- 1School of Medicine, National University of Ireland (NUI) Galway, Galway, Ireland
- 2Department of Haematology-Oncology, National University Health System, Singapore, Singapore
- 3The N.1 Institute for Health, National University of Singapore, Singapore, Singapore
- 4Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 5School of Nursing, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, Hong Kong SAR, China
- 6Department of Medical Oncology, International University of Health and Welfare Narita Hospital, Chiba, Japan
- 7National University Cancer Institute, National University Health System, Singapore, Singapore
- 8Department of Radiation Oncology, National University Health System, Singapore, Singapore
Background: Paclitaxel-induced peripheral neuropathy (PIPN) is a disabling side effect of paclitaxel with few effective preventive strategies. We aim to determine the efficacy of pharmacological and non-pharmacological neuroprotective interventions in preventing PIPN incidence.
Methods: Biomedical literature databases were searched from years 2000 to 2021 for trials comparing neuroprotective interventions and control. Meta-analysis was performed using the random-effects model. The primary outcome was the incidence of PIPN.
Results: Of 24 relevant controlled trials, 14 were eligible for meta-analysis. Pooled results from seven non-pharmacological trials were associated with a statistically significant 48% relative reduction of PIPN risk with low heterogeneity. Conversely, pooled results from six pharmacological trials were associated with a significant 20% relative reduction of PIPN risk with moderate heterogeneity. Both pharmacological and non-pharmacological approaches appear effective in reducing PIPN incidence in the treatment arm compared to control (pooled RR < 1).
Conclusion: Current evidence suggests that both interventions may reduce PIPN risk. Non-pharmacological interventions appear more effective than pharmacological interventions.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN), a severe dose-dependent toxicity, often results in chemotherapy dose reduction or cessation, adversely affecting efficacy of chemotherapy itself. The resulting neurotoxicity profoundly impacts the quality of life of survivors. With increasing survival rates of cancer patients, there has been an emerging research interest in addressing the detrimental dose-limiting and long-term effects of CIPN (1, 2).
Paclitaxel, a taxane, is used to treat various cancers, including ovarian, breast, and lung carcinomas. Paclitaxel-induced peripheral neuropathy (PIPN) presents as predominantly sensory peripheral neuropathy, while motor and autonomic neuropathy occur to a lesser extent. A recent study showed that up to 80% of patients had neuropathic symptoms for up to 2 years post-treatment, with approximately 25% reporting severe symptoms of numbness and/or discomfort in their hands and feet (3, 4). Existing treatment is limited to dose management and symptomatic cure i.e. pain killers (1). Prevention of these toxic neuropathies impels clinical impact by delivery of the appropriate chemotherapy dose and improved quality of life.
Neuroprotective strategies are being explored to address this unmet medical need. Several interventions have been reported for preventing PIPN. Despite extensive research efforts and successfully reported preclinical studies, there is paucity of high-quality, consistent evidences of clinical successes (2). Trial designs vary in several parameters. Moreover, the absence of a standardized CIPN assessment approach contributes to the lack of clarity of outcomes reported (3).
Prevention of PIPN is crucial to ensure effective delivery, hence maximal benefit, of paclitaxel. PIPN is often irreversible and deteriorates the quality of life of affected patients financially (unemployment and medications), psychologically, and physically, leading to economic and healthcare costs (5). Interventions reducing incidence of CIPN could be key adjuncts to future oncological treatment using such neurotoxic chemotherapeutic agents.
The objective of this systematic review and meta-analysis was to determine the effectiveness of pharmacological and non-pharmacological interventions in reducing the incidence of PIPN. This meta-analysis is designed to better assess the potential efficacy of such neuroprotective interventions currently tested in humans and provides a better understanding of the current state of neuroprotectants. With more interventions (pharmacological and non-pharmacological) gaining recent focus towards the prevention of this disabling side effect, this systematic review aims to consolidate available evidence to help identify promising directions and address factors that would propel development of more wholesome future clinical trials for PIPN prevention.
Methods
Inclusion Criteria
Only controlled trials were included—randomized controlled trials (RCTs) and internal controls (to accommodate non-pharmacological studies). Studies with adult participants, of either sex and diagnosed with any cancer or stage, and scheduled to undergo paclitaxel chemotherapy were included. The intervention arm would include pharmacological or non-pharmacological interventions. The control arm comprised participants assigned to the placebo or control arm. Self-controlled studies were also included, where an intervention was administered unilaterally/one limb and the PIPN burden compared between the two limbs, e.g., frozen gloves applied to one hand per participant. The primary outcome was incidence of PIPN. Studies that investigated symptomatic treatment of pre-existing peripheral neuropathy were excluded. i.e., only studies that excluded patients with pre-existing grade 1 neuropathy were accepted. Studies that investigated combinations of antineoplastic drugs (which included paclitaxel) were excluded.
Search Method
We searched MEDLINE (via PubMed), Embase, and Scopus from January 2000 to Sept 2021. The search strategy included the medical subject headings “paclitaxel neuropathy” with “clinical” and “oncology” filter. The full search string may be found in Supplementary Table 1. The results were hand-searched for eligible studies, and their reference lists were searched for any other relevant studies.
Data Collection and Analysis
Selection of Studies
All titles and abstracts retrieved by electronic search were downloaded to a reference management database, duplicates were removed, and the remaining references were examined independently by two review authors (AL and DY). Studies that failed to meet the eligibility criteria were excluded and copies of the full texts of potentially relevant references were obtained. The eligibility of the retrieved papers was assessed independently, and disagreements were resolved by discussion between the two authors.
Data Extraction and Management
Two reviewers (AL and DY) independently extracted the data on characteristics of patients (inclusion criteria, age, primary cancer histology, paclitaxel administration schedule, and number enrolled in each arm) and interventions (pharmacological or non-pharmacological therapies, dose and administration routes of pharmacological agents), risk of bias, duration of follow-up, and primary outcome for all included studies.
For the primary outcome, the number of patients in the treatment and control arms who experienced PIPN and that of patients assessed at the endpoint was extracted to estimate a risk ratio (RR) and 95% confidence interval (CI). For studies that reported participant numbers at different chemotherapy cycles, the last data point was extracted. To avoid heterogeneity introduced by studies that used different CIPN evaluation questionnaires, the participant numbers across neuropathic grades were summed for studies that reported patient numbers stratified by grade of neuropathy.
Assessment of Risk of Bias
Two authors independently assessed risk of bias of included studies using Cochrane Collaboration’s risk of bias tool on the following domains: allocation sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data and selective reporting (6). Differences were resolved by discussion.
Assessment of Heterogeneity
Visual inspection of forest plots, chi-square tests, and the I2 statistic assessed heterogeneity between studies. A p-value higher than 0.10 for the chi-square test and an I2 value lower than 25% was interpreted as a low level of heterogeneity.
Data Synthesis
When sufficient clinically similar studies were available, their results were pooled in the meta-analysis. For the primary outcome, the RR for each study was calculated and combined using the random-effects model based on the Mantel-Haenzsel method (7). An RR of less than 1 indicated an advantage for the neuroprotective intervention.
Subgroup Analyses
Subgroup analyses determined a priori were performed for routes of administration of pharmacological agents (oral versus parenteral). Additional subgroup analyses were conducted including studies deemed to have clinically similar interventions. Any statistically significant differences in primary outcome measure between subgroups was determined by testing for heterogeneity.
Quality of Summarized Evidence
We determined the quality of evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for the following domains: risk of bias of included studies; inconsistency; indirectness, imprecision, and publication bias.
