also highlighted the challenges with transitioning back home after rehabilitation, and a desire to have more connectivity and more technology in a home setting. For example, sEMG could play a role during time at home outside therapy, or as a means to monitor and prevent further medical complications resulting from inactivity (Table 3, Quotes 9, 10). Both caregivers also noted the challenges that arise with the transition home, whether that be fatigue, logistics, or time for technology or other recovery activities (Table 3, Quotes 11, 12). Ultimately, for both treatment decisions as well as technology decisions, participants stressed the importance of involving the stroke survivor at every step, even in the early stages, to self-advocate for their needs (Table 3, Quotes 13, 14).
All participants discussed insurance frustrations during their stroke recovery. Over half the participants (n = 6) also noted that data describing these incremental changes noted during recovery could be essential for improving policy and access to neurorehabilitation services as well as technology such as sEMG. However, it was clear to participants that if sEMG will become a justifiable and viable rehabilitation technology, that it must demonstrate its significance to both users and insurance companies (Table 3, Quote 15, 16).
Theme 3: “Neurorehabilitation Technology Is Cumbersome”
The third theme reflects experiences of stroke survivors with other types of muscle tracking technology, as well as perceptions of current sEMG systems. Participants highlighted issues of cost and funding coverage, time, set-up logistics, and the tension between their desire to optimize time in therapy sessions vs. a willingness to try new technologies that may (or may not) enhance recovery. For example, even for a participant whose previous job was to create and test activity monitors, cost and accuracy were issues (Table 4, Quote 1).
All participants had purchased some type of rehabilitation technology out of pocket, and described similar sentiments about cost, maintenance, and reuse of supplies (Table 4, Quotes 2-4). Overall, the participants described a “buy and try” mentality, resulting in both successes and failures with technology (Table 4, Quotes 5-7). However, despite a willingness to search out and try, most participants (n = 7) expressed a preference for lower tech adaptive devices, or more ubiquitously available lay technology. Over half (n = 6) of the participants had tried wrist-worn commercial fitness trackers but reported mixed results. Those that responded favorably noted the device's potential for motivation, and those with less favorable opinions cited a lack of accuracy or customizability (Table 4, Quotes 8, 9). When sharing their experiences with sEMG or related technologies such as electrical stimulation, participants described the challenging logistics and cumbersome nature of these rehab technologies as a major barrier to use. Finding the correct sensor placement and need for caregiver assistance for setup were mentioned by half (n = 5) the participants (Table 4, Quotes 10-12). Training on proper placement and use of muscle technologies was also reported as a barrier by all the participants who had previously used wearable sensing devices (n = 5), even when self-cueing was performed by taking photographs or drawing on the skin. Participants noted the need for refresher training with their therapists when using this technology (Table 4, Quote 13).
Trends emerged, however no consensus from the participants was reached when discussing the benefits and drawbacks perceived from the demonstration of four sEMG systems. In general, participants felt the Delsys Trigno™ was a better research tool and impractical for clinical or home use, which reflects what it and other research-grade systems were designed for. Participants appreciated the flexibility and low profile of the ESS tape sensors and the Biostamp®, but preferred the Myo™ in terms of simplicity and ease of placement without the use of stickers. Half the participants raised the issue of difficulty removing sticker backing and applying sensors one-handed (Table 4, Quote 14). Aesthetics was less of an issue, with only one participant commenting about the sensor appearance (Table 4, Quote 15). Consensus was reached, however, in regard to user-centered feedback. All participants felt that a significant improvement for the future of sEMG systems would be the ability to incorporate real-time, multisensory feedback with flexible methods for receiving signal data outputs that are meaningful to the user (Table 4, Quotes 16-18).
Participants also had some lofty goals for the future of creative sEMG system design and multifunctional capacity. Design ideas included built-in lighting, navigation systems, and greater connectivity between body and technology, envisioned as a social media-style account for muscle tracking (Table 4, Quotes 19-21). Ultimately, participants were excited about the potential for sEMG technology to play a meaningful role in their stroke recoveries, despite the existing barriers of current systems and a lack of cost-effective, adaptable, user-centered systems. As one participant notably summarized, the issue with sEMG in rehabilitation populations is largely about product development stage, user knowledge, availability, and impact in a specialized market (Table 4, Quote 22).
