- Department of Haematology, Jeffrey Cheah Biomedical Centre, Wellcome Trust/MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge, United Kingdom
Zeta-chain-associated protein kinase-70 (ZAP-70) is a tyrosine kinase mainly expressed in T cells, NK cells and a subset of B cells. Primarily it functions in T cell receptor (TCR) activation through its tyrosine kinase activity. Aberrant expression of ZAP-70 has been evidenced in different B cell malignancies, with high expression of ZAP-70 in a subset of patients with Chronic Lymphocytic Leukemia (CLL), associating with unfavorable disease outcomes. Previous studies to understand the mechanisms underlying this correlation have been focused on tumor intrinsic mechanisms, including the activation of B cell receptor (BCR) signaling. Recent evidence also suggests that ZAP-70, intrinsically expressed in tumor cells, can modulate the cross-talk between malignant B cells and the immune environment, implying a more complex role of ZAP-70 in the pathogenesis of B cell malignancies. Meanwhile, the indispensible roles of ZAP-70 in T cell and NK cell activation also demonstrate that the autologous expression of ZAP-70 in the immune environment can be a central target in modulation of tumor immunity. Here we review the evidences of the link between ZAP-70 and tumor immunology in the microenvironment in B cell malignancies. Considering an emerging role of immunotherapies in treating these conditions, understanding the distinct molecular functions of ZAP-70 in a broader cellular context could ultimately benefit patient care.
Introduction
Zeta-chain-associated protein kinase-70 (ZAP-70) is a tyrosine kinase mainly expressed in T cells and NK cells (1, 2). The function of ZAP-70 in T cell receptor (TCR) activation through its tyrosine kinase activity has been well-studied through pioneering works by the Weiss laboratory and others [for review see (3)]. In the early 2000s, the aberrant high expression of ZAP-70 was identified in a subset of Chronic Lymphocytic Leukemia (CLL) patients (4), which turned out to also reflect an unfavorable clinical outcome (5). Much work has been done to establish ZAP-70 as a prognostic marker in CLL, assuming that assessment of its expression was somehow less time and labor-consuming than IGHV mutation analyses (6). However, the variation of expression levels and the lack of harmonized tests have hampered this development (7), consequently ZAP-70 expression is not routinely assessed to guide clinical decisions. Subsequent studies further revealed the expression of ZAP-70 in other B cell malignancies, such as Acute Lymphoblastic Leukemia (ALL), Burkitt-lymphoma and Mantle Cell Lymphoma (MCL) (8, 9). Although studies have shown the involvement of ZAP-70 in IgM-mediated B cell receptor (BCR) signaling in CLL, the role of ZAP-70 in the pathogenesis of CLL and other B cell malignancies is still arguable. Recently studies have implied that tumor intrinsic ZAP-70 expression modulates the cross-talk between malignant B cells and their environment, suggesting a new angle to understand the role of ZAP-70 in these diseases. We will review here how ZAP-70 expression in malignant B cells has an impact on cell migration, innate immune response, and T cell infiltration. In contrast, its expression in T cells and NK cells can affect tumor immune responses. Therefore, targeting ZAP-70 may exert anti-tumor effects not only through the modulation of signaling cascades in malignant B cells, but also through inhibition of cells resident or recruited to the tumor microenvironment.
ZAP-70 Expression in B Cell Malignancies
The expression of ZAP-70 in B cell malignancies was first detected in CLL with 20–80% of leukemic B cells having ZAP-70 expression levels equivalent to autologous CD3+ T cells in patients, correlating with unmutated IGHV gene and poor clinical outcomes (5, 6, 10, 11). Notably, the expression of ZAP-70 in CLL cells frequently varies across the entire clone and a somewhat arbitrary threshold of >20% is required to classify a patient by flow-cytometry as “ZAP-70-positive.” Importantly, the expression levels of ZAP-70 in CLL cells are relatively stable over time (6, 10, 12). The aberrant ZAP-70 expression has further been found to associate with sIgM expression in CLL (13), which further suggested an essential role of ZAP-70 in CLL pathogenesis and progression. Importantly, discordant cases of ZAP-70 expression in IGHV- mutated CLL indicated that it possesses a higher predictive value for a poor clinical outcome and therefore strongly suggest that it may actively contribute to the pathogenesis (5, 6). In addition to CLL, ZAP-70 is also expressed in a fraction of B-ALL cases, including most of the childhood pre-B cells ALL (14, 15) and adult ALL cases with different maturation phenotypes (9, 16). Notably, ZAP-70 level in ALL is associated with CD38 expression, but no correlation was observed to specific cytogenetic abnormalities (9, 17). Moreover, ZAP-70 expression was identified in a subset of other B cell malignancies, including, Follicular Lymphoma (FL), Mantle Cell Lymphoma (MCL), Hairy Cell Leukemia (HCL), and Diffuse Large B-cell Lymphoma (DLBCL) by western blotting, flow cytometry (14) and immunohistochemistry assessment (8), and in very rare cases of classic Hodgkin lymphoma (18).
