- 1Department of Psychiatry, The Jikei University School of Medicine, Tokyo, Japan
- 2Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, United Kingdom
It has been a clinically important, long-standing challenge to accurately localize epileptogenic focus in drug-resistant focal epilepsy because more intensive intervention to the detected focus, including resection neurosurgery, can provide significant seizure reduction. In addition to neurophysiological examinations, neuroimaging plays a crucial role in the detection of focus by providing morphological and neuroanatomical information. On the other hand, epileptogenic lesions in the brain may sometimes show only subtle or even invisible abnormalities on conventional MRI sequences, and thus, efforts have been made for better visualization and improved detection of the focus lesions. Recent advance in neuroimaging has been attracting attention because of the potentials to better visualize the epileptogenic lesions as well as provide novel information about the pathophysiology of epilepsy. While the progress of newer neuroimaging techniques, including the non-Gaussian diffusion model and arterial spin labeling, could non-invasively detect decreased neurite parameters or hypoperfusion within the focus lesions, advances in analytic technology may also provide usefulness for both focus detection and understanding of epilepsy. There has been an increasing number of clinical and experimental applications of machine learning and network analysis in the field of epilepsy. This review article will shed light on recent advances in neuroimaging for focal epilepsy, including both technical progress of images and newer analytical methodologies and discuss about the potential usefulness in clinical practice.
Introduction
Epilepsy is a common chronic brain disease, which affects around 50 million people all over the world (Leonardi and Ustun, 2002; GBD 2016 Epilepsy Collaborators, 2019). The burden of epilepsy includes recurrent seizures, their physical and psychosocial problems, and various comorbidities (GBD 2016 Epilepsy Collaborators, 2019). While seizures can be controlled by anti-seizure medicine in over 60% of patients with epilepsy (Kwan and Brodie, 2000; Chen et al., 2018b), the rest of them experience drug-resistant seizures, which may result in poorer quality of life (Kubota and Awaya, 2010). Epilepsy surgery is a well-established option to remediate patients with drug-resistant epilepsy, and particularly accurate localization of epileptogenic focus has a key role for the successful surgical resection in focal epilepsy (Rathore and Radhakrishnan, 2015).
Neuroimaging is an essential examination for epilepsy, and one of its major roles is to visualize epileptogenic lesions, particularly in patients with drug-resistant focal seizures (Bernasconi et al., 2019). However, a part of cases with focal epilepsy show visually normal MRI, which is called “MRI-negative” epilepsy (So and Lee, 2014), and the proportion of MRI-negative cases was supposed to be up to 30% in temporal lobe epilepsy (Muhlhofer et al., 2017). Since unsuccessful localization of focus by MRI may lead to poorer surgical seizure outcome (So and Lee, 2014), accurate visualization of epileptogenic lesions by neuroimaging techniques has been a long-standing challenge in epilepsy.
Thus, the current review will shed light on recent advanced neuroimaging techniques for focus detection as well as conventional standard and quantitative analysis.
Conventionally “Visible” Structural Lesions
Even though a lot of quantitative methodologies have been developed, visual inspection is still an important and standard approach for focus detection. Figure 1 presents an overview of conventionally visible epileptogenic lesions, including hippocampal sclerosis, focal cortical dysplasia and other malformation of cortical development, neoplasms, vascular malformations, and cerebrovascular diseases. Before discussing about MRI-negative epilepsy, epileptologists should be aware of these common epileptogenic lesions. Particularly, the two common etiologies, i.e., hippocampal sclerosis and focal cortical dysplasia, may need careful and specific attention for detection, as only subtle abnormalities may sometimes be found (Bernasconi et al., 2019). Additionally, meningoencephalocele has been recently recognized as another etiology in drug-resistant focal epilepsy, which may sometimes show only subtle abnormalities (Saavalainen et al., 2015; Tse et al., 2020). In cases with encephalocele, constructive interference in steady-state (CISS) imaging may be helpful for detection by enhancing the contrast between brain parenchyma and cerebrospinal fluid (Wang et al., 2017) (Figure 2). On the other hand, we need to keep in mind that the detected abnormalities may not always cause the seizures, in cases with incidental lesions.
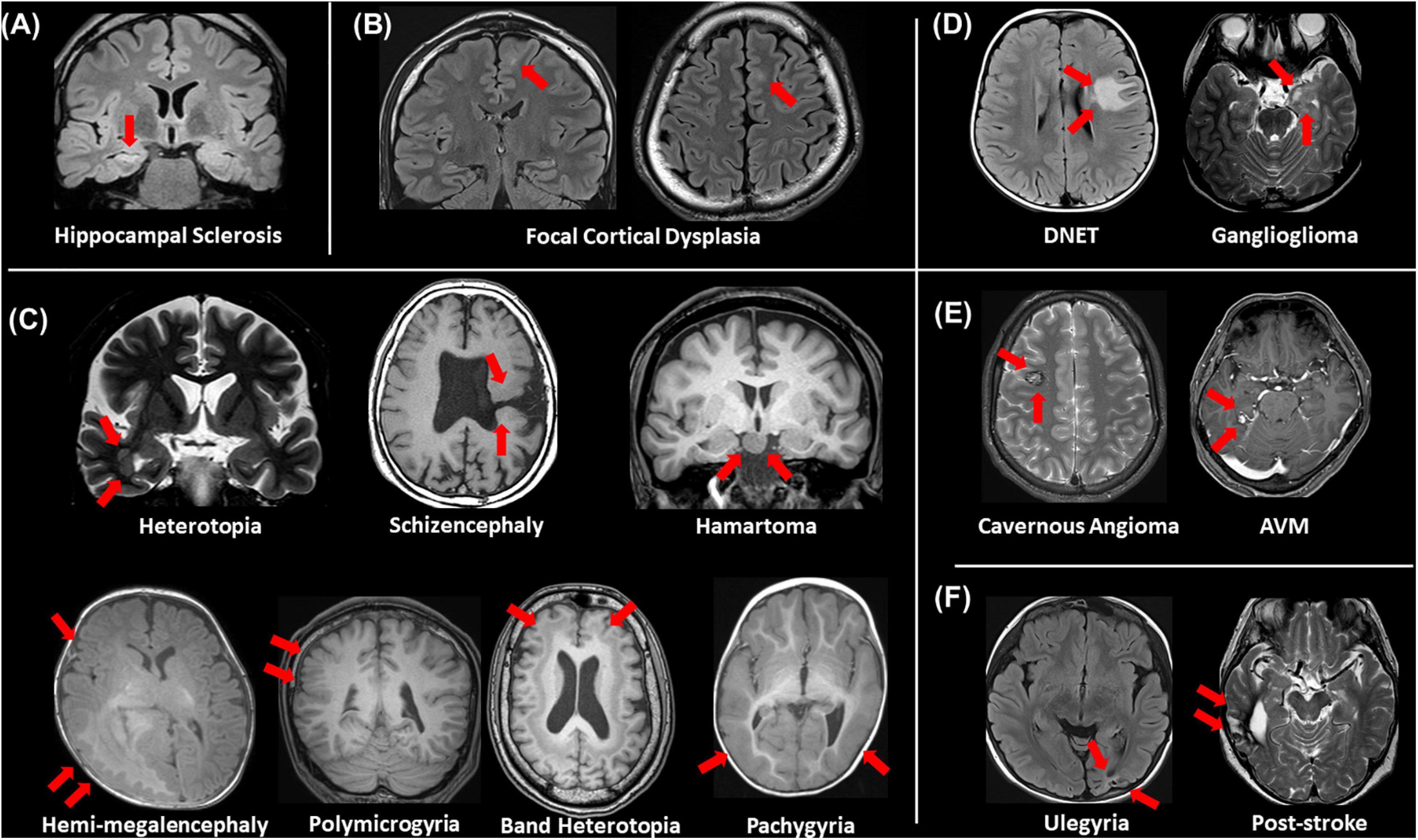
Figure 1. An overview of visible epileptogenic lesions (red arrows). (A) Hippocampal sclerosis, (B) focal cortical dysplasia, (C) other malformations of cortical development, (D) neoplasms, (E) vascular malformations, and (F) cerebrovascular lesions.
