- 1Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 3Department of Medicine, Faculty of Medicine, Chiangmai University, Chiang Mai, Thailand
- 4Department of Medicine, Faculty of Medicine, Khon Kaen Hospital. Khon Kaen University, Khon Kaen, Thailand
- 5Department of Medicine, Faculty of Medicine, Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand
- 6Department of Medicine, Rajavithi Hospital, Bangkok, Thailand
- 7Holistic Center for Cancer Study and Care (HOCC-PSU) and Department of Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- 8 Department of Medicine, Faculty of Medicine, Chulalongkorn University and The King Chulalongkorn Memorial Hospital, Bangkok, Thailand
Introduction: The mainstay systemic treatment for non-oncogenic addictive advanced stage non-small cell lung cancer is chemotherapy. Anti-angiogenic agents are additive compounds that enhance disease control and lead to improvement of overall survival benefit. Recently PD-(L)1 blockage, a checkpoint inhibitor, has been adopted as another line of treatment. A sequential strategy to enhance the efficacy of combination docetaxel and nintedanib after immunotherapy, correlated with genomic mutation, has been explored.
Method: A retrospective cohort study of 56 patients from 8 centers in Thailand who received combination docetaxel and nintedanib via the Thai nintedanib Named Patient Use program was conducted. Demographic characteristics, treatment details, and treatment responses were retrieved from medical records.
Results: The majority of patients were male (62.5%) with adenocarcinoma subtype (88%). Thirty-five percent had sensitizing EGFR mutation. Combination docetaxel and nintedanib was given as second to fourth line of treatment. Median PFS of docetaxel/nintedanib was 5.6 months [95% CI 4.8-6.9]. Median OS of the entire cohort was 22.5 months [95% CI 20.2-31.1]. Among them, only four patients received this combination after immunotherapy which limited the validity of efficacy analysis. Median PFS of those four patients was 7.9 months [range 5.2-9.1] which was slightly higher than the remaining cohort (median PFS 4.5 months, 95% CI: 4.0-6.0, p-value 0.09). Among the adenocarcinoma subtype, a relapse-time of platinum-doublet chemotherapy of more than 6 months was solely indicated as a benefit of combination docetaxel/nintedanib treatment compared to the relapse-time of platinum-doublet chemotherapy of less than 6 months by multivariate HR of PFS 0.32 [95% CI: 0.14-0.68, p-value 0.003].
Conclusion: Combination docetaxel and nintedanib provided more benefit in relapse-time of platinum-doublet chemotherapy of more than 6 months in advanced stage adenocarcinoma lung cancer. Neither EGFR nor ALK alteration influenced the outcome of treatment.
Introduction
The development of current standard treatments of advanced non-small cell lung cancer has led to the improvement of survival outcome. Novel strategies adopting predictive biomarkers have guided treatment towards an era of precision medicine. Biomarker discoveries to define more targeted therapies have been explored in many clinical studies. For non-targetable advanced non-small cell lung cancer, which has no targeted therapy option, there seems to be fewer treatment opportunities and worse prognosis outcome (1). Combination antiangiogenic therapies and chemotherapy has improved the efficacy of treatment in non-small cell carcinoma lung cancer by normalizing abnormal tumor vasculature and enhancing tumor shrinkage. A combination of docetaxel/nintedanib was approved by the USFDA as a subsequent treatment after platinum-resistance in advanced adenocarcinoma lung cancer patients. Significant improvement in progression-free survival (PFS) with a median of 3.4 months vs. 2.7 months compared to placebo and docetaxel has been shown in the phase III LUME-lung 1 global study (2). Furthermore, PD-(L)1 blockage, a novel immunotherapy, has shown benefits for improving survival outcomes, either by monotherapy or combination with chemotherapy (3–5). Chemotherapy enhances the effect of immunotherapy by increasing recognition, eliminating tumor cells by the host immune system, and reducing the immunosuppressive microenvironment (6). Furthermore, preclinical reports revealed that VEGFR blockage inhibits suppressive immune cells (MDSC, Treg, macrophages) and increases mature dendritic cell results in delayed tumor growth (7, 8). Combination anti-angiogenic and PD-(L)1 blockage has shown significant tumor control (9). Sequence of immunotherapy before subsequent docetaxel/nintedanib treatment has also shown improved response to treatment in a retrospective cohort (10, 11).