The presence of ZAP-70 in subsets of B cell malignancies also with immature phenotypes may reflect their cellular origin, since ZAP-70 expression is also evidenced in normal B cells, especially developing and differentiating B cells. Using a ZAP-70 deficient mouse model, the protein was found to be expressed in pro-B and pre-B cells and to play a role in the process of pro-B to pre-B cells transition in the bone marrow through engaging in the pre-BCR complex formation (19). Notably, ZAP-70 and SYK were functionally redundant in B cell development, since only mice with both ZAP-70 and SYK deficiency displayed a complete B cell developmental block (19, 20). This finding was further supported by a study analyzing B cell populations from human bone marrow, peripheral blood, and tonsils, which found ZAP-70 expression in pro-B and pre-B cells but not in the majority of normal mature B cells (9). Notably, similar to malignant B cells, ZAP-70 expression in normal B cell populations is also modulated by phosphorylation upon BCR activation (14, 21).
Since ZAP-70 is normally not expressed in mature B cells, its expression in CLL and other mature B cell-derived neoplasms likely points to their different cellular origin (9, 15). Interestingly, point mutations in ZAP-70, which can result in the lack of ZAP-70 protein expression in human T cells, were not identified in normal human B cells and ZAP-70 negative malignant B cells (9). Therefore, the down-regulation of ZAP-70 through B cell development may represent a physiological process of B cell maturation.
The aberrant high ZAP-70 expression found in some mature B cell malignancies may be caused by epigenetic modulation and clonal evolution during tumor transformation. In CLL, hypomethylation on CpG sites in the ZAP-70 gene 5′ regulatory regions have been identified to be associated with high ZAP-70 expression and predictive of a poor disease outcome (22–24). Alternative mechanisms leading to the aberrant expression of ZAP-70 relate to tumor-microenvironment mediated induction of ZAP-70: In B cells derived from peripheral blood, which have consistently low ZAP-70 levels, BCR-activating stimuli (e.g., anti-IgM, sCD40L, IL-4, IL-6, and IL-10) upregulate the expression of ZAP-70 (14). Unmethylated CpG oligodeoxynucleotides, which can trigger an innate immune response through TLR9 activation, promote proliferation in a subset of CLL cells, accompanied by ZAP-70 induction (25, 26).
Tumor ZAP-70 Expression Modulates the Tumor- and Immune Microenvironment
Efforts have been made to understand the molecular role of tumor-intrinsic ZAP-70 expression in B cell malignancies. In CLL, ZAP-70 expression is associated with enhanced BCR signaling upon IgM activation, evidenced by a positive correlation between ZAP-70 expression, phosphorylation of SYK, BLNK, and PLCγ2 and calcium response (4, 27). Notably, the kinase activity of ZAP-70 is dispensable for BCR signaling in CLL, since the phosphorylation of ZAP-70 catalytic sites appears negligible compared to that of SYK (28). In addition an introduced mutation abrogating kinase activity of the ZAP-70 catalytic site had no significant effect on IgM-mediated BCR signaling activation (29). This suggests that the role of ZAP-70 in B cell malignancies is different from that in T cells. Interestingly, despite the dispensable nature of its kinase activity, ectopic expression of ZAP-70 in the Burkitt lymphoma line BJAB enhanced the phosphorylation and activation of BCR-related signaling cascades under conditions of IgM activation (28). These findings have led to the suggestion that ZAP-70 acts mainly as an adaptor protein to recruit downstream protein kinases, such as PI3K, c-Cbl, Cbl-b, and Shc (28). In contrast, in B-ALL, ZAP-70 is constitutively phosphorylated, suggesting the tyrosine kinase activity is continuously involved in ALL biology (16). However, the detailed role of ZAP-70 in B-ALL is still unknown.
In addition to engaging in tumor cell intrinsic signaling, likely improving the cellular fitness of tumor cells, evidence suggest that ZAP-70 expression is also involved in the cross-talk between malignant B cells and their microenvironment (Figure 1).
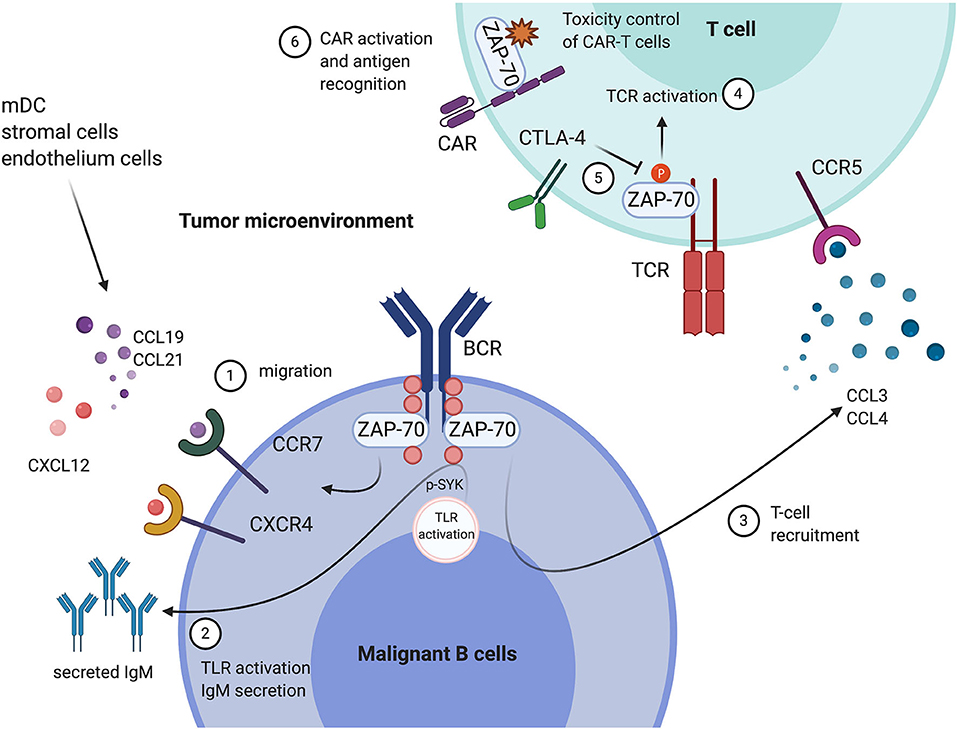