Discussion
Despite a significant body of research that describes the benefits that sEMG technology may provide in better understanding stroke recovery and enhancing neurorehabilitation outcomes, as well as standards for systematically implementing sEMG, translation to clinical and community settings has been limited (16, 18, 35, 41, 42). One aspect of this complex landscape is patient technology acceptance. Thus, it is important to more closely examine the perspectives of stroke survivors and their families, as central stakeholders in neurorehabilitation, in regard to sEMG technology features and functions. The recovery stories, setbacks and successes, and perceptions and presence of technologies represented in the themes that emerged from this study can play a central role in improving the translational capacity of sEMG systems.
First, our participants noted that little or no muscle monitoring technology was used during the acute and subacute phases of their recovery, these experiences underscore previous work documenting slow uptake of sEMG technologies outside research environments (15, 16). However, stroke survivors and their families indicated that this would be an ideal time to trial sEMG technology, when frustration and fear about return of muscle function is most prevalent and sEMG may detect muscle activity that is not visible or palpable. This early technology intervention is also supported in the literature, and capitalizes on the principles of neuroplasticity routinely cited as drivers of clinical practice in current stroke rehabilitation (43, 44). Further, given that detectable sEMG activity has been seen in flaccid limbs of stroke survivors days after stroke and prior to onset of voluntary muscle contraction, having this resource more readily available in acute recovery phases could serve to build hope and motivation for stroke survivors, in addition to providing information to the medical team about neural pathway integrity (45). Interestingly, participants in this study also discussed the relative “downtime” during recovery, despite their willingness to work on exercises or activities outside of scheduled therapy visits. Rehabilitation clinicians have similarly noted these challenges that come with a relatively passive institutional rehabilitation culture, where stroke survivors have limited opportunities to practice real-world functional skills during down time from therapy or medical cares—which could signal a clinical practice gap in which sEMG may offer novel and individually tailored activity challenges to promote recovery (22).
Second, at home and in the community, stroke survivors demonstrated creativity, and resilience in adapting to their changing abilities, using a combination of high and low technology to improve participation and access. This is consistent with previous literature that describes the processes by which physical resilience is demonstrated following stroke, in part by participating in the hard work of rehabilitation, as well as capitalizing on technologies that may provide access or motivation during recovery (46, 47). However, while technology use with low tech tools such as adapted cutting boards or assistive mobility devices was ubiquitous, perceptions, and use of high-tech tools, including lay technologies for tracking fitness and activity, were quite mixed. These results are also consistent with previous literature describing general interest and excitement combined with skepticism about the features and function of rehabilitation technology (19–21). Further, while most participants were exposed to muscle tracking and training technologies such as electrical stimulation or biofeedback, use was inconsistent and often abandoned due to personal cost, cumbersome set-up, or lack of progress, which is also a common theme in previous work. This presents a unique challenge to clinicians to critically examine how and when these technologies are introduced, as well as to designers and manufacturers of sEMG to understand user and clinician perspectives in early development stages, respond to the relative simplicity and aesthetic appeal of lay technologies, and simultaneously address the precision and adaptive requirements to meet rehabilitation needs.
Third, the participants most strongly highlighted the need for technology to provide both significant and meaningful results. Current sEMG technology had potential in their eyes, and many participants were willing to try, but the financial investment and learning curve were possible barriers, especially if it did not result in impactful information or change from their own point of view. From a perspective of stroke survivor as consumer, this issue is likely one of the most important considerations for future sEMG system design and function. Interestingly, a lack of meaningful outcomes is a frequently cited reason for rehabilitation technology abandonment, however, user-centered, participatory design and implementation strategies are still not widely used in the development of such technologies, especially with older adults (48–51). It is important to consider, however, that sEMG systems may be less like traditional rehabilitation technologies but more closely resemble other wearable biomedical sensors that are engrained in routine clinical care, such as those that monitor heart rhythm (EKG) or brain activity (EEG). These systems also provide valuable results to clinicians and users, but do not require any action by the user aside from passively wearing the sensors. There appears to be a gap in research exploring user acceptance of these similar devices (52), but it remains unclear whether acceptance is not viewed as a major concern, whether differing perspectives may be due to the nuance of sEMG having the potential to elicit action or provide real-time feedback to users, or whether it is simply representative of a unique timeline and trajectory for clinical translation that sEMG may come to enjoy in the future. Regardless, this further highlights the need for additional collaboration between users, clinicians, and engineers during technology development and deployment processes.