The Thai non-squamous cell carcinoma of the lung has up to 57% predominated EGFR mutation (12), contrary to the Western non-squamous lung cancer population, which has less than 10% prevalence of EGFR mutation. Comparing the efficacy in our country to a global study that enrolled a majority of Caucasian patients might help us to understand the real benefits of treatment of Asian patients. We report a retrospective cohort study of advanced stage non-small lung cancer patients who received subsequent treatment of docetaxel/nintedanib after platinum-resistant advanced stage lung cancer to explore treatment efficacy in terms of EGFR/ALK alteration status and efficacy of treatment following immunotherapy.
Materials and Methods
Study Participants
A retrospective study of fifty-six advanced non-small cell lung cancer patients who enrolled in the Thai nintedanib Named Patient Use program from eight centers across Thailand was conducted to evaluate the treatment efficacy of combination nintedanib and docetaxel as a treatment after platinum-doublet chemotherapy during 2017-2018. These eight centers included four hospitals in Bangkok: The King Chulalongkorn Memorial Hospital, Siriraj Hospital, Ramathibodi Hospital, and Rajavithi Hospital, and four provincial hospitals: Maharaj Nakorn Chiang Mai Hospital, Chiangmai, Srinakarin Hospital, Khon Kaen Hospital, and Songklanagarind Hospital, Songkhla. This study is a collaborative project of the Thai Society of Clinical Oncology: Lung Cancer Working Group.
All patients had either a cytologic or histologic confirmed diagnosis of NSCLC. Demographic characteristics were obtained from individual patients. Treatment decision, assessments, and follow-up were obtained from individual physicians as standard practice per institute through medical records. Patient death date was validated from The Bureau of Registration Administration, Ministry of Interior, Bangkok, Thailand. This study was approved by the Ethics Committee of each local institution: Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB 536/62), Faculty of Medicine, Siriraj Hospital, Mahidol University [IRB 349/2563 (EC4)], Faculty of Medicine, Khon Kaen University (HE631180), Faculty of Medicine, Ramathibodi Hospital, Mahidol University (IRB MURA2020/794), Faculty of Medicine, Chiang Mai University (IRB MED-2563-07205). Written informed consent was waived from individual study participants as permission from the director of each hospital was granted. Objective response and progression of disease were determined by local investigators using RECIST version 1.1 (13).
Statistical Analysis
Mann-Whitney U test was used to assess the difference between groups of non-parametric distributed variables. Chi-square or Fisher’s exact tests were used for categorical variables. There were varying lines of combination docetaxel/nintedanib treatment from second to fourth. Then progression free survival of docetaxel/nintedanib was defined as duration from start of docetaxel/nintedanib treatment to disease progression or death. Overall disease control rate was defined as the best response evaluation of complete remission and partial response by the provided physician. We applied RECIST criteria version 1.1 as the standard oncology practice in Thailand. Overall survival was defined as the duration from diagnosis of cancer to death from any cause or at censored time which was defined on December 31, 2019. A Survival curve comparison was performed using the Kaplan-Meier method and log-rank test. The cox proportion hazards regression analysis was used to estimate multivariate hazard ratios of progression-free survival and overall survival. A two-sided p-value of less than 0.05 was defined as statistically significant. All statistical analyses were carried out using R version 3.3.0.
