- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 2College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
- 3Head and Neck Cancer Surveillance & Research Group, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 4Otolaryngology Head and Neck Surgery, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 5Department of Electrical Engineering, Yuan Ze University, Taoyuan, Taiwan
- 6Division of Medical Oncology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 7Graduate Institute of Medicine, Yuan Ze University, Taoyuan, Taiwan
- 8Institute of Traditional Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 9Division of Radiation Oncology, Department of Radiology, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 10School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 11Division of Thoracic Surgery, Department of Surgery, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 12Medical Device Innovation and Translation Center, National Yang Ming Chiao Tung University, Taipei, Taiwan
Malignancies of the head and neck (HN) region and esophagus are among the most common cancers worldwide. Due to exposure to common carcinogens and the theory of field cancerization, HN cancer patients have a high risk of developing second primary tumors (SPTs). In our review of 28 studies with 51,454 HN cancer patients, the prevalence of SPTs was 12%. The HN area is the most common site of SPTs, followed by the lungs and esophagus, and 13% of HN cancer patients have been reported to have esophageal high-grade dysplasia or invasive carcinoma. The prognosis of HN cancer patients with concomitant esophageal SPTs is poor, and therefore identifying esophageal SPTs as early as possible is of paramount importance for risk stratification and to guide the treatment strategy. Image-enhanced endoscopy, especially using narrow-band imaging endoscopy and Lugol’s chromoendoscopy, has been shown to improve the diagnostic performance in detecting esophageal neoplasms at an early stage. Moreover, the early detection and minimally invasive endoscopic treatment of early esophageal neoplasm has been shown to improve the prognosis. Well-designed prospective studies are warranted to establish appropriate treatment and surveillance programs for HN cancer patients with esophageal SPTs.
Introduction
Malignancies of the head and neck (HN) region and esophagus are among the most common cancers worldwide (1). In parallel with the advances in diagnostic modalities for cancer screening and surveillance, an increasing number of second primary tumors (SPTs) are being detected. SPTs may develop into any kind of malignancy, including malignancy of multicentric origins in the HN region, lungs and esophagus, particularly in HN cancer patients (2–5). This cancerization field known as the upper aerodigestive tract (UADT) is exposed to common carcinogens, particularly cigarette smoke, alcohol, and betel quid. The occurrence of SPTs in the UADT, either synchronously or metachronously, and single or multiple, in HN cancer patients is associated with worse survival despite appropriate management of the primary index HN tumor (2, 3, 6, 7). Of these SPTs, esophageal cancer is associated with a worse prognosis than other sites of the UADT (2, 3). Moreover, esophageal SPTs are easily overlooked as many are diagnosed at asymptomatic early stages (8–12). Therefore, the early identification of esophageal neoplasms and treatment of the primary index cancer and esophageal SPTs is of paramount importance to improve the overall outcomes of HN cancer patients. In this review, we describe the association between HN and esophageal cancers, and propose a screening strategy for esophageal SPTs among HN cancer patients.
Disease Burden of HN Cancer and Esophageal Cancer
Head and neck cancers are the sixth and seventh most common cancers in Taiwan and worldwide, respectively (1, 13). Globally, HN cancer was the fifth most common cancer in men and the 12th most common cancer in women, accounting for an estimated 8,170 and 888,000 new cases in Taiwan and worldwide, respectively, in 2018 (1, 13). The incidence is higher in males, especially middle-aged males, with a male-to-female incidence ratio of 3:1, and most (about 70%) new cases occur in low- and middle-income countries (1). Regarding mortality from HN cancer, there were an estimated 3,027 and 453,000 deaths in Taiwan (the fifth leading cause of cancer deaths) and worldwide, respectively, in 2018 (1, 13). A Canadian study examined the 25-year survival outcomes of 1,657 patients, and reported 2, 5, 15 and 25-year HN cancer-specific survival rates of 74%, 63%, 53% and 49%, respectively (14). In addition, an Italian study of 801 cases reported a 5-year overall survival for HN cancer of 62%, including 55% for cancer of the oral cavity, 53% for the oropharynx, 41% for the hypopharynx, and 71% for the larynx (15). In Taiwan, the 5-year overall survival for HN cancer during the past decade ranged from 40~60%, and the standardized death growth rate in men was 7.7% (13).
Esophageal cancer is the eighth most common cancer (sixth in Taiwanese males) and the sixth most common cause of cancer deaths (ninth in Taiwan) worldwide (1, 13). Malignancy of the esophagus has two main histological subtypes, namely esophageal squamous cell carcinoma (ESCC), and esophageal adenocarcinoma (EAC). ESCC accounts for the majority (93.13% in Taiwan, 87% globally) of all esophageal cancer cases (1, 13). In 2012, there were an estimated 398,000 and 52,000 new cases of ESCC and EAC, respectively, worldwide (1). In Taiwan, 2,436 and 84 new cases of ESCC and EAC were reported in 2018 (13). The male-to-female incidence ratio is 2.7:1 for ESCC and 4.4:1 for EAC (1). Similar to HN cancer, about half (52.71%) of esophageal cancers develop in patients aged between 40~60 years in Taiwan (13). The overall prognosis of esophageal cancer is poor because most cases are diagnosed at a late stage with obstructive symptoms. Only 15.93% of esophageal cancer patients are diagnosed at stage 0/I, compared to 69.83% at stage III/IV in Taiwan (13). The overall 5-year survival rate for esophageal cancer is less than 10~20%, and lower than 5% in low- and middle-income countries (1, 13, 16). In Taiwan, the standardized death growth rate of esophageal cancer during the past decade was 15.5% (13).
The incidence rates of both HN and esophageal cancers are increasing and the prognosis is unsatisfactory, especially for esophageal cancer. Most cases occur in middle-aged males with a great impact on cancer-related morbidity and mortality. Consequently, early detection through screening programs for patients at high risk is crucial to improve their prognosis.
Association Between HN and Esophageal Cancers
Common Risk Factors and the Epidemiology for HN Cancer and Esophageal SPTs
The risk factors for HN cancer include male sex, infectious agents [human papillomaviruses (HPV), Epstein–Barr virus], exposure to carcinogens (tobacco or marijuana use, alcohol consumption, betel quid chewing), poor oral hygiene, history of esophageal cancer, drinking hot beverages such as maté, occupational exposure (metal smelting and textile production), and consumption of preserved foods with high nitrosamine content (1, 13, 17–19). In addition, genetic factors have also been associated with the development of HN cancer. Among non-HPV-related HN cancers, TP53 and cyclin-dependent kinase inhibitor 2A (CDKN2A) are the most affected genes, while the genetic changes in HPV-related tumors are in the phosphoinositide 3-kinase (PI3K) pathway, particularly involving activating mutations and amplifications of the PIK3CA oncogene (1, 6). Alcohol-metabolizing enzyme gene polymorphisms have also been associated with a higher risk of HN cancer (19, 20). For ESCC, the risk factors are older age, male sex, low body mass index, lower socioeconomic status, exposure to carcinogens (alcohol consumption, cigarette smoking, and betel quid chewing), low fruit/vegetable consumption, high meat/high temperature beverage intake, family members with esophageal cancer, history of HN cancer, poor oral hygiene, genetic polymorphism of alcohol-dehydrogenase-1B (ADH1B) and aldehyde dehydrogenase-2 (ALDH2), and motor disorders of the esophagus (e.g., achalasia) (7, 19, 21). For EAC, the most important risk factors are obesity, gastroesophageal reflux disease and Barrett’s esophagus (7). Mutations of tumor suppressor genes, multiple allelic losses, hypermethylation of promoter genes, genetic overexpression, and changes in miRNA expression profile have also been reported in both EAC and ESCC (7).
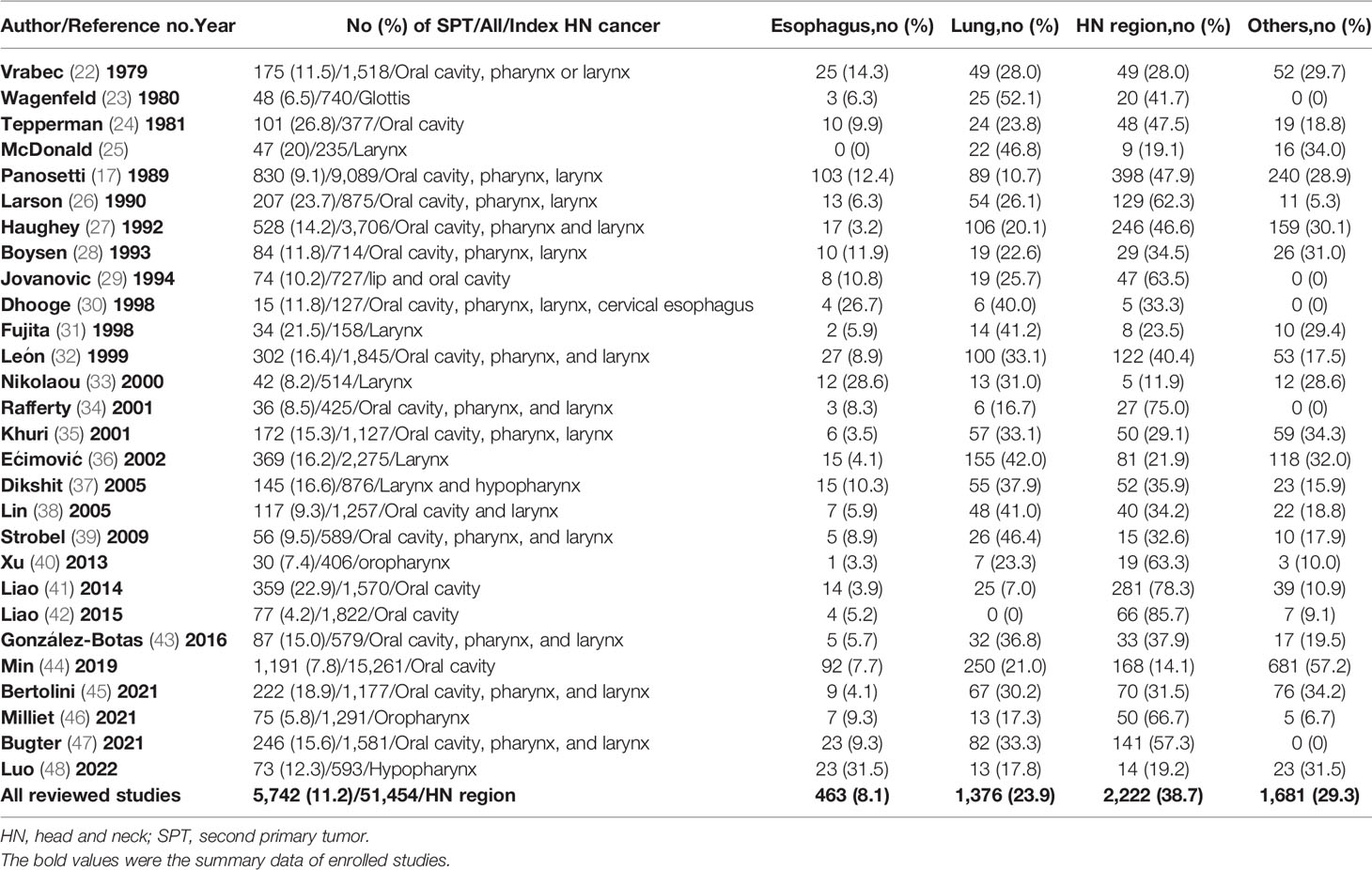
There are many common risk factors for the development of HN cancer and ESCC. The squamous epithelium of both the HN region and esophagus are exposed to common environmental factors, particularly carcinogens. Consequently, with underlying genetic alterations such as polymorphisms in alcohol-metabolizing enzyme genes, those with accumulating exposure to carcinogens may develop both HN cancer and ESCC (Figure 1). Several epidemiology studies have demonstrated an increased risk of synchronous and metachronous SPTs among HN cancer patients. We used keywords including “head and neck” AND “ esophageal cancer” AND “ second tumor” AND “screening” for literature review on PubMed. Exclusion criteria were as followings: studies without data upon incidence of esophageal SPTs, review article, case reports and number of HN cancer patients less than one-hundred (Figure 2). In our review of 28 studies with 51,454 HN cancer patients, the estimated prevalence of SPTs was 12% (95% CI, 10-15% with a random effects model). The index primary cancer, sites of SPT, and screening modalities in these 51,454 HN cancer patients are shown in Table 1 and Figure 3 (3, 8, 11, 12, 17, 22–45, 47–51). One 10-year follow-up study of 6,258 HN cancer patients reported that 21.8% presented with SPTs, with the highest excess absolute risk (EAR) for SPTs of the lungs, followed by those located at the HN region and esophagus (52). Similar results were reported in a population-based cohort study of 64,673 HN cancer patients in the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry between 1979 and 2008, in which the standardized incidence ratio (SIR) of synchronous SPTs was 5.0, with the highest excess risk of a second cancer at the HN region (SIR, 41.4), followed by the esophagus (SIR, 21.8), and lungs (SIR, 7.4) (53). In addition, a meta-analysis reported an SIR for metachronous SPTs, which were defined as occurring six months after the primary index tumor, of 2.04 (95% CI, 1.61~2.59) (9). The highest risk for metachronous SPTs located at the HN region was for the oropharynx (SIR, 17.82; 95% CI, 6.79–46.77), followed by the hypopharynx (SIR, 9.17; 95% CI, 3.51–23.98) and larynx (SIR, 4.12; 95% CI, 2.87–5.90), while the highest risk for SPTs located outside the HN area was for the esophagus (SIR, 4.64; 95% CI, 3.12–6.89), followed by the salivary glands (SIR, 8.30; 95% CI, 2.37–29.09) and thyroid (SIR, 1.47; 95% CI, 1.22–1.76) (9). In a study that defined a metachronous SPT as occurring 2 months after the primary HN cancer, an increased risk for metachronous SPTs of the lungs (SIR, 4.32; 95% CI 2.15-8.68) was also noted (9). Another systematic review of 456,130 HN cancer patients from 61 articles with a minimum follow-up of 22 months reported a mean incidence of SPTs of 13.2% (95% CI, 11.56-14.84), including 5.3% for synchronous SPTs (95% CI, 4.24-6.36) and 9.4% for metachronous SPTs (95% CI, 7.9-10.9) (54). In addition, the most common site of SPTs was the HN area, followed by the lungs and esophagus, which is similar to other studies (54). Metachronous SPTs are more prevalent than synchronous SPTs, and therefore, surveillance programs including investigations for SPTs are of paramount importance to improve the long-term care of HN cancer patients (17, 55).
