- 1Department of Medical Oncology, Euromedica General Clinic of Thessaloniki, Thessaloniki, Greece
- 2Department of Surgery, Queen Elizabeth Hospital, Lewisham and Greenwich NHS Trust, London, United Kingdom
- 3Department of Surgery, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 4Department of Medical Oncology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
Hereditary breast cancer accounts for 5%–10% of breast cancer cases. The majority of familial cases have been linked to germline mutations in BRCA1 and BRCA2 genes, though other high penetrance susceptibility genes have also been identified through genomic testing advances. Optimal surgical treatment for these patients, who are of a younger age, has several challenges as it usually involves aggressive therapeutic and risk reducing interventions. At the same time, the therapeutic armamentarium for BRCA1/2 mutation carriers apart from platinum salts, has been enriched with the addition of poly-ADP ribose polymerase (PARP) inhibitors with promising outcomes. In this review we provide a succinct and comprehensive overview of the surgical and systemic treatment options for patients with BRCA1/2 mutation related breast cancer and an update on the most recent systemic treatment advances.
Introduction
Breast cancer (BC) is the most common female malignancy, with more than 2 million cases being diagnosed world-wide annually (1). Hereditary syndromes account for approximately 5-10% of the cases and are associated with the presence of germ-line mutations. The majority of hereditary breast cancer cases result from mutations in BRCA1 and BRCA2 genes, whereas the rest have been linked to less frequent germline mutations in other high penetrance genes such as TP53, STK11, PTEN, CDH1, and PALB2, as well as moderate penetrance genes like ATM and CHEK2 (2).
Both BRCA1 and BRCA2 are tumour suppressor genes encoding proteins involved in homologous recombination repair (3). Pathogenic variants in both genes affect 1 in 400 persons in the general population and 1 in 40 in the Ashkenazi Jewish population. They get inherited by an autosomal dominant pattern and carry a lifetime cumulative breast cancer risk of 72% for BRCA1 and 69% for BRCA2 (4).
This review will focus on the surgical and systemic treatment of hereditary breast cancer with a particular focus on BRCA1 and BRCA2 mutations.
Surgical Treatment
Surgery on Locoregional Disease
The optimal surgical treatment for operable BC in BRCA1/2 mutation carriers depends on several factors and remains a topic of debate. Although breast conserving surgery (BCS) is the preferred surgical treatment for early stage disease in sporadic breast cancer, its oncological safety in BRCA1/2 mutation carriers has not been extensively studied. A meta-analysis of 10 studies, demonstrated a significantly higher risk for ipsilateral breast recurrence (IBR) in BCRA1/2 mutation carriers compared to non-carriers following BCS at a median follow up greater than 7 years, but no difference for shorter follow up periods (5). The risk for contralateral breast cancer was also found to be increased in BRCA1/2 mutation carriers (5). Although BCS is associated with higher IBR risk compared to mastectomy in BRCA1/2 mutation carriers, no difference was found between the two treatment options for overall survival, breast cancer death, or distant recurrence (Table 1) (5–8). Data from a meta-analysis indicate that the risk of IBR in BRCA1/2 mutation carriers who have undergone BCS was found to be reduced with adjuvant chemotherapy (RR 0.51, 95%CI 0.31–0.84) and oophorectomy (RR 0.42, 95%CI 0.22–0.81) (5).