Finally, the results of this study point critically to issues of knowledge and understanding of current rehabilitation evidence. Participants discussed the need for further information and education- both for themselves and their families, but also for their healthcare providers- in regard to the benefits and potential outcomes that may be enhanced by sEMG technology. Lack of knowledge or training, time, and self-confidence, as well as a need for meaningful therapeutic outcomes surrounding use of sEMG systems are indeed themes that have been reported previously from the perspective of clinician stakeholders, who often become technology gatekeepers during stroke rehabilitation (28, 35). This is concerning, given the extensive body of literature and standardized guidelines from the SENIAM project that support sEMG as a valuable rehabilitation tool, upon which comprehensive clinician training programs could be built (17, 18). Practical solutions to fill this knowledge gap for clinicians already exist, such as the American Board of Physical Therapy Specialties certification in Clinical Electrophysiology, but could also take the form of international multidisciplinary working groups, further expansion of electrophysiology content in professional rehabilitation training, and vetted teaching and learning modules that extend to a greater number of clinical practice areas.
In addition to technology recommendations from members of their rehabilitation team, a majority of participants largely sought out solutions on their own or at the suggestion of other stroke survivors in their peer groups, which are viewed as an important aspect of recovery (53). Improving education and information sharing among clinicians and stroke survivors appears to be a pathway by which sEMG could achieve greater uptake, provided a clear and compelling message about its utility during recovery could be delivered. This will be a challenge, given the difficulties with system change and novel technology implementation that have been reported in healthcare literature (36, 37). However, there is a unique opportunity to learn from these perspectives and use them to drive product and process improvements. By listening to stakeholders, it is possible for a re-branding of the potential of sEMG technology as a valuable tool that has the capability to provide a rapid, non-invasive, and data-driven look at post-stroke muscle activity which can impact prognostic outcomes, service recommendations, education, and empowerment for stroke survivors and their families.
Study Limitations
Although the stories shared by stroke survivors and their families who participated in this research provide a much-needed perspective on sEMG technology, there are several limitations to this study. First, the participants represent a small, convenience sample contained within a single metropolitan area, and may not represent the diverse perspectives of a larger or randomized group of stroke survivors. While there was a wide range of ages, genders, stroke types, and rehabilitation courses represented among our participants, all but one individual was Caucasian. Additionally, while a standard set of factual information was shared about four sEMG systems, this is not representative of all available sEMG technologies, so participant perspectives presented here are limited to these systems only. Further, given interview time constraints and to avoid fatiguing the participants, the interviewer did not fully connect or operate the systems in real-time. Participants had the opportunity to physically interact with the sensors, observe signal printouts, and verbally or visually attend to a feature comparison chart. Further work in this area should aim to mitigate these limitations, by intentionally recruiting racially diverse participants, conducting interviews across a wider geographic area, and involving the users in real-time set up and implementation of the sEMG systems over a longer study period to obtain perspectives after full immersion in the processes. Future research should also combine user and clinician perspectives together during rehabilitation care and further assess clinician training in neurorehabilitation technology to determine how closely perspectives align and how therapeutic relationships may affect responses to sEMG technology. Finally, while these perspectives are useful, further user, clinician, and engineering collaboration before technology development and deployment will strengthen resulting outcomes, as it is less helpful to constructively critique sEMG systems once they are already commercially deployed.
Conclusion
Perspectives of individuals with neurologic injuries and their caregivers are one central piece of a broader discussion of factors influencing improved translation of sEMG technology use into clinical settings. The stroke survivors in this study felt that sEMG would be a useful tool for motivation and acquisition of objective data, but that the user interface would have to be simple, available in multiple formats based on the preferences of the user, and provide meaningful feedback for participation in real-world activities, not just exercises. Participants highlighted essential features of sEMG systems, including low cost, flexibility, intuitive and independent use and interpretation, disposability, and comfort. Further translation of sEMG technology for neurorehabilitation into clinical and community environments holds promise, but sEMG system design and user interface needs refinement, and training and education opportunities for clinicians to leverage sEMG technology throughout all phases of rehabilitation following stroke is warranted. Including stroke survivors directly in translational efforts, particularly in creating sEMG system outputs and feedback that is meaningful and motivating to users, is essential to improve uptake in both clinical and community environments.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The University of Washington Human Subjects Division. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed meaningfully to the preparation of this manuscript. HF conducted all the interviews. HF and CP completed primary data analysis. KS and KP completed secondary data analysis. All authors contributed to the writing and editing of the manuscript and equipment used in the study is owned by the Steele Lab in the Department of Mechanical Engineering at the University of Washington. Photos of sEMG systems were taken by HF and are provided courtesy of the Steele Lab. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funding from the NIH NIBIB R01EB021935 (PI Steele). Support for the time and effort of HF was also funded by the NIH NCATS KL2 TR002317.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the stroke survivors and spouses/caregivers that participated in this study for contributing their meaningful perspectives and sharing their experiences, and acknowledge the work of Shannon Chung and Carlie Morishita for their assistance with transcription.
