- 1Section of Biotechnologies, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy
- 2Medical Genetics and Neurogenetics Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 3Section of Pharmacology, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy
Iron homeostasis is an essential prerequisite for metabolic and neurological functions throughout the healthy human life, with a dynamic interplay between intracellular and systemic iron metabolism. The development of different neurodegenerative diseases is associated with alterations of the intracellular transport of iron and heavy metals, principally mediated by Divalent Metal Transporter 1 (DMT1), responsible for Non-Transferrin Bound Iron transport (NTBI). In addition, DMT1 regulation and its compartmentalization in specific brain regions play important roles during aging. This review highlights the contribution of DMT1 to the physiological exchange and distribution of body iron and heavy metals during aging and neurodegenerative diseases. DMT1 also mediates the crosstalk between central nervous system and peripheral tissues, by systemic diffusion through the Blood Brain Barrier (BBB), with the involvement of peripheral iron homeostasis in association with inflammation. In conclusion, a survey about the role of DMT1 and iron will illustrate the complex panel of interrelationship with aging, neurodegeneration and neuroinflammation.
Introduction
Interest in the contribution of iron and DMT1 to both aging and neurodegeneration comes from relevant studies performed during the course of the last 20 years. As Knutson (2017) importantly highlighted, last year was the 20th anniversary of the discovery of DMT1, the first reported mammalian divalent metal transporter. DMT1 principally mediates the transport of ferrous iron and heavy metals in systemic iron homeostasis, from the plasma membrane or endosomes to the intracellular labile pool, mostly sustaining Non-Transferrin Bound Iron transport (NTBI). Although according to recent highlights, the uptake of NTBI into the liver seems to be mediated by ZIP14/SLC39A14 (Jenkitkasemwong et al., 2012) and hepatocyte DMT1 was shown to be dispensable for hepatic iron accumulation (Wang and Knutson, 2013). Interestingly, 59Fe-NTBI uptake was impaired in ZIP14/SLC39A14 null mice when fed with iron-loaded diet or crossed with mouse models of hereditary hemochromatosis, while DMT1 siRNA reduced iron loading in a mouse model of hereditary hemochromatosis (Wang et al., 2019), thus highlighting the role of both NTBI transporters in the complex homeostasis of iron overload, with cerebral implications. Noteworthy, the comparison of these two NTBI-transporters activity, in RNA injected Xenopus oocytes, showed a maximum at pH 7.5 for mouse ZIP14, compared to human DMT1 (Pinilla-Tenas et al., 2011), a proton co-transporter, shows an optimum ferrous iron uptake at pH 5.5 (Garrick et al., 2006; Mackenzie et al., 2007), which could exacerbate iron uptake during the pathological-associated extracellular acidosis. Other non-transferrin-bound iron transporters were later identified, such as ZIP14 (SLC39A14), ZIP8 (SLC39A8), L-type calcium channels (LTCCs) and T-type calcium channels (TTCCs), and TRPC6 (Martinez et al., 1999; Mwanjewe and Grover, 2004; He et al., 2006; Liuzzi et al., 2006; Jenkitkasemwong et al., 2012; Striessnig et al., 2014). However, here attention will be given into the complex structure and function of DMT1, underpinning the equilibrium of iron and heavy metals homeostasis, as an essential prerequisite for normal metabolic and neurological functions in relationship to heterogeneous pathophysiological regulation. In this regard, iron and DMT1 significantly contribute to the development of different neurodegenerative diseases, assuming the role of a common participant, as later described. In this review, we discuss the growing body of findings and the emerging concepts about DMT1 and NTBI involvement in both physiological aging and neurodegeneration/neuroinflammation, as found in Parkinson’s disease (PD), ischemia, Neurodegeneration with Brain Iron Accumulation (NBIA), and Alzheimer’s disease (AD). To this purpose, focus will be given to the potential role of DMT1 as an interesting target for the development of future neuroprotective strategies in the field.
Ferrous Iron Transporter DMT1 and Redox Equilibrium in the Life-Sustaining Cellular Homeostasis
Divalent Metal Transporter 1 (or Nramp2 gene) was first isolated by low stringency Nramp1 homology screening (Gruenheid et al., 1995) and expression cloning of a rat duodenal cDNA library (Gunshin et al., 1997). Subsequently, Lee et al. (1998) firstly defined the structure of the 3′UTR splice variant without-IRE and Kishi and Tabuchi (1997) performed functional characterization in yeast of human DMT1 cDNA (Tabuchi et al., 1999). DMT1 expression and function are strictly dependent on this complex structure, with four different isoforms, generated by two alternative splicing (Garrick et al., 2006; Mackenzie et al., 2007). The 5′UTR splicing produces two different promoter regions, 1A and 1B. The 1A promoter is responsive to hypoxia in PC12 rat cells (Lis et al., 2005) and HIF-2 alpha in Caco-2/TC7 cells (Mastrogiannaki et al., 2009). Conversely, the 1B isoform is responsive to NF-κB in P19 mouse embryonic carcinoma cells, mouse primary cortical neurons (Paradkar and Roth, 2006a, 2006b; Ingrassia et al., 2012) and to HIF-1 alpha in HepG2 cells (Wang et al., 2010; Qian et al., 2011). The second splicing, at 3′UTR, implies that either 1A and 1B isoforms possess or do not possess an Iron Responsive Element (IRE), that is sensitive to feedback regulation by intracellular iron levels, through IRE/IRP system (Hubert and Hentze, 2002; Pantopoulos, 2004). Moreover, in Caco-2 cell line, both 1A isoforms and (+) IRE 1B isoform show the predicted mRNAs up- and down-regulation in iron deficiency or overload, respectively, while the (–) IRE 1B isoform is regulated by iron-independent mechanisms (Hubert and Hentze, 2002). Particularly, Hubert and Hentze showed that, in Caco-2 cell line, the Transferrin Receptor (TfR) and (+) IRE DMT1 isoform are down-regulated by intracellular iron overload, due to the canonical IRE/IRP post-transcriptional control. In this respect, while (+) IRE DMT1 isoforms do not contribute to the increased uptake of ferrous iron during iron overload, (–) IRE DMT1 isoforms are not influenced by intracellular iron perturbation. Conversely, (–) IRE DMT1 can be regulated at the transcriptional level (Paradkar and Roth, 2006a, 2006b; Ingrassia et al., 2012; Figure 1), post-translationally, via impairment of proteasome degradation (Garrick et al., 2012; Jia et al., 2014) and by autophagy (Ingrassia et al., 2017). In this regard, IRP1, IRP2, (+) IRE and (–) IRE DMT1 proteins are up-regulated in lesioned rat hippocampus after kainate treatment and expressed in GFAP positive astrocytes (Huang et al., 2006); the (–) IRE DMT1 increased expression leads to argue that mechanisms other than IRP regulation modulate DMT1 expression.
Importantly, (–) IRE DMT1, which co-localizes with TfR on early endosomes, is also involved in the life-sustaining Transferrin Receptor cycle (Tabuchi et al., 2002, 2010), where the release of ferric iron from transferrin is triggered by endosomal acidic pH, followed by its reduction to ferrous iron with loading by DMT1, whose activity is maximally stimulated at low pH (Garrick et al., 2006; Mackenzie et al., 2007).
Divalent Metal Transporter 1 isoforms play multiple roles and are characterized by complex subcellular distribution; in fact, they localize at the cell membrane, in the cytoplasm and at the nuclear and outer mitochondrial membranes (Roth et al., 2000; Lis et al., 2004; Wolff et al., 2014a, 2014b), where they were recently found to play a role in mitochondrial iron and manganese uptake (Wolff et al., 2018). Through this heterogenous distribution, a tight control of ferrous iron homeostasis is guaranteed at intracellular level, avoiding, for example, excess of ferrous iron that may catalyze the formation of free radicals through the Haber–Weiss and Fenton reactions, and lead to cell damage (Connor and Ghio, 2009). In this context, pharmacological manipulation of the Fenton reaction, by preventing cellular oxidative damage and the formation of hydrogen peroxide and hydroxyl radicals, could represent a useful targeting system leading to potential therapeutic strategies in the treatment of neurodegenerative disorders (Youdim, 2018). Moreover, a physiological pathway for NTBI uptake and regulation of systemic iron homeostasis in different tissues has been extensively described (Knutson, 2018). In this scenario, DMT1 supports a crosstalk between peripheral districts and the central nervous system, with iron transport into the blood stream at the Blood Brain Barrier. According to previous results, DMT1 gene expression in the mouse brain presumably is mostly accounted for by 1B isoform, with both (+)/(–) IRE splicing, which has also been reported to be expressed in rat brain. Whereas no evidence for expression of 1A isoform was found (Hubert and Hentze, 2002; Ke et al., 2005). DMT1 mRNA was also found up-regulated in the cerebellum of ceruloplasmin-knockout mice, particularly in Purkinje and deep nuclei neurons (Jeong and David, 2006), in the frontal cortex of both wild-type and APPSWE/PS1ΔE9 Alzheimer mouse model (Xian-hui et al., 2015), and (+)/(–) IRE DMT1 isoforms significantly increased in rat cortex, striatum, hippocampus and substantia nigra (Lu et al., 2017). DMT1 is also controversially reported to be expressed not only in neurons, but also in non-neuronal cells, such as astrocytes, microglia, oligodendrocytes, and over the brain capillary endothelial cells and choroid plexus cells, that form the brain barrier (Moos and Morgan, 2004; Mills et al., 2010; Skjørringe et al., 2015). Indeed, rat brain capillaries in vivo and isolated endothelial cells, with Blood Brain Barrier (BBB) competence, express DMT1, transferrin receptor, ferroportin, ceruloplasmin, ferrireductases STEAP2-3, and hephestin. This complex machinery sustains the distribution of iron in the central nervous system with epithelial cells of the choroid plexus expressing large amounts of DMT1 mRNA, in order to regulate metal transport across the BBB (Yang W.M. et al., 2011; Burkhart et al., 2016). Divalent metal transport was also studied in cerebral hemorrhage models subjected to iron chelation by deferoxamine (Li et al., 2017), in induced pluripotent stem cell (iPSC)-derived brain endothelial cells (huECs) and a human BBB cellular model where transferrin, hepcidin, and DMT1 sustain iron transport and release (Chiou et al., 2018).