Figure 1. Tumor expression of ZAP-70 modulates the immune microenvironment, and the functional roles of ZAP-70 in environmental T cells. In Chronic Lymphocytic Leukemia (CLL) cells, ZAP-70 mediates BCR signal transduction through its kinase activity or as a scaffold protein recruiting other tyrosine kinases. In addition, ZAP-70 modulates the communication between malignant cells and their tumor microenvironment. (1) ZAP-70 is involved in the regulation of CCR7, CXCR4 expression on CLL cells, which promote the migration of tumor cells toward an environment niche, secreting CCL19, CCL21, and CXCL12; (2) ZAP-70 in CLL is engaged in TLR9 activation-mediated anti-apoptotic effects and cell proliferation, likely through mediating SYK activation and IgM secretion; (3) ZAP-70 expression in CLL cells is associated with high levels of CCL3 and CCL4 secretion, which engage in the recruitment of T cells. In T cells in the tumor microenvironment, (4) ZAP-70 expression and phosphorylation is essential for TCR-mediated T cell activation; (5) CTLA-4 is a negative regulator of ZAP-70 phosphorylation, thus suppressing T cell activation; (6) ZAP-70 expression in CAR-T cells is important for cell proliferation and antigen recognition. This Figure has been created with Biorender.com.
Cell Migration
C-C chemokine receptor type 7 (CCR7) and C-X-C chemokine receptor type 4 (CXCR4) expression on B cells is essential for cell migration and homing during B cell development through the binding to their putative chemokine ligands CCL19/CCL21 and CXCL12, respectively (30). High receptor expression on malignant B cells correlates with advanced disease stage in CLL (31), and in Diffuse Large B cell Lymphoma (DLBCL), associated with increased bone marrow infiltration and poor outcomes (32). Other studies have shown ZAP-70 expression correlates with enhanced T- and B cells migration and chemotaxis in the microenvironment. In a recent study deciphering the molecular cues which modulate inflammation-dependent oligomerization of the chemokine receptor CCR7 in dendritic- and T cells, ZAP-70 has been identified as an interactor of CCR7 under chemokine stimulation, suggesting a role of ZAP-70 in CCR7 related cell migration and chemotaxis (33). This finding is consistent with previous studies showing ZAP-70 expression in CLL cells correlates with CCR7 expression, induced by IgM-mediated ERK activation, thus enhancing the migratory ability to CCL19 and CCL21 (34, 35). A recent study has further evidenced this in CLL patients, and observed that ZAP-70 positive CLL cells migrated more to CCL19, CCL21, and CXCL12 by controlling the chemokine-driven clustering of the integrins VLA-4 and LFA-1 (36). Moreover, ZAP-70 expression also correlates with CCR7/CXCR4 expression in B cell precursor ALL disease and here promotes migration toward CCL19/CXCL12 in the central nervous system (37).
Innate Immune Responses
Besides BCR mediated signals, Toll-like receptor (TLR) signaling, which can bridge innate and adaptive immune responses, has been found to play a role in CLL activation and proliferation (38, 39). Interestingly, ZAP-70 appears to play a role to determine the environmental triggered TLR response in CLL: A recent study from our lab has elucidated that expression of ZAP-70 in CLL is strongly predictive of TLR9 agonists-mediated anti-apoptotic effects and cell proliferation, likely through mediating SYK activation, IgM secretion and Bim degradation (26).
T Cell Infiltration
Mounting evidence indicates that the ZAP-70 expressed in tumor cells has ramifications for the composition of immune cells in the microenvironment, especially for the number of infiltrating T cells. In studies comparing the immune-phenotype of ZAP-70 positive and ZAP-70 negative CLL patients, tumor ZAP-70 expression was associated with increased CD4 central memory T cells and CD3/CD69+ T cells with decreased CD4/CD8 ratio in the peripheral blood (40–42). However, since high ZAP-70 expression is normally observed in only a subpopulation of CLL cells and varies substantially between patients, it is possible that subtle changes in different T cell populations between ZAP-70 positive and negative patients are partly impacted by inconsistencies in the definition of ZAP-70 positive in these studies. Interestingly, studies have evidenced that CLL cells secrete the C-C motif chemokine ligands, CCL3 and CCL4, which enable the recruitment of T cells and monocytes, under the stimulation of IgM and in co-culture with nurse-like cells (NLC) (43). In addition, in CLL, ZAP-70 positive patients have significantly higher CCL3 and CCL4 plasma levels (43), and CCL3 plasma levels correlate with other risk factors (44). These findings suggest a potential role of tumor autologous ZAP-70, mediating immune-responses and fostering a tumor-supportive microenvironment through modulation of the expression of T cell chemokines.