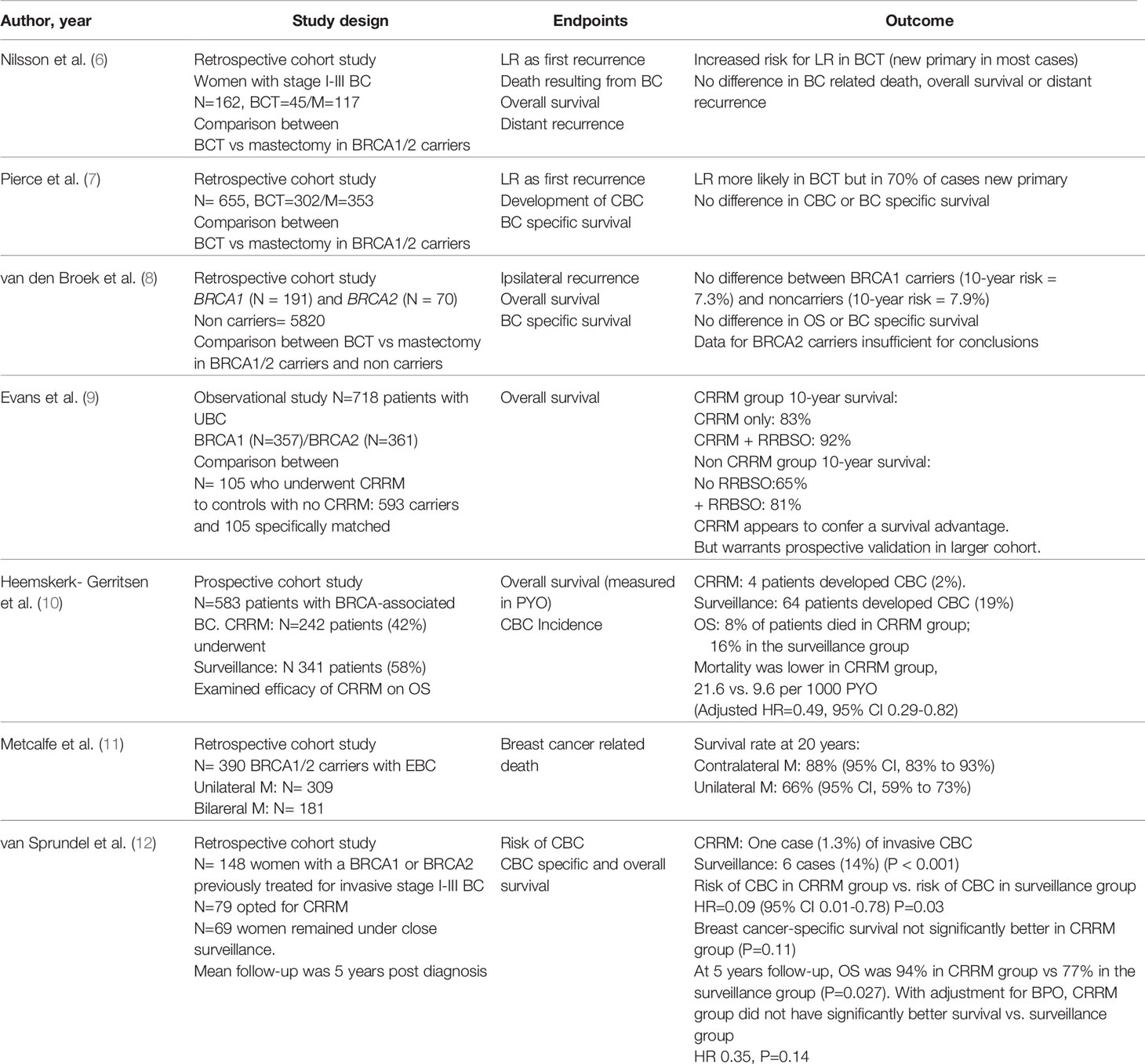
Table 1 Summary of main studies investigating the role of breast conserving surgery and risk reducing mastectomy in breast cancer patients with BRCA1/2 mutations.
BCS could be considered a safe and reasonable option for BRCA1/2 mutation carriers but this should be discussed on an individual basis and further factors need to be taken into account. These include patient’s understanding of the increased risk for an ipsilateral new primary breast cancer with all potential emotional implications, as well as their ability to undergo appropriate breast surveillance.
International guidelines recommend that early breast cancer patients carrying mutations in moderate penetrance breast cancer susceptibility genes, should be offered BCS if appropriate. However, patients carrying TP53 germline mutations should avoid BCS followed by radiation as they are at high risk of developing radiation induced malignancies such us angiosarcoma (13).
Risk Reducing Mastectomy
The term “risk reducing” has been deemed more appropriate than “prophylactic” in recent times as no mastectomy can remove all breast tissue. Several studies demonstrated a reduction in the risk of breast cancer by ~95% in BRCA1/2 mutation carriers who underwent bilateral risk reducing mastectomy (BRRM) in combination with oophorectomy and by ~90% in those with intact ovaries (14–17). A recent systematic review confirms the benefit of BRRM in reducing both incidence and mortality from breast cancer in high risk patients, such as BRCA1/2 carriers, but calls for rigorous prospective studies due to methodological flaws of the existing literature (18). Data for contralateral risk reducing mastectomy (CRRM) for patients who have had breast cancer in one breast are less conclusive as existing studies show a reduction in the incidence of contralateral breast cancer but no definitive survival benefit (Table 1) (9–12, 18).