DMT1 and Iron Up-Regulation During Aging
Several studies demonstrated how (–)/(+)IRE DMT1 mRNAs expression levels are significantly increased during aging in rat brain cortex, hippocampus, striatum and substantia nigra (Ke et al., 2005; Lu et al., 2017). The authors of these studies showed how (–)/(+)IRE DMT1 mRNAs have a peculiar compartmentalization in both early development and aging, with significant up-regulation at 3 post-natal weeks in the cortex and at 28 post-natal weeks in the substantia nigra, that is surprisingly not influenced by dietary intake in rats, in spite of the expected iron-dependent response. DMT1 mRNA was then found up-regulated in the cerebellum of 24-month-old ceruloplasmin-knockout mice, and DMT1 protein was found increased in Purkinje and deep nuclei neurons (Jeong and David, 2006). DMT1 mRNA was also higher in the frontal cortex of 12-month-old wild-type mice and, with significant increase, in the APPSWE/PS1ΔE9 Alzheimer mouse model (Xian-hui et al., 2015). However, (+)/(–) IRE DMT1 isoforms were found significantly up-regulated at the protein level in the cortex, striatum, hippocampus and substantia nigra of 3-, 12-, and 24-month-old rats, where a significant increase of hepcidin was also described by immunofluorescence (Lu et al., 2017), potentially sustaining a block of iron export. Interestingly, DMT1 up-regulation in the substantia nigra during aging links the transporter to the pathogenesis of other neurodegenerative diseases, such as PD, Parkinsonisms and NBIA, in which enhanced iron accumulation into the basal ganglia occurs. In this regard, in an NBIA mouse model with a mutation in phospholipase A2 beta (PLA2G6), aging significantly up-regulates IRPs and (+) IRE DMT1 in the cortex, striatum, substantia nigra and cerebellum of 100-weeks-old mice, with respect to age-matched wild-type controls (Beck et al., 2015). Furthermore, beside DMT1 increase during aging in the central nervous system, which is highly subjected to damage by iron-dependent oxidative stress, hepatic DMT1 protein was also found up-regulated by Deferoxamine treatment in 24-month-old rats, when compared to 3-month-old rats (Bloomer et al., 2014).
DMT1 and Iron Up-Regulation in the Neurodegeneration
Oxidative stress leads to DNA damage and to the polymerization and denaturation of proteins that, together, can form insoluble structures typically known as plaques, a hallmark of neurodegenerative diseases (Kell, 2009), with altered cellular proteostasis. Arising from the previous reasons, the intracellular iron level and traffic need a tight control to avoid perturbations in the expression of DMT1, which may induce downstream cellular damage and dysfunctions in iron metabolism, contributing to the pathogenesis of several neurodegenerative disorders (Biasiotto et al., 2016). In this respect, DMT1 mRNA localization in rat central nervous system by in situ hybridization showed a restricted pattern throughout different areas, as reported by Gunshin et al. (1997), with prominent DMT1 labeling in cerebellar granule cells, hippocampal pyramidal and granule cells in the preoptic nucleus and pyramidal cells of the piriform cortex, as well as in the substantia nigra. The authors also detected DMT1 labeling in the ventral area of the anterior olfactory bulb and in the olfactory epithelium, an important route for the delivery of environmental metals to the brain. The intranasal drug delivery may exert highly efficient effects in the central nervous system (Henkin, 2011) and at the circulatory level, inducing an effective systemic immunity (McGhee, 2011). In fact, the olfactory epithelium can be considered as a gateway for vaccines or peptide hormones administration, also in light of the high neuroprotective potential showed by intranasal administration of iron chelators (Guo et al., 2015). Accordingly, intranasal administration of neurotoxins in rodents, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), has been used to develop an animal model suitable for testing neuroprotective drugs against PD (Prediger et al., 2011).
An important aspect concerns the role of DMT1 in the pathogenesis of several neurodegenerative diseases induced by known neurotoxic stimuli such as kainate, N-methyl-D-aspartate (NMDA), agonist of the NMDA Receptors of the family of ionotropic glutamate receptors, levodopa or L-3,4-dihydroxyphenylalanine (L-DOPA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA) and hypoxia, as discussed later. DMT1 also plays a role in neuronal cell death in the hippocampus by excitotoxic glutamatergic stimuli, such as kainate and NMDA. DMT1, in fact, was shown to be up-regulated by kainate in rat hippocampus, both by an IRP-dependent and -independent mechanism, being the (+)IRE and the (–)IRE isoforms up-regulated and mostly expressed in GFAP positive astrocytes (Huang et al., 2006). The kainate-dependent DMT1 up-regulation and hippocampal injury were then associated with higher cerebral levels of lead and cadmium in rats, when treated with these metals dissolved in the drinking water (Ong et al., 2006). Interestingly, other reports suggested a possible role of DMT1 in the hippocampal spatial memory formation, due to the evidence of NMDA-dependent plasticity induction by 1B/(+)IRE DMT1 in rat primary hippocampal neurons, and evidence of its block by the transcription inhibitor Actinomycin D and the NMDA receptor antagonist MK-801 (Haeger et al., 2010). Thus, NMDA receptor can influence neurodegenerative diseases through DMT1 enhancement, with consequent iron overload-dependent NMDA-Receptor mediated synaptic plasticity. The NMDARs-dependent iron overload also challenges increased lysosomal iron release. In this regard, it was well reported that (–)/(+)IRE DMT1 isoforms have different subcellular localization (Tabuchi et al., 2002, 2010), characterized by peculiar sorting and recycling kinetics into endolysosomal compartment (White et al., 2016; Xu et al., 2017). In fact, (+)IRE DMT1 has prevalent surface expression, it is internalized from the plasma membrane without efficient recycling and targeted to lysosomes, while (–) IRE DMT1 is efficiently sorted to recycling endosomes upon internalization (Lam-Yuk-Tseung and Gros, 2006). Moreover, it was shown that expression of (–) IRE DMT1, increased by L-DOPA, could have a role in the development of L-DOPA toxicity, significantly counteracted by (–) IRE DMT1 silencing in primary cortical neurons (Du et al., 2009). Again, iron and DMT1 are involved in the pathogenesis of neurodegenerations with multifactorial origin, such as idiopathic PD, as shown by Salazar et al. (2008). They reported an increase of iron and DMT1 levels in the substantia nigra of post-mortem brain of PD patients and in the ventral mesencephalon of the PD mouse model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication. Furthermore, the authors importantly showed that the mk/mk microcytic mice and the Belgrade rat model, both harboring a DMT1 point mutation G185R with consequent impairment of iron uptake (Fleming et al., 1997, 1998), were protected from neurotoxicity induced by MPTP or by 6-hydroxidopamine (6-OHDA), respectively, thus highlighting an iron- and DMT1-dependence of the toxins mechanism of action. Again, in MES23.5 dopaminergic neurons and in the substantia nigra of the 6-OHDA lesioned rats, as a PD model, iron accumulation was associated to NMDA receptors activation, through the up-regulation of (+)IRE DMT1 and down-regulation of the iron exporter ferroportin (FPN1) (Xu et al., 2018). In this model, iron increase was counteracted by the non-competitive NMDA antagonist MK-801 and by the selective NMDA antagonist (2R)-amino-5-phosphonovaleric acid (AP5). Accordingly, iron and DMT1 protein accumulation were also found in the substantia nigra of the NF-κB/c-Rel knockout mice, a model of neurodegeneration with Parkinsonism (Baiguera et al., 2012). Ferrous iron and DMT1 up-regulation are also linked to neuroinflammatory signaling pathways, downstream to the NF-κB/RelA Lys310 acetylation-mediated cell death, during the early phase of brain ischemia, as demonstrated in the well-studied models of in vivo mouse brain ischemic neurodegeneration, subjected to transient middle cerebral artery occlusion tMCAO, and in mouse primary cortical neurons subjected to oxygen-glucose-deprivation (OGD) (Ingrassia et al., 2012). Moreover, (–) IRE DMT1 was shown to contribute to increased ferrous iron uptake subsequent to treatment with 1-methyl-4-phenylpyridinium, MPP (+), in the MES23.5 model of dopaminergic neurons, fully antagonized by iron chelation with Desferal (Zhang et al., 2009), known to prevent dopaminergic neuronal death in MPTP-treated mice (Guo et al., 2016). Incidentally, the conditional overexpression of H-ferritin in TH positive neurons of the substantia nigra counteracted the increased level of iron and DMT1 in the transgenic mice, upon treatment with the proteasome inhibitor lactacystin, thus providing a genetic model of iron chelation (Zhu et al., 2010). In this regard, not only the epigenetic modulation but also the post-translational proteasomal degradation of DMT1 plays a role in DMT1-associated neurodegeneration. In fact, the E3-ubiquitin ligase Parkin, when overexpressed in SH-SY5Y neuroblastoma cells, increased proteasomal degradation of 1B DMT1 for both (+) IRE and (–) IRE isoforms (Roth et al., 2010). The authors also showed an accumulation of both (+) IRE and (–) IRE DMT1 isoforms in human lymphocytes of early onset patients with familial PD, bearing homozygous deletion of Parkin exon 4, and in the mouse brain of the E3 ubiquitin ligase Parkin knockout. Incidentally, another form of familial PD was identified with a mutation in the gene coding for the Vacuolar Protein Sorting 35, VPS35 (Zavodszky et al., 2014), which is responsible for the correct endosomal recycling and trafficking of DMT1 (Tabuchi et al., 2010), underpinning the derangement of DMT1 in autophagosomal localization as a basal mechanism involved in the disease.
