40 Years of CSF Toxicity Studies in ALS: What Have We Learnt About ALS Pathophysiology?
- 1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 2West Midlands Academic Foundation Programme, University of Birmingham, Birmingham, United Kingdom
- 3UK Dementia Research Institute at University of Edinburgh, Edinburgh, United Kingdom
- 4Euan MacDonald Centre for MND Research, University of Edinburgh, Edinburgh, United Kingdom
- 5MRC Edinburgh Brain Bank, Academic Department of Neuropathology, University of Edinburgh, Edinburgh, United Kingdom
- 6Edinburgh Pathology, University of Edinburgh, Edinburgh, United Kingdom
- 7Anne Rowling Regenerative Neurology Clinic, University of Edinburgh, Edinburgh, United Kingdom
- 8Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 9Centre for Brain Development and Repair, InStem, Bengaluru, India
- 10Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Based on early evidence of in vitro neurotoxicity following exposure to serum derived from patients with amyotrophic lateral sclerosis (ALS), several studies have attempted to explore whether cerebrospinal fluid (CSF) obtained from people with ALS could possess similar properties. Although initial findings proved inconclusive, it is now increasingly recognized that ALS-CSF may exert toxicity both in vitro and in vivo. Nevertheless, the mechanism underlying CSF-induced neurodegeneration remains unclear. This review aims to summarize the 40-year long history of CSF toxicity studies in ALS, while discussing the various mechanisms that have been proposed, including glutamate excitotoxicity, proteotoxicity and oxidative stress. Furthermore, we consider the potential implications of a toxic CSF circulatory system in the pathophysiology of ALS, and also assess its significance in the context of current ALS research.
Introduction
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a neurodegenerative condition that is pathologically characterized by the accumulation of ubiquitinated intracellular inclusions containing TAR DNA-binding protein 43 (TDP-43) (Neumann et al., 2006). Clinically, it initially manifests as focal weakness, rapidly progressing to widespread paralysis, with the resulting impairment in respiratory function usually being fatal. About 90% of ALS cases are believed to be sporadic, while monogenetic causes account for the remaining occurrences (Brown and Al-Chalabi, 2017). To date, various mechanisms such as glutamate excitotoxicity, impaired proteostasis and dysregulation of RNA metabolism have been implicated in ALS pathophysiology, with neurodegeneration believed to arise from their highly complex interplay (Hardiman et al., 2017).
Early Suggestions of a Circulating Toxin
Although the pathophysiology of ALS was comparatively little understood in the 1970s, it had nevertheless already been recognized as a fatal neurodegenerative disease involving the selective loss of motor neurons (Wolfgram and Myers, 1973). Amongst various proposed explanations for the etiology of ALS was the existence of a circulating toxin—one thought to be particularly hostile toward motor neurons. Based on this premise, in vitro experiments were conducted, in which anterior horn cells were exposed to diluted serum from ALS patients (Wolfgram and Myers, 1973). Two important observations emerged from this study. First, ALS serum was considerably more toxic to anterior horn cells compared to serum obtained from patients with other neurodegenerative diseases. Second, the observed toxicity did not appear to extend to non-neuronal cells. The inability of the toxic factor to undergo dialysis prompted the authors to suggest the possibility of it being a protein, a particularly interesting proposal given that ALS was at the time not known to be a proteinopathy. The authors further noted a lack of association between patient demographics and serum toxicity.
Given the potential promise of these results in explaining the pathogenesis of ALS, subsequent experiments employing a range of culture systems, including motor neurons and neuroblastoma cells, were performed in the 1970s, in an attempt to reproduce previous findings (Horwich et al., 1974; Liveson et al., 1975; Lehrich and Couture, 1978; Viktorov and Bunina, 1979). These, however, failed to demonstrate evidence of serum toxicity in ALS, and it was only almost a decade later that findings of ALS serum toxicity were successfully replicated (Roisen et al., 1982). Intriguingly, the study investigators also observed increased neurotoxicity following exposure to serum from the relatives of ALS patients as well as from a number of neurological controls, suggesting that serum toxicity was not restricted to the ALS phenotype. In addition to confirming the specific toxicity of ALS serum toward anterior horn cells, the authors further concluded that the toxic factor was both non-dialysable and heat-labile. Other studies have also investigated the effect of ALS serum on various enzymatic reactions, with mixed results (Touzeau and Kato, 1983, 1986; Doherty et al., 1986; Maher et al., 1987; Van der Neut et al., 1991).
From Serum to Cerebrospinal Fluid
Cerebrospinal fluid (CSF), which is often considered as an ultrafiltrate of plasma, is a clear colorless liquid that surrounds most of the central nervous system (CNS). In addition to providing buoyant support and protection to the brain, it also serves a regulatory role, made possible by substantial exchange of material between CSF and interstitial fluid (ISF). In view of the close proximity of CSF to neuronal cells, it was hypothesized that the toxin could also be present in the CSF circulation. The first study to assess the toxicity of ALS-CSF thus emerged in 1981 (Askanas et al., 1981). Measuring the levels of neuron-specific enolase (NSE), a marker of living neurons, in neuronal cultures treated with CSF obtained from ALS patients, however, revealed no significant toxicity. Two subsequent studies were also unable to demonstrate strong evidence of cytotoxicity following exposure to ALS-CSF, although a qualitative decrease in neuronal survival was observed in one study when exposure time was increased (Silani et al., 1987; Swift et al., 1988). Initial evidence of ALS-CSF toxicity arose only in 1993, with the observed toxicity found to be dependent on CSF concentration (Couratier et al., 1993). While additional studies were performed in the same decade (Iwasaki et al., 1995; Gredal et al., 1996; Terro et al., 1996; Smith et al., 1998), it could be argued that evidence for ALS-CSF toxicity remained mostly mixed, similar to serum toxicity.
From 2000 onward, however, evidence grew strongly in favor of the neurotoxic properties of ALS-CSF (see Table 2 in Ng Kee Kwong et al., 2020a). This was despite heterogenous study conditions, including different culture models, CSF concentrations and exposure times (see Table 1 in Ng Kee Kwong et al., 2020a). Notable was the finding that CSF from patients with both sporadic and familial forms of ALS caused cytotoxicity when incubated with neurons in culture. Although these results were predominantly based on rat neuron cultures, as well as the NSC-34 cell line (a mouse spinal cord-neuroblastoma hybrid cell line), recent evidence involving motor neurons derived from human induced pluripotent stem cells (iPSCs) and human embryonic stem cells (hESCs), indicates that CSF-mediated toxicity may also be common to human neurons (Sumitha et al., 2019; Brauer et al., 2020). Complementing in vitro evidence, several in vivo studies have also been performed, demonstrating a range of pathological changes upon CSF injection. In the following sections, we elaborate on the various in vitro and in vivo observations, and discuss the potential underlying mechanisms (Figure 1).
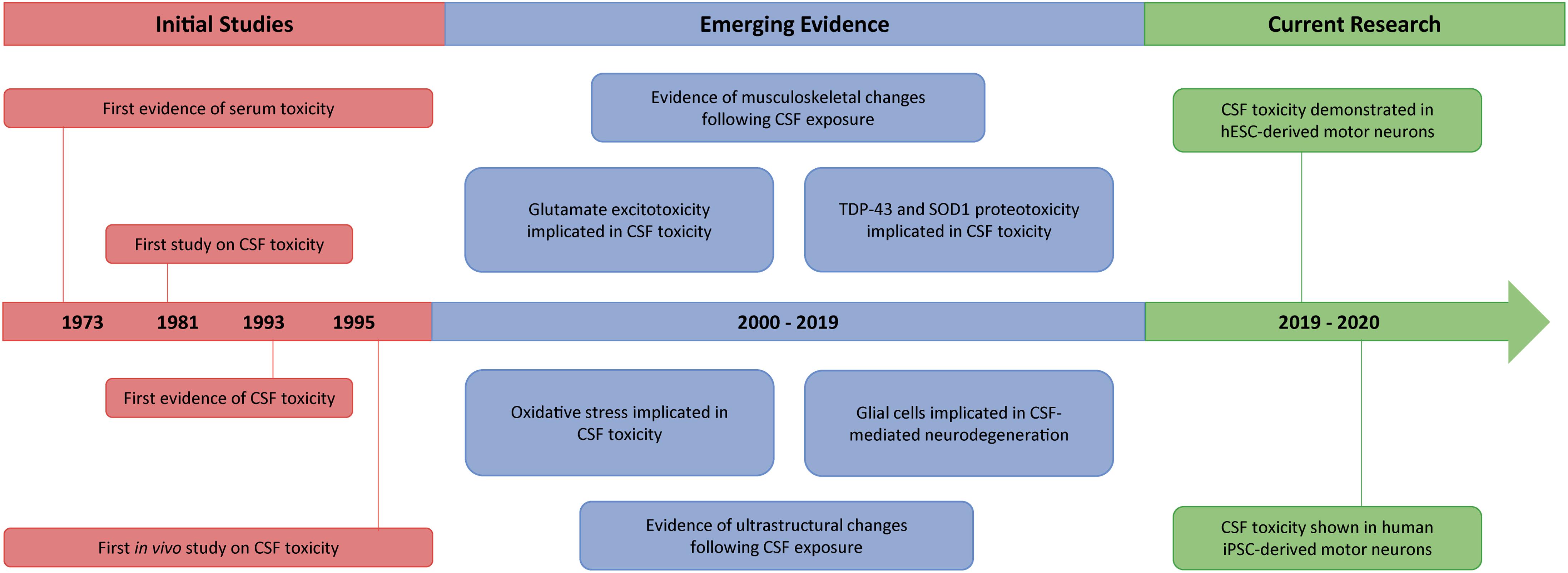
Figure 1. Timeline summarizing the key events from 40 years of CSF toxicity research in ALS. Following evidence of serum toxicity in ALS in the early 1970s, several studies started to explore the potential toxicity of ALS-CSF. By 1995, findings of CSF toxicity had been reported both in vitro and in vivo, although results were initially inconclusive. Subsequently, however, different mechanisms such as glutamate excitotoxicity, proteotoxicity and oxidative stress became implicated in CSF-mediated neurodegeneration, with a glial cell contribution also being demonstrated. Concurrently documented were various downstream pathological features, including ultrastructural, as well as musculoskeletal, changes. More recently, the toxicity of ALS-CSF has been found to extend to both hESC-derived and human iPSC-derived motor neurons.