For high risk patients such as BRCA1/2 mutation carriers, international guidelines recommend RRM with appropriate counselling on risks and benefits. When assessing the risk for developing contralateral breast cancer (CBC) the following factors need to be taken into account: age at diagnosis of primary breast cancer, family history, ability to undergo indicated surveillance imaging, prognosis from this or other malignancies, comorbidities and life expectancy (13, 19). RRM cannot completely eliminate the risk of breast cancer and can have a negative impact on body image and quality of life due to potential complications such as multiple surgeries, chronic pain, sexual dysfunction and poor cosmetic outcomes (20). Women considering this procedure should be well informed and weigh the risks and benefits compared to other alternatives such as risk reducing bilateral salpingo-oophorectomy, chemoprevention and intensive screening. For women who wish to avoid or delay RRM, MRI-based breast screening is a reasonable option (19, 21). For patients who undergo RRM, skin sparing mastectomy with or without preservation of the nipple-areolar complex has been found to be a safe option for BRCA carriers while achieving better cosmesis (22, 23).
There is a lack of data in the existing literature on the risk for CBC in breast cancer patients carrying mutations in cancer susceptibility genes other than BRCA1/2. Limited data exist for the CHEK2 1100elC frameshift mutation which is associated with a 3-fold increase in the risk of CBC (24). Decisions on CRRM for patients with moderate risk mutations should not be extrapolated from existing data on BRCA1/2, but should be balanced on several factors (age at diagnosis of primary breast cancer, family history, ability to undergo surveillance imaging) and involve appropriate patient counselling (13).
Risk Reducing Bilateral Salpingo-Oophorectomy
Risk reducing bilateral salpingo-oophorectomy (rrSBO) is recommended for female BRCA1/2 carriers who have completed childbearing and should be completed by age 35 to 40 for BRCA1, 40 to 45 for BRCA2 carriers or earlier as per patient’s relevant family history (25). It has been demonstrated that rrBSO reduces the risk for ovarian cancer by 80% and all-cause mortality by 68% in female BRCA1/2 carriers (26, 27). The beneficial effect of rrBSO on breast cancer risk reduction has also been assessed but current data are less conclusive. Some prospective studies confirmed that rrBSO reduces BC risk for both BRCA1/2 carriers (25, 28). However, a large case-control study showed a benefit for rrBSO only for BRCA1 carriers when performed before the age of 40, while a more recent study identified a benefit only for BRCA2 carriers when performed prior to 50 years old (29). Oophorectomy has been associated with a significant decrease in the risk of IBR and CBC (5).
Systemic Treatment
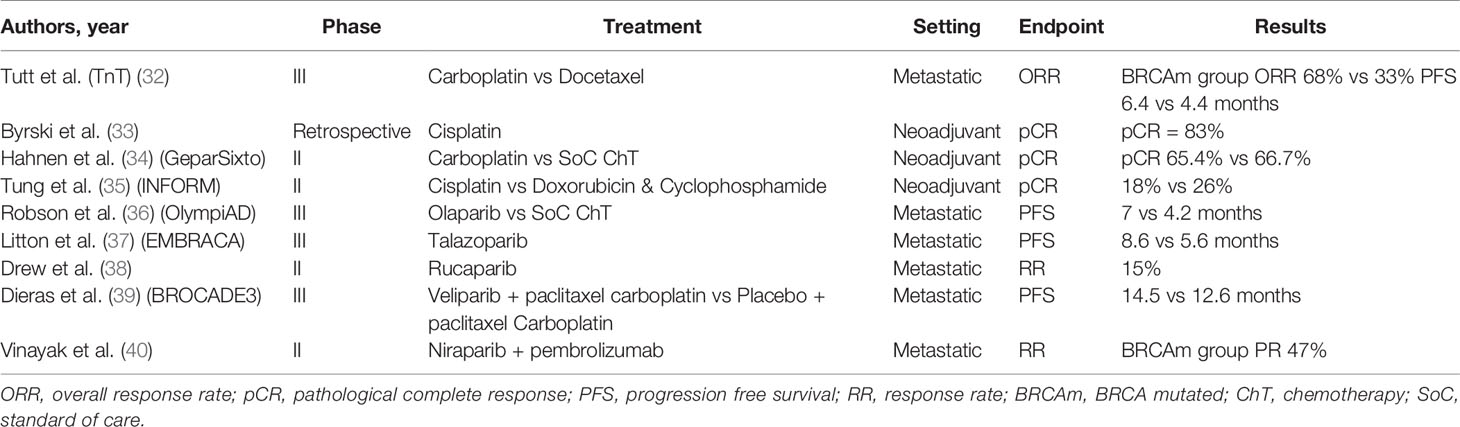
Germline mutations of BRCA1 and BRCA2 genes lead to the decreased capacity of the cell to repair double strand breaks (DSBs), as they are key elements of the homologous recombination (HR), one of the two main mechanisms of DSB repair (30, 31). This formed the basis for the development of new therapeutic strategies and the development of novel treatments for this specific breast cancer patient subgroup (Table 2).