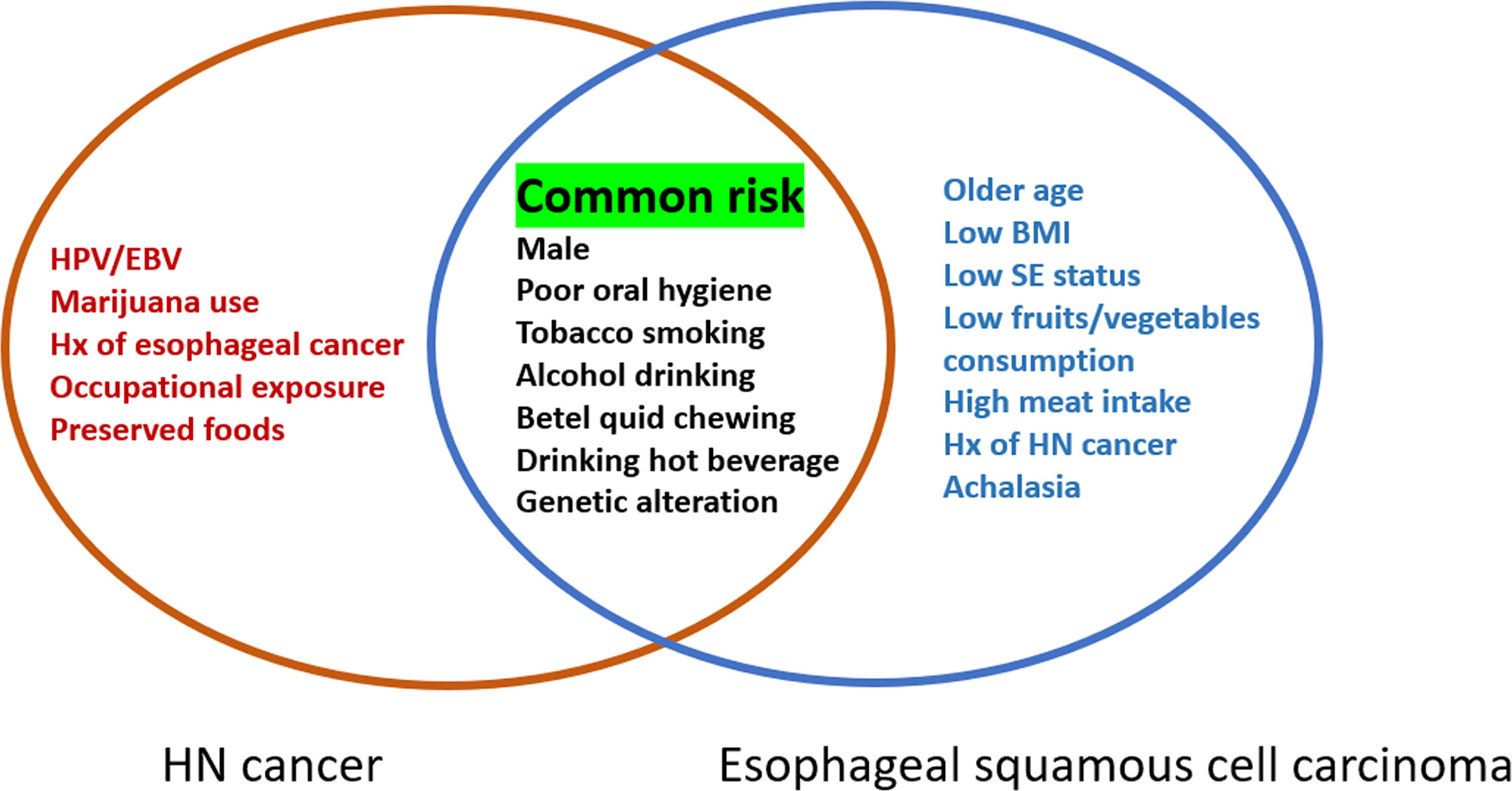
Figure 1 Risk factors for head and neck cancer and esophageal squamous cell carcinoma. HPV, human papillomavirus; EBV, Epstein-Barr virus; Hx, history; BMI, body mass index; SE, socioeconomic.
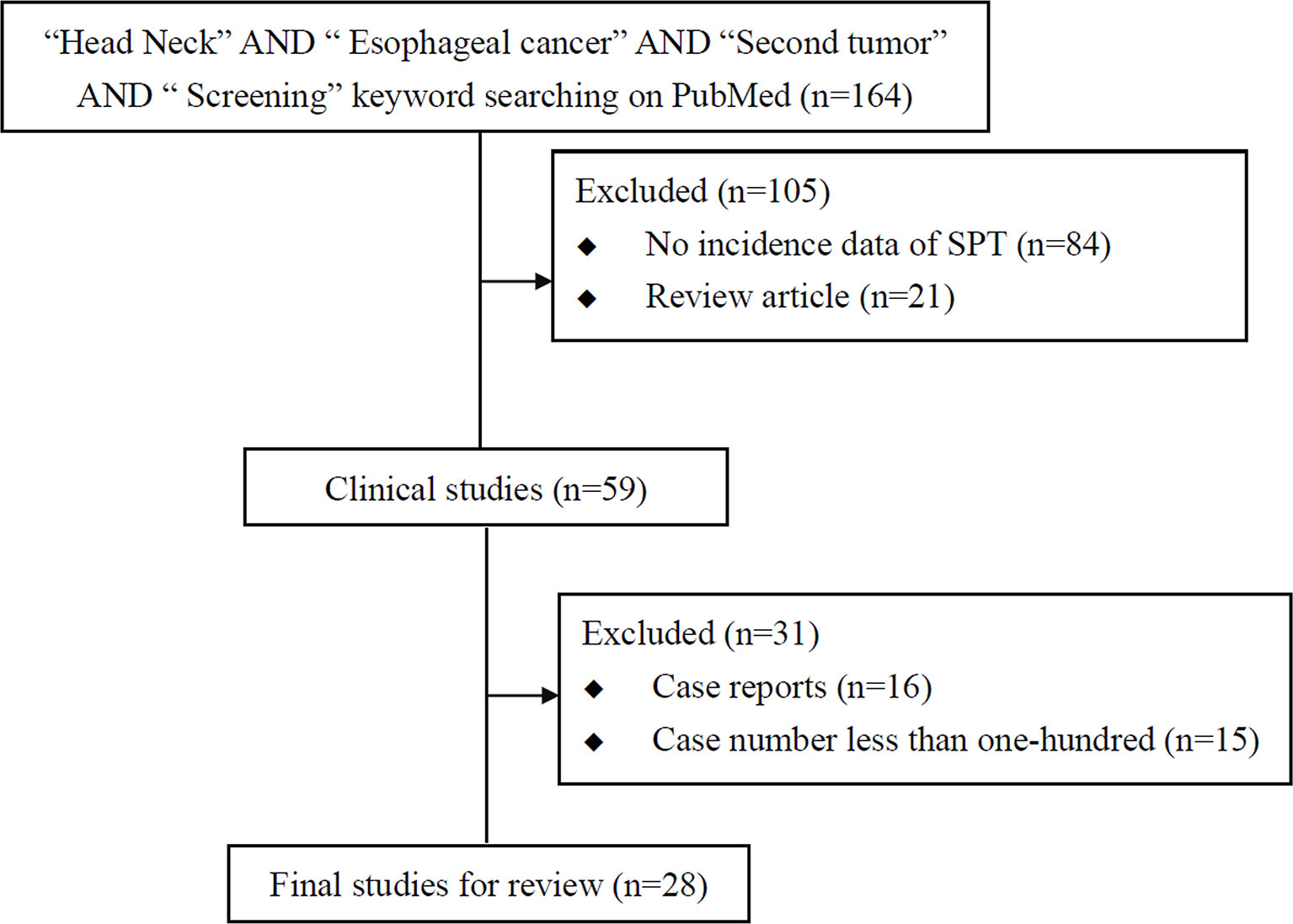
Figure 2 Flowchart of literature review of studies on screening esophageal second primary tumor (SPT) in head and neck cancer patients.