Evidence of Excitotoxic Mechanisms
Glutamate excitotoxicity has been subject to longstanding interest in ALS research, and several studies have attempted to assess its contribution to CSF toxicity. Early suggestions of the possible involvement of excitotoxic mechanisms include the finding that 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainite receptor, could protect against CSF-induced neurodegeneration (Couratier et al., 1993). Although the same study failed to demonstrate neuroprotection via blocking of N-methyl-D-aspartate (NMDA) receptors (Couratier et al., 1993), results from other groups suggest that both NMDA receptors and AMPA/kainate receptors may be implicated in CSF-induced neurodegeneration (Tikka et al., 2002; Sen et al., 2005). It was further shown that CSF-mediated neurodegeneration was preceded by an increase in intracellular calcium levels, with the rise being more prominent in motor neurons compared to other spinal neurons (Sen et al., 2005). Thus, glutamate receptor activation appears to be much more pronounced in neurons treated with ALS-CSF, with the variation across studies possibly arising from differences in glutamate receptor composition amongst employed neuronal cultures.
Concomitant experiments assessing glutamate levels in ALS-CSF as well as glutamate receptor activation following exposure to ALS-CSF have produced mixed evidence, with suggestions that elevated glutamate levels may be a feature of only a subgroup of ALS patients (Spreux-Varoquaux et al., 2002; Fiszman et al., 2010). Whether significantly raised glutamate levels are necessary to promote excitotoxicity is also unclear. In one study, the approximately threefold increase in glutamate levels observed in ALS patients was found to be sufficient to induce apoptosis and reduce viability in CSF-exposed cells (Cid et al., 2003). In another study, although ALS-CSF contained higher glutamate levels, and could promote neurotoxicity via ionotropic glutamate receptors, increased glutamate concentrations did not correlate with the degree of induced neurotoxicity (Fiszman et al., 2010). Conflicting findings were also reported by a different group, in which ALS-CSF was still shown to trigger excitotoxicity, as suggested by the protective effect of AMPA/kainate receptor antagonists, despite glutamate levels not being significantly elevated (Tikka et al., 2002).
Although it appears that glutamate excitotoxicity may form part of the overall neurodegenerative pathway, suggestions that elevated glutamate levels could be a driver of CSF-mediated neurodegeneration are not supported by other lines of evidence. First, neurodegeneration secondary to ALS-CSF exposure could not be reproduced by administration of L-glutamate in in vitro experiments (Anneser et al., 2006). Second, more recent results show that glutamate levels considerably higher than those found in ALS-CSF are required to produce neurotoxicity (Yanez et al., 2011). It is also worth noting that early findings demonstrating a possible neuroprotective effect by riluzole (Couratier et al., 1994), which remains the only globally licensed drug for ALS, could not be replicated (Yanez et al., 2011). Instead, the observed neurotoxicity could be inhibited by resveratrol, a natural antioxidant found in grapes, possibly by suppressing elevation of cytosolic calcium levels (Yanez et al., 2011). Other compounds found to reduce ALS-CSF toxicity include memantine, minocycline and lithium (Yanez et al., 2014). Intriguingly, the influence of all four neuroprotective factors appeared to be counteracted by the presence of riluzole. While the mechanistic underpinnings of these findings remain unclear, with riluzole potentially exerting both neuroprotective and neurotoxic influences in ALS, the authors suggest a possible confounding effect from riluzole in ALS clinical trials (Yanez et al., 2014). Lastly, we note that dialysis experiments, from which additional insights into the contribution of glutamate to CSF toxicity could potentially arise from, remain sparsely evidenced.
Glial Contributions to CSF-Mediated Neurodegeneration
In line with the increasingly acknowledged role of astrocytes and microglia in ALS pathophysiology (Philips and Rothstein, 2014; Zhao et al., 2019; Clarke and Patani, 2020; Franklin et al., 2020), CSF toxicity has also been argued to possess a cell non-autonomous component. Suggestive findings arose as early as 1987 when in vitro experiments showed that ALS-CSF exposure triggered an increase in GFAP-positive cells, consistent with astrocyte proliferation (Silani et al., 1987). The cause of astrogliosis is unclear, although further results showed that it could follow from stimulation of mGluRs in response to ALS-CSF exposure, as suggested by the anti-proliferative effect of the mGluR group I antagonist, 1-aminoindan-1,5-dicarboxylic acid (AIDA) (Anneser et al., 2004). Complementing in vitro observations, ALS-CSF has also been found to promote increased GFAP immunoreactivity when injected in rats (Shahani et al., 1998). This time, the resulting astrogliosis was shown to be reduced by administration of the immunosuppressive agent, cyclophosphamide, or the selective monoamine oxidase B (MAO-B) inhibitor, (-)-deprenyl (Shahani et al., 2001, 2004). Nevertheless, given that a proportion of astrocytes may only become GFAP-positive following insult (Sofroniew and Vinters, 2010), increases in GFAP-positive cells could potentially be independent of astrocyte proliferation.
As the possible involvement of astrocytes further prompted researchers to investigate their role in glutamate excitotoxicity, one of the emergent findings was downregulation of the expression of glutamate transporter-1 (GLT-1/SLC1A2/EAAT2), which is expressed by astrocytes and is involved in glutamate uptake, following exposure to ALS-CSF (Shobha et al., 2007). Various mechanisms, such as abnormal mRNA processing and lipid peroxidation, have been proposed for the decreased GLT-1 expression. Expression of glutamate aspartate transporter (GLAST/SLC1A3/EAAT1) was, however, not found to be affected. More recent evidence showed that the reduction in glutamate uptake was also accompanied by a significant increase in the release of glutamate (Mishra et al., 2016). Nevertheless, whether the raised extracellular glutamate levels resulting from reduced glutamate uptake could contribute to neuronal death is still unclear.
Early findings have also implicated microglia in CSF-mediated neurodegeneration. Upon administration of minocycline, which blocks microglial activation, a neuroprotective effect was observed following CSF exposure (Tikka et al., 2002). Given that antagonists of NMDA receptors and AMPA/kainate receptors could also suppress the observed neurotoxicity, but without influencing microglial activation, the authors proposed that ALS-CSF may contain toxic factors that promote microglial activation, which, in turn, leads to the activation of ionotropic glutamate receptors and neuronal death. Nevertheless, the variable proportion of astrocytes and microglia across studies, which could range from five percent to thirty percent glial constitution, makes their exact contribution to CSF-induced neurodegeneration difficult to establish (Couratier et al., 1993; Tikka et al., 2002). In one study where microglia made up less than one percent of the total cell count for instance, neurodegeneration via a glutamate-dependent pathway could still be observed (Anneser et al., 2006).
The pro-inflammatory properties of ALS-CSF are further highlighted by its ability to upregulate in astrocytes the expression of various inflammatory factors, including interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), while increasing production of nitric oxide (NO) and reactive oxygen species (ROS) (Mishra et al., 2016). Expression of anti-inflammatory cytokines such as interleukin 10 (IL-10) was also found to be downregulated. The possible contribution of these changes to a neurotoxic environment is supported by the finding that conditioned medium from CSF-exposed astrocytes could promote neurodegeneration (Mishra et al., 2016). Similar observations have been made regarding microglia, with changes in the expression levels of both pro-inflammatory and anti-inflammatory cytokines, along with increased toxicity of microglia-conditioned medium toward neurons (Mishra et al., 2017).