Platinum Salts
Since the introduction of cisplatin in the 1970s, platinum compounds have been the cornerstone in the treatment of various tumour types. Platinum agents form intra-strand adducts by binding with the purines leading to DSBs. This triggers various repair mechanisms including that of homologous recombination (HR) (41). Consequently, cells with HR deficiency can be particularly sensitive to platinum compounds (42, 43).
In a small phase II open label study, 20 BRCA1 mutation carriers with metastatic breast cancer (mBC) received cisplatin 75 mg/m2 on a 3-weekly basis with 35% achieving partial response and 45% complete response with acceptable toxicity profile (44). In the Phase II TBCR009 trial, 86 previously treated triple negative mBC patients received either cisplatin or carboplatin. Response rates in the BRCA1/2 mutation carrier patient subgroup were significantly higher compared to the total study population (54% versus 26%) (45).
The triple negative breast cancer trial (TNT) was the largest trial examining the role of platinum compounds in the treatment of triple negative and BRCA1/2 mutated mBC patients. In this Phase III study, 376 mBC patients were randomised to receive first line chemotherapy with carboplatin or docetaxel. In the BRCA1/2 mutation subgroup the overall response rates were higher for the carboplatin group (68% vs 33%). Similarly, PFS was also improved in the BRCA1/2 mutation carriers who received carboplatin (6.4 vs 4.4 months) (32).
The use of platinum compounds has also been assessed in the neoadjuvant setting. In 2010, Byrski et al. reported a pathological complete response (pCR) rate of 83% for women with BRCA1 positive BC treated with neoadjuvant cisplatin (33). This was further echoed in the findings of a single arm study including 107 BC patients carrying BRCA1 mutation who were treated with 4 cycles of neoadjuvant chemotherapy with 61% achieving pCR (46).
In GeparSixto, a phase II randomised trial, triple negative stage II-III breast cancer patients were given anthracycline and taxane based neoadjuvant chemotherapy with or without carboplatin (47). In a secondary analysis, BRCA1/2 mutation carriers did not gain any additional benefits in terms of pCR with the addition of carboplatin (65.4% vs 66.7%) with similar impact on DFS. On the contrary, carboplatin conferred significant improvement in response rates to non-carriers (34). In the phase II CALBG 40603 trial, although the addition of carboplatin to NACT achieved superior pCR rates in patients with II-III triple negative BC, an improvement in long term survival outcomes was not demonstrated (48). Results from the recent randomized Phase II INFORM trial, demonstrated that in BRCA1/2 carriers with HER2 negative stage I-III BC, neoadjuvant single agent cisplatin did not achieve better pCR compared to doxorubicin and cyclophosphamide (AC) (35). All things considered, the use of platinum compounds as part of neoadjuvant chemotherapy does not clearly improve the rates of pCR in breast cancer patients carrying BRCA1/2 mutations.
PAPR Inhibitors
The concept that some genes can be “synthetically lethal” has been well known since early preclinical studies. In order for two genes to be synthetically lethal, both have to carry mutations leading to cell death. As a result, the targeting of one gene, combined with a known genetic mutation could be a tempting field for the development of new anticancer drugs (49).
Under this scope, the inhibition of single strand (SS) DNA repair with the use of the enzyme poly (ADP) ribose polymerase (PARP) inhibitors, in combination with known homologous recombination (HR) deficiency, can result in cell death (50).