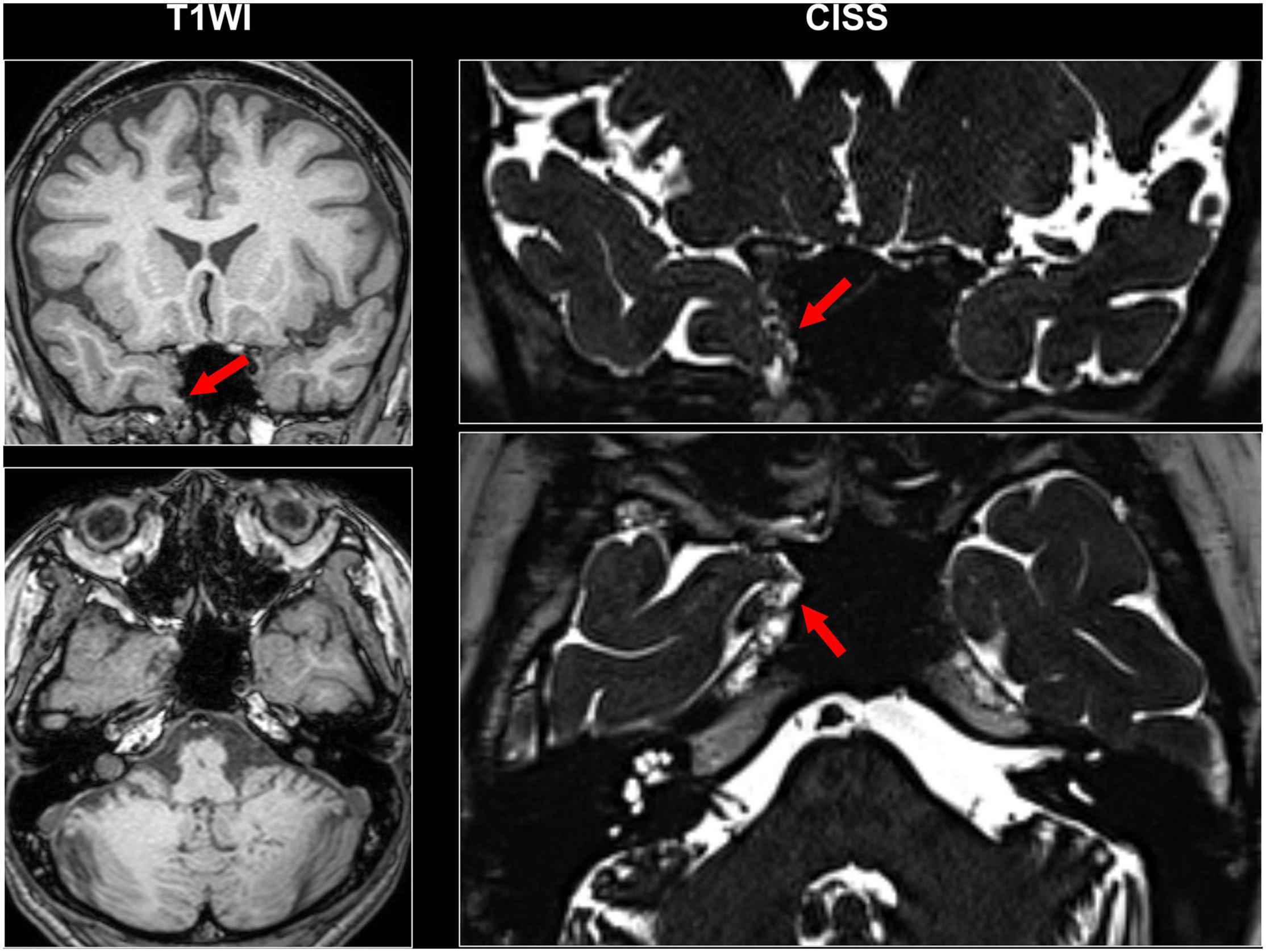
Figure 2. A case with drug-resistant temporal lobe epilepsy and encephalocele. Constructive interference in steady-state (CISS) imaging was helpful for detection by enhancing the contrast between brain parenchyma and cerebrospinal fluid.
It is also important to differentiate epileptogenic lesions, particularly focal cortical dysplasia, from other findings, such as unspecific aging-related changes showing T2 hyperintensity. For that, we need to consider the main features of focal cortical dysplasia, including cortical thickening, blurring of gray–white matter junction, cortical or white matter T2 hyperintensity, and transmantle sign (De Vito et al., 2021) (Figures 1B, 3). To detect hippocampal sclerosis, which is the most common etiology of temporal lobe epilepsy (Thom, 2014), attention should be paid to hippocampal atrophy and T2 hyperintensity, and thinning and blurring of the molecular layer (Bernasconi et al., 2019; De Vito et al., 2021) (Figure 4). As described, epileptogenic lesions are sometimes subtle, and 3D acquisition with reformats is important (De Vito et al., 2021). Therefore, we should be careful about motion artifact and quality control.
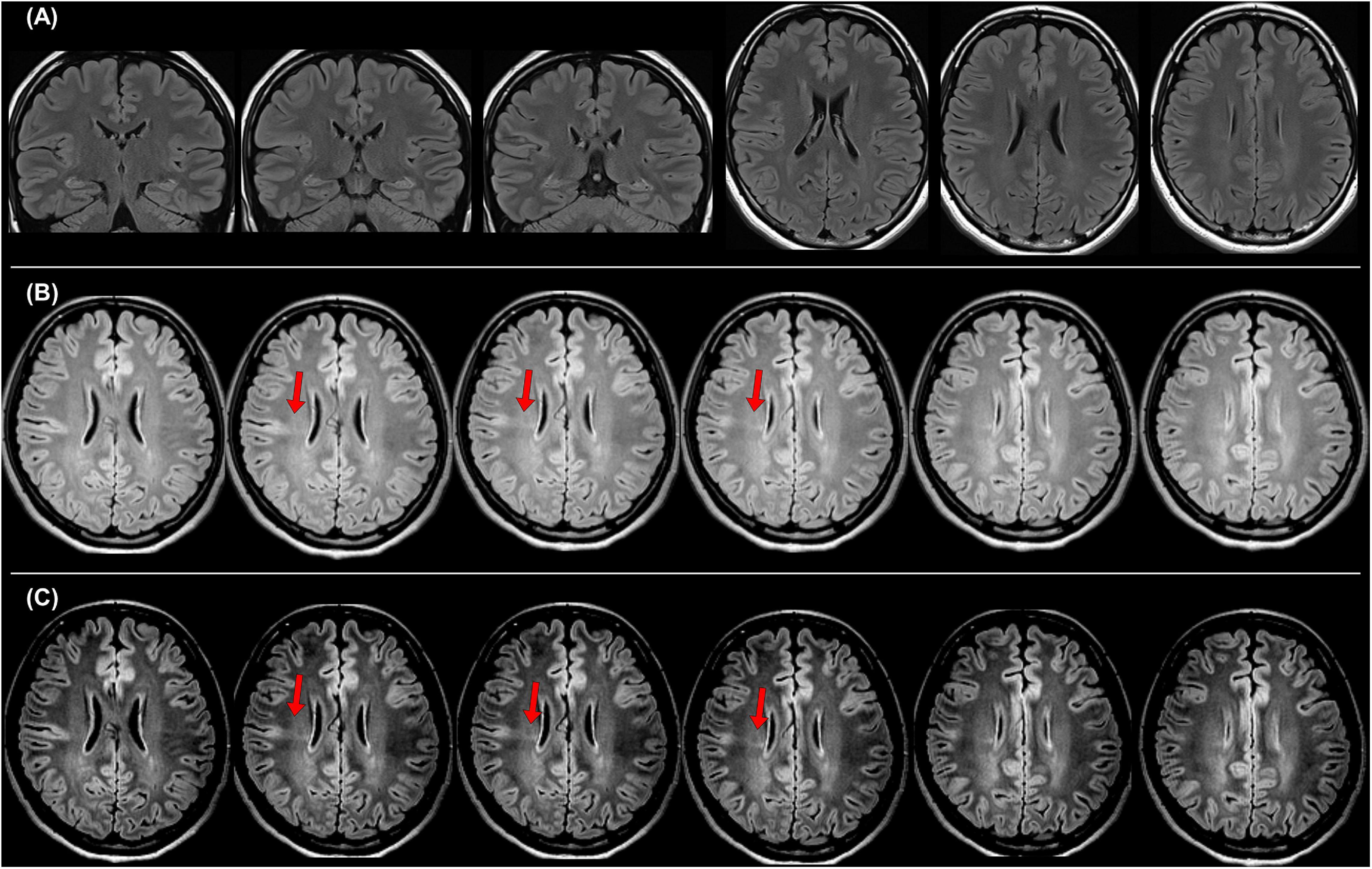
Figure 3. A case with drug-resistant focal epilepsy, who benefited from the official standard protocol for epilepsy. It was impossible to detect any abnormalities in both coronal and axial 2D fluid-attenuated inversion recovery (FLAIR) images with 3-mm slice thickness (A), but the 3D FLAIR images revealed findings of a bottom-of-sulcus-type focal cortical dysplasia with transmantle sign (B), and changing the signal range is sometimes helpful to clearly visualize the lesion (C). The pathological finding was focal cortical dysplasia type IIb.