Although many potentially toxic factors have emerged from studying the effects of ALS-CSF on glial cells, chitotriosidase-1 (CHIT-1), an inflammatory product specific to microglia, has been proposed to play a particularly important role in CSF-mediated neurodegeneration (Varghese et al., 2020). Proteomic analysis found CHIT-1 to be significantly upregulated in ALS-CSF compared to control CSF, suggesting its possible role as a biomarker (Varghese et al., 2013). This was subsequently substantiated by findings from the same group demonstrating its high specificity and sensitivity as a diagnostic biomarker (Varghese et al., 2020). It is worth commenting that the function of CHIT-1 in the human nervous system has still not been established, with CHIT-1 also being implicated in other neurodegenerative diseases, such as Alzheimer’s disease and multiple sclerosis (Varghese et al., 2020). Notwithstanding this, the particularly elevated levels of CHIT-1 in ALS-CSF, which have been found to promote astrogliosis and microgliosis, as well as neuronal loss, support its possible participation in CSF-mediated neurodegeneration (Varghese et al., 2020).
There are also intriguing observations derived from co-culture studies that suggest that the glial influence may be more complex than a direct contribution to neuronal loss. Although exposure of motor neuron mono-cultures to both ALS-CSF and control-CSF resulted in neuronal death, motor neurons co-cultured with glia experienced no significant decrease in survival following ALS-CSF exposure, with administration of control-CSF even leading to an increase in cell count (Barber et al., 2011). As well as emphasizing the role of glia in CSF-mediated neurodegeneration, these findings, as the authors proposed, suggest the presence of factors that may have a neuroprotective effect, in addition to toxic components in CSF.
ALS as a Proteinopathy
While ALS is now recognized as a proteinopathy, proteotoxicity initially received comparatively little attention as a possible underlying mechanism in both ALS pathophysiology and CSF-mediated neurodegeneration. In fact, it was found that CSF toxicity could neither be suppressed by prior heating nor by using a 5 kDa filter, thus appearing to exclude the possibility of the toxic agent being a protein (Anneser et al., 2006). Much more recent evidence, however, supports the involvement of key ALS proteins, including superoxide dismutase 1 (SOD1) and TDP-43. Although CSF concentrations of SOD1 in ALS patients are not known to be significantly different from other individuals (Zetterstrom et al., 2011; Winer et al., 2013), misfolded SOD1 present in CSF obtained from patients with sporadic ALS has been shown to be highly toxic to NSC-34 cells, with antibody-mediated depletion to remove the misfolded SOD1 found to produce a significant reduction in neurotoxicity (Tokuda et al., 2019). Whether misfolding of the SOD1 protein is a phenomenon occurring inside cells or within the CSF circulation, as well as the factors promoting its occurrence, remain, however, incompletely understood.
With TDP-43 known to be present in ALS-CSF (Majumder et al., 2018), several attempts have also been made to establish the impact of ALS-CSF exposure on TDP-43 aggregation, and the associated downstream features. Intraventricular injection of CSF from ALS patients into rats resulted in the formation of cytosolic inclusions of TDP-43 co-localizing with ubiquitin (Gomez-Pinedo et al., 2018). In a later study, ALS-CSF injection was also found to induce TDP-43 proteinopathy as well as motor and cognitive disability in hTDP43 mice (Mishra et al., 2020). TDP-43 proteinopathy was, however, not observed in normal mice, although mild motor deficits were described. The authors further noted increased vulnerability with age, with 8-month-old mice experiencing a greater degree of muscle impairment when compared to younger mice. Earlier in vitro findings of TDP-43 mislocalization were again observed following ALS-CSF exposure, a feature which has previously been shown to be partially reversed by vascular endothelial growth factor (VEGF) supplementation (Shantanu et al., 2017). Other intriguing findings to emerge from the same study were the mislocalization of fused in sarcoma/translocated in liposarcoma (FUS/TLS) and formation of stress granules secondary to ALS-CSF exposure, with reversibility by VEGF further being demonstrated (Shantanu et al., 2017).
A more recent study employing human iPSC-derived motor neurons, however, failed to observe such changes following ALS-CSF exposure (Brauer et al., 2020). Additionally, although CSF exposure produced significant neuronal loss, this effect did not appear to be specific to cells treated with ALS-CSF. Nevertheless, exposure to ALS-CSF resulted in significantly higher Golgi fragmentation rates, possibly suggestive of an early sign of neurodegeneration. It has therefore been suggested that a dose-dependent effect may underlie the induction of pathological changes in cells (Brauer et al., 2020; Mishra et al., 2020). In support of this is the finding that TDP-43 mislocalisation and aggregation could be observed only when human glioma U251 cells were exposed to CSF from patients with both ALS and frontotemporal dementia (FTD), while CSF from ALS and control patients failed to induce similar pathology (Ding et al., 2015), consistent with the greater TDP-43 pathology seen in ALS-FTD.
Although additional evidence is required to establish the contribution of TDP-43 and SOD1 to the neurotoxicity of ALS-CSF, these findings could have important implications in our understanding of ALS pathophysiology. Of note is the possibility already suggested by others that the CSF circulation could constitute a pathway for the spread of pathology, which various lines of evidence are now hinting at (Smith et al., 2015; Gomez-Pinedo et al., 2018; Mishra et al., 2020). This could in turn help to potentially account for many of the unexplained features of ALS, one of which is the non-contiguous spreading pattern occasionally observed (Smith et al., 2015; Ng Kee Kwong et al., 2020b).
Other Potential Candidates
Beyond the different factors already described, other components of ALS-CSF could also be responsible for its toxicity toward cells. Levels of 4-hydroxynonenal (HNE), a marker of lipid peroxidation, when raised to that of ALS-CSF, have been found to induce neuronal loss in vitro (Smith et al., 1998). Potentially elevated concentrations of 3-nitrotyrosine, as seen in patients with sporadic ALS, could also indicate oxidative stress by peroxynitrite, which has been shown to activate astrocytes and promote neurodegeneration (Tohgi et al., 1999; Cassina et al., 2002). While the contribution of oxidative stress as an underlying mechanism for CSF-mediated neurodegeneration remains unclear, with definite conclusions from studies measuring levels of oxidative stress biomarkers in ALS-CSF not being possible, antioxidants such as allopurinol and vitamin E have been found to suppress in vitro neurotoxicity following exposure to ALS-CSF (Terro et al., 1996). In addition to the various inflammatory cytokines with potentially neurotoxic properties, such as IL-6 and TNF-α (Moreau et al., 2005), immunoglobulin G (IgG) from ALS patients could be yet another contributing factor to CSF toxicity, given their previously evidenced role in promoting neurodegeneration and microgliosis (Obal et al., 2001; Pullen et al., 2004; Demestre et al., 2005). Finally, the possibility that accumulation of other metabolic waste products could result in a neurotoxic extracellular environment and CSF constitution cannot be excluded.
Additional Downstream Effects of ALS-CSF Exposure
Several pathological changes have been observed across both in vitro and in vivo models in response to ALS-CSF administration. During the 1990s, increased neurofilament phosphorylation was reported when chick spinal cord neurons were exposed to ALS-CSF (Nagaraja et al., 1994), a finding subsequently reproduced in vivo, with the change being considerably more pronounced in ventral horn motor neurons (Rao et al., 1995). Despite being repeatedly linked to ALS-CSF exposure, the cause of neurofilament phosphorylation, as well as its contribution to CSF-mediated neurodegeneration, is still poorly understood (Vijayalakshmi et al., 2009). Ultrastructural changes following exposure to ALS-CSF also include Golgi fragmentation and endoplasmic reticulum stress, the latter being supported by free polyribosomes and fragmented ER cisternae being observed microscopically (Ramamohan et al., 2007; Vijayalakshmi et al., 2011; Brauer et al., 2020). Mitochondrial and lysosomal dysregulation have also been described, hinting at possible oxidative stress (Sharma et al., 2016). While apoptosis is another downstream feature of ALS-CSF exposure (Vijayalakshmi et al., 2011), it is again unclear whether this could be a direct consequence of the various ultrastructural observations.