Results
Demographic Characteristics and Treatment Overview
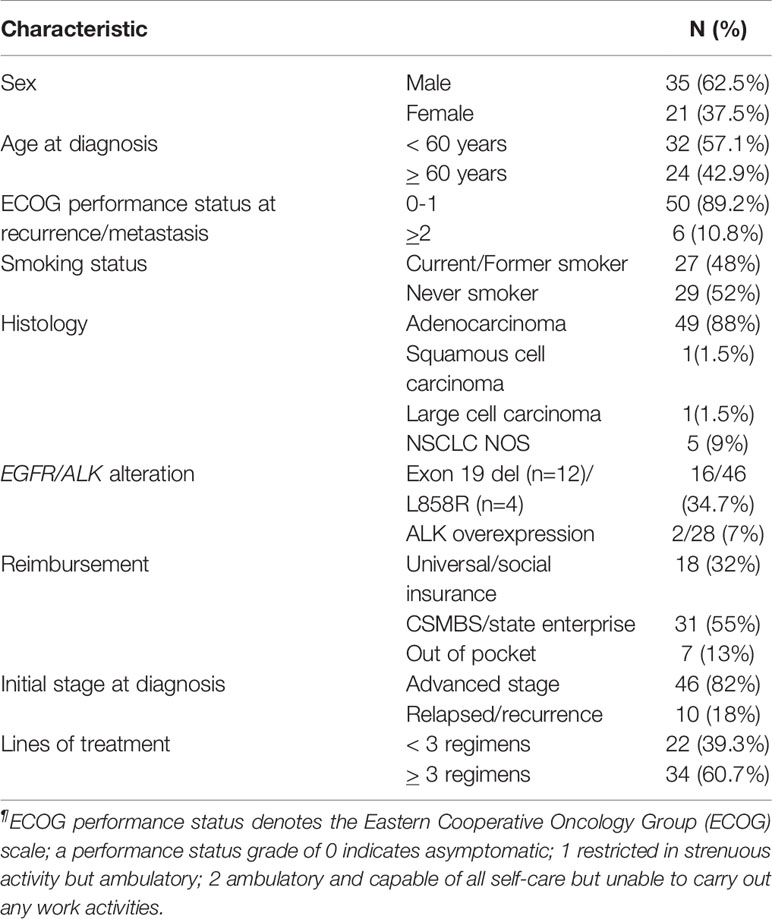
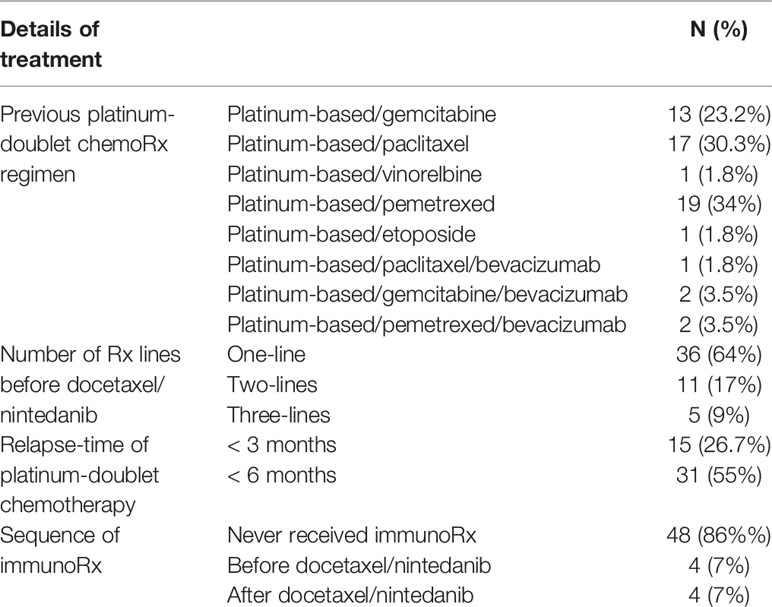
From January 2017 to October 2018, 56 patients from eight centers who received combination docetaxel/nintedanib treatment in advanced stage were enrolled in this retrospective study. Patients in this retrospective cohort received nintedanib via Named Patient Use program following the criteria of having advanced non-small cell lung cancer with disease progression after platinum-doublet chemotherapy. Among them, 62.5% of patients were male with majority ECOG performance status of 0-1 (89.2%) and adenocarcinoma cell type (88%). Demographic characteristics and patient treatments are shown in Tables 1, 2. EGFR and ALK testing were performed in 82.1% and 50%, respectively by using a standard platform of testing according to each institute. 35% had a sensitizing EGFR mutation that was composed of EGFR exon 19 deletion (n=12; 75%) and L858R (n=4; 25%). 61% of patients received more than three lines of treatment which included chemotherapy, EGFR TKI, and immunotherapy as combination or single agent. Pemetrexed, paclitaxel, and gemcitabine were commonly used as part of platinum-doublet chemotherapy at 34%, 30.3% and 23.2%, respectively. 88% of patients received 60 mg/m2 instead of 75 mg/m2 of docetaxel as the common Thai standard practice in advanced stage disease. Eight patients (14.2%) received immunotherapy as a line of standard treatment in advanced stage. Among them, four patients received immunotherapy before docetaxel/nintedanib combination treatment (Table 2).
Outcome of Platinum-Doublet Chemotherapy
The majority of patients (80%) received platinum-doublet chemotherapy as a first-line metastatic setting. Among them, five patients received bevacizumab as part of a combination and maintenance treatment. The median cycle of platinum-doublet chemotherapy was five cycles [range 1-14]. Median PFS of platinum-based doublet chemotherapy was 5.6 months [range 0.5-48, 95% CI: 4.9-6.8]. 31 patients (55%) had disease progression in less than 6 months since the start of treatment, while 15 patients (26.7%) had disease progression in less than 3 months.