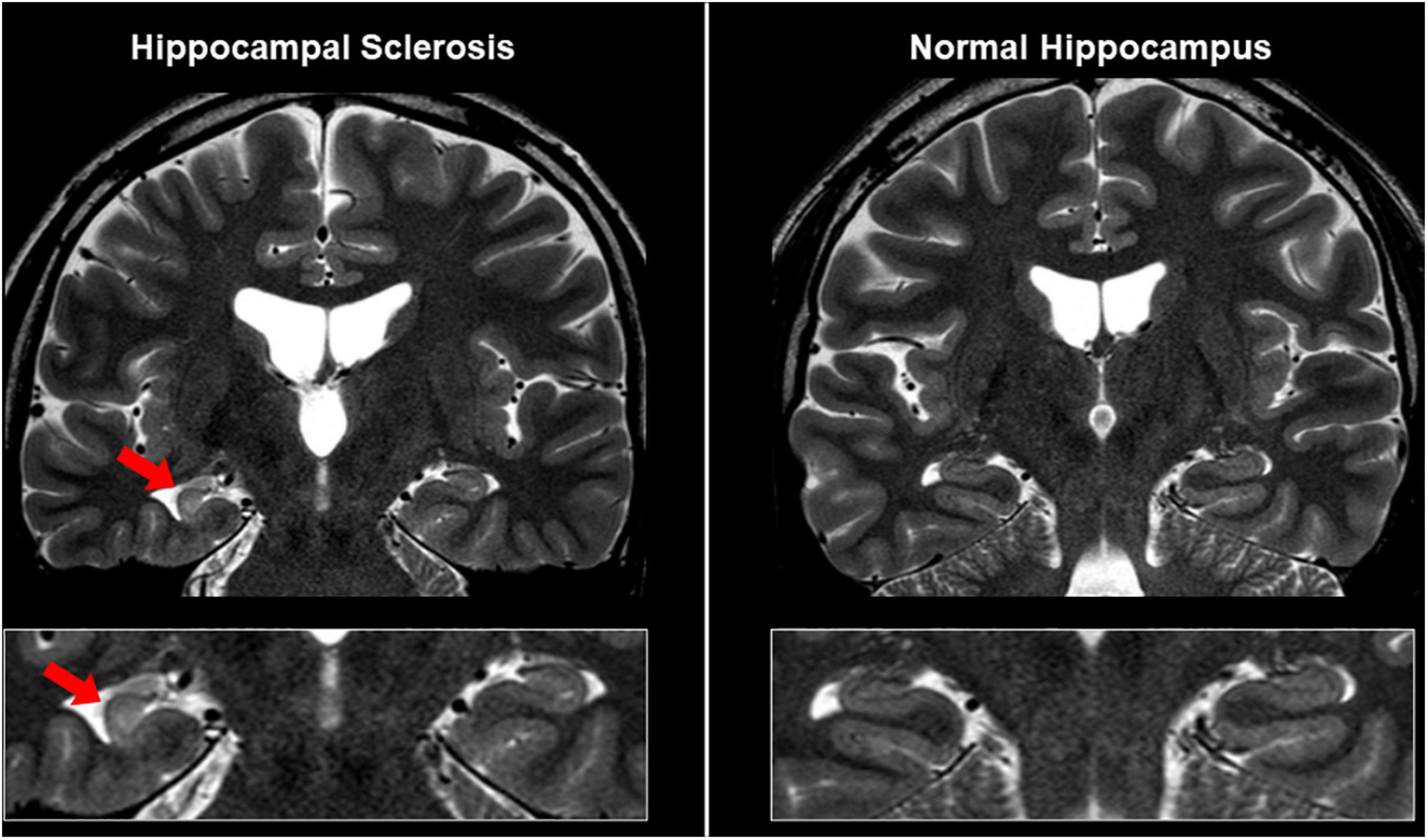
Figure 4. MRI findings in a case with unilateral hippocampal sclerosis (left). The affected hippocampus showed hippocampal atrophy and T2 hyperintensity, and thinning and blurring of the molecular layer, compared with the contralateral side or normal case (right).
Recommendation of the Official Standard Protocol for Epilepsy
In 2019, the International League Against Epilepsy (ILAE) published the official recommendation of structural MRI for epilepsy (Bernasconi et al., 2019). In that, the following protocols were recommended as a standard: 3D millimetric T1-weighted images (T1WI) and fluid-attenuated inversion recovery (FLAIR) images, and 2D submillimetric coronal T2-weighted images (T2WI). Figure 3 shows a representative case with drug-resistant focal epilepsy, who benefited from 3D millimetric FLAIR images. It was impossible to detect any abnormalities in both coronal and axial 2D FLAIR images with 3-mm slice thickness (Figure 3A), but the 3D FLAIR images revealed findings of a bottom-of-sulcus-type focal cortical dysplasia with transmantle sign (Figure 3B), and changing the signal range may sometimes be helpful to clearly visualize the lesion (Figure 3C). The patient underwent surgical resection, and the pathological result was focal cortical dysplasia type IIb. Thus, the optimal MRI protocol for epilepsy may be able to make the previously invisible lesions visible.
However, even with such optimized protocols, we sometimes encounter patients with visually normal MRI. To detect the conventionally invisible epileptogenic lesions, efforts have been made to seek for useful advanced neuroimaging techniques in drug-resistant focal epilepsy (Bernasconi and Wang, 2021).
Advanced Structural Imaging
Beyond the recommended MRI protocol, newer structural MRI sequences have been suggested to provide additional usefulness. Double inversion recovery (DIR), which shows a high contrast between gray and white matters (Ryan, 2016), has been increasingly reported as a useful sequence to detect epileptogenic lesions in temporal lobe epilepsy (TLE) and extratemporal focal epilepsy (Li et al., 2011; Morimoto et al., 2013a, b; Granata et al., 2016; Wong-Kisiel et al., 2016; Wychowski et al., 2016; Sone et al., 2021). In TLE, the superiority of DIR to FLAIR for the detection of anterior temporal white matter abnormalities in the focus side in TLE was reported by both qualitative and quantitative evaluations (Morimoto et al., 2013a; Sone et al., 2021). Figure 5 describes a case of conventionally MRI-negative PET-positive unilateral TLE, in which increased DIR signals can be found in the focus side, while it was difficult to detect on FLAIR, T1WI, and T2WI. More recently, fluid and white matter suppression (FLAWS) has been reported for better visualization of focal cortical dysplasia even in conventionally MRI-negative cases (Chen et al., 2018a; Sun et al., 2021). FLAWS suppresses the white matter and cerebrospinal fluid signals and then generate gray matter-specific images (Tanner et al., 2012; Chen et al., 2018a). Thus, the enhanced contrast between gray and white matters by these newer sequences may improve the visualization of epileptic foci. In addition, edge-enhancing gradient echo (EDGE) imaging was reported to allow us to detect focal cortical dysplasia by directly visualizing the boundary between gray and white matters (Middlebrooks et al., 2020).
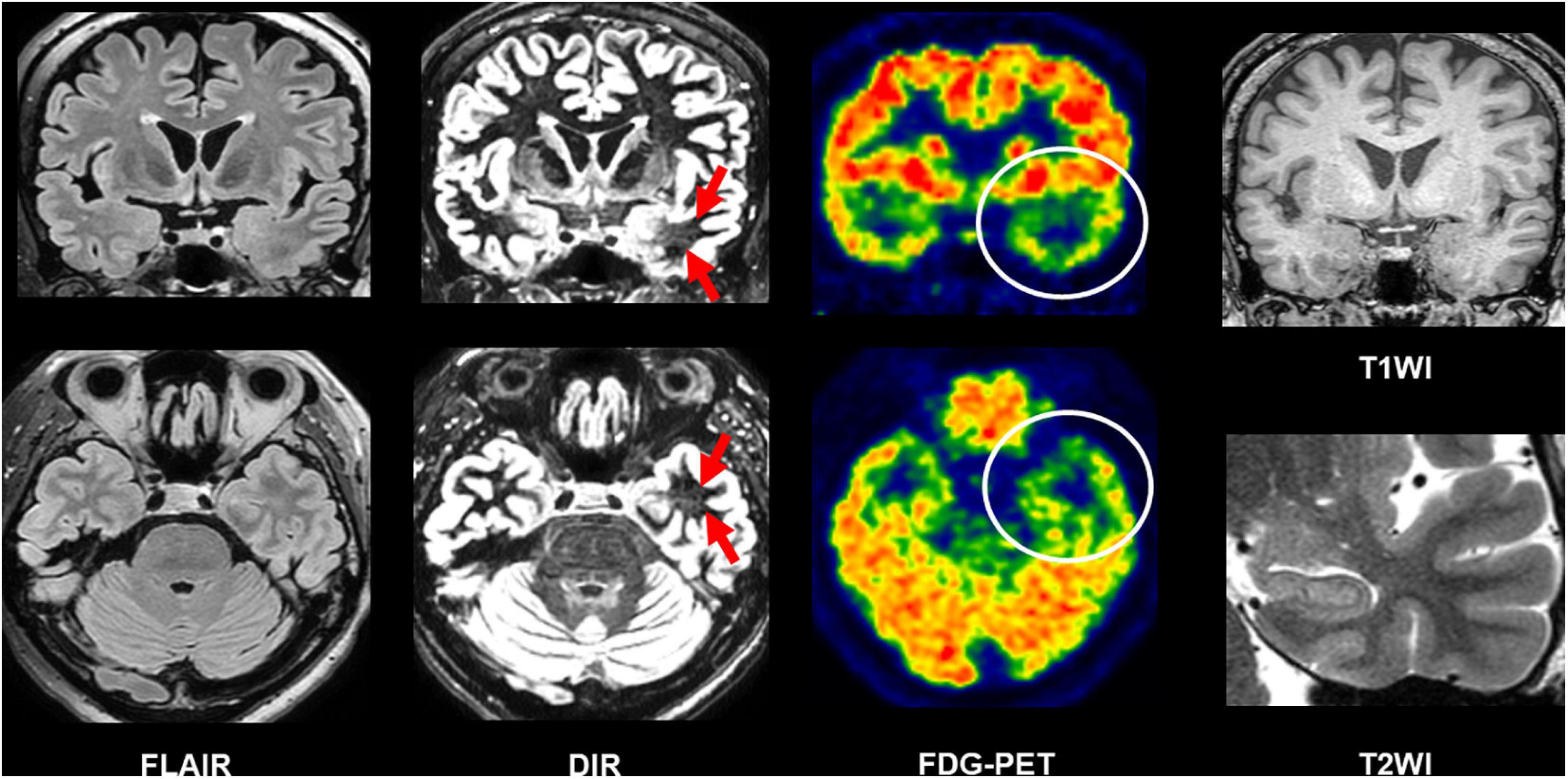
Figure 5. A case of conventionally MRI-negative PET-positive temporal lobe epilepsy (TLE). While it was difficult to detect abnormalities on FLAIR, double inversion recovery (DIR) visualized hyperintensity within the anterior temporal white matter of the focus side. T1-weighted images (T1WI) and T2WI were also intact including the hippocampus.