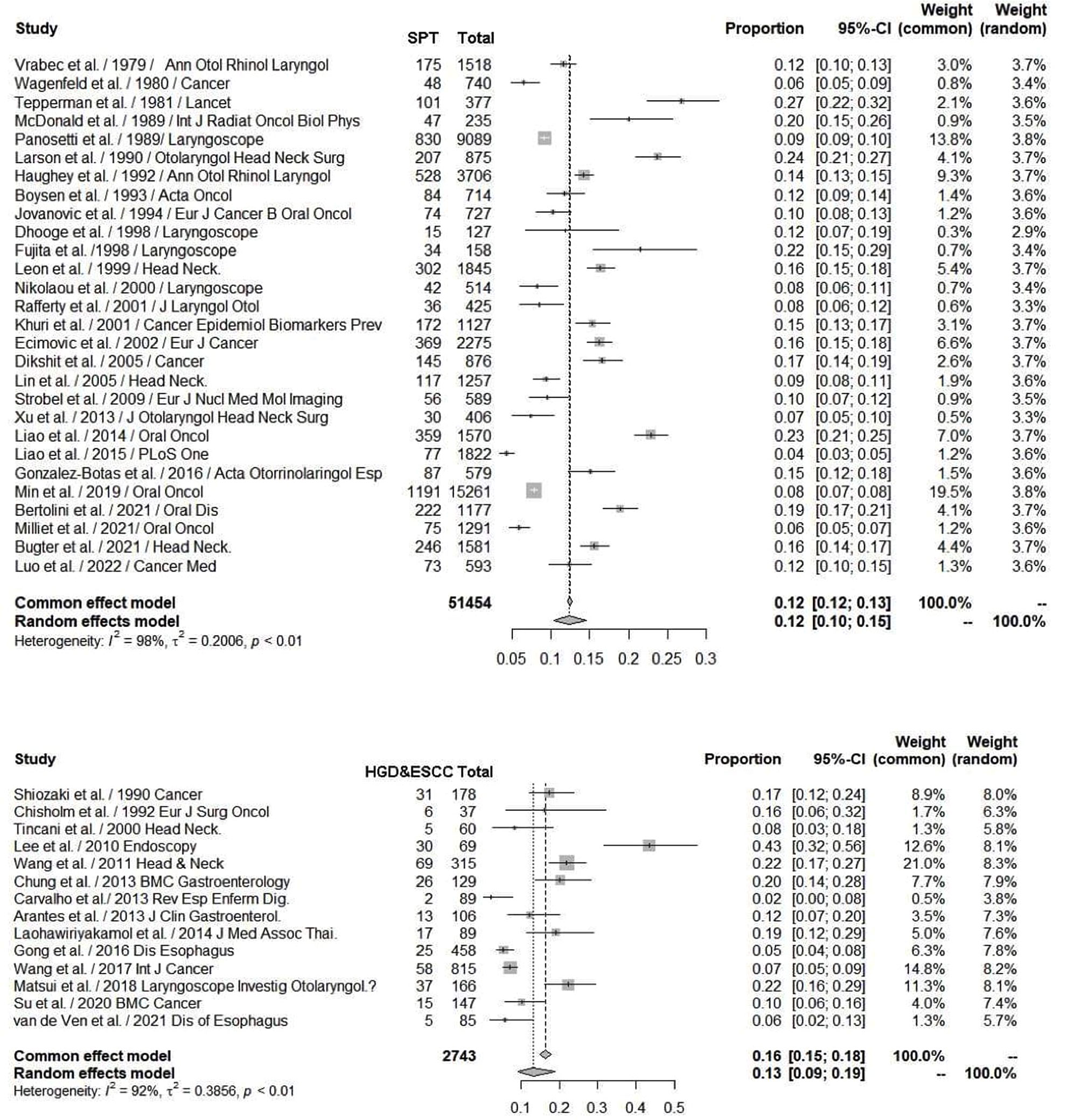
Figure 3 Upper: Forest plots showing the reported proportion of SPTs among head and neck cancers with a random effect models due to significant heterogeneity, the overall SPT rate was 12% (95% CI, 10-15%). Lower: Forest plots showing a reported 13% incidence rate of HGD and ESCC (95% CI, 9-19%) by image-enhanced endoscopy screening among head and neck cancer patients. ESCC, esophageal squamous cell carcinoma; HGD, high-grade dysplasia; SPT, second primary tumor.
Different Risk for Esophageal SPTs According to the Primary Site of HN Cancer
The risk factors for SPTs are different depending on the primary site of the index HN cancer. One study of 75,087 HN cancer patients in the SEER database reported the highest risk for SPTs for primary hypopharyngeal cancer (SIR, 3.5; EAR, 307.1 per 10,000 person-years) and the lowest for laryngeal cancer (SIR, 1.9; EAR, 147.8 per 10,000 person-years) (56). Nasopharyngeal cancer (NPC) arises from a unique site with a large number of resident leukocytes, predominantly T-cells, together with other stromal cells. Therefore, the pathophysiology and tumor phenotype of NPC is quite different from other HN cancers, and the reported association between NPC and ESCC is lower than for other primary sites in the HN region. One large retrospective study of a cohort of 1,549 NPC patients following radiotherapy in Taiwan reported increased risks of developing SPTs in the HN region (SIR, 16.5; 95% CI, 10.0~26.8), stomach (SIR, 5.5; 95% CI, 2.2~11.4) and leukemia (SIR, 9; 95% CI, 1.9~26.3) (57). In a multicenter study of 8,947 NPC patients, 167 (1.9%) patients developed SPTs with increased risks of tongue cancer, non-Hodgkin’s lymphoma, brain cancer, myeloid leukemia and non-melanoma skin cancer (58). Interestingly, the risk of developing SPTs has been shown to vary between different histological subtypes among NPC patients. A cross-sectional study of 1,175 NPC patients reported that SPTs, and especially those located in the HN region and UADT, were more prevalent in keratinizing NPC compared to non-keratinizing NPC (59). Another multicenter study of 3,166 NPC patients also reported significantly higher risks of cancer in the oral cavity, sarcoma, oropharynx, paranasal sinus, salivary gland, thyroid, skin and lungs (60).
Of note, a significantly lower risk of SPTs has been demonstrated among patients with oropharyngeal SCC in the HPV infection era (annual percentage change in EAR, -4.6%; p = 0.03), and that routine panendoscopy examinations are not even recommended in some studies (56, 61). A Canadian retrospective study of 406 oropharyngeal cancer patients reported a significantly lower incidence rate of SPTs in those who were p16-positive, which is indicative of HPV-related oropharyngeal cancer patients (0.7 per 100 patient-years vs. 8.5 in p16-negative patients, p < 0.0001) (40). In addition, the yield rate of field cancerization work-up (2.8% vs. 10.2%, p = 0.02) was lower in the HPV-positive than in the HPV-negative oropharyngeal cancer patients (40). Moreover, multivariate analysis from a multicenter study of 1,291 HN cancer patients showed that p16-negative tumor status (p = 0.003), tobacco/alcohol consumption (p = 0.005), and soft palate tumor site (p = 0.009) were significantly associated with a higher risk of metachronous SPTs (46). Furthermore, a higher proportion of metachronous SPTs arising outside the UADT was found in HPV-positive than in HPV-negative patients (46).
Second Primary Tumors of HN Region in Primary Esophageal Cancer Patients
Second primary neoplasms occur mutually in patients with UADT cancers. Patients with primary ESCC are also at risk of SPTs in the HN region. Analysis of data from a mean follow-up period of 76 months in a study of 285 ESCC patients showed 5-year cumulative occurrence rates of metachronous SPTs of the esophagus, HN region and stomach of 14.0%, 2.8% and 4.1%, respectively (62). Another study of 439 superficial esophageal cancer patients reported that 53 metachronous HN cancers developed in 40 (9.1%) patients after a median follow-up period of 46 months, and the cumulative incidence rates of metachronous HN cancers at 3, 5, and 7 years were 5.3%, 9.7%, and 17.2%, respectively (63). A systematic review of 6,483 ESCC patients from 12 studies in Japan revealed a pooled prevalence of HN SPTs of 6.7% (95% CI, 4.9~8.4%), including 48.2% synchronous and 51.8% metachronous SPTs, 85.3% at an early stage, and 60.3% located in the hypopharynx (18).
Prognosis of HN Cancer Patients With Esophageal SPTs
Esophageal SPTs not only occur synchronously or metachronously, but also have a negative impact on the prognosis of HN cancer patients (64). The 15-year survival rate of HN cancer patients with SPTs is lower than in those without SPTs (22% vs. 54%), and the prognosis is especially poor with a 5-year survival rate of only 6% in those with esophageal SPTs (vs. 25% in those with all SPTs) (2, 3, 26). Another study also demonstrated lower 5-year (68% vs. 76%) and 10-year (26% vs. 57%) overall survival rates in laryngeal cancer patients who developed SPTs (p = 0.003) (31). A nationwide analysis of 93,891 HN cancer patients from the Taiwan Cancer Registry reported that 9,996 (10.6%) patients presented with SPTs, and that those with SPTs had a significantly lower survival rate (univariate analysis: HR, 2.59; 95% CI, 2.53-2.65; multivariate analysis: HR, 2.34; 95% CI, 2.28-2.40) (65).
To summarize, the risk and distribution of SPTs differ significantly according to the subsite of the index primary HN cancer, with a lower risk in laryngeal and HPV-positive oropharyngeal cancer patients. About 11.2% of HN cancer patients develop either synchronous or metachronous SPTs at the HN region (38.7%), lung and bronchus (23.9%), and esophagus (8.1%) (Table 1). The occurrence of ESCC is especially associated with a poor prognosis, and thus identifying esophageal SPTs is crucial in screening and surveillance programs for HN cancer patients.
Image-Enhanced Endoscopic Screening and Risk Factors for Esophageal SPTs in HN Cancer Patients
Esophagogastroduodenoscopy is the most reliable diagnostic tool for esophageal neoplasms, especially using an image-enhanced endoscopy (IEE) system, which is composed of optical- and dye-based technology (49, 66, 67). Among several IEE techniques, narrow-band imaging (NBI) and chromoendoscopy with Lugol’s solution are widely used for screening ESCC (49, 66–68). By using narrow-bandwidth filters to remove red light and narrow wavelengths of green (540 nm) and blue (415 nm) light, NBI can improve visualization of hemoglobin-rich vascular microstructures (Figure 4) (49). Because the color of gastrointestinal mucosa is primarily determined by hemoglobin, and neovascularization occurs in neoplastic squamous epithelium of the esophagus, the light emitted from NBI is absorbed by neoplastic mucosa more than healthy mucosa. Therefore, early neoplasms, which usually have a flat morphology, can be differentiated from normal mucosa by dark brownish discoloration compared with the greenish color of healthy mucosa under NBI (Figure 5). In addition, when combining a magnifying endoscope with an NBI system, the microvascular pattern of neoplastic squamous cell epithelium can be well delineated (Figure 6) (49, 67, 69). These microvessels seen under magnifying NBI, so-called intra-epithelial papillary capillary loops, can also predict tumor invasion depth with accuracy of 90.5% (69). Among dye-based IEE, iodine-containing Lugol’s solution is commonly used for ESCC screening. Normal glycogen-abundant squamous epithelium reacts with Lugol’s solution, while dysplastic mucosa with diminished or absent glycogen remains unstained (67, 68, 70). By spraying Lugol’s solution on esophageal mucosa, unstained areas are indicative of dysplastic or cancerous parts. Moreover, when unstained mucosa turns pink within a few minutes, high-grade dysplasia or squamous cell carcinoma can be diagnosed with a sensitivity of 91.9% and specificity of 94.0% (Figure 7) (68).
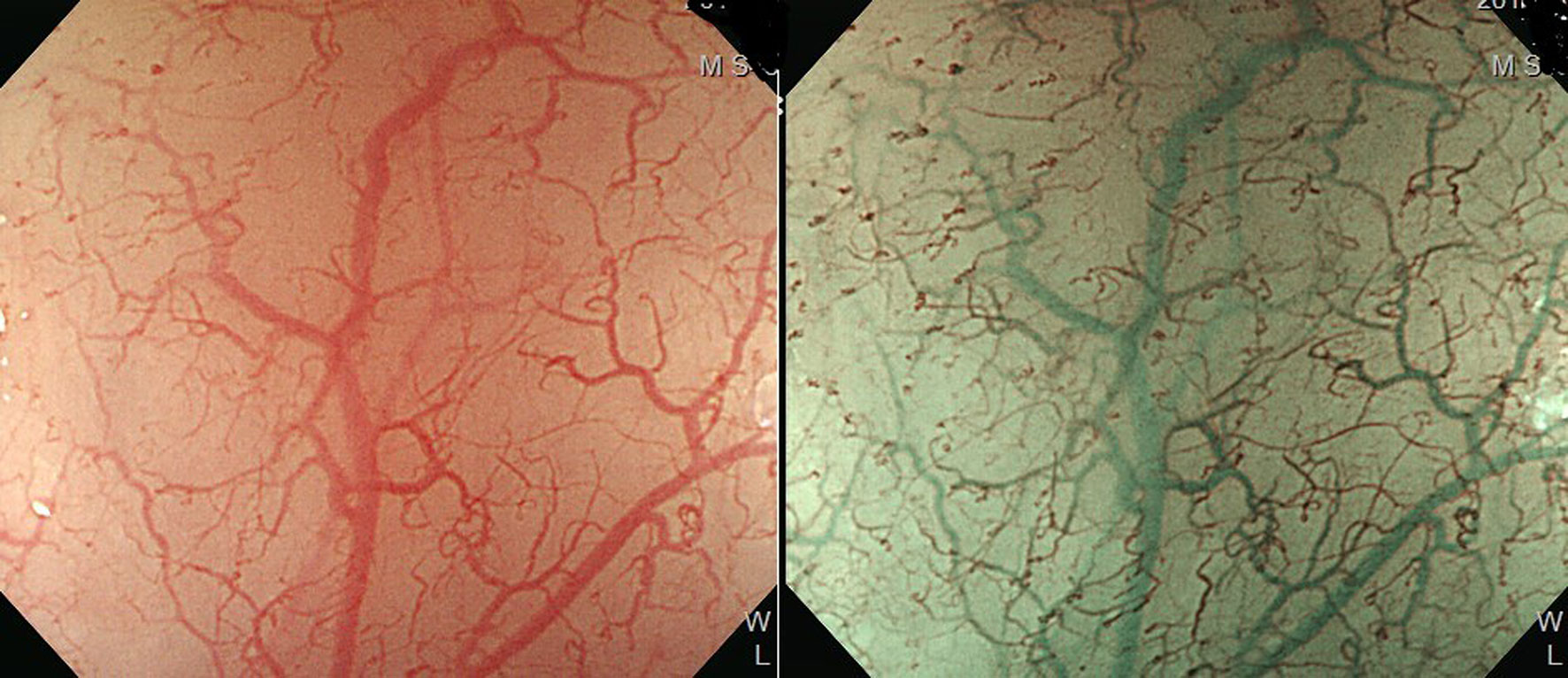
Figure 4 Improved visualization of microvascular structure under narrow-band imaging endoscopy (Left: conventional white-light imaging. Right: narrow-band imaging.).