Results
Search Strategy Results
Database search identified 2,473 records, of which following de-duplication and screening, 46 full-text articles were retrieved for further assessment and 24 RCTs met the inclusion criteria (Figure 1). Of these, 14 (seven pharmacological and seven non-pharmacological) were included in quantitative synthesis (meta-analysis).
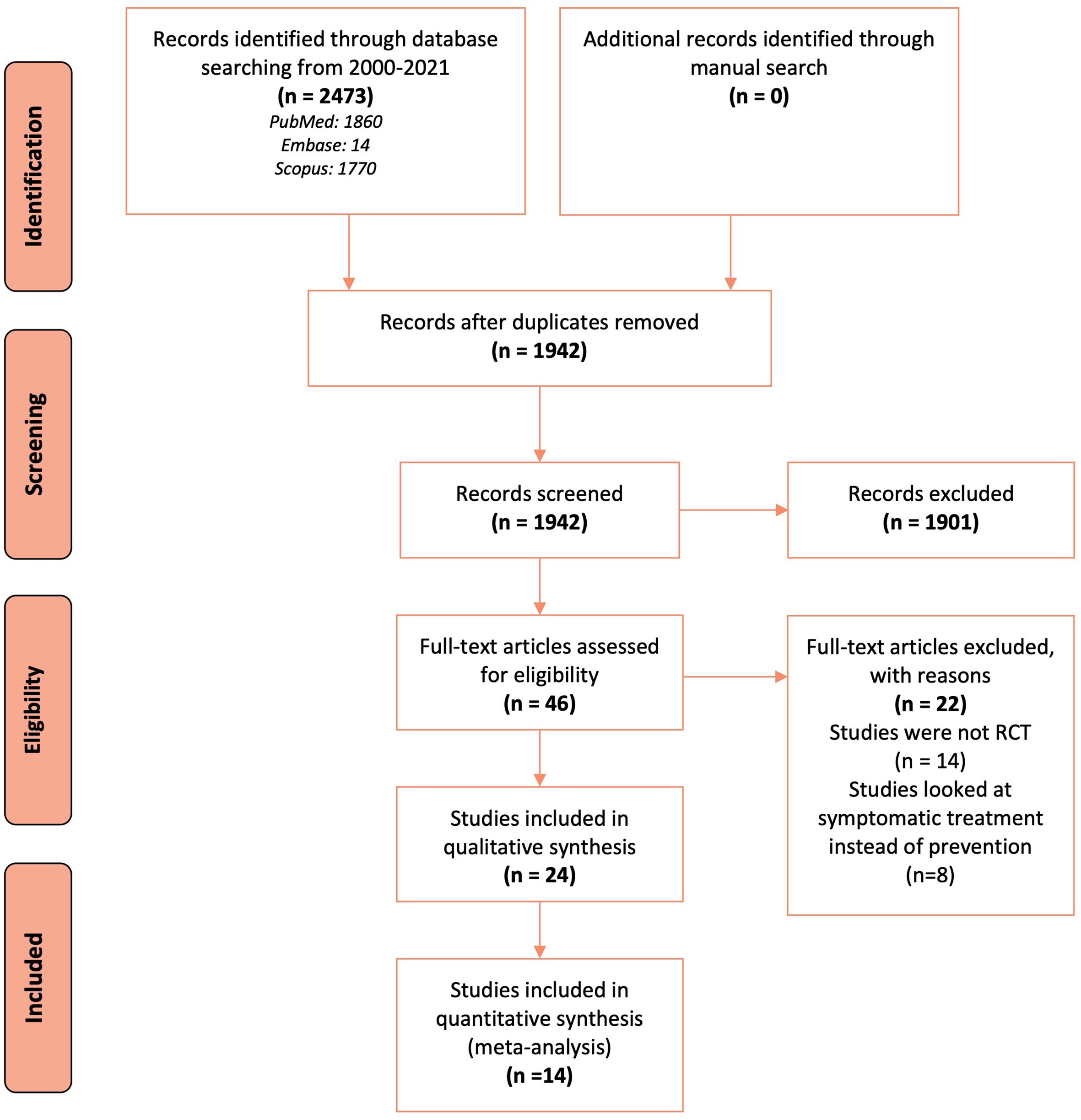
Figure 1 Flow chart describing the search and selection of studies according to the PRISMA guidelines.
To evaluate the presence of neuropathy, 11 studies used validated questionnaires based on self-reported symptoms (8–18). Seven studies used objective measures such as nerve conduction studies, electrophysiological studies, sensory testing, or balance scores (19–25). Six studies used a combination of questionnaires and objective measures (26–31). The studies primarily included breast cancer patients treated with paclitaxel.
Characteristics of Included Studies
Of the 24 included studies, 12 investigated pharmacological agents while the other 12 investigated non-pharmacological interventions (Tables 1 and 2).
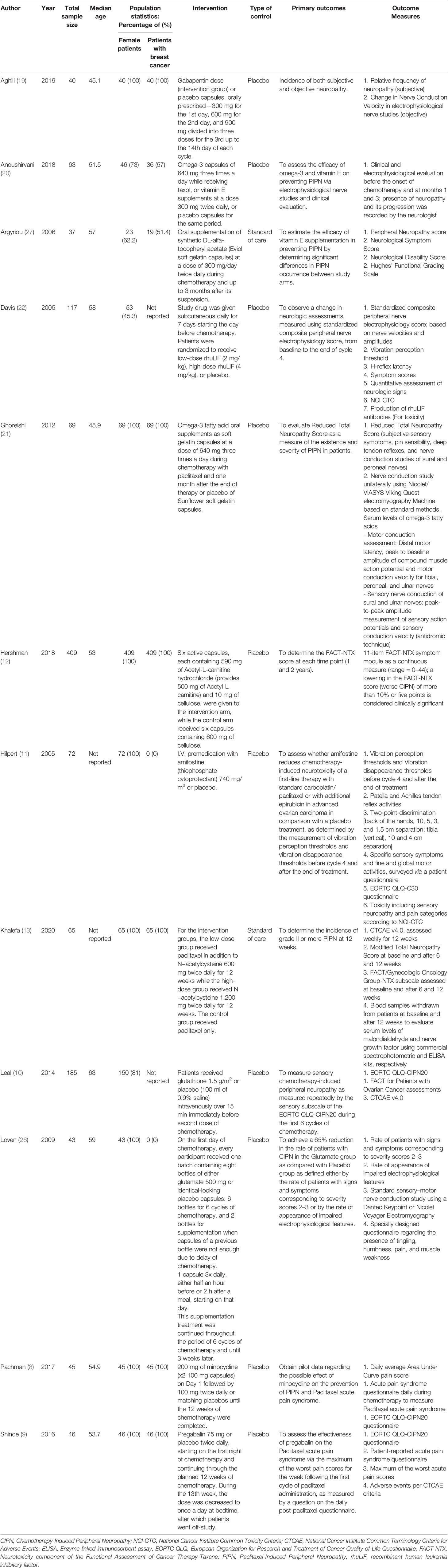
Table 1 Characteristics of included studies describing pharmacological interventions to reduce the incidence of paclitaxel-induced peripheral neuropathy.