In addition to cellular changes, intrathecal and intraventricular infusion of ALS-CSF in rat models were also found to result in motor impairment and other musculoskeletal features (Sankaranarayani et al., 2010, 2014; Sumitha et al., 2014; Shanmukha et al., 2018). These could possibly be attributed to altered neuronal activity in the motor cortex, in which increased neuronal excitability was observed (Sankaranarayani et al., 2010, 2014; Shanmukha et al., 2018). It has previously been shown that ALS-CSF exposure could lead to reduced expression levels of ion channels, including Nav1.6 and Kv1.6 channels (Gunasekaran et al., 2009), although their exact association with motor changes is yet to be explored. The expression of miR-206, which is involved in muscle development, was also found to be altered by ALS-CSF infusion (Sumitha et al., 2014). A more recent study reported significant damage to the neuromuscular junction following intrathecal injection of ALS-CSF, along with mitochondrial and sarcoplasmic reticular defects (Shanmukha et al., 2018). The cellular changes produced by intrathecal administration of ALS-CSF have also been found to reflect the degree of motor impairment (Das et al., 2020). Overall, these findings suggest that mechanisms common to both ALS-CSF toxicity and ALS pathophysiology may exist beyond the cellular level.
Neuroprotection From CSF Toxicity
Intriguingly, several neuroprotective factors have also emerged from CSF toxicity studies. VEGF, which has previously been implicated in ALS pathophysiology (Oosthuyse et al., 2001; Lambrechts et al., 2003; Azzouz et al., 2004; Storkebaum et al., 2005), has also been shown to counteract the neurotoxicity of ALS-CSF, reversing morphological changes and decreasing aggregation of phosphorylated neurofilaments (Kulshreshtha et al., 2011). In a later study, VEGF supplementation was further found to reduce caspase-3 levels and restore the expression of calbindin-D28K expression following ALS-CSF exposure, the latter possibly contributing to improved calcium buffering capacity (Vijayalakshmi et al., 2015). It is also worth pointing out that the expression of various growth factors, including brain-derived neurotrophic factor (BDNF), fibroblast growth factor 2 (FGF2) and insulin-like growth factor 1 (IGF1), is downregulated upon ALS-CSF infusion (Deepa et al., 2011). While the exact downstream consequences remain unclear, administration of BDNF, ciliary neurotrophic factor (CNTF) and platelet-derived growth factor (PDGF) has been shown to offer neuroprotection (Gunasekaran et al., 2009; Deepa et al., 2011; Chen et al., 2014; Shruthi et al., 2017).
Is There a Consensus Regarding CSF Toxicity in ALS?
Despite the increasing recognition that ALS-CSF may promote neurodegeneration, with various studies having attempted to characterize its toxicity, synthesizing the current body of literature remains challenging (Box 1). Most prominently, substantial heterogeneity exists across studies with regard to the employed disease models and CSF exposure conditions (Ng Kee Kwong et al., 2020a). For instance, many studies have been performed using rat culture systems and NSC-34 cells, although results have recently been reproduced in human iPSC-derived and hESC-derived motor neurons (Sumitha et al., 2019; Brauer et al., 2020). We have also previously described considerable variation in how results are reported and interpreted across studies (Ng Kee Kwong et al., 2020a). Additionally, different patient and control populations are often recruited, the latter including subjects with wide-ranging conditions which could influence their CSF composition. However, we acknowledge the ethical implications of obtaining CSF from healthy controls. Along this line, it is also often not feasible to recruit larger patient populations in such studies.
Box 1 | Outstanding questions regarding CSF toxicity in ALS.
• What is the nature of the circulating toxin responsible for CSF-induced neurodegeneration?
• What is the cellular origin of this circulating toxin?
• What is/are the mechanism/s by which it triggers neurodegeneration?
• Do astrocytes and microglia play a role in mediating CSF toxicity?
• To what extent does CSF composition reflect that of ISF?
• What is the contribution of CSF toxicity toward ALS pathophysiology?
• How accurately do the models employed to study CSF toxicity recapitulate human in vivo conditions?
• Could similar mechanisms explain CSF toxicity in sporadic and familial forms of ALS?
It is still unclear why conflicting findings have been obtained in early toxicity studies, both in those involving serum and CSF. Studies concomitantly investigating the toxicity of serum and CSF in ALS patients have, to our knowledge, not been conducted, and could potentially reveal additional insights into their association. Furthermore, no significant correlation has to date been observed by studies assessing the possible relationship between CSF toxicity and demographic and clinical variables, suggesting that CSF toxicity may not represent a possible biomarker for disease stratification (Tikka et al., 2002; Anneser et al., 2006; Barber et al., 2011; Yanez et al., 2011; Galan et al., 2017). Although the underlying reasons for this lack of association deserve further investigation, they could nonetheless help to confirm the nature of ALS as a highly heterogeneous disease. This would, notwithstanding methodological considerations, be consistent with recent evidence demonstrating that CSF cytotoxicity is not a feature of all ALS patients (Galan et al., 2017).
In spite of this, there are a number of advantages to considering the neurotoxicity of CSF in an ALS model. First, CSF toxicity, which has been observed across both sporadic and familial forms of ALS, could potentially form part of a common pathogenic pathway in ALS, thus helping to reconcile the two forms of ALS. Although its contribution in ALS pathophysiology has not yet been established, ALS-CSF toxicity appears to possess many common features, including an apparent predilection for motor neurons and an important cell non-autonomous component. As previously mentioned, the presence of a circulating toxin in CSF could also offer possible explanations for the spread of pathology in ALS (Smith et al., 2015). Future research may benefit from additional insights into the cellular origin of this circulating toxin, which has remained elusive to date. The possibility that toxicity may be a feature acquired within the CSF circulation, as has been proposed in the case of SOD1 misfolding (Tokuda et al., 2019), however, cannot be excluded.
While drawing definite conclusions at this stage may not be possible, we believe, based on the numerous observations derived from past studies, that ALS-CSF contains both toxic and protective factors, and is able to promote neurodegeneration either directly or indirectly via a process mediated by both astrocytes and microglia (Figure 2). The neurodegenerative process may be driven by proteotoxicity or neuroinflammation, with possible contribution from other toxic components of CSF. Although evidence suggests that endogenous glutamate is unlikely to initiate the neurodegenerative process, glutamate excitotoxicity is likely to be an important step in the overall pathway. Other downstream consequences leading to the death of motor neurons may include Golgi fragmentation, mitochondrial dysfunction, oxidative stress and apoptosis.
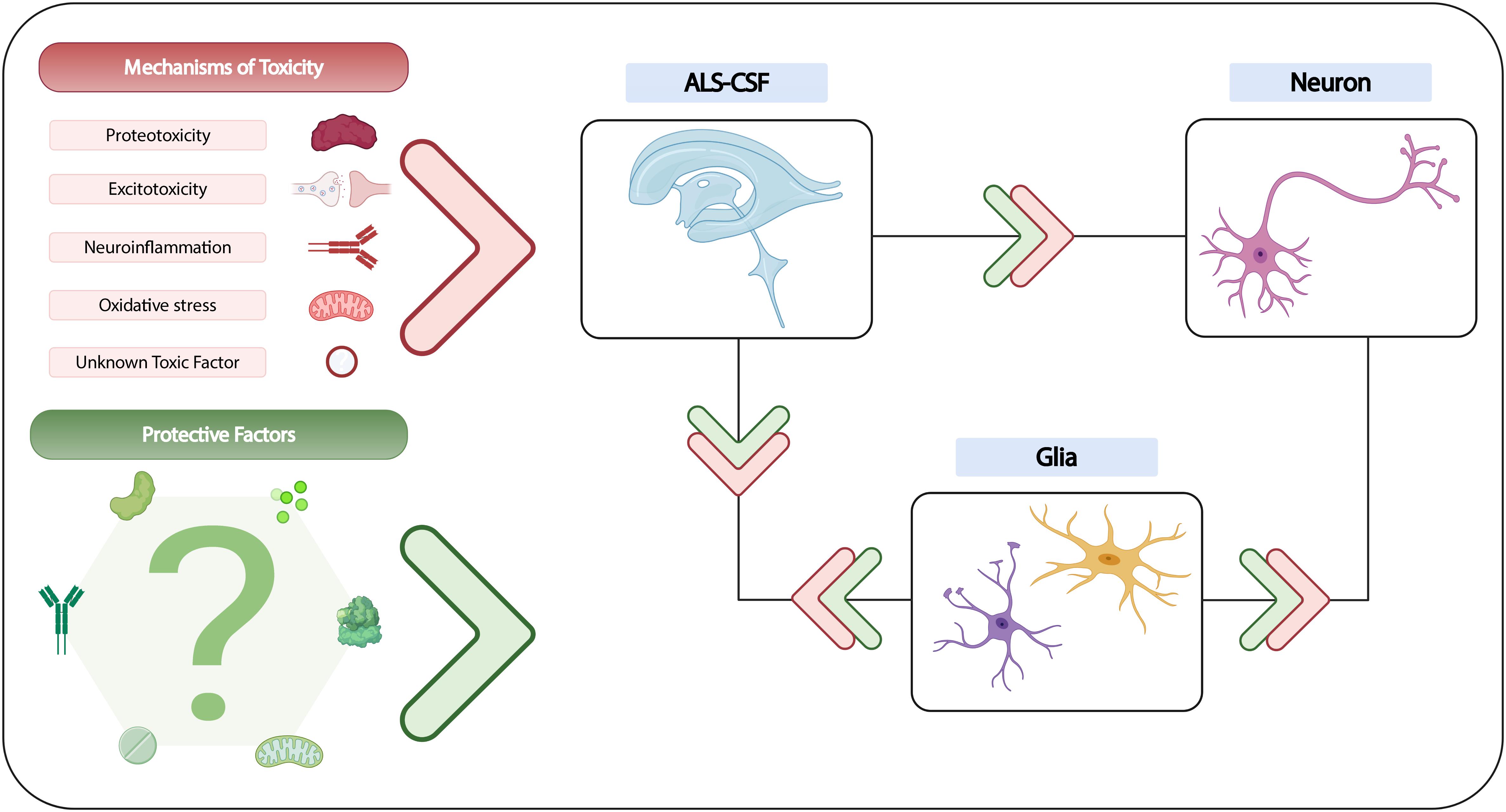
Figure 2. Summary of current understanding of CSF toxicity in ALS. While the exact cause of CSF-induced neurodegeneration remains to be established, various mechanisms of neurotoxicity have been evidenced, and could potentially be attributed to several candidate factors, of which some may not yet be known. The neuroprotective qualities of certain growth factors, glutamate receptor antagonists and antioxidants have also been demonstrated in vitro, although the contribution of protective factors in in vivo models is still unclear. Nevertheless, the existing literature suggests that a highly complex interplay may exist between ALS-CSF, glial cells and neurons, with the involvement of both toxic and protective factors that could directly or indirectly influence neuronal degeneration.