ZAP-70 Expression in T Cells and NK Cells, and Their Roles in B Cell Malignancies
Immunotherapy, including checkpoint blockade inhibition and cell-based immunotherapies, is a fast developing area in cancer treatment. Such treatment modalities have been applied to treat B cell malignancies and demonstrated significant improved outcomes in smaller subsets of patients, who previously relapsed from chemotherapies and targeted therapies (45). Notably, despite showing some promising effects, the molecular mechanisms that inhibit T cell and NK cell activation in B cell malignancies and block anti-tumor immunity are far from being comprehensively described.
Because of its indispensible role for TCR activation, deficiency, or aberrantly high expression of ZAP-70 in T cells expectedly result in immune deficiency [ZAP-70-related severe combined immunodeficiency syndromes (SCID)] (46) and autoimmunity, respectively (47). To date, it remains an important but unanswered question whether ZAP-70 expression levels in T and NK cells are associated with patient responses to immunotherapies, but an increasing amount of evidence suggests that ZAP-70 deficiency or inhibition can contribute to impaired tumor-surveillance (Figure 2).
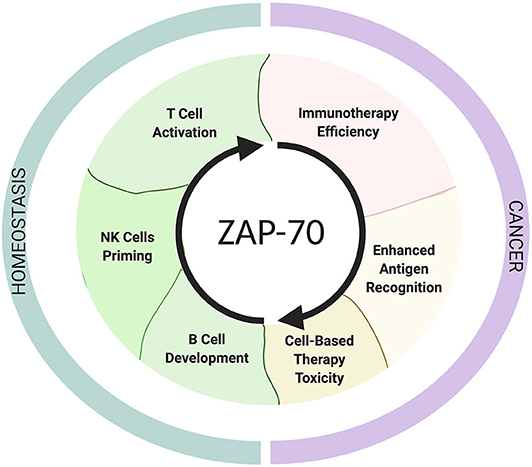
Figure 2. The roles of ZAP-70 in homeostasis and cancer. The schematic shows ZAP-70 involvement in physiological contexts and in cancer. This Figure has been created with Biorender.com.
T Cells
Structurally, ZAP-70 has been found to play a central role in immunological synapse formation in cytotoxic T lymphocytes (CTL) (48). CTLA-4 is a well-established inhibitory checkpoint for T cell activation (49). It has been suggested that the inhibition of ZAP-70 tyrosine-phosphorylation is a mechanism of CTLA-4 mediated suppression of CD4+ T activation, indicating a central role of ZAP-70 kinase activity in T cell activation and anti-tumor immune responses (50). In a recent study the GTPase-activating protein (GAP) Rasal1, which inhibits ZAP-70, has been identified to suppress anti-tumor immune-responses. Antagonizing ZAP-70 inhibition by siRNA against Rasal1 increased the number of CD8+ tumor-infiltrating T-cells expressing granzyme B and interferon gamma (no ‘1') and enhanced tumor killing (51). Notably, in a DLBCL case, ZAP-70 deficiency caused complete ablation of the CD8 population in the tumor environment (52), suggesting a profound effect of ZAP-70 in tumor immune-responses.
NK Cells
Recently, NK cells have been applied for cancer-immunotherapies, benefitting from its antigen-independent host immune responses and cytotoxicity against malignant cells (53). ZAP-70 is a kinase that is also involved in the activation of NK cells upon engaging with ligands on targeted cells (54) and downregulation of ZAP-70 is associated with inhibition of NK cell responses under prolonged activation and continuous DNA damage stress (55). A very recent study revealed that ZAP-70 is engaged in immunomodulatory drug pomalidomide induced granzyme-B secretion and cytolytic activity of NK cells (56). However, ZAP-70-independent pathways exist which modulate NK cell mediated cytotoxicity, primarily through signaling modulated by non-ITAM-based receptors, like NKG2D (57). It has also been described that NK cells from SYK−/− ZAP-70−/− mice still maintain natural cytotoxicity, which suggests a redundant role of ZAP-70 in this process, despite driving the activation of NK cell receptor signaling (58).
CAR-T Cells
Chimeric antigen receptor T cells (CAR-T) are T cells expressing artificial T cell receptors which contain both tumor specific- as well as T cell activating motifs (59). Promising results from clinical trials had led to several CAR-T cell therapies approved by the United States Food and Drug Administration and European Medicines Agency for treating relapsed or refractory B cell malignancies (60). In spite of the similarity between chimeric antigen receptors (CARs) and natural TCRs, reduced efficiency of antigen-recognition and affinity remain major issues in CAR-T cell therapies. Third generation CAR-T cells are potentially more efficient than second generation through engaging additional co-stimulatory molecules. Evidence from a comparative study indicates that activation and phosphorylation of ZAP-70 in CAR-T cells is associated with enhanced cell proliferation and expansion of third generation CARs, containing both CD28 and 4-1BB motifs, compared to second generation CARs (61). The importance of ZAP-70 in CAR-T activation has been further addressed by a very recent study: Using quantitative single-molecule live-cell imaging, CAR-T cells have been shown to have ~1,000 times reduced antigen sensitivity compared to normal T cells, and data suggest that the underlying mechanism relates to reduced recruitment of ZAP-70 to CARs. This study enlightens the importance of ZAP-70 in CAR-T activation and suggests it as a promising target for improving CAR-T antigen recognition (62).