Advanced Diffusion Imaging
The progress in diffusion MRI has been an emerging topic in the field of neurology and psychiatry. Particularly, multi-shell protocols of diffusion MRI, including diffusion kurtosis imaging (DKI), q-space imaging (QSI), restriction spectrum imaging (RSI), and neurite orientation dispersion and density imaging (NODDI), have provided further information on brain microstructures (Cohen and Assaf, 2002; Jensen et al., 2005; White et al., 2013; Sone, 2019). In the field of epilepsy, NODDI and RSI have been repeatedly reported for their usefulness (Winston et al., 2014; Loi et al., 2016; Reyes et al., 2018; Rostampour et al., 2018; Sone et al., 2018; Lorio et al., 2020; Winston et al., 2020; Shao et al., 2021). Neurite orientation dispersion and density imaging allows us to investigate neurite density and orientation dispersion of the brain microstructures, and reduced neurite density has been consistently found in visible focal cortical dysplasia (Winston et al., 2014; Lorio et al., 2020). Neurite orientation dispersion and density imaging may also visualize neurite abnormalities within the focus even in MRI-negative cases (Sone et al., 2018). Figure 6 represents two cases with conventionally MRI-negative PET-positive unilateral TLE, which showed reduced neurite density within the anterior temporal lobe of the focus side. In TLE with hippocampal sclerosis, reductions of neurite orientation dispersion as well as neurite density were reported (Sone et al., 2018). Additionally, NODDI could help in better visualization of cortical tubers in tuberous sclerosis (Shao et al., 2021). RSI is another advanced diffusion MRI using multi-shell, reduced neurite density, and its correlation with clinical symptoms in epilepsy was also confirmed by RSI (Loi et al., 2016; Reyes et al., 2018). Thus, advances in diffusion MRI may be a promising tool for patients with drug-resistant focal epilepsy and invisible lesions on conventional MRI.
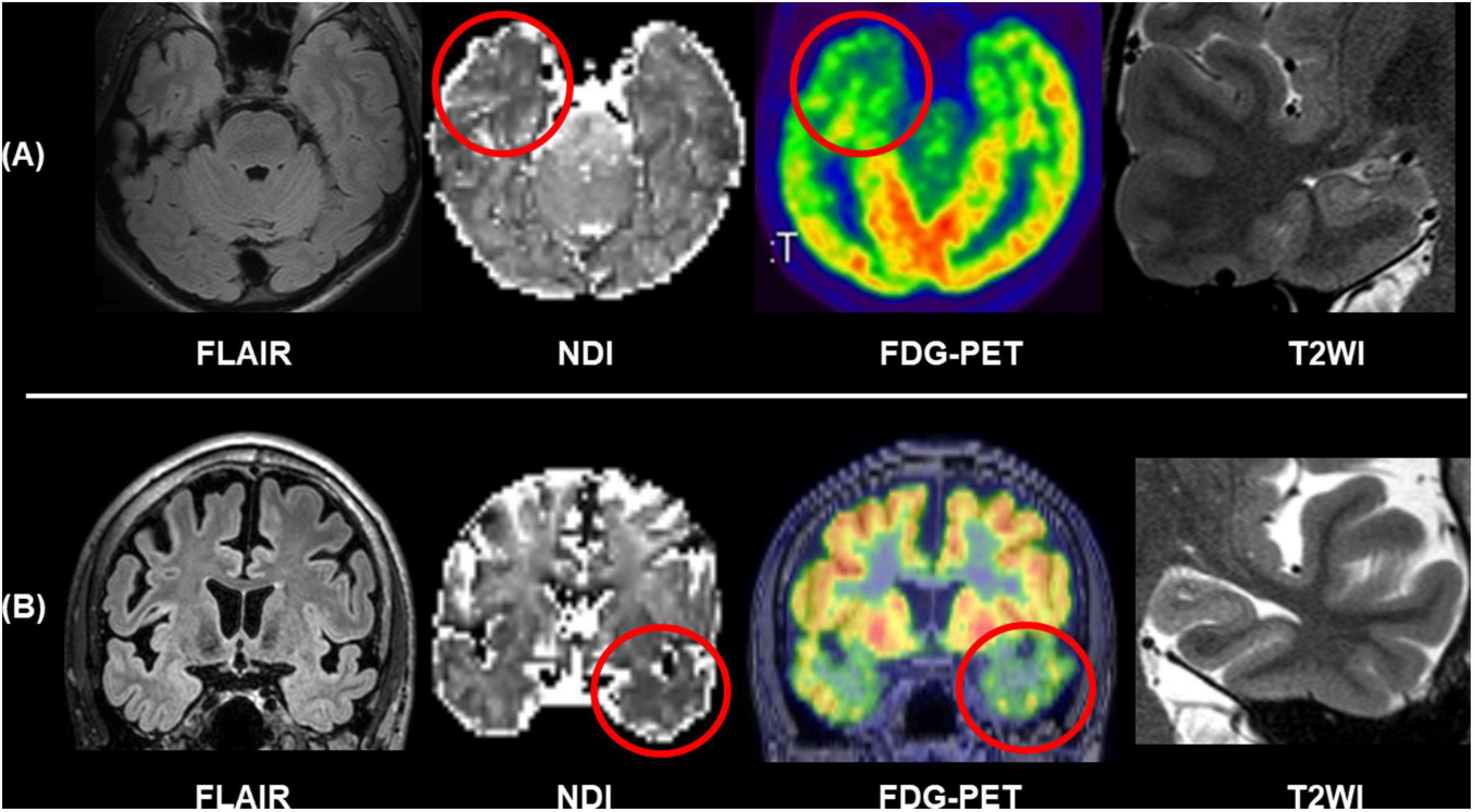
Figure 6. Two cases of conventionally MRI-negative PET-positive TLE. While no abnormalities were found in FLAIR and T2WI including the hippocampus, neurite orientation dispersion and density imaging (NODDI) revealed reduced neurite density of the focus side. (A) Modified from Sone et al. (2018). (B) Modified from Sone (2019).
Advanced Functional Neuroimaging
Interictal reduction of glucose metabolisms in 18F-FDG PET and ictal hyperperfusion detected by SPECT are traditional and established biomarkers for the detection of focus in drug-resistant epilepsy and often effective for MRI-negative cases (Kumar and Chugani, 2013; Shigemoto et al., 2020). In addition to nuclear imaging, recent advances in functional neuroimaging may further improve the detection of focus. Arterial spin labeling (ASL) is a non-invasive method to visualize brain perfusion by MRI (Haller et al., 2016) and, thus, expected to detect abnormal cerebral blood flow, particularly interictal reduction, around the epileptogenic foci in epilepsy (Figure 7) (Boscolo Galazzo et al., 2016; Shang et al., 2018; Wang et al., 2018; Sone et al., 2019; Lam et al., 2020). Although ASL might not surpass 18F-FDG PET in terms of detectability of focus (Sone et al., 2019), its non-invasive nature and wide availability will guarantee a supplemental role in clinical practice. Functional MRI triggered by electroencephalogram (EEG-fMRI) is another newer tool of functional imaging for focus detection. EEG-fMRI can non-invasively detect the hemodynamic signals related with interictal epileptic discharges on EEG (van Graan et al., 2015), and then it can be utilized to visualize the epileptogenic zone and its propagations (Khoo et al., 2017, 2018).