The recent development of a transgenic mouse model of DMT1 overexpression has highlighted the relevant point that DMT1 overexpression alone is not enough to cause neurodegeneration (Zhang et al., 2017). In fact, DMT1 transgenic mice showed selective iron accumulation in the substantia nigra at 18 months of age, only when fed with an iron-supplemented diet. DMT1 expressing mice also showed increased levels of Parkin, as a compensative neuroprotection mechanism. However, DMT1 overexpression in the double mutant mice with Parkin null background, generated to avoid Parkin neuroprotective effect, showed no vulnerability against dietary iron intake, while exposure to 6-hydroxydopamine led to increased neurotoxicity.
Interestingly, with respect to brain iron compartmentalization in PD, post-mortem brain sections of PD patients showed a peculiar redistribution, with decreased levels of iron and iron transporters in the temporal cortex, if compared with the substantia nigra (Yu et al., 2013). Furthermore, several reports have recently highlighted that, during aging, iron and DMT1 accumulate in the frontal cortex of the APPSWE/PS1ΔE9 transgenic mouse model (Xian-hui et al., 2015), where both (–)/(+) IRE DMT1 were also increased in the cortex and hippocampus compared with wild type-control (Zheng et al., 2009), potentially contributing to increasing to the risk of developing AD. Interestingly, (–)/(+) IRE DMT1 up-regulation in the cortex and hippocampus of the APPSWE/PS1ΔE9 transgenic mouse model was recently shown as a consequence of down-regulation of Ndfip1, the NEDD4 family interacting protein 1 (Tian et al., 2018), which modulates DMT1 degradation through ubiquitination pathway (Foot et al., 2011). Again, (–)IRE DMT1 up-regulation was associated with a quantitative, age-dependent increase of heavy metals in the frontal cortex of a ”natural” rodent model of AD, named Octodon degus (Braidy et al., 2017), with impaired lysosomal function. Interestingly, in support for the presence of common mechanisms involving iron metabolism in different neurodegenerative diseases, the knockout mice lacking the ferroxidase Ceruloplasmin at 6 months of age showed Parkinsonian neurodegeneration with nigral iron accumulation and neuronal loss, which was prevented by iron chelation (Ayton et al., 2014). However, more recently, it was reported that Alzheimer models were induced in the knockout mouse of Ceruloplasmin either by injection of A into the lateral ventricle of the brain or by transgenic APP expression, with a consequential increase in memory impairment and hippocampal iron accumulation mediated by (–) IRE DMT1 and changes in ROS levels (Zhao et al., 2018).
In conclusion, these findings highlight the role of DMT1 in the pathogenesis of several, multifactorial and genetic neurodegenerative diseases induced by known neurotoxic stimuli.
Neurodegeneration With Brain Iron Accumulation (NBIA)
Neurodegeneration with Brain Iron Accumulation (NBIA) encompasses a heterogeneous group of rare disorders characterized by abnormal progressive iron accumulation in specific brain areas. Their prevalence is estimated between 1 and three individuals in a million (Gregory and Hayflick, 2013). Clinical signs include dystonia, tremor, bradykinesia, rigidity, postural instability, optic atrophy or retinal degeneration, neuropsychiatric abnormalities ranging from rapid neurodevelopmental regression in infancy to minor cognitive impairment in adulthood (Gregory and Hayflick, 2013). To date, 12 disease-associated genes leading to NBIA have been identified; however, around 20% of cases are still genetically undefined (Arber et al., 2016; Levi and Tiranti, 2019). Only two genes, namely FTL (ferritin light chain) and CP (ceruloplasmin), are directly associated with iron homeostasis (Levi and Rovida, 2015; Kassubek et al., 2017). While the other ten genes encode proteins either with various functions in lipid metabolism, lysosomal activity and autophagic processes or with still unknown mechanisms: PANK2 (Pantothenate kinase 2), COASY (Coenzyme A synthase), PLA2G6 (Phospholipase A2), C19orf12 (Chromosome 19 open reading frame, with transcript of unknown function), FA2H (fatty acid 2-hydroxylase), ATP13A2 (ATPase cation transporting 13A2), WDR45 (WD repeat containing protein 45, beta-propeller protein), DCAF17 (DDB1 and CUL4-associated factor 17), SCP2 (sterol carrier protein 2) and GTPBP2 (GTP binding protein 2). Although demonstration of iron accumulation in the brain is essential for the diagnosis of NBIA, the impairment of iron metabolism in the different NBIA subclasses has not been clarified. Ingrassia et al. (2017) have recently shown an altered pattern of iron transporters with iron overload in patients’ fibroblasts with WDR45 mutations, a NBIA gene with a predicted role in autophagy (Hayflick et al., 2013). PLA2G6 knockout mice (Beck et al., 2015) and fibroblasts of a patient with Neuroferritinopathy (Barbeito et al., 2010) also showed altered expression of iron transporters with DMT1 up-regulation.
DMT1 and Signaling Contributions to Neurodegeneration
Of abiding interest is the influence of inflammatory signaling pathways consequential upon heavy metal intoxication, which induces cytokine production and pro-inflammatory stimuli activation. Inflammation, concomitant to oxidative stress, is present in several neurodegenerative diseases. Noteworthy, the NF-κB signaling induces a transient rise of intracellular iron immediately after a short stimulation with Lipopolysaccharide (LPS), with consequential NF-κB nuclear activation already present at 15 min, as revealed in hepatic macrophages by electromobility shift assay, with a kB specific probe (Xiong et al., 2003). Intriguingly, later findings showed how LPS and NF-κB can regulate specific DMT1 isoforms, as later discussed, with apparently divergent responses at the post-translational level, due the activity of both NEDD4 family interacting protein 1 (Ndfip1) and E3 ubiquitin ligase Parkin. This critical regulatory system may represent a useful target in the prevention of metal accumulation, by DMT1 post-translational degradation, and neuronal cell death. In fact, overexpression of Ndfip1 was demonstrated to have a neuroprotective effect from metal toxicity with ubiquitination and degradation of DMT1 in human primary cortical neurons (Howitt et al., 2009) and after acute cortical brain injury (Sang et al., 2006). Accordingly, Ndfip1 knockout mice revealed a significant increase of intestinal DMT1 with higher serum iron levels, transferrin saturation and inflammation (Foot et al., 2011). Moreover, ferrous iron and LPS induced Ndfip1 protein expression in MES23.5 dopaminergic cell lines, were counteracted by iron chelation with deferoxamine (Xu et al., 2013). Xu and colleagues described Ndfip1 regulatory mechanism mediated by NF-κB activation, in agreement with previous results obtained with LPS stimulation (Xiong et al., 2003), demonstrating how BAY11-7802, an inhibitor of NF-κB activation, significantly decreased mRNA expression levels of Ndfip1 in response to both ferrous iron and LPS.