References
1. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery. Stroke. (2016) 47:e98–169. doi: 10.1161/STR.0000000000000098
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics 2019 update: a report from the american heart association. Circulation. (2019) 139:e56–28. doi: 10.1161/CIR.0000000000000659
3. Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:439–58. doi: 10.1016/S1474-4422(19)30034-1
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Annal Neurol. (2017) 81:479–84. doi: 10.1002/ana.24897
5. Haley WE, Roth DL, Hovater M, Clay OJ. Long-term impact of stroke on family caregiver well-being: a population-based case-control study. Neurology. (2015) 84:1323–9. doi: 10.1212/WNL.0000000000001418
6. Grant JS, Clay OJ, Keltner NL, Haley WE, Wadley VG, Perkins MM, et al. Does caregiver well-being predict stroke survivor depressive symptoms? A mediation analysis. Top Stroke Rehabil. (2013) 20:44–51. doi: 10.1310/tsr2001-44
7. Merletti R, Farina D. Surface Electromyography: Physiology, Engineering, and Applications. Hoboken, NJ: John Wiley & Sons (2016). doi: 10.1002/9781119082934
8. Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. (1951) 74:443–80. doi: 10.1093/brain/74.4.443
9. Scheving LE, Pauly JE. An electromyographic study of some muscles acting on the upper extremity of man. Anatomical Record. (1959) 135:239–45. doi: 10.1002/ar.1091350402
10. Becker S, Bergamo F, Schnake KJ, Schreyer S, Rembitzki IV, Disselhorst-Klug C. The relationship between functionality and erector spinae activity in patients with specific low back pain during dynamic and static movements. Gait Posture. (2018) 66:208–13. doi: 10.1016/j.gaitpost.2018.08.042
11. Wolf S, Basmajian J. Assessment of Paraspinal Activity in Normal Subjects and in Chronic Back Pain Patients Using a Muscle Biofeedback Device. International Series on Biomechanics VI-B. (1979). p. 319–23.
12. Dewald JPA, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. (1995) 118:495–510. doi: 10.1093/brain/118.2.495
13. Li S, Liu J, Bhadane M, Zhou P, Rymer WZ. Activation deficit correlates with weakness in chronic stroke: evidence from evoked and voluntary EMG recordings. Clin Neurophysiol. (2014) 125:2413–7. doi: 10.1016/j.clinph.2014.03.019
14. Merlo A, Campanini I. Impact of instrumental analysis of stiff knee gait on treatment appropriateness and associated costs in stroke patients. Gait Posture. (2019) 72:195–201. doi: 10.1016/j.gaitpost.2019.06.009
15. Hogrel J-Y. Clinical applications of surface electromyography in neuromuscular disorders. Neurophysiol Clin Neurophysiol. (2005) 35:59–71. doi: 10.1016/j.neucli.2005.03.001
16. Cram JR. The history of surface electromyography. Appl Psychophysiol Biofeedback. (2003) 28:81–91. doi: 10.1023/A:1023802407132