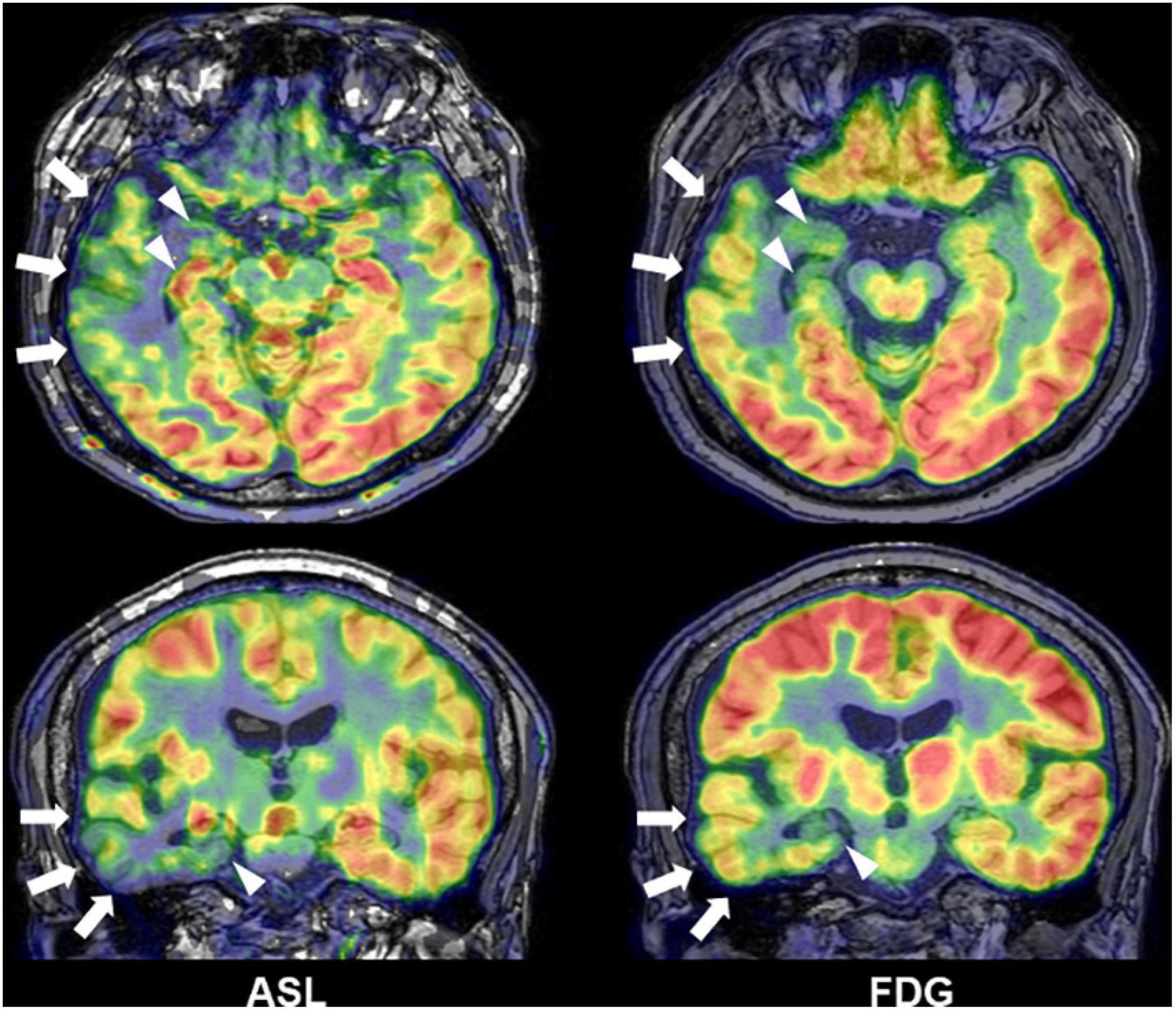
Figure 7. A case of temporal lobe epilepsy with hippocampal sclerosis. Both Arterial spin labeling (ASL) and 18F-FDG PET showed reduced signals around the temporal lobe of focus side (modified from Sone et al., 2019).
Quantitative Analysis and Post-Processing
Another solution for MRI-negative drug-resistant epilepsy is quantitative analysis and post-processing of images. It is known that quantitative hippocampal volumetry and signal analysis improve the visual detectability of hippocampal sclerosis (Coan et al., 2014), and better segmentation and detailed hippocampal profiling methods have also been developed (Winston et al., 2013; Vos et al., 2020). The Morphometric Analysis Program (MAP) is a well-investigated software to generate voxel-based morphometric maps, which can visualize subtle blurring of the gray–white boundary or abnormal cortical surface, using 3D T1WI. In fact, many studies confirmed the usefulness of MAP for the detection of focal cortical dysplasia (Kassubek et al., 2002; Huppertz et al., 2005; Wagner et al., 2011; Wang et al., 2015; Lin et al., 2018; Demerath et al., 2020) or band heterotopia (Huppertz et al., 2008). In addition to T1WI, usefulness of quantitative FLAIR or DIR analysis was also reported (Rugg-Gunn et al., 2006; Focke et al., 2009).
Machine learning is an emerging topic in this field; the advantage of machine-learning may include the accurate, automated, and fast pattern learning, which could be utilized to develop and/optimize clinical algorithms. Currently, studies on machine learning and epilepsy imaging reported its usefulness in the lateralization of TLE (Pustina et al., 2015; Bennett et al., 2019; Beheshti et al., 2020a, b) or automated detection of focal cortical dysplasia (Hong et al., 2014; Hong et al., 2016; Adler et al., 2017; Tan et al., 2018). While machine leaning has provided promising results for the detection of focus in epilepsy, we may need to develop and validate consistent methodology given the diversity of methods (Sone and Beheshti, 2021). Furthermore, network analysis is another trend in epilepsy (Bernhardt et al., 2015), and literature suggested that network metrics derived from neuroimaging could also be used for focus detection when combined with machine learning (Chiang et al., 2015; Yang et al., 2015; Kamiya et al., 2016; Fallahi et al., 2020).
Multimodal Imaging
Combination of multimodal imaging is also important for precise localization of focus (Kurian et al., 2007). Concordance across different modalities supports successful epilepsy surgery (Rathore and Radhakrishnan, 2015), and in addition, coregistered images would improve visual detectability of epileptogenic foci, which was demonstrated by a study using MRI and 18F-FDG PET (Salamon et al., 2008). Multimodal imaging is also a topic in machine learning studies (Pustina et al., 2015; Bennett et al., 2019). Given the importance of multiple modalities in epilepsy, developing a platform for fusion of data (Marecek et al., 2021) would become a significant work for the future.
Seven-Tesla MRI
Seven-tesla (7T) MRI is expected to yield improved detectability over 3T MRI, by the ultra-high-field magnetic strength (van Lanen et al., 2021). Despite the still limited access to 7T MRI, there have been several studies reporting its usefulness in epilepsy (De Ciantis et al., 2016; Veersema et al., 2017; Bartolini et al., 2019; Feldman et al., 2019). On the other hand, diagnostic gain of 7T over conventional MRI has been variable, ranging from 8 to 67% (van Lanen et al., 2021), and thus, further studies would be needed to establish the utility of 7T MRI for clinical use in patients with epilepsy.
Establishment of Clinical MRI Standards for Epilepsy
While this review focused on the recent progress in newer imaging techniques, uniformity of the MRI protocols is of great relevance in clinical epileptology. To establish a practical standard, various aspects need to be considered, including magnetic field strength, imaging resolution, and acquisition time.
Regarding the magnetic field strength, 1.5- or 3-T MRI scanners are currently utilized in clinical practice. In principle, 3-T MRI provides a better signal-to-noise ratio and higher resolution of images, although we need to pay more careful attention to flow and motion artifact in 3-T scanners (Martinez-Rios et al., 2016; De Vito et al., 2021). Indeed, some previous studies reported better identification of epileptogenic lesions by 3- than by 1.5-T MRI, and the use of 3-T MRI may improve the clinical decision making (Knake et al., 2005; Zijlmans et al., 2009; Mellerio et al., 2014; Rubinger et al., 2016). The imaging resolution should be along with the official recommendation of ILAE (Bernasconi et al., 2019), i.e., 3D isotropic T1WI and FLAIR images with millimetric voxels (1 × 1 × 1 mm3), and 2D submillimetric T2WI designed for hippocampal evaluation. More advanced techniques, which were reviewed in this article, may be considered as additional imaging. On the other hand, however, such additions usually require longer acquisition time, which may become a trade-off dilemma for clinically acceptable epilepsy imaging. Thus, those advanced imaging methods need to become more established, particularly by robustly revealing the clinical usefulness, e.g., long-term prognosis of surgery. The manufacturer of MRI scanners is another important factor for the uniformity of epilepsy protocols, as some newer sequences have been developed by each specific manufacturer.