However, while Ndfip1-mediated post-translational regulation was mostly found associated to pan-DMT1 response, a parallel DMT1 isoform-specific, translational and post-translational, regulation has arisen. In fact, epigenetic studies contributed to elucidate the acetylome-dependent NF-κB regulation of 1B DMT1 promoter. Particularly, the acetylation of NF-κB/RelA at Lys310 activates the NF-κB-dependent response, a well-studied epigenetic regulation in post-ischemic injury (Lanzillotta et al., 2010), with consequent 1B/(–)IRE DMT1 up-regulation in models of differentiated human neuroblastoma and in mouse primary cortical neurons exposed to OGD, or transient middle cerebral artery occlusion (tMCAO) (Ingrassia et al., 2012), and also in undifferentiated P19 embryonic carcinoma cells (Paradkar and Roth, 2006a,Paradkar and Roth, 2006b). In this regard, the isoform-specific regulation of DMT1 could possibly explain the apparent discrepancy emerged between translational and post-translational regulation of Ndfip1. In fact, the Parkin-dependent post-translational degradation of 1B DMT1 isoform was shown to influence metal transport in SH-SY5Y, human neuroblastoma cells, overexpressing wild-type and mutant forms (Park-T240R) of human Parkin, while 1A DMT1 isoform resulted unaffected (Roth et al., 2010). Roth et al. (2010) importantly showed similar findings in human B lymphocyte cell lines derived from a PD patient carrying the homozygous deletion of Parkin exon 4, and in Parkin knockout mice where a clear (–) IRE DMT1 isoform specific degradation occurs, while the (+) IRE isoform expression is not altered. To this purpose, clear-cut data obtained from studies on HEK293 cells highlighted the isoform-dependence in DMT1 post-translational regulation (Garrick et al., 2012). Taking advantage of the peculiar expression level of 1A DMT1 isoform, which is high in HEK293 cell lines compared to neuronal cells, Garrick and colleagues clearly showed that in HEK293, 1A DMT1 is not a target for Parkin ubiqitination. In fact, transient transfection of Parkin in two HEK293F cell lines, with tetracycline-dependent overexpression of 1A/(+)IRE DMT1 and 1B/(–)IRE DMT1 isoforms respectively, allowed to detect a 1B isoform isoform-specificity of Parkin ubiquitination. In agreement with these findings, the Ndfip1 knockout mice showed a rise of intestinal pan-DMT1 (Foot et al., 2011), a district with predominant expression of 1A DMT1, further supporting the Parkin specificity for 1B/(–)IRE DMT1 isoform. Altogether, these results highlighted the heterogeneous post-translational regulation of DMT1 isoforms. On the other hand, data collected so far clearly claims the need for further studies in this regard. In fact, while Parkin induces DMT1 degradation in several models of PD, the loss of Ndfip1 was associated to DMT1 increase in ventral mesencephalic neuronal cultures of Ndfip1 knockout mice (Howitt et al., 2014). However, Howitt and colleagues concomitantly found increased Ndfip1 levels in the substantia nigra of PD patients, associated with the increase of (+) IRE DMT1. The authors suggested that this DMT1 up-regulation, parallel to Ndfip1 increase, could be accounted to the prevalence of translational regulation, respect to the post-translational one, with an increase of DMT1 isoforms due to iron exposure (Salazar et al., 2008), hypoxia (Lis et al., 2005), and NF-κB activation (Paradkar and Roth, 2006a,Paradkar and Roth, 2006b; Ingrassia et al., 2012).
In addition, signaling derangement with DMT1 misregulation was also presents in Alzheimer models. In fact, the decrease of Ndfip1 in the cortex and hippocampus of APPSWE/PS1ΔE9 transgenic mice was associated with the up-regulation of both (–) and (+) IRE DMT1 isoforms (Tian et al., 2018). Tian et al. (2018) also showed how the down-regulation of DMT1, with reduced iron uptake and decreased Aβ(1–42) peptide secretion, occurs after transient overexpression of Ndfip1 in SH-SY5Y human neuroblastoma cells stably over-expressing APPsw. Accordingly, in immortalized microglia cells, the Aβ (1–42) treatment induced pan-DMT1 up-regulation (McCarthy et al., 2018). Comprehensively, all these evidences lead to hypothesize a signaling mechanism accounting for the activation of specific ubiquitination pathways in the regulation of DMT1 isoforms.
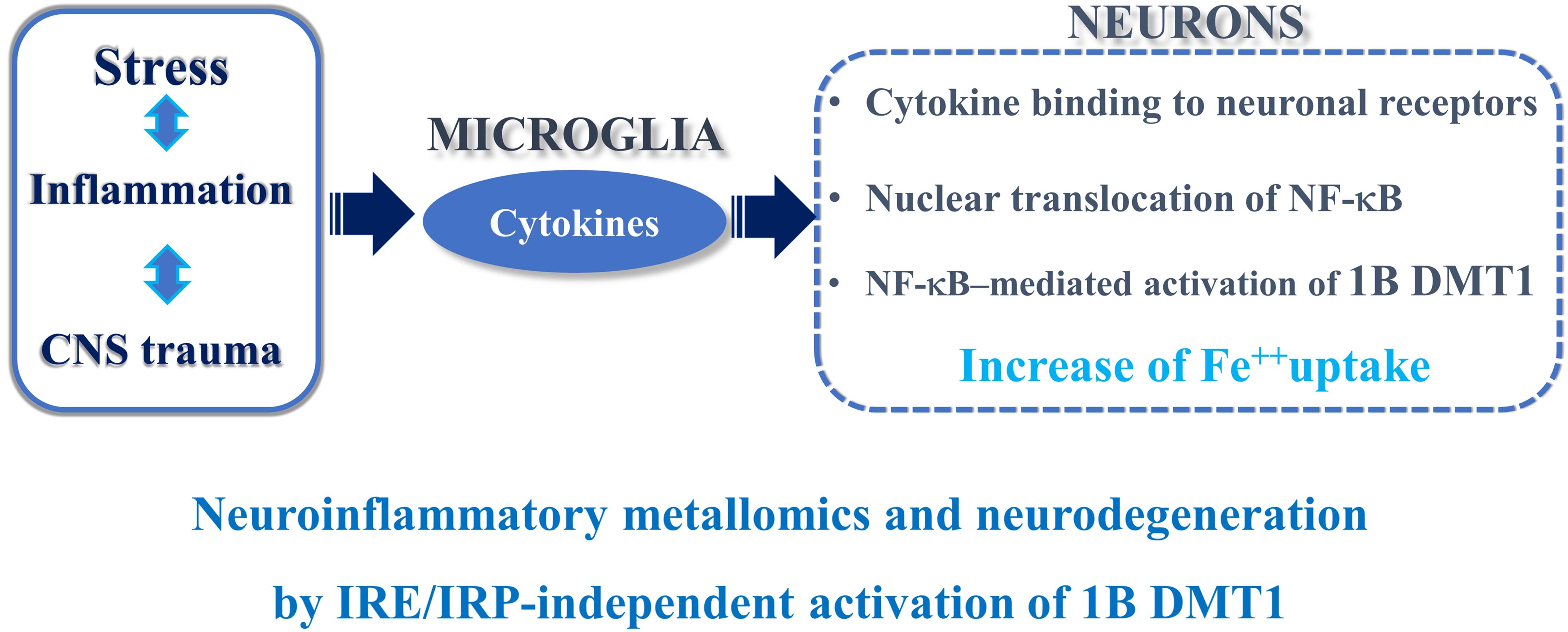
In conclusion, the complex transcriptional and post-translational coordination of DMT1 isoforms may be underpinning to divalent metals exposure and neuroinflammation in PD, AD or any other trauma to the CNS. To this purpose, evident signs of gliosis and microglia activation (Toklu et al., 2018), or a mild inflammatory profile at pre-motor stage in the NF-κB/c-Rel–/– mouse model of late-onset Parkinsonism, without gliosis at 18 months of age, were described (Porrini et al., 2017). Interestingly, the damage to the substantia nigra in PD induces TNF alpha and other cytokines that bind to their neuronal receptors with consequent NF-κB translocation to the nucleus, 1B DMT1 up-regulation and iron accumulation with cellular damage (Roth, 2009). In PD, iron and (+) IRE DMT1 are up-regulated in the dopaminergic, neuromelanin positive neurons of the substantia nigra in PD patients (Salazar et al., 2008). In accordance with the previous evidence, the early signs of neuroinflammation present in the NF-κB/c-Rel knockout mice, considered as a model of late-onset Parkinsonism (Baiguera et al., 2012), induced an increase of nigral iron and DMT1 gene expression in the striatum and mesencephalon. An analogous mechanism is involved during the early phase of brain ischemia, both in vitro and in vivo (Ingrassia et al., 2012). Accordingly, pretreatment with Tanshinone IIA, a phenanthrenequinone derivative, with anti-inflammatory potential against the NF-κB pathway, exerted neuroprotection against ischemic and hypoxic damage with down-regulation of DMT1 and TfR in cerebral ischemic rats subjected to in vivo tMCAO (Yang L. et al., 2011).
Furthermore, a close relationship between inflammation and ferrous iron increase, mediated by enhanced DMT1 gene expression, emerges in primary hippocampal astrocytes upon interferon gamma treatment (Pelizzoni et al., 2013). In addition, the inflammation-dependent increase of NTBI via up-regulated expression of pan-DMT1 is evident in immortalized mouse brain microglia cells, where LPS treatment up-regulates both DMT1 mRNA and protein levels, with concomitant extracellular acidification, due to metabolic changes. This leads to a maximum DMT1 uptake, and treatment with Amiloyd β (1-42), increases DMT1 mRNA and NTBI uptake (McCarthy et al., 2018). Moreover, inflammatory stimuli led to DMT1 up-regulation and ferroportin down-regulation, at both mRNA and protein levels in primary rat hippocampal neurons (Urrutia et al., 2013), in which a significant increase in cell death and oxidative stress was observed after treatment with Aβ-treated astrocyte- or microglia-conditioned media (Urrutia et al., 2017). These evidences show a complex panel about the contribution of the altered homeostasis of DMT1 regulation and iron accumulation, closely related to neuroinflammation, in several neurodegenerative disorders.