17. Merletti R. Surface electromyography: the SENIAM project. Eur J Phys Rehabil Med. (2000) 36:167.
18. Stegeman D, Hermens H. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles. SENIAM (2007).
19. Alt Murphy M, Bergquist F, Hagström B, Hernández N, Johansson D, Ohlsson F, et al. An upper body garment with integrated sensors for people with neurological disorders – early development and evaluation. BMC Biomed Eng. (2019) 1:3. doi: 10.1186/s42490-019-0002-3
20. Fisher JM, Hammerla NY, Rochester L, Andras P, Walker RW. Body-worn sensors in Parkinson's disease: evaluating their acceptability to patients. Telemed e-Health. (2016) 22:63–9. doi: 10.1089/tmj.2015.0026
21. Crosbie JH, Lennon S, McNeill MD, McDonough SM. Virtual reality in the rehabilitation of the upper limb after stroke: the user's perspective. CyberPsychol Behav. (2006) 9:137–41. doi: 10.1089/cpb.2006.9.137
22. Eng XW, Brauer SG, Kuys SS, Lord M, Hayward KS. Factors affecting the ability of the stroke survivor to drive their own recovery outside of therapy during inpatient stroke rehabilitation. Stroke Res Treat. (2014) 2014:626538. doi: 10.1155/2014/626538
23. Lewis GN, Woods C, Rosie JA, Mcpherson KM. Virtual reality games for rehabilitation of people with stroke: perspectives from the users. Disabil Rehabil. (2011) 6:453–63. doi: 10.3109/17483107.2011.574310
24. Luker J, Lynch E, Bernhardsson S, Bennett L, Bernhardt J. Stroke survivors' experiences of physical rehabilitation: a systematic review of qualitative studies. Arch Phys Med Rehabil. (2015) 96:1698–708. e10. doi: 10.1016/j.apmr.2015.03.017
25. Barker R, Brauer S. Upper limb recovery after stroke: the stroke survivors' perspective. Disabil Rehabil. (2005) 27:1213–23. doi: 10.1080/09638280500075717
26. Brown EVD, McCoy SW, Fechko AS, Price R, Gilbertson T, Moritz CT. Preliminary investigation of an electromyography-controlled video game as a home program for persons in the chronic phase of stroke recovery. Arch Phys Med Rehabil. (2014) 95:1461–9. doi: 10.1016/j.apmr.2014.02.025
27. Sørensen HV, Lendal S, Schultz-Larsen K, Uhrskov T. Stroke rehabilitation: assistive technology devices and environmental modifications following primary rehabilitation in hospital—a therapeutic perspective. Assist Technol. (2003) 15:39–48. doi: 10.1080/10400435.2003.10131888
28. Langan J, Subryan H, Nwogu I, Cavuoto L. Reported use of technology in stroke rehabilitation by physical and occupational therapists. Disabil Rehabil. (2018) 13:641–7. doi: 10.1080/17483107.2017.1362043
29. White JH, Bartley E, Janssen H, Jordan L-A, Spratt N. Exploring stroke survivor experience of participation in an enriched environment: a qualitative study. Disabil Rehabil. (2015) 37:593–600. doi: 10.3109/09638288.2014.935876
30. Power V, O'Sullivan L, de Eyto A, Schülein S, Nikamp C, Bauer C, et al. Exploring user requirements for a lower body soft exoskeleton to assist mobility. In: Proceedings of the 9th ACM International Conference on PErvasive Technologies Related to Assistive Environments. (2016). doi: 10.1145/2910674.2935827
31. Morone G, Paolucci S, Cherubini A, De Angelis D, Venturiero V, Coiro P, et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat. (2017) 13:1303. doi: 10.2147/NDT.S114102
32. Wolff J, Parker C, Borisoff J, Mortenson WB, Mattie J. A survey of stakeholder perspectives on exoskeleton technology. J Neuroeng Rehabil. (2014) 11:169. doi: 10.1186/1743-0003-11-169
33. Bragoni M, Broccoli M, Iosa M, Morone G, De Angelis D, Venturiero V, et al. Influence of psychologic features on rehabilitation outcomes in patients with subacute stroke trained with robotic-aided walking therapy. Am J Phys Med Rehabil. (2013) 92:e16–25. doi: 10.1097/PHM.0b013e3182a20a34
34. Brown EVD, Dudgeon BJ, Gutman K, Moritz CT, McCoy SW. Understanding upper extremity home programs and the use of gaming technology for persons after stroke. Disabil Health J. (2015) 8:507–13. doi: 10.1016/j.dhjo.2015.03.007
35. Feldner HA, Howell D, Kelly VE, McCoy SW, Steele KM. “Look, Your Muscles Are Firing!”: A Qualitative Study of Clinician Perspectives on the Use of Surface Electromyography in Neurorehabilitation. Arch Phys Med Rehabil. (2019) 100:663–75. doi: 10.1016/j.apmr.2018.09.120
36. Berg M, Langenberg C, Vd Berg I, Kwakkernaat J. Considerations for sociotechnical design: experiences with an electronic patient record in a clinical context. Int J Med Inform. (1998) 52:243–51. doi: 10.1016/S1386-5056(98)00143-9
37. Pals RA, Hansen UM, Johansen CB, Hansen CS, Jørgensen ME, Fleischer J, et al. Making sense of a new technology in clinical practice: a qualitative study of patient and physician perspectives. BMC Health Services Res. (2015) 15:402. doi: 10.1186/s12913-015-1071-1
38. Merriam SB, Tisdell EJ. Qualitative Research: A Guide to Design and Implementation. San Francisco, CA: Jossey-Bass, A Wiley Brand (2015).