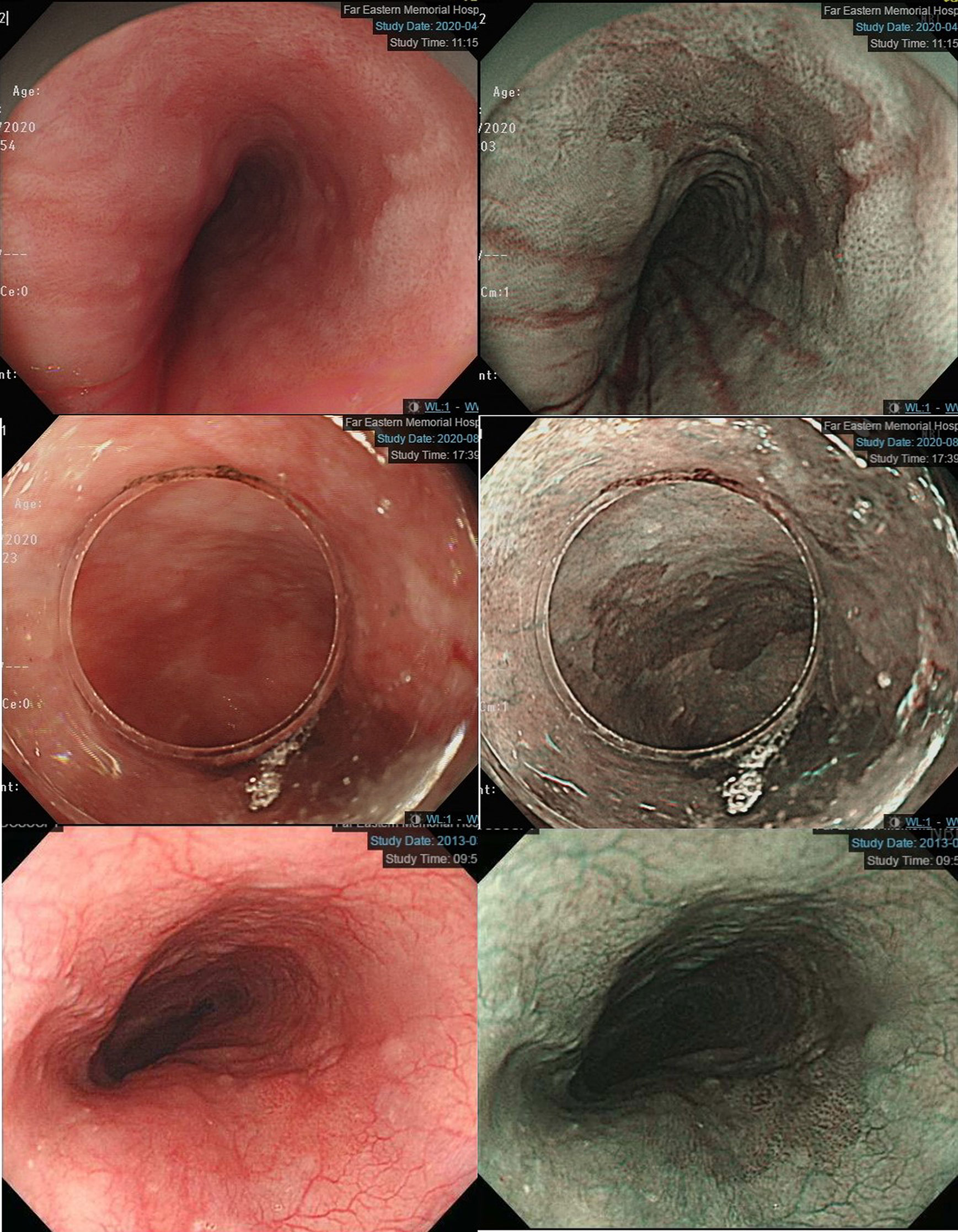
Figure 5 Left panels: Early esophageal neoplasm with barely visible flat morphology under conventional white-light endoscopy. Right panels: Dark brownish color compared with the greenish color of healthy mucosa under narrow-band imaging endoscopy.
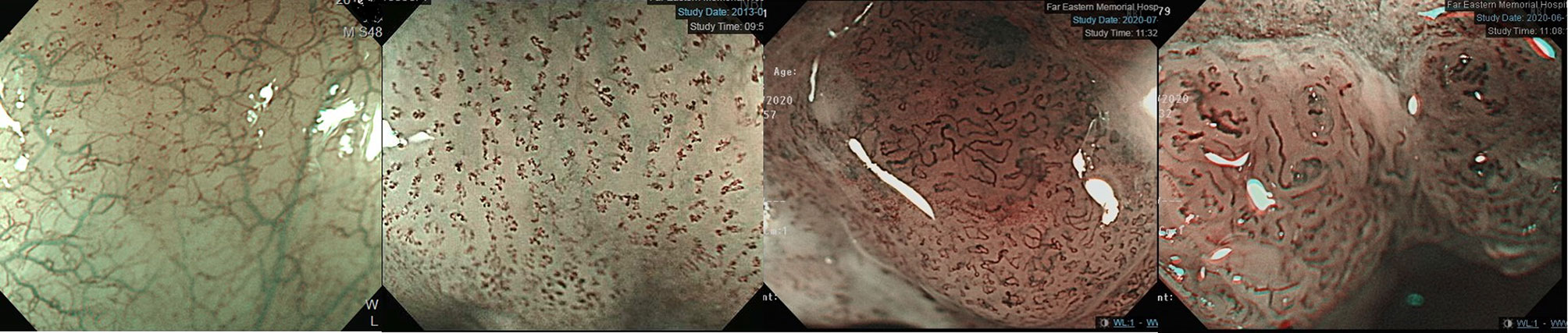
Figure 6 JES classification of microvessel morphology of IPCL. From left to right: JES type A- Normal IPCL without irregularity. JES type B1- Abnormal microvessels with severe irregularity, meandering caliber or highly dilated proliferative abnormal vessels with a loop-like formation. JES type B2- Abnormal microvessels with severe irregularity, meandering calibers or highly dilated proliferative abnormal vessels without a loop-like formation. JES type B3- Highly dilated microvessels with three times as many calibers than usual type B2 vessels. IPCL, intraepithelial papillary capillary loop; JES, Japanese Esophageal Society.
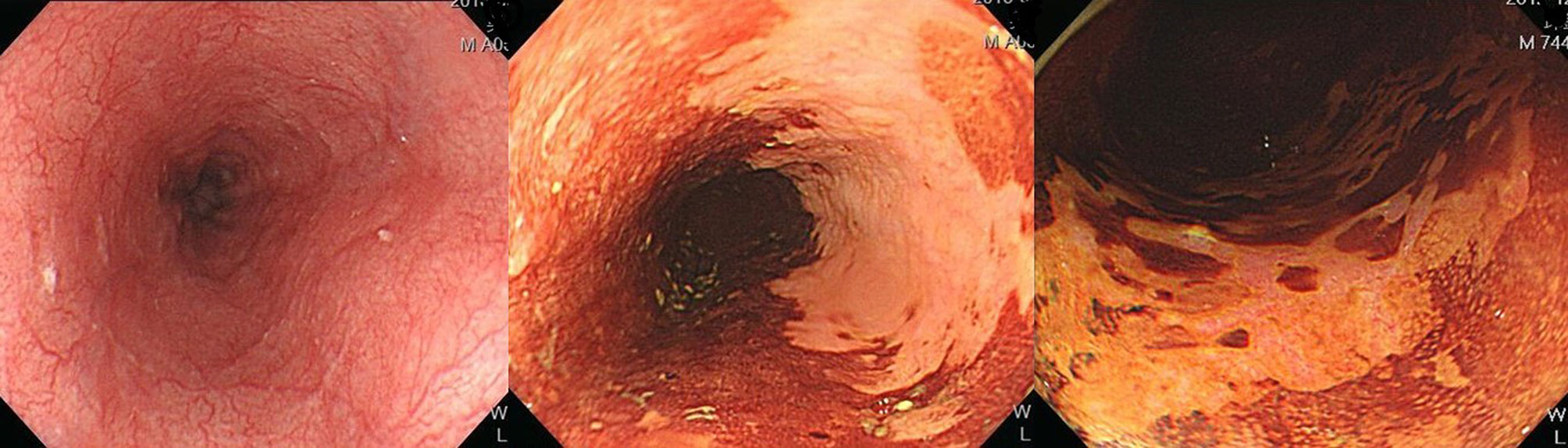
Figure 7 Esophageal high-grade dysplastic lesion. Left: Normal appearance upon white-light endoscopy. Middle: Lugol-voiding unstained mucosa. Right: The color of Lugol-unstained mucosa turns pink in a few minutes.
Both NBI and Lugol’s chromoendoscopy (LCE) are effective real-time screening endoscopic techniques for the early detection of esophageal neoplasms. A meta-analysis of 4,918 esophageal and HN cancer patients from 16 prospective and randomized trials showed that NBI and LCE had better diagnostic performance than conventional white-light imaging, with pooled sensitivity, specificity and area under the receiver operating characteristic curve of 87% (95% CI, 83~90%) and 88% (95% CI, 85~91%) versus 53% (95% CI, 48~59%), 99% (95% CI, 98~99%) and 95% (95% CI, 94~96%) versus 63% (95% CI, 61~66%), and 97% and 82% versus 66%, respectively (66). Given that most esophageal SPTs detected in HN cancer patients are at asymptomatic premalignant or early cancer stages, these lesions might be overlooked by white-light imaging or even advanced cross-sectional and radionuclide imaging modalities. In a study of 147 HN cancer patients, suspicious esophageal SPTs were identified by position emission tomography/computed tomography (PET/CT) in 8 (5.4%) and by NBI endoscopy in 35 (23.8%) patients (71). In addition, the diagnostic sensitivity of NBI endoscopy (100.0%) was superior to whole body PET/CT (33.3%) in detecting esophageal SPTs (71). In a review of 14 studies with 2,743 HN cancer patients, IEE screening identified esophageal high grade dysplasia or invasive carcinoma in 13% (95% CI, 9-19% with a random effects model) of the patients (Table 2, Figure 3) (8, 10–12, 70–79). Most of the esophageal SPTs were at an early stage without tumor-related obstructive symptoms. Therefore, if these esophageal SPTs had not been identified, the patients may have had a poor prognosis from esophageal cancer.