Noteworthy, iron overload induces cellular oxidative stress with increased lipid peroxidation, protein and nucleic acid modifications associated to neurodegenerative diseases (Liu et al., 2019). Importantly, under pathological conditions with inflammation, Nitric Oxide (NO) production is increased and aberrantly S-nitrosylated proteins can induce neurodegeneration, like the endogenous S-nitrosylation of DMT1 found in the substantia nigra of post-mortem PD brains (Liu et al., 2018). Interestingly, Liu et al. (2018) pointed the attention on the important aspect that, like extracellular acidification, the increased S-nitrosylation may enhance DMT1 uptake and cell death in the substantia nigra. Moreover, intra-nigral injection of LPS induced NO production and increased neuronal cell death, specifically blocked by the NO synthase inhibitor L-NAME or by Ebselen, a selective inhibitor of ferrous iron uptake by DMT1. Interestingly, lipids peroxidation also increased in the striatum of 100 week aged knockout mice for calcium-independent phospholipase A2 beta (iPLA2β), an enzyme that catalyzes the hydrolysis of membrane glycerophospholipids into free fatty acids and lysophospholipids, as a model of PLA2G6/NBIA (Beck et al., 2015).
Again, inflammation, through the altered expressions of DMT1, ferroportin and hepcidin, induces iron accumulation in the central nervous system (Urrutia et al., 2013; You et al., 2017; Ganz, 2018).
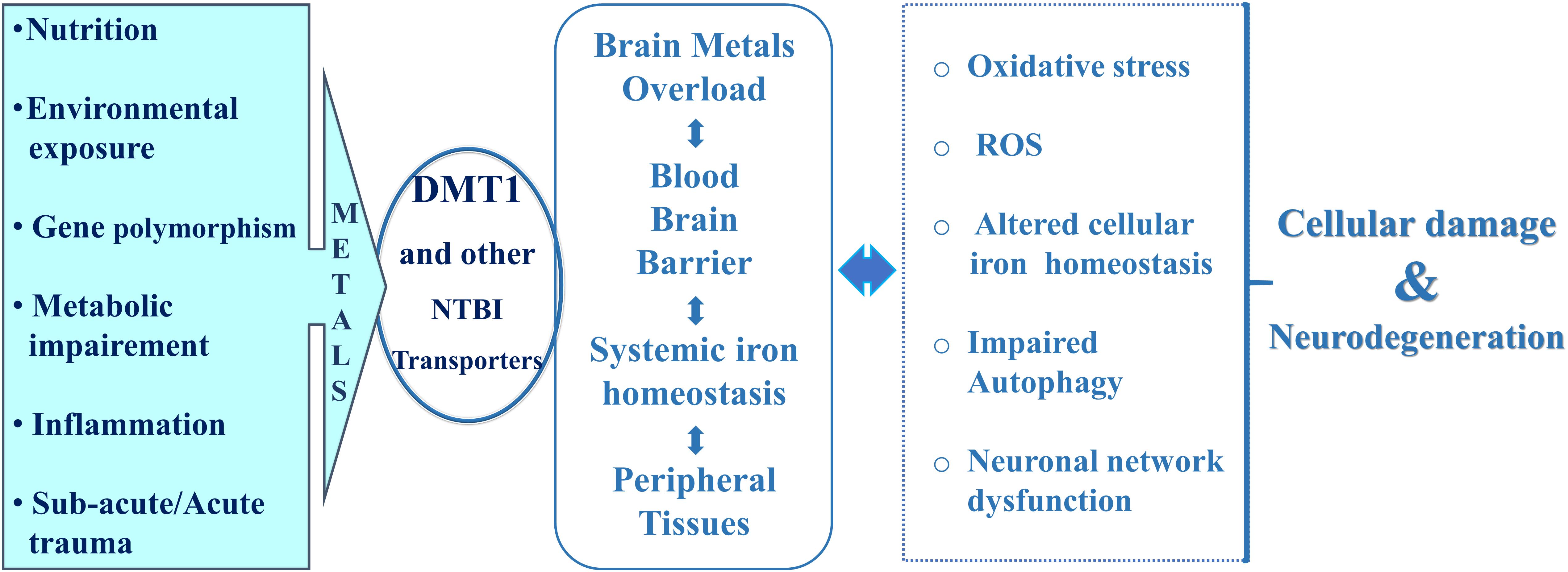
In this respect, hepcidin, the liver-derived peptide hormone, with iron-sensing competence through post-translational degradation of ferroportin, the only known iron-exporter so far identified, plays a remarkable role in the control of systemic iron homeostasis as its expression is up-regulated by inflammation (Ganz, 2013). Hepcidin significantly influences transferrin-bound-iron transport (Tf-Fe) and NTBI homeostasis with the involvement of the respective transporters (Du et al., 2015; Zhou et al., 2017). In addition, hepcidin deficiency has also been reported to be associated with iron overload in hereditary hemochromatosis (Papanikolaou et al., 2005). Recent evidences highlighted that hepcidin expression in the brain is low, according to Burkhart et al. (2016), and is upregulated in the central nervous system during pathophysiological inflammation, exerting a paracrine action on neurons through down-regulation of ferroportin expression (Urrutia et al., 2013; You et al., 2017; Vela, 2018). Expression of ferroportin was assessed in endothelial cells of the blood–brain barrier, in neurons, oligodendrocytes, astrocytes, in the choroid plexus and ependymal cells, as well as being identified in the synaptic vesicles fraction of purified rat brain synaptosomes (Wu et al., 2004). However, the iron exporter ferroportin is a hepcidin target in neurons, astrocytes and microglial cells (Urrutia et al., 2013; You et al., 2017). During inflammation, astrocytes drive iron influx from blood flow to the brain, with neuronal iron accumulation through microglia and astrocyte activity, resulting in neurocytotoxicity and neurodegeneration. Systemic hepcidin can cross the blood brain barrier, particularly when BBB is damaged by pathological conditions such as intracerebral hemorrhage and acute inflammation (Ostergaard et al., 2009; Xiong et al., 2016). It is noteworthy that hepcidin serum levels were significantly increased in PD patients after deep brain stimulation (Kwiatek-Majusuak et al., 2018) and in AD patients (Sternbreg et al., 2017). Therefore, the role of heavy metals absorption and accumulation in the central nervous system may represent a central event for several neurodegenerative diseases of both idiopathic and genetic origin, which arise from nutrition, lifestyle, environmental exposure, genetic polymorphisms, traumatic and neuroinflammatory events affecting the central nervous system (Figure 2).
Author Contributions
RI, BG, and MM contributed to the writing of the manuscript. RI reviewed and edited the manuscript.
Funding
The authors acknowledge financial support by research funds from the University of Brescia and from private donation to the corresponding author.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arber, C. E., Li, A., Houlden, H., and Wray, S. (2016). Insights into molecular mechanisms of disease in neurodegeneration with brain iron accumulation: unifying theories. Neuropathol. Appl. Neurobiol. 42, 220–241. doi: 10.1111/nan.12242
Ayton, S., Lei, P., Adlard, P. A., Volitakis, I., Cherny, R. A., Bush, A. I., et al. (2014). Iron accumulation confers neurotoxicity to a vulnerable population of nigral neurons: implications for Parkinson’s disease. Mol. Neurodegener. 9:27. doi: 10.1186/1750-1326-9-27
Baiguera, C., Alghisi, M., Pinna, A., Bellucci, A., De Luca, M. A., Frau, L., et al. (2012). Late-onset parkinsonism in NFκ B/c-Rel-deficient mice. (2012). Brain 135(Pt 9), 2750–2765. doi: 10.1093/brain/aws193
Barbeito, A. G., Levade, T., Delisle, M. B., Ghetti, B., and Vidal, R. (2010). Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy. Mol. Neurodenegener. 5:50. doi: 10.1186/1750-1326-5-50
Beck, G., Shinzawa, K., Hayakawa, H., Baba, K., Yasuda, T., Sumi-Akamaru, H., et al. (2015). Deficiency of calcium-independent phospholipase A2 Beta induces brain iron accumulation through upregulation of divalent metal transporter 1. PLoS One 10:e0141629. doi: 10.1371/journal.pone.0141629
Biasiotto, G., Di Lorenzo, D., Archetti, S., and Zanella, I. (2016). Iron and neurodegeneration: is ferritinophagy the link? Mol. Neurobiol. 53, 5542–5574. doi: 10.1007/s12035-015-9473-y
Bloomer, S. A., Han, O., Kregel, K. C., and Brown, K. E. (2014). Altered expression of iron regulatory proteins with aging is associated with transient hepatic iron accumulation after environmental heat stress. Blood Cells Mol. Dis. 52, 19–26. doi: 10.1016/j.bcmd.2013.07.002
Braidy, N., Poljak, A., Marjo, C., Rutlidge, H., Rich, A., Jugder, B. E., et al. (2017). Identification of cerebral metal ion imbalance in the brain of aging Octodon degus. Front. Aging Neurosci. 9:66. doi: 10.3389/fnagi.2017.00066
Burkhart, A., Skjorringe, T., Johnsen, K. B., Siupka, P., Thomsen, L. B., Nielsen, M. S., et al. (2016). Espression of iron-related proteins at the neurovascular unit supports reduction and reoxidation of iron for transport through the blood brain barrier. Mol. Neurobiol. 53, 7237–7253. doi: 10.1007/s12035-015-9582-7
Chiou, B., Neal, E. H., Bowman, A. B., Lippmann, E. S., Simpson, I. A., and Connor, J. R. (2018). Endothelial cells are critical regulators of iron transport in a model of the human blood-brain barrier. J. Cereb. Blood Flow Metab. [Epub ahead of print].