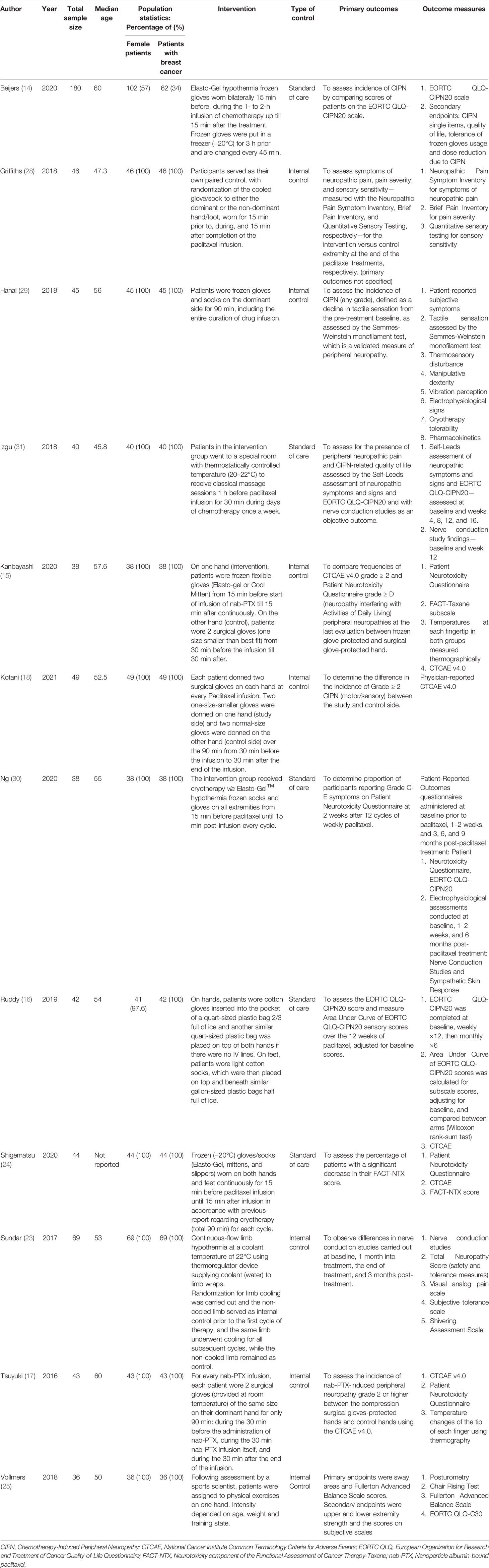
Table 2 Characteristics of included studies describing non-pharmacological interventions to reduce the incidence of paclitaxel-induced peripheral neuropathy.
For the 12 pharmacological RCTs, median sample size was 64. The age of included participants ranged from 30 to 74 years. Six RCTs included only female patients with breast cancer. The pharmacological interventions include gabapentin (19), omega-3 fatty acids (20, 21), vitamin E supplementation (alfa-tocopheryl acetate) (20, 27), minocycline (8), pregabalin (9), glutathione (10), glutamate (26), amifostine (11), recombinant human leukemia inhibitory factor (rhuLIF) (22), Acetyl-L-carnitine (12), and N-acetylcysteine (13).
For the 12 non-pharmacological controlled trials, median sample size was 43.5. The age of included participants ranged from 23 to 74 years. Nine trials included only female patients with breast cancer. The non-pharmacological interventions included cryotherapy (14–16, 23, 24, 28–30), classical massage (31), compression therapy (17, 18) and sensorimotor exercises (25).
Risk of Bias of Included Studies
The risk of bias for the selected studies is summarized in Figures 2 and 3.
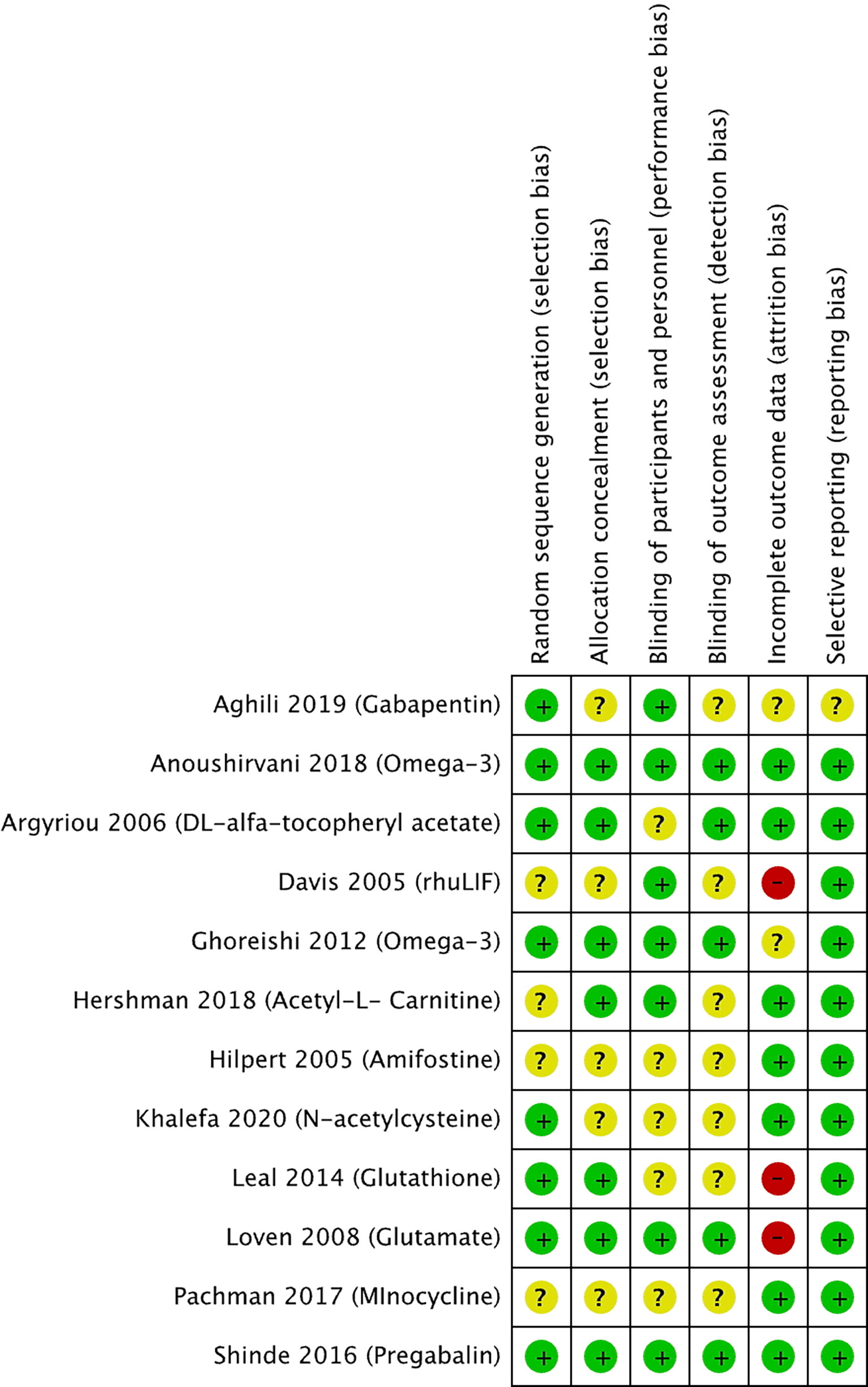
Figure 2 Risk of bias summary for studies investigating pharmacological approaches to prevent PIPN. Green circles represent low risk of bias, yellow circles represent unclear risk of bias, and red circles represent high risk of bias for their corresponding component categories.