Limitation and Future Challenge
As noted above, compared with conventionally established sequences, the usefulness of advanced imaging still needs to be more robustly elucidated. Although most studies reported potentials of better focus detection, long-term seizure outcomes after resection of the abnormal areas are rarely investigated, so far. Additionally, the cost effectiveness of acquisition time should be kept in mind. Thus, future studies should include more comprehensive and robust comparisons between imaging modalities and clinical parameters, as well as consideration of time efficiency. Another important topic in epilepsy imaging is the preclinical MRI studies to identify the underlying mechanism and time course of epileptogenesis (Immonen et al., 2019; Reddy et al., 2019). Advanced neuroimaging methods may provide further information for basic research on epilepsy. Eventually, in addition to focus detection, neuroimaging could contribute to elucidation of the neurobiological mechanisms, brain functions, and longitudinal brain changes in epilepsy (Galovic et al., 2019; Bernasconi and Wang, 2021). Thus, clinical and basic applications of advanced neuroimaging would be promising for better understanding and improved clinical practice for epilepsy.
Conclusion
There have been various, continuous efforts to better visualize epileptogenic foci in drug-resistant focal epilepsy. The promising advances in structural, diffusion, and functional neuroimaging, as well as quantitative processing and machine learning, may provide critical information for epilepsy surgery and benefit patients with drug-resistant focal epilepsy.
Author Contributions
DS was the sole author of this manuscript and contributed to all aspects.
Funding
This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Number JP21K15720) to DS.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I am grateful to the colleagues in the Department of Radiology, National Center of Neurology and Psychiatry, Tokyo, Japan.
References
Adler, S., Wagstyl, K., Gunny, R., Ronan, L., Carmichael, D., Cross, J. H., et al. (2017). Novel surface features for automated detection of focal cortical dysplasias in paediatric epilepsy. Neuroimage Clin. 14, 18–27. doi: 10.1016/j.nicl.2016.12.030
Bartolini, E., Cosottini, M., Costagli, M., Barba, C., Tassi, L., Spreafico, R., et al. (2019). Ultra-High-Field targeted imaging of focal cortical dysplasia: the intracortical black line sign in type IIb. AJNR Am. J. Neuroradiol. 40, 2137–2142. doi: 10.3174/ajnr.A6298
Beheshti, I., Sone, D., Maikusa, N., Kimura, Y., Shigemoto, Y., Sato, N., et al. (2020a). FLAIR-Wise machine-learning classification and lateralization of MRI-Negative (18)F-FDG PET-Positive temporal lobe epilepsy. Front. Neurol. 11:580713. doi: 10.3389/fneur.2020.580713
Beheshti, I., Sone, D., Maikusa, N., Kimura, Y., Shigemoto, Y., Sato, N., et al. (2020b). Pattern analysis of glucose metabolic brain data for lateralization of MRI-negative temporal lobe epilepsy. Epilepsy Res. 167:106474. doi: 10.1016/j.eplepsyres.2020.106474
Bennett, O. F., Kanber, B., Hoskote, C., Cardoso, M. J., Ourselin, S., Duncan, J. S., et al. (2019). Learning to see the invisible: a data-driven approach to finding the underlying patterns of abnormality in visually normal brain magnetic resonance images in patients with temporal lobe epilepsy. Epilepsia 60, 2499–2507. doi: 10.1111/epi.16380
Bernasconi, A., Cendes, F., Theodore, W. H., Gill, R. S., Koepp, M. J., Hogan, R. E., et al. (2019). Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: a consensus report from the international league against epilepsy neuroimaging task force. Epilepsia 60, 1054–1068. doi: 10.1111/epi.15612
Bernasconi, N., and Wang, I. (2021). Emerging trends in neuroimaging of epilepsy. Epilepsy Curr. 21, 79–82. doi: 10.1177/1535759721991161
Bernhardt, B. C., Bonilha, L., and Gross, D. W. (2015). Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 50, 162–170. doi: 10.1016/j.yebeh.2015.06.005
Boscolo Galazzo, I., Mattoli, M. V., Pizzini, F. B., De Vita, E., Barnes, A., Duncan, J. S., et al. (2016). Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of (18)F-FDG PET and arterial spin labeling. Neuroimage Clin. 11, 648–657. doi: 10.1016/j.nicl.2016.04.005
Chen, X., Qian, T., Kober, T., Zhang, G., Ren, Z., Yu, T., et al. (2018a). Gray-matter-specific MR imaging improves the detection of epileptogenic zones in focal cortical dysplasia: a new sequence called fluid and white matter suppression (FLAWS). Neuroimage Clin. 20, 388–397. doi: 10.1016/j.nicl.2018.08.010
Chen, Z., Brodie, M. J., Liew, D., and Kwan, P. (2018b). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-Year longitudinal cohort study. JAMA Neurol. 75, 279–286. doi: 10.1001/jamaneurol.2017.3949
Chiang, S., Levin, H. S., and Haneef, Z. (2015). Computer-automated focus lateralization of temporal lobe epilepsy using fMRI. J. Magn. Reson. Imaging 41, 1689–1694. doi: 10.1002/jmri.24696
Coan, A. C., Kubota, B., Bergo, F. P., Campos, B. M., and Cendes, F. (2014). 3T MRI quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. AJNR Am. J. Neuroradiol. 35, 77–83. doi: 10.3174/ajnr.A3640
Cohen, Y., and Assaf, Y. (2002). High b-value q-space analyzed diffusion-weighted MRS and MRI in neuronal tissues - a technical review. NMR Biomed. 15, 516–542. doi: 10.1002/nbm.778
De Ciantis, A., Barba, C., Tassi, L., Cosottini, M., Tosetti, M., Costagli, M., et al. (2016). 7T MRI in focal epilepsy with unrevealing conventional field strength imaging. Epilepsia 57, 445–454. doi: 10.1111/epi.13313
De Vito, A., Mankad, K., Pujar, S., Chari, A., Ippolito, D., and D’Arco, F. (2021). Narrative review of epilepsy: getting the most out of your neuroimaging. Transl. Pediatr. 10, 1078–1099. doi: 10.21037/tp-20-261
Demerath, T., Rubensdorfer, L., Schwarzwald, R., Schulze-Bonhage, A., Altenmuller, D. M., Kaller, C., et al. (2020). Morphometric MRI analysis: improved detection of focal cortical dysplasia using the MP2RAGE sequence. AJNR Am. J. Neuroradiol. 41, 1009–1014. doi: 10.3174/ajnr.A6579
Fallahi, A., Pooyan, M., Lotfi, N., Baniasad, F., Tapak, L., Mohammadi-Mobarakeh, N., et al. (2020). Dynamic functional connectivity in temporal lobe epilepsy: a graph theoretical and machine learning approach. Neurol. Sci. 42, 2379–2390. doi: 10.1007/s10072-020-04759-x
Feldman, R. E., Delman, B. N., Pawha, P. S., Dyvorne, H., Rutland, J. W., Yoo, J., et al. (2019). 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One 14:e0213642. doi: 10.1371/journal.pone.0213642
Focke, N. K., Bonelli, S. B., Yogarajah, M., Scott, C., Symms, M. R., and Duncan, J. S. (2009). Automated normalized FLAIR imaging in MRI-negative patients with refractory focal epilepsy. Epilepsia 50, 1484–1490. doi: 10.1111/j.1528-1167.2009.02022.x
Galovic, M., van Dooren, V. Q. H., Postma, T., Vos, S. B., Caciagli, L., Borzi, G., et al. (2019). Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol. 76, 1230–1239. doi: 10.1001/jamaneurol.2019.1708
GBD 2016 Epilepsy Collaborators (2019). Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18, 357–375. doi: 10.1016/S1474-4422(18)30454-X
Granata, F., Morabito, R., Mormina, E., Alafaci, C., Marino, S., Lagana, A., et al. (2016). 3T double inversion recovery magnetic resonance imaging: diagnostic advantages in the evaluation of cortical development anomalies. Eur. J. Radiol. 85, 906–914. doi: 10.1016/j.ejrad.2016.02.018
Haller, S., Zaharchuk, G., Thomas, D. L., Lovblad, K. O., Barkhof, F., and Golay, X. (2016). Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 281, 337–356. doi: 10.1148/radiol.2016150789
Hong, S. J., Bernhardt, B. C., Schrader, D. S., Bernasconi, N., and Bernasconi, A. (2016). Whole-brain MRI phenotyping in dysplasia-related frontal lobe epilepsy. Neurology 86, 643–650. doi: 10.1212/wnl.0000000000002374
Hong, S. J., Kim, H., Schrader, D., Bernasconi, N., Bernhardt, B. C., and Bernasconi, A. (2014). Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology 83, 48–55. doi: 10.1212/wnl.0000000000000543
Huppertz, H. J., Grimm, C., Fauser, S., Kassubek, J., Mader, I., Hochmuth, A., et al. (2005). Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 67, 35–50. doi: 10.1016/j.eplepsyres.2005.07.009
Huppertz, H. J., Wellmer, J., Staack, A. M., Altenmuller, D. M., Urbach, H., and Kroll, J. (2008). Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia 49, 772–785. doi: 10.1111/j.1528-1167.2007.01436.x
Immonen, R., Harris, N. G., Wright, D., Johnston, L., Manninen, E., Smith, G., et al. (2019). Imaging biomarkers of epileptogenecity after traumatic brain injury - preclinical frontiers. Neurobiol. Dis. 123, 75–85. doi: 10.1016/j.nbd.2018.10.008
Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H., and Kaczynski, K. (2005). Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440. doi: 10.1002/mrm.20508
Kamiya, K., Amemiya, S., Suzuki, Y., Kunii, N., Kawai, K., Mori, H., et al. (2016). Machine learning of DTI structural brain connectomes for lateralization of temporal lobe epilepsy. Magn. Reson. Med. Sci. 15, 121–129. doi: 10.2463/mrms.2015-2027
Kassubek, J., Huppertz, H. J., Spreer, J., and Schulze-Bonhage, A. (2002). Detection and localization of focal cortical dysplasia by voxel-based 3-D MRI analysis. Epilepsia 43, 596–602. doi: 10.1046/j.1528-1157.2002.41401.x
Khoo, H. M., Hao, Y., von Ellenrieder, N., Zazubovits, N., Hall, J., Olivier, A., et al. (2017). The hemodynamic response to interictal epileptic discharges localizes the seizure-onset zone. Epilepsia 58, 811–823. doi: 10.1111/epi.13717
Khoo, H. M., von Ellenrieder, N., Zazubovits, N., He, D., Dubeau, F., and Gotman, J. (2018). The spike onset zone: the region where epileptic spikes start and from where they propagate. Neurology 91, e666–e674. doi: 10.1212/WNL.0000000000005998
Knake, S., Triantafyllou, C., Wald, L. L., Wiggins, G., Kirk, G. P., Larsson, P. G., et al. (2005). 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology 65, 1026–1031.