ZAP-70 as Therapeutic Target
Considering the importance of ZAP-70 in T cell and NK cell activation, great effort has been put to target ZAP-70 in order to control diseases derived from abnormal T or NK cell activation, such as immune disorders and autoimmune diseases (3, 63). ZAP-70 has been found not only to function through its kinase activity, but also as an important scaffold protein to associate with TCR or BCR related molecules, independent of its catalytic activity (28, 64), suggesting that kinase-inhibition may not completely abolish protein function. Several in vitro studies have previously investigated inhibitors which can suppress ZAP-70 kinase activity or disrupt its protein-binding ability to access downstream TCR related activators (65, 66). These inhibitors have been well-described in a recent review (63).
Although the expression of ZAP-70 in tumor cells has been linked to a dismal outcome, there have only been few attempts to inhibit ZAP-70 as a treatment, partly because the biological functions of ZAP-70 in B cell malignancies remain elusive. Tyrosine kinase inhibitors have been assessed to treat ZAP-70 positive CLL, for example, gefitinib has been tested for inducing apoptosis of ZAP-70 positive CLL cells and cell lines in vitro. These studies demonstrated that gefitinib inhibits the basal and BCR activation-mediated phosphorylation of ZAP-70 at the micromolar level and that ZAP-70 expression sensitizes cells to gefitinib induced cell apoptosis (67). However, it is arguable whether these pro-apoptotic effects of gefitinib were achieved through the inhibition of ZAP-70 or other related tyrosine kinases, such as SYK.
While ZAP-70 constitutes an interesting and attractive target for therapeutic interventions in cancer patients, especially in those with aberrant expression in B cell malignancies, the simultaneous inhibition of T and NK cells appears to be inevitable and may be less desirable and potentially even harmful. While T cell subsets may promote tumorigenesis (e.g., through CD40 stimulation) and their inhibition may therefore be therapeutically beneficial, blockage of cytotoxic T cells and NK cells may be less so. Whether different immune cells display different susceptibilities to ZAP-70 inhibition, thus allowing for a wide-enough therapeutic window of antagonists to be beneficial, is unknown, but at least seems possible. We believe this is a substantial problem to be considered in the design of ZAP-70 directed therapies.
Immunotherapies, including CAR-T cells, immune checkpoint blockade, and adaptive T cell therapies, have been applied in clinical treatment for B cell malignancies. The safe and precise control of over-reactions of anti-tumor immune responses has been a major issue for the toxicity of cell-based immunotherapies (68). Based on the essential role of ZAP-70 in TCR activation, some studies suggest targeting ZAP-70 in order to control effector T cells, which could potentially be applied for developing safer adaptive T cell therapies (Figure 1). A recent study defined ZAP-70 as a target to control the toxicity caused by over-reacting CAR-T cells such as cytokine release syndrome (CRS). Dasatinib, a tyrosine kinase inhibitor has been found to attenuate CAR-T toxicity by suppressing ZAP-70 activation (69). However, inhibition of other kinases by dasatinib, such as Abl and Src tyrosine kinases, may likely contribute. An engineered ZAP-70 construct has been established by the Weiss lab to specifically study the role and requirement of ZAP-70 kinase activity in different biological processes. The so-called analog-sensitive ZAP-70 mutant (ZAP-70 AS), which contains an engineered binding pocket around the kinase domain, sensitive to an analog of the small molecule kinase inhibitor PP1, conserves the normal ZAP-70 catalytic activity and can be specifically inhibited (70). This specificity has a great potential to be applied for the safe control of adaptive cell-based immunotherapies (71).
Conclusion
ZAP-70 is not only critical for T cell and NK cell activation, but also associated with poor outcomes of B cell malignancies, especially in CLL. Tumor intrinsic expression of ZAP-70 in B cell malignancies has been shown to enhance cellular signals under ligand stimulated BCR activation. However, the underlying mechanisms known so far cannot fully explain the correlation between ZAP-70 expression and dismal outcome. More evidence has pointed to ZAP-70 driven environmental changes, which may play a central role for triggering innate immune responses and immune cell infiltration (Figure 1 and Table 1).
Growing evidence also indicates that the modulation of ZAP-70 activity can be applied to control T cell activation, which has translational potential to mitigate the toxicity associated with cell-based immunotherapies (Figure 1). However, since most of the evidence has only been compiled from in vitro experiments, more in vivo studies are needed to fully characterize whether such therapies can be applied in a clinical setting.
A thorough review of the published evidences focusing on defining the role of ZAP-70 in health and disease clearly indicates that it remains an attractive target for therapeutic interventions, more than ever. More experimental evidence is needed to fully understand the biology behind ZAP-70 in B cell malignances in a holistic cellular approach. The simultaneous targeting of ZAP-70 in tumor cells, T and NK cells, may be beneficial in some instances, but also bears the risk to promote tumor growth through impairing immune surveillance.
Author Contributions
JC and IR designed and wrote this review. AM provided critical editing on the manuscript and the graphs. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Cancer Research UK (CRUK; C49940/A17480). IR is a senior CRUK fellow. JC is funded by the Kay Kendall Foundation (KKLF) (KKL1070).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Esther Baena for supporting the graphical design of Figure 2.