Connor, J. R., and Ghio, A. J. (2009). The impact of host iron homeostasis on disease. Biochim. Biophys. Acta 1790, 581–582. doi: 10.1016/j.bbagen.2009.05.004
Du, F., Qian, Z. M., Luo, Q., Yung, W. H., and Ke, Y. (2015). Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Mol. Neurobiol. 52, 101–114. doi: 10.1007/s12035-014-8847-x
Du, F., Qian, Z. M., Zhu, L., Wu, X. M., Yung, W. H., Tsim, T. Y., et al. (2009). L-DOPA neurotoxicity is mediated by up-regulation of DMT1-IRE expression. PLoS One 4:e4593. doi: 10.1371/journal.pone.0004593
Fleming, M. D., Romano, M. A., Su, M. A., Garrick, L. M., Garrick, M. D., and Andrews, N. C. (1998). Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. U.S.A. 95, 1148–1153. doi: 10.1073/pnas.95.3.1148
Fleming, M. D., Trenor, C. C. III, Su, M. A., Foernzler, D., Beier, D. R., Dietrich, W. F., et al. (1997). Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 16, 383–386. doi: 10.1038/ng0897-383
Foot, N. J., Leong, Y. A., Dorstyn, L. E., Dalton, H. E., Ho, K., Zhao, L., et al. (2011). Ndfip1-deficient mice have impaired DMT1 regulation and iron homeostasis. Blood 117, 638–646. doi: 10.1182/blood-2010-07-295287
Ganz, T. (2013). Systemic iron homeostasis. Physiol. Rev. 93, 1721–1741. doi: 10.1152/physrev.00008.2013
Garrick, M. D., Kuo, H. C., Vargas, F., Singleton, S., Zhao, L., Smith, J. J., et al. (2006). Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochem. J. 398, 539–546. doi: 10.1042/bj20051987
Garrick, M. D., Zhao, L., Roth, J. A., Jiang, H., Feng, J., Foot, N. J., et al. (2012). Isoform specific regulation of divalent metal (ion) transporter (DMT1) by proteasomal degradation. Biometals 25, 787–793. doi: 10.1007/s10534-012-9522-1
Gregory, A., and Hayflick, S. (2013). “Neurodegeneration with brain iron accumulation disorders overview,” in GeneReviews®, eds M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. H. Bean, K. Stephens, et al. (Seattle, WA: University of Washington).
Gruenheid, S., Cellier, M., Vidal, S., and Gros, P. (1995). Identification and characterization of a second mouse Nramp gene. Genomics 25, 514–525. doi: 10.1016/0888-7543(95)80053-o
Gunshin, H., Mackenzie, B., Berger, U. V., Gunshin, Y., Romero, M. F., Boron, W. F., et al. (1997). Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488. doi: 10.1038/41343
Guo, C., Hao, L. J., Yang, Z. H., Chai, R., Zhang, S., Gu, Y., et al. (2016). Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp. Neurol. 280, 13–23. doi: 10.1016/j.expneurol.2016.03.016
Guo, C., Zhang, Y. X., Wang, T., Zhong, M. L., Yang, Z. H., Hao, L. J., et al. (2015). Intranasal deferoxamine attenuates synapse loss via up-regulating the P38/HIF-1α pathway on the brain of APP/PS1 transgenic mice. Front. Aging Neurosci. 7:104. doi: 10.3389/fnagi.2015.00104
Haeger, P., Alvarez, A., Leal, N., Adasme, T., Nunez, M. T., and Hidalgo, C. (2010). Increased hippocampal expression of divalent metal transporter 1 (DMT1) mRNA variants 1B and +IRE and DMT1 protein after NMDA-receptor stimulation or spatial memory training. Neurotox. Res. 17, 238–247. doi: 10.1007/s12640-009-9096-z
Hayflick, S. J., Kruer, M. C., Gregory, A., Haack, T. B., Kurian, M. A., Houlden, H. H., et al. (2013). β-Propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation. Brain 136(Pt 6), 1708–1717.
He, L., Girijashanker, K., Dalton, T. P., Reed, J., Li, H., Soleimani, M., et al. (2006). ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol. Pharmacol. 70, 171–180.
Howitt, J., Gysbers, A. M., Ayton, S., Carew-Jones, F., Putz, U., Finkelstein, D. I., et al. (2014). Increased Ndfip1 in the substantia nigra of Parkinsonian brains is associated with elevated iron levels. PLoS One 9:e87119. doi: 10.1371/journal.pone.0087119
Howitt, J., Putz, U., Lackovic, J., Doan, A., Dorstyn, L., Cheng, H., et al. (2009). Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons. Proc. Natl. Acad. Sci. U.S.A. 106, 15489–15494. doi: 10.1073/pnas.0904880106
Huang, E., Ong, W. Y., and Connor, J. R. (2006). Upregulation of iron regulatory proteins and divalent metal transporter-1 isoforms in the reat hippocampus after kainite induced neuronal injury. Exp. Brain Res. 170, 376–386. doi: 10.1007/s00221-005-0220-x
Hubert, N., and Hentze, M. W. (2002). Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc. Natl. Acad. Sci. U.S.A. 17, 12345–12350. doi: 10.1073/pnas.192423399
Ingrassia, R., Lanzillotta, A., Sarnico, I., Benarese, M., Blasi, F., Borgese, L., et al. (2012). 1B/(-)IRE DMT1 expression during brain ischemia contributes to cell death mediated by NF-κB/RelA acetylation of Lys310. PLoS One 7:e38019. doi: 10.1371/journal.pone.0038019
Ingrassia, R., Memo, M., and Garavaglia, B. (2017). Ferrous iron up-regulation in fibroblasts of patients with beta propeller protein-associated neurodegeneration (BPAN). Front. Genet. 8:18. doi: 10.3389/fgene.2017.00018
Jenkitkasemwong, S., Wang, C. Y., Mackenzie, B., and Knutson, M. D. (2012). Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25, 643–655. doi: 10.1007/s10534-012-9526-x
Jeong, S. Y., and David, S. (2006). Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J. Neurosci. 26, 9810–9819. doi: 10.1523/jneurosci.2922-06.2006
Jia, W., Xu, H., Du, X., Jiang, H., and Xie, J. (2014). Ndfip1 attenuated 6-OHDA-induced iron accumulation via regulating the degradation of DMT1. Neurobiol. Aging 36, 1183–1193. doi: 10.1016/j.neurobiolaging.2014.10.021
Kassubek, R., Uttner, I., Schönfeldt-Lecuona, C., Kassubek, J., and Connemann, B. J. (2017). Extending the aceruloplasminemia phenotype: NBIA on imaging and acanthocytosis, yet only minor neurological findings. J. Neurol. Sci. 376, 151–152. doi: 10.1016/j.jns.2017.03.019
Ke, Y., Chang, Y. Z., Duan, X. L., Du, J. R., Zhu, L., Wang, K., et al. (2005). Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol. Aging 26, 739–748. doi: 10.1016/j.neurobiolaging.2004.06.002
Kell, D. B. (2009). Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genomics 8:2. doi: 10.1186/1755-8794-2-2
Kishi, F., and Tabuchi, M. (1997). Complete nucleotide sequence of human NRAMP2 cDNA. Mol. Immunol. 34, 839–842. doi: 10.1016/s0161-5890(97)00110-7
Knutson, M. D. (2017). Iron transport proteins: gateways of cellular and systemic iron homeostasis. J. Biol. Chem. 292, 12735–12743. doi: 10.1074/jbc.R117.786632
Knutson, M. D. (2018). Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 133, 101–111. doi: 10.1016/j.freeradbiomed.2018.10.413
Kwiatek-Majusuak, J., Geremek, M., Koziorowski, D., Tomasiuk, R., Szlufik, S., and Friedman, A. (2018). Higher serum pro-hepcidin in patients with Parkinson’s disease treated with deep brain stimulation. Neurosci. Lett. 684, 205–209. doi: 10.1016/j.neulet.2018.06.031
Lam-Yuk-Tseung, S., and Gros, P. (2006). Distinct targeting and recycling properties of two isoforms of the iron transporter DMT1 (NRAMP2, Slc11A2). Biochemistry 45, 2294–2301. doi: 10.1021/bi052307m
Lanzillotta, A., Sarnico, I., Ingrassia, R., Boroni, F., Benarese, M., Faraco, G., et al. (2010). The acetylation of RelA at Lys310 dictates the NF-κB-dependent response in post-ischemic injury. Cell Death Dis. 1:e96. doi: 10.1038/cddis.2010.76
Lee, P. L., Gelbart, T., West, C., Halloran, C., and Beutler, E. (1998). The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol. Dis. 24, 199–215. doi: 10.1006/bcmd.1998.0186
Levi, S., and Rovida, E. (2015). Neuroferritinopathy: from ferritin structure modification to pathogenetic mechanism. Neurobiol. Dis. 81, 134–143. doi: 10.1016/j.nbd.2015.02.007
Levi, S., and Tiranti, V. (2019). Neurodegeneration with brain iron accumulation disorders: valuable models aimed at understanding the pathogenesis of iron deposition. Pharmaceuticals (Basel) 12:E27. doi: 10.3390/ph12010027
Li, Y., Yang, H., Ni, W., and Gu, Y. (2017). Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS One 12:e0172784. doi: 10.1371/journal.pone.0172784
Lis, A., Barone, T. A., Paradkar, P. N., Plunkett, R. J., and Roth, J. A. (2004). Expression and localization of different forms of DMT1 in normal and tumor astroglial cells. Brain Res. Mol. Brain Res. 17, 62–70. doi: 10.1016/j.molbrainres.2003.11.023
Lis, A., Paradkar, P. N., Singleton, S., Kuo, H. C., Garrick, M. D., and Roth, J. A. (2005). Hypoxia induces changes in expression of isoforms of the divalent metal transporter (DMT1) in rat pheochromocytoma (PC12) cells. Biochem. Pharmacol. 69, 1647–1655. doi: 10.1016/j.bcp.2005.03.023
Liu, C., Liang, M. C., and Soong, T. W. (2019). Nitric oxide, iron and neurodegeneration. Front. Neurosci. 13:114. doi: 10.3389/fnins.2019.00114