39. Tolley EE, Ulin PR, Mack N, Robinson ET, Succop SM. Qualitative Methods in Public Health. 2nd Edn. Hoboken, NJ: John Wiley & Sons (2016)
40. Davidsen AS. Phenomenological approaches in psychology and health sciences. Qual Res Psychol. (2013) 10:318–39. doi: 10.1080/14780887.2011.608466
41. Woodford HJ, Price CI. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst Rev. (2007) 2010:CD004585. doi: 10.1002/14651858.CD004585.pub2
42. Meekins GD, So Y, Quan D. American association of neuromuscular & electrodiagnostic medicine evidenced-based review: use of surface electromyography in the diagnosis and study of neuromuscular disorders. Muscle Nerve. (2008) 38:1219–24. doi: 10.1002/mus.21055
43. da Silva Cameirão M, Bermudez i Badia S, Duarte E, Verschure PF. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system. Restor Neurol Neurosci. (2011) 29:287–98. doi: 10.3233/RNN-2011-0599
44. Kaminsky T, Oldham D, Ferree C, Robert A, Yee A, Tomlin G. Effectiveness of everyday technology use in upper-extremity stroke rehabilitation. Am J Occupat Ther. (2019) 73(4_Supplement_1):7311505179p1-p1. doi: 10.5014/ajot.2019.73S1-PO7019
45. Papazian C, Baicoianu N, Peters K, Feldner H, Steele K. Electromyography recordings reveal muscle activity in flaccid arm during initial days after stroke. Arch Phys Med Rehabil. (2019) 100:e178. doi: 10.1016/j.apmr.2019.10.047
46. Michael K. Call to action: enhancing poststroke resilience. Top Geriatr Rehabil. (2014) 30:195–8. doi: 10.1097/TGR.0000000000000018
47. White J, Janssen H, Jordan L, Pollack M. Tablet technology during stroke recovery: a survivor's perspective. Disabil Rehabil. (2015) 37:1186–92. doi: 10.3109/09638288.2014.958620
48. Duque E, Fonseca G, Vieira H, Gontijo G, Ishitani L editors. A systematic literature review on user centered design and participatory design with older people. In: Proceedings of the 18th Brazilian Symposium on Human Factors in Computing Systems. (2019). doi: 10.1145/3357155.3358471
49. Mawson S, Nasr N, Parker J, Zheng H, Davies R, Mountain G. Developing a personalised self-management system for post stroke rehabilitation; utilising a user-centred design methodology. Disabil Rehabil. (2014) 9:521–8. doi: 10.3109/17483107.2013.840863
50. Sugawara AT, Ramos VD, Alfieri FM, Battistella LR. Abandonment of assistive products: assessing abandonment levels and factors that impact on it. Disabil Rehabil. (2018) 13:716–23. doi: 10.1080/17483107.2018.1425748
51. Hughes A-M, Burridge JH, Demain SH, Ellis-Hill C, Meagher C, Tedesco-Triccas L, et al. Translation of evidence-based assistive technologies into stroke rehabilitation: users' perceptions of the barriers and opportunities. BMC Health Services Res. (2014) 14:124. doi: 10.1186/1472-6963-14-124
52. Fensli R, Pedersen P, Gundersen T, Hejlesen O. Sensor acceptance model–measuring patient acceptance of wearable sensors. Methods Inform Med. (2008) 47:89–95. doi: 10.3414/ME9106
Keywords: surface electromyography, stroke, qualitative research, perceptions of technology, rehabilitation
Citation: Feldner HA, Papazian C, Peters K and Steele KM (2020) “It's All Sort of Cool and Interesting…but What Do I Do With It?” A Qualitative Study of Stroke Survivors' Perceptions of Surface Electromyography. Front. Neurol. 11:1037. doi: 10.3389/fneur.2020.01037
Received: 05 June 2020; Accepted: 10 August 2020;
Published: 17 September 2020.
Edited by:
Roberto Merletti, Politecnico di Torino, ItalyReviewed by:
William Zev Rymer, Shirley Ryan AbilityLab, United StatesCristiano De Marchis, Roma Tre University, Italy
Sybele Williams, Rehabilitations- und Präventionstechnik, RWTH Aachen, Germany
Copyright © 2020 Feldner, Papazian, Peters and Steele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heather A. Feldner, hfeldner@uw.edu
 Heather A. Feldner
Heather A. Feldner Christina Papazian
Christina Papazian Keshia Peters2
Keshia Peters2 Katherine M. Steele
Katherine M. Steele