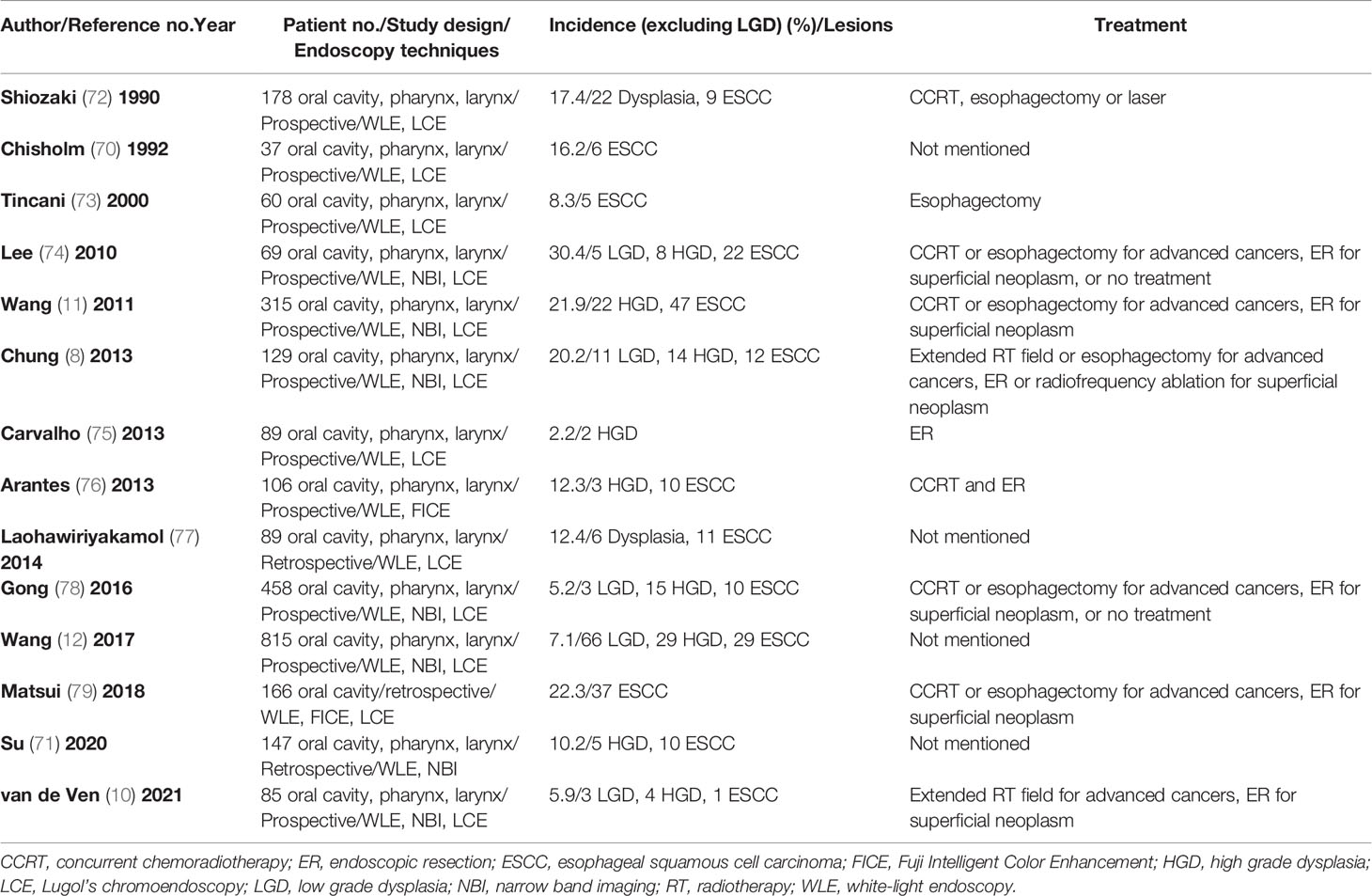
Table 2 Image-enhanced endoscopic screening of synchronous or metachronous esophageal neoplasm in HN cancer patients.
There are many common risk factors for HN and esophageal cancers. Among environmental factors, alcohol is one of the most important carcinogens for esophageal cancer (1, 19, 21). The results from a meta-analysis of 8 cohort and 11 case-control studies showed that alcohol drinking was associated with significantly increased risk of UADT SPTs (RR, 2.97; 95% CI, 1.96~4.50), and that every increase of 10 g/day in alcohol intake resulted in a significantly increased RR of 1.09 (95% CI, 1.04-1.14) for UADT SPTs in a dose-response relationship (80). Alcohol metabolizing enzyme genes are disease modifiers which are responsible for the increased risk of cancer after alcohol consumption (81). Ethanol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH), then converted to acetate by acetaldehyde dehydrogenase (ALDH). The intermediate metabolized product, acetaldehyde is not only associated with unpleasant disulfiram-like reactions such as facial flushing, nausea, vomiting, tachycardia and hypotension, but also increased oxidant stress, inflammation and reactions with deoxynucleosides, leading to the formation of deoxyribonucleic acid adducts and subsequently cancerization (19, 81, 82). The results from a case-control study of 120 HN cancer and 138 ESCC patients in Taiwan demonstrated that the minor alleles of ADHB (rs1229984) and ALDH2 (rs671) were associated with an increased risk of UADT cancers (OR, 3.53 and 2.59; 95% CI, 2.14~5.80 and 1.79~3.75), and also that they potentiated the carcinogenic effects of alcohol (OR, 53.44 and 70.08; 95% CI, 25.21~113.29 and 33.65~145.95) (19). In addition, the haplotypes GAGC and CCAATG on chromosome 4 and 12, respectively, have been associated with a higher risk of HN and esophageal cancers (19). Another case-control study with age- and gender-matched 164 HN cancer patients showed that polymorphisms in ADH1B (OR, 2.09; 95% CI, 1.15~3.18; p < 0.05) and ALDH2 (OR, 5.19; 95% CI, 2.44~11.00; p < 0.001) increased the risk of developing multiple SPTs (20). Thus, HN cancer patients who are alcohol drinkers have a higher risk of esophageal SPT, particularly those carrying risk genetic polymorphisms of alcohol-metabolizing enzymes.
Primary sites of HN cancer are associated with different risk of developing esophageal SPTs. Compared with oral cavity and nasopharyngeal cancers, primary malignancy of the hypopharynx, HPV-negative oropharynx, and larynx are more likely to have esophageal SPTs (8, 11, 12, 50, 53, 54, 71). Other demographic data, including older age, comorbidities, lower body mass index, advanced stages of primary HN cancer and alcohol flushing syndrome have also been associated with a higher risk of esophageal SPTs (8, 12, 47). A systematic review identified 51 genes that were significantly associated with an increased risk of SPTs among HN cancer patients (83). In addition, the presence of multiple Lugol-voiding lesions, which are indicative of dysplastic or cancerous lesions in the esophagus, has also been reported to be a significant risk factor for developing both synchronous and metachronous SPTs (62, 84). A 13-year follow-up study of 682 patients with esophageal dysplasia reported that 23.7%, 50% and 73.9% of patients with low-grade, moderate, and high-grade dysplasia (HGD) developed invasive carcinoma (85). The molecular changes in Lugol-voiding mucosa precede the cancerization process, and the hotspot p53 mutation has been identified in 20% and 40% of non-dysplastic and dysplastic Lugol-voiding mucosa (84). Therefore, when multiple Lugol-unstained areas are noted after LCE screening, a shorter interval of IEE surveillance for metachronous esophageal SPTs is mandatory.
For HN cancer patients at risk of esophageal neoplasms, endoscopic screening and surveillance, especially using IEE techniques with NBI endoscopy and LCE, are crucial to identify esophageal SPTs. Before the development of obstructive symptoms from advanced esophageal neoplasms, the early detection of esophageal SPTs is one of the most important management strategies to improve the overall prognosis of HN cancer patients (Figure 8).
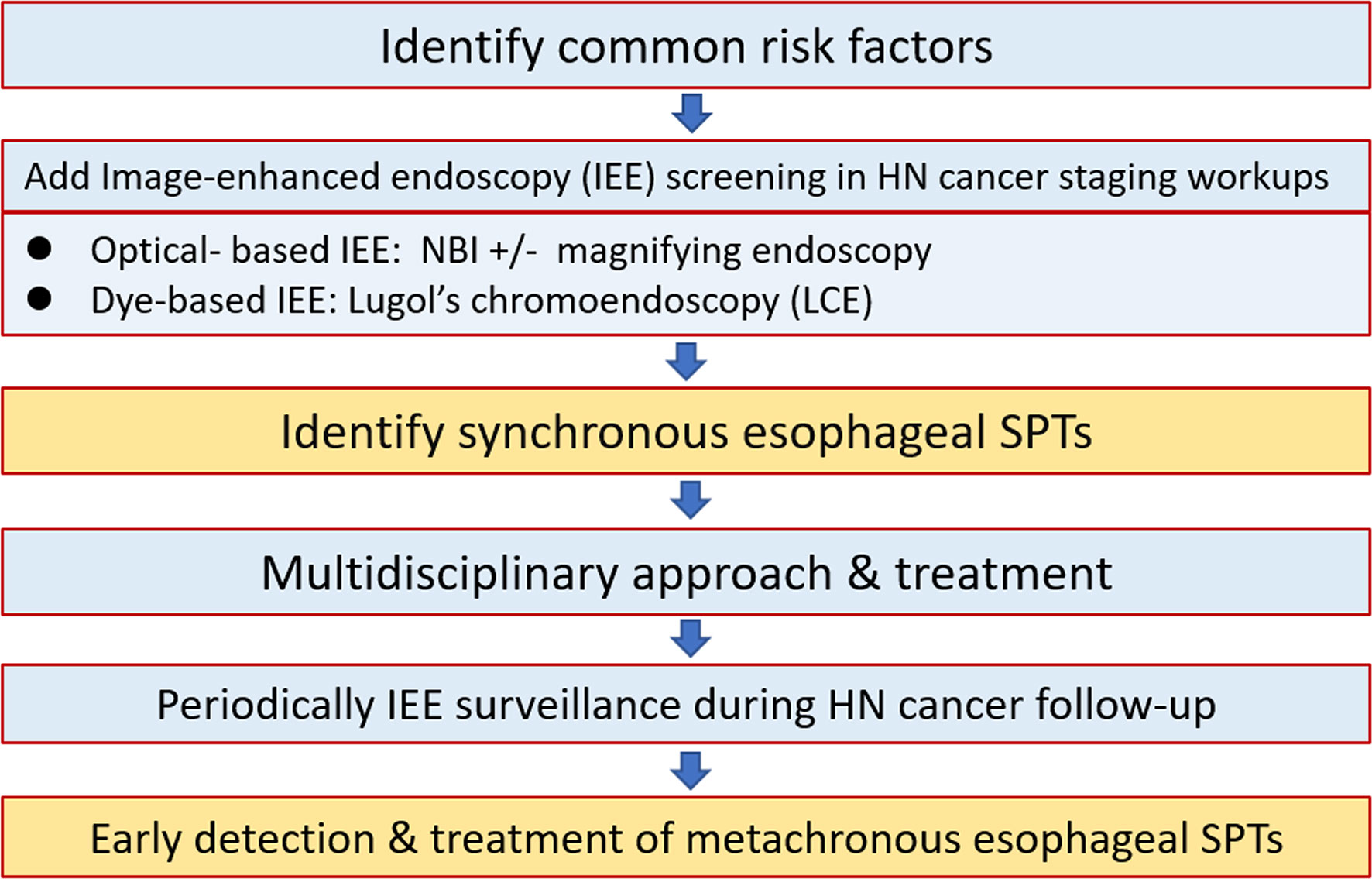
Figure 8 Approach algorithm for head and neck cancer patients at risk of esophageal second primary tumors. HN, head and heck; IEE, image-enhanced endoscopy; LCE, Lugol’s chromoendoscopy; NBI, narrow-band imaging; SPT, second primary tumor.