Liu, C., Zhang, C. W., Lo, S. Q., Ang, S. T., Chew, K. C. M., Yu, D., et al. (2018). S-nitrosylation of divalent metal transporter 1 enhances iron uptake to mediate loss of dopaminergic neurons and motoric deficit. J. Neurosci. 38, 8364–8377. doi: 10.1523/JNEUROSCI.3262-17.2018
Liuzzi, J. P., Aydemir, F., Nam, H., Knutson, M. D., and Cousins, R. J. (2006). Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 103, 13612–13617. doi: 10.1073/pnas.0606424103
Lu, L. N., Qian, Z. M., Wu, K. C., Yung, W. H., and Ke, Y. (2017). Expression of iron transporters and pathological hallmarks of Parkinson’s and Alzheimer’s diseases in the brain of young, adult, and aged rats. Mol. Neurobiol. 54, 5213–5224. doi: 10.1007/s12035-016-0067-0
Mackenzie, B., Takanaga, H., Hubert, N., Rolfs, A., and Hediger, M. A. (2007). Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem. J. 403, 59–69. doi: 10.1042/bj20061290
Martinez, M. L., Heredia, M. P., and Delgado, C. (1999). Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J. Mol. Cell. Cardiol. 31, 1617–1625. doi: 10.1006/jmcc.1999.0998
Mastrogiannaki, M., Matak, P., Keith, B., Simon, M. C., Vaulont, S., and Peyssonnaux, C. (2009). HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Invest. 119, 1159–1166. doi: 10.1172/JCI38499
McCarthy, R. C., Sosa, J. C., Gardeck, A. M., Baez, A. S., Lee, C. H., and Wessling-Resnick, M. (2018). Inflammation-induced iron transport and metabolism by brain microglia. J. Biol. Chem. 293, 7853–7863. doi: 10.1074/jbc.RA118.001949
McGhee, J. R. (2011). A mucosal gateway for vaccines. Nat. Biotechnol. 29, 136–138. doi: 10.1038/nbt.1766
Mills, E., Dong, X. P., Wang, F., and Xu, H. (2010). Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med. Chem. 2, 51–64. doi: 10.4155/fmc.09.140
Moos, T., and Morgan, E. H. (2004). The significance of the mutated divalent metal transporter (DMT1) on iron transport into the Belgrade rat brain. J. Neurochem. 88, 233–245. doi: 10.1046/j.1471-4159.2003.02142.x
Mwanjewe, J., and Grover, A. K. (2004). Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochem. J. 378(Pt 3), 975–982. doi: 10.1042/bj20031187
Ong, W. Y., Chua, L. H., and Cong, C. N. (2006). Increased uptake of divalent metals lead and cadmium into the brain after kainite-induced neuronal injury. Exp. Brain Res. 173, 468–474. doi: 10.1007/s00221-006-0390-1
Ostergaard, C., Sandvang, D., Frimodt-Møller, N., and Kristensen, H. H. (2009). High cerebrospinal fluid (CSF) penetration and potent bactericidal activity in CSF of NZ2114, a novel plectasin variant, during experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 53, 1581–1585. doi: 10.1128/AAC.01202-08
Pantopoulos, K. (2004). Iron metabolism and the IRE/IRP regulatory system: an update. Ann. N. Y. Acad. Sci. 1012, 1–13. doi: 10.1196/annals.1306.001
Papanikolaou, G., Tzilianos, M., Christakis, J. I., Bogdanos, D., Tsimirika, K., MacFarlane, J., et al. (2005). Hepcidin in iron overload disorders. Blood 105, 4103–4105. doi: 10.1182/blood-2004-12-4844
Paradkar, P. N., and Roth, J. A. (2006a). Nitric oxide transcriptionally downregulates specific isoforms of divalent metal transporter (DMT1) via NFkappaB. J. Neurochem. 96, 1768–1777. doi: 10.1111/j.1471-4159.2006.03702.x
Paradkar, P. N., and Roth, J. A. (2006b). Post-translational and transcriptional regulation of DMT1 during P19 embryonic carcinoma cell differentiation by retinoic acid. Biochem. J. 394(Pt 1), 173–183. doi: 10.1042/bj20051296
Pelizzoni, I., Zacchetti, D., Campanella, A., Grohovaz, F., and Codazzi, F. (2013). Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochim. Biophys. Acta 1832, 1326–1333. doi: 10.1016/j.bbadis.2013.04.007
Pinilla-Tenas, J. J., Sparkman, B. K., Shawki, A., Illing, A. C., Mitchell, C. J., Zhao, N., et al. (2011). Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862–C871. doi: 10.1152/ajpcell.00479.2010
Porrini, V., Mota, M., Parrella, E., Bellucci, A., Benarese, M., Faggi, L., et al. (2017). Mild inflammatory profile without gliosis in the c-Rel deficient mouse modeling in a late-onset Parkinsonism. Front. Aging Neurosci. 9:229. doi: 10.3389/fnagi.2017.00229
Prediger, R. D., Aguiar, A. S. Jr., Moreira, E. L., Matheus, F. C., Castro, A. A., Walz, R., et al. (2011). The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson’s disease. Curr. Pharm. Des. 17, 489–507. doi: 10.2174/138161211795164095
Qian, Z. M., Wu, X. M., Fan, M., Yang, L., Du, F., Yung, W. H., et al. (2011). Divalent metal transporter 1 is a hypoxia-inducible gene. J. Cell. Physiol. 226, 1596–1603. doi: 10.1002/jcp.22485
Roth, J. A. (2009). Are there common biochemical and molecular mechanisms controlling manganism and parkinsonism. Neuromol. Med. 11, 281–296. doi: 10.1007/s12017-009-8088-8
Roth, J. A., Horbinski, C., Feng, L., Dolan, K. G., Higgins, D., and Garrick, M. D. (2000). Differential localization of divalent metal transporter 1 with and without iron response element in rat PC12 and sympathetic neuronal cells. J. Neurosci. 20, 7595–7601. doi: 10.1523/jneurosci.20-20-07595.2000
Roth, J. A., Singleton, S., Feng, J., Garrick, M., and Paradkar, P. N. (2010). Parkin regulates metal transport via proteasomal degradation of 1B isoform od divalent metal transporter 1. J. Neurochem. 113, 454–464. doi: 10.1111/j.1471-4159.2010.06607.x
Salazar, J., Mena, N., Hunot, S., Prigent, A., Alvarez-Fisher, D., Arredondo, M., et al. (2008). Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 105, 18578–18583. doi: 10.1073/pnas.0804373105
Sang, Q., Kim, M. H., Kumar, S., Bye, N., Morganti-Kossman, M. C., Gunnersen, J., et al. (2006). Nedd4-WW domain-binding protein 5 (Ndfip1) is associated with neuronal survival after acute cortical brain injury. J. Neurosci. 26, 7234–7244. doi: 10.1523/jneurosci.1398-06.2006
Skjørringe, T., Burkhart, A., Johnsen, K. B., and Moos, T. (2015). Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front. Mol. Neurosci. 8:19. doi: 10.3389/fnmol.2015.00019
Sternbreg, Z., Hu, Z., Sternberg, D., Waesh, S., Quinn, J. F., Wild, K., et al. (2017). Serum hepcidin levels, iron dyshomeostasis and cognitive loss in Alzheimer’s disease. Aging Dis. 8, 215–227. doi: 10.14336/AD.2016.0811
Striessnig, J., Pinggera, A., Kaur, G., Bock, G., and Tuluc, P. (2014). L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 3, 15–38. doi: 10.1002/wmts.102
Tabuchi, M., Tanaka, N., Nishida-Kitayama, J., Ohno, H., and Kishi, F. (2002). Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Mol. Biol. Cell 13, 4371–4387. doi: 10.1091/mbc.e02-03-0165
Tabuchi, M., Yanatori, I., Kawai, Y., and Kishi, F. (2010). Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. J. Cell Sci. 123(Pt 5), 756–766. doi: 10.1242/jcs.060574
Tabuchi, M., Yoshida, T., Takegawa, K., and Kishi, F. (1999). Functional analysis of the human NRAMP family expressed in fission yeast. Biochem. J. 344, 211–219. doi: 10.1042/0264-6021:3440211
Tian, J., Zheng, W., Li, X. L., Cui, Y. H., and Wang, Z. Y. (2018). Lower expression of Ndfip1 is associated with Alzheimer disease pathogenesis through decreasing DMT1 degradation and increasing iron influx. Front. Aging Neurosci. 10:165. doi: 10.3389/fnagi.2018.00165
Toklu, H. Z., Yang, Z., Oktay, S., Sakaraya, Y., Kirichenko, N., Matheny, M. K., et al. (2018). Overpressure blast injury-induced oxidative stress and neuroinflamamtion response in rat frontal cortex and cerebellum. Behav. Brain Res. 340, 14–22. doi: 10.1016/j.bbr.2017.04.025
Urrutia, P., Aguirre, P., Esparza, A., Tapia, V., Mena, N. P., Arredondo, M., et al. (2013). Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 126, 541–549. doi: 10.1111/jnc.12244
Urrutia, P. J., Hirsch, E. C., González-Billault, C., and Núñez, M. T. (2017). Hepcidin attenuates amyloid beta-induced inflammatory and pro-oxidant responses in astrocytes and microglia. J. Neurochem. 142, 140–152. doi: 10.1111/jnc.14005
Vela, D. (2018). The dual role of hepcidin in brain iron load and inflammation. Front. Neurosci. 12:740. doi: 10.3389/fnins.2018.00740
Wang, C. Y., and Knutson, M. D. (2013). Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology 58, 788–798. doi: 10.1002/hep.26401
Wang, D., Wang, L. H., Zhao, Y., Lu, Y. P., and Zhu, L. (2010). Hypoxia regulates the ferrous iron uptake and reactive oxygen species level via divalent metal transporter 1 (DMT1) Exon1B by hypoxia-inducible factor-1. IUBMB Life 62, 629–636. doi: 10.1002/iub.363
Wang, X., Zhang, M., Flores, S. R. L., Woloshun, R. R., Yang, C., Yin, L., et al. (2019). Oral gavage of ginger nanoparticle-derived lipid vectors carrying Dmt1 siRNA blunts iron loading in murine hereditary hemochromatosis. Mol. Ther [Epub ahead of print].