Outcome of Combination Docetaxel and Nintedanib
The median number of treatment cycles of docetaxel and nintedanib as part of combination docetaxel/nintedanib were 6 [range 1-10] and 5 cycles [range 0-43], respectively. Overall disease control rate (DCR) of combination docetaxel/nintedanib was 57%. The median PFS was 5.6 months [range 0.25-45, 95% CI: 4.8-6.9] (Figure 1B) which was longer than median PFS in the LUME-lung 1 study (median PFS 3.4 months [95% CI 2.9-3.9]. Three patients (5%) stopped docetaxel/nintedanib after the first cycle due to intolerance/toxicities and response of treatment could not be evaluated. 10 patients (17%) and 20 patients (35%) had either interrupted or reduced doses of docetaxel and nintedanib, respectively. The prevalence of dose modification of nintedanib in our study was higher than the LUME lung I study (18.6%). Three patients continuing maintenance nintedanib beyond eight cycles of docetaxel and were censored on December 31, 2019.
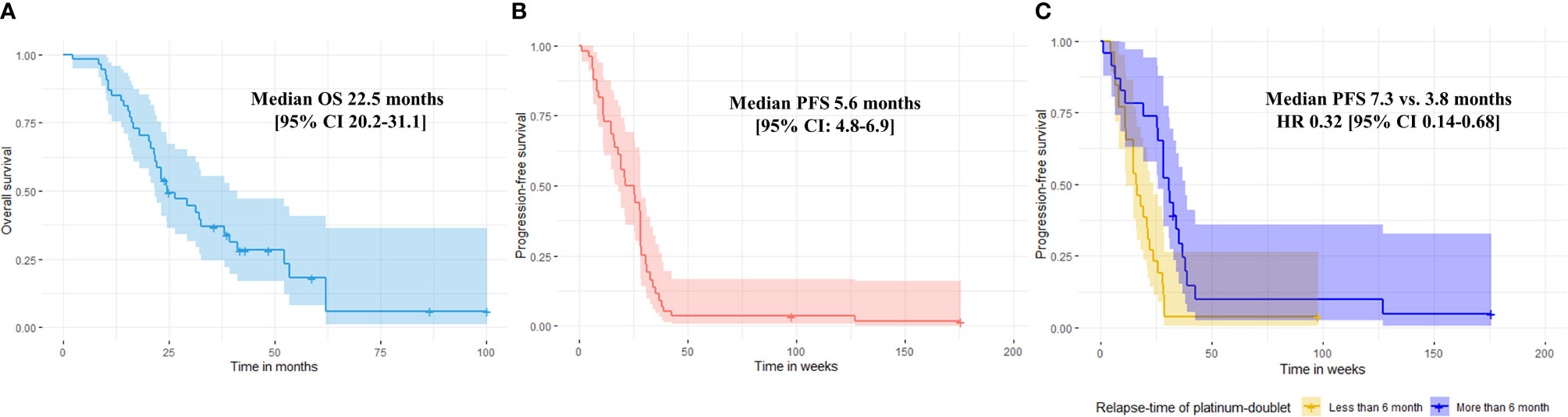
Figure 1 Median overall survival (A) and median progression-free survival of combination docetaxel/nintedanib of the entire population (B). Progression-free survival of combination docetaxel and nintedanib according to relapse-time of platinum-doublet chemotherapy. Median PFS were 7.3 months vs. 3.8 months for relapse-time of platinum doublet chemotherapy ≥ 6 months vs. < 6 months, respectively (multivariate HR 0.32 [95% CI: 0.14-0.68], p-value 0.003) (C).
Analysis According to Relapse-Time of Platinum-Double Chemotherapy
The efficacy of docetaxel/nintedanib disease control was categorized by relapse-time of platinum-doublet chemotherapy i.e. rapid (less than 3 months) or slow progressor (more than 3 months). For excluded patients who could not tolerate treatment, median PFS of combination docetaxel/nintedanib for rapid relapse-time of platinum-doublet chemotherapy was 3.6 months [range 1-3; 95% CI: 2.5-5.5] which was significantly shorter than slow progressor which had a median PFS of 6.4 months [range 1.2-43.9; 95% CI: 4.9-7.4, p-value 0.03]. Using a relapse-time of 6 months also represented shorter disease control from combination docetaxel/nintedanib than relapse-time of more than 6 months. Median PFS for patients who had a relapse-time of platinum-doublet chemotherapy of less than 6 months and more than 6 months were 3.8 months [range 1-24.4; 95% CI: 3.2-5.2] and 7.3 months [range 1.2-43.9; 95% CI: 5.1-8.6, p-value = 0.01), respectively (Figure 1C).