Screening and Treatment Strategy of Esophageal SPTs for HN Cancer Patients
After screening for esophageal SPTs, HN cancer patients who are free from synchronous esophageal SPTs have the best outcomes (16). Thus, before starting treatment of newly diagnosed HN cancers, risk stratification and identification of synchronous esophageal SPTs could modify the oncological treatment plan (8). When considering ESCC treatment, surgical esophagectomy was the traditional curative therapeutic option. However, in the early 20th century, with advances in minimally invasive endoscopic resection techniques, early esophageal neoplasms could be managed by endoscopic submucosal dissection (ESD) and radiofrequency ablation (RFA) (86, 87). Due to the low risk of nodal or distant metastasis of superficial esophageal neoplasms, ESD can be considered as the first-line therapy for HGD or ESCC limited to the epithelium and lamina propria without lymphovascular invasion, while RFA can be considered for flat-type esophageal HGD or ESCC confined above the lamina propria (86–88). The overall curative resection and recurrence rates of esophageal neoplasms for ESD have been reported to be 78~100% and 0~2.6%, respectively, with complete remission and recurrence rates of 50~100% and 0~50% for RFA (86, 88). Five-year overall, disease-specific and metastasis-free survival rates above 90% have been reported after ESD for early esophageal neoplasms (86, 89, 90). Compared with surgical intervention, ESD (relative hazard, 0.89; 95% CI, 0.51~1.56; p = 0.68) has comparable long-term outcomes for early esophageal neoplasms, with a better quality of life and lower rate of adverse events (86, 90, 91). However, stricture complications are one of the most important concerns after ESD for large size neoplasms or those which involve more than 75% of the circumference (86, 90, 91). Most post-ESD strictures can be managed by endoscopic balloon dilation or prophylactic steroid therapy. As a result, identifying early esophageal SPTs in HN cancer patients could be a triage for screening and surveillance programs, and could also provide a chance for minimally invasive endoscopic resection with curative intent of early esophageal SPTs.
When considering the treatment strategy, the curability of both primary and secondary neoplasms must be carefully evaluated and discussed with a multidisciplinary approach. In HN cancer patients, prior treatment of the primary cancer often affects the treatment of esophageal SPTs. Trismus, malnutrition with cancer cachexia, performance status, the location of the esophageal SPT, and patient preference are important factors which should be taken into account. The treatment for esophageal SPTs, including endoscopic resection, concurrent chemoradiotherapy (CCRT), surgical intervention or no treatment, varies between studies due to the heterogeneous characteristics of HN cancer patients (Table 2). Cox proportional regression analysis of the SEER database which enrolled 3,038 HN cancer patients showed that those with SPTs of the HN region who underwent conservative surgery with radiation had the best 5-year overall survival rate (22.6%), those with lung SPTs who underwent radical surgery had the best 2-year overall survival rate (60.8%), and that there was no difference in the prognosis between treatment groups in those with esophageal SPTs (64). However, in a prospective study with long-term outcome analysis of 145 HN cancer patients, those with early esophageal SPTs who underwent aggressive treatment of both primary and secondary neoplasms had similar overall survival compared to HN cancer patients without esophageal SPTs (p = 0.47) (92). Definitive CCRT of esophageal cancer patients with synchronous HN SPTs can also safely be offered to improve overall survival, and those who receive CCRT have been shown to have better survival than those with radiotherapy alone (93).
Screening of esophageal SPTs by IEE should be performed in every newly diagnosed HN cancer patient, and regular IEE surveillance is also important to detect metachronous esophageal neoplasms. After identifying esophageal SPTs in HN cancer patients, management of neoplasms at the primary and secondary sites is quite complex and should be individualized according to the patient’s condition. It depends on the stage and survival of the primary and secondary tumors, prior treatments, expertise in endoscopic resection techniques and CCRT, as well as the patient’s performance and preference. Close cooperation between medical staff members including HN surgeon, gastroenterologist, endoscopist, oncologist and radio-oncologist are essential in a multidisciplinary approach.
Summary
The development of synchronous or metachronous SPTs is more frequently being identified due to advances in diagnostic modalities, and it is an emerging issue in oncology medicine. SPTs are not uncommon among HN cancer patients, particularly those located in the HN region, lungs and esophagus. Patients with HN cancer and concomitant esophageal SPTs have the worst prognosis. Therefore, identifying esophageal SPTs in HN cancer patients is of paramount importance for risk stratification and to guide the treatment strategy. IEE, especially using NBI endoscopy and LCE, improves the diagnostic performance in detecting early esophageal neoplasms. Several studies have demonstrated a high diagnostic yield of IEE to identify esophageal SPTs at an early stage in HN cancer patients, particularly in patients at high risk, such those with primary sites of the hypopharynx and larynx, alcoholism with flushing syndrome, older age, and advanced stage primary HN cancer. In addition, with minimally invasive endoscopic resection and radiotherapy techniques, HN cancer patients with early esophageal neoplasms can be managed without surgical interventions to allow for a better quality of life. However, there are currently no standardized surveillance protocols with regards to the interval and therapeutic options for primary HN cancers and esophageal SPTs. In terms of personalized medicine, the treatment strategy should be individualized and discussed by a multidisciplinary team involving gastroenterologists, endoscopists, oncologists, radiologists, and HN and chest surgeons. Most of the enrolled studies in this review were retrospective or case-control design and the results might be influenced by the bias upon independent literature review. More well-designed prospective studies are warranted to establish the most appropriate treatment and surveillance programs to improve overall outcomes for HN cancer patients with esophageal SPTs.
Author Contributions
Conceptualization, C-SC, L-JL and P-WS; Methodology, C-SC, L-JL and P-WS; Software, C-SC and L-JL; Validation, C-SC, L-JL, C-YW, W-CL, C-HH, T-HL, C-YL and P-WS; Formal analysis, C-SC and L-JL; Investigation, C-SC, L-JL, C-YW, W-CL, C-HH, T-HL, C-YL and P-WS; Resources, C-SC, L-JL, C-YW, W-CL, C-HH, T-HL, C-YL and P-WS; Data curation, C-SC, L-JL and P-WS; Writing—original draft preparation, C-SC; Writing—review and editing, C-SC, L-JL, D-YK and P-WS; Visualization, C-SC, L-JL, C-YW, W-CL, C-HH, T-HL, C-YL and P-WS; Supervision, C-SC, L-JL and P-WS; Project administration, C-SC; Funding acquisition, C-SC and P-WS. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. CP WILD, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer (2020). Available at: http://publications.iarc.fr/586.Licence.
2. Chen MC, Chen PT, Chan CH, Yang CT, Chen CC, Huan CE, et al. Second Primary Esophageal or Lung Cancer in Patients With Head and Neck Carcinoma in Taiwan: Incidence and Risk in Relation to Primary Index Tumor Site. J Cancer Res Clin Oncol (2011) 137(1):115–23. doi: 10.1007/s00432-010-0865-0
3. León X, Ferlito A, Myer CM 3rd, Saffiotti U, Shaha AR, Bradley PJ, et al. Second Primary Tumors in Head and Neck Cancer Patients. Acta Otolaryngol (2002) 122(7):765–78. doi: 10.1080/003655402/000028048
4. Slaughter DP, Southwick HW, Smejkal W. Field Cancerization in Oral Stratified Squamous Epithelium; Clinical Implications of Multicentric Origin. Cancer (1953) 6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q
5. Coyte A, Morrison DS, McLoone P. Second Primary Cancer Risk - the Impact of Applying Different Definitions of Multiple Primaries: Results From a Retrospective Population-Based Cancer Registry Study. BMC Cancer (2014) 14:272. doi: 10.1186/1471-2407-14-272
6. Chung CS, Lee YC, Wang CP, Ko JY, Wang WL, Wu MS, et al. Secondary Prevention of Esophageal Squamous Cell Carcinoma in Areas Where Smoking, Alcohol, and Betel Quid Chewing are Prevalent. J Formos Med Assoc (2010) 109(6):408–21. doi: 10.1016/S0929-6646(10)60072-1
7. Chung CS, Lee YC, Wu MS. Prevention Strategies for Esophageal Cancer: Perspectives of the East vs. West. Best Pract Res Clin Gastroenterol (2015) 29(6):869–83. doi: 10.1016/j.bpg.2015.09.010
8. Chung CS, Liao LJ, Lo WC, Chou YH, Chang YC, Lin YC, et al. Risk Factors for Second Primary Neoplasia of Esophagus in Newly Diagnosed Head and Neck Cancer Patients: A Case-Control Study. BMC Gastroenterol (2013) 13:154. doi: 10.1186/1471-230X-13-154
9. Hoxhaj I, Hysaj O, Vukovic V, Leoncini E, Amore R, Pastorino R, et al. Occurrence of Metachronous Second Primary Cancer in Head and Neck Cancer Survivors: A Systematic Review and Meta-Analysis of the Literature. Eur J Cancer Care (Engl) (2020) 29(5):e13255. doi: 10.1111/ecc.13255
10. van de Ven S, de Graaf W, Bugter O, Spaander MCW, Nikkessen S, de Jonge PJF, et al. Screening for Synchronous Esophageal Second Primary Tumors in Patients With Head and Neck Cancer. Dis Esophagus (2021) 11(10):doab037. doi: 10.1093/dote/doab037
11. Wang WL, Lee CT, Lee YC, Hwang TZ, Wang CC, Hwang JC, et al. Risk Factors for Developing Synchronous Esophageal Neoplasia in Patients With Head and Neck Cancer. Head Neck (2011) 33(1):77–81. doi: 10.1002/hed.21397
12. Wang YK, Chuang YS, Wu TS, Lee KW, Wu CW, Wang HC, et al. Endoscopic Screening for Synchronous Esophageal Neoplasia Among Patients With Incident Head and Neck Cancer: Prevalence, Risk Factors, and Outcomes. Int J Cancer (2017) 141(10):1987–96. doi: 10.1002/ijc.30911
13. Wang YW. 2018 Annual Report of Health Promotion Administration. Health Promotion Administration, Ministry of Health and Welfare. (2018).
14. Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA. 25 Year Survival Outcomes for Squamous Cell Carcinomas of the Head and Neck: Population-Based Outcomes From a Canadian Province. Oral Oncol (2014) 50(7):651–6. doi: 10.1016/j.oraloncology.2014.03.009
15. Leoncini E, Vukovic V, Cadoni G, Pastorino R, Arzani D, Bosetti C, et al. Clinical Features and Prognostic Factors in Patients With Head and Neck Cancer: Results From a Multicentric Study. Cancer Epidemiol (2015) 39(3):367–74. doi: 10.1016/j.canep.2015.02.004
16. Chung CS, Lo WC, Chen KC, Lin CL, Wen MH, Hsieh CH, et al. Clinical Benefits From Endoscopy Screening of Esophageal Second Primary Tumor for Head and Neck Cancer Patients: Analysis of a Hospital-Based Registry. Oral Oncol (2019) 96:27–33. doi: 10.1016/j.oraloncology.2019.06.038
17. Panosetti E, Luboinski B, Mamelle G, Richard JM. Multiple Synchronous and Metachronous Cancers of the Upper Aerodigestive Tract: A Nine-Year Study. Laryngoscope (1989) 99:1267–73. doi: 10.1288/00005537-198912000-00011
18. van de Ven S, Bugter O, Hardillo JA, Bruno MJ, Baatenburg de Jong RJ, Koch AD. Screening for Head and Neck Second Primary Tumors in Patients With Esophageal Squamous Cell Cancer: A Systematic Review and Meta-Analysis. United European Gastroenterol J (2019) 7(10):1304–11. doi: 10.1177/2050640619856459
19. Chung CS, Lee YC, Liou JM, Wang CP, Ko JY, Lee JM, et al. Tag Single Nucleotide Polymorphisms of Alcohol-Metabolizing Enzymes Modify the Risk of Upper Aerodigestive Tract Cancers: HapMap Database Analysis. Dis Esophagus (2014) 27(5):493–503. doi: 10.1111/j.1442-2050.2012.01437.x
20. Chien HT, Young CK, Chen TP, Liao CT, Wang HM, Cheng SD, et al. Alcohol-Metabolizing Enzymes’ Gene Polymorphisms and Susceptibility to Multiple Head and Neck Cancers. Cancer Prev Res (Phila) (2019) 12(4):247–54. doi: 10.1158/1940-6207.CAPR-18-0449
21. Lee CH, Lee JM, Wu DC, Hsu HK, Kao EL, Huang HL, et al. Independent and Combined Effects of Alcohol Intake, Tobacco Smoking and Betel Quid Chewing on the Risk of Esophageal Cancer in Taiwan. Int J Cancer (2005) 113(3):475–82. doi: 10.1002/ijc.20619
22. Vrabec DP. Multiple Primary Malignancies of the Upper Aerodigestive System. Ann Otol Rhinol Laryngol (1979) 88:846–54. doi: 10.1177/000348947908800620
23. Wagenfeld DJ, Harwood AR, Bryce DP, van Nostrand AW, DeBoer G. Second Primary Respiratory Tract Malignancies in Glottic Carcinoma. Cancer (1980) 46(8):1883–6. doi: 10.1002/1097-0142(19801015)46:8<1883::AID-CNCR2820460830>3.0.CO;2-G
24. Tepperman BS, Fitzpatrick PJ. Second Respiratory and Upper Digestive Tract Cancers After Oral Cancer. Lancet (1981) 2(8246):547–9. doi: 10.1016/S0140-6736(81)90938-7
25. McDonald S, Haie C, Rubin P, Nelson D, Divers LD. Second Malignant Tumors in Patients With Laryngeal Carcinoma: Diagnosis, Treatment, and Prevention. Int J Radiat Oncol Biol Phys (1989) 17(3):457–65. doi: 10.1016/0360-3016(89)90095-3
26. Larson JT, Adams GL, Fattah HA. Survival Statistics for Multiple Primaries in Head and Neck Cancer. Otolaryngol Head Neck Surg (1990) 103(1):14–24. doi: 10.1177/019459989010300103
27. Haughey BH, Gates GA, Arfken CA, Harvey J. Meta-Analysis of Second Malignant Tumors in Head and Neck Cancer: The Case for an Endoscopic Screening Protocol. Ann Otol Rhinol Laryngol (1992) 102:105–12. doi: 10.1177/000348949210100201
28. Boysen M, Loven JO. Second Malignant Neoplasms in Patients With Head and Neck Squamous Cell Carcinomas. Acta Oncol (1993) 32(3):283–8. doi: 10.3109/02841869309093596
29. Jovanovic A, van der Tol IG, Kostense PJ, Schulten EA, de Vries N, Snow GB, et al. Second Respiratory and Upper Digestive Tract Cancer Following Oral Squamous Cell Carcinoma. Eur J Cancer B Oral Oncol (1994) 30B(4):225–9. doi: 10.1016/0964-1955(94)90001-9
30. Dhooge IJ, De Vos M, Van Cauwenberge PB. Multiple Primary Malignant Tumors in Patients With Head and Neck Cancer: Results of a Prospective Study and Future Perspectives. Laryngoscope (1998) 108(2):250–6. doi: 10.1097/00005537-199802000-00017
31. Fujita M, Rudoltz MS, Canady DJ, Patel P, Machtay M, Pittard MQ, et al. Second Malignant Neoplasia in Patients With T1 Glottic Cancer Treated With Radiation. Laryngoscope (1998) 108(12):1853–5. doi: 10.1097/00005537-199812000-00016
32. León X, Quer M, Diez S, Orús C, López-Pousa A, Burgués J. Second Neoplasm in Patients With Head and Neck Cancer. Head Neck (1999) 21(3):204–10. doi: 10.1002/(SICI)1097-0347(199905)21:3<204::AID-HED4>3.0.CO;2-7
33. Nikolaou AC, Markou CD, Petridis DG, Daniilidis IC. Second Primary Neoplasms in Patients With Laryngeal Carcinoma. Laryngoscope (2000) 110(1):58–64. doi: 10.1097/00005537-200001000-00012
34. Rafferty MA, O’Dwyer TP. Secondary Primary Malignancies in Head and Neck Squamous Cell Carcinoma. J Laryngol Otol (2001) 115(12):988–91. doi: 10.1258/0022215011909567
35. Khuri FR, Kim ES, Lee JJ, Winn RJ, Benner SE, Lippman SM, et al. The Impact of Smoking Status, Disease Stage, and Index Tumor Site on Second Primary Tumor Incidence and Tumor Recurrence in the Head and Neck Retinoid Chemoprevention Trial. Cancer Epidemiol Biomarkers Prev (2001) 10(8):823–9. PMID: 11489748.
36. Ećimović P, Pompe-Kirn V. Second Primary Cancers in Laryngeal Cancer Patients in Slovenia, 1961-1996. Eur J Cancer (2002) 38(9):1254–60. doi: 10.1016/S0959-8049(02)00012-6
37. Dikshit RP, Boffetta P, Bouchardy C, Merletti F, Crosignani P, Cuchi T, et al. Risk Factors for the Development of Second Primary Tumors Among Men After Laryngeal and Hypopharyngeal Carcinoma. Cancer (2005) 103(11):2326–33. doi: 10.1002/cncr.21051
38. Lin K, Patel SG, Chu PY, Matsuo JMS, Singh B, Wong RJ, et al. Second Primary Malignancy of the Aerodigestive Tract in Patients Treated for Cancer of the Oral Cavity and Larynx. Head Neck (2005) 27(12):1042–8. doi: 10.1002/hed.20272
39. Strobel K, Haerle SK, Stoeckli SJ, Schrank M, Soyka JD, Veit-Haibach P, et al. Head and Neck Squamous Cell Carcinoma (HNSCC)–detection of Synchronous Primaries With (18)F-FDG-PET/Ct. Eur J Nucl Med Mol Imaging (2009) 36(6):919–27. doi: 10.1007/s00259-009-1064-6
40. Xu CC, Biron VL, Puttagunta L, Seikaly H. HPV Status and Second Primary Tumours in Oropharyngeal Squamous Cell Carcinoma. J Otolaryngol Head Neck Surg (2013) 42(1):36. doi: 10.1186/1916-0216-42-36
41. Liao CT, Wallace CG, Lee LY, Hsueh C, Lin CY, Fan KH, et al. Clinical Evidence of Field Cancerization in Patients With Oral Cavity Cancer in a Betel Quid Chewing Area. Oral Oncol (2014) 50(8):721–31. doi: 10.1016/j.oraloncology.2014.04.010
42. Liao CT, Fan KH, Kang CJ, Lin CY, Chang JTC, Tsang NM, et al. Clinical Outcomes of Patients With Resected Oral Cavity Cancer and Simultaneous Second Primary Malignancies. PLoS One (2015) 10(9):e0136918. doi: 10.1371/journal.pone.0136918
43. González-Botas JH, Vázquez PV, Barro CV. Second Primary Tumours in Head and Neck Cancer. Acta Otorrinolaringol Esp (2016) 67(3):123–9. doi: 10.1016/j.otoeng.2016.04.005
44. Min SK, Choi SW, Lim J, Park JY, Jung KW, Won YJ. Second Primary Cancers in Patients With Oral Cavity Cancer Included in the Korea Central Cancer Registry. Oral Oncol (2019) 95:16–28. doi: 10.1016/j.oraloncology.2019.05.025
45. Bertolini F, Trudu L, Banchelli F, Schipilliti F, Napolitano M, Alberici MP, et al. Second Primary Tumors in Head and Neck Cancer Patients: The Importance of a “Tailored” Surveillance. Oral Dis (2021) 27(6):1412–20. doi: 10.1111/odi.13681
46. Milliet F, Bozec A, Schiappa R, Viotti J, Modesto A, Dassonville O, et al. Metachronous Second Primary Neoplasia in Oropharyngeal Cancer Patients: Impact of Tumor HPV Status. A GETTEC Multicentric Study. Oral Oncol (2021) 122:105503. doi: 10.1016/j.oraloncology.2021.105503
47. Bugter O, van Iwaarden DLP, van Leeuwen N, Nieboer D, Dronkers EAC, Hardillo JAU, et al. A Cause-Specific Cox Model for Second Primary Tumors in Patients With Head and Neck Cancer: A RONCDOC Study. Head Neck (2021) 43(6):1881–9. doi: 10.1002/hed.26666
48. Luo X, Huang X, Liu S, Wang X, Luo J, Xiao J, et al. Evaluation of the Prevalence of Metachronous Second Primary Malignancies in Hypopharyngeal Carcinoma and Their Effect on Outcomes. Cancer Med (2022) 11(4):1059–67. doi: 10.1002/cam4.4501
49. Asge Technology Committee, Song LMWK, Adler DG, Conway JD, Diehl DL, Farraye FA, et al. Narrow Band Imaging and Multiband Imaging. Gastrointest Endosc (2008) 67(4):581–9. doi: 10.1016/j.gie.2008.01.013
50. Boakye EA, Buchanan P, Hinyard L, Osazuwa-Peters N, Schootman M, Piccirillo JF. Incidence and Risk of Second Primary Malignant Neoplasm After a First Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg (2018) 144(8):727–37. doi: 10.1001/jamaoto.2018.0993
51. Mroueh R, Nevala A, Haapaniemi A, Pitkäniemi J, Salo T, Mäkitie AA. Risk of Second Primary Cancer in Oral Squamous Cell Carcinoma. Head Neck (2020) 42(8):1848–58. doi: 10.1002/hed.26107
52. Jégu J, Binder-Foucard F, Borel C, Velten M. Trends Over Three Decades of the Risk of Second Primary Cancer Among Patients With Head and Neck Cancer. Oral Oncol (2013) 49(1):9–14. doi: 10.1016/j.oraloncology.2012.06.018
53. Jain KS, Sikora AG, Baxi SS, Morris LGT. Synchronous Cancers in Patients With Head and Neck Cancer: Risks in the Era of Human Papillomavirus-Associated Oropharyngeal Cancer. Cancer (2013) 119(10):1832–7. doi: 10.1002/cncr.27988
54. Coca-Pelaz A, Rodrigo JP, Suárez C, Nixon IJ, Makitie A, Sanabria A, et al. The Risk of Second Primary Tumors in Head and Neck Cancer: A Systematic Review. Head Neck (2020) 42(3):456–66. doi: 10.1002/hed.26016
55. Yamashita T, Araki K, Tomifuji M, Tanaka Y, Harada E, Suzuki T, et al. Clinical Features and Treatment Outcomes of Japanese Head and Neck Cancer Patients With a Second Primary Cancer. Asia Pac J Clin Oncol (2017) 13(3):172–8. doi: 10.1111/ajco.12599
56. Morris LGT, Sikora AG, Patel SG, Hayes PB, Ganly I. Second Primary Cancers After an Index Head and Neck Cancer: Subsite-Specific Trends in the Era of Human Papillomavirus-Associated Oropharyngeal Cancer. J Clin Oncol (2011) 29(6):739–46. doi: 10.1200/JCO.2010.31.8311
57. Wang CC, Chen ML, Hsu KH, Lee SP, Chen TC, Chang YS, et al. Second Malignant Tumors in Patients With Nasopharyngeal Carcinoma and Their Association With Epstein-Barr Virus. Int J Cancer (2000) 87(2):228–31. doi: 10.1002/1097-0215(20000715)87:2<228::AID-IJC12>3.0.CO;2-T
58. Scélo G, Boffetta P, Corbex M, Chia KS, Hemminki K, Friis S, et al. Second Primary Cancers in Patients With Nasopharyngeal Carcinoma: A Pooled Analysis of 13 Cancer Registries. Cancer Causes Control (2007) 18(3):269–78. doi: 10.1007/s10552-006-0101-z
59. Ooft ML, van Ipenburg J, Braunius WW, Stegeman I, Wegner I, de Bree R, et al. A Nation-Wide Epidemiological Study on the Risk of Developing Second Malignancies in Patients With Different Histological Subtypes of Nasopharyngeal Carcinoma. Oral Oncol (2016) 56:40–6. doi: 10.1016/j.oraloncology.2016.02.009
60. Chow JCH, Tam AHP, Cheung KM, Lee VHF, Chiang CL, Tong M, et al. Second Primary Cancer After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Territory-Wide Study by HKNPCSG. Oral Oncol (2020) 111:105012. doi: 10.1016/j.oraloncology.2020.105012
61. Valentin A, Goetz M, Hetzel J, Reinert S, Hoefert S. Routine Panendoscopy in Oral Squamous Cell Cancer Patients: Mandatory or Facultative? Clin Oral Investig (2021) 25(3):1245–54. doi: 10.1007/s00784-020-03429-8
62. Onochi K, Shiga H, Takahashi S, Watanabe N, Fukuda S, Ishioka M, et al. Risk Factors Linking Esophageal Squamous Cell Carcinoma With Head and Neck Cancer or Gastric Cancer. J Clin Gastroenterol (2019) 53(4):e164–70. doi: 10.1097/MCG.0000000000001019
63. Kato M, Ishihara R, Hamada K, Tonai Y, Yamasaki Y, Matsuura N, et al. Endoscopic Surveillance of Head and Neck Cancer in Patients With Esophageal Squamous Cell Carcinoma. Endosc Int Open (2016) 4(7):E752–755. doi: 10.1055/s-0042-106720
64. Wang X, Mauer EA, Christos P, Manzerova J, Wernicke AG, Parashar B. First Clinical Report on Comparative Treatment and Survival Outcomes in Second Cancers After Primary Head and Neck Cancer: A Population-Based Study. Cureus (2017) 9(5):e1284. doi: 10.7759/cureus.1284
65. Liao LJ, Chou HW, Wang CT, Chung CS, Lai MS. The Impact of Second Primary Malignancies on Head and Neck Cancer Survivors: A Nationwide Cohort Study. PLoS One (2013) 8(4):e62116. doi: 10.1371/journal.pone.0062116
66. Chung CS, Lo WC, Lee YC, Wu MS, Wang HP, Liao LJ. Image-Enhanced Endoscopy for Detection of Second Primary Neoplasm in Patients With Esophageal and Head and Neck Cancer: A Systematic Review and Meta-Analysis. Head Neck (2016) 38 Suppl 1:E2343–2349. doi: 10.1002/hed.24277
67. Chung CS, Wang HP. Screening for Precancerous Lesions of Upper Gastrointestinal Tract: From the Endoscopists’ Viewpoint. Gastroenterol Res Pract (2013) 2013:681439. doi: 10.1155/2013/681439
68. Shimizu Y, Omori T, Yokoyama A, Yoshida T, Hirota J, Ono Y, et al. Endoscopic Diagnosis of Early Squamous Neoplasia of the Esophagus With Iodine Staining: High-Grade Intra-Epithelial Neoplasia Turns Pink Within a Few Minutes. J Gastroenterol Hepatol (2008) 23(4):546–50. doi: 10.1111/j.1440-1746.2007.04990.x
69. Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, et al. Prediction of the Invasion Depth of Superficial Squamous Cell Carcinoma Based on Microvessel Morphology: Magnifying Endoscopic Classification of the Japan Esophageal Society. Esophagus (2017) 14(2):105–12. doi: 10.1007/s10388-016-0527-7
70. Chisholm EM, Williams SR, Leung JW, Chung SC, van Hasselt CA, Li AK. Lugol’s Iodine Dye-Enhanced Endoscopy in Patients With Cancer of the Oesophagus and Head and Neck. Eur J Surg Oncol (1992) 18(6):550–2.