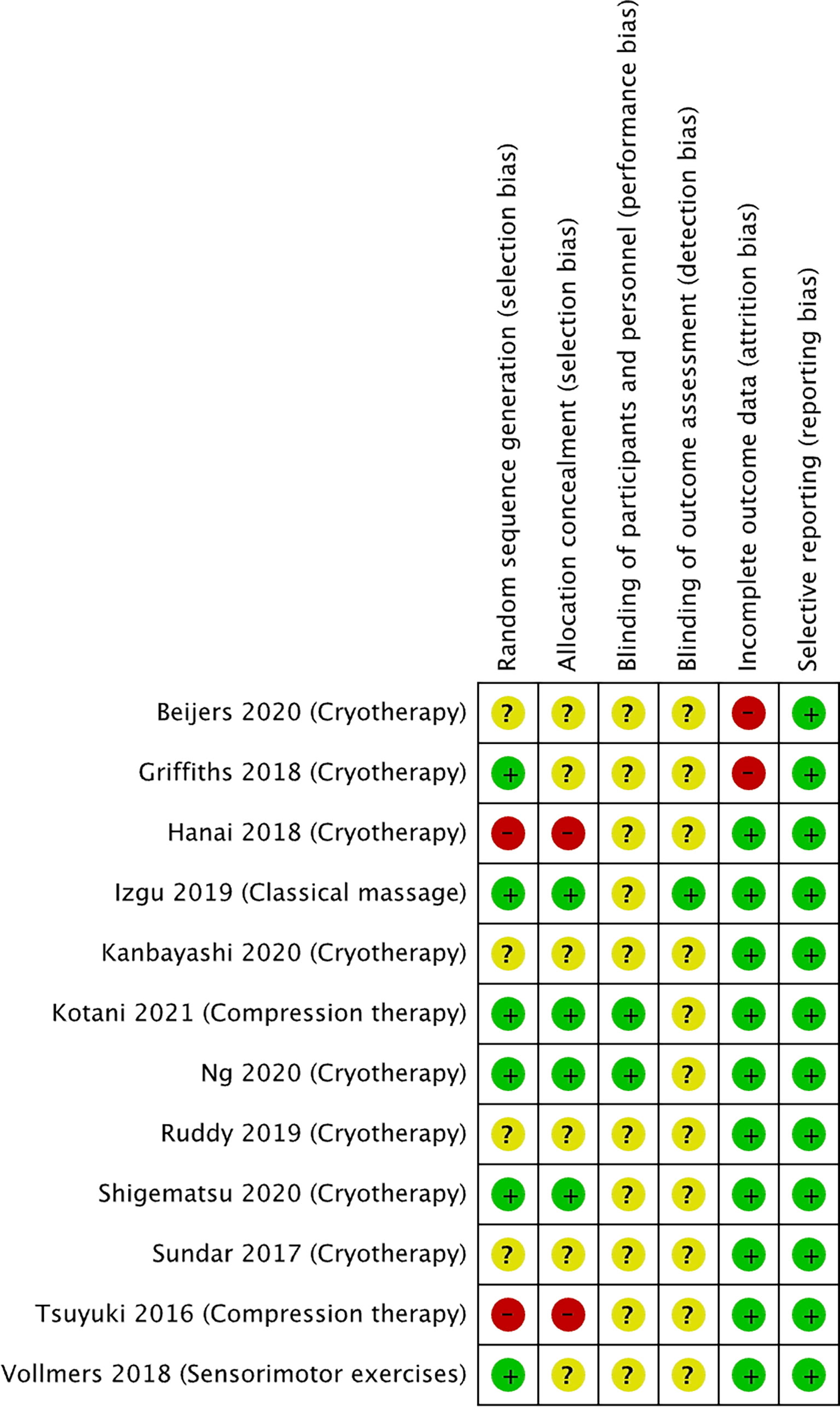
Figure 3 Risk of bias summary for studies investigating non-pharmacological approaches to prevent PIPN. Green circles represent low risk of bias, yellow circle represents unclear risk of bias, and red circle represents high risk of bias for their corresponding component categories.
Of the 12 selected pharmacological RCTs (Figure 2), four had unclear risk of selection bias as their methods for random sequence generation were not reported, while five had an unclear risk of selection bias with respect to the lack of reporting on allocation concealment. Blinding of participants and personnel was not reported in five trials, hence judged to have unclear risk of performance bias. Similarly, seven trials had an unclear risk of detection bias as blinding of outcome assessors was not reported. Two trials had an unclear risk, and three trials a high risk of attrition bias as more than 30% of the study participants were not included in the analysis for various reasons including loss of follow-up or disease progression. All trials were judged to have low risk of reporting bias, except one with an unclear risk.
Of the 12 non-pharmacological trials (Figure 3), two studies were at high risk of selection bias as the intervention was conducted on the participants’ dominant hands. Two studies were at high risk of selection bias as allocation was not concealed. All except one study had an unclear risk of performance bias as the blinding of the participants and study personnel was not reported. All except two studies had an unclear risk of detection bias the studies did not describe the blinding of outcome assessment. Two of the studies were at high risk of attrition bias as both studies had a 34% dropout rate, while all the other studies were at low risk. All the studies were at low risk of reporting bias.
Pharmacological Interventions
12 controlled trials investigated the effect of pharmacological interventions on the prevention of neuropathy (Table 1).
Meta-Analysis
A meta-analysis including seven of the 12 pharmacological controlled trials was pursued (10, 12, 13, 20, 21, 26, 27) as they reported the number of participants corresponding to each grade of neuropathy, which allowed pooling of data. Three studies reported a significant reduction in the risk of neuropathy, while four studies did not find any statistically significant reduction. Pooling these estimates, we found a small reduction in the risk of peripheral neuropathy (RR 0.80, 95% CI 0.60–1.06) (Figure 4). The GRADE quality was judged to be low due to high risk of bias in the methodological components of the included studies and inconsistency and imprecision for the pooled results (Table 3). There was significant heterogeneity between individual trial results (chi-square p value = 0.003, I2 = 69%). There were insufficient studies to pursue sub-group analysis stratified by intervention method (oral and parenteral).
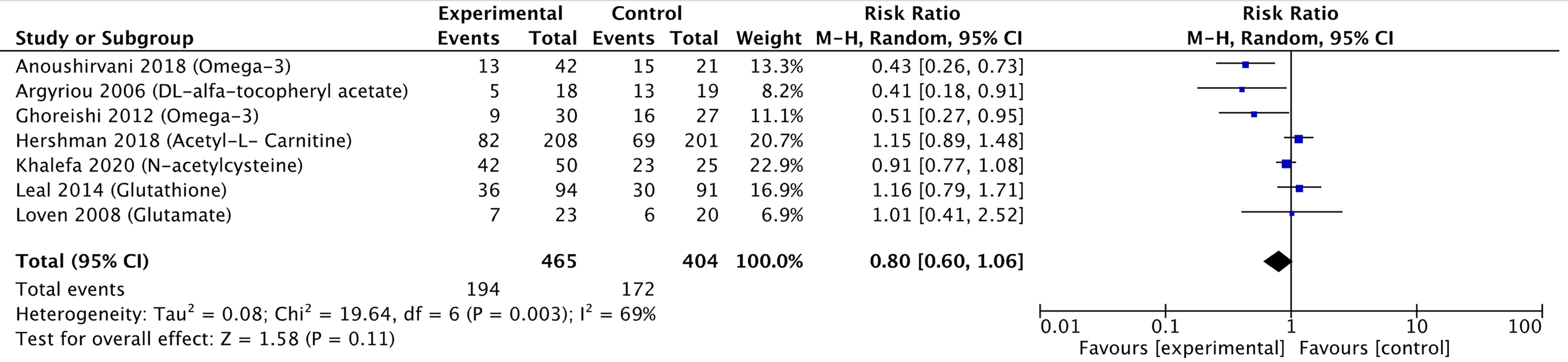
Figure 4 Forest plot comparing the incidence of PIPN between the experimental (pharmacological approaches) and control (placebo) arms. Black diamonds are the estimated pooled hazard ratio for each meta-analysis; blue box sizes reflect the relative weight apportioned to studies in the meta-analysis.