Kubota, H., and Awaya, Y. (2010). Assessment of health-related quality of life and influencing factors using QOLIE-31 in Japanese patients with epilepsy. Epilepsy Behav. 18, 381–387. doi: 10.1016/j.yebeh.2010.04.045
Kumar, A., and Chugani, H. T. (2013). The role of radionuclide imaging in epilepsy, Part 1: sporadic temporal and extratemporal lobe epilepsy. J. Nucl. Med. 54, 1775–1781. doi: 10.2967/jnumed.112.114397
Kurian, M., Spinelli, L., Delavelle, J., Willi, J. P., Velazquez, M., Chaves, V., et al. (2007). Multimodality imaging for focus localization in pediatric pharmacoresistant epilepsy. Epileptic Disord. 9, 20–31. doi: 10.1684/epd.2007.0070
Kwan, P., and Brodie, M. J. (2000). Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319. doi: 10.1056/NEJM200002033420503
Lam, J., Tomaszewski, P., Gilbert, G., Moreau, J. T., Guiot, M. C., Albrecht, S., et al. (2020). The utility of arterial spin labeling in the presurgical evaluation of poorly defined focal epilepsy in children. J. Neurosurg. Pediatr. 25, 1–10. doi: 10.3171/2020.7.PEDS20397
Leonardi, M., and Ustun, T. B. (2002). The global burden of epilepsy. Epilepsia 43, (Suppl. 6), 21–25.
Li, Q., Zhang, Q., Sun, H., Zhang, Y., and Bai, R. (2011). Double inversion recovery magnetic resonance imaging at 3 T: diagnostic value in hippocampal sclerosis. J. Comput. Assist. Tomogr. 35, 290–293. doi: 10.1097/RCT.0b013e3182073c56
Lin, Y., Fang, Y. D., Wu, G., Jones, S. E., Prayson, R. A., Moosa, A. N. V., et al. (2018). Quantitative positron emission tomography-guided magnetic resonance imaging postprocessing in magnetic resonance imaging-negative epilepsies. Epilepsia 59, 1583–1594. doi: 10.1111/epi.14474
Loi, R. Q., Leyden, K. M., Balachandra, A., Uttarwar, V., and Hagler, D. J. Jr., et al. (2016). Restriction spectrum imaging reveals decreased neurite density in patients with temporal lobe epilepsy. Epilepsia 57, 1897–1906. doi: 10.1111/epi.13570
Lorio, S., Adler, S., Gunny, R., D’Arco, F., Kaden, E., Wagstyl, K., et al. (2020). MRI profiling of focal cortical dysplasia using multi-compartment diffusion models. Epilepsia 61, 433–444. doi: 10.1111/epi.16451
Marecek, R., Riha, P., Bartonova, M., Kojan, M., Lamos, M., Gajdos, M., et al. (2021). Automated fusion of multimodal imaging data for identifying epileptogenic lesions in patients with inconclusive magnetic resonance imaging. Hum. Brain Mapp. 42, 2921–2930. doi: 10.1002/hbm.25413
Martinez-Rios, C., McAndrews, M. P., Logan, W., Krings, T., Lee, D., and Widjaja, E. (2016). MRI in the evaluation of localization-related epilepsy. J. Magn. Reson. Imaging 44, 12–22. doi: 10.1002/jmri.25269
Mellerio, C., Labeyrie, M. A., Chassoux, F., Roca, P., Alami, O., Plat, M., et al. (2014). 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia 55, 117–122. doi: 10.1111/epi.12464
Middlebrooks, E. H., Lin, C., Westerhold, E., Okromelidze, L., Vibhute, P., Grewal, S. S., et al. (2020). Improved detection of focal cortical dysplasia using a novel 3D imaging sequence: edge-Enhancing gradient echo (3D-EDGE) MRI. Neuroimage Clin. 28:102449. doi: 10.1016/j.nicl.2020.102449
Morimoto, E., Kanagaki, M., Okada, T., Yamamoto, A., Mori, N., Matsumoto, R., et al. (2013a). Anterior temporal lobe white matter abnormal signal (ATLAS) as an indicator of seizure focus laterality in temporal lobe epilepsy: comparison of double inversion recovery. FLAIR and T2W MR imaging. Eur. Radiol. 23, 3–11. doi: 10.1007/s00330-012-2565-2564
Morimoto, E., Okada, T., Kanagaki, M., Yamamoto, A., Fushimi, Y., Matsumoto, R., et al. (2013b). Evaluation of focus laterality in temporal lobe epilepsy: a quantitative study comparing double inversion-recovery MR imaging at 3T with FDG-PET. Epilepsia 54, 2174–2183. doi: 10.1111/epi.12396
Muhlhofer, W., Tan, Y. L., Mueller, S. G., and Knowlton, R. (2017). MRI-negative temporal lobe epilepsy-what do we know? Epilepsia 58, 727–742. doi: 10.1111/epi.13699
Pustina, D., Avants, B., Sperling, M., Gorniak, R., He, X., Doucet, G., et al. (2015). Predicting the laterality of temporal lobe epilepsy from PET, MRI, and DTI: a multimodal study. Neuroimage Clin. 9, 20–31. doi: 10.1016/j.nicl.2015.07.010
Rathore, C., and Radhakrishnan, K. (2015). Concept of epilepsy surgery and presurgical evaluation. Epileptic Disord. 17, 19–31; quiz31. doi: 10.1684/epd.2014.0720
Reddy, S. D., Younus, I., Sridhar, V., and Reddy, D. S. (2019). Neuroimaging biomarkers of experimental epileptogenesis and refractory epilepsy. Int. J. Mol. Sci. 20:220. doi: 10.3390/ijms20010220
Reyes, A., Uttarwar, V. S., Chang, Y. A., Balachandra, A. R., Pung, C. J., Hagler, D. J., et al. (2018). Decreased neurite density within frontostriatal networks is associated with executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 78, 187–193. doi: 10.1016/j.yebeh.2017.09.012
Rostampour, M., Hashemi, H., Najibi, S. M., and Oghabian, M. A. (2018). Detection of structural abnormalities of cortical and subcortical gray matter in patients with MRI-negative refractory epilepsy using neurite orientation dispersion and density imaging. Phys. Med. 48, 47–54. doi: 10.1016/j.ejmp.2018.03.005
Rubinger, L., Chan, C., D’Arco, F., Moineddin, R., Muthaffar, O., Rutka, J. T., et al. (2016). Change in presurgical diagnostic imaging evaluation affects subsequent pediatric epilepsy surgery outcome. Epilepsia 57, 32–40. doi: 10.1111/epi.13229
Rugg-Gunn, F. J., Boulby, P. A., Symms, M. R., Barker, G. J., and Duncan, J. S. (2006). Imaging the neocortex in epilepsy with double inversion recovery imaging. Neuroimage 31, 39–50. doi: 10.1016/j.neuroimage.2005.11.034
Ryan, M. E. (2016). Utility of double inversion recovery sequences in MRI. Pediatr. Neurol. Briefs 30:26. doi: 10.15844/pedneurbriefs-30-4-1
Saavalainen, T., Jutila, L., Mervaala, E., Kalviainen, R., Vanninen, R., and Immonen, A. (2015). Temporal anteroinferior encephalocele: an underrecognized etiology of temporal lobe epilepsy? Neurology 85, 1467–1474. doi: 10.1212/WNL.0000000000002062
Salamon, N., Kung, J., Shaw, S. J., Koo, J., Koh, S., Wu, J. Y., et al. (2008). FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 71, 1594–1601. doi: 10.1212/01.wnl.0000334752.41807.2f
Shang, K., Wang, J., Fan, X., Cui, B., Ma, J., Yang, H., et al. (2018). Clinical value of hybrid TOF-PET/MR imaging-based multiparametric imaging in localizing seizure focus in patients with MRI-Negative temporal lobe epilepsy. AJNR Am. J. Neuroradiol. 39, 1791–1798. doi: 10.3174/ajnr.A5814
Shao, X., Zhang, X., Xu, W., Zhang, Z., Zhang, J., Guo, H., et al. (2021). Neurite orientation dispersion and density imaging parameters may help for the evaluation of epileptogenic tubers in tuberous sclerosis complex patients. Eur. Radiol. 31, 5605–5614. doi: 10.1007/s00330-020-07626-7627