Additional studies are nevertheless required to improve our understanding of CSF toxicity based on both its constitution and dynamics. Although proteomic and lipidomic studies have revealed altered levels of inflammatory markers as well as a different lipid profile in ALS-CSF (Barschke et al., 2017; Blasco et al., 2017; Hayashi et al., 2019), the literature surrounding proteomics in the study of CSF toxicity remains sparse (Varghese et al., 2013), despite its potential in revealing additional candidates for the toxicity of ALS-CSF. CSF biomarker studies have shown many ALS-associated proteins, including TDP-43 and neurofilaments, to be significantly elevated in ALS-CSF (Schreiber et al., 2018; Kasai et al., 2019). While it is unclear whether this may be due to increased production or reduced clearance, the contribution of CSF in their regulation may deserve investigation. Given the currently limited understanding of CSF dynamics, we also propose that investigating the interplay between the extracellular space and the CSF circulation, in which the glymphatic system has been shown to play an important role (Jessen et al., 2015; Plog and Nedergaard, 2018), may help to establish how accurately CSF constitution reflects that of the interstitium. Ependymal cells and astrocytes, which are in direct contact with the CSF, could potentially be vulnerable to its toxicity in ALS patients. Finally, we posit, based on recent evidence of CSF-mediated neurodegeneration in multiple sclerosis and Parkinson’s disease (Kong et al., 2015; Wentling et al., 2019), that CSF toxicity may well be a feature of other neurodegenerative diseases, thus adding to the growing list of common mechanisms that appear to underpin their pathophysiology.
Conclusion
From initial findings of serum toxicity to current in vivo and in vitro evidence of CSF toxicity, striking similarities have been observed between CSF-mediated neurodegeneration and ALS pathophysiology. Although it would be difficult at this stage to argue for the role CSF toxicity as a driver of the disease process, we believe that the potential promise of CSF toxicity in capturing the broader pathophysiological picture of ALS may warrant its additional attention as an area of active research.
Author Contributions
KN and AM conceptualized the work. KN wrote the first draft of the manuscript, supervised by AM. All authors provided critical input and revised subsequent drafts, before approving the final manuscript
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
AM is a Lady Edith Wolfson Clinical Fellow and is jointly funded by the Medical Research Council (MRC) and the Motor Neurone Disease Association (MR/R001162/1). He also acknowledges support from the Rowling Scholars scheme, administered by the Anne Rowling Regenerative Neurology Clinic (ARRNC), University of Edinburgh, and a seedcorn grant from The Chief Scientist Office and the RS Macdonald Charitable Trust via the Scottish Neurological Research Fund, administered by the University of St Andrews. The Hardingham and Chandran laboratories are supported by the Euan MacDonald Centre for Motor Neurone Disease Research, and the UK Dementia Research Institute (DRI), which receives its funding from UK DRI Ltd., funded by the MRC, Alzheimer’s Society and Alzheimer’s Research UK.
Acknowledgments
Figure 2 was created via BioRender.com.
Abbreviations
AIDA, 1-aminoindan-1,5-dicarboxylic acid; ALS, amyotrophic lateral sclerosis; ALS-CSF, cerebrospinal fluid obtained from people with amyotrophic lateral sclerosis; AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; BDNF, brain-derived neurotrophic factor; CHIT-1, chitotriosidase-1; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; CNS, central nervous system; CNTF, ciliary neurotrophic factor; CSF, cerebrospinal fluid; FGF2, fibroblast growth factor 2; FTD, frontotemporal dementia; FUS, fused in sarcoma; GLAST, glutamate aspartate transporter; GLT-1, glutamate transporter-1; hESC, human embryonic stem cell; HNE, 4-hydroxynonenal; IGF1, insulin-like growth factor 1; IgG, immunoglobulin G; IL-6, interleukin 6; IL-10, interleukin 10; iPSC, induced pluripotent stem cell; ISF, interstitial fluid; MAO-B, monoamine oxidase B; mGluR, metabotropic glutamate receptor; MND, motor neuron disease; NMDA, N-methyl -D-aspartate; NO, nitric oxide; NSC-34, mouse spinal cord-neuroblastoma hybrid cell line; NSE, neuron-specific enolase; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; TDP-43, TAR DNA-binding protein 43; TLS, translocated in liposarcoma; TNF- α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
References
Anneser, J. M. H., Chahli, C., Ince, P. G., Borasio, G. D., and Shaw, P. J. (2004). Glial proliferation and metabotropic glutamate receptor expression in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 63, 831–840. doi: 10.1093/jnen/63.8.831
Anneser, J. M., Chahli, C., and Borasio, G. D. (2006). Protective effect of metabotropic glutamate receptor inhibition on amyotrophic lateral sclerosis-cerebrospinal fluid toxicity in vitro. Neuroscience 141, 1879–1886. doi: 10.1016/j.neuroscience.2006.05.044
Askanas, V., Marangos, P. J., and Engel, W. K. (1981). CSF from amyotrophic lateral sclerosis patients applied to motor neurons in culture fails to alter neuron-specific enolase. Neurology 31, 1196–1197. doi: 10.1212/WNL.31.9.1196
Azzouz, M., Ralph, G. S., Storkebaum, E., Walmsley, L. E., Mitrophanous, K. A., Kingsman, S. M., et al. (2004). VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429, 413–417. doi: 10.1038/nature02544
Barber, S. C., Wood-Allum, C. A., Sargsyan, S. A., Walsh, T., Cox, L. E., Monk, P. N., et al. (2011). Contrasting effects of cerebrospinal fluid from motor neuron disease patients on the survival of primary motor neurons cultured with or without glia. Amyotroph. Lateral Scler. 12, 257–263. doi: 10.3109/17482968.2011.560672
Barschke, P., Oeckl, P., Steinacker, P., Ludolph, A., and Otto, M. (2017). Proteomic studies in the discovery of cerebrospinal fluid biomarkers for amyotrophic lateral sclerosis. Expert Rev. Proteomics 14, 769–777. doi: 10.1080/14789450.2017.1365602
Blasco, H., Veyrat-Durebex, C., Bocca, C., Patin, F., Vourc’h, P., Kouassi Nzoughet, J., et al. (2017). Lipidomics reveals cerebrospinal-fluid signatures of ALS. Sci. Rep. 7:17652. doi: 10.1038/s41598-017-17389-9
Brauer, S., Gunther, R., Sterneckert, J., Glass, H., and Hermann, A. (2020). Human spinal motor neurons are particularly vulnerable to cerebrospinal fluid of amyotrophic lateral sclerosis patients. Int. J. Mol. Sci. 21:3564. doi: 10.3390/ijms21103564
Brown, R. H., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172. doi: 10.1056/NEJMra1603471
Cassina, P., Peluffo, H., Pehar, M., Martinez-Palma, L., Ressia, A., Beckman, J. S., et al. (2002). Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 67, 21–29. doi: 10.1002/jnr.10107
Chen, Y. Y., Liu, X. W., Gong, T. X., Zhang, Z. Y., Liu, Y. E., Zhang, Y. B., et al. (2014). Role of platelet derived growth factor (PDGF) in reverting neuronal nuclear and soma size alterations in NSC-34 cells exposed to cerebrospinal fluid from amyotrophic lateral sclerosis patients. Clin. Neurol. Neurosurg. 120, 1–5. doi: 10.1016/j.clineuro.2014.02.004