Over the past 6 years multiple PARP inhibitors have been approved for the treatment of ovarian cancer (51). Olaparib is the PARP inhibitor which has been studied more extensively in breast cancer patients with BRCA1/2 mutations. In the early phase clinical trial olaparib showed efficacy in advanced solid tumors with 22 patients having breast cancer and 9 of them being BRCA1/2 mutant (52). In a proof of concept trial 54 pretreated metastatic breast cancer patients with BRCA1/2 mutation were treated with olaparib 400 mg twice daily (BD) or 100 mg BD. On the 400 mg BD arm, overall response rate was 41% and 22% in the cohort of 100 mg BD with acceptable toxicity profile (53). In another phase II basket trial 62 women with advanced breast cancer received olaparib. ORR was achieved in 13% of patients and stable disease for more than 8 weeks was observed in 47% (54). The ORR was lower in patients with previous exposure to platinum compounds suggesting that there is cross-resistance with PAPR inhibitors.
In the randomized open label phase III OlympiAD trial, olaparib 300 mg BD monotherapy was compared with standard chemotherapy (eribulin, capecitabine, gemcitabine) in 302 patients with metastatic, HER2 negative, BRCA1/2 related breast cancer. All patients had received anthracycline and taxane based chemotherapy. Median progression free survival was significantly improved for the olaparib arm (7 months vs 4.2 months). The response rates were 59.9% for the olaparib group and 28.8% for the chemotherapy group (36). Of note, olaparib was not compared to cisplatin or carboplatin.
Talazoparib is a potent PARP inhibitor which has been studied for the treatment of BRCA1/2 mutation related breast cancer. In the early clinical trial, talazoparib showed promising activity in BRCA1/2 mutation related solid tumors including patients with breast cancer (55). EMBRACA was a phase III open label clinical trial, which randomised 431 metastatic breast cancer patients with germline BRCA1/2 mutations to talazoparib or physician’s choice chemotherapy. Median PFS was significantly improved in the talazoparib arm (8.6 months vs. 5.6 months) (37). ABRAZO was a phase II, trial assessing the efficacy of talazoparib in germline BRCA1/2 mutant breast cancer patients with previous response to platinum-based chemotherapy or in patients with 3 or more previous lines of cytotoxic treatment and demonstrated promising anti-tumour activity (56).
Talazoparib has also been tested in the early breast cancer setting. After the promising results of a feasibility study in which 2 months of neoadjuvant treatment with talazoparib before the initiation of standard neoadjuvant chemotherapy, showed median decrease in tumor size of 88% (57), a separate pilot study was organized. Twenty patients with germline BRCA1/2 mutant HER2 negative breast cancer received 6 months of neoadjuvant treatment with talazoparib before proceeding with surgery. Pathological complete response was achieved in 53% of the patients with acceptable toxicity (58).
Another PARP inhibitor, rucaparib has been evaluated for the treatment of patients with metastatic breast cancer. In a phase II, open-label, multicentre trial of rucaparib in BRCA1/2 mutation carriers with advanced breast or ovarian cancer, the range of dosing schedules, safety and tolerability were assessed. The treatment schedule included intravenous and subsequently oral rucaparib. In the intravenous only schedule response rated was only 2%, with 15% on the continuous oral schedule. The authors concluded that in order to achieve optimal response continuous dosing schedule is required (38).
Veliparib has also been tested in germline BRCA1/2 mutation carrier breast cancer patients. In a phase II trial, veliparib was given as a monotherapy at 400 mg BD and at the time of progression carboplatin at a dose of AUC5 was added. Partial response rate was 17% for BRCA1 and 23% for BRCA2 mutation carries who had at least 4 cycles of follow-up (59).
Recently the results of phase III BROCADE3 trial were presented. In this trial 509 germline BRCA1/2 mutation carriers with metastatic breast cancer were randomised 2:1 to receive paclitaxel/carboplatin plus intermittent veliparib or paclitaxel/carboplatin plus placebo. Median PFS was improved by 1.9 months (14.5 vs 12.6 months) (39).
The results of a phase II open label trial of niraparib in combination with pembrolizumab were recently announced (40). In this study, 55 women with triple negative metastatic breast cancer were treated with niraparib at a dose of 200 mg once daily combined with pembrolizumab 200 mg every 3 weeks. Fifteen patients had somatic or germline BRCA1/2 mutation with 7 achieving partial response (47%).