Table 3 GRADE score on the quality of summarized evidence comparing the incidence of peripheral neuropathy between the experimental (pharmacological approaches) with control (placebo) arms.
Interventions That Reduced the Incidence of PIPN
The study by Aghili et al. (19), which investigated the effect of gabapentin, showed a statistically significant difference between intervention and placebo groups in terms of neuropathic grade based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 questionnaire (CTCAE v4.0) and sensory nerve conduction. However, patient-reported symptoms varied across the different chemotherapy cycles. The study by Anoushirvani et al. (20) reported a significant difference between patients receiving Omega-3 or Vitamin E as compared to placebo, although no significant difference was seen in the electrophysiological variables measured and the study was limited by the small sample size (n = 21) per intervention. The study by Argyriou et al. (27) showed that patients who received Vitamin E were at lower risk of developing PIPN, although it is worth noting that the trial was limited by its small intervention sample size (n = 18) and lack of placebo. The study by Ghoreishi et al. (21) showed that patients who received Omega-3 fatty acid supplements were at lower risk of incidence of peripheral neuropathy, and a significantly higher (better) sural sensory action potential amplitude. It is worth noting, however, that the trial was limited by its relatively small sample size in the intervention group (n = 35). The study by Khalefa et al. (13) showed that patients who received N-acetylcysteine had a statistically significant reduction in the incidence of Grade 3 peripheral neuropathy. Similarly, however, the study was also limited by small intervention sample size (n = 42).
Interventions That Did Not Reduce the Incidence of PIPN
The study by Davis et al. (22), which investigated the effect of rhuLIF, showed that it was not effective in preventing, delaying, or diminishing CIPN caused by carboplatin and paclitaxel. The study by Hilpert et al. (11) showed that while Amifostine did show some protective effects in that a significantly delayed onset and accelerated recovery was observed, there were no differences in self-reported sensory or motor symptoms, which led the authors to conclude that Amifostine should not be routinely recommended. Leal et al. (10) showed that Glutathione did not have any statistically significant effect on the development of CIPN. Loven et al. (26) showed that long-term glutamate supplementation did not have any statistically significant effect on neurological examinations, questionnaires and nerve conduction studies. The study by Pachman et al. (8) showed that minocycline was able to reduce the pain score attributed to Paclitaxel acute pain syndrome, but no statistically significant difference was seen in CIPN scores as defined by European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire CIPN20 (EORTC QLQ-CIPN20) sensory scores. Similarly, Shinde et al. (9) showed that patients treated with pregabalin did not find a statistically significant difference in the pain score associated with Paclitaxel acute pain syndrome and CIPN as determined by EORTC QLQ-CIPN20.
Interventions That Increased the Incidence of PIPN
Only one study showed that patients were at higher risk of PIPN. Hershman et al. (12) showed that patients who were co-administered Acetyl-L-Carnitine during chemotherapy on average had worse peripheral neuropathy as determined by the neurotoxicity component of the Functional Assessment of Cancer Therapy-Taxane (FACT-NTX) symptom module. The CIPN also persisted throughout the two-year period following Acetyl-L-Carnitine discontinuation.
Non-Pharmacological Interventions
12 controlled trials investigated the effect of non-pharmacological interventions on the prevention of neuropathy (14–18, 23–25, 28–31) (Table 2).
Meta-Analysis
A meta-analysis was pursued for studies that used non-pharmacological interventions, as per our protocol. Seven studies were included in the meta-analysis as they reported the number of participants corresponding to each grade of neuropathy. Six studies reported a reduced relative risk of neuropathy, while one study did not find any difference. Pooling these estimates, we found that non-pharmacological interventions were able to halve the risk of developing neuropathy (pooled RR = 0.52, 95% CI = 0.38–0.70, p < 0.001, I2 = 29%) (Figure 5A).
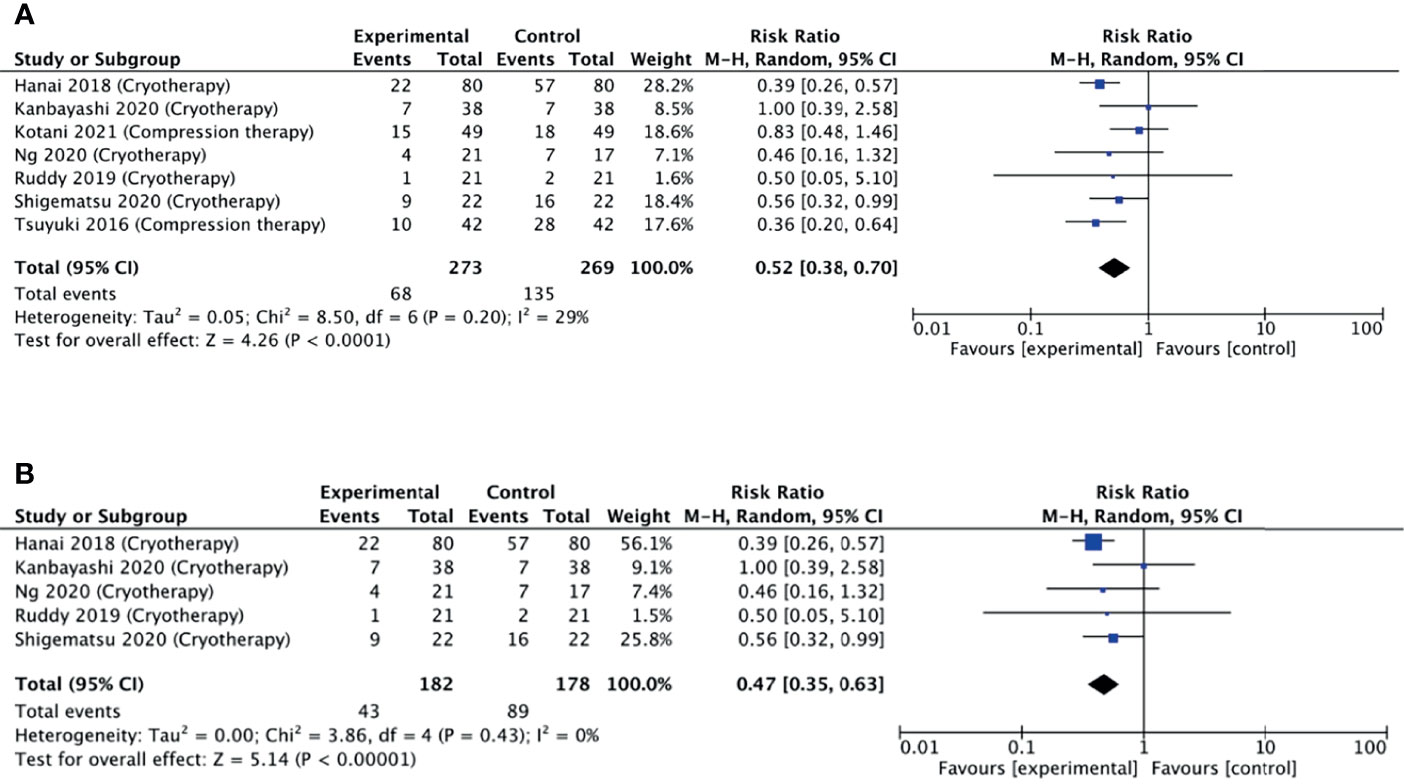
Figure 5 (A) Forest plot comparing the incidence of PIPN between the experimental (non-pharmacological approaches) with control (placebo) arms. (B) Forest plot comparing the incidence of PIPN between experimental (cryotherapy) and control (placebo) arms. Black diamonds are the estimated pooled hazard ratio for each meta-analysis; blue box sizes reflect the relative weight apportioned to studies in the meta-analysis.