References
1. Brumbaugh KM, Binstadt BA, Billadeau DD, Schoon RA, Dick CJ, Ten RM, et al. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J Exp Med. (1997) 186:1965–74. doi: 10.1084/jem.186.12.1965
2. Chan AC, Irving BA, Fraser JD, Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci USA. (1991) 88:9166–70. doi: 10.1073/pnas.88.20.9166
3. Au-Yeung BB, Shah NH, Shen L, Weiss A. ZAP-70 in signaling, biology, and disease. Annu Rev Immunol. (2018) 36:127–56. doi: 10.1146/annurev-immunol-042617-053335
4. Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. (2002) 100:4609–14. doi: 10.1182/blood-2002-06-1683
5. Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. (2003) 101:4944–51. doi: 10.1182/blood-2002-10-3306
6. Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. (2004) 351:893–901. doi: 10.1056/NEJMoa040857
7. Vroblova V, Smolej L, Krejsek J. Pitfalls and limitations of ZAP-70 detection in chronic lymphocytic leukemia. Hematology. (2012) 17:268–74. doi: 10.1179/1607845412Y.0000000015
8. Carreras J, Villamor N, Colomo L, Moreno C, Ramon y Cajal S, Crespo M, et al. Immunohistochemical analysis of ZAP-70 expression in B-cell lymphoid neoplasms. J Pathol. (2005) 205:507–13. doi: 10.1002/path.1727
9. Crespo M, Villamor N, Gine E, Muntanola A, Colomer D, Marafioti T, et al. ZAP-70 expression in normal pro/pre B cells, mature B cells, and in B-cell acute lymphoblastic leukemia. Clin Cancer Res. (2006) 12:726–34. doi: 10.1158/1078-0432.CCR-05-1531
10. Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. (2003) 348:1764–75. doi: 10.1056/NEJMoa023143
11. Orchard JA, Ibbotson RE, Davis Z, Wiestner A, Rosenwald A, Thomas PW, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. (2004) 363:105–11. doi: 10.1016/S0140-6736(03)15260-9
12. Durig J, Nuckel H, Cremer M, Fuhrer A, Halfmeyer K, Fandrey J, et al. ZAP-70 expression is a prognostic factor in chronic lymphocytic leukemia. Leukemia. (2003) 17:2426–34. doi: 10.1038/sj.leu.2403147
13. Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. (2003) 101:1087–93. doi: 10.1182/blood-2002-06-1822
14. Scielzo C, Camporeale A, Geuna M, Alessio M, Poggi A, Zocchi MR, et al. ZAP-70 is expressed by normal and malignant human B-cell subsets of different maturational stage. Leukemia. (2006) 20:689–95. doi: 10.1038/sj.leu.2404138
15. Wandroo F, Bell A, Darbyshire P, Pratt G, Stankovic T, Gordon J, et al. ZAP-70 is highly expressed in most cases of childhood pre-B cell acute lymphoblastic leukemia. Int J Lab Hematol. (2008) 30:149–57. doi: 10.1111/j.1751-553X.2007.00915.x
16. Guillaume N, Alleaume C, Munfus D, Capiod JC, Touati G, Pautard B, et al. ZAP-70 tyrosine kinase is constitutively expressed and phosphorylated in B-lineage acute lymphoblastic leukemia cells. Haematologica. (2005) 90:899–905. Available online at: https://www.haematologica.org/article/view/3587
17. Chakupurakal G, Bell A, Griffiths M, Wandroo F, Moss P. Analysis of ZAP70 expression in adult acute lymphoblastic leukaemia by real time quantitative PCR. Mol Cytogenet. (2012) 5:22. doi: 10.1186/1755-8166-5-22
18. Sup SJ, Domiati-Saad R, Kelley TW, Steinle R, Zhao X, Hsi ED. ZAP-70 expression in B-cell hematologic malignancy is not limited to CLL/SLL. Am J Clin Pathol. (2004) 122:582–7. doi: 10.1309/WVQPVDF8UF7AV21X
19. Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. (2003) 18:523–33. doi: 10.1016/S1074-7613(03)00082-7
20. Fallah-Arani F, Schweighoffer E, Vanes L, Tybulewicz VL. Redundant role for Zap70 in B cell development and activation. Eur J Immunol. (2008) 38:1721–33. doi: 10.1002/eji.200738026
21. Nolz JC, Tschumper RC, Pittner BT, Darce JR, Kay NE, Jelinek DF. ZAP-70 is expressed by a subset of normal human B-lymphocytes displaying an activated phenotype. Leukemia. (2005) 19:1018–24. doi: 10.1038/sj.leu.2403726
22. Claus R, Lucas DM, Ruppert AS, Williams KE, Weng D, Patterson K, et al. Validation of ZAP-70 methylation and its relative significance in predicting outcome in chronic lymphocytic leukemia. Blood. (2014) 124:42–8. doi: 10.1182/blood-2014-02-555722
23. Claus R, Lucas DM, Stilgenbauer S, Ruppert AS, Yu L, Zucknick M, et al. Quantitative DNA methylation analysis identifies a single CpG dinucleotide important for ZAP-70 expression and predictive of prognosis in chronic lymphocytic leukemia. J Clin Oncol. (2012) 30:2483–91. doi: 10.1200/JCO.2011.39.3090
24. Corcoran M, Parker A, Orchard J, Davis Z, Wirtz M, Schmitz OJ, et al. ZAP-70 methylation status is associated with ZAP-70 expression status in chronic lymphocytic leukemia. Haematologica. (2005) 90:1078–88. Available online at: https://haematologica.org/article/view/3628
25. Bekeredjian-Ding I, Doster A, Schiller M, Heyder P, Lorenz HM, Schraven B, et al. TLR9-activating DNA up-regulates ZAP70 via sustained PKB induction in IgM+ B cells. J Immunol. (2008) 181:8267–77. doi: 10.4049/jimmunol.181.12.8267