There are no data to support the use of systemic treatments in patients with moderate-risk breast cancer susceptibility mutations. This is currently investigated in a Phase II clinical trial which explores the effectiveness of olaparib in mBC patients with somatic or germline mutations in DNA repair genes. Preliminary data shown efficacy in patient with somatic BRCA1/2 and germline PALB2 mutations but not in those with ATM or CHEK2 mutations (60).
Conclusion
Treating hereditary breast cancer entails more challenges than sporadic cases. High risk patients such as BRCA1/2 germline mutation carriers, present at a young age and their optimal surgical management yet remains an individualized and debatable area. BRCA1/2 mutation carriers face more aggressive surgical interventions for therapeutic and risk reducing purposes due to their high risk of developing primary or contralateral breast cancer. Breast conserving surgery as well as skin sparing mastectomies with or without preservation of the nipple-areolar complex have been proven to be safe and achieve better cosmesis. Selecting the best surgical approach for this patient population requires taking into account several factors including patient’s genetic risk, family history, previous BC biology, as well as patient’s own preferences.
Due to defects in homologous recombination, BRCA1/2 related BC is highly susceptible to treatment with platinum compounds. Several clinical trials demonstrated higher response rates with platinum in BRCA1/2 mutation carriers with metastatic BC. However, this finding was not replicated in the neoadjuvant setting, where an additive benefit of platinum compounds in achieving pCR has not been demonstrated for BRCA1/2 mutation carriers.
The therapeutic landscape of BRCA1/2 related breast cancer has been enriched with the addition of PARP inhibitors which led to improvements in survival outcomes. Olaparib and talazoparib have already gained regulatory approval while other such as niraparib and rucaparib and veliparib are undergoing clinical trial assessment. Combinatorial strategies involving PARP inhibitors with chemotherapy or immunotherapy are also being under investigation and hold promise for the future management of BRCA1/2 related breast cancer.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
N, number; BCT, breast conserving treatment; UBC, unilateral breast cancer; M, mastectomy; BC, breast cancer; CRRM, Contralateral risk reducing mastectomy; OS, Overall survival;CBC, contralateral breast cancer; PYO, person years of observation; LR, local recurrence; EBC, early breast cancer; BPO, bilateral salpingo-oophorectomy.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Lu HM, Li S, Black MH, Lee S, Hoiness R, Wu S, et al. Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol (2019) 5:51–7. doi: 10.1001/jamaoncol.2018.2956
3. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer (2004) 4:665–76. doi: 10.1038/nrc1431
4. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA (2017) 317:2402–16. doi: 10.1001/jama.2017.7112
5. Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat (2014) 144:443–55. doi: 10.1007/s10549-014-2890-1
6. Nilsson MP, Hartman L, Kristoffersson U, Johannsson OT, Borg A, Henriksson K, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat (2014) 147:571–8. doi: 10.1007/s10549-014-3115-3
7. Pierce LJ, Phillips K-A, Griffith KA, Buys S, Gaffney DK, Moran MS, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat (2010) 121:389–98. doi: 10.1007/s10549-010-0894-z
8. van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Oldenburg HSA, Rutgers EJ, Russell NS, et al. Prognostic Impact of Breast-Conserving Therapy Versus Mastectomy of BRCA1/2 Mutation Carriers Compared With Noncarriers in a Consecutive Series of Young Breast Cancer Patients. Ann Surg (2019) 270:364–72. doi: 10.1097/SLA.0000000000002804
9. Evans DG, Ingham SL, Baildam A, Ross GL, Lalloo F, Buchan I, et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat (2013) 140:135–42. doi: 10.1007/s10549-013-2583-1
10. Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, Ausems MG, Collée JM, Jansen L, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer (2015) 136:668–77. doi: 10.1002/ijc.29032
11. Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ (Clin Res ed.) (2014) 348:g226. doi: 10.1136/bmj.g226
12. van Sprundel TC, Schmidt MK, Rookus MA, Brohet R, van Asperen CJ, Rutgers EJ, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer (2005) 93:287–92. doi: 10.1038/sj.bjc.6602703
13. Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol (2020) 38:2080–106. doi: 10.1200/JCO.20.00299
14. Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol (2004) 22:1055–62. doi: 10.1200/JCO.2004.04.188
15. Hartmann LC, Lindor NM. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. New Engl J Med (2016) 374:454–68. doi: 10.1056/NEJMra1503523
16. Meijers-Heijboer H, van Geel B, van Putten WL, Henzen-Logmans SC, Seynaeve C, Menke-Pluymers MB, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. New Engl J Med (2001) 345:159–64. doi: 10.1056/NEJM200107193450301
17. Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst (2001) 93:1633–7. doi: 10.1093/jnci/93.21.1633
18. Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev (2018) 4:CD002748–CD002748. doi: 10.1002/14651858.CD002748.pub4
19. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: Breast and ovarian. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdfhttps://www.nccn.org/ (Accessed on February 15, 2020).