71. Su HA, Hsiao SW, Hsu YC, Wang LY, Yen HH. Superiority of NBI Endoscopy to PET/CT Scan in Detecting Esophageal Cancer Among Head and Neck Cancer Patients: A Retrospective Cohort Analysis. BMC Cancer (2020) 20(1):69. doi: 10.1186/s12885-020-6558-4
72. Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, et al. Endoscopic Screening of Early Esophageal Cancer With the Lugol Dye Method in Patients With Head and Neck Cancers. Cancer (1990) 66(10):2068–71. doi: 10.1002/1097-0142(19901115)66:10<2068::AID-CNCR2820661005>3.0.CO;2-W
73. Tincani AJ, Brandalise N, Altemani A, Scanavini RC, Valério JB, Lage HT, et al. Diagnosis of Superficial Esophageal Cancer and Dysplasia Using Endoscopic Screening With a 2% Lugol Dye Solution in Patients With Head and Neck Cancer. Head Neck (2000) 22(2):170–4. doi: 10.1002/(sici)1097-0347(200003)22:2<170::aid-hed9>3.0.co;2-7
74. Lee CT, Chang CY, Lee YC, Tai CM, Wang WL, Tseng HP, et al. Narrow-Band Imaging With Magnifying Endoscopy for the Screening of Esophageal Cancer in Patients With Primary Head and Neck Cancers. Endoscopy (2010) 42(8):613–9. doi: 10.1055/s-0030-1255514
75. Carvalho R, Areia M, Brito D, Saraiva S, Alves S, Cadime AT. Diagnostic Accuracy of Lugol Chromoendoscopy in the Oesophagus in Patients With Head and Neck Cancer. Rev Esp Enferm Dig (2013) 105(2):79–83. doi: 10.4321/s1130-01082013000200004
76. Arantes V, Albuquerque W, Salles JMP, Dias CAF, Alberti LR, Kahaleh M, et al. Effectiveness of Unsedated Transnasal Endoscopy With White-Light, Flexible Spectral Imaging Color Enhancement, and Lugol Staining for Esophageal Cancer Screening in High-Risk Patients. J Clin Gastroenterol (2013) 47(4):314–21. doi: 10.1097/MCG.0b013e3182617fc1
77. Laohawiriyakamol S, Sunpaweravong S, Leelamanit V, Pruegsanusak K, Sinkijcharoenchai W. Evaluating Synchronous Esophageal Cancer in Head and Neck Cancer Patients Using Lugol Dye Chromoendoscopy. J Med Assoc Thai (2014) 97(11):1164–70. PMID: 25675681.
78. Gong EJ, Kim DH, Ahn JY, Choi KS, Jung KW, Lee JH, et al. Routine Endoscopic Screening for Synchronous Esophageal Neoplasm in Patients With Head and Neck Squamous Cell Carcinoma: A Prospective Study. Dis Esophagus (2016) 29(7):752–29. doi: 10.1111/dote.12404
79. Matsui T, Okada T, Kawada K, Okuda M, Ogo T, Nakajima Y, et al. Detection of Second Primary Malignancies of the Esophagus and Hypophraynx in Oral Squamous Cell Carcinoma Patients. Laryngoscope Investig Otolaryngol (2018) 3(4):263–7. doi: 10.1002/lio2.179
80. Druesne-Pecollo N, Keita Y, Touvier M, Chan DSM, Norat T, Hercberg S, et al. Alcohol Drinking and Second Primary Cancer Risk in Patients With Upper Aerodigestive Tract Cancers: A Systematic Review and Meta-Analysis of Observational Studies. Cancer Epidemiol Biomarkers Prev (2014) 23(2):324–31. doi: 10.1158/1055-9965.EPI-13-0779
81. Vaca CE, Fang JL, Schweda EK. Studies of the Reaction of Acetaldehyde With Deoxynucleosides. Chem Biol Interact (1995) 98(1):51–67. doi: 10.1016/0009-2797(95)03632-V
82. Harada H, Shinohara S, Takebayashi S, Kikuchi M, Fujiwara K, Michida T, et al. Facial Flushing After Alcohol Intake as a Predictor for a High Risk of Synchronous or Metachronous Cancer of the Upper Gastrointestinal Tract. Jpn J Clin Oncol (2017) 47(12):1123–8. doi: 10.1093/jjco/hyx150
83. Hoxhaj I, Vukovic V, Boccia S, Pastorino R. Single Nucleotide Polymorphisms and the Risk of Developing a Second Primary Cancer Among Head and Neck Cancer Patients: A Systematic Literature Review and Meta-Analysis. BMC Cancer (2021) 21(1):660. doi: 10.1186/s12885-021-08335-0
84. Kaneko K, Katagiri A, Konishi K, Kurahashi T, Ito H, Kumekawa Y, Yamamoto T, et al. Study of P53 Gene Alteration as a Biomarker to Evaluate the Malignant Risk of Lugol-Unstained Lesion With non-Dysplasia in the Oesophagus. Br J Cancer (2007) 96(3):492–8. doi: 10.1038/sj.bjc.6603582
85. Wang GQ, Abnet CC, Shen Q, Lewin KL, Sun SD, Roth MJ, et al. Histological Precursors of Oesophageal Squamous Cell Carcinoma: Results From a 13 Year Prospective Follow Up Study in a High Risk Population. Gut (2005) 54(2):187–92. doi: 10.1136/gut.2004.046631
86. Aadam AA, Abe S. Endoscopic Submucosal Dissection for Superficial Esophageal Cancer. Dis Esophagus (2018) 31(7). doi: 10.1093/dote/doy021
87. Wang WL, Chang IW, Chen CC, Chang CY, Mo LR, Lin JT, et al. Radiofrequency Ablation Versus Endoscopic Submucosal Dissection in Treating Large Early Esophageal Squamous Cell Neoplasia. Medicine (Baltimore) (2015) 94(49):e2240. doi: 10.1097/MD.0000000000002240
88. Lei S, Shrestha SM, Shi R. Radiofrequency Ablation for Early Superficial Flat Esophageal Squamous Cell Neoplasia: A Comprehensive Review. Gastroenterol Res Pract (2020) 2020:4152453. doi: 10.1155/2020/4152453
89. Nagami Y, Ominami M, Shiba M, Minamino H, Fukunaga S, Kameda N, et al. The Five-Year Survival Rate After Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Cell Neoplasia. Dig Liver Dis (2017) 49(4):427–33. doi: 10.1016/j.dld.2016.12.009
90. Oda I, Shimizu Y, Yoshio T, Katada C, Yokoyama T, Yano T, et al. Long-Term Outcome of Endoscopic Resection for Intramucosal Esophageal Squamous Cell Cancer: A Secondary Analysis of the Japan Esophageal Cohort Study. Endoscopy (2020) 52(11):967–75. doi: 10.1055/a-1185-9329
91. Das A, Singh V, Fleischer DE, Sharma VK. A Comparison of Endoscopic Treatment and Surgery in Early Esophageal Cancer: An Analysis of Surveillance Epidemiology and End Results Data. Am J Gastroenterol (2008) 103(6):1340–5. doi: 10.1111/j.1572-0241.2008.01889.x
92. Chung CS, Lo WC, Wen MH, Hsieh CH, Lin YC, Liao LJ. Long Term Outcome of Routine Image-Enhanced Endoscopy in Newly Diagnosed Head and Neck Cancer: A Prospective Study of 145 Patients. Sci Rep (2016) 6:29573. doi: 10.1038/srep29573
Keywords: head neck cancer, esophageal cancer, second primary tumor, cancer screening, image-enhanced endoscopy, narrow-band imaging, Lugol’s chromoendoscopy
Citation: Chung C-S, Liao L-J, Wu C-Y, Lo W-C, Hsieh C-H, Lee T-H, Liu C-Y, Kuo D-Y and Shueng P-W (2022) Endoscopic Screening for Second Primary Tumors of the Esophagus Among Head and Neck Cancer Patients. Front. Oncol. 12:906125. doi: 10.3389/fonc.2022.906125
Received: 28 March 2022; Accepted: 11 May 2022;
Published: 07 June 2022.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Wei-Kuo Chang, Tri-Service General Hospital, TaiwanYu-Hsuan Kuo, Chi Mei Medical CenterTaiwan
Copyright © 2022 Chung, Liao, Wu, Lo, Hsieh, Lee, Liu, Kuo and Shueng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Wei Shueng, shuengsir@gmail.com
†These authors have contributed equally to this work
 Chen-Shuan Chung1,2,3†
Chen-Shuan Chung1,2,3† Chia-Yun Wu
Chia-Yun Wu Wu-Chia Lo
Wu-Chia Lo Chen-Hsi Hsieh
Chen-Hsi Hsieh Chao-Yu Liu
Chao-Yu Liu Pei-Wei Shueng
Pei-Wei Shueng