Cid, C., Alvarez-Cermeno, J. C., Regidor, I., Salinas, M., and Alcazar, A. (2003). Low concentrations of glutamate induce apoptosis in cultured neurons: implications for amyotrophic lateral sclerosis. J. Neurol. Sci. 206, 91–95. doi: 10.1016/S0022-510X(02)00339-8
Clarke, B. E., and Patani, R. (2020). The microglial component of amyotrophic lateral sclerosis. Brain 143, 3526–3539. doi: 10.1093/brain/awaa309
Couratier, P., Hugon, J., Sindou, P., Vallat, J. M., and Dumas, M. (1993). Cell culture evidence for neuronal degeneration in amyotrophic lateral sclerosis being linked to glutamate AMPA/kainate receptors. Lancet 341, 265–268. doi: 10.1016/0140-6736(93)92615-Z
Couratier, P., Sindou, P., Esclaire, F., Louvel, E., and Hugon, J. (1994). Neuroprotective effects of riluzole in ALS CSF toxicity. Neuroreport 5, 1012–1014. doi: 10.1097/00001756-199404000-00040
Das, S., Nalini, A., Laxmi, T. R., and Raju, T. R. (2020). ALS-CSF-induced structural changes in spinal motor neurons of rat pups cause deficits in motor behaviour. Exp. Brain Res. 239, 315–327. doi: 10.1007/s00221-020-05969-7
Deepa, P., Shahani, N., Alladi, P. A., Vijayalakshmi, K., Sathyaprabha, T. N., Nalini, A., et al. (2011). Down regulation of trophic factors in neonatal rat spinal cord after administration of cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients. J. Neural Transm. (Vienna) 118, 531–538. doi: 10.1007/s00702-010-0520-6
Demestre, M., Pullen, A., Orrell, R. W., and Orth, M. (2005). ALS-IgG-induced selective motor neurone apoptosis in rat mixed primary spinal cord cultures. J. Neurochem. 94, 268–275. doi: 10.1111/j.1471-4159.2005.03184.x
Ding, X., Ma, M., Teng, J., Teng, R. K., Zhou, S., Yin, J., et al. (2015). Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure. Oncotarget 6, 24178–24191. doi: 10.18632/oncotarget.4680
Doherty, P., Dickson, J. G., Flanigan, T. P., Kennedy, P. G., and Walsh, F. S. (1986). Effects of amyotrophic lateral sclerosis serum on cultured chick spinal neurons. Neurology 36, 1330–1334. doi: 10.1212/WNL.36.10.1330
Fiszman, M. L., Ricart, K. C., Latini, A., Rodriguez, G., and Sica, R. E. (2010). In vitro neurotoxic properties and excitatory aminoacids concentration in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Relationship with the degree of certainty of disease diagnoses. Acta Neurol. Scand. 121, 120–126. doi: 10.1111/j.1600-0404.2009.01200.x
Franklin, H., Clarke, B. E., and Patani, R. (2020). Astrocytes and microglia in neurodegenerative diseases: lessons from human in vitro models. Prog. Neurobiol. (in press). doi: 10.1016/j.pneurobio.2020.101973
Galan, L., Matias-Guiu, J., Matias-Guiu, J. A., Yanez, M., Pytel, V., Guerrero-Sola, A., et al. (2017). Cerebrospinal fluid cytotoxicity does not affect survival in amyotrophic lateral sclerosis. Acta Neurol. Scand. 136, 212–216. doi: 10.1111/ane.12717
Gomez-Pinedo, U., Galan, L., Yanez, M., Matias-Guiu, J., Valencia, C., Guerrero-Sola, A., et al. (2018). Histological changes in the rat brain and spinal cord following prolonged intracerebroventricular infusion of cerebrospinal fluid from amyotrophic lateral sclerosis patients are similar to those caused by the disease. Neurologia 33, 211–223. doi: 10.1016/j.nrleng.2016.07.002
Gredal, O., Witt, M. R., Dekermendjian, K., Moller, S. E., and Nielsen, M. (1996). Cerebrospinal fluid from amyotrophic lateral sclerosis has no effect on intracellular free calcium in cultured cortical neurons. Mol. Chem. Neuropathol. 29, 141–152. doi: 10.1007/BF02814998
Gunasekaran, R., Narayani, R. S., Vijayalakshmi, K., Alladi, P. A., Shobha, K., Nalini, A., et al. (2009). Exposure to cerebrospinal fluid of sporadic amyotrophic lateral sclerosis patients alters Nav1.6 and Kv1.6 channel expression in rat spinal motor neurons. Brain Res. 1255, 170–179. doi: 10.1016/j.brainres.2008.11.099
Hardiman, O., Al-Chalabi, A., Chio, A., Corr, E. M., Logroscino, G., Robberecht, W., et al. (2017). Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3:17085. doi: 10.1038/nrdp.2017.71
Hayashi, N., Doi, H., Kurata, Y., Kagawa, H., Atobe, Y., Funakoshi, K., et al. (2019). Proteomic analysis of exosome-enriched fractions derived from cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurosci. Res. 160, 43–49. doi: 10.1016/j.neures.2019.10.010
Horwich, M. S., Engel, W. K., and Chauvin, P. B. (1974). Amyotrophic lateral sclerosis sera applied to cultured motor neurons. Arch. Neurol. 30, 332–333. doi: 10.1001/archneur.1974.00490340060015
Iwasaki, Y., Ikeda, K., Shiojima, T., Tagaya, M., and Kinoshita, M. (1995). Amyotrophic lateral sclerosis cerebrospinal fluid is not toxic to cultured spinal motor neurons. Neurol. Res. 17, 393–395. doi: 10.1080/01616412.1995.11740349
Jessen, N. A., Munk, A. S., Lundgaard, I., and Nedergaard, M. (2015). The glymphatic system: a beginner’s guide. Neurochem. Res. 40, 2583–2599. doi: 10.1007/s11064-015-1581-6
Kasai, T., Kojima, Y., Ohmichi, T., Tatebe, H., Tsuji, Y., Noto, Y.-I., et al. (2019). Combined use of CSF NfL and CSF TDP-43 improves diagnostic performance in ALS. Ann. Clin. Translational Neurol. 6, 2489–2502. doi: 10.1002/acn3.50943
Kong, P., Zhang, B. S., Lei, P., Kong, X. D., Zhang, S. S., Li, D., et al. (2015). Neurotoxicity of cerebro-spinal fluid from patients with Parkinson’s disease on mesencephalic primary cultures as an in vitro model of dopaminergic neurons. Mol. Med. Rep. 12, 2217–2224. doi: 10.3892/mmr.2015.3575
Kulshreshtha, D., Vijayalakshmi, K., Alladi, P. A., Sathyaprabha, T. N., Nalini, A., and Raju, T. R. (2011). Vascular endothelial growth factor attenuates neurodegenerative changes in the NSC-34 motor neuron cell line induced by cerebrospinal fluid of sporadic amyotrophic lateral sclerosis patients. Neurodegener. Dis. 8, 322–330. doi: 10.1159/000323718
Lambrechts, D., Storkebaum, E., Morimoto, M., Del-Favero, J., Desmet, F., Marklund, S. L., et al. (2003). VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 34, 383–394. doi: 10.1038/ng1211
Lehrich, J. R., and Couture, J. (1978). Amyotrophic lateral sclerosis sera are not cytotoxic to neuroblastoma cells in tissue culture. Ann. Neurol. 4:384. doi: 10.1002/ana.410040419
Liveson, J., Frey, H., and Bornstein, M. B. (1975). The effect of serum from ALS patients on organotypic nerve and muscle tissue cultures. Acta Neuropathol. 32, 127–131. doi: 10.1007/BF00689566
Maher, I., Pouplard-Barthelaix, A., and Emile, J. (1987). Cytotoxicity of serum from amyotrophic lateral sclerosis patients on spinal cord cells in culture. Adv. Exp. Med. Biol. 209, 75–77. doi: 10.1007/978-1-4684-5302-7_12
Majumder, V., Gregory, J. M., Barria, M. A., Green, A., and Pal, S. (2018). TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. 18:90. doi: 10.1186/s12883-018-1091-7
Mishra, P. S., Boutej, H., Soucy, G., Bareil, C., Kumar, S., Picher-Martel, V., et al. (2020). Transmission of ALS pathogenesis by the cerebrospinal fluid. Acta Neuropathol. Commun. 8:65. doi: 10.1186/s40478-020-00943-4
Mishra, P. S., Dhull, D. K., Nalini, A., Vijayalakshmi, K., Sathyaprabha, T. N., Alladi, P. A., et al. (2016). Astroglia acquires a toxic neuroinflammatory role in response to the cerebrospinal fluid from amyotrophic lateral sclerosis patients. J. Neuroinflammation 13:212. doi: 10.1186/s12974-016-0698-0