26. Wagner M, Oelsner M, Moore A, Gotte F, Kuhn PH, Haferlach T, et al. Integration of innate into adaptive immune responses in ZAP-70-positive chronic lymphocytic leukemia. Blood. (2016) 127:436–48. doi: 10.1182/blood-2015-05-646935
27. Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. (2005) 105:2036–41. doi: 10.1182/blood-2004-05-1715
28. Gobessi S, Laurenti L, Longo PG, Sica S, Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. (2007) 109:2032–9. doi: 10.1182/blood-2006-03-011759
29. Chen L, Huynh L, Apgar J, Tang L, Rassenti L, Weiss A, et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. (2008) 111:2685–92. doi: 10.1182/blood-2006-12-062265
30. McHeik S, Van Eeckhout N, De Poorter C, Gales C, Parmentier M, Springael JY. Coexpression of CCR7 and CXCR4 during B cell development controls CXCR4 responsiveness and bone marrow homing. Front Immunol. (2019) 10:2970. doi: 10.3389/fimmu.2019.02970
31. Ghobrial IM, Bone ND, Stenson MJ, Novak A, Hedin KE, Kay NE, et al. Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma. Mayo Clin Proc. (2004) 79:318–25. doi: 10.4065/79.3.318
32. Du H, Zhang L, Li G, Liu W, Tang W, Zhang H, et al. CXCR4 and CCR7 expression in primary nodal diffuse large B-cell lymphoma-A clinical and immunohistochemical study. Am J Med Sci. (2019) 357:302–10. doi: 10.1016/j.amjms.2019.01.008
33. Hauser MA, Schaeuble K, Kindinger I, Impellizzieri D, Krueger WA, Hauck CR, et al. Inflammation-induced CCR7 oligomers form scaffolds to integrate distinct signaling pathways for efficient cell migration. Immunity. (2016) 44:59–72. doi: 10.1016/j.immuni.2015.12.010
34. Calpe E, Codony C, Baptista MJ, Abrisqueta P, Carpio C, Purroy N, et al. ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. (2011) 118:4401–10. doi: 10.1182/blood-2011-01-333682
35. Richardson SJ, Matthews C, Catherwood MA, Alexander HD, Carey BS, Farrugia J, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL). Blood. (2006) 107:3584–92. doi: 10.1182/blood-2005-04-1718
36. Laufer JM, Lyck R, Legler DF. ZAP70 expression enhances chemokine-driven chronic lymphocytic leukemia cell migration and arrest by valency regulation of integrins. FASEB J. (2018) 32:4824–35. doi: 10.1096/fj.201701452RR
37. Alsadeq A, Fedders H, Vokuhl C, Belau NM, Zimmermann M, Wirbelauer T, et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica. (2017) 102:346–55. doi: 10.3324/haematol.2016.147744
38. Arvaniti E, Ntoufa S, Papakonstantinou N, Touloumenidou T, Laoutaris N, Anagnostopoulos A, et al. Toll-like receptor signaling pathway in chronic lymphocytic leukemia: distinct gene expression profiles of potential pathogenic significance in specific subsets of patients. Haematologica. (2011) 96:1644–52. doi: 10.3324/haematol.2011.044792
39. Decker T, Schneller F, Kronschnabl M, Dechow T, Lipford GB, Wagner H, et al. Immunostimulatory CpG-oligonucleotides induce functional high affinity IL-2 receptors on B-CLL cells: costimulation with IL-2 results in a highly immunogenic phenotype. Exp Hematol. (2000) 28:558–68. doi: 10.1016/S0301-472X(00)00144-2
40. Correia RP, Matos ESFA, Bacal NS, Campregher PV, Hamerschlak N, Amarante-Mendes GP. ZAP-70 expression is associated with increased CD4 central memory T cells in chronic lymphocytic leukemia: cross-sectional study. Hematol Transfus Cell Ther. (2018) 40:317–25. doi: 10.1016/j.htct.2018.03.008
41. Grywalska E, Bartkowiak-Emeryk M, Pasiarski M, Olszewska-Bozek K, Mielnik M, Podgajna M, et al. Relationship between the expression of CD25 and CD69 on the surface of lymphocytes T and B from peripheral blood and bone marrow of patients with chronic lymphocytic leukemia and established prognostic factors of this disease. Adv Clin Exp Med. (2018) 27:987–99. doi: 10.17219/acem/74437
42. Rivkina A, Holodnuka-Kholodyuk I, Murovska M, Soloveichika M, Lejniece S. Peripheral blood lymphocyte phenotype of ZAP-70(+) and ZAP-70(-) patients with B-cell chronic lymphocytic leukaemia. Exp Oncol. (2015) 37:73–6. doi: 10.31768/2312-8852.2015.37(1):73-76
43. Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. (2009) 113:3050–8. doi: 10.1182/blood-2008-07-170415
44. Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, et al. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood. (2011) 117:1662–9. doi: 10.1182/blood-2010-09-307249
45. Kansara RR, Speziali C. Immunotherapy in hematologic malignancies. Curr Oncol. (2020) 27:S124–31. doi: 10.3747/co.27.5117
46. Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. (1994) 264:1596–9. doi: 10.1126/science.8202712
47. Chan AY, Punwani D, Kadlecek TA, Cowan MJ, Olson JL, Mathes EF, et al. A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP-70. J Exp Med. (2016) 213:155–65. doi: 10.1084/jem.20150888