The GRADE quality was judged to be moderate due to serious risk of bias in the methodological components of included studies but there were no serious inconsistencies between studies (Table 4).

Table 4 GRADE score on the quality of summarized evidence comparing the incidence of peripheral neuropathy between the experimental (non-pharmacological approaches) with control (placebo) arms.
Cryotherapy
Limb cryotherapy was the most investigated intervention, with eight studies included in this analysis (14–16, 23, 24, 28–30). Six of these studies used frozen or cooled gloves to cool limbs (14, 15, 24, 28–30), one study used a thermoregulatory device (23), and one study involved immersing the patient’s cotton gloved hands into an ice bucket (16). Of these, one study did not find any difference in the incidence of neuropathy, while four studies found a reduced incidence (Figure 5B).
A subgroup meta-analysis including five of the eight studies was conducted. Pooling these estimates (Figure 5B), it was found that non-pharmacological interventions were able to significantly reduce the incidence of neuropathy (pooled RR = 0.47, 95% CI = 0.35–0.63, p < 0.001, I2 = 0%). Additional sensitivity analysis also revealed that no single study had a substantial effect on the CI.
Compression Therapy
Two studies explored the effect of peripheral compression (through wearing smaller glove sizes) during chemotherapy infusion (17, 18). The study by Tsuyuki et al. (17) found that hands compressed using surgical gloves had significantly lower mean grade of neuropathy as compared to non-treated hands. Conversely, the study by Kotani et al. (18) found that surgical glove compression therapy was not effective in preventing the incidence of peripheral neuropathy. The limited number of studies precluded a meta-analysis.
Kanbayashi et al. (15) evaluated efficacy of compression therapy via surgical glove and cryotherapy via frozen gloves, and found that they showed comparable efficacy in preventing CIPN.
Other Therapies
Izgu et al. showed that classical massage therapy prior to chemotherapy infusion showed a significantly reduced relative incidence of neuropathy at week 12 of infusion, although the effect was not preserved at week 16 (31). Vollmers et al. showed that sensorimotor exercise during treatment period improved strength and balance, although the improvements were not statistically significant (25).
Discussion
CIPN is a critical issue affecting delivery of chemotherapy and quality of life of survivors; hence, prevention is imperative. This meta-analysis assesses clinically explored neuroprotectants for reducing incidence of PIPN, aiming to interpret their therapeutic efficacy and identify promising directions. Importantly, this study includes newer non-pharmacological trials not included in previous similar reviews. This study evidences a relatively higher efficacy of non-pharmacological than pharmacological interventions in reducing the incidence of PIPN in the treatment arm compared to the control.
Overall, when comparing the forest plots, non-pharmacological interventions displayed a lower pooled RR of 0.52 compared to 0.80 for the pharmacological (Figures 4 and 5), supporting higher efficacy of non-pharmacological interventions in reducing PIPN incidence. Among the non-pharmacological interventions, there was a notable benefit observed by the subgroup of studies investigating cryotherapy with a pooled RR of 0.47. This suggests that cryotherapy, through its vasoconstrictive effects, may have the potential to reduce the incidence of peripheral neuropathy by limiting initial exposure damage during the chemotherapy infusion.
The inconsistency in study design across the pharmacological and non-pharmacological studies was substantial. Though the evaluated studies demonstrated low heterogeneity, a low GRADE score was observed when comparing the incidence of PIPN in the treatment to control arms for pharmacological interventions. However, a high GRADE score was observed for the non-pharmacological interventions (Tables 3 and 4). Placebo control was a commonly used standard in all pharmacological studies except one. In the non-pharmacological studies, most used internal controls, while one used a placebo to demonstrate efficacy. The non-possibility of relevant placebos in physical interventions is important to recognize and consider.
A variety of outcome measures was used to assess PIPN incidence and treatment efficacy in preventing PIPN. Of the included pharmacological studies, the most commonly reported outcome measure was nerve conduction studies, while the most commonly used outcome in non-pharmacological studies was the EORTC QLQ-CIPN20 questionnaire. Majority of the studies reported adverse events as per CTCAE criteria. The diversity of CIPN assessment methods is concerning, with implications in clinical trial design, result interpretation, and practice (3, 32). Several consortiums have been formed to address this and propose a standardized approach (33). This study further emphasizes the importance of a gold standard objective approach to assess and report CIPN outcomes.
Apart from the efficacy of the interventions in the prevention of neuropathy, it is also important to consider the limitations, in terms of their safety and tolerability. In this review, majority of studies did not observe a large dropout rate due to direct concerns of safety. However, in the field of cryotherapy, there have been reports of frozen gloves used for limb hypothermia being recalled over concerns of frostbite and patient intolerance (34). In the studies that investigated compression therapy, limitations such as the patient physique and amount of required compression were raised (18). Thus, further studies investigating the safety, tolerability, and reproducibility (across various population cohorts) of the proposed interventions are imperative.
Study Limitations
While mitigated through a systematic search, there is a risk of sampling bias due to exclusion of gray or non-English literature. Studies that investigated combinations of antineoplastic drugs (which included paclitaxel) were not included. The included studies often reported the incidence of PIPN using varying assessment tools. This precluded further investigation of the effect of these interventions on a reduction in the severity of neuropathy. Furthermore, methodological heterogeneity was observed across studies and inconsistencies in reporting were observed, which limits the conclusion strength for pharmacological interventions.
Practice Implications
The included neuroprotective interventions are promising approaches in development. Low-to-moderate quality evidence suggests that non-pharmacological interventions are more efficacious than pharmacological agents explored in this study in reducing the incidence of PIPN. Specifically, subgroup analysis has revealed that limb cryotherapy appears to hold promise as a potential intervention for the prevention of PIPN. However, the strength of evidence is weak due to the small number of evaluable studies with small sample size. Considering the diversity in clinical and methodological aspects, the results should be interpreted with caution, and no absolute or general recommendations can be made.
Research Implications
Relatively few clinical studies have explored non-pharmacological interventions for PIPN prevention. Some emerging ones include combination therapies such as cryocompression, and behavioral interventions such as acupuncture and exercise therapy (35–38). Larger multi-center, well-designed trials should investigate the effects of these promising agents for more conclusive evidence. Future trials may also consider the combination of strategies—pharmacological, non-pharmacological, or both, in the prevention of peripheral neuropathy. Trials should use standardized objective assessment methods and well-defined primary and secondary outcomes to ensure validity. Experimental studies uncovering the mechanisms of action of these methods are required. These can advance treatment by incorporation of optimal therapeutic parameters.