Analysis According to Sequence of Immunotherapy Treatment
The efficacy of docetaxel/nintedanib according to the sequence of immunotherapy, either before or after, was explored. Four patients who received combination docetaxel/nintedanib after immunotherapy had a median PFS of 7.9 months [range 5.2-9.1]. This duration seemed longer than the median PFS of the remaining patients (median PFS 4.5 months, range 0.25-43.9; 95% CI: 4.0-6.0, p-value 0.09). A response assessment was done in three patients and revealed a partial response rate of 50% and stable disease rate of 25%. One patient who received only one cycle of docetaxel/nintedanib after immunotherapy was not evaluated for response due to toxicity from treatment.
Cox Proportional Hazards Regression Model for Prognostic and Predictive Factors
The median OS of the entire cohort was 22.5 months [range 2.2-100.1; 95% CI 20.2-31.1] (Figure 1A). We evaluated prognostic factors of overall survival using cox proportional hazards regression model and applied it to all potential factors including age, ECOG, smoking status, histology, oncogenic alteration, relapse-time of platinum-doublet chemotherapy, line of treatment, and sequence of docetaxel/nintedanib after immunotherapy (Table 3) and found that none of them prognosticated survival in our study.
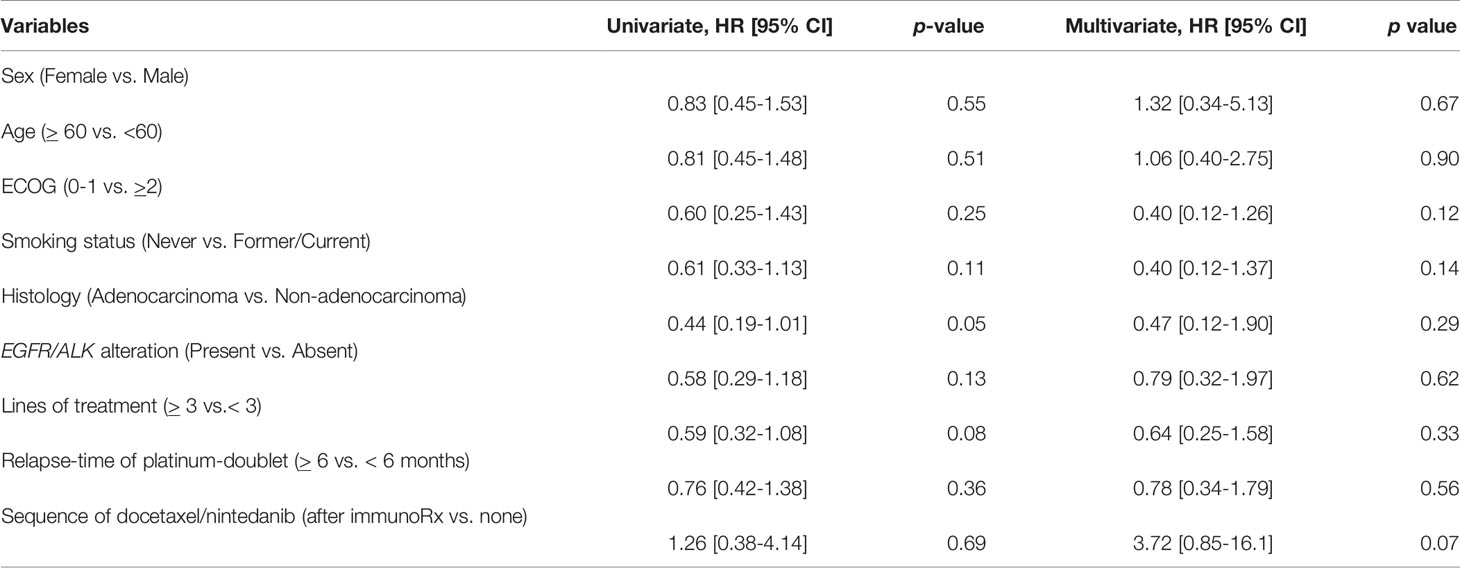
Table 3 Univariate and multivariate analysis of prognostic factors to overall survival benefit including demographic characteristics, treatment by using Cox proportional hazards regression model.