48. Jenkins MR, Stinchcombe JC, Au-Yeung BB, Asano Y, Ritter AT, Weiss A, et al. Distinct structural and catalytic roles for Zap70 in formation of the immunological synapse in CTL. eLife. (2014) 3:e01310. doi: 10.7554/eLife.01310.018
49. Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. (1995) 182:459–65. doi: 10.1084/jem.182.2.459
50. Guntermann C, Alexander DR. CTLA-4 suppresses proximal TCR signaling in resting human CD4(+) T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a potential role for tyrosine phosphatases. J Immunol. (2002) 168:4420–9. doi: 10.4049/jimmunol.168.9.4420
51. Thaker YR, Raab M, Strebhardt K, Rudd CE. GTPase-activating protein Rasal1 associates with ZAP-70 of the TCR and negatively regulates T-cell tumor immunity. Nat Commun. (2019) 10:4804. doi: 10.1038/s41467-019-12544-4
52. Newell A, Dadi H, Goldberg R, Ngan BY, Grunebaum E, Roifman CM. Diffuse large B-cell lymphoma as presenting feature of Zap-70 deficiency. J Allergy Clin Immunol. (2011) 127:517–20. doi: 10.1016/j.jaci.2010.09.016
53. Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. (2015) 14:487–98. doi: 10.1038/nrd4506
54. Chiesa S, Mingueneau M, Fuseri N, Malissen B, Raulet DH, Malissen M, et al. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. (2006) 107:2364–72. doi: 10.1182/blood-2005-08-3504
55. Pugh JL, Nemat-Gorgani N, Norman PJ, Guethlein LA, Parham P. Human NK cells downregulate Zap70 and syk in response to prolonged activation or DNA damage. J Immunol. (2018) 200:1146–58. doi: 10.4049/jimmunol.1700542
56. Hideshima T, Ogiya D, Liu J, Harada T, Kurata K, Bae J, et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia. (2020). doi: 10.1038/s41375-020-0809-x. [Epub ahead of print].
57. Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, et al. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. (2003) 4:565–72. doi: 10.1038/ni930
58. Colucci F, Schweighoffer E, Tomasello E, Turner M, Ortaldo JR, Vivier E, et al. Natural cytotoxicity uncoupled from the Syk and ZAP-70 intracellular kinases. Nat Immunol. (2002) 3:288–94. doi: 10.1038/ni764
59. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548
60. Halim L, Maher J. CAR T-cell immunotherapy of B-cell malignancy: the story so far. Ther Adv Vaccines Immunother. (2020) 8:2515135520927164. doi: 10.1177/2515135520927164
61. Karlsson H, Svensson E, Gigg C, Jarvius M, Olsson-Stromberg U, Savoldo B, et al. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS ONE. (2015) 10:e0144787. doi: 10.1371/journal.pone.0144787
62. Gudipati V, Rydzek J, Doel-Perez I, Goncalves VDR, Scharf L, Konigsberger S, et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat Immunol. (2020) 21:848–56. doi: 10.1038/s41590-020-0719-0
63. Kaur M, Singh M, Silakari O. Insight into the therapeutic aspects of 'Zeta-Chain associated protein kinase 70 kDa' inhibitors: a review. Cell Signal. (2014) 26:2481–92. doi: 10.1016/j.cellsig.2014.06.017
64. Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. (2010) 11:1085–92. doi: 10.1038/ni.1955
65. Nishikawa K, Sawasdikosol S, Fruman DA, Lai J, Songyang Z, Burakoff SJ, et al. A peptide library approach identifies a specific inhibitor for the ZAP-70 protein tyrosine kinase. Mol Cell. (2000) 6:969–74. doi: 10.1016/S1097-2765(05)00085-7
66. Visperas PR, Wilson CG, Winger JA, Yan Q, Lin K, Arkin MR, et al. Identification of inhibitors of the association of ZAP-70 with the T cell receptor by high-throughput screen. SLAS Discov Adv Life Sci R D. (2017) 22:324–31. doi: 10.1177/1087057116681407
67. Dielschneider RF, Xiao W, Yoon JY, Noh E, Banerji V, Li H, et al. Gefitinib targets ZAP-70-expressing chronic lymphocytic leukemia cells and inhibits B-cell receptor signaling. Cell Death Dis. (2014) 5:e1439. doi: 10.1038/cddis.2014.391
68. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
69. Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Translat Med. (2019) 11:eaau5907. doi: 10.1126/scitranslmed.aau5907
70. Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. (2008) 283:15419–30. doi: 10.1074/jbc.M709000200
Keywords: ZAP-70, tumor microenvironment, immunotherapy, B cell lymphoma, CLL
Citation: Chen J, Moore A and Ringshausen I (2020) ZAP-70 Shapes the Immune Microenvironment in B Cell Malignancies. Front. Oncol. 10:595832. doi: 10.3389/fonc.2020.595832
Received: 17 August 2020; Accepted: 17 September 2020;
Published: 27 October 2020.
Edited by:
Jérôme Paggetti, Luxembourg Institute of Health, LuxembourgReviewed by:
Elisa Ten Hacken, Dana–Farber Cancer Institute, United StatesMarek Mraz, Central European Institute of Technology (CEITEC), Czechia
Copyright © 2020 Chen, Moore and Ringshausen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingo Ringshausen, ir279@cam.ac.uk
 Jingyu Chen
Jingyu Chen Andrew Moore
Andrew Moore Ingo Ringshausen
Ingo Ringshausen