White, R. S., Bhattacharya, A. K., Chen, Y., Byrd, M., McMullen, M. F., Siegel, S. J., et al. (2016). Lysosomal iron modulates NMDA receptor-mediated excitation via small GTPase, Dexras1. Mol. Brain 9:38. doi: 10.1186/s13041-016-0220-8
Wolff, N. A., Garrick, M. D., Zhao, L., Garrick, L. M., Ghio, A. J., and Thévenod, F. (2018). A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 8:211. doi: 10.1038/s41598-017-18584-4
Wolff, N. A., Garrick, L. M., Zhao, L., Garrick, M. D., and Thevenod, F. (2014a). Mitochondria represent another locale for the divalent metal transporter 1 (DMT1). Channels (Austin) 8, 458–466. doi: 10.4161/19336950.2014.956564
Wolff, N. A., Ghio, A. J., Garrick, L. M., Zhao, L., Fenton, R. A., and Thavenod, F. (2014b). Evidence for mitochondrial localization of divalent metal transporter 1 (DMT1). FASEB J. 28, 2134–2145. doi: 10.1096/fj.13-240564
Wu, L. J., Leenders, A. G., Cooperman, S., Meyron-Holtz, E., Smith, S., Land, W., et al. (2004). Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 1001, 108–117. doi: 10.1016/j.brainres.2003.10.066
Xian-hui, D., Wei-juan, G., Tie-mei, S., Hong-lin, X., Jiang-tao, B., Jing-yi, Z., et al. (2015). Age-related changes of brain iron load changes in the frontal cortex in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. J. Trace Elem. Med. Biol. 30, 118–123. doi: 10.1016/j.jtemb.2014.11.009
Xiong, S., She, H., Takeuchi, H., Han, B., Engelhardt, J. F., Barton, C. H., et al. (2003). Signaling role of intracellular iron in NF-kappaB activation. J. Biol. Chem. 278, 17646–17654.
Xiong, X. Y., Liu, L., Wang, F. X., Yang, Y. R., Hao, J. W., Wang, P. F., et al. (2016). Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation 134, 1025–1038. doi: 10.1161/circulationaha.116.021881
Xu, H., Chang, Q., Jia, W., Jiang, H., Sun, P., and Xie, J. (2013). Iron status and lipopolysaccharide regulate Ndfip1 by activation of nuclear factor-kappa B. Biometals 26, 981–988. doi: 10.1007/s10534-013-9674-7
Xu, H., Jiang, H., and Xie, J. (2017). New insight into the crosstalk between NMDARs and Iron: implications for understanding of neurological diseases. Front. Mol. Neurosci. 10:71. doi: 10.3389/fnmol.2017.00071
Xu, H., Liu, X., Xia, J., Yu, T., Qu, Y., Jiang, H., et al. (2018). Activation of NMDA receptors mediated iron accumulation via modulating iron transporters in Parkinson’s disease. FASEB J. 32, 6100–6111. doi: 10.1096/fj.201800060RR
Yang, L., Zhang, B., Yin, L., Cai, B., Shan, H., Zhang, L., et al. (2011). Tanshinone IIA prevented brain iron dyshomeostasis in cerebral ischemic rats. Cell Physiol. Biochem. 27, 23–30. doi: 10.1159/000325202
Yang, W. M., Jung, K. J., Lee, M. O., Lee, Y. S., Lee, Y. H., Nakagawa, S., et al. (2011). Transient expression of iron transport proteins in the capillary of the developing rat brain. Cell Mol. Neurobiol. 31, 93–99. doi: 10.1007/s10571-010-9558-0
You, L. H., Yan, C. Z., Zheng, B. J., Ci, Y. Z., Chang, S. Y., Yu, P., et al. (2017). Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis. 8:e2676. doi: 10.1038/cddis.2017.93
Youdim, M. B. H. (2018). Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J. Neural Transm. (Vienna) 125, 1719–1733. doi: 10.1007/s00702-018-1942-9
Yu, X., Du, T., Song, N., He, Q., Shen, Y., Jiang, H., et al. (2013). Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology 29, 492–495. doi: 10.1212/wnl.0b013e31827f0ebb
Zavodszky, E., Seaman, M. N., Moreau, K., Jimenez-Sanchez, M., Breusegem, S. Y., Harbour, M. E., et al. (2014). Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 5:3828. doi: 10.1038/ncomms4828
Zhang, C. W., Tai, Y. K., Chai, B. H., Chew, K. C. M., Ang, E. T., Tsang, F., et al. (2017). Transgenic mice overexpressing divalent metal transporter 1 exhibit iron accumulation and enhanced Parkin expression in the Brain. Neuromol. Med. 19, 375–386. doi: 10.1007/s12017-017-8451-0
Zhang, S., Wang, J., Song, N., Xie, J., and Jang, H. (2009). Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP (+)-induced apoptosis in MES23.5 cells. Neurobiol. Aging 30, 1466–1476. doi: 10.1016/j.neurobiolaging.2007.11.025
Zhao, Y. S., Zhang, L. H., Yu, P. P., Gou, Y. J., Zhao, J., You, L. H., et al. (2018). Ceruloplasmin, a potential therapeutic agent for Alzheimer’s disease. Antioxid. Redox Signal. 28, 1323–1337. doi: 10.1089/ars.2016.6883
Zheng, W., Xin, N., Chi, Z. H., Zhao, B. L., Zhang, J., Li, J. Y., et al. (2009). Divalent metal transporter 1 is involved in amyloid precursor protein processing and Abetageneration. FASEB J. 23, 4207–4217. doi: 10.1096/fj.09-135749
Zhou, Y. F., Zhang, C., Yang, G., Qian, Z. M., Zhang, M. W., Ma, J., et al. (2017). Hepcidin protects neuron from hemin-mediated injury by reducing iron. Front. Physiol. 23:332. doi: 10.3389/fphys.2017.00332
Keywords: iron homoeostasis, neurodegenerative diseases, aging, transport of iron and heavy metals, divalent metal transporter 1 up-regulation, non-transferrin bound iron transport, neurodegeneration with brain iron accumulation
Citation: Ingrassia R, Garavaglia B and Memo M (2019) DMT1 Expression and Iron Levels at the Crossroads Between Aging and Neurodegeneration. Front. Neurosci. 13:575. doi: 10.3389/fnins.2019.00575
Received: 25 October 2018; Accepted: 20 May 2019;
Published: 05 June 2019.
Edited by:
Massimiliano Filosto, Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia, ItalyReviewed by:
Torben Moos, Aalborg University, DenmarkGladys Oluyemisi Latunde-Dada, King’s College London, United Kingdom
Gabriela Alejandra Salvador, Universidad Nacional del Sur, Argentina
Copyright © 2019 Ingrassia, Garavaglia and Memo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosaria Ingrassia, rosaria.ingrassia@unibs.it
 Rosaria Ingrassia
Rosaria Ingrassia Barbara Garavaglia
Barbara Garavaglia Maurizio Memo3
Maurizio Memo3