Mishra, P. S., Vijayalakshmi, K., Nalini, A., Sathyaprabha, T. N., Kramer, B. W., Alladi, P. A., et al. (2017). Etiogenic factors present in the cerebrospinal fluid from amyotrophic lateral sclerosis patients induce predominantly pro-inflammatory responses in microglia. J. Neuroinflammation 14:251. doi: 10.1186/s12974-017-1028-x
Moreau, C., Devos, D., Brunaud-Danel, V., Defebvre, L., Perez, T., Destee, A., et al. (2005). Elevated IL-6 and TNF-alpha levels in patients with ALS: inflammation or hypoxia? Neurology 65, 1958–1960. doi: 10.1212/01.wnl.0000188907.97339.76
Nagaraja, T. N., Gourie-Devi, M., Nalini, A., and Raju, T. R. (1994). Neurofilament phosphorylation is enhanced in cultured chick spinal cord neurons exposed to cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neuropathol. 88, 349–352. doi: 10.1007/BF00310378
Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., et al. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133. doi: 10.1126/science.1134108
Ng Kee Kwong, K. C., Gregory, J. M., Pal, S., Chandran, S., and Mehta, A. R. (2020a). Cerebrospinal fluid cytotoxicity in amyotrophic lateral sclerosis: a systematic review of in vitro studies. Brain Commun. 2:fcaa121. doi: 10.1093/braincomms/fcaa121
Ng Kee Kwong, K. C., Mehta, A. R., Nedergaard, M., and Chandran, S. (2020b). Defining novel functions for cerebrospinal fluid in ALS pathophysiology. Acta Neuropathol. Commun. 8:140. doi: 10.1186/s40478-020-01018-0
Obal, I., Jakab, J. S., Siklos, L., and Engelhardt, J. I. (2001). Recruitment of activated microglia cells in the spinal cord of mice by ALS IgG. Neuroreport 12, 2449–2452. doi: 10.1097/00001756-200108080-00032
Oosthuyse, B., Moons, L., Storkebaum, E., Beck, H., Nuyens, D., Brusselmans, K., et al. (2001). Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28, 131–138. doi: 10.1038/88842
Philips, T., and Rothstein, J. D. (2014). Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 262(Pt B), 111–120. doi: 10.1016/j.expneurol.2014.05.015
Plog, B. A., and Nedergaard, M. (2018). The glymphatic system in central nervous system health and disease: past, present, and future. Annu. Rev. Pathol. Mech. Dis. 13, 379–394. doi: 10.1146/annurev-pathol-051217-111018
Pullen, A. H., Demestre, M., Howard, R. S., and Orrell, R. W. (2004). Passive transfer of purified IgG from patients with amyotrophic lateral sclerosis to mice results in degeneration of motor neurons accompanied by Ca2+ enhancement. Acta Neuropathol. 107, 35–46. doi: 10.1007/s00401-003-0777-z
Ramamohan, P. Y., Gourie-Devi, M., Nalini, A., Shobha, K., Ramamohan, Y., Joshi, P., et al. (2007). Cerebrospinal fluid from amyotrophic lateral sclerosis patients causes fragmentation of the Golgi apparatus in the neonatal rat spinal cord. Amyotroph. Lateral Scler. 8, 79–82. doi: 10.1080/08037060601145489
Rao, M. S., Devi, M. G., Nalini, A., Shahani, N., and Raju, T. R. (1995). Neurofilament phosphorylation is increased in ventral horn neurons of neonatal rat spinal cord exposed to cerebrospinal fluid from patients with amyotrophic lateral sclerosis. Neurodegeneration 4, 397–401. doi: 10.1006/neur.1995.0048
Roisen, F. J., Bartfeld, H., Donnenfeld, H., and Baxter, J. (1982). Neuron specific in vitro cytotoxicity of sera from patients with amyotrophic lateral sclerosis. Muscle Nerve 5, 48–53. doi: 10.1002/mus.880050109
Sankaranarayani, R., Nalini, A., Rao Laxmi, T., and Raju, T. R. (2010). Altered neuronal activities in the motor cortex with impaired motor performance in adult rats observed after infusion of cerebrospinal fluid from amyotrophic lateral sclerosis patients. Behav. Brain Res. 206, 109–119. doi: 10.1016/j.bbr.2009.09.009
Sankaranarayani, R., Raghavan, M., Nalini, A., Laxmi, T. R., and Raju, T. R. (2014). Reach task-associated excitatory overdrive of motor cortical neurons following infusion with ALS-CSF. J. Neural Transm. (Vienna) 121, 49–58. doi: 10.1007/s00702-013-1071-4
Schreiber, S., Spotorno, N., Schreiber, F., Acosta-Cabronero, J., Kaufmann, J., Machts, J., et al. (2018). Significance of CSF NfL and tau in ALS. J. Neurol. 265, 2633–2645. doi: 10.1007/s00415-018-9043-0
Sen, I., Nalini, A., Joshi, N. B., and Joshi, P. G. (2005). Cerebrospinal fluid from amyotrophic lateral sclerosis patients preferentially elevates intracellular calcium and toxicity in motor neurons via AMPA/kainate receptor. J. Neurol. Sci. 235, 45–54. doi: 10.1016/j.jns.2005.03.049
Shahani, N., Gourie-Devi, M., Nalini, A., and Raju, T. R. (2001). Cyclophosphamide attenuates the degenerative changes induced by CSF from patients with amyotrophic lateral sclerosis in the neonatal rat spinal cord. J. Neurol. Sci. 185, 109–118. doi: 10.1016/S0022-510X(01)00479-8
Shahani, N., Gourie-Devi, M., Nalini, A., Rammohan, P., Shobha, K., Harsha, H. N., et al. (2004). (-)-Deprenyl alleviates the degenerative changes induced in the neonatal rat spinal cord by CSF from amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 5, 172–179. doi: 10.1080/14660820410017037
Shahani, N., Nalini, A., Gourie-Devi, M., and Raju, T. R. (1998). Reactive astrogliosis in neonatal rat spinal cord after exposure to cerebrospinal fluid from patients with amyotrophic lateral sclerosis. Exp. Neurol. 149, 295–298. doi: 10.1006/exnr.1997.6651
Shanmukha, S., Narayanappa, G., Nalini, A., Alladi, P. A., and Raju, T. R. (2018). Sporadic amyotrophic lateral sclerosis (SALS) – skeletal muscle response to cerebrospinal fluid from SALS patients in a rat model. Dis. Model Mech. 11:dmm031997. doi: 10.1242/dmm.031997
Shantanu, S., Vijayalakshmi, K., Shruthi, S., Sagar, B. K. C., Sathyaprabha, T. N., Nalini, A., et al. (2017). VEGF alleviates ALS-CSF induced cytoplasmic accumulations of TDP-43 and FUS/TLS in NSC-34 cells. J. Chem. Neuroanat. 81, 48–52. doi: 10.1016/j.jchemneu.2017.01.007
Sharma, A., Varghese, A. M., Vijaylakshmi, K., Sumitha, R., Prasanna, V. K., Shruthi, S., et al. (2016). Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces mitochondrial and lysosomal dysfunction. Neurochem. Res. 41, 965–984. doi: 10.1007/s11064-015-1779-7
Shobha, K., Vijayalakshmi, K., Alladi, P. A., Nalini, A., Sathyaprabha, T. N., and Raju, T. R. (2007). Altered in-vitro and in-vivo expression of glial glutamate transporter-1 following exposure to cerebrospinal fluid of amyotrophic lateral sclerosis patients. J. Neurol. Sci. 254, 9–16. doi: 10.1016/j.jns.2006.12.004
Shruthi, S., Sumitha, R., Varghese, A. M., Ashok, S., Chandrasekhar Sagar, B. K., Sathyaprabha, T. N., et al. (2017). Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. Neurodegener. Dis. 17, 44–58. doi: 10.1159/000447559
Silani, V., Pizzuti, A., Redaelli, L. M., Bassani, R., Causarano, I. R., Buscaglia, M., et al. (1987). ALS cerebrospinal fluid enhances human foetal astroglial cell proliferation in vitro. Adv. Exp. Med. Biol. 209, 79–81. doi: 10.1007/978-1-4684-5302-7_13
Smith, R. G., Henry, Y. K., Mattson, M. P., and Appel, S. H. (1998). Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann. Neurol. 44, 696–699. doi: 10.1002/ana.410440419