20. Gahm J, Wickman M, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer–prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast (Edinburgh Scotland) (2010) 19:462–9. doi: 10.1016/j.breast.2010.05.003
21. Warner E. Screening BRCA1 and BRCA2 Mutation Carriers for Breast Cancer. Cancers (2018) 10:477. doi: 10.3390/cancers10120477
22. Jakub JW, Peled AW, Gray RJ, Greenup RA, Kiluk JV, Sacchini V, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg (2018) 153:123–9. doi: 10.1001/jamasurg.2017.3422
23. Lanitis S, Tekkis PP, Sgourakis G, Dimopoulos N, Al Mufti R, Hadjiminas DJ. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg (2010) 251:632–9. doi: 10.1097/SLA.0b013e3181d35bf8
24. Kriege M, Hollestelle A, Jager A, Huijts PEA, Berns EM, Sieuwerts AM, et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: impact of adjuvant chemotherapy. Br J Cancer (2014) 111:1004–13.
25. Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. New Engl J Med (2002) 346:1609–15. doi: 10.1056/NEJMoa020119
26. Marchetti C, De Felice F, Palaia I, Perniola G, Musella A, Musio D, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Women’s Health (2014) 14:150. doi: 10.1186/s12905-014-0150-5
27. Finch AP, Lubinski J, Moller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol (2014) 32:1547–53. doi: 10.1200/JCO.2013.53.2820
28. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA (2010) 304:967–75. doi: 10.1001/jama.2010.1237
29. Kotsopoulos J, Huzarski T, Gronwald J, Singer CF, Moller P, Lynch HT, et al. Bilateral Oophorectomy and Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer Inst (2017) 109(1):djw177. doi: 10.1093/jnci/djw177
30. Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature (2012) 481:287–94. doi: 10.1038/nature10760
31. Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell (2001) 7:263–72. doi: 10.1016/S1097-2765(01)00174-5
32. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med (2018) 24:628–37. doi: 10.1038/s41591-018-0009-7
33. Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol (2010) 28:375–9. doi: 10.1200/JCO.2008.20.7019
34. Hahnen E, Lederer B, Hauke J, Loibl S, Kröber S, Schneeweiss A, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol (2017) 3:1378–85. doi: 10.1001/jamaoncol.2017.1007
35. Tung N, Arun B, Hacker MR, Hofstatter E, Toppmeyer DL, Isakoff SJ, et al. TBCRC 031: Randomized Phase II Study of Neoadjuvant Cisplatin Versus Doxorubicin-Cyclophosphamide in Germline BRCA Carriers With HER2-Negative Breast Cancer (the INFORM trial). J Clin Oncol (2020) 38:1539–48. doi: 10.1200/JCO.19.03292
36. Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New Engl J Med (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
37. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. New Engl J Med (2018) 379:753–63. doi: 10.1056/NEJMoa1802905
38. Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer (2016) 114:723–30. doi: 10.1038/bjc.2016.41
39. Dieras VC, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub J, et al. Phase III study of Veliparib with Carboplatin and Paclitaxel in HER2 negative advanced/metastatic gBRCA- associated breast cancer. Ann Oncol (2019) 30:v851–934. doi: 10.1093/annonc/mdz394.008
40. Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol (2019) 5:1132–40. doi: 10.1001/jamaoncol.2019.1029
41. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene (2012) 31:1869–83. doi: 10.1038/onc.2011.384
42. Garutti M, Pelizzari G, Bartoletti M, Malfatti MC, Gerratana L, Tell G, et al. Platinum Salts in Patients with Breast Cancer: A Focus on Predictive Factors. Int J Mol Sci (2019) 20(14):3390. doi: 10.3390/ijms20143390
43. Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem (2000) 275:23899–903. doi: 10.1074/jbc.C000276200
44. Byrski T, Dent R, Blecharz P, Foszczynska-Kloda M, Gronwald J, Huzarski T, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res (2012) 14:R110. doi: 10.1186/bcr3231
45. Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol (2015) 33:1902–9. doi: 10.1200/JCO.2014.57.6660
46. Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat (2014) 147:401–5. doi: 10.1007/s10549-014-3100-x
47. von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol (2014) 15:747–56. doi: 10.1016/S1470-2045(14)70160-3
48. Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol (2015) 33:13–21. doi: 10.1200/JCO.2014.57.0572
49. Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer (2005) 5:689–98. doi: 10.1038/nrc1691
50. Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol (2008) 26:3785–90. doi: 10.1200/JCO.2008.16.0812
51. Cook SA, Tinker AV. PARP Inhibitors and the Evolving Landscape of Ovarian Cancer Management: A Review. BioDrugs Clin immunotherapeutics biopharmaceuticals Gene Ther (2019) 33:255–73. doi: 10.1007/s40259-019-00347-4
52. Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. New Engl J Med (2009) 361:123–34. doi: 10.1056/NEJMoa0900212
53. Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet (London Engl) (2010) 376:235–44. doi: 10.1016/S0140-6736(10)60892-6
54. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol (2015) 33:244–50. doi: 10.1200/JCO.2014.56.2728
55. Mina L, Ramanathan R, Wainberg Z, Byers L, Chugh R, Sachdev J, et al. Abstract P2-09-02: BMN 673 is a PARP inhibitor in clinical development for the treatment of breast cancer patients with deleterious germline BRCA 1 and 2 mutations. Cancer Res (2013) 73:P2–09-02-P2-09-02. doi: 10.1158/0008-5472.SABCS13-P2-09-02
56. Turner NC, Telli ML, Rugo HS, Mailliez A, Ettl J, Grischke EM, et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). J Clin Oncol (2019) 25:2717–24. doi: 10.1158/1078-0432.CCR-18-1891
57. Litton JK, Scoggins M, Ramirez DL, Murthy RK, Whitman GJ, Hess KR, et al. A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity. NPJ Breast Cancer (2017) 3:49. doi: 10.1038/s41523-017-0052-4
58. Litton JK, Scoggins ME, Hess KR, Adrada BE, Murthy RK, Damodaran S, et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J Clin Oncol (2019) 38:388–94. doi: 10.1200/JCO.19.01304
59. Somlo G, Frankel PH, Luu TH, Ma C, Arun B, Garcia A, et al. Phase II trial of single agent PARP inhibitor ABT-888 (veliparib [vel]) followed by postprogression therapy of vel with carboplatin (carb) in patients (pts) with stage BRCA-associated metastatic breast cancer (MBC): California Cancer Consortium trial PHII-96. J Clin Oncol (2014) 32:1021–1. doi: 10.1200/jco.2014.32.15_suppl.1021
60. Tung NM, Robson ME, Ventz S, Santa-Maria CA, Marcom PK, Nanda R, et al. TBCRC 048: A phase II study of olaparib monotherapy in metastatic breast cancer patients with germline or somatic mutations in DNA damage response (DDR) pathway genes (Olaparib Expanded). J Clin Oncol (2020) 38:1002–2. doi: 10.1200/JCO.2020.38.15_suppl.1002
Keywords: genes, hereditary, breast cancer, BRCA1, BRCA2, surgical management, systemic treatment
Citation: Pouptsis A, Swafe L, Patwardhan M and Stavraka C (2020) Surgical and Systemic Treatment of Hereditary Breast Cancer: A Mini-Review With a Focus on BRCA1 and BRCA2 Mutations. Front. Oncol. 10:553080. doi: 10.3389/fonc.2020.553080
Received: 17 April 2020; Accepted: 30 September 2020;
Published: 20 October 2020.
Edited by:
Conxi Lazaro, Catalan Institute of Oncology, SpainReviewed by:
Alexandra Easson, University of Toronto, CanadaRaksha Bhat, University of Houston, United States
Copyright © 2020 Pouptsis, Swafe, Patwardhan and Stavraka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chara Stavraka, chara.stavraka@kcl.ac.uk
†These authors have contributed equally to this work
 Athanasios Pouptsis
Athanasios Pouptsis Leyla Swafe
Leyla Swafe Maneesha Patwardhan
Maneesha Patwardhan Chara Stavraka
Chara Stavraka