Conclusion
This meta-analysis assessed neuroprotectants for preventing PIPN and highlights the emergence of non-pharmacological interventions (especially cryotherapy and compression therapy). Evidence from the selected trials demonstrate a greater efficacy of non-pharmacological than pharmacological interventions in reducing the incidence of PIPN. The quality of evidence from the evaluated studies is overall low, and sample size is small. Meticulous planning of trial design and standardizing CIPN assessment techniques will greatly improve outcome reporting and ease judgement of prospective interventions for incorporation into clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
AL and DY contributed equally to the study. RS, YS, and AB conceptualized the research. AL, DY, CT, BT, and YS carried out the data curation, analysis, and interpretation. AL, DY, SF, and AB drafted the manuscript. RS, AM, HI, and YS reviewed the manuscript and provided critical feedback. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Medical Research Council under its Clinician Scientist—Individual Research Grant (NMRC/CNIG/1167/2017); the National University Health System under its NUHS Summit Research Program—Cancer (NCSP N-171-000-493-001); and the National University of Singapore under its N.1 Institute for Health’s Translational Core.
Conflict of Interest
RS has received honoraria from Bristol-Myers Squibb, Lilly, Roche, Taiho, Astra Zeneca, DKSH, and MSD; has advisory activity with Bristol-Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD, and AstraZeneca; received research funding from MSD and Paxman Coolers; and has received travel grants from AstraZeneca, Eisai, Roche, and Taiho Pharmaceutical. AB reports grants from Paxman Coolers Ltd., outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.763229/full#supplementary-material
References
1. Loprinzi CL. Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. In: UpToDate (2017). Available at: https://www.uptodate.com/contents/prevention-and-treatment-of-chemotherapy-induced-peripheral-neuropathy.
2. Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2014) 32(18):1941–67. doi: 10.1200/JCO.2013.54.0914
3. Molassiotis A, Cheng HL, Lopez V, Au JS, Chan A, Bandla A, et al. Are We Mis-Estimating Chemotherapy-Induced Peripheral Neuropathy? Analysis of Assessment Methodologies From a Prospective, Multinational, Longitudinal Cohort Study of Patients Receiving Neurotoxic Chemotherapy. BMC Cancer (2019) 19(1):132. doi: 10.1186/s12885-019-5302-4
4. Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-Induced Peripheral Neurotoxicity (CIPN): An Update. Crit Rev Oncol Hematol (2012) 82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012
5. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare Costs and Workloss Burden of Patients With Chemotherapy-Associated Peripheral Neuropathy in Breast, Ovarian, Head and Neck, and Nonsmall Cell Lung Cancer. Chemother Res Pract (2012) 2012:913848. doi: 10.1155/2012/913848
6. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. Br Med J (2019) 366:4898. doi: 10.1136/bmj.l4898
8. Pachman DR, Dockter T, Zekan PJ, Fruth B, Ruddy KJ, Ta LE, et al. A Pilot Study of Minocycline for the Prevention of Paclitaxel-Associated Neuropathy: ACCRU Study RU221408I. Support Care Cancer (2017) 25(11):3407–16. doi: 10.1007/s00520-017-3760-2
9. Shinde SS, Seisler D, Soori G, Atherton PJ, Pachman DR, Lafky J, et al. Can Pregabalin Prevent Paclitaxel-Associated Neuropathy?–An ACCRU Pilot Trial. Support Care Cancer (2016) 24(2):547–53. doi: 10.1007/s00520-015-2807-5
10. Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber C, et al. NCCTG N08CA (Alliance): The Use of Glutathione for Prevention of Paclitaxel/Carboplatin Induced Peripheral Neuropathy: A Phase III Randomized, Double-Blind Placebo-Controlled Study. Cancer (2014) 120(12):1890–7. doi: 10.1002/cncr.28654
11. Hilpert F, Stahle A, Tome O, Burges A, Rossner D, Spathe K, et al. Neuroprotection With Amifostine in the First-Line Treatment of Advanced Ovarian Cancer With Carboplatin/Paclitaxel-Based Chemotherapy–a Double-Blind, Placebo-Controlled, Randomized Phase II Study From the Arbeitsgemeinschaft Gynakologische Onkologoie (AGO) Ovarian Cancer Study Group. Support Care Cancer (2005) 13(10):797–805. doi: 10.1007/s00520-005-0782-y
12. Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, et al. Two-Year Trends of Taxane-Induced Neuropathy in Women Enrolled in a Randomized Trial of Acetyl-L-Carnitine (SWOG S0715). J Natl Cancer Inst (2018) 110(6):669–76. doi: 10.1093/jnci/djx259
13. Khalefa HG, Shawki MA, Aboelhassan R, El Wakeel LM. Evaluation of the Effect of N-Acetylcysteine on the Prevention and Amelioration of Paclitaxel-Induced Peripheral Neuropathy in Breast Cancer Patients: A Randomized Controlled Study. Breast Cancer Res Treat (2020) 183(1):117–25. doi: 10.1007/s10549-020-05762-8
14. Beijers AJM, Bonhof CS, Mols F, Ophorst J, de Vos-Geelen J, Jacobs EMG, et al. Multicenter Randomized Controlled Trial to Evaluate the Efficacy and Tolerability of Frozen Gloves for the Prevention of Chemotherapy-Induced Peripheral Neuropathy. Ann Oncol (2020) 31(1):131–6. doi: 10.1016/j.annonc.2019.09.006
15. Kanbayashi Y, Sakaguchi K, Ishikawa T, Ouchi Y, Nakatsukasa K, Tabuchi Y, et al. Comparison of the Efficacy of Cryotherapy and Compression Therapy for Preventing Nanoparticle Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy: A Prospective Self-Controlled Trial. Breast (2020) 49:219–24. doi: 10.1016/j.breast.2019.12.011
16. Ruddy KJ, Le-Rademacher J, Lacouture ME, Wilkinson M, Onitilo AA, Vander Woude AC, et al. Randomized Controlled Trial of Cryotherapy to Prevent Paclitaxel-Induced Peripheral Neuropathy (RU221511I); an ACCRU Trial. Breast (2019) 48:89–97. doi: 10.1016/j.breast.2019.09.011
17. Tsuyuki S, Senda N, Kanng Y, Yamaguchi A, Yoshibayashi H, Kikawa Y, et al. Evaluation of the Effect of Compression Therapy Using Surgical Gloves on Nanoparticle Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy: A Phase II Multicenter Study by the Kamigata Breast Cancer Study Group. Breast Cancer Res Treat (2016) 160(1):61–7. doi: 10.1007/s10549-016-3977-7
18. Kotani H, Terada M, Mori M, Horisawa N, Sugino K, Kataoka A, et al. Compression Therapy Using Surgical Gloves Does Not Prevent Paclitaxel-Induced Peripheral Neuropathy: Results From a Double-Blind Phase 2 Trial. BMC Cancer (2021) 21(1):548. doi: 10.1186/s12885-021-08240-6