We further analyzed predictive factors of combination docetaxel/nintedanib to define which patient subgroup might benefit most from this treatment. Nevertheless, we restricted predictive factor analysis for combination docetaxel/nintedanib in only the adenocarcinoma subtype following the USFDA approval indication. Relapse-time of platinum-doublet of more than 6 months was correlated with longer PFS with the HR of 0.36 [95% CI 0.19-0.69]. It was also an independent predictive factor of progression-free survival from docetaxel/nintedanib with the multivariate HR of 0.32 [95% CI: 0.14-0.68, p-value 0.003] (Table 4). Relapse-time of platinum-doublet chemotherapy of more than 6 months provided benefits of combination docetaxel/nintedanib treatment compared to the relapse-time of platinum-doublet chemotherapy of less than 6 months.
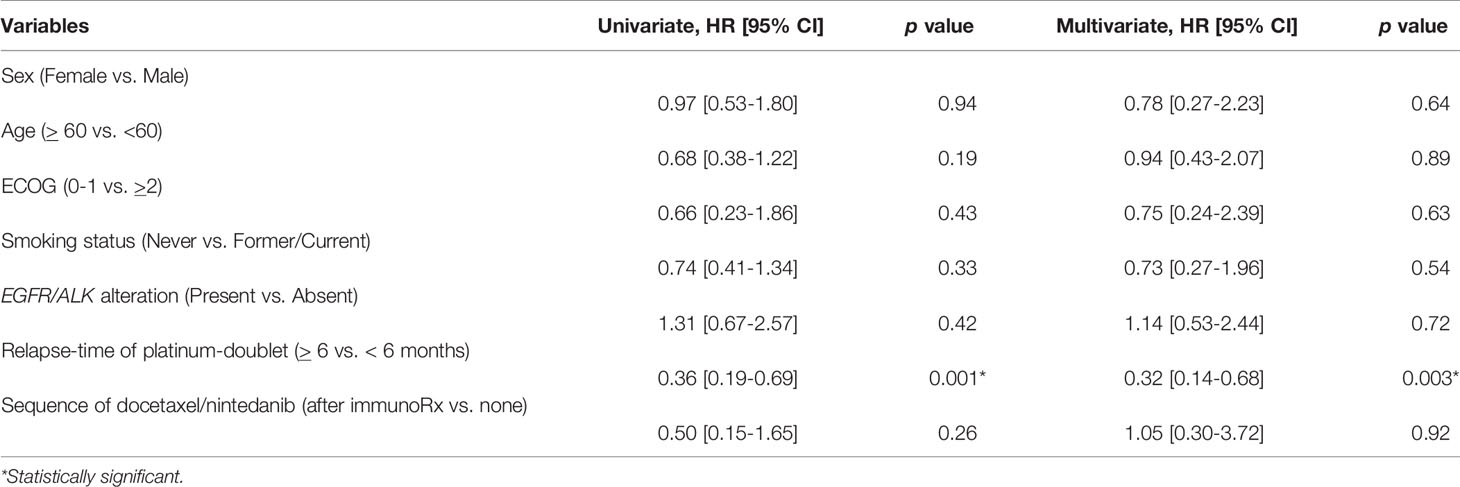
Table 4 Univariate and multivariate analyses of predictive factors of combination docetaxel/nintedanib treatment for adenocarcinoma subtype including demographic characteristics and treatment by using Cox proportional hazards regression model.