Smith, R., Myers, K., Ravits, J., and Bowser, R. (2015). Amyotrophic lateral sclerosis: Is the spinal fluid pathway involved in seeding and spread? Med. Hypotheses 85, 576–583. doi: 10.1016/j.mehy.2015.07.014
Sofroniew, M. V., and Vinters, H. V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35. doi: 10.1007/s00401-009-0619-8
Spreux-Varoquaux, O., Bensimon, G., Lacomblez, L., Salachas, F., Pradat, P. F., Le Forestier, N., et al. (2002). Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J. Neurol. Sci. 193, 73–78. doi: 10.1016/S0022-510X(01)00661-X
Storkebaum, E., Lambrechts, D., Dewerchin, M., Moreno-Murciano, M. P., Appelmans, S., Oh, H., et al. (2005). Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat. Neurosci. 8, 85–92. doi: 10.1038/nn1360
Sumitha, R., Manjunatha, V. M., Sabitha, R. K., Alladi, P. A., Nalini, A., Rao, L. T., et al. (2019). Cerebrospinal fluid from patients with sporadic amyotrophic lateral sclerosis induces degeneration of motor neurons derived from human embryonic stem cells. Mol. Neurobiol. 56, 1014–1034. doi: 10.1007/s12035-018-1149-y
Sumitha, R., Sidhu, R. J., Sathyaprabha, T. N., Nalini, A., Raju, T. R., and Alladi, P. A. (2014). Differential expression of microRNA-206 in the gastrocnemius and biceps brachii in response to CSF from sporadic amyotrophic lateral sclerosis patients. J. Neurol. Sci. 345, 254–256. doi: 10.1016/j.jns.2014.07.005
Swift, T. R., Gulati, A. K., and Rivner, M. H. (1988). ALS CSF: effect on cultured neurons. Muscle Nerve 11, 278. doi: 10.1002/mus.880110107
Terro, F., Lesort, M., Viader, F., Ludolph, A., and Hugon, J. (1996). Antioxidant drugs block in vitro the neurotoxicity of CSF from patients with amyotrophic lateral sclerosis. Neuroreport 7, 1970–1972. doi: 10.1097/00001756-199608120-00022
Tikka, T. M., Vartiainen, N. E., Goldsteins, G., Oja, S. S., Andersen, P. M., Marklund, S. L., et al. (2002). Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain 125, 722–731. doi: 10.1093/brain/awf068
Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Ishizaki, E., and Isobe, C. (1999). Remarkable increase in cerebrospinal fluid 3-nitrotyrosine in patients with sporadic amyotrophic lateral sclerosis. Ann. Neurol. 46, 129–131. doi: 10.1002/1531-8249(199907)46:1<129::AID-ANA21>3.0.CO;2-Y
Tokuda, E., Takei, Y., Ohara, S., Fujiwara, N., Hozumi, I., and Furukawa, Y. (2019). Wild-type Cu/Zn-superoxide dismutase is misfolded in cerebrospinal fluid of sporadic amyotrophic lateral sclerosis. Mol. Neurodegener. 14:42. doi: 10.1186/s13024-019-0341-5
Touzeau, G., and Kato, A. C. (1983). Effects of amyotrophic lateral sclerosis sera on cultured cholinergic neurons. Neurology 33, 317–322. doi: 10.1212/WNL.33.3.317
Touzeau, G., and Kato, A. C. (1986). ALS serum has no effect on three enzymatic activities in cultured human spinal cord neurons. Neurology 36, 573–576. doi: 10.1212/WNL.36.4.573
Van der Neut, R., Gispen, W. H., and Bar, P. R. (1991). Serum from patients with amyotrophic lateral sclerosis induces the expression of B-50/GAP-43 and neurofilament in cultured rat fetal spinal neurons. Mol. Chem. Neuropathol. 14, 247–258. doi: 10.1007/BF03159940
Varghese, A. M., Ghosh, M., Bhagat, S. K., Vijayalakshmi, K., Preethish-Kumar, V., Vengalil, S., et al. (2020). Chitotriosidase, a biomarker of amyotrophic lateral sclerosis, accentuates neurodegeneration in spinal motor neurons through neuroinflammation. J. Neuroinflammation 17:232. doi: 10.1186/s12974-020-01909-y
Varghese, A. M., Sharma, A., Mishra, P., Vijayalakshmi, K., Harsha, H. C., Sathyaprabha, T. N., et al. (2013). Chitotriosidase – a putative biomarker for sporadic amyotrophic lateral sclerosis. Clin. Proteomics 10:19. doi: 10.1186/1559-0275-10-19
Vijayalakshmi, K., Alladi, P. A., Ghosh, S., Prasanna, V. K., Sagar, B. C., Nalini, A., et al. (2011). Evidence of endoplasmic reticular stress in the spinal motor neurons exposed to CSF from sporadic amyotrophic lateral sclerosis patients. Neurobiol. Dis. 41, 695–705. doi: 10.1016/j.nbd.2010.12.005
Vijayalakshmi, K., Alladi, P. A., Sathyaprabha, T. N., Subramaniam, J. R., Nalini, A., and Raju, T. R. (2009). Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces degeneration of a cultured motor neuron cell line. Brain Res. 1263, 122–133. doi: 10.1016/j.brainres.2009.01.041
Vijayalakshmi, K., Ostwal, P., Sumitha, R., Shruthi, S., Varghese, A. M., Mishra, P., et al. (2015). Role of VEGF and VEGFR2 receptor in reversal of ALS-CSF induced degeneration of NSC-34 motor neuron cell line. Mol. Neurobiol. 51, 995–1007. doi: 10.1007/s12035-014-8757-y
Viktorov, I. V., and Bunina, T. L. (1979). [Cytotoxic effect of the serum of amyotrophic lateral sclerosis patients on spinal cord motoneurons in tissue culture]. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova 79, 169–171.
Wentling, M., Lopez-Gomez, C., Park, H.-J., Amatruda, M., Ntranos, A., Aramini, J., et al. (2019). A metabolic perspective on CSF-mediated neurodegeneration in multiple sclerosis. Brain 142, 2756–2774. doi: 10.1093/brain/awz201
Winer, L., Srinivasan, D., Chun, S., Lacomis, D., Jaffa, M., Fagan, A., et al. (2013). SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol. 70, 201–207. doi: 10.1001/jamaneurol.2013.593
Wolfgram, F., and Myers, L. (1973). Amyotrophic lateral sclerosis: effect of serum on anterior horn cells in tissue culture. Science 179, 579–580. doi: 10.1126/science.179.4073.579
Yanez, M., Galan, L., Matias-Guiu, J., Vela, A., Guerrero, A., and Garcia, A. G. (2011). CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 1423, 77–86. doi: 10.1016/j.brainres.2011.09.025
Yanez, M., Matias-Guiu, J., Arranz-Tagarro, J. A., Galan, L., Vina, D., Gomez-Pinedo, U., et al. (2014). The neuroprotection exerted by memantine, minocycline and lithium, against neurotoxicity of CSF from patients with amyotrophic lateral sclerosis, is antagonized by riluzole. Neurodegener. Dis. 13, 171–179. doi: 10.1159/000357281
Zetterstrom, P., Andersen, P. M., Brannstrom, T., and Marklund, S. L. (2011). Misfolded superoxide dismutase-1 in CSF from amyotrophic lateral sclerosis patients. J. Neurochem. 117, 91–99. doi: 10.1111/j.1471-4159.2011.07177.x
Keywords: amyotrophic lateral sclerosis, cerebrospinal fluid, motor neuron disease, neurodegeneration, pathophysiology, toxicity
Citation: Ng Kee Kwong KC, Harbham PK, Selvaraj BT, Gregory JM, Pal S, Hardingham GE, Chandran S and Mehta AR (2021) 40 Years of CSF Toxicity Studies in ALS: What Have We Learnt About ALS Pathophysiology? Front. Mol. Neurosci. 14:647895. doi: 10.3389/fnmol.2021.647895
Received: 30 December 2020; Accepted: 24 February 2021;
Published: 18 March 2021.
Edited by:
Wenting Guo, VIB-KU Leuven Center for Brain and Disease Research, BelgiumReviewed by:
Vincenzo Silani, University of Milan, ItalyJose-Luis Gonzalez De Aguilar, Université de Strasbourg, France
Copyright © 2021 Ng Kee Kwong, Harbham, Selvaraj, Gregory, Pal, Hardingham, Chandran and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arpan R. Mehta, amehta@exseed.ed.ac.uk
 Koy Chong Ng Kee Kwong
Koy Chong Ng Kee Kwong Pratap K. Harbham
Pratap K. Harbham Bhuvaneish T. Selvaraj
Bhuvaneish T. Selvaraj Jenna M. Gregory
Jenna M. Gregory Suvankar Pal
Suvankar Pal Giles E. Hardingham3,4,8
Giles E. Hardingham3,4,8  Arpan R. Mehta
Arpan R. Mehta