19. Aghili M, Zare M, Mousavi N, Ghalehtaki R, Sotoudeh S, Kalaghchi B, et al. Efficacy of Gabapentin for the Prevention of Paclitaxel Induced Peripheral Neuropathy: A Randomized Placebo Controlled Clinical Trial. Breast J (2019) 25(2):226–31. doi: 10.1111/tbj.13196
20. Anoushirvani AA, Poorsaadat L, Aghabozorgi R, Kasravi M. Comparison of the Effects of Omega 3 and Vitamin E on Palcitaxel-Induced Peripheral Neuropathy. Open Access Macedonian J Med Sci (2018) 62018:1857–61. doi: 10.3889/oamjms.2018.333
21. Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega-3 Fatty Acids are Protective Against Paclitaxel-Induced Peripheral Neuropathy: A Randomized Double-Blind Placebo Controlled Trial. BMC Cancer (2012) 12:355. doi: 10.1186/1471-2407-12-355
22. Davis ID, Kiers L, MacGregor L, Quinn M, Arezzo J, Green M, et al. A Randomized, Double-Blinded, Placebo-Controlled Phase II Trial of Recombinant Human Leukemia Inhibitory Factor (rhuLIF, Emfilermin, AM424) to Prevent Chemotherapy-Induced Peripheral Neuropathy. Clin Cancer Res (2005) 11(5):1890–8. doi: 10.1158/1078-0432.CCR-04-1655
23. Sundar R, Bandla A, Tan SSH, Liao LD, Kumarakulasinghe NB, Jeyasekharan AD, et al. Limb Hypothermia for Preventing Paclitaxel-Induced Peripheral Neuropathy in Breast Cancer Patients: A Pilot Study. Front Oncol (2017) 6(JAN). doi: 10.3389/fonc.2016.00274
24. Shigematsu H, Hirata T, Nishina M, Yasui D, Ozaki S. Cryotherapy for the Prevention of Weekly Paclitaxel-Induced Peripheral Adverse Events in Breast Cancer Patients. Support Care Cancer (2020) 28(10):5005–11. doi: 10.1007/s00520-020-05345-9
25. Vollmers PL, Mundhenke C, Maass N, Bauerschlag D, Kratzenstein S, Rocken C, et al. Evaluation of the Effects of Sensorimotor Exercise on Physical and Psychological Parameters in Breast Cancer Patients Undergoing Neurotoxic Chemotherapy. J Cancer Res Clin Oncol (2018) 144(9):1785–92. doi: 10.1007/s00432-018-2686-5
26. Loven D, Levavi H, Sabach G, Zart R, Andras M, Fishman A, et al. Long-Term Glutamate Supplementation Failed to Protect Against Peripheral Neurotoxicity of Paclitaxel. Eur J Cancer Care (Engl) (2009) 18(1):78–83. doi: 10.1111/j.1365-2354.2008.00996.x
27. Argyriou AA, Chroni E, Koutras A, Iconomou G, Papapetropoulos S, Polychronopoulos P, et al. Preventing Paclitaxel-Induced Peripheral Neuropathy: A Phase II Trial of Vitamin E Supplementation. J Pain Symptom Manage (2006) 32(3):237–44. doi: 10.1016/j.jpainsymman.2006.03.013
28. Griffiths C, Kwon N, Beaumont JL, Paice JA. Cold Therapy to Prevent Paclitaxel-Induced Peripheral Neuropathy. Support Care Cancer (2018) 26(10):3461–9. doi: 10.1007/s00520-018-4199-9
29. Hanai A, Ishiguro H, Sozu T, Tsuda M, Yano I, Nakagawa T, et al. Effects of Cryotherapy on Objective and Subjective Symptoms of Paclitaxel-Induced Neuropathy: Prospective Self-Controlled Trial. J Natl Cancer Inst (2018) 110(2):141–8. doi: 10.1093/jnci/djx178
30. Ng DQ, Tan CJ, Soh BC, Tan MML, Loh SY, Tan YE, et al. Impact of Cryotherapy on Sensory, Motor, and Autonomic Neuropathy in Breast Cancer Patients Receiving Paclitaxel: A Randomized, Controlled Trial. Front Neurol (2020) 11. doi: 10.3389/fneur.2020.604688
31. Izgu N, Metin ZG, Karadas C, Ozdemir L, Váetin N, Demirci U. Prevention of Chemotherapy-Induced Peripheral Neuropathy With Classical Massage in Breast Cancer Patients Receiving Paclitaxel: An Assessor-Blinded Randomized Controlled Trial. Eur J Oncol Nurs (2019) 40:36–43. doi: 10.1016/j.ejon.2019.03.002
32. Yeo F, Ng CC, Loh KWJ, Molassiotis A, Cheng HL, Au JSK, et al. Minimal Clinically Important Difference of the EORTC QLQ-CIPN20 for Worsening Peripheral Neuropathy in Patients Receiving Neurotoxic Chemotherapy. Support Care Cancer (2019) 27(12):4753–62. doi: 10.1007/s00520-019-04771-8
33. Alberti P, Cavaletti G, Cornblath DR. Toxic Neuropathies: Chemotherapy Induced Peripheral Neurotoxicity. Curr Opin Neurol (2019) 32(5):676–83. doi: 10.1097/WCO.0000000000000724
34. Medical Device Recall Notice Hypothermia Caps, Mittens and Slippers [Press Release]. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=162228.
35. Bao T, Seidman AD, Piulson L, Vertosick E, Chen X, Vickers AJ, et al. A Phase IIA Trial of Acupuncture to Reduce Chemotherapy-Induced Peripheral Neuropathy Severity During Neoadjuvant or Adjuvant Weekly Paclitaxel Chemotherapy in Breast Cancer Patients. Eur J Cancer (2018) 101:12–9. doi: 10.1016/j.ejca.2018.06.008
36. Molassiotis A, Suen LKP, Cheng HL, Mok TSK, Lee SCY, Wang CH, et al. A Randomized Assessor-Blinded Wait-List-Controlled Trial to Assess the Effectiveness of Acupuncture in the Management of Chemotherapy-Induced Peripheral Neuropathy. Integr Cancer Ther (2019) 182019:1–4. doi: 10.1177/1534735419836
37. Kleckner IR, Kamen C, Gewandter JS, Mohile NA, Heckler CE, Culakova E, et al. Effects of Exercise During Chemotherapy on Chemotherapy-Induced Peripheral Neuropathy: A Multicenter, Randomized Controlled Trial. Support Care Cancer (2018) 26(4):1019–28. doi: 10.1007/s00520-017-4013-0
Keywords: chemotherapy-induced peripheral neuropathy (CIPN), taxane, prevention, neuroprotection, paclitaxel-induced peripheral neuropathy, non-invasive
Citation: Leen AJ, Yap DWT, Teo CB, Tan BKJ, Molassiotis A, Ishiguro H, Fan SWX, Sundar R, Soon YY and Bandla A (2022) A Systematic Review and Meta-Analysis of the Effectiveness of Neuroprotectants for Paclitaxel-Induced Peripheral Neuropathy. Front. Oncol. 11:763229. doi: 10.3389/fonc.2021.763229
Received: 23 August 2021; Accepted: 07 December 2021;
Published: 05 January 2022.
Edited by:
Raquel Abalo, Rey Juan Carlos University, SpainReviewed by:
Yang Li, The Ohio State University, United StatesHelmar Lehmann, University of Cologne, Germany
Laura Gilchrist, St. Catherine University, United States
Copyright © 2022 Leen, Yap, Teo, Tan, Molassiotis, Ishiguro, Fan, Sundar, Soon and Bandla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aishwarya Bandla, lsiab@nus.edu.sg; Yu Yang Soon, yu_yang_soon@nuhs.edu.sg; Raghav Sundar, mdcragh@nus.edu.sg
†These authors have contributed equally to this work and share first authorship
 Alisha Joan Leen
Alisha Joan Leen Dominic Wei Ting Yap4†
Dominic Wei Ting Yap4† Alex Molassiotis
Alex Molassiotis Hiroshi Ishiguro
Hiroshi Ishiguro Raghav Sundar
Raghav Sundar Yu Yang Soon
Yu Yang Soon Aishwarya Bandla
Aishwarya Bandla