Discussion
Novel strategies to define treatment by adopting predictive biomarkers are currently accepted as standard practice. However, in the setting of subsequent treatment after disease progression, there are limitations of biomarker usage. Subsequent immunotherapy after platinum-doublet chemotherapy were explored in several randomized phase III trials to improve patient survival benefit and ensure quality of life (5, 14, 15). There was more progression disease and shorter PFS compared to the standard treatment arm of docetaxel. This could imply that a novel immune checkpoint inhibitor did have efficacy of long term durability and disease control in a limited number of patients in a second-line setting (11). Adding anti-angiogenesis such as nintedanib to docetaxel is another option that has been approved by the USFDA as second-line treatment after platinum-resistance in advanced NSCLC patients with adenocarcinoma subtype (2). However, there is no comparative efficacy of this combination to immunotherapy. The strategy to enhance treatment efficacy by modulating the sequence of treatment requires further elucidation. There are potential high objective response rates and PFS reports for the nintedanib/docetaxel treatment combination after immunotherapy in a case series from the Spanish Named patient used program (ORR 36%, median PFS 3.2 months [95%CI: 1.4-14.6]) (16) and the prospective non-interventional VARGADI cohort study (ORR 58%, PFS 5.5 months [95% CI: 1.9-8.7]) (10). In our series, albeit a small sample size to validate the results, the median PFS for patients who received combination nintedanib/docetaxel after immunotherapy (median PFS 7.9 months, range 5.2-9.1) was longer than the rest of the patients in this retrospective cohort (median PFS 4.5 months, range 0.25-43.9; 95% CI: 4.0-6.0, p-value 0.09). Among the adenocarcinoma subtype, the Cox proportional hazard regression analysis did not indicate superiority of sequential docetaxel/nintedanib after immunotherapy in terms of PFS with HR 0.50 [95% CI: 0.15-1.65, p-value 0.26] by univariate analysis and HR 1.05 [95% CI: 0.30-3.72, p-value 0.92] by multivariate analysis.
Advanced stage adenocarcinoma histology lung cancer patients who had rapid progression of platinum-doublet chemotherapy within 9 months had better outcomes when adding nintedanib to docetaxel as a subsequent treatment, which can be translated to survival outcome (17, 18). Advanced stage non-small cell lung cancer with rapid progressive disease might not be fruitful for immunotherapy (19). However, none of our patients who received immunotherapy had rapid progression from platinum-doublet chemotherapy. This limits our ability to explore this issue. Furthermore, there was a smaller proportion than the general prevalence of sensitizing EGFR mutation in this cohort (34.7%) which represented physician selections of preferred non-oncogenic addicted advanced stage lung cancer for anti-angiogenic treatment. However, among the adenocarcinoma subtype, neither EGFR nor ALK alteration impacts the outcome of this combination. A relapse time of platinum-doublet chemotherapy of more than 6 months solely indicated the benefit of combination docetaxel/nintedanib treatment compared to the relapse time of platinum-doublet chemotherapy of less than 6 months by multivariate HR of PFS 0.32 [95% CI: 0.14-0.68, p-value 0.003].
We would like to declare our study limitations. First, the retrospective cohort prohibits us to retrieve complete information, for example, toxicity of treatment and precise time of imaging evaluation. There might be variations among each center that provided treatment for patients. The treatment lines of combination nintedanib/docetaxel varied from second to forth line. Heavy pretreatment chemotherapy might evolve resistance clones than limited-line treatment. Moreover, the median PFS of combination docetaxel/nintedanib in our study (5.6 months, 95% CI 4.8-6.9) was longer than the LUME lung-1 study (3.4 months, 95% CI 2.9-3.9). The higher frequency of imaging evaluation in LUME lung-1 [first at 4-weeks and then every 6- weeks after randomization compared to our usual standard practice (every 9-weeks)] probably explains this finding. Lastly, even though the general global recommended dosage of docetaxel is 75 mg/m2, in Thailand, the standard used is 60 mg/m2. No direct comparison between dosage efficacy has been reported, however, results from a prospective randomized phase IIb (SENECA study) revealed less toxicity, such as afebrile neutropenia and mucositis of combination nintedanib/docetaxel from the lower dosage (33 mg/m2 D1,D8) of docetaxel, without any compromise of efficacy (20).
Data Availability Statement
Due to confidentiality agreements, supporting data can only be made available to bona fide researchers subject to a non-disclosure agreement. Please contact the corresponding author (chanida.vi@chula.ac.th).
Ethics Statement
This study was approved by the Ethics Committee of each local institution: Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB 536/62), Faculty of Medicine, Siriraj Hospital, Mahidol University (IRB 349/2563 (EC4)), Faculty of Medicine, Khon Kaen University (HE631180), Faculty of Medicine, Ramathibodi Hospital, Mahidol University (IRB MURA2020/794), and Faculty of Medicine, Chiang Mai University (IRB MED-2563-07205). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and design: CV. Administrative support: CV. Provision of study materials or patients: KK, PD, TR, BC, JC, KM, CS, and LT. Collection and assembly of data: KK and CV. Data analysis and interpretation: CV. Manuscript writing: all authors. Final approval of manuscript: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Thai Society of Clinical Oncology: Lung Cancer Working Group.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.572740/full#supplementary-material
Abbreviations
FDA, Food and Drug Administration; MDSC, Myeloid-derived suppressor cell; VEGFR, Vascular endothelial growth factor receptor.
References
1. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA (2014) 311:1998–2006. doi: 10.1001/jama.2014.3741
2. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol (2014) 15:143–55. doi: 10.1016/S1470-2045(13)70586-2
3. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
5. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
6. Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat (2019) 46:100644. doi: 10.1016/j.drup.2019.100644
7. Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PloS One (2009) 4:e7669. doi: 10.1371/journal.pone.0007669
8. Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res (2007) 13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374
9. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
10. Grohe C, Gleiber W, Haas S, Losem C, Mueller-Huesmann H, Schulze M, et al. Nintedanib plus docetaxel after progression on immune checkpoint inhibitor therapy: insights from VARGADO, a prospective study in patients with lung adenocarcinoma. Future Oncol (2019) 15:2699–706. doi: 10.2217/fon-2019-0262
11. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol (2017) 35:3924–33. doi: 10.1200/JCO.2017.74.3062
12. Sriuranpong V, Chantranuwat C, Huapai N, Chalermchai T, Leungtaweeboon K, Lertsanguansinchai P, et al. High frequency of mutation of epidermal growth factor receptor in lung adenocarcinoma in Thailand. Cancer Lett (2006) 239:292–7. doi: 10.1016/j.canlet.2005.08.029
13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst (2000) 92:205–16. doi: 10.1093/jnci/92.3.205
14. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
15. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
16. Corral J, Majem M, Rodriguez-Abreu D, Carcereny E, Cortes AA, Llorente M, et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol (2019) 21:1270–9. doi: 10.1007/s12094-019-02053-7
17. Gaschler-Markefski B, Sikken P, Heymach JV, Gottfried M, Mellemgaard A, Novello S, et al. Time since start of first-line therapy as a predictive clinical marker for nintedanib in patients with previously treated non-small cell lung cancer. ESMO Open (2017) 2:e000102. doi: 10.1136/esmoopen-2016-000102
18. Gottfried M, Bennouna J, Bondarenko I, Douillard JY, Heigener DF, Krzakowski M, et al. Efficacy and Safety of Nintedanib Plus Docetaxel in Patients with Advanced Lung Adenocarcinoma: Complementary and Exploratory Analyses of the Phase III LUME-Lung 1 Study. Target Oncol (2017) 12:475–85. doi: 10.1007/s11523-017-0517-2
19. Garde-Noguera J, Martin-Martorell P, De Julian M, Perez-Altozano J, Salvador-Coloma C, Garcia-Sanchez J, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol (2018) 20:1072–9. doi: 10.1007/s12094-017-1829-5
Keywords: docetaxel, nintedanib, non-small cell lung cancer, sequential treatment, anti-angiogenesis therapy
Citation: Korphaisarn K, Danchaivijitr P, Reungwetwattana T, Chewaskulyong B, Thongthieang L, Chindaprasirt J, Maneenil K, Sathitruangsak C and Vinayanuwattikun C (2021) Efficacy of Combination Docetaxel and Nintedanib in Advanced Non-Small Cell Lung Cancer in Thailand: A Multicenter Study. Front. Oncol. 11:572740. doi: 10.3389/fonc.2021.572740
Received: 15 June 2020; Accepted: 23 March 2021;
Published: 29 April 2021.
Edited by:
Wenhua Liang, First Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Sibo Tian, Emory University, United StatesMargarita Majem, Hospital de la Santa Creu i Sant Pau, Spain
Copyright © 2021 Korphaisarn, Danchaivijitr, Reungwetwattana, Chewaskulyong, Thongthieang, Chindaprasirt, Maneenil, Sathitruangsak and Vinayanuwattikun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanida Vinayanuwattikun, Chanida.Vi@chula.ac.th
†These authors share first authorship
 Krittiya Korphaisarn
Krittiya Korphaisarn Pongwut Danchaivijitr1†
Pongwut Danchaivijitr1† Thanyanan Reungwetwattana
Thanyanan Reungwetwattana Busayamas Chewaskulyong
Busayamas Chewaskulyong Luangyot Thongthieang
Luangyot Thongthieang Jarin Chindaprasirt
Jarin Chindaprasirt Kunlatida Maneenil
Kunlatida Maneenil Chirawadee Sathitruangsak
Chirawadee Sathitruangsak Chanida Vinayanuwattikun
Chanida Vinayanuwattikun