Shigemoto, Y., Sone, D., Kimura, Y., Sato, N., and Matsuda, H. (2020). Nuclear imaging in epilepsy: principles and progress. Epilepsy Seizure 12, 40–48. doi: 10.3805/eands.12.40
So, E. L., and Lee, R. W. (2014). Epilepsy surgery in MRI-negative epilepsies. Curr. Opin. Neurol. 27, 206–212. doi: 10.1097/WCO.0000000000000078
Sone, D. (2019). Neurite orientation and dispersion density imaging: clinical utility, efficacy, and role in therapy. Rep. Med. Imag. 12, 17–29. doi: 10.2147/RMI.S194083
Sone, D., and Beheshti, I. (2021). Clinical application of machine learning models for brain imaging in epilepsy: a review. Front. Neurosci. 15:684825. doi: 10.3389/fnins.2021.684825
Sone, D., Maikusa, N., Sato, N., Kimura, Y., Ota, M., and Matsuda, H. (2019). Similar and differing distributions between 18F-FDG-PET and arterial spin labeling imaging in temporal lobe epilepsy. Front. Neurol 10:318. doi: 10.3389/fneur.2019.00318
Sone, D., Sato, N., Kimura, Y., Maikusa, N., Shigemoto, Y., and Matsuda, H. (2021). Quantitative analysis of double inversion recovery and FLAIR signals in temporal lobe epilepsy. Epilepsy Res. 170:106540. doi: 10.1016/j.eplepsyres.2020.106540
Sone, D., Sato, N., Ota, M., Maikusa, N., Kimura, Y., and Matsuda, H. (2018). Abnormal neurite density and orientation dispersion in unilateral temporal lobe epilepsy detected by advanced diffusion imaging. Neuroimage Clin. 20, 772–782. doi: 10.1016/j.nicl.2018.09.017
Sun, K., Yu, T., Yang, D., Ren, Z., Qiao, L., Ni, D., et al. (2021). Fluid and white matter suppression imaging and voxel-based morphometric analysis in conventional magnetic resonance imaging-negative epilepsy. Front. Neurol. 12:651592. doi: 10.3389/fneur.2021.651592
Tan, Y. L., Kim, H., Lee, S., Tihan, T., Ver Hoef, L., Mueller, S. G., et al. (2018). Quantitative surface analysis of combined MRI and PET enhances detection of focal cortical dysplasias. Neuroimage 166, 10–18. doi: 10.1016/j.neuroimage.2017.10.065
Tanner, M., Gambarota, G., Kober, T., Krueger, G., Erritzoe, D., Marques, J. P., et al. (2012). Fluid and white matter suppression with the MP2RAGE sequence. J. Magn. Reson. Imaging 35, 1063–1070. doi: 10.1002/jmri.23532
Thom, M. (2014). Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol. Appl. Neurobiol. 40, 520–543. doi: 10.1111/nan.12150
Tse, G. T., Frydman, A. S., O’Shea, M. F., Fitt, G. J., Weintrob, D. L., Murphy, M. A., et al. (2020). Anterior temporal encephaloceles: elusive, important, and rewarding to treat. Epilepsia. 61, 2675–2684. doi: 10.1111/epi.16729
van Graan, L. A., Lemieux, L., and Chaudhary, U. J. (2015). Methods and utility of EEG-fMRI in epilepsy. Quant. Imaging Med. Surg. 5, 300–312. doi: 10.3978/j.issn.2223-4292.2015.02.04
van Lanen, R., Colon, A. J., Wiggins, C. J., Hoeberigs, M. C., Hoogland, G., Roebroeck, A., et al. (2021). Ultra-high field magnetic resonance imaging in human epilepsy: a systematic review. Neuroimage Clin. 30:102602. doi: 10.1016/j.nicl.2021.102602
Veersema, T. J., Ferrier, C. H., van Eijsden, P., Gosselaar, P. H., Aronica, E., Visser, F., et al. (2017). Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. Epilepsia Open 2, 162–171. doi: 10.1002/epi4.12041
Vos, S. B., Winston, G. P., Goodkin, O., Pemberton, H. G., Barkhof, F., Prados, F., et al. (2020). Hippocampal profiling: localized magnetic resonance imaging volumetry and T2 relaxometry for hippocampal sclerosis. Epilepsia 61, 297–309. doi: 10.1111/epi.16416
Wagner, J., Weber, B., Urbach, H., Elger, C. E., and Huppertz, H. J. (2011). Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain 134(Pt 10), 2844–2854. doi: 10.1093/brain/awr204
Wang, Y. H., An, Y., Fan, X. T., Lu, J., Ren, L. K., Wei, P. H., et al. (2018). Comparison between simultaneously acquired arterial spin labeling and (18)F-FDG PET in mesial temporal lobe epilepsy assisted by a PET/MR system and SEEG. Neuroimage Clin. 19, 824–830. doi: 10.1016/j.nicl.2018.06.008
Wang, Z. I., Jones, S. E., Jaisani, Z., Najm, I. M., Prayson, R. A., Burgess, R. C., et al. (2015). Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann. Neurol. 77, 1060–1075. doi: 10.1002/ana.24407
Wang, Z. I., McBride, A., Grinenko, O., Blumcke, I., Overmyer, M., Bingaman, W., et al. (2017). Utility of CISS sequence in detecting anteroinferior temporal encephalocele. J. Neurol. Sci. 381, 59–61. doi: 10.1016/j.jns.2017.08.004
White, N. S., Leergaard, T. B., D’Arceuil, H., Bjaalie, J. G., and Dale, A. M. (2013). Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum. Brain Mapp. 34, 327–346. doi: 10.1002/hbm.21454
Winston, G. P., Cardoso, M. J., Williams, E. J., Burdett, J. L., Bartlett, P. A., Espak, M., et al. (2013). Automated hippocampal segmentation in patients with epilepsy: available free online. Epilepsia 54, 2166–2173. doi: 10.1111/epi.12408
Winston, G. P., Micallef, C., Symms, M. R., Alexander, D. C., Duncan, J. S., and Zhang, H. (2014). Advanced diffusion imaging sequences could aid assessing patients with focal cortical dysplasia and epilepsy. Epilepsy Res. 108, 336–339. doi: 10.1016/j.eplepsyres.2013.11.004
Winston, G. P., Vos, S. B., Caldairou, B., Hong, S. J., Czech, M., Wood, T. C., et al. (2020). Microstructural imaging in temporal lobe epilepsy: diffusion imaging changes relate to reduced neurite density. Neuroimage Clin. 26:102231. doi: 10.1016/j.nicl.2020.102231
Wong-Kisiel, L. C., Britton, J. W., Witte, R. J., Kelly-Williams, K. M., Kotsenas, A. L., Krecke, K. N., et al. (2016). Double inversion recovery magnetic resonance imaging in identifying focal cortical dysplasia. Pediatr. Neurol. 61, 87–93. doi: 10.1016/j.pediatrneurol.2016.04.013
Wychowski, T., Hussain, A., Tivarus, M. E., Birbeck, G. L., Berg, M. J., and Potchen, M. (2016). Qualitative analysis of double inversion recovery MRI in drug-resistant epilepsy. Epilepsy Res. 127, 195–199. doi: 10.1016/j.eplepsyres.2016.09.001
Yang, Z., Choupan, J., Reutens, D., and Hocking, J. (2015). Lateralization of temporal lobe epilepsy based on resting-state functional magnetic resonance imaging and machine learning. Front. Neurol. 6:184. doi: 10.3389/fneur.2015.00184
Keywords: focal epilepsy, magnetic resonance imaging, advanced neuroimaging, structural neuroimaging, diffusion neuroimaging, functional neuroimaging
Citation: Sone D (2021) Making the Invisible Visible: Advanced Neuroimaging Techniques in Focal Epilepsy. Front. Neurosci. 15:699176. doi: 10.3389/fnins.2021.699176
Received: 23 April 2021; Accepted: 28 June 2021;
Published: 27 July 2021.
Edited by:
Ahmad Raza Khan, Centre of Bio-Medical Research (CBMR), IndiaReviewed by:
Vivek Tiwari, Indian Institute of Science, IndiaShilpi Modi, Institute of Nuclear Medicine & Allied Sciences (DRDO), India
Copyright © 2021 Sone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daichi Sone, d.sone@ucl.ac.uk
 Daichi Sone